Abstract
Colorectal cancer (CRC), a multifactorial disease, is usually induced and developed through complex mechanisms, including impact of diet and lifestyle, genomic abnormalities, change of signaling pathways, inflammatory response, oxidation stress, dysbiosis, and so on. As natural polyphenolic phytochemicals that exist primarily in tea, tea polyphenols (TPs) have been shown to have many clinical applications, especially as anticancer agents. Most animal studies and epidemiological studies have demonstrated that TPs can prevent and treat CRC. TPs can inhibit the growth and metastasis of CRC by exerting the anti-inflammatory, anti-oxidative or pro-oxidative, and pro-apoptotic effects, which are achieved by modulations at multiple levels. Many experiments have demonstrated that TPs can modulate several signaling pathways in cancer cells, including the mitogen-activated protein kinase pathway, phosphatidylinositol-3 kinase/Akt pathway, Wnt/β-catenin pathway, and 67 kDa laminin receptor pathway, to inhibit proliferation and promote cell apoptosis. In addition, novel studies have also suggested that TPs can prevent the growth and metastasis of CRC by modulating the composition of gut microbiota to improve immune system and decrease inflammatory responses. Molecular pathological epidemiology, a novel multidisciplinary investigation, has made great progress on CRC, and the further molecular pathological epidemiology research should be developed in the field of TPs and CRC. This review summarizes the existing in vitro and in vivo animal and human studies and potential mechanisms to examine the effects of tea polyphenols on CRC.
Keywords: Tea polyphenols, Colorectal cancer, Gut microbiota, Signal pathway, Anti-inflammation, Mechanisms
Core tip: Colorectal cancer (CRC) has become the third most common cancer and the fourth most common cause of cancer-related death, which is involved in a series of complex mechanisms. Professors pay attention to searching for reasonable methods to prevent and treat CRC. Tea polyphenols, as natural polyphenolic phytochemicals in tea, have been demonstrated to prevent and treat CRC effectively. They may become a novel medicine applied in CRC to prevent cancer and reduce the side effects of chemotherapy medicines in the future. This review summarizes the molecular mechanism of tea polyphenols acting on CRC.
INTRODUCTION
Colorectal cancer
Colorectal cancer (CRC) has become the third most common cancer and the fourth most common cause of cancer-related death[1]. In the past several decades, the mortality rates and the incidence of CRC have declined, and death count has maintained a long-standing decrease from a peak of 57644 in 1995 to 51651 in 2014[2]. However, for adults aged < 50 years, evidence has shown that the CRC incidence rate has risen by 1.6% from 2000 to 2013, and mortality has increased by 13% from 2000 to 2014[2]. Usually, the outcome of CRC depends on the phase at diagnosis[3]. Therefore, early detection and strong protective measures can effectively decrease the mortality and morbidity of CRC[4]. The occurrence of CRC is related to many factors, including inflammatory bowel disease, dietary factors, and lifestyle[5,6]. Furthermore, genomic mutation and an imbalance in gut microbiota are also viewed as significant pathogenic factors that can induce tumorigenesis[1,3].
Molecular mechanisms of CRC
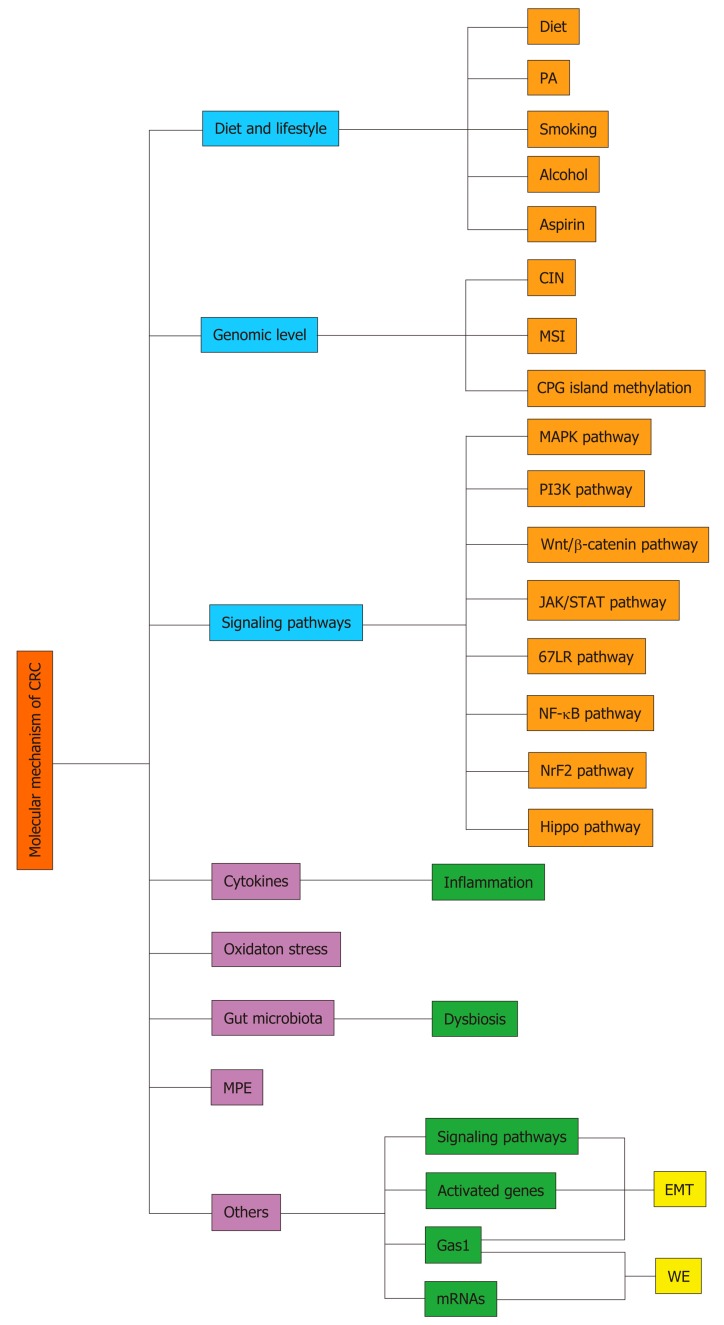
Several mechanisms, including those summarized below, have been related to the onset and metastasis of CRC (Figure 1).
Figure 1.
The general molecular mechanisms of colorectal cancer. CRC: Colorectal cancer; MPE: Molecular pathological epidemiology; PA: Physical activity; CIN: Chromosomal instability; 67LR: 67 kDa laminin receptor; MSI: Microsatellite instability; EMT: Epithelial-to-mesenchymal transition; WE: Warburg effect.
Diet and lifestyle: CRC is generally reported as diet- and lifestyle-related pathology and is associated with several main factors: Diet, physical activity, consumption of alcohol, cigarettes and aspirin[7].
Diet: Findings from a systematic review have demonstrated that various foods are associated with CRC, positively or negatively[8]. In general, the higher or lower risk of CRC is related to the proinflammatory or anti-inflammatory property of the food, respectively[9]. Moreover, different foods can exert the function via different mechanisms. We will take some of these foods as examples to explain the mechanism that they act on CRC, briefly.
(1) Red and processed meats: There are several systematic reviews and epidemiological studies indicating that intake of red and processed meats will increase the risk of CRC[10-12]. Besides, a study demonstrated that the consumption of red and processed meats was associated more strongly with an increased risk of CRC with KRAS-wildtype, indicating that the potential mechanism should be studied[12]. Intake of red and processed meats at high temperatures results in the formation of heterocyclic amines and polycyclic aromatic hydrocarbons, and then allows the formation of DNA adducts that subsequently cause DNA damage to promote tumorigenesis[13,14]. In red meat, heme is present in high concentrations in the form of myoglobin and a large amount of heme iron intake has been associated with a higher risk of CRC[14]. Heme iron from red and processed meats can stimulate the metabolism of nitrate/nitrite and the formation of N-nitroso compounds, and induce oxidative stress and lipid peroxidation (LPO) to trigger inflammatory response, and thereby promote the development of CRC[14,15].
(2) Dietary fats: Dietary fats are also associated with CRC. A high intake of ω-6 polyunsaturated fatty acid (PUFA) and saturated fat has tumor-enhancing effects[16]. Rapid metabolism of arachidonic acid (AA), increased activities of phospholipases, and elevated levels of cyclooxygenase (COX) and lipoxygenase (LPO) may suggest the potential mechanism of fatty acid promoting the incident of CRC[17]. However, ω-3 PUFA intake can reduce the risk of CRC, particularly with microsatellite instability (MSI)-high cancer subtype or high FOXP3+ regulatory T cell (Treg cell) counts[18,19]. ω-3 PUFA exerts the effect of anticancer through several potential molecular mechanisms, including suppression of AA-derived eicosanoid biosynthesis, impact on transcription factor activity, gene expression, and signal transduction pathways, increased or decreased production of free radicals and reactive oxygen species (ROS), and so on[20]. In addition, marine ω-3 PUFA also protects against CRC through inhibition of the T cell-suppressive activity of Treg cells[19]. In addition, oleic acid, the main monounsaturated fatty acid in olive oil also exerts a protective effect on CRC[21,22]. A competitive inhibition by oleic acid of the ∆6-desaturase will suppress the eicosanoid biosynthesis of AA to disrupt the tumor growth progress[21].
(3) Vitamin D: Vitamin D can inhibit the development of CRC, particularly of some specific subtypes of CRC. The beneficial survival association of high vitamin D level is stronger for CRC with lower-level peritumoral lymphocytic reaction than for carcinoma with higher-level reaction[23]. Vitamin D is hydroxylated in the liver to produce 25(OH)D that serves as a standard indicator of vitamin D activity[23]. And, then, 25(OH)D is hydroxylated further in the kidneys to produce a hormonally active metabolite, 1,25-dihydroxyvitamin D[23]. Vitamin D and its metabolites exert their antineoplastic effect by binding with the transcription factor vitamin D receptor[24]. Vitamin D may suppress signaling pathways and cytokines and modulate adaptive immune cells, such as B cells, helper T cells (Th cells) and Treg cells[24]. Moreover, vitamin D diet can also cause significant changes in the fecal microbial community structure. During the development of CRC, vitamin D deficiency can not only cause a sharp decrease in Akkermansia muciniphila but also induce changes in the expression of mucus and goblet-cell associated genes, so that the gut barrier integrity is destroyed[25].
(4) Dietary fiber: A high intake of dietary fiber, particularly derived from vegetables and fruit, was inversely associated with CRC risk[26,27]. This association was driven mainly by the position of the tumor, which was stronger for the risk of rectal cancer[28]. However, a new study also indicated that the relationship of fiber and risk of CRC is independent of tumor subsite or molecular marker[29]. In addition, higher intake of dietary fiber was more strongly associated with lower risk for Fusobacterium nucleatum (F. nucleatum)-positive CRC, but not F. nucleatum-negative CRC[30]. The findings suggest a potential role for intestinal microbiota in mediating the association between fiber and CRC[30]. Fiber can be fermented by the gut bacteria into short-chain fatty acids, such as butyrate, acetate, and propionate, that possess a diversity of tumorsuppressive effects[31]. High level of short-chain fatty acids produced from fiber might alter pH, increase transit time of gut contents, and lead to differences in local immune surveillance, and thereby reduce the growth of harmful species, such as F. nucleatum[30]. In addition, the potential mechanism underlying fiber inhibiting the development of CRC also contains other following aspects: increasing the stool bulk; shortening the bowel transit time; diluting the effect of potential carcinogens; and altering bile acid metabolism[26,32].
(5) Selenium (Se): Epidemiological investigation has demonstrated that higher Se levels were inversely associated with the risk to develop CRC in Europeans[33]. Usually, dietary Se intake is essential for synthesizing selenoproteins that are important in inhibiting oxidative and inflammatory processes linked to colorectal carcinogenesis[34]. Se supply might play an important role in regulating expression of some selenoproteins, such as glutathione peroxidases (i.e., GPX1), selenoprotein F (SELENOF), selenoprotein P (SELENOP), selenoprotein K (SELENOK), and components of the thioredoxin reductase system (TXNRD1-3), to reduce the oxidative stress and inflammatory response[34,35]. However, some studies also suggested that the selenoprotein expression may affect CRC development independent of the Se status, even leading to the development of CRC with suboptimal Se status[34,35]. In addition, a study also indicated that intake of Se nanoparticles can activate autophagy to promote cancer cell death, through up-regulation of beclin 1-related signaling pathways[36].
(6) Folic acid: Accumulating evidence displays that folic acid can also be an effective chemopreventive agent for CRC[37,38]. Supplemental folic acid has been shown to prevent the loss of heterozygosity of the tumor suppressor gene that is deleted in CRC and to stabilize its protein in normal appearing rectal mucosa of patients with colorectal adenomas[38]. In addition, deficiency folic acid may lead to inadequate purine and pyrimidine synthesis and changes in methylation, with a concomitant impact on DNA replication and cell division due to the disruption of folate cycle[39]. Thus, deficiency of folic acid can promote epidermal growth factor receptor (EGFR) expression through reducing methylation of CpG sequences within its promoter[38].
And (7) Others: Other foods are also associated with CRC positively or negatively. High sugar foods and spicy foods might have a positive association with CRC risk; however, vegetables, soy bean/soy products, seafood, and vitamins C, E and B12 play a protective role against CRC risk[6-8]. These foods exert the promotive or protective effect on CRC through modulating the inflammatory response, insulin resistance, and the composition of gut microbiota mainly[40,41].
Physical activity: Physical inactivity has also been well demonstrated as a lifestyle risk factor for CRC. There are many epidemiological studies indicating that physical activity (PA) is associated with a statistically significant reduction in CRC risk[42-45]. Moreover, the association may depend on the location of tumor and gender[42-45]. Some meta-analysis and systematic reviews indicated that PA is associated with reduced risk of both proximal colon and distal colon cancer, but the difference was observed between the colon and the rectum[42,43]. There is even no association observed between PA and rectal cancer[43]. And, gender is another factor to impact the relationship between the PA and CRC. A systematic review has observed an apparent interaction between sex and PA in relation to CRC risk, that being a statistically significant reduced risk among men but statistically nonsignificant reduced risk among women[45]. In addition, there is a potential interactive effect of PA and sedentary time on CRC risk. The benefits of moderate to strenuous-vigorous PA on CRC risk are observed most clearly among those with more sedentary time because these individuals have lower total activity[45]. Several plausible biological mechanisms have been proposed, including changes in endogenous sexual and metabolic hormone levels and growth factors, decreased obesity and central adiposity, and possibly changes in immune function and so on[46]. Peroxisome proliferator-activated receptor gamma coactivator 1α (PGC-1α) is a mitochondrial regulator in a wide variety of biological processes, such as thermogenesis, circadian rhythm, fatty acid oxidation, glucose metabolism, mitochondrial organization, and biogenesis[46]. PA, as a stressor that demands energy, stimulates PGC-1α expression, increasing biological processes of CRC and suppressing the development of CRC[47].
Consumption of alcohol: High consumption of alcoholic beverages may lead to an increasing risk of CRC[48,49]. Consumption of alcohol is also relative to molecular subtypes of CRC. Alcohol intake was positively related to risk of BRAF-tumors but not to risk of BRAF-positive tumors, irrespective of their KRAS status[50]. Similarly, a study has demonstrated that higher alcohol consumption was associated with risk of CRC with insulin-like growth factor 2 (IGF2) differentially methylated region-0 (DMR0) hypomethylation but not risk of cancer with high-level IGF2 DMR0 methylation[51]. IGF2 up-regulation by DMR0 hypomethylation caused by alcohol may promote tumorigenesis in colorectal tissue[51]. Alcohol can also interfere with one-carbon metabolism, a complex network of interrelated biochemical reactions that involve the transfer of one-carbon (methyl) groups from one compound to another[52]. Excess alcohol can antagonize methyl donors, including vitamin B6, vitamin B12, methionine, and folate, leading to a lower concentration of S-adenosylmethionine in the liver, and thereby cause abnormal DNA methylation[51]. Thus, alcohol can impair the bioavailability of dietary folate as well as folate-dependent intermediary metabolisms to cause carcinogenesis[52]. Besides, monocyte chemoattractant protein-1 (MCP-1) is a chemokine that plays an important role in regulating tumor microenvironment and metastasis[53]. Alcohol can increase the expression of MCP-1 and its receptor CCR2 at both protein and mRNA levels[53]. The study demonstrated that alcohol may promote the metastasis of CRC through modulating the glycogen synthase kinase 3β (GSK3β)/β-catenin/MCP-1 pathway[53].
Cigarette: Cigarette smoke is considered as a risk factor for CRC. A study found that individuals with heavy, long-term cigarette smoke exposure were significantly younger at the time of CRC diagnosis compared to lifelong never-smokers[54]. And, smoking is also correlative to some specific subtypes of CRC, such as MSI-high, CpG island methylator phenotype (CIMP)-positive, and BRAF mutation-positive subtypes[55]. This finding from the study also indicated that epigenetic modification may be involved in smoking-related carcinogenesis[55]. In general, heterocyclic aromatic amines and polycyclic aromatic hydrocarbons may play an important role in CRC associated with smoking[56,57]. N-Acetyltransferases 1 and 2 (NAT1 and NAT2) are also considered to participate in the metabolism of aromatic and heterocyclic aromatic amines[56]. Glutathione S-transferases (GSTs), particularly GSTM1, GSTT1 and GSTP1, are detoxification enzymes that have been known to metabolize a wide range of carcinogens from cigarette smoke, such as heterocyclic aromatic amines and polycyclic aromatic hydrocarbons[57]. Thus, NAT1 and NAT2, and GSTs gene polymorphisms may be involved in cigarette smoking-CRC risk[56,57]. A study demonstrated that individuals with fast acetylation capacity achieved by NAT1 and NAT2, may more efficiently activate heterocyclic aromatic amines, thereby increasing the induction of DNA damage and, consequently, increasing susceptibility to CRC[56]. Besides, GST gene polymorphisms influence interindividual susceptibility to smoking-associated CRC, which can play an important role in the detoxification of colorectal carcinogenesis during smoking[57]. A novel opinion is that smoking may increase cancer cell survival and induce some events associated to epithelial-to-mesenchymal transition (EMT) process[58]. Smoking may reduce cell necrosis, deregulate Claudin-1 and E-cadherin expression and enhance the expression of miR-21 to induce EMT[58].
Aspirin: Abundant evidence indicates that regular use of aspirin is associated with a significant reduction in the incidence of CRC[59-62]. Not only that, the beneficial function of aspirin may be emphasized in some specific molecular subtypes of CRC. Several studies have indicated that regular use of aspirin is associated with better prognosis and clinical outcome in COX-2-positive and PIK3CA-mutated CRC[61,62]. Aspirin might inhibit the expression of COX-2 to reduce the prostaglandin (PG)E2 synthesis, and thereby to reduce the inflammatory response and suppress cancer cell proliferation and survival[59,61]. As to the status of PIK3CA mutation, pho-sphatidylinositol-3 kinase (PI3K) and the downstream Akt pathway can be activated to enhance COX-2 activity and PGE2 synthesis, resulting in inhibition of apoptosis in CRC cells[61]. Therefore, aspirin can attenuate PI3K activity through inhibiting PGE2 signaling[59,61,63]. Meanwhile, aspirin might inhibit mTOR, a downstream effector of the PI3K pathway, by activation of adenosine monophosphate-activated protein kinase (AMPK) in CRC[61]. In addition, aspirin may also inhibit Wnt signaling either directly or through down-regulation of PGE2 to suppress the onset of CRC[63].
Genomic level: Genomic instability is an essential feature that underlies CRC. There are three aspects to achieving genomic instability that can contribute to CRC: Chromosomal instability, MSI, and CpG island methylation[1]. First, chromosomal instability, a common and efficient mechanism, can lead to the physical loss of tumor suppressor genes, such as adenomatous polyposis coli (APC), P53, and SMAD family member 4, and the activation of oncogenes, such as KRAS and PI3KCA[64,65]. These changes can transform the normal phenotype into a malignant phenotype[1,64,65]. Second, MSI can silence mismatch repair genes, such as MLH1, in patients with hereditary nonpolyposis colon cancer, who then have an even higher risk of developing CRC[65]. Finally, the aberrant methylation of CpG islands has been demonstrated to result in the CIMP or CIMP-high, which accounts for 15% of CRC cases and exists in nearly all CRC tumors with aberrant methylation of MLH1[64].
In addition to genomic mutation, microRNAs (miRNAs) and long noncoding RNAs (commonly referred to as lncRNAs) are also expressed abnormally in CRC. Existing evidence indicates that miRNAs, such as miR-93 and miR-328, are aberrantly expressed in CRC and regulate the proliferation and metastasis of cancer stem cells[66,67]. MiR-200c can promote the EMT to induce proliferation and metastasis[68]. Moreover, the up-regulation of a novel lncRNA, colorectal neoplasia differentially expressed (CRNDE), has been observed in the early stages of colorectal neoplasia (> 90%), except for CRNDE-d[69].
Modification of the signaling pathways: It has been demonstrated that tumor cells in CRC are maintained by the deregulation of specific signaling pathways[70]. Genetic events are also part of a larger network that alters signal pathways, resulting in an increase in tumor cell proliferation and a decrease in tumor cell death[70]. The onset and migration of CRC involves several signaling pathways, including the mitogen-activated protein kinase (MAPK) pathway, PI3K pathway, Wnt/β-catenin pathway, Janus-activated kinase/signal transducers and activators of transcription (JAK/STAT) pathway, 67 kDa laminin receptor (67LR) pathway, nuclear factor-kappa B (NF-κB) pathway, and nuclear factor-erythroid 2-related factor (Nrf2) pathway. Moreover, the crosstalk between pathways can promote the development and invasion of CRC and increase its resistance to drugs[71]. The author details these specific pathways in the following section. Another novel signaling pathway, the Hippo pathway, is responsible for cell proliferation, differentiation, apoptosis, and tumorigenesis and exists in many malignant tumors, including CRC[72,73]. The Hippo pathway was initially defined as a tumor suppressor pathway, but its major effector, Yes-associated protein (YAP1), is viewed as an oncogene; therefore, the down-regulation of the Hippo pathway is connected to CRC initiation and progression[72,74]. It has been emphasized that the interaction between the Hippo pathway and the Wnt/β-catenin pathway is crucial in the development of CRC[74].
Cytokines: Chronic inflammation can promote the development of CRC[75]. In chronic inflammation, immune cells such as lymphocytes, plasma cells, macrophages, and neutrophils infiltrate the colon and enrich ROS and reactive nitrogen species (RNS)[76]. In addition to exogenous mutagens, ROS and RNS can also cause DNA damage, which facilitates the initiation of cancer, as observed in a mouse model[76]. Infiltrating inflammatory cells can also produce high levels of protumorigenic cytokines that drive tumor progression[76].
Cytokines are low-molecular-weight proteins that can mediate cell-to-cell communication and induce cell transformation and malignancy[77]. Many proinflammatory cytokines, such as tumor necrosis factor (TNF)-α and interleukin (IL)-6, are involved in the creation of the tumor microenvironment[77]. Furthermore, there are immunosuppressive cells in the tumor microenvironment that secrete vascular endothelial growth factor (VEGF), IL-6, IL-10, transforming growth factor-beta (TGF-β), soluble FasL, and indolamine-2,3-dioxygenase to promote the growth and metastasis of CRC by generating ROS and RNS, potentiating the EMT, and inducing angiogenesis[77,78]. Following the activation of these cytokines, some signaling pathways that can induce inflammation and stress are activated to induce the proliferation of cancer cells[75].
Some enzymes, such as Rab GTPase, can also have an essential function in the growth and metastasis of CRC, and they are correlated with cytokines. Rab GTPases, a large family of Ras small GTPases, play a crucial role in normal human physiology by controlling membrane identity and vesicle traffic[79]. A study using a metastatic mouse model suggested that high Rab3C expression in patients might increase the migration and invasion ability of colon cancer as a result of IL-6 secretion and JAK2/STAT3 signaling pathway activation[80]. Besides the Rab3C-IL-6-STAT3 axis, another Rab GTPase, Rab25, is also linked to CRC[81]. The loss of Rab25 is associated with a poor prognosis in CRC because Rab25 can influence the trafficking and recycling of numerous key regulators of polarity and signaling that are involved in transformation[81].
Oxidative stress: Another factor that promotes the development of CRC is oxidative damage, which is characterized by elevated ROS levels and accumulated mutations that cause oxidative DNA damage[82]. ROS, which includes superoxide (O2−), the hydroxyl radical (•OH), and hydrogen peroxide (H2O2), act as crucially important mediators in multiple cell signaling pathways[82]. Sustained and excessive ROS are not only strongly correlated with the tumorigenic potential of cancer cells but also render cancer cells resistant to anticancer drugs[83]. Studies have demonstrated that both gut microbiota and inflammation can cause oxidative stress[84]. Gut microbiota can generate reactive metabolites and induce chronic mucosal inflammation[84]. Inflammatory cells can mediate immediate cellular stress responses through the activation of NF-κB, signal transducer and STAT3, hypoxia-inducible factor-1α, activator protein-1 (AP-1), and Nrf2[84]. Meanwhile, LPO, protein oxidation, nitric oxide (NO) production, enzymatic activity alteration and DNA damage can be mediated by oxidative stress, to injure cells, induce gene mutation, and influence signaling pathways and transcription factors[84].
Gut microbiota: Gut microbiota is viewed as a forgotten organ that participates as an essential contributing factor in the initiation and development of CRC[85]. The balance of gut microbiota is conducive to the metabolism of nutrients, maintenance of the intestinal barrier, modulation of the immune system, and protection from pathogens[86]. However, some bacterial species have been identified and suspected to play a role in colorectal carcinogenesis; these include Helicobacter pylori, Bacteroides fragilis, F. nucleatum, and so on[85]. Dysbiosis is characterized by reduced Firmicutes to Bacteroidetes ratio (known as FIR/BAC), depletion of short-chain fatty acid-producing members of Lachnospiraceae and Ruminococcaceae, and the presence of putative pathobionts of oral origin[87]. Dysbiosis contributes to increased mucosal permeability, bacterial translocation, and increased activation of components of the innate and adaptive immune system. These changes promote chronic inflammation and further downstream changes that promote colon carcinogenesis[88].
Gut microbiota can regulate some immune cells of the immune system to impact the development of CRC.
(1) T lymphocytes: Gut microbiota can exert an important effect on T lymphocytes to modulate the progression of CRC. On the one hand, gut microbiota plays an important role in triggering chemokines production, such as that of CCL3, CCL4, CCL5, CCL20 and CXCL10, ultimately leading to T lymphocyte recruitment in tumor tissues and improved prognosis of CRC[89]. Bacteria-induced chemokine gene expression may also be initiated by Toll-like receptor (TLR) triggering on CRC cells[89]. On the other hand, gut microbiota can regulate the differentiation of T lymphocytes. Different T lymphocytes can exert different effects on CRC. Th1 cytokine interferon gamma (IFNγ) plays an antitumorigenic role, whereas the Th2/Treg cytokines IL-4, IL-5, and IL-10 mediate a protumorigenic role[90]. Besides, Th17 cells are known to be protumorigenic in CRC, and IL-17A is also linked to the gut microbiota[90]. Gut microbiota depletion can increase numbers of antitumor IFNγ-secreting T cells and decrease numbers of protumor IL-17A and IL-10 secreting immune populations to reduce the development of CRC[90]. Another study also demonstrated that a remodel of the gut microbiota can enhance anti-inflammatory capacity through promoting the induction of Tregs[91]. In addition, F. nucleatum, a proinflammatory bacterial species in tumor tissue but rarely found in normal intestinal microbiota, is associated with increased lymph node metastases and a worse outcome in CRC patients[92,93]. F. nucleatum is likely to possess immunosuppressive activities through its inhibition of human T cell responses[94,95]. F. nucleatum has been shown to expand myeloid-derived immune cells, which inhibit T cell proliferation and induce T cell apoptosis in CRC[95,96]. F. nucleatum also expresses the virulence factor FadA on their bacterial cell surface, which has been shown to activate the Wnt signaling pathway and down-regulate the T cell-mediated antitumor immune response[92]. Similarly, F. nucleatum can recruit proinflammatory cytokines, such as IL-17A, TNF, and CCL20, which induce inflammation and suppress immunity[97]. Meanwhile, rats with depletion of gut microbiota also show an increase in cytotoxic T lymphocyte cells[98]. Finally, a study demonstrated that fecal bacteria from CRC patients can up-regulate degranulation and cytotoxicity of CD8+T cells[99].
(2) B lymphocytes: The human gut homeostasis requires microbiota coated by both secretory immunoglobulin M (SIgM) and secretory immunoglobulin A (SIgA) emerging from B lymphocytes[100]. SIgA deficiency will cause dysbiosis, which may promote the development of CRC[100]. The study indicated that SIgM may emerge from pre-existing memory B cells and could help SIgA anchor highly diverse commensal communities to intestinal mucus[100]. Meanwhile, IL-33 might participate in modulating the IgA-microbiota axis to prevent IL-1α-dependent colitis and tumorigenesis[101]. IL-33 can promote IgA production to maintain gut microbial homoeostasis and inhibit IL-1α-mediated inflammation to prevent the onset of CRC[101]. Similarly, bacteria in CRC can also induce the production of IL-17, which promotes influx of intratumor B cells that promote tumor growth and progression[102].
(3) Natural killer (NK) cells: Some certain bacteria may favor recruitment of immune cells such as NK cells other than T cells, to achieve a favorable prognosis[89]. NK cells and CD8+T cell crosstalk in the tumor microenvironment may benefit patient outcome[103]. Nlrp3 inflammasome components exacerbate liver CRC metastatic growth by impairing IL-18 signaling and further impacting maturation of hepatic NK cells[104]. In addition, Nlrp3 activation might be mediated by a microbial ligand derived from the remaining intestinal microbiota[104].
(4) Neutrophils: Neutrophils are also believed to modulate growth of colon tumors, and correlate with outcomes of patients with colon cancer[102]. It has been indicated that neutrophil depletion is correlated with increased numbers of bacteria in tumors and proliferation of tumor cells, and an inflammatory response mediated by IL-17, thereby inducing the development of CRC[102].
(5) Eosinophils: Eosinophils in CRC patients are strongly linked with a decreased disease risk, better prognosis, and extended patient survival[105]. Dysbiosis might impair eosinophil-driven responses to promote the development of CRC[105]. However, the specific mechanism is not clear.
(6) Macrophages: Macrophages are also involved in the development of CRC. Monocytes/macrophages may polarize as M1 or M2 cells[106]. Overall, M1 macrophages display a pro-inflammatory potential mediating antitumor activities, while M2 macrophage display an anti-inflammatory promoting cancer cell growth[106,107]. In the tumor microenvironment, tumor-associated macrophages undergo polarization into M1 and M2 phenotypes[108]. The specific interaction of gut microbiota and macrophages on CRC is still required to investigate further. Some studies have provided insights into the relative mechanism. A metastasis-related secretory protein, cathepsin K, activated by the imbalance of intestinal microbiota, stimulates CRC progression through accelerating M2 polarization of tumor-associated macrophages through a TLR4-mTOR-dependent pathway[108]. Besides, another study also indicated that defects in the subepithelial band of lamina propria-indigenous macrophages barrier in inflammatory bowel disease encourage the trespassing of the gut microflora into the host, thereby destabilizing host immunity and promoting the development of CRC[109]. High amounts of F. nucleatum intratumorally are correlated with increased macrophage infiltration and CDKN2A promoter methylation in MSI-H CRC[110]. Although it can be hypothesized that the repression of CDKN2A via promoter methylation may be connected with the increased M2 macrophages in F. nucleatum-high CRC, the M2 macrophage density was not significantly associated with F. nucleatum status in MSI-H CRCs, as displayed by the study[110]. Besides, a strong association between lower frequency of macrophages, increased Firmicutes, and decreased tumorigenesis was also observed in CRC[111].
(7) Dendritic cells: Dendritic cells play critical roles in maintaining tolerance and immune homeostasis in the gut[112]. And, some species in the gut can also induce dendritic cell maturation and the induction of Tregs and IL-10 production to regulate tumorigenesis[112]. Overall, the specific immune-microbiota mechanism needs to be investigated by more animal studies and epidemiological studies before it is proven.
Molecular pathological epidemiology: Molecular pathological epidemiology (MPE) has emerged as an integration of molecular pathology and epidemiology, to address the need to investigate the inherent heterogeneity of pathogenic processes even for a single disease entity[113,114]. Overall, MPE discusses the interrelationship between exogenous and endogenous factors, tumoral molecular signatures, and tumor progression[114]. On the one hand, MPE can uncover potential risk factors that are not detectable in conventional epidemiological research without using molecular pathology methods[115]. On the other hand, MPE can help us refine the association between exogenous or endogenous factors and validate specific etiological hypotheses, thereby augmenting causal inference[113-116]. Meanwhile, MPE study can provide novel etiologic and pathogenic insights, potentially contributing to precision medicine for personalized prevention and treatment[116,117]. In addition, MPE can also integrate several disciplines to evolve subfields of MPE, including pharmaco-MPE, immuno-MPE and microbial MPE, to provide novel opinions into underlying etiologic mechanisms[116].
Some progression has been made in CRC. The MPE research has determined the strength of the association for between the exposures and the specific subtypes of CRC, which can help to establish causality and speculation on the relative mechanism of exposure acting on CRC. A MPE study has demonstrated that both obesity and physical inactivity are associated with a higher risk of CTNNB1 (β-catenin)-negative CRC but not with CTNNB1-positive cancer risk[118]. Hence, the study implied that energy balance and metabolism status might exert impact on the development of CRC independent of WNT/β-catenin activation[118]. Then, pharmaco-MPE, integrating MPE into pharmacoepidemiology, will play a vital role in identifying target individuals who will most likely benefit from use of a particular drug, clinically[116]. MPE studies have demonstrated that regular use of aspirin can reduce the risk of CRC with overexpression of COX-2 but not of that with weak or absent expression of COX-2[119,120]. Many pharmaco-MPE studies have shown that regular aspirin use was associated with lower risk of BRAF-wildtype and PIK3CA-mutated CRC but not with BRAF-mutated and PIK3CA-wildtype CRC[121,122].
Immuno-MPE, the integration of immunology and MPE, can mainly discuss exposures impacting CRC through regulating the immune system and disease-immune interactions[117]. An MPE research project has revealed that the association of aspirin use with CRC survival is stronger in patients with the programmed cell death ligand 1 (PD-L1)-low tumors than the PD-L1-high CRC[123]. It indicated that PD-L1 expression might serve as a biomarker that predicts resistance to aspirin use[123]. In addition, microbial MPE is also studied in CRC. Typically, a high level of F. nucleatum might be associated with molecular features of CRC, including MSI-high and CIMP-high[124,125]. Meanwhile, another MPE study demonstrated that a greater amount of F. nucleatum was associated with a lower density of CD3+T cells in CRC, indicating that the interaction of target microbiota and immune system should be discussed further for CRC prevention and precision treatment[126]. In addition to F. nucleatum, other components of gut microbiota need to be investigated in the future.
Although the MPE has many strengths, the pitfalls and challenges should be considered. Challenges in MPE mainly include sample size selection, need for rigorous validation of molecular assays and study findings, and paucities of interdisciplinary experts, education programs, international forums, and standardized guidelines[113]. In addition, MPE research needs to face the issue of multiple hypothesis testing, so it is necessary to form a priori hypotheses based on earlier exploratory findings or on potential biological mechanisms[114]. Similarly, MPE also may create a higher chance of yielding spurious findings[113].
Other mechanisms: The EMT and the Warburg effect (WE) are considered to be involved in tumor metastasis. EMT occurs when polarized epithelial cells lose their adhesion property and obtain mesenchymal cell phenotypes as a result of the loss of membrane E-cadherin expression[127]. Although the exact mechanism in CRC is not clear, EMT-related molecular mechanisms have been described, including the activation of many signaling pathways, such as the TGF-β/Wnt pathway and PTEN/Akt/HIF-1α pathway, as well as many activated genes, such as APC and Akt[128]. The WE is the result of pyruvate being directed away from the tricarboxylic acid cycle and metabolized to lactate, resulting in a buildup of glycolytic intermediates[129]. Briefly, the WE is the process of aerobic glycolysis[129]. Many mechanisms can inhibit the WE in CRC to affect the metastasis of cancer; these include some miRNAs[130,131], such as miRNA-98 and Pim1[132]. Gas1, a tumor suppressor, can inhibit both EMT and the WE in CRC through AMPK activation and the mTOR pathway[133].
Tea polyphenols
Because of the drug resistance and side effect that can arise in targeted therapy of CRC, studies have investigated treatments that involve natural bioactivate materials found in various foods, such as tea polyphenols (TPs)[134,135]. We have learned that TPs may become a novel medicine to prevent and treat disease with fewer side effects than traditional medicines[135]. Also, the combination of chemotherapeutic drugs and TPs could synergistically enhance treatment efficacy and reduce the adverse side effects of anticancer drugs[136]. Over the past several decades, we have learned that TPs can be utilized effectively as chemopreventive and chemotherapeutic agents for some diseases, including obesity[137], diabetes mellitus[138], Alzheimer’s disease[139], Parkinson’s disease[140], cardiovascular disease[141], and cancers[142]. TPs can play an essential role in the treatment of most cancers by causing G0/G1 phase cell cycle arrest and inhibiting angiogenesis[143,144]. One study demonstrated that green TPs could suppress the pathological formation of new blood vessels by inhibiting members of the VEGF family[144].
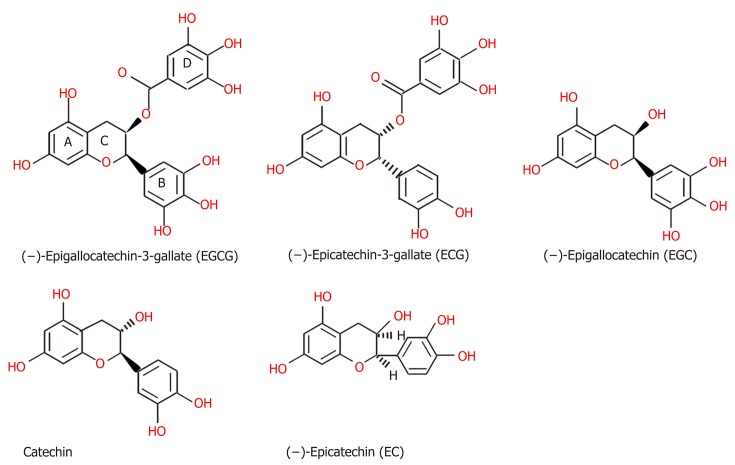
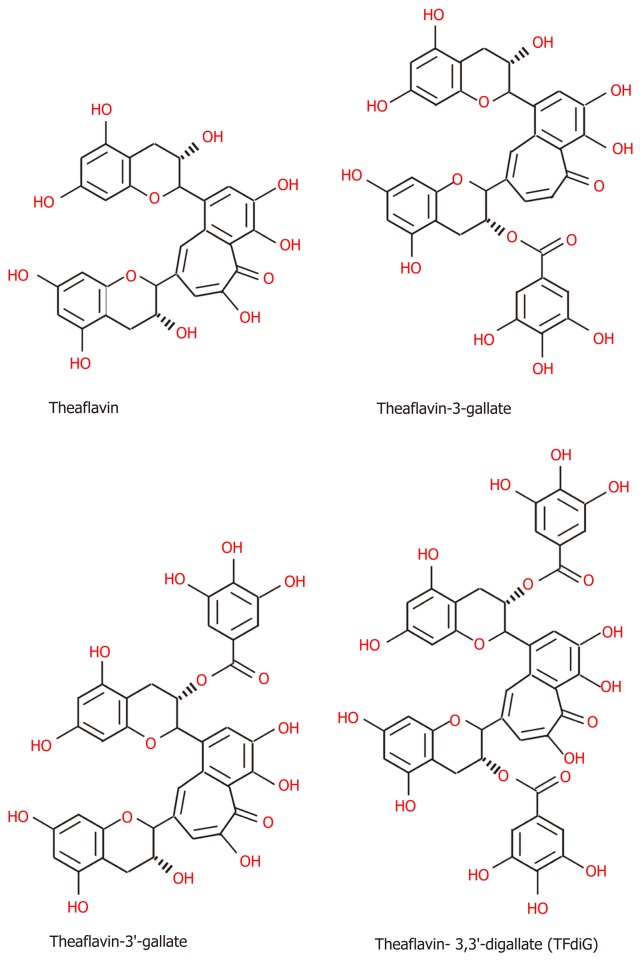
Chemical structure of TPs: Tea, which originates from the plant species Camellia sinensis, has become the second most commonly consumed beverage following water, with teas such as green tea, black tea, and oolong tea among those frequently consumed[145]. The main difference between these three kinds of tea is the fermentation level, which leads to the presence of different TPs[145]. Green tea is made from dry tea leaves, which do not undergo the process of fermentation[145]. Thus, green TPs contain more oligomeric polyphenols, with the main content comprising flavan-3-ols or tea catechins (approximately 59%), including (-)-epigallocatechin (EGC), (-)-epigallocatechin-3-gallate (EGCG), (-)-epicatechin (EC), and (-)-epicatechin-3-gallate (ECG) (Figure 2), among which EGCG is the most abundant polyphenol in green tea[145-147]. Although gallic acid (GA) can also be found in green tea, it is mostly contained in the fully fermented Pu-erh tea, usually[148]. Black tea must undergo high or full fermentation, and the level of fermentation of oolong tea falls in the middle of this range[136,149]. Therefore, black tea contains lower monomeric polyphenol content (3%-10% of solids) and higher concentrations of polymeric polyphenols (23-25% of solids), such as theaflavin (TF), theaflavin-3-gallate (TF2a), theaflavin-3’-gallate (TF2b), theaflavin-3,3’-digallate (TF3 or TFdiG), and thearubigin (Figure 3)[147,150]. Oolong tea polyphenol content includes epitheflagallin (ETG) and EGCG, among others[149].
Figure 2.
The structures of green tea polyphenols, including (−)-epigallocatechin-3-gallate, (−)-epicatechin-3-gallate, (−)-epigallocatechin, catechin, (−)-epicatechin[146].
Figure 3.
The structures of black tea polyphenols, including, theaflavin, theaflavin-3-gallate, theaflavin-3'-gallate and theaflavin- 3,3'-digallate[150].
Oligomeric and polymeric polyphenols undergo mutual transformation. The formation of black TPs involves two steps: Oxidation and polymerization, which are regarded as the fermentation of green tea[151]. In the first step, catechins are partially oxidized to quinones as a result of the enzymatic catalysis of polyphenol oxidase or peroxidase, which exist in nature[151]. Subsequently, polymerization produces gallocatechin quinones, and further oxidation and rearrangement lead to the synthesis of the core of black TPs, namely, benzotropolone[151]. For instance, EC and EGC form TF1, ECG and EGC form TF2a, EC and EGCG form TF2b, and ECG and EGCG form TF3[151]. Therefore, the chemical structure of black TPs and green TPs share some similarities. All oligomeric polyphenols have the same basic chemical structure of two aromatic rings (A and B) linked by three carbons that usually form an oxygenated heterocycle (C ring), which consists of a C6–C3–C6 skeleton[152,153]. In the B ring, OH or OCH3 groups usually occupy up to three positions[153]. In flavan-3-ols, the C ring, as the activated center, is a saturated heterocycle with a hydroxyl group that provides different arrangements of hydroxy, methoxy, and glycosidic groups and bonds with other monomers[153]. Moreover, the chemical structures of TPs are not simple linear oligomers because they contain gallate groups[153].
Bioavailability of TPs: After tea is consumed, TPs can be decomposed into different fractions and absorbed in the gut, which is considered to be a complex physiological process. Bioavailability is used to describe the extent of absorption that an ingested compound is released from food, and its fate in the organism[154]. Furthermore, many studies have investigated the kinetics and extent of polyphenol absorption by measuring plasma concentrations and/or urinary excretion after the ingestion of TPs[155]. A number of studies have demonstrated that TPs have poor bioavailability from in vivo and in vitro gastrointestinal digestion[154,156]. In nature, most flavan-3-ols undergo epimerization and exist as stereoisomers in a cis or trans configuration [(-)-epicatechin or (+)-catechin, respectively][150]. Different stereoisomers have different bioavailability. The bioavailability of the stereoisomers has been ranked as (-)-epicatechin > (+)-epicatechin = (+)-catechin > (-)-catechin[150]. Also, the in vivo effects of flavan-3-ols, major components of green TPs, rely on their absorption and metabolism in the gastrointestinal tract[157]. Thus, we should discuss the health effects of not only TPs but also their metabolites.
The different metabolites can be found in the small intestine and large intestine. The absorption and metabolism of TPs, including the processes of methylation, glucuronidation, and sulphation, mainly transpire in the small intestine[150]. Among the TPs, EGCG is the only known polyphenol present in plasma with a large proportion (77%-90%) in the free form[158]. Others are highly conjugated with glucuronic acid and/or sulfate groups, such as epicatechin-3’-glucuronide and 4’-O-methylepicatechin-3’-glucuronide, among others, after being metabolized[155]. One study observed that the conjugated forms of two major phenolic catabolites, (-)-5-(3’,4’,5’-trihydroxyphenyl)-gamma-valerolactone (M4) and (-)-5-(3’,4’-dihydroxyphenyl)-gamma-valerolactone (M6), which accounted for up to 40% of the amount of ingested pure EGC and EC, could be detected in plasma, urine, and feces[159]. 4’,4’’-di-methyl-EGCG was also detected in human plasma and urine following green tea ingestion[160]. Although the metabolism of black TPs has been researched less, the metabolites might contain 3-methylgallic acid, 4-methylgallic acid, and 3,4-di-methylgallic acid, which also exist in green TPs[150].
In the large intestine, the metabolic fate of TPs after in vitro gastrointestinal digestion was studied, and bioaccessibility activity of TPs was shown to be higher in the colon than in the duodenum, suggesting that, in vivo, the gut microbiota might be able to metabolize dietary polyphenols, resulting in an increase in their beneficial effects in the large intestine[136,161]. The study demonstrated that green tea catechins were more bioavailable when colonic ring fission metabolites were taken into consideration[158]. A possible ring-fission metabolite, (-)-5-(3’,5’-dihydroxyphenyl)-γ-valerolactone (M6’), was detected in human urine after green tea ingestion[160]. The fraction of flavan-3-ols that is not absorbed in the small intestine reaches the large intestine, where it can undergo several microbial processes that finally lead to smaller molecules that can be absorbed and reach the liver and, subsequently, the systemic circulation[158]. Studies have indicated that catechin and epicatechin, which can enter the portal vein at a relatively high concentration as a result of ileal transfer, can be further metabolized to methylated and glucuronidated forms by phase I and II metabolism in the liver[162]. From the action of microbiota, ingested flavan-3-ols can be converted to C6-C5 phenylvalerolactones and phenylvaleric acids, which undergo side-chain shortening to produce C6-C1 phenolic and aromatic acids that enter the bloodstream and are excreted in urine[150].
THE STUDY OF TPS AND CRC
Cytological studies
TPs have been studied to assess their ability to prevent CRC cells in vitro. For example, the inhibitory effect of EGCG was observed in caco-2 colorectal adenocarcinoma cells and Hs578T breast ductal carcinoma cells[163]. In HT-29 cells, fermented Pu-erh tea showed stronger anticancer function than unfermented Pu-erh tea and green tea due to increased GA[148]. In addition to the effects exerted by the TPs themselves, their metabolic products can also have an antiproliferative effect. A study reported that black tea, green tea, and some phenolic acids, such as 4-hy-droxyphenylacetic acid (4-HPAA), 3-hydroxyphenylacetic acid (3-HPAA), 3-O-methylgallic acid (3OMGA), and polyhydroxy- valerolactones, all exhibited an antiproliferative effect in HCT-116 cells, in which 3OMGA exhibited the strongest antiproliferative activity among the phenolic acids[164]. The in vitro study also demonstrated that the combination of EGCG and 3,4-dihydroxyphenylacetic acid (3,4-DHPAA) significantly increased the antiproliferative activity compared with EGCG or DHPAA alone[164]. Moreover, many in vitro studies have been conducted to elucidate the specific mechanisms of the preventive and therapeutic effects of TPs on CRC. The concentrations of TPs used in cell line studies are usually much higher than the levels that are achievable in vivo because of their low bioavailability.
Some studies have demonstrated that a concentration-dependent relationship exists between TPs and CRC. For instance, in human colon cancer cell lines, EGCG might exert a therapeutic effect by inhibiting Met signaling in a concentration-dependent manner[165]. At concentrations of 0.5, 1 and 5 μmol/L, EGCG markedly suppressed the activation of Met in the presence of hepatocyte growth factor. Concentrations of 10 μmol/L EGCG and below generated low amounts of H2O2 (< 1.5 μmol/L), whereas higher H2O2 concentrations (> 5 μmol/L) were required to directly increase the phosphorylation of Met[165]. Green TPs (> 800 mg/mL) can trigger apoptosis of preneoplastic and neoplastic intestinal epithelial cells in a dose- and time-dependent manner[166]. Cellular studies also play an important role in understanding the relationship between TPs and chemotherapeutic drugs. A study on HCT-116 and SW-480 cells indicated that the combination of panaxadiol and EGCG significantly increased the pro-apoptotic activity of panaxadiol compared with the drug alone (P < 0.01) because panaxadiol and EGCG can bind to two different sites of annexin V, an endogenous protein, to mediate cell death[167].
Animal studies
By establishing related animal models of CRC, we can further identify the inhibitory effect of TPs on CRC and further understand the manner in which TPs act on CRC. A study demonstrated that a green TP fraction could inhibit the development of azoxymethane (AOM)-induced colon carcinogenesis in male Fischer rats and F344 rats, but no dose-dependent relationship was found in male Fischer rats[168-171]. Moreover, after investigating 129 female F344 rats, Narisawa et al [172] suggested that GTP, even at a very low dose (0.002% solution), also had an inhibitory effect on N-methyl-N-nitrosourea-induced colon carcinogenesis. In addition, the antitumor effect of white tea has been observed. A study demonstrated that in the ileum, the major site of tumor formation in Apc(min) mice, white tea was more effective than green tea, and white tea in combination with non-steroidal anti-inflammatory drugs (commonly known as NSAIDs), such as sulindac, provided greater tumor suppression than tea or sulindac treatment alone[173]. It has been demonstrated that black TPs can inhibit 1,2-dimethylhydrazine-induced oxidative DNA damage in the colon mucosa of Fisher 344 rats because 1,2-dimethylhydrazine can cause DNA oxidative damage and is a colon carcinogen[174]. Moreover, the modification (beneficial or harmful) effect of green TPs on CRC may depend on their dosage. The results showed that 0.5% and 1% green TP could exert a potential effect by increasing the expression of IL-1β and MIF, but 0.1% might have had a suppressive effect[175]. Similarly, Ju et al [176] confirmed that EGCG in drinking fluid in the range of 0.02%-0.32% dose-dependently inhibited small tumorigenesis in Apc(min)/+ mice (with a significant negative linear relationship, P < 0.01).
Epidemiological studies
An increasing number of clinical trials have been carried out to confirm the relationship between TPs and CRC. A randomized, placebo-controlled, multicenter trial demonstrated the effect of diet supplementation with green tea extract (GTE) containing 300 mg EGCG on the recurrence of colon adenomas[177]. Scientists have also concentrated more on the specific dose of TPs to inhibit CRC. A dose-response meta-analysis was performed to evaluate the relationship between tea consumption and CRC risk by analyzing 29 qualifying studies. The results of the dose–response analysis showed that there was a significant inverse association between an increment of 1 cup/d of tea consumption and CRC risk in the green tea-drinking (odds ratio of 0.98, 95%CI: 0.96-1.01) and female subgroups (odds ratio of 0.68, 95%CI: 0.56-0.81)[178]. A randomized trial demonstrated the preventive effect of GTE supplements on metachronous colorectal adenomas by raising the green tea consumption in the target population from an average of 6 cups (1.5 g GTE) daily to 10 cups equivalent (2.5 g GTE) in supplemental GTE tablets[179]. Additionally, a total of 12 studies (5 cohort and 7 case-control studies) involving 17481 cases and 740859 controls indicated that high intake of flavonols, such as quercetin, might reduce the risk of colon cancer, and high intake of flavones (such as apigenin) might reduce the risk of rectal cancer[180].
In another study, the plasma concentration of TPs was observed to have a suppressive effect on cancer cells, but no association was found between urinary tea catechins and the risk of rectal cancer[180]. However, a cohort study of 18244 men demonstrated that those with high prediagnostic urinary EGC levels and 4’-MeEGC had a lower risk of colon cancer, with a statistically significant difference observed[181]. Some previous studies have not found a statistically significant decrease in the risk of CRC with the administration of TPs[182-184]. There are many reasons that might cause these discrepancies among studies. Sex may be regarded as one of the factors. A sex hormone-mediated pathway may be involved in the observed positive association between green tea intake and late-stage CRC, which seems to be restricted to males[182]. Moreover, there also exists some bias in study participants. For example, we cannot avoid effects of lifestyles, such as cigarette smoking and alcohol consumption, or other diseases, such as diabetes[182,183]. In addition, one of the studies did not divide tea consumption into specific subcategories (such as 5-9 cups and 10 or more cups), so the amount of tea consumption might have affected the result of the experiments[184].
THE RELATIVE MECHANISM OF TPS IN CRC
There are many studies that have proposed mechanisms by which TPs prevent the formation and migration of CRC. However, many doubts surround the specific mechanism of the prevention of cancer formation and metastasis. In this section, the author aims to explain the mechanisms by which TPs prevent the formation and metastasis of CRC.
Diet and lifestyle
The impact of diet and lifestyle should be considered in elucidating the relationship between TPs and CRC risk. In a dietary pattern, tea should be combined with other foods. The combination may enhance or diminish the relationship of tea and CRC. Nowadays, existing studies have strongly demonstrated the relation of tea and some foods with CRC. A study suggested that green tea and black tea can inhibit the formation of heterocyclic amine produced from red and processed meat to suppress colonic aberrant crypt foci (ACF) in the rat[185]. Besides, tea can also induce cytochromes P450 and phase II enzymes in a manner consistent with the rapid metabolism and excretion of heterocyclic amines[185]. Meanwhile, tea has been shown to block N-nitroso compounds’ formation from red meat, thereby exerting the protective effect of CRC[186]. A study demonstrated that combination of Se and green tea is more effective in suppressing CRC than either agent alone[187]. The preventive effect of combination diet on CRC has been evidenced by restoring SFRP5 gene expression, increasing histone H3 acetylation and reducing DNA methyltransferase (DNMT) 1 expression, inhibiting β-catenin nuclear accumulation, and reducing cyclin D1 expression and cell proliferation in normal-appearing crypts[187].
Other foods should also be considered when discussing the protective effect of tea on CRC. Further studies should be undertaken to understand the relationship and mechanism profoundly. Variations in lifestyle factors might be the cause of inconsistent findings regarding green tea intake and CRC risk in several epidemiological studies. One study has shown that high green tea consumption (≥ 25.50 g/d) was associated with a decreased risk of CRC, with or without considering lifestyle factors[188]. However, moderate green tea consumption increased the risk of CRC among ever-smokers, ever-drinkers and the high-inflammatory diet group[188]. Similarly, another study conducted in Shanghai also demonstrated that regular consumption of green tea may reduce CRC risk among non-smokers but no significant association was found among smokers[189]. However, we cannot obtain the relative studies to understand the interaction of PA or aspirin, and tea, on CRC. Furthermore, the relative mechanism of this interaction between lifestyle and CRC should also be investigated in the future.
Effects on the signaling pathway
TPs can modulate several signaling pathways to exert a suppressive effect on the growth and metastasis of CRC.
The MAPK pathway: One of the functions of MAPK signaling is the regulation of gene expression in response to extracellular stimuli to suppress cell proliferation and induce apoptosis[190]. Among the MAPKs, Jun amino-terminal kinases (JNKs) and p38 are involved in stress-induced apoptosis, and extracellular signal-related kinases (ERKs) are connected to cell proliferation in CRC[191]. In the ERK/MAPK pathway, mutation and overexpression of Ras genes have been found in various cancers to dysregulate stem cells[192]. In CRC, the Ras signaling pathway involves the activation of two protein kinases, MAPKKs (Raf) and MAPKKs (MEK), which lead to the phosphorylation of ERK1 and ERK2 at threonine and tyrosine residues to cause dimerization, nuclear translocation, and the induction of target genes, such as KRas, NRas, and BRAF[193,194]. In addition, Ras can also activate JNK and p38 by similarly activating the protein kinase phosphorylation cascade through rac and cdc42, which are small GTP-binding proteins[194]. In the JNK/MAPK pathway, JNK is activated in cells exposed to environmental stress or treated with proinflammatory cytokines, and targets of the signaling pathway include the transcription factors ATF-2, Elk-1, c-Jun, and NFAT4[195].
MAPKKs, together with members of the MEK kinase (MEKK) and mixed-lineage kinase (MLK) groups of MAPKKKs, regulate the activity of JNK (Figure 4)[195]. In CRC, the p38 MAPK pathway is involved in sustaining tumor growth and chemoresistance, and it is classified as a “stress-activated” kinase pathway that is activated by a variety of extracellular stimuli[196]. Four genes have been identified that encode p38 MAPKs: MAPK14 (p38α), MAPK11 (p38β), MAPK12 (p38γ), and MAPK13 (p38δ)[197]. MKK3/6 are activated by their upstream kinases, such as MTK1 (or MEKK4) and apoptosis signal-regulating kinase 1 (Figure 4)[196]. In normal and CRC cells, the manipulation of p38-interacting protein and p38α, a negative regulator of autophagy, was found to alter the localization of mATG9, a protein required for autophagosome formation, which suggests that p38α could provide a link to nutrient-dependent signaling cascades that are activated during autophagy[196]. Normally, the balance between ERK1,2 and p38 pathways is sustained by PP1/PP2A protein phosphatases[198]. Once the balance is disturbed, some diseases, including CRC, can be induced[198].
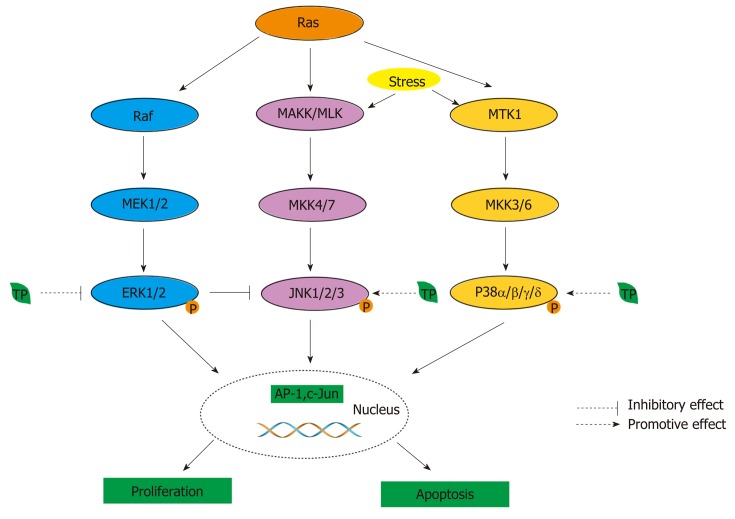
Figure 4.
MAPK activity is regulated through three-tiered cascades composed of a MAPK, MAPK kinase (MAPKK, MKK, or MEK), and an MAPKK kinase or MEK kinase (MAPKKK or MEKK)[190]. Four groups of MAPKs are extracellular signal-related kinase (ERK)-1/2, Jun amino-terminal kinase (JNK1/2/3), p38 proteins (p38α/β/γ/δ), and ERK5, which are activated by specific MAPKKs: MEK1/2, MKK4/7 (JNKK1/2), MKK3/6, and MEK5, respectively[190]. MAPKKKs, the upstream targets of MAPKKs, contain Raf in the ERK pathway, MEK kinase (MEKK) and mixed-lineage kinase (MLK) in the JNK pathway, and MTK1 (or MEKK4) and apoptosis signal-regulating kinase 1 in the p38 pathway. Moreover, phosphorylated MAPKs can activate transcription factors, such as AP-1. Tea polyphenols (TPs) can modulate MAPK pathways. In the ERK pathway, TPs can inhibit the activity of transcription factors, such as AP-1, by inhibiting the phosphorylation of ERK1/2. In the JNK and p38 pathway, TPs can promote the activation of JNK1/2 and p38 to induce cell death. In addition, the blockade of ERK can also lead to the activation of the JNK pathway.
TPs can modulate MAPK pathways to inhibit the onset of CRC. Although EGCG inhibits the ERK signaling pathway in activated cells to induce death, EGCG can increase the phosphorylation levels of ERK1/2 and Akt in resting cells[199]. In addition, phosphorylated MAPKs can activate transcription factors, such as AP-1[200]. Thus, green and black TPs, except for EC, can inhibit AP-1 activity by inhibiting phospho-ERK and phospho-c-Jun formation and, subsequently, decreasing the levels of c-Jun and fra-1 by EGCG and TFdiG, respectively (Figure 4)[200]. The inhibitory effect may depend on the presence of the galloyl structure on the B ring and the gallate moiety[200]. In addition, the JNK pathway plays a pivotal role in EGCG-induced cell death[191]. EGCG can induce the activation of JNK to promote the release of cytochrome c, leading to apoptosis in the Bax-dependent condition (Figure 4)[191,199]. One study reported a linkage between the ERK and JNK pathways, and an inhibitor of ERK can cause JNK activation, which induces cell death[191]. In the p38 pathway, EGCG can activate phospho-p38α, p38γ, and p38δ to mediate the p38 MAPK pathway, thus affecting CRC growth (Figure 4)[199].
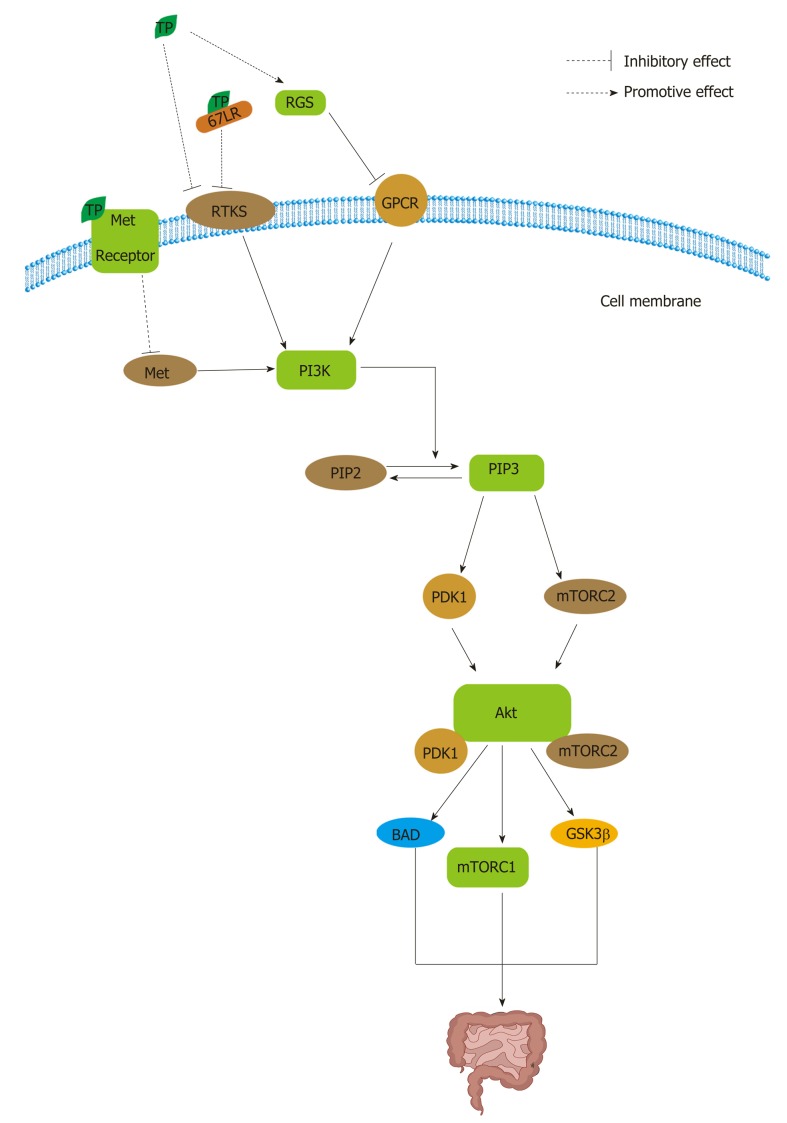
PI3K/Akt signaling: PI3K/Akt signaling is also involved in the reduction of apoptosis, stimulation of cell growth, and an increase in proliferation[201]. Normally, Akt plays central but diverse roles in the responses of various cell types and tissues to hormones, growth factors, cytokines, and neurotrophic factors, among other stimuli[201]. Pathologically, multiple genetic lesions confer hyperactivation of Akt in solid human tumors[201]. In CRC, the array of downstream pro-survival and pro-growth effects of Akt signaling, including changes in cellular metabolism, are likely to contribute to its role in tumor growth and progression[201]. Receptor tyrosine kinases (RTKs), such as EGFR, belong to a family of transmembrane receptors that display tyrosine kinase activity and trigger the activation of downstream signaling pathways that are mainly involved in cell proliferation and survival, similar to the effects of lipid rafts (Figure 5)[202]. The canonical pathway leading to Akt activation is initiated by the stimulation of RTKs or G-protein-coupled receptors, leading to plasma membrane recruitment and the activation of one or more isoforms of the class I PI3K family (Figure 5)[201]. Phosphorylated Akt activates a multitude of downstream targets, such as the mTOR complex 1 (mTORC1), BAD, CASP9, various FOXO proteins, GSK3β, MDM2, and TSC1, which regulate proliferation, apoptosis, and other processes (Figure 5)[201,203].
Figure 5.
The class I PI3Ks activated by receptor tyrosine kinases, such as epidermal growth factor receptor, can phosphorylate PI 4,5-bisphosphate to yield PI 3,4,5-triphosphate in the cell membrane[203]. The activation of PI3K results in the phosphorylation of two key residues on Akt1, T308 in the activation loop (or T-loop) of the catalytic protein kinase core and S473 in a C-terminal hydrophobic motif[201]. PDK1 at T308 and mTOR complex 2 (mTORC2) at S473 can activate Akt[203]. Phosphorylated Akt activates a multitude of downstream targets, such as the mTOR complex 1 (mTORC1), BAD, GSK3β. Tea polyphenols (TPs) can inhibit PI3K/Akt signaling by interrupting receptor tyrosine kinases (RTKs) and G protein-coupled receptors. TPs can inhibit RTKs not only directly but also indirectly by binding to 67 kDa laminin receptor. Moreover, TPs can activate the regulators of G protein signaling to negatively regulate the pathway. In addition, TPs can bind to the Met receptor to inhibit PI3K activation. PIP2: PI 4,5-bisphosphate; PIP3: PI 3,4,5-triphosphate; TP: Tea polyphenol; RGS: Regulators of G protein signaling; 67LR: 67 kDa laminin receptor; GPCR: G protein-coupled receptor.
TPs can inhibit RTKs and G-protein-coupled receptors in PI3K/Akt pathways (Figure 5). TPs can inhibit RTKs in many ways. On the one hand, EGCG can inhibit EGF binding and EGFR activation directly and indirectly by modulating multiple components in cell membranes to cause anti-angiogenic effects[204]. Low concentration and high concentration of EGCG can modify the structure of lipid bilayers and reduce the bilayer stiffness, respectively[204]. On the other hand, EGCG can bind to the 67 LR protein and thus further inhibit lipid rafts[204]. EGCG inhibits not only EGFR but also other RTKs[204]. Studies have demonstrated that EGCG can down-regulate the expression of VEGF by blocking ERK-1 and ERK-2 activation so that it can suppress the growth of CRC[205]. The Akt pathway is also activated by Met. Met, as the receptor for hepatocyte growth factor, mediates the proliferation, motility, and invasion of CRC cells in metastasis[206]. Met activation has docking sequences for several SH2/SH3-containing molecules, including Gab1, Src, Grb2, and PI3K, which in turn are capable of activating a number of downstream signaling components, including the Akt pathway (Figure 5)[206]. However, EGCG can inhibit Met signaling independent of H2O2-related mechanisms[165,207]. Enzyme kinetic studies have identified the gallate moiety as a key structural feature of TPs’ ability to bind to the kinase domain of the Met receptor[208]. Moreover, the diverse effects of TPs that are mediated by the G-signaling pathway may be attributable to their selective effect on the regulators of G protein signaling (RGS); RGS can negatively induce G protein signaling and control the expression of downstream target inflammatory genes, such as COX-2, inducible nitric oxide synthase (iNOS), and matrix metalloproteinase (MMP)-9, possibly through NF-κB (Figure 5)[208]. TF-2, TF-3, and EGCG, all of which contain a gallate group, can induce the expression of RGS10 and RGS14 selectively to inhibit tumorigenesis (Figure 5)[209].
The Wnt/β-catenin pathway: Another signaling pathway is the Wnt/β-catenin pathway. The Wnt pathway also plays a key role in stem-cell differentiation and cellular growth, and it is activated aberrantly at the bottom of intestinal crypts[209]. Usually, in the absence of Wnt stimulation, β-catenin is sequentially phosphorylated within the destruction complex by casein kinase 1 (CK1) and GSK3α/β[210]. Phosphorylated β-catenin is then recognized by β-TrCP, a component of the E3 ubiquitin ligase complex, and ubiquitinated within the destruction complex[210]. Once Wnt ligands bind to the frizzled (FZD) and low-density-lipoprotein-related protein 5/6 (LRP5/6) coreceptor complex, they cause the accumulation and nuclear translocation of β-catenin to activate the Wnt transcriptional program (Figure 6)[210]. The aberrant up-regulation of Wnt signaling has been found to exist in CRC, not only influencing the frequent mutation of some tumor suppressor genes, such as APC, AXIN2, and β-catenin, but also disturbing the epigenetic silencing of some Wnt inhibitors and negative regulators, such as SFRP1, WIF1, DKK1, and DKK3[209].
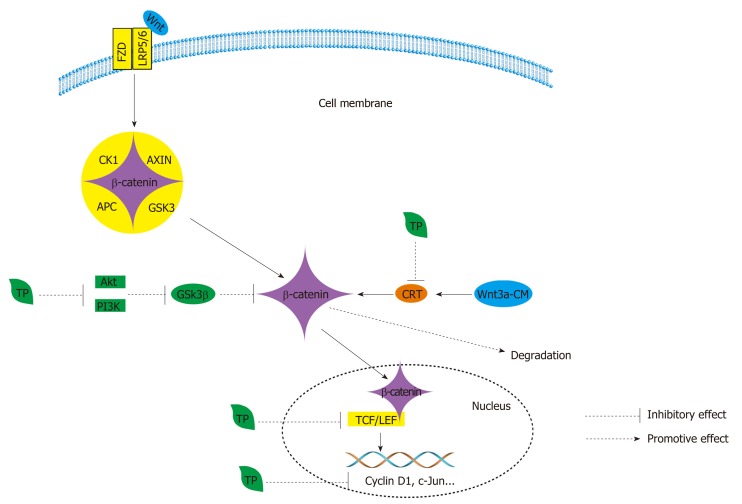
Figure 6.
Wnt signaling is regulated by the cytoplasmic β-catenin destruction complex, which consists of the core proteins AXIN, adenomatous polyposis coli, casein kinase 1, and glycogen synthase kinase 3[209]. Wnt ligands bind to the frizzled and low-density-lipoprotein-related protein 5/6 coreceptor complex and cause the accumulation and nuclear translocation of β-catenin. In the nucleus, β-catenin engages the T cell factor (TCF)/lymphoid enhancer-binding factor transcription factors to activate the Wnt transcriptional program[210]. In the Wnt/β-catenin pathway, Tea polyphenols (TPs) can inhibit β-catenin/TCF-4 activity and reduce β-catenin protein expression and further reduce the expression of two downstream signaling targets, namely, cyclin D1 and c-Jun. Black TPs can also suppress the accumulation of β-catenin by suppressing the phosphorylation of GSK3β by decreasing the activation of PI3Knase and Akt. Furthermore, TPs also promote the degradation of β-catenin by inhibiting β-catenin response transcription. APC: Adenomatous polyposis coli; CK1: Casein kinase 1; GSK3: Glycogen synthase kinase 3; TCF: T cell factor; TP: Tea polyphenol; LEF: Lymphoid enhancer-binding factor; LRP5/6: Low-density lipoprotein-related protein 5/6; CRT: β-catenin response transcription.
TPs have an inhibitory effect on the expression of β-catenin (Figure 6); they can inhibit β-catenin/TCF-4 activity and reduce β-catenin protein expression (Figure 6)[173]. Similarly, they can also reduce the expression of two downstream signaling targets, namely, cyclin D1 and c-Jun (Figure 6)[173]. When black TPs inhibit the Wnt/β-catenin pathway, they can decrease the nuclear accumulation of β-catenin by suppressing the phosphorylation of GSK3β at serine 9, and the suppression is achieved by decreasing the activation of PI3Knase and Akt[211]. As a result of the decrease in the accumulation of β-catenin, there is a decrease in related proteins, such as c-MYC and COX-2, and p21, induced by black TPs[211]. Additionally, EGCG suppresses β-catenin response transcription, activated by Wnt3a-conditioned medium, to promote the degradation of intracellular β-catenin through a mechanism that is independent of GSK-3β and PP2A (Figure 6)[212].
The 67 kDa laminin receptor pathway: The 67 kDa laminin receptor (67LR), a non-integrin cell surface receptor for the extracellular matrix molecule laminin, is over-expressed in CRC and plays a role in the growth and metastasis of tumor cells and resistance to chemotherapy[213,214]. EGCG, as the target ligand of 67LR, can bind to 67LR to exert a series of effects, ultimately achieving antitumor effects[215]. First, EGCG can mediate 67LR-dependent cell death by eliciting Akt/endothelial nitric oxide synthase/NO/soluble guanylate cyclase/cGMP/protein kinase Cδ/acid sphingomyelinase signaling in CRC[213]. After EGCG binds to 67LR, Akt is phosphorylated, and endothelial nitric oxide synthase is activated, leading to an increase in NO[215]. The production of NO causes an increase in cGMP by activating NO-dependent soluble guanylate cyclase, which is viewed as a rate-determining process, and then protein kinase Cδ and acid sphingomyelinase are activated[214]. Acid sphingomyelinase is known to be part of the signaling cascade that participates in apoptosis[215]. In addition, the 67LR pathway can also mediate the anti-inflammatory effects of EGCG in lipopolysaccharide-stimulated human colorectal cells[216]. 67LR signaling can activate the Toll-interacting protein, which is a negative regulator of TLRs, to prevent the inflammatory response, further suppressing the onset of CRC[216].
The NF-κB pathway: In CRC, the NF-κB pathway is responsible for suppressing cell proliferation, apoptosis, inflammation, angiogenesis, and metastasis[217]. There are two distinct but interacting arms of the NF-κB pathway: The canonical pathway activated by TNF-α, TLR ligands, and IL-1, and the noncanonical pathway activated by the TNF superfamily members BAFF, CD40, receptor-activated NF-κB ligand, and lymphotoxin β[217]. The canonical NF-κB pathway can be activated by the inhibitor of κB (IκB) kinase (IKK) complex[218]. The proteasomal degradation of IκB is accompanied by the translocation of NF-κB to the nucleus, where it facilitates gene transcription[219]. The noncanonical pathway is activated in response to β-catenin and also involved in tumor proliferation and growth, angiogenesis, and invasiveness by up-regulating the PI3K/Akt cascade, COX-2, and VEGF, among others[219]. As a transcription factor that is necessary for iNOS induction, NF-κB can be inhibited by TPs, and TF3 might have a greater inhibitory effect than other TPs[220]. There is evidence that has indicated that TPs can block the phosphorylation of IKKβ in the cytosolic fraction and reduce the nuclear accumulation of the transcription factor NF-κB[220]. TF3 decreases the protein levels of inducible NO synthase by reducing the expression of iNOS mRNA, and the reduction may be the result of preventing the activation of NF-κB, thereby inhibiting the induction of iNOS transcription[220].
The JAK/STAT pathway: One study analyzed 65 human CRC samples, and the results showed that phosphorylated-STAT3 was correlated with vasculogenic mimicry, which refers to the process by which highly invasive cancer cells mimic endothelial cells by forming blood channels[221]. STAT3, a key member of the JAK/STAT pathway, is constitutively activated in CRC, activated by IL-6[221]. STAT3 is also a convergence point for multiple signaling pathways. In cases of CRC, evidence has demonstrated that the activation of STAT3 and overexpression of cyclin D1 have a relationship and that STAT3 is correlated with both survivin and bcl-x[222]. TPs have the ability to suppress the JAK/STAT pathway[223]. EGCG can decrease elevated levels of phosphorylated STAT1 and STAT3 proteins in a dose-dependent manner[223]. By blocking the JAK/STAT pathway, the cytokine-mediated up-regulation of iNOS and intercellular adhesion molecule-1 (commonly referred to as ICAM-1) can be inhibited[223].
The Nrf2-related factor pathway: Another pathway associated with CRC is the Nrf2 pathway. Usually, the Nrf2 pathway plays a dual role in CRC[224]. On the one hand, Nrf2 prevents tumor initiation, progression and cancer metastasis by eliminating carcinogens[224]. On the other hand, Nrf2 hyperactivity due to the accumulation of DNA damage can lead to the up-regulated expression of downstream genes, which consequently induce metabolic reprogramming and an improved cell proliferation rate[224]. Because of its antioxidative effect, the Kelch-like ECH-associated protein 1 (Keap 1)-Nrf2-antioxidant responsive element signaling pathway could be targeted in CRC for cancer chemoprevention[225]. The evidence indicates that TPs can activate the Nrf2 pathway to prevent CRC[226,227]. Yuan et al[226] elucidated that the protein and mRNA levels of Nrf2 were significantly increased in EGCG-treated mice compared with that in the control group (all P < 0.01). Another study demonstrated that activation of the Nrf2 signaling pathway was probably induced by the up-regulation of p62 and the inhibition of Keap1[227]. In addition, polymeric black TPs can also modulate the Nrf2-antioxidant responsive element pathway in both hepatic and colorectal tissues to induce of the phase II enzymes NAD(P)H quinone oxi-doreductase-1 and GST to accelerate 1,2-dimethylhydrazine metabolism and decrease DNA damage in CRC[211].
In sum, although TPs have the ability to modulate the NF-κB pathway, JAK/STAT pathway and Nrf2 pathway, we do not clearly understand how to regulate this pathway in CRC specifically, so further studies are required.
Anti-inflammatory effect
Some cytokines, such as TNF and IL-6, which are secreted by immune cells (monocyte/macrophage lineages, mast cells, T and B lymphocytes, natural killer cells, and neutrophils), can further activate other factors to induce tumorigenesis[228-230]. TPs can inhibit the production of TNF-α and IL-6 to inhibit the inflammation response[166]. Moreover, the excessive cytokines can modulate the expression of target genes, such as COX-2[219]. COX is modulated by cytokines, and COX-2, especially, can mediate inflammation. COX-2 has been found to be overexpressed in early and advanced CRC tissues, which is associated with a poor prognosis[229]. COX-1 has been hypothesized to function as a housekeeping gene for the production of cytoprotective PGs in the gastrointestinal tract, whereas COX-2 is an immediate early gene that is thought to be involved in inflammation, mitogenesis, specialized signal transduction mechanisms, or a combination of these processes[231]. NF-κB and Wnt/β-catenin signaling have both been shown to regulate the expression of COX-2 (under hypoxic conditions) and TNF-α[231]. Similarly, Ras and PI3K signaling are also involved in the expression of COX-2 at a transcriptional and post-transcriptional level[231].
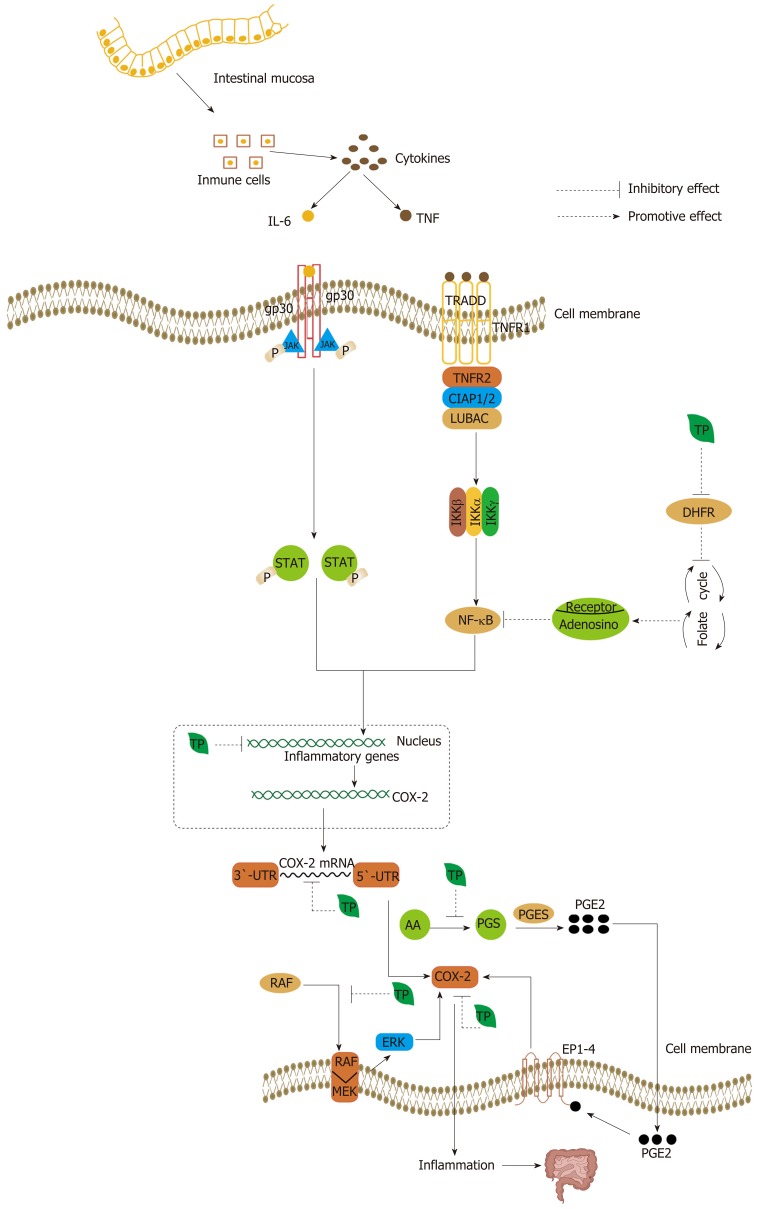
Since COX-2 signaling plays a crucial role in colorectal carcinogenesis, the reduction of COX-2 can reduce inflammation and prevent tumorigenesis[232]. TPs inhibit the expression of COX-2 in a multilevel process. TF2 can inhibit the expression of COX-2 at both the mRNA and protein level (Figure 7)[233,234]. In addition, TNF-α, iNOS, ICAM-1, and NF-κB are strongly down-regulated[234]. The EGCG-induced inhibition of COX-2 occurs at the mRNA transcriptional level. EGCG can decrease the stability of COX-2 mRNA through the COX-2 3’-untranslated region (Figure 7)[235]. Similarly, at the post-transcriptional level, EGCG can also block the translocation of RAF-1 from the cytosol to the plasma membrane, thereby disrupting the association of MEK-1 and RAF-1. The inhibition of MEK-1 activation can affect downstream ERK, so that it can contribute to a decrease in the expression of COX-2 (Figure 7)[235]. Besides, fermented Pu-erh tea X has also shown an anti-inflammation effect, via decreased expression of NF-κB, iNOS and COX-2 messenger RNA, and increased expression of IκB-α[148]. By inhibiting COX-2 directly and indirectly by TPs, the secretion of cytokines by immune cells is suppressed indirectly, and then the inflammatory response can be controlled. In addition, the anti-inflammatory property of EGCG is linked to antifolate activity. EGCG is known as an “antifolate” because it can inhibit dihydrofolate reductase, and this interferes with DNA biosynthesis[236]. As a result, tumor cells are more sensitive to inhibitors of their formation and development[236]. EGCG can mediate the release of adenosine following the disruption of the folate cycle. The release of adenosine can inhibit the Akt and NF-κB pathway by binding to specific receptors that are produced by a significant increase by EGCG[236].
Figure 7.
Tumor necrosis factor from immune cells can activate nuclear factor-kappa B, a central activator that induces the expression of inflammatory genes, such as COX-2, tumor necrosis factor-alpha, inducible nitric oxide synthase, and ICAM-1, and tumor cell survival, proliferation, invasion, angiogenesis, and metastasis are affected when tumor necrosis factor binds to tumor necrosis factor receptor 1 and assembles with tumor necrosis factor receptor 1-associated death domain protein, tumor necrosis factor receptor-associated factor 2, cellular inhibitor of apoptosis protein 1 or 2, and linear ubiquitin chain assembly complex[219,230]. Similarly, another cytokine, interleukin (IL)-6, is also secreted in the tumor microenvironment. The binding of IL-6 to the IL-6 receptor activates STAT3, a major oncogenic transcription factor[229]. After binding to the IL-6 receptor at the cell surface, gp130 dimerization and activation and the transphosphorylation of JAKs are achieved and then contribute to the phosphorylation of STAT3, causing its translocation from the cytoplasm to the nucleus, where it modulates the expression of a variety of genes[229]. Tea polyphenols (TPs) can inhibit inflammatory gene expression caused by cytokines secreted by immune cells in colorectal cancer, with the suppression of COX-2 being particularly important. TPs can inhibit COX-2 in a multilevel process, majorly at the transcriptional and post-transcriptional level. In addition, (-)-epigallocatechin-3-gallate (EGCG) can suppress the expression of nuclear factor-kappa B (NF-κB) through interruption of folate cycle. EGCG can inhibit dihydrofolate reductase and thereby interfere with the folate cycle, causing the release of adenosine. Adenosine binding to specific receptors inhibits the activation of NF-κB. In addition, prostaglandin E2 (PGE2) can also activate COX-2 by stimulating EP receptor (EP1-4) signaling, with the subsequent induction of colorectal cancer onset. Moreover, TPs can inhibit the synthesis of PGE2 and thereby suppress the expression of COX-2. TNF: Tumor necrosis factor; TRADD: TNFR1-associated death domain protein; TRAF2: TNFR-associated factor 2; LUBAC: Linear ubiquitin chain assembly complex; IL-6: Interleukin 6; TP: Tea polyphenol.
In addition, PGs, being cyclooxygenase metabolites of arachidonic acid, are abundantly produced in tumor tissues, and PGE2 is predominant[237,238]. Many types of cells, including tumor cells, fibroblasts, endothelial cells, and immune cells, produce PGE2 in response to the activation of COX-2 and PGE synthase (Figure 7)[237,238]. Moreover, PGE2 can also activate COX-2 by stimulating EP receptor (EP1-4) signaling, with subsequent enhancement of cellular proliferation, promotion of angiogenesis, inhibition of apoptosis, stimulation of invasion/motility, and suppression of immune responses in CRC (Figure 7)[239]. TPs can inhibit COX and LPO-dependent arachidonic acid metabolism in human colonic tissues to reduce risk of CRC, with ECG showing the strongest inhibitory effects[240]. Reduced PGE2 levels can also decrease the synthesis of COX-2 (Figure 7). It has been reported that green tea can inhibit PGE2 synthesis and thus suppress the development and metastasis of CRC in colorectal mucosa in the population[241]. Surprisingly, the formation of the PGE2 metabolites thromboxane (TBX) and 12-hydroxyheptadecatrienoic acid is also inhibited by TPs, because they may affect the interaction of COX-2 with other microsomal factors in tumor microsomes[240].
Anti-oxidation and pro-oxidation
Oxidation stress is viewed as the major mechanism to initiate CRC. The antitumor effects of TPs are undoubtedly tied to pro- and anti-oxidative properties. Anti-oxidation and pro-oxidation by TPs, especially EGCG, have been recently studied in CRC. Depending on their chemical structure, TPs can act as anti-oxidants, pro-oxidants, or both[242]. On the one hand, the effect of anti-oxidation depends on the chemical structure of TPs. The phenol rings of TPs can act as electron traps and scavengers of free radicals, inhibiting the formation of ROS and reducing the harm caused by oxidative stress[242]. Compared with monomeric catechin, the reducing power of synthetic polycatechins is lower[243]. Reducing power is associated with molecular weight[243]. The reducing power was found to be lower with increased molecular weight[243]. In addition, TPs can also enhance the expression of anti-oxidant enzymes, including superoxide dismutase and glutathione peroxidase[244]. The polycatechins showed great amplification of superoxide scavenging activity, xanthine oxidase inhibitory activity, and compared to monomeric catechin: These activities were proportional to their molecular weights[243]. Similarly, because of its phenolic hydroxyl group, GA can inhibit LPO, decrease LPO products, and deplete the levels of antioxidants in CRC[245]. GA can also suppress ROS and enhance the level of GSH[245]. All of these studies have indicated that GA has anti-oxidative properties. On the other hand, pro-oxidative activity is usually the result of the auto-oxidation of TPs in high concentrations[242]. TPs can be oxidized by superoxide anion (O2-) radicals, which affect their stability[246]. TPs can work directly or indirectly to promote cytotoxicity as part of their antitumor activity; they promote it directly by producing hydrogen peroxide and indirectly by reducing Fe(III) to Fe(II), which induces the creation of more potent ROS by the Fenton reaction[247].
In CRC, TPs can also exert the effect of pro-oxidation to inhibit carcinogenesis. By producing ROS, TP can modulate the expression of MMPs. MMPs are a family of tightly regulated zinc-dependent endopeptidases that can degrade nearly all components of the extracellular matrix and basement membrane and mediate the EMT of primary epithelial tumors and their subsequent metastatic capacity[248,249]. In CRC, MMP2 and MMP7 were shown to have a significant association with a decreased risk of developing CRC, but MMP9 showed a significant association with an increased risk[250]. Therefore, MMP7 can be tested to explore whether it is reduced in CRCs. EGCG, acting as a pro-oxidant, can exert a promotive effect on pro-MMP7 production in a dose- and time-dependent manner by generating ROS, activating ERK 1/2, JNK 1/2, and c-JUN/c-FOS, and potentiating AP-1 transcription by increasing the activity of the Jun/FOS protein superfamily[251].
Pro-apoptotic effect
Apoptosis is divided into the intrinsic pathway and the extrinsic pathway. Both the extrinsic and intrinsic pathways converge in the same terminal execution pathway, which is initiated by the cleavage of caspase-3 and results in the fragmentation of DNA, degradation of cytoskeletal and nuclear proteins, cross-linking of proteins, formation of apoptotic bodies, and expression of ligands for phagocytic cell receptors, and the cell fragments are finally taken up by phagocytic cells[252]. The extrinsic apoptotic signaling pathway is initiated by transmembrane receptor-mediated interactions, which include best-matched ligands and their death receptors, such as FasL/FasR, TNF-α/TNFR1, Apo3L/DR3, tumor necrosis factor-related apoptosis-inducing ligand (TRAIL, also called Apo2L)/DR4, and Apo2L/DR5[252,253]. The intrinsic apoptotic signaling pathway, also known as the mitochondrial pathway of apoptosis, involves a variety of stimuli that act on multiple targets within the cell[254]. Two main groups of normally sequestered pro-apoptotic proteins, such as cytochrome c, are released in response to the stimuli[255]. The Bcl-2 family of proteins can regulate these apoptotic mitochondrial pathways positively or negatively. This family includes four anti-apoptotic proteins (Bcl-xL, Bcl-w, A1, and Mcl1) and two groups of proteins that promote cell death (the Bax and the BH3-only families, such as PUMA) (Figure 8)[256]. The tumor suppressor protein p53 can regulate Bcl-2 family proteins, such as Bax, Noxa, or PUMA (Figure 8)[257].
Figure 8.
Both extrinsic and intrinsic pathways converge in the same terminal execution pathway, which is initiated by the cleavage of caspase-3. In the extrinsic pathway, FasL/FasR and tumor necrosis factor-alpha (TNF-α)/ tumor necrosis factor receptor (TNFR)1 associate with the adapter protein called Fas-associated death domain (FADD), and procaspase-8 and TNF-α/TNFR1 also need to bind to TNFR-associated death domain to initiate the execution pathway[253]. In the intrinsic pathway, all stimuli can change the inner mitochondrial membrane to initiate the mitochondrial permeability transition and release two main groups of normally sequestered pro-apoptotic proteins, such as cytochrome c, from the mitochondria into the cytosol[255]. These proteins activate procaspase-9 and caspase-9 to activate the caspase-dependent mitochondrial pathway[253]. Tea polyphenols (TPs) can promote apoptosis of tumor cells. In the intrinsic pathway, TPs can up-regulate p53, Bax, and PUMA, which are proteins that promote cell death. In addition, TPs can also induce the release of cytochrome c from the mitochondria into the cytosol. In the extrinsic pathway, TPs induce apoptosis, mediated by caspase-8, through a FADD-dependent pathway. FADD: Fas-associated death domain; TRADD: TNFR-associated death domain; TP: Tea polyphenol.
TPs can promote both the intrinsic and extrinsic pathways of apoptosis in CRC cells. In the extrinsic apoptotic pathway, green TPs induce apoptosis in intestinal epithelia mediated by caspase-8 through a FADD-dependent pathway. They can increase caspase activities, block NF-κB activation, and lead to the activation of FADD and recruitment of the Fas/CD95 domain[143,166]. Moreover, TPs can also directly bind to Fas death receptor to initiate caspase-8, which mediates apoptosis[191]. In addition, EGCG can prevent TRAIL-induced tumor cell death by antagonizing the TRAIL pathway by decreasing the binding of DR4 and DR5 and activating autophagy (Figure 8)[258]. Usually, autophagic flux is involved in TRAIL-induced apoptosis, and the activation of autophagy can protect against TRAIL-induced cell death[258]. In the intrinsic pathway, p53 and Bax are up-regulated, and the permeabilization of mitochondria is mediated by TPs[234]. Fermented Pu-erh tea can induce intrinsic apoptosis by increased expression of Bax, caspase-9, and caspase-3 messenger RNA and decreased expression of Bcl-2[148]. Green TPs can induce the expression of PUMA by inhibiting the activities of ERK1/2[259,260]. The release of cytochrome c and the pro-apoptotic protein Bax can be induced by TPs (Figure 8)[233]. Moreover, EGCG can activate AMPK signaling to inhibit COX-2 by generating ROS, which are activation signals upstream of AMPK, leading to pro-apoptosis and antitumor effects (Figure 8)[261].
The NSAID activated gene NAG-1 was identified a pro-apoptotic and antitumorigenic protein with homology to members of the TGF-β superfamily[262]. In the induction of NAG-1, even though the presence of a hydroxyl group may play a pivotal role, the function of ECG, which lacks a hydroxyl group in the B ring, is more potent than EGCG, indicating that the mechanisms by which the two types of TPs induce NAG-1 are different[262]. EGCG can induce NAG-1 through a p53-dependent mechanism (Figure 8)[262]. However, ECG-induced NAG-1 expression is mediated by ATF3 in a p53-independent manner[262]. The over-expression of ATF3 may inhibit cell growth and slow cell cycle progression from the G1 phase to the S phase[263]. In the process of NAG-1 induction by ECG, thrombospondin-1 induced by ECG is an early effector in the suppression of angiogenesis and modulation of the activity of TGF-β1[262].
Modulation of gut microbiota
TPs might alter the human intestinal and oral microbiomes that are relevant to intestinal dysbiosis, which has been associated with colorectal carcinogenesis[87]. On the one hand, TPs can modulate the composition of gut microbiota. A study demonstrated that green TPs could induce elevated levels of short-chain fatty acids producing so-called “beneficial” bacterial genera, such as Faecalibacterium, Blautia, Bifidobacterium, Roseburia, Eubacterium, and Coprococcus, and reduce the functional markers of inflammation[87]. Short-chain fatty acids have potent anti-inflammatory properties, including the promotion of colonic Treg expansion and the production of bile acid products, such as ursodeoxycholic acid, to protect hosts from inflammation[264]. Biofilms are massive bacterial invasions of the mucus layer and consist of many different types of bacteria (and even fungi), such as Bacteroidetes, to promote the formation of CRC[264]. TPs can induce the irreversible elevation of Fimicutes, reduction of Bacteroidetes, and elevation of FIR/BAC to reduce the formation of biofilms[87]. In addition, green TPs can also deplete Peptostreptococcaceae, which has been previously linked to the CRC phenotype in a dose-dependent manner, and related microbial genes may be modified[265]. Similarly, green TPs also can reduce the abundance of Fusobacterium[87]. On the other hand, TPs are also metabolized by esterase and glucosidase, and the demethylation, dehydroxylation, and decarboxylation activities of bacteria can further facilitate the absorption of TPs by intestinal mucosa[265].
TPs might regulate the immune system through modulating the gut microbiota, in the tumor microenvironment. It has been hypothesized that TPs may promote T lymphocyte proliferation and reduce M2 macrophages. Besides, other immune cells such as B lymphocytes, NK cells, neutrophils, eosinophils and dendritic cells may be regulated to some extent respectively. However, there is not enough evidence to confirm these speculations. Further research on the specific mechanisms of TPs regulating immune cells through gut microbiota should be undertaken. Not only that, different forms of TPs might exert different impacts on lymphocytes. In bulk form, TPs were shown to produce a statistically significant reduction in DNA damage in lymphocytes[266,267]. In contrast, in NP form, TPs, although initially causing a reduction, were shown to produce a statistically significant increase in DNA damage in the lymphocytes[267]. This finding may support the notion that TPs can exert both anti-oxidant and pro-oxidant functions. This study also suggested whether different forms of TPs can play different roles in other immune cells, such as macrophages, granulocytes and different subtypes of lymphocytes. More investigations are also required to be carried out in the future.
MPE
Nowadays, there is still a lack of MPE studies on TPs for prevention and treatment of CRC. However, in actuality, MPE studies on TPs and CRC are worthy carrying out. These studies can uncover many aspects, not only for different kinds of TPs but also for different subtypes of CRC. In addition, whether other endogenous or exogenous factors will impact the effect of TPs on CRC remains a question, and MPE studies may deal with this issue. Moreover, the pharmaco-MPE, immuno-MPE, and microbial MPE should be considered in the studies of impact of TPs on CRC. These are novel and important directions for investigators in the future. MPE studies are beneficial to understanding potential causal mechanisms of TPs acting on CRC. MPE studies are also helpful for the diagnosis and treatment of CRC using TPs earlier and more precisely in clinic. Overall, MPE studies might represent an essential direction for studying the TPs and CRC in the future, to satisfy the requirement of precision medicine.
Epigenetic mechanism
Epigenetic gene silencing, which involves DNMTs and histone deacetylases (HDACs), plays an important role in the formation and progression of CRC and can lead to the silencing of some normally regulated genes, such as the mismatch repair and tumor suppressor genes[268]. EGCG can promote the degradation of DNMT3A and HDAC3 to disrupt the expression of genes by reducing the interaction between the E3 ubiquitin ligase UHRF1 and DNM3A[268]. Thereby, gene silencing actions of HDACs and DNMTs are inhibited by EGCG[268]. Butyrate (NaB), one of the principal products of dietary fiber fermentation, induces differentiation in colon cancer cell lines by inhibiting HDACs[269]. EGCG and EC can also inhibit NaB-induced differentiation in the human colon adenocarcinoma HT29 cells[269]. The effect could be due to an interaction between NaB and EGCG and EC that prevents the entry and cellular action of NaB. EGCG and EC might inhibit H+-coupled monocarboxylate transporter 1-mediated NaB transport by altering lipid raft organization[269]. RXRα expression is decreased in malignant human colorectal tumors as a result of the silencing of regulatory genes through the hypermethylation of CpG islands[270]. EGCG can restore activity levels of the nuclear transcription factor RXRα and reduce RXRα promoter methylation. Subsequently, nuclear β-catenin and cyclin D1 levels decrease, and cell proliferation is disrupted[270].
Other factors
Some enzymes are modulated by TPs and as a result prevent the growth and metabolism of CRC. Telomerase, as a cancer-associated enzyme, has a major function in the maintenance of telomeres and the extension of cellular life span, as well as DNA repair and anti-apoptotic activities[271,272]. EGCG can block telomerase and limit the growth of tumor cells[273]. By inhibiting telomerase activity, STAT3 and STAT1 interactions are induced to reduce the cancer stem cell phenotype[274]. EGCG can also inhibit topoisomerase II activity, and Ardisia compressa tea more effectively inhibits topoisomerase II activity and thus suppresses the proliferation of cancer cells[275]. Class II topoisomerase inhibitors inhibit either the DNA binding or DNA cleavage step of the enzymatic reaction; thus, DNA breaks are not generated, and tumor cells with defective decatenation checkpoints fail to arrest their cell cycle in the G2 phase and enter the M phase[276].
Moreover, green TPs have been reported to inhibit tumors by preventing protein carbonylation in the tumor tissue environment, which depends on the pH of the medium surrounding the tissue, the type of tumor, the stage of dysregulation of lipid peroxidation, and the stage of carcinoma development[277].
CONCLUSION AND FURTHER DIRECTION
Overall, most studies, both in vitro and in vivo, support the notion that drinking tea can be beneficial to health and that TPs play a predominant role in the promotion of health. Worldwide, many scientists have indicated that TPs can inhibit the growth and metastasis of CRC in a comprehensive multi-process mechanism. Diet and lifestyle not only play an important role in the onset of CRC but also enhance or diminish the impact of TPs on CRC. TPs can inhibit the proliferation of tumor cells by the modulation of signaling pathways, such as the MAPK pathway, PI3K/Akt pathway, Wnt/β-catenin pathway, and JAK/STAT pathway. TPs can exert their anti-oxidative or pro-oxidative properties to suppress the growth of CRC. Meanwhile, TPs can promote tumor cell apoptosis by facilitating extrinsic and intrinsic apoptosis. TPs have also been shown to be able to induce cell autophagy by inhibiting TRAIL-mediated apoptosis. In addition, TPs can also modulate the components of gut microbiota to improve immunity, regulate immune cells and decrease the inflammatory response by increasing beneficial bacteria, and reducing harmful bacteria. Inflammation is also inhibited by suppressing the expression of COX-2 in a multilevel process. Finally, TPs serve a role in epigenetic mechanisms by promoting the degradation of DNMT3A and HDAC3 and restoring RXRα activity levels. TPs can also decrease chemoresistance in combination with chemotherapy medicines. Therefore, TPs have widespread roles in the prevention and treatment of CRC.
At present, the properties and application of EGCG are attaining increasing interest but there are not enough studies to determine whether other TPs can be applied in the same way. Therefore, further investigation should be carried out to confirm the specific mechanisms of each TP. In addition, because of the poor oral bioavailability of TPs, it is difficult to judge the specific amount of TPs that should be recommended to the population to promote health. To date, many clinical trials have been conducted to demonstrate the function of TPs in the mitigation of many diseases, such as obesity and breast cancer[278,279]. However, more randomized, double-blind, crossover, and placebo-controlled clinical trials with large samples from multiple centers are required to identify the effect and concentration of TPs in even more diseases. The route of injection may affect bioavailability. A novel study examined transdermal EGCG[280] and additional studies are required to identify the efficacy and risk. The interaction of TPs and other foods and lifestyle on CRC should be discussed more deeply in the future. Although MPE studies have made great progress on CRC, there is still a lack of relative investigations between different subtypes of CRC and different kinds of TPs. MPE can satisfy the requirement of precision medicine and provide a novel direction for further research. It will be helpful to prevent and treat CRC using TPs by gaining more precise information for clinical application. Overall, TPs, as newly applied biological agents, still have many unknown fields that are waiting to be explored. Not only animal studies but also epidemiological studies will be indispensable.
Footnotes
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
Conflict-of-interest statement: We declare no conflicts of interest.
Peer-review started: October 19, 2019
First decision: December 4, 2019
Article in press: January 14, 2020
P-Reviewer: Ogino S S-Editor: Gong ZM L-Editor: Filipodia E-Editor: Zhang YL
Contributor Information
Shi-Tong Wang, Department of Cardiovascular Ultrasound, First Affiliated Hospital of China Medical University, Shenyang 110001, Liaoning Province, China.
Wen-Qi Cui, Department of Neurology, Shengjing Hospital, Affiliated Hospital of China Medical University, Shenyang 110004, Liaoning Province, China.
Dan Pan, Department of Geriatrics, First Affiliated Hospital of China Medical University, Shenyang 110001, Liaoning Province, China.
Min Jiang, Department of Gastroenterology, First Affiliated Hospital of China Medical University, Shenyang 110001, Liaoning Province, China.
Bing Chang, Department of Gastroenterology, First Affiliated Hospital of China Medical University, Shenyang 110001, Liaoning Province, China.
Li-Xuan Sang, Department of Geriatrics, First Affiliated Hospital of China Medical University, Shenyang 110001, Liaoning Province, China. sanglixuan2008@163.com.
References
- 1.Mármol I, Sánchez-de-Diego C, Pradilla Dieste A, Cerrada E, Rodriguez Yoldi MJ. Colorectal Carcinoma: A General Overview and Future Perspectives in Colorectal Cancer. Int J Mol Sci. 2017;18 doi: 10.3390/ijms18010197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Siegel RL, Miller KD, Fedewa SA, Ahnen DJ, Meester RGS, Barzi A, Jemal A. Colorectal cancer statistics, 2017. CA Cancer J Clin. 2017;67:177–193. doi: 10.3322/caac.21395. [DOI] [PubMed] [Google Scholar]
- 3.Brenner H, Kloor M, Pox CP. Colorectal cancer. Lancet. 2014;383:1490–1502. doi: 10.1016/S0140-6736(13)61649-9. [DOI] [PubMed] [Google Scholar]
- 4.Yiu AJ, Yiu CY. Biomarkers in Colorectal Cancer. Anticancer Res. 2016;36:1093–1102. [PubMed] [Google Scholar]
- 5.Dulai PS, Sandborn WJ, Gupta S. Colorectal Cancer and Dysplasia in Inflammatory Bowel Disease: A Review of Disease Epidemiology, Pathophysiology, and Management. Cancer Prev Res (Phila) 2016;9:887–894. doi: 10.1158/1940-6207.CAPR-16-0124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Van Blarigan EL, Meyerhardt JA. Role of physical activity and diet after colorectal cancer diagnosis. J Clin Oncol. 2015;33:1825–1834. doi: 10.1200/JCO.2014.59.7799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Conti L, Del Cornò M, Gessani S. Revisiting the impact of lifestyle on colorectal cancer risk in a gender perspective. Crit Rev Oncol Hematol. 2019;145:102834. doi: 10.1016/j.critrevonc.2019.102834. [DOI] [PubMed] [Google Scholar]
- 8.Azeem S, Gillani SW, Siddiqui A, Jandrajupalli SB, Poh V, Syed Sulaiman SA. Diet and Colorectal Cancer Risk in Asia--a Systematic Review. Asian Pac J Cancer Prev. 2015;16:5389–5396. doi: 10.7314/apjcp.2015.16.13.5389. [DOI] [PubMed] [Google Scholar]
- 9.Steck SE, Guinter M, Zheng J, Thomson CA. Index-based dietary patterns and colorectal cancer risk: a systematic review. Adv Nutr. 2015;6:763–773. doi: 10.3945/an.115.009746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Alexander DD, Weed DL, Miller PE, Mohamed MA. Red Meat and Colorectal Cancer: A Quantitative Update on the State of the Epidemiologic Science. J Am Coll Nutr. 2015;34:521–543. doi: 10.1080/07315724.2014.992553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wada K, Oba S, Tsuji M, Tamura T, Konishi K, Goto Y, Mizuta F, Koda S, Hori A, Tanabashi S, Matsushita S, Tokimitsu N, Nagata C. Meat consumption and colorectal cancer risk in Japan: The Takayama study. Cancer Sci. 2017;108:1065–1070. doi: 10.1111/cas.13217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Carr PR, Jansen L, Bienert S, Roth W, Herpel E, Kloor M, Bläker H, Chang-Claude J, Brenner H, Hoffmeister M. Associations of red and processed meat intake with major molecular pathological features of colorectal cancer. Eur J Epidemiol. 2017;32:409–418. doi: 10.1007/s10654-017-0275-6. [DOI] [PubMed] [Google Scholar]
- 13.Ananthakrishnan AN, Du M, Berndt SI, Brenner H, Caan BJ, Casey G, Chang-Claude J, Duggan D, Fuchs CS, Gallinger S, Giovannucci EL, Harrison TA, Hayes RB, Hoffmeister M, Hopper JL, Hou L, Hsu L, Jenkins MA, Kraft P, Ma J, Nan H, Newcomb PA, Ogino S, Potter JD, Seminara D, Slattery ML, Thornquist M, White E, Wu K, Peters U, Chan AT. Red meat intake, NAT2, and risk of colorectal cancer: a pooled analysis of 11 studies. Cancer Epidemiol Biomarkers Prev. 2015;24:198–205. doi: 10.1158/1055-9965.EPI-14-0897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Demeyer D, Mertens B, De Smet S, Ulens M. Mechanisms Linking Colorectal Cancer to the Consumption of (Processed) Red Meat: A Review. Crit Rev Food Sci Nutr. 2016;56:2747–2766. doi: 10.1080/10408398.2013.873886. [DOI] [PubMed] [Google Scholar]
- 15.Etemadi A, Sinha R, Ward MH, Graubard BI, Inoue-Choi M, Dawsey SM, Abnet CC. Mortality from different causes associated with meat, heme iron, nitrates, and nitrites in the NIH-AARP Diet and Health Study: population based cohort study. BMJ. 2017;357:j1957. doi: 10.1136/bmj.j1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hodge AM, Williamson EJ, Bassett JK, MacInnis RJ, Giles GG, English DR. Dietary and biomarker estimates of fatty acids and risk of colorectal cancer. Int J Cancer. 2015;137:1224–1234. doi: 10.1002/ijc.29479. [DOI] [PubMed] [Google Scholar]
- 17.Jones R, Adel-Alvarez LA, Alvarez OR, Broaddus R, Das S. Arachidonic acid and colorectal carcinogenesis. Mol Cell Biochem. 2003;253:141–149. doi: 10.1023/a:1026060426569. [DOI] [PubMed] [Google Scholar]
- 18.Song M, Nishihara R, Wu K, Qian ZR, Kim SA, Sukawa Y, Mima K, Inamura K, Masuda A, Yang J, Fuchs CS, Giovannucci EL, Ogino S, Chan AT. Marine ω-3 polyunsaturated fatty acids and risk of colorectal cancer according to microsatellite instability. J Natl Cancer Inst. 2015;107 doi: 10.1093/jnci/djv007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Song M, Nishihara R, Cao Y, Chun E, Qian ZR, Mima K, Inamura K, Masugi Y, Nowak JA, Nosho K, Wu K, Wang M, Giovannucci E, Garrett WS, Fuchs CS, Ogino S, Chan AT. Marine ω-3 Polyunsaturated Fatty Acid Intake and Risk of Colorectal Cancer Characterized by Tumor-Infiltrating T Cells. JAMA Oncol. 2016;2:1197–1206. doi: 10.1001/jamaoncol.2016.0605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Larsson SC, Kumlin M, Ingelman-Sundberg M, Wolk A. Dietary long-chain n-3 fatty acids for the prevention of cancer: a review of potential mechanisms. Am J Clin Nutr. 2004;79:935–945. doi: 10.1093/ajcn/79.6.935. [DOI] [PubMed] [Google Scholar]
- 21.Escrich E, Moral R, Grau L, Costa I, Solanas M. Molecular mechanisms of the effects of olive oil and other dietary lipids on cancer. Mol Nutr Food Res. 2007;51:1279–1292. doi: 10.1002/mnfr.200700213. [DOI] [PubMed] [Google Scholar]
- 22.Pelucchi C, Bosetti C, Negri E, Lipworth L, La Vecchia C. Olive oil and cancer risk: an update of epidemiological findings through 2010. Curr Pharm Des. 2011;17:805–812. doi: 10.2174/138161211795428920. [DOI] [PubMed] [Google Scholar]
- 23.Hamada T, Liu L, Nowak JA, Mima K, Cao Y, Ng K, Twombly TS, Song M, Jung S, Dou R, Masugi Y, Kosumi K, Shi Y, da Silva A, Gu M, Li W, Keum N, Wu K, Nosho K, Inamura K, Meyerhardt JA, Nevo D, Wang M, Giannakis M, Chan AT, Giovannucci EL, Fuchs CS, Nishihara R, Zhang X, Ogino S. Vitamin D status after colorectal cancer diagnosis and patient survival according to immune response to tumour. Eur J Cancer. 2018;103:98–107. doi: 10.1016/j.ejca.2018.07.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Al-Ghafari AB, Balamash KS, Al Doghaither HA. Relationship between Serum Vitamin D and Calcium Levels and Vitamin D Receptor Gene Polymorphisms in Colorectal Cancer. Biomed Res Int. 2019;2019:8571541. doi: 10.1155/2019/8571541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhou X, Chen C, Zhong YN, Zhao F, Hao Z, Xu Y, Lai R, Shen G, Yin X. Effect and mechanism of vitamin D on the development of colorectal cancer based on intestinal flora disorder. J Gastroenterol Hepatol. 2019 doi: 10.1111/jgh.14949. [DOI] [PubMed] [Google Scholar]
- 26.Zhong X, Fang YJ, Pan ZZ, Lu MS, Zheng MC, Chen YM, Zhang CX. Dietary fiber and fiber fraction intakes and colorectal cancer risk in Chinese adults. Nutr Cancer. 2014;66:351–361. doi: 10.1080/01635581.2013.877496. [DOI] [PubMed] [Google Scholar]
- 27.Bradbury KE, Appleby PN, Key TJ. Fruit, vegetable, and fiber intake in relation to cancer risk: findings from the European Prospective Investigation into Cancer and Nutrition (EPIC) Am J Clin Nutr. 2014;100 Suppl 1:394S–398S. doi: 10.3945/ajcn.113.071357. [DOI] [PubMed] [Google Scholar]
- 28.Terry P, Giovannucci E, Michels KB, Bergkvist L, Hansen H, Holmberg L, Wolk A. Fruit, vegetables, dietary fiber, and risk of colorectal cancer. J Natl Cancer Inst. 2001;93:525–533. doi: 10.1093/jnci/93.7.525. [DOI] [PubMed] [Google Scholar]
- 29.He X, Wu K, Zhang X, Nishihara R, Cao Y, Fuchs CS, Giovannucci EL, Ogino S, Chan AT, Song M. Dietary intake of fiber, whole grains and risk of colorectal cancer: An updated analysis according to food sources, tumor location and molecular subtypes in two large US cohorts. Int J Cancer. 2019;145:3040–3051. doi: 10.1002/ijc.32382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mehta RS, Nishihara R, Cao Y, Song M, Mima K, Qian ZR, Nowak JA, Kosumi K, Hamada T, Masugi Y, Bullman S, Drew DA, Kostic AD, Fung TT, Garrett WS, Huttenhower C, Wu K, Meyerhardt JA, Zhang X, Willett WC, Giovannucci EL, Fuchs CS, Chan AT, Ogino S. Association of Dietary Patterns With Risk of Colorectal Cancer Subtypes Classified by Fusobacterium nucleatum in Tumor Tissue. JAMA Oncol. 2017;3:921–927. doi: 10.1001/jamaoncol.2016.6374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Song M, Wu K, Meyerhardt JA, Ogino S, Wang M, Fuchs CS, Giovannucci EL, Chan AT. Fiber Intake and Survival After Colorectal Cancer Diagnosis. JAMA Oncol. 2018;4:71–79. doi: 10.1001/jamaoncol.2017.3684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vulcan A, Brändstedt J, Manjer J, Jirström K, Ohlsson B, Ericson U. Fibre intake and incident colorectal cancer depending on fibre source, sex, tumour location and Tumour, Node, Metastasis stage. Br J Nutr. 2015;114:959–969. doi: 10.1017/S0007114515002743. [DOI] [PubMed] [Google Scholar]
- 33.Hughes DJ, Fedirko V, Jenab M, Schomburg L, Méplan C, Freisling H, Bueno-de-Mesquita HB, Hybsier S, Becker NP, Czuban M, Tjønneland A, Outzen M, Boutron-Ruault MC, Racine A, Bastide N, Kühn T, Kaaks R, Trichopoulos D, Trichopoulou A, Lagiou P, Panico S, Peeters PH, Weiderpass E, Skeie G, Dagrun E, Chirlaque MD, Sánchez MJ, Ardanaz E, Ljuslinder I, Wennberg M, Bradbury KE, Vineis P, Naccarati A, Palli D, Boeing H, Overvad K, Dorronsoro M, Jakszyn P, Cross AJ, Quirós JR, Stepien M, Kong SY, Duarte-Salles T, Riboli E, Hesketh JE. Selenium status is associated with colorectal cancer risk in the European prospective investigation of cancer and nutrition cohort. Int J Cancer. 2015;136:1149–1161. doi: 10.1002/ijc.29071. [DOI] [PubMed] [Google Scholar]
- 34.Fedirko V, Jenab M, Méplan C, Jones JS, Zhu W, Schomburg L, Siddiq A, Hybsier S, Overvad K, Tjønneland A, Omichessan H, Perduca V, Boutron-Ruault MC, Kühn T, Katzke V, Aleksandrova K, Trichopoulou A, Karakatsani A, Kotanidou A, Tumino R, Panico S, Masala G, Agnoli C, Naccarati A, Bueno-de-Mesquita B, Vermeulen RCH, Weiderpass E, Skeie G, Nøst TH, Lujan-Barroso L, Quirós JR, Huerta JM, Rodríguez-Barranco M, Barricarte A, Gylling B, Harlid S, Bradbury KE, Wareham N, Khaw KT, Gunter M, Murphy N, Freisling H, Tsilidis K, Aune D, Riboli E, Hesketh JE, Hughes DJ. Association of Selenoprotein and Selenium Pathway Genotypes with Risk of Colorectal Cancer and Interaction with Selenium Status. Nutrients. 2019;11 doi: 10.3390/nu11040935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hughes DJ, Kunická T, Schomburg L, Liška V, Swan N, Souček P. Expression of Selenoprotein Genes and Association with Selenium Status in Colorectal Adenoma and Colorectal Cancer. Nutrients. 2018;10 doi: 10.3390/nu10111812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Huang G, Liu Z, He L, Luk KH, Cheung ST, Wong KH, Chen T. Autophagy is an important action mode for functionalized selenium nanoparticles to exhibit anti-colorectal cancer activity. Biomater Sci. 2018;6:2508–2517. doi: 10.1039/c8bm00670a. [DOI] [PubMed] [Google Scholar]
- 37.Moazzen S, Dolatkhah R, Tabrizi JS, Shaarbafi J, Alizadeh BZ, de Bock GH, Dastgiri S. Folic acid intake and folate status and colorectal cancer risk: A systematic review and meta-analysis. Clin Nutr. 2018;37:1926–1934. doi: 10.1016/j.clnu.2017.10.010. [DOI] [PubMed] [Google Scholar]
- 38.Majumdar AP, Kodali U, Jaszewski R. Chemopreventive role of folic acid in colorectal cancer. Front Biosci. 2004;9:2725–2732. doi: 10.2741/1431. [DOI] [PubMed] [Google Scholar]
- 39.Jennings BA, Willis G. How folate metabolism affects colorectal cancer development and treatment; a story of heterogeneity and pleiotropy. Cancer Lett. 2015;356:224–230. doi: 10.1016/j.canlet.2014.02.024. [DOI] [PubMed] [Google Scholar]
- 40.Cho YA, Lee J, Oh JH, Shin A, Kim J. Dietary Inflammatory Index and Risk of Colorectal Cancer: A Case-Control Study in Korea. Nutrients. 2016;8 doi: 10.3390/nu8080469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.De Almeida CV, de Camargo MR, Russo E, Amedei A. Role of diet and gut microbiota on colorectal cancer immunomodulation. World J Gastroenterol. 2019;25:151–162. doi: 10.3748/wjg.v25.i2.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Boyle T, Keegel T, Bull F, Heyworth J, Fritschi L. Physical activity and risks of proximal and distal colon cancers: a systematic review and meta-analysis. J Natl Cancer Inst. 2012;104:1548–1561. doi: 10.1093/jnci/djs354. [DOI] [PubMed] [Google Scholar]
- 43.Robsahm TE, Aagnes B, Hjartåker A, Langseth H, Bray FI, Larsen IK. Body mass index, physical activity, and colorectal cancer by anatomical subsites: a systematic review and meta-analysis of cohort studies. Eur J Cancer Prev. 2013;22:492–505. doi: 10.1097/CEJ.0b013e328360f434. [DOI] [PubMed] [Google Scholar]
- 44.Mok Y, Jeon C, Lee GJ, Jee SH. Physical Activity Level and Colorectal Cancer Mortality. Asia Pac J Public Health. 2016;28:638–647. doi: 10.1177/1010539516661761. [DOI] [PubMed] [Google Scholar]
- 45.Eaglehouse YL, Koh WP, Wang R, Aizhen J, Yuan JM, Butler LM. Physical activity, sedentary time, and risk of colorectal cancer: the Singapore Chinese Health Study. Eur J Cancer Prev. 2017;26:469–475. doi: 10.1097/CEJ.0000000000000369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Friedenreich CM, Orenstein MR. Physical activity and cancer prevention: etiologic evidence and biological mechanisms. J Nutr. 2002;132:3456S–3464S. doi: 10.1093/jn/132.11.3456S. [DOI] [PubMed] [Google Scholar]
- 47.de Souza-Teixeira F, Alonso-Molero J, Ayán C, Vilorio-Marques L, Molina AJ, González-Donquiles C, Dávila-Batista V, Fernández-Villa T, de Paz JA, Martín V. PGC-1α as a Biomarker of Physical Activity-Protective Effect on Colorectal Cancer. Cancer Prev Res (Phila) 2018;11:523–534. doi: 10.1158/1940-6207.CAPR-17-0329. [DOI] [PubMed] [Google Scholar]
- 48.Fardet A, Druesne-Pecollo N, Touvier M, Latino-Martel P. Do alcoholic beverages, obesity and other nutritional factors modify the risk of familial colorectal cancer? A systematic review. Crit Rev Oncol Hematol. 2017;119:94–112. doi: 10.1016/j.critrevonc.2017.09.001. [DOI] [PubMed] [Google Scholar]
- 49.Klarich DS, Brasser SM, Hong MY. Moderate Alcohol Consumption and Colorectal Cancer Risk. Alcohol Clin Exp Res. 2015;39:1280–1291. doi: 10.1111/acer.12778. [DOI] [PubMed] [Google Scholar]
- 50.Jayasekara H, MacInnis RJ, Williamson EJ, Hodge AM, Clendenning M, Rosty C, Walters R, Room R, Southey MC, Jenkins MA, Milne RL, Hopper JL, Giles GG, Buchanan DD, English DR. Lifetime alcohol intake is associated with an increased risk of KRAS+ and BRAF-/KRAS- but not BRAF+ colorectal cancer. Int J Cancer. 2017;140:1485–1493. doi: 10.1002/ijc.30568. [DOI] [PubMed] [Google Scholar]
- 51.Nishihara R, Wang M, Qian ZR, Baba Y, Yamauchi M, Mima K, Sukawa Y, Kim SA, Inamura K, Zhang X, Wu K, Giovannucci EL, Chan AT, Fuchs CS, Ogino S, Schernhammer ES. Alcohol, one-carbon nutrient intake, and risk of colorectal cancer according to tumor methylation level of IGF2 differentially methylated region. Am J Clin Nutr. 2014;100:1479–1488. doi: 10.3945/ajcn.114.095539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nan H, Lee JE, Rimm EB, Fuchs CS, Giovannucci EL, Cho E. Prospective study of alcohol consumption and the risk of colorectal cancer before and after folic acid fortification in the United States. Ann Epidemiol. 2013;23:558–563. doi: 10.1016/j.annepidem.2013.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Xu M, Wang S, Qi Y, Chen L, Frank JA, Yang XH, Zhang Z, Shi X, Luo J. Role of MCP-1 in alcohol-induced aggressiveness of colorectal cancer cells. Mol Carcinog. 2016;55:1002–1011. doi: 10.1002/mc.22343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Peppone LJ, Mahoney MC, Cummings KM, Michalek AM, Reid ME, Moysich KB, Hyland A. Colorectal cancer occurs earlier in those exposed to tobacco smoke: implications for screening. J Cancer Res Clin Oncol. 2008;134:743–751. doi: 10.1007/s00432-007-0332-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Limsui D, Vierkant RA, Tillmans LS, Wang AH, Weisenberger DJ, Laird PW, Lynch CF, Anderson KE, French AJ, Haile RW, Harnack LJ, Potter JD, Slager SL, Smyrk TC, Thibodeau SN, Cerhan JR, Limburg PJ. Cigarette smoking and colorectal cancer risk by molecularly defined subtypes. J Natl Cancer Inst. 2010;102:1012–1022. doi: 10.1093/jnci/djq201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lilla C, Verla-Tebit E, Risch A, Jäger B, Hoffmeister M, Brenner H, Chang-Claude J. Effect of NAT1 and NAT2 genetic polymorphisms on colorectal cancer risk associated with exposure to tobacco smoke and meat consumption. Cancer Epidemiol Biomarkers Prev. 2006;15:99–107. doi: 10.1158/1055-9965.EPI-05-0618. [DOI] [PubMed] [Google Scholar]
- 57.Koh WP, Nelson HH, Yuan JM, Van den Berg D, Jin A, Wang R, Yu MC. Glutathione S-transferase (GST) gene polymorphisms, cigarette smoking and colorectal cancer risk among Chinese in Singapore. Carcinogenesis. 2011;32:1507–1511. doi: 10.1093/carcin/bgr175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dino P, D'Anna C, Sangiorgi C, Di Sano C, Di Vincenzo S, Ferraro M, Pace E. Cigarette smoke extract modulates E-Cadherin, Claudin-1 and miR-21 and promotes cancer invasiveness in human colorectal adenocarcinoma cells. Toxicol Lett. 2019;317:102–109. doi: 10.1016/j.toxlet.2019.09.020. [DOI] [PubMed] [Google Scholar]
- 59.Tougeron D, Sha D, Manthravadi S, Sinicrope FA. Aspirin and colorectal cancer: back to the future. Clin Cancer Res. 2014;20:1087–1094. doi: 10.1158/1078-0432.CCR-13-2563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Frouws MA, van Herk-Sukel MPP, Maas HA, Van de Velde CJH, Portielje JEA, Liefers GJ, Bastiaannet E. The mortality reducing effect of aspirin in colorectal cancer patients: Interpreting the evidence. Cancer Treat Rev. 2017;55:120–127. doi: 10.1016/j.ctrv.2016.12.008. [DOI] [PubMed] [Google Scholar]
- 61.Paleari L, Puntoni M, Clavarezza M, DeCensi M, Cuzick J, DeCensi A. PIK3CA Mutation, Aspirin Use after Diagnosis and Survival of Colorectal Cancer. A Systematic Review and Meta-analysis of Epidemiological Studies. Clin Oncol (R Coll Radiol) 2016;28:317–326. doi: 10.1016/j.clon.2015.11.008. [DOI] [PubMed] [Google Scholar]
- 62.Li P, Wu H, Zhang H, Shi Y, Xu J, Ye Y, Xia D, Yang J, Cai J, Wu Y. Aspirin use after diagnosis but not prediagnosis improves established colorectal cancer survival: a meta-analysis. Gut. 2015;64:1419–1425. doi: 10.1136/gutjnl-2014-308260. [DOI] [PubMed] [Google Scholar]
- 63.Drew DA, Cao Y, Chan AT. Aspirin and colorectal cancer: the promise of precision chemoprevention. Nat Rev Cancer. 2016;16:173–186. doi: 10.1038/nrc.2016.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Markowitz SD, Bertagnolli MM. Molecular origins of cancer: Molecular basis of colorectal cancer. N Engl J Med. 2009;361:2449–2460. doi: 10.1056/NEJMra0804588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ponz de Leon M, Percesepe A. Pathogenesis of colorectal cancer. Dig Liver Dis. 2000;32:807–821. doi: 10.1016/s1590-8658(00)80361-8. [DOI] [PubMed] [Google Scholar]
- 66.Yu XF, Zou J, Bao ZJ, Dong J. miR-93 suppresses proliferation and colony formation of human colon cancer stem cells. World J Gastroenterol. 2011;17:4711–4717. doi: 10.3748/wjg.v17.i42.4711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Xu XT, Xu Q, Tong JL, Zhu MM, Nie F, Chen X, Xiao SD, Ran ZH. MicroRNA expression profiling identifies miR-328 regulates cancer stem cell-like SP cells in colorectal cancer. Br J Cancer. 2012;106:1320–1330. doi: 10.1038/bjc.2012.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lu YX, Yuan L, Xue XL, Zhou M, Liu Y, Zhang C, Li JP, Zheng L, Hong M, Li XN. Regulation of colorectal carcinoma stemness, growth, and metastasis by an miR-200c-Sox2-negative feedback loop mechanism. Clin Cancer Res. 2014;20:2631–2642. doi: 10.1158/1078-0432.CCR-13-2348. [DOI] [PubMed] [Google Scholar]
- 69.Graham LD, Pedersen SK, Brown GS, Ho T, Kassir Z, Moynihan AT, Vizgoft EK, Dunne R, Pimlott L, Young GP, Lapointe LC, Molloy PL. Colorectal Neoplasia Differentially Expressed (CRNDE), a Novel Gene with Elevated Expression in Colorectal Adenomas and Adenocarcinomas. Genes Cancer. 2011;2:829–840. doi: 10.1177/1947601911431081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Pennel KAF, Park JH, McMillan DC, Roseweir AK, Edwards J. Signal interaction between the tumour and inflammatory cells in patients with gastrointestinal cancer: Implications for treatment. Cell Signal. 2019;54:81–90. doi: 10.1016/j.cellsig.2018.11.013. [DOI] [PubMed] [Google Scholar]
- 71.Zhou S, Xu H, Tang Q, Xia H, Bi F. Dipyridamole Enhances the Cytotoxicities of the Trametinib Against Colon Cancer Cells Through Combined Targeting HMGCS1 and MEK Pathway. Mol Cancer Ther. 2019 doi: 10.1158/1535-7163.MCT-19-0413. [DOI] [PubMed] [Google Scholar]
- 72.Zhao B, Li L, Lei Q, Guan KL. The Hippo-YAP pathway in organ size control and tumorigenesis: an updated version. Genes Dev. 2010;24:862–874. doi: 10.1101/gad.1909210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Liu H, Jiang D, Chi F, Zhao B. The Hippo pathway regulates stem cell proliferation, self-renewal, and differentiation. Protein Cell. 2012;3:291–304. doi: 10.1007/s13238-012-2919-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wierzbicki PM, Rybarczyk A. The Hippo pathway in colorectal cancer. Folia Histochem Cytobiol. 2015;53:105–119. doi: 10.5603/FHC.a2015.0015. [DOI] [PubMed] [Google Scholar]
- 75.Ray AL, Berggren KL, Restrepo Cruz S, Gan GN, Beswick EJ. Inhibition of MK2 suppresses IL-1β, IL-6, and TNF-α-dependent colorectal cancer growth. Int J Cancer. 2018;142:1702–1711. doi: 10.1002/ijc.31191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Meira LB, Bugni JM, Green SL, Lee CW, Pang B, Borenshtein D, Rickman BH, Rogers AB, Moroski-Erkul CA, McFaline JL, Schauer DB, Dedon PC, Fox JG, Samson LD. DNA damage induced by chronic inflammation contributes to colon carcinogenesis in mice. J Clin Invest. 2008;118:2516–2525. doi: 10.1172/JCI35073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Landskron G, De la Fuente M, Thuwajit P, Thuwajit C, Hermoso MA. Chronic inflammation and cytokines in the tumor microenvironment. J Immunol Res. 2014;2014:149185. doi: 10.1155/2014/149185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Koido S, Ohkusa T, Homma S, Namiki Y, Takakura K, Saito K, Ito Z, Kobayashi H, Kajihara M, Uchiyama K, Arihiro S, Arakawa H, Okamoto M, Gong J, Tajiri H. Immunotherapy for colorectal cancer. World J Gastroenterol. 2013;19:8531–8542. doi: 10.3748/wjg.v19.i46.8531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Stenmark H. Rab GTPases as coordinators of vesicle traffic. Nat Rev Mol Cell Biol. 2009;10:513–525. doi: 10.1038/nrm2728. [DOI] [PubMed] [Google Scholar]
- 80.Chang YC, Su CY, Chen MH, Chen WS, Chen CL, Hsiao M. Secretory RAB GTPase 3C modulates IL6-STAT3 pathway to promote colon cancer metastasis and is associated with poor prognosis. Mol Cancer. 2017;16:135. doi: 10.1186/s12943-017-0687-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Goldenring JR, Nam KT. Rab25 as a tumour suppressor in colon carcinogenesis. Br J Cancer. 2011;104:33–36. doi: 10.1038/sj.bjc.6605983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lin J, Chuang CC, Zuo L. Potential roles of microRNAs and ROS in colorectal cancer: diagnostic biomarkers and therapeutic targets. Oncotarget. 2017;8:17328–17346. doi: 10.18632/oncotarget.14461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.He J, Jiang BH. Interplay between Reactive oxygen Species and MicroRNAs in Cancer. Curr Pharmacol Rep. 2016;2:82–90. doi: 10.1007/s40495-016-0051-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Mandal P. Potential biomarkers associated with oxidative stress for risk assessment of colorectal cancer. Naunyn Schmiedebergs Arch Pharmacol. 2017;390:557–565. doi: 10.1007/s00210-017-1352-9. [DOI] [PubMed] [Google Scholar]
- 85.Gao R, Gao Z, Huang L, Qin H. Gut microbiota and colorectal cancer. Eur J Clin Microbiol Infect Dis. 2017;36:757–769. doi: 10.1007/s10096-016-2881-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Chen J, Pitmon E, Wang K. Microbiome, inflammation and colorectal cancer. Semin Immunol. 2017;32:43–53. doi: 10.1016/j.smim.2017.09.006. [DOI] [PubMed] [Google Scholar]
- 87.Yuan X, Long Y, Ji Z, Gao J, Fu T, Yan M, Zhang L, Su H, Zhang W, Wen X, Pu Z, Chen H, Wang Y, Gu X, Yan B, Kaliannan K, Shao Z. Green Tea Liquid Consumption Alters the Human Intestinal and Oral Microbiome. Mol Nutr Food Res. 2018;62:e1800178. doi: 10.1002/mnfr.201800178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Keku TO, Dulal S, Deveaux A, Jovov B, Han X. The gastrointestinal microbiota and colorectal cancer. Am J Physiol Gastrointest Liver Physiol. 2015;308:G351–G363. doi: 10.1152/ajpgi.00360.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Cremonesi E, Governa V, Garzon JFG, Mele V, Amicarella F, Muraro MG, Trella E, Galati-Fournier V, Oertli D, Däster SR, Droeser RA, Weixler B, Bolli M, Rosso R, Nitsche U, Khanna N, Egli A, Keck S, Slotta-Huspenina J, Terracciano LM, Zajac P, Spagnoli GC, Eppenberger-Castori S, Janssen KP, Borsig L, Iezzi G. Gut microbiota modulate T cell trafficking into human colorectal cancer. Gut. 2018;67:1984–1994. doi: 10.1136/gutjnl-2016-313498. [DOI] [PubMed] [Google Scholar]
- 90.Sethi V, Kurtom S, Tarique M, Lavania S, Malchiodi Z, Hellmund L, Zhang L, Sharma U, Giri B, Garg B, Ferrantella A, Vickers SM, Banerjee S, Dawra R, Roy S, Ramakrishnan S, Saluja A, Dudeja V. Gut Microbiota Promotes Tumor Growth in Mice by Modulating Immune Response. Gastroenterology. 2018;155:33–37.e6. doi: 10.1053/j.gastro.2018.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Yao X, Zhang C, Xing Y, Xue G, Zhang Q, Pan F, Wu G, Hu Y, Guo Q, Lu A, Zhang X, Zhou R, Tian Z, Zeng B, Wei H, Strober W, Zhao L, Meng G. Remodelling of the gut microbiota by hyperactive NLRP3 induces regulatory T cells to maintain homeostasis. Nat Commun. 2017;8:1896. doi: 10.1038/s41467-017-01917-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Castellarin M, Warren RL, Freeman JD, Dreolini L, Krzywinski M, Strauss J, Barnes R, Watson P, Allen-Vercoe E, Moore RA, Holt RA. Fusobacterium nucleatum infection is prevalent in human colorectal carcinoma. Genome Res. 2012;22:299–306. doi: 10.1101/gr.126516.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Yang Z, Ji G. Fusobacterium nucleatum-positive colorectal cancer. Oncol Lett. 2019;18:975–982. doi: 10.3892/ol.2019.10433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Shuwen H, Xi Y, Quan Q, Yuefen P, Miao D, Qing Z. Relationship between intestinal microorganisms and T lymphocytes in colorectal cancer. Future Oncol. 2019;15:1655–1666. doi: 10.2217/fon-2018-0595. [DOI] [PubMed] [Google Scholar]
- 95.Nosho K, Sukawa Y, Adachi Y, Ito M, Mitsuhashi K, Kurihara H, Kanno S, Yamamoto I, Ishigami K, Igarashi H, Maruyama R, Imai K, Yamamoto H, Shinomura Y. Association of Fusobacterium nucleatum with immunity and molecular alterations in colorectal cancer. World J Gastroenterol. 2016;22:557–566. doi: 10.3748/wjg.v22.i2.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kostic AD, Chun E, Robertson L, Glickman JN, Gallini CA, Michaud M, Clancy TE, Chung DC, Lochhead P, Hold GL, El-Omar EM, Brenner D, Fuchs CS, Meyerson M, Garrett WS. Fusobacterium nucleatum potentiates intestinal tumorigenesis and modulates the tumor-immune microenvironment. Cell Host Microbe. 2013;14:207–215. doi: 10.1016/j.chom.2013.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ye X, Wang R, Bhattacharya R, Boulbes DR, Fan F, Xia L, Adoni H, Ajami NJ, Wong MC, Smith DP, Petrosino JF, Venable S, Qiao W, Baladandayuthapani V, Maru D, Ellis LM. Fusobacterium Nucleatum Subspecies Animalis Influences Proinflammatory Cytokine Expression and Monocyte Activation in Human Colorectal Tumors. Cancer Prev Res (Phila) 2017;10:398–409. doi: 10.1158/1940-6207.CAPR-16-0178. [DOI] [PubMed] [Google Scholar]
- 98.Vannucci L, Stepankova R, Kozakova H, Fiserova A, Rossmann P, Tlaskalova-Hogenova H. Colorectal carcinogenesis in germ-free and conventionally reared rats: different intestinal environments affect the systemic immunity. Int J Oncol. 2008;32:609–617. [PubMed] [Google Scholar]
- 99.Lu Y, Wang L, Zhang J, Li J, Wan G. Induction of CD8 T cell cytotoxicity by fecal bacteria from healthy individuals and colorectal cancer patients. Biochem Biophys Res Commun. 2019;516:1007–1012. doi: 10.1016/j.bbrc.2019.06.078. [DOI] [PubMed] [Google Scholar]
- 100.Magri G, Comerma L, Pybus M, Sintes J, Lligé D, Segura-Garzón D, Bascones S, Yeste A, Grasset EK, Gutzeit C, Uzzan M, Ramanujam M, van Zelm MC, Albero-González R, Vazquez I, Iglesias M, Serrano S, Márquez L, Mercade E, Mehandru S, Cerutti A. Human Secretory IgM Emerges from Plasma Cells Clonally Related to Gut Memory B Cells and Targets Highly Diverse Commensals. Immunity. 2017;47:118–134.e8. doi: 10.1016/j.immuni.2017.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Malik A, Sharma D, Zhu Q, Karki R, Guy CS, Vogel P, Kanneganti TD. IL-33 regulates the IgA-microbiota axis to restrain IL-1α-dependent colitis and tumorigenesis. J Clin Invest. 2016;126:4469–4481. doi: 10.1172/JCI88625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Triner D, Devenport SN, Ramakrishnan SK, Ma X, Frieler RA, Greenson JK, Inohara N, Nunez G, Colacino JA, Mortensen RM, Shah YM. Neutrophils Restrict Tumor-Associated Microbiota to Reduce Growth and Invasion of Colon Tumors in Mice. Gastroenterology. 2019;156:1467–1482. doi: 10.1053/j.gastro.2018.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Sconocchia G, Eppenberger S, Spagnoli GC, Tornillo L, Droeser R, Caratelli S, Ferrelli F, Coppola A, Arriga R, Lauro D, Iezzi G, Terracciano L, Ferrone S. NK cells and T cells cooperate during the clinical course of colorectal cancer. Oncoimmunology. 2014;3:e952197. doi: 10.4161/21624011.2014.952197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Dupaul-Chicoine J, Arabzadeh A, Dagenais M, Douglas T, Champagne C, Morizot A, Rodrigue-Gervais IG, Breton V, Colpitts SL, Beauchemin N, Saleh M. The Nlrp3 Inflammasome Suppresses Colorectal Cancer Metastatic Growth in the Liver by Promoting Natural Killer Cell Tumoricidal Activity. Immunity. 2015;43:751–763. doi: 10.1016/j.immuni.2015.08.013. [DOI] [PubMed] [Google Scholar]
- 105.Loktionov A. Eosinophils in the gastrointestinal tract and their role in the pathogenesis of major colorectal disorders. World J Gastroenterol. 2019;25:3503–3526. doi: 10.3748/wjg.v25.i27.3503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Sconocchia G, Zlobec I, Lugli A, Calabrese D, Iezzi G, Karamitopoulou E, Patsouris ES, Peros G, Horcic M, Tornillo L, Zuber M, Droeser R, Muraro MG, Mengus C, Oertli D, Ferrone S, Terracciano L, Spagnoli GC. Tumor infiltration by FcγRIII (CD16)+ myeloid cells is associated with improved survival in patients with colorectal carcinoma. Int J Cancer. 2011;128:2663–2672. doi: 10.1002/ijc.25609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Isidro RA, Appleyard CB. Colonic macrophage polarization in homeostasis, inflammation, and cancer. Am J Physiol Gastrointest Liver Physiol. 2016;311:G59–G73. doi: 10.1152/ajpgi.00123.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Li R, Zhou R, Wang H, Li W, Pan M, Yao X, Zhan W, Yang S, Xu L, Ding Y, Zhao L. Gut microbiota-stimulated cathepsin K secretion mediates TLR4-dependent M2 macrophage polarization and promotes tumor metastasis in colorectal cancer. Cell Death Differ. 2019;26:2447–2463. doi: 10.1038/s41418-019-0312-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Rubio CA, Schmidt PT. Severe Defects in the Macrophage Barrier to Gut Microflora in Inflammatory Bowel Disease and Colon Cancer. Anticancer Res. 2018;38:3811–3815. doi: 10.21873/anticanres.12664. [DOI] [PubMed] [Google Scholar]
- 110.Park HE, Kim JH, Cho NY, Lee HS, Kang GH. Intratumoral Fusobacterium nucleatum abundance correlates with macrophage infiltration and CDKN2A methylation in microsatellite-unstable colorectal carcinoma. Virchows Arch. 2017;471:329–336. doi: 10.1007/s00428-017-2171-6. [DOI] [PubMed] [Google Scholar]
- 111.Bader JE, Enos RT, Velázquez KT, Carson MS, Nagarkatti M, Nagarkatti PS, Chatzistamou I, Davis JM, Carson JA, Robinson CM, Murphy EA. Macrophage depletion using clodronate liposomes decreases tumorigenesis and alters gut microbiota in the AOM/DSS mouse model of colon cancer. Am J Physiol Gastrointest Liver Physiol. 2018;314:G22–G31. doi: 10.1152/ajpgi.00229.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Owen JL, Mohamadzadeh M. Microbial activation of gut dendritic cells and the control of mucosal immunity. J Interferon Cytokine Res. 2013;33:619–631. doi: 10.1089/jir.2013.0046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Ogino S, Nishihara R, VanderWeele TJ, Wang M, Nishi A, Lochhead P, Qian ZR, Zhang X, Wu K, Nan H, Yoshida K, Milner DA, Jr, Chan AT, Field AE, Camargo CA, Jr, Williams MA, Giovannucci EL. Review Article: The Role of Molecular Pathological Epidemiology in the Study of Neoplastic and Non-neoplastic Diseases in the Era of Precision Medicine. Epidemiology. 2016;27:602–611. doi: 10.1097/EDE.0000000000000471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Ogino S, Chan AT, Fuchs CS, Giovannucci E. Molecular pathological epidemiology of colorectal neoplasia: an emerging transdisciplinary and interdisciplinary field. Gut. 2011;60:397–411. doi: 10.1136/gut.2010.217182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Ogino S, Nowak JA, Hamada T, Milner DA, Jr, Nishihara R. Insights into Pathogenic Interactions Among Environment, Host, and Tumor at the Crossroads of Molecular Pathology and Epidemiology. Annu Rev Pathol. 2019;14:83–103. doi: 10.1146/annurev-pathmechdis-012418-012818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Hamada T, Keum N, Nishihara R, Ogino S. Molecular pathological epidemiology: new developing frontiers of big data science to study etiologies and pathogenesis. J Gastroenterol. 2017;52:265–275. doi: 10.1007/s00535-016-1272-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Ogino S, Nowak JA, Hamada T, Phipps AI, Peters U, Milner DA, Jr, Giovannucci EL, Nishihara R, Giannakis M, Garrett WS, Song M. Integrative analysis of exogenous, endogenous, tumour and immune factors for precision medicine. Gut. 2018;67:1168–1180. doi: 10.1136/gutjnl-2017-315537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Morikawa T, Kuchiba A, Lochhead P, Nishihara R, Yamauchi M, Imamura Y, Liao X, Qian ZR, Ng K, Chan AT, Meyerhardt JA, Giovannucci E, Fuchs CS, Ogino S. Prospective analysis of body mass index, physical activity, and colorectal cancer risk associated with β-catenin (CTNNB1) status. Cancer Res. 2013;73:1600–1610. doi: 10.1158/0008-5472.CAN-12-2276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Chan AT, Ogino S, Fuchs CS. Aspirin and the risk of colorectal cancer in relation to the expression of COX-2. N Engl J Med. 2007;356:2131–2142. doi: 10.1056/NEJMoa067208. [DOI] [PubMed] [Google Scholar]
- 120.Chan AT, Ogino S, Fuchs CS. Aspirin use and survival after diagnosis of colorectal cancer. JAMA. 2009;302:649–658. doi: 10.1001/jama.2009.1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Liao X, Lochhead P, Nishihara R, Morikawa T, Kuchiba A, Yamauchi M, Imamura Y, Qian ZR, Baba Y, Shima K, Sun R, Nosho K, Meyerhardt JA, Giovannucci E, Fuchs CS, Chan AT, Ogino S. Aspirin use, tumor PIK3CA mutation, and colorectal-cancer survival. N Engl J Med. 2012;367:1596–1606. doi: 10.1056/NEJMoa1207756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Nishihara R, Lochhead P, Kuchiba A, Jung S, Yamauchi M, Liao X, Imamura Y, Qian ZR, Morikawa T, Wang M, Spiegelman D, Cho E, Giovannucci E, Fuchs CS, Chan AT, Ogino S. Aspirin use and risk of colorectal cancer according to BRAF mutation status. JAMA. 2013;309:2563–2571. doi: 10.1001/jama.2013.6599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Hamada T, Cao Y, Qian ZR, Masugi Y, Nowak JA, Yang J, Song M, Mima K, Kosumi K, Liu L, Shi Y, da Silva A, Gu M, Li W, Keum N, Zhang X, Wu K, Meyerhardt JA, Giovannucci EL, Giannakis M, Rodig SJ, Freeman GJ, Nevo D, Wang M, Chan AT, Fuchs CS, Nishihara R, Ogino S. Aspirin Use and Colorectal Cancer Survival According to Tumor CD274 (Programmed Cell Death 1 Ligand 1) Expression Status. J Clin Oncol. 2017;35:1836–1844. doi: 10.1200/JCO.2016.70.7547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Mima K, Nishihara R, Qian ZR, Cao Y, Sukawa Y, Nowak JA, Yang J, Dou R, Masugi Y, Song M, Kostic AD, Giannakis M, Bullman S, Milner DA, Baba H, Giovannucci EL, Garraway LA, Freeman GJ, Dranoff G, Garrett WS, Huttenhower C, Meyerson M, Meyerhardt JA, Chan AT, Fuchs CS, Ogino S. Fusobacterium nucleatum in colorectal carcinoma tissue and patient prognosis. Gut. 2016;65:1973–1980. doi: 10.1136/gutjnl-2015-310101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Lee DW, Han SW, Kang JK, Bae JM, Kim HP, Won JK, Jeong SY, Park KJ, Kang GH, Kim TY. Association Between Fusobacterium nucleatum, Pathway Mutation, and Patient Prognosis in Colorectal Cancer. Ann Surg Oncol. 2018;25:3389–3395. doi: 10.1245/s10434-018-6681-5. [DOI] [PubMed] [Google Scholar]
- 126.Mima K, Sukawa Y, Nishihara R, Qian ZR, Yamauchi M, Inamura K, Kim SA, Masuda A, Nowak JA, Nosho K, Kostic AD, Giannakis M, Watanabe H, Bullman S, Milner DA, Harris CC, Giovannucci E, Garraway LA, Freeman GJ, Dranoff G, Chan AT, Garrett WS, Huttenhower C, Fuchs CS, Ogino S. Fusobacterium nucleatum and T Cells in Colorectal Carcinoma. JAMA Oncol. 2015;1:653–661. doi: 10.1001/jamaoncol.2015.1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Chen T, You Y, Jiang H, Wang ZZ. Epithelial-mesenchymal transition (EMT): A biological process in the development, stem cell differentiation, and tumorigenesis. J Cell Physiol. 2017;232:3261–3272. doi: 10.1002/jcp.25797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Gurzu S, Silveanu C, Fetyko A, Butiurca V, Kovacs Z, Jung I. Systematic review of the old and new concepts in the epithelial-mesenchymal transition of colorectal cancer. World J Gastroenterol. 2016;22:6764–6775. doi: 10.3748/wjg.v22.i30.6764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Krisher RL, Prather RS. A role for the Warburg effect in preimplantation embryo development: metabolic modification to support rapid cell proliferation. Mol Reprod Dev. 2012;79:311–320. doi: 10.1002/mrd.22037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Zhu W, Huang Y, Pan Q, Xiang P, Xie N, Yu H. MicroRNA-98 Suppress Warburg Effect by Targeting HK2 in Colon Cancer Cells. Dig Dis Sci. 2017;62:660–668. doi: 10.1007/s10620-016-4418-5. [DOI] [PubMed] [Google Scholar]
- 131.Wang J, Wang H, Liu A, Fang C, Hao J, Wang Z. Lactate dehydrogenase A negatively regulated by miRNAs promotes aerobic glycolysis and is increased in colorectal cancer. Oncotarget. 2015;6:19456–19468. doi: 10.18632/oncotarget.3318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Zhang M, Liu T, Sun H, Weng W, Zhang Q, Liu C, Han Y, Sheng W. Pim1 supports human colorectal cancer growth during glucose deprivation by enhancing the Warburg effect. Cancer Sci. 2018;109:1468–1479. doi: 10.1111/cas.13562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Li Q, Qin Y, Wei P, Lian P, Li Y, Xu Y, Li X, Li D, Cai S. Gas1 Inhibits Metastatic and Metabolic Phenotypes in Colorectal Carcinoma. Mol Cancer Res. 2016;14:830–840. doi: 10.1158/1541-7786.MCR-16-0032. [DOI] [PubMed] [Google Scholar]
- 134.Fang L, Jiang Y, Yang Y, Zheng Y, Zheng J, Jiang H, Zhang S, Lin L, Zheng J, Zhang S, Zhuang X. Determining the optimal 5-FU therapeutic dosage in the treatment of colorectal cancer patients. Oncotarget. 2016;7:81880–81887. doi: 10.18632/oncotarget.11980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Chung MY, Lim TG, Lee KW. Molecular mechanisms of chemopreventive phytochemicals against gastroenterological cancer development. World J Gastroenterol. 2013;19:984–993. doi: 10.3748/wjg.v19.i7.984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Cao J, Han J, Xiao H, Qiao J, Han M. Effect of Tea Polyphenol Compounds on Anticancer Drugs in Terms of Anti-Tumor Activity, Toxicology, and Pharmacokinetics. Nutrients. 2016;8 doi: 10.3390/nu8120762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Hursel R, Viechtbauer W, Westerterp-Plantenga MS. The effects of green tea on weight loss and weight maintenance: a meta-analysis. Int J Obes (Lond) 2009;33:956–961. doi: 10.1038/ijo.2009.135. [DOI] [PubMed] [Google Scholar]
- 138.Yang WS, Wang WY, Fan WY, Deng Q, Wang X. Tea consumption and risk of type 2 diabetes: a dose-response meta-analysis of cohort studies. Br J Nutr. 2014;111:1329–1339. doi: 10.1017/S0007114513003887. [DOI] [PubMed] [Google Scholar]
- 139.Wobst HJ, Sharma A, Diamond MI, Wanker EE, Bieschke J. The green tea polyphenol (-)-epigallocatechin gallate prevents the aggregation of tau protein into toxic oligomers at substoichiometric ratios. FEBS Lett. 2015;589:77–83. doi: 10.1016/j.febslet.2014.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Qi H, Li S. Dose-response meta-analysis on coffee, tea and caffeine consumption with risk of Parkinson's disease. Geriatr Gerontol Int. 2014;14:430–439. doi: 10.1111/ggi.12123. [DOI] [PubMed] [Google Scholar]
- 141.Serban C, Sahebkar A, Ursoniu S, Andrica F, Banach M. Effect of sour tea (Hibiscus sabdariffa L.) on arterial hypertension: a systematic review and meta-analysis of randomized controlled trials. J Hypertens. 2015;33:1119–1127. doi: 10.1097/HJH.0000000000000585. [DOI] [PubMed] [Google Scholar]
- 142.Wang LX, Shi YL, Zhang LJ, Wang KR, Xiang LP, Cai ZY, Lu JL, Ye JH, Liang YR, Zheng XQ. Inhibitory Effects of (-)-Epigallocatechin-3-gallate on Esophageal Cancer. Molecules. 2019;24:pii: E954. doi: 10.3390/molecules24050954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Ahmad N, Cheng P, Mukhtar H. Cell cycle dysregulation by green tea polyphenol epigallocatechin-3-gallate. Biochem Biophys Res Commun. 2000;275:328–334. doi: 10.1006/bbrc.2000.3297. [DOI] [PubMed] [Google Scholar]
- 144.Rashidi B, Malekzadeh M, Goodarzi M, Masoudifar A, Mirzaei H. Green tea and its anti-angiogenesis effects. Biomed Pharmacother. 2017;89:949–956. doi: 10.1016/j.biopha.2017.01.161. [DOI] [PubMed] [Google Scholar]
- 145.Sharma P, Montes de Oca MK, Alkeswani AR, McClees SF, Das T, Elmets CA, Afaq F. Tea polyphenols for the prevention of UVB-induced skin cancer. Photodermatol Photoimmunol Photomed. 2018;34:50–59. doi: 10.1111/phpp.12356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Xu J, Xu Z, Zheng W. A Review of the Antiviral Role of Green Tea Catechins. Molecules. 2017;22:pii: E1337. doi: 10.3390/molecules22081337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Henning SM, Choo JJ, Heber D. Nongallated compared with gallated flavan-3-ols in green and black tea are more bioavailable. J Nutr. 2008;138:1529S–1534S. doi: 10.1093/jn/138.8.1529S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Zhao X, Song JL, Kim JD, Lee JS, Park KY. Fermented Pu-erh tea increases in vitro anticancer activities in HT-29 cells and has antiangiogenetic effects on HUVECs. J Environ Pathol Toxicol Oncol. 2013;32:275–288. doi: 10.1615/jenvironpatholtoxicoloncol.2013007074. [DOI] [PubMed] [Google Scholar]
- 149.Rothenberg DO, Zhou C, Zhang L. A Review on the Weight-Loss Effects of Oxidized Tea Polyphenols. Molecules. 2018;23:pii: E1176. doi: 10.3390/molecules23051176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Clifford MN, van der Hooft JJ, Crozier A. Human studies on the absorption, distribution, metabolism, and excretion of tea polyphenols. Am J Clin Nutr. 2013;98:1619S–1630S. doi: 10.3945/ajcn.113.058958. [DOI] [PubMed] [Google Scholar]
- 151.Li S, Lo CY, Pan MH, Lai CS, Ho CT. Black tea: chemical analysis and stability. Food Funct. 2013;4:10–18. doi: 10.1039/c2fo30093a. [DOI] [PubMed] [Google Scholar]
- 152.Fraga CG, Galleano M, Verstraeten SV, Oteiza PI. Basic biochemical mechanisms behind the health benefits of polyphenols. Mol Aspects Med. 2010;31:435–445. doi: 10.1016/j.mam.2010.09.006. [DOI] [PubMed] [Google Scholar]
- 153.Fraga CG, Oteiza PI. Dietary flavonoids: Role of (-)-epicatechin and related procyanidins in cell signaling. Free Radic Biol Med. 2011;51:813–823. doi: 10.1016/j.freeradbiomed.2011.06.002. [DOI] [PubMed] [Google Scholar]
- 154.D'Archivio M, Filesi C, Di Benedetto R, Gargiulo R, Giovannini C, Masella R. Polyphenols, dietary sources and bioavailability. Ann Ist Super Sanita. 2007;43:348–361. [PubMed] [Google Scholar]
- 155.Manach C, Williamson G, Morand C, Scalbert A, Rémésy C. Bioavailability and bioefficacy of polyphenols in humans. I. Review of 97 bioavailability studies. Am J Clin Nutr. 2005;81:230S–242S. doi: 10.1093/ajcn/81.1.230S. [DOI] [PubMed] [Google Scholar]
- 156.Peters CM, Green RJ, Janle EM, Ferruzzi MG. Formulation with ascorbic acid and sucrose modulates catechin bioavailability from green tea. Food Res Int. 2010;43:95–102. doi: 10.1016/j.foodres.2009.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Tzounis X, Vulevic J, Kuhnle GG, George T, Leonczak J, Gibson GR, Kwik-Uribe C, Spencer JP. Flavanol monomer-induced changes to the human faecal microflora. Br J Nutr. 2008;99:782–792. doi: 10.1017/S0007114507853384. [DOI] [PubMed] [Google Scholar]
- 158.Del Rio D, Calani L, Cordero C, Salvatore S, Pellegrini N, Brighenti F. Bioavailability and catabolism of green tea flavan-3-ols in humans. Nutrition. 2010;26:1110–1116. doi: 10.1016/j.nut.2009.09.021. [DOI] [PubMed] [Google Scholar]
- 159.Li C, Lee MJ, Sheng S, Meng X, Prabhu S, Winnik B, Huang B, Chung JY, Yan S, Ho CT, Yang CS. Structural identification of two metabolites of catechins and their kinetics in human urine and blood after tea ingestion. Chem Res Toxicol. 2000;13:177–184. doi: 10.1021/tx9901837. [DOI] [PubMed] [Google Scholar]
- 160.Meng X, Sang S, Zhu N, Lu H, Sheng S, Lee MJ, Ho CT, Yang CS. Identification and characterization of methylated and ring-fission metabolites of tea catechins formed in humans, mice, and rats. Chem Res Toxicol. 2002;15:1042–1050. doi: 10.1021/tx010184a. [DOI] [PubMed] [Google Scholar]
- 161.Annunziata G, Maisto M, Schisano C, Ciampaglia R, Daliu P, Narciso V, Tenore GC, Novellino E. Colon Bioaccessibility and Antioxidant Activity of White, Green and Black Tea Polyphenols Extract after In Vitro Simulated Gastrointestinal Digestion. Nutrients. 2018;10:pii: E1711. doi: 10.3390/nu10111711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Kuhnle G, Spencer JP, Schroeter H, Shenoy B, Debnam ES, Srai SK, Rice-Evans C, Hahn U. Epicatechin and catechin are O-methylated and glucuronidated in the small intestine. Biochem Biophys Res Commun. 2000;277:507–512. doi: 10.1006/bbrc.2000.3701. [DOI] [PubMed] [Google Scholar]
- 163.Chen ZP, Schell JB, Ho CT, Chen KY. Green tea epigallocatechin gallate shows a pronounced growth inhibitory effect on cancerous cells but not on their normal counterparts. Cancer Lett. 1998;129:173–179. doi: 10.1016/s0304-3835(98)00108-6. [DOI] [PubMed] [Google Scholar]
- 164.Henning SM, Wang P, Abgaryan N, Vicinanza R, de Oliveira DM, Zhang Y, Lee RP, Carpenter CL, Aronson WJ, Heber D. Phenolic acid concentrations in plasma and urine from men consuming green or black tea and potential chemopreventive properties for colon cancer. Mol Nutr Food Res. 2013;57:483–493. doi: 10.1002/mnfr.201200646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Larsen CA, Dashwood RH. Suppression of Met activation in human colon cancer cells treated with (-)-epigallocatechin-3-gallate: minor role of hydrogen peroxide. Biochem Biophys Res Commun. 2009;389:527–530. doi: 10.1016/j.bbrc.2009.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Oz HS, Ebersole JL. Green tea polyphenols mediated apoptosis in intestinal epithelial cells by a FADD-dependent pathway. J Cancer Ther. 2010;1:105–113. doi: 10.4236/jct.2010.13018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Du GJ, Wang CZ, Qi LW, Zhang ZY, Calway T, He TC, Du W, Yuan CS. The synergistic apoptotic interaction of panaxadiol and epigallocatechin gallate in human colorectal cancer cells. Phytother Res. 2013;27:272–277. doi: 10.1002/ptr.4707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Yamane T, Hagiwara N, Tateishi M, Akachi S, Kim M, Okuzumi J, Kitao Y, Inagake M, Kuwata K, Takahashi T. Inhibition of azoxymethane-induced colon carcinogenesis in rat by green tea polyphenol fraction. Jpn J Cancer Res. 1991;82:1336–1339. doi: 10.1111/j.1349-7006.1991.tb01801.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Hao X, Xiao H, Ju J, Lee MJ, Lambert JD, Yang CS. Green Tea Polyphenols Inhibit Colorectal Tumorigenesis in Azoxymethane-Treated F344 Rats. Nutr Cancer. 2017;69:623–631. doi: 10.1080/01635581.2017.1295088. [DOI] [PubMed] [Google Scholar]
- 170.Xiao H, Hao X, Simi B, Ju J, Jiang H, Reddy BS, Yang CS. Green tea polyphenols inhibit colorectal aberrant crypt foci (ACF) formation and prevent oncogenic changes in dysplastic ACF in azoxymethane-treated F344 rats. Carcinogenesis. 2008;29:113–119. doi: 10.1093/carcin/bgm204. [DOI] [PubMed] [Google Scholar]
- 171.Weisburger JH, Rivenson A, Aliaga C, Reinhardt J, Kelloff GJ, Boone CW, Steele VE, Balentine DA, Pittman B, Zang E. Effect of tea extracts, polyphenols, and epigallocatechin gallate on azoxymethane-induced colon cancer. Proc Soc Exp Biol Med. 1998;217:104–108. doi: 10.3181/00379727-217-44211. [DOI] [PubMed] [Google Scholar]
- 172.Narisawa T, Fukaura Y. A very low dose of green tea polyphenols in drinking water prevents N-methyl-N-nitrosourea-induced colon carcinogenesis in F344 rats. Jpn J Cancer Res. 1993;84:1007–1009. doi: 10.1111/j.1349-7006.1993.tb02792.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Orner GA, Dashwood WM, Blum CA, Díaz GD, Li Q, Dashwood RH. Suppression of tumorigenesis in the Apc(min) mouse: down-regulation of beta-catenin signaling by a combination of tea plus sulindac. Carcinogenesis. 2003;24:263–267. doi: 10.1093/carcin/24.2.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Lodovici M, Casalini C, De Filippo C, Copeland E, Xu X, Clifford M, Dolara P. Inhibition of 1,2-dimethylhydrazine-induced oxidative DNA damage in rat colon mucosa by black tea complex polyphenols. Food Chem Toxicol. 2000;38:1085–1088. doi: 10.1016/s0278-6915(00)00109-5. [DOI] [PubMed] [Google Scholar]
- 175.Kim M, Murakami A, Miyamoto S, Tanaka T, Ohigashi H. The modifying effects of green tea polyphenols on acute colitis and inflammation-associated colon carcinogenesis in male ICR mice. Biofactors. 2010;36:43–51. doi: 10.1002/biof.69. [DOI] [PubMed] [Google Scholar]
- 176.Ju J, Hong J, Zhou JN, Pan Z, Bose M, Liao J, Yang GY, Liu YY, Hou Z, Lin Y, Ma J, Shih WJ, Carothers AM, Yang CS. Inhibition of intestinal tumorigenesis in Apcmin/+ mice by (-)-epigallocatechin-3-gallate, the major catechin in green tea. Cancer Res. 2005;65:10623–10631. doi: 10.1158/0008-5472.CAN-05-1949. [DOI] [PubMed] [Google Scholar]
- 177.Stingl JC, Ettrich T, Muche R, Wiedom M, Brockmöller J, Seeringer A, Seufferlein T. Protocol for minimizing the risk of metachronous adenomas of the colorectum with green tea extract (MIRACLE): a randomised controlled trial of green tea extract versus placebo for nutriprevention of metachronous colon adenomas in the elderly population. BMC Cancer. 2011;11:360. doi: 10.1186/1471-2407-11-360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Chen Y, Wu Y, Du M, Chu H, Zhu L, Tong N, Zhang Z, Wang M, Gu D, Chen J. An inverse association between tea consumption and colorectal cancer risk. Oncotarget. 2017;8:37367–37376. doi: 10.18632/oncotarget.16959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Shimizu M, Fukutomi Y, Ninomiya M, Nagura K, Kato T, Araki H, Suganuma M, Fujiki H, Moriwaki H. Green tea extracts for the prevention of metachronous colorectal adenomas: a pilot study. Cancer Epidemiol Biomarkers Prev. 2008;17:3020–3025. doi: 10.1158/1055-9965.EPI-08-0528. [DOI] [PubMed] [Google Scholar]
- 180.Chang H, Lei L, Zhou Y, Ye F, Zhao G. Dietary Flavonoids and the Risk of Colorectal Cancer: An Updated Meta-Analysis of Epidemiological Studies. Nutrients. 2018;10 doi: 10.3390/nu10070950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Yuan JM, Gao YT, Yang CS, Yu MC. Urinary biomarkers of tea polyphenols and risk of colorectal cancer in the Shanghai Cohort Study. Int J Cancer. 2007;120:1344–1350. doi: 10.1002/ijc.22460. [DOI] [PubMed] [Google Scholar]
- 182.Sun CL, Yuan JM, Koh WP, Lee HP, Yu MC. Green tea and black tea consumption in relation to colorectal cancer risk: the Singapore Chinese Health Study. Carcinogenesis. 2007;28:2143–2148. doi: 10.1093/carcin/bgm171. [DOI] [PubMed] [Google Scholar]
- 183.Wang ZJ, Ohnaka K, Morita M, Toyomura K, Kono S, Ueki T, Tanaka M, Kakeji Y, Maehara Y, Okamura T, Ikejiri K, Futami K, Maekawa T, Yasunami Y, Takenaka K, Ichimiya H, Terasaka R. Dietary polyphenols and colorectal cancer risk: the Fukuoka colorectal cancer study. World J Gastroenterol. 2013;19:2683–2690. doi: 10.3748/wjg.v19.i17.2683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Suzuki Y, Tsubono Y, Nakaya N, Koizumi Y, Suzuki Y, Shibuya D, Tsuji I. Green tea and the risk of colorectal cancer: pooled analysis of two prospective studies in Japan. J Epidemiol. 2005;15:118–124. doi: 10.2188/jea.15.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Dashwood RH, Xu M, Hernaez JF, Hasaniya N, Youn K, Razzuk A. Cancer chemopreventive mechanisms of tea against heterocyclic amine mutagens from cooked meat. Proc Soc Exp Biol Med. 1999;220:239–243. doi: 10.1046/j.1525-1373.1999.d01-41.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Hughes R, Pollock JR, Bingham S. Effect of vegetables, tea, and soy on endogenous N-nitrosation, fecal ammonia, and fecal water genotoxicity during a high red meat diet in humans. Nutr Cancer. 2002;42:70–77. doi: 10.1207/S15327914NC421_10. [DOI] [PubMed] [Google Scholar]
- 187.Hu Y, McIntosh GH, Le Leu RK, Nyskohus LS, Woodman RJ, Young GP. Combination of selenium and green tea improves the efficacy of chemoprevention in a rat colorectal cancer model by modulating genetic and epigenetic biomarkers. PLoS One. 2013;8:e64362. doi: 10.1371/journal.pone.0064362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Kim H, Lee J, Oh JH, Chang HJ, Sohn DK, Shin A, Kim J. Protective Effect of Green Tea Consumption on Colorectal Cancer Varies by Lifestyle Factors. Nutrients. 2019;11:pii: E2612. doi: 10.3390/nu11112612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Yang G, Zheng W, Xiang YB, Gao J, Li HL, Zhang X, Gao YT, Shu XO. Green tea consumption and colorectal cancer risk: a report from the Shanghai Men's Health Study. Carcinogenesis. 2011;32:1684–1688. doi: 10.1093/carcin/bgr186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Chang L, Karin M. Mammalian MAP kinase signalling cascades. Nature. 2001;410:37–40. doi: 10.1038/35065000. [DOI] [PubMed] [Google Scholar]
- 191.Chen C, Shen G, Hebbar V, Hu R, Owuor ED, Kong AN. Epigallocatechin-3-gallate-induced stress signals in HT-29 human colon adenocarcinoma cells. Carcinogenesis. 2003;24:1369–1378. doi: 10.1093/carcin/bgg091. [DOI] [PubMed] [Google Scholar]
- 192.Lee MH, Yoon DS. A Phenotype-Based RNAi Screening for Ras-ERK/MAPK Signaling-Associated Stem Cell Regulators in C. elegans. Methods Mol Biol. 2017;1622:207–221. doi: 10.1007/978-1-4939-7108-4_15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Temraz S, Mukherji D, Shamseddine A. Dual Inhibition of MEK and PI3K Pathway in KRAS and BRAF Mutated Colorectal Cancers. Int J Mol Sci. 2015;16:22976–22988. doi: 10.3390/ijms160922976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Denhardt DT. Signal-transducing protein phosphorylation cascades mediated by Ras/Rho proteins in the mammalian cell: the potential for multiplex signalling. Biochem J. 1996;318(Pt 3):729–747. doi: 10.1042/bj3180729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Whitmarsh AJ, Cavanagh J, Tournier C, Yasuda J, Davis RJ. A mammalian scaffold complex that selectively mediates MAP kinase activation. Science. 1998;281:1671–1674. doi: 10.1126/science.281.5383.1671. [DOI] [PubMed] [Google Scholar]
- 196.Grossi V, Peserico A, Tezil T, Simone C. p38α MAPK pathway: a key factor in colorectal cancer therapy and chemoresistance. World J Gastroenterol. 2014;20:9744–9758. doi: 10.3748/wjg.v20.i29.9744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Obergasteiger J, Frapporti G, Pramstaller PP, Hicks AA, Volta M. A new hypothesis for Parkinson's disease pathogenesis: GTPase-p38 MAPK signaling and autophagy as convergence points of etiology and genomics. Mol Neurodegener. 2018;13:40. doi: 10.1186/s13024-018-0273-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Li SP, Junttila MR, Han J, Kähäri VM, Westermarck J. p38 Mitogen-activated protein kinase pathway suppresses cell survival by inducing dephosphorylation of mitogen-activated protein/extracellular signal-regulated kinase kinase1,2. Cancer Res. 2003;63:3473–3477. [PubMed] [Google Scholar]
- 199.Cerezo-Guisado MI, Zur R, Lorenzo MJ, Risco A, Martín-Serrano MA, Alvarez-Barrientos A, Cuenda A, Centeno F. Implication of Akt, ERK1/2 and alternative p38MAPK signalling pathways in human colon cancer cell apoptosis induced by green tea EGCG. Food Chem Toxicol. 2015;84:125–132. doi: 10.1016/j.fct.2015.08.017. [DOI] [PubMed] [Google Scholar]
- 200.Chung JY, Huang C, Meng X, Dong Z, Yang CS. Inhibition of activator protein 1 activity and cell growth by purified green tea and black tea polyphenols in H-ras-transformed cells: structure-activity relationship and mechanisms involved. Cancer Res. 1999;59:4610–4617. [PubMed] [Google Scholar]
- 201.Manning BD, Toker A. AKT/PKB Signaling: Navigating the Network. Cell. 2017;169:381–405. doi: 10.1016/j.cell.2017.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Abou-Fayçal C, Hatat AS, Gazzeri S, Eymin B. Splice Variants of the RTK Family: Their Role in Tumour Progression and Response to Targeted Therapy. Int J Mol Sci. 2017;18 doi: 10.3390/ijms18020383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Danielsen SA, Eide PW, Nesbakken A, Guren T, Leithe E, Lothe RA. Portrait of the PI3K/AKT pathway in colorectal cancer. Biochim Biophys Acta. 2015;1855:104–121. doi: 10.1016/j.bbcan.2014.09.008. [DOI] [PubMed] [Google Scholar]
- 204.Adachi S, Nagao T, Ingolfsson HI, Maxfield FR, Andersen OS, Kopelovich L, Weinstein IB. The inhibitory effect of (-)-epigallocatechin gallate on activation of the epidermal growth factor receptor is associated with altered lipid order in HT29 colon cancer cells. Cancer Res. 2007;67:6493–6501. doi: 10.1158/0008-5472.CAN-07-0411. [DOI] [PubMed] [Google Scholar]
- 205.Jung YD, Kim MS, Shin BA, Chay KO, Ahn BW, Liu W, Bucana CD, Gallick GE, Ellis LM. EGCG, a major component of green tea, inhibits tumour growth by inhibiting VEGF induction in human colon carcinoma cells. Br J Cancer. 2001;84:844–850. doi: 10.1054/bjoc.2000.1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Bigelow RL, Cardelli JA. The green tea catechins, (-)-Epigallocatechin-3-gallate (EGCG) and (-)-Epicatechin-3-gallate (ECG), inhibit HGF/Met signaling in immortalized and tumorigenic breast epithelial cells. Oncogene. 2006;25:1922–1930. doi: 10.1038/sj.onc.1209227. [DOI] [PubMed] [Google Scholar]
- 207.Larsen CA, Dashwood RH. (-)-Epigallocatechin-3-gallate inhibits Met signaling, proliferation, and invasiveness in human colon cancer cells. Arch Biochem Biophys. 2010;501:52–57. doi: 10.1016/j.abb.2010.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Lu J, Gosslau A, Liu AY, Chen KY. PCR differential display-based identification of regulator of G protein signaling 10 as the target gene in human colon cancer cells induced by black tea polyphenol theaflavin monogallate. Eur J Pharmacol. 2008;601:66–72. doi: 10.1016/j.ejphar.2008.10.044. [DOI] [PubMed] [Google Scholar]
- 209.Novellasdemunt L, Antas P, Li VS. Targeting Wnt signaling in colorectal cancer. A Review in the Theme: Cell Signaling: Proteins, Pathways and Mechanisms. Am J Physiol Cell Physiol. 2015;309:C511–C521. doi: 10.1152/ajpcell.00117.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Li VS, Ng SS, Boersema PJ, Low TY, Karthaus WR, Gerlach JP, Mohammed S, Heck AJ, Maurice MM, Mahmoudi T, Clevers H. Wnt signaling through inhibition of β-catenin degradation in an intact Axin1 complex. Cell. 2012;149:1245–1256. doi: 10.1016/j.cell.2012.05.002. [DOI] [PubMed] [Google Scholar]
- 211.Patel R, Ingle A, Maru GB. Polymeric black tea polyphenols inhibit 1,2-dimethylhydrazine induced colorectal carcinogenesis by inhibiting cell proliferation via Wnt/beta-catenin pathway. Toxicol Appl Pharmacol. 2008;227:136–146. doi: 10.1016/j.taap.2007.10.009. [DOI] [PubMed] [Google Scholar]
- 212.Oh S, Gwak J, Park S, Yang CS. Green tea polyphenol EGCG suppresses Wnt/β-catenin signaling by promoting GSK-3β- and PP2A-independent β-catenin phosphorylation/degradation. Biofactors. 2014;40:586–595. doi: 10.1002/biof.1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Tsukamoto S, Yamashita S, Kim YH, Kumazoe M, Huang Y, Yamada K, Tachibana H. Oxygen partial pressure modulates 67-kDa laminin receptor expression, leading to altered activity of the green tea polyphenol, EGCG. FEBS Lett. 2012;586:3441–3447. doi: 10.1016/j.febslet.2012.07.065. [DOI] [PubMed] [Google Scholar]
- 214.Kumazoe M, Sugihara K, Tsukamoto S, Huang Y, Tsurudome Y, Suzuki T, Suemasu Y, Ueda N, Yamashita S, Kim Y, Yamada K, Tachibana H. 67-kDa laminin receptor increases cGMP to induce cancer-selective apoptosis. J Clin Invest. 2013;123:787–799. doi: 10.1172/JCI64768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Negri A, Naponelli V, Rizzi F, Bettuzzi S. Molecular Targets of Epigallocatechin-Gallate (EGCG): A Special Focus on Signal Transduction and Cancer. Nutrients. 2018;10 doi: 10.3390/nu10121936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Byun EB, Kim WS, Sung NY, Byun EH. Epigallocatechin-3-Gallate Regulates Anti-Inflammatory Action Through 67-kDa Laminin Receptor-Mediated Tollip Signaling Induction in Lipopolysaccharide-Stimulated Human Intestinal Epithelial Cells. Cell Physiol Biochem. 2018;46:2072–2081. doi: 10.1159/000489447. [DOI] [PubMed] [Google Scholar]
- 217.Patel M, Horgan PG, McMillan DC, Edwards J. NF-κB pathways in the development and progression of colorectal cancer. Transl Res. 2018;197:43–56. doi: 10.1016/j.trsl.2018.02.002. [DOI] [PubMed] [Google Scholar]
- 218.Vaiopoulos AG, Athanasoula KCh, Papavassiliou AG. NF-κB in colorectal cancer. J Mol Med (Berl) 2013;91:1029–1037. doi: 10.1007/s00109-013-1045-x. [DOI] [PubMed] [Google Scholar]
- 219.Kalliolias GD, Ivashkiv LB. TNF biology, pathogenic mechanisms and emerging therapeutic strategies. Nat Rev Rheumatol. 2016;12:49–62. doi: 10.1038/nrrheum.2015.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Lin YL, Tsai SH, Lin-Shiau SY, Ho CT, Lin JK. Theaflavin-3,3'-digallate from black tea blocks the nitric oxide synthase by down-regulating the activation of NF-kappaB in macrophages. Eur J Pharmacol. 1999;367:379–388. doi: 10.1016/s0014-2999(98)00953-4. [DOI] [PubMed] [Google Scholar]
- 221.Han C, Sun B, Zhao X, Zhang Y, Gu Q, Liu F, Zhao N, Wu L. Phosphorylation of STAT3 Promotes Vasculogenic Mimicry by Inducing Epithelial-to-Mesenchymal Transition in Colorectal Cancer. Technol Cancer Res Treat. 2017;16:1209–1219. doi: 10.1177/1533034617742312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Ye TH, Yang FF, Zhu YX, Li YL, Lei Q, Song XJ, Xia Y, Xiong Y, Zhang LD, Wang NY, Zhao LF, Gou HF, Xie YM, Yang SY, Yu LT, Yang L, Wei YQ. Correction: Inhibition of Stat3 signaling pathway by nifuroxazide improves antitumor immunity and impairs colorectal carcinoma metastasis. Cell Death Dis. 2019;10:722. doi: 10.1038/s41419-019-1955-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Senggunprai L, Kukongviriyapan V, Prawan A, Kukongviriyapan U. Quercetin and EGCG exhibit chemopreventive effects in cholangiocarcinoma cells via suppression of JAK/STAT signaling pathway. Phytother Res. 2014;28:841–848. doi: 10.1002/ptr.5061. [DOI] [PubMed] [Google Scholar]
- 224.Sadeghi MR, Jeddi F, Soozangar N, Somi MH, Samadi N. The role of Nrf2-Keap1 axis in colorectal cancer, progression, and chemoresistance. Tumour Biol. 2017;39:1010428317705510. doi: 10.1177/1010428317705510. [DOI] [PubMed] [Google Scholar]
- 225.Saw CL, Kong AN. Nuclear factor-erythroid 2-related factor 2 as a chemopreventive target in colorectal cancer. Expert Opin Ther Targets. 2011;15:281–295. doi: 10.1517/14728222.2011.553602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Yuan JH, Li YQ, Yang XY. Inhibition of epigallocatechin gallate on orthotopic colon cancer by upregulating the Nrf2-UGT1A signal pathway in nude mice. Pharmacology. 2007;80:269–278. doi: 10.1159/000106447. [DOI] [PubMed] [Google Scholar]
- 227.Bi W, He CN, Li XX, Zhou LY, Liu RJ, Zhang S, Li GQ, Chen ZC, Zhang PF. Ginnalin A from Kujin tea (Acer tataricum subsp. ginnala) exhibits a colorectal cancer chemoprevention effect via activation of the Nrf2/HO-1 signaling pathway. Food Funct. 2018;9:2809–2819. doi: 10.1039/c8fo00054a. [DOI] [PubMed] [Google Scholar]
- 228.Borish LC, Steinke JW. 2. Cytokines and chemokines. J Allergy Clin Immunol. 2003;111:S460–S475. doi: 10.1067/mai.2003.108. [DOI] [PubMed] [Google Scholar]
- 229.Klampfer L. Cytokines, inflammation and colon cancer. Curr Cancer Drug Targets. 2011;11:451–464. doi: 10.2174/156800911795538066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Bradley JR. TNF-mediated inflammatory disease. J Pathol. 2008;214:149–160. doi: 10.1002/path.2287. [DOI] [PubMed] [Google Scholar]
- 231.Brown JR, DuBois RN. COX-2: a molecular target for colorectal cancer prevention. J Clin Oncol. 2005;23:2840–2855. doi: 10.1200/JCO.2005.09.051. [DOI] [PubMed] [Google Scholar]
- 232.Liu Y, Sun H, Hu M, Zhang Y, Chen S, Tighe S, Zhu Y. The Role of Cyclooxygenase-2 in Colorectal Carcinogenesis. Clin Colorectal Cancer. 2017;16:165–172. doi: 10.1016/j.clcc.2016.09.012. [DOI] [PubMed] [Google Scholar]
- 233.Lu J, Ho CT, Ghai G, Chen KY. Differential effects of theaflavin monogallates on cell growth, apoptosis, and Cox-2 gene expression in cancerous versus normal cells. Cancer Res. 2000;60:6465–6471. [PubMed] [Google Scholar]
- 234.Gosslau A, En Jao DL, Huang MT, Ho CT, Evans D, Rawson NE, Chen KY. Effects of the black tea polyphenol theaflavin-2 on apoptotic and inflammatory pathways in vitro and in vivo. Mol Nutr Food Res. 2011;55:198–208. doi: 10.1002/mnfr.201000165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Peng G, Dixon DA, Muga SJ, Smith TJ, Wargovich MJ. Green tea polyphenol (-)-epigallocatechin-3-gallate inhibits cyclooxygenase-2 expression in colon carcinogenesis. Mol Carcinog. 2006;45:309–319. doi: 10.1002/mc.20166. [DOI] [PubMed] [Google Scholar]
- 236.Navarro-Perán E, Cabezas-Herrera J, Sánchez-Del-Campo L, García-Cánovas F, Rodríguez-López JN. The anti-inflammatory and anti-cancer properties of epigallocatechin-3-gallate are mediated by folate cycle disruption, adenosine release and NF-kappaB suppression. Inflamm Res. 2008;57:472–478. doi: 10.1007/s00011-008-8013-x. [DOI] [PubMed] [Google Scholar]
- 237.Subbaramaiah K, Dannenberg AJ. Cyclooxygenase 2: a molecular target for cancer prevention and treatment. Trends Pharmacol Sci. 2003;24:96–102. doi: 10.1016/S0165-6147(02)00043-3. [DOI] [PubMed] [Google Scholar]
- 238.Kobayashi K, Omori K, Murata T. Role of prostaglandins in tumor microenvironment. Cancer Metastasis Rev. 2018;37:347–354. doi: 10.1007/s10555-018-9740-2. [DOI] [PubMed] [Google Scholar]
- 239.Wang D, Dubois RN. Prostaglandins and cancer. Gut. 2006;55:115–122. doi: 10.1136/gut.2004.047100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Hong J, Smith TJ, Ho CT, August DA, Yang CS. Effects of purified green and black tea polyphenols on cyclooxygenase- and lipoxygenase-dependent metabolism of arachidonic acid in human colon mucosa and colon tumor tissues. Biochem Pharmacol. 2001;62:1175–1183. doi: 10.1016/s0006-2952(01)00767-5. [DOI] [PubMed] [Google Scholar]
- 241.August DA, Landau J, Caputo D, Hong J, Lee MJ, Yang CS. Ingestion of green tea rapidly decreases prostaglandin E2 levels in rectal mucosa in humans. Cancer Epidemiol Biomarkers Prev. 1999;8:709–713. [PubMed] [Google Scholar]
- 242.Tipoe GL, Leung TM, Hung MW, Fung ML. Green tea polyphenols as an anti-oxidant and anti-inflammatory agent for cardiovascular protection. Cardiovasc Hematol Disord Drug Targets. 2007;7:135–144. doi: 10.2174/187152907780830905. [DOI] [PubMed] [Google Scholar]
- 243.Chung JE, Kurisawa M, Kim YJ, Uyama H, Kobayashi S. Amplification of antioxidant activity of catechin by polycondensation with acetaldehyde. Biomacromolecules. 2004;5:113–118. doi: 10.1021/bm0342436. [DOI] [PubMed] [Google Scholar]
- 244.Li Y, Zhao S, Zhang W, Zhao P, He B, Wu N, Han P. Epigallocatechin-3-O-gallate (EGCG) attenuates FFAs-induced peripheral insulin resistance through AMPK pathway and insulin signaling pathway in vivo. Diabetes Res Clin Pract. 2011;93:205–214. doi: 10.1016/j.diabres.2011.03.036. [DOI] [PubMed] [Google Scholar]
- 245.Giftson JS, Jayanthi S, Nalini N. Chemopreventive efficacy of gallic acid, an antioxidant and anticarcinogenic polyphenol, against 1,2-dimethyl hydrazine induced rat colon carcinogenesis. Invest New Drugs. 2010;28:251–259. doi: 10.1007/s10637-009-9241-9. [DOI] [PubMed] [Google Scholar]
- 246.Chu C, Deng J, Man Y, Qu Y. Green Tea Extracts Epigallocatechin-3-gallate for Different Treatments. Biomed Res Int. 2017;2017:5615647. doi: 10.1155/2017/5615647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Kim HS, Quon MJ, Kim JA. New insights into the mechanisms of polyphenols beyond antioxidant properties; lessons from the green tea polyphenol, epigallocatechin 3-gallate. Redox Biol. 2014;2:187–195. doi: 10.1016/j.redox.2013.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Díez-Torre A, Díaz-Núñez M, Eguizábal C, Silván U, Aréchaga J. Evidence for a role of matrix metalloproteinases and their inhibitors in primordial germ cell migration. Andrology. 2013;1:779–786. doi: 10.1111/j.2047-2927.2013.00109.x. [DOI] [PubMed] [Google Scholar]
- 249.Yadav L, Puri N, Rastogi V, Satpute P, Ahmad R, Kaur G. Matrix metalloproteinases and cancer - roles in threat and therapy. Asian Pac J Cancer Prev. 2014;15:1085–1091. doi: 10.7314/apjcp.2014.15.3.1085. [DOI] [PubMed] [Google Scholar]
- 250.Banday MZ, Sameer AS, Mir AH, Mokhdomi TA, Chowdri NA, Haq E. Matrix metalloproteinase (MMP) -2, -7 and -9 promoter polymorphisms in colorectal cancer in ethnic Kashmiri population - A case-control study and a mini review. Gene. 2016;589:81–89. doi: 10.1016/j.gene.2016.05.028. [DOI] [PubMed] [Google Scholar]
- 251.Kim M, Murakami A, Kawabata K, Ohigashi H. (-)-Epigallocatechin-3-gallate promotes pro-matrix metalloproteinase-7 production via activation of the JNK1/2 pathway in HT-29 human colorectal cancer cells. Carcinogenesis. 2005;26:1553–1562. doi: 10.1093/carcin/bgi104. [DOI] [PubMed] [Google Scholar]
- 252.Elmore S. Apoptosis: a review of programmed cell death. Toxicol Pathol. 2007;35:495–516. doi: 10.1080/01926230701320337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Ashkenazi A, Dixit VM. Death receptors: signaling and modulation. Science. 1998;281:1305–1308. doi: 10.1126/science.281.5381.1305. [DOI] [PubMed] [Google Scholar]
- 254.D'Arcy MS. Cell death: a review of the major forms of apoptosis, necrosis and autophagy. Cell Biol Int. 2019;43:582–592. doi: 10.1002/cbin.11137. [DOI] [PubMed] [Google Scholar]
- 255.Saelens X, Festjens N, Vande Walle L, van Gurp M, van Loo G, Vandenabeele P. Toxic proteins released from mitochondria in cell death. Oncogene. 2004;23:2861–2874. doi: 10.1038/sj.onc.1207523. [DOI] [PubMed] [Google Scholar]
- 256.Cory S, Adams JM. The Bcl2 family: regulators of the cellular life-or-death switch. Nat Rev Cancer. 2002;2:647–656. doi: 10.1038/nrc883. [DOI] [PubMed] [Google Scholar]
- 257.Schuler M, Green DR. Mechanisms of p53-dependent apoptosis. Biochem Soc Trans. 2001;29:684–688. doi: 10.1042/0300-5127:0290684. [DOI] [PubMed] [Google Scholar]
- 258.Kim SW, Moon JH, Park SY. Activation of autophagic flux by epigallocatechin gallate mitigates TRAIL-induced tumor cell apoptosis via down-regulation of death receptors. Oncotarget. 2016;7:65660–65668. doi: 10.18632/oncotarget.11597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Dudgeon C, Yu J. Green tea and PUMA: a deadly combination? Cancer Biol Ther. 2008;7:909–910. doi: 10.4161/cbt.7.6.6317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Wang X, Wang R, Hao MW, Dong K, Wei SH, Lin F, Ren JH, Zhang HZ. The BH3-only protein PUMA is involved in green tea polyphenol-induced apoptosis in colorectal cancer cell lines. Cancer Biol Ther. 2008;7:902–908. doi: 10.4161/cbt.7.6.5911. [DOI] [PubMed] [Google Scholar]
- 261.Hwang JT, Ha J, Park IJ, Lee SK, Baik HW, Kim YM, Park OJ. Apoptotic effect of EGCG in HT-29 colon cancer cells via AMPK signal pathway. Cancer Lett. 2007;247:115–121. doi: 10.1016/j.canlet.2006.03.030. [DOI] [PubMed] [Google Scholar]
- 262.Baek SJ, Kim JS, Jackson FR, Eling TE, McEntee MF, Lee SH. Epicatechin gallate-induced expression of NAG-1 is associated with growth inhibition and apoptosis in colon cancer cells. Carcinogenesis. 2004;25:2425–2432. doi: 10.1093/carcin/bgh255. [DOI] [PubMed] [Google Scholar]
- 263.Fan F, Jin S, Amundson SA, Tong T, Fan W, Zhao H, Zhu X, Mazzacurati L, Li X, Petrik KL, Fornace AJ, Jr, Rajasekaran B, Zhan Q. ATF3 induction following DNA damage is regulated by distinct signaling pathways and over-expression of ATF3 protein suppresses cells growth. Oncogene. 2002;21:7488–7496. doi: 10.1038/sj.onc.1205896. [DOI] [PubMed] [Google Scholar]
- 264.Drewes JL, Housseau F, Sears CL. Sporadic colorectal cancer: microbial contributors to disease prevention, development and therapy. Br J Cancer. 2016;115:273–280. doi: 10.1038/bjc.2016.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Wang J, Tang L, Zhou H, Zhou J, Glenn TC, Shen CL, Wang JS. Long-term treatment with green tea polyphenols modifies the gut microbiome of female sprague-dawley rats. J Nutr Biochem. 2018;56:55–64. doi: 10.1016/j.jnutbio.2018.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Etxeberria U, Fernández-Quintela A, Milagro FI, Aguirre L, Martínez JA, Portillo MP. Impact of polyphenols and polyphenol-rich dietary sources on gut microbiota composition. J Agric Food Chem. 2013;61:9517–9533. doi: 10.1021/jf402506c. [DOI] [PubMed] [Google Scholar]
- 267.Alotaibi A, Bhatnagar P, Najafzadeh M, Gupta KC, Anderson D. Tea phenols in bulk and nanoparticle form modify DNA damage in human lymphocytes from colon cancer patients and healthy individuals treated in vitro with platinum-based chemotherapeutic drugs. Nanomedicine (Lond) 2013;8:389–401. doi: 10.2217/nnm.12.126. [DOI] [PubMed] [Google Scholar]
- 268.Moseley VR, Morris J, Knackstedt RW, Wargovich MJ. Green tea polyphenol epigallocatechin 3-gallate, contributes to the degradation of DNMT3A and HDAC3 in HCT 116 human colon cancer cells. Anticancer Res. 2013;33:5325–5333. [PMC free article] [PubMed] [Google Scholar]
- 269.Sánchez-Tena S, Vizán P, Dudeja PK, Centelles JJ, Cascante M. Green tea phenolics inhibit butyrate-induced differentiation of colon cancer cells by interacting with monocarboxylate transporter 1. Biochim Biophys Acta. 2013;1832:2264–2270. doi: 10.1016/j.bbadis.2013.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Morris J, Moseley VR, Cabang AB, Coleman K, Wei W, Garrett-Mayer E, Wargovich MJ. Reduction in promotor methylation utilizing EGCG (epigallocatechin-3-gallate) restores RXRα expression in human colon cancer cells. Oncotarget. 2016;7:35313–35326. doi: 10.18632/oncotarget.9204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Bodnar AG, Ouellette M, Frolkis M, Holt SE, Chiu CP, Morin GB, Harley CB, Shay JW, Lichtsteiner S, Wright WE. Extension of life-span by introduction of telomerase into normal human cells. Science. 1998;279:349–352. doi: 10.1126/science.279.5349.349. [DOI] [PubMed] [Google Scholar]
- 272.Fu W, Begley JG, Killen MW, Mattson MP. Anti-apoptotic role of telomerase in pheochromocytoma cells. J Biol Chem. 1999;274:7264–7271. doi: 10.1074/jbc.274.11.7264. [DOI] [PubMed] [Google Scholar]
- 273.Naasani I, Oh-Hashi F, Oh-Hara T, Feng WY, Johnston J, Chan K, Tsuruo T. Blocking telomerase by dietary polyphenols is a major mechanism for limiting the growth of human cancer cells in vitro and in vivo. Cancer Res. 2003;63:824–830. [PubMed] [Google Scholar]
- 274.Chung SS, Adekoya D, Enenmoh I, Clarke O, Wang P, Sarkyssian M, Wu Y, Vadgama JV. Salinomycin Abolished STAT3 and STAT1 Interactions and Reduced Telomerase Activity in Colorectal Cancer Cells. Anticancer Res. 2017;37:445–453. doi: 10.21873/anticanres.11336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.de Mejía EG, Chandra S, Ramírez-Mares M, Wang W. Catalytic inhibition of human DNA topoisomerase by phenolic compounds in Ardisia compressa extracts and their effect on human colon cancer cells. Food Chem Toxicol. 2006;44:1191–1203. doi: 10.1016/j.fct.2006.01.015. [DOI] [PubMed] [Google Scholar]
- 276.Jain CK, Roychoudhury S, Majumder HK. Selective killing of G2 decatenation checkpoint defective colon cancer cells by catalytic topoisomerase II inhibitor. Biochim Biophys Acta. 2015;1853:1195–1204. doi: 10.1016/j.bbamcr.2015.02.021. [DOI] [PubMed] [Google Scholar]
- 277.Beretta G, Furlanetto S, Regazzoni L, Zarrella M, Facino RM. Quenching of alpha,beta-unsaturated aldehydes by green tea polyphenols: HPLC-ESI-MS/MS studies. J Pharm Biomed Anal. 2008;48:606–611. doi: 10.1016/j.jpba.2008.05.036. [DOI] [PubMed] [Google Scholar]
- 278.Huang LH, Liu CY, Wang LY, Huang CJ, Hsu CH. Effects of green tea extract on overweight and obese women with high levels of low density-lipoprotein-cholesterol (LDL-C): a randomised, double-blind, and cross-over placebo-controlled clinical trial. BMC Complement Altern Med. 2018;18:294. doi: 10.1186/s12906-018-2355-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Samavat H, Ursin G, Emory TH, Lee E, Wang R, Torkelson CJ, Dostal AM, Swenson K, Le CT, Yang CS, Yu MC, Yee D, Wu AH, Yuan JM, Kurzer MS. A Randomized Controlled Trial of Green Tea Extract Supplementation and Mammographic Density in Postmenopausal Women at Increased Risk of Breast Cancer. Cancer Prev Res (Phila) 2017;10:710–718. doi: 10.1158/1940-6207.CAPR-17-0187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Lambert JD, Kim DH, Zheng R, Yang CS. Transdermal delivery of (-)-epigallocatechin-3-gallate, a green tea polyphenol, in mice. J Pharm Pharmacol. 2006;58:599–604. doi: 10.1211/jpp.58.5.0004. [DOI] [PubMed] [Google Scholar]