Abstract
Background:
Physical activity is associated with reduced risk of Parkinson’s disease (PD). The explanations for this association are not completely elucidated. We use long-term PD-incidence data from long-distance skiers to study the relationship between exercise and PD.
Objective:
We aimed to investigate if physical activity is associated with long-term lower risk of PD and if this association could be explained by physically active people being able to sustain more PD neuropathology before clinical symptoms, a motor reserve.
Methods:
Using a prospective observational design, we studied whether long-distance skiers of the Swedish Vasaloppet (n = 197,685), exhibited reduced incidence of PD compared to matched individuals from the general population (n = 197,684) during 21 years of follow-up (median 10, interquartile range (IQR) 5–15 years).
Results:
Vasaloppet skiers (median age 36.0 years [IQR 29.0–46.0], 38% women) had lower incidence of PD (HR: 0.71; 95 % CI 0.56–0.90) compared to non-skiers. When reducing risk for reverse causation by excluding PD cases within the first five years from race participation, there was still a trend for lower risk of PD (HR: 0.80; 95 % CI 0.62–1.03). Further, the PD prevalence converged between skiers and non-skiers after 15 years of follow-up, which is more consistent with a motor reserve in the physically active rather than neuroprotection.
Conclusions:
A physical active lifestyle is associated with reduced risk for PD. This association weakens with time and might be explained by a motor reserve among the physically active.
Keywords: Physical activity, exercise, Parkinson’s disease, motor reserve
INTRODUCTION
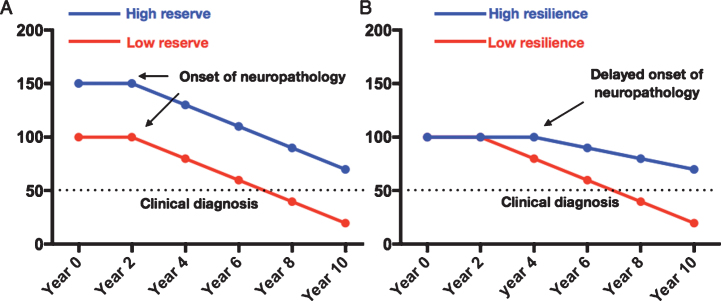
Parkinson’s disease (PD) is the most common neurodegenerative movement disorder and with an aging population more people will be afflicted [1]. The risk of PD is determined by an interaction of environmental and genetic factors [2]. Physical activity is known to be associated with lower risk of PD [3, 4] with several possible explanations. First, it could be reverse-causation, i.e., people with prodromal PD reduce their activity level. However, the protective effect of physical activity remains even if you exclude people who develop PD within 8 years, as reported by Yang and colleagues in a comprehensive study [3]. Second, it could be misdiagnosis, for example exercise could protect against vascular parkinsonism which is often misdiagnosed as PD. Only 80–90% of patients with clinical PD have the diagnosis confirmed post-mortem [5, 6]. Third, it could of course be a real protective effect on dopaminergic neurons, we could call this “brain resilience” [7]. In contrast to resilience exercise could confer a greater reserve against PD, henceforth termed motor reserve. A patient with a high motor reserve would be able to sustain a higher amount of PD brain pathology before the onset of overt symptoms. Thus, the diagnosis of PD could be delayed in those with high motor reserves (Fig. 1A).
Fig.1.
Two possible mechanisms of protection. A) High cognitive/motor reserve where the brain can sustain more neuropathological damage before the onset of overt clinical symptoms. B) High brain resilience may delay onset of neuropathology and then slow the rate of decline.
In this report we study the risk of PD among participants in Vasaloppet, an up to 90 km annual cross-country ski race compared to age matched non-skiers. We use participation in Vasaloppet as a proxy for physical activity similar to previous studies [8]. First, we investigate whether physical activity is associated with lower risk of PD and if it can be explained by reverse causation. We then investigate whether this association is more likely to be mediated by inhibiting brain pathology (brain resilience, Fig. 1B) or by a greater motor reserve.
MATERIALS AND METHODS
Swedish national patient registry
Data on PD diagnoses were retrieved from the Swedish National Patient Registry, which since 1987 provides information on all primary and secondary diagnoses in patients attending hospital-based care in Sweden. The register covers 99% of all hospital-based diagnoses, both somatic and psychiatric, and includes hospital-based outpatient visits since 2001. Primary care diagnoses are not included in the registry. PD was defined according to the International Classification of Diseases, tenth revision (ICD10) or ninth revision (ICD9). Diagnoses included are Parkinson’s disease (G20, 332A, 3420).
Vasaloppet cohort
The Vasaloppet study population comprises all Swedish participators in the world’s largest long distance (30, 45, or 90 km) cross-country ski race (Vasaloppet) between 1989 and 2010 (n = 197,685), together with frequency-matched individuals from the general population (n = 197,684). Although Vasaloppet started already in 1922 it was not until 1989 that the personal number of the participants was registered which made the present study possible. Frequency matching was done from the population registry according to age group (five-year intervals), sex, region of residency, and year of participation in ski race as previously described [9]. On average, Vasaloppet skiers have higher leisure time physical activity, smoke less, have a healthier diet, and lower mortality than the general Swedish population [10]. To reduce bias due to inability to participate in the race because of poor health, individuals with severe disease (e.g., cardiovascular and respiratory diseases) were excluded as previously described [11] (for more information, see the Supplementary Material including Supplementary Figure 1 and Supplementary Table 1). Information on date of birth, sex, and education level were derived from Swedish registries [9] The total study cohort (n = 395,369) was followed in the Swedish National Patient Registry throughout 2010. The Ethical Review Board in Uppsala, Sweden, approved the Vasaloppet study.
Statistical analyses
We used R statistical software package for analyses. Two-tailed p-values <0.05 were considered statistically significant. Demographic data are presented as median and interquartile range (IQR) or numbers (n) and percent (%). Numeric and categorical group differences were estimated with Mann-Whitney U test and Pearson’s χ2 test, respectively. Cox regression models were used to compare risk of PD for skiers vs. non-skiers. For the cox regression models, the time variable was calculated as years between participation in Vasaloppet (and the same year for the matched non-skier) and event or censoring. The event was PD. Censoring appeared when subjects died or at time of register outtake. Information on date of death for deceased study individuals was available through the Causes of Death Register, held at the National Board of Health and Welfare. Risk of PD is presented as hazard ratio (HR) with 95% confidence intervals (CI). We present both a crude model and an age-, sex-, and education-adjusted model. Education was categorized as noted in Table 1. We modeled Schoenfeld residuals graphically to confirm the proportionality assumption. Figure data were constructed using Kaplan Meier curves. The same time and event variables were used as in the Cox regressions, and the hazards are presented for skiers vs. non-skiers. Numbers at risk were derived from survival tables specifying number of individuals entering each five-year interval, as presented in the graph (Fig. 2). Since there is evidence suggesting that patients may have motor symptoms 5 years before the diagnosis of PD [12], we decided to set five years as a cut-off for sensitivity analyses.
Table 1.
Characteristics of the Vasaloppet study population
| All n = 395 369 | Skiers n = 197 685 | Non-skiers n = 197 684 | |
| Characteristics 1989–2010 | Median (IQR) or n (%) | Median (IQR) or n (%) | Median (IQR) or n (%) |
| Age at baseline, y | 36.0 (29.0–46.0) | 36.0 (29.0–46.0) | 36.0 (29.0–46.0) |
| Women | 149796 (38) | 74897 (38) | 74899 (38) |
| Education: | |||
| Primary/elementary school (≤8 y) | 49344 (13) | 14538 (7.4) | 34806 (18)*** |
| Secondary school/high school (9–12 y) | 176571 (45) | 76635 (39) | 99936 (51) |
| Higher education/university (≥13 y) | 166133 (42) | 106147 (54) | 59986 (31) |
| Diagnoses at follow-up | N events | ||
| Parkinson’s Disease | 283 | 119 | 164 |
Characteristics of the Vasaloppet study population presented for the whole cohort and by skiers and non-skiers separately. ***p < 0.001. Group difference between skiers and non-skiers, estimated with Mann-Whitney U test (numeric variables) and Pearson’s χ2 test (categorical variables). Only significant differences are noted in the table.
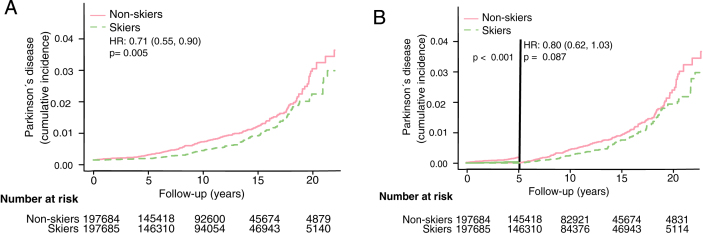
Fig.2.
Cumulative incidence of PD among skiers and non-skiers. A) Cumulative incidence of PD among all skiers and non-skiers. B) Cumulative incidence of PD among all Vasaloppet participants with exclusion of diagnoses set within the first five years after baseline. HR represents hazard ratios from an unadjusted cox regression.
RESULTS
Vasaloppet skiers have a lower cumulative incidence of PD
Demographic data for the Vasaloppet cohort is presented in Table 1. After a median follow-up of 10 years (IQR 5–15 years), 283 PD diagnoses were identified. The overall risk of developing PD was significantly lower among those who had participated in Vasaloppet compared to those who had not (Fig. 2A, Hazard ratio (HR) 0.71, confidence interval (CI) 0.56–0.9). When excluding individuals diagnosed with PD within the first five years from baseline HR rose to 0.8 (Fig. 2B, HR 0.80, CI 0.62–1.03). Adjusting for age, sex and education level did not alter the results (see Table 2).
Table 2.
Association between physical activity and incident dementia in the Vasaloppet cohort
| PD incidence | ||
| Physical activity | HR (95% CI) | p |
| Unadjusted model | 283 events | |
| Non-skiers (Reference) | 1 | |
| Skiers | 0.71 (0.56–0.90) | 0.005 |
| Adjusted model | 275 events | |
| Non-skiers (Reference) | 1 | |
| Skiers | 0.73 (0.57–0.93) | 0.01 |
| Excluding PD cases <5 years | ||
| Unadjusted model | 246 events | |
| Non-skiers (Reference) | 1 | |
| Skiers | 0.80 (0.62–1.03) | 0.087 |
| Adjusted model | 239 events | |
| Non-skiers (Reference) | 1 | |
| Skiers | 0.80 (0.62–1.04) | 0.099 |
Association between physical activity and PD incidence in the Vasaloppet cohort, based on participation in a long-distance ski race (skiers) compared to non-skiers. Cox regression models showing hazard ratio (HR) with 95% confidence interval (CI) for risk of PD. Adjusted model for age, sex, and education.
The differential of cumulative PD-incidence between participants and non-participants decrease with time
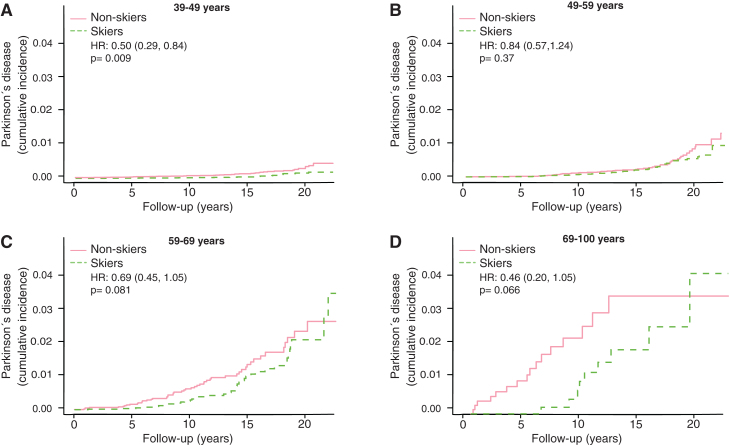
The motor reserve hypothesis predicts that the cumulative incidences would converge between skiers and non-skiers in the older age groups. We therefore broke the results down by age of subject at participation in Vasaloppet (Fig. 3). In the age group 18–39 there was barely any incidence of PD (data not shown). In age group 39–49, the incidence of PD was significantly lower among the skiers (Fig. 3A, HR 0.50, CI 0.29–0.84). Given the up to 20-year follow-up the oldest participants here could be 69 at the end of follow-up (Fig. 3A). In age-group 49–59 years, we observed no difference in PD prevalence between skiers and non-skiers (Fig. 3B, HR 0.84, CI 0.57–1.24). In age group 59–69 the prevalence of PD was lower at early follow-up period but converged at later follow-ups (Fig. 3C, HR 0.69, CI 0.45–1.05). In the oldest age group, 69–100, we observed the same pattern, with a lower PD prevalence among skiers to begin with but convergence at the end (Fig. 3D, HR 0.46, CI 0.20–1.05).
Fig.3.
Kaplan Meier plots of cumulative incidence of Parkinson’s disease in subgroups with different age at participation, 39–49 years (A), 49–59 year (B), 59–69 years (C) and 69–100 years (D). HR represents hazard ratios from an unadjusted cox regression. HR represents hazard ratios from an unadjusted cox regression.
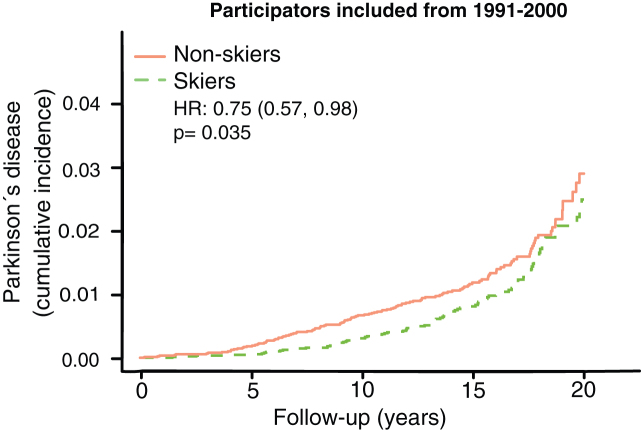
To further look at this convergence of PD-prevalence with time we specifically looked at the group with longest follow-up times, participating in the ski race 1991–2000. In this group we see a convergence in prevalence after 15+ years (Fig. 4). It should also be noted that 90 % of all events (PD-cases) come from this group.
Fig.4.
Kaplan Meier plot of Parkinson’s disease prevalence among those with the longest follow-up time. HR represents hazard ratios from an unadjusted cox regression.
The differential of cumulative incidence of PD between participants and non-participants in men and women separately
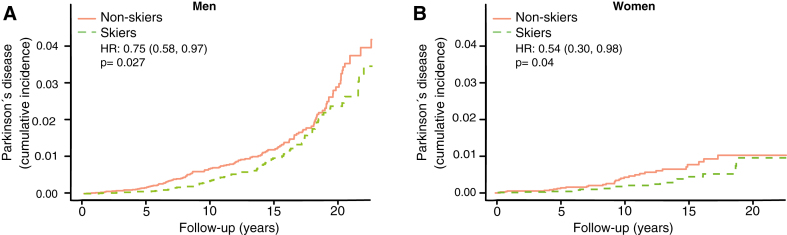
The incidence of PD plateaus at an earlier age among women than men [13]. The motor reserve hypothesis thus predicts a greater convergence of cumulative incidence among female skiers than male skiers. We therefore performed a subgroup analysis for men and women separately to test this aspect of the hypothesis. We observed a greater convergence in cumulative incidence towards the end of follow-up between skiers and non-skiers in women (Fig. 5B, HR 0.54, CI 0.30–0.98) compared to men (Fig. 5A, HR 0.75, CI 0.58–0.97).
Fig.5.
Cumulative incidence of Parkinson’s disease among skiers vs. non-skiers in men (A) and women (B) separately. HR represents hazard ratios from an unadjusted cox regression.
DISCUSSION
In the current study, we aimed to investigate if physical exercise is correlated to the risk of PD and if this correlation could be explained by a motor reserve built up over years of physical activity.
We found that physical activity, as measured by participation in a long-distance ski race, was associated with lower incidence of PD. This effect was weakened when we excluded those who were diagnosed within 5 years after inclusion, indicating some degree of reverse-causation due to people with pre-morbid PD exercising less. The association between physical activity and lower incidence of PD in our study are in concordance with earlier studies showing a protective effect of exercise against PD [3, 4].
There are principally three possible protective mechanisms of physical activity against PD: 1) greater resilience of the neurons against the neuropathology of PD; 2) less neuropathology; and 3) a greater motor reserve, so that the brain can sustain more damage before symptoms become apparent, a phenomenon analogous to the cognitive reserve concept in Alzheimer’s disease [14]. It has been shown that the negative correlation between Alzheimer’s disease and education is more consistent with a greater cognitive reserve rather than greater resilience or less neuropathology [15]. In this study, we have no possibility of distinguishing between mechanisms 1 and 2, but the concept of motor reserve makes some predictions that can be examined in our material. If the sole reason for the negative correlation between PD and exercise was direct protection from neuropathology, then you would expect a lower incidence of PD at all ages and time-points. This is not what we observed. If it were instead a motor reserve protecting against PD you would expect converging cumulative incidence with longer follow-up time and older age. That is what we observed. In both the 59–69 and 69–100 age group we see an initial lower cumulative incidence of PD, but at the end of the more than 20 years of follow-up the cumulative incidence of PD converged (Fig. 3C, D). Among those who had the longest follow-up times (ski race participation between 1991–2000), we also observe a convergence of PD prevalence with time (Fig. 4). This convergence somewhat depends on the relation between PD-incidence and age. If PD-incidence always increased with age, the cumulative incidence of PD would converge between those with a low and high motor reserve as long as the incidence to prevalence ratio was declining, but the convergence would not be complete. If the incidence of PD declines after a certain age we would move towards complete convergence between the high and low motor reserve groups. The data on PD-incidence among those over 80 is not as robust as for lower ages but there seems to be an incidence decline after 79 [16]. Interestingly the incidence decline in PD with age is significantly more pronounced among women [13]. Thus, the motor reserve hypothesis also predicts a greater convergence of cumulative PD-incidence among women skiers than male skiers, which is what we observe (Fig. 5B).
Though outside the scope of our study the motor reserve hypothesis also predicts milder motor symptoms for every given level of neuropathology/neuronal death in those with a high motor reserve. It should thus be noted that PD patients with a higher premorbid exercise activity have better motor scores relative to their dopamine levels compared to sedentary peers [17]. Further, PD is often unilateral at onset with persistent asymmetries. It has been shown that patients with PD on their dominant side have better motor scores than those affected on their non-dominant side, possibly due to a greater motor reserve in the dominant hemisphere [18].
Interestingly, our cohort has previously shown that the level of physical activity can affect the risk for amyotrophic lateral sclerosis (ALS) [8], specifically a four-fold risk-increase among elite-skiers and a moderate risk-decrease in recreational skiers. In that study, the motor reserve did not seem to have the same compensatory effect in ALS. ALS is a very aggressive disease affecting upper and lower motor neurons. The primary cause of death is respiratory failure due to degeneration of respiratory muscles [19] and the median survival may be as low as 2 years [20]. Thus any motor reserve in ALS would delay the disease by months rather than years which would make it difficult to detect. This makes it more likely that the associations seen between ALS and exercise are more directly related to the neuropathology.
Our study includes limitations such as the lack of data on physical activity among the non-skiers. Thereby, the non-skier group also includes physically active individuals to some extent and this may attenuate the true association. Skiers were assumed to be physically active since it is necessary to prepare for such a demanding ski race with regular physical training, as demonstrated by a previous study [10]. We excluded individuals with severe diseases to reduce bias due to inability to participate in the ski race. However, bias due to inability to participate due to poor health is not completely eliminated since it is not possible to exclude all diagnoses that might indirectly affect participation (type I error). Furthermore, other lifestyle factors, such as diet, smoking and education differs between the skiers and non-skiers [10]. However, adjusting for education did not significantly alter our results. It is both a strength and a weakness that we do not adjust for additional confounders. It is a weakness as other factors such as smoking habits (smoking is more prevalent among non-skiers) and diet could independently of exercise affect the risk of PD. However, smoking has been associated with lower incidence of PD [4] and could thus contribute to an underestimation of the true association between physical activity and PD (type II error). In addition, we did not compensate for possible immortal time bias. This skiing population has been shown to live longer than the control population [10], which should increase their risk of getting PD (type II error). However, adjusting for confounders can also increase the risk of type I errors, particularly when the measurement of the confounder is not exact, diet for example is difficult to retrospectively measure [21]. By not adjusting for confounders, we therefore decrease the risk of type I error at the expense of less certainty in what exact factor among the Vasaloppet skiers that decreases the risk of PD. Nevertheless the most salient differential characteristic among the skiers is their higher level of exercise [10]. Our skiing population has been characterized before and it is known that the majority exercise for at least 4 hours a week, which was not the case for the general population [10, 22]. Our data thus points to a protective effect of physical activity against PD.
Conclusion
In summary we observe a lower incidence of PD among skiers, likely mediated by physical activity. This association dissipates with time and is consistent with a greater motor reserve among the well-trained. Thus, skiers may suffer as much brain pathology but take longer to develop clinical PD than non-skiers. However, studies confirming these findings in other contexts as well as elucidating the mechanisms behind it are needed in order to draw more general conclusions.
CONFLICTS OF INTEREST
The authors have no conflict of interest to report.
Supplementary Material
ACKNOWLEDGMENTS
We are grateful to Johan Österman, without whom the Vasaloppet study on Parkinson’s disease would not have been initiated. We are also grateful to Vasaloppet Registry for providing us with the research material. We were funded by the Strategic Research Area MultiPark (Multidisciplinary Research in neurodegenerative diseases) at Lund University, the Swedish Alzheimer foundation, the Swedish Brain Foundation, Crafoord Foundation, Swedish Dementia Association, G&J Kock Foundation, Olle Engkvist Foundation, the Swedish Medical Research Council. Swedish Parkinson Foundation and A.E. Berger Foundation.
SUPPLEMENTARY MATERIAL
The supplementary material is available in the electronic version of this article: https://dx.doi.org/10.3233/JPD-191762.
REFERENCES
- [1]. de Lau LML, Breteler MMB (2006) Epidemiology of Parkinson’s disease. Lancet Neurol 5, 525–535. [DOI] [PubMed] [Google Scholar]
- [2]. Nalls MA, McLean CY, Rick J, Eberly S, Hutten SJ, Gwinn K, Sutherland M, Martinez M, Heutink P, Williams NM, Hardy J, Gasser T, Brice A, Price TR, Nicolas A, Keller MF, Molony C, Gibbs JR, Chen-Plotkin A, Suh E, Letson C, Fiandaca MS, Mapstone M, Federoff HJ, Noyce AJ, Morris H, Deerlin VMV, Weintraub D, Zabetian C, Hernandez DG, Lesage S, Mullins M, Conley ED, Northover CAM, Frasier M, Marek K, Day-Williams AG, Stone DJ, Ioannidis JPA, Singleton AB (2015) Diagnosis of Parkinson’s disease on the basis of clinical and genetic classification: A population-based modelling study. Lancet Neurol 14, 1002–1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3]. Yang F, Trolle Lagerros Y, Bellocco R, Adami H-O, Fang F, Pedersen NL, Wirdefeldt K (2015) Physical activity and risk of Parkinson’s disease in the Swedish National March Cohort. Brain 138, 269–275. [DOI] [PubMed] [Google Scholar]
- [4]. Bellou V, Belbasis L, Tzoulaki I, Evangelou E, Ioannidis JPA (2016) Environmental risk factors and Parkinson’s disease: An umbrella review of meta-analyses. Parkinsonism Relat Disord 23, 1–9. [DOI] [PubMed] [Google Scholar]
- [5]. Hughes AJ, Daniel SE, Lees AJ (2001) Improved accuracy of clinical diagnosis of Lewy body Parkinson’s disease. Neurology 57, 1497–1499. [DOI] [PubMed] [Google Scholar]
- [6]. Tolosa E, Wenning G, Poewe W (2006) The diagnosis of Parkinson’s disease. Lancet Neurol 5, 75–86. [DOI] [PubMed] [Google Scholar]
- [7]. Tillerson JL, Caudle WM, Reverón ME, Miller GW (2003) Exercise induces behavioral recovery and attenuates neurochemical deficits in rodent models of Parkinson’s disease. Neuroscience 119, 899–911. [DOI] [PubMed] [Google Scholar]
- [8]. Fang F, Hållmarker U, James S, Ingre C, Michaëlsson K, Ahlbom A, Feychting M (2016) Amyotrophic lateral sclerosis among cross-country skiers in Sweden. Eur J Epidemiol 31, 247–253. [DOI] [PubMed] [Google Scholar]
- [9]. Hållmarker U, Lindbäck J, Michaëlsson K, Ärnlöv J, Åsberg S, Wester P, Hellberg D, Lagerqvist B, James S (2018) Survival and incidence of cardiovascular diseases in participants in a long-distance ski race (Vasaloppet, Sweden) compared with the background population. Eur Heart J Qual Care Clin Outcomes 4, 91–97. [DOI] [PubMed] [Google Scholar]
- [10]. Farahmand BY, Ahlbom A, Ekblom Ö, Ekblom B, Hållmarker U, Aronson D, Brobert GP (2003) Mortality amongst participants in Vasaloppet: A classical long-distance ski race in Sweden. J Intern Med 253, 276–283. [DOI] [PubMed] [Google Scholar]
- [11]. Hållmarker U, Michaëlsson K, Ärnlöv J, Hellberg D, Lagerqvist B, Lindbäck J, James S (2016) Risk of recurrent ischaemic events after myocardial infarction in long-distance ski race participants. Eur J Prev Cardiol 23, 282–290. [DOI] [PubMed] [Google Scholar]
- [12]. Darweesh SKL, Verlinden VJA, Stricker BH, Hofman A, Koudstaal PJ, Ikram MA (2017) Trajectories of prediagnostic functioning in Parkinson’s disease. Brain 140, 429–441. [DOI] [PubMed] [Google Scholar]
- [13]. Van Den Eeden SK, Tanner CM, Bernstein AL, Fross RD, Leimpeter A, Bloch DA, Nelson LM (2003) Incidence of Parkinson’s disease: Variation by age, gender, and race/ethnicity. Am J Epidemiol 157, 1015–1022. [DOI] [PubMed] [Google Scholar]
- [14]. Bennett DA, Wilson RS, Schneider JA, Evans DA, Mendes de Leon CF, Arnold SE, Barnes LL, Bienias JL (2003) Education modifies the relation of AD pathology to level of cognitive function in older persons. Neurology 60, 1909–1915. [DOI] [PubMed] [Google Scholar]
- [15]. Stern Y, Albert S, Tang MX, Tsai WY (1999) Rate of memory decline in AD is related to education and occupation: Cognitive reserve? Neurology 53, 1942–1947. [DOI] [PubMed] [Google Scholar]
- [16]. Hirsch L, Jette N, Frolkis A, Steeves T, Pringsheim T (2016) The incidence of Parkinson’s disease: A systematic review and meta-analysis. Neuroepidemiology 46, 292–300. [DOI] [PubMed] [Google Scholar]
- [17]. Sunwoo MK, Lee JE, Hong JY, Ye BS, Lee HS, Oh JS, Kim JS, Lee PH, Sohn YH (2017) Premorbid exercise engagement and motor reserve in Parkinson’s disease. Parkinsonism Relat Disord 34, 49–53. [DOI] [PubMed] [Google Scholar]
- [18]. Ham JH, Lee JJ, Kim JS, Lee PH, Sohn YH (2015) Is dominant-side onset associated with a better motor compensation in Parkinson’s disease? Mov Disord 30, 1921–1925. [DOI] [PubMed] [Google Scholar]
- [19]. Pattinson KTS, Turner MR (2016) A wider pathological network underlying breathlessness and respiratory failure in amyotrophic lateral sclerosis. Eur Respir J 47, 1632–1634. [DOI] [PubMed] [Google Scholar]
- [20]. Knibb JA, Keren N, Kulka A, Leigh PN, Martin S, Shaw CE, Tsuda M, Al-Chalabi A (2016) A clinical tool for predicting survival in ALS. J Neurol Neurosurg Psychiatry 87, 1361–1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21]. Westfall J, Yarkoni T (2016) Statistically controlling for confounding constructs is harder than you think. PLoS One 11, e0152719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22]. Carlsson S, Olsson L, Farahmand BY, Hållmarker U, Ahlbom A (2007) Skiers in the long-distance ski race invest in their health. Lakartidningen 104, 670–671. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.