Abstract
Hypermobile Ehlers-Danlos syndrome (hEDS) is a hereditary disorder of connective tissue, often presenting with complex symptoms can include chronic pain, fatigue, and dysautonomia. Factors influencing functional disability in the pediatric hEDS population are incompletely studied. This study's aims were to assess factors that affect quality of life in children and adolescents with hEDS. Individuals with hEDS between the ages 12–20 years and matched parents were recruited through retrospective chart review at two genetics clinics. Participants completed a questionnaire that included the Pediatric Quality of Life Inventory (PedsQLTM), PedsQL Multidimentional Fatigue Scale, Functional Disability Inventory, Pain-Frequency-Severity-Duration Scale, the Brief Illness Perception Questionnaire, measures of anxiety and depression, and helpful interventions. Survey responses were completed for 47 children and adolescents with hEDS/hypermobility spectrum disorder (81% female, mean age 16 years), some by the affected individual, some by their parent, and some by both. Clinical data derived from chart review were compared statistically to survey responses. All outcomes correlated moderately to strongly with each other. Using multiple regression, general fatigue and pain scores were the best predictors of the PedsQL total score. Additionally, presence of any psychiatric diagnosis was correlated with a lower PedsQL score. Current management guidelines recommend early intervention to prevent disability from deconditioning; these results may help identify target interventions in this vulnerable population.
Keywords: adolescents, children, health-related quality of life, hypermobile Ehlers-Danlos syndrome, pediatric
1 |. INTRODUCTION
Hypermobile Ehlers-Danlos syndrome (hEDS) is a heterogeneous symptom complex. Previously, hEDS was defined by Villefranche criteria (Beighton, De Paepe, Steinman, Tsipouras, & Wenstrup, 1998; Grahame, Bird, & Child, 2000) and was clinically indistinguishable from joint hypermobility syndrome (JHS) (Castori et al., 2017; B. T. Tinkle et al., 2009; Zoppi, Chiarelli, Binetti, Ritelli, & Colombi, 2018). In 2017, revised nosology for hEDS was introduced; patients not meeting updated hEDS criteria but with joint hypermobility symptoms are diagnosed as hypermobility spectrum disorder (HSD), often with similar symptom and functional complications as those diagnosed with hEDS (Malfait et al., 2017). Prior estimates note the increased prevalence of joint hypermobility in 5–65% of all younger children (Armon, 2015; Cattalini, Khubchandani, & Cimaz, 2015; Murray, 2006). As generalized joint hypermobility is a major component of hEDS diagnostic criteria, referrals for children with suspected hEDS/HSD have been increasingly sent to genetics and other specialties, and present a public health concern due to the high prevalence and healthcare utilization of this population (Gunz, Canizares, Mackay, & Badley, 2012). The pediatric community from generalists to a broad range of specialists is increasingly challenged to address this growing and complex patient population on a local, national, and international scale.
Characteristic symptoms of hEDS include joint hypermobility and instability, recurrent joint subluxations or dislocations, chronic widespread joint pain, skin manifestations, and a wide variety of associated symptoms and comorbidities, including migraine headaches, functional bowel disorders, orthostatic intolerance, and chronic fatigue, among many others (Bulbena et al., 2017; De Wandele et al., 2013, 2014; Fikree, Chelimsky, Collins, Kovacic, & Aziz, 2017; Hakim, De Wandele, O'Callaghan, Pocinki, & Rowe, 2017; Malfait et al., 2017; P. C. Rowe et al., 1999). Although these findings are usually present by childhood or adolescence, the evaluation and treatment of hEDS/HSD in the pediatric population is hindered by a lack of provider familiarity with pediatric hEDS-related symptomatology as well as the complex medical needs of this population. These factors can contribute to a delay in diagnosis and treatment, with some pediatric cohorts studied reporting 2–3 years from symptom onset to intervention (Adib, Davies, Grahame, Woo, & Murray, 2005). Management guidelines for adults with hEDS/HSD focus on symptomatic treatment with physical therapy and other interventions, with the goal to improve daily function and to prevent deconditioning (Chopra et al., 2017; Engelbert et al., 2017; Ericson & Wolman, 2017; Hakim, O'Callaghan, et al., 2017). However, there is no consensus for treatment of hEDS/HSD manifestations in the pediatric population, or which aspects of management to target to optimize quality of life.
Health-related quality of life (HRQOL) optimization is increasingly understood as a goal of healthcare, with important underlying factors including pain, fatigue, and sleep. Studies are ongoing to improve quality of life in children with chronic healthcare conditions for better clinical and long-term outcomes related to their medical health (Frakking et al., 2018). There is a shortage of studies looking specifically at complications and HRQOL predictors in the pediatric and adolescent hEDS/HSD population, and important additional HRQOL factors such as daily function, sleep, school attendance, sense of wellness, and illness representation are not well addressed by prior studies. This present study aimed to address these important factors in children and adolescents with hEDS/HSD, as perceived by both the affected individuals as well as their parents.
2 |. MATERIALS AND METHODS
2.1 |. Participant recruitment
Potential participants were identified through retrospective review of patients seen in the genetics clinics at Johns Hopkins University (JHU) and Greater Baltimore Medical Center (GBMC) from 02/2014 through 02/2017. The recruitment list was then generated based on the following inclusion criteria: individuals who were between the ages of 10–18 years at time of evaluation (up to age 20 years by February 28, 2017), had a Beighton score of 4/9 or higher (which has been validated in children) (Smits-Engelsman, Klerks, & Kirby, 2011), and met clinical diagnostic criteria for hypermobile type EDS as defined by the Brighton criteria for JHS and the Villefranche criteria for hypermobile EDS (Beighton et al., 1998; Grahame et al., 2000). As all patients were evaluated prior to the revised 2017 hEDS classification (Malfait et al., 2017), we will use the combined terms “hypermobile Ehlers-Danlos syndrome/hypermobility spectrum disorder” (hEDS/HDS) to refer to the range of clinical phenotypes experienced by our participants under the current nomenclature. Exclusion criteria included other unrelated comorbid diagnoses as of 02/2017 suggesting a separate genetic etiology for which joint hypermobility is one component, such as multiple congenital anomalies, intellectual disability or autism. In this manuscript, “patient participant” will refer to the participants with a diagnosis of hEDS/HSD aged 12–20 years as defined by the above inclusion criteria, and “parent participant” will refer to the parent of these individuals.
Invitation letters to participate in a Qualtrics online survey were sent 220 eligible patient participants and their parents, including 88 at JHU and 132 at GBMC, each with an assigned unique identifier to link responses to the clinical information collected from retrospective chart review. For individuals with hEDS/HSD younger than age 18 years, online informed consent was obtained from their parent on the first page of the Qualtrics survey. For these, the recruitment letter was addressed to the parent with instructions to complete the questionnaire about their child with hEDS/HSD prior to helping the younger child complete the questionnaire, as to reduce bias in the parental responses. For individuals with hEDS/HSD 18 years or older, informed consent was obtained separately from individual as well as from one of their parents. Participants with hEDS/HSD 18 years or older were instructed to complete the questionnaire separately from their parents. Two reminder letters or emails were sent and a paper survey was mailed to participants without email addresses.
This study was approved by the Institutional Review Boards of the Johns Hopkins University School of Medicine and GBMC.
2.2 |. Clinical genetics evaluation
All patient participants were previously evaluated by clinical geneticists. Clinical genetic testing for classical EDS was offered if clinically indicated; in this sample none of the participants had positive genetic testing for pathogenic variants associated with vascular EDS (COL3A1) or classical EDS (COL5A1 and COL5A2). Investigators used specific search terms to acquire data from the following sections of the electronic chart: demographics, problem list, and the documentation of the clinical genetics evaluation. The following data were collected for the analysis: clinical genetic diagnosis, presence or absence of family history of any type of EDS, Beighton score at the last clinical exam date (highest discrete number if a range was noted), and Karnofsky Performance Status (KPS) Scale (lower score if a range was noted) (Karnofsky, Abelmann, Craver, & Burchenal, 1948) at the last clinical exam date if obtained. Height and weight taken from the last clinical genetics visit and BMI percentile was calculated using the CDC BMI Percentile Calculator for Child and Teen. Additionally, the following variables were noted: the presence or absence of mast cell activation syndrome (MCAS) symptoms; specific gastrointestinal diagnoses (eosinophilic esophagitis, gastroparesis, and/or dysmotility); suspected or confirmed postural orthostatic tachycardia syndrome (POTS); chronic headaches and/or migraines; specific neurosurgical complications (Chiari Type I malformation, tethered cord, cranio-cervical or cervical instability, syringomyelia); past or present psychiatric diagnoses for which the patient was or is receiving treatment (including counseling and therapy); and other pertinent diagnoses or features (such as urinary incontinence, chronic regional pain syndrome or arachnodactyly).
2.3 |. Functional impairment, psychosocial factors, and quality of life
Functional impairment was estimated cross-sectionally using the Functional Disability Inventory (FDI) (Claar & Walker, 2006; Kashikar-Zuck et al., 2011; Walker & Greene, 1991) as well as retrospectively using the KPS Scale (Karnofsky et al., 1948). Quality of life was estimated using the corresponding Pediatric Quality of Life Inventory (PedsQLTM) for individuals 10–12 years, 13–17 years, and 18–20 years—subcomponents analyzed included physical, emotional, social, school, and psychosocial functioning (Varni, Burwinkle, Seid, & Skarr, 2003). Fatigue was measured using the PedsQL Multidimentional Fatigue Scale (PedsQL MFS) (Varni, Burwinkle, & Szer, 2004). Psychosocial and psychiatric factors were estimated using select questions for anxiety and depression agreed upon via Delphi method (see Supporting Information Appendix for specific questions) (Linstone & Turoff, 1975), the Pain-Frequency-Severity-Duration Scale (PFSD) (Salamon, Davies, Fuentes, Weisman, & Hainsworth, 2014), the Herth Hope Index (Herth, 1992), as well as the psychosocial components of the PedsQL. Illness perception was evaluated using the Brief Illness Perception Questionnaire (BIPQ) (Broadbent, Petrie, Main, & Weinman, 2006). Participants also were asked to provide qualitative responses for any helpful interventions for their symptoms. See Supporting Information Appendix for additional information about each scale.
2.4 |. Statistical methods
Scores for the primary variables were compared among patient participants with hEDS/HSD, among parents of individuals with hEDS/HSD, and also for each matched parent-child dyad. Paired t-test comparison to compare primary characteristics (age, Beighton score, Karnofsky, etc.) and percentages of comorbidities between the JHU and GBMC populations. The distributions were compared by symptom severity using scatterplots and correlation analysis.
In order to determine which demographic variables, clinical diagnoses, and survey outcomes in our cohort had the greatest impact on patient-reported HRQOL, we used Bayesian Multiple Regression. When the number of independent variables is large and the number of observations is relatively small, penalized regression (aka regularization) methods like horseshoe (Carvalho, Polson, & Scott, 2010) and elastic net (Zou & Hastie, 2005) can be used to eliminate variables that have little impact on prediction. All predictors were standardized to give comparable regression coefficients. For missing data (<2% missing data overall), multiple imputation was performed using the MICE (Multivariate Imputation by Chained Equations in R) package. FDI scores were not included due to high degree of correlation with the physical subscore of the PedsQL (R > 0.9). The subscores of the PedsQL were also not included in the regression model. Horseshoe regression models were estimated using the empirical Bayes approach, and were compared against use of the half-Cauchy and Jeffreys priors using the horseshoe package (van der Pas, Scott, Chakraborty, & Bhattacharya, 2016). These results were qualitatively compared to elastic net regression using the glmnet package, with the tuning parameter chosen by 10-fold cross validation (Friedman, Hastie, & Tibshirani, 2010). The relative importance of the variables was the primary concern of this study, so quantitative inferences from the β coefficients were not made. Data analysis was completed using R version 3.4.1 software (R Core Team 2017).
2.5 |. Qualitative analysis
As part of the BIPQ, participants were asked about the three most important factors that influence their/their child's illness in free-text format. Responses on causal beliefs (the last item of the illness perception questionnaire) were coded using thematic content analysis. Codebook development was influenced by the self-determination theory, which separates beliefs into intrinsic and extrinsic, and has been studied to have importance in rehabilitation in similar pediatric populations with functional limitations (Meyns, Roman de Mettelinge, van der Spank, Coussens, & Van Waelvelde, 2018). Codes were then modified based on open-ended coding. Two coders separately coded all responses using the same codebook, and reconciled any discrepancies through discussion.
3 |. RESULTS
Responses were obtained from 68 participants out of 220 eligible participants (total response rate 31%). Fourteen responses had been excluded because they completed <25% of survey questions, and were not counted in the overall response rate. There were a total of 104 respondees, of whom 47 were a patient and 57 were a parent (patient participant response rate 21%). There were 36 parent-p dyads (72 responses), 11 with only a patient (child) response, and 21 with only a parent response. For the purposes of this report, we focused on self-reported responses of the patient participants (n = 47).
3.1 |. Demographics
Table 1 describes the characteristics of the study population in detail. Participants included 81% female and 19% male patient participants, with a mean age of 16.1 years and average body mass index percentile of 59.3% at time of data collection. Regarding race, 93.6% of patients self-identified as White; 6.4% as White and Other, Other, or Pacific Islander. Regarding ethnicity, 8.5% self-identified as Hispanic or Latino. Median Beighton score was 6 (range 4–9); median Karnofsky performance index was 70 (range 40–100). About a third (36.2%) had at least one affected sibling. The majority of the patients had at least one comorbidity. Comorbid diagnoses reported by the patient participants included headaches or migraines in 35 out of 47 (76.1%), POTS symptoms in 26 (56.5%), a psychiatric disorder (most commonly anxiety and depression) in 19 (41.3%), gastrointestinal symptoms in 17 (37.0%), MCAS in 12 (26.1%), neurosurgical complications in 10 (21.7%), and urinary incontinence in 5 (11.6%).
TABLE 1.
Study participant demographics and primary characteristics
| Study population demographics | |
|---|---|
| Total participants | 47 |
| n | |
| Female participants | 38 (80.9) |
| n (%) | |
| Age | 16.1 (2.90, 10–20) |
| Mean (SD, range) | |
| BMI centile | 59.3 (30, 0.27–99.2) |
| Mean (SD, range) | |
| Presence of affected sibling | 17 (36.2) |
| n (%) | |
| Karnofsky Performance Status | 70(40–100) |
| Median (SD, range) | |
| Beighton score | 6 (4–9) |
| Median (range) |
Note. BMI, body mass index; GI, gastrointestinal; MCAS, mast cell activation syndrome; POTS, postural orthostatic tachycardia syndrome.
Statistical comparison of the two centers (Mann-Whitney U test) showed no statistically significant differences in demographics and comorbid diagnoses, with the exception of a trend toward participants at GBMC being more likely to have an affected sibling (p = 0.01), have a diagnosis of mast cell activation disorder (p = 0.02), and having a lower Karnofsky score (p = 0.03).
3.2 |. Primary outcome measures
As detailed in Table 2, PedsQL scores in this population were notably lower than mean scores for healthy controls (Peter C. Rowe et al., 2014) especially in the areas of physical (mean was 49.5, SD 23.5) and emotional subscores (mean 54.7, SD 25.1). Fatigue scores were also low (mean 48.5, SD 25.8), indicating higher fatigue-related disability. Mean functional disability score was 19.1 (SD 13.2), suggesting a notable but not severe pain-related disability.
TABLE 2.
Outcome measures in pediatric and adolescent hEDS/HSD population
| Primary outcome variables |
||||
|---|---|---|---|---|
| Mean | SD | Healthya Mean |
Healthy SD |
|
| FDI | 19.1 | 13.2 | 1.7 | 3.2 |
| PedsQL multidimensional fatigue total score | 48.5 | 25.8 | 85.0 | 12.6 |
| General fatigue | 45.0 | 30.0 | 89.2 | 11.9 |
| Sleep/rest fatigue | 47.4 | 26.4 | 78.3 | 15.7 |
| Cognitive fatigue | 52.9 | 29.2 | 89.4 | 13.6 |
| PedsQL 4.0 generic core total score | 56.7 | 21.4 | 90.2 | 10.1 |
| Physical functioning | 49.5 | 23.5 | 91.9 | 9.4 |
| Emotional functioning | 54.7 | 25.1 | 84.8 | 17.4 |
| Social functioning | 70.2 | 21.2 | 95.2 | 8.9 |
| School functioning | 56.5 | 26.1 | 87.4 | 12.5 |
| Psychosocial functioning | 60.5 | 21.9 | 88.5 | 11.9 |
| Pain (PFSD) | 53.7 | 41.3 | b | |
| Herth Hope Index total | 37.6 | 6.87 | b | |
| BPQI | 45.8 | 11.8 | b | |
Note. BIPQ, Brief Illness Perception Questionnaire; FDI, Functional Disability Inventory; hEDS, hypermobile Ehlers-Danlos syndrome; HRQOL, health-related quality of life; HSD, hypermobility spectrum disorder; PedsQL: Pediatric Quality of Life; PFSD, Pain-Frequency- Severity-Duration.
Pediatric and adolescent participants with hEDS/HSD (n = 47) completed scales measuring functional impairment, psychosocial factors and quality of life.
Data for the healthy controls on the FDI, PedsQL, and the Peds QL Multidimensional Fatigue Scale represent responses from 48 healthy individuals age 10–20 years from the study by Rowe et al. (2014).
There are few studies on healthy control values for these measures.
All survey scores correlated moderately to strongly with each other and with pain (range 0.41–0.95 or 0.38–0.89). There were weaker correlations of some scores with age and incontinence (range 0.31–0.52 or 0.29–0.51); see Supporting Information Appendix for details.
3.3 |. Multiple regression analysis
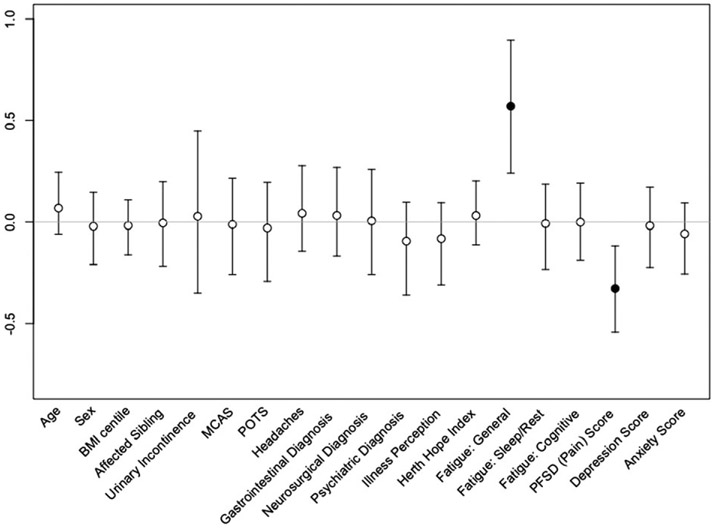
In order to determine which variables in our cohort had the greatest impact on patient-reported HRQOL as measured, we performed horseshoe regression with PedsQL total score as the dependent variable. FDI scores were not included due to high degree of correlation with the physical subscore of the PedsQL (r > 0.9). The horseshoe regression, selecting from all demographic variables, diagnoses, and survey scores (except for subscores of the PedsQL) resulted in the selection of general fatigue (Beta = 0.57, 95% confidence interval [CI] 0.21–0.89) and pain (Beta = −0.33, 95% CI −0.55 to −0.11) as the strongest predictors of PedsQL total score (r2 = 0.901), as seen in Figure 1. This result was confirmed by elastic net regression which returned similar coefficients. This result suggests that best model for predicting patient-reported HRQOL in our cohort including only the general fatigue score and pain score, explains 90% of the variance in the PedsQL total score. A third variable, BIPQ total score, was selected by elastic net but not by the horseshoe regression.
FIGURE 1.
Predictors of quality of life in pediatric and adolescent hEDS/HSD population. Beta (regression) coefficients and 95% confidence intervals from the regression model with horseshoe priors. Black dots indicate variables included in the final model, white dots indicate variables dropped by the model. BMI, body mass index; hEDS, hypermobile EDS; HSD, hypermobility spectrum disorder; MCAS, mast cell activation syndrome; PFSD, Pain-Frequency-Severity-Duration; POTS, postural orthostatic tachycardia syndrome
3.4 |. Comorbidities comparison against primary outcome variables
Seven subsets of patient participants with the following diagnoses were compared to those without the diagnosis from their medical record: neurosurgical diagnosis, POTS, gastrointestinal diagnosis, psychiatric diagnosis, MCAS, headaches, or urinary incontinence. Those with a psychiatric diagnosis had a lower PedsQL social score (mean 58.9 vs 77.8, 95% CI 7.5–30.3, p < 0.005) and were older (mean 17.5 vs 15.1 years, 95% CI −0.84 to 3.8, p < 0.05). There were lower Karnofsky scores in those with MCAS (mean 65.6 vs 76.6, 95% CI 1.6–20.6, p < 0.01), POTS (mean 65.9 vs 79.3, 95% CI 4.6–22.1, p < 0.001), headaches (mean 67.4 vs 82.2, 95% CI 6.6–23.0, p < 0.005), psychiatric diagnosis (mean 65.5 vs 76.7, 95% CI 1.6–20.6, p < 0.01), or neurosurgical diagnosis (mean 60.0 vs 74.8, 95% CI 3.2–26.4, p < 0.05) compared to those without. Other than these findings, there were no significant differences in the mean demographics, diagnosis rates, or survey scores between those with and without each diagnosis.
3.5 |. Interventions tried
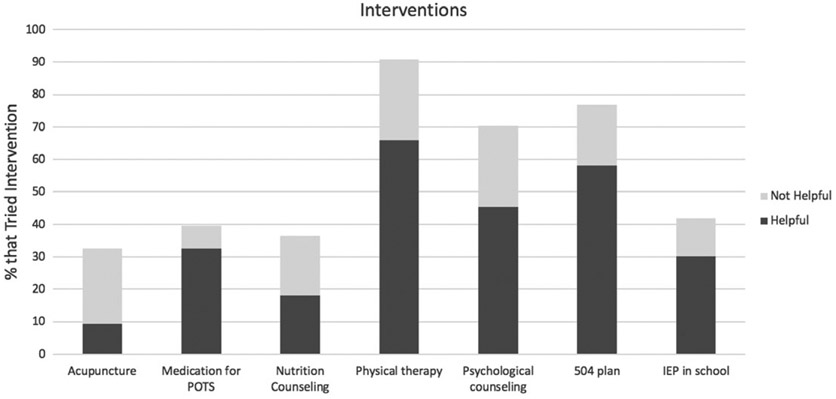
As indicated in Figure 2, patients with hEDS/HSD reported physical therapy as the most common intervention tried (89%, of whom 74% reported as helpful). The next most common interventions tried were 504 plan (77% tried, of whom 73% reported as helpful), psychological counseling (74% tried, of whom 73% reported as helpful), individualized education plan (IEP) as part of a school system (45% tried, of whom 76% reported as helpful), medication for POTS (44% tried, of whom 71% reported as helpful), nutritional counseling (35% tried, of whom 48% reported as helpful), and acupuncture (31% tried, of whom 25% reported as helpful). In the United States, the 504 plan covers any accommodations a student with a handicap may need, while the IEP is a more specific learning plan for students who require special education because of a specific type of disability.
FIGURE 2.
Interventions tried by participants with hEDS/HSD. Forty-seven children and adolescents completed responses on a list of selected interventions, and whether these were found to be helpful, reported as a stacked bar graph. hEDS, hypermobile Ehlers-Danlos syndrome; HSD, hypermobility spectrum disorder; IEP, individualized education plan; POTS, postural orthostatic tachycardia syndrome
3.6 |. Illness representation
Table 3 describes coded causal beliefs of patients with EDS, derived from the last free-text response of the BIPQ. In response to what caused their illness, 95% of patient participants reported an intrinsic factor (outside of their realm of control) and 51% reported an extrinsic factor (within their locus of control). In the category of intrinsic factors, the most common responses were “genes” or “genetics” in 85% of respondents, followed by events of “undergoing puberty” or “a growth spurt” in 15% of respondents. In the category of extrinsic factors, 21% reported too much physical activity as an instigating factor, while another not mutually exclusive 21% reported triggering events, such as illnesses or injuries. Finally, 5% of respondents cited a comorbid diagnosis as having an impact on illness causation.
TABLE 3.
Causal attributions of hEDS/HSD symptoms in pediatric and adolescent population
| Causal beliefs summary |
|||
|---|---|---|---|
| N (n = 39) | % | Illustrative quotes | |
| Intrinsic factors | 37 | 95 | |
| Genes | 33 | 84.6 | “genetic predisposition’’ |
| Puberty/development | 6 | 15.4 | “puberty started moving my bones around as I grew, and if feels like they’ll never to back to not feeling like they’re stabbing me.” |
| Family history | 2 | 5.1 | “my mom has it” |
| Specific physical characteristics | 2 | 5.1 | “Weak joints” |
| Extrinsic factors | 20 | 51.3 | |
| Too much physical activity | 8 | 20.5 | “overuse of joints during dance and other activities” |
| Too little physical activity | 1 | 2.6 | “to much time in bed [sic]” |
| Viral/postviral illness | 6 | 15.4 | “viral illness in 2009” |
| Delay in diagnosis/treatment | 3 | 7.7 | “Going undiagnosed for more than 17 years” |
| Psychosocial factors | 2 | 5.1 | “attitude” |
| Comorbidities | 2 | 5.1 | |
Note. hEDS, hypermobile Ehlers-Danlos syndrome; HSD, hypermobility spectrum disorder.
Responses were completed by 39 patient participants as part of the Brief Illness Perception Questionnaire as free text, which was coded; codes were not mutually exclusive.
4 |. DISCUSSION
Symptoms of hEDS/HSD in the pediatric and adolescent population can range from minimal effects to significant pain, fatigue and medical complications leading to disability. Although much has been written about both chronic pain and chronic fatigue and their effects on quality of life and management of both in hEDS and related disorders (Carter & Threlkeld, 2012; Castori et al., 2012; Chopra et al., 2017), little has been published on these complications in youth (Pacey, Tofts, Adams, Munns, & Nicholson, 2015). In addition, there have been no specific recommendations to maximize quality of life in the pediatric and adolescent population with hEDS/HSD.
In this study, 21% of 220 children, adolescents and young adults with hEDS/HSD ages 12–20 years evaluated within the previous 3 years responded to our questionnaire. These patients were evaluated at a large academic urban institution (JHU) and a private community hospital with a national EDS Center (GBMC), with equal numbers of participants from each center and with well-matched characteristics. In general, HRQOL scores were notably worse in participants with hEDS/HSD compared to healthy controls (Peter C. alRowe et al., 2014), especially when comparing the physical, emotional and school functioning components of the PedsQL, as well as fatigue scores.
In comparison with healthy controls, children with hEDS/HSD (previously overlapping with JHS) have been previously reported to have overall decreased QOL scores (Fatoye, Palmer, MacMillan, Rowe, & Van Der Linden, 2012; Pacey et al., 2015), with possibly contributing factors including increased musculoskeletal injury (Rombaut, Malfait, Cools, De Paepe, & Calders, 2010; Stern et al., 2017), pain intensity and duration (Fatoye et al., 2012; Stern et al., 2017), increased fatigue (Scheper, Nicholson, Adams, Tofts, & Pacey, 2017), and higher risk for multisystem complaints including orthostatic intolerance and headaches (Rombaut et al., 2010; Scheper et al., 2017). Moreover, these children had decreased physical activity and participation in daily life activities (Schubert-Hjalmarsson, Ohman, Kyllerman, & Beckung, 2012). A recent study of 89 Australian children with JHS and one of their parents revealed that children with JHS reported poor HRQOL and disabling fatigue. Pain, fatigue, and stress incontinence accounted for 75% of the variance in child-reported HRQOL (Pacey et al., 2015). Another study of 29 children with JHS reported significantly lower QOL scores and significantly higher pain intensity scores in affected children when compared with age-matched controls (Fatoye et al., 2012).
The primary result of this study is that fatigue and pain scores were the main predictors of child-reported HRQOL: 90% of the variance in the PedsQL total score could be explained by these two variables alone, without any other demographic factor, diagnosis, or other survey score. In other words, the selection algorithm dropped all the other variables, including the three-item anxiety and depression scores. Our model suggested that the relative strength of effect of fatigue and pain on HRQOL was 2:1, respectively. This supports the results of previous studies (Pacey et al., 2015), emphasizing the importance of assessing and treating fatigue and pain to improve HRQOL. While Pacey et al. noted stress incontinence to be a predictor of HRQOL, we did not have a significant enough proportion of our patient population reporting stress incontinence to evaluate this effect. Unique to our study are that presence of any psychiatric diagnosis was correlated with a lower PedsQL score as well as a lower KPS Scale. Further studies are needed on the effect of psychiatric and emotional symptoms in youth with a diagnosis of hEDS/HSD.
We previously reviewed surveys from a pediatric cohort with hypermobile and classical EDS assessed at the National Institute of Aging followed prospectively (Muriello et al., 2018). In this group, all patients had poor sleep quality with respect to historical controls, pain severity had a strong correlation with pain interference similar to other disorders with chronic pain, and both pain and sleep were significant issues in this population. Compared to these studies, our results similarly showed that fatigue and pain are the best predictors of HRQOL; however, the number of participants reporting urinary incontinence was too low (11.6%) to make a meaningful comparison. Interestingly, while several diagnoses were associated with a lower Karnofsky score, no comorbid diagnosis had a significant impact on any survey outcome with the exception of a psychiatric diagnosis on the PedsQL social component.
Management of children and adolescents with hEDS can be challenging due to chronic pain and multisystem complaints, and often requires multidisciplinary care. The majority of patient participants who tried any interventions found them helpful (Figure 2). Physical therapy was the most commonly tried intervention, and has been found effective in clinical trials in patients with hEDS (Engelbert et al., 2017). The majority of children and adolescents treated for POTS also found this helpful, highlighting the importance of making this frequently comorbid diagnosis. While some studies have suggested acupuncture may be helpful in treating pain in patients with connective tissue disorders (Siminovich-Blok, 2016), most who tried acupuncture in this study did not find it helpful (71% reporting as “not helpful”).
Casual beliefs are known to influence an individual's health status, management behaviors, lifestyle decisions, and disease outcomes (Caqueo-Urizar, Boyer, Baumstarck, & Gilman, 2015; Fleary & Ettienne, 2014; Petrie, Jago, & Devcich, 2007; Wisdom & Green, 2004). The majority of child participants felt that factors beyond their control, such as genetics, were responsible for their symptoms; this is not surprising, given that most patients are counseled that hEDS is a genetic disorder. However, half also felt that extrinsic factors, such as excessive activity or psychological outlook, also contributed, aligning with more recent hypotheses about the multifactorial etiology of hEDS and HSD (B. Tinkle et al., 2017). This latter population attributing potentially “controllable” factors to illness development is of particular interest, as this belief could lead to more active coping strategies and adherence to management guidelines. Similar studies of causal attribution in chronic fatigue and chronic pain find that patients frequently attributed symptoms to physical rather than psychological causes, and that this physical attribution (suggesting a more medically deterministic view, with a de-emphasis on psychological coping) may lead to increased levels of functional disability (Cho, Bhugra, & Wessely, 2008; Keating et al., 2017). Future studies could investigate the impact of causal beliefs in hEDS/HSD on interest in trying physical therapy, and eventual health outcomes.
Limitations of this study include that due to the relatively small size, cross-sectional analysis, and retrospective nature of this survey, participants may have been evaluated up to 3 years prior to taking the survey, and therefore may have had notable improvement or worsening of symptoms and function, or received additional comorbid diagnoses that were not noted upon the chart review. Furthermore, this referred group of children and adolescents with hEDS may not be representative of the general pediatric and adolescent hEDS/HSD population. Not all participants were able to be clinically ascertained for each comorbidity. Additionally, the proxy questions for anxiety and depression were not validated.
In conclusion, fatigue and pain are important determinants of HRQOL in children and adolescents with hEDS/HSD. Future work on this data will include investigation of the relationship and impact of paired parent-child responses to HRQOL. Additionally, future research is needed to continue investigating the underlying mechanisms of hEDS, genomic studies to find causative or associated genetic variants, and clinical trials to address optimal approaches to treatment.
Supplementary Material
ACKNOWLEDGMENTS
We thank our patients and their families for participating. We would also like to thank Dimitri Avramopoulis for statistical analysis assistance. This research was funded by the Research Accelerator and Mentorship Program (RAMP) through the Johns Hopkins Clinical Research Network (JHCRN). This publication was made possible by the Johns Hopkins Institute for Clinical and Translational Research (ICTR), which is funded in part by Grant Number UL1 TR001079 from the National Center for Advancing Translational Sciences (NCATS), a component of the National Institutes of Health (NIH), and NIH Roadmap for Medical Research. Its contents are solely the responsibility of the authors and do not necessarily represent the official view of the Johns Hopkins ICTR, NCATS or NIH.
Funding information
Johns Hopkins Clinical Research Network, Grant/Award Number: N/A; NIH Roadmap for Medical Research; National Institutes of Health; National Center for Advancing Translational Sciences, Grant/Award Number: UL1 TR001079
Footnotes
CONFLICT OF INTEREST
The authors received no internal or external funding for the data pertaining to this manuscript and have no conflicts of interest to declare.
SUPPORTING INFORMATION
Additional supporting information may be found online in the Supporting Information section at the end of the article.
REFERENCES
- Adib N, Davies K, Grahame R, Woo P, & Murray KJ (2005). Joint hypermobility syndrome in childhood. A not so benign multisystem disorder? Rheumatology, 44(6), 744–750. 10.1093/rheumatology/keh557 [DOI] [PubMed] [Google Scholar]
- Armon K (2015). Musculoskeletal pain and hypermobility in children and young people: Is it benign joint hypermobility syndrome? Archives of Disease in Childhood, 100(1), 2–3. 10.1136/archdischild-2014-306556 [DOI] [PubMed] [Google Scholar]
- Beighton P, De Paepe A, Steinman B, Tsipouras P, & Wenstrup RJ (1998). Ehlers-Danlos syndromes: Revised nosology, Villefranche, 1997. American Journal of Medical Genetics, 77, 31–37. 10.1002/(SICI)1096-8628(19980428)77 [DOI] [PubMed] [Google Scholar]
- Broadbent E, Petrie KJ, Main J, & Weinman J (2006). The Brief Illness Perception Questionnaire. Journal of Psychosomatic Research, 60(6), 631–637. 10.1016/j.jpsychores.2005.10.020 [DOI] [PubMed] [Google Scholar]
- Bulbena A, Baeza-Velasco C, Bulbena-Cabré A, Pailhez G, Critchley H, Chopra P, … Porges S (2017). Psychiatric and psychological aspects in the Ehlers-Danlos syndromes. American Journal of Medical Genetics, Part C: Seminars in Medical Genetics, 175(1), 237–245. 10.1002/ajmg.c.31544 [DOI] [PubMed] [Google Scholar]
- Caqueo-Urizar A, Boyer L, Baumstarck K, & Gilman SE (2015). The relationships between patients' and caregivers' beliefs about the causes of schizophrenia and clinical outcomes in Latin American countries. Psychiatry Research, 229(1–2), 440–446. 10.1016/j.psychres.2015.06.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter BD, & Threlkeld BM (2012). Psychosocial perspectives in the treatment of pediatric chronic pain. Pediatric Rheumatology, 10(5), 15 10.1186/1546-0096-10-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvalho CM, Polson NG, & Scott JG (2010). The horseshoe estimator for sparse signals. Biometrika, 97(2), 465–480. 10.1093/biomet/asq017 [DOI] [Google Scholar]
- Castori M, Morlino S, Celletti C, Celli M, Morrone A, Colombi M, … Grammatico P (2012). Management of pain and fatigue in the joint hypermobility syndrome (a.k.a. Ehlers-Danlos syndrome, hypermobility type): Principles and proposal for a multidisciplinary approach. American Journal of Medical Genetics: Part A, 158A(8), 2055–2070. 10.1002/ajmg.a.35483 [DOI] [PubMed] [Google Scholar]
- Castori M, Tinkle B, Levy H, Grahame R, Malfait F, & Hakim A (2017). A framework for the classification of joint hypermobility and related conditions. American Journal of Medical Genetics, Part C: Seminars in Medical Genetics, 175(1), 148–157. 10.1002/ajmg.c.31539 [DOI] [PubMed] [Google Scholar]
- Cattalini M, Khubchandani R, & Cimaz R (2015). When flexibility is not necessarily a virtue: A review of hypermobility syndromes and chronic or recurrent musculoskeletal pain in children. Pediatric Rheumatology Online Journal, 13(1), 40 10.1186/s12969-015-0039-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho HJ, Bhugra D, & Wessely S (2008). “Physical or psychological?”— A comparative study of causal attribution for chronic fatigue in Brazilian and British primary care patients. Acta Psychiatrica Scandinavica, 118(1), 34–41. 10.1111/j.1600-0447.2008.01200.x [DOI] [PubMed] [Google Scholar]
- Chopra P, Tinkle B, Hamonet C, Brock I, Gompel A, Bulbena A, & Francomano C (2017). Pain management in the Ehlers-Danlos syndromes. American Journal of Medical Genetics, Part C: Seminars in Medical Genetics, 175(1), 212–219. 10.1002/ajmg.c.31554 [DOI] [PubMed] [Google Scholar]
- Claar RL, & Walker LS (2006). Functional assessment of pediatric pain patients: Psychometric properties of the Functional Disability Inventory. Pain, 121(1–2), 77–84. 10.1016/j.pain.2005.12.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Wandele I, Calders P, Peersman W, Rimbaut S, De Backer T, Malfait F, … Rombaut L (2014). Autonomic symptom burden in the hypermobility type of Ehlers-Danlos syndrome: A comparative study with two other EDS types, fibromyalgia, and healthy controls. Seminars in Arthritis and Rheumatism, 44(3), 353–361. 10.1016/j.semarthrit.2014.05.013 [DOI] [PubMed] [Google Scholar]
- De Wandele I, Rombaut L, Malfait F, De Backer T, De Paepe A, & Calders P (2013). Clinical heterogeneity in patients with the hypermobility type of Ehlers-Danlos syndrome. Research in Developmental Disabilities, 34(3), 873–881. 10.1016/j.ridd.2012.11.018 [DOI] [PubMed] [Google Scholar]
- Engelbert RHH, Juul-Kristensen B, Pacey V, de Wandele I, Smeenk S, Woinarosky N,… Simmonds JV (2017). The evidence-based rationale for physical therapy treatment of children, adolescents, and adults diagnosed with joint hypermobility syndrome/hypermobile Ehlers Danlos syndrome. American Journal of Medical Genetics, Part C: Seminars in Medical Genetics, 175(1), 158–167. 10.1002/ajmg.c.31545 [DOI] [PubMed] [Google Scholar]
- Ericson WB, & Wolman R (2017). Orthopaedic management of the Ehlers-Danlos syndromes. American Journal of Medical Genetics, Part C: Seminars in Medical Genetics, 175(1), 188–194. 10.1002/ajmg.c.31551 [DOI] [PubMed] [Google Scholar]
- Fatoye F, Palmer S, MacMillan F, Rowe P, & Van Der Linden M (2012). Pain intensity and quality of life perception in children with hypermobility syndrome. Rheumatology International, 32(5), 1277–1284. 10.1007/s00296-010-1729-2 [DOI] [PubMed] [Google Scholar]
- Fikree A, Chelimsky G, Collins H, Kovacic K, & Aziz Q (2017). Gastrointestinal involvement in the Ehlers-Danlos syndromes. American Journal of Medical Genetics, Part C: Seminars in Medical Genetics, 175(1), 181–187. 10.1002/ajmg.c.31546 [DOI] [PubMed] [Google Scholar]
- Fleary SA, & Ettienne R (2014). Inherited or behavior? What causal beliefs about obesity are associated with weight perceptions and decisions to lose weight in a US sample? International Scholarly Research Notices, 2014, 1–10. 10.1155/2014/632940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frakking TT, Waugh J, Teoh HJ, Shelton D, Moloney S, Ward D, … Weir K (2018). Integrated children's clinic care (ICCC) versus a self-directed care pathway for children with a chronic health condition: A multi-centre randomised controlled trial study protocol. BMC Pediatrics, 18(1), 1–9. 10.1186/s12887-018-1034-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman J, Hastie T, & Tibshirani R (2010). Regularlization paths for generalized linear models via coordinate descent. Journal of Statistical Software, 33(1), 1–22. [PMC free article] [PubMed] [Google Scholar]
- Grahame R, Bird HA, & Child AH (2000). The revised (Brighton 1998) criteria for the diagnosis of benign joint hypermobility syndrome (BJHS). The Journal of Rheumatology, 27(7), 1777–1779. [PubMed] [Google Scholar]
- Gunz AC, Canizares M, Mackay C, & Badley EM (2012). Magnitude of impact and healthcare use for musculoskeletal disorders in the paediatric: A population-based study. BMC Musculoskeletal Disorders, 13 (1), 98 10.1186/1471-2474-13-98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hakim A, De Wandele I, O'Callaghan C, Pocinki A, & Rowe P (2017). Chronic fatigue in Ehlers-Danlos syndrome—Hypermobile type. American Journal of Medical Genetics, Part C: Seminars in Medical Genetics, 175(1), 175–180. 10.1002/ajmg.c.31542 [DOI] [PubMed] [Google Scholar]
- Hakim A, O'Callaghan C, De Wandele I, Stiles L, Pocinki A, & Rowe P (2017). Cardiovascular autonomic dysfunction in Ehlers-Danlos syndrome—Hypermobile type. American Journal of Medical Genetics, Part C: Seminars in Medical Genetics, 175(1), 168–174. 10.1002/ajmg.c.31543 [DOI] [PubMed] [Google Scholar]
- Herth K (1992). Abbreviated instrument to measure Hope: Development and psychometric evaluation. Journal of Advanced Nursing, 17(10), 1251–1259. 10.1111/j.1365-2648.1992.tb01843.x [DOI] [PubMed] [Google Scholar]
- Karnofsky DA, Abelmann WH, Craver LF, & Burchenal JH (1948). The use of the nitrogen mustards in the palliative treatment of carcinoma. Cancer, 1(4), 634–656. [DOI] [Google Scholar]
- Kashikar-Zuck S, Flowers SR, Claar RL, Guite JW, Logan DE, Lynch-Jordan AM, … Wilson AC (2011). Clinical utility and validity of the Functional Disability Inventory among a multicenter sample of youth with chronic pain. Pain, 152(7), 1600–1607. 10.1016/j.pain.2011.02.050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keating EM, Antiel RM, Weiss KE, Wallace D, Antiel SJ, Fischer PR … Harbeck-Weber C (2017). Parental perceptions of pediatric pain and POTS-related disability. Clinical Pediatrics, 56(13), 1185–1192. 10.1177/0009922816681137 [DOI] [PubMed] [Google Scholar]
- Linstone HA, & Turoff M (1975). The Delphi method-Techniques and applications. London: Addison-Wesley; 10.2307/1268751 [DOI] [Google Scholar]
- Malfait F, Francomano C, Byers P, Belmont J, Berglund B, Black J, … Tinkle B (2017). The 2017 international classification of the Ehlers-Danlos syndromes. American Journal of Medical Genetics, Part C: Seminars in Medical Genetics, 175(1), 8–26. 10.1002/ajmg.c31552 [DOI] [PubMed] [Google Scholar]
- Meyns P, Roman de Mettelinge T, van der Spank J, Coussens M, & Van Waelvelde H (2018). Motivation in pediatric motor rehabilitation: A systematic search of the literature using the self-determination theory as a conceptual framework. Developmental Neurorehabilitation, 21 (6), 317–390. 10.1080/17518423.2017.1295286 [DOI] [PubMed] [Google Scholar]
- Muriello M, Clemens JL, Mu W, Tran PT, Rowe PC, Smith CH, … Kline AD (2018). Pain and sleep quality in children with non-vascular Ehlers-Danlos syndromes. American Journal of Medical Genetics: Part A, 176(9), 1858–1864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray KJ (2006). Hypermobility disorders in children and adolescents. Best Practice and Research: Clinical Rheumatology, 20(2), 329–351. 10.1016/j.berh.2005.12.003 [DOI] [PubMed] [Google Scholar]
- Pacey V, Tofts L, Adams RD, Munns CF, & Nicholson LL (2015). Quality of life prediction in children with joint hypermobility syndrome. Journal of Paediatrics and Child Health, 51(7), 689–695. 10.1111/jpc.12826 [DOI] [PubMed] [Google Scholar]
- Petrie K, Jago LA, & Devcich DA (2007). The role of illness perceptions in patients with medical conditions. Current Opinion in Psychiatry, 20(2), 163–167. 10.1097/YCO.0b013e328014a871 [DOI] [PubMed] [Google Scholar]
- Rombaut L, Malfait F, Cools A, De Paepe A, & Calders P (2010). Musculoskeletal complaints, physical activity and health-related quality of life among patients with the Ehlers-Danlos syndrome hypermobility type. Disability and Rehabilitation, 32(16), 1339–1345. 10.3109/09638280903514739 [DOI] [PubMed] [Google Scholar]
- Rowe PC, Barron DF, Calkins H, Maumenee IH, Tong PY, & Geraghty MT (1999). Orthostatic intolerance and chronic fatigue syndrome associated with Ehlers-Danlos syndrome. The Journal of Pediatrics, 135(4), 494–499. 10.1016/S0022-3476(99)70173-3 [DOI] [PubMed] [Google Scholar]
- Rowe PC, Marden CL, Flaherty MAK, Jasion SE, Cranston EM, Johns AS, … Violand RL (2014). Impaired range of motion of limbs and spine in chronic fatigue syndrome. Journal of Pediatrics, 165(2), 360–366. 10.1016/j.jpeds.2014.04.051 [DOI] [PubMed] [Google Scholar]
- Salamon KS, Davies WH, Fuentes MR, Weisman SJ, & Hainsworth KR (2014). The Pain Frequency-Severity-Duration Scale as a measure of pain: Preliminary validation in a pediatric chronic pain sample. Pain Research and Treatment, 2014, 653592 10.1155/2014/653592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheper MC, Nicholson LL, Adams RD, Tofts L, & Pacey V (2017). The natural history of children with joint hypermobility syndrome and Ehlers-Danlos hypermobility type: A longitudinal cohort study. Rheumatology (Oxford), 56(12), 2073–2083. 10.1093/rheumatology/kex148 [DOI] [PubMed] [Google Scholar]
- Schubert-Hjalmarsson E, Öhman A, Kyllerman M, & Beckung E (2012). Pain, balance, activity, and participation in children with hypermobility syndrome. Pediatric Physical Therapy, 24(4), 339–344. 10.1097/PEP.0b013e318268e0ef [DOI] [PubMed] [Google Scholar]
- Siminovich-Blok B (2016). Treating connective tissue disorders with accupuncture: The case for Ehlers-Danlos syndrome. The Journal of Alternative and Complementary Medicine, 22(1), A42. [Google Scholar]
- Smits-Engelsman B, Klerks M, & Kirby A (2011). Beighton score: A valid measure for generalized hypermobility in children. Journal of Pediatrics, 158(1), 119–123. 10.1016/j.jpeds.2010.07.021 [DOI] [PubMed] [Google Scholar]
- Stern CM, Pepin MJ, Stoler JM, Kramer DE, Spencer SA, & Stein CJ (2017). Musculoskeletal conditions in a pediatric population with Ehlers-Danlos syndrome. Journal of Pediatrics, 181, 261–266. 10.1016/j.jpeds.2016.10.078 [DOI] [PubMed] [Google Scholar]
- Tinkle B, Castori M, Berglund B, Cohen H, Grahame R, Kazkaz H, & Levy H (2017). Hypermobile Ehlers-Danlos syndrome (a.k.a. Ehlers-Danlos syndrome Type III and Ehlers-Danlos syndrome hypermobility type): Clinical description and natural history. American Journal of Medical Genetics, Part C: Seminars in Medical Genetics, 175(1), 48–69. 10.1002/ajmg.c.31538 [DOI] [PubMed] [Google Scholar]
- Tinkle BT, Bird HA, Grahame R, Lavallee M, Levy HP, & Sillence D (2009). The lack of clinical distinction between the hypermobility type of Ehlers-Danlos syndrome and the joint hypermobility syndrome (a.k.a. hypermobility syndrome). American Journal of Medical Genetics: Part A, 149(11), 2368–2370. 10.1002/ajmg.a.33070 [DOI] [PubMed] [Google Scholar]
- van der Pas S, Scott J, Chakraborty A, & Bhattacharya A (2016). horseshoe: Implementation of the horseshoe prior (R package version 0.1.0). https://CRAN.R-project.org/package=horseshoe.
- Varni JW, Burwinkle TM, Seid M, & Skarr D (2003). The PedsQL TM 4.0 as a pediatric population health measure: Feasibility, reliability, and validity. Ambulatory Pediatrics, 3(6), 329–341. 10.1007/s11136-005-1388-z [DOI] [PubMed] [Google Scholar]
- Varni JW, Burwinkle TM, & Szer IS (2004). The PedsQL Multidimensional Fatigue Scale in pediatric rheumatology: Reliability and validity. The Journal of Rheumatology, 31(12), 2494–2500. [PubMed] [Google Scholar]
- Walker LS, & Greene JW (1991). The Functional Disability Inventory: Measuring a neglected dimension of child health status. Journal of Pediatric Psychology, 16, 39–58. [DOI] [PubMed] [Google Scholar]
- Wisdom JP, & Green CA (2004). “Being in a funk”: Teens' efforts to understand their depressive experiences. Qualitative Health Research, 14(9), 1227–1238. 10.1177/1049732304268657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoppi N, Chiarelli N, Binetti S, Ritelli M, & Colombi M (2018). Dermal fibroblast-to-myofibroblast transition sustained by αvß3 integrin-ILK-Snail1/Slug signaling is a common feature for hypermobile Ehlers-Danlos syndrome and hypermobility spectrum disorders. Biochimica et Biophysica Acta (BBA)–Molecular Basis of Disease, 1864(4), 1010–1023. 10.1016/j.bbadis.2018.01.005 [DOI] [PubMed] [Google Scholar]
- Zou H, & Hastie T (2005). Regularization and variable selection via the elastic net. Journal of the Royal Statistical Society: Series B (Statistical Methodology), 2, 301–320. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.