Abstract
Long non-coding RNA (lncRNA) colon cancer-associated transcript-1 (CCAT1) has been reported to play important roles in the development and progression of multiple human malignancies. However, the functional role and molecular mechanism of CCAT1 on gefitinib resistance in non-small cell lung cancer (NSCLC) are largely unclear. The aim of this study is to explore the roles of CCAT1 on gefitinib resistance in NSCLC and to explore the underlying mechanisms. The quantitative real-time PCR (qRT-PCR) analysis was to investigate the expression pattern of CCAT1 in gefitinib-resistant NSCLC patient tissues and cell lines, and then the effects of CCAT1 on gefitinib resistance of NSCLC in vitro and in vivo. Furthermore, bioinformatics online program predictions and luciferase reporter assay were used to validate the association of CCAT1 and miR-218 in NSCLC cells. In this study, CCAT1 was observed to be upregulated in gefitinib-resistant patient tissues and cell lines. In vitro and in vivo experiments demonstrated that CCAT1 knockdown impaired cell proliferation and promoted the gefitinib-induced cell apoptosis. Furthermore, we demonstrated that CCAT1 acts as a sponge for miR-218, and verified that HOXA1 is a novel target of miR-218. These results suggest that CCAT1 may serve as a promising therapeutic target for the treatment of epidermal growth factor receptor (EGFR) plus NSCLC patients.
Keywords: lncRNA, CCAT1, NSCLC, gefitinib, ceRNA
Introduction
Lung cancer is the leading cause of cancer-associated mortality worldwide, of which more than 80% of cases are non-small cell lung cancer (NSCLC).1 Despite the sustained breakthroughs in the clinical diagnosis and treatment of lung cancer, mortality rates continue to rise. Over the last few decades, epidermal growth factor receptor (EGFR) tyrosine kinase inhibitors (TKIs) have significantly improved survival of patients with NSCLC.2,3 However, TKI resistance may be an obstacle for NSCLC. Therefore, further studies are required to determine the mechanisms of TKI resistance in NSCLC so that treatment options can be improved and effective treatments selected for individual patients with NSCLC.
Long non-coding RNA (lncRNA) is a category of endogenous non-coding RNAs longer than 200 nt, which is a hotspot in current research.4,5 Recently, several lncRNAs have been shown to act as important regulatory molecules that participate in cellular processes and drug resistance in several types of cancers, including NSCLC.6 lncRNAs have various functions and complicated regulative mechanisms, and take part in almost all physiological or pathological cytobiological characteristics, including apoptosis, cell proliferation, and chemotherapy resistance.7 Recently, lncRNAs have been demonstrated to function as competing endogenous RNAs (ceRNAs) by competitively binding common microRNAs (miRNAs).8,9 lncRNA colon cancer-associated transcript-1 (CCAT1), located on chromosome 8q24.21, was first identified as an oncogene in colorectal cancer.10 The overexpression of CCAT1 was recently proven to activate the initiation and progression in a variety of cancers.11, 12, 13 However, the functional role and precise mechanisms of CCAT1 in TKI resistance of NSCLC remain unclear.
Results
CCAT1 Is Significantly Upregulated in Gefitinib-Resistant NSCLC Cell Lines and NSCLC Patients
To determine the expression levels of CCAT1 in NSCLC, we analyzed the lung squamous cell carcinoma (LUSC) and the lung adenocarcinoma (LUAD) dataset from The Cancer Genome Atlas (TCGA) database and found that the level of CCAT1 was significantly higher in 486 LUSC tissues than 338 normal tissues (p < 0.05; Figure 1A). Kaplan-Meier survival analysis from TCGA datasets suggested that TCGA expression is significantly associated with worse overall survival (OS) (log rank test, p = 0.035; Figure 1B); however, TCGA expression is not significantly associated with disease-free survival (DFS) (log rank test, p = 0.24; Figure 1C). In addition, we performed the qRT-PCR analysis to measure the levels of CCAT1 in clinically enrolled NSCLC tissue.
Figure 1.
CCAT1 Is Significantly Upregulated in Gefitinib Resistant NSCLC Cell Lines and NSCLC Patients
(A) CCAT1 expression in lung cancer and normal samples from the TCGA RCC dataset. (B) Kaplan-Meier analyses of the correlations between CCAT1 expression and OS of lung cancer patients from the TCGA RCC dataset. (C) Kaplan-Meier analyses of the correlations between CCAT1 expression and progression-free survival (PFS) of lung cancer patients from the TCGA RCC dataset. (D) After nuclear and cytosolic separation, RNA expression levels were measured by qRT-PCR. GAPDH was used as a cytosolic marker, and U6 was used as a nuclear marker. The results suggested that the cytoplasm location for CCAT1 mainly existed in PC9 cells. (E) Relative expression of CCAT1 in the gefitinib-sensitive group and gefitinib-resistant group in NSCLC patients. (F) Relative expression of CCAT1 in a panel of NSCLC cell lines. All tests were performed at least three times. Data were expressed as mean ± SD. ***p < 0.001; **p < 0.01.
Next, we performed a subcellular fractionation location assay to investigate CCAT1 location. The results suggested that the cytoplasm location for CCAT1 mainly existed in PC9 cells (Figure 1D). Consistent with the above results, qPCR assay stated that CCAT1 expression was highly observed in the NSCLC samples compared with the normal adjacent lung tissue. Furthermore, we discovered that CCAT1 expression was upregulated in the NSCLC patients who were diagnosed with gefitinib resistance (N = 28) compared with those who were sensitive to the gefitinib chemotherapy (N = 24) (p < 0.001; Figure 1E). We further examined the different expression levels of CCAT1 in NSCLC cell lines and found that CCAT1 expression was enhanced in all NSCLC cells compared with that in 16HBE cells (p < 0.01). Among these NSCLC cells, the expression of CCAT1 was significantly higher in the gefitinib-resistant cell lines, HCC827GR and PC9GR (p < 0.001; Figure 1F). The correlations between CCAT1 expression and clinical pathological characteristics of NSCLC were investigated using univariate and multivariate analysis. Lower CCAT1 expression in NSCLC tissues occurred much more often in patients with more malignant behaviors, including tumor size, high TNM stage, and poor differentiation (p < 0.05; Table 1), but excluding age, sex, and lymphatic metastasis. Those results provided initial evidence that CCAT1 might be involved in the genesis and progression of NSCLC, but not in metastasis. Those results suggested that CCAT1 might play a role in genesis and gefitinib resistance of NSCLC, and higher levels of CCAT1 might be predictive of unfavorable prognosis in NSCLC.
Table 1.
Correlation between CCAT1 Expression and Clinicopathologic Characteristics of NSCLC Patients
| Overall (n = 52) | CCAT1 |
p | ||
|---|---|---|---|---|
| Low (n = 26) | High (n = 26) | |||
| Sex | ||||
| Male | 31 | 17 | 14 | 0.572 |
| Age | ||||
| ≥60 y | 28 | 12 | 16 | 0.404 |
| Differentiation | ||||
| Poor | 18 | 4 | 14 | 0.007 |
| Tumor Size | ||||
| ≥4 | 15 | 3 | 12 | 0.013 |
| Lymphatic Metastasis | ||||
| Positive | 12 | 2 | 10 | 0.018 |
| TNM Stage | ||||
| III + IV | 10 | 1 | 9 | 0.011 |
CCAT1 Inhibited the Proliferation Ability and Advanced Cell Apoptosis of NSCLC In Vitro
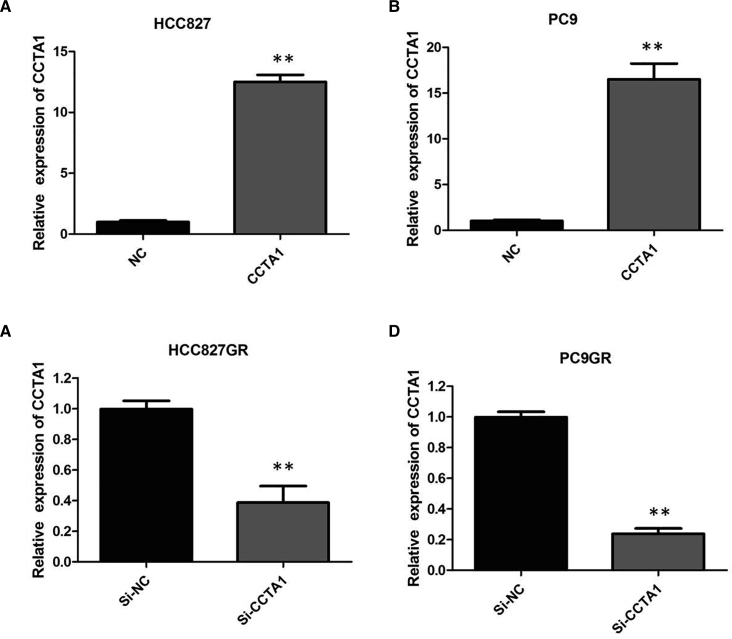
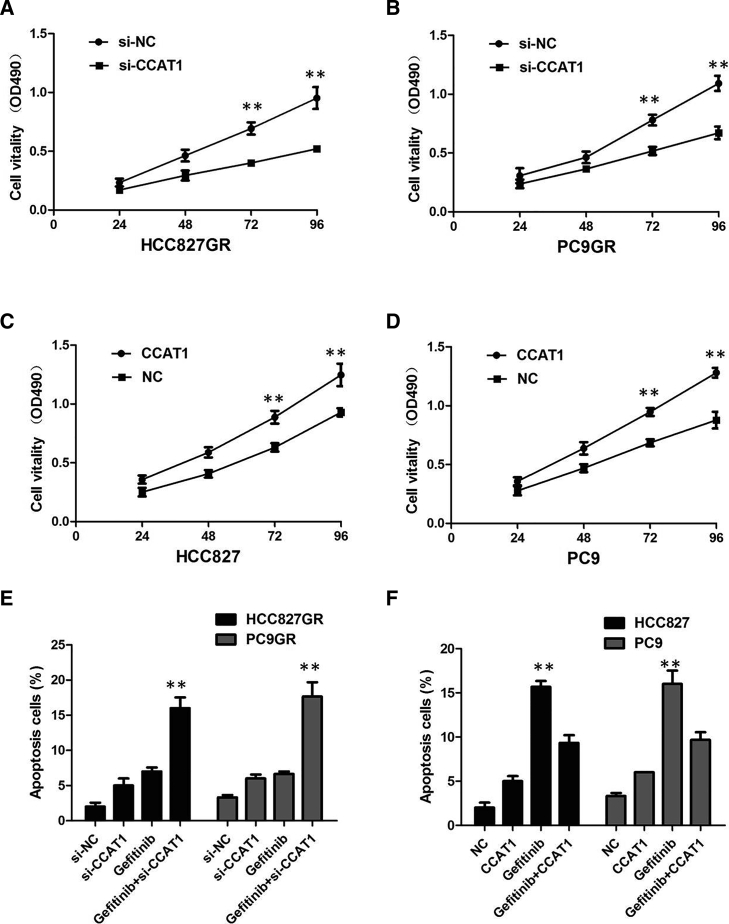
To elucidate the mechanism of CCAT1 function, we assessed the effect of CCAT1 on several biologic properties of NSCLC cells in vitro. First, plasmid-mediated overexpression was used for exogenously manipulating the expression of CCAT1 in HCC827 and PC9 cells (p < 0.01; Figures 2A and 2B), and we used lentivirus (LV)-anti-CCAT1-mediated knockdown to interfere with CCAT1 expression in HCC827GR and PC9GR cells (p < 0.01; Figures 2C and 2D). Then we examined cellular proliferation by Cell Counting Kit-8 (CCK-8) assays, cell cycle by propidium iodide (PI), and cell apoptosis by Annexin V-fluorescein isothiocyanate (FITC) and PI. CCAT1 silencing suppressed the proliferation of HCC827GR and PC9GR cells in the presence of 1 μM gefitinib (p < 0.01; Figures 3A and 3B), implying that CCAT1 promotes proliferation in gefitinib-resistant cells. However, after overexpression of CCAT1, the growth rates of HCC827 and PC9 cells were significantly increased compared with the control group (p < 0.01; Figures 3C and 3D).
Figure 2.
Knockdown and Overexpression of CCAT1 in NSCLC Cells
(A) Plasmid-mediated CCAT1 overexpression in HCC827 cells. (B) Plasmid-mediated CCAT1 overexpression in PC9 cells. (C) LV-anti-CCAT1-mediated CCAT1 knockdown in HCC827GR cells. (D) LV-anti-CCAT1-mediated CCAT1 knockdown in PC9GR cells. All tests were performed at least three times. Data were expressed as mean ± SD. ***p < 0.001; **p < 0.01.
Figure 3.
CCAT1 Inhibited the Proliferation Ability and Advanced Cell Apoptosis of NSCLC In Vitro
(A) CCK-8 assay showed that CCAT1 knockdown inhibited growth rate in HCC827GR cells. (B) CCK-8 assay showed that CCAT1 knockdown inhibited growth rate in PC9GR cells. (C) CCK-8 assay showed that CCAT1 overexpression promoted growth rate in HCC827 cells. (D) CCK-8 assay showed that CCAT1 overexpression promoted growth rate in PC9 cells. (E) Knockdown of CCAT1 increased the gefitinib-induced cell apoptosis of HCC827GR and PC9GR cells. (F) CCAT1 overexpression decreased the gefitinib-induced cell apoptosis of HCC827 and PC9 cells. All tests were performed at least three times. Data were expressed as mean ± SD. ***p < 0.001; **p < 0.01.
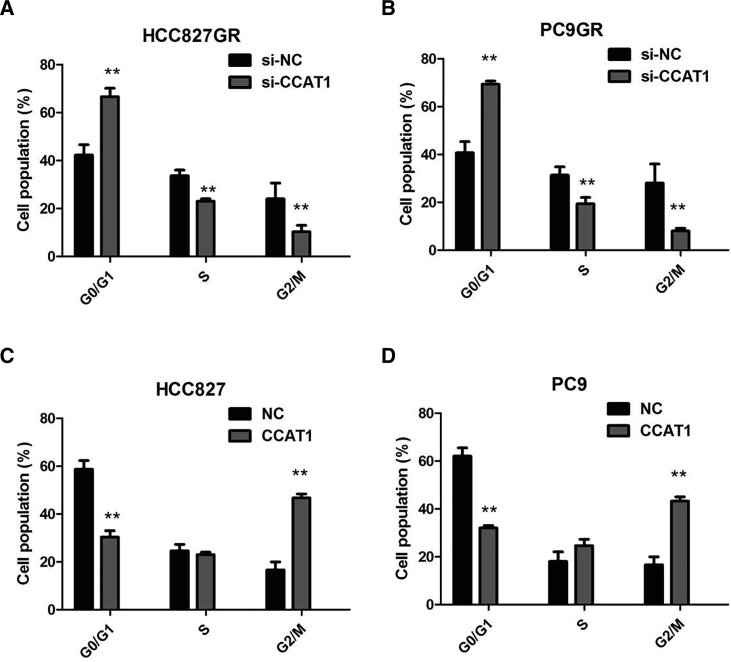
In the presence of 1 μM gefitinib, silencing of CCAT1 promoted the gefitinib-induced cell apoptosis of HCC827GR and PC9GR cells (p < 0.01; Figure 3E). However, cell apoptosis assays revealed that following CCAT1 overexpression, the apoptosis of HCC827 and PC9 cells in the presence of 1 μM gefitinib was significantly decreased compared with the control group (p < 0.01; Figure 3F). We proved that knockdown of CCAT1 caused significant G1 phase cell-cycle arrest of HCC827GR and PC9GR cells in the presence of 1 μM gefitinib (p < 0.01; Figures 4A and 4B). Enhanced CCAT1 expression increased the S phase percentage and decreased G0/G1 phase percentage of HCC827 and PC9 cells in the presence of 1 μM gefitinib (p < 0.01; Figures 4C and 4D). Collectively, these results demonstrated that CCAT1 promotes NSCLC cell growth by enhancing cellular proliferation and cell-cycle progression, while reducing apoptosis.
Figure 4.
CCAT1 Increased the S-phase Percentage and Decreased G0/G1 Phase Percentage of NSCLC Cells
(A) Knockdown of CCAT1 caused significant G1 phase cell-cycle arrest of HCC827GR cells in the presence of 1 μM gefitinib. (B) Knockdown of CCAT1 caused significant G1 phase cell-cycle arrest of PC9GR cells in the presence of 1 μM gefitinib. (C) CCAT1 overexpression increased the S phase percentage and decreased the G0/G1 phase percentage of HCC827 cells in the presence of 1 μM gefitinib. (D) CCAT1 overexpression increased the S phase percentage and decreased the G0/G1 phase percentage of PC9 cells in the presence of 1 μM gefitinib. All tests were performed at least three times. Data were expressed as mean ± SD. **p < 0.01.
Knockdown of CCAT1 Enhances Gefitinib Sensitivity In Vivo
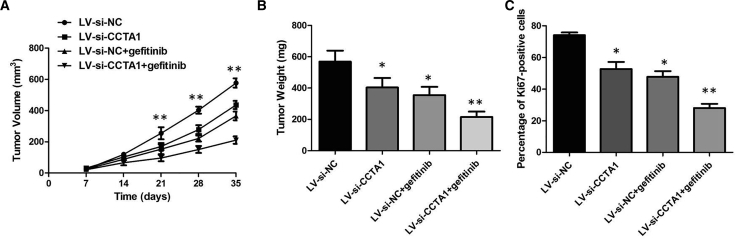
To evaluate the function of CCAT1 in gefitinib resistance in vivo, we first generated a stable CCAT1 knockdown cell line by transfecting PC9GR cells with a LV-anti-CCAT1 vector to downregulate CCAT1 expression. Then we implanted these infected PC9GR cells into nude mice. LV-si-CCAT1 decreased tumor volumes and weights compared with the LV-si-NC plus gefitinib group, and there was no significance of tumor volumes and weights between the LV-si-CCAT1 group and LV-si-NC group without gefitinib treatment (Figures 5A and 5B). Consistent with this, LV-si-CCAT1 plus gefitinib decreased the percentage of proliferating Ki67-positive cells compared with the LV-si-NC plus gefitinib group (Figure 5C). These findings suggested that CCAT1 could modulate gefitinib sensitivity in vivo.
Figure 5.
Knockdown of CCAT1 Enhances Gefitinib Sensitivity In Vivo
(A) The tumor volume curve of nude mice was analyzed. (B) The tumor weights of nude mice were measured. (C) LV-si-CCAT1 plus gefitinib decreased the percentage of proliferating Ki67-positive cells compared with the LV-si-NC plus gefitinib group. **p < 0.01.
CCAT1 Functioned as a Molecular Sponge of miR-218 in NSCLC Cells
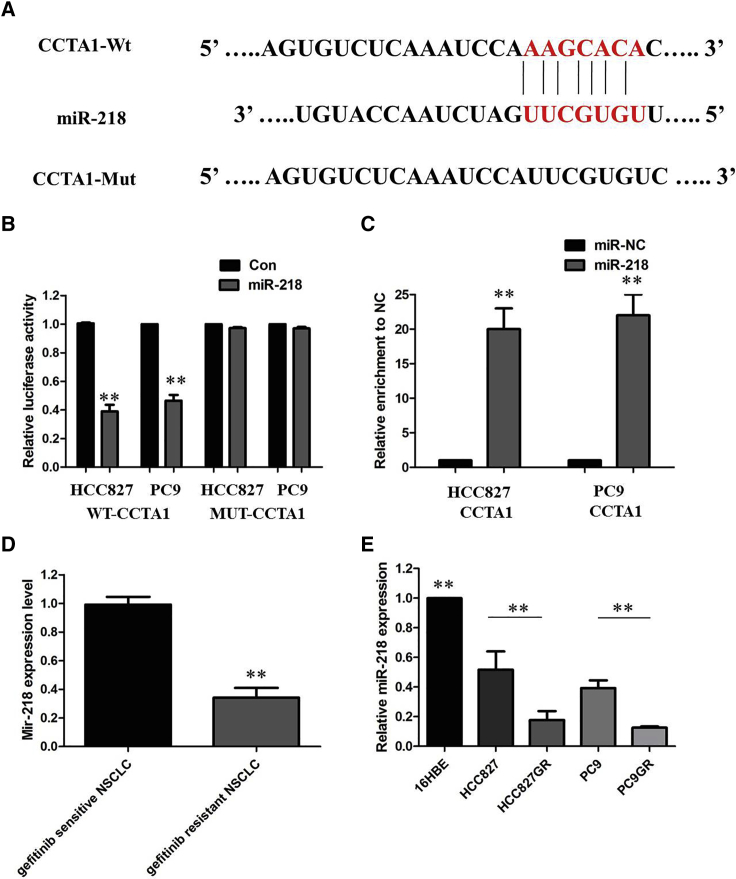
Up to now, accumulating evidence indicated that lncRNAs exerted the function by interacting with miRNAs. Therefore, we examined the potential miRNAs associated with CCAT1, and miR-218 was predicated to be the possible miRNA targeting CCAT1 from miRTarBase (http://mirtarbase.mbc.nctu.edu.tw/php/search.php), microRNA.org (http://www.microrna.org/microrna/microrna/getMirnaForm.do), TargetScan (http://www.targetscan.org/vert_71/), and mirDIP (http://ophid.utoronto.ca/mirDIP/). As shown in Figure 6A, miR-218 harbor the complementary binding sequence of CCAT1. In order to further validate the interaction, CCAT1 sequence containing the putative or mutated miR-218 binding site was cloned into the downstream of luciferase reporter gene, generating wild-type (WT)-CCAT1 or MUT-CCAT1 luciferase reporter plasmids. Then the effect of miR-218 on WT-CCAT1 or MUT-CCAT1 luciferase reporter systems was determined. The results showed that miR-218 mimic considerably reduced the luciferase activity of the WT-CCAT1 luciferase reporter vector compared with negative control, whereas miR-218 mimic did not pose any impact on the luciferase activity of MUT-CCAT1-transfected cells (p < 0.01; Figure 6B). In a further RNA immunoprecipitation (RIP) experiment, CCAT1 and miR-218 simultaneously existed in the production precipitated by anti-AGO2 (p < 0.01; Figure 6C), suggesting that miR-218 is CCAT1-targeting miRNA. These outcomes indicated that the interaction of CCAT1 and miR-218 was realized by the putative binding site.
Figure 6.
CCAT1 Functioned as a Molecular Sponge of miR-218 in NSCLC Cells
(A) starBase v.2.0 results showing the sequence of CCAT1 with highly conserved putative miR-218 binding sites. (B) miR-218 mimic considerably reduced the luciferase activity of the WT-CCAT1 luciferase reporter vector compared with negative control, whereas miR-218 mimic did not pose any impact on the luciferase activity of MUT-CCAT1-transfected cells. (C) CCAT1 and miR-218 simultaneously existed in the production precipitated by anti-AGO2. (D) Relative expression of miR-218 in the gefitinib-sensitive group and gefitinib-resistant group in NSCLC patients. (E) Relative expression of miR-218 in a panel of NSCLC cell lines. All tests were performed at least three times. Data were expressed as mean ± SD. **p < 0.01.
We next identified the expression of miR-218 in NSCLC patient tissues and cell lines, and explored its tumor-suppressing effect. As shown in Figures 6D and 6E, miR-218 showed lower expression in gefitinib-resistant NSCLC patients compared with gefitinib-sensitive NSCLC patients. The expression of miR-218 was obviously decreased in gefitinib-resistant cells compared with that in gefitinib-sensitive cells, indicating the opposite result to CCAT1 expression. Subsequently, the effect of CCAT1 on miR-218 expression was also observed in NSCLC cells. The results manifested that knockdown or overexpression of CCAT1 significantly affected miR-218 expression (Figures 7A and 7B). Moreover, we performed antagonist effects experiments by cotransfecting HCC827GR and PC9GR cells with a LV-anti-CCAT1 vector and miR-218 inhibitor. Results revealed that the inhibitory action of the LV-anti-CCAT1 vector on proliferation could be partially reversed by the miR-218 inhibitor (Figures 7C and 7D). Collectively, these data indicate that the CCAT1/miR-218 pathway contributes to gefitinib resistance in gefitinib-resistant cell lines that overexpress CCAT1.
Figure 7.
CCAT1/miR-218 Pathway Contributes to Gefitinib Resistance
(A) Silencing of CCAT1 increased the expression level of miR-218 in PC9GR and HCC827GR cells. (B) Overexpression of CCAT1 decreased the expression level of miR-218 in PC9 and HCC827 cells. (C) CCK-8 assay showing the inhibitory action of the LV-anti-CCAT1 vector on cell proliferation of HCC827GR could be partially reversed by the miR-218 inhibitor. (D) CCK-8 assay showing the inhibitory action of the LV-anti-CCAT1 vector on cell proliferation of PC9GR could be partially reversed by the miR-218 inhibitor. All tests were performed at least three times. Data were expressed as mean ± SD. **p < 0.01.
CCAT1 Induces the Expression of HOXA1 by Sponging miR-218
The downstream mechanism of the circEIF4G2/miR-218 axis was further investigated. Following in silico prediction analysis, HOXA1 was identified to be a potential target of miR-218 (Figure 8A). To examine the association between miR-218 and HOXA1, we performed dual-luciferase reporter assay, and the data suggested that transfection with miR-218 mimic significantly suppressed the luciferase activity of HOXA1-WT plasmid compared with the negative control; however, the luciferase activity of HOXA1-MUT plasmid was not affected (Figure 8B). We examined HOXA1 expression in NSCLC tissues and cell lines. The results of immunohistochemistry (IHC) showed that HOXA1 expression in gefitinib-resistant NSCLC patient tissues was significantly upregulated compared with that in gefitinib-sensitive NSCLC patient tissues (p < 0.01; Figure 8C). The expression of HOXA1 was obviously increased in gefitinib-resistant cells compared with that in gefitinib-sensitive cells (p < 0.01; Figure 8D). Subsequently, we explored whether CCAT1 regulated HOXA1 expression in NSCLC cells. As expected, CCAT1 knockdown decreased the mRNA expression of HOXA1 in PC9GR cells, whereas miR-218 inhibitor could restore this inhibition (p < 0.01; Figure 8E).
Figure 8.
CCAT1 Induces the Expression of HOXA1 by Sponging miR-218
(A) Bioinformatics analysis revealed the predicted binding sites between HOXA1 and miR-218. (B) Luciferase reporter assay demonstrated miR-218 mimics significantly decreased the luciferase activity of HOXA1-WT in NSCLC cells. (C) IHC analysis of HOXA1 in the gefitinib-sensitive group in NSCLC patients and the gefitinib-resistant group. (D) Relative expression of HOXA1 in a panel of NSCLC cell lines. (E) CCAT1 knockdown decreased the mRNA expression of HOXA1 in PC9GR cells, whereas miR-218 inhibitor could restore this inhibition. All tests were performed at least three times. Data were expressed as mean ± SD. **p < 0.01.
Discussion
Lately, increasing evidence has demonstrated that lncRNAs were generally dysregulated in various cancers and involved in cancer progression, implying that lncRNAs may be a new kind of potential biomarker for cancers.14 Moreover, recent studies have demonstrated that lncRNAs could serve as ceRNA by competitive binding to MREs to regulate gene transcription. Among hundreds of lncRNAs, CCAT1 is an intriguing target because it plays a role in cell-cycle regulation and may be involved in tumor development.15 CCAT1 is also a biomarker for identifying colorectal cancer patients who are likely to benefit from bromodomain and extra-terminal motif (BET) inhibitors, indicating that CCAT1 could serve as an indicator of drug sensitivity.16 Previous studies have shown that lncRNA-CCAT1 was upregulated and acted as an oncogenic lncRNA in several types of human cancers.17 Ma et al.13 showed that CCAT1 promotes gallbladder cancer development via negative modulation of miRNA-218-5p. Zhang et al.18 also indicated that CCAT1 was upregulated in breast cancer and was associated with OS, as well as progression-free survival, suggesting that CCAT1 could be a potential prognostic biomarker for breast cancer progression. However, the function of CCAT1 in gefitinib resistance in NSCLC has not been investigated.
It was reported that CCAT1 increased CDDP resistance in NSCLC cell lines by targeting SOX4.19 In nasopharynx cancer, CCAT1 modulates paclitaxel sensitivity via the miR-181a/CPEB2 axis.20 CCAT1 was also shown to act as an oncogene and promoted chemoresistance in docetaxel-resistant LUAD cells.21 In the present study, we observed that CCAT1 expression was markedly higher in gefitinib-resistant cells and gefitinib-resistant patient tissues than that in gefitinib-sensitive cells and gefitinib-sensitive patient tissues. Besides, high expression of CCAT1 indicated shorter OS of NSCLC patients, which was verified with Kaplan-Meier analysis and log rank test. To further validate the effect of CCAT1 on gefitinib resistance, we performed loss-of-function studies by knocking down CCAT1 in gefitinib-resistant cells HCC827GR and PC9GR. Meanwhile, we upregulated the CCAT1 expression in gefitinib-sensitive cells HCC827 and PC9 by establishing CCAT1-overexpressing cell lines. Suppression of CCAT1 significantly reduced cell growth and promoted cell apoptosis of gefitinib-resistant cells in the presence of 1 μM gefitinib, compared with negative-control-transfected cells. However, CCAT1 overexpression promoted the gefitinib-induced cell apoptosis and cell mobility of gefitinib-sensitive cells under gefitinib treatment.
Recently, it has been demonstrated that lncRNA could participate in post-transcriptional regulation by interfering with the miRNA pathways by acting as ceRNAs. These ceRNAs are associated with many biological processes, and disruption of the balance between lncRNAs and miRNAs is crucial for tumorigenesis. More importantly, we further demonstrated that CCAT1 functioned as a ceRNA of miR-218 in NSCLC cells. To further confirm the underlying molecular mechanisms involved, we performed the RIP and luciferase assays and found the direct binding ability of the miR-218 response elements on the full-length CCAT1 transcript.
Many studies have documented that dysregulation of miRNA is closely associated with tumorigenesis. miRNAs inhibit target protein translation by interacting with the untranslated region (3′ UTR) of target mRNAs.22 To date, miRNAs have been proved to act as either tumor suppressors or oncogenes in cancer initiation and development of NSCLC.23 Our results showed that miR-218 expression was also significantly decreased in gefitinib-resistant NSCLC patients compared with gefitinib-sensitive NSCLC patients. Moreover, repression of miR-218 could reverse the inhibition of cell proliferation caused by CCAT1 knockdown. Further analysis demonstrated that miR-218 can regulate HOXA1 expression by direct targeting with the 3′ UTR of HOXA1. Taken together, our results suggested that CCAT1 promoted gefitinib resistance of NSCLC by acting as a ceRNA of miR-218 to regulate HOXA1 expression.
In conclusion, our results demonstrated that CCAT1 promotes gefitinib resistance in NSCLC via acting as a ceRNA against miR-218, which further upregulated HOXA1 expression. Our study discovered a novel mechanism of drug resistance in NSCLC cells mediated by lncRNA CCAT1, which further suggests that CCAT1 may be a potential target for esophageal cancer therapy.
Materials and Methods
TCGA Dataset Analysis
The data and the corresponding clinical information of patients were collected from TCGA database (https://www.cancer.gov/about-nci/organization/ccg/research/structural-genomics/tcga). We used the edgeR package of R packages to perform the difference analysis (http://www.bioconductor.org/packages/release/bioc/html/edgeR.html) and used the pheatmap package of R packages to perform the cluster analysis (https://cran.r-project.org/web/packages/pheatmap/index.html). Sva R package was used to remove the batch effect. Genes with adjusted p values <0.05 and absolute fold changes (FCs) >1.5 were considered differentially expressed genes. Kaplan-Meier survival curves were drawn to analyze the relationships between genes and OS in the survival package. The corresponding statistical analysis and graphics were performed in R software (R version 3.3.2).
Clinical Specimens
A total of 52 fresh NSCLC tissues and paired adjacent noncancerous lung tissues were collected after obtaining informed consent from patients at The First Hospital of Jilin University. Histological and pathological diagnostics for NSCLC patients were evaluated based on the Revised International System for Staging Lung Cancer. Patients received neither chemotherapy nor radiotherapy before tissue sampling. The samples were snap-frozen in liquid nitrogen and stored at −80°C prior to RNA extraction. This study was approved by the Ethics Committee of The First Hospital of Jilin University.
Cell Culture
The NSCLC cell lines (HCC827 and PC9) and human bronchial epithelial cells (16HBE) were obtained from the Cell Culture Center, Chinese Academy of Medical Sciences (Beijing, China). HCC827GR and PC9GR cells were generated by continually exposing to stepwise increased concentration of gefitinib over a period of 24 months. Cell lines were cultured in DMEM or RPMI 1640 (Thermo Fisher Scientific) supplemented with 10% fetal bovine serum (FBS; HyClone), penicillin, and streptomycin (Thermo Fisher Scientific) at 37°C in 5% CO2.
Subcellular Fractionation Location
The separation of the nuclear and cytosolic fractions was performed using a PARIS Kit (AM1921; Life Technologies) according to the manufacturer’s instructions.
RNA Isolation and Quantitative Real-Time PCR
RNA extraction and qRT-PCR total RNA were extracted from cells with TRIzol reagent (Invitrogen, Thermo Fisher Scientific). For qRT-PCR, RNA was reverse transcribed to cDNA using HiScript II First-Strand cDNA Synthesis Kit (Vazyme Biotech). Real-time PCR analyses were performed with SYBR Green (Vazyme Biotech). Results were normalized to the expression of GAPDH or U6. Primers were chemically synthesized by SprinGen Biotech.
Cell Transfection
We transfected oligonucleotides and plasmids using Lipofectamine 2000 (Invitrogen) following the manufacturer’s protocol. LV was used to infect cells in the presence of polybrene. HCC827GR and PC9GR cells were transfected with LV-anti-CCAT1 or LV-NC, and HCC827 and PC9 cells were transfected for stable overexpression of CCAT1 vector or with control vector. Cells were transiently transfected using miRNA mimics or miRNA inhibitors. The efficiency of knockdown and overexpression was determined by qRT-PCR.
Cell Proliferation Assay
CCK-8 (DOJINDO, Japan) was used for the assay, as previously described. In brief, cells were plated in 96-well plates at 5.0 × 103/well and treated with the indicated concentration of gefitinib and/or mimics or plasmid for 24 h after transfection. To test the cell proliferation, 10 μL of CCK-8 reagent was added to each well and incubated for 2 h at 37°C. Then the absorption was evaluated by a microplate reader at 450 nm (Tecan, Switzerland).
Flow Cytometric Analysis of Cell Cycle and Apoptosis
For cell-cycle assay, we collected transfected cells and fixed them with 75% cold ethanol at −20°C overnight. Cells were stained with 50 μg/mL PI in a 1 mg/mL RNase solution for 30 min at 4°C. Cell-cycle distributions were analyzed using a FACS flow cytometer (BD Biosciences). Apoptosis was conducted with an Annexin V-FITC/PI Apoptosis Detection Kit or an Annexin V-APC/PI Apoptosis Detection Kit (KeyGene Biotech) according to the manufacturer’s instructions. Cell Quest Pro Software (BD Biosciences) was used to analyze cellular apoptosis.
Xenograft Assay
Five-week-old male BALB/c nude mice were raised in specific pathogen-free conditions and manipulated in line with protocols authorized by the animal center of The First Hospital of Jilin University. Mice were randomly divided into two groups (n = 4/group): control group and shUCA1 group. Tumor volumes (π/6 × minor axis2 × major axis) were inspected every 7 days as the implantations began to develop bigger. All mice were killed after 5 weeks of injection, and the tumors were excised, weighed, and paraffin embedded. All experimental procedures took place at the animal center of The First Hospital of Jilin University and were approved by the Institutional Animal Care and Use Committee.
IHC
For each patient sample, three paraffin sections of 5 μm were prepared, one for hematoxylin and eosin (HE) staining and the other two for immunohistochemical staining. PBS instead of primary antibodies was used for negative control, and the breast cancer tissue was used for positive control. Sections were dewaxed using xylene, followed by hydration with ethanol solutions and addition of EDTA for antigen retrieval. Later, sections were blocked with normal goat serum for 30 min to eliminate non-specific binding. Sections were incubated with anti-human HOXA1 polyclonal antibody (1:1,000; Abcam, Cambridge, MA, USA). Sections were then incubated with biotin-labeled secondary antibodies for 30 min at room temperature, followed by staining with diaminobenzidine (DAB). Finally, the sections were counterstained with hematoxylin. The result of staining was determined by two doctors who did not know the clinical condition of patients. The proportions of positive cells of 0%, 1%–5%, 6%–25%, 26%–75%, and 76%–100% were assigned with scores of 0, 1, 2, 3, and 4, respectively. Scores of 0–2 were considered as negative expression, and scores of 3–4 were considered as positive expression.
Luciferase Reporter Assay
Cells (5 × 103) were seeded into 96-well plates and co-transfected with corresponding plasmids and miRNA mimics or inhibitors using the Lipofectamine 2000 transfection reagent. Luciferase activity was measured using the dual-luciferase reporter assay system (Promega, Madison, WI, USA) after 48 h of incubation according to the manufacturer’s instructions. Independent experiments were performed in triplicate. Relative luciferase activity was normalized to the Renilla luciferase internal control.
RIP Assay
RIP assay was performed using an EZ-Magna RiP Kit (Millipore, Billerica, MA, USA) in accordance with the manufacturer’s instructions. Cells were lysed at 70%–80% confluence in RIP lysis buffer and then incubated with magnetic beads conjugated with human anti-Ago2 antibody (Millipore) and normal mouse IgG control (Millipore) in RIP buffer. The RNAs in the immunoprecipitates were isolated with TRIzol reagent and analyzed by qRT-PCR.
Western Blot
Protein was extracted by lysing with RIPA buffer (Beyotime, Shanghai, China), and the concentration was determined using the BCA assay (Beyotime, Shanghai, China) according to the manufacturer’s instructions. A total of 30 μg of protein was subjected to 10% SDS-PAGE and transferred to a 0.22-μm PVDF membrane. After being incubated with specific HOXA1 antibodies (Abcam, USA) on a shaker overnight at 4°C, signals were visualized using the infrared labeled antibody and scanned with the Dual Color Infra-red Laser Imaging System (Gene, HK, China) by being normalized to the inference gene of GAPDH.
Statistical Analysis
SPSS 21.0 software (IBM, Somers, NY, USA) was selected to perform the statistical analysis. Results are presented expressed as mean ± SD (standard deviation). For the comparison of differences, paired Student’s t test was used. The association between CCAT1 expression and pathological grades was assessed using one-way ANOVA and binary logistic regression. The OS rate was calculated by the Kaplan-Meier method. A p value <0.05 means a significant difference.
Author Contributions
X.J. and Y.G. performed primers design and experiments. X.L. contributed flow cytometry assay and animal experiments. Z.Z. collected and classified the human tissue samples. X.J. analyzed the data. Y.G. wrote the paper. All authors read and approved the final manuscript.
Conflicts of Interest
The authors declare no competing interests.
References
- 1.Saintigny P., Burger J.A. Recent advances in non-small cell lung cancer biology and clinical management. Discov. Med. 2012;13:287–297. [PubMed] [Google Scholar]
- 2.Tassinari D., Carloni F., Santelmo C., Tamburini E., Lazzari Agli L., Tombesi P., Sartori S. Second line treatments in advanced platinum-resistant non small cell lung cancer. A critical review of literature. Rev. Recent Clin. Trials. 2009;4:27–33. doi: 10.2174/157488709787047549. [DOI] [PubMed] [Google Scholar]
- 3.Gridelli C., De Marinis F., Di Maio M., Cortinovis D., Cappuzzo F., Mok T. Gefitinib as first-line treatment for patients with advanced non-small-cell lung cancer with activating epidermal growth factor receptor mutation: Review of the evidence. Lung Cancer. 2011;71:249–257. doi: 10.1016/j.lungcan.2010.12.008. [DOI] [PubMed] [Google Scholar]
- 4.Gupta R.A., Shah N., Wang K.C., Kim J., Horlings H.M., Wong D.J., Tsai M.C., Hung T., Argani P., Rinn J.L. Long non-coding RNA HOTAIR reprograms chromatin state to promote cancer metastasis. Nature. 2010;464:1071–1076. doi: 10.1038/nature08975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee N., Moss W.N., Yario T.A., Steitz J.A. EBV noncoding RNA binds nascent RNA to drive host PAX5 to viral DNA. Cell. 2015;160:607–618. doi: 10.1016/j.cell.2015.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Prensner J.R., Iyer M.K., Sahu A., Asangani I.A., Cao Q., Patel L., Vergara I.A., Davicioni E., Erho N., Ghadessi M. The long noncoding RNA SChLAP1 promotes aggressive prostate cancer and antagonizes the SWI/SNF complex. Nat. Genet. 2013;45:1392–1398. doi: 10.1038/ng.2771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang K.C., Chang H.Y. Molecular mechanisms of long noncoding RNAs. Mol. Cell. 2011;43:904–914. doi: 10.1016/j.molcel.2011.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yuan J.H., Yang F., Wang F., Ma J.Z., Guo Y.J., Tao Q.F., Liu F., Pan W., Wang T.T., Zhou C.C. A long noncoding RNA activated by TGF-β promotes the invasion-metastasis cascade in hepatocellular carcinoma. Cancer Cell. 2014;25:666–681. doi: 10.1016/j.ccr.2014.03.010. [DOI] [PubMed] [Google Scholar]
- 9.Yoon J.H., You B.H., Park C.H., Kim Y.J., Nam J.W., Lee S.K. The long noncoding RNA LUCAT1 promotes tumorigenesis by controlling ubiquitination and stability of DNA methyltransferase 1 in esophageal squamous cell carcinoma. Cancer Lett. 2018;417:47–57. doi: 10.1016/j.canlet.2017.12.016. [DOI] [PubMed] [Google Scholar]
- 10.Nissan A., Stojadinovic A., Mitrani-Rosenbaum S., Halle D., Grinbaum R., Roistacher M., Bochem A., Dayanc B.E., Ritter G., Gomceli I. Colon cancer associated transcript-1: a novel RNA expressed in malignant and pre-malignant human tissues. Int. J. Cancer. 2012;130:1598–1606. doi: 10.1002/ijc.26170. [DOI] [PubMed] [Google Scholar]
- 11.Arunkumar G., Murugan A.K., Prasanna Srinivasa Rao H., Subbiah S., Rajaraman R., Munirajan A.K. Long non-coding RNA CCAT1 is overexpressed in oral squamous cell carcinomas and predicts poor prognosis. Biomed. Rep. 2017;6:455–462. doi: 10.3892/br.2017.876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang H., Zhong J., Bian Z., Fang X., Peng Y., Hu Y. Long non-coding RNA CCAT1 promotes human retinoblastoma SO-RB50 and Y79 cells through negative regulation of miR-218-5p. Biomed. Pharmacother. 2017;87:683–691. doi: 10.1016/j.biopha.2017.01.004. [DOI] [PubMed] [Google Scholar]
- 13.Ma M.Z., Chu B.F., Zhang Y., Weng M.Z., Qin Y.Y., Gong W., Quan Z.W. Long non-coding RNA CCAT1 promotes gallbladder cancer development via negative modulation of miRNA-218-5p. Cell Death Dis. 2015;6:e1583. doi: 10.1038/cddis.2014.541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Arancio W., Pizzolanti G., Genovese S.I., Baiamonte C., Giordano C. Competing endogenous RNA and interactome bioinformatic analyses on human telomerase. Rejuvenation Res. 2014;17:161–167. doi: 10.1089/rej.2013.1486. [DOI] [PubMed] [Google Scholar]
- 15.Guo X., Hua Y. CCAT1: an oncogenic long noncoding RNA in human cancers. J. Cancer Res. Clin. Oncol. 2017;143:555–562. doi: 10.1007/s00432-016-2268-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim T., Cui R., Jeon Y.J., Lee J.H., Lee J.H., Sim H., Park J.K., Fadda P., Tili E., Nakanishi H. Long-range interaction and correlation between MYC enhancer and oncogenic long noncoding RNA CARLo-5. Proc. Natl. Acad. Sci. USA. 2014;111:4173–4178. doi: 10.1073/pnas.1400350111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McCleland M.L., Mesh K., Lorenzana E., Chopra V.S., Segal E., Watanabe C., Haley B., Mayba O., Yaylaoglu M., Gnad F., Firestein R. CCAT1 is an enhancer-templated RNA that predicts BET sensitivity in colorectal cancer. J. Clin. Invest. 2016;126:639–652. doi: 10.1172/JCI83265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang X.F., Liu T., Li Y., Li S. Overexpression of long non-coding RNA CCAT1 is a novel biomarker of poor prognosis in patients with breast cancer. Int. J. Clin. Exp. Pathol. 2015;8:9440–9445. [PMC free article] [PubMed] [Google Scholar]
- 19.Hu B., Zhang H., Wang Z., Zhang F., Wei H., Li L. LncRNA CCAT1/miR-130a-3p axis increases cisplatin resistance in non-small-cell lung cancer cell line by targeting SOX4. Cancer Biol. Ther. 2017;18:974–983. doi: 10.1080/15384047.2017.1385679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang Q., Zhang W., Hao S. LncRNA CCAT1 modulates the sensitivity of paclitaxel in nasopharynx cancers cells via miR-181a/CPEB2 axis. Cell Cycle. 2017;16:795–801. doi: 10.1080/15384101.2017.1301334. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 21.Ansari M.H., Irani S., Edalat H., Amin R., Mohammadi Roushandeh A. Deregulation of miR-93 and miR-143 in human esophageal cancer. Tumour Biol. 2016;37:3097–3103. doi: 10.1007/s13277-015-3987-9. [DOI] [PubMed] [Google Scholar]
- 22.Hu T.B., Chen H.S., Cao M.Q., Guo F.D., Cheng X.Y., Han Z.B., Li M.Q. MicroRNA-421 inhibits caspase-10 expression and promotes breast cancer progression. Neoplasma. 2018;65:49–54. doi: 10.4149/neo_2018_170306N159. [DOI] [PubMed] [Google Scholar]
- 23.Wang M., Wang W., Wang J., Zhang J. MiR-182 promotes glucose metabolism by upregulating hypoxia-inducible factor 1α in NSCLC cells. Biochem. Biophys. Res. Commun. 2018;504:400–405. doi: 10.1016/j.bbrc.2018.06.035. [DOI] [PubMed] [Google Scholar]