Abstract
Performance of commercially available human papillomavirus (HPV) assays (approved for cervical HPV detection) is unknown for detecting HPV-related oropharyngeal cancer (HPV-OPC). Assays for detection of HPV DNA [ELISA (DEIA) and Cobas], and RNA (Aptima) in oral rinse samples, and serum HPV oncogene antibodies were evaluated. Sensitivity and specificity of each test was explored among HPV-OPC cases and controls. Biomarker prevalence was evaluated among 294 "at-risk" people (screening) and 133 "high-risk" people [known to previously have oral oncogenic HPV (oncHPV) DNA and/or HPV16 E6/E7 antibodies detected]. HPV16 E6 antibodies had the best overall test performance with sensitivity of 88%, compared with oral HPV16 DNA sensitivity of 51% by DEIA and 43% by Cobas (each P < 0.001). Specificity was comparable in each of these tests (≥98%). When positivity for any oncHPV type was compared with HPV16 for the same test, sensitivity was comparable (60% vs. 51%, 40% vs. 43%, and 92% vs. 88% for DEIA, Cobas, and E6 antibodies, respectively), but specificity was reduced (93%–97%). Aptima had poor sensitivity (23%). Sensitivity decreased when cotesting HPV16 oral rinse DNA and E6 antibodies (37%–48%), or multiple E antibodies (69%–72%). HPV16 DNA were detected in ~2% of the at-risk by either DEIA or Cobas and up to 15% of the high-risk population. HPV16 E6 seroprevalence was 2.3% and 2.4% in the at-risk and high-risk populations, respectively. Oral rinse HPV testing had moderate-to-poor sensitivity for HPV-OPC, suggesting many true positives would be missed in a potential screening scenario. HPV16 E6 serum antibody was the most promising biomarker evaluated.
Introduction
Human papillomavirus–related oropharynx squamous cell cancers (HPV-OPC) are dramatically increasing in incidence in the United States and globally (1-3). Oral HPV infection (4) and circulating antibodies (5-7) to HPV oncogenes precede the development of HPV-OPC. However, natural history data from the detection of infection to the pivotal carcinogenic events that herald this malignant transformation remain lacking. In contrast, cervical neoplasia studies have robust long-term natural history data that have elucidated the progression (and risk factors) for incident and persistent cervical HPV infection, dysplasia, and malignancy (8, 9).
Cervical HPV testing has clinical utility with validated performance properties. The U.S. Preventive Services Task Force now recommends the use of oncogenic HPV testing alone every 5 years with or without cytology as an alternative to cytology alone every 3 years (10); clinical laboratories use validated, FDA-approved, and commercially available tests for cervical HPV detection (11). In contrast, there are no analogous FDA-approved tests to detect clinically relevant oral HPV infections.
Indeed, for oral HPV, studies to date are limited to large cross-sectional and small size or short-term natural history studies (12-14) in which HPV detection has been performed by PCR and linear array and/or quantitative PCR in research laboratories. Use of either oral HPV detection (15) or HPV serum antibodies (16) in screening scenarios remains investigational. However, current data support the potential role of oral or plasma HPV DNA in clinical surveillance of patients with oropharyngeal cancer posttreatment (for recurrence) (17-21). A roadblock at present for investigational and clinical purposes is the lack of universally accepted or FDA-approved oral HPV detection method. Therefore, we sought to determine the performance of two commercially available cervical HPV assays applied to oral rinse among individuals with newly diagnosed HPV-OPC and at-risk for HPV-OPC. There are currently no commercially available HPV serum antibody detection tests, but as these markers have been suggested to have utility for HPV-OPC detection in previous research (5-7) they were similarly explored in this study.
Materials and Methods
As described below, samples from the biorepository of multiple cohort studies were included in this study. These studies were Institutional Review Board (IRB)-approved and all participants provided informed consent. This study was conducted in accordance with the U.S. common rule.
HOTSPOT study population (OPC case and controls)
To evaluate sensitivity and specificity of the HPV biomarkers a pilot study was performed among 133 incident HPV-OPC and 134 noncancer controls from the HOTSPOT study (22). Noncancer controls from HOTSPOT included 101 partners/spouses of HPV-OPC cases as well as a convenience sample of 33 healthy volunteers enrolled from free oral cancer screening events. All 133 cases had confirmed oncogenic HPV-positive oropharyngeal cancer; many did not have HPV-type-specific tumor testing, but 68 were confirmed to be HPV16-positive (22).
MOUTH study population (at-risk and high-risk groups)
From a different cohort, the MOUTH (Men and 93 women Understanding Throat HPV) study (23), individuals at increased risk of oncogenic oral HPV infection and HPV-OPC were enrolled (called the at-risk group hereafter). Eligibility included: (i) men 35–69 years old with 3 or more lifetime oral sex partners (11), (ii) women with history of cervical dysplasia and/or their partners, and (iii) partners of patients with HPV-OPC. The MOUTH cohort is actively enrolling; this analysis included samples from the first 294 individuals enrolled 2017–2018.
In addition, 133 high-risk participants in a cohort of HIV-infected and HIV at-risk individuals in the MACS WIHS Combined Cohort study or CCS (24) who had persistent HPV biomarkers at the end of a previous research study (13, 25) were also enrolled into MOUTH study during the same time period and included in this analysis (called the high-risk group hereafter). This included 119 participants who had persistent oncogenic oral HPV infection and 14 participants with HPV16 E6 and/or E7 oncogene antibodies detected during the previous study. These participants were analyzed separately given their higher risk profile.
Participants in the at-risk group were primarily male, white, middle aged, with at least a college education (Table 1). Participants enrolled in the high-risk group included racially diverse men and women, most with HIV infection, many with only a high-school education and current cigarette use (Table 1).
Table 1.
Description of study participants at MOUTH (or HOTSPOT) study baseline, by study population
| N (%) | |||||
|---|---|---|---|---|---|
| HOTSPOT Study |
MOUTH Study |
||||
| HPV-OPC Cases N = 133 |
Controlsa N = 134 |
At-risk (MOUTH screening) N = 294 |
High-risk (HIV-positive CCS)b N = 100 |
High-risk (HIV-negative CCS) N = 33 |
|
| Sex | |||||
| Male | 119 (89.5%) | 18 (13.4%) | 261 (88.8%) | 49 (49.0%) | 17 (51.5%) |
| Female | 14 (10.5%) | 116 (86.6%) | 33 (11.2%) | 51 (51.0%) | 16 (48.5%) |
| Age in years: | |||||
| Median (IQR) | 55 (50, 60) | 51 (45, 59) | 55 (48, 62) | 56 (50, 61) | 57 (48, 62) |
| Race | |||||
| White | 122 (91.7%) | 115 (85.8%) | 238 (81.0%) | 36 (36.0%) | 12 (36.4%) |
| Black | 6 (4.5%) | 7 (5.2%) | 38 (12.9%) | 56 (56.0%) | 18 (54.6%) |
| Other | 0 (0.0%) | 6 (4.5%) | 18 (6.1%) | 8 (8.0%) | 3 (9.1%) |
| Missing | 5 (3.8%) | 6 (4.5%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) |
| Education level | |||||
| <High-school | 4 (3.0%) | 3 (2.2%) | 8 (2.7%) | 18 (18.0%) | 6 (18.2%) |
| High school/GED | 52 (39.1%) | 40 (29.9%) | 77 (26.2%) | 48 (48.0%) | 14 (42.4%) |
| College | 47 (35.3%) | 49 (36.6%) | 83 (28.2%) | 20 (20.0%) | 6 (18.2%) |
| Adv/Degree | 17 (12.8%) | 34 (25.4%) | 124 (42.2%) | 14 (14.0%) | 6 (18.2%) |
| Missing | 13 (9.8%) | 8 (6.0%) | 2 (0.7%) | 0 (0.0%) | 1 (3.0%) |
| Marital status | |||||
| Married | 107 (80.5%) | 116 (86.6%) | 224 (76.2%) | 31 (31.0%) | 13 (39.4%) |
| Sep/Div/Widow | 13 (9.8%) | 5 (3.7%) | 39 (13.3%) | 21 (21.0%) | 11 (33.3%) |
| Single | 8 (6.0%) | 12 (9.0%) | 29 (9.9%) | 48 (48.0%) | 9 (27.3%) |
| Missing | 5 (3.8%) | 1 (0.7%) | 2 (0.7%) | 0 (0.0%) | 0 (0.0%) |
| Smoking status | |||||
| Never | 66 (49.6%) | 67 (50.0%) | 174 (59.2%) | 31 (31.0%) | 11 (33.3%) |
| Former | 43 (32.3%) | 45 (33.6%) | 95 (32.3%) | 27 (27.0%) | 9 (27.3%) |
| Current | 17 (12.8%) | 12 (9.0%) | 23 (7.8%) | 41 (41.0%) | 13 (39.4%) |
| Missing | 7 (5.3%) | 10 (7.5%) | 2 (0.7%) | 1 (1.0%) | 0 (0.0%) |
| Ever alcohol use | |||||
| No | 23 (17.3%) | 46 (34.3%) | 45 (15.3%) | 38 (38.0%) | 8 (24.2%) |
| Yes | 105 (78.9%) | 78 (58.2%) | 246 (83.7%) | 56 (56.0%) | 23 (69.7%) |
| Missing | 5 (3.8%) | 10 (7.5%) | 3 (1.0%) | 6 (6.0%) | 2 (6.1%) |
| Number of lifetime oral sexual partners | |||||
| 0 | 2 (1.5%) | 6 (4.5%) | 3 (1.0%) | 10 (10.0%) | 2 (6.1%) |
| 1 | 9 (6.8%) | 18 (13.4%) | 11 (3.7%) | 4 (4.0%) | 1 (3.0%) |
| 2–5 | 42 (31.6%) | 60 (44.8%) | 115 (39.1%) | 16 (16.0%) | 9 (27.3%) |
| 6–10 | 18 (13.5%) | 19 (14.2%) | 64 (21.8%) | 15 (15.0%) | 1 (3.0%) |
| 11–15 | 16 (12.0%) | 8 (6.0%) | 30 (10.2%) | 8 (8.0%) | 1 (3.0%) |
| 16–24 | 11 (8.3%) | 6 (4.5%) | 25 (8.5%) | 4 (4.0%) | 4 (12.1%) |
| 25 or more | 18 (13.5%) | 4 (3.0%) | 44 (15.0%) | 43 (43.0%) | 15 (45.5%) |
| Missing | 17 (12.8%) | 13 (9.7%) | 2 (0.7%) | 0 (0.0%) | 0 (0.0%) |
| Year baseline | |||||
| sample collected: | 2011 | 2011 | 2018 | 2017 | 2017 |
| Median (range) | (2009–2013) | (2009–2013) | (2017–2018) | (2017–2018) | (2017–2018) |
The HOTSPOT control group included 33 convenience controls and 101 spouses/partners of HPV-positive OPC patients, all of whom had a head and neck examination ruling out cancer.
HIV-positive participants had a median current CD4 cell count of 458.5 (IQR = 273–660.5).
Oral rinse sample collection and processing
Oral rinse samples were collected using 10 mL saline (MOUTH study) or Scope (HOTSPOT study) by 30-second oral rinse and gargle. Samples were stored at 4°C until processed. Samples were collected from HOTSPOT cases around time of HPV-OPC diagnosis and before any therapy was received.
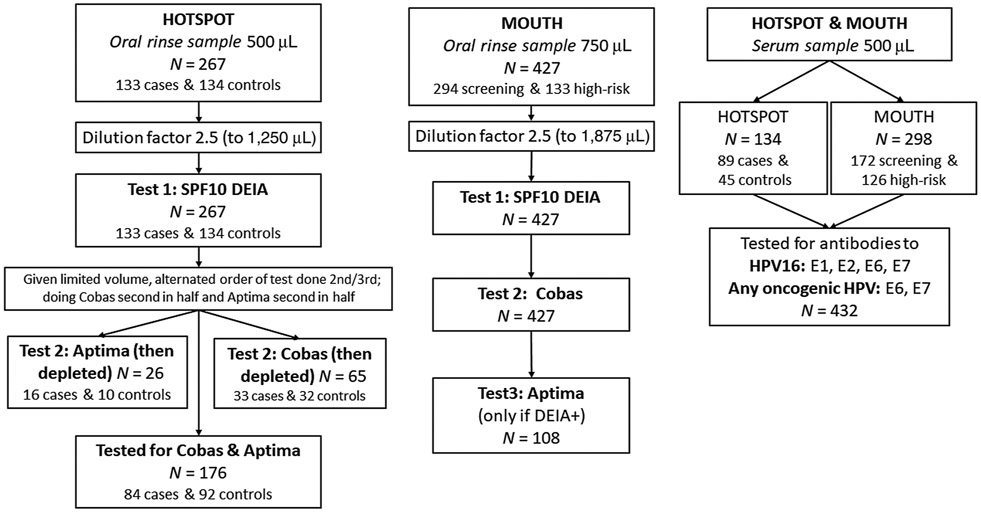
For the MOUTH study, sample processing was performed centrally by the Johns Hopkins Biospecimen Repository. Samples were centrifuged at 3,000 × g for 10 minutes, pellet was resuspended in 10 mL saline, recentrifuged at 3,000 × g for 10 minutes, and pellet resuspend in 1.5 mL of normal PBS. Samples were then divided in two equal volume aliquots (750 μL each) and half to be tested was stored at −80°C until shipped to DDL (on dry ice). Oral rinse samples were tested by DDL where they were vortexed then diluted 2.5 times PreservCyt medium, from starting volume of 750 μL, in a 13 mL Sarstedt tube. Samples that for any reason had a lower volume (500 to <750 μL) were instead diluted 3.5-fold to have sufficient input volume for all assays. This dilution factor was based on pilot testing performed at DDL to ensure reproducibility after dilution (Fig. 1).
Figure 1.
Flowchart of sample dilution and testing, by sample type (oral rinse and serum) and study population (HOTSPOT and MOUTH).
HPV-OPC cases and control samples from the HOTSPOT study biorepository were similarly processed except these pellets had been previously resuspended in 1.0 mL of PBS and aliquoted into 500 μL instead (22). The same sample dilution factor of 2.5 was used for these HOTSPOT samples, although their original volume was 500 μL (Fig. 1).
Oral HPV detection
A schematic of sample testing is shown in Fig. 1. All MOUTH and HOTSPOT oral rinse HPV results reported in this paper were tested centrally by DDL Diagnostic Laboratory using study biorepository samples. DNA was extracted using the NucliSENS easyMAG. DNA specimens were tested for the presence of HPV DNA by PCR amplification using the SPF10 primer system (version 1 system Labo Biomedical Products). HPV SPF10 PCR products were detected using the DNA Enzyme Immuno-assay (called DEIA hereafter) detection system. All samples which tested positive for HPV DNA by SPF10 DEIA were then tested by SPF10 LIPA to detect the following 13 oncogenic HPV types: 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, and 66, as well as 12 nononcogenic HPV types: 6, 11, 34, 40, 42, 43, 44, 53, 54, 68/73, 70, and 74 not reported on in this article (26, 27).
The remaining oral rinse sample volume was used to perform additional HPV DNA testing by both Cobas (Roche Cobas HPV Test) and E6/E7 mRNA detection using Aptima (Hologic) according to the manufacturer's standard instructions for cervical cytology specimens in PreservCytSolution (Hologic Corp). The Cobas test identifies 14 oncogenic HPV types, including: HPV 16 and 18 specifically as well as a combined result for other oncogenic HPV types: 31, 33, 35, 39, 45, 51,52, 56, 58, 59, 66, and 68. Note the Cobas definition of oncogenic is similar to that used for DEIA but not identical as the Cobas probe includes type 68, which the DEIA study definition did not. Aptima identifies an oncogenic combined result for any of the same 14 oncogenic types as Cobas used: HPV16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 66, and 68. As 150 (99.3%) of 151 DEIA negative samples for the HOTSPOT study were also negative by Aptima, samples that were DEIA negative in the MOUTH study were assumed to be Aptima negative. Samples that were positive by DEIA for HPV DNA were tested by Aptima (when remaining volume allowed). HPV 68 is included in the classification of oncogenic types by Cobas and Aptima, but was not considered oncogenic in our DEIA analysis (28).
Blood collection and processing
Participants had blood collected in an SST tube at study enrollment. After collection, tubes were inverted five times to allow clot formation and stored at 4°C until ready to process. Samples were spun at 1,942 × g for 15 minutes at room temperature, serum layer was removed and stored at −80°C until tested.
Serology HPV antibody detection
Serum for MOUTH were tested for E1, E2 antibodies to HPV types 16, 18, and E6 and E7 oncogene antibodies to HPV types 16, 18, 31, 33, 35, 45, 52, and 58. HOTSPOT serum was similarly tested, but did not include testing for HPV 35 or 52). All MOUTH and HOTSPOT antibody testing was performed centrally by the German Cancer Research Center (DKFZ) using multiplex serology, an antibody detection method based on glutathione S-transferase (GST) capture ELISA, in combination with fluorescent bead-based technology, in a procedure that has been described previously (29). Median fluorescence intensity (MFI) values were dichotomized as antibody positive or negative, using lab established cut-off values (6).
Statistical analysis
Sensitivity for HPV-OPC was calculated as the number of individuals positive by each HPV biomarker of interest out of those known to be diagnosed with HPV-OPC (HOTSPOT cases). Specificity was calculated as the number of individuals negative for HPV by each biomarker out of those not diagnosed with HPV-OPC (this "noncancer control group" included HOTSPOT controls as well as the MOUTH at-risk group). Prevalence of each HPV biomarker was explored alone and in some cotesting scenarios, and pattern of positivity across tests was compared. Analysis was performed considering prevalence of any oncogenic HPV type (called oncHPV here after), as well as to HPV16 specifically.
Results
To understand whether detection of HPV biomarkers in oral exfoliated cells and serum could discern participants with HPV-OPC from participants without known malignancy, sensitivity, and specificity were calculated (Table 2). The sensitivity of oral HPV16 DNA to detect HPV-OPC cases was only moderate, by DEIA and Cobas (51% vs. 43%, P = 0.19). Specificity of HPV16 DNA was high by both DEIA (99%) and Cobas (98%; Table 2).
Table 2.
Sensitivity and specificity of oncogenic HPV biomarkers in oral exfoliated cells or serum for HPV-related OPC
| n/N (%) | ||
|---|---|---|
| Sensitivity for HPV-positive OPC | Specificity for noncancer "controls"b | |
| Oral rinse HPV | ||
| HPV16 | ||
| SPF10 DEIA | 68/133 (51%) | 422/428 (99%) |
| Cobas DNA | 50/117 (43%) | 410/417 (98%) |
| Any oncogenic typea | ||
| SPF10 DEIA | 80/133 (60%) | 415/428 (97%) |
| Cobas DNA | 57/117 (49%) | 403/417 (97%) |
| Aptima RNA | 23/100 (23%) | 394/396 (99%) |
| HPV serum antibodies | ||
| HPV16 | ||
| E1 antibody | 61/89 (69%) | 212/217 (98%) |
| E2 antibody | 69/89 (78%) | 216/217 (100%) |
| E6 antibody | 78/89 (88%) | 213/217 (98%) |
| E7 antibody | 67/89 (75%) | 206/217 (95%) |
| Any oncogenic typea | ||
| E6 antibody | 82/89 (92%) | 201/217 (93%) |
| E7 antibody | 69/89 (78%) | 201/217 (93%) |
| Cotesting | ||
| Cotesting by HPV16 oral rinse DNA and serum antibodies: | ||
| BOTH: HPV16 DEIA and E6 antibody | 43/89 (48%) | 217/217 (100%) |
| BOTH: HPV16 Cobas and E6 antibody | 29/79 (37%) | 214/214 (100%) |
| EITHER: HPV16 DEIA and/or E6 antibody | 81/89 (91%) | 210/217 (97%) |
| EITHER: HPV16 Cobas and/or E6 antibody | 72/79 (91%) | 206/214 (96%) |
| Cotesting with multiple HPV16 E6 serum antibodies: | ||
| BOTH: HPV16E6 and E7 antibodies | 61/89 (69%) | 217/217 (100%) |
| ≥3 of these 4: HPV16 E1, E2, E6, E7 | 64/89 (72%) | 217/217 (100%) |
| EITHER: HPV16 E6 and/or E7 antibodies | 84/89 (94%) | 202/217 (93%) |
| ANY OF: HPV16 E1, E2, E6, and/or E7 | 85/89 (96%) | 197/217 (91%) |
| Cotesting with multiple OncHPV E6 serum antibodies: | ||
| BOTH: OncHPV E6 and E7 antibodies | 65/89 (73%) | 215/217 (99%) |
| EITHER: OncHPV E6 and/or E7 antibodies | 86/89 (97%) | 187/217 (86%) |
Oral rinse and serum included these 8 oncogenic HPV types: 16, 18, 31, 33, 35, 45, 52, 58; In addition, oral rinse (but not serum) test also included in oncogenic definition these 6 types: 39, 51, 56, 59, 66, (and type 68 for Cobas and Aptima but not DEIA).
The non-cancer "control" group included HOTSPOT controls and individuals from the MOUTH screening study (see columns 2 and 3 in Supplementary Table S1; The high-risk group (MACS WIHS CCS) were not included in this comparison group given the non-representative nature of this population. Results were similar when using only the 134 HOTSPOT noncancer controls (excluding the MOUTH screening) as the negative reference group, with a specificity of: 100% (oncogenic SPF10-DEIA 134/134); 100% (Cobas 124/124); 99.0% (Aptima 101/102); and 95.6% (43/45 HPV16 E6 and/or E7 seropositive).
Test characteristics of oral rinse biomarkers were similar when considering any oncogenic HPV (oncHPV). Sensitivity of oncHPV DNA for identification of HPV-OPC was similar to that for HPV16 (DEIA: 60% vs. 51%, P = 0.14; Cobas: 49% vs. 43%, P = 0.36). Specificity of oncHPV was consistently high using oncHPV or HPV16 by eitherDEIA (97% vs. 99%, P = 0.10) or Cobas (97% vs. 98% P = 0.12). Sensitivity was low when tested by Aptima as only 23% of HPV-OPC cases had oncHPV E6/E7 mRNA detected (P < 0.001). Given heterogeneity in study populations (Table 1), the same analysis was performed when restricted to only men, and the test characteristics were the same (Supplementary Table S1).
When considering HPV oncogene antibodies in serum, sensitivity and specificity were generally high. HPV16 E6 antibodies had a sensitivity of 88% and specificity of 98%, which was the best overall test performance of any of the biomarkers evaluated (Table 2). Inclusion of E7 did not improve performance properties (Table 2). Seropositivity for any of four HPV16 oncogenes (E1, E2, E6, and/or E7) had marginally higher sensitivity than HPV16 E6 seropositivity alone (96% vs. 88% P = 0.06) but significantly lower specificity (91% vs. 98%. P < 0.001). In contrast, requiring positivity for three of four HPV16 oncogenes (≥3 of E1, E2, E6, and E7) had lower sensitivity than HPV16 alone (72% vs. 88%, P = 0.009) but increased specificity (100% vs. 98%, P = 0.04).
When considering the ability of oral HPV16 biomarkers to correctly categorize cases and controls, E6 antibodies (88%) had the highest sensitivity relative to oral rinse DEIA (51%), or Cobas (43%; each P < 0.001), and specificity was similar (98%, 99%, and 98% for HPV16 E6 antibodies, DEIA and Cobas respectively, P ≥ 0.67). HPV16 E6 seropositivity had significantly higher specificity than when considering E6 antibodies to any oncHPV type (98% vs. 93%, P = 0.006), while sensitivity remained similarly high (88% vs. 92%, P = 0.32). In the scenario of HPV16 cotesting, when requiring biomarker positivity by both oral rinse and serum tests, sensitivity decreased (P < 0.001), but specificity increased to 100% (Table 2). Considering biomarker positivity for either oral rinse or serum biomarkers yielded high sensitivities (each >90%, Table 2), but it was not significantly higher than when using HPV16 E6 antibody alone. For example, HPV16 detection by either E6 antibody and/or DEIA DNA resulted in sensitivity of 91% versus E6 antibody alone which was 88% (P = 0.47). Specificity was similar when cotesting with HPV16 E6 antibody and/or DEIA (97%) or, both E6 antibody and DEIA (100%, P = 0.01) or E6 antibody alone (98%, P = 0.36; Table 2).
Next, prevalence of each HPV biomarker was explored within the at-risk population (Table 3). Most HPV16 biomarkers were detected in <2.5% of at-risk participants screened, including when tested for in oral rinse by DEIA (2.0%) or Cobas (2.4%), or in serum by HPV16 E6 antibodies (2.3%). Seroprevalence of HPV16 E6 and E7 antibodies were 2.3% and 5.2%, respectively (Table 3). When considering prevalence of any oncHPV type among at-risk participants, biomarker prevalence remained <7%, including by DEIA (4.4%), Cobas (4.8%), mRNA by Aptima (0.3%), or serum E6 antibodies (6.4%).
Table 3.
Prevalence of HPV biomarkers in oral exfoliated cells and serum among healthy individuals in the MOUTH cohort in the screening (at-risk) population, and in the higher risk of MACS/WIHS participants enrolled because they had HPV biomarkers detected in a previous study
| Prevalence | ||
|---|---|---|
| Biomarkers | At-Risk (MOUTH Screening) | High-risk (MACS/WIHS CCS) |
| Oral rinse HPV | N = 294 | N = 133 |
| HPV16 | ||
| SPF10 DEIA | 2.0% | 10.5% |
| Cobas DNA | 2.4% | 15.0% |
| Any oncogenic typea | ||
| SPF10 DEIA | 4.4% | 39.9% |
| Cobas DNA | 4.8% | 45.9% |
| Aptima RNAb | 0.3% | 6.0% |
| Any HPV type: SPF10 DEIA | 12.6% | 53.4% |
| HPV serum antibodiesc | N = 172 | N = 126 |
| HPV16 | ||
| E1 antibody | 2.3% | 1.6% |
| E2 antibody | 0.6% | 0.8% |
| E6 antibody | 2.3% | 2.4% |
| E7 antibody | 5.2% | 8.7% |
| E6 and E7 antibodies | 0.0% | 0.8% |
| E6 and/or E7 antibodies | 7.6% | 10.3% |
| E1, E2, E6, E7 (≥3 of these 4) | 0.0% | 0.8% |
| E1, E2, E6, and/or E7 (any) | 9.9% | 11.1% |
| Any oncogenic typea | ||
| E6 antibody | 6.4% | 11.1% |
| E7 antibody | 7.0% | 15.9% |
| E6 and E7 antibodies | 0.6% | 4.0% |
| E6 and/or E7 antibodies | 12.8% | 23.0% |
| Cotesting of HPV16 | ||
| Cotesting by oral rinse DNA and serum antibodies | ||
| Both: HPV16 DEIA and E6 antibody | 0.0% | 1.6% |
| Either: HPV16 DEIA and/or E6 antibody | 4.1% | 11.9% |
| Any of: HPV16 DEIA, Cobas, and/or E6 antibody | 4.7% | 16.7% |
| Any of: HPV16 DEIA, Cobas, E6 and/or E7 antibodies | 9.4% | 24.6% |
Oral rinse and serum included these 8 oncogenic HPV types: 16, 18, 31, 33, 35, 45, 52, 58; In addition, oral rinse (but not serum) test also included in oncogenic definition these 6 types: 39, 51, 56, 59, 66, (and type 68 for Cobas and Aptima but not DEIA).
Among MOUTH participants, Aptima testing was only performed among those who were HPV positive by DEIA - other samples assumed to be negative by Aptima for calculation of prevalence (based on dual testing of 151 HOTSPOT HPV-OPC cases and controls by both DEIA and Aptima, showing that 150 (99.3%) of 151 DEIA negative samples were negative by Aptima.
Serum antibody results were only available in a subset of participants at the time this paper was written as the next batch of test results were not yet completed.
These same biomarkers were also explored in the high-risk population of 133 individuals from the MACS WIHS Combined Cohort Study (CCS) who previously had HPV biomarkers detected, on average, 2.5 years prior to the first MOUTH study visit (IQR of 1.3–7.1 years). Prevalence of oral HPV16 biomarkers was elevated in the high-risk group compared with the at-risk group, as expected (Table 3), by both DEIA (10.5% vs. 2.0%) and Cobas (15.0% vs. 2.4%). HPV16 E6 antibody prevalence was similar in the high-risk and at-risk groups (2.4% vs. 2.3%, P = 0.98). Although these participants had oncHPV detected by DEIA at their previous study visit, more than half of these participants now tested negative for oncHPV DNA using DEIA (Table 3).
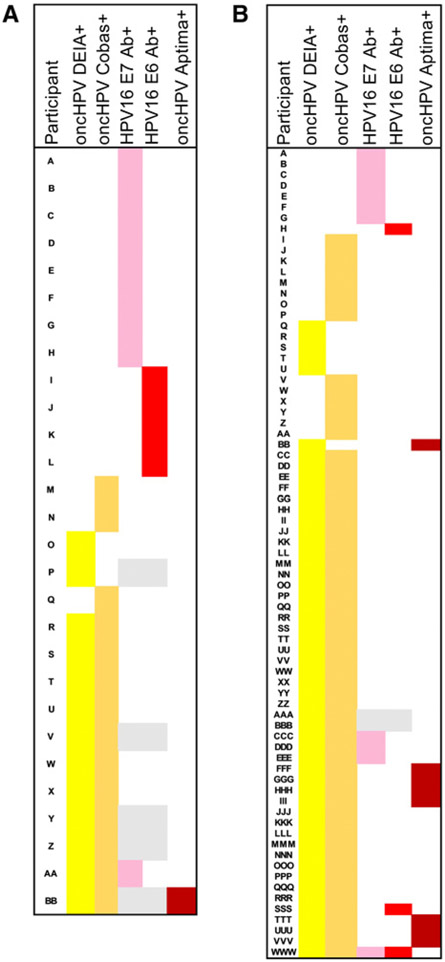
Concordance of results across biomarkers was explored to inform the potential for misclassification (false positives or negatives). Among 294 at-risk participants, there was poor agreement across biomarkers (Fig. 2A). Only one participant (0.3%) had HPV16 biomarkers detected in both oral rinse and serum (i.e., was positive for HPV16 by DEIA, Cobas, and E6 antibody). Only one (0.3%) at-risk participant had oral oncHPV mRNA detected (not the same individual). Consistent with the similar sensitivity and specificity of Cobas and DEIA, there was good concordance of these tests for HPV16 (kappa = 0.92) or oncHPV (kappa = 0.81). Seven (of 293, 2.4%) at-risk participants had oral HPV16 DNA detected by either DEIA or Cobas, of which 6 of 7 (86%) had oral HPV16 detected by both tests. Eleven (3.7%) at-risk participants had oral oncHPV DNA detected by both DEIA and Cobas, and another five had discrepant oncHPV findings by Cobas and DEIA (Fig. 2A).
Figure 2.
Pattern of HPV biomarker detection among subjects positive to at least one of the following 5 biomarkers in the at-risk (MOUTH screening population, A) and high-risk (MACS/WIHS population, B), with color indicating a positive result to each assay, including: (i) DEIA oncogenic oral HPV DNA (yellow), (ii) Cobas oncogenic oral HPV DNA (orange), (iii) HPV16 E7 serum antibodies (pink), (iv) HPV16 E6 serum antibodies (bright red), (v) Aptima oncogenic HPV RNA (dark red). Shaded gray areas represent biomarker results not tested/available.
There was a lack of a clear pattern of positivity across biomarkers studied with few at-risk participants having multiple biomarker positivity. Indeed, there was poor concordance of HPV16 E6 seropositivity and oral HPV16 DNA detection. Although 90% of subjects were negative by both tests, most (12/13, 92%) of those with HPV16 E6 (n = 4) or E7 (n = 9) antibodies detected had no oral HPV16 infections detected (Fig. 2). To further explore patterns of biomarker positivity, results were compared across tests among subjects positive for any oncHPV in the at-risk (Fig. 2A) or high-risk (Fig. 2B) populations. The majority of those with oral oncHPV DNA detected by either DEIA (7/8, 88%) or Cobas (9/10, 90%) did not have HPV16 E6 or E7 antibodies (Fig. 2A). A similar pattern of biomarker positivity was found among the 133 high-risk participants (Fig. 2B). Having oncHPV biomarkers in both oral rinse and serum was rare (4%, 5/133), as was having HPV mRNA (Aptima: 6%, 8/133). Only 70% (47/67) of the oral oncHPV infections detected by DEIA or Cobas were consistently detected by both assays. Oral oncHPV DNA was detected by DEIA but not Cobas in 6 (4.5%) participants, and by Cobas but not by DEIA in 14 (10.5%) participants. Among the high-risk participants, there were 5 subjects with both oral oncHPV infection and HPV16 E6 or E7 antibodies, representing 38% (5/13) of those with antibodies and 7.5% (5/67) of those with oral oncHPV infection (Fig. 2B).
Discussion
While there may be a future opportunity to screen for HPV-OPC, no validated biomarkers have yet been identified to reliably detect HPV-OPC at an early stage. Evidence of strong performance characteristics for biomarkers would be needed to support any future screening strategies. This study suggests that oral rinses tested for oncHPV (or HPV16) DNA or mRNA with commercially available tests each have good specificity but moderate to poor sensitivity for HPV-OPC, implying they would miss many true-positives. HPV16 E6 serum antibody appears to be the most promising biomarker of those evaluated and was the only one with high sensitivity and specificity.
When considering potential biomarkers for screening, it is important to contemplate the ideal properties of a screening test (30). First, high specificity is critical for screening the general population, given the potential harm of a false positive test. High sensitivity, although often considered less critical than specificity when screening healthy populations, is also important so that affected individuals are not falsely reassured by a false negative test. That is why some screening scenarios use sequential screening with a high sensitivity test first to catch most cases and a high specificity test second to reduce the number of false-positives. To be useful, a screening test should be validated, widely available, safe to administer, and have a reasonable cost. Given these criteria, of the biomarkers evaluated in this study, HPV16 E6 antibodies have sufficiently high specificity and sensitivity.
Disappointingly, oral rinse HPV biomarkers, although easier than the venipuncture required for antibody detection, had inferior sensitivity. HPV16 E6 antibody, however, appeared favorable in the context of these screening principles as a promising biomarker, and had sensitivity and specificity for HPV-OPC comparable with that observed for HPV biomarkers used in the established cervical cancer screening program (31-34). Cervical cancer and HPV-OPC have a shared viral etiology, and the success of the U.S. cervical screening program, which includes HPV detection, provides promise for its oropharyngeal counterpart. Given that there are tradeoffs in identifying optimal screening tests, the properties of tests currently used for cervical cancer screening may provide a benchmark for consideration of OPC screening biomarkers. This and other articles (15) begin to serve as validation for the performance of these tests, although more analysis is needed and no HPV biomarker tests are currently FDA approved (or otherwise sufficiently validated to have been endorsed by professional practice) for HPV-OPC screening.
Few studies have explored the test characteristics of HPV biomarkers for HPV-OPC. To our knowledge, this is the first study to formally evaluate the sensitivity and specificity of Cobas and Aptima in oral rinse samples to detect HPV-OPC. Utility of oncogenic HPV DNA detected by DEIA was evaluated in a recent systematic review (by our group) in 7 studies of OPSCC (n = 5) or HNSCC (n = 2) patients; our findings are consistent with the estimates in these heterogeneous studies, which found sensitivity of 55% and specificity of 94% for HPV-OPC (15). Some recent studies of incident HPV-OPC have reported high prevalence of oral oncHPV among cases, suggesting higher sensitivity than that found here (35). Performance of HPV16 E6 antibodies for HPV-OPC has been explored in a few studies previously, which reported high sensitivity (90%–96%) and specificity (96%–98%), comparable to that reported here (16, 36, 37).
The performance of oral and serum HPV markers among healthy individuals has not previously been compared, although both oral HPV DNA (38, 39) and HPV serum antibody (36, 40, 41) detection have emerged as candidate screening biomarkers for HPV-OPC. HPV16 biomarkers in both rinse and serum were rare among the at-risk population screened, and the pattern of biomarker detection was not consistent. Indeed, there was poor agreement of these biomarkers among healthy individuals in a screening population. While HPV16 E6 antibody testing had the best performance of the biomarkers evaluated, this marker is rare even among the selected at-risk and high-risk populations included in this study. As most individuals with HPV16 E6 antibodies are not expected to have clinically evident disease, the number needed to screen to detect a case of HPV-OPC cancer is expected to be very high, decreasing enthusiasm (42). However, these data support the prospective evaluation of serologic HPV antibody detection in at-risk populations to determine whether appropriate surveillance of HPV16 E6-positive individuals might lead early detection and improved treatment outcomes, which remains unknown.
It was notable that the performance of HPV16-specific, not any oncogenic biomarkers had the best performance for HPV-OPC, which may reflect that most (~90%) HPV-OPC in the United States are HPV16-positive tumors (43). This is in contrast to the cervical HPV paradigm where the distribution of oncogenic types in premalignancy and malignancy is broad. While HPV-type–specific infection was unavailable for some HPV-OPC cases in this study, it is assumed that the vast majority of tumors were HPV16-positive. As non-HPV16 oncogenic infections are responsible for the minority of an already rare cancer, detection of other oncogenic oral HPV infections appears to be less predictive, as supported by HPV16 biomarkers having better tests characteristics for HPV-OPC. The addition of nonHPV16 oncogenic types by antibody did not appear to yield improvement by sensitivity; however, decreased specificity. Consideration of adding nonHPV16 oncogenic oral rinses to HPV16 E6 also did not improve the properties of the standalone biomarker. Therefore, going forward it appears that rather than trying to modestly improve sensitivity by detection of any oncogenic infection, HPV16 detection alone may yield the highest number of cases of interest, because having high specificity is critical when the prevalence is rare.
This study had several limitations and strengths. The serologic HPV antibody test evaluated is not commercially available and is not an FDA-approved test. Strengths include that samples were centrally processed and tested and samples were prospectively collected within the same research protocols. Two of the tests included (Cobas and Aptima) are commercially available HPV tests, FDA-approved for cervical HPV testing but not oral HPV detection.
Screening in the general population is clearly not presently warranted, given the potential harm of identifying screen-positive individuals without detectable cancer for whom surveillance, risk, and benefit are unclear. There is at this time unclear benefit from screening to outweigh to psychologic harm and potential false positives for screening for HPV-OPC, even with this promising biomarker. Identification of healthy individuals with HPV16 E6 may be harmful given the absence of evidence-based surveillance guidelines, lack of knowledge about the lead time between biomarker positivity and cancer, and no current data to support improvement in survival outcomes, reduction in therapy, or morbidity from those detected earlier by this biomarker.
Furthermore, HPV16 E6 serum antibodies are known to also be elevated in individuals with anal premalignancy or cancer, although interestingly for cervical cancer they are often absent or detected later and at a low titer. The fact that HPV16 E6 antibody seropositivity may have utility for screening for anal cancer (44) as well as OPC is an important consideration for appropriate triage/evaluation of biomarker positive individuals. Like HPV-OPC, anal cancer is another HPV-related cancer for which optimal screening approaches remain unclear (45-47), and research suggests these same HPV DNA biomarkers (DEIA and Cobas) also have high specificity and moderate sensitivity for anal precancer (48, 49). The low prevalence of E6 antibodies, even among the high-risk group of HIV-infected individuals and men who have sex with men in this study, supports a possible higher positive predictive value of this marker in highlighting those as risk. Some studies have suggested higher antibody titers are associated with cancer progression, suggesting titer level or cutoff should also be explored.
While general population screening is not warranted, screening among select higher-risk groups may be appropriate if future clinical studies demonstrate that the harm to benefit ratio is favorable, natural history of disease is changed, and that outcomes are improved by earlier detection. For example, groups with higher oncogenic oral HPV prevalence, such as middle aged men who smoke and have a higher number of lifetime oral sexual partners (11) would have a higher positive predictive value from these tests and have a higher HPV-OPC risk. Groups with high anxiety about HPV-OPC and a risk profile indicating increased cancer risk, such as spouses or partners of patients with HPV-OPC and women with a history of cervical dysplasia or cancer and their spouses or partners, might also benefit. These questions warrant further investigation within clinical trials.
Supplementary Material
Acknowledgments
This work was supported by National Institute of Child Health and Human Development (grant no. R35DE026631). Data in this manuscript were collected by the Multicenter AIDS Cohort Study (MACS) and Women's Interagency HIV Study (WIHS), now the MACS-WIHS Combined Cohort Study. Study funding (principal investigators) includes: Johns Hopkins University Bloomberg School of Public Health (to Joseph Margolick and Todd Brown), U01-AI35042; North-western University (to Steven Wolinsky), U01-AI35039; University of California, Los Angeles (Roger Detels, Otoniel Martinez-Maza, Otto Yang), U01-AI35040; University of Pittsburgh (Charles Rinaldo, Lawrence Kingsley, Jeremy Martinson), U01-AI35041; the Center for Analysis and Management of MACS, Johns Hopkins University Bloomberg School of Public Health (Lisa Jacobson, Gypsyamber D'Souza), UM1-AI35043; UAB-MS WIHS (Mirjam-Colette Kempf and Deborah Konkle-Parker), U01-AI-103401; Atlanta WIHS (Ighovwerha Ofotokun and Gina Wingood), U01-AI-103408; Bronx WIHS (Kathryn Anastos and Anjali Sharma), U01-AI-035004; Brooklyn WIHS (Howard Minkoff and Deborah Gustafson), U01-AI-031834; Chicago WIHS (Mardge Cohen and Audrey French), U01-AI-034993; Metropolitan Washington WIHS (Seble Kassaye), U01-AI-034994; Miami WIHS (Margaret Fischl and Lisa Metsch), U01-AI-103397; UNC WIHS (Adaora Adimora), U01-AI-103390; Connie Wofsy Women's HIV Study, Northern California (Ruth Greenblatt, Bradley Aouizerat, and Phyllis Tien), U01-AI-034989; WIHS Data Management and Analysis Center (Stephen Gange and Elizabeth Golub). The MACS & WIHS are funded primarily by the National Institute of Allergy and Infectious Diseases (NIAID), with additional co-funding from the National Cancer Institute (NCI), the National Institute on Drug Abuse (NIDA), the National Institute of Mental Health (NIMH), the Eunice Kennedy Shriver NICHD, and the National Institute on Drug Abuse (NIDA).
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked advertisement in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
Footnotes
Disclosure of Potential Conflicts of Interest
D.J. Wiley reports receiving other commercial research support from Copan Italia (specimen swabs), Roche Molecular Systems (Cobas HPV test kits), Hologic, Inc (HPV-APTIMA kits), and Qiagen, Inc. (HPV HC2 kits). R.I. Haddad has unpaid consultant/advisory board relationship with BMS, MERCK, PFIZER, GENENTECH, LOXO, BAYER, IMMUNOMIC, NANOBIOTIX, GSK. No potential conflicts of interest were disclosed by the other authors.
Supplementary data for this article are available at Cancer Prevention Research Online (http://cancerprevres.aacrjournals.org/).
Disclaimer
The contents of this publication are solely the responsibility of the authors and do not represent the official views of the National Institutes of Health (NIH).
References
- 1.Osazuwa-Peters N, Simpson MC, Massa ST, Adjei Boakye E, Antisdel JL, Varvares MA. 40-year incidence trends for oropharyngeal squamous cell carcinoma in the United States. Oral Oncol 2017;74:90–7. [DOI] [PubMed] [Google Scholar]
- 2.Tota JE, Anderson WF, Coffey C, Califano J, Cozen W, Ferris RL, et al. Rising incidence of oral tongue cancer among white men and women in the United States, 1973–2012. Oral Oncol 2017; 67:146–52. [DOI] [PubMed] [Google Scholar]
- 3.Hussein AA, Helder MN, de Visscher JG, Leemans CR, Braakhuis BJ, de Vet HCW, et al. Global incidence of oral and oropharynx cancer in patients younger than 45 years versus older patients: a systematic review. Eur J Cancer 2017;82:115–27. [DOI] [PubMed] [Google Scholar]
- 4.Agalliu I, Gapstur S, Chen Z, Wang T, Anderson RL, Teras L, et al. Associations of oral α-, β-, and γ-human papillomavirus types with risk of incident head and neck cancer. JAMA Oncol 2016;2: 599–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang Y, Waterboer T, Pawlita M, Sugar E, Minkoff H, Cranston RD, et al. Human Papillomavirus (HPV) 16 E6 seropositivity is elevated in subjects with oral HPV16 infection. Cancer Epidemiol 2016;43:30–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kreimer AR, Johansson M, Waterboer T, Kaaks R, Chang-Claude J, Drogen D, et al. Evaluation of human papillomavirus antibodies and risk of subsequent head and neck cancer. J Clin Oncol 2013; 31:2708–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Anderson KS, Wallstrom G, Langseth H, Posner M, Cheng JN, Alam R, et al. Pre-diagnostic dynamic HPV16 IgG seropositivity and risk of oropharyngeal cancer. Oral Oncol 2017;73: 132–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rodríguez AC, Schiffman M, Herrero R, Hildesheim A, Bratti C, Sherman ME, et al. Longitudinal study of human papillomavirus persistence and cervical intraepithelial neoplasia grade 2/3: critical role of duration of infection. J Natl Cancer Inst 2010;102: 315–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Koshiol J, Lindsay L, Pimenta JM, Poole C, Jenkins D, Smith JS. Persistent human papillomavirus infection and cervical neoplasia: a systematic review and meta-analysis. Am J Epidemiol 2008; 168:123–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.US Preventive Services Task Force, Curry SJ, Krist AH, Owens DK, Barry MJ, Caughey AB, et al. Screening for cervical cancer: US Preventive Services Task Force Recommendation Statement. JAMA 2018;320:674–86. [DOI] [PubMed] [Google Scholar]
- 11.D'Souza G, McNeel TS, Fakhry C. Understanding personal risk of oropharyngeal cancer: risk-groups for oncogenic oral HPV infection and oropharyngeal cancer. Ann Oncol 2017;28: 3065–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pierce Campbell CM, Kreimer AR, Lin H-Y, Fulp W, O'Keefe MT, Ingles DJ, et al. Long-term persistence of oral human papillomavirus type 16: the HPV Infection in Men (HIM) study. Cancer Prev Res 2015;8:190–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Beachler DC, Sugar EA, Margolick JB, Weber KM, Strickler HD, Wiley DJ, et al. Risk factors for acquisition and clearance of oral human papillomavirus infection among HIV-infected and HIV-uninfected adults. Am J Epidemiol 2015;181:40–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tam S, Fu S, Xu L, Krause KJ, Lairson DR, Miao H, et al. The epidemiology of oral human papillomavirus infection in healthy populations: a systematic review and meta-analysis. Oral Oncol 2018;82:91–9. [DOI] [PubMed] [Google Scholar]
- 15.Gipson BJ, Robbins HA, Fakhry C, D'Souza G. Sensitivity and specificity of oral HPV detection for HPV-positive head and neck cancer. Oral Oncol 2018;77:52–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Holzinger D, Wichmann G, Baboci L, Michel A, Höfler D, Wiesenfarth M, et al. Sensitivity and specificity of antibodies against HPV16 E6 and other early proteins for the detection of HPV16-driven oropharyngeal squamous cell carcinoma. Int J Cancer 2017;140:2748–57. [DOI] [PubMed] [Google Scholar]
- 17.Mirghani H, Lang Kuhs KA, Waterboer T. Biomarkers for early identification of recurrences in HPV-driven oropharyngeal cancer. Oral Oncol 2018;82:108–14. [DOI] [PubMed] [Google Scholar]
- 18.Rettig EM, Wentz A, Posner MR, Gross ND, Haddad RI, Gillison ML, et al. Prognostic implication of persistent human papillomavirus type 16 DNA detection in oral rinses for human papillomavirus-related oropharyngeal carcinoma. JAMA Oncol 2015; 1:907–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fakhry C, Qualliotine JR, Zhang Z, Agrawal N, Gaykalova DA, Bishop JA et al. Serum antibodies to HPV16 early proteins warrant investigation as potential biomarkers for risk stratification and recurrence of HPV-associated oropharyngeal cancer. Cancer Prev Res 2016;9:135–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Spector ME, Sacco AG, Bellile E, Taylor JMG, Jones T, Sun K, et al. E6 and E7 antibody levels are potential biomarkers of recurrence in patients with advanced-stage human papillomavirus-positive oropharyngeal squamous cell carcinoma. Clin Cancer Res 2017; 23:2723–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ahn SM, Chan JYK, Zhang Z, Wang H, Khan Z, Bishop JA, et al. Saliva and plasma quantitative polymerase chain reaction-based detection and surveillance of human papillomavirus-related head and neck cancer. JAMA Otolaryngol Head Neck Surg 2014;140:846–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.D'Souza G, Gross ND, Pai SI, Haddad R, Anderson KS, Rajan S, et al. Oral human papillomavirus (HPV) infection in HPV-positive patients with oropharyngeal cancer and their partners. J Clin Oncol 2014;32:2408–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.NIDCR. Men and women offering understanding of throat HPV (MOUTH Study) [Internet]; 2018. September 12 Available from: http://bit.ly/MOUTHStudy.
- 24.MACS/WIHS Combined Cohort Study [Internet]; 2019. Available from: https://mwccs.org/.
- 25.Beachler DC, Guo Y, Xiao W, Burk RD, Minkoff H, Strickler HD, et al. High oral human papillomavirus type 16 load predicts long-term persistence in individuals with or at risk for HIV infection. J Infect Dis 2015;212:1588–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kleter B, van Doorn LJ, Schrauwen L, Molijn A, Sastrowijoto S, ter Schegget J, et al. Development and clinical evaluation of a highly sensitive PCR-reverse hybridization line probe assay for detection and identification of anogenital human papillomavirus. J Clin Microbiol 1999;37:2508–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kleter B, van Doorn LJ, ter Schegget J, Schrauwen L, van Krimpen K, Burger M, et al. Novel short-fragment PCR assay for highly sensitive broad-spectrum detection of anogenital human papillomaviruses. Am J Pathol 1998;153:1731–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Arbyn M, Tommasino M, Depuydt C, Dillner J. Are 20 human papillomavirus types causing cervical cancer? J Pathol 2014;234: 431–5. [DOI] [PubMed] [Google Scholar]
- 29.Waterboer T, Sehr P, Michael KM, Franceschi S, Nieland JD, Joos TO, et al. Multiplex human papillomavirus serology based on in situ-purified glutathione s-transferase fusion proteins. Clin Chem 2005;51:1845–53. [DOI] [PubMed] [Google Scholar]
- 30.Herman C What makes a screening exam "good"? Virtual Mentor VM 2006;8:34–7. [DOI] [PubMed] [Google Scholar]
- 31.Castle PE, Stoler MH, Wright TC, Sharma A, Wright TL, Behrens CM. Performance of carcinogenic human papillomavirus (HPV) testing and HPV16 or HPV18 genotyping for cervical cancer screening of women aged 25 years and older: a subanalysis of the ATHENA study. Lancet Oncol 2011;12: 880–90. [DOI] [PubMed] [Google Scholar]
- 32.Ge Y, Christensen P, Luna E, Armylagos D, Xu J, Schwartz MR, et al. Aptima human papillomavirus E6/E7 mRNA test results strongly associated with risk for high-grade cervical lesions in follow-up biopsies. J Low Genit Tract Dis 2018;22:195–200. [DOI] [PubMed] [Google Scholar]
- 33.Ge Y, Christensen P, Luna E, Armylagos D, Schwartz MR, Mody DR. Performance of Aptima and Cobas HPV testing platforms in detecting high-grade cervical dysplasia and cancer. Cancer Cytopathol 2017;125:652–7. [DOI] [PubMed] [Google Scholar]
- 34.Forslund O, Miriam Elfström K, Lamin H, Dillner J. HPV-mRNA and HPV-DNA detection in samples taken up to seven years before severe dysplasia of cervix uteri. Int J Cancer 2019;144: 1073–81. [DOI] [PubMed] [Google Scholar]
- 35.Martin-Gomez L, Giuliano AR, Fulp WJ, Caudell J, Echevarria M, Sirak B, et al. Human papillomavirus genotype detection in oral gargle samples among men with newly diagnosed oropharyngeal squamous cell carcinoma. JAMA Otolaryngol Head Neck Surg 2019;145: 460–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lang Kuhs KA, Kreimer AR, Trivedi S, Holzinger D, Pawlita M, Pfeiffer RM, et al. Human papillomavirus 16 E6 antibodies are sensitive for human papillomavirus-driven oropharyngeal cancer and are associated with recurrence. Cancer 2017;123:4382–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lang Kuhs KA, Pawlita M, Gibson SP, Schmitt NC, Trivedi S, Argiris A, et al. Characterization of human papillomavirus antibodies in individuals with head and neck cancer. Cancer Epidemiol 2016;42:46–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rosenthal M, Huang B, Katabi N, Migliacci J, Bryant R, Kaplan S, et al. Detection of HPV related oropharyngeal cancer in oral rinse specimens. Oncotarget 2017;8:109393–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yoshida H, Murono S, Ueno T, Nakanishi Y, Tsuji A, Hatano M, et al. Usefulness of human papillomavirus detection in oral rinse as a biomarker of oropharyngeal cancer. Acta Otolaryngol (Stockh) 2017;137:773–7. [DOI] [PubMed] [Google Scholar]
- 40.Kreimer AR, Johansson M, Yanik EL, Katki HA, Check DP, Lang Kuhs KA, et al. Kinetics of the human papillomavirus type 16 E6 antibody response prior to oropharyngeal cancer. J Natl Cancer Inst 2017;109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dahlstrom KR, Anderson KS, Field MS, Chowell D, Ning J, Li N, et al. Diagnostic accuracy of serum antibodies to human papillomavirus type 16 early antigens in the detection of human papillomavirus-related oropharyngeal cancer. Cancer 2017; 123:4886–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gillison ML, Chaturvedi AK, Anderson WF, Fakhry C. Epidemiology of human papillomavirus-positive head and neck squamous cell carcinoma. J Clin Oncol 2015;33:3235–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chaturvedi AK, Engels EA, Pfeiffer RM, Hernandez BY, Xiao W, Kim E, et al. Human papillomavirus and rising oropharyngeal cancer incidence in the United States. J Clin Oncol 2011;29: 4294–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kreimer AR, Brennan P, Lang Kuhs KA, Waterboer T, Clifford G, Franceschi S, et al. Human papillomavirus antibodies and future risk of anogenital cancer: a nested case-control study in the European prospective investigation into cancer and nutrition study. J Clin Oncol 2015;33:877–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hillman RJ, Berry-Lawhorn JM, Ong JJ, Cuming T, Nathan M, Goldstone S, et al. International Anal Neoplasia Society Guidelines for the Practice of Digital Anal Rectal Examination. J Low Genit Tract Dis 2019;23:138–46. [DOI] [PubMed] [Google Scholar]
- 46.Godfrey C, Firnhaber CS, D'Souza G, Heard I. Anal dysplasia in HIV-infected women: a commentary on the field. Int J STD AIDS 2017;28:543–9. [DOI] [PubMed] [Google Scholar]
- 47.Wasserman P, Rubin DS, Turett G. Review: anal intraepithelial neoplasia in HIV-infected men who have sex with men: is screening and treatment justified? AIDS Patient Care STDs 2017;31:245–53. [DOI] [PubMed] [Google Scholar]
- 48.Wentzensen N, Follansbee S, Borgonovo S, Tokugawa D, Sahasrabuddhe VV, Chen J, et al. Analytic and clinical performance of cobas HPV testing in anal specimens from HIV-positive men who have sex with men. J Clin Microbiol 2014;52:2892–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jin F, Roberts JM, Grulich AE, Poynten IM, Machalek DA, Cornall A, et al. The performance of human papillomavirus biomarkers in predicting anal high-grade squamous intraepithelial lesions in gay and bisexual men. AIDS 2017;31:1303–11. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.