Abstract
Introduction
Gastrointestinal dysfunction (GDF) is one of the primary causes of morbidity and mortality in critically ill patients. Intensive care interventions, such as intravenous fluids and enteral feeding, can exacerbate GDF. There exists a paucity of high-quality literature on the interaction between these two modalities (intravenous fluids and enteral feeding) as a combined therapy on its impact on GDF.
Aim
To review the impact of intravenous fluids and enteral nutrition individually on determinants of gut function and implications in clinical practice.
Methods
Randomized controlled trials on intravenous fluids and enteral feeding on GDF were identified by a comprehensive database search of MEDLINE and EMBASE. Extraction of data was conducted for study characteristics, provision of fluids or feeding in both groups and quality of studies was assessed using the Cochrane criteria. A random-effects model was applied to estimate the impact of these interventions across the spectrum of GDF severity.
Results
Restricted/ goal-directed intravenous fluid therapy is likely to reduce ‘mild’ GDF such as vomiting (p = 0.03) compared to a standard/ liberal intravenous fluid regime. Enterally fed patients experienced increased episodes of vomiting (p = <0.01) but were less likely to develop an anastomotic leak (p = 0.03) and peritonitis (p = 0.03) compared to parenterally fed patients. Vomiting (p = <0.01) and anastomotic leak (p = 0.04) were significantly lower in the early enteral feeding group.
Conclusions
There is less emphasis on the combined approach of intravenous fluid resuscitation and enteral feeding in critically ill patients. Conservative fluid resuscitation and aggressive enteral feeding are presumably key factors contributing to severe life-threatening GDF. Future trials should evaluate the impact of cross-interaction between conservative and aggressive modes of these two interventions on the severity of GDF.
Keywords: gastrointestinal dysfunction, gastrointestinal failure, critical illness, surgical, intravenous fluids, resuscitation, enteral feeding
Introduction
Gut dysfunction (GDF) is a common problem in critically ill patients. It is the leading cause of multiple organ dysfunction syndrome/failure (MODS/MOF) and a significant cause of mortality and morbidity in critically ill patients [1]. In addition to this, the treatment of acute and critical illness can exacerbate GDF. Commonly used ICU interventions such as intravenous fluid resuscitation, early aggressive enteral feeding and vasopressor therapy are key factors leading to a secondary gut injury. In critical illness, intravenous fluid is the mainstay of early management for hemo-dynamic instability. It is vital to resuscitate a patient before commencing vasopressor therapy, particularly to delay the onset of an ischemic insult commonly occurring in hemodynamically unstable patients [2]. On the flip side, over-resuscitation can lead to bowel oedema leading to an ileus, while under-resuscitation with persistent splanchnic and peripheral vasoconstriction can trigger intestinal mucosal ischemia [3]. Although, enteral nutrition is the preferred approach to meet nutritional and modest fluid requirements in these patients, the delivery of early but aggressive enteral nutrition (EN) in hemodynamically unstable patients can precipitate the development of severe GDF, potentially leading to non-occlusive mesenteric ischemia which increases the chance of sepsis, multi-organ failure and mortality [4]. Intravenous fluid and enteral nutrition are two sides of the same coin and play a crucial role in determining the outcome of GDF if used wisely. However, very few studies have evaluated the role of these two modalities, thus making it difficult to understand their relationship with relevance to the severity of GDF. The aim was to review the evidence of the impact of intravenous fluid resuscitation and enteral nutrition individually on determinants of gut function and the implications in clinical practice.
Methods
Search Criteria and Study Identification
Electronic databases (MEDLINE and EMBASE) were searched using keywords on ‘gastrointestinal dysfunction in adult intensive care unit (ICU) /surgical patients on enteral feeding and intravenous fluids. The databases screened for all publications from the earliest available until 16th October 2018 (Appendix A).
Randomised controlled trials were searched by applying the keywords. Any additional studies on the impact of ‘intravenous fluid’ and ‘enteral feeding’ were included in the screening for the systematic review and meta-analysis. The search identification, screening and selection were conducted by the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) flow chart (Fig1) [5]. The study selection criteria were as follows.
The inclusion criteria were:
Study design: all randomised controlled trials (intravenous fluids and enteral feeding on GDF);
Study population: Adult surgical and critically ill patients
Disease state: critical illness and postoperative conditions
Intervention: enteral feeding: route of feeding (enteral vs parenteral); timing of feeding (early vs delayed); feeding vs nil-by-mouth and intravenous fluids: restricted vs liberal regime, goal-directed vs standard/conventional, low-infusions vs high-infusion or controlled vs rapid fluid therapy; intravenous fluids type: crystalloid fluid (normal saline or plasmalyte or ringer’s lactate) or colloid fluid (hydroxyethyl starch, albumin, gelofusion).
Study outcome: the occurrence of gastrointestinal dysfunction
The studies were excluded if they were:
non-ICU or non-surgical patients
paediatric population
animal studies
published in non-English languages
conducted on healthy volunteers
non-randomized trials (intravenous fluid therapy and enteral feeding)
not relevant to either of the interventions planned to study pattern of feeding (bolus vs continuous), comparative feed compositions (standard vs immune-enriched), related routes of feeding (nasogastric vs nasojejunal or jejunal) and studies addressing medications (e.g. prokinetic therapy).
Data Extraction: Data were extracted and independently recorded by two authors using predesigned data collection forms on Microsoft Excel.
Study characteristics included baseline demographic data such as author, publication year, study setting (ICU or surgical ward), admission diagnosis, study population, the total number of patients, fluid or enteral feeding interventions applied to experimental and control groups. The effect of fluid therapy and enteral feeding on GDF was analysed by separating the severity of GDF outcomes: 1) mild to moderate and 2) moderate to severe. All studies were stratified into the Clavien-Dindo classification [6] depending on the variability of clinical aetiology and interventions applied. Any additional studies derived from other sources and reference lists of included articles were screened and included if relevant. Data were independently reviewed and cross-checked by two authors (V.A. and A.B.). Any inconsistencies or disagreements were discussed between the two authors (V.A. and A.B.), and differences of opinion were further clarified by the senior author (J.A.W.).
Methodological quality
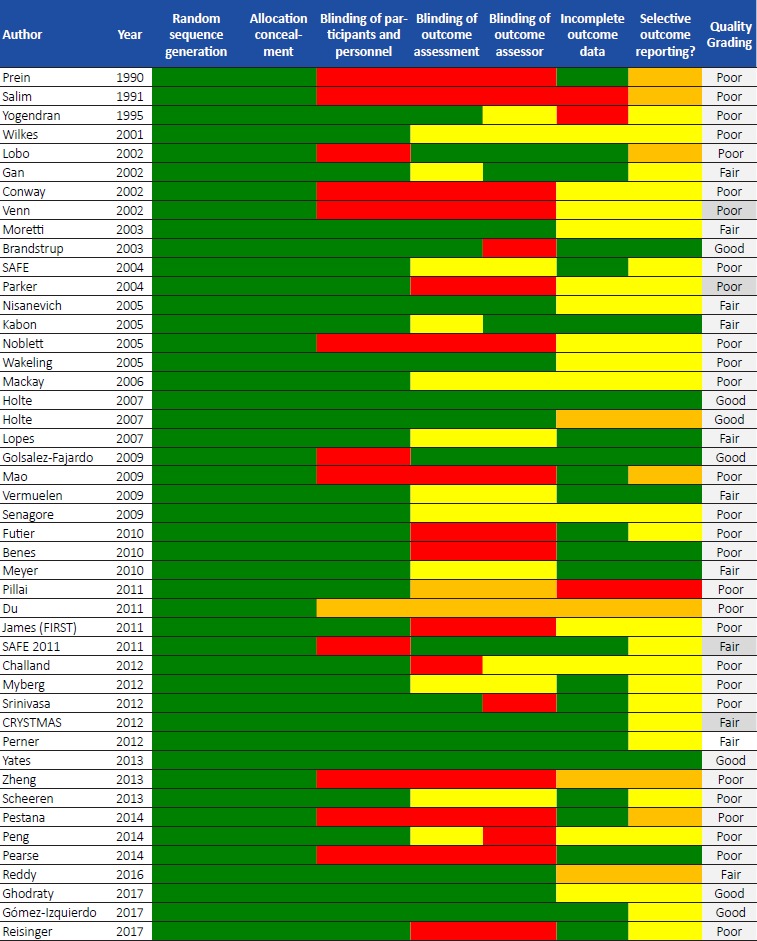
The methodological quality of included randomised controlled trials was assessed according to the Cochrane recommendations (The Nordic Cochrane Centre, The Cochrane Collaboration, 2008) [7]. These included systematic differences between groups (selection bias and performance bias), blinding of study participants and assessors, sequence allocation and concealment of allocated groups, the validity of findings and data withdrawal, incomplete outcome data (attrition and detection bias), and differences between data reporting or unreported data. The risk of bias assessment was presented according to the Cochrane collaboration recommendations. The overall quality of the study was graded as ‘poor’, ‘fair’ and ‘good’ based on the classification in the Cochrane’s quality assessment tool.
Statistical Analysis
All data were presented as the number of episodes of GDF in patients. Data analysis and interpretation were performed using Revman 5.3 (Revman, Version 5.3 for Windows; Copenhagen, Denmark: the Nordic Cochrane Centre, The Cochrane Collaboration, 2008) [7]. The nature of the analysis was not suitable for a pooled data analysis. Within each class of interventions (intravenous fluid and enteral feeding), a meta-analysis of GDF events was performed. Quantitative data meta-analysis was performed with at least two studies reporting on GDF as the primary or secondary outcome. Studies that did not have GDF as a primary or secondary outcome were excluded from the meta-analyses (Fig 1).
Heterogeneity was assessed by using I2 and classified as < 25% - low ; 25 – 50% - moderate and > 75% as high heterogeneity (heterogeneity and subgroup analysis in Cochrane consumers and communication group reviews) [8]. Regardless of the presence or absence of heterogeneity, a random-effects model was used to provide the most conservative estimate. Pooled effects for classes of interventions were calculated as weighted mean difference (MD) with 95% confidence interval (CI). P-value < 0.05 was considered statistically significant for all analyses. Ethical approval was not necessary for a review of published trials.
Results
Study Selection and Characteristics
A total of 103 studies including intravenous fluids (n = 46) and enteral feeding (n = 57) were eligible for inclusion in the systematic review, of which 43 (n = 22 intravenous fluid; n = 21 enteral feeding) studies were included in the final meta-analyses.
In studies on intravenous fluid therapy [9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54], 46 randomised controlled trials’ including 20,780 patients were systematically reviewed, of which 22 studies (n = 2696) were included in the final meta-analysis. Ten studies included mechanical ventilated critically ill patients, and the remaining 36 studies included post-operative patients. The intervention group received either restricted, goal-directed, low-infusion fluids or a controlled-expansion fluid regime given as crystalloid fluid (normal saline or plasmalyte) or colloid fluid (hydroxyethyl starch). The control group included standard, liberal, conventional, high-infusion fluids or rapid-expansion fluid regimes given as crystalloid fluids (ringers lactate, plasmalyte and saline). Five studies compared more than two groups of fluid regimes. Fifteen studies included critically ill, trauma and surgical patients with a grading of IV as per the Clavien-Dindo classification (Appendix B). The remaining studies included postoperative and acutely ill patients with Clavien-Dindo grading of II and III (Tables 1-3).
Table 1.
Study Characteristics of 'good' quality studies on the impact of intravenous fluid therapy on gut dysfunction included in the systematic review
| Author | Year | Study Population | Study Setting |
Study type |
Study patients |
Admission diagnosis | Experimental | Intravenous fluid |
Control | Intravenous fluid |
Dindo-Clavien Classification* |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Brandstrup9 | 2003 | elective colorectal resection | surgery | RCT | 141 | postsurgical | 69 | restricted | 72 | standard | |
| Holte10 | 2007 | elective surgery | surgery | RCT | 32 | elective colorectal surgery | 16 | restricted | 16 | liberal | |
| Holte11 | 2007 | post-surgery | surgery | RCT | 48 | knee arthroplasty | 24 | restricted | 24 | liberal | |
| Gonsalez-Fajardo12 | 2009 | post-surgery | surgery | RCT | 40 | vascular surgery transperitoneal aorto-iliac | 20 | restricted | 20 | standard | |
| Yates13 | 2013 | elective surgery | surgery | RCT | 206 | elective colorectal surgery | 104 | starch | 98 | crystalloid | |
| Ghodraty14 | 2017 | post-surgery | surgery | RCT | 91 | abdominal surgery | 46 | HES | 45 | ringers lactate | |
| Gómez-Izquierdo15 | 2017 | post-surgery | surgery | RCT | 128 | colorectal surgery | 4 | GDFT | 64 | control |
Abbreviations: HES- hydroxyethyl starch; GDFT-goal-directed fluid therapy; RCT-randomised controlled trial. * Appendix C
Table 3.
Study Characteristics of 'poor ' quality studies on the impact of intravenous fluid therapy on gut dysfunction.
| Author | Year | Study Population | Study Setting |
Study type |
Study patients |
Admission diagnosis | Experimental | Intravenous fluid |
Control | Intravenous fluid | Dindo-Clavien Classification* |
|||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Prein27 | 1990 | post-surgery | surgery | RCT | 18 | modified Whipple's | 6- ringers' lactate; 6– starch; 6-albumin | I | II | III | IV | |||
| Salim28 | 1991 | elective surgery | surgery | RCT | 130 | Hartmann's procedure +/- cholecystectomy | 71 | early oral | 59 | conventional intravenous | ||||
| Yogendran29 | 1995 | elective surgery | surgical | RCT | 200 | surgical | 100 | Low-infusion | 100 | High infusion | ||||
| Wilkes30 | 2001 | elective, open surgical | surgical | RCT | 47 | surgical | 23 | Balanced | 24 | Saline | ||||
| Lobo 31 | 2002 | post-surgery | surgery | RCT | 20 | colorectal surgery | 10 | restricted | 10 | liberal | ||||
| Conway32 | 2002 | major bowel surgery | surgical | RCT | 57 | surgical | 28 | GDFT | 39 | Standard | ||||
| Venn33 | 2002 | hip fracture surgery | surgical | RCT | 90 | surgical | 29CON- VF ; CVP guided FT- 3 1 ; Doppler-guided FT- 30 | |||||||
| SAFE34 | 2004 | ICU | ICU | RCT | 6997 | ICU | 3497 | Colloid | 3500 | Crystalloid | ||||
| Parker35 | 2004 | hip fracture surgery | surgical | RCT | 396 | surgical | 198 | Colloid | 198 | Crystalloid | ||||
| Noblett36 | 2005 | elective colorectal resection | surgical | RCT | 108 | surgical | 54 | GDFT | 54 | Standard | ||||
| Wakeling37 | 2005 | large bowel surgery | surgical | RCT | 128 | surgical | 64 | GDFT | 64 | Standard | ||||
| Mackay38 | 2006 | elective colorectal surgery | surgical | RCT | 80 | surgical | 41 | Restricted | 39 | Standard | ||||
| En-quiang38 | 2009 | critically ill | S-ICU | RCT | 76 | severe acute pancreatitis | 30 | controlled fluid expansion | 30 | rapid fluid expansion | ||||
| Senagore40 | 2009 | laparoscopic colectomy |
surgical | RCT | 64 | surgical | 21 GDFT/LR; 21 GDFT/HS;2 22 standard | |||||||
| Futier41 | 2010 | major abdominal surgery | surgery | RCT | 70 | postsurgical | 36 | Restricted-GDFT | 34 | Conservative GDFT | ||||
| Benes42 | 2010 | elective intraabdominal surgery |
surgery | RCT | 120 | ICU surgical | 60 | GDFT | 60 | Standard | ||||
| Pillai43 | 2011 | post-surgery | surgery | RCT | 66 | radical cystectomy | 34 | intervention | 32 | control | ||||
| Du44 | 2011 | critically ill | ICU | RCT | 41 | severe acute pancreatitis | 20 | starch | 21 | ringers' lactate | ||||
| James45 | 2011 | Blunt and penetrating trauma |
surgical | RCT | 109 | surgical | Penetrating trauma- HES 36 ; SAL 31 Blunt trauma- HES20 ; SAL 22 | |||||||
| Challand46 | 2012 | major elective colorectal surgery |
surgical | RCT | 179 | surgical | 90 | GDFT | 89 | Standard | ||||
| Myberg47 | 2012 | ICU | ICU | RCT | 7000 | ICU | 3500 | Colloid | 3500 | Crystalloid | ||||
| Srinivasa48 | 2012 | elective colectomy | surgical | RCT | 85 | surgical | 37 | GDFT Restricted | 37 | Restricted | ||||
| Zheng49 | 2013 | post-surgery | surgery | RCT | 60 | gastrointestinal surgery | 30 | GDFT | 30 | control | ||||
| Scheeren50 | 2013 | High-risk surgery | ICU | RCT | 52 | ICU | 26 | GDFT | 26 | Control | ||||
| Pestana51 | 2014 | post-surgery | S-ICU | RCT | 142 | abdominal surgery | 70 | GDFT | 72 | control | ||||
| Pearse52 | 2014 | Major Gastrointestinal Surgery |
surgery | RCT | 734 | surgical | 368 | GDFT | 366 | Standard | ||||
| Peng53 | 2014 | elective surgery | surgery | RCT | 80 | orthopaedic surgery | 40 | GDFT | 40 | standard | ||||
| Reisinger54 | 2017 | elective colorectal resection for malignancy |
surgery | RCT | 58 | postsurgical | 27 | GDFT | 31 | Standard | ||||
Abbreviations: : HES- hydroxyethyl starch ; HS- hetastach; SAL- saline; LR- lactate ringers; GDFT-goal-directed fluid therapy; ICU - intensive care unit ; S-ICU -surgical ICU; CON-IVF- conventional intravenous fluid therapy; CVP- central venous pressure; FT-fluid therapy RCT-randomised controlled trial; * Appendix C
In studies on enteral feeding [55, 56, 57, 58, 59, 60, 61, 62, 63, 64, 65, 66, 67, 68, 69, 70, 71, 72, 73, 74, 75, 76, 77, 78, 79, 80, 81, 82, 83, 84, 85, 86, 87, 88, 89, 90, 91, 92, 93, 94, 95, 96, 97, 98, 99, 100, 101, 102, 103, 104, 105, 106, 107, 108, 109, 110, 111], 57 randomised controlled trials’, included nearly 50% of the cohort as critically ill patients while the remaining were admitted as acute or elective surgical patients with variable admission diagnoses. The experimental group included enteral feeding delivered based on the route of feeding (enteral vs parenteral; nasogastric vs nasojejunal or jejunostomy), the timing of feeding (early vs delayed), the pattern of feeding (bolus vs continuous), or enteral feeding vs nil-by-mouth (NBM) with/without intravenous fluid. Twenty-four studies included critically ill, multiple trauma or sepsis patients with a Clavien-Dindo grading of IV. The remaining studies included post-operative and acutely ill patients with Clavien-Dindo grading of II and III (Tables 4-6).
Table 4.
Study Characteristics of studies on the impact of enteral feeding on gut dysfunction included in the systematic review
| Author | Year | Study Population | Study Setting | Study type | Study patients | Admission diagnosis | Experimental | Control | Dindo-Clavien Classification# | Quality Grading* | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| I | II | III | IV | ||||||||||
| Hoover55 | 1980 | surgical | surgical | RCT | 48 | surgical | 26 EF | 22IVF | Poor | ||||
| Adams56 | 1986 | ICU surgical | ICU | RCT | 46 | multiple trauma | 23 (EN) | 23(PN) | Poor | ||||
| Moore57 | 1986 | major abdo trauma | surgical | RCT | 59 | surgical | 29 (EN) | 30 (PN) | Poor | ||||
| Bower58 | 1986 | surgical | surgery | RCT | 20 | GI/pancreato-biliary surgery | 10 (EN-JeJ) | 10 (PN) | Poor | ||||
| Hamoui59 | 1989 | surgical | surgical | RCT | 19 | major GI surgery | 11EN | 8PN | Poor | ||||
| Von Meyenfeldt60 | 1992 | surgical | surgery | RCT | 101 | GI/colon cancer | 50 (EN) | 51(PN) | Poor | ||||
| Montecalvo61 | 1992 | surgical | surgical | RCT | 38 | surgical | 19 NG | 19 NJ | Poor | ||||
| Dunham62 | 1994 | critically ill | ICU | RCT | 37 | trauma | 12 (EN)+ 15 (PN) + 10(EN+PN) | Poor | |||||
| Borzotta63 | 1994 | trauma | surgical trauma | RCT | 48 | trauma | 27 (EN) | 21(PN) | Poor | ||||
| Daly64 | 1995 | surgical | surgical | RCT | 60 | surgical | 18ENSD; 12SD-IP; 19 ENSD-IP-OP; 11 EN-IP | Poor | |||||
| Carr65 | 1996 | post-surgical | surgery | RCT | 28 | intestinal resection | 14(EEN) | 14(CEN) | Poor | ||||
| Beier-Holgersen66 | 1996 | post-surgical | surgery | RCT | 60 | major abdominal surgery | 30(EEN) | 30 (placebo) | Poor | ||||
| Baigrie67 | 1996 | post-surgical | surgery | RCT | 97 | oesophagectomy/gastrectomy | 50 (EN) | 47(PN) | Poor | ||||
| VanBerge68 | 1997 | post-surgical | surgery | RCT | 57 | pancreatoduodenectomy | 30 (CON) | 27(CYC) | Poor | ||||
| Kalfarentzos69 | 1997 | critically ill | ICU | RCT | 38 | Severe acute pancreatitis | 18(EN) | 20 (PN) | |||||
| Heslin70 | 1997 | surgical | surgery | RCT | 195 | upper GI malignancy | 97 (EN) | 98(IVF) | Poor | ||||
| Reynolds71 | 1997 | major upper GI surgery | surgical | RCT | 67 | surgical | 33 (EN) | 34(PN) | Poor | ||||
| Stewart72 | 1998 | elective surgical | surgery | RCT | 80 | colorectal resections | 40 (EOF) | 40 (COF) | Poor | ||||
| Windsor73 | 1998 | surgical | surgical | RCT | 34 | acute pancreatitis | 16 EN | 18PN | Poor | ||||
| Singh74 | 1998 | surgical | surgical | RCT | 43 | surgical | 22JEJ | 21IVF | Poor | ||||
| Braga75 | 1998 | surgical | surgical | RCT | 166 | surgical | 55 STD-EN; 55 -STD-EN enriched; 56 TPN | Poor | |||||
| Taylor76 | 1999 | critically ill | ICU | RCT | 82 | head injury | 41TRO | 41 EN | Fair | ||||
| Pupelis77 | 2000 | critically ill | S-ICU | RCT | 60 | severe pancreatitis/peritonitis | 30 (JEN) | 30 (Control) | Poor | ||||
| Minard78 | 2000 | critically ill | ICU | RCT | 27 | head injury/trauma | 12(EEN) | 15(DEN) | Poor | ||||
| Powell79 | 2000 | critically ill | ICU | RCT | 27 | severe acute pancreatitis | 13 (EN) | 14(NBM) | Poor | ||||
| Kearns80 | 2000 | critically ill | ICU | RCT | 44 | critically ill | 23 G | 21 SI | Poor | ||||
| Bozzetti81 | 2001 | elective surgery | surgery | RCT | 317 | GIcancer | 159(EN) | 158(PN) | Poor | ||||
| Braga82 | 2001 | surgical | surgery | RCT | 257 | GIcancer | 126(EEN) | 131(PN) | Poor | ||||
| Montejo83 | 2002 | critically ill | ICU | RCT | 101 | critically ill | 50 (JEN) | 51(GEN) | Poor | ||||
| Davies84 | 2002 | critically ill | ICU | RCT | 73 | critically ill | 34 (NJ) | 39 (NG) | Poor | ||||
| Bertolini85 | 2003 | critically ill | ICU | RCT | 39 | Sepsis | 18(EN) | 17 (PN) | Poor | ||||
| Kompan86 | 2004 | critically ill | ICU | RCT | 52 | multiple trauma | 27(EEN) | 21(DEN) | Poor | ||||
| Malhotra87 | 2004 | post-surgical | surgery | RCT | 164 | perforated gut and peritonitis | 83 (EN) | 81(NBM) | Poor | ||||
| Kumar88 | 2006 | Surgical | surgical | RCT | 31 | surgical | 15 NG | 16 NJ | Poor | ||||
| Nguyen89 | 2007 | critically ill | ICU | RCT | 31 | critically ill | 23 (NJ) | 28 (NJ) | Poor | ||||
| Han-Guerts90 | 2007 | post-surgical | surgery | RCT | 150 | oesophagectomy | 71 (ND) | 79 (JEJ) | Poor | ||||
| Descahy91 | 2008 | critically ill | ICU | RCT | 100 | ICU | 50EEN | 50CEN | Poor | ||||
| Tien92 | 2009 | critically ill | ICU | RCT | 200 | ICU | 98TRO | 102 EN | Poor | ||||
| Barlow93 | 2011 | Surgical | surgery | RCT | 121 | upper GI malignancy | 64 (EN) | 57(NBM+IVF) | Poor | ||||
| Altintas94 | 2011 | critically ill | ICU | RCT | 71 | ICU | 30 (EN) | 41 (PN) | Poor | ||||
| Rice95 | 2011 | Surgical | surgical | RCT | 247 | surgical | EN 123 | 124 IVF | Poor | ||||
| Davies96 | 2013 | critically ill | ICU | RCT | 181 | ICU | 91 NJ | 89 NG | Poor | ||||
| Zhu97 | 2013 | post-surgical | surgery | RCT | 68 | pancreaticoduodenectomy | 34(JT) | 34(NJT) | Poor | ||||
| Sun98 | 2013 | critically ill | S-ICU | RCT | 60 | severe acute pancreatitis | 30(EEN) | 30(DEN) | Poor | ||||
| Kadamani99 | 2014 | critically ill | ICU | RCT# | 15 | critically ill | 15 (CON) | 15 (BOL) | Poor | ||||
| Boelens100 | 2014 | elective surgical | surgery | RCT | 123 | rectal surgery | 61(EEN) | 62(EPN) | Poor | ||||
| Harvey101 | 2014 | critically ill | ICU | RCT | 2388 | critically ill | 1197(EN) | 1191(PN) | Poor | ||||
| Ma102 | 2015 | acute surgical | surgery | RCT | 35 | acute pancreatitis | 17 (NTF) | 18(NPO) | Poor | ||||
| Bing Li103 | 2015 | post-surgical | surgery | RCT | 400 | gastrectomy | 200(EEN) | 200 (PN) | Poor | ||||
| Taylor104 | 2016 | critically ill | ICU | RCT | 50 | critically ill | 25 (NJ) | 25(NG + ProK) | Poor | ||||
| Ozen105 | 2016 | critically ill | ICU | RCT | 51 | critically ill | 26(no-GRV's) | 25(GRV's) | Poor | ||||
| Van Barneveld106 | 2016 | elective surgical | surgery | RCT | 123 | rectal ca malignancy | 61 (EEN) | 62(EPN) | Good | ||||
| Malik107 | 2016 | critically ill | ICU | RCT | 60 | critically ill | 30 (EF) | 30 (placebo) | Poor | ||||
| Fan108 | 2016 | critically ill | ICU | RCT | 80 | Severe TBI | 40 (EN) | 40 (PN) | Poor | ||||
| Stimac109 | 2016 | acute pancreatitis | pancreatitis | RCT | 214 | acute pancreatitis | 107 EN | 107 IVF | Poor | ||||
| Hongyin110 | 2017 | acute surgical | surgery | RCT | 161 | acute pancreatitis | 83 (APD)/61 EN) | 78(non-APD)/68(EN) | Poor | ||||
| Reigner111 | 2018 | critically ill | ICU | RCT | 2410 | shock | 1202(EN) | 1208(PN) | Fair | ||||
Abbreviations : EEN— early enteral feeding; CEN— conventional enteral feeding; EN— enteral nutrition; PN parenteral nutrition; CON— continuous enteral feeding; CYC — cyclic enteral feeding; EOF — early oral feeding ; COF — conventional oral feeding ;JEN—jejunal enteral nutrition; DEN— delayed enteral nutrition ; NBM — nil by mouth; GEN — gastric enteral nutrition ; NJ — nasojejunal; NG nasogastric; ND — nasoduodenal; JEJ- jejunostomy ; JT —jejeunostomy tube ; NJT — nasojejunal tube; BOL —bolus ; EPN — early parenteral nutrition; NTF — nasogastric tube feeding; NPO —nil per oral; ProK—prokinetics; GRV— gastric residual volumes; APD — abdominalparacentesis drainage ;ICU — intensive care unit ; S-ICU — surgical ICU; RCT — randomised controlled trial, # - pseudo-RCT; GI— gastrointestinal; TBI — traumatic brain injury; IVF — intravenous fluids; TRO- trophic feeding; ENSD — enteral nutrition with supplemented diet; IP inpatient; OP — outpatient; STD — standard; # D-C classification Appendix C; * Thresholds for Converting the Cochrane Risk of Bias Tool.
Table 6.
Impact of enteral feeding on variables of gut dysfunction as classified by feeding categories
| Symptoms of GDF§ | Intervention Enteral | Control Parenteral | Odds [95% Ratio CI]* | P Trend | I2 [%]# |
|---|---|---|---|---|---|
| A. Route of feeding | |||||
| Vomiting | 605/2388 | 350/2598 | 2.02 (1.74, 2.35) | <0.01 | 0 |
| Diarrhoea | 190/1508 | 421/1515 | 1.75 (0.39, 7.86) | 0.46 | 92 |
| Abdominal distension | 123/1386 | 90/1390 | 1.51 (0.93, 2.45) | 0.10 | 28 |
| Ileus | 52/347 | 65/347 | 0.97 (0.34, 2.76) | 0.96 | 58 |
| Anastomotic leak | 28/540 | 54/545 | 0.54 (0.31, 0.95) | 0.03 | 14 |
| Intestinal ischaemia | 33/2493 | 16/2495 | 1.87 (0.72, 4.87) | 0.20 | 42 |
| Peritonitis | 5/265 | 18/268 | 0.31 (0.11, 0.87) | 0.03 | 0 |
| B. Timing of feeding | Early | Delayed | |||
| Vomiting | 3/56 | 19/54 | 0.11 (0.03, 0.41) | <0.01 | 0 |
| Diarrhoea | 27/39 | 23/40 | 2.45 (0.26, 22.75) | 0.43 | 69 |
| Abdominal Distension | 12/66 | 21/69 | 0.51 (0.22, 1.91) | 0.12 | 0 |
| C. Enteral feeding vs Nil-by-mouth (NBM) | Enteral | NBM | |||
| Vomiting | 21/220 | 22/219 | 0.72 (0.18, 2.90) | 0.65 | 0 |
| Abdominal Distension | 66/242 | 48/240 | 1.40 (0.75, 2.64) | 0.29 | 33 |
| GI bleed | 2/133 | 2/133 | 0.99 (0.17, 5.86) | 0.99 | 0 |
| Anastomotic leak | 12/244 | 24/236 | 0.46 (0.22, 0.95) | 0.04 | 0 |
*CI - Confidence interval used; Significant P values (<0.05) are shown in bold; #I2 - heterogeneity between studies expressed as percentages; § GDF - gut dysfunction
Quality assessment
The quality of studies was graded based on the Cochrane Quality assessment tool for randomised controlled trials for intravenous fluid (Tables 1-3) and enteral feeding (Table 4) studies (Appendix C and D). All studies met the criteria for randomisation and allocation concealment, but a wide variability existed between studies for other domains (blinding of participants and personnel, blinding of outcome assessment and assessor, incomplete outcome data and selective reporting). In the intravenous fluid group, quality assessment for 7 studies [9, 10, 11, 12, 13, 14, 15] (15%) scored ‘good’ (Table 1), 11 studies [16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26] (22%) scored ‘fair’ (Table 2), and more than half (63%) of the studies [27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54] were ‘poor’ (Table 3). In the enteral feeding group, the majority (95%) of the studies [55, 56, 57, 58, 59, 60, 61, 62, 63, 64, 65, 66, 67, 68, 69, 70, 71, 72, 73, 74, 75,77, 78, 79, 80, 81, 82, 83, 84, 85, 86, 87, 88, 89, 90, 91, 92, 93, 94, 95, 96, 97, 98, 99, 100, 101, 102, 103, 104, 105,107, 108, 109, 110] scored ‘poor’; two studies scored ‘fair’ [76, 111] and 1 study [106] was of ‘good’ quality (Table 4).
Table 2.
Study Characteristics of 'fair ' quality studies on the impact of intravenous fluid therapy on gut dysfunction.
| Author | Year | Study Population | Study Setting |
Study type |
Study patients |
Admission diagnosis |
Experimental | Intravenous fluid |
Control | Intravenous fluid |
Dindo-Clavien Classification* |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Gan16 | 2002 | major elective general, urologic, or gynaecologic surgery | surgery | RCT | 100 | postsurgical | 50 | GDFT | 50 | Standard | |
| Moretti1 7 | 2003 | Major elective cardiac surgery | surgery | RCT | 90 | postsurgical | 30 - HetaStarch normal saline; 30 Heta Starch Balanced salt; 30 Lactated Ringers |
||||
| Nisanevich18 | 2005 | elective intraabdominal surgery | surgery | RCT | 157 | postsurgical | 77 | Restrictive | 75 | Liberal | |
| Kabon19 | 2005 | open colonic resection | surgery | RCT | 253 | ICU surgical | 124 | Small volume | 129 | Large Volume | |
| Lopes20 | 2007 | High-risk surgery | surgery | RCT | 33 | ICU surgical | 17 | GDFT | 16 | Control | |
| Vermuelen21 | 2009 | elective major abdominal surgical procedures | surgical | RCT | 62 | surgical | 30 | Restricted | 32 | Standard | |
| Mayer22 | 2010 | major abdominal surgery | surgery | RCT | 60 | ICU surgical | 30 | GDFT | 30 | Standard | |
| SAFE 23 | 2011 | ICU | ICU | RCT | 1218 | ICU | 603 | Colloid | 615 | Crystalloid | |
| Guidet24 | 2012 | severe sepsis | ICU | RCT | 196 | ICU | 100 | Colloid | 96 | Crystalloid | |
| Perner25 | 2012 | severe sepsis | ICU | RCT | 798 | ICU | 398 | Colloid | 400 | Crystalloid | |
| Reddy26 | 2016 | critically ill | ICU | RCT | 69 | critically ill | 35 | plasmalyte | 34 | saline | |
Abbreviations: GDFT-goal-directed fluid therapy; ICU -intensive care unit; S-ICU -surgical ICU; RCT-randomised controlled trial; * Appendix C
Quantitative data analysis
Impact of intravenous fluid therapy on GDF
Twenty-two randomised controlled trials [9,10,13, 14, 15, 16,18, 19, 20, 21, 22,26,28,31,41, 42, 43,49,51, 52, 53, 54] evaluated mild to moderate (nausea, vomiting and ileus) and moderate to severe (GI bleed, anastomotic leak, perforation and intestinal obstruction) GDF in 7368 patients, of which, 3682 (50%) were randomised to the intervention group (goal-directed/ restricted/ balanced intravenous fluids) and the remaining to the control group (liberal/ standard intravenous fluid). In the intervention group, no significant difference was observed for nausea, ileus, GI bleed, anastomotic leak, perforation or intestinal obstruction, in the intervention group in comparison to the control group. However, restricted/goal-directed fluid therapy in the form of colloids (starch/albumin) or a balanced fluid solution (plasmalyte /ringers lactate) was likely to reduce ‘mild’ GDF such as vomiting (p = 0.03) in critically ill and major surgical patients compared to a standard/liberal intravenous fluid regime (Table 5). Heterogeneity between studies ranged from 0 - 45 %.
Table 5.
Impact of intravenous fluid therapy on variables of gut dysfunction
| Symptoms of GDF § | Interventional | Control | Odds Ratio [95% CI]* | P Trend | I2 (%)# |
|---|---|---|---|---|---|
| Nausea | 88/ 274 | 90/278 | 0.98 (0.67, 1.44) | 0.92 | 0 |
| Vomiting | 62/462 | 94/447 | 0.51 (0.28, 0.94) | 0.03 | 45 |
| Ileus | 66/832 | 80/828 | 0.83 (0.52, 1.32) | 0.42 | 23 |
| GI bleed | 15/592 | 10/587 | 1.48 (0.66, 3.35) | 0.34 | 0 |
| Anastomotic leak | 44/833 | 43/867 | 1.03 (0.54, 1.96) | 0.93 | 31 |
| Perforation | 7/238 | 6/234 | 1.05 (0.36, 3.09) | 0.92 | 0 |
| Intestinal obstruction | 5/451 | 11/445 | 0.53 (0.20, 1.45) | 0.22 | 0 |
a: restricted, goal-directed, low-infusions or a controlled-expansion fluid therapy given as crystalloid fluid (normal saline or plasmalyte) or colloid fluid (hydroxyethyl starch)
b: standard, liberal, conventional, high-infusions or rapid-expansion fluid regimes given as crystalloid fluids (ringers lactate, plasmalyte and saline).
*CI - Confidence interval used; Significant P values (<0.05) are shown in bold; #I2 - heterogeneity between studies expressed as percentages; § GDF - gut dysfunction
Impact of enteral feeding on GDF
Twenty-one randomised controlled trials’ [60,63,67, 70,71, 72, 73,75,78,81,85, 86, 87,91,93,94,100,101,106, 108,111] enrolled 18,543 patients of which, 50% (n = 9260) patients were randomised to the enteral nutrition groups. The remaining half (n = 9283) were randomised to the parenteral nutrition group, delayed enteral feeding or nil-by-mouth group. Mild to moderate GDF (vomiting, diarrhoea, abdominal distention and ileus) and moderate to severe (GI bleed, anastomotic leak, intestinal ischaemia, peritonitis) are presented in sub-groups (route of feeding – enteral vs. parenteral; the timing of feeding – early vs. delayed and feeding vs no feeding – enteral vs. nil-by-mouth) demonstrated in Table 6.
(i). Route of feeding (enteral vs parenteral)
In the EN group, a significant increase in vomiting episodes was observed compared to in parenteral nutrition group (p < 0.01). The EN group showed a trend in fewer events for anastomotic leaks (p = 0.03) and peritonitis (p = 0.03) compared to the parenteral nutrition group. Other variables of GDF, including diarrhoea, abdominal distension and intestinal ischemia, presented with no significant differences between the two groups (Table 6). Heterogeneity between studies ranged from 0 – 92 %.
(ii). Timing of feeding (early vs delayed)
Four randomised controlled trials’ enrolled 324 patients, of which 50% of patients were allocated to the early enteral nutrition group and the other half to the delayed/conventional enteral nutrition group. A significant decrease in the vomiting episodes was observed in the early enteral nutrition group compared to delayed/conventional enteral nutrition group (p < 0.01). No differences were observed between groups for diarrhoea and abdominal distension. Heterogeneity between studies ranged from 0 – 69 %.
(iii). Enteral feeding vs nil-by-mouth (NBM)
Six randomised controlled trials’ enrolled 1667 patients, of which 50% was randomised to the intervention group. There was a tendency of reduced anastomotic leaks in patients receiving enteral feeding (p = 0.04) compared to patients on a nil-by-mouth regimen. However, no differences were observed for events on vomiting, abdominal distension and GI bleed. (Table 6). Heterogeneity between studies ranged from 0 – 33 %.
Discussion
The results of the meta-analysis demonstrate that restricted/goal-directed fluid therapy regardless of the type of fluid reduces mild GDF (vomiting) but not other complications associated with GDF. Enteral feeding, on the other hand, significantly increased vomiting episodes compared to parenteral nutrition but ‘early’ enteral nutrition significantly reduced the incidence of vomiting compared to delayed feeding. Enteral feeding was likely to reduce severe gut complications such as anastomotic leak and peritonitis compared with parenteral nutrition or an NBM status. Other mild to moderate variables of GDF (i.e. nausea, abdominal distension, ileus or diarrhoea) and moderate to severe complications (i.e. GI bleed, perforation, intestinal obstruction or intestinal ischaemia) were not associated with significant changes in outcomes. The results suggest that although the beneficial effects of restricted/goal-directed intravenous fluids and enteral feeding are essential to reduce some form of GDF, the impact is not prevalent for other variables of GDF (e.g. ileus and intestinal ischemia) associated with poor clinical outcomes. This may reflect the paucity of high-quality literature on the interaction between intravenous fluid (resuscitation) and enteral feeding as a combined therapy on the impact of GDF. The role of these two modalities in combination should be regarded as an important aspect in identifying the impact on the severity of GDF in acute surgical and critically ill patients.
Intravenous fluid therapy is frequently the first line of treatment in acute surgical and critically ill patients but hypervolemia and hypovolemia, both, are deemed detrimental. A revival of interest emerged almost two decades ago when hypovolemia in the form of restrictive fluid therapy was associated with improved post-operative clinical outcomes [9,16,18,31]. These studies suggested that a preferred approach of ‘zero’ or ‘neutral’ fluid balance not only improves outcomes related to gut motility but also may prevent adverse long-term outcomes. The current study demonstrated that mild GDF, i.e. vomiting, was significantly lower in patients on a restrictive/ targeted intravenous fluid regime. Studies have also reported similar results when colloids have been administered postoperatively [14,17]. The benefit of this outcome may be explained by cumulative administration of smaller volumes (of colloids) compared to crystalloids. Hypervolemia from excessive or liberal fluid administration, particularly crystalloids is associated with poor outcomes in postoperative [9,18] and in critically ill patients [25,47]. It can precipitate intestinal oedema leading to an ileus, delayed gastric emptying, feeding intolerance and hence sub-optimal nutrition delivery. Another school of thought indicates that complex surgical patients with high-risk surgeries possibly require judicious amounts of fluids to avoid complications associated with circulatory failure and gut mucosal ischemia [41,54,116,119]. This may be particularly relevant when liberal intravenous fluids are necessary to resuscitate patients after massive haemorrhagic losses for haemodynamic stability. In recent decades, goal-directed fluids have been advocated to prevent tissue hypovolemia [20] but maintain euvolemia by using targeted fluid approach raising the possibility of improved clinical outcomes in high-risk patients [22, 42, 84,121]. Hence, it is expected that a modest amount of fluids might be necessary to prevent anastomotic hypoperfusion, gut mucosal ischemia and reduce postoperative complications. Although the benefit of goal-directed fluid therapy is projected at improving organ perfusion without the onset of tissue oedema [54,117,122]; a paucity of studies exists warranting more research in this area [15, 41, 52, 118].
Enteral Nutrition forms an integral part of overall fluid administration in addition to intravenous fluids. Enteral nutrition and intravenous fluids combined play a crucial role in GDF outcomes, but due to a paucity of studies, this area has not received due attention. Enteral nutrition is invariably the first choice of nutrition compared to parenteral nutrition over decades [112]. The current study demonstrated that mild GDF, i.e. vomiting significantly increased in patients receiving enteral nutrition but reduced significantly when enteral feeding was commenced earlier. This is possible because ‘early’ enteral nutrition has multiple advantages over parenteral nutrition [75,82,114,121], and these benefits are evident in high-risk surgical and critically ill patients [70,124]. The initiation of enteral feeding is known to stimulate gut motility which reduces the incidence of GDF symptoms such as nausea and vomiting postoperatively. However, a significant difference for ileus between groups was not observed, although the number of events were lower in the enterally fed group. In cases of gut failure, when enteral feeding is contraindicated, parenteral nutrition becomes the sole choice of feeding and may be commenced within 24 hrs of ICU admission or post-surgery [120]. Administering parenteral nutrition appears to be a logical clinical decision, especially if enteral feeding raises the suspicion of non-occlusive mesenteric ischemia in the critically ill, with haemodynamic compromise. Our review showed no differences for intestinal 'ischaemia' between groups, although the events were half in the control group compared to the intervention (enteral nutrition) arm. Considering that the current review included a heterogeneous mix of patients, it is evident that in a sub-set of patients, i.e. post-cardiac surgery, severe acute pancreatitis or septic shock, administration of early enteral nutrition may potentially pose more risk than benefit by increasing the risk of bowel ischemia.
Nevertheless, the use of trophic enteral feeding has been suggested in haemodynamically unstable patients to maintain gut integrity [4]. Authors have argued that enteral nutrition comes with its risks such as aspiration, pneumonia, intestinal obstruction, necrosis and pneumonitis intestinalis. However, the present study demonstrated no such differences for any of these complications. For gastrointestinal complications, a significant reduction in anastomotic leaks in the enteral nutrition group suggesting its benefits irrespective of the feeding route was observed. It is common practice in some areas, particularly intensive care, to commence patients on parenteral nutrition with anastomotic leaks before a trial of enteral nutrition. However, it should be acknowledged that a correct assessment for an enteral nutrition challenge can be countered in patients on parenteral nutrition with significant complications (e.g. anastomotic leaks), hence lowering the threshold of initiating enteral nutrition. Barlow et al. [93] found a lower incidence (2 vs 7) of anastomotic leaks in the early enteral nutrition group. They attributed a three-day shorter length of stay and reduced postoperative complications from installing early enteral nutrition. A similar effect was confirmed by a Cochrane review [115] in which enteral nutrition reduced the risk of anastomotic leaks from 27% in the standard group to 13% in early enteral group. These results affirmed with the present findings. It is hypothesised that enteral nutrition may improve perfusion at the anastomosis site, which promotes mucosal wound healing and prevents further leaks.
In comparison, Lewis et al. (2009) did not support this finding and observed mortality of 50% in the intervention group (enteral group) with anastomotic leaks [114]. However, it is likely that a smaller sample size may result in a false positive rate for mortality, thus exaggerating the magnitude of the negative result. The benefit of enteral feeding in complications such as perforation and peritonitis has been confirmed by several reports, which resonated with our findings. Early enteral feeding seems to maintain gut integrity by improving mucosal circulation and oxygen delivery that may reduce the risk of peritonitis [74, 87,113].
The present study is not without limitations:
The severity score in majority of the studies including surgical patients was low (ranging between I to III) hence the overall effect may be confounded by the clinical severity of the cohort. The majority of studies were conducted in stable postoperative patients and results may not be generalisable to a high-risk group, e.g. septic shock.
Critically ill patients are a heterogeneous group, and the effect on gut function can differ with specific sub-population. Such high-risk heterogenous patients need to be assessed in robust, well-designed, and randomised controlled trials. A possible stumbling block may be the ethical dilemma of implementing clinical trials using regimented interventions in these patients is often challenging for institutions and ethics committees.
Individualised unit protocols were variable with prescription of fluid and enteral feeding regimes possibly confounding the overall impact on GDF outcomes.
Most studies included small numbers of patients and were single-centred studies.
Postoperative morbidity manifested as GDF may be associated with the type of surgical procedure or manipulation of the bowel during surgery which may be associated with inducing a surgical stress response. However, this is expected to be low in our study, considering that the majority of the cohort included stable postoperative patients.
The majority of our studies found no differences between long-term endpoints (mortality and length of stay) but the occurrence of GDF was excluded from primary endpoints.
Most importantly, it was difficult to define or classify gut dysfunction because, until now, there is no valid, objective or a reliable scoring system to assess gut function in intensive care patients [125]. This suggests the need to develop a novel scoring tool to address this concern in future trials. Due to fewer studies on the effect of intravenous fluids and enteral nutrition on GDF, our meta-analyses may have been underpowered to see significant outcomes on GDF. Overall, studies on intravenous fluid remain mostly inconclusive, and potentially the impact of intravenous fluids may project variable outcomes when applied to a homogenous cohort instead of heterogeneous patient groups.
Further, inconclusive results from large-scale fluid and enteral feeding trials raise the suspicion that GDF may be the missing link, which perhaps may be associated with long-term outcomes. This dimension is often ignored when evaluating endpoints. To observe a difference in the key outcome, we first need to understand the combined effects of intravenous fluids and enteral nutrition in influencing clinical outcomes, including GDF. It is expected that as a result of the potential interaction between these two modalities, patients receiving liberal fluid resuscitation and early aggressive feeding are more likely to be at risk of severe GDF. More work is required to understand the implications of intravenous fluids and enteral nutrition on GDF and how this may impact overall patient outcomes. Future studies should evaluate this potential interaction and assess the combined impact of these two modalities on GDF in surgical and critically ill patients.
Conclusion
A restricted/goal-directed fluid regime and early enteral feeding compared to parenteral or a nil-by-mouth regime may reduce the risk on mild GDF in some, but not all complications of severe GDF. Because of a preventive strategy, we need to first understand the interaction between both (intravenous fluids and enteral feeding) and their impact on the gut so its implications can be translated into clinical practice eventually. Hence, it can be hypothesised that conservative fluid resuscitation and aggressive enteral feeding may potentially be the fundamental cause of developing severe life-threatening GDF (i.e. intestinal ischemia) and complications that can delay recovery and affect clinical outcomes in acute surgical and critically ill patients. Future research should evaluate and focus on an extended conceptual framework on the cross-interaction of conservative and aggressive modes across these two interventions and its impact on various levels of severity of GDF.
Acknowledgements
Varsha M. Asrani is currently a PhD candidate with the University of Auckland and holds a New Zealand Health Research Council Fellowship Award.
Appendix A.
Search Strategy
| # Searches Results |
|---|
| 1 Gastrointestinal Diseases/ |
| 2 ((gastrointestinal or intestin* or digestive) adj3 (dysfunction* or failure or disorder* or injur* or disease*)).mp. |
| 3 ((abdominal or gut or bowel or intestin*) adj3 (perforat* or infarct* or obstruct* or failure or ischemi*)).mp. |
| 4 gastroparesis.mp. or Gastroparesis/ |
| 5 gastrointestinal motilit*.mp. or exp Gastrointestinal Motility/ |
| 6 (dysmotilit* or intestinal motilit*).mp. 5645 |
| 7 Intra-Abdominal Hypertension/ |
| 8 (abdominal compartment syndrome* or intra abdominal hypertension or intraabdominal hypertension).mp. |
| 9 feed* intolerance.mp. |
| 10 ileus.mp. or Ileus/ |
| 11 Intestinal Obstruction/ or Intestinal Pseudo-Obstruction/ or pseudo obstruction.mp. or ogilvie’s syndrome.mp. |
| 12 (mesenteric or peritonitis).mp. 91360 |
| 13 or/1-12 282880 |
| 14 enteral nutrition/ or parenteral nutrition/ |
| 15 Parenteral Nutrition, Total/ |
| 16 ((enteral or parenteral) adj3 (feed* or nutrition)).mp. |
| 17 Fluid Therapy/ or intravenous fluid*.mp. |
| 18 (fluid* adj3 therap*).mp. |
| 19 (resuscitation adj3 fluid*).mp. |
| 20 vasoactive.mp. |
| 21 Vasoconstrictor Agents/ or vasoconstrictor*.mp. or vasopressor*.mp. |
| 22 inotrope*.mp. |
| 23 or/14-22 |
| 24 intensive care/ or critical illness/ |
| 25 Intensive Care Units/ |
| 26 General Surgery/ |
| 27 Postoperative Complications/ or Postoperative Care/ |
| 28 (intensive care or ICU or critical care or critical* ill*).mp. |
| 29 (surgery or surgical or postoperative).mp. |
| 30 or/24-29 |
| 31 randomized controlled trial.pt. |
| 32 controlled clinical trial.pt. |
| 33 randomized.ab. |
| 34 placebo.ab. 35 drug therapy.fs. |
| 36 randomly.ab. |
| 37 trial.ab. 38 groups.ab. |
| 39 or/31-38 |
| 40 adult/ or aged/ or “aged, 80 and over”/ or frail elderly/ or middle aged/ or (adult* or middle aged or older or old or aged or |
| elderly or geriatric* or frail).mp. |
| 41 13 and 23 and 30 and 39 and 40 |
| 42 exp animals/ not humans.sh |
| 43 41 not 42 |
Appendix B.
Dindo-Clavien Classification
| Grade | Grade Definition |
|---|---|
| Grade I | Any deviation from the normal postoperative course without the need for pharmacological treatment or surgical, endoscopic, and radiological interventions Allowed therapeutic regimens are: drugs as antiemetics, antipyretics, analgesics, diuretics, electrolytes, and physiotherapy. This grade also includes wound infections opened at the bedside |
| Grade II | Requiring pharmacological treatment with drugs other than such allowed for grade I complications, Blood transfusions and total parenteral nutrition are also included |
| Grade III | Requiring surgical, endoscopic or radiological intervention Grade IIIa Intervention not under general anaesthesia Grade IIIb Intervention under general anaesthesia |
| Grade IV | Life-threatening complication (including CNS complications)* requiring IC/ICU management Grade IVa Single organ dysfunction (including dialysis) Grade IVb Multiorgan dysfunction) |
Dindo D, Demartines N, Clavien PA. Classification of Surgical Complications: A New Proposal with Evaluation in a Cohort of 6336 Patients and Results of a Survey. Annals of Surgery. 2004; 240 (2): 205-213.
Appendix C.
Quality assessment for studies on the effect of intravenous fluid therapy on gut dysfunction (Cochrane quality grading for randomised controlled trials)7
|
*Thresholds for Converting the Cochrane Risk of Bias Tool: Good quality: All criteria met (i.e. low for each domain); Fair quality: One criterion not met (i.e. high risk of bias for one domain) or two criteria unclear, and the assessment that this was unlikely to have biased the outcome, and there is no known important limitation that could invalidate the results Poor quality: One criterion not met (i.e. high risk of bias for one domain) or two criteria unclear, and the assessment that this was likely to have biased the outcome, and there are significant limitations that could invalidate the results OR Two or more criteria listed as high or unclear risk of bias
Appendix D.
Quality assessment for studies on the effect of enteral feeding on gut dysfunction (Cochrane quality grading for randomised controlled trials)7
|
*Thresholds for Converting the Cochrane Risk of Bias Tool: Good quality: All criteria met (i.e. low for each domain); Fair quality: One criterion not met (i.e. high risk of bias for one domain) or two criteria unclear, and the assessment that this was unlikely to have biased the outcome, and there is no known significant limitation that could invalidate the results; Poor quality: One criterion not met (i.e. high risk of bias; for one domain) or two criteria unclear, and the assessment that this was likely to have biased the outcome, and there are significant limitations that could invalidate the results OR Two or more criteria listed as high or unclear risk of bias
Footnotes
Author contributions
V Asrani and JA Windsor contributed to the conception and design of the research. V Asrani performed the literature search, extracted, analysed and interpreted data, and drafted the manuscript. A Brown contributed to the literature search, data acquisition and analysis, and co-reviewed the data. JA Windsor and I Bissett critically revised the manuscript and supervised the project. All authors read and approved the final version of the manuscript and agree to be fully accountable for ensuring the integrity and accuracy of the manuscript.
Conflict of Interest
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest concerning the research, authorship, or publication of this article.
References
- 1.Klingensmith NJ, Coopersmith CM. The gut as the motor of multiple organ dysfunction in critical illness. Crit. Care Clin. 2016;32:203–12. doi: 10.1016/j.ccc.2015.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Perner A, Juntila E, Haney M. Scandinavian clinical practice guideline on choice of fluid in resuscitation of critically ill patients with acute circulatory failure. Acta Anaesthesiol Scand. 2015;59:274–85. doi: 10.1111/aas.12429. et al. [DOI] [PubMed] [Google Scholar]
- 3.Codner PA, Patel J, Rosenthal M. Fluid management, volume overload, and gastrointestinal tolerance in the perioperative period. Curr Surg Rep. 2016;4:12. et al. [Google Scholar]
- 4.Flordelís Lasierra J, Pérez-Vela J, Montejo González J. Enteral nutrition in the hemodynamically unstable critically ill patient. Med Intensiva. 2015;39(1):40–48. doi: 10.1016/j.medin.2014.04.002. [DOI] [PubMed] [Google Scholar]
- 5.Moher D, Liberati A, Tetzlaf J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151:264–9. doi: 10.7326/0003-4819-151-4-200908180-00135. W64. [DOI] [PubMed] [Google Scholar]
- 6.Dindo D, Demartines N, Clavien PA. Classification of surgical complications : A new proposal with evaluation in a cohort of 6336 patients and results of a survey. Annals of Surgery. 2004;240(2):205–213. doi: 10.1097/01.sla.0000133083.54934.ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Higgins JP, Altman DG, Gotzsche PC. The Cochrane collaboration’s tool for assessing risk of bias in randomised trials Br Med J. 2011;343:d5928. doi: 10.1136/bmj.d5928. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ryan R. Cochrane Consumers and Communication Review Group. Heterogeneity and subgroup analysis in Cochrane consumers and communication group reviews: planning the analysis at protocol stage. 2016. http://cccrg.cochrane.org.ezproxy.auckland.ac.nz
- 9.Brandstrup B, Tonnesen H, Beier-Holgersen R. Danish Study Group on Perioperative Fluid Therapy: Effects of intravenous fluid restriction on postoperative complications: Comparison of two perioperative fluid regimens: A randomized assessor-blinded multicenter trial. Ann Surg. 2003;238:641–8. doi: 10.1097/01.sla.0000094387.50865.23. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Holte K, Kristensen BB, Valentiner L, Foss NB, Husted H, Kehlet H. Liberal versus restrictive fluid management in knee arthroplasty: a randomized, double blind study: Anesthesia & Analgesia. 2007;105(2):465–474. doi: 10.1213/01.ane.0000263268.08222.19. [DOI] [PubMed] [Google Scholar]
- 11.Holte K, Foss NB, Andersen J. Liberal or restrictive fluid administration in fast-track colonic surgery: a randomized, double-blind study. British Journal of Anaesthesia. 2007;99(4):500–508. doi: 10.1093/bja/aem211. et al. [DOI] [PubMed] [Google Scholar]
- 12.González-Fajardo JA, Mengibar L, Brizuela JA, Castrodeza J, Vaquero-Puerta C. effect of postoperative restrictive fluid therapy in the recovery of patients with abdominal vascular surgery. European Journal of Vascular and Endovascular Surgery. 2009;37(5):538–543. doi: 10.1016/j.ejvs.2009.01.010. [DOI] [PubMed] [Google Scholar]
- 13.Yates DRA, Davies SJ, Milner HE, Wilson RJT. Crystalloid or colloid for goal-directed fluid therapy in colorectal surgery. British Journal of Anaesthesia. 2014;112(2):281–289. doi: 10.1093/bja/aet307. [DOI] [PubMed] [Google Scholar]
- 14.Ghodraty MR, Rokhtabnak F, Dehghan HR. Crystalloid versus colloid fluids for reduction of postoperative ileus after abdominal operation under combined general and epidural anesthesia. Surgery. 2017;162(5):1055–1062. doi: 10.1016/j.surg.2017.06.014. et al. [DOI] [PubMed] [Google Scholar]
- 15.Gómez-Izquierdo JC, Trainito A, Mirzakandov D. Goal-directed fluid therapy does not reduce primary postoperative ileus after elective laparoscopic colorectal surgery: A randomized controlled trial. Anesthesiology. 2017;127(1):36–49. doi: 10.1097/ALN.0000000000001663. et al. [DOI] [PubMed] [Google Scholar]
- 16.Gan TJ, Soppit A, Maroof M. Goal-directed intraoperative fluid administration reduces length of hospital stay after major surgery. Anesthesiology. 2002;97:820. doi: 10.1097/00000542-200210000-00012. et al. [DOI] [PubMed] [Google Scholar]
- 17.Moretti EW, Robertson KM, El-Moalem H, Gan TJ. Intraoperative colloid administration reduces postoperative nausea and vomiting and improves postoperative outcomes compared with crystalloid administration. Anesth Analg. 2003;96:611–7. doi: 10.1097/00000539-200302000-00056. [DOI] [PubMed] [Google Scholar]
- 18.Nisanevich V, Felsenstein I, Almogy G, Weissman C, Einav S, Matot I. Effect of intraoperative fluid management on outcome after intraabdominal surgery. Anesthesiology. 2005;103:25–32. doi: 10.1097/00000542-200507000-00008. [DOI] [PubMed] [Google Scholar]
- 19.Kabon B, Akça O, Taguchi A. Supplemental intravenous crystalloid administration does not reduce the risk of surgical wound infection: Anesthesia & Analgesia. 2005;101(5):1546–1553. doi: 10.1213/01.ANE.0000180217.57952.FE. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lopes MR, Oliveira MA, Pereira V, Lemos I, Auler J, Michard F. Goal-directed fluid management based on pulse pressure variation monitoring during high-risk surgery: a pilot randomized controlled trial. Critical Care. 2007;11(5):R100. doi: 10.1186/cc6117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vermeulen H, Hofland J, Legemate DA, Ubbink DT. Intravenous fluid restriction after major abdominal surgery: a randomized blinded clinical trial. Trials. 2009;10(1):50. doi: 10.1186/1745-6215-10-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mayer J, Boldt J, Mengistu AM, Röhm KD, Suttner S. Goal-directed intraoperative therapy based on autocalibrated arterial pressure waveform analysis reduces hospital stay in high-risk surgical patients: a randomized, controlled trial. Critical Care. 2010;14(1):R18. doi: 10.1186/cc8875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.The SAFE Study Investigators. Impact of albumin compared to saline on organ function and mortality of patients with severe sepsis. Intensive Care Medicine. 2011;37(1):86–96. doi: 10.1007/s00134-010-2039-6. [DOI] [PubMed] [Google Scholar]
- 24.Guidet B, Martinet O, Boulain T. Assessment of hemodynamic efficacy and safety of 6% hydroxyethylstarch 130/0.4 vs. 0.9% NaCl fluid replacement in patients with severe sepsis: The CRYSTMAS study. Critical Care. 2012;16(3):R94. doi: 10.1186/cc11358. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Perner A, Haase N, Gutormsen AB. Hydroxyethyl Starch 130/0.42 versus Ringer’s Acetate in Severe Sepsis. New England Journal of Medicine. 2012;367(2):124–134. doi: 10.1056/NEJMoa1204242. et al. [DOI] [PubMed] [Google Scholar]
- 26.Reddy S, Bailey M, Beasley R. Effect of saline 0.9% or Plasma-Lyte 148 therapy on feeding intolerance in patients receiving nasogastric enteral nutrition. Critical Care and Resuscitation. 2016;18(3):7. et al. [PubMed] [Google Scholar]
- 27.Prien T, Backhaus N, Pelster F, Pircher W, Büe H, Lawin P. Effect of intraoperative fluid administration and colloid osmotic pressure on the formation of intestinal edema during gastrointestinal surgery. Journal of Clinical Anesthesia. 1990;2(5):317–323. doi: 10.1016/0952-8180(90)90077-g. [DOI] [PubMed] [Google Scholar]
- 28.Salim AW. Duration of intravenous fluid replacement after abdominal surgery: a prospective randomised study. Annals of the Royal College of Surgeons of England. 1991;73:119–123. [PMC free article] [PubMed] [Google Scholar]
- 29.Yogendran S, Asokumar B, Cheng DC, Chung F. A prospective randomized double-blinded study of the effect of intravenous fluid therapy on adverse outcomes of outpatient surgery. Anaesth Analg. 1995;80:682–6. doi: 10.1097/00000539-199504000-00006. [DOI] [PubMed] [Google Scholar]
- 30.Wilkes NJ, Woolf R, Mutch M. The Effects of Balanced Versus Saline-Based Hetastarch and Crystalloid Solutions on Acid-Base and Electrolyte Status and Gastric Mucosal Perfusion in Elderly Surgical Patients: Anesthesia & Analgesia. 2001;93(4):811–816. doi: 10.1097/00000539-200110000-00003. et al. [DOI] [PubMed] [Google Scholar]
- 31.Lobo DN, Bostock KA, Neal KR, Perkins AC, Rowlands BJ, Allison SP. Effect of salt and water balance on recovery of gastrointestinal function after elective colonic resection: a randomised controlled trial. The Lancet. 2002;359(9320):1812–1818. doi: 10.1016/S0140-6736(02)08711-1. [DOI] [PubMed] [Google Scholar]
- 32.Conway DH, Mayall R, Abdul-Latif MS, Gilligan S, Tackaberry C. Randomised controlled trial investigating the influence of intravenous fluid titration using oesophageal Doppler monitoring during bowel surgery. Anaesthesia. 2002;57(9):845–849. doi: 10.1046/j.1365-2044.2002.02708.x. [DOI] [PubMed] [Google Scholar]
- 33.Venn R, Steele A, Richardson P, Poloniecki J, Grounds M, Newman P. Randomized controlled trial to investigate influence of the fluid challenge on duration of hospital stay and perioperative morbidity in patients with hip fractures. British Journal of Anaesthesia. 2002;88(1):65–71. doi: 10.1093/bja/88.1.65. [DOI] [PubMed] [Google Scholar]
- 34.The SAFE Study Investigators. A comparison of albumin and saline for fluid resuscitation in the intensive care unit. N Eng J Med. 2004;350:2247. doi: 10.1056/NEJMoa040232. –. [DOI] [PubMed] [Google Scholar]
- 35.Parker MJ, Grifiths R, Boyle A. Preoperative saline versus gelatin for hip fracture patients; a randomized trial of 396 patients. British Journal of Anaesthesia. 2004;92(1):67–70. doi: 10.1093/bja/aeh006. [DOI] [PubMed] [Google Scholar]
- 36.Noblet SE, Snowden CP, Shenton BK, Horgan AF. Randomized clinical trial assessing the effect of Doppler-optimized fluid management on outcome after elective colorectal resection. British Journal of Surgery. 2006;93(9):1069–1076. doi: 10.1002/bjs.5454. [DOI] [PubMed] [Google Scholar]
- 37.Wakeling HG, McFall MR, Jenkins CS. Intraoperative oesophageal Doppler guided fluid management shortens postoperative hospital stay after major bowel surgery. British Journal of Anaesthesia. 2005;95(5):634–642. doi: 10.1093/bja/aei223. et al. [DOI] [PubMed] [Google Scholar]
- 38.MacKay G, Fearon K, McConnachie A, Serpell MG, Molloy RG, O’Dwyer PJ. Randomized clinical trial of the effect of postoperative intravenous fluid restriction on recovery after elective colorectal surgery. British Journal of Surgery. 2006;93(12):1469–1474. doi: 10.1002/bjs.5593. [DOI] [PubMed] [Google Scholar]
- 39.En-qiang MA, Yao-qing T, Jian F, Shuai Q, Jun W, Lei L, Dong M, Sheng-dao Z. Fluid therapy for severe acute pancreatitis in acute response stage. Chin Med J. 2009;122(2):169–173. [PubMed] [Google Scholar]
- 40.Senagore AJ, Emery T, Luchtefeld M, Kim D, Dujovny N, Hoedema R. Fluid Management for Laparoscopic Colectomy: A Prospective. Randomized Assessment of Goal-Directed Administration of Balanced Salt Solution or Hetastarch Coupled with an Enhanced Recovery Program: Diseases of the Colon & Rectum. 2009;52(12):1935–1940. doi: 10.1007/DCR.0b013e3181b4c35e. [DOI] [PubMed] [Google Scholar]
- 41.Futier E. Conservative vs restrictive individualized goal-directed fluid replacement strategy in major abdominal surgery: A prospective randomized trial. Archives of Surgery. 2010;145(12):1193. doi: 10.1001/archsurg.2010.275. [DOI] [PubMed] [Google Scholar]
- 42.Benes J, Chytra I, Altmann P. Intraoperative fluid optimization using stroke volume variation in high risk surgical patients: results of prospective randomized study. Critical Care. 2010;14(3):R118. doi: 10.1186/cc9070. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pillai P, McEleavy I, Gaughan M. A double-blind randomized controlled clinical trial to assess the effect of doppler optimized intraoperative fluid management on outcome following radical cystectomy. Journal of Urology. 2011;186(6):2201–2206. doi: 10.1016/j.juro.2011.07.093. et al. [DOI] [PubMed] [Google Scholar]
- 44.Du X-J, Hu W-M, Xia Q. Hydroxyethyl starch resuscitation reduces the risk of intra-abdominal hypertension in severe acute pancreatitis: Pancreas. 2011;40(8):1220–1225. doi: 10.1097/MPA.0b013e3182217f17. et al. [DOI] [PubMed] [Google Scholar]
- 45.James MFM, Michell WL, Joubert IA, Nicol AJ, Navsaria PH, Gillespie RS. Resuscitation with hydroxyethyl starch improves renal function and lactate clearance in penetrating trauma in a randomized controlled study: the FIRST trial (Fluids in Resuscitation of Severe Trauma) British Journal of Anaesthesia. 2011;107(5):693–702. doi: 10.1093/bja/aer229. [DOI] [PubMed] [Google Scholar]
- 46.Challand C, Struthers R, Sneyd JR. Randomized controlled trial of intraoperative goal-directed fluid therapy in aerobically fit and unfit patients having major colorectal surgery. British Journal of Anaesthesia. 2012;108(1):53–62. doi: 10.1093/bja/aer273. et al. [DOI] [PubMed] [Google Scholar]
- 47.Myburgh JA, Finfer S, Bellomo R. Hydroxyethyl starch or saline for fluid resuscitation in intensive care. New England Journal of Medicine. 2012;367(20):1901–1911. doi: 10.1056/NEJMoa1209759. et al. [DOI] [PubMed] [Google Scholar]
- 48.Srinivasa S, Taylor MHG, Singh PP, Yu T-C, Soop M, Hill AG. Randomized clinical trial of goal-directed fluid therapy within an enhanced recovery protocol for elective colectomy: Goal-directed fluid therapy within an enhanced recovery protocol for elective colectomy. British Journal of Surgery. 2013;100(1):66–74. doi: 10.1002/bjs.8940. [DOI] [PubMed] [Google Scholar]
- 49.Zheng H, Guo H, Ye J, Chen L, Ma H. Goal-directed fluid therapy in gastrointestinal surgery in older coronary heart disease patients: randomized trial. World Journal of Surgery. 2013;37(12):2820–2829. doi: 10.1007/s00268-013-2203-6. [DOI] [PubMed] [Google Scholar]
- 50.Scheeren TWL, Wiesenack C, Gerlach H, Marx G. Goal-directed intraoperative fluid therapy guided by stroke volume and its variation in high-risk surgical patients: a prospective randomized multicentre study. Journal of Clinical Monitoring and Computing. 2013;27(3):225–233. doi: 10.1007/s10877-013-9461-6. [DOI] [PubMed] [Google Scholar]
- 51.Pestaña D, Espinosa E, Eden A. Perioperative goal-directed hemodynamic optimization using noninvasive cardiac output monitoring in major abdominal surgery: a prospective, randomized, multicenter, pragmatic trial. POEMAS Study (PeriOperative goal-directed thErapy in Major Abdominal Surgery) Anesthesia and Analgesia. 2014;119(3):579–587. doi: 10.1213/ANE.0000000000000295. et al. [DOI] [PubMed] [Google Scholar]
- 52.Pearse RM, Harrison DA, MacDonald N. Effect of a perioperative, cardiac output–guided hemodynamic therapy algorithm on outcomes following major gastrointestinal surgery: a randomized clinical trial and systematic review. JAMA. 2014;311(21):2181. doi: 10.1001/jama.2014.5305. et al. [DOI] [PubMed] [Google Scholar]
- 53.Peng K, Li J, Cheng H, Ji F. Goal-directed fluid therapy based on stroke volume variations improves fluid management and gastrointestinal perfusion in patients undergoing major orthopedic surgery. Medical Principles and Practice. 2014;23(5):413–420. doi: 10.1159/000363573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Reisinger KW, Willigers HM, Jansen J. Doppler-guided goal-directed fluid therapy does not affect intestinal cell damage but increases global gastrointestinal perfusion in colorectal surgery: a randomized controlled trial. Colorectal Disease. 2017;19(12):1081–1091. doi: 10.1111/codi.13923. et al. [DOI] [PubMed] [Google Scholar]
- 55.Hoover HC, Ryan JA, Anderson EJ, Fischer JE. Nutritional benefits of immediate postoperative jejunal feeding of an elemental diet. The American Journal of Surgery. 1980;139(1):153–159. doi: 10.1016/0002-9610(80)90245-7. [DOI] [PubMed] [Google Scholar]
- 56.Adam S, Batson S. A study of problems associated with the delivery of enteral feed in critically ill patients in five ICUs in the UK. Intensive Care Medicine. 1997;23(3):261–266. doi: 10.1007/s001340050326. [DOI] [PubMed] [Google Scholar]
- 57.Moore EE. Jones TN. Benefit of immediate jejunostomy feeding after major abdominal trauma – a prospective , randomized study. J of Trauma. 1986;26(10):874–881. doi: 10.1097/00005373-198610000-00003. and. [DOI] [PubMed] [Google Scholar]
- 58.Bower RH. Postoperative Enteral vs Parenteral Nutrition: A Randomized Controlled Trial. Archives of Surgery. 1986;121(9):1040. doi: 10.1001/archsurg.1986.01400090070011. [DOI] [PubMed] [Google Scholar]
- 59.Hamaoui E, Lefkowitz R, Olender L. Enteral nutrition in the early postoperative period: a new semi-elemental formula versus total parenteral nutrition. Journal of Parenteral and Enteral Nutrition. 1990;14(5):501–507. doi: 10.1177/0148607190014005501. et al. [DOI] [PubMed] [Google Scholar]
- 60.Von Meyenfeldt MF, Meijerink WJHJ, Rouflart MMJ, Builmaassen MTHJ, Soeters PB. Perioperative nutritional support: a randomised clinical trial. Clinical Nutrition. 1992;11(4):180–186. doi: 10.1016/0261-5614(92)90026-m. [DOI] [PubMed] [Google Scholar]
- 61.Montecalvo MA, Steger KA, Harrison WF. the critical research team. Nutritional outcome and pneumonia in critical care patients randomized to gastric versus jejunal tube feedings. Crit Care Med. 1992;20(10):1377–1387. doi: 10.1097/00003246-199210000-00004. et al. [DOI] [PubMed] [Google Scholar]
- 62.Dunham CM, Frankenfield D, Belzberg H, Wiles C, Cushing B, Grant Z. Gut Failure – predictor of or contributor to mortality in mechanically ventilated blunt trauma patients? J of Trauma. 1994;37(1):31–35. doi: 10.1097/00005373-199407000-00007. [DOI] [PubMed] [Google Scholar]
- 63.Borzoto AP, Pennings J, Papasadero B. Enteral versus parenteral nutrition after severe closed head injury. J of Trauma. 1994;37(1):459–468. doi: 10.1097/00005373-199409000-00022. et al. [DOI] [PubMed] [Google Scholar]
- 64.Daly JM, Weintraub FN, Shou J, Rosato EF, Lucia M. Enteral nutrition during multimodality therapy in upper gastrointestinal cancer patients: Annals of Surgery. 1995;221(4):327–338. doi: 10.1097/00000658-199504000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Carr SC, Eddie ling KD, Boulos P, Singer M. Randomised trial of safety and efficacy of immediate postoperative enteral feeding in patients undergoing gastrointestinal resection. BMJ. 1996;312:869. doi: 10.1136/bmj.312.7035.869. –. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Beier-Holgersen R, Boesby S. Influence of postoperative enteral nutrition on postsurgical infections. Gut. 1996;39(6):833–835. doi: 10.1136/gut.39.6.833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Baigrie RJ, Devit PG, Watkin DS. Enteral versus parenteral nutrition after oesophagogastric surgery: a prospective randomized comparison. Aust N Z J Surg. 1996;66:668. doi: 10.1111/j.1445-2197.1996.tb00714.x. –. [DOI] [PubMed] [Google Scholar]
- 68.Van Berge Henegouwen MI, Akkermans LMA, van Gulik TM. Prospective, randomized trial on the effect of cyclic versus continuous enteral nutrition on postoperative gastric function after pylorus-preserving pancreatoduodenectomy: Annals of Surgery. 1997;226(6):677–687. doi: 10.1097/00000658-199712000-00005. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kalfarentzos F, Kehagias J, Mead N, Kokkinis K, Gogos CA. Enteral nutrition is superior to parenteral nutrition in severe acute pancreatitis: results of a randomized prospective trial. British Journal of Surgery. 1997;84(12):1665–1669. [PubMed] [Google Scholar]
- 70.Heslin MJ, Latkany L, Leung D. A prospective, randomized trial of early enteral feeding after resection of upper gastrointestinal malignancy: Annals of Surgery. 1997;226(4):567–580. doi: 10.1097/00000658-199710000-00016. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Reynolds JV, Kanwar S, Welsh FKS. Does the route of feeding modify gut barrier function and clinical outcome in patients after major gastrointestinal surgery? JPEN. 1997;21(4):196–201. doi: 10.1177/0148607197021004196. et al. [DOI] [PubMed] [Google Scholar]
- 72.Stewart BT, Woods RJ, Collopy BT, Fink RJ, Mackay JR, Keck JO. Early feeding after elective open colorectal resections: a prospective randomized trial. ANZ Journal of Surgery. 1998;68(2):125–128. doi: 10.1111/j.1445-2197.1998.tb04721.x. [DOI] [PubMed] [Google Scholar]
- 73.Windsor AC, Kanwar S, Li AG. Compared with parenteral nutrition, enteral feeding attenuates the acute phase response and improves disease severity in acute pancreatitis. Gut. 1998;42:431. doi: 10.1136/gut.42.3.431. et al. –. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Singh G, Ram RP, Khanna SK. Early postoperative enteral feeding in patients with nontraumatic intestinal perforations and peritonitis. J Am Coll Surg. 1998;187:142–146. doi: 10.1016/s1072-7515(98)00154-9. [DOI] [PubMed] [Google Scholar]
- 75.Braga M, Gianoti L, Gentilini O, Parisi V, Salis C, Di Carlo V. Early postoperative enteral nutrition improves gut oxygenation and reduces costs compared with total parenteral nutrition: Crit Care Med. 2001;29(2):242–248. doi: 10.1097/00003246-200102000-00003. [DOI] [PubMed] [Google Scholar]
- 76.Taylor SJ, Allan K, McWilliam H. A randomised controlled feasibility and proof-of-concept trial in delayed gastric emptying when metoclopramide fails: We should revisit nasointestinal feeding versus dual prokinetic treatment. Clinical Nutrition ESPEN. 2016;14:1–8. doi: 10.1016/j.clnesp.2016.04.020. et al. [DOI] [PubMed] [Google Scholar]
- 77.Pupelis G, Selga G, Austrums E, Kaminski A. Jejunal feeding, even when instituted late, improves outcomes in patients with severe pancreatitis and peritonitis. Nutrition. 2001;17(2):91–94. doi: 10.1016/s0899-9007(00)00508-6. [DOI] [PubMed] [Google Scholar]
- 78.Minard G, Kudsk KA, Melton S, Paton JH, Tolley EA. Early Versus Delayed Feeding with an Immune-Enhancing Diet in Patients with Severe Head Injuries. Journal of Parenteral and Enteral Nutrition. 2000;24(3):145–149. doi: 10.1177/0148607100024003145. [DOI] [PubMed] [Google Scholar]
- 79.Powell JJ, Murchison JT, Fearon KCH, Ross JA, Siriwardena AK. Randomized controlled trial of the effect of early enteral nutrition on markers of the inflammatory response in predicted severe acute pancreatitis: Early enteral nutrition in acute pancreatitis. British Journal of Surgery. 2000;87(10):1375–1381. doi: 10.1046/j.1365-2168.2000.01558.x. [DOI] [PubMed] [Google Scholar]
- 80.Kearns PJ, Chin D, Mueller L, Wallace K, Jensen WA, Kirsch CM. The incidence of ventilator-associated pneumonia and success in nutrient delivery with gastric versus small intestinal feeding: A randomized clinical trial: Crit Care Med. 2000;28(6):1742–1746. doi: 10.1097/00003246-200006000-00007. [DOI] [PubMed] [Google Scholar]
- 81.Bozzeti F, Braga M, Gianoti L, Gavazzi C, Mariani L. Postoperative enteral versus parenteral nutrition in malnourished patients with gastrointestinal cancer: a randomised multicentre trial. The Lancet. 2001;358:6. doi: 10.1016/S0140-6736(01)06578-3. [DOI] [PubMed] [Google Scholar]
- 82.Dickerson R. Early postoperative enteral nutrition improves gut oxygenation and reduces costs compared with total parenteral nutrition. Nutr Clin Prac. 2001;16(6):363. doi: 10.1097/00003246-200102000-00003. [DOI] [PubMed] [Google Scholar]
- 83.Montejo JC, Grau T, Acosta J. Multicenter, prospective, randomized, single-blind study comparing the efficacy and gastrointestinal complications of early jejunal feeding with early gastric feeding in critically ill patients: Crit Care Med. 2002;30(4):796–800. doi: 10.1097/00003246-200204000-00013. et al. [DOI] [PubMed] [Google Scholar]
- 84.Davies AR, Froomes PRA, French CJ. Randomized comparison of nasojejunal and nasogastric feeding in critically ill patients. Crit Care Med. 2002;30(3):586–590. doi: 10.1097/00003246-200203000-00016. et al. [DOI] [PubMed] [Google Scholar]
- 85.Bertolini G, Iapichino G, Radrizzani D. Early enteral immunonutrition in patients with severe sepsis: Results of an interim analysis of a randomized multicentre clinical trial. Intensive Care Medicine. 2003;29(5):834–840. doi: 10.1007/s00134-003-1711-5. et al. [DOI] [PubMed] [Google Scholar]
- 86.Kompan L, Vidmar G, Spindler-Vesel A, Pecă r J. Is early enteral nutrition a risk factor for gastric intolerance and pneumonia? Clinical Nutrition. 2004;23(4):527–532. doi: 10.1016/j.clnu.2003.09.013. [DOI] [PubMed] [Google Scholar]
- 87.Malhotra A, Mathur A K, Gupta S. Early enteral nutrition after surgical treatment of gut perforations: A prospective randomised study. J Postgrad Med. 2004;50:102–6. [PubMed] [Google Scholar]
- 88.Kumar A, Singh N, Prakash S, Saraya A, Joshi YK. Early enteral nutrition in severe acute pancreatitis: a prospective randomized controlled trial comparing nasojejunal and nasogastric routes. Journal of Clinical Gastroenterology. 2006;40(5):431–434. doi: 10.1097/00004836-200605000-00013. [DOI] [PubMed] [Google Scholar]
- 89.Nguyen NQ, Fraser RJ, Chapman MJ. Feed intolerance in critical illness is associated with increased basal and nutrient-stimulated plasma cholecystokinin concentrations. Crit Care Med. 2007;35(1):82–88. doi: 10.1097/01.CCM.0000250317.10791.6C. et al. [DOI] [PubMed] [Google Scholar]
- 90.Han-Geurts IJM, Hop WC, Verhoef C, Tran KTC, Tilanus HW. Randomized clinical trial comparing feeding jejunostomy with nasoduodenal tube placement in patients undergoing oesophagectomy. British Journal of Surgery. 2007;94(1):31–35. doi: 10.1002/bjs.5283. [DOI] [PubMed] [Google Scholar]
- 91.Desachy A, Clavel M, Vuagnat A, Normand S, Gissot V, François B. Initial efficacy and tolerability of early enteral nutrition with immediate or gradual introduction in intubated patients. Intensive Care Medicine. 2008;34(6):1054–1059. doi: 10.1007/s00134-007-0983-6. [DOI] [PubMed] [Google Scholar]
- 92.Tien Y-W, Yang C-Y, Wu Y-M, Hu R-H, Lee P-H. Enteral nutrition and biliopancreatic diversion effectively minimize impacts of gastroparesis after pancreaticoduodenectomy. Journal of Gastrointestinal Surgery. 2009;13(5):929–937. doi: 10.1007/s11605-009-0831-9. [DOI] [PubMed] [Google Scholar]
- 93.Barlow R, Price P, Reid TD. Prospective multicentre randomised controlled trial of early enteral nutrition for patients undergoing major upper gastrointestinal surgical resection. Clinical Nutrition. 2011;30(5):560–566. doi: 10.1016/j.clnu.2011.02.006. et al. [DOI] [PubMed] [Google Scholar]
- 94.Altintas ND, Aydin K, Turkoglu MA, Abbasoglu O, Topeli A. Effect of enteral versus parenteral nutrition on outcome of medical patients requiring mechanical ventilation. Nutrition in Clinical Practice. 2011;26(3):322–329. doi: 10.1177/0884533611405790. [DOI] [PubMed] [Google Scholar]
- 95.Rice TW, Mogan S, Hays MA, Bernard GR, Jensen GL, Wheeler AP. Randomized trial of initial trophic versus full-energy enteral nutrition in mechanically ventilated patients with acute respiratory failure: Critical Care Medicine. 2011;39(5):967–974. doi: 10.1097/CCM.0b013e31820a905a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Davies SJ, Francis J, Dilley J. Measuring outcomes after major abdominal surgery during hospitalization: reliability and validity of the postoperative morbidity survey. Periop Med. 2013;2:1. doi: 10.1186/2047-0525-2-1. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Zhu X, Wu Y, Qiu Y, Jiang C, Ding Y. Comparative analysis of the efficacy and complications of nasojejunal and jejunostomy on patients undergoing pancreaticoduodenectomy. Journal of Parenteral and Enteral Nutrition. 2014;38(8):996–1002. doi: 10.1177/0148607113500694. [DOI] [PubMed] [Google Scholar]
- 98.Sun J, Li W, Ke L. Early enteral nutrition prevents intraabdominal hypertension and reduces the severity of severe acute pancreatitis compared with delayed enteral nutrition: A prospective pilot study. World Journal of Surgery. 2013;37(9):2053–2060. doi: 10.1007/s00268-013-2087-5. et al. [DOI] [PubMed] [Google Scholar]
- 99.Kadamani I, Itani M, Zahran E, Taha N. Incidence of aspiration and gastrointestinal complications in critically ill patients using continuous versus bolus infusion of enteral nutrition: A pseudo-randomised controlled trial. Australian Critical Care. 2014;27(4):188–193. doi: 10.1016/j.aucc.2013.12.001. [DOI] [PubMed] [Google Scholar]
- 100.Boelens PG, Heesakkers FFBM, Luyer MDP. Reduction of postoperative ileus by early enteral nutrition in patients undergoing major rectal surgery: prospective, randomized, controlled trial. Annals of Surgery. 2014;259(4):649–655. doi: 10.1097/SLA.0000000000000288. et al. [DOI] [PubMed] [Google Scholar]
- 101.Harvey SE, Parrot F, Harrison DA. Trial of the route of early nutritional support in critically ill adults. New England Journal of Medicine. 2014;371(18):1673–1684. doi: 10.1056/NEJMoa1409860. et al. [DOI] [PubMed] [Google Scholar]
- 102.Ma J, Pendharkar SA, O’Grady G, Windsor JA, Petrov MS. Effect of Nasogastric Tube Feeding vs Nil per Os on Dysmotility in Acute Pancreatitis: Results of a Randomized Controlled Trial. Nutrition in Clinical Practice. 2016;31(1):99–104. doi: 10.1177/0884533615603967. [DOI] [PubMed] [Google Scholar]
- 103.Li Bing, Hong-Yi Liu, Shao-Hua Guo, Peng Sun, Fang-Ming Gong,. Bao-Qing Jia. The postoperative clinical outcomes and safety of early enteral nutrition in operated gastric cancer patients. JBUON. 2015;20(2):468–472. and. [PubMed] [Google Scholar]
- 104.Taylor SJ, Allan K, McWilliam H, Manara A, Brown J, Greenwood R, Toher D. A randomised controlled feasibility and proof-of-concept trial in delayed gastric emptying when metoclopramide fails: We should revisit nasointestinal feeding versus dual prokinetic treatment Achieving goal nutrition in critical illness and delayed gastric emptying: Trial of nasointestinal feeding versus nasogastric feeding plus prokinetics. Clinical Nutrition ESPEN. 2016;14:1–8. doi: 10.1016/j.clnesp.2016.04.020. [DOI] [PubMed] [Google Scholar]
- 105.Ozen N, Tosun N, Yamanel L, Altintas ND, Kilciler G, Ozen V. Evaluation of the effect on patient parameters of not monitoring gastric residual volume in intensive care patients on a mechanical ventilator receiving enteral feeding: A randomized clinical trial. Journal of Critical Care. 2016;33:137–144. doi: 10.1016/j.jcrc.2016.01.028. [DOI] [PubMed] [Google Scholar]
- 106.Van Barneveld KWY, Smeets BJJ, Heesakkers FFBM. Beneficial effects of early enteral nutrition after major rectal surgery: a possible role for conditionally essential amino acids? results of a randomized clinical trial. Critical Care Medicine. 2016;44(6):e353–e361. doi: 10.1097/CCM.0000000000001640. et al. [DOI] [PubMed] [Google Scholar]
- 107.Malik AA, Rajandram R, Tah PC, Hakumat-Rai V-R, Chin K-F. Microbial cell preparation in enteral feeding in critically ill patients: A randomized, double-blind, placebo-controlled clinical trial. Journal of Critical Care. 2016;32:182–188. doi: 10.1016/j.jcrc.2015.12.008. [DOI] [PubMed] [Google Scholar]
- 108.Fan M, Wang Q, Fang W. Early enteral combined with parenteral nutrition treatment for severe traumatic brain injury: effects on immune function, nutritional status and outcomes. Chinese Medical Sciences Journal. 2016;31(4):213–220. doi: 10.1016/s1001-9294(17)30003-2. et al. [DOI] [PubMed] [Google Scholar]
- 109.Stimac D, Poropat G, Hauser G. Early nasojejunal tube feeding versus nil-by-mouth in acute pancreatitis: A randomized clinical trial. Pancreatology. 2016;16(4):523–528. doi: 10.1016/j.pan.2016.04.003. et al. [DOI] [PubMed] [Google Scholar]
- 110.Hongyin L, Zhu H, Tao W. Abdominal paracentesis drainage improves tolerance of enteral nutrition in acute pancreatitis: a randomized controlled trial. Scandinavian Journal of Gastroenterology. 2017;52(4):389–395. doi: 10.1080/00365521.2016.1276617. et al. [DOI] [PubMed] [Google Scholar]
- 111.Reignier J, Bensaid S, Perrin-Gachadoat D, Burdin M, Boiteau R, Tenaillon A. Erythromycin and early enteral nutrition in mechanically ventilated patients: Critical Care Medicine. 2002;30(6):1237–1241. doi: 10.1097/00003246-200206000-00012. [DOI] [PubMed] [Google Scholar]
- 112.Gramlich L, Kichian K, Pinilla J, Rodych NJ, Dhaliwal R, Heyland DK. Does enteral nutrition compared to parenteral nutrition result in beter outcomes in critically ill adult patients? A systematic review of the literature. Nutrition. 2004;20:843–848. doi: 10.1016/j.nut.2004.06.003. [DOI] [PubMed] [Google Scholar]
- 113.Kaur N, Gupta MK, Minocha VR. Early enteral feeding by nasoenteric tubes in patients with perforation peritonitis. World J Surg. 2005;29:1023–1028. doi: 10.1007/s00268-005-7491-z. [DOI] [PubMed] [Google Scholar]
- 114.Lewis SJ, Andersen HK, Thomas S. Early Enteral Nutrition Within 24 h of Intestinal Surgery Versus Later Commencement of Feeding: A systematic review and meta-analysis. J Gastrointest Surg. 2009;13:569–575. doi: 10.1007/s11605-008-0592-x. [DOI] [PubMed] [Google Scholar]
- 115.Andersen HK, Lewis SJ, Thomas S. Early enteral nutrition within 24h of colorectal surgery versus later commencement of feeding for postoperative complications. Cochrane Colorectal Cancer Group, ed. Cochrane Database of Systematic Reviews. 2006;(Issue 4) doi: 10.1002/14651858.CD004080.pub2. [DOI] [PubMed] [Google Scholar]
- 116.Bennet-Guerrero E, Welsby I, Dunn TJ. The use of a postoperative morbidity survey to evaluate patients with prolonged hospitalization after routine, moderate-risk, elective surgery. Anesth Analg. 1999;89(2):514–519. doi: 10.1097/00000539-199908000-00050. et al. [DOI] [PubMed] [Google Scholar]
- 117.Cecconi M, Corredor C, Arulkumaran N. Clinical review: Goal-directed therapy-what is the evidence in surgical patients? The effect on diferent risk groups. Crit Care. 2013;17(2):209. doi: 10.1186/cc11823. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Compton FD, Zukunft B, Hoffman C, Zidek W, Schaefer JH. Performance of a minimally invasive uncalibrated cardiac output monitoring system (Flotrac/Vigileo) in haemodynamically unstable patients. Br J Anaesth. 2008;100:451–456. doi: 10.1093/bja/aem409. [DOI] [PubMed] [Google Scholar]
- 119.Schnuriger B, Inaba K, Wu T, Eberle BM, Belzberg H, Demetriades D. Crystalloids after primary colon resection and anastomosis at initial trauma laparotomy: excessive volumes are associated with anastomotic leakage. J Trauma. 2011;70(3):603–610. doi: 10.1097/TA.0b013e3182092abb. [DOI] [PubMed] [Google Scholar]
- 120.Doig Gordon S. Early parenteral nutrition in critically ill patients with short-term relative contraindications to early enteral nutrition: A randomized controlled trial. JAMA. 2013;309(20):2130. doi: 10.1001/jama.2013.5124. [DOI] [PubMed] [Google Scholar]
- 121.Moore FA. Andrassy RJ. Early enteral feedings, compared with parenteral, reduces postoperative septic complications. The results of a meta-analysis. Ann Surg. 1991;216:172–183. doi: 10.1097/00000658-199208000-00008. Feliciano. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Rivers E, Nguyen B, Havstad S. et al– For the early goal-directed therapy collabortive group. Early goal-directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med. 2001;345(19):1368–1377. doi: 10.1056/NEJMoa010307. [DOI] [PubMed] [Google Scholar]
- 123.Grocott MPW, Mythen MG, Gan TJ. Perioperative fluid management and clinical outcomes in adults. Anaesth Analg. 2005;100:1093–106. doi: 10.1213/01.ANE.0000148691.33690.AC. [DOI] [PubMed] [Google Scholar]
- 124.Peter JV, Moran JL, Phillips-Hughes J. A metaanalysis of treatment outcomes of early enteral versus early parenteral nutrition in hospitalised patients. Crit Care Med. 2005;33(1):213–220. doi: 10.1097/01.ccm.0000150960.36228.c0. [DOI] [PubMed] [Google Scholar]