Summary
Background
Since influenza often presents non-specifically in infancy, we aimed to assess the extent to which existing respiratory surveillance platforms might underestimate the frequency of severe influenza disease among infants.
Methods
The Influenza and Respiratory Syncytial Virus in Infants (IRIS) study was a prospective observational study done at four hospitals in Albania, Jordan, Nicaragua, and the Philippines. We included acutely ill infants aged younger than 1 year admitted to hospital within 10 days or less of illness onset during two influenza seasons (2015–16 and 2016–17) in Albania, Jordan, and Nicaragua, and over a continuous 34 week period (2015–16) in the Philippines. We assessed the frequency of influenza virus infections by real-time RT-PCR (rRT-PCR) and serology. The main study outcome was seroconversion, defined as convalescent antibody titres more than or equal to four-fold higher than acute sera antibody titres, and convalescent antibody titres of 40 or higher. Seroconverison was confirmed by haemagglutination inhibition assay for influenza A viruses, and by hemagglutination inhibition assay and microneutralisation for influenza B viruses.
Findings
Between June 27, 2015, and April 21, 2017, 3634 acutely ill infants were enrolled, of whom 1943 were enrolled during influenza seasons and had complete acute-convalescent pairs and thus were included in the final analytical sample. Of the 1943 infants, 94 (5%) were influenza-positive by both rRT-PCR and serology, 58 (3%) were positive by rRT-PCR-only, and 102 (5%) were positive by serology only. Seroconversion to at least one of the influenza A or B viruses was observed among 196 (77%) of 254 influenza-positive infants. Of the 254 infants with influenza virus, 84 (33%) only had non-respiratory clinical discharge diagnoses (eg, sepsis, febrile seizures, dehydration, or other non-respiratory viral illness). A focus on respiratory diagnoses and rRT-PCR-confirmed influenza underdetects influenza-associated hospital admissions among infants by a factor of 2·6 (95% CI 2·0–3·6). Findings were unchanged when syndromic severe acute respiratory infection criteria were applied instead of clinical diagnosis.
Interpretation
If the true incidence of laboratory-confirmed influenza-associated hospital admissions among infants is at least twice that of previous estimates, this substantially increases the global burden of severe influenza and expands our estimates of the preventive value of maternal and infant influenza vaccination programmes.
Funding
US Centers for Disease Control and Prevention.
Introduction
Although global rates of acute upper and lower respiratory disease are highest among infants aged younger than 1 year 1,2 and influenza virus infections are among the leading contributors to this burden,1–5 existing studies are likely to underestimate the frequency of influenza-associated hospital admissions in at least two ways. First, existing research and surveillance platforms, such as those focused on severe acute respiratory infections (SARI), often overlook non-febrile and non-respiratory manifestations of influenza disease.6–8 Second, since complications from influenza, such as pneumonia and bronchiolitis, often occur days after the primary infection, individuals might no longer be shedding influenza virus at the time of hospital admission.9–12 If substantial gaps in influenza diagnosis exist, the findings could be relevant to immunisation policy decisions, especially in low-income and middle-income countries (LMICs), where data about the potential preventive value of the influenza vaccine are scarce.13,14 Even in high-income countries, misperceptions about the risk of severe influenza during infancy might be associated with the underuse of influenza vaccination during pregnancy15,16 and among infants aged 6 months and older.17
In this prospective study,18 we aimed to assess the frequency of influenza virus infection among infants aged younger than 1 year who were admitted to hospital in four middle-income countries. Here, we report the frequency of influenza virus infections identified by serological detection among infants who were influenza-negative by traditional molecular methods, describe the proportion of influenza virus infections that did not present as an acute respiratory illness, and report the frequency of influenza confirmed by real-time RT-PCR (rRT-PCR) among non-ill infants enrolled during the study period from the same communities.
Methods
Study design and participants
Detailed methods of the Influenza and Respiratory Syncytial Virus in Infants Study (IRIS), including the use of a common protocol have been published previously18 (appendix pp 2–5). The four hospitals included in the IRIS study were selected intentionally from middle-income countries and from tropical and temperate climates (Albania, Jordan, Nicaragua, and the Philippines); collaborators were selected from a relatively small number of research organisations with experience enrolling infants in both hospital and community settings, following infants prospectively, and collecting both respiratory and sera specimens from infants.
Infants aged younger than 1 year who had been admitted to hospital were enrolled at study hospitals during two influenza seasons (2015–16 and 2016–17) in Albania, Jordan, and Nicaragua, and over a continuous 34 week period (April, 2016–December, 2016) in the Philippines, on the basis of previous regional influenza surveillance (appendix pp 6, 7).
All acutely ill infants admitted to hospital regardless of symptoms were identified from hospital admission records (including presenting complaints and preliminary diagnoses) and were eligible for enrolment within 24 h of admission if their family lived in the hospital catchment area (to facilitate follow-up) and they had been admitted to hospital within 10 days or less of illness onset.
We also enrolled a control of non-ill infants at routine immunisation clinics and other settings (appendix p 6); enrolled non-ill infants were stratified by age (0–5 months vs 6–11 months) to reflect the mixture of ages observed among infants admitted to hospital.
The study protocol and procedures have been approved by Abt Associates (the organisation coordinating the study) and by Institutional Review Boards at each study site: Medical Ethics Committee, Ministry of Health, Tirana, Albania; Jordanian Ministry of Health Institutional Review Board; Comite Institucional de Revision Etica, Ministerio de Salud, Nicaragua; and Research Institute for Tropical Medicine Institutional Review Board. Written informed consent was obtained from the parents or guardians of all infants in the parent or guardian’s language.
Procedures
After obtaining written informed consent, parents were interviewed to document sociodemographic characteristics, medical history, maternal and infant vaccination history, and symptoms. We extracted clinician-measured temperature, respiration rate, oxygen saturation, and clinical discharge diagnoses from hospital records. Combined nasal and oropharyngeal swabs and acute sera were collected within 24 h of hospital admission. A convalescent blood draw was collected 3–5 weeks after enrolment.
For the enrolment of non-ill infants, screening questions confirmed that these infants were not currently ill and had not been acutely ill within the past 7 days with nasal congestion or discharge, new or worsening cough, shortness of breath, difficulty breathing, wheezing, fever, chills, or diarrhoea. Combined nasal and oropharyngeal swabs were collected. To assess whether non-ill infants developed illness symptoms after swab collection, during the second year of the study period, parents of non-ill infants were contacted 4–10 days after enrolment.
All study laboratories used singleplex rRT-PCR influenza assays and reagents, which were validated by the US Centers for Disease Control and Prevention (CDC) after completion of annual influenza proficiency panels and quality assurance testing (appendix p 3). Haemagglutination inhibition testing was done by Battelle Laboratory (Aberdeen, MA, USA) after the completion of proficiency panels (appendix p 3). Haemagglutination inhibition assays for the detection of antibodies against influenza A H1N1pdm09 and B influenza antigens were done using turkey erythrocytes, and those for the detection of antibodies against influenza A (H3N2) viruses were done using guinea pig erythrocytes in the presence of oseltamivir carboxylate to eliminate the interference by neuraminidase. For influenza B serology, ether treated antigens were used. Microneutralisation assays against influenza B viruses were done at a CDC laboratory (Atlanta, GA, USA; appendix p 2). Influenza serology was done on paired acute and convalescent sera against egg-grown viruses antigenically similar to those circulating during 2015–16 and 2016–17 influenza seasons: A/California/07/2009 (H1N1), A/Switzerland/9715293/2014 (H3N2), A/Hong Kong/ 4801/2014 (H3N2), B/Brisbane/60/2008/Victoria, and B/Phuket/3073/2013/Yamagata (appendix p 2).
Outcomes
The main outcome was seroconversion for influenza virus, with convalescent antibody titres more than or equal to four-fold higher than acute sera titres, and convalescent antibody titres of 40 or higher.19 For influenza A viruses, serological confirmation was defined as seroconversion by haemagglutination inhibition only. Since hemagglutination inhibition for influenza B viruses has high sensitivity but moderate specificity for ether-treated B antigens,20 serological confirmation of B virus infection required achievement of seroconversion by both haemagglutination inhibition and by microneutralisation.
Statistical analysis
The analytic sample was limited to infants enrolled between the illness onset dates of the first and last infants with rRT-PCR-confirmed influenza to be admitted to hospital at each site. To assess possible selection and information biases, we calculated the proportion of all potentially eligible infants whose parent refused screening or consent and the proportion of infants without convalescent sera, by study year, site, age group, sex, hospital ward, discharge diagnosis, and rRT-PCR influenza positivity. We used multivariable logistic regression to examine the joint association between these covariates and study refusal or sera collection.
To estimate the proportion of infants with influenza, we calculated percentages and 95% CIs assuming a binomial distribution. We compared these percentages by infant and illness characteristics and by influenza type or subtype using a χ2 or Fisher’s exact test of association. For parent-reported infant health, values were missing for 2·2% of participants, and therefore we linearly imputed missing values as a function of site, year, and infant age. Since the study included two diagnostic indicators (molecular and serological diagnosis of influenza), we calculated the sensitivity and specificity of each indicator with the other indicator considered the so-called gold standard. For example, the sensitivity of serology was calculated using rRT-PCR as the gold standard whereby sensitivity was defined as the percentage of all rRT-PCR positives that were serologically positive; specificity of serology defined as the percentage of all rRT-PCR-negatives that were also serological-negatives. All analyses were done using SAS (version 9.4).
To summarise the frequency of influenza virus infections identified by serological detection among infants who were influenza-negative by traditional molecular methods, and the proportion of influenza virus infections that did not present as an acute respiratory illness, we calculated an underdetection multiplier, similar to the approach used in estimating population-level influenza disease burden;21 the multiplier was the ratio of influenza-positives (by either rRT-PCR or seroconversion) with any clinical diagnosis of an acute illness to rRT-PCR-confirmed influenza-positives with respiratory diagnoses. We assumed that this multiplier would vary by site and season due to differences in participant age, disease presentation, and circulating influenza viruses; thus, we estimated the multiplier and 95% CI using a beta-binomial model with maximum likelihood estimation using the SAS NLMIXED with BETABIN macro, whereby the ratio follows a beta distribution drawn from seven study site seasons (three sites with two seasons plus one season for the Philippines) using previously published methods21 (appendix pp 4, 5).
Role of the funding source
The funder of the study was involved in study design, data analysis, data interpretation, and writing of the report. The corresponding author had full access to all the data in this study and had final responsibility for the decision to submit for publication.
Results
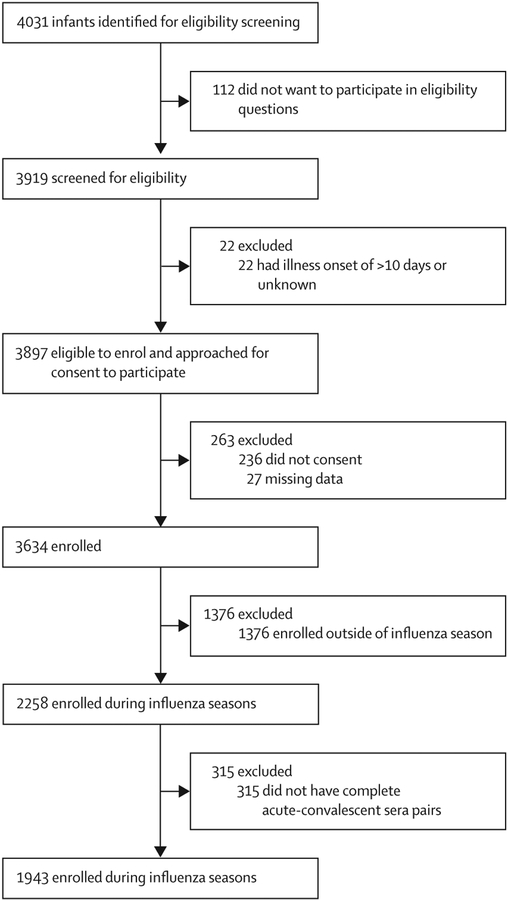
Parental consent to screening and enrolment was high across all four sites (appendix p 22); the parents of 3919 (97%) of 4031 infants consented to screening (range across sites 95–99%), and 3634 (93%) of 3897 eligible infants were enrolled (range 76–99%). Consent to screening and enrolment were lower in the first year and at the Philippines site (appendix p 8). After exclusion of 1376 infants enrolled outside of influenza seasons, and 315 infants without complete acute-convalescent pairs, 1943 infants were included in the final analytic sample (figure 1). In a multivariate model (appendix p 9), loss to follow-up was higher in the first year than the second year (p=0·050), higher at the Nicaragua and Philippines sites than the Albania and Philippines sites (p<0·0001), higher among infants admitted to the general ward than the intensive care unit (p=0·020), and higher among infants with non-respiratory diagnoses than respiratory diagnoses (p=0·0020), but did not differ significantly by age or influenza positivity (appendix p 9).
Figure 1:
Study profile
Characteristics of the 1943 infants included in the analytical sample are presented in table 1. The age distribution of infants varied across sites; 988 (51%) of 1943 infants were aged 0–2 months (range across sites 32–71%), 364 (19%) were aged 3–5 months (range 14–24%), and 591 (30%) were aged 6–11 months (range 15–49%). 1363 (70%) of 1943 infants were described by their parents as in very good or excellent overall health before the acute illness (range 56–84% across sites). 162 (8%) of 1943 infants had one or more parent-reported chronic medical conditions (range 5–11%), and 356 (18%) had previously been admitted to hospital (range 10–33%). No infants had previously received influenza vaccination; 45 (15%) of 307 mothers in Nicaragua, three (<1%) of 664 mothers in Jordan, and no mothers in Albania and Philippines had received influenza vaccination during pregnancy.
Table 1:
Characteristics of acutely ill infants admitted to hospital, by study site
| All infants (n=1943) | Albania (n=638) | Jordan (n=664) | Nicaragua (n=307) | Philippines (n=334) | p value* | |
|---|---|---|---|---|---|---|
| Dichotomous age, months | ||||||
| 0–5 | 1352 (70%) | 412 (65%) | 566 (85%) | 202 (66%) | 172 (51%) | <0·0001 |
| 6–11 | 591 (30%) | 226 (35%) | 98 (15%) | 105 (34%) | 162 (49%) | ·· |
| Catergorical age, months | ||||||
| 0–2 | 988 (51%) | 258 (40%) | 474 (71%) | 148 (48%) | 108 (32%) | <0·0001 |
| 3–5 | 364 (19%) | 154 (24%) | 92 (14%) | 54 (18%) | 64 (19%) | ·· |
| 6–8 | 328 (17%) | 126 (20%) | 61 (9%) | 54 (18%) | 87 (26%) | ·· |
| 9–11 | 263 (14%) | 100 (16%) | 37 (6%) | 51 (17%) | 75 (22%) | ·· |
| Study year | ||||||
| 2015–16 | 937 (48%) | 216 (34%) | 439 (66%) | 75 (24%) | 207 (62%) | <0·0001 |
| 2016–17 | 1006 (52%) | 422 (66%) | 225 (34%) | 232 (76%) | 127 (38%) | ·· |
| Sex | ||||||
| Boys | 1121 (58%) | 355 (56%) | 375 (56%) | 178 (58%) | 213 (64%) | 0·087 |
| Girls | 822 (42%) | 283 (44%) | 289 (44%) | 129 (42%) | 121 (36%) | ·· |
| Clinically preterm (gestational age <37 weeks)† | 163 (8%) | 49 (8%) | 77 (12%) | 22 (7%) | 15 (4%) | 0·00090 |
| Chronic medical condition(s)‡ | 162 (8%) | 40 (6%) | 71 (11%) | 16 (5%) | 35 (10%) | <0·0001 |
| Previous hospital admission | 356 (18%) | 66 (10%) | 107 (16%) | 100 (33%) | 83 (25%) | <0·0001 |
| Parent-reported infant healths§ | ||||||
| Poor or fair | 167 (9%) | 17 (3%) | 91 (14%) | 29 (9%) | 30 (9%) | <0·0001 |
| Good | 413 (21%) | 86 (13%) | 135 (20%) | 102 (33%) | 90 (27%) | ·· |
| Excellent or very good | 1363 (70%) | 535 (84%) | 438 (66%) | 203 (66%) | 187 (56%) | ·· |
| Influenza vaccination | ||||||
| Mother vaccinated during pregnancy | 48 (2%) | 0 (0) | 3 (0) | 45 (15%) | 0 (0) | <0·0001 |
| Infant vaccinated at age ≥6 months | 0 | 0 | 0 | 0 | 0 | NA |
| No influenza vaccination | 1895 (98%) | 638 (100%) | 661 (100%) | 262 (85%) | 334 (100%) | NA |
| Time since onset of illness, days | ||||||
| 0–2 | 780 (40%) | 143 (22%) | 298 (45%) | 129 (42%) | 210 (63%) | <0·0001 |
| 3–4 | 707 (36%) | 298 (47%) | 214 (32%) | 107 (35%) | 88 (26%) | |
| 5–10 | 456 (23%) | 197 (31%) | 152 (23%) | 71 (23%) | 36 (11%) | |
| Mean (SD) | 3·3 (2·0) | 3·9 (1·8) | 3·2 (2·1) | 3·3 (2·2) | 2·3 (1·7) | <0·0001 |
| SARI status¶ | ||||||
| No SARI | 1109 (57%) | 336 (53%) | 429 (65%) | 187 (61%) | 157 (47%) | <0·0001 |
| Met SARI definition | 834 (43%) | 302 (47%) | 235 (35%) | 120 (39%) | 177 (53%) | |
| Hospital department‖ | ||||||
| General care ward | 1416 (73%) | 398 (62%) | 413 (62%) | 288 (94%) | 317 (95%) | <0·0001 |
| Intensive care unit | 527 (27%) | 240 (38%) | 251 (38%) | 19 (6%) | 17 (5%) | |
| Antibiotic treatment after hospital admission | ||||||
| No | 367 (19%) | 48 (8%) | 80 (12%) | 70 (23%) | 169 (51%) | <0·0001 |
| Yes | 1576 (81%) | 590 (92%) | 584 (88%) | 237 (77%) | 165 (49%) | |
| Length of hospital stay, days | 5·9 (3·2) | 5·5 (3·2) | 6·5 (6·1) | 5·3 (4·2) | 6·0 (3·6) | 0·0010 |
| Time from illness onset to convalescence, days | 27·8 (11·2) | 30·6 (16·9) | 26·2 (5·2) | 29·1 (9·1) | 24·3 (4·9) | 0·0010 |
| Discharge diagnoses | ||||||
| Respiratory (or mixed) | 1191 (61%) | 482 (76%) | 347 (52%) | 168 (55%) | 194 (58%) | <0·0001 |
| Non-respiratory only | 752 (39%) | 156 (24%) | 317 (48%) | 140 (46%) | 140 (42%) | |
| Respiratory diagnoses** | ||||||
| Acute respiratory distress syndrome | 13 (1%) | 0 (0) | 13 (2%) | 0 (0) | 0 (0) | <0·0001 |
| Bronchiolitis or asthma | 366 (19%) | 294 (46%) | 37 (6%) | 26 (8%) | 9 (3%) | <0·0001 |
| Pertussis-like syndrome | 71 (4%) | 1 (0) | 57 (9%) | 13 (4%) | 0 (0) | <0·0001 |
| Pneumonia | 668 (34%) | 140 (22%) | 215 (32%) | 124 (40%) | 189 (57%) | <0·0001 |
| Viral or other | 182 (9%) | 101 (16%) | 35 (5%) | 31 (10%) | 15 (4%) | <0·0001 |
| Non-respiratory diagnoses** | ||||||
| Dehydration | 149 (8%) | 69 (11%) | 6 (1%) | 1 (0) | 73 (22%) | <0·0001 |
| Febrile seizures | 43 (2%) | 21 (3%) | 8 (1%) | 0 (0) | 14 (4%) | 0·00020 |
| Meningitis | 26 (1%) | 2 (0) | 22 (3%) | 0 (0) | 2 (1%) | <0·0001 |
| Sepsis | 320 (16%) | 6 (1%) | 260 (39%) | 10 (3%) | 44 (13%) | <0·0001 |
| Urinary tract infection | 56 (3%) | 6 (1%) | 34 (5%) | 2 (1%) | 14 (4%) | <0·0001 |
| Viral or other | 703 (36%) | 213 (33%) | 194 (29%) | 142 (46%) | 154 (46%) | <0·0001 |
| Influenza status by rRT-PCR | ||||||
| Negative | 1791 (92%) | 577 (90%) | 612 (92%) | 287 (93%) | 315 (94%) | NA |
| A or B positive | 152 (8%) | 61 (10%) | 52 (8%) | 20 (7%) | 19 (6%) | 0·15 |
| A positive†† | 106/152 (70%) | 38/61 (62%) | 42/52 (81%) | 17/20 (85%) | 9/19 (47%) | 0·075 |
| A(H1N1)pdm09 positive‡‡ | 28/106 (26%) | 10/38 (26%) | 7/42 (17%) | 8/17 (47%) | 3/9 (33%) | 0·26 |
| A(H3N2) positive‡‡ | 73/106 (69%) | 24/38 (63%) | 34/42 (81%) | 9/17 (53%) | 6/9 (67%) | 0·055 |
| B positive†† | 46/152 (30%) | 23/61 (38%) | 10/52 (19%) | 3/20 (15%) | 10/19 (53%) | 0·023 |
| B (Victoria) positive§§ | 43/46 (93%) | 23/23 (100%) | 10/10 (100%) | 2/3 (67%) | 8/10 (80%) | 0·013 |
| B (Yamagata) positive§§ | 2/46 (4%) | 0 | 0 | 0 | 2/10 (20%) | 0·054 |
Data are n (%), n/N (%), or mean (SD). NA=not applicable. SARI=severe acute respiratory infection. rRT-PCR=real-time RT-PCR.
χ2, Fisher’s exact test, or one-way ANOVA test for differences between study sites.
Infants were considered full term if they were born at known gestational age ≥37 weeks; infants born with a calculated gestational age of <37 weeks were not considered preterm if parents did not report them as such in questionnaire.
40 infants had no chronic conditions reported because their status was classified as unknown or their parents refused to provide this information.
2% with missing values were imputed as a linear function of infant age, country, study year, and other demographic variables.
An acute respiratory infection with history of fever or measured fever of ≥38°C and cough with onset within the last 10 days that required hospital admission.
General ward includes infants who required treatment in the intensive care unit but were placed in a general ward.
654 infants had >1 present or primary discharge diagnosis.
Denominator is the total influenza A or B positives by RT-PCR.
Denominator is the influenza A positives, including those that could not be subtyped.
Denominator is out of influenza B positives, including those with indeterminate lineage.
1487 (76%) of 1943 infants were admitted to hospital within 4 days of illness onset (range 69–89% across sites). Albania and Jordan had a greater volume of admissions and availability of intensive care; 240 (38%) of 638 enrolled infants in Albania and 251 (38%) of 664 enrolled infants in Jordan had been admitted to the intensive care unit compared with 19 (6%) of 307 enrolled infants in Nicaragua and 17 (5%) of 334 infants in the Philippines. Mean time since illness onset at admission was 3·3 days for both infants on general wards and infants in intensive care units. The mean length of hospital stay was 5·9 days (SD 3·2). Influenza antiviral drugs were not available at study hospitals.
Across sites and seasons, 152 (8%, 95% CI 6·8–9·1) of 1943 infants admitted to hospital were influenza-positive by rRT-PCR (range across sites 5·7–9·6%). Influenza A (H1N1)pdm09, influenza A (H3N2), and influenza B (Victoria lineage) viruses were identified at all sites (table 1).
Acute serum was collected at a mean of 3·3 days (SD 2·0) after illness onset, and convalescent serum was collected at a mean of 27·8 days (SD 11·2) after onset. Seroconversion to at least one of the influenza A or B viruses was observed among 196 infants. Of the 1943 infants, 94 (5%) were influenza-positive by both rRT-PCR and serology, 58 (3%) were positive by rRT-PCR-only, and 102 (5%) were positive by serology only. Using rRT-PCR as the gold standard, seroconversion had a sensitivity of 62% (95% CI 54–70; 94/152); conversely, using seroconversion as the gold standard, rRT-PCR had a sensitivity of 48% (41–55; 94/196; appendix p 10). Specificity was high for both serology (94%, 95% CI 93–95; 1689/1791) and for rRT-PCR (97%, 96–98; 1689/1747).
254 (13%) of 1943 infants were influenza-positive by either rRT-PCR or serology (table 2). The proportion of influenza-positive infants confirmed by rRT-PCR or serology appeared to vary by study site (from 9% in the Philippines to 16% in Albania) and was lower among infants aged 0–2 months (87 [9%] of 988 infants) than older infants (range 16–20%; p<0·0001). Infants described by their parents as in excellent or very good health before the illness were more likely to be influenza-positive (198 [15%] of 1363 infants) than those in good, fair, or poor health (56 [10%] of 580; p=0·010). The proportion of infants with influenza was highest among infants with parent-reported fever (p<0·0001), clinician-measured temperature of 39°C or higher (p<0·0001), or a diagnosis of febrile seizures (p<0·0001). Influenza positivity was not associated with number of days since illness onset. The proportion of infants admitted to intensive care units was similar for influenza-negative and influenza-positive infants (28% vs 22%; p=0·072).
Table 2:
Influenza status by diagnostic method
| Influenza-negative by both PCR and serology, n (%) | Influenza-positive | Proportion of confirmed cases identified by serology but not rRT-PCR* | |||||
|---|---|---|---|---|---|---|---|
| By rRT-PCR, n (%) | By serology only, n (%) | By rRT-PCR or serology | % (95% CI) | p value† | |||
| n (%) | p value‡ | ||||||
| Infants (n=1943) | 1689 (87%) | 152 (8%) | 102 (5%) | 254 (13%) | 40% (34 to 46) | ·· | |
| Dichotomous age, months | |||||||
| 0–5 (n=1352) | 1206 (89%) | 80 (6%) | 66 (5%) | 146 (11%) | <0·0001 | 45% (37 to 53) | 0·056 |
| 6–11 (n=591) | 483 (82%) | 72 (12%) | 36 (6%) | 108 (18%) | 33% (24 to 42) | ·· | |
| Categorical age, months | |||||||
| 0–2 (n=988) | 901 (91%) | 48 (5%) | 39 (4%) | 87 (9%) | <0·0001 | 45% (34 to 55) | 0·30 |
| 3–5 (n=364) | 305 (84%) | 32 (9%) | 27 (7%) | 59 (16%) | ·· | 46% (33 to 58) | ·· |
| 6–8 (n=328) | 263 (80%) | 43 (13%) | 22 (7%) | 65 (20%) | ·· | 34% (22 to 45) | ·· |
| 9–11 (n=263) | 220 (84%) | 29 (11%) | 14 (5%) | 43 (16%) | ·· | 33% (19 to 47) | ·· |
| Enrolment year | |||||||
| 2015–16 (n=937) | 815 (87%) | 72 (8%) | 50 (5%) | 122 (13%) | 0·950 | 41% (32 to 50) | 0·08 |
| 2016–17 (n=1006) | 874 (87%) | 80 (8%) | 52 (5%) | 132 (13%) | ·· | 39% (31 to 48) | ·· |
| Enrolment site | |||||||
| Albania (n=638) | 533 (84%) | 61 (10%) | 44 (7%) | 105 (16%) | 0·0045 | 42% (32 to 51) | 0·93 |
| Jordan (n=664) | 577 (87%) | 52 (8%) | 35 (5%) | 87 (13%) | ·· | 40% (30 to 51) | ·· |
| Nicaragua (n=307) | 276 (90%) | 20 (7%) | 11 (4%) | 31 (10%) | ·· | 35% (19 to 52) | ·· |
| Philippines (n=334) | 303 (91%) | 19 (6%) | 12 (4%) | 31 (9%) | ·· | 39% (22 to 56) | ·· |
| Sex | |||||||
| Boys (n=1121) | 979 (87%) | 80 (7%) | 62 (6%) | 142 (13%) | 0·540 | 44% (36 to 52) | 0·20 |
| Girls (n=822) | 710 (86%) | 72 (9%) | 40 (5%) | 112 (14%) | ·· | 36% (27 to 45) | ·· |
| Clinically preterm and gestational age <37 weeks§ (n=163) | 146 (90%) | 13 (8%) | 4 (2%) | 17 (10%) | 0·300 | 24% (3 to 44) | 0·15 |
| Chronic medical condition(s)¶ (n=162) | 147 (91%) | 8 (5%) | 6 (4%) | 14 (9%) | 0·400 | 43% (17 to 69) | 0·46 |
| Previous hospital admission (n=356) | 308 (87%) | 32 (9%) | 16 (4%) | 48 (13%) | 0·800 | 33% (20 to 47) | 0·28 |
| Parent-reported infant health‖ | |||||||
| Poor or fair (n=167) | 150 (90%) | 8 (5%) | 10 (6%) | 18 (11%) | 0·010 | 56% (33 to 79) | 0·087 |
| Good (n=413) | 375 (91%) | 18 (4%) | 20 (5%) | 38 (9%) | ·· | 53% (37 to 69) | ·· |
| Excellent or very good (n=1363) | 1165 (85%) | 126 (9%) | 72 (5%) | 198 (15%) | ·· | 36% (30 to 43) | ·· |
| Time since illness onset, days | |||||||
| 0–2 (n=1194) | 687 (58%) | 65 (5%) | 28 (2%) | 93 (8%) | 0·310 | 30% (21 to 39) | 0·039 |
| 3–4 (n=1194) | 604 (51%) | 54 (5%) | 49 (4%) | 103 (9%) | ·· | 48% (38 to 57) | ·· |
| 5–10 (n=749) | 398 (53%) | 33 (4%) | 25 (3%) | 58 (8%) | ·· | 43% (30 to 56) | ·· |
| Mean (SD) | 3·3 (2·0) | 3·2 (1·8) | 3·6 (2·0) | 3·4 (2) | 0·528 | ·· | ·· |
| SARI status** | |||||||
| No SARI (n=1109) | 1012 (91%) | 50 (5%) | 47 (4%) | 97 (9%) | <0·0001 | 48% (39 to 58) | 0·034 |
| Met SARI definition (n=834) | 677 (81%) | 102 (12%) | 55 (7%) | 157 (19%) | ·· | 35% (28 to 42) | ·· |
| Hospital department†† | |||||||
| General ward (n=1416) | 1219 (86%) | 128 (9%) | 69 (5%) | 197 (14%) | 0·072 | 35% (28 to 42) | 0·0020 |
| Intensive care unit (n=527) | 470 (89%) | 24 (5%) | 33 (6%) | 57 (11%) | ·· | 58% (45 to 71) | ·· |
| Antibiotic treatment after hospital admission | |||||||
| No (n=368) | 333 (90%) | 24 (7%) | 11 (3%) | 35 (10%) | 0·030 | 31% (16 to 47) | 0·26 |
| Yes (n=1575) | 1356 (86%) | 128 (8%) | 91 (6%) | 219 (14%) | 42% (35 to 48) | ·· | |
| Mean length of hospital stay, days | 5·9 (4·7) | 5·9 (4·1) | 6·0 (3·7) | 5·9 (4) | 0·978 | ·· | ·· |
| Mean time from onset to convalescence, days | 27·5 (7·9) | 29·6 (9·4) | 287 (8·6) | 29·2 (16) | 0·262 | ·· | ·· |
| Parent reported symptoms | |||||||
| Cough (n=128) | 1101 (86%) | 111 (9%) | 73 (6%) | 184 (14%) | 0·022 | 40% (33 to 47) | 0·80 |
| Other respiratory symptoms (n=1348) | 1155 (86%) | 119 (9%) | 74 (5%) | 193 (14%) | 0·010 | 38% (31 to 45) | 0·29 |
| Fever, felt hot, or chills (n=1049) | 854 (81%) | 129 (12%) | 66 (6%) | 195 (19%) | <0·0001 | 34% (27 to 40) | 0·0020 |
| Vomiting (n=584) | 520 (89%) | 31 (5%) | 33 (6%) | 64 (11%) | 0·070 | 52% (39 to 64) | 0·031 |
| Diarrhoea (n=464) | 407 (88%) | 27 (6%) | 30 (6%) | 57 (12%) | 0·560 | 53% (40 to 66) | 0·029 |
| Vital signs | |||||||
| High temperature‡‡ (n=252) | 186 (74%) | 42 (17%) | 24 (10%) | 66 (26%) | <0·0001 | 36% (25 to 48) | 0·47 |
| Hypoxaemia§§ (n=238) | 163 (68%) | 32 (13%) | 43 (18%) | 75 (32%) | 0·932 | 57% (46 to 69) | 0·0030 |
| Tachypnoea¶¶(n=387) | 332 (86%) | 29 (7%) | 26 (7%) | 55 (14%) | 0·624 | 47% (34 to 60) | 0·31 |
| Discharge diagnoses | |||||||
| Respiratory (or mixed)‖‖ (n=1191) | 1021 (86%) | 98 (8%) | 72 (6%) | 170 (14%) | 0·048 | 42% (35 to 50) | 0·31 |
| Non-respiratory (n=752) | 668 (89%) | 54 (7%) | 30 (4%) | 84 (11%) | ·· | 36% (25 to 46) | ·· |
| Common respiratory diagnoses | |||||||
| Acute respiratory distress syndrome (n=13) | 13 (100%) | 0 | 0 | 0 | 0·160 | ·· | NA |
| Bronchiolitis or asthma (n=422) | 366 (87%) | 26 (6%) | 30 (7%) | 56 (13%) | 0·160 | 54% (41 to 67) | 0·020 |
| Pertussis-like syndrome (n=76) | 71 (93%) | 2 (3%) | 3 (4%) | 5 (7%) | 0·120 | 60% (17 to 103) | 0·39 |
| Pneumonia (n=757) | 668 (88%) | 48 (6%) | 41 (5%) | 89 (12%) | 0·810 | 46% (36 to 56) | 0·16 |
| Viral or other (n=220) | 182 (83%) | 32 (15%) | 6 (3%) | 38 (17%) | 0·001 | 16% (4 to 27) | 0·0010 |
| Non-respiratory diagnoses | |||||||
| Dehydration (n=160) | 149 (93%) | 5 (3%) | 6 (4%) | 11 (7%) | 0·033 | 55% (25 to 84) | 0·36 |
| Febrile seizures (n=59) | 43 (73%) | 12 (20%) | 4 (7%) | 16 (27%) | <0·0001 | 25% (4 to 46) | 0·20 |
| Meningitis (n=30) | 26 (87%) | 4 (13%) | 0 | 4 (13%) | 0·720 | ·· | 0·15 |
| Sepsis (n=358) | 320 (89%) | 27 (8%) | 11 (3%) | 38 (11%) | 0·490 | 29% (15 to 43) | 0·13 |
| Urinary tract infection (n=62) | 56 (90%) | 4 (6%) | 2 (3%) | 6 (10%) | 0·590 | 33% (−4 to 71) | 0·31 |
| Viral or other (n=764) | 703 (92%) | 33 (4%) | 28 (4%) | 61 (8%) | <0·0001 | 46% (33 to 58) | 0·29 |
| Influenza status | |||||||
| A Influenza positive (n=1943) | ·· | 106 (5%) | 89 (5%) | 198 (10%) | ·· | ·· | ·· |
| A(H1N1)pdm09 positive (n=1943) | ·· | 29 (1%) | 22 (1%) | 51 (3%) | ·· | 43% (30 to 57) | 0·63 |
| A(H3N2) positive (n=1943) | ·· | 76 (4%) | 67 (3%) | 143 (7%) | ·· | 47% (39 to 55) | 0·014 |
| B Influenza positive (n=1943) | ·· | 46 (2%) | 16 (1%) | 62 (3%) | ·· | ·· | ·· |
| B (Victoria) positive (n=1943) | ·· | 43 (2%) | 14 (1%) | 57 (3%) | ·· | 25% (13 to 36) | 0·0020 |
Data are n, or n/N (%), unless otherwise specified.
CIs calculated using binomial method.
χ2 or Fisher’s exact test of association between each variable and influenza-positive infants diagnosed only by serology but not by rRT-PCR.
χ2, Fisher’s exact test, or one-way ANOVA test of association between each variable and influenza positivity (by rRT-PCR or serology).
Infants were considered full term if they were born at known gestational age ≥37 weeks; infants born with a calculated gestational age of <37 weeks were not considered preterm if parents did not report them as such in questionnaire.
40 infants had no chronic conditions reported because their status was classified as unknown or their parents refused to provide this information.
Some values imputed to complete dataset. Imputations carried using a linear combination of other variables (infant age, country, study year, and depravation).
An acute respiratory infection with history of fever or measured fever of ≥38°C and cough with onset within the last 10 days that required hospital admission.
Includes infants who required treatment in the intensive care unit but were placed in a general ward.
Temperature ≥39°C; 1935 infants had data for this variable.
For infants aged 0–2 months, respiration rate >60 breaths per min, for infants aged 2–12 months, respiration rate >50 breaths per min; 1310 infants had data for this variable.
Oxygen saturation <93%; 1605 infants had data for this variable.
Includes reports that listed the named discharge diagnosis as primary or other secondary cause of hospital admission.
Of the 254 infants with confirmed influenza (according to rRT-PCR, serology, or both), 102 (40%, 95% CI 34–46) were positive by serology only. The diagnosis was established by serology only, but not by rRT-PCR, in 67 (47%) of 143 infants with influenza A (H3N2), 22 (43%) of 51 with influenza A H1N1pdm09, and 14 (25%) of 57 with influenza B(Victoria) viruses (table 2). The contribution of serological diagnosis increased with days since illness onset (p=0·039); the proportion of influenza-positive infants confirmed by serology but missed by rRT-PCR was lower among those admitted within 0–2 days of illness onset (28 [30%] of 93) than among those admitted 3–4 days after onset (49 [48%] of 103). Diagnosis by serology only was higher among infants admitted to the intensive care unit (33 [58%] of 57) than infants admitted to the general ward (69 [35%] of 197; p=0·0020). Diagnosis by serology only was also more common among infants with hypoxaemia at admission than those without (p=0·0030) or a clinical diagnosis of bronchiolitis or asthma (p=0·020). The proportion of serology-only diagnoses among all influenza-positive infants did not differ significantly by study site, age, demographic, health, or clinical characteristics, length of hospital stay, or days between illness onset and convalescent sera collection (table 2).
Of the 254 influenza-positive infants, 167 (66%) had only respiratory clinical discharge diagnoses, three (1%) had both respiratory and non-respiratory diagnoses, and 84 (33%) had only non-respiratory discharge diagnoses. The most common respiratory discharge diagnoses were pneumonia, bronchiolitis, and other respiratory viral disease. The most common non-respiratory diagnoses were sepsis, febrile seizures, dehydration, and other non-respiratory viral diseases.
No statistically significant differences in the proportion of influenza-positive infants with non-respiratory discharge diagnoses were identified by age, demographic, or health characteristics (table 3). However, the proportion of influenza-positive infants with non-respiratory diagnoses differed by study site, with statistically significant differences identified between Albania (17 [16%] of 105 infants) and Jordan (47 [54%] of 87 infants; p<0·0001). This difference corresponded with differences in clinical diagnoses between sites: 260 (39%) of 664 infants in Jordan had a sepsis discharge diagnosis compared with less than 14% at the other sites (table 1). Differences observed in the proportion of influenza-positive infants with non-respiratory diagnoses across study sites were smaller and not statistically significant when stratified by age group (table 4). The proportion of infants with non-respiratory diagnoses was higher among those infected with influenza A (H3N2) than those with other influenza A or B viruses (p=0·019; table 3).
Table 3:
Influenza virus positives by clinician discharge diagnosis and proportion of all influenza-positive infants with non-respiratory diagnoses
| Respiratory or mixed diagnoses, n (%) | Non-respiratory diagnosis, n (%) | Proportion of influenza-positive cases with non-respiratory diagnosis, % (95% CI)* | p value† | |
|---|---|---|---|---|
| Influenza-positive infants (n=254) | 170 (67%) | 84 (33%) | 33 (27 to 39) | ·· |
| Dichotomous age, months | ||||
| 0–5 (n=146) | 93 (64%) | 53 (36%) | 36 (29 to 44) | 0·20 |
| 6–11 (n=108) | 77 (71%) | 31 (29%) | 29 (20 to 37) | ·· |
| Catergorical age, months | ||||
| 0–2 (n=87) | 51 (59%) | 36 (41%) | 41 (31 to 52) | 0·091 |
| 3–5 (n=59) | 42 (71%) | 17 (29%) | 29 (17 to 40) | ·· |
| 6–8 (n=65) | 50 (77%) | 15 (23%) | 23 (13 to 33) | ·· |
| 9–11 (n=43) | 27 (63%) | 16 (37%) | 37 (23 to 52) | ·· |
| Enrolment year | ||||
| 2015–16 (n=122) | 86 (70%) | 36 (30%) | 30 (21 to 38) | 0·25 |
| 2016–17 (n=132) | 84 (64%) | 48 (36%) | 36 (28 to 45) | ·· |
| Enrolment site | ||||
| Albania (n=105) | 88 (84%) | 17 (16%) | 16 (9 to 23) | <0·0001 |
| Jordan (n=87) | 40 (46%) | 47 (54%) | 54 (44 to 64) | ·· |
| Nicaragua (n=31) | 18 (58%) | 13 (42%) | 42 (25 to 59) | ·· |
| Philippines (n=31) | 24 (77%) | 7 (23%) | 23 (8 to 37) | ·· |
| Sex | ||||
| Boys (n=142) | 97 (68%) | 45 (32%) | 32 (24 to 39) | 0·60 |
| Girls (n=112) | 73 (65%) | 39 (35%) | 35 (26 to 44) | ·· |
| Clinical preterm and gestational age <37 weeks‡ (n=17) | 13 (76%) | 4 (24%) | 24 (3 to 44) | 0·39 |
| Chronic medical condition(s)§ (n=14) | 12 (86%) | 2 (14%) | 14 (−4 to 33) | 0·11 |
| Previous hospital admission (n=48) | 34 (71%) | 14 (29%) | 29 (16 to 42) | 0·52 |
| Parent reported infant health¶ | ||||
| Poor or fair (n=18) | 13 (72%) | 5 (28%) | 28 (7 to 48) | 0·79 |
| Good (n=38) | 26 (68%) | 12 (32%) | 32 (17 to 46) | ·· |
| Excellent or very good (n=198) | 131 (66%) | 67 (34%) | 34 (27 to 40) | ·· |
| Time since illness onset, days | ||||
| 0–2 days (n=93) | 51 (55%) | 42 (45%) | 45 (35 to 55) | 0·0075 |
| 3–4 days (n=103) | 77 (75%) | 26 (25%) | 25 (17 to 34) | ·· |
| 5–10 days (n=58) | 42 (72%) | 16 (28%) | 28 (16 to 39) | ·· |
| Mean (SD) | 3·5 (1·8) | 3·0 (2·0) | 0·11 | |
| SARI status‖ | ||||
| No SARI (n=97) | 38 (39%) | 59 (61%) | 61 (51 to 71) | <0·0001 |
| Met SARI definition (n=157) | 132 (84%) | 25 (16%) | 16 (10 to 22) | ·· |
| Hospital department** | ||||
| General ward (n=197) | 133 (68%) | 64 (32%) | 32 (26 to 39) | 0·71 |
| Intensive care unit (n=57) | 37 (65%) | 20 (35%) | 35 (23 to 47) | ·· |
| Antibiotic treatment after hospital admission | ||||
| No (n=35) | 20 (57%) | 15 (43%) | 43 (26 to 59) | 0·19 |
| Yes (n=219) | 150 (68%) | 69 (32%) | 32 (25 to 38) | ·· |
| Mean length of hospital stay, days (SD) | 6·2 (4·0) | 5·3 (3·7) | 0·75 | |
| Meant time from illness onset to convalescence, days (SD) | 30·1 (9·5) | 27·2 (7·5) | 0·47 | |
| Parent reported symptoms | ||||
| Cough (n=184) | 155 (84%) | 29 (16%) | 16 (10 to 21) | <0·0001 |
| Other respiratory symptoms (n=193) | 156 (81%) | 37 (19%) | 19 (14 to 25) | <0·0001 |
| Fever, felt hot, or chills (n=195) | 135 (69%) | 60 (31%) | 31 (24 to 37) | 0·16 |
| Vomiting (n=64) | 41 (64%) | 23 (36%) | 36 (24 to 48) | 0·57 |
| Diarrhoea (n=57) | 28 (49%) | 29 (51%) | 51 (38 to 64) | 0·0012 |
| Vital signs | ||||
| High temperature (≥39°C; n=66) | 41 (62%) | 25 (38%) | 38 (26 to 50) | 0·065 |
| Hypoxaemia†† (n=75) | 58 (77%) | 17 (23%) | 23 (13 to 32) | 0·38 |
| Tachypnoea‡‡ (n=55) | 48 (87%) | 7 (13%) | 13 (4 to 22) | 0·0026 |
| Respiratory diagnoses | ||||
| Acute respiratory distress syndrome (n=0) | 0 | 0 | ·· | NA |
| Bronchiolitis or asthma (n=56) | 56 (100%) | 0 | ·· | <0·0001 |
| Pertussis-like syndrome (n=5) | 5 (100%) | 0 | ·· | 0·11 |
| Pneumonia (n=89) | 87 (98%) | 2 (2%) | 2·2 (−1 to 5·3) | <0·0001 |
| Viral or other (n=38) | 33 (87%) | 5 (13%) | 13 (2 to 24) | 0·0021 |
| Non-respiratory diagnoses | ||||
| Dehydration (n=11) | 1 (9%) | 10 (91%) | 91 (74 to 108) | <0·0001 |
| Febrile seizures (n=16) | 6 (38%) | 10 (62%) | 63 (39 to 86) | 0·0097 |
| Meningitis (n=4) | 1 (25%) | 3 (75%) | 75 (33 to 117) | 0·11 |
| Sepsis (n=38) | 12 (32%) | 26 (68%) | 68 (54 to 83) | <0·0001 |
| Urinary tract infection (n=6) | 0 | 6 (100%) | 100 (100 to 100) | 0·0012 |
| Viral or other (n=61) | 16 (26%) | 45 (74%) | 74 (63 to 85) | <0·0001 |
| Influenza status by rRT-PCR or serology | ||||
| A Influenza positive (n=198) | 127 (64%) | 71 (36%) | 36 (29 to 43) | 0·075 |
| A(H1N1)pdm09 positive (n=51) | 36 (71%) | 15 (29%) | 29 (17 to 42) | 0·53 |
| A(H3N2) positive (n=143) | 87 (61%) | 56 (39%) | 39 (31 to 47) | 0·019 |
| B Influenza positives (n=62) | 47 (76%) | 15 (24%) | 24 (14 to 35) | 0·088 |
| B (Victoria) positive (n=57) | 42 (74%) | 15 (26%) | 26 (15 to38) | 0·21 |
Data are n or n/N (%), unless otherwise specified. SARI=severe acute respiratory infection. rRT-PCR=real-time RT-PCR.
CIs calculated using binomial method.
χ2, Fisher’s exact test, or one-way ANOVA test of association between each variable and respiratory diagnosis, among all influenza-positive samples.
Infants were considered full term if they were born at known gestational age ≥37 weeks; infants born with a calculated gestational age of <37 weeks were not considered preterm if parents did not report them as such in questionnaire.
40 infants had no chronic conditions reported because their status was classified as unknown or their parents refused to provide this information.
Some values imputed to complete dataset, using a linear combination of other variables (infant age, country, study year and depravation).
An acute respiratory infection with history of fever or measured fever of ≥38°C and cough with onset within the last 10 days that required hospital admission.
General ward includes infants who required treatment in the intensive care unit but were placed in a general ward.
Oxygen saturation <93%.
For infants aged 0–2 months, respiration rate >60 breaths per min; for infants aged 2–12 months, respiration rate >50 breaths per min.
Table 4:
All influenza virus positives by respiratory versus non-respiratory discharge diagnosis and underdetection multiplier by age
| All rRT-PCR positives (n=152) | Positive by serology only (n=102) | Underdetection multiplier*, X (95% CI) | |||
|---|---|---|---|---|---|
| Respiratory or mixed, n (%) | Non- respiratory, n (%) | Respiratory or mixed, n (%) | Non-respiratory, n (%) | ||
| Influenza-positive infants (n=254) | 98 (39%) | 54 (21%) | 72 (28%) | 30 (12%) | 2·6 (2·0–3·6)† |
| Age, months | |||||
| 0–5 (n=146) | 45 (31%) | 35 (24%) | 48 (33%) | 18 (12%) | 3·0 (2·1–5·0)† |
| 6–11 (n=108) | 53 (49%) | 19 (18%) | 24 (22%) | 12 (11%) | 2·0 (1·7–2·5)† |
| Hospital department | |||||
| General ward (n=197) | 84 (43%) | 44 (22%) | 49 (25%) | 20 (10%) | 2·4 (1·8–3·4)† |
| Intensive care unit (n=57) | 14 (25%) | 10 (18%) | 23 (40%) | 10 (18%) | 4·1 (2·7–9·1)† |
| Influenza subtype | |||||
| A(H1N1) pdm09 positive (n=51) | 22 (43%) | 7 (14%) | 14 (27%) | 8 (16%) | 2·3 (1·6–4·0)† |
| A(H3N2) positive (n=143) | 39 (27%) | 37 (26%) | 48 (34%) | 19 (13%) | 4·0 (2·6–8·4)† |
| Blq B (Victoria) positive (n=57) | 32 (56%) | 12 (21%) | 10 (18%) | 3 (5%) | 1·8 (1·4–2·3)† |
| Enrolment site | |||||
| Albania (n=105) | 53 (50%) | 8 (8%) | 35 (33%) | 9 (9%) | 2·0 (1·5–2·8) |
| Jordan (n=87) | 19 (22%) | 33 (38%) | 21 (24%) | 14 (16%) | 4·6 (3·3–7·7) |
| Nicaragua (n=31) | 12 (39%) | 8 (26%) | 6 (19%) | 5 (16%) | 2·6 (1·8–4·8) |
| Philippines (n=31) | 14 (45%) | 5 (16%) | 10 (32%) | 2 (6%) | 2·2 (1·6–3·6) |
| Age 0–5 months by site | |||||
| Albania (n=53) | 23 (43%) | 2 (4%) | 23 (43%) | 5 (9%) | 2·2 (1·5–4·2) |
| Jordan (n=63) | 11 (17%) | 25 (40%) | 16 (25%) | 11 (17%) | 5·7 (3·7–12·7) |
| Nicaragua (n=20) | 8 (40%) | 5 (25%) | 5 (25%) | 2 (10%) | 2·3 (1·4–10·8) |
| Philippines (n=10) | 3 (30%) | 3 (30%) | 4 (40%) | 0 | 3·3 (1·7–57·4) |
| Age 6–11 months by site | |||||
| Albania (n=52) | 30 (58%) | 6 (12%) | 12 (23%) | 4 (8%) | 1·7 (1·4–2·3) |
| Jordan (n=24) | 8 (33%) | 8 (33%) | 5 (21%) | 3 (13%) | 3·0 (1·9–7·5) |
| Nicaragua (n=11) | 4 (36%) | 3 (27%) | 1 (9%) | 3 (27%) | 2·8 (1·5–12·6) |
| Philippines (n=21) | 11 (52%) | 2 (10%) | 6 (29%) | 2 (10%) | 1·9 (1·4–3·2) |
Estimates pooled by year unless otherwise indicated. Some percentages do not equal 100 because of rounding.
Pooled estimates calculated using beta binomial models with maximum likelihood estimation.
Pooled by site and year.
The proportion of influenza-positive infants with non-respiratory diagnoses was highest among infants admitted within 0–2 days of illness onset (42 [45%] 93), and was significantly higher than that among infants admitted 3–4 days after onset (26 [25%] of 103; p=0·0080; table 3). Infants with respiratory diagnoses were more likely to have cough (p<0·0001) and tachypnoea (p<0·0001), but less likely to have diarrhoea (p=0·0012). Among both respiratory and non-respiratory diagnosis groups, the proportion of infants with parent-reported fever was similar (135 [79%] of 170 vs 60 [71%] of 84), and the proportion of infants with a temperature of 39°C or higher was similar (41 [24%] of 170 vs 25 [30%] of 84). The proportion of infants admitted to intensive care units compared with general wards was also similar in both diagnosis groups. A substantial proportion of influenza-positive infants discharged with only non-respiratory diagnoses had a cough (29 [35%] of 84) or other respiratory symptoms (37 [45%] of 84) at admission; 17 (21%) of 84 infants had signs of hypoxaemia and seven (9%) of 84 infants had signs of tachypnoea.
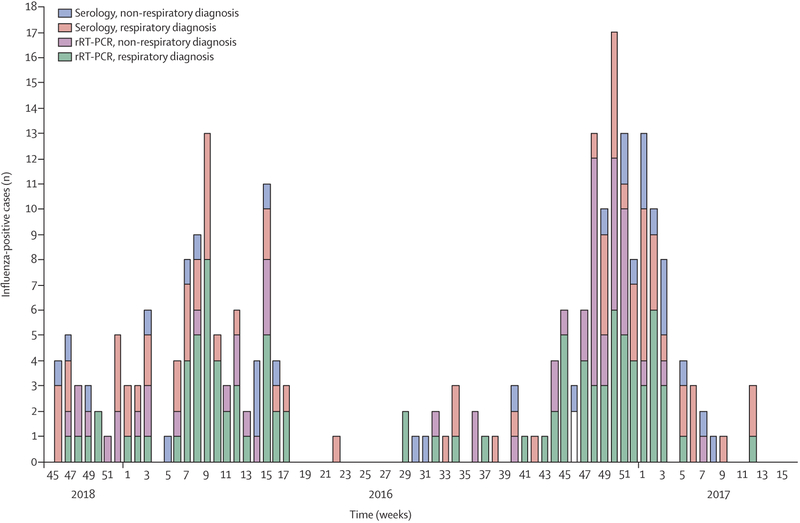
The 98 infants with rRT-PCR confirmed influenza who had an acute respiratory illness discharge diagnosis accounted for only 39% of the 254 infants with any acute illness and influenza virus infection confirmed by either rRT-PCR or serology. This indicates that a focus on respiratory PCR-positive influenza disease underdetects influenza-associated hospital admissions among infants by a factor of 2·6 (95% CI 2·0–3·6), accounting for study site and season variation (table 4). The point estimate of this adjusted multiplier was higher for infants aged 0–5 months (3·0, 95% CI 2·1–5·0) than infants aged 6–11 months (2·0, 1·7–2·5), but the 95% CIs overlapped. The contribution of serological and non-respiratory diagnoses to the total number of influenza-positive infants observed across sites is shown in figure 2; site-specific information is presented in the appendix (pp 18–21).
Figure 2: Number of influenza-positive infants by diagnostic method and clinical discharge diagnosis across study sites.
rRT-PCR=real-time RT-PCR.
Of the 1467 infants screened for inclusion in the non-ill group, 107 (7%) were ineligible because of current or recent illness (onset ≤7 days) and 24 (2%) did not complete enrolment or their parents refused consent; of the remaining 1335 non-ill infants, 745 were enrolled during the influenza study period (appendix pp 11, 12). Influenza virus infection was detected by rRT-PCR in 21 (3%, 95% CI 1·6–4·0) of 745 infants. Similar to the acutely ill infants, a mixture of influenza types and subtypes were identified.
During year 2 (between May 2016, and April 2017), the parents of 400 non-ill infants were contacted a mean 7·5 days (SD 2·3) after respiratory swab collection. Of the 15 infants who were not ill during enrolment but later tested positive for influenza by rRT-PCR, four (27%) subsequently developed one or more respiratory or non-respiratory illness symptoms. This proportion was similar to the that of non-ill rRT-PCR-negative infants (18%) who subsequently developed illness symptoms (appendix p 13).
Across all sites and seasons, 834 (43%) of 1943 infants met the case definition for SARI (cough and fever) at hospital admission (range across sites 35–53%; table 1). Of the 254 influenza-positive infants (by rRT-PCR or serology), 157 (62%) met the case definition at admission; thus, 97 (38%) were non-SARI admissions (table 2). The proportion of influenza-positive infants admitted to hospital with non-SARI was higher among infants aged 0–2 months (45 [52%] of 97) than those aged 6–11 months (33 [31%] of 108; p=0·0027; appendix pp 15, 16). After adjusting for study site and season, assessment of rRT-PCR-confirmed influenza presenting with SARI underdetected influenza-associated hospital admissions among infants by a factor of 2·6 (95% CI 2·0–3·5; appendix p 17).
Similarly, although we focused on clinical diagnoses at discharge, these diagnoses were consistent with admission diagnoses; most infants (1793 [92%] of 1943) remained in the same diagnostic category from admission to discharge (appendix p 17).
Discussion
In our multicentre, prospective study, we found that the incidence of influenza-associated hospital admissions among infants younger than 1 year was 2·6 times higher than would have been identified by rRT-PCR testing of acute respiratory illnesses. Relying on molecular diagnostics missed 40% of the 254 influenza virus infections identified through a combination of rRT-PCR and serology. Furthermore, 33% of influenza-positives were identified among acutely ill infants without respiratory discharge diagnoses.
Our study exclusively focused on infants and simultaneously assessed both clinical and diagnostic sources of underdetection. Our findings were similar to those of previous studies that examined these issues separately among children across a broader age range. In two studies of pneumonia aetiology, the number of influenza-positive infants identified only by serology constituted 41% of all children admitted to hospital with influenza (61 of 149 with A or B viruses by either rRT-PCR or serology) in US children aged younger than 18 years11 and 47% of children admitted to hospital with influenza among children aged younger than 5 years in Thailand.12 Studies done in emergency departments in France of children aged 0–11 months6 and 0–35 months7 found that one-third of children identified as influenza-positive by rRT-PCR had predominantly non-respiratory symptoms (30% and 33%, respectively).
The implications of our findings partly depend on how the discordance between rRT-PCR and serological diagnoses of influenza virus infection is interpreted. As expected, the proportion of infants with influenza identified by serology only was lowest among those admitted within 0–2 days of illness onset, when viral shedding would be highest, but even in this group, about one-third of all influenza cases were rRT-PCR negative. Since testing upper respiratory specimens with the rRT-PCR assay is typically considered to be a highly sensitive and specific method, it is surprising that rRT-PCR assays were only 48% sensitive to seroconversions and thus missed 102 of 254 influenza cases. Such underdetection was documented despite our study using rRT-PCR assays in CDC-approved laboratories on combed nasal and oropharyngeal specimens22 collected within 4 days of illness onset for most infants. The use of rRT-PCR as a gold standard emerged from studies comparing the assay with virus culture and immunofluorescence rather than measures of immune response, and previous research22 suggests the sensitivity of rRT-PCR can reduce to 80% depending on specimen type and other differences in methodology. It is also possible that sampling from the upper respiratory tract might have missed virus concentrated in the lower respiratory tract. Further research needs to examine whether the shedding or concentration of virus varies by type or subtype and among infants and young children. The similar sensitivity (62%) of seroconversion to rRT-PCR-confirmed influenza among infants in our study is less surprising considering that multiple factors could have prevented seroconversion, including known limits in measurement sensitivity of haemagglutination inhibition,23,24 elevated titres among young infants from maternal antibodies, and the inability of young and immune-challenged infants to mount a robust antibody response to infection.25,26
Nosocomial influenza virus infection among children is not uncommon.27 Thus, some of the infants in our study who were rRT-PCR-influenza-negative at admission but who had a more than or equal to four-fold increase in influenza antibody titres about a month later could have been infected during their stay in hospital. Although the development of increased antibody concentrations after infection is hypothesised to take at least 2 weeks,19 it is also possible that some infants were infected shortly after hospital discharge. In our study, the proportion of infants with influenza detected by serology only was similar across study hospitals and was not higher among chronically ill or premature infants, those with prolonged hospital stays, or those with prolonged periods between acute and convalescent sera collection. However, the proportion of infants with influenza detected by serology only was higher among infants admitted to the intensive care unit than general wards, which raises the question of whether this could be partly due to nosocomial infections in intensive care units associated with increased health vulnerabilites, increased exposure to aerosol-producing medical procedures, or other risks. Future studies that repeatedly swab infants and test for influenza by rRT-PCR during and after hospital admission are needed to clarify how many of the serology-confirmed influenza infections were acquired after hospital admission.
It is important to note that the absence of a respiratory diagnosis by a clinician among one-third of influenza cases does not imply the disease had no respiratory involvement. Indeed, almost half of the infants with only non-respiratory discharge diagnoses had cough or other respiratory signs and symptoms at admission. Since our study relied on clinician-recorded vital signs and diagnoses, it is possible that clinicians misdiagnosed some disease or were simply more focused on other manifestations of diseases, such as sepsis and febrile seizures. Since study hospitals did not have routine molecular clinical testing, clinicians rarely knew the underlying pathogens. Thus, across sites, clinicians referred to clinical syndromes and observable signs; “other viral illness” was the most commonly abstracted respiratory and non-respiratory diagnosis. Clearly, future research with clinicians trained to use standard diagnostic procedures are needed to describe clinical features with greater detail and consistency. As expected, testing all acutely ill infants identified a substantial proportion of influenza-positive infants who would have been missed by an exclusive focus on respiratory diagnoses, and this was consistent across seasons and study sites. Re-examination of our findings using a syndromic definition, SARI, showed that rRT-PCR testing of patients with this condition at admission underdetected the total number of infants with influenza by 2·6 times, which is identical to our findings using clinical discharge diagnoses.
Our sample of infants admitted to hospital with influenza is among the largest to date, especially outside of high-income countries. Nonetheless, future studies with even larger samples are needed to explore the heterogeneity we observed by age, disease severity, and viruses. Several findings suggest underdetection of influenza might be higher for young infants (aged <6 months), more severely ill infants including those admitted to intensive care units, and infants with influenza A (H3N2) virus infections; however, these trends were not statistically significant in pooled models that accounted for variations by study year and site. Since hospital admission can be driven by medical and social factors and admission and diagnostic practices can vary greatly within and across countries (eg, Albania and Jordan had a greater volume of admissions and availability of intensive care),4,5,28 the fact that the underdetection multiplier trended higher (at 4·1-fold) among the most severely ill infants suggests that the pooled effect across all infants is likely to be a conservative estimate of true effect size.
Although IRIS was designed to inform and improve interpretation of studies of influenza burden, our study was not designed to provide burden estimates. The extent to which our pooled site and study year estimates can be generalised to other countries is unclear. Variation in influenza incidence estimates among children even within countries during the same seasons is common.29,30 Thus, mathematical models that can account for multiple sources of heterogeneity are needed to estimate influenza burden and the potential preventive value of influenza vaccine programmes.21,31 Knowledge about the fraction of influenza virus infections among infants that might have atypical disease presentations or might be missed by molecular diagnostics could also be relevant to infection control measures in hospitals27 and to characterising trans mission dynamics using mathematical models.
Our study had many strengths, including the use of a common protocol, tools, and laboratory methods. Systematic enrolment and high participation and follow-up rates reduced the risk of selection biases. However, the causative role of influenza virus infections in disease aetiology and hospital admissions must be interpreted with caution. Our finding that 3% of non-ill infants enrolled simultaneously from the local communities tested positive for influenza by rRT-PCR is a reminder of the complexity of interpreting diagnostic results. Although studies of attributable risk have concluded that influenza has a relatively strong association with clinical illness,5,32 further research focused on infants is needed. The fact that during year 2 of our study (2016–17), four (27%) of 15 of non-ill infants with rRT-PCR-influenza became ill in the days after swabbing highlights the challenges in making causal attributions given that some presumably non-ill infants might not be truly asymptomatic. Furthermore, our study is limited by a lack of information on possible coinfections with other pathogens, pending further laboratory assessments. As with all influenza studies, the generalisability of our findings are limited by the specific influenza viruses in circulation during the study seasons and at specific sites; however, our study included infants infected with a mixture of influenza A (H1N1)pdm09, influenza A (H3N2), and influenza B(Victoria) viruses across all study sites. Moreover, serological methods differed for influenza A and influenza B viruses due to the circulating viruses and measurement best practices.19 For influenza A (H3N2), serological positivity was indicated by seroconversion to either of two circulating influenza A (H3N2) viruses. For influenza B viruses, seroconversion by both haemagglutination inhibition and microneutralisation was required, which was likely to have improved specificity but might have reduced sensitivity to infection. The specific laboratory methods required for this study might limit the generalisability of findings to a season with different circulating influenza viruses. Further analyses of antibody response to influenza virus infections from IRIS are planned.
If the true burden of laboratory-confirmed influenza-associated hospital admissions among infants is at least twice that of previous estimates, this could have implications for ongoing debates about the preventive value of introducing or expanding influenza vaccination programmes for pregnant women and infants aged 6 months or older. Justifying the preventive value of influenza vaccines in LMICs is especially challenging considering the many obstacles to implementation and competing public health priorities.13,14 Even in high-income countries, a better understanding of the vaccine-preventable burden of severe influenza among infants is needed to inform future research and policy with regard to optimal vaccine types and strategies for pregnant women and infants aged 6 months or older.33
Supplementary Material
Research in context.
Evidence before this study
Although influenza is an established cause of morbidity and mortality among infants worldwide, determining the burden of influenza is challenging since infants might present with atypical symptoms or be admitted to the hospital later in the illness, when they no longer test positive by molecular testing. We searched PubMed from database inception to Dec 31, 2018, for studies published in English using the search term “influenza” and filters for “human” and ages “newborn”, “infant”, or “child”; within these results, we applied the search terms “acute respiratory”, “burden”, “hospital”, or “serology”. We identified two studies of pneumonia aetiology that assessed influenza virus infection by both real-time RT-PCR (rRT-PCR) and serology among children younger than 18 years in the USA and among children younger than 5 years in Thailand. Both studies focused on acute respiratory disease only and data on infants were scarce. We identified two studies of children aged 0–11 months and aged 0–35 months done in emergency departments in France, which tested all acutely ill infants for influenza by rRT-PCR only. With the exception of the study in Thailand, all relevant previous studies were done in high-income countries with temperate climates.
Added value of this study
Our study is unique in its exclusive focus on infants aged younger than 1 year and the simultaneous assessment of both clinical and diagnostic sources of underdetection. Our study of infants admitted to hospital with influenza in four middle-income countries (including two countries with tropical climates) is among the largest to date. We found that molecular diagnostics missed more than one-third of influenza virus infections identified by a combination of rRT-PCR and serology. Furthermore, one-third of influenza-positive cases were identified among acutely ill infants without respiratory clinical discharge codes. To our knowledge, this is the first study to estimate the magnitude of influenza underdetection in this population using a broad case definition and applying both molecular and serological diagnostic methods.
Implications of all the available evidence
The global burden of severe influenza would be substantially underestimated if the true incidence of laboratory-confirmed influenza-associated hospital admissions among infants is at least twice that of previous estimates using existing respiratory surveillance platforms. This incremental increase in burden estimates might expand estimates of the preventive value of maternal and infant influenza vaccination programmes, which is especially relevant to low-income and-middle-income countries considering the many obstacles to implementation and competing health priorities. Even in high-income countries, a better understanding of the vaccine-preventable burden of severe influenza among infants is needed to inform future research and policy with regard to optimal vaccine types and strategies for pregnant women and infants aged 6 months and older. Further research is warranted to examine the extent to which underdetection of influenza varies by age groups, disease severity, and virus type or subtype.
Acknowledgments
This study was funded by the US Center for Disease Control and Prevention (CDC) through contract HHSD2002013M53890B and task 200-2014-F-60406 to Abt Associates. We wish to thank other IRIS Network investigators from the US CDC (Brett Whitaker, Teresa C T Peret, Sue Gerber), Abt Associates (Rebecca Fink, Laura Edwards), Nicaragua (Guillermina Kuan, Nery Sanchez, Sergio Ojeda, Karla Patricia Membreño), Jordan (Aktham Haddadin, Mahmoud Al-Gazo), and the Philippines (Lei Lanna Mendoza - Dancel and Karen Lana Cruz). The authors would also like to thank the following from the US CDC (Alicia Fry, Jerome Tokars, Marc-Alain Widdowson, Melissa Rolfes) and Nicaragua (Karena Vega, Eveling Aguilar, Jasmina Ampie, Vilmaricia Cerda, Yexica Chavez, Heydi Rodriguez, Wismar Whitman Ubau Andino, Noritza Martinez, Roger Lopez, Andrea Nuñez, Ubania Vargas, Jose Victor Zambrana, William Aviles, Raquel Burger-Calderon, Douglas Elizondo, Brenda Lopez). Authors from all study sites wish to thank local collaborators at participating study hospitals and health centres, and all study participants and their families.
Footnotes
See Online for appendix
Declaration of interests
We declare no competing interests. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the US Centers for Disease Control and Prevention.
Data sharing
We intend to make data freely available in the public domain after the publication of major findings. Researchers interested in collaborations should contact the corresponding author. We welcome proposals to use this data from potential collaborators or from researchers interested in investigating specific questions independently.
References
- 1.Nair H, Brooks WA, Katz M, et al. Global burden of respiratory infections due to seasonal influenza in young children: a systematic review and meta-analysis. Lancet 2011; 378: 1917–30. [DOI] [PubMed] [Google Scholar]
- 2.O’Brien MA, Uyeki TM, Shay DK, et al. Incidence of outpatient visits and hospitalizations related to influenza in infants and young children. Pediatrics 2004; 113: 585–93. [DOI] [PubMed] [Google Scholar]
- 3.Chaves SS, Perez A, Farley MM, et al. The burden of influenza hospitalizations in infants from 2003 to 2012, United States. Pediatr Infect Dis J 2014; 33: 912–19. [DOI] [PubMed] [Google Scholar]
- 4.Silvennoinen H, Peltola V, Vainionpaa R, Ruuskanen O, Heikkinen T. Incidence of influenza-related hospitalizations in different age groups of children in Finland: a 16-year study. Pediatr Infect Dis J 2011; 30: e24–28. [DOI] [PubMed] [Google Scholar]
- 5.Shi T, McLean K, Campbell H, Nair H. Aetiological role of common respiratory viruses in acute lower respiratory infections in children under five years: a systematic review and meta-analysis. J Glob Health 2015; 5: 010408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ploin D, Liberas S, Thouvenot D, et al. Influenza burden in children newborn to eleven months of age in a pediatric emergency department during the peak of an influenza epidemic. Pediatr Infect Dis J 2003; 22 (10 suppl): S218–22. [DOI] [PubMed] [Google Scholar]
- 7.Ploin D, Gillet Y, Morfin F, et al. Influenza burden in febrile infants and young children in a pediatric emergency department. Pediatr Infect Dis J 2007; 26: 142–47. [DOI] [PubMed] [Google Scholar]
- 8.Marcone DN, Durand LO, Azziz-Baumgartner E, et al. Incidence of viral respiratory infections in a prospective cohort of outpatient and hospitalized children aged ≤5 years and its associated cost in Buenos Aires, Argentina. BMC Infect Dis 2015; 15: 447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Katz MA, Tharmaphornpilas P, Chantra S, et al. Who gets hospitalized for influenza pneumonia in Thailand? Implications for vaccine policy. Vaccine 2007; 25: 3827–33. [DOI] [PubMed] [Google Scholar]
- 10.Feikin DR, Njenga MK, Bigogo G, et al. Additional diagnostic yield of adding serology to PCR in diagnosing viral acute respiratory infections in Kenyan patients 5 years of age and older. Clin Vaccine Immunol 2013; 20: 113–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jain S, Williams DJ, Arnold SR, et al. Community-acquired pneumonia requiring hospitalization among U.S. children. N Engl J Med 2015; 372: 835–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sawatwong P, Chittaganpitch M, Hall H, et al. Serology as an adjunct to polymerase chain reaction assays for surveillance of acute respiratory virus infections. Clin Infect Dis 2012; 54: 445–46. [DOI] [PubMed] [Google Scholar]
- 13.Fell DB, Azziz-Baumgartner E, Baker MG, et al. Influenza epidemiology and immunization during pregnancy: final report of a World Health Organization working group. Vaccine 2017; 35: 5738–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Peasah SK, Azziz-Baumgartner E, Breese J, et al. Influenza cost and cost-effectiveness studies globally—a review. Vaccine 2013; 31: 5339–48. [DOI] [PubMed] [Google Scholar]
- 15.Wong VW, Lok KY, Tarrant M. Interventions to increase the uptake of seasonal influenza vaccination among pregnant women: a systematic review. Vaccine 2016; 34: 20–32. [DOI] [PubMed] [Google Scholar]
- 16.Arriola CS, Vasconez N, Bresee J, et al. Knowledge, attitudes and practices about influenza vaccination among pregnant women and healthcare providers serving pregnant women in Managua,Nicaragua. Vaccine 2018; 36: 3686–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yeung KHT, Tarrant M, Chan KCC, Tam WH, Nelson EAS. Increasing influenza vaccine uptake in children: a randomised controlled trial. Vaccine 2018; 36: 5524–35. [DOI] [PubMed] [Google Scholar]
- 18.Thompson MG, Hunt DR, Arbaji AK, et al. Influenza and respiratory syncytial virus in infants study (IRIS) of hospitalized and non-ill infants aged <1 year in four countries: study design and methods. BMC Infect Dis 2017; 17: 222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Katz JM, Hancock K, Xu X. Serologic assays for influenza surveillance, diagnosis and vaccine evaluation. Expert Rev Anti Infect Ther 2011; 9: 669–83. [DOI] [PubMed] [Google Scholar]
- 20.Reber A, Katz J. Immunological assessment of influenza vaccines and immune correlates of protection. Expert Rev Vaccines 2013; 12: 519–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reed C, Chaves SS, Daily Kirley P, et al. Estimating influenza disease burden from population-based surveillance data in the United States. PLoS One 2015; 10: e0118369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Spencer S, Gaglani M, Naleway A, et al. Consistency of influenza A virus detection test results across respiratory specimen collection methods using real-time reverse transcription-PCR. J Clin Microbiol 2013; 51: 3880–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Haaheim LR, Katz JM. Immune correlates of protection against influenza: challenges for licensure of seasonal and pandemic influenza vaccines, Miami, FL, USA, March 1–3, 2010. Influenza Other Respir Viruses 2011; 5: 288–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thompson MG, Gaglani MJ, Naleway AL, et al. Reduced serologic sensitivity to influenza A virus illness among inactivated influenza vaccinees. Vaccine 2016; 34: 3443–46. [DOI] [PubMed] [Google Scholar]
- 25.Dowling DJ, Levy O. Ontogeny of early life immunity. Trends Immunol 2014; 35: 299–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Munoz FM. Influenza virus infection in infancy and early childhood. Paediatr Respir Rev 2003; 4: 99–104. [PubMed] [Google Scholar]
- 27.Maltezou HC, Drancourt M. Nosocomial influenza in children. J Hosp Infect 2003; 55: 83–91. [DOI] [PubMed] [Google Scholar]
- 28.Beck AF, Florin TA, Campanella S, Shah SS. Geographic variation in hospitalization for lower respiratory tract infections across one county. JAMA Pediatr 2015; 169: 846–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hirve S, Krishnan A, Dawood FS, et al. Incidence of influenza-associated hospitalization in rural communities in western and northern India, 2010–2012: a multi-site population-based study. J Infect 2014; 70: 160–70. [DOI] [PubMed] [Google Scholar]
- 30.Gessner BD, Shindo N, Briand S. Seasonal influenza epidemiology in sub-Saharan Africa: a systematic review. Lancet Infect Dis 2011; 11: 223–35. [DOI] [PubMed] [Google Scholar]
- 31.Gessner BD. Acute lower respiratory infection in the developing world. Expert Rev Respir Med 2011; 5: 459–63. [DOI] [PubMed] [Google Scholar]
- 32.Furuse Y, Tamaki R, Okamoto M, et al. Association between preceding viral respiratory infection and subsequent respiratory illnesses among Children: a prospective cohort study in the Philippines. J Infect Dis 2019; 219: 197–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Grohskopf LA, Sokolow LZ, Broder KR, Walter EB, Fry AM, Jernigan DB. Prevention and control of seasonal influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices—United States, 2018–19 Influenza Season. MMWR Recomm Rep 2018; 67: 1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.