Abstract
Objective:
To compare the effectiveness of an integrated rehabilitation programme with an existing rehabilitation programme in patients with chronic low back pain.
Design:
A single-centre, pragmatic, two-arm parallel, randomized controlled trial (1:1 ratio).
Setting:
A rheumatology inpatient rehabilitation centre in Denmark.
Subjects:
A total of 165 adults (aged ⩾ 18 years) with chronic low back pain.
Interventions:
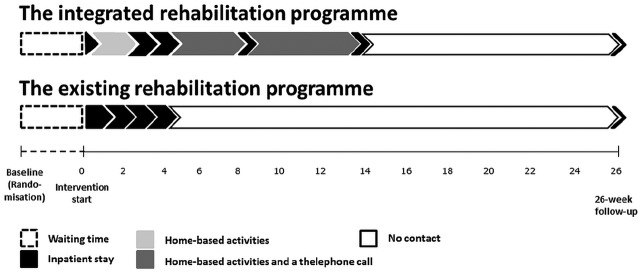
An integrated rehabilitation programme comprising an alternation of three weeks of inpatient stay and 12 weeks of home-based activities was compared with an existing rehabilitation programme of four weeks of inpatient stay.
Main measures:
Patient-reported outcomes were collected at baseline and at the 26-week follow-up. The primary outcome was back-specific disability (Oswestry Disability Index). Secondary outcomes included pain intensity (Numerical Rating Scale), pain self-efficacy (Pain Self-Efficacy Questionnaire), health-related quality of life (EuroQol-5 Domain 5-level (EQ-5D)), and depression (Major Depression Inventory). A complete case analysis was performed.
Results:
A total of 303 patients were assessed for eligibility of whom 165 (mean age: 50 years (SD 13) and mean Oswestry Disability Index score 42 (SD 11)) were randomized (83 to existing rehabilitation programme and 82 to integrated rehabilitation programme). Overall, 139 patients provided the 26-week follow-up data. Baseline demographic and clinical characteristics were comparable between programmes. The between-group difference in the Oswestry Disability Index score when adjusting for the corresponding baseline score was −0.28 (95% confidence interval (CI): −4.02, 3.45) which was neither statistically nor clinically significant. No significant differences were found in the secondary outcomes.
Conclusion:
An integrated rehabilitation programme was no more effective than an existing rehabilitation programme at the 26-week follow-up.
Keywords: Chronic low back pain, multidisciplinary rehabilitation, biopsychosocial approach, complex interventions
Introduction
Multidisciplinary rehabilitation is recommended as a second-line treatment for patients with chronic low back pain who do not respond to first-line treatments.1,2 Multidisciplinary rehabilitation is a multifaceted intervention targeting the wide range of modifiable factors known to contribute to chronic low back pain and it is usually based on the widely accepted biopsychosocial approach.1–4 There are different ways of delivering multidisciplinary rehabilitation. A Cochrane review included 12 randomized controlled trials comparing at least two different multidisciplinary rehabilitation approaches,3 but it did not compare the effectiveness of different delivery modes. Thus, the optimal approach, dose, content, or structure of a multidisciplinary rehabilitation programme is not known.3
To optimize the effectiveness of multidisciplinary rehabilitation, it is generally considered important for the patient to integrate the new knowledge, skills, and behaviours gained from an inpatient rehabilitation programme into their daily life. Approaches to support this integration include taking the patient’s environment into account5,6 and ensuring regular interaction over time between the patient and the multidisciplinary team via scheduled booster sessions.7 One trial included in the Cochrane review3 assessed the effect of adding booster sessions (phone calls) to a four-week inpatient rehabilitation programme.8 The trial found a small, but not statistically significant, benefit compared with the same inpatient rehabilitation programme without booster sessions.8
From a theoretical point of view, it seems reasonable to combine the biopsychosocial approach5,9 with the Chronic Care Model7 in terms of supporting integration of knowledge, skills, and behaviours gained from an inpatient rehabilitation programme into the patient’s own environment and daily life.10
No trials have yet tested whether an approach like that is more effective than an existing inpatient rehabilitation programme. Therefore, we designed an integrated multidisciplinary rehabilitation programme (integrated programme) that comprised a two-week inpatient stay, followed by home-based activities plus two further inpatient booster sessions (each lasting two days).10 We hypothesized that the integrated programme, combining inpatient interventions supported by a multidisciplinary team with home-based activities to better integrate knowledge, skills, and behaviours in the patient’s daily life, would be superior to an existing multidisciplinary inpatient rehabilitation programme (existing programme).
Therefore, in patients with chronic low back pain, the aim of this trial was to compare the effectiveness of the integrated programme with the existing programme in terms of back-specific disability.
Methods
The Central Denmark Region Committees on Biomedical and Research Ethics approved the trial (journal number: 1-10-72-117-16), and the trial was registered (ClinicalTrials.gov: identifier NCT02884466). The trial was funded by Sano, Aarhus University, the Danish Rheumatism Association, and Familien Hede Nielsens Fond, and reported according to the Consolidated Standards of Reporting Trials (CONSORT) 2010 Statement.11
A consultative and collaborative approach was used when involving stakeholders (patients, providers, administrative, and management staff) in the development, feasibility testing, and evaluation of the trial. The short form of ‘Guidance for Reporting Involvement of Patients and the Public’12 was used to structure the reporting of patient and public involvement (Supplemental Material 1). A process evaluation was integrated into the trial as advocated for complex interventions13 (Supplemental Material 2).
This was a single-centre, pragmatic, two-arm parallel randomized controlled trial conducted in a rheumatology rehabilitation centre in Aarhus, Denmark.10 Patients were referred to the rehabilitation centre where the trial was conducted from general practitioners or hospital departments.10 The rheumatologist at that centre identified potentially eligible patients based on the clinical problem detailed on the referral request and a list of International Classification of Diseases, 10th revision (ICD-10) diagnosis codes for diseases, signs, and symptoms related to chronic low back pain. Before inclusion, a research assistant performed eligibility checks by telephoning potentially eligible patients. Written information and an informed consent form were emailed by the research assistant, and if a signed version was returned, the patient was included. Patients then waited until the next available rehabilitation programme group was scheduled, as this is usual practice at the centre. The final eligibility checking was performed by the rheumatologist on the first inpatient day.
Patients were eligible if they had chronic low back pain for more than 12 months (with or without sciatica and/or with or without widespread pain) and if they were 18 years or older. The exclusion criteria were (1) severe systemic diseases (American Society of Anesthesiologists physical status classification system ⩾3),14 (2) a diagnosis of axial spondyloarthritis, (3) spinal fracture within the last three months, (4) severe osteoporosis, (5) active cancer, (6) severe psychiatric disease, (7) pregnancy, (8) lack of fluency in Danish, and (9) minimal back-specific disability (Oswestry Disability Index score <21).15
A computer-generated randomization with 1:1 allocation in random blocks of six ensuring allocation concealment was performed by the research assistant. Randomization was stratified on the basis of disability at baseline using the Oswestry Disability Index score with cutoff at 4115 in order to achieve approximate balance in mean disability levels in the arms of the trial. The research assistant informed patients about intervention allocation and the dates for their allocated rehabilitation programme. Blinding of patients and providers was not possible due to the nature of the interventions. In order to ensure patients had equal expectations about each rehabilitation programme, we attempted to blind participants to the hypothesis by informing them that the trial aimed to compare two rehabilitation programmes that meet current recommendations.1,2 The researcher who performed the statistical analysis was blinded.
A secure electronic database was used to email questionnaires and store data. Patients were emailed the questionnaires 10 days prior to the inpatient stay. A reminder was emailed after five and eight days if required. If they were not completed, the research assistant ensured completion of questionnaires on an electronic tablet on the first inpatient day. Patients who were unable to complete the electronic questionnaires completed a paper version.
Patients excluded between baseline and before the start of their rehabilitation programme (due to exclusion criteria), patients who, following baseline, subsequently reported they did not wish to participate, or patients who dropped out of their rehabilitation programme, did not receive further questionnaires.
Data on sex, age, marital status, smoking, leg pain, employment status and education level were collected at baseline. The outcome measures were collected (1) before randomization (baseline), (2) before the start of the rehabilitation programme, and (3) at the 26-week follow-up (26 weeks after the start of the rehabilitation programme).
The choice of outcome domains and outcome measures was based on patient and public involvement in combination with international recommendations.16–18 The primary outcome was back-specific disability assessed by the Oswestry Disability Index version 2.1a.15 Secondary outcomes were back pain intensity assessed by a Numerical Rating Scale,18 pain self-efficacy measured by the Pain Self-Efficacy Questionnaire,19 health-related quality of life measured by the EuroQol-5 Domain 5-level (EQ-5D 5L),20 depression measured by the Major Depression Inventory,21 and physical activity assessed by three questions.22
Cases of adverse events and death were collected from the electronic health records. Adherence was extracted from the electronic health records and defined as attending ⩾80% of the scheduled inpatient days. Thus, adherence was defined as attending ⩾17 inpatient days in the existing programme and attending ⩾12 inpatient days in the integrated programme. Adherence to the home-based activities was not assessed.
In brief, both rehabilitation programmes comprised multidisciplinary inpatient rehabilitation based on the biopsychosocial approach and included the same 38 clinical activities, the same providers, and the same contact hours between patients and providers. An inpatient day consisted of 8–10 hours per day alternating between (1) group lecture and dialogue, (2) supervised group sessions, (3) unsupervised group sessions, (4) individual counselling, and (5) unsupervised individual exercise. Full details about clinical activities, providers and setting have been described previously.10 The key difference between the two rehabilitation programmes was in the way in which they were delivered.
Patients in the integrated programme participated in (1) preadmission day, (2) two-week home-based activities, (3) two-week inpatient stay, (4) four-week home-based activities, (5) first two-day inpatient booster session, (6) six-week home-based activities, (7) second two-day inpatient booster session, and (8) 26-week follow-up (a total of 15 inpatient days) (Figure 1). The integrated programme was developed and feasibility tested according to the Medical Research Council’s guidance on developing and evaluating complex interventions13 as previously described.10
Figure 1.
The integrated programme and the existing programme being compared.
Patients in the existing programme were offered a four-week inpatient stay and 26-week follow-up (a total of 21 inpatient days). The existing programme has been usual practice for more than 15 years in the setting under study.
Statistical analysis
The sample size calculation was based on a hypothesis of superiority of the integrated programme over the existing programme for back-specific disability (using the Oswestry Disability Index). A difference of 4 points has been suggested as a minimum clinically important difference.15 The trial was powered to be able to detect a standardized mean difference of at least 0.5 between the rehabilitation programmes, assuming a decrease of 10 points on the Oswestry Disability Index at 26 weeks’ follow-up in the integrated programme compared with a decrease of 5 points in the existing programme. The standard deviation was informed by a feasibility test with 12 patients attending the existing programme (standard deviation of 10). With 80% power and a significance level of 0.05, 64 patients were required in each arm of the trial, and allowing for a loss to follow-up of 20%, a total of 160 patients was needed.
A statistical analysis plan was completed prior to data analyses. Baseline demographic and clinical characteristics were descriptively summarized and presented as the mean (SD) or number (%) according to patients allocated at baseline and patients providing 26-week follow-up. In addition, differences in sex, age, and ICD-10 diagnosis codes are presented for those patients randomized and those declining to participate.
The primary analysis was a modified intention-to-treat analysis according to originally allocated intervention arms, excluding patients with missing outcome data at the 26-week follow-up (=complete case analysis). The between-group difference in change scores from baseline to the 26-week follow-up was analysed by multiple linear regression for continuous outcomes using change scores as the dependent variable, rehabilitation programme as the independent variable, and the corresponding baseline score as a covariate. Categorical outcomes were compared using Wilcoxon Rank-Sum Tests. For the secondary analysis, the within-group changes from baseline to the 26-week follow-up were presented descriptively. Furthermore, the robustness of the modified intention-to-treat analysis in terms of the primary outcome was checked by an intention-to-treat analysis using imputed data with the last value carried forward. A per-protocol analysis was also conducted excluding patients with low adherence to their rehabilitation programme (defined as <80% attendance). An exploratory analysis including waiting time (days between randomization (baseline) and the start of the rehabilitation programme) as a covariate was performed, as the process evaluation revealed that this variable by chance differed between the two rehabilitation programmes. P-values ⩽ 0.05 were considered statistically significant. For statistical analysis, STATA, versoin 15 was used.
Results
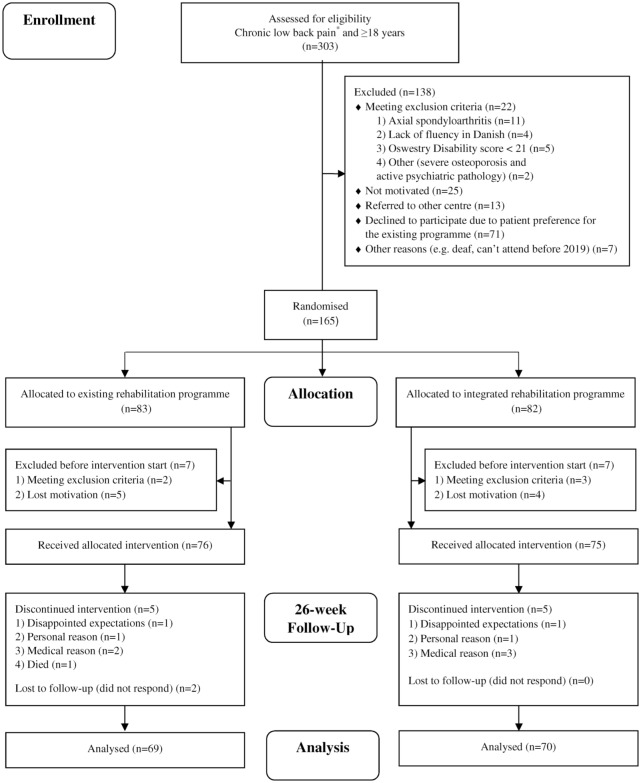
Participant recruitment started in February 2016 and ended in August 2018. The first rehabilitation programme commenced in September 2016, and the last rehabilitation programme reached the 26-week follow-up in May 2019. The flow of participants is shown in Figure 2. The 71 patients who declined to participate did not differ from those willing to participate with respect to age (mean age 50, age range: 22–79), sex (68% women), or diagnosis (data not presented). Baseline demographic and clinical characteristics were comparable between arms (Table 1). Adherence to the inpatient days (those attending ⩾80%) was excellent in both arms of the trial (100% in the existing programme and 99% in the integrated programme). Mean waiting time was 141 days (SD 10) in the existing programme and 105 days (SD 9) in the integrated programme. There were no related adverse events or deaths.
Figure 2.
Flow-chart of participants through the trial.
*The following ICD-10 diagnosis codes for diseases, signs, and symptoms related to chronic low back pain were used: DM40, DM41, DM42, DM43 (not 43.3, 43.4, 43.6), DM47, DM48, DM51, DM53 (not 53.0, 53.1), DM54 (not 54.0, 54.2, 54.6), DM96.1, and DT91.0.
Table 1.
Characteristics of the population at baseline.
| Patients allocated at baseline |
Patients providing 26-week follow-up data |
|||
|---|---|---|---|---|
| Existing programme, n = 83 | Integrated programme, n = 82 | Existing programme, n = 69 | Integrated programme, n = 70 | |
| Sex (women), n (%) | 60 (73) | 60 (72) | 49 (71) | 52 (74) |
| Age (years) | ||||
| Mean (SD) | 51 (13) | 49 (13) | 52 (12) | 50 (12) |
| Range | 25–84 | 22–72 | 25–84 | 28–72 |
| Marital status, n (%) | ||||
| Married | 58 (70) | 60 (73) | 50 (72) | 51 (73) |
| Single/widowed | 25 (30) | 22 (27) | 19 (28) | 19 (27) |
| Smokers, n (%) | ||||
| Yes | 28 (34) | 24 (29) | 23 (33) | 17 (24) |
| No | 55 (66) | 58 (71) | 46 (67) | 62 (76) |
| Leg pain, n (%) | ||||
| Yes | 59 (71) | 65 (79) | 49 (71) | 55 (79) |
| No | 17 (21) | 12 (15) | 14 (20) | 12 (17) |
| Do not know | 7 (8) | 5 (6) | 6 (9) | 3 (4) |
| Employment statusa, n (%) | ||||
| Self-supporting | 16 (25) | 17 (26) | 15 (29) | 17 (30) |
| Temporary social benefits | 11 (17) | 9 (14) | 8 (15) | 7 (13) |
| Permanent social benefits | 27 (42) | 29 (45) | 20 (39) | 24 (43) |
| Age-related pension | 10 (16) | 8 (12) | 9 (17) | 7 (13) |
| Others | 0 (0) | 2 (3) | 0 (0) | 1 (2) |
| Education levela, n (%) | ||||
| Low (⩽12 years) | 14 (22) | 10 (15) | 11 (21) | 6 (11) |
| Middle (⩽16 years) | 44 (69) | 44 (68) | 36 (69) | 40 (71) |
| High (>16 years) | 6 (9) | 11 (17) | 5 (10) | 10 (18) |
| Disabilityb ODI (0–100) | ||||
| Mean (SD) | 43 (11) | 42 (10) | 43 (12) | 41 (11) |
| Range | 24–72 | 20–68 | 24–72 | 20–68 |
| Back pain intensityc NRS (0–10) | ||||
| Mean (SD) | 6 (2) | 6 (2) | 6 (2) | 6 (2) |
| Pain Self-efficacy PSEQ (0–60) | ||||
| Mean (SD) | 27 (10) | 28 (11) | 27 (11) | 28 (12) |
| Quality of life EQ-5D 5L (–0.624 to 1) | ||||
| Mean (SD) | 0.603 (0.118) | 0.567 (0.157) | 0.599 (0.126) | 0.578 (0.153) |
| Depression MDI (0–50) | ||||
| Mean (SD) | 20 (11) | 20 (12) | 22 (11) | 19 (11) |
| Physical activity, n (%) | ||||
| Minutes spent on physical exercise during a week | ||||
| <30 | 50 (60) | 42 (51) | 41 (59) | 34 (49) |
| ⩾30 ⩽ 120 | 31 (37) | 32 (39) | 26 (38) | 28 (40) |
| >120 | 2 (3) | 8 (10) | 2 (3) | 8 (11) |
| Minutes spent on physical activity during a week, n (%) | ||||
| <30 | 20 (24) | 13 (16) | 17 (25) | 9 (13) |
| ⩾30 ⩽ 300 | 55 (66) | 52 (63) | 45 (65) | 45 (64) |
| >300 | 8 (10) | 17 (21) | 7 (10) | 16 (23) |
| Hours spent sitting during 24 hours, n (%) | ||||
| ⩾10 | 14 (17) | 9 (11) | 6 (9) | 7 (10) |
| <10 ⩾ 4 | 52 (63) | 53 (65) | 51 (74) | 47 (67) |
| <4 | 17 (20) | 20 (24) | 12 (17) | 16 (23) |
Columns 1 and 2 show patients randomized (n = 165) and columns 3 and 4 show patients providing 26-week follow-up data (n = 139).
ODI: Oswestry Disability Index; NRS: Numerical Rating Scale; PSEQ: Pain Self-Efficacy Questionnaire; EQ-5D 5L: EuroQol-5 Domain 5-level; MDI: Major Depression Inventory.
Due to technical issues in the database, data on employment status and education level was only available in ≈75% of the patients.
Due to technical issues in the database, one patient with an ODI score of 20 was included.
Mean back pain intensity for the last two weeks.
The Oswestry Disability Index scores decreased on average in those allocated to the integrated programme from 41 (SD 11) at baseline to 36 (SD 14) at the 26-week follow-up, and in those allocated to the existing programme from 43 (SD 12) at baseline to 37 (SD 16) at the 26-week follow-up. The adjusted between-group difference was −0.28 (95% confidence interval: −4.02, 3.45) which was neither statistically nor clinically significant (Table 2). Data on physical activity were not presented, as the analysis revealed low quality of the data.
Table 2.
Summary of 26-week follow-up data on primary and secondary outcomes: between-group and within-group change – complete case analysis.
| Between-groupa |
Within-group |
|||
|---|---|---|---|---|
| Mean (95% CI) | P-value | Existing programme (n = 69) |
Integrated programme (n = 70) |
|
| Mean (95% CI) | Mean (95% CI) | |||
| Primary outcome | ||||
| Disability (ODI) | −0.28 (−4.02, 3.45) | 0.881 | −5.64 (−8.45, −2.83) | −5.76 (−8.31, −3.20) |
| Secondary outcomes | ||||
| Pain intensityb (NRS) | −0.02 (−0.64, 0.59) | 0.937 | −0.64 (−1.08, −0.19) | −0.76 (−1.21, −0.31) |
| Pain Self-Efficacy (PSEQ) | 0.05 (−3.47, 3.57) | 0.978 | 6.22 (3.63, 8.80) | 6.01 (3.48, 8.80) |
| Quality of life (EQ-5D 5L) | 0.01 (−0.03, 0.05) | 0.670 | 0.03 (0.00, 0.07) | 0.05 (0.02, 0.08) |
| Depression (MDI) | 0.62 (−1.98, 3.21) | 0.639 | −4.57 (−6.52, −2.62) | −3.3 (−5.27, −1.24) |
CI: confidence interval; ODI: Oswestry Disability Index; NRS: Numerical Rating Scale; PSEQ: Pain Self-Efficacy Questionnaire; EQ-5D 5L: EuroQol-5 Domain 5-level; MDI: Major Depression Inventory.
Adjusted for corresponding baseline value. Existing programme as reference group.
Mean pain intensity for the last two weeks.
No statistically significant differences were found between the rehabilitation programmes in any of the secondary outcomes (Table 2). The data in Table 2 show that on average, patients in both arms of the trial improved from baseline to the 26-week follow-up on all outcomes. Intention-to-treat analysis with the last value carried forward did not change the conclusions from the primary analysis (data not presented). As only one patient had poor adherence (attending <80% of the inpatient days), the per-protocol analysis was deemed unnecessary. The exploratory analysis, including waiting time as a covariate, did not change the trial conclusion (data not presented).
Discussion
This trial provides convincing evidence that changing the way in which a multidisciplinary rehabilitation programme is delivered by alternating inpatient stays with home-based activities and booster sessions, did not lead to better outcomes for patients allocated to an integrated programme compared with patients allocated to an existing programme at the 26-week follow-up. As expected, given existing clinical practice guidelines,12 and systematic review evidence,3 on average, patients in both rehabilitation programmes improved over time.
There are several potential reasons for our results. One explanation relates to the lack of sufficient difference between the two rehabilitation programmes in the trial. The clinical activities and contact hours with the providers were the same in the two rehabilitation programmes; the key difference was the way in which the rehabilitation programmes were delivered. Furthermore, the process evaluation revealed difficulties with implementing elements of the integrated programme. In order to support integration of knowledge, skills, and behaviours into daily life, patients in the integrated programme received a preparation pamphlet as well as a phone call before each booster session.10 The pamphlet was requested and developed by the providers delivering the rehabilitation programmes, but despite that, the pamphlet was infrequently provided to patients, and instruction in, and follow-up on, patient’s reflections reported in the pamphlet was often forgotten. The providers mentioned lack of time and unclear responsibility for conducting the phone calls as possible barriers to implementation. The implementation difficulties could potentially have served to attenuate any difference in outcomes between the two rehabilitation programmes, as these elements were essential parts of the integrated programme.
A further explanation for the results relates to the Oswestry Disability Index as the primary outcome measure. The Oswestry Disability Index is a measure of back-related disability and is not a measure of successful integration of knowledge, skills, and behaviours in the daily life of patients, which was the intended target of the integrated programme. As a measure of disability, the Oswestry Disability Index was expected to be a proxy for this integration, but the relationship between disability and integration of knowledge, skills, and behaviours is unknown. The reasons for choosing the Oswestry Disability Index as the primary outcome measure were a combination of patient and public involvement and international recommendations about core outcome sets for trials in the field of low back pain.16–18 On the contrary, the domain of pain self-efficacy may be somewhat closer to the domain of integrating knowledge, skills, and behaviours. However, we also observed no difference between the arms of the trial on this outcome, although we did not power the trial to detect differences on this outcome. To our knowledge, no single outcome measure has been developed and validated to measure the domain of integrating knowledge, skills, and behaviours into daily life.
We identified three further trials23–25 in addition to the 12 trials already included in the most recent Cochrane review3 that compared two or more multidisciplinary rehabilitation programmes. Of all 15 trials identified, only two used the Oswestry Disability Index as their primary outcome measure.24,25 The changes they observed in back-specific disability are similar to those we observed with a within-group decrease of between 7 and 9 points at the 12-week follow-up24 and a within-group decrease between 2 and 5 points at the 52-week follow-up.25 Neither of these trials found significant between-group differences,24,25 similar to our results.
There are conflicting results of adding booster sessions to interventions for musculoskeletal pain. Only one trial involving patients with chronic low back pain has assessed the effect of booster sessions and found no additional benefit.8 A review including three trials26 and a further single trial27 in patients with hip and/or knee osteoarthritis, showed beneficial effects of adding booster sessions to exercise therapy. The opposite was found in two other trials with patients with hip and/or knee osteoarthritis.28,29 These conflicting results question the effectiveness of adding booster sessions.
The strengths of this trial include the randomized parallel design, comparability of patients in the two arms at baseline, and high adherence to the scheduled inpatient days. We also reached our target sample size and had high follow-up rates. A small proportion of patients did not complete the trial (12 out of 82/83 ≈ 15%), the majority of whom disengaged from the trial before the start of their rehabilitation programme (7 out of 12 = 58%), largely due to the waiting time. Those patients not completing the programme were balanced in numbers and baseline characteristics between the two arms. The thorough development and feasibility testing of the integrated programme according to the Medical Research Council’s guidance for complex interventions,13 including patient and public involvement (Supplemental Material 1) as well as the integrated process evaluation (Supplemental Material 2) are considered further strengths.
The trial also had some limitations. The lack of measurement of adherence to the home-based activities is a limitation. Data on adherence to home-based activities could have allowed us to better assess if the hoped-for integration of knowledge, skills, and behaviours was different in patients in the integrated programme compared with those in the existing programme. Measuring adherence to home-based activities is a methodological challenge; hence, we opted to simply capture data on inpatient attendance. A second limitation is the potential risk of contamination. Both rehabilitation programmes were managed by the same providers, in the same centre and at the same time, meaning that patients inevitably met each other. Thus, both the patients and the providers would have had the opportunity to compare and discuss the two rehabilitation programmes allowing for patients in the existing programme to potentially be inspired to integrate knowledge, skills, and behaviours in their daily life, diluting any differences between the two rehabilitation programmes. Finally, it is considered a limitation that a cost-utility analysis was not conducted.
This trial has contributed new knowledge regarding the delivery of rehabilitation programmes; as long as the content is the same, it appears that the way in which a rehabilitation programme is delivered does not impact clinical outcomes at least in the medium term as assessed in this article. Given this, factors such as patient preferences and/or the costs of the different rehabilitation programmes should perhaps drive decisions about the delivery approach. Patients, clinicians, researchers, and stakeholders need to continue to collaborate about development, evaluation, and implementation of effective second-line treatments for patients with chronic low back pain. Future research needs to investigate the long-term outcomes from different approaches to the delivery of rehabilitation programmes.
Clinical messages.
Introducing an integrated rehabilitation programme aiming to better integrate new knowledge, skills, and behaviours into the daily life of the patient with chronic low back pain, did not lead to better back-related disability compared with an existing rehabilitation programme at the 26-week follow-up.
Patients in both rehabilitation programmes reported improvements in the primary outcome (disability) over the 26 weeks, and those improvements were of clinically relevant size.
Supplemental Material
Supplemental material, Supplemental_Material_ for The effect of an integrated multidisciplinary rehabilitation programme alternating inpatient interventions with home-based activities for patients with chronic low back pain: a randomized controlled trial by Anne Mette Schmidt, Berit Schiøttz-Christensen, Nadine E Foster, Trine Bay Laurberg and Thomas Maribo in Clinical Rehabilitation
Acknowledgments
The authors are grateful to all patients, providers, administrative, and management staff at Sano who made the trial possible. Dr Martyn Lewis is thanked for his helpful advice on statistical issues.
Footnotes
Author contributions: All authors have contributed substantially to (1) conception and design, or analysis and interpretation of data; (2) drafting or revising the article critically for important intellectual content; and (3) final approval of the version to be published.
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: N.E.F. is a National Institute for Health Research (NIHR) senior investigator, in the United Kingdom (UK). The views expressed in this publication are those of the authors and not necessarily those of the UK National Health Service (NHS), NIHR, or the Department of Health and Social Care. The trial was funded by Sano, Aarhus University, the Danish Rheumatism Association, and Familien Hede Nielsens Fond. None of the funders had any influence on the trial.
ORCID iD: Anne Mette Schmidt  https://orcid.org/0000-0002-3077-4985
https://orcid.org/0000-0002-3077-4985
Supplemental material: Supplemental material for this article is available online.
References
- 1. Foster NE, Anema JR, Cherkin D, et al. Prevention and treatment of low back pain: evidence, challenges, and promising directions. Lancet 2018; 391(10137): 2368–2383. [DOI] [PubMed] [Google Scholar]
- 2. Vlaeyen JWS, Maher CG, Wiech K, et al. Low back pain. Nat Rev Dis Primers 2018; 4: 52. [DOI] [PubMed] [Google Scholar]
- 3. Kamper SJ, Apeldoorn AT, Chiarotto A, et al. Multidisciplinary biopsychosocial rehabilitation for chronic low back pain: Cochrane systematic review and meta-analysis. BMJ 2015; 350: h444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Waddell G. The biopsychosocial model. In: Waddell G. (ed.) The back pain revolution. 2nd ed London: Churchill Livingstone, 2004, pp.265–282. [Google Scholar]
- 5. Wade D. Rehabilitation – a new approach. Part two: the underlying theories. Clin Rehabil 2015; 29(12): 1145–1154. [DOI] [PubMed] [Google Scholar]
- 6. Dunn P, Conard S. Chronic Care Model in research and in practice. Int J Cardiol 2018; 258: 295–296. [DOI] [PubMed] [Google Scholar]
- 7. Wagner EH, Austin BT, Davis C, et al. Improving chronic illness care: translating evidence into action. Health Aff 2001; 20(6): 64–78. [DOI] [PubMed] [Google Scholar]
- 8. Mangels M, Schwarz S, Worringen U, et al. Evaluation of a behavioral-medical inpatient rehabilitation treatment including booster sessions: a randomized controlled study. Clin J Pain 2009; 25(5): 356–364. [DOI] [PubMed] [Google Scholar]
- 9. World Health Organization. International classification of functioning, disability and health (ICF). Geneva: World Health Organization, 2001, pp.1–26. [Google Scholar]
- 10. Schmidt AM, Terkildsen Maindal H, Laurberg TB, et al. The Sano study: justification and detailed description of a multidisciplinary biopsychosocial rehabilitation programme in patients with chronic low back pain. Clin Rehabil 2018; 32(11): 1431–1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Schulz KF, Altman DG, Moher D. CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. J Clin Epidemiol 2010; 63: 834–840. [DOI] [PubMed] [Google Scholar]
- 12. Staniszewska S, Brett J, Simera I, et al. GRIPP2 reporting checklists: tools to improve reporting of patient and public involvement in research. BMJ 2017; 358: j3453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Council TMR. Developing and evaluating complex interventions: new guidance, https://mrc.ukri.org/documents/pdf/complex-interventions-guidance/ (2008, accessed 6 February 2019).
- 14. American Society of Anesthesiologists (ASA). ASA physical status classification system, https://www.asahq.org/resources/clinical-information/asa-physical-status-classification-system (accessed 10 September 2018).
- 15. Fairbank JC, Pynsent PB. The Oswestry Disability Index. Spine 2000; 25: 2940–2952; discussion 2952. [DOI] [PubMed] [Google Scholar]
- 16. Chiarotto A, Boers M, Deyo RA, et al. Core outcome measurement instruments for clinical trials in nonspecific low back pain. Pain 2018; 159(3): 481–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Chiarotto A, Deyo RA, Terwee CB, et al. Core outcome domains for clinical trials in non-specific low back pain. Eur Spine J 2015; 24: 1127–1142. [DOI] [PubMed] [Google Scholar]
- 18. Chapman JR, Norvell DC, Hermsmeyer JT, et al. Evaluating common outcomes for measuring treatment success for chronic low back pain. Spine 2011; 36(21 suppl): S54–S68. [DOI] [PubMed] [Google Scholar]
- 19. Nicholas MK. The pain self-efficacy questionnaire: taking pain into account. Eur J Pain 2007; 11(2): 153–163. [DOI] [PubMed] [Google Scholar]
- 20. Group TE. EQ-5D-5L, https://euroqol.org/eq-5d-instruments/eq-5d-5l-about/ (accessed 7 February 2019).
- 21. Olsen LR, Jensen DV, Noerholm V, et al. The internal and external validity of the Major Depression Inventory in measuring severity of depressive states. Psychol Med 2003; 33(2): 351–356. [DOI] [PubMed] [Google Scholar]
- 22. Fysioterapeuter D. Hvordan spørger du ind til patienternes aktivitetsniveau? https://www.fysio.dk/nyheder/2014/Hvordan-sporger-du-ind-til-patienternes-aktivitetsniveau (2014, accessed 12 June 2019).
- 23. Linden M, Scherbe S, Cicholas B. Randomized controlled trial on the effectiveness of cognitive behavior group therapy in chronic back pain patients. J Back Musculoskelet Rehabil 2014; 27(4): 563–568. [DOI] [PubMed] [Google Scholar]
- 24. Iversen VM, Vasseljen O, Mork PJ, et al. Resistance band training or general exercise in multidisciplinary rehabilitation of low back pain? A randomized trial. Scand J Med Sci Spor 2018; 28: 2074–2083. [DOI] [PubMed] [Google Scholar]
- 25. Verra ML, Angst F, Brioschi R, et al. Effectiveness of subgroup-specific pain rehabilitation: a randomized controlled trial in patients with chronic back pain. Eur J Phys Rehabil Med 2018; 54(3): 358–370. [DOI] [PubMed] [Google Scholar]
- 26. Pisters MF, Veenhof C, van Meeteren NL, et al. Long-term effectiveness of exercise therapy in patients with osteoarthritis of the hip or knee: a systematic review. Arthritis Rheum 2007; 57: 1245–1253 [DOI] [PubMed] [Google Scholar]
- 27. Abbott JH, Chapple CM, Fitzgerald GK, et al. The incremental effects of manual therapy or booster sessions in addition to exercise therapy for knee osteoarthritis: a randomized clinical trial. J Orthop Sports Phys Ther 2015; 45(12): 975–983. [DOI] [PubMed] [Google Scholar]
- 28. Fitzgerald GK, Fritz JM, Childs JD, et al. Exercise, manual therapy, and use of booster sessions in physical therapy for knee osteoarthritis: a multi-center, factorial randomized clinical trial. Osteoarthr Cartilage 2016; 24(8): 1340–1349. [DOI] [PubMed] [Google Scholar]
- 29. Bennell KL, Kyriakides M, Hodges PW, et al. Effects of two physiotherapy booster sessions on outcomes with home exercise in people with knee osteoarthritis: a randomized controlled trial. Arthritis Care Res 2014; 66(11): 1680–1687. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, Supplemental_Material_ for The effect of an integrated multidisciplinary rehabilitation programme alternating inpatient interventions with home-based activities for patients with chronic low back pain: a randomized controlled trial by Anne Mette Schmidt, Berit Schiøttz-Christensen, Nadine E Foster, Trine Bay Laurberg and Thomas Maribo in Clinical Rehabilitation