Abstract
Background
Adult-onset immunodeficiency associated with interferon-γ autoantibody (IGA) is an emerging disease. The majority of patients require both antimicrobial and immunosuppressive treatments. However, anti-CD20 therapy is not fully accessible in a resource-limited setting to date.
Background
The objectives of this work were to study the efficacy of cyclophosphamide treatment and the role of laboratory biomarkers for disease progression monitoring.
Methods
A prospective pilot cohort study was conducted among patients with anti-interferon-γ autoantibodies (IGA) who had recurrent infections and required long-term antimicrobial therapy between 2015 and 2018. The patients were categorized into 2 groups: receipt of intravenous cyclophosphamide (IVCY) and receipt of anti-CD20 therapy (RTX). Clinical and laboratory data were determined.
Results
A total of 17 IGA patients were enrolled. Prolonged fever was the most common manifestation, and the most common infection identified was nontuberculous mycobacterial infections. Both were found in 88.24% of all patients.
After completion of IVCY, 9/11 patients achieved complete remission and tended to reach remission faster compared with individuals in the RTX group. The median duration from treatment initiation to remission (interquartile range) was 84 (42–154) days in the IVCY group and 99 (51–202) days in the RTX group. In remission patients, the biomarkers of interest had normalized after treatment, except interferon γ autoantibody titers. There were no differences in adverse events among the 2 groups.
Conclusion
IVCY may be considered as alternative therapy in this population, especially in resource-limited countries. A comparable clinical outcome to RTX may support its use on a larger scale. However, further study is encouraged.
Keywords: nontuberculous mycobacterial infection, interferon-γ autoantibodies, cyclophosphamide, adult-onset immunodeficiency
Adult-onset immunodeficiency associated with interferon-? autoantibody is an emerging disease. The majority of patients require both antimicrobial and immunosuppressive treatments. Intravenous cyclophosphamide seems to be an appropriate option, especially in a resource-limited setting. However, further study is encouraged.
Adult-onset immunodeficiency associated with anti-interferon-γ autoantibodies (IGA) is a rare condition causing a considerable burden to affected individuals, particularly in Asia. According to previous studies, a large proportion of patients presented with a wide range of opportunistic infections from nontuberculous mycobacteria (NTM) disseminated to other pathogens including Salmonella spp., etc. [1–4]. Despite intensive antimicrobial treatment, more than half of the patients had a persistent or relapsed infection and experienced multiple adverse drug effects [3].
Fundamentally, this syndrome is related to abnormal production of anti-interferon (IFN)–γ autoantibodies, which can block the IFN-γ signaling pathway, resulting in an immunodeficiency state [5, 6]. To control this condition, the use of immunosuppressive drugs, particularly anti-CD20 monoclonal antibody, has been reported to be associated with favorable clinical outcomes [7, 8]. However, rituximab is a high-cost drug that can only be used by a certain group of patients. Searches for alternative lower-cost treatment have been conducted. To date, there has been very limited data on the clinical benefits of other potent immunosuppressive drugs, for example, cyclophosphamide [9].
Phosphoramide mustard, an active metabolite of cyclophosphamide, when in the human body forms DNA crosslinks within DNA strands at guanine N-7 positions and leads to cell apoptosis at the final step [10]. Cyclophosphamide therefore impacts adaptive immunity via elimination of regulatory T cells and induction of T-cell growth factor [11]. Nonetheless, the efficacy of cyclophosphamide among IGA patients has never been largely elucidated.
We now present our experience in using intravenous cyclophosphamide with favorable outcomes as an adjunctive treatment in IGA patients compared with anti-CD20 therapy.
Objectives
The primary objective was to describe the clinical outcomes of the patients with IGA who received intravenous cyclophosphamide therapy (IVCY) in comparison with rituximab/biosimilar products (RTX). The secondary objective was to identify the potential parameters that predict disease progression for IGA patients.
METHODS
A prospective pilot cohort study was conducted among 37 patients at Ramathibodi Hospital, Mahidol University, Bangkok, Thailand, who had been diagnosed with difficult-to-treat infections associated with IGA in 2015–2018. Infectious disease specialists managed the antimicrobial regimens initially and referred the patients to the immunologists for immune-modifying treatment justification. After careful counseling regarding the study protocol to all participants, the patients were divided into 2 groups solely based on patients’ preferences: 1) receipt of antimicrobial agents and RTX and 2) receipt of antimicrobial agents and IVCY. The investigators had no influence on immunosuppressive treatment selection in every patient. Twenty patients who opted to receive only supportive care were excluded from the study.
Ethical approval was granted by the Research Ethics Committee of the Faculty of Medicine Ramathibodi Hospital, Mahidol University, approval No. MURA2019/1091. The committee approved the use of patient samples and data for the publication of this study. Written informed consent was obtained from every patient.
In the IVCY group, enrolled patients were given intravenous cyclophosphamide at the different dosing regimens. The first dose was given at 5 mg/kg and gradually increased at the following cycles every 3 weeks targeting 15 mg/kg/dose unless significant adverse events (eg, severe nausea/vomiting, diarrhea, bone marrow suppression, etc) were observed. In the RTX group, intravenous rituximab was given at a dose of 500 mg intravenously on days 1 and 15 initially. The need for subsequent doses was justified depending on the clinical responses defined in the “Definition” section. The regimen was adopted from Vital et al. [7, 12]. Patients’ demographic data, clinical presentations, clinical outcomes, and routine laboratory tests and biomarkers—namely erythrocyte sedimentation rate (ESR), C-reactive protein (CRP), IFN-γ autoantibody (both qualitative and quantitative methods), flow cytometry, immunoglobulin levels, and cytokine profiles—were determined. All patients were monitored by health care providers for at least 6 months after completion of immunosuppressive therapy, and the longest follow-up time was 2 years.
Definitions
The “difficult-to-treat infections” were defined as active or relapsed infections despite at least a 3-month course of intensive antimicrobial therapy. The “complete remission stage” represented a controlled infection that previously affected each individual and met all of the following criteria: 1) recovery of all of the symptoms reported before immunosuppressive therapy, 2) resolution of previously positive findings on physical examination compatible with an infection of interest, 3) resolution or stabilization of the radiographic findings in the patients with previously abnormal radiographic findings consistent with an infection of interest, and 4) antibiotic-free for at least 3 months. “Partial remission” referred to an individual who met at least 1 criterion previously described, and 1 of these had to be criterion number 4. “Active infection” referred to the patients who did not meet all the criteria stated.
The resolution of leukocytosis was defined as the decline of a previously elevated white blood cell count to <10 000 cells/mm3. Normalization of ESR and CRP was defined as a decline of previously elevated ESR to <20 mm/h and CRP to <5 mg/L.
Cytokine Analyses
LEGENDplex Human Inflammation Panel (BioLegend), a bead-based multiplex assay, was used for the quantification of 13 human inflammatory cytokines/chemokines, including interleukin (IL)-1β, IFN-α, IFN-γ, tumor necrosis factor (TNF)–α, monocyte chemoattractant protein (MCP)–1 (chemokine ligand 2 [CCL2]), IL-6, IL-8 (chemokine ligand 8 [CXCL8]), IL-10, IL-12p70, IL-17A, IL-18, IL-23, and IL-33. EDTA blood was drawn from each patient and processed as per the package insert [13, 14].
Statistical Analysis
Data were analyzed using Statistical Package for the Social Sciences for Windows (SPSS), version 24. P values <.05 were considered statistically significant. Descriptive analysis was applied to demographic data. The Mann-Whitney test and Fisher exact test were used to compare the parameters of the 2 independent, unrelated patient groups. The Wilcoxon signed rank test was applied for the follow-up period analysis.
RESULTS
A total of 17 patients diagnosed with IGA were enrolled. Eleven patients were included in the IVCY group, and 6 patients were with the RTX group. Most of them were from the upper region of the country.
Patients’ Demographics
Table 1 demonstrates patients’ demographics, clinical presentations, and isolated microorganisms. In the IVCY group, 7 of 11 patients (63.64%) were male. The median age of disease onset (interquartile range [IQR]) was 52 (35–64) years. The median durations from initial symptoms to the first visit to the infectious disease clinic and to the diagnosis of IGA made by infectious disease specialists (IQR) were 322 (91–1460) days and 59 (21–882) days, respectively. Generalized lymphadenopathy, weight loss, and prolonged fever were the most common presentations. In regards to abnormal skin lesions, Sweet’s syndrome was identified in 8/9 patients (88.89%). Only 2/9 patients (22.22%) had true NTM cutaneous skin/soft tissue infections. Disseminated NTM infection was the most common opportunistic infection found in all patients. Eight of 11 patients were infected with multiple microorganisms. Mycobacterium abscessus was the most common NTM identified. Two of 11 patients (18.18%) were infected with >1 type of NTM.
Table 1.
Demographic Data and Clinical Presentations Among IVCY and RTX Groups
| IVCY Group | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Microorganisms | Clinical Manifestations and Organ Involvements | ||||||||||||||||
| Case No. | Gender | Age at Disease Onset, y | NTM | Other | Prolonged Fever | Weight Loss | Lymphadenopathy | Skin | Pulmonary | Septicemia | Musculoskeletal | Miscellaneous | Duration From Disease Onset to IGA Diagnosis, d | Duration From Disease Onset to Enrollment, d | Prior Treatment | Cumulative Dose of IVCY/ Cycles, mg | Outcome of Treatment |
| 1 | F | 35 | M. avium complex, M. fortuitum | None | X | X | X | X | X | X | X | 39 | 1926 | ABx + IVCY (NIH regimen) × 7 cycles | 11 250/13 | R | |
| 2 | M | 59 | M. kansasii | Salmonella spp., HSV, Cryptococcus spp. | X | X | X | X | X | X | X | X | 882 | 1747 | ABx + plasmaphe- resis × 3 d | 7500/10 | R |
| 3 | F | 56 | NTM | None | X | X | X | X | 81 | 540 | ABx | 4100/8 | R | ||||
| 4 | M | 59 | M. abscessus | HZV | X | X | X | X | X | X | 21 | 1086 | ABx | 4600/6 | R | ||
| 5 | M | 40 | M. abscessus | None | X | X | X | X | 571 | 1600 | ABx | 7400/10 | R | ||||
| 6 | F | 47 | M. manteneii | Cryptococcus spp. | X | X | X | X | X | X | X | 158 | 907 | ABx | 3350/6 | PR | |
| 7 | F | 45 | M. parascofula- ceum, M. abscessus | None | X | X | X | X | X | X | X | 53 | 835 | ABx + IVCY (NIH regimen) × 6 cycles | 3650/5 | PR | |
| 8 | M | 62 | M. abscessus | Salmonella spp., HSV, Cryptococcus spp. | X | X | X | X | X | X | 29 | 163 | ABx | 2800/5 | R | ||
| 9 | M | 46 | M. abscessus | None | X | X | X | X | X | X | 59 | 659 | ABx | 6050/7 | R | ||
| 10 | M | 52 | M. abscessus | Cryptococcus spp., cytomegalovirus | X | X | X | X | X | X | X | 252 | 488 | ABx | 4700/6 | R | |
| 11 | M | 64 | M. fortuitum | Salmonella spp. | X | X | X | X | X | X | 36 | 213 | ABx | 3900/6 | R | ||
| RTX Group | |||||||||||||||||
| Case No. | Gender | Age at Disease Onset, y | Microorganisms | Clinical Manifestations and Organ Involvements | Duration From Disease Onset to IGA Diagnosis, d | Duration From Disease Onset to Enrollment, d | Prior Treatment | Cumulative Dose of RTX/Cycles, mg/m2 | Outcome of Treatment | ||||||||
| NTM | Other | Prolonged Fever | Weight Loss | Lymphadenopathy | Skin | Pulmonary | Septicemia | Musculoskeletal | Miscellaneous | ||||||||
| 1 | F | 66 | None | Salmonella spp., HSV, HZV | X | X | X | X | 108 | 250 | ABx | 345/6 | PR | ||||
| 2 | F | 48 | M. avium complex | None | X | X | X | X | 1275 | 1997 | ABx | 339/4 | R | ||||
| 3 | F | 44 | M. szulgai, M. angelicum | MTB | X | X | X | X | X | 128 | 231 | ABx | 359//4 | PR | |||
| 4 | F | 79 | M. avium complex | None | X | 180 | 383 | ABx | 326/2 | R | |||||||
| 5 | M | 51 | M. avium complex | None | 279 | 574 | ABx | 319/2 | R | ||||||||
| 6 | M | 62 | None | Salmonella spp. | X | X | X | X | X | 174 | 876 | ABx | 286/2 | R | |||
Abbreviations: ABx, antibiotics; F, female; HSV, herpes simplex virus; HZV, herpes zoster virus; IGA, adult-onset immunodeficiency associated with interferon-γ autoantibody; IVCY, intravenous cyclophosphamide; M, male; MKD, mg/kg/dose; MTB, M. tuberculosis; NIH, National Institutes of Health; NTM, nontuberculous mycobacteria; PR, partial remission; R, remission; RTX, rituximab.
In the RTX group, a total of 6 patients were included. Four of these (66.67%) were female. The median age of disease onset (IQR) was 56.5 (44–79) years. The median durations from the onset of initial symptoms to the first visit to the infectious disease clinic and to diagnosis of IGA made by an infectious disease specialist (IQR) were 147.0 (16–413) days and 177.0 (108–1275) days, respectively.
The most common complaint was a prolonged fever in 4/6 patients (66.67%). Pulmonary system and skin/soft tissue (Sweet’s syndrome) were mostly involved and were found in 3/6 patients (50.0%) each. Disseminated NTM infection was the most common opportunistic infection, in 4/6 patients (66.67%), followed by salmonellosis in 2/6 patients (33.33%). Two of 6 patients (33.33%) had multiple infections.
Basic Laboratory Tests
During the active infection stage, all patients had leukocytosis and elevated ESR. More than half of the patients had anemia, thrombocytosis, elevated CRP, hypoalbuminemia, and elevated alkaline phosphatase levels in both groups. Complete laboratory results are displayed in Table 2.
Table 2.
Comparison of Laboratory Results Among all Patients Before and After Treatment
| IVCY Group (n = 11) | RTX Group (n = 6) | |||||
|---|---|---|---|---|---|---|
| Active | Remission | P - Value | Active | Remission | P - Value | |
| Basic investigations (normal range) | ||||||
| WBC (4.00–10.00), ×103/cumm | 20.12 (17.13–38.61)a | 7.55 (4.16–10.91)a | <.001* | 14.77 (10.80–36.62)a | 7.60 (5.11–9.23)a | .007* |
| Neutrophils (40.00–74.00), % | 78.23 (±9.92)b | 44.78 (±8.27)b | <.001* | 67.40 (±10.07)b | 52.57 (±9.53)b | .013* |
| Hemoglobin (12.00–16.00), g/dL | 9.80 (±1.69)b | 12.40 (±1.34)b | <.001* | 8.75 (±1.95)b | 11.99 (±1.05)b | .050* |
| Platelets (140.0–450.0), ×103/cumm | 478.00 (358–654)a | 273.00 (172–412)a | <.001* | 247.00 (105–821)a | 223.00 (179–291)a | .417 |
| ESR (4.0–20.0), mm/h | 97.78 (±25.69)b | 47.95 (±18.00)b | <.001* | 91.14 (±11.04)b | 52.00 (±18.99)b | .004* |
| C-reactive protein (<5), mg/dL | 37.63 (8.08–272.00)a | 3.34 (1.00–17.63)a | <.001* | 57.45 (4.26–99.28)a | 3.02 (1.59–4.68)a | .009* |
| Serum albumin (35–50) (±SD), g/L | 28.85 (±6.44)b | 38.55 (±2.64)b | <.001* | 25.46 (±8.52)b | 35.55 (±1.62)b | .333 |
| Alkaline phosphatase (40–150), U/L | 135.0 (83–671)a | 84.5 (36.7–154.0)a | .016* | 405.0 (112.00–1191.0)a | 71.0 (57.0–108.0)a | .012* |
Abbreviations: ESR, erythrocyte sedimentation rate; IQR, interquartile range; IVCY, intravenous cyclophosphamide; RTX, rituximab; WBC, white blood cell count.
aMedian (IQR).
bMean (SD).
Immunological Investigations
Complete immunological laboratory tests were performed in the patients in both groups during active disease but in none during remission. In the IVCY group, the median proportion of the CD19 cell population (IQR) was 7.45% (3.9%–21.4%), with a median absolute B-cell count (IQR) of 0.25 (0.1–0.38) K/μL. The mean levels of IgG, IgM, and IgA were within the normal ranges. Delayed hypersensitivity skin test by antigens including the tuberculin, tetanus toxoid, and measles, mumps, and rubella vaccines showed the wheal reaction after 72 hours in all patients. Six of 11 patients (54.54%) had IFN-γ inhibition assay titers >1:50 000 during active infection.
In the RTX group, complete T-cell subset analyses were performed in 5 of 6 patients. The median CD19 cell proportion (IQR) was 7.2% (2.5%–19.3%), and the median absolute B-cell count (IQR) was 0.2 (0.05–0.5) K/μL. Three of 4 patients (75%) had IFN-γ inhibition assay titers >1:50 000 during the active infection phase. Cytokine analyses in both groups were within normal ranges.
Treatment Regimens
In the IVCY group, the median duration from the onset of disease to IVCY (IQR) was 871 (163–1926) days. The mean dose of intravenous cyclophosphamide given was 10.93 ± 1.54 mg/kg/dose every 3 weeks. The total course of treatment given ranged from 5 to 13 cycles.
In the RTX group, the median duration from the onset of disease to RTX (IQR) was 478.5 (231–1997) days. The total course of RTX varied from 2 to 6 cycles.
Clinical Responses to Treatment
After IVCY, symptoms of prolonged fever, weight loss, lymphadenopathy, and reactive dermatosis had subsided in all patients. At the 6-month evaluation, 9/11 patients (81.82%) had complete remission, whereas 2/11 patients (18.18%) had partial remission and still required antimicrobial treatment for persistent soft tissue infection from Cryptococcus spp. and M. abscessus and the addition of RTX did not reverse the disease progression. The median time from treatment initiation to clinical remission (IQR) was 84 (42–154) days, and the median duration of antibiotic treatment (IQR) was 415.5 (153–911) days, as demonstrated in Table 3.
Table 3.
Comparison of Clinical Responses Between IVCY and RTX Groups
| IVCY | RTX | P Value | 95% CI | |
|---|---|---|---|---|
| Median duration from immunosuppressive treatment initiation to clinical remission, d | 84 (42–154)a | 99 (51–202)a | .491 | –95.63 to 49.13 |
| Median duration of antimicrobial treatment used, d | 415.5 (153–911)a | 203 (118–1040)a | .432 | –218.00 to 477.92 |
| Median duration of remaining in clinical remission, d | 613 (231–944)a | 334 (225–790)a | .418 | –274.35 to 588.35 |
Abbreviations: CI, confidence interval; IQR, interquartile range; IVCY, intravenous cyclophosphamide; RTX, rituximab.
aMean (IQR).
During the follow-up period, 1 of 9 patients (11.11%) developed relapsed NTM infection at 252 days after treatment discontinuation, although 8/9 patients (88.89%) remained stable until the end of the study, with a median duration of remission retainment (IQR) of 613 (231–944) days.
In the RTX group, 4/6 patients (66.67%) achieved complete remission after 6 months of RTX initiation, whereas 2/6 patients (33.33%) had partial remission. The patients with partial remission still required antimicrobial therapy, and they did not achieve complete remission despite additional RTX. The median duration from RTX initiation to complete remission (IQR) was 99 (51–202) days, and the median duration of antibiotic treatment (IQR) was 203 (118–1040) days.
During the follow-up period, every patient with an initial complete remission status had relapsed infections. The median duration from complete remission to relapsed infection (IQR) was 334 (225–790) days.
Changes in Basic Investigations and Immunological Studies
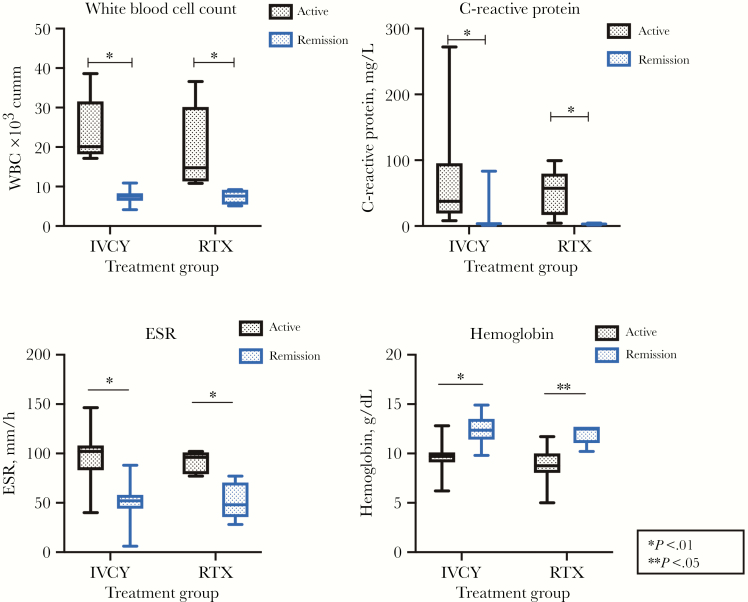
At 6 months after the first dose of IVCY, normalization of basic laboratory values was documented, as the median WBC count (IQR) had decreased to 7.55×103/mm3 (4.16×103–10.91×103), the mean hemoglobin was 12.40 ± 1.34 g/dL, and the median platelet count (IQR) was 273×103/mm3 (172×103–412×103). The resolution of hypoalbuminemia and elevated alkaline phosphatase levels were observed, as the mean serum albumin level had risen to 38.55 + 2.64 g/L and the median alkaline phosphatase level (IQR) had declined to 84.5 (36.7–154) IU/L. The normalization of CRP was noticed in 7/11 patients (63.64%), with a median CRP level (IQR) of 3.34 (1.00–17.63) mg/L, but ESR remained elevated in all patients, with a mean ESR of 47.95 ± 18.00 mm/h. Changes in all parameters reached statistical significance, at a P value <.05, as demonstrated in Figure 1.
Figure 1.
Changes in markers before and after treatement among intravenous cyclophosphamide and rituximab groups. Abbreviations: ESR, erythrocyte sedimentation rate; IVCY, intravenous cyclophosphamide; RTX, rituximab; WBC, white blood cell count.
After 3 months, an absolute depletion of CD19 cells was not observed. A decline of IFN-γ inhibition assay titers correlated with disease remission only in 4/8 patients (50%). The median values of IFN-α, MCP-1, IL-6, IL-8, and IL-17A (IQR) had decreased to 17.53 (2.24–31.75) pg/mL, 189.84 (135.35–762.65) pg/mL, 8.61 (2.06–28.70) pg/mL, 18.34 (5.20–50.99) pg/mL, and 17.58 (4.51–41.95) pg/mL, respectively. However, the median IL-18 value (IQR) had increased to 338.71 (93.48–603.23) pg/mL. The changes in all cytokine parameters did not reach statistical significance.
In the RTX group, normalization of basic laboratory values was documented after 6 months of RTX initiation, as demonstrated in Table 2 except for ESR, which remained elevated with a mean value of 52.00 ± 18.99 mm/h. Changes in all parameters had reached statistical significance, except for the changes in platelet count and serum albumin level. Changes in all cytokine parameters did not reach statistical significance.
An absolute depletion of CD20 cells was observed at 3 months after RTX in all patients, and this was concordant to the decline of IFN-γ inhibition assay titers, but all were still positive results.
Safety and Complications
Grade I adverse side effects, especially nausea and vomiting during the first few days after IVCY, were observed in every patient, and all improved with supportive treatment. No adverse side effects other than grade I were observed. However, 5/11 patients (45.45%) developed adverse drug events associated with antibiotic use; hearing impairment associated with prolonged aminoglycoside use (4/11 patients), and anemia was associated with linezolid use (1/11 patients).
In the RTX group, grade I adverse side effects were documented in all patients with fatigue. However, no specific treatment was necessary. No adverse side effects other than grade I were observed.
DISCUSSION
IGA is an emerging condition that is primarily endemic in Southeast Asia [1–8]. In our study, the majority of patients were from the Northern/Northeastern part rather than other regions of the country. To date, it is unclear whether maternal geographic residence explains IGA pathogenesis. Disruption of the IL-12-IFN-γ signaling pathway caused by anticytokine antibodies plays a crucial role in the pathogenesis and explains the immunodeficiency state causing difficult-to-control infection in affected individuals [2, 5].
The median age of onset of disease in this study (IQR) was 52 (35–79) years, compared with 48 (44.8–60.0) years in a previous report [3]. There was no gender predilection among this population. The lag time between the onset of disease and diagnosis of IGA was >2 months in all patients. This may reflect that affected individuals potentially received delayed treatment. To date, there have been no clinical studies to explore the reasons for this delay. However, several possibilities might explain this phenomenon; these include lack of a good understanding of this condition among health care providers either in diagnostic modalities or available treatment options and low socioeconomic status in affected individuals, leading to difficulty obtaining appropriate medical care, etc.
NTM was the most common opportunistic infection identified among our patients, and the majority of patients presented with lymphadenopathy, similar to prior reports [2, 3, 15]. Interestingly, the incidence of bacteremia was much lower than in the study of Browne et al., who reported that nearly 40% of patients were bacteremic [2]. Among the patients with abnormal skin lesions, Sweet’s syndrome rather than true skin/soft tissue infection was reported most frequently, similar to prior studies, as well as the unique characteristic of increased absolute natural killer (NK) cell count [3, 16, 17]. This feature indicates that NK cells are necessary for NTM eradication.
However, other immune cells may be required to achieve complete disease control, as it was witnessed in our population that infections were difficult to control with only the presence of NK cells. Another important finding was a relatively scant absolute B-cell count since enrollment in all patients despite high titers of IFN-γ inhibition assay with active infection. This may reflect that excessive antibody production could have been from various sources of immune cells besides CD20 cells, for example, plasma cells.
Anti-CD20 monoclonal antibody treatment has been reported to be associated with favorable treatment outcomes among patients with IGA who are co-infected with difficult-to-treat infection [7, 8, 18]. However, this treatment may not be of interest in resource-limited settings given that the therapy is high cost and has not been shown to warrant a cure in all patients. Therefore, IVCY may be considered a good alternative in these cases, based on its mechanism of action targeting T-cell proliferation, compromising B-cell and plasma cell functions as a consequence. More importantly, IVCY is more accessible in less-developed countries. However, the use of IVCY at higher dosages potentially carries multiple toxicities, including bone marrow suppression, severe nausea, and vomiting, etc., which can restrict its use as a routine practice [19].
Baerlecken et al. [9] reported successful treatment of an IGA patient who experienced severe osteomyelitis due to Mycobacterium avium complex infection. Plasmapheresis and pulse cyclophosphamide were prescribed, in addition to antimicrobial therapy aiming to decrease the circulating autoantibodies against IFN-γ, which resulted in remission at 3-year follow-up. The average dose of cyclophosphamide utilized in our study was higher than in prior studies and resulted in a higher proportion of patients being antibiotic-free after complete remission up to 944 days.
After either IVCY or RTX, there was no significant difference between the 2 groups in achieving remission/partial remission at 6 months post-treatment. However, IVCY tended to provide faster remission than RTX (84 vs 99 days; 95% confidence interval [CI], –95.63 to 49.13; P = .491). Moreover, the patients in the IVCY group appeared to have a longer duration of remission and a lower incidence of relapsed infection compared with those in the RTX group. Our hypothesis to explain the long duration of remission attainment in the IVCY group was based on the mechanism of cyclophosphamide, which has an impact on multiple aspects of the immune system, either T cells, B cells, plasma cells, or dendritic cells [20–22]. Therefore, ICVY’s ability to suppress autoantibody production is possibly superior to that of anti-CD20 therapy alone. And this may also explain that additional RTX did not provide remission in several circumstances. Nonetheless, the median duration of antimicrobial use was longer in the IVCY group after entering the remission stage. This might partly explain the longer clinical remission in the IVCY group as well.
In this study, we were not able to explain the real pathogenesis among affected patients who had achieved only partial remission instead of complete remission despite similar treatment modality. The explanation with regards to the nonsuppressible antibody titers after either IVCY or RTX in multiple subjects was not fully elucidated. Identification of other potential sources of antibody production is the next crucial step to describe the disease pathogenesis and to be able to justify the appropriate treatment option. Nonetheless, several other factors could have impacted patients’ clinical responses, for example, various genetic factors and lack of standard treatment guidelines for certain infected organisms such as M. manteneii and M. parascrofulaceum, which were isolated from the patients with partial remission.
Various parameters were used to monitor infection status in real clinical practice, for example, clinical signs and symptoms, but these provided less accuracy in several circumstances. WBC count, Hb, ESR, and CRP are considered the basic laboratory tests, and they have been adopted widely among clinicians to identify patient status. Declines in WBC count, percentage of neutrophils, Hb, CRP, ESR, and alkaline phosphatase levels were documented in the remission stage and remained good parameters in monitoring the patients, instead of using more complex immunological investigations. These findings are consistent with prior reports and confirm the benefit of common parameters in disease monitoring [3, 23].
The inflammatory process is a complex mechanism composed of activation of both innate and adaptive immunity. This leads to a release of cytokines and chemokines including MCP-1 that induces intracellular calcium reflux and promotes production of tissue factor and pro-inflammatory cytokines, for example, IL-1 and IL-6 [24–26]. In this study, we explored uncommon tests that have never been studied, aiming to explain the real dynamic changes in affected individuals while on treatment. Patients who were in the partial remission/remission stage tended to have lower levels of IL-6 and MCP-1 compared with patients with active disease. However, these levels did not reach statistical significance, partly due to the small sample size.
One strength of the current study is a long follow-up period for each patient. The natural history of the patients in this population and clinical outcomes have been described after a standard treatment regimen vs newly proposed therapy. The major weaknesses of this study are its small population size and incomplete laboratory analyses in some patients, including lack of a comprehensive immunological study to identify other potential sources of excessive antibody production, particularly plasma cells. Therefore, a complete explanation of disease pathogenesis, appropriate treatment, and certain parameters to distinguish patients at each stage cannot be made.
CONCLUSIONS
In conclusion, this is the first comprehensive clinical and immunological study that has demonstrated the benefit of IVCY in IGA patients for better control of the infectious process. Furthermore, this therapy demonstrated the ability to avoid unnecessary and prolonged antibiotic use in several patients without significant adverse side effects compared with more well-known anti-CD20 therapy. This is considered a novel approach in treating IGA patients, especially in resource-limited countries. However, future studies on a larger scale and identification of other sources of excessive antibody production are encouraged for better care in this population.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Acknowledgments
The authors gratefully acknowledge the invaluable help given by Supa Oncham, MSc, in performing laboratory tests and handling the data.
Financial support. This work was supported by the Faculty of Medicine Ramathibodi Hospital (grant number CF_61004).
Potential conflicts of interest. All authors: no reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Author contributions. W.L. and P.R. conceived the study question and design and drafted the manuscript; W.L. extracted data and performed statistical analysis; P.P., P.N., and P.R. reviewed the analysis for accuracy. T.S. was responsible for the laboratory analyses. P.R. takes responsibility for the integrity of the data and the accuracy of the analysis. All authors had full access to the data (including statistical reports and tables) in the study and approved the final manuscript.
References
- 1. Kampitak T, Suwanpimolkul G, Browne S, Suankratay C. Anti-interferon-γ autoantibody and opportunistic infections: case series and review of the literature. Infection 2011; 39:65–71. [DOI] [PubMed] [Google Scholar]
- 2. Browne SK, Burbelo PD, Chetchotisakd P, et al. Adult-onset immunodeficiency in Thailand and Taiwan. N Engl J Med 2012; 367:725–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Chi CY, Lin CH, Ho MW, et al. Clinical manifestations, course, and outcome of patients with neutralizing anti-interferon-γ autoantibodies and disseminated nontuberculous mycobacterial infections. Medicine (Baltimore) 2016; 95:e3927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hase I, Morimoto K, Sakagami T, et al. Patient ethnicity and causative species determine the manifestations of anti-interferon-gamma autoantibody-associated nontuberculous mycobacterial disease: a review. Diagn Microbiol Infect Dis 2017; 88:308–15. [DOI] [PubMed] [Google Scholar]
- 5. Döffinger R, Helbert MR, Barcenas-Morales G, et al. Autoantibodies to interferon-gamma in a patient with selective susceptibility to mycobacterial infection and organ-specific autoimmunity. Clin Infect Dis 2004; 38:e10–4. [DOI] [PubMed] [Google Scholar]
- 6. Höflich C, Sabat R, Rosseau S, et al. Naturally occurring anti-IFN-gamma autoantibody and severe infections with Mycobacterium cheloneae and Burkholderia cocovenenans. Blood 2004; 103:673–5. [DOI] [PubMed] [Google Scholar]
- 7. Browne SK, Zaman R, Sampaio EP, et al. Anti-CD20 (rituximab) therapy for anti-IFN-γ autoantibody-associated nontuberculous mycobacterial infection. Blood 2012; 119:3933–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Czaja CA, Merkel PA, Chan ED, et al. Rituximab as successful adjunct treatment in a patient with disseminated nontuberculous mycobacterial infection due to acquired anti-interferon-γ autoantibody. Clin Infect Dis 2014; 58:e115–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Baerlecken N, Jacobs R, Stoll M, et al. Recurrent, multifocal Mycobacterium avium-intercellulare infection in a patient with interferon-gamma autoantibody. Clin Infect Dis 2009; 49:e76–8. [DOI] [PubMed] [Google Scholar]
- 10. Giraud B, Hebert G, Deroussent A, et al. Oxazaphosphorines: new therapeutic strategies for an old class of drugs. Expert Opin Drug Metab Toxicol 2010; 6:919–38. [DOI] [PubMed] [Google Scholar]
- 11. Sistigu A, Viaud S, Chaput N, et al. Immunomodulatory effects of cyclophosphamide and implementations for vaccine design. Semin Immunopathol 2011; 33:369–83. [DOI] [PubMed] [Google Scholar]
- 12. Vital EM, Rawstron AC, Dass S, et al. Reduced-dose rituximab in rheumatoid arthritis: efficacy depends on degree of B cell depletion. Arthritis Rheum 2011; 63:603–8. [DOI] [PubMed] [Google Scholar]
- 13. Bremm M, Huenecke S, Zimmermann O, et al. In-vitro influence of mycophenolate mofetil (MMF) and ciclosporin A (CsA) on cytokine induced killer (CIK) cell immunotherapy. J Transl Med 2016; 14:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lefort N, LeBlanc R, Surette ME. Dietary buglossoides arvensis oil increases circulating n-3 polyunsaturated fatty acids in a dose-dependent manner and enhances lipopolysaccharide-stimulated whole blood interleukin-10-a randomized placebo-controlled trial. Nutrients 2017; 9(3):1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Valour F, Perpoint T, Sénéchal A, et al. ; Lyon TB study group Interferon-γ autoantibodies as predisposing factor for nontuberculous mycobacterial infection. Emerg Infect Dis 2016; 22:1124–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Chan JF, Trendell-Smith NJ, Chan JC, et al. Reactive and infective dermatoses associated with adult-onset immunodeficiency due to anti-interferon-gamma autoantibody: Sweet’s syndrome and beyond. Dermatology 2013; 226:157–66. [DOI] [PubMed] [Google Scholar]
- 17. Chruewkamlow N, Mahasongkram K, Pata S, et al. Immune alterations in patients with anti-interferon-γ autoantibodies. PLoS One 2016; 11:e0145983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Koizumi Y, Sakagami T, Nishiyama N, et al. Rituximab restores IFN-γ-STAT1 function and ameliorates disseminated Mycobacterium avium infection in a patient with anti-interferon-γ autoantibody. J Clin Immunol 2017; 37:644–9. [DOI] [PubMed] [Google Scholar]
- 19. Emadi A, Jones RJ, Brodsky RA. Cyclophosphamide and cancer: golden anniversary. Nat Rev Clin Oncol 2009; 6:638–47. [DOI] [PubMed] [Google Scholar]
- 20. Madondo MT, Quinn M, Plebanski M. Low dose cyclophosphamide: mechanisms of T cell modulation. Cancer Treat Rev 2016; 42:3–9. [DOI] [PubMed] [Google Scholar]
- 21. Fassbinder T, Saunders U, Mickholz E, et al. Differential effects of cyclophosphamide and mycophenolate mofetil on cellular and serological parameters in patients with systemic lupus erythematosus. Arthritis Res Ther 2015; 17:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Nakahara T, Uchi H, Lesokhin AM, et al. Cyclophosphamide enhances immunity by modulating the balance of dendritic cell subsets in lymphoid organs. Blood 2010; 115:4384–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wongkulab P, Wipasa J, Chaiwarith R, Supparatpinyo K. Autoantibody to interferon-gamma associated with adult-onset immunodeficiency in non-HIV individuals in Northern Thailand. PLoS One 2013; 8:e76371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Rubartelli A, Lotze MT. Inside, outside, upside down: damage-associated molecular-pattern molecules (DAMPs) and redox. Trends Immunol 2007; 28:429–36. [DOI] [PubMed] [Google Scholar]
- 25. Chen L, Deng H, Cui H, et al. Inflammatory responses and inflammation-associated diseases in organs. Oncotarget 2018; 9:7204–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Mukaida N, Harada A, Matsushima K. Interleukin-8 (IL-8) and monocyte chemotactic and activating factor (MCAF/MCP-1), chemokines essentially involved in inflammatory and immune reactions. Cytokine Growth Factor Rev 1998; 9:9–23. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.