Abstract
Previous work of ours and others has documented regressive changes in neuronal architecture and function in the medial prefrontal cortex (mPFC) of male rats following chronic stress. As recent focus has shifted toward understanding whether chronic stress effects on mPFC are sexually dimorphic, here we undertake a comprehensive analysis to address this issue. First, we show that chronic variable stress (14-day daily exposure to different challenges) resulted in a comparable degree of adrenocortical hyperactivity, working memory impairment, and dendritic spine loss in mPFC pyramidal neurons in both sexes. Next, exposure of female rats to 21-day regimen of corticosterone resulted in a similar pattern of mPFC dendritic spine attrition and increase in spine volume. Finally, we examined the effects of another widely used regimen, chronic restraint stress (CRS, 21-day of daily 6-h restraint), on dendritic spine changes in mPFC in both sexes. CRS resulted in response decrements in adrenocortical output (habituation), and induced a pattern of consistent, but less widespread, dendritic spine loss similar to the foregoing challenges. Our data suggest that chronic stress or glucocorticoid exposure induces a relatively undifferentiated pattern of structural and functional alterations in mPFC in both males and females.
Keywords: corticosterone, delayed alternation, dendritic spine, prelimbic, sex differences
Introduction
Stress responses entail a constellation of physiological and behavioral changes to any challenge that overwhelms, or is perceived to overwhelm, selective homeostatic systems of the individual (Selye 1980; Day 2005). Integral to the stress response is the induction of neuroendocrine (hypothalamo–pituitary–adrenal or HPA) and autonomic (sympatho-adrenal) systems for the mobilization and redistribution of bodily resources. The extent to which one or both systems are recruited may vary depending on the particular challenge at hand (Mason et al. 1976), although each is geared toward the facilitation and complementation of situation-specific behavioral adaptations. Glucocorticoid end products of the HPA axis (cortisol in humans, corticosterone in rodents) are potent hormonal mediators that act broadly, and may do so over extended time periods, to help prepare the individual for adaptation ranging from acute challenges or protracted threats (Sapolsky 1992; de Kloet et al. 2005; Ulrich-Lai and Herman 2009; McEwen and Gianaros 2011).
Multiple limbic cortical regions undergo dendritic and synaptic remodeling in response to repeated stressors (Christoffel et al. 2011b; McEwen and Gianaros 2011; McEwen et al. 2015; Radley et al. 2015b). This synaptic reorganization is regarded as integral to the concepts of allostasis and allostatic load (and similar to Selye’s heterostasis; Selye, 1973) following chronic stress (McEwen and Wingfield 2003), whereby such modifications promote adaptation for survival but at the cost of increased susceptibility to psychiatric illnesses (de Kloet et al. 2005; Christoffel et al. 2011b; McEwen and Gianaros 2011). In this regard, evidence that women are more likely than men to develop stress-related psychiatric disorders (Holden 2005; Marcus et al. 2005; Kendler et al. 2006; Grigoriadis and Robinson 2007; Seo et al. 2017) has led to increasing scrutiny in animal studies over whether chronic stress has differential effects on limbic cortical network function in males and females (McLaughlin et al. 2009; Bourke et al. 2012; Shansky and Woolley 2016; Shors 2016). However, evidence demonstrating greater susceptibility to chronic stress in female rodents has not been particularly forthcoming, and existing data suggest that the female hippocampus and medial prefrontal cortex (mPFC) may in fact show resistance to the adverse effects of chronic stress that have been widely observed in male rats (McLaughlin et al. 2005; Garrett and Wellman 2009; Shansky et al. 2010; Rico et al. 2015; Moench and Wellman 2017).
Here we provide the first comprehensive analysis of dendritic spine density and morphology in mPFC neurons of female and male rats following exposure to chronic stress. In a first experiment, we evaluated the effects of chronic variable stress (CVS; daily exposure to different stressors at unpredictable times over 14 days) on dendritic spine morphology in layer 2/3 neurons in the prelimbic cortex (PL) and spatial working memory in male and female rats. Ancillary experiments involved subjecting female rats to different regimens known to induce regressive structural changes in mPFC neurons in males, such as chronic glucocorticoid exposure for 21 days, and chronic restraint stress (CRS; 6 h/day, 21 days). Following CVS exposure, males and females displayed a comparable degree of impairment in spatial working memory. Furthermore, male and female rats showed a similar extent of dendritic spine remodeling and attrition in PL neurons following CVS, exposure to exogenous glucocorticoids, or CRS. These studies extend our previous work by showing that the regressive effects of chronic stress on prefrontal structural and functional plasticity are evident in female as well as male rats and endorse the idea that allostatic changes resulting from repeated stress or glucocorticoid exposure tend to promote a similar set of adaptations regardless of sex.
Materials and Methods
Animals
The animals used in this study were 3-month-old male Sprague Dawley albino rats (Charles River Laboratories). Rats were pair-housed, unless otherwise noted, and maintained on a 12:12 h light/dark cycle (lights on at 0600), with free access to food and water. All experimental protocols were approved by the Institutional Animal Care and Use Committee of the University of Iowa. After 7 days of acclimatization in the animal housing facility, rats were habituated to human contact by handling each for 5 min each day, over at least 7 days before the initiation of experiments.
Experiment 1: Evaluation of Sex Differences in the Effects of CVS on Prefrontal Functioning and Dendritic Spine Density and Morphology in PL Neurons
General Procedures
In this experiment, separate cohorts of rats were used to assess CVS effects on a prefrontal-dependent behavioral task and dendritic spine morphology in prefrontal neurons (Fig. 1A). The rationale for not examining prefrontal function and spine morphology in the same cohort is based upon consideration that the effects and learning may have interactive effects on prefrontal spine morphology (Knott et al. 2002; Holtmaat et al. 2008; Dumitriu et al. 2010; Liston et al. 2013) that could potentially confound the interpretation of CVS effects.
Figure 1.
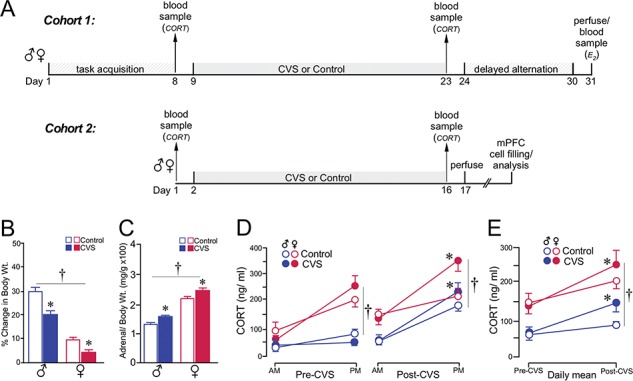
CVS results in a comparable degree of adrenocortical hyperactivity in both sexes. (A) Timelines of the first experiment, displaying individual timelines for each cohort (1 and 2). (B, C) Both groups of rats showed decreases in body weight gain (B) and adrenal hypertrophy (C) through the course of the 14-day CVS period. (D) Graph depicting mean ± SEM plasma CORT levels at AM and PM sampling of CORT as a function of sex and treatment group. Measurements were taken in the same rats prior (left) to and following (right) CVS. CVS resulted in significantly increased PM levels of CORT (*, P < 0.05), regardless of sex. (E) Graph showing changes in the daily mean values of CORT levels pre- and post-CVS as a function of sex and treatment group. CVS increased levels of CORT in both sexes (*, P < 0.05), whereas levels of CORT were higher in female rats (†, P < 0.05). N = 10–11/group.
In cohort 1, male (N = 22) and female (N = 20) rats were first submitted to repeated blood sampling for assessment of baseline adrenocortical activity. Rats were then placed on a restricted diet (~20 g of chow per day for male and ~ 15 g/day for female rats) and trained in a prefrontal-dependent spatial working memory task, delayed alternation using a T maze (Divac 1970; Ramos et al. 2003; Anderson et al. 2014). In order to appropriately control for food intake, rats in this experiment were single-housed. Delayed alternation training ensued over a 6-day period, whereafter a subset of rats (N = 10/sex) were subjected to 14 days of CVS. AM (0500) and PM (1700) blood samples were collected for assessment of plasma corticosterone (CORT) 1 day prior to CVS exposure in both CVS and control groups. Controls were handled but not subjected to any of the stress procedures. During the 14-day CVS period, both CVS and control groups were provided food and water ad libitum. On the day following CVS, AM and PM blood samples were again collected in CVS and control groups for the assessment of CORT via radioimmunoassay. One male rat that was removed since it underwent significantly greater weight decreases following CVS exposure relative to the cohort.
In cohort 2, analysis of sex and CVS effects on prefrontal dendritic spine density and morphology were conducted in groups of male (N = 16) and female (N = 40) rats. Each group was divided in half and then either subjected to 14 days of CVS or remained as unstressed controls. AM and PM blood samples were collected for assessment of plasma CORT 1 day prior to CVS exposure in both CVS and control groups. A second series of blood samples were collected in both groups of rats on the evening on the last day of CVS (PM time point). The AM sample was collected on the morning of day 15, and rats were perfused thereafter. Brains were harvested and tissue was prepared for intracellular dye injection of prefrontal neurons. Attrition in the final sample sizes reported in the results are based upon the strict criteria required for inclusion of neuronal segments into the dendritic spine analysis (described below) and the sensitivity of the cell loading procedure to optimal fixation conditions (males: N = 5 control; N = 5 CVS; females: N = 10 control; N = 13 CVS).
CVS
CVS involved daily exposure to either 2 brief or 1 sustained stressor over a 14-day period, in semirandomized order, presented at different times each day. Brief stressors included open-field exposure for 10 min, placement of cage on orbital shaker for 30 min at 100 rpm, tail suspension for 10 min, forced swim for 10 min in 23 °C water, and cold exposure for 4 h (4 °C). Sustained stressors included overnight exposure (12–16 h duration) to wet bedding or isolation.
Assessment of Prefrontal Functionality Using Delayed Alternation
The training period began by initially habituating rats to a T maze (90 × 65 cm; 40-cm opaque walls surrounding the perimeter) over a several day period using miniature chocolate chips as a food reward for running to either goal arm in under 60 s. Next, rats were subjected to a forced alternation period of training where they were only rewarded with chocolate after entering the opposite goal arm that they were in previously. Between trials, the maze was wiped clean with 95% ethanol to prevent olfactory cues from determining choice. After 3 consecutive days of 10 trials/day, rats were tested for spontaneous alternation (i.e., chocolate was present in both goal arms, although rats were only rewarded for entering the opposite arm from the previous trial). The delay between trials was increased until animals could successfully alternate at a 15-s interval with > 80% accuracy for 10 trials. After all rats reached the same criterion level of performance, some were subjected to 14 days of CVS. On the second day following CVS exposure, rats were tested in delayed alternation, involving 8 trials/day over 6 consecutive days. The delays between each trial were semirandomly varied between 30, 60, or 120 s (on a given day the same pattern was used for all animals). During delay, rats were placed in the holding cage and the maze was cleaned with 95% ethanol. The percentage of correct choice alternations made at each delay interval (30, 60, and 120 s) were obtained for each animal, and overall averages were expressed as a function of treatment group.
Experiment 2: Effect of Prolonged CORT Exposure on Dendritic Spine Structural Alterations in PL Neurons in Female Rats
Female rats were implanted with slow-release CORT (200 mg) to clamp circulating CORT levels of this hormone to constant levels that approximate the circadian zenith (250–300 ng/mL) over a 21-day period. Rats were anesthetized with isoflurane and were implanted subcutaneously in the interscapular region with 200-mg (N = 12) CORT pellets that provide a constant and slow release into the general circulation for up to 3 weeks (Innovative Research of America). A second group of rats (N = 12) was implanted with inert cholesterol pellets of weight equal to the CORT pellets. CORT pellet efficacy and baseline adrenocortical activity in CORT-replaced and sham rats, respectively, were confirmed by collection of repeated blood samples at AM and PM times for radioimmunoassay of CORT, and all rats were perfused on the morning of day 22. Two rats containing CORT implants were excluded from the study due to ineffective CORT pellet functioning. Additional rats were excluded from the data analysis due to insufficient fixation parameters or quality of dye filling, yielding final group sizes of N = 7 CORT and N = 5 control.
Experiment 3: Effect of CRS on Dendritic Spine Structural Alterations in PL Neurons in Female Rats
Male and female rats (N = 10/sex) were subjected to 6 h of restraint (beginning at 0800) for a 21-day period. Restraint was performed in Plexiglas restrainers (Braintree Scientific) that have adjustable enclosures to account for individual differences in sizes and rates of growth. Control animals (N = 10/sex) were left undisturbed during throughout this period. AM and PM blood samples were collected for assessment of plasma CORT 1 day prior to CRS exposure in both CRS and control groups. A second series of blood samples were collected in both groups of rats on the evening on the last day of CRS, and the AM sample collected on the morning of day 22. All rats perfused on the morning of day 22, and brain tissue was prepared for intracellular dye injection of prefrontal neurons. Final group sizes due to attrition from the cell loading procedure were as follows: males: N = 8 control, N = 6 CRS; females: N = 8 control, N = 7 CRS.
Blood Collection and Radioimmunoassay
Basal adrenocortical activity was measured by obtaining repeated blood samples from the tail vein of rats at morning (0500) and evening time points (1700) before and after the chronic stress/CORT treatments in each experiment. Rats were briefly restrained (15–30 s), and a small longitudinal incision was made at the distal tip of the tail with a sterile blade. Blood samples (~200 uL) were collected into chilled plastic microfuge tubes containing EDTA and aprotinin, centrifuged, and fractionated for storage of plasma at −80 °C until assayed. Plasma corticosterone (CORT) was measured without extraction, using an antiserum raised in rabbits against a CORT-BSA conjugate, and 125I-CORT-BSA as tracer (MP Biomedicals). Assay sensitivity was 8 ng/mL; intra- and interassay coefficients of variation were 5% and 10%, respectively.
Gonadal steroid levels were measured by obtaining blood from cardiac punctures on the final day of each experiment, immediately prior to perfusion. Plasma estradiol levels were measured using a 17β-estradiol radioimmunoassay (MP Biomedicals) with 125I-17β-estradiol as tracer. The 17β-estradiol antibody cross-reacts 100% with 17β-estradiol, 20% with estrone, 2% with estriol, 1% with 17α-estradiol, but not with progesterone, testosterone, cortisol, aldosterone, or cholesterol (<0.01%). The minimum detectable 17β-estradiol concentration was 10 pg/mL, and the intra- and interassay coefficients of variation were 4% and 9%, respectively. Plasma testosterone was measured via testosterone radioimmunoassay (MP Biomedicals) with 125I-testosterone as tracer. The testosterone antibody cross-reacts 3% with 5α-DHT, 2% with 5α-androstane-3β,17β-diol, and 2% with 11-oxotestosterone, but does not cross-react with progesterone, estrogen, or the glucocorticoids (all < 0.01%). The minimum detectable testosterone concentration was 0.1 ng/mL and the intra- and interassay coefficients of variation were 5% and 8%, respectively.
Histology and Tissue Processing
Rats were anesthetized with Fatal-Plus (100 mg/kg, i.p.) and perfused via the ascending aorta with 100-mL 1% PFA and 0.125% glutaraldehyde in 0.1-M phosphate buffered saline (PBS, pH 7.4), followed by 500 mL of 4% PFA and 0.125% glutaraldehyde in 0.1-M PBS, 7.4, at a flow rate of 55 mL/min. The descending aorta was clamped to limit the flow of fixative to the head and upper extremities, and to prevent fixation of the adrenal glands. Blood was taken via cardiac puncture before perfusion and centrifuged and fractionated for storage of plasma at −80 °C for later radioimmunoassay of estradiol and testosterone. Immediately following perfusion, the adrenal glands were extracted and weighed, and brains were removed and postfixed for 4 h. After postfixation, the pregenual pole of the cortex was sectioned coronally into 250 μm–thick slabs using an oscillating tissue slicer (VT-1000S, Leica) and stored in 0.1-M PBS containing 0.1% sodium azide at 4 °C until the time of cell loading.
Intracellular Dye Injection Procedure
The procedures used in these experiments are based on previous reports using the same methodology (Radley et al. 2006; Anderson et al. 2014). Coronal tissue slabs were treated in the DNA-binding fluorescent stain DAPI (Invitrogen) to distinguish between nuclear lamination patterns that distinguish PL from other adjacent-lying prefrontal cortical subfields. DAPI-treated sections were mounted on nitrocellulose filter paper and submerged in a tissue culture dish containing PBS and viewed under fluorescence using a fixed-stage microscope (Leica DM5500). Injections of 5% Lucifer yellow (LY; Invitrogen) were made by iontophoresis through micropipettes (1–2 μm inner diameter) under a DC current of 1–6 nA for 5–10 min. Neurons in superficial and deep layers of PL were selected for the dye injection procedure based upon the distinguishing cytoarchitectonic features of this region, notably a more densely packed layer 2 and a broader layer 5 relative to adjacent-lying cortical subfields. The general technique for cell filling involved carefully observing the passive diffusion of LY resulting from application of a negligibly small amount of current from the advancing micropipette tip under 40× magnification; LY diffuses amorphously until hitting a dendritic process or cell body, whereby the dye becomes restricted intracellularly. After several neurons were filled intracellularly, tissue sections were mounted onto glass slides and coverslipped in Vectashield (Vector Laboratories).
Imaging and Dendritic Spine Morphometric Analyses
An experimenter unaware of the treatment condition for each animal performed neuronal reconstructions and data analyses. Pyramidal neuron dendritic arbors were reconstructed in 3D using a computer-assisted morphometry system consisting of a Leica DM4000R equipped with an Applied Scientific Instrumentation MS-2000 XYZ computer-controlled motorized stage, a QImaging Blue digital camera, a Dell computer, and morphometry software (MBF Biosciences). Neurons were visualized, and the dendritic tree was reconstructed using a Leica Apochromat 40X objective with a numerical aperture of 1.4 and Neurolucida software (MBF Biosciences). To be considered for dendritic spine morphometric analyses, LY-filled PL neurons had to exhibit complete filling of the dendritic tree, as evidenced by well-defined endings. However, because the dye-filling procedure performed in the sections were only 250-μm thick, and it was virtually impossible to retain an entirely intact apical dendritic arbor with no truncations, we focused our analysis on dendritic spine density and morphology.
All fluorescent dye-filled neurons were analyzed by individuals unaware of the experimental treatment. Two-dimensional renderings for each neuron were obtained using Neurolucida software, and radial distances at 150-μm and 250-μm increments from the soma were selected as a boundary delineating proximal, distal, and tufted portions of the apical dendritic tree. Sampling of basal dendrites was only carried out within the proximal region (<150 μm). Within these regions, branches were randomly selected for imaging from each neuron for an average of 3–5 segments per neuron and 5–7 neurons for each animal. The selection criteria for confocal imaging of dendritic segments are based upon previous reports (Radley et al. 2006; Radley et al. 2013; Anderson et al. 2014): (1) possess a diameter of < 3 μm, as larger diameter dendrites in PL pyramidal neurons exhibit greater variability in spine density values; (2) reside within a depth of 50 μm from the top surface of the section, due to the limited working distance of the optical system; (3) to be either parallel to, or course gently relative to, the coronal surface of the section (i.e., this helps to minimize z-axis distortion and facilitate the unambiguous identification of spines); and (4) have no overlap with other branches that would obscure visualization of spines. z-Stacks were collected on a Leica SP5 confocal laser-scanning microscope equipped with an argon laser and a 100X, 1.4 NA oil-immersion objective, using voxel dimensions of 0.1 × 0.1 × 0.1 μm3. Settings for pinhole size (1 airy disc), gain, and offset were optimized initially and then held relatively constant throughout the study to ensure that all images were digitized under similar illumination conditions at a resolution of 512 × 512 pixels.
Images were deconvolved with AutoDeblur (Media Cybernetics), and spine analyses were performed using the semiautomated software NeuronStudio (Rodriguez et al. 2006; Radley et al. 2008; Anderson et al. 2014) (http://research.mssm.edu/cnic/tools-ns.html), which analyzes in 3D dendritic length, spine density, and morphometric features (i.e., head/neck diameter, volume, subtype) for each dendritic spine. Spines were classified as thin or mushroom if the ratio of the head diameter-to-neck diameter was > 1.1. If their ratio exceeded this value, spines with a maximum head diameter >0.4 μm were classified as mushroom, or else were classified as thin. Spines with head-to-neck diameter ratios < 1.1 were also classified as thin if the ratio of spine length-to-neck diameter was > 2.5; otherwise, they were classified as stubby. A fourth category, filopodial spines, exhibited a long and thin shape with no enlargement at the distal tip. Since these were very seldom observed, they were classified as thin subtypes. Finally, data readouts from the spine analysis algorithm were visually compared by the experimenter for each optical stack to verify accurate subtype classifications for dendritic spines.
As delineated in each experimental description above, the final group sizes for the spine morphology analyses are lower than our starting sample sizes (~60% yield). This was due to the fact that the success rate from perfused rats that needed to provide at least 5 high-quality fluorescently labeled neurons for inclusion into the analysis. The judgment of whether individual cases did not meet our standard of quality for dendritic spine labeling or contained a sufficient number of dendritic segments imaged was made by an experimenter unaware of the specific treatment.
Statistics
Delayed alternation performance was compared using a mixed-design analysis of variance (ANOVA) with delay interval (30, 60, and 120 s) as the within-subjects variable, and stress treatment and sex as between-subjects variables. Performance at individual delay intervals was further evaluated using a 2-way ANOVA. Group data from the CORT radioimmunoassay were compared with a mixed-design ANOVA with time (i.e., time of day or date of blood sample) as the within-subjects variable, and sex and stress as between-subjects variables. Density values were obtained as the number of spines per unit micron of dendritic segment from each animal (i.e., 3–5 segments/neuron, 5–7 neurons/rat), and compared using a mixed-design ANOVA, with treatment and sex as between-subjects variables, and distance as the within-subjects variable. Significance was set at P < 0.05. In instances where main effects were observed, follow-up analyses were performed using a 2-way ANOVA (with treatment and sex as factors), at each dendritic distance (apical: < 150 μm, 150–250 μm, > 250 μm; basal: < 150 μm), with mean values composed of all segments from each radial distance within each rat. Taking into consideration the possibility that the analysis of density within any of the 4 dendritic distances increased the risk of producing a type 1 error, alpha was set at 0.05 ÷ 4, requiring that P < 0.0125 for significance. Data are expressed at mean ± standard error of the mean (SEM).
Population analyses of spine volume as a function of subtype and experimental treatment were analyzed via comparison of cumulative frequency distributions using the Kolmogorov–Smirnov (K-S) test with MATLAB software (MathWorks). Our main objective with this analysis was to make within-sex comparisons of treatment effects to better understand sex-specific patterns of variability. However, since the K-S test only allows comparisons of only 2 distributions, we performed a correction for multiple comparisons, using an expanded Bonferroni approximation (Brittain 1987). Thus for an alpha level of 0.05, based on this correction, the number of possible comparisons from 4 groups yields P < 0.0099 (we round off to 0.01). A further correction is made for K-S tests run within dendritic distances based upon dividing the alpha level by the number of dendritic distances (i.e., 0.01 ÷ 4 = 0.0025). Finally, permutation tests were performed for all significant K-S test results as an additional evaluative measure. For this analysis, group labels from the population analyses were randomly re-assigned to each spine (i.e., shuffled) and a K-S test was performed on these groups. This process was repeated 10 000 times to generate a distribution of K-S test statistics (D). These tests verified that the probability of randomly obtaining a D statistic of the same or greater magnitude approximated those reported in the original K-S comparisons.
Results
Experiment 1: Evaluation of CVS on Prefrontal Functioning and Dendritic Spine Density and Morphology in PL Neurons in Male and Female Rats
CVS Exposure Induces Adrenocortical Hyperactivity in Both Sexes
CVS and similar repeated stress regimens (a.k.a., chronic unpredictable stress, chronic mild stress, chronic intermittent stress) are known to produce a constellation of allostatic physiological, endocrine, and behavioral alterations (Ottenweller et al. 1989; Papp et al. 1991; Herman et al. 1995; Willner 1997; Grippo et al. 2003) among which are decreased body weight gain and adrenocortical hyperactivity. Thus, for each cohort of rats (Fig. 1A), we assayed for changes in pre- and post-CVS body weight gain and plasma levels of CORT, and we measured adrenal weights postmortem to obtain adrenal-to-body weight ratios. CVS produced reliable decrements in the percentage of weight gain (F1,38 = 86.8, P < 0.05) in both sexes over the 14-day stress period as compared with unstressed control rats (Fig. 1B). Significant increases in adrenal weight (grams) by percentage of body weight (kilograms) (F1,38 = 16.1, P < 0.05) were also noted in all CVS-exposed rats regardless of sex (Fig. 1C). Main effects of sex were noted in each of these analyses (weight gain: F1,38 = 946.1, P < 0.05; adrenal weight: F1,38 = 138.6, P < 0.05) but sex by stress interactions were not observed for weight gain (F1,38 = 1.3, P = 0.3), or adrenal weight (F1,38 = 0.4, P = 0.5).
Comparison of AM and PM CORT levels prior to CVS exposure revealed main effects of time of day (F1,38 = 20.2, P < 0.05), sex (F1,38 = 15.2, P < 0.05), and interaction for time and sex (F1,38 = 12.1, P < 0.05) (Fig. 1D, left). These main effects are consistent with the expectations that evening levels of plasma CORT should be substantially higher than in the morning, and that female rats exhibit higher basal levels of plasma CORT relative to males. Following CVS, plasma levels of CORT were elevated in CVS-treated male and female rats, most notably at the PM time point. Main effects were observed for sex (F1,38 = 18.5, P < 0.05), time of day (F1,38 = 36.9, P < 0.05), and interaction of time of day with CVS exposure (F1,38 = 5.6, P < 0.05) (Fig. 1D, right). Assessment of mean daily values of AM and PM CORT between pre- and poststress days of measurement also revealed main effects for sex (F1,38 = 27.8, P < 0.05), day (F1,38 = 25.8, P < 0.05), and interaction between day with CVS exposure (F1,38 = 4.1, P < 0.05) (Fig. 1E). The general trend highlighted by analysis of averaged CORT is that, although females display higher levels of CORT than male rats, CVS reliably enhances adrenocortical output in both sexes.
CVS Impairs Spatial Working Memory in Both Sexes
To assess the effects of sex and CVS on prefrontal functioning, rats were trained and tested in a delayed alternation task using a T maze (Fig. 2A). This task has been shown to be dependent upon intact mPFC functioning (Divac 1970; Ramos et al. 2003; Hinwood et al. 2012), and a stable population of dendritic spines in pyramidal neurons in PL (Hains et al. 2009; Anderson et al. 2014; Radley et al. 2015a). Rats were first trained to an equivalent level of performance (i.e., 80% choice accuracy at a 15-s delay) prior to CVS exposure and then tested for working memory function in the days following the completion of the CVS regimen. Acquisition was evaluated based upon the number of training sessions required for each animal to reach criterion (Ramos et al. 2003). Female rats required less time to reach the criterion level for task acquisition than males (F1,40 = 3.3, P < 0.05) (Fig. 2B), with the mean number of sessions (1 per day) for a female of 4.2 and males 5.5 days. After the 14-day CVS epoch, both stress and control groups were tested in the delayed alternation task at increasing delay intervals (30, 60, or 120 s) over a course of 3 days. Mixed-design ANOVA revealed main effects for delay interval (F2,74 = 90.4, P < 0.05), stress treatment (F1,37 = 7.2, P < 0.05), and interactions between delay and stress treatment (F2,37 = 7.0, P < 0.05) and delay and sex (F2,37 = 13.1, P < 0.05). Whereas overall choice accuracy decreases with increased delay intervals, the interaction between delay interval and stress treatment reveals a significant influence of CVS in further downward effects on this index in both male and female rats (Fig. 2C). The interaction between delay interval and sex is largely accounted for by decreasing choice accuracy through longer delay intervals, regardless of stress treatment (Fig. 2C). Consideration of treatment effects at individual time points demonstrated CVS-induced decreases in displayed alternation at the 60-s delay interval (F1,37 = 6.9, P < 0.05), and both effects of CVS (F1,37 = 10.8) and sex (F1,37 = 12.1) in reducing choice accuracy at the 120-s delay (P < 0.05 for each), most notably in female rats. Comparison of individual values for delay interval as a function of CORT levels (AM, PM, or integrated values), or estradiol levels (Table 1) using linear regression, each failed to reveal any reliable correlation as a function of sex or stress (data not shown).
Figure 2.
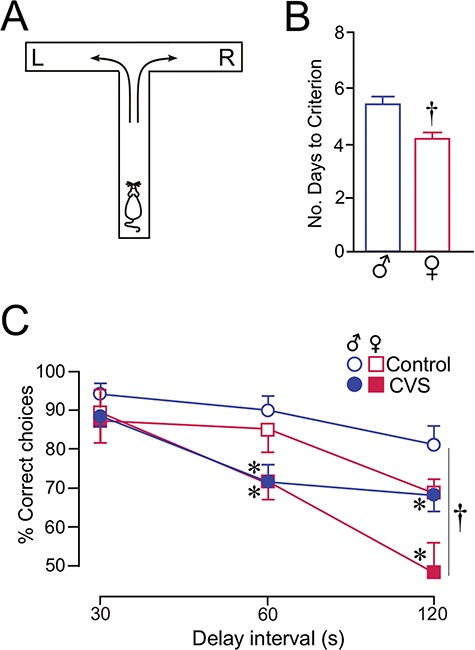
CVS impairs spatial working memory in both sexes. (A) Schematic diagram of the T maze (90 × 65 cm) used for delayed alternation. Rats are placed in the starting location (as shown) and were rewarded for selecting the opposite goal arm (e.g., right, R) from the previous trial (left, L). As the delay interval between each trial is increased, the percentage of correct choices provides a measure of spatial working memory. (B) Histogram shows the average number of days that males and females required to reach an equivalent level of performance in the delayed alternation task (≥80% choice accuracy at a 15-s delay). Female rats took fewer days to reach criterion than males (*, P < 0.05). N = 20–21/group. (C) Graph demonstrating the percentage of correct responses at increasing delay intervals. Behavioral impairments were noted as both a function of increasing delay interval and as a function of CVS (*, P < 0.05). Females performed at lower levels of choice accuracies at the 120-s time point (†, P < 0.05), although this showed no interaction with the effects of CVS (P = 0.3). N = 10–11 rats/group.
Table 1.
Plasma levels of gonadal steroids of rats from each experiment. Mean ± SEM estradiol and testosterone values, as based upon radioimmunoassay of plasma samples collected prior to perfusion in rats from each experiment. Whereas mean values of sex steroids are lower following each experimental treatment, none of these were significant
| Experiment 1 | Control | CVS | P value |
|---|---|---|---|
| Female, estradiol (E2) | 156 ± 17 pg/mL | 137 ± 9 pg/mL | 0.3 |
| Male, testosterone (T) | – | – | – |
| Experiment 2 | Sham | CORT | |
| Female, E2 | 146 ± 27 pg/mL | 129 ± 11 pg/mL | 0.6 |
| Experiment 3 | Control | CRS | |
| Female, E2 | 153 ± 21 pg/mL | 127 ± 9 pg/mL | 0.4 |
| Male, T | 2.2 ± 0.6 ng/mL | 1.5 ± 0.3 ng/mL | 0.3 |
CVS Exposure Induces Dendritic Spine Loss in Adult Male and Female Rats
A separate cohort of rats were used to assess CVS effects on dendritic spine morphology in prefrontal neurons. The rationale for using a separate cohort of rats to assess PL spine morphology is based upon evidence of the exquisite degree of sensitivity of dendritic spines to environmental experience and learning (Knott et al. 2002; Holtmaat et al. 2008; Dumitriu et al. 2010; Liston et al. 2013) that could have interactive effects with CVS. Adrenocortical hyperactivity in CVS-treated male and female rats was verified as described above. On the day after CVS, all rats were perfused, and tissue was prepared for intracellular dye injections in pyramidal neurons in layer 2/3 of the PL (Fig. 3A). Different regions of the dendritic tree (apical: proximal, < 150 μm; medial, 150–250 μm; distal, > 250 μm; basal: < 150 μm) were selected for high-resolution confocal laser scanning microscopic imaging of dendritic segments (Fig. 3B). Digital renderings (z-stacks) of dendritic segments made in 3 dimensions were deconvolved, followed by the analysis of spine density and morphology using the semi-automated software NeuronStudio. In this study, a total of 504 dendritic segments were digitally imaged and approximately 80 000 dendritic spines were reconstructed and analyzed.
Figure 3.
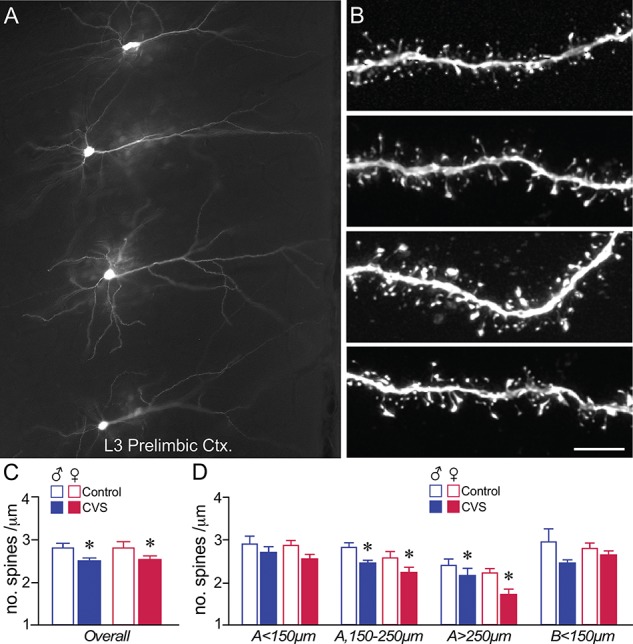
CVS induces dendritic spine loss in PL pyramidal neurons in male and female rats. (A) Darkfield photomicrograph depicting several layer 3 pyramidal neurons in PL targeted for intracellular dye-injection with Lucifer Yellow. (B) Examples of deconvolved images of dendritic segments from layer 2/3 pyramidal neurons in PL. Scale bar = 75 μm (A), 5 μm (B). (C, D) Mean + SEM for overall (i.e., summated and averaged across the 4 different regions of the arbor) dendritic spine density (C) and density as a function of the specific dendritic compartment sampled (D). CVS decreased overall spine density that was accounted for by downward trends throughout both apical and basal dendrites, and significant decreases in distal apical dendrites. *, indicates significant difference between control and CVS; P < 0.05 (C), P < 0.0125 (D). Males: N = 5, control; N = 5 CVS; females: N = 10 control; N = 13 CVS.
Mixed-design ANOVA revealed a main effect of CVS (F1,29 = 15.4, P = 0.01), dendritic distance (F3,87 = 20.6, P < 0.001), and sex (F1,29 = 4.5, P = 0.047), but no interaction effect of CVS by sex (F1,29 = 0.1, P = 0.75), and no interaction effects as a function of dendritic distance (i.e., distance by sex: F3,87 = 1.56, P = 0.21; distance by treatment, F3,87 = 0.24, P = 0.87, or distance by sex by treatment, F3,87 = 0.99, P = 0.41), on dendritic spine density. CVS induced an 11% decrease (11.5% in males, 10% in females) in overall dendritic spine density in PL neurons in following 14 days of CVS exposure (Fig. 3C). Comparisons made between CVS and control animals revealed significant dendritic spine loss more distally in apical dendrites following CVS (apical 150–250 μm: F1,31 = 12.1, P = 0.002; apical > 250 μm: F1,31 = 8.1, P = 0.011), and downward trends in more proximal aspect of the dendritic tree (apical < 150 μm: F1,31 = 3.9, P = 0.057; basal < 150 μm: F1,31 = 6.3, P = 0.018) (Fig. 3D). No main effects of sex or sex by CVS interactions on PL dendritic spine density were noted with the exception of a downward trend of sex effect in distal apical (i.e., > 250 μm) dendrites (F1,31 = 6.7, P = 0.014), marked by decreases in both control and CVS groups of female rats (Fig. 3D).
Effects of CVS on PL Dendritic Spine Morphology
Dendritic spines can be distinguished by geometric characteristics (i.e., thin, mushroom, stubby) that have proved to be useful for inferring synaptic structure–function relationships (Kasai et al. 2003; Bourne and Harris 2007; Yang et al. 2009; Dumitriu et al. 2010; Lee et al. 2012). For example, dendritic spines in cortical neurons classified as thin represent the majority of the population (60–70%; Bourne and Harris 2007), and previous studies have identified this subtype as important for long-term potentiation and learning-related plasticity (Arnsten et al. 2010; Anderson et al. 2014). Mushroom spines comprise 10–20% of the synaptic population, and are representative of larger volume, stable, and mature synaptic phenotypes (Harris and Stevens 1989; Kasai et al. 2003; Holtmaat et al. 2005; Knott et al. 2006; Yasumatsu et al. 2008). We utilized NeuronStudio software to partition spines into subtypes as based upon spine head–neck and head–length ratios to account for whether CVS-induced dendritic spine attrition is manifest within specific subtypes (see Materials and Methods) (Fig. 4A).
Figure 4.

Effects of CVS on dendritic spine morphology in male and female rats. (A) Example of high-resolution deconvolved optical z-stack of a dendritic segment used for spine analysis with NeuronStudio Software. Open colored circles designate spine subtypes based upon user-defined parameters in the software. Scale bar = 5 μm. (B–G) Mean + SEM of dendritic spine subtypes. When considered as overall averages, thin (B) and mushroom (D) subtypes displayed significant decreases following CVS, with thin spines significantly decreased in distal apical (>250 μm) dendrites (C), but no differences in any distances with mushroom subtypes (E). (F, G) Since overall densities for stubby spine subtypes did not show significant decreases in any variable (F), no post hoc tests were performed at dendritic distances (G). *, indicates significant difference between control and CVS; P < 0.05 (B, D), P < 0.0125 (C). Males: N = 5, control; N = 5 CVS; females: N = 10 control; N = 13 CVS. (H–K) Cumulative frequency distributions of overall spine volume in PL neurons reveal graded leftward shifts (i.e., decrease) in spine volume in male rats (H) and rightward shifts (i.e., increase) in female rats (J) following CVS exposure. Plots in I and K display the difference between each function in H and J, respectively, with negative values indicating that spines up to that volume make up a smaller proportion of the CVS than control group, and positive values the opposite. K-S, Kolmogorov–Smirnov test, significance set at P < 0.01.
Thin spines underwent overall decreases following CVS (F1,29 = 5.8, P = 0.026; 8.5%), and showed main effects for dendritic distance (F3,87 = 17.8, P < 0.001), and sex (F1,29 = 4.9, P = 0.039), but no main effects of any possible interactions (CVS by sex, F1,29 = 0.11, P = 0.75; distance by sex: F3,87 = 3.55, P = 0.075; distance by treatment, F3,87 = 0.07, P = 0.79; distance by sex by treatment, F3,87 = 2.29, P = 0.15) (Fig. 4B). Consideration of how thin spine loss was spatially distributed throughout the dendritic tree revealed significant CVS-induced decreases in distal apical dendrites (>250 μm, F1,31 = 8.05; P = 0.011), and consistent nonsignificant downward trends in all other apical distances (apical < 150 μm, F1,31 = 4.23, P = 0.047; apical 150–250 μm, F1,31 = 3.62, P = 0.067) (Fig. 4C). No main effects of sex or sex by CVS interactions in thin spine density were noted in any dendritic compartment, except for a nonsignificant downward trend in sex at the > 250-μm apical distance (F1,31 = 7.26, P = 0.014).
Overall mushroom spine densities showed significant main effects as a function of CVS (F1,29 = 4.57, P = 0.046), dendritic distance (F3,87 = 5.93, P = 0.01), but no main effect of sex or among any of the interaction terms (F and P values not shown) (Fig. 4D). However, no effects of CVS, sex, or interaction were noted on mushroom spine densities within any dendritic distance, except for a nonsignificant trend at the apical 150–250 μm distance (F1,31 = 6.56, P = 0.016) (Fig. 4E). Overall densities of stubby subtypes showed main effects only distance (F3,87 = 3.49, P = 0.021) and a significant downward trend for CVS (F1,29 = 3.35, P = 0.083) (Fig. 4F). Illustration of the decreasing trend of stubby spine density in PL neurons following CVS is displayed as a function of dendritic distances in Fig. 4G. We gave consideration to whether filopodial spine densities in PL neurons varied as a function of CVS or sex; however, these were too seldom observed to allow for quantitative analysis. Comparison of individual values of spine densities (overall or subtype) as a function of CORT levels (AM, PM, or integrated values) using linear regression failed to reveal any reliable correlation as a function of sex or stress (data not shown).
Finally, analyses of several spine parameters (length, head diameter, volume) did not reveal any group differences as a function of CVS (data not shown). Nevertheless, population analysis of dendritic spine volumes showed differential effects of stress and sex on spine size. CVS produced a downward shift (i.e., leftward) in spine volume as compared with unstressed control male rats (D = 0.020, P = 0.008) (Fig. 4H, I), in line with our previous observation (Radley et al. 2008) and effects following chronic glucocorticoid exposure (Liston and Gan 2011; Anderson et al. 2016). By contrast, female rats subjected to CVS displayed upward trends in spine volume (i.e., rightward shift in the cumulative distribution frequency curve) related to unstressed controls (D = 0.026, P = 6.9 × 10−5) (Fig. 4J, K). The increases in spine volume in CVS-treated females could not be accounted for by the possibility that smaller volume (thin) subtypes may have been the target of CVS effects, as population analyses within thin subtypes also revealed a similar trend toward increases (K-S test, P = 0.004).
Experiment 2: Effect of Prolonged CORT Exposure on Dendritic Spine Structural Alterations in PL Neurons in Female Rats
Previous studies have shown that glucocorticoids play a prominent role in chronic stress-induced limbic and neocortical structural alterations in male rodents, by itself, and in evoking reorganization of prefrontal synaptic networks following chronic stress exposure (Magariños and McEwen 1995a; Wellman 2001; Cerqueira et al. 2005; Cerqueira et al. 2007; Liu and Aghajanian 2008; Liston and Gan 2011; Gourley et al. 2013; Anderson et al. 2016). Thus, we next examined whether sustained increases in CORT in female rats are capable of inducing a similar pattern of alterations in prefrontal dendritic spine density and morphology as we and others have previously observed in male rats (Liu and Aghajanian 2008; Gourley et al. 2013; Anderson et al. 2016).
Characterization of Plasma CORT Levels
Blood samples were collected from the tail vein of CORT-implanted and sham female rats at times in the morning (0500) and evening (1700) to verify that CORT pellets were producing elevated concentrations and a flattened rhythm of CORT relative to control animals over the 21-day period of exposure (Fig. 5A). Comparison of AM and PM CORT revealed main effects for time of day (F(1,20) = 56.8, P < 0.05), interaction between CORT implant and time of day (F(1,20) = 37.2, P < 0.05), and no main effect for CORT implant alone (F(1,20) = 0.5, P = 0.5). CORT-implanted female rats showed a very small average deviation between AM and PM levels of CORT (18 ± 15 ng/mL), as compared with controls (171 ± 19 ng/mL, P < 0.05) (Fig. 5B). The expected lack of circadian fluctuation of endogenous CORT was typified by elevated AM levels of CORT, which were nearly 2-fold higher than sham-implanted control female rats (P < 0.05), whereas no significant difference between the groups was noted at the PM time point (P = 0.5).
Figure 5.
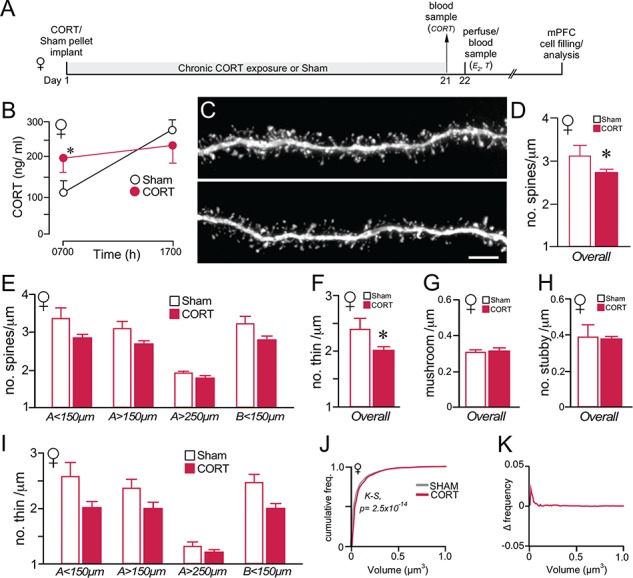
Chronic CORT effects on PL dendritic spine morphology in female rats. (A) Timeline of the second experiment; this is same design as in a previous experiment of ours comparing the effects of chronic CORT exposure on PL dendritic spine morphology in male rats (Anderson et al., 2016). (B) Graph depicting mean ± SEM plasma CORT levels at AM and PM sampling of plasma CORT levels on day 21 of the experiment. Blood samples were collected to verify CORT implant (s.c.) efficacy relative to sham rats implanted with cholesterol pellets. (C) Examples of deconvolved images of dendritic segments from layer 2/3 pyramidal neurons in PL. Scale bar = 5 μm. (D, E) Overall (D) spine densities are decreased following chronic CORT exposure, along with nonsignificant downward trends across dendritic distances (E). (F–H) Chronic CORT treatment decreased thin spine (F), but not mushroom (G) or stubby (H) subtype density. (I) Breakdown of thin spine loss following CORT treatment as a function of different dendritic compartments, illustrates trends but no significant decreases at all distances. *, P < 0.05 (N = 7 CORT; N = 5 Sham). (J) Cumulative distribution frequencies of overall spine volume in PL neurons reveal rightward shifts (i.e., increase) in the CORT versus control group. (K) Graph displaying the difference between each function in J, with positive values indicating that spines up to that volume comprise a greater proportion of the CORT than control group. K-S, Kolmogrov-Smirnov test, significance set at P < 0.01.
Chronic Elevations in Plasma CORT Induce Dendritic Spine Remodeling and Attrition in PL Pyramidal Neurons of Female Rats
Analysis of approximately 43 000 dendritic spines throughout dendritic compartments in PL pyramidal neurons in female rats revealed a 14% decrease in overall spine density following chronic CORT treatment (main effect for CORT treatment: F1,9 = 7.42, P = 0.021) (Fig. 5, C–D). The other main effect was for CORT treatment by dendritic distance (F1,30 = 3.58, P = 0.047), but there was no effect for distance alone (F1,30 = 0.59, P = 0.93). Comparisons made between CORT and control animals at different dendritic distances revealed consistent, nonsignificant downward trends (0.05 > P > 0.02 for all; see Methods for details), suggestive of widespread spine loss throughout PL pyramidal neurons in female rats following prolonged CORT exposure (Fig. 5E). Analysis of spine subtypes most affected by the effects of prolonged CORT exposure revealed the greatest vulnerability in thin spine subtypes. Main effects on thin spines were observed for CORT treatment (F1,11 = 7.28, P = 0.022), but not for dendritic distance or distance by treatment (Fig. 5F), whereas no main effects were observed in mushroom and stubby spine densities (Fig. 5H). Thin spine densities showed downward trends across all dendritic regions analyzed; however, these trends were nonsignificant (0.05 > P > 0.02) (Fig. 5I). By contrast, no trends were noted in any dendritic distance in mushroom or stubby spine subtypes (data not shown). The effects of elevated CORT on estradiol levels were considered as a possible predictor of alterations in overall dendritic spine density or subtype (Table 1); however, no correlations were noted.
Analysis of dendritic spine volumes revealed hypertrophic effects on the overall dendritic population following prolonged CORT treatment in female rats. Cumulative distribution frequencies in chronically CORT-treated female rats showed a rightward shift relative to controls for the overall population (D = 0.026, P = 2.5 × 10−14) (Fig. 5J, K).
Experiment 3: Effect of CRS On Dendritic Spine Structural Alterations in PL Neurons in Female Rats
Characterization of CRS Effects on Adrenocortical Activity
Thus far, our results largely suggest that chronic stress leads to prefrontal functional and structural impairments that are not well-differentiated between male and female rats. Prolonged exposure to exogenous CORT in each sex was also observed to recapitulate the dendritic spine modifications in prefrontal neurons following CVS (Anderson et al., 2014), suggestive of a role for CVS-induced HPA adrenocortical hyperactivity in prefrontal dendritic spine attrition. Nevertheless, much of the existing information in support for sex differences in chronic stress-induced limbic cortical structural alterations has been derived from a different stress regimen—21-day CRS (Watanabe et al. 1992; Luine et al. 1994; Magariños and McEwen 1995a; Galea et al. 1997; Bowman et al. 2001; Bowman et al. 2002; Vyas et al. 2002; Cook and Wellman 2004; Radley et al. 2004; Radley et al. 2006; Garrett and Wellman 2009; Shansky et al. 2010; Conrad et al. 2012). Although the general nature of allostatic changes in limbic cortical neurons following CVS and CRS is considered to be similar (e.g., Magariños and McEwen 1995a; Radley et al. 2006; Radley et al. 2013), CRS is contrasted by declining adrenocortical responses through the course of repeated stress exposure and the normalization of basal CORT levels (Watanabe et al. 1992; Grissom and Bhatnagar 2009; Goel et al. 2014; Radley and Sawchenko 2015). Moreover, the lack of any direct comparison between these paradigms has propagated uncertainty regarding whether sex differences in response to chronic stress may vary as a function of the specific stress regimen employed.
In the last experiment, we assessed the generality of effects of chronic stress on dendritic spine alterations in PL neurons in male and female rats by employing a commonly used variation of CRS, involving exposure of rats to restraint for 6 h/day for 21 days (Watanabe et al. 1992; Luine et al. 1994; Galea et al. 1997; Bowman et al. 2001; Bowman et al. 2002; Radley et al. 2004; Radley et al. 2006; Radley et al. 2008) (Fig. 6A). As shown in previous studies (Radley et al. 2006), we verified that 21 days of CRS significantly decreased the percentage of weight gain over the 21-day epoch (F1,36 = 23.9, P < 0.05). On average, females also displayed reduced weight gains than in males, regardless of treatment condition (F1,36 = 216.4, P < 0.05); however, there was no interaction between CRS and sex (F1,36 = 2.5, P = 0.1) (Fig. 6B). Similar trends were observed with changes in adrenal-to-body weight ratios, with CRS leading to significant decreases (F1,36 = 6.4, P < 0.05; sex: F1,36 = 65.8, P < 0.05; CRS by sex interaction: F1,36 = 1.2, P = 0.3) (Fig. 6C).
Figure 6.
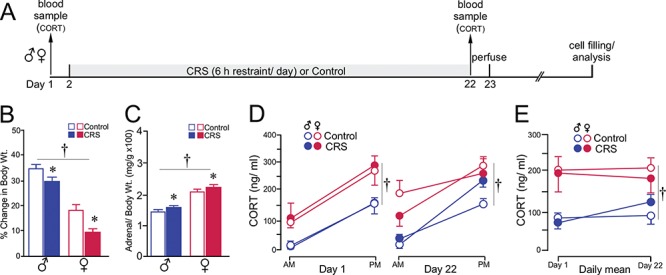
CRS produces a comparable adrenocortical signature in both sexes. (A) Timeline of the third experiment. (B, C) Both groups of rats showed decreases in body weight gain (B) and adrenal hypertrophy (C) through the course of the 21-day CRS period. (D) Graph depicting mean ± SEM plasma CORT levels at AM and PM sampling of CORT as a function of sex and treatment group. Measurements were taken in the same rats prior (day 1) to and following (day 22) CRS. (E) Graph showing changes in the daily mean values of plasma CORT levels pre- and post-CRS as a function of sex and treatment group. Although sex differences in plasma CORT were evident (†, P < 0.05), CRS did not result in any significant increase in CORT levels, as contrasted by the effects of CVS (i.e., compare with Fig. 1E). N = 6–8/group.
In contrast to elevated levels of basal CORT following CVS exposure, measurement of AM and PM CORT prior to (day 1) and after (day 22) CRS did not reveal any increase in adrenocortical activity. Comparison of CORT levels prior to CRS exposure revealed increased titers between AM and PM time points in both sexes (time of day: F1,36 = 79.7, P < 0.05) and overall higher levels in females (sex: F1,36 = 15.8, P < 0.05) (Fig. 6D, left). At the end of the 21-day period of CRS, main effects were again noted for time of day of CORT sample and sex (F1,36 = 67.7 and 10.3, respectively, P < 0.05 for each) (Fig. 6D, right). However, no effects were noted for CRS exposure or any interaction between CRS and sex. Assessment of daily mean values of AM and PM plasma CORT from pre- and poststress days of measurement again revealed main effects only for sex (F1,36 = 17.4, P < 0.05), with females displaying higher average CORT values than males regardless of stress or day of measurement (Fig. 6E). No main effects were noted for average CORT values across time (F(1,36) = 1.4, P = 0.2), stress (F(1,36) = 0.4, P = 0.9), or any interaction. Although a visual inspection of the histograms for the PM time point of the CORT data on day 21 is suggestive of an upward trend in male rats at the PM time point, direct comparison of control and CRS males was not significant (P = 0.1).
Repeated Restraint Induces Similar Effects on Dendritic Spine Density and Morphology in PL Neurons Regardless of Sex
Analysis of approximately 58 000 dendritic spines throughout dendritic compartments in PL pyramidal neurons revealed an 11% decrease in overall spine density following CRS exposure (F1,25 = 4.4, P = 0.046). The only other main effect that was noted was for dendritic distance (F3,75 = 57.09, P < 0.001), but no main effects of sex or any interactions were observed (Fig. 7C). CRS resulted in significant decreases in dendritic spine density in the < 150-μm apical distance (F1,27 = 11.49, P = 0.002), and nonsignificant downward trends in all other distances (P = 0.05–0.02) (Fig. 7D). Much of the decreases in PL neuronal dendritic spine density observed following CRS were due to attrition of thin subtypes, which showed a main effect of treatment (F1,25 = 7.90, P = 0.009), and dendritic distance (F3,75 = 60.52, P < 0.001), but no main effects of sex or any interactions (Fig. 7E). Decreases in overall thin spine densities were most prominent in apical < 150 μm (F1,27 = 13.5, P = 0.001), and nonsignificant trends were noted in other distances (P = 0.1–0.04) (Fig. 7F). For mushroom spine densities, main effects were noted only for dendritic distance (F3,75 = 7.76, P < 0.001) (Fig. 7G), whereas the mixed-design ANOVA for stubby spines failed to reveal any main effects or interactions (Fig. 7H). No correlations were noted between gonadal steroids (Table 1) and any of the dendritic spine indices surveyed (data not shown).
Figure 7.
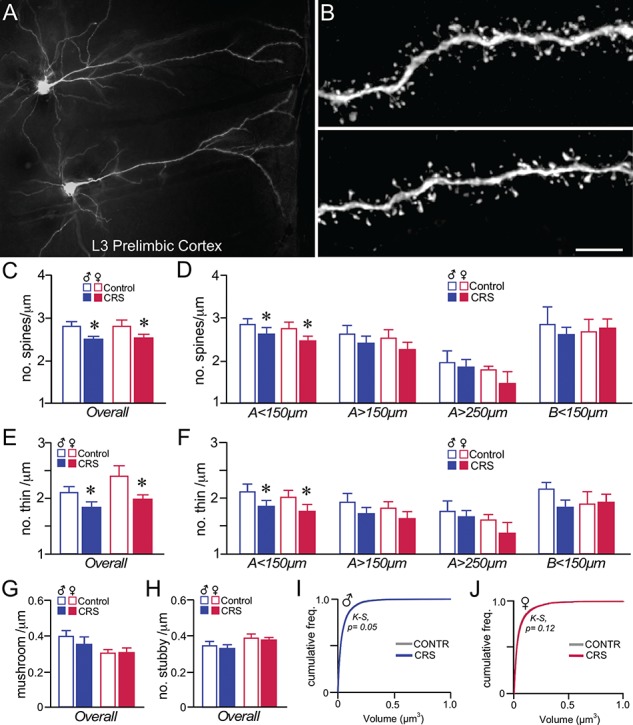
CRS induces dendritic spine loss in PL pyramidal neurons in male and female rats. (A) Darkfield photomicrograph illustrating examples of Lucifer Yellow dye-filled layer 3 pyramidal neurons in PL. (B) Examples of deconvolved images of dendritic segments from layer 2/3 pyramidal neurons in PL. Scale bar = 50 μm (A), 5 μm (B). (C, D) Mean + SEM for overall dendritic spine density (C) and density values within specific dendritic compartments (D). CRS decreased overall spine density in both sexes, and was accounted for by downward trends throughout all apical dendritic compartments, and significant decreases in the < 150-μm apical distance. (E, F) Both the overall density of thin spine subtypes (E) and density values within the specific dendritic compartments sampled (F) illustrate an attrition in this subtype following CRS that accounts for much of the overall decreases above. *, indicates significant difference between control and CVS; P < 0.05 (C, E), P < 0.0125 (D, F). N = 6–8/group. (G, H) CRS had no effect on mushroom (G) or stubby (H) subtypes in PL neurons. (I–J) Cumulative frequency distributions of overall spine volume in PL neurons do not reveal any significant population shifts in spine volume. K-S, Kolmogrov-Smirnov test, significance set at P < 0.01.
Population analysis of cumulative distribution frequencies in CRS-treated versus control rats failed to reveal any population differences on overall dendritic spine volume, based upon analyses within male (K-S test, P = 0.05) and female rats (K-S test, P = 0.12) (Fig. 7I, J).
Discussion
This study addresses the question of whether the chronic stress-induced neuroplastic changes in the rodent PL subregion of mPFC are sexually dimorphic. We observed comparable degrees of impairment in a PL-dependent cognitive task, spatial working memory, in both males and females following 14-day CVS exposure. Male and female rats showed a similar extent of dendritic spine remodeling and attrition in PL pyramidal neurons, and most notably in distal apical dendrites, following chronic stress exposure. These structural changes are likely to involve at least some glucocorticoid dependency, as male rats (Liu and Aghajanian 2008; Gourley et al. 2013; Anderson et al. 2016) and female rats (presented here) showed a similar pattern of regressive structural plasticity in PL neurons following chronic CORT exposure. Both CVS and chronic CORT exposure produced sexually differentiated patterns in dendritic spine volumes, with decreases observed in male and increases in female rats. However, these changes in volume do not appear to account for the comparable working memory deficits observed in CVS-exposed male and female rats, which are more likely due to dendritic spine loss. Moreover, the absence of pronounced changes in spine volume following CRS exposure, which itself is marked by HPA axis habituation, suggests that these morphometric changes are more critically dependent on elevated glucocorticoids than dendritic spine loss. Collectively, these results justify consideration of the possibility that the allostatic adjustments in the mPFC following chronic stress exposure are fundamentally similar across male and female rats.
Comparison With Past Studies
An abundance of research has been conducted over the past 30 years examining the effects of chronic stress on neuronal morphology in the rodent hippocampus, and more recently, mPFC. The general finding from these studies is chronic stress is capable of imposing regressive alterations to pyramidal neurons throughout multiple subregions of the mPFC (anterior cingulate, PL, and infralimbic regions) and hippocampus (CA3c, CA1) (Watanabe et al. 1992; Sousa et al. 2000; Cook and Wellman 2004; Radley et al. 2006; Perez-Cruz et al. 2007; Liu and Aghajanian 2008; Shansky et al. 2009; Farrell et al. 2013). Nevertheless, much of this literature has been carried out in male rodents, whereas the few studies that have been carried out in female rodents have found that chronic stress may lead to either regressive (Sarkar and Kabbaj 2016; Wohleb et al. 2018), hypertrophic changes (Garrett and Wellman 2009; Shansky et al. 2010), or no change (Galea et al. 1997) in hippocampal or prefrontal neuronal morphology. Instances where female rodents displayed increases in neuromorphological indices following chronic stress have been interpreted in the context that responses to chronic stress may be sexually dimorphic. It should be noted that much of the work highlighting hypertrophic changes in dendritic arborization or spine densities have been observed following ovariectomy and estrogen replacement (McLaughlin et al. 2005; Shansky et al. 2010). While evidence of sexually dimorphic responses to stress in rodents is well supported by an extensive literature (Goel and Bale 2010; Bourke et al. 2012; Goel et al. 2014; Moench and Wellman 2015; Shors 2016), there are several important problems with the methodology used in much of the morphology work that we propose accounts for the lack of reliability across studies.
One major issue concerns the analysis of dendritic morphology. Much of this problem owes to the fact that dendritic trees cannot be comprehensively reconstructed in a thin brain section (i.e., 100–250 μm) with dye-filling procedures such as used here and by others (Perez-Cruz et al. 2007; Liu and Aghajanian 2008; Dumitriu et al. 2012). For instance, we generally observed decreases in apical dendritic indices following our experimental treatments regardless of sex, although the high incidence of truncated branches prohibited their inclusion in the final data set. One assumption embedded in dendritic arbor analyses is that truncations are random and, as such, will on average produce a relatively similar degree of variability across animals and treatment groups. However, it is noteworthy that studies with conflicting findings often report pyramidal neuron apical dendritic lengths of 800–1500 μm, which are 50–75% below the average lengths of what should be predicted for apical dendrites in layer 2/3 pyramidal neurons in cortical structures. Although there have been few attempts to directly compare Golgi to dye-filling methods, one study found that neurobiotin-filled neurons in the basolateral amygdala displayed 2-fold larger dendritic arbors compared with labeling using the Golgi–Cox staining procedure (Klenowski et al. 2017).
A second problem concerns the methodology used for imaging and analyzing dendritic spine density. Much of the information that has guided the field has been based on studies analyzing dendritic spines in Golgi-stained preparations. Data gathered from 3D electron microscopic approaches (Harris and Stevens 1989), and high-resolution confocal imaging and analytic approaches used in the present study and by others (Perez-Cruz et al. 2007; Liu and Aghajanian 2008; Bloss et al. 2011; Christoffel et al. 2011a), have shown that the Golgi technique vastly undersamples dendritic spine density by 65–75% and is biased against thin subtypes (Shen et al. 2009; Dumitriu et al. 2011). The problem of biased sampling against thin spines makes it impossible to accurately or reliably assess treatment or sex effects on dendritic spine alterations. This problem is remedied by employing analyses on fluorescently labeled material, such as dye-filling procedures used here and by others (Perez-Cruz et al. 2007; Liu and Aghajanian 2008; Shansky et al. 2009; Dumitriu et al. 2012), lipophilic dye labeling (Shen et al. 2009), or labeling from transgenic reporter lines (Wohleb et al. 2018). Time-lapse imaging of spines provides less resolution of morphometry, yet gives invaluable information about how stress and stress hormones can alter dendritic spine plasticity, relative to the aforementioned approaches (Liston and Gan 2011; Liston et al. 2013; Chen et al. 2018). Until a more rigorous set of standards are adopted for the analysis of neuromorphometry, a consensus for understanding how these indices are affected by chronic stress as a function of sex will remain elusive.
Methodological Considerations
There are several limitations to the current study that warrant discussion. The first is that our quantification of dendritic spines is intended to serve as an index for the entire population of axospinous synapses, although at least a small minority of immature spines in the adult cortex have been shown to lack a presynaptic bouton (Knott et al. 2006). Nevertheless, immature spines tend to have a filopodial morphology, and these were seldom observed in any of our control or experimental treatment groups. While dendritic spine loss in prefrontal neurons have been increasingly regarded in the pathogenesis of chronic stress-related psychiatric disorders (Duman and Aghajanian 2012; Leuner and Shors 2013; Moench and Wellman 2015; Duman et al. 2016; Qiao et al. 2017), recent evidence suggests a role for altered patterns of signaling at inhibitory synapses made with pyramidal neurons that cannot be measured with these approaches (McKlveen et al. 2016; Chen et al. 2018). A final consideration is that our analysis of dendritic spines was focused on pyramidal neurons in layers 2/3 of PL. Although similar chronic stress-induced alterations have been observed in other prefrontal subregions and lamina in male rats (Liu and Aghajanian 2008; Goldwater et al. 2009; Holmes and Wellman 2009; Shansky et al. 2009; Liston and Gan 2011; Chen et al. 2018), evidence that neurons in layer 2/3 may be more highly plastic than neurons in other cortical laminae raises the possibility that these effects may not be as generalizable to other laminae in females (Trachtenberg et al. 2000; Wallace and Bear 2004; Bitzenhofer et al. 2017). Finally, our analysis did not account for the fact that there is circuit-specificity in the effects of chronic stress on dendritic spine plasticity in PL neurons (Radley et al., 2013), and in particular as a function of ovarian hormonal status (Shansky et al. 2010), that could account for sex differences that were not observed here.
Functional Considerations
Our assessment dendritic spine morphology at multiple dendritic distances was designed to offer some insight into how input into these neurons may be differentially altered following chronic stress or glucocorticoid exposure. The dendritic trees of layer 2/3 neurons each contain on the order of 1 × 104 excitatory synapses and span a broad region within the cortex, with apical dendrites undergoing extensive branching throughout layer 1 to the pial surface, and basal dendrites extending into layer 4. Thus, each neuron is situated to receive a vast amount of input from cortical (with local and distant input received in proximal and distal aspects of the dendrites) and subcortical (more distally in layer 1) regions. Although postsynaptic signaling events in neurons are appreciated to be distance-dependent and thus more distal regions may have less influence on somatic voltages, large apical dendrites may utilize a variety of different mechanisms that may not only offset this attenuation, but may also enhance their overall influence. Although speculative, our results may suggest that CVS- and chronic CORT-induced spine loss disrupts processes in distal apical dendrites (e.g., voltage-dependent spikes, clustering events, e.g., see Stuart and Sakmann 1994; Magee and Johnston 1995; Hoffman et al. 1997; Bloss et al. 2018) that may be critical for learning-related plasticity. Since CVS and chronic CORT are distinguished from CRS by increased circulating glucocorticoids, it seems reasonable to speculate that physiological disruptions due to dendritic spine loss in distal apical dendrites are glucocorticoid-dependent.
In all 3 experiments, the majority of the spine loss is accounted for by decrements in thin subtypes. Thin spines show a much higher degree of plasticity compared with other spine phenotypes (Knott et al. 2006; Bourne and Harris 2007), and have been implicated in optimal prefrontal functioning as well as working memory (Kasai et al. 2003; Arnsten et al. 2010; Anderson et al. 2014). Our results indicate that thin spines are vulnerable to different regimens of stress exposure (CVS and CRS) as well as to 21 days of glucocorticoid exposure in both male and female rats. As the majority of the aforementioned studies have been carried out in male rats, this extends the body of literature supporting the vulnerability of thin spines in females as well.
A number of studies have observed impairments in working memory tasks following chronic stress (Arnsten 2000; Liston et al. 2006; Dias-Ferreira et al. 2009; Hains et al. 2009; Yuen et al. 2012), albeit nearly all of these previous studies were carried out in male rodents. Here we show that females also display comparable deficits in working memory following CVS exposure. At first glance, our results may be considered to contradict past work in female rats that have showed either no impairment, or even enhanced hippocampal functioning, following chronic stress exposure (Bowman et al. 2001; Bowman et al. 2002; Kitraki et al. 2004; McLaughlin et al. 2009; Conrad et al. 2012; Wei et al. 2014). Yet, it is plausible that prefrontal and hippocampal functions may be differentially susceptible to stress in female rodents. Although more work is needed to reinforce these findings, they collectively may suggest a teleological value for the preservation of at least hippocampal-related functions in females under chronic stress conditions.
One contrasting finding from our results is the bi-directional effect of chronic stress or glucocorticoid effects on dendritic spine volumes. As verified from previous work (Liston and Gan 2011; Anderson et al. 2014; Anderson et al. 2016), we observed reductions in spine volume in male rats following prolonged increases in CORT. However, our study appears to be the first demonstration that female rats show overall increases in prefrontal dendritic spine volume following chronic stress or CORT exposure. Although we did not compare PL dendritic spine alterations in the same rats that underwent delayed alternation testing (see Materials and Methods), one inference that may be gleaned is that changes in prefrontal dendritic spine volume are not critically important for working memory. As both male and female rats showed working memory impairments following CVS, any adaptive gain from enlarged PL dendritic spine volumes in females would not appear to compensate for the spine loss that they experience.
One idea that has gained support in the literature is that estrogen may play a neuroprotective role in buffering limbic cortical networks from the effects chronic stress (see McLaughlin et al. 2009; Luine 2016). From the standpoint of dendritic spine number, our data do not appear to support this idea; however, protective effects in mPFC neurons could instead be mediated through increases in dendritic spine volume. Larger spine volumes are associated with increased synaptic efficacy and stability (Harris et al. 1992; Grutzendler et al. 2002; Nimchinsky et al. 2002; Mizrahi and Katz 2003; Nimchinsky et al. 2004; Holtmaat et al. 2005; Ashby et al. 2006), such that prefrontal dendritic spine loss in females may be compensated for by increases in spine volume that help to stabilize the extant population. As one important function of spines is the sequestration of Ca2+, larger average volumes of dendritic spines could also help to limit excitotoxicity (Segal 1995; Nimchinsky et al. 2002; Jeanneteau et al. 2018). In male rats, chronic stress increases vulnerability to excitotoxicity or inflammation in cortical structures (Munhoz et al. 2006; Conrad et al. 2007; Tynan et al. 2013). Taken together, chronic stress or glucocorticoid-induced increases in mPFC dendritic spine volume that occurs in female rats may offer a structural basis for a greater resistance to such insults.
Funding
National Institutes of Health (R56 MH-095972, R21 MH115673, R01 MH119106); Brain and Behavior Research Foundation NARSAD Independent Investigator Grant.
References
- Anderson RM, Birnie AK, Koblesky NK, Romig-Martin SA, Radley JJ. 2014. Adrenocortical status predicts the degree of age-related deficits in prefrontal structural plasticity and working memory. The Journal of Neuroscience. 34:8387–8397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson RM, Glanz RM, Johnson SB, Miller MM, Romig-Martin SA, Radley JJ. 2016. Prolonged corticosterone exposure induces dendritic spine remodeling and attrition in the rat medial prefrontal cortex. The Journal of Comparative Neurology. 524:3729–3746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnsten AF. 2000. Stress impairs prefrontal cortical function in rats and monkeys: role of dopamine D1 and norepinephrine a-1 receptor mechanisms. Progress in Brain Research. 126:183–192. [DOI] [PubMed] [Google Scholar]
- Arnsten AF, Paspalas CD, Gamo NJ, Yang Y, Wang M. 2010. Dynamic network connectivity: a new form of neuroplasticity. Trends in Cognitive Sciences. 14:365–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashby MC, Maier SR, Nishimune A, Henley JM. 2006. Lateral diffusion drives constitutive exchange of AMPA receptors at dendritic spines and is regulated by spine morphology. The Journal of Neuroscience. 26:7046–7055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bitzenhofer SH, Ahlbeck J, Wolff A, Wiegert JS, Gee CE, Oertner TG, Hanganu-Opatz IL. 2017. Layer-specific optogenetic activation of pyramidal neurons causes beta-gamma entrainment of neonatal networks. Nature Communications. 8: 14563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloss EB, Cembrowski MS, Karsh B, Colonell J, Fetter RD, Spruston N. 2018. Single excitatory axons form clustered synapses onto CA1 pyramidal cell dendrites. Nature Neuroscience. 21:353–363. [DOI] [PubMed] [Google Scholar]
- Bloss EB, Janssen WG, Ohm DT, Yuk FJ, Wadsworth S, Saardi KM, McEwen BS, Morrison JH. 2011. Evidence for reduced experience-dependent dendritic spine plasticity in the aging prefrontal cortex. The Journal of Neuroscience. 31:7831–7839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourke CH, Harrell CS, Neigh GN. 2012. Stress-induced sex differences: adaptations mediated by the glucocorticoid receptor. Hormones and Behavior. 62:210–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourne J, Harris KM. 2007. Do thin spines learn to be mushroom spines that remember? Current Opinion in Neurobiology. 17:381–386. [DOI] [PubMed] [Google Scholar]
- Bowman EM, Ferguson D, Luine V. 2002. Effects of chronic restraint stress and estradiol on open field activity, spatial memory, and monoaminergic neurotransmitters in ovariectomized rats. Neuroscience. 113:401–441. [DOI] [PubMed] [Google Scholar]
- Bowman RE, Zrull MC, Luine VN. 2001. Chronic restraint stress enhances radial arm maze performance in female rats. Brain Research. 904:279–289. [DOI] [PubMed] [Google Scholar]
- Brittain EH. 1987. P-values for the multi-sample kolmogorov-smirnov test using the expanded bonferroni appoximation. Communications in Statistics-Theory and Methods. 16:821–835. [Google Scholar]
- Cerqueira JJ, Mailliet F, Almeida OF, Jay TM, Sousa N. 2007. The prefrontal cortex as a key target of the maladaptive response to stress. The Journal of Neuroscience. 27:2781–2787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerqueira JJ, Pego JM, Taipa R, Bessa JM, Almeida OF, Sousa N. 2005. Morphological correlates of corticosteroid-induced changes in prefrontal cortex-dependent behaviors. The Journal of Neuroscience. 25:7792–7800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CC, Lu J, Yang R, Ding JB, Zuo Y. 2018. Selective activation of parvalbumin interneurons prevents stress-induced synapse loss and perceptual defects. Molecular Psychiatry. 23:1614–1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christoffel DJ, Golden SA, Dumitriu D, Robison AJ, Janssen WG, Ahn HF, Krishnan V, Reyes CM, Han MH, Ables JL et al. 2011a. IkappaB kinase regulates social defeat stress-induced synaptic and behavioral plasticity. The Journal of Neuroscience. 31:314–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christoffel DJ, Golden SA, Russo SJ. 2011b. Structural and synaptic plasticity in stress-related disorders. Reviews in the Neurosciences. 22:535–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrad CD, McLaughlin KJ, Harman JS, Foltz C, Wieczorek L, Lightner E, Wright RL. 2007. Chronic glucocorticoids increase hippocampal vulnerability to neurotoxicity under conditions that produce CA3 dendritic retraction but fail to impair spatial recognition memory. The Journal of Neuroscience. 27:8278–8285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrad CD, McLaughlin KJ, Huynh TN, El-Ashmawy M, Sparks M. 2012. Chronic stress and a cyclic regimen of estradiol administration separately facilitate spatial memory: relationship with hippocampal CA1 spine density and dendritic complexity. Behavioral Neuroscience. 126:142–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook SC, Wellman CL. 2004. Chronic stress alters dendritic morphology in rat medial prefrontal cortex. Journal of Neurobiology. 60:236–248. [DOI] [PubMed] [Google Scholar]
- Day TA. 2005. Defining stress as a prelude to mapping its neurocircuitry: no help from allostasis. Progress in Neuro-psychopharmacology & Biological Psychiatry. 29:1195–1200. [DOI] [PubMed] [Google Scholar]
- de Kloet ER, Joels M, Holsboer F. 2005. Stress and the brain: from adaptation to disease. Nature Reviews Neuroscience. 6:463–475. [DOI] [PubMed] [Google Scholar]
- Dias-Ferreira E, Sousa JC, Melo I, Morgado P, Mesquita AR, Cerqueira JJ, Costa RM, Sousa N. 2009. Chronic stress causes frontostriatal reorganization and affects decision-making. Science. 325:621–625. [DOI] [PubMed] [Google Scholar]
- Divac I. 1970. Frontal lobe system and spatial reversal in the rat. Neuropsychologia. 9:175–183. [DOI] [PubMed] [Google Scholar]
- Duman RS, Aghajanian GK. 2012. Synaptic dysfunction in depression: potential therapeutic targets. Science. 338:68–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duman RS, Aghajanian GK, Sanacora G, Krystal JH. 2016. Synaptic plasticity and depression: new insights from stress and rapid-acting antidepressants. Nature Medicine. 22:238–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumitriu D, Hao J, Hara Y, Kaufmann J, Janssen WG, Lou W, Rapp PR, Morrison JH. 2010. Selective changes in thin spine density and morphology in monkey prefrontal cortex correlate with aging-related cognitive impairment. The Journal of Neuroscience. 30:7507–7515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumitriu D, Laplant Q, Grossman YS, Dias C, Janssen WG, Russo SJ, Morrison JH, Nestler EJ. 2012. Subregional, dendritic compartment, and spine subtype specificity in cocaine regulation of dendritic spines in the nucleus accumbens. The Journal of Neuroscience. 32:6957–6966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumitriu D, Rodriguez A, Morrison JH. 2011. High-throughput, detailed, cell-specific neuroanatomy of dendritic spines using microinjection and confocal microscopy. Nature Protocols. 6:1391–1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrell MR, Sengelaub DR, Wellman CL. 2013. Sex differences and chronic stress effects on the neural circuitry underlying fear conditioning and extinction. Physiology & Behavior. 122:208–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galea LA, McEwen BS, Tanapat P, Deak T, Spencer RL, Dhabhar FS. 1997. Sex differences in dendritic atrophy of CA3 pyramidal neurons in response to chronic restraint stress. Neuroscience. 81:689–697. [DOI] [PubMed] [Google Scholar]
- Garrett JE, Wellman CL. 2009. Chronic stress effects on dendritic morphology in medial prefrontal cortex: sex differences and estrogen dependence. Neuroscience. 162:195–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goel N, Bale TL. 2010. Sex differences in the serotonergic influence on the hypothalamic-pituitary-adrenal stress axis. Endocrinology. 151:1784–1794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goel N, Workman JL, Lee TT, Innala L, Viau V. 2014. Sex differences in the HPA axis. Comprehensive Physiology. 4:1121–1155. [DOI] [PubMed] [Google Scholar]
- Goldwater DS, Pavlides C, Hunter RG, Bloss EB, Hof PR, McEwen BS, Morrison JH. 2009. Structural and functional alterations to rat medial prefrontal cortex following chronic restraint stress and recovery. Neuroscience. 164:798–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gourley SL, Swanson AM, Koleske AJ. 2013. Corticosterone-induced neural remodeling predicts behavioral vulnerability and resilience. The Journal of Neuroscience. 33:3107–3112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grigoriadis S, Robinson GE. 2007. Gender issues in depression. Annals of Clinical Psychiatry. 19:247–255. [DOI] [PubMed] [Google Scholar]
- Grippo AJ, Beltz TG, Johnson AK. 2003. Behavioral and cardiovascular changes in the chronic mild stress model of depression. Physiology & Behavior. 78:703–710. [DOI] [PubMed] [Google Scholar]
- Grissom N, Bhatnagar S. 2009. Habituation to repeated stress: get used to it. Neurobiology of Learning and Memory. 92:215–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grutzendler J, Kasthuri N, Gan WB. 2002. Long-term dendritic spine stability in the adult neocortex. Nature. 420:812–816. [DOI] [PubMed] [Google Scholar]
- Hains AB, Vu MA, Maciejewski PK, van Dyck CH, Gottron M, Arnsten AF. 2009. Inhibition of protein kinase C signaling protects prefrontal cortex dendritic spines and cognition from the effects of chronic stress. Proceedings of the National Academy of Sciences of the United States of America. 106:17957–17962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris KM, Jensen FE, Tsao B. 1992. Three-dimensional structure of dendritic spines and synapses in rat hippocampus (CA1) at postnatal day 15 and adult ages: implications for the maturation of synaptic physiology and long-term potentiation. The Journal of Neuroscience. 12:2685–2705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris KM, Stevens JK. 1989. Dendritic spines of CA 1 pyramidal cells in the rat hippocampus: serial electron microscopy with reference to their biophysical characteristics. The Journal of Neuroscience. 9:2982–2997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman JP, Adams D, Prewitt C. 1995. Regulatory changes in neuroendocrine stress-integrative circuitry produced by a variable stress paradigm. Neuroendocrinology. 61:180–190. [DOI] [PubMed] [Google Scholar]
- Hinwood M, Morandini J, Day TA, Walker FR. 2012. Evidence that microglia mediate the neurobiological effects of chronic psychological stress on the medial prefrontal cortex. Cerebral Cortex. 22:1442–1454. [DOI] [PubMed] [Google Scholar]
- Hoffman DA, Magee JC, Colbert CM, Johnston D. 1997. K+ channel regulation of signal propagation in dendrites of hippocampal pyramidal neurons. Nature. 387:869–875. [DOI] [PubMed] [Google Scholar]
- Holden C. 2005. Sex and the suffering brain. Science. 308:1574–1581. [DOI] [PubMed] [Google Scholar]
- Holmes A, Wellman CL. 2009. Stress-induced prefrontal reorganization and executive dysfunction in rodents. Neuroscience and Biobehavioral Reviews. 33:773–783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtmaat A, De Paola V, Wilbrecht L, Knott GW. 2008. Imagining of experience-dependent structural plasticity in the mouse neocortex in vivo. Behavioural Brain Research. 192:20–25. [DOI] [PubMed] [Google Scholar]
- Holtmaat AJ, Trachtenberg JT, Wilbrecht L, Shepherd GM, Zhang X, Knott GW, Svoboda K. 2005. Transient and persistent dendritic spines in the neocortex in vivo. Neuron. 45:279–291. [DOI] [PubMed] [Google Scholar]
- Jeanneteau F, Barrere C, Vos M, De Vries CJM, Rouillard C, Levesque D, Dromard Y, Moisan MP, Duric V, Franklin TC et al. 2018. The stress-induced transcription factor NR4A1 adjusts mitochondrial function and synapse number in prefrontal cortex. The Journal of Neuroscience. 38:1335–1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasai H, Matsuzaki M, Noguchi J, Yasumatsu N, Nakahara H. 2003. Structure–stability–function relationships of dendritic spines. Trends in Neurosciences. 26:360–368. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Gatz M, Gardner CO, Pedersen NL. 2006. A swedish national twin study of lifetime major depression. The American Journal of Psychiatry. 163:109–114. [DOI] [PubMed] [Google Scholar]
- Kitraki E, Kremmyda O, Youlatos D, Alexis MN, Kittas C. 2004. Gender-dependent alterations in corticosteroid receptor status and spatial performance following 21 days of restraint stress. Neuroscience. 125:47–55. [DOI] [PubMed] [Google Scholar]
- Klenowski PM, Wright SE, Mu EWH, Noakes PG, Lavidis NA, Bartlett SE, Bellingham MC, Fogarty MJ. 2017. Investigating methodological differences in the assessment of dendritic morphology of basolateral amygdala principal neurons—a comparison of golgi-cox and neurobiotin electroporation techniques. Brain Sciences. 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knott GW, Holtmaat A, Wilbrecht L, Welker E, Svoboda K. 2006. Spine growth precedes synapse formation in the adult neocortex in vivo. Nature Neuroscience. 9:1117–1124. [DOI] [PubMed] [Google Scholar]
- Knott GW, Quairiaux C, Genoud C, Welker E. 2002. Formation of dendritic spines with GABAergic synapses induced by whisker stimulation in adult mice. Neuron. 34:265–273. [DOI] [PubMed] [Google Scholar]
- Lee KF, Soares C, Beique JC. 2012. Examining form and function of dendritic spines. Neural Plasticity. 2012: 704103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leuner B, Shors TJ. 2013. Stress, anxiety, and dendritic spines: what are the connections? Neuroscience. 251:108–119. [DOI] [PubMed] [Google Scholar]
- Liston C, Cichon JM, Jeanneteau F, Jia Z, Chao MV, Gan WB. 2013. Circadian glucocorticoid oscillations promote learning-dependent synapse formation and maintenance. Nature Neuroscience. 16:698–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liston C, Gan WB. 2011. Glucocorticoids are critical regulators of dendritic spine development and plasticity in vivo. Proceedings of the National Academy of Sciences of the United States of America. 108:16074–16079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liston C, Miller MM, Goldwater DS, Radley JJ, Rocher AB, Hof PR, Morrison JH, McEwen BS. 2006. Stress-induced alterations in prefrontal cortical dendritic morphology predict selective impairments in perceptual attentional set-shifting. The Journal of Neuroscience. 26:7870–7874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu RJ, Aghajanian GK. 2008. Stress blunts serotonin- and hypocretin-evoked EPSCs in prefrontal cortex: role of corticosterone-mediated apical dendritic atrophy. Proceedings of the National Academy of Sciences of the United States of America. 105:359–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luine V. 2016. Estradiol: mediator of memories, spine density and cognitive resilience to stress in female rodents. The Journal of Steroid Biochemistry and Molecular Biology. 160:189–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luine VN, Vilegas M, Martinez C, McEwen BS. 1994. Repeated stress causes reversible impairments of spatial memory performance. Brain Research. 639:167–170. [DOI] [PubMed] [Google Scholar]
- Magariños AM, McEwen BS. 1995a. Stress-induced apical dendrites of hippocampal CA3 neurons: involvement of glucocorticoid secretion and excitatory amino acid receptors. Neuroscience. 69:89–98. [DOI] [PubMed] [Google Scholar]
- Magariños AM, McEwen BS. 1995b. Stress-induced atrophy of apical dendrites of hippocampal CA3c neurons: comparison of stressors. Neuroscience. 69:83–88. [DOI] [PubMed] [Google Scholar]
- Magee JC, Johnston D. 1995. Synaptic activation of voltage-gated channels in the dendrites of hippocampal pyramidal neurons. Science. 268:301–304. [DOI] [PubMed] [Google Scholar]
- Marcus SM, Young EA, Kerber KB, Kornstein S, Farabaugh AH, Mitchell J. 2005. Gender differences in depression: findings from the STAR*D study. Journal of Affective Disorders. 87:141–150. [DOI] [PubMed] [Google Scholar]
- Mason JW, Maher JT, Hartley LH, Mougey EH, Perlow MJ, Jones LG. 1976. Selectivity of corticosteroid and catecholamine responses to various natural stimuli. New York: Springer. [Google Scholar]
- McEwen BS, Wingfield JC. 2003. The concept of allostasis in biology and biomedicine. Hormones and behavior. 43:2–15. [DOI] [PubMed] [Google Scholar]
- McEwen BS, Bowles NP, Gray JD, Hill MN, Hunter RG, Karatsoreos IN, Nasca C. 2015. Mechanisms of stress in the brain. Nature Neuroscience. 18:1353–1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS, Gianaros PJ. 2011. Stress- and allostasis-induced brain plasticity. Annual Review of Medicine. 62:431–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKlveen JM, Morano RL, Fitzgerald M, Zoubovsky S, Cassella SN, Scheimann JR, Ghosal S, Mahbod P, Packard BA, Myers B et al. 2016. Chronic stress increases prefrontal inhibition: a mechanism for stress-induced prefrontal dysfunction. Biological Psychiatry. 80:754–764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin KJ, Baran SE, Conrad CD. 2009. Chronic stress- and sex-specific neuromorphological and functional changes in limbic structures. Molecular Neurobiology. 40:166–182. [DOI] [PubMed] [Google Scholar]
- McLaughlin KJ, Baran SE, Wright RL, Conrad CD. 2005. Chronic stress enhances spatial memory in ovariectomized female rats despite CA3 dendritic retraction: possible involvement of CA1 neurons. Neuroscience. 135:1045–1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizrahi A, Katz LC. 2003. Dendritic stability in the adult olfactory bulb. Nature Neuroscience. 6:1201–1207. [DOI] [PubMed] [Google Scholar]
- Moench KM, Wellman CL. 2015. Stress-induced alterations in prefrontal dendritic spines: implications for post-traumatic stress disorder. Neuroscience Letters. 601:41–45. [DOI] [PubMed] [Google Scholar]
- Moench KM, Wellman CL. 2017. Differential dendritic remodeling in prelimbic cortex of male and female rats during recovery from chronic stress. Neuroscience. 357:145–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munhoz CD, Lepsch LB, Kawamoto EM, Malta MB, Lima Lde S, Avellar MC, Sapolsky RM, Scavone C. 2006. Chronic unpredictable stress exacerbates lipopolysaccharide-induced activation of nuclear factor-kappaB in the frontal cortex and hippocampus via glucocorticoid secretion. The Journal of Neuroscience. 26:3813–3820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nimchinsky EA, Sabatini BL, Svoboda K. 2002. Structure and function of dendritic spines. Annual Review of Physiology. 64:313–353. [DOI] [PubMed] [Google Scholar]
- Nimchinsky EA, Yasuda R, Oertner TG, Svoboda K. 2004. The number of glutatmate receptors opened by synaptic stimulation in single hippocampal spines. The Journal of Neuroscience. 24:2054–2064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ottenweller E, Natelson BH, Pitman DL, Drastal SD. 1989. Adrenocortical and behavioral response to repeated stressors: toward an animal model of chronic stress and stress-related mental illness. Biological Psychiatry. 26:829–841. [DOI] [PubMed] [Google Scholar]
- Papp M, Willner P, Muscat R. 1991. An animal model of anhedonia: attenuation of sucrose consumption and place preference conditioning by chronic unpredictable mild stress. Psychopharmacology. 104:255–259. [DOI] [PubMed] [Google Scholar]
- Perez-Cruz C, Muller-Keuker JI, Heilbronner U, Fuchs E, Flugge G. 2007. Morphology of pyramidal neurons in the rat prefrontal cortex: lateralized dendritic remodeling by chronic stress. Neural Plasticity. 2007: 46276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiao H, An SC, Xu C, Ma XM. 2017. Role of proBDNF and BDNF in dendritic spine plasticity and depressive-like behaviors induced by an animal model of depression. Brain Research. 1663:29–37. [DOI] [PubMed] [Google Scholar]
- Radley JJ, Anderson RM, Cosme CV, Glanz RM, Miller MC, Romig-Martin SA, LaLumiere RT. 2015a. The contingency of cocaine administration accounts for structural and functional medial prefrontal deficits and increased adrenocortical activation. The Journal of Neuroscience. 35:11897–11910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radley J, Morilak D, Viau V, Campeau S. 2015b. Chronic stress and brain plasticity: mechanisms underlying adaptive and maladaptive changes and implications for stress-related CNS disorders. Neuroscience and Biobehavioral Reviews. 58:79–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radley JJ, Anderson RM, Hamilton BA, Alcock JA, Romig-Martin SA. 2013. Chronic stress-induced alterations of dendritic spine subtypes predict functional decrements in an hypothalamo-pituitary-adrenal-inhibitory prefrontal circuit. The Journal of Neuroscience. 33:14379–14391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radley JJ, Rocher AB, Miller M, Janssen WG, Liston C, Hof PR, McEwen BS, Morrison JH. 2006. Repeated stress induces dendritic spine loss in the rat medial prefrontal cortex. Cerebral Cortex. 16:313–320. [DOI] [PubMed] [Google Scholar]
- Radley JJ, Rocher AB, Rodriguez A, Ehlenberger DB, Dammann M, McEwen BS, Morrison JH, Wearne SL, Hof PR. 2008. Repeated stress alters dendritic spine morphology in the rat medial prefrontal cortex. The Journal of Comparative Neurology. 507:1141–1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radley JJ, Sawchenko PE. 2015. Evidence for involvement of a limbic paraventricular hypothalamic inhibitory network in hypothalamic-pituitary-adrenal axis adaptations to repeated stress. The Journal of Comparative Neurology. 523:2769–2787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radley JJ, Sisti HM, Hao J, Rocher AB, McCall T, Hof PR, McEwen BS, Morrison JH. 2004. Chronic behavioral stress induces apical dendritic reorganization in pyramidal neurons of the medial prefrontal cortex. Neuroscience. 125:1–6. [DOI] [PubMed] [Google Scholar]
- Ramos BP, Birnbaum SG, Lindenmayer I, Newton SS, Duman RS, Arnsten AF. 2003. Dysregulation of protein kinase A signaling in the aged prefrontal cortex: new strategy for treating age-related cognitive decline. Neuron. 40:835–845. [DOI] [PubMed] [Google Scholar]
- Rico AM, Mendoza AL, Duran DA, Torres Hde L, Mendoza GA, Gomez AB. 2015. The effects of chronic restraint on the morphology of ventral CA1 neurons in female Long Evans rats. Stress. 18:67–75. [DOI] [PubMed] [Google Scholar]
- Rodriguez A, Ehlenberger DB, Hof PR, Wearne SL. 2006. Rayburst sampling, an algorithm for automated three-dimensional shape analysis from laser scanning microscopy images. Nature Protocols. 1:2152–2161. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM. 1992. Stress, the aging brain, and the mechanisms of neuron death. Cambridge, Massachusetts: MIT Press [Google Scholar]
- Sarkar A, Kabbaj M. 2016. Sex differences in effects of ketamine on behavior, spine density, and synaptic proteins in socially isolated rats. Biological Psychiatry. 80:448–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segal M. 1995. Dendritic spines for neuroprotection: a hypothesis. Trends in Neurosciences. 18:468–471. [DOI] [PubMed] [Google Scholar]
- Selye H. 1973. Homeostasis and heterostasis. Perspectives in Biology and Medicine. 16:441–445. [DOI] [PubMed] [Google Scholar]
- Selye H. 1980. Stress in health and disease. Boston: Butterworths [Google Scholar]
- Seo D, Ahluwalia A, Potenza MN, Sinha R. 2017. Gender differences in neural correlates of stress-induced anxiety. Journal of Neuroscience Research. 95:115–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shansky RM, Hamo C, Hof PR, Lou W, McEwen BS, Morrison JH. 2010. Estrogen promotes stress sensitivity in a prefrontal cortex-amygdala pathway. Cerebral Cortex. 20:2560–2567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shansky RM, Hamo C, Hof PR, McEwen BS, Morrison JH. 2009. Stress-induced dendritic remodeling in the prefrontal cortex is circuit specific. Cerebral Cortex. 19:2479–2484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shansky RM, Woolley CS. 2016. Considering sex as a biological variable will be valuable for neuroscience research. The Journal of Neuroscience. 36:11817–11822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen HW, Toda S, Moussawi K, Bouknight A, Zahm DS, Kalivas PW. 2009. Altered dendritic spine plasticity in cocaine-withdrawn rats. The Journal of Neuroscience. 29:2876–2884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shors TJ. 2016. A trip down memory lane about sex differences in the brain. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences. 371: 20150124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sousa N, Lukoyanov NV, Madeira MD, Almeida OF, Paula-Barbosa MM. 2000. Reorganization of the morphology of hippocampal neurites and synapses after stress-induced damage correlates with behavioral improvement. Neuroscience. 97:253–266. [DOI] [PubMed] [Google Scholar]
- Stuart GJ, Sakmann B. 1994. Active propagation of somatic action potentials into neocortical pyramidal cell dendrites. Nature. 367:69–72. [DOI] [PubMed] [Google Scholar]
- Trachtenberg JT, Trepel C, Stryker MP. 2000. Rapid extragranular plasticity in the absence of thalamocortical plasticity in the developing primary visual cortex. Science. 287:2029–2032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tynan RJ, Beynon SB, Hinwood M, Johnson SJ, Nilsson M, Woods JJ, Walker FR. 2013. Chronic stress-induced disruption of the astrocyte network is driven by structural atrophy and not loss of astrocytes. Acta Neuropathologica. 126:75–91. [DOI] [PubMed] [Google Scholar]
- Ulrich-Lai YM, Herman JP. 2009. Neural regulation of endocrine and autonomic stress responses. Nature Reviews Neuroscience. 10:397–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vyas A, Bernal S, Chattarji S. 2002. Effects of chronic stress on dendritic arborization in the central and extended amgydala. Brain Research. 965:290–294. [DOI] [PubMed] [Google Scholar]
- Wallace W, Bear MF. 2004. A morphological correlate of synaptic scaling in visual cortex. The Journal of Neuroscience. 24:6928–6938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe Y, Gould E, McEwen BS. 1992. Stress induces atrophy of apical dendrites of hippocampal CA3 pyramidal neurons. Brain Research. 588:341–345. [DOI] [PubMed] [Google Scholar]
- Wei J, Yuen EY, Liu W, Li X, Zhong P, Karatsoreos IN, McEwen BS, Yan Z. 2014. Estrogen protects against the detrimental effects of repeated stress on glutamatergic transmission and cognition. Molecular Psychiatry. 19:588–598. [DOI] [PubMed] [Google Scholar]
- Wellman CL. 2001. Dendritic reorganization in pyramidal neurons in medial prefrontal cortex after chronic corticosterone administration. Journal of Neurobiology. 49:245–253. [DOI] [PubMed] [Google Scholar]
- Willner P. 1997. Validity, reliability and utility of the chronic mild stress model of depression: a 10-year review and evaluation. Psychopharmacology. 134:319–329. [DOI] [PubMed] [Google Scholar]
- Wohleb ES, Terwilliger R, Duman CH, Duman RS. 2018. Stress-induced neuronal colony stimulating factor 1 provokes microglia-mediated neuronal remodeling and depressive-like behavior. Biological Psychiatry. 83:38–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang G, Pan F, Gan WB. 2009. Stably maintained dendritic spines are associated with lifelong memories. Nature. 462:920–924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasumatsu N, Matsuzaki M, Miyazaki T, Noguchi J, Kasai H. 2008. Principles of long-term dynamics of dendritic spines. The Journal of Neuroscience. 28:13592–13608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuen EY, Wei J, Liu W, Zhong P, Li X, Yan Z. 2012. Repeated stress causes cognitive impairment by suppressing glutamate receptor expression and function in prefrontal cortex. Neuron. 73:962–977. [DOI] [PMC free article] [PubMed] [Google Scholar]


