Abstract
Objectives
Respiratory syncytial virus (RSV) is a major cause of severe lower respiratory tract infections in infants, and there is no vaccine available. In early life, the most important contributors to protection against infectious diseases are the innate immune response and maternal antibodies. However, antibody‐mediated protection against RSV disease is incompletely understood, as both antibody levels and neutralisation capacity correlate poorly with protection. Since antibodies also mediate natural killer (NK) cell activation, we investigated whether this functionality correlates with RSV disease.
Methods
We performed an observational case–control study including infants hospitalised for RSV infection, hernia surgery or RSV‐negative respiratory viral infections. We determined RSV antigen‐specific antibody levels in plasma using a multiplex immunoassay. Subsequently, we measured the capacity of these antibodies to activate NK cells. Finally, we assessed Fc‐glycosylation of the RSV‐specific antibodies by mass spectrometry.
Results
We found that RSV‐specific maternal antibodies activate NK cells in vitro. While concentrations of RSV‐specific antibodies did not differ between cases and controls, antibodies from infants hospitalised for severe respiratory infections (RSV and/or other) induced significantly less NK cell interferon‐γ production than those from uninfected controls. Furthermore, NK cell activation correlated with Fc‐fucosylation of RSV‐specific antibodies, but their glycosylation status did not significantly differ between cases and controls.
Conclusion
Our results suggest that Fc‐dependent antibody function and quality, exemplified by NK cell activation and glycosylation, contribute to protection against severe RSV disease and warrant further studies to evaluate the potential of using these properties to evaluate and improve the efficacy of novel vaccines.
Keywords: antibody‐dependent cell‐mediated cytotoxicity, Fc‐mediated effector functions, fucosylation, interferon‐gamma, NK cell, respiratory syncytial virus
Respiratory syncytial virus (RSV) is a global public health concern, and vaccine development is hampered by the lack of a well‐defined correlate of protection. Here, we show that RSV‐specific antibodies from children hospitalised for severe viral respiratory infections induce significantly less natural killer (NK) cell interferon‐gamma production than those from uninfected controls. In addition, we show that NK cell activation by RSV‐specific antibodies correlates with their Fc‐glycosylation status.

Introduction
Respiratory syncytial virus (RSV) is a major cause of severe lower respiratory tract disease in young children, with an estimated 118 000 deaths worldwide in children below 5 years of age annually.1, 2 Despite decades of research, there are currently no market‐approved vaccines available against this virus and their development is hampered by the lack of a well‐defined correlate of protection. Severe RSV disease is most prevalent in the first six months of life,3, 4 when infants mainly rely on their innate immune system and maternal antibodies for protection against infectious diseases. Considering that maternal vaccination is a potential strategy to prevent RSV disease in infants, it is of pivotal importance to obtain a thorough understanding of the mechanisms by which maternal antibodies might protect against disease, both directly and through their interaction with innate immune cells.
The role of maternal antibodies in RSV infection and disease is unclear. Some studies show that high RSV‐specific maternal antibody titres are associated with protection against RSV infection or (severe) disease.5, 6, 7, 8 In contrast, other studies do not show a protective effect9, 10, 11, 12 or even indicate an association between high maternal antibody titres and an increased risk of recurrent wheezing.13 Strikingly, the vast majority of studies investigating the role of (maternal) antibodies in RSV infection only look at in vitro binding or neutralisation capacity, while additional antibody effector functions are not taken into account.
Natural killer (NK) cells are important innate immune cells in the early response to viral infection (for reviews, see Vivier et al. 14 and Lodoen and Lanier15), and their activity is tightly regulated, for example via their interaction with antigen‐specific antibodies.16 Engagement of the main NK cell Fc gamma receptor (FcγRIIIa) by antibodies bound to virus‐infected cells leads to the release of cytotoxic granules containing perforins and granzymes, a process known as antibody‐dependent cell‐mediated cytotoxicity (ADCC).17 In addition, antibody‐dependent activation of NK cells is known to result in the secretion of pro‐inflammatory cytokines, including interferon‐gamma (IFN‐γ).18
Several groups have shown clearance of RSV‐infected cells by peripheral blood mononuclear cells (PBMCs) in the presence of monoclonal antibodies, or antibodies from different natural sources, including breastmilk, cord blood, nasopharyngeal secretions and serum.19, 20, 21, 22, 23 However, none of these studies shows whether killing was specifically NK cell‐mediated. In addition, antibodies from RSV patients and controls have to our knowledge never been compared regarding functional properties other than neutralisation, except for antibody‐dependent enhancement of infection.24
In recent years, it has become increasingly clear that the glycan present at the conserved N‐linked glycosylation site in the Fc‐tail of immunoglobulin G (IgG) is variable and significantly affects antibody functionality.25 Moreover, in some immune responses, such as with HIV,26 dengue27 and alloimmune diseases,28, 29 reduced fucosylation of the elicited antigen‐specific antibodies can affect disease outcome by increasing the affinity for FcγRIIIa. In autoimmune diseases, IgG antibodies can display atypical galactosylation, which affects disease severity through mechanisms that are not yet understood.30 The potential impact of IgG glycosylation on (protection from) RSV disease has not been studied to date.
Here, we used samples from an observational case–control study of infants who were hospitalised for RSV, an inguinal hernia surgery or viral respiratory infections other than RSV. We first measured plasma levels of antigen‐specific IgG against the RSV attachment (G) and fusion (F) proteins, including both the pre‐ and post‐fusion conformation of the latter (pre‐F and post‐F, respectively). We then assessed the capacity of RSV‐specific antibodies to activate NK cells in vitro as measured by CD107a surface expression, which is a marker for degranulation, and IFN‐γ production. Finally, we assessed the Fc‐glycosylation status of total and RSV‐specific antibodies by mass spectrometry. Our findings suggest that antibody‐mediated NK cell activation is one of the factors contributing to protection against severe RSV disease and should be further investigated as a potential co‐correlate of protection to use for the evaluation and improvement of vaccine efficacy.
Results
Clinical characteristics of study subjects
Plasma samples were obtained from a total number of 84 infants below 7 months of age that were hospitalised for RSV infection (cases), hernia surgery (uninfected controls) or viral respiratory infections other than RSV (RSV‐negative infected controls). Since we were primarily interested in the antibodies already present at the time of initial infection, we used RSV‐specific IgA as a hallmark for newly elicited antibody production. Five children had IgA against at least two RSV antigens (AU mL−1 > 0.2) and were excluded (Figure 1a). In addition to IgA positivity, we excluded two children who had presumably received palivizumab (a prophylactic monoclonal antibody), based on the fact that they displayed unusually high IgG levels for both pre‐F and post‐F, while G‐specific levels were low or undetectable.
Figure 1.
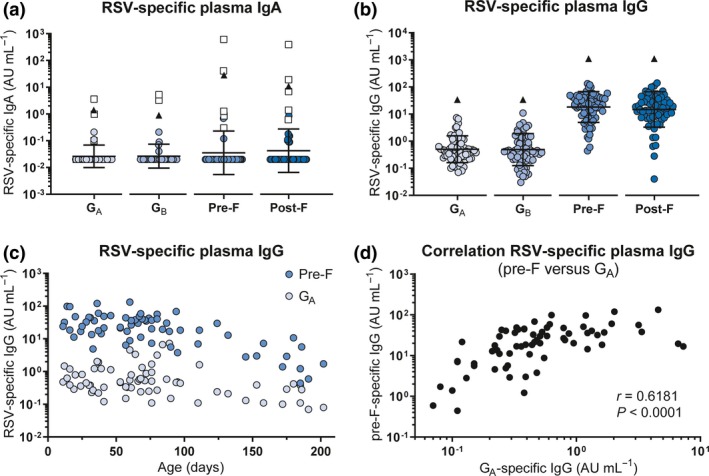
Characterisation of the infant RSV‐specific (maternal) antibody repertoire. An RSV‐specific multiplex immunoassay was performed to quantify the concentration of IgA (n = 66) and IgG (n = 69) specific for RSV G (A‐ and B‐strain), pre‐F and post‐F in infant plasma. (a) Concentration of plasma IgA for each of the RSV antigens. Children who tested positive for IgA (AU mL−1 > 0.2) against a minimum of 2 RSV antigens are excluded for subsequent analyses (n = 5, white squares). (b) Concentration of plasma IgG for each of the RSV antigens. Black triangles indicate the IgA/IgG levels of the adult reference serum pool. Since arbitrary concentrations were determined based on separate standard curves, concentrations cannot be directly compared between antigens. All samples were measured in a single run for each isotype. Graphs depict the geometric mean and SD. (c) Concentration of RSV pre‐F‐ and GA‐specific plasma IgG plotted against age. (d) Correlation analysis of pre‐F‐ and GA‐specific maternal antibody levels. Nonparametric Spearman's correlation analysis was used to assess correlations. AU, arbitrary units; IgA, immunoglobulin A; IgG, immunoglobulin G; pre‐F, pre‐fusion F protein; post‐F, post‐fusion F protein; RSV, respiratory syncytial virus; SD, standard deviation.
The clinical and demographic characteristics of the children included in further analyses are depicted in Table 1. There was no significant difference in age, gestational age or breastfeeding between the three groups. However, there was a significant difference in sex between the three groups (P = 0.0459). A higher percentage of infants was infected with RSV‐A (70%) compared to RSV‐B (30%). The group of RSV patients contained both RSV mono‐infections (n = 28) and co‐infections (n = 15) with other respiratory viral pathogens, with rhinovirus being the most prevalent.
Table 1.
Demographic and clinical characteristics of study subjects
|
RSV cases (n = 43) |
Uninfected controls (n = 16) |
RSV‐negative controls (n = 18) |
P‐value | |
|---|---|---|---|---|
| Age in days (range) | 70 (11–185) | 79 (28–175)a | 89 (16–202) | 0.3260b |
| Gestational age in weeks (range)c | 38 (34–41) | NA | 38 (35–41) | 0.9749d |
| Sex (M/F) | 22/21 | 12/4 | 5/11e | 0.0459f |
| Breastfeeding (%)g | 21 (50%) | NA | 7 (39%) | 0.8246f |
| RSV types | ||||
| A | 30 (70%) | NA | NA | |
| B | 13 (30%) | NA | NA | |
| Co‐infections (%) | 15 (35%) | NA | NA | |
Age of one infant was not recorded.
Comparisons between RSV patients, uninfected controls and RSV‐negative controls; the Kruskal–Wallis test followed by Dunn's test to adjust for multiple comparisons.
Gestational age of 1 RSV patient, all uninfected controls and 3 RSV‐negative controls were not recorded.
Comparisons between RSV patients, uninfected controls and RSV‐negative controls; the Mann–Whitney test.
Sex of two infants was not recorded.
Comparisons between RSV patients, uninfected controls (only for sex) and RSV‐negative controls; the chi‐square test.
Breastfeeding was not recorded for 1 RSV patient, all the uninfected controls and 3 RSV‐negative controls.
The concentration of RSV‐specific IgG does not differ between RSV cases and controls
We used a multiplex immunoassay to quantify the concentration of IgG specific for the RSV attachment (G) and fusion (F) proteins.31 G protein from both an A‐ and B‐strain was included, as these sequences are highly variable between the two RSV subtypes.32 Both the pre‐ and post‐fusion conformations of the F protein were included, as these harbour partly overlapping but also distinct epitopes.33 We could detect antibodies to all four antigens in all samples and observed substantial spread between individuals (Figure 1b). Antibody levels were lower than those in the adult reference serum pool (triangles) in all samples. Despite the cross‐sectional design of the study, we could observe a gradual waning of antibody concentrations with increasing age, as exemplified by antibodies targeting pre‐F and GA (Figure 1c). As expected, there was a positive correlation between the concentrations of antibodies targeting different RSV antigens, as exemplified for pre‐F and GA (Figure 1d). The concentrations of antigen‐specific antibodies did not significantly differ between RSV‐infected cases, uninfected controls and RSV‐negative infected controls (Figure 2a–d), confirming previous findings in a subset of this cohort.12
Figure 2.
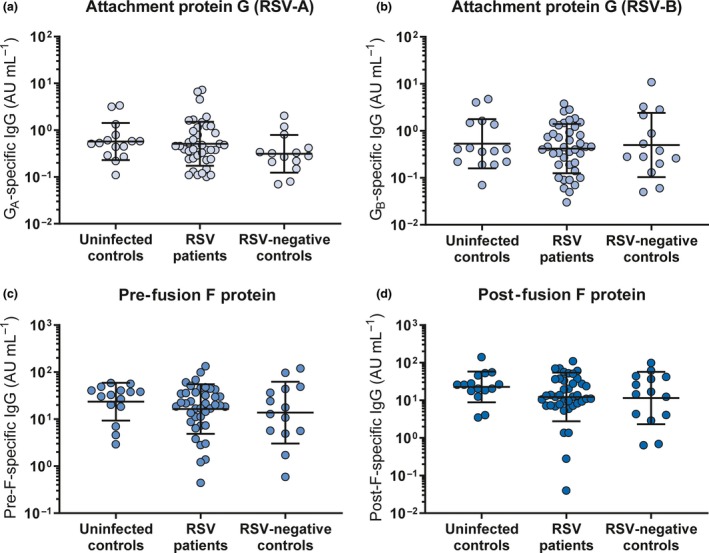
RSV‐specific antibody concentrations do not differ between RSV cases and controls. An RSV‐specific multiplex immunoassay was performed to quantify the amount of RSV antigen‐specific IgG in infant plasma. Comparison of the antibody concentration against RSV‐A attachment protein G (a), RSV‐B attachment protein G (b), pre‐fusion F protein (c) and post‐fusion F protein (d) between uninfected controls (n = 15), RSV patients (n = 40) and RSV‐negative infected controls (n = 14). All samples were measured in a single run. All graphs depict the geometric mean and SD. The Kruskal–Wallis test with Dunn's multiple comparisons test was used for comparison between multiple groups, and no significant differences were found. AU, arbitrary units; IgG, immunoglobulin G; RSV, respiratory syncytial virus; SD, standard deviation.
RSV‐specific maternal antibodies activate NK cells in vitro
As NK cells play an important role in the control of viral infections,14, 15 we next assessed whether RSV‐specific maternal antibodies are capable of activating NK cells in an in vitro assay (schematically depicted in Figure 3a). To control for RSV specificity, the assay was set up with a cord blood plasma pool that had been depleted for RSV‐specific antibodies by incubation with RSV‐infected cells, or with mock‐infected cells as a control. Successful depletion was confirmed by multiplex immunoassay (Figure 3b). Incubation of primary NK cells with complexes of intact RSV particles (antigen) and RSV‐specific antibodies formed with a dilution series of mock‐depleted cord blood plasma, naturally containing RSV‐specific maternal antibodies, resulted in a concentration‐dependent activation as measured by IFN‐γ production (Figure 3c) and CD107a surface expression (Figure 3d), the latter being an established marker for NK cell degranulation.34 Importantly, cord blood plasma largely lacking RSV‐specific antibodies (RSV‐depleted) showed a strongly reduced NK cell activation (Figure 3c and d).
Figure 3.
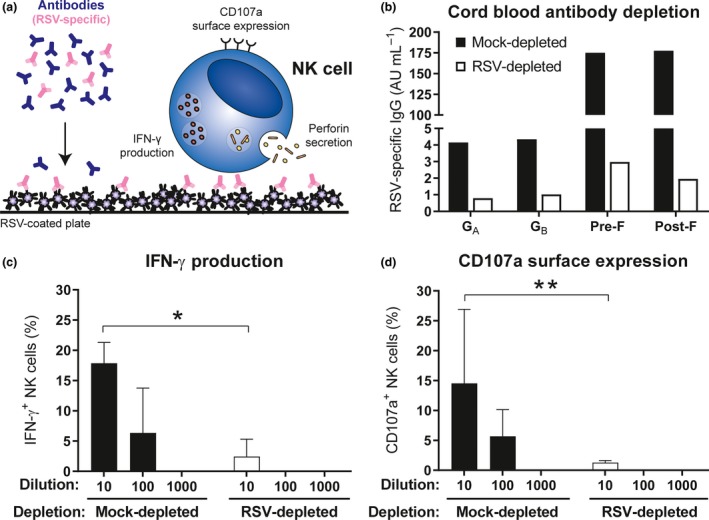
RSV‐specific maternal antibodies activate NK cells in vitro. (a) Schematic representation of the NK cell activation assay. (b) RSV‐specific multiplex immunoassay of mock‐depleted (black) compared to RSV‐depleted (white) cord blood plasma. Samples were measured in a single run. NK cell‐specific IFN‐γ production (c) and CD107a surface expression (d) of different PBMC donors (n = 3) after 4‐hour incubation with viral particles opsonised with mock‐depleted or RSV‐depleted cord blood plasma. Data are from three independent experiments. Graphs depict the geometric mean and SD. The Kruskal–Wallis test with Dunn's multiple comparisons test was used for comparison between mock‐ and RSV‐depleted cord blood plasma with the same dilution (*P < 0.05, **P < 0.01). AU, arbitrary units; CB, cord blood; IFN‐γ, interferon‐gamma; NK cells, natural killer cells; RSV, respiratory syncytial virus.
RSV‐specific antibodies from infants with severe viral respiratory tract infections induce less IFN‐γ production by NK cells than those from uninfected controls
Next, we assessed whether the capacity of RSV‐specific antibodies to activate NK cells differed between RSV cases and controls. Since we observed considerable donor variation in primary NK cell activation in our initial experiments, we normalised the assay for individual PBMC donors (Supplementary figure 2). To this end, NK cell activation by the mock‐depleted cord blood pool was set to 100 arbitrary units (AU). Activation by patient samples was expressed as AU relative to the mock‐depleted cord blood pool for each individual PBMC donor. Finally, the overall response induced by each patient sample is obtained by averaging the normalised responses for all PBMC donors tested. Interestingly, we found that RSV‐specific antibodies from both RSV patients and RSV‐negative infected controls showed significantly decreased induction of NK cell IFN‐γ production compared to uninfected controls (Figure 4a). Slightly less induction of CD107a expression was also observed for plasma from both RSV patients and RSV‐negative infected controls compared to those from uninfected controls, but this difference was not statistically significant (Figure 4b). Since NK cell CD107a surface expression is used as a proxy for degranulation, we assessed its correlation with perforin secretion in the supernatant of the NK cell activation assay for one PBMC donor and found a strong positive correlation (Figure 4c). Finally, since the capacity of antibodies to activate NK cells is expected to be related to their concentration, we assessed the correlation between antibody levels and IFN‐γ production. As exemplified for pre‐F‐specific antibody concentrations, there was only a moderate correlation with IFN‐γ production (Figure 4d), where some samples excelled in antibody concentrations and others in antibody functionality. In addition, the combination of these two factors does not provide a clear separation between cases and controls. Together, this suggests that additional factors besides concentration contribute to the functional capacity of these antibodies and ultimately protection from severe disease.
Figure 4.
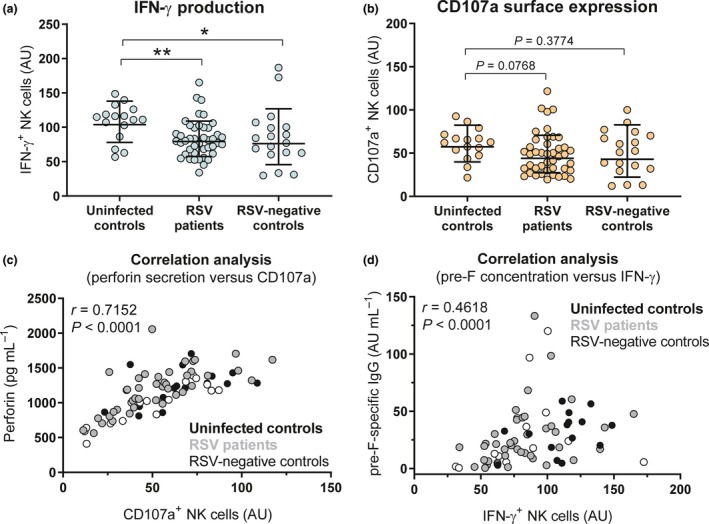
NK cell activation in RSV cases compared to controls. Normalised NK cell IFN‐γ production (a) and CD107a surface expression (b) after 4‐hour incubation of PBMCs (≤6 individual donors per sample) with viral particles opsonised with 10‐fold diluted plasma samples from uninfected controls (n = 16), RSV patients (n = 43) or RSV‐negative infected controls (n = 18). Two independent experiments were performed, with three PBMC donors per experiment. Graphs depict the geometric mean and SD. The Kruskal–Wallis test with Dunn's multiple comparisons test was used for comparison between multiple groups (*P < 0.05, **P < 0.01). (c) Correlation analysis between perforin secretion and CD107a surface expression of one PBMC donor. (d) Correlation analysis between RSV pre‐F‐specific antibody levels and IFN‐γ production. Uninfected controls are indicated in black (n = 15), RSV patients in grey (n = 40) and RSV‐negative infected controls in white (n = 14). The nonparametric Spearman correlation analysis was used to assess correlations. IFN‐γ, interferon‐gamma; NK cells, natural killer cells; RSV, respiratory syncytial virus; SD, standard deviation.
RSV‐specific antibodies are differently glycosylated than total infant antibody pool
We next assessed the Fc‐glycosylation status of total and RSV‐specific antibodies in our cohort. To this end, we enriched plasma samples for RSV‐specific antibodies by adsorption to inactivated RSV‐coated plates. Subsequently, we determined the levels of IgG1 Fc‐glycosylation using mass spectrometry for the eluted RSV‐specific antibodies and purified total IgG. The IgG Fc‐tail contains one conserved N‐linked glycosylation site that harbours an invariable core glycan with variable presence of fucose, galactose, sialic acid and bisecting N‐acetylglucosamine (bisection). A schematic representation of Fc‐glycosylation is presented in Figure 5a. Since Fc‐glycosylation patterns have previously been shown to differ between antibodies of maternal and infant origin,35, 36, 37 we first compared the total antibody pool (a combination of maternal and infant origin) to the RSV‐specific antibodies (maternal origin). As expected, we found a significantly higher abundance of galactosylation and sialylation in RSV‐specific antibodies compared to total antibodies (Figure 5b). Notably, there appears to be greater variation in the levels of glycosylation of RSV‐specific antibodies compared to the total antibody pool. Because maternal antibodies are gradually replaced by infant antibodies over time, we next assessed whether we could observe changes in glycosylation patterns with increasing age. Despite the cross‐sectional design of the study, we noticed that for both RSV‐specific and total antibodies, the abundance of fucosylation increased with age (Figure 5c‐d), while the abundance of galactosylation, sialylation and bisecting N‐acetylglucosamine decreased with age (Figure 5e–j).
Figure 5.
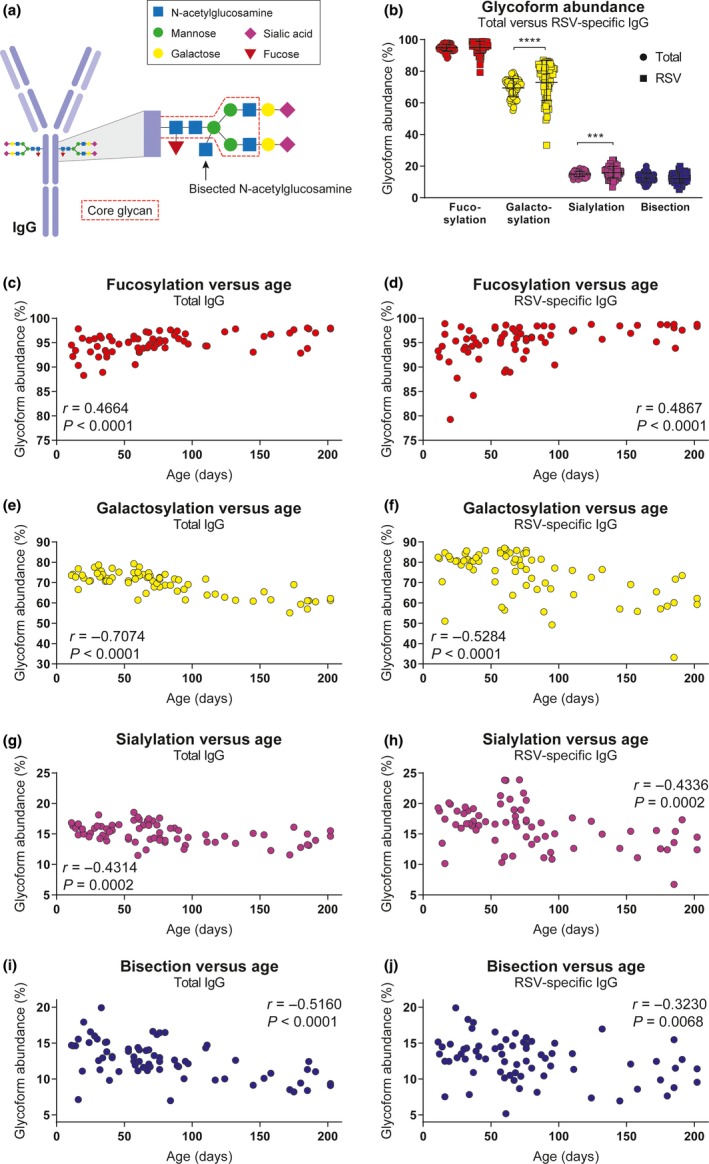
Fc‐glycosylation pattern of total and RSV‐specific antibodies. (a) Schematic representation of IgG Fc‐glycosylation. (b) Glycoform abundance of total compared to RSV‐specific antibodies in infant samples (n = 70). All samples were measured in a single run. The graph depicts the geometric mean and SD. The nonparametric Wilcoxon matched‐pairs test was used for comparison between total and RSV‐specific antibodies (***P < 0.001, ****P < 0.0001). Correlation analysis between glycoform abundance (fucosylation, galactosylation, sialylation, bisecting N‐acetylglucosamine) and age for total antibodies (c, e, g, i) and RSV‐specific antibodies (d, f, h, j). The nonparametric Spearman correlation analysis was used to assess correlations. AU, arbitrary units; IFN‐γ, interferon‐gamma; NK cells, natural killer cells; RSV, respiratory syncytial virus; SD, standard deviation.
Fucosylation of RSV‐specific antibodies correlates with NK cell activation
The absence of fucose on the core Fc‐glycan has been shown to increase binding affinity for FcγRIIIa, thereby increasing the capacity of the antibody for NK cell activation.25, 38 Similarly, galactosylation is known to enhance FcγRIIIa binding, albeit to a lesser extent than afucosylation, and this is particularly evident in the case of afucosylated IgG.25, 39 We therefore asked whether fucosylation of RSV‐specific antibodies correlated with the antibody‐induced NK cell activation observed in our assay. Indeed, although not very strong, we observed a significant negative correlation between fucosylation and the induction of NK cell IFN‐γ production (Figure 6a) and CD107a surface expression (Figure 6b). Galactosylation of RSV‐specific antibodies did not significantly correlate with IFN‐γ production (Figure 6c), but it did show a small but significant positive correlation with CD107a surface expression (Figure 6d). Sialylation showed a small but significant positive correlation with both IFN‐γ production (Figure 6e) and CD107a surface expression (Figure 6f). Bisection did not show a significant correlation with NK cell activation. As RSV‐specific antibodies from infants with severe respiratory tract infections induced significantly less NK cell IFN‐γ production compared to antibodies from uninfected controls, we asked whether this difference was reflected in the glycosylation status of these antibodies. However, none of the glycoforms showed a significant difference in abundance between cases and controls (Figure 7a–d).
Figure 6.
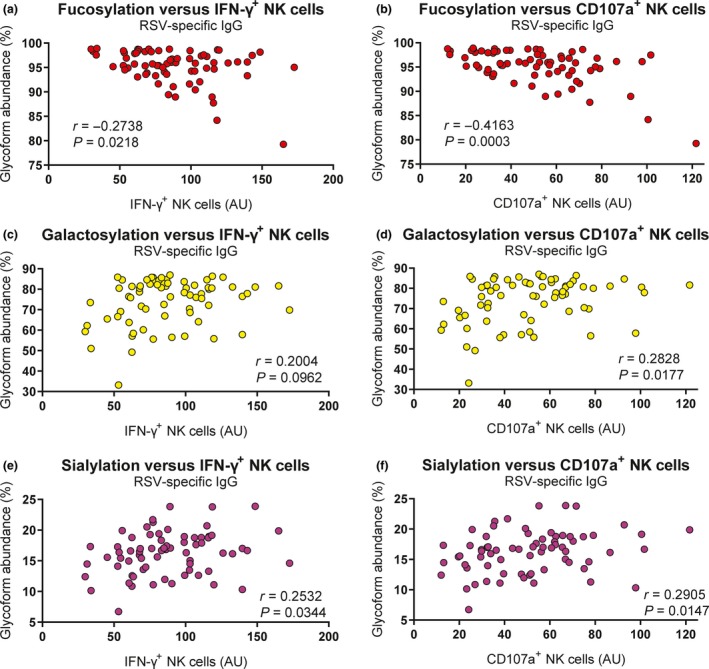
Fc‐glycosylation correlates with NK cell activation. Correlation analysis between glycoform abundance of RSV‐specific antibodies (fucosylation, galactosylation and sialylation) and the capacity to induce IFN‐γ production (a, c, e) and CD107a surface expression (b, d, f) of infant plasma samples (n = 70). All samples were measured in a single run. The nonparametric Spearman correlation analysis was used to assess correlations. AU, arbitrary units; IFN‐γ, interferon‐gamma; IgG, immunoglobulin G; RSV, respiratory syncytial virus.
Figure 7.
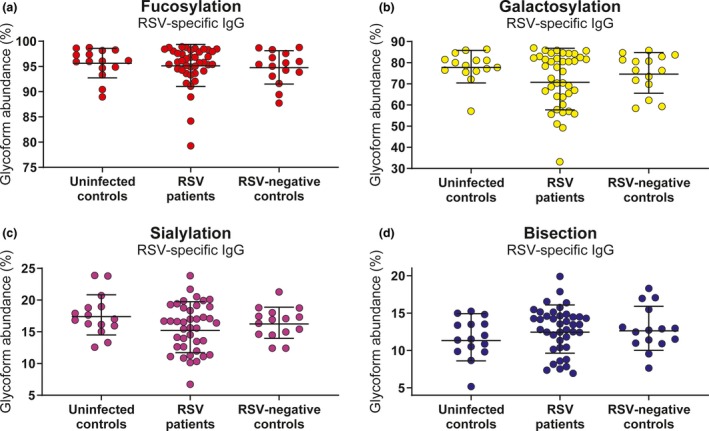
Fc‐glycosylation does not differ between RSV cases and controls. Comparison of the glycosylation profile of RSV‐specific antibodies between uninfected controls (n = 15), RSV patients (n = 40) and RSV‐negative infected controls (n = 15). Abundance of fucosylation (a), galactosylation (b), sialylation (c) and bisecting N‐acetylglucosamine (d) is shown. All samples were measured in a single run. Graphs depict the geometric mean and SD. The Kruskal–Wallis test with Dunn's multiple comparisons test was used for comparison between multiple groups, and no significant differences were found. IgG, immunoglobulin G; RSV, respiratory syncytial virus; SD, standard deviation.
Discussion
Studies investigating the role of (maternal) antibodies in RSV infection mostly focus on neutralisation and binding titres. Whereas antibody‐mediated protection against RSV disease likely depends (at least in part) on virus neutralisation, there is increasing evidence that Fc‐mediated effector functions also play an important role during RSV infection (reviewed in van Erp et al. 40 and Acevedo et al. 41). In two independent in vivo studies, modification of the Fc‐domain of RSV‐specific antibodies has shown major effects on lung viral titre.42, 43 These results strongly support the idea that Fc‐dependent mechanisms contribute to the protective efficacy of RSV‐specific antibodies. In the present study, we found that RSV‐specific antibodies from infants hospitalised for RSV and/or other respiratory viral infections less potently induced NK cell IFN‐γ production than those from uninfected controls. In addition, we found that NK cell activation significantly correlated with RSV antigen‐specific antibody concentration and fucosylation, although neither of these factors significantly differed between cases and controls.
The development of an effective RSV vaccine would greatly benefit from the identification of a correlate of protection by directing rational vaccine design, facilitating the evaluation of vaccine efficacy and limiting the required size of clinical trials. Both binding and neutralisation titres of serum IgG do not adequately predict disease susceptibility for RSV,9, 10, 11, 12 highlighting the need for the identification of additional (co‐)correlates of protection. Although we found a statistically significant difference in antibody‐mediated NK cell IFN‐γ production between infants with respiratory viral infections and uninfected controls, this difference was modest and cannot serve as a correlate of protection by itself. Also the combination of antibody binding titres and NK cell activation does not provide a clear separation between disease groups. Since pre‐F‐specific antibody titres strongly correlate with in vitro neutralisation titres,31, 33 it is expected that the combination of neutralisation and NK cell activation will reveal a similar pattern.
It is important to realise that antibody functionality, and ultimately protective efficacy, is determined by a wide range of factors, including concentration, antigen and epitope specificity, avidity, localisation, glycosylation, isotype, and subclass. Each of these factors contributes to a greater or lesser extent to protection, which might explain why we did find a modest but significant difference between cases and uninfected controls in antibody‐mediated NK cell IFN‐γ production, but not in CD107a surface expression, antibody concentration, and glycosylation. The multitude of factors contributing to antibody‐mediated protection from severe RSV disease complicates the definition of a correlate of protection for this pathogen. The use of a systems serology approach for RSV may offer an unbiased and comprehensive way to systematically investigate antibody characteristics and effector functions on a multidimensional level. It has already proven effective in identifying antibody features that contribute to protection against various other (viral) pathogens.44, 45, 46
Antibody‐mediated NK cell activation has gained increasing attention for its role in protection against a variety of infectious diseases. The capacity of antibodies to induce NK cell ADCC correlates with the control of mycobacterium tuberculosis45 and the killing of chlamydia trachomatis.47 For HIV, ADCC‐inducing antibodies have been identified as a key correlate of protection in the RV144 HIV vaccine trial.48, 49, 50 For influenza, the role of ADCC was shown to be protective in some studies,51, 52 but others point to the involvement of ADCC in exaggeration of the immune response.53, 54, 55 Taken together, these examples show that the capacity of pathogen‐specific antibodies to engage NK cells can significantly contribute to the outcome of infection. Regarding RSV infection, in mice, NK cells have been shown to be sufficient to eliminate infection56 and NK cell depletion was found to increase viral titres.57 However, NK cells have also been found to contribute to inflammatory lung injury in mice.57, 58 In humans, NK cells have been reported to be both decreased59, 60 and increased61, 62 compared to controls upon RSV infection. Thus, the exact role of NK cells during RSV infection remains to be established, but our results suggest that antibody‐mediated activation of NK cells contributes to a favorable outcome of infection.
We found that RSV‐specific antibodies from both RSV‐infected and RSV‐negative infected controls showed a decreased propensity to activate NK cells compared to those from uninfected controls. Since we only have samples that were obtained during hospitalisation, and not before infection, we cannot exclude that the difference we observe is a result of the inflammation that these infants are experiencing. Although it has been shown that chronic infection and inflammation can affect functionality of the newly produced antibody repertoire (for a review, see Alter et al. 63), acute inflammation is unlikely to affect the antibodies that are already in circulation. A prospective study in which children are sampled before and during infection would provide more insight into the possible effects of acute inflammation.
In addition to antibody functionality, we assessed the glycosylation status of both the total antibody pool and the RSV‐specific antibodies. Glycosylation, including galactosylation, sialylation, fucosylation and bisection, is expressed as a percentage of the measured antibodies and varies within a certain (narrow) range depending on the specific modification, with subtle differences between maternal and infant antibodies.35 As expected, analysis of the total antibody pool revealed changes related to infant age, where especially galactosylation showed a strong negative correlation with age. In contrast, fucosylation showed a slight but significant increase with age. These observations are in line with the gradual replacement of maternal with infant antibodies. Altered glycosylation during pregnancy and preferential transplacental transfer result in increased IgG Fc‐galactosylation of maternal antibodies.35, 36, 64 In addition, like all adult antibodies, maternal antibodies have lower fucosylation (94%) compared to highly fucosylated infant antibodies, which reach adult levels around the age of 20 years.35
Surprisingly, we observed similar correlations between glycosylation and age for RSV‐specific antibodies. This suggests that, despite the young age of the infants, our analysis may not be entirely restricted to (RSV‐specific) maternal antibodies. The enriched antibody samples used for the glycosylation analysis might still contain small amounts of infant‐derived antibodies (of varying specificity), the relative abundance of which might increase with age as the concentrations of RSV‐specific maternal antibodies decrease. The increased galactosylation of RSV‐specific antibodies compared to the total antibody pool, consisting of both infant and maternal antibodies, and the gradual waning of RSV‐specific antibody concentrations seen with age do suggest that we are looking primarily at maternal antibodies. However, this can only be firmly concluded when general glycosylation patterns of RSV‐specific antibodies made by the infant are known. An alternative explanation for the age‐related changes in glycosylation of RSV‐specific antibodies is extracellular glycan modification of antibodies, but this remains speculative and has thus far only been shown for sialylation.65 Even for sialylation, most of the incorporated sialic acid is still expected to be assembled in the B cells, as antigen‐specific antibodies formed in certain immune responses can deviate markedly from total IgG.28
To focus on the antibodies that were already present at the start of the infection, we excluded those infants who were positive for RSV‐specific IgA. We considered the possibility that the detected IgA was derived from breastmilk. However, according to current knowledge, breastmilk IgA is not transferred to the infant circulation.66 Indeed, of the 5 infants who had detectable levels of IgA, only 2 were recorded to be breastfed, while 2 others were recorded not to be breastfed and 1 was of unknown breastfeeding status. Combined with the fact that a considerable proportion of the infants who had undetectable RSV‐specific IgA levels were breastfed, we concluded that it is unlikely that the detected IgA was of maternal origin.
Although it is too early to draw definite conclusions, our results are in line with the idea that antibody‐mediated NK cell activation contributes to protection against severe RSV disease and warrant further investigations into the potential of using Fc‐mediated effector functions and their regulation for evaluating and improving the effectiveness of future RSV vaccines. A thorough understanding of the protective capacity, in its broadest sense, of (maternal) RSV‐specific antibodies will be invaluable for the development of a safe and effective vaccine against this elusive pathogen.
Methods
Study design
Plasma samples from hospitalised infants were collected during an observational case–control study in 2010–2014 and have been described before.24 For the current study, hospitalised infants below 7 months of age with PCR‐confirmed RSV infections were included as RSV cases. Age‐matched infants admitted for inguinal hernia repair surgery were included as uninfected controls, whereas infants admitted to the hospital for viral respiratory tract infections other than RSV were included as RSV‐negative infected controls. The ePlex System (Genmark Dx, Carlsbad) was used for identification of respiratory viral pathogens and RSV subtype in nasopharyngeal aspirates. No RSV was detected in uninfected controls and RSV‐negative infected controls. Blood samples were collected in heparin tubes within 24 h after admission. Patients with congenital heart or lung disease, immunodeficiency or glucocorticoid use were excluded. The study protocols were approved by the Regional Committee on Research Involving Human Subjects Arnhem‐Nijmegen (serving as the IRB) and were conducted in accordance with the principles of the Declaration of Helsinki. Written informed consent was obtained from the parents of all infants.
Cells and viruses
Peripheral blood mononuclear cells were obtained from healthy adult volunteers at the National Institute for Public Health and the Environment (RIVM, the Netherlands). Blood was collected in heparin tubes, and the mononuclear fraction was isolated by density gradient centrifugation (Lymphoprep; Alere Technologies AS, Oslo). Isolated cells were cultured in Roswell Park Memorial Institute medium (RPMI; Gibco, Thermo Fisher Scientific, Waltham) supplemented with 10% heat‐inactivated foetal calf serum (hiFCS; Hyclone; GE Healthcare Life Sciences, Marlborough), 1% penicillin/streptomycin/glutamine (PSG; Gibco) and 5 ng mL−1 recombinant human IL‐15 (BioLegend, San Diego). Before use, PBMCs were rested overnight at a density of 1 × 106 PBMCs mL−1 at 37°C and 5% CO2. Vero cells (ATCC CCL‐81) were cultured in Dulbecco's modified Eagle's medium (DMEM; Gibco), supplemented with 5% hiFCS and 1% PSG.
Recombinant RSV‐X (GenBank FJ948820), used as a coat in the NK cell activation assay, was propagated in Vero cells and purified between layers of 10% and 50% sucrose by ultracentrifugation. The 50% tissue culture infectious dose (TCID50) per mL was determined on Vero cells using the Spearman–Karber method.67 RSV‐A2 (ATCC VR1540), used as a coat for the enrichment of RSV‐specific antibodies for mass spectrometry, was propagated in Vero cells, purified and concentrated by filtration, and inactivated by the addition of 2% Triton X‐100.
Control sera/plasma
The International Standard for Antiserum to Respiratory Syncytial Virus (16/284; National Institute for Biological Standards and Control (NIBSC), Potters Bar) was used as control in the NK cell activation assay and glycosylation study. An in‐house adult reference serum pool (RIVM) was used as standard in the multiplex immunoassay. Additionally, cord blood plasma pooled from > 10 individual donors was (mock‐)depleted for RSV‐specific antibodies by incubation with mock‐ or RSV‐infected Vero cells, respectively. Depletion of RSV‐specific antibodies was confirmed by multiplex immunoassay as described below. Cord blood samples were collected from umbilical cords of healthy neonates born by caesarean delivery at Radboudumc Nijmegen (the Netherlands). All mothers provided written informed consent.
RSV‐specific multiplex immunoassay
To quantify the concentration of RSV‐specific IgG and IgA, a multiplex immunoassay was performed as described before.31 Briefly, diluted plasma samples were incubated with RSV antigen‐coupled beads, including GA, GB, post‐F and pre‐F (DS‐CAV1 mutant).68 All antigens were produced in eukaryotic cells. Captured antibodies were detected with secondary R‐phycoerythrin‐labelled goat anti‐human IgA F(ab')2 (Southern Biotech, Birmingham) or IgG F(ab')2 (Jackson ImmunoResearch Laboratories, Ely). IgG was measured on a Bio‐Plex 200 (Luminex Corporation, Austin) in combination with Bio‐Plex Manager software version 6.1 (Bio‐Rad, Hercules). IgA was measured on a Flexmap 3D (Luminex Corporation) in combination with Xponent version 4.2 (Luminex Corporation). For each analyte, median fluorescence intensity (MFI) was converted to arbitrary units (AU) mL−1 by interpolation from a 5‐parameter logistic standard curve from an in‐house reference serum pool. Whereas all samples were tested in the NK cell activation assay, for some samples not enough volume remained to be tested in the IgG (n = 8) and/or IgA (n = 11) multiplex immunoassay. These samples were dispersed equally across the three different groups.
NK cell activation assay
Sterile Immulon ELISA plates (Thermo Scientific, Waltham) were coated with intact RSV‐X particles at a concentration of 1.7 × 105 TCID50 per well. After overnight coating at 4°C, wells were washed with phosphate‐buffered saline (PBS) and blocked with 10% hiFCS in PBS for 30 min at RT. After washing with PBS, coated plates were incubated for 2 h at 37°C with 10‐fold diluted plasma, to allow for antigen‐specific immune complex formation. Subsequently, unbound antibodies and other plasma constituents were washed away and 1 × 106 PBMCs were added per well, together with brefeldin A (BD Bioscience, San Jose) and anti‐human CD107a‐PerCP/Cy5.5 (clone H4A3; BioLegend). PBMCs were incubated with the opsonised virions for 4 hours at 37°C, after which the cells were stained for flow cytometric analysis as described below. Incubation of PBMCs in RSV‐coated plates in the absence of antibodies was used to determine the background signal. The PBMC supernatants were stored at −80°C and used for detection of perforin secretion by ELISA (Mabtech, Nacka Strand). The ELISA was performed following the manufacturer's protocol, and optical density was measured at 450 nm using an ELISA reader (BioTek, Winooski).
Due to the limited amount of fresh PBMCs per donor, we were unable to test all samples using cells of all 6 donors. For this reason, we could test 63 of the 84 plasma samples on cells from all 6 PBMC donors. For 18 samples, we were able to test with PBMCs from 2‐5 different donors. Three samples were only tested on a single donor; these were all RSV‐negative infected controls. Excluding these 3 samples had no effect on the outcome of subsequent statistical analysis.
Flow cytometric analysis
PBMCs that were incubated with opsonised virions as described above were stained for flow cytometric analysis. Extracellular staining was performed with anti‐human CD3‐FITC (clone UCHT1; BioLegend), anti‐human CD56‐PE (clone HCD56; BioLegend) and Fixable Viability Staining‐eFluor 780 (eBioscience, Thermo Fisher Scientific). After fixation and permeabilisation, cells were intracellularly stained with anti‐human IFN‐γ‐PECy7 (clone B27; BioLegend). Flow cytometric analysis was performed using the FACS LSRFortessa X20 (BD Bioscience). In all experiments, NK cells were gated as the CD3–, CD56+ population. The gating strategy is depicted in Supplementary figure 1. FlowJo software V10 (FlowJo, LLC, Ashland) was used for data analysis.
Purification of plasma antibodies for glycosylation analysis
Total IgG antibodies were captured from 2 µL of plasma using Protein G Sepharose 4 Fast Flow beads (GE Healthcare Life Sciences) in a 96‐well filter plate (Millipore Multiscreen, Amsterdam) as described previously.69 To isolate RSV‐specific antibodies from plasma, MaxiSorp ELISA plates were coated with inactivated RSV‐A2 and washed with PBS containing 0.05% Tween‐20 (PBST). Next, plasmas were diluted five times in PBST and incubated for 1 h at room temperature while shaking. WHO RSV standard serum and adalimumab in 1% bovine serum albumin in PBST were used as a positive and negative control, respectively. Plates were then washed once with PBST, twice with PBS and twice with 200 µL ammonium bicarbonate. Elution of RSV‐specific antibodies was performed by incubating with 100 mm formic acid for 5 min.
Mass spectrometric IgG Fc‐glycosylation analysis
Eluates containing either total or RSV‐specific IgG were collected in V‐bottom plates, dried by vacuum centrifugation for 2.5 h at 50°C and then dissolved in 20 µL RA buffer (0.4% sodium deoxycholate, 10 mm tris(2‐carboxyethyl)phosphine (TCEP), 40 mm chloroacetamide, 100 mm tris(hydroxymethyl)aminomethane (TRIS), pH 8.5), followed by shaking for 10 min and heating for 5 min at 95°C. Antibody samples were then subjected to proteolytic cleavage by adding 20 µL trypsin (10 ng µL−1) and incubating for 20 h at 37°C.
Analysis of IgG Fc‐glycosylation was performed with nanoLC reverse phase (RP)‐electrospray (ESI)‐MS on an Ultimate 3000 RSLCnano system (Dionex/Thermo Scientific, Breda) coupled to a Maxis HD quadrupole time‐of‐flight MS equipped with a nanoBooster (Bruker Daltonics, Billerica) using acetonitrile‐doped nebulising gas. (Glyco)peptides were trapped with 100% solvent A (0.1% trifluoroacetic acid in water) and separated on a 4.5‐min 3.0‐21.7% solvent B (95% acetonitrile, 5% water) linear gradient.70 In the current study, we focused on IgG1, without analysing IgG3 because of its possible interference with IgG2 and IgG4 at the glycopeptide level.69 Mass spectrometry results were extracted and evaluated using Skyline software.71 The levels of galactosylation, sialylation, bisecting N‐acetylglucosamine (bisection) and fucosylation were calculated on the basis of the normalised intensities of IgG1 Fc glycopeptides according to the following formulas: galactosylation = (G1F + G1FN + G1FS + G1FNS + G1) * 0.5 + (G2F + G2FN + G2FS + G2FNS + G2 + G2S); sialylation = (G1FS + G2FS + G1FNS + G2FNS + G1S + G2S + G2NS) * 0.5 + (G2S2 + G2FS2); bisection = G0FN + G1FN + G2FN + G1FNS + G2FNS; and fucosylation = G0F + G1F + G2F + G0FN + G1FN + G2FN + G1FS + G2FS. The glycosylation levels were presented as percentages of the total normalised signal intensity.
Statistical analysis
Comparison of two groups or data points was performed by using a nonparametric Mann–Whitney test. Multiple comparisons were analysed by using a nonparametric Kruskal–Wallis test, followed by Dunn's multiple comparisons test. Multiple comparisons of categorical values were tested by the chi‐square test. Correlations were assessed using a nonparametric Spearman's rank‐order correlation. Paired samples were analysed using a nonparametric Wilcoxon matched‐pairs test. P‐values < 0.05 were considered statistically significant. Statistical analyses were performed and graphs were produced with Prism 8 software (GraphPad, San Diego).
Conflict of Interest
The authors declare no conflicts of interest.
Supporting information
Acknowledgments
We thank Oliver Wicht for performing the cord blood depletion assay, Ronald Jacobi and Annelies Mesman for information regarding the NK cell activation assay protocol and Lie Mulder for assistance with virus production. RSV post‐F protein for the RSV multiplex immunoassay was kindly provided by Mark Esser from AstraZeneca. This work was supported by a ZonMw Off Road grant (451001021), the Dutch Ministry of Health, Welfare and Sport (SPR S/112008), the Virgo Consortium (FES0908), the Netherlands Genomics Initiative (050‐060‐452), Sanquin (PPOC‐15‐12), and the Landsteiner Foundation for Blood Research (LSBR 1721).
References
- 1. Shi T, McAllister DA, O'Brien KL et al Global, regional, and national disease burden estimates of acute lower respiratory infections due to respiratory syncytial virus in young children in 2015: a systematic review and modelling study. Lancet 2017; 390: 946–958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Pneumonia Etiology Research for Child Health Study G . Causes of severe pneumonia requiring hospital admission in children without HIV infection from Africa and Asia: the PERCH multi‐country case‐control study. Lancet 2019; 394: 757–779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hall CB, Weinberg GA, Iwane MK et al The burden of respiratory syncytial virus infection in young children. N Engl J Med 2009; 360: 588–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Geoghegan S, Erviti A, Caballero MT et al Mortality due to respiratory syncytial virus. Burden and risk factors. Am J Respir Crit Care Med 2017; 195: 96–103. [DOI] [PubMed] [Google Scholar]
- 5. Glezen WP, Taber LH, Frank AL, Kasel JA. Risk of primary infection and reinfection with respiratory syncytial virus. Am J Dis Child (1960) 1986; 140: 543–546. [DOI] [PubMed] [Google Scholar]
- 6. Eick A, Karron R, Shaw J et al The role of neutralizing antibodies in protection of American Indian infants against respiratory syncytial virus disease. Pediatr Infect Dis J 2008; 27: 207–212. [DOI] [PubMed] [Google Scholar]
- 7. Chu HY, Steinhoff MC, Magaret A et al Respiratory syncytial virus transplacental antibody transfer and kinetics in mother‐infant pairs in Bangladesh. J Infect Dis 2014; 210: 1582–1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Capella C, Chaiwatpongsakorn S, Gorrell E et al Prefusion F, postfusion F, G antibodies and disease severity in infants and young children with acute respiratory syncytial virus infection. J Infect Dis 2017; 216: 1398–1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Vieira SE, Gilio AE, Durigon EL, Ejzenberg B. Lower respiratory tract infection caused by respiratory syncytial virus in infants: the role played by specific antibodies. Clinics (Sao Paulo) 2007; 62: 709–716. [DOI] [PubMed] [Google Scholar]
- 10. Chu HY, Tielsch J, Katz J et al Transplacental transfer of maternal respiratory syncytial virus (RSV) antibody and protection against RSV disease in infants in rural Nepal. J Clin Virol 2017; 95: 90–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Nyiro JU, Sande CJ, Mutunga M et al Absence of Association between Cord Specific Antibody Levels and Severe Respiratory Syncytial Virus (RSV) disease in early infants: a case control study from coastal Kenya. PLoS One 2016; 11: e0166706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Jans J, Wicht O, Widjaja I et al Characteristics of RSV‐specific maternal antibodies in plasma of hospitalized, acute RSV patients under three months of age. PLoS One 2017; 12: e0170877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Stensballe LG, Ravn H, Kristensen K et al Respiratory syncytial virus neutralizing antibodies in cord blood, respiratory syncytial virus hospitalization, and recurrent wheeze. J Allergy Clin Immunol 2009; 123: 398–403. [DOI] [PubMed] [Google Scholar]
- 14. Vivier E, Tomasello E, Baratin M, Walzer T, Ugolini S. Functions of natural killer cells. Nat Immunol 2008; 9: 503–510. [DOI] [PubMed] [Google Scholar]
- 15. Lodoen MB, Lanier LL. Natural killer cells as an initial defense against pathogens. Curr Opin Immunol 2006; 18: 391–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Culley FJ. Natural killer cells in infection and inflammation of the lung. Immunology 2009; 128: 151–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Smyth MJ, Cretney E, Kelly JM et al Activation of NK cell cytotoxicity. Mol Immunol 2005; 42: 501–510. [DOI] [PubMed] [Google Scholar]
- 18. Lopez‐Montanes M, Alari‐Pahissa E, Sintes J, Martinez‐Rodriguez JE, Muntasell A, Lopez‐Botet M. Antibody‐dependent NK cell activation differentially targets EBV‐infected cells in lytic cycle and bystander B lymphocytes bound to viral antigen‐containing particles. J Immunol 2017; 199: 656–665. [DOI] [PubMed] [Google Scholar]
- 19. Scott R, de Landazuri MO, Gardner PS, Owen JJ. Human antibody‐dependent cell‐mediated cytotoxicity against target cells infected with respiratory syncytial virus. Clin Exp Immunol 1977; 28: 19–26. [PMC free article] [PubMed] [Google Scholar]
- 20. Meguro H, Kervina M, Wright PF. Antibody‐dependent cell‐mediated cytotoxicity against cells infected with respiratory syncytial virus: characterization of in vitro and in vivo properties. J Immunol 1979; 122: 2521–2526. [PubMed] [Google Scholar]
- 21. Kaul TN, Welliver RC, Ogra PL. Development of antibody‐dependent cell‐mediated cytotoxicity in the respiratory tract after natural infection with respiratory syncytial virus. Infect Immun 1982; 37: 492–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gupta N, LeGoff J, Chamat S et al Affinity‐purified respiratory syncytial virus antibodies from intravenous immunoglobulin exert potent antibody‐dependent cellular cytotoxicity. PLoS One 2013; 8: e69390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Cortjens B, Yasuda E, Yu X et al Broadly reactive anti‐respiratory syncytial virus G antibodies from exposed individuals effectively inhibit infection of primary airway epithelial cells. J Virol 2017; 91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. van Erp EA, van Kasteren PB, Guichelaar T et al In vitro enhancement of respiratory syncytial virus infection by maternal antibodies does not explain disease severity in infants. J Virol 2017; 91: e00851-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Dekkers G, Treffers L, Plomp R et al Decoding the human immunoglobulin G‐glycan repertoire reveals a spectrum of Fc‐receptor‐ and complement‐mediated‐effector activities. Front Immunol 2017; 8: 877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ackerman ME, Crispin M, Yu X et al Natural variation in Fc glycosylation of HIV‐specific antibodies impacts antiviral activity. J Clin Invest 2013; 123: 2183–2192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wang TT, Sewatanon J, Memoli MJ et al IgG antibodies to dengue enhanced for FcgammaRIIIA binding determine disease severity. Science 2017; 355: 395–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kapur R, Kustiawan I, Vestrheim A et al A prominent lack of IgG1‐Fc fucosylation of platelet alloantibodies in pregnancy. Blood 2014; 123: 471–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kapur R, Della Valle L, Sonneveld M et al Low anti‐RhD IgG‐Fc‐fucosylation in pregnancy: a new variable predicting severity in haemolytic disease of the fetus and newborn. Br J Haematol 2014; 166: 936–945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Dekkers G, Rispens T, Vidarsson G. Novel concepts of altered immunoglobulin G galactosylation in autoimmune diseases. Front Immunol 2018; 9: 553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Schepp RM, de Haan CAM, Wilkins D et al Development and standardization of a high‐throughput multiplex immunoassay for the simultaneous quantification of specific antibodies to five respiratory syncytial virus proteins. mSphere 2019; 4: e00236-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Johnson PR, Spriggs MK, Olmsted RA, Collins PL. The G glycoprotein of human respiratory syncytial viruses of subgroups A and B: extensive sequence divergence between antigenically related proteins. Proc Natl Acad Sci USA 1987; 84: 5625–5629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ngwuta JO, Chen M, Modjarrad K et al Prefusion F‐specific antibodies determine the magnitude of RSV neutralizing activity in human sera. Sci Transl Med 2015; 7: 309ra162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Alter G, Malenfant JM, Altfeld M. CD107a as a functional marker for the identification of natural killer cell activity. J Immunol Methods 2004; 294: 15–22. [DOI] [PubMed] [Google Scholar]
- 35. de Haan N, Reiding KR, Driessen G, van der Burg M, Wuhrer M. Changes in healthy human IgG Fc‐glycosylation after birth and during early childhood. J Proteome Res 2016; 15: 1853–1861. [DOI] [PubMed] [Google Scholar]
- 36. Selman MH, Derks RJ, Bondt A et al Fc specific IgG glycosylation profiling by robust nano‐reverse phase HPLC‐MS using a sheath‐flow ESI sprayer interface. J Proteomics 2012; 75: 1318–1329. [DOI] [PubMed] [Google Scholar]
- 37. Martinez DR, Fong Y, Li SH et al Fc characteristics mediate selective placental transfer of IgG in HIV‐infected women. Cell 2019; 178: 190–201.e111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Shields RL, Lai J, Keck R et al Lack of fucose on human IgG1 N‐linked oligosaccharide improves binding to human FcgammaRIII and antibody‐dependent cellular toxicity. J Biol Chem 2002; 277: 26733–26740. [DOI] [PubMed] [Google Scholar]
- 39. Lippold S, Nicolardi S, Dominguez‐Vega E et al Glycoform‐resolved FcRIIIa affinity chromatography‐mass spectrometry. MAbs 2019; 11: 1191–1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. van Erp EA, Luytjes W, Ferwerda G, van Kasteren PB. Fc‐mediated antibody effector functions during respiratory syncytial virus infection and disease. Front Immunol 2019; 10: 548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Acevedo OA, Díaz FE, Beals TE et al Contribution of Fcγ receptor‐mediated immunity to the pathogenesis caused by the human respiratory syncytial virus. Front Cell Infect Microbiol 2019; 9: 75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Hiatt A,Bohorova N,Bohorov O et al Glycan variants of a respiratory syncytial virus antibody with enhanced effector function and in vivo efficacy. Proc Natl Acad Sci USA 2014; 111: 5992–5997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Boyoglu‐Barnum S, Chirkova T, Todd SO et al Prophylaxis with a respiratory syncytial virus (RSV) anti‐G protein monoclonal antibody shifts the adaptive immune response to RSV rA2‐line19F infection from Th2 to Th1 in BALB/c mice. J Virol 2014; 88: 10569–10583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Saphire EO, Schendel SL, Fusco ML et al Systematic analysis of monoclonal antibodies against ebola virus GP defines features that contribute to protection. Cell 2018; 174: 938–952 e913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Lu LL, Chung AW, Rosebrock TR et al A functional role for antibodies in tuberculosis. Cell 2016; 167: 433–443 e414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Ackerman ME, Barouch DH, Alter G. Systems serology for evaluation of HIV vaccine trials. Immunol Rev 2017; 275: 262–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Moore T, Ananaba GA, Bolier J et al Fc receptor regulation of protective immunity against Chlamydia trachomatis . Immunology 2002; 105: 213–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Bonsignori M, Pollara J, Moody MA et al Antibody‐dependent cellular cytotoxicity‐mediating antibodies from an HIV‐1 vaccine efficacy trial target multiple epitopes and preferentially use the VH1 gene family. J Virol 2012; 86: 11521–11532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Haynes BF, Gilbert PB, McElrath MJ et al Immune‐correlates analysis of an HIV‐1 vaccine efficacy trial. N Engl J Med 2012; 366: 1275–1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Corey L, Gilbert PB, Tomaras GD, Haynes BF, Pantaleo G, Fauci AS. Immune correlates of vaccine protection against HIV‐1 acquisition. Sci Transl Med 2015; 7: 310rv317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Jegaskanda S, Luke C, Hickman HD et al Generation and protective ability of influenza virus‐specific antibody‐dependent cellular cytotoxicity in humans elicited by vaccination, natural infection, and experimental challenge. J Infect Dis 2016; 214: 945–952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Vanderven HA, Liu L, Ana‐Sosa‐Batiz F et al Fc functional antibodies in humans with severe H7N9 and seasonal influenza. JCI Insight 2017; 2: 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Khurana S, Loving CL, Manischewitz J et al Vaccine‐induced anti‐HA2 antibodies promote virus fusion and enhance influenza virus respiratory disease. Sci Transl Med 2013; 5: 200ra114. [DOI] [PubMed] [Google Scholar]
- 54. Co MD, Terajima M, Thomas SJ et al Relationship of preexisting influenza hemagglutination inhibition, complement‐dependent lytic, and antibody‐dependent cellular cytotoxicity antibodies to the development of clinical illness in a prospective study of A(H1N1)pdm09 Influenza in children. Viral Immunol 2014; 27: 375–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Ye ZW, Yuan S, Poon KM et al Antibody‐dependent cell‐mediated cytotoxicity epitopes on the hemagglutinin head region of pandemic H1N1 influenza virus play detrimental roles in H1N1‐infected mice. Front Immunol 2017; 8: 317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Hussell T, Openshaw PJ. IL‐12‐activated NK cells reduce lung eosinophilia to the attachment protein of respiratory syncytial virus but do not enhance the severity of illness in CD8 T cell‐immunodeficient conditions. J Immunol 2000; 165: 7109–7115. [DOI] [PubMed] [Google Scholar]
- 57. Harker JA, Godlee A, Wahlsten JL et al Interleukin 18 coexpression during respiratory syncytial virus infection results in enhanced disease mediated by natural killer cells. J Virol 2010; 84: 4073–4082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Li F, Zhu H, Sun R, Wei H, Tian Z. Natural killer cells are involved in acute lung immune injury caused by respiratory syncytial virus infection. J Virol 2012; 86: 2251–2258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Welliver TP, Garofalo RP, Hosakote Y et al Severe human lower respiratory tract illness caused by respiratory syncytial virus and influenza virus is characterized by the absence of pulmonary cytotoxic lymphocyte responses. J Infect Dis 2007; 195: 1126–1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Larranaga CL, Ampuero SL, Luchsinger VF et al Impaired immune response in severe human lower tract respiratory infection by respiratory syncytial virus. Pediatr Infect Dis J 2009; 28: 867–873. [DOI] [PubMed] [Google Scholar]
- 61. Tripp RA, Moore D, At Barskey et al Peripheral blood mononuclear cells from infants hospitalized because of respiratory syncytial virus infection express T helper‐1 and T helper‐2 cytokines and CC chemokine messenger RNA. J Infect Dis 2002; 185: 1388–1394. [DOI] [PubMed] [Google Scholar]
- 62. Kerrin A, Fitch P, Errington C et al Differential lower airway dendritic cell patterns may reveal distinct endotypes of RSV bronchiolitis. Thorax 2017; 72: 620–627. [DOI] [PubMed] [Google Scholar]
- 63. Alter G, Ottenhoff THM, Joosten SA. Antibody glycosylation in inflammation, disease and vaccination. Semin Immunol 2018; 39: 102–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Jennewein MF, Goldfarb I, Dolatshahi S et al Fc glycan‐mediated regulation of placental antibody transfer. Cell 2019; 178: 202–215 e214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Jones MB, Oswald DM, Joshi S, Whiteheart SW, Orlando R, Cobb BA. B‐cell‐independent sialylation of IgG. Proc Natl Acad Sci USA 2016; 113: 7207–7212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Van de Perre P. Transfer of antibody via mother's milk. Vaccine 2003; 21: 3374–3376. [DOI] [PubMed] [Google Scholar]
- 67. Hierholzer JC, Killington RA. Virus isolation and quantitation In: Mahy BWJ, Kangro HO. eds. Virology methods manual. London and San Diego: Academic Press, 1996: 24–32. [Google Scholar]
- 68. McLellan JS, Chen M, Joyce MG et al Structure‐based design of a fusion glycoprotein vaccine for respiratory syncytial virus. Science 2013; 342: 592–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Sonneveld ME, Koelewijn J, de Haas M et al Antigen specificity determines anti‐red blood cell IgG‐Fc alloantibody glycosylation and thereby severity of haemolytic disease of the fetus and newborn. Br J Haematol 2017; 176: 651–660. [DOI] [PubMed] [Google Scholar]
- 70. Falck D, Jansen BC, de Haan N, Wuhrer M. High‐throughput analysis of IgG Fc glycopeptides by LC‐MS. Methods Mol Biol 2017; 1503: 31–47. [DOI] [PubMed] [Google Scholar]
- 71. MacLean B, Tomazela DM, Shulman N et al Skyline: an open source document editor for creating and analyzing targeted proteomics experiments. Bioinformatics 2010; 26: 966–968. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


