Key Points
Question
What is the comparative effect on in-hospital mortality of using proton pump inhibitors (PPIs) vs histamine-2 receptor blockers (H2RBs) for stress ulcer prophylaxis among adults requiring invasive mechanical ventilation in the intensive care unit (ICU)?
Findings
In this cluster crossover randomized clinical trial, 26 982 patients requiring invasive mechanical ventilation within 24 hours of ICU admission were randomized by site at 50 ICUs in 5 countries to a PPI strategy or an H2RB strategy for stress ulcer prophylaxis. In-hospital mortality was 18.3% for patients treated at sites randomized to PPI use vs 17.5% for those treated at sites randomized to H2RB use, a difference that did not reach statistical significance (P = .054). Among patients treated at sites randomized to PPIs, 4.1% received H2RBs; among patients treated at sites randomized to H2RBs, 20.1% received PPIs.
Meaning
A strategy of use with PPIs vs H2RBs for stress ulcer prophylaxis among adults requiring mechanical ventilation did not result in a statistically significant difference for in-hospital mortality, but study interpretation may be limited by crossover in medication use.
Abstract
Importance
Proton pump inhibitors (PPIs) or histamine-2 receptor blockers (H2RBs) are often prescribed for patients as stress ulcer prophylaxis drugs in the intensive care unit (ICU). The comparative effect of these drugs on mortality is unknown.
Objective
To compare in-hospital mortality rates using PPIs vs H2RBs for stress ulcer prophylaxis.
Design, Setting, and Participants
Cluster crossover randomized clinical trial conducted at 50 ICUs in 5 countries between August 2016 and January 2019. Patients requiring invasive mechanical ventilation within 24 hours of ICU admission were followed up for 90 days at the hospital.
Interventions
Two stress ulcer prophylaxis strategies were compared (preferential use with PPIs vs preferential use with H2RBs). Each ICU used each strategy sequentially for 6 months in random order; 25 ICUs were randomized to the sequence with use of PPIs and then use of H2RBs and 25 ICUs were randomized to the sequence with use of H2RBs and then use of PPIs (13 436 patients randomized by site to PPIs and 13 392 randomized by site to H2RBs).
Main Outcomes and Measures
The primary outcome was all-cause mortality within 90 days during index hospitalization. Secondary outcomes were clinically important upper gastrointestinal bleeding, Clostridioides difficile infection, and ICU and hospital lengths of stay.
Results
Among 26 982 patients who were randomized, 154 opted out, and 26 828 were analyzed (mean [SD] age, 58 [17.0] years; 9691 [36.1%] were women). There were 26 771 patients (99.2%) included in the mortality analysis; 2459 of 13 415 patients (18.3%) in the PPI group died at the hospital by day 90 and 2333 of 13 356 patients (17.5%) in the H2RB group died at the hospital by day 90 (risk ratio, 1.05 [95% CI, 1.00 to 1.10]; absolute risk difference, 0.93 percentage points [95% CI, −0.01 to 1.88] percentage points; P = .054). An estimated 4.1% of patients randomized by ICU site to PPIs actually received H2RBs and an estimated 20.1% of patients randomized by ICU site to H2RBs actually received PPIs. Clinically important upper gastrointestinal bleeding occurred in 1.3% of the PPI group and 1.8% of the H2RB group (risk ratio, 0.73 [95% CI, 0.57 to 0.92]; absolute risk difference, −0.51 percentage points [95% CI, −0.90 to −0.12 percentage points]; P = .009). Rates of Clostridioides difficile infection and ICU and hospital lengths of stay were not significantly different by treatment group. One adverse event (an allergic reaction) was reported in 1 patient in the PPI group.
Conclusions and Relevance
Among ICU patients requiring mechanical ventilation, a strategy of stress ulcer prophylaxis with use of proton pump inhibitors vs histamine-2 receptor blockers resulted in hospital mortality rates of 18.3% vs 17.5%, respectively, a difference that did not reach the significance threshold. However, study interpretation may be limited by crossover in the use of the assigned medication.
Trial Registration
anzctr.org.au Identifier: ACTRN12616000481471
This cluster randomized trial compares the effects of proton pump inhibitors (PPIs) vs histamine-2 receptor blockers (H2RBs) on 90-day mortality among patients requiring invasive mechanical ventilation within 24 hours of admission to the intensive care unit (ICU).
Introduction
Data collected during 2013 and 2014 indicated an estimated 2.5% of adults acutely admitted to an intensive care unit (ICU) developed upper gastrointestinal bleeding and, in an attempt to prevent this bleeding, 70% were prescribed stress ulcer prophylaxis.1 Although proton pump inhibitors reportedly reduced bleeding risk,2,3 and were prescribed most commonly according to data collected during 2014, some clinicians prescribed histamine-2 receptor blockers.4,5 This practice variation depended on clinician preference or hospital policy.3,5 A meta-analysis6 of randomized clinical trials concluded that proton pump inhibitors might be more effective than histamine-2 receptor blockers in preventing upper gastrointestinal bleeding; however, the robustness of this conclusion was limited by the paucity of available data, the methodological limitations of the trials included, and possible publication bias.
The uncertainty about which class of drug to use, a decision that affects an estimated 2.5 million critically ill patients per year in high-income countries alone,7 was increased when proton pump inhibitor use compared with histamine-2 receptor blocker use was associated with greater risk of nosocomial pneumonia8,9,10 and Clostridioides difficile infection.8 Proton pump inhibitors have been reported to exert a range of immunosuppressive effects11 that could potentially increase the risk of death from commonly occurring infection-related complications among patients in the ICU. These immunosuppressive effects include inhibition of natural killer cell activity,12 neutrophil chemotaxis, and superoxide generation.13 The relative effect of different classes of stress ulcer prophylaxis drugs on mortality rates is unknown because adequately powered clinical trials including patients in the ICU and comparing proton pump inhibitors and histamine-2 receptor blockers have not been performed.
Accordingly, the Proton Pump Inhibitors vs Histamine-2 Receptor Blockers for Ulcer Prophylaxis Treatment in the Intensive Care Unit (PEPTIC) trial was conducted to compare 2 approaches for stress ulcer prophylaxis among adults in the ICU requiring invasive mechanical ventilation. The primary aim was to compare the risk of all-cause mortality during index hospitalization up to 90 days.
Methods
Trial Design
This trial was an international open-label, cluster crossover, registry-embedded randomized clinical trial comparing 2 approaches for stress ulcer prophylaxis implemented in the ICU among adults requiring mechanical ventilation. It was designed by the trial’s management committee and was overseen by an independent data and safety monitoring committee.
The trial protocol, which was reported14 before enrollment was completed and appears in Supplement 1, was approved by the health research ethics committee responsible for each participating institution. The terms of the ethics approvals differed by jurisdiction; some jurisdictions granted a full waiver of consent and others required surviving participants be given the opportunity to opt out of having their health information included in the study. Health information was used in accordance with relevant laws in all participating countries.
Patients
Patients aged 18 years or older requiring invasive mechanical ventilation within 24 hours of ICU admission were eligible for the study. Patients who had an ICU admission diagnosis of upper gastrointestinal bleeding were excluded.
Randomization and Study Treatment
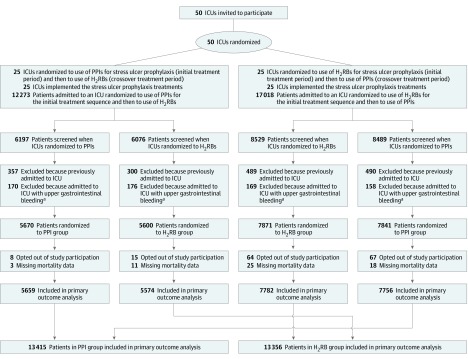
This study compared 2 standard approaches for stress ulcer prophylaxis among adults requiring mechanical ventilation. One approach was to use proton pump inhibitors by default and the other was to use histamine-2 receptor blockers by default when stress ulcer prophylaxis was prescribed. Each ICU used one approach for a 6-month treatment period and then switched to the alternative approach for the next 6 months (Figure 1).
Figure 1. Screening, Randomization, and Follow-up of Participants in the PEPTIC Randomized Trial.
One intensive care unit (ICU) randomized to the sequence of proton pump inhibitors (PPIs) first and then histamine-2 receptor blockers (H2RBs) contributed 46 patients to the H2RB group but did not contribute patients to the PPI group because it did not contribute to the registry for treatment period 1. As shown in eFigure 2 in Supplement 2, patients in a small number of sites received both PPIs and H2RBs, and a small number of patients distributed across multiple sites received no stress ulcer prophylaxis.
aDefined by the presence of a registry ICU admission code indicating that the patient was admitted to the ICU with upper gastrointestinal bleeding.
Participating ICUs were randomized to the order of treatments in a 1:1 ratio when ethics and regulatory approvals were in place at each study site. Randomization was performed using computer-generated random numbers and was stratified by region and time period with a minimum of 4 ICUs randomized at a time.
Study treatments were administered open-label in this unblinded trial. Clinicians decided whether individual patients would receive stress ulcer prophylaxis. When clinicians chose to prescribe stress ulcer prophylaxis, the default prescription of either a proton pump inhibitor or a histamine-2 receptor blocker was determined by ICU randomization status. However, irrespective of the therapy assigned to the ICU, either a proton pump inhibitor or a histamine-2 receptor blocker could be used for a particular patient if the treating clinician considered this preferable.
Patients who remained in the ICU through a crossover period continued to receive their originally assigned treatment. No washout occurred between crossover periods. The duration of study treatment was until death, ICU discharge, development of a clinically important upper gastrointestinal bleeding event, or until the treating clinician considered stress ulcer prophylaxis was no longer indicated. If upper gastrointestinal bleeding occurred, a proton pump inhibitor was administered at clinician discretion in accordance with standard practice, irrespective of treatment randomization status.
Individual patient-level data on the stress ulcer prophylaxis used were not collected for all trial participants. Such data were obtained for trial participants from Canada and Ireland. For participants from Australia, England, and New Zealand, information on stress ulcer prophylaxis used was obtained from the medication charts on 1 day of each month for any adults requiring mechanical ventilation in the ICU. These data were supplemented, when available, with additional electronic prescribing and pharmacy dispensing data. Additional details appear in the eMethods in Supplement 2.
Outcome Measures
The primary outcome was in-hospital all-cause mortality during the index hospitalization up to 90 days from the date of the index ICU admission. When this trial was registered, the primary end point was a composite comprising clinically important upper gastrointestinal bleeding, Clostridioides difficile infections, and episodes of mechanical ventilation lasting longer than 10 days. It was changed in March 2017 to in-hospital mortality because it was determined that giving mortality primacy was preferable to combining components of a composite end point that were not necessarily equally important to patients and which might move in opposite directions. The change was made prior to any site completing recruitment and prior to reviewing any data.
The secondary outcomes were clinically important upper gastrointestinal bleeding,1 Clostridioides difficile infection, and ICU and hospital lengths of stay. The occurrence of ventilator-associated conditions15 and the duration of invasive mechanical ventilation were tertiary outcomes. The definitions of the outcome measures appear in the statistical analysis plan posted online16 and appear in Supplement 1. The primary outcome data were obtained from registries. Other outcomes were ascertained using a combination of registry and trial-specific data sources as outlined in eTable 1 and eFigure 1 in Supplement 2.
Adverse Event Reporting
Because proton pump inhibitors and histamine-2 receptor blockers have been in widespread use in ICUs for many years (as outlined in the eMethods in Supplement 2), the usual methods for reporting of suspected adverse reactions to licensed medicines were used rather than trial-specific adverse event reporting methods.
Statistical Analysis
The statistical analysis plan was posted online16 before the study database was locked and appears in Supplement 1. Patients were analyzed according to their randomization group; however, some patients opted out of allowing use of their data. Using previously described methods,17 we calculated the study would have 80% power at a significance level of .05 using a 2-sided test to detect an absolute reduction of 2.4% for in-hospital mortality from a baseline mortality of 15%, corresponding to a relative risk reduction of 16%.14 This calculation assumed a mean cluster period size of 310 patients with a coefficient of variation value of 0.50, a within-cluster within-period correlation of 0.035, and a within-cluster between-period correlation of 0.025, yielding a cluster autocorrelation of 0.71 (0.025/0.035). Additional information appears in Supplement 2.
The analysis of the primary outcome used individual patient-level data and generalized estimating equations with a logarithmic link function, an exchangeable working correlation matrix, and robust standard errors using the ICU as the clustering unit. Because randomization was performed in batches of ICUs, covariate adjustment for randomization batch, the order of administration of the treatments, and batch × order interaction was performed to allow for separate order and secular time effects occurring in each of the randomization batches.
The treatment effect was partitioned into its within-ICU and between-ICU components by including the proportion of patients assigned to the proton pump inhibitor group at each ICU as a covariate together with the treatment group.18 The within-ICU treatment effect estimate, which was not confounded by the differences between ICUs and was represented by the main effect of treatment group in these models, is reported. Treatment comparisons are presented as risk ratios (RRs) and 95% CIs from the generalized estimating equation analysis, and as absolute risk differences and 95% CIs obtained by marginalizing (ie, standardizing) of the RR model.19
The approach to the analyses of the secondary end points appears in the eMethods in Supplement 2. The main sensitivity analyses for the effect of missing data on the primary and secondary outcomes involved imputing outcomes under worst-best and best-worst case scenarios. In the worst-best scenario for a binary outcome, a worst outcome event (eg, in-hospital death within 90 days) was assigned to all patients with missing data for the outcome in one treatment group, and a best outcome event (eg, survival to hospital discharge within 90 days) was assigned to all patients with missing data for the outcome in the other treatment group. The best-worst scenario was the opposite assignment of outcomes.
Preplanned analyses assessed heterogeneity of treatment effects on the primary and secondary outcomes for the following subgroups: (1) patients admitted to the ICU after cardiac surgery vs any other reason, (2) patients admitted after emergency vs elective surgery, and (3) patients by geographic region. Post hoc subgroup analyses classified patients into groups by ICU using adherence (low, medium, or high) tertiles during the histamine-2 receptor blocker period based on the estimated frequency of proton pump inhibitor use in patients admitted during the histamine-2 receptor blocker period, and quartiles of illness severity using the Acute Physiology and Chronic Health Evaluation score. Subgroup analyses included treatment × subgroup interaction terms in respective regression models.
All statistical analyses were completed using Stata version 15 (StataCorp) with statistical significance indicated by a P value of .05 and with the use of a 2-sided hypothesis test. Because of the potential for type I error due to multiple comparisons, findings for the analyses of the secondary end points should be interpreted as exploratory.
Results
Patient Characteristics
From August 2016 through January 2019, 26 982 patients from 50 ICUs in Australia, Canada, England, Ireland, and New Zealand were enrolled. A total of 154 patients opted out of participation resulting in a population of 26 828, of whom 13 436 were assigned to proton pump inhibitors as the default treatment for stress ulcer prophylaxis and 13 392 were assigned to histamine-2 receptor blockers. The mean (SD) age was 58 (17.0) years; 9691 (36.1%) were women; 8815 (32.9%) were admitted to the ICU after elective surgery and 4946 (18.4%) after emergency surgery. The study groups had similar characteristics at baseline (Table 1; eTables 2-4 in Supplement 2). Primary outcome data were missing for 57 participants (Figure 1; eTables 5 and 6 in Supplement 2).
Table 1. Baseline Characteristics.
| Proton Pump Inhibitors (n = 13 436) |
Histamine-2 Receptor Blockers (n = 13 392) |
|
|---|---|---|
| Age, mean (SD), y | 58.6 (17.0) | 58.2 (17.1) |
| Sex, No. (%) | ||
| Male | 8577 (63.8) | 8560 (63.9) |
| Female | 4859 (36.2) | 4832 (36.1) |
| APACHE II chronic comorbidities, No./total No. (%) | ||
| Respiratory | 821/13 425 (6.1) | 798/13 375 (6.0) |
| Cardiovascular | 872/13 425 (6.5) | 804/13 375 (6.0) |
| Hepatic | 182/13 425 (1.4) | 191/13 371 (1.4) |
| Kidney | 252/13 425 (1.9) | 277/13 375 (2.1) |
| Immunosuppression | 783/13 425 (5.8) | 872/13 375 (6.5) |
| Metastatic cancer | 367/13 425 (2.7) | 340/13 371 (2.5) |
| Source of admission to ICU, No. (%) | ||
| Emergency department | 4026 (30.0) | 4026 (30.1) |
| Hospital ward | 1479 (11.0) | 1406 (10.5) |
| Transfer from another ICU | 322 (2.4) | 342 (2.6) |
| Transfer from another hospital (except from another ICU) | 1012 (7.5) | 993 (7.4) |
| After elective surgery | 4356 (32.4) | 4459 (33.3) |
| After emergency surgery | 2490 (18.5) | 2456 (18.3) |
| Unknown | 14 (0.1) | 22 (0.2) |
| Admitted to ICU with lower gastrointestinal bleeding, No. (%) | 8 (0.06) | 6 (0.04) |
| APACHE II score | ||
| No. of patients | 13 374 | 13 339 |
| Mean (SD)a | 18.7 (8.3) | 18.7 (8.4) |
| APACHE III score | ||
| No. of patients | 11 214 | 11 382 |
| Mean (SD)b | 65.2 (29.9) | 65.5 (29.5) |
| Risk of death for participants living in Australia and New Zealand onlyc | ||
| No. of patients | 8818 | 9078 |
| Mean (SD), %d | 14.1 (22.4) | 13.9 (22.0) |
| Median (interquartile range), %d | 3.2 (0.7-16.1) | 3.1 (0.8-16.0) |
| ICNARC risk of death for participants living in Ireland and England onlyc | ||
| No. of patients | 2212 | 1993 |
| Mean (SD), %d | 31.6 (31.4) | 31.5 (31.1) |
| Median (interquartile range), %d | 19.8 (4.3-55.7) | 19.7 (4.5-54.7) |
| Patients per site, median (interquartile range)e | 193 (130-393) | 175 (109-416) |
| By region, No. (%) | ||
| Australia and New Zealand | 8826 (65.7) | 9088 (67.9) |
| Canada | 2217 (16.5) | 2148 (16.0) |
| Ireland and England | 2393 (17.8) | 2156 (16.1) |
Abbreviations: APACHE, Acute Physiology and Chronic Health Evaluation; ICNARC, Intensive Care National Audit and Research Centre; ICU, intensive care unit.
Scores range from 0 to 71; higher scores indicate more severe disease and a higher risk of death.
Scores range from 0 to 299; higher scores indicate more severe disease and a higher risk of death.
Combines physiology, age, diagnosis, and comorbidities collected during the first 24 hours in the ICU to create predicted risk of death at the hospital.
Scores range from 0% to 99.9%.
Further details appear in eTables 2 and 3 in Supplement 2.
Use of Stress Ulcer Prophylaxis
The median time in the ICU (ie, the time available for exposure to stress ulcer prophylaxis) was 2.8 days (interquartile range, 1.2-5.7 days) in the proton pump inhibitor group and 2.7 days (interquartile range, 1.2-5.8 days) in the histamine-2 receptor blocker group. Among patients in the proton pump inhibitor group, an estimated 82.5% received proton pump inhibitor, 4.1% received histamine-2 receptor blocker, 1.9% received both proton pump inhibitor and histamine-2 receptor blocker, and 11.5% received neither.
Among patients in the histamine-2 receptor blocker group, an estimated 63.6% received histamine-2 receptor blocker, 20.1% received proton pump inhibitor, 5.1% received both histamine-2 receptor blocker and proton pump inhibitor, and 11.2% received neither. The use of stress ulcer prophylaxis drugs from each class (proton pump inhibitor or histamine-2 receptor blocker) for each ICU during each treatment period appears in eFigure 2 and eTables 7-56 in Supplement 2.
Primary Outcome
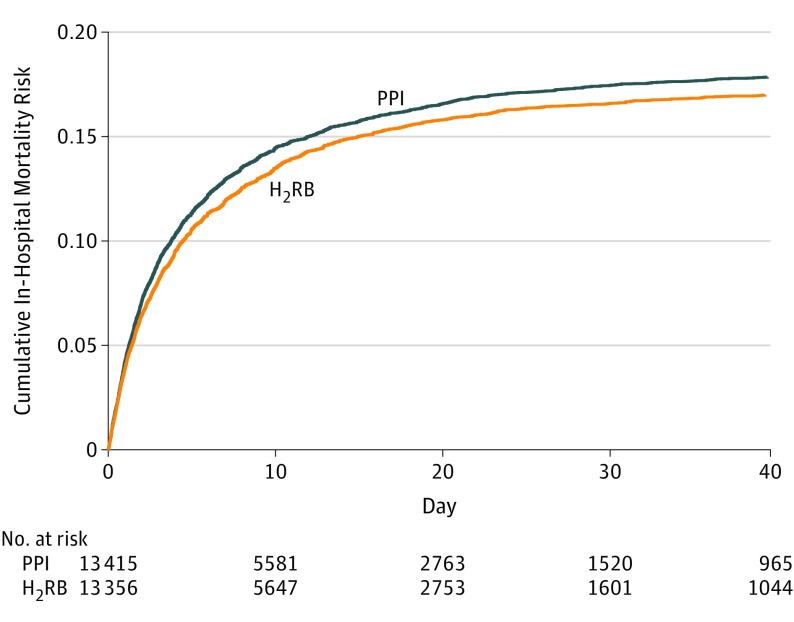
A total of 2459 of 13 415 patients (18.3%) in the proton pump inhibitor group died at the hospital by day 90 and 2333 of 13 356 patients (17.5%) in the histamine-2 receptor blocker group died at the hospital by day 90 (RR, 1.05 [95% CI, 1.00 to 1.10]; absolute risk difference, 0.93 percentage points [95% CI, −0.01 to 1.88 percentage points]; P = .054) (Table 2 and Figure 2; eTables 57-60 in Supplement 2). The primary outcome result was robust when applying different analysis methods and small sample corrections and when using the best-case and worst-case imputation of missing data (eTables 58 and 61 in Supplement 2).
Table 2. Primary, Secondary, and Tertiary Outcomes.
| Proton Pump Inhibitors |
Histamine-2 Receptor Blockers |
Estimate (95% CI) | Absolute Risk Difference (95% CI) | P Value | |
|---|---|---|---|---|---|
| Primary Outcome | |||||
| Died at the hospital by 90 d, No./total No. (%) | 2459/13 415 (18.3) | 2333/13 356 (17.5) | RR, 1.05 (1.00 to 1.10) | 0.93 (−0.01 to 1.88) percentage points | .054 |
| Secondary Outcomes | |||||
| Types of complications, No./total No. (%) | |||||
| Clinically important upper gastrointestinal bleedinga | 172/13 436 (1.3) | 239/13 392 (1.8) | RR, 0.73 (0.57 to 0.92) | −0.51 (−0.90 to −0.12) percentage points | .009 |
| Clostridioides difficile infectionb | 40/13 436 (0.30) | 57/13 392 (0.43) | RR, 0.74 (0.51 to 1.09) | −0.11 (−0.25 to 0.03) percentage points | .13 |
| Length of stay variables and duration of ventilation | |||||
| Days until discharged alive from the ICU | |||||
| No. of patients | 13 425 | 13 384 | |||
| Median (interquartile range)c | 3.6 (1.6 to 10.4) | 3.3 (1.5 to 10.0) | ROM, 1.00 (0.97 to 1.03)d | .85 | |
| Days until discharged alive from the hospital | |||||
| No. of patients | 13 418 | 13 370 | |||
| Median (interquartile range)c | 12.2 (6.0 to 40.0) | 12.0 (6.0 to 39.3) | ROM, 1.01 (0.98 to 1.03)d | .66 | |
| Tertiary Outcomes | |||||
| Hours until removed alive from mechanical ventilation | |||||
| No. of patients | 6047 | 5438 | |||
| Median (interquartile range) | 48.0 (12.1 to 271) | 48.0 (14.3 to 265) | ROM, 0.98 (0.92 to 1.04)d | .43 | |
| Ventilator-associated conditions, No./total No. (%)e | 143/2217 (6.5) | 124/2148 (5.8) | RR, 1.18 (0.87 to 1.59) | 1.11 (−0.89 to 3.11) percentage points | .28 |
Abbreviations: ICU, intensive care unit; ROM, ratio of median time to discharge (or extubation); RR, risk ratio.
Defined as overt upper gastrointestinal bleeding (eg, hematemesis, melaena, or frank blood in the nasogastric tube or upper gastrointestinal endoscopy) developing as a complication in the ICU and accompanied by 1 or more of the following features within 24 hours: (1) a spontaneous decline in systolic, diastolic, or mean arterial pressure of 20 mm Hg or greater; (2) initiation of a vasopressor or a 20% increase in dose of ongoing vasopressor; (3) a decrease in hemoglobin level of 20 g/L or greater; or (4) a transfusion of at least 2 U of packed red blood cells.
Defined as a new toxin or culture-positive stool sample collected during an ICU admission (excluding any patients who had positive test results from specimens collected prior to ICU admission).
Calculated from cumulative incidence functions with mortality regarded as a competing risk. A total of 15.1% of patients who received proton pump inhibitors and 13.9% of patients who received histamine-2 receptor blockers died during their index ICU admission; a total of 18.5% and 17.6%, respectively, died during their index hospital admission. Among patients with duration of ventilation data present, a total of 18.5% of patients who received proton pump inhibitors and 17.8% of patients who received histamine-2 receptor blockers died prior to extubation or were determined to have been extubated with palliative intent.
Estimated using censored linear regression models with logarithm of time to discharge as the dependent variable. Patients who died were censored at their time of death. Adjustment was made for the same variables as for binary outcomes. Heterogeneity of variance by treatment group and country was accommodated for and robust standard errors were used to account for clustering by ICU.
Only available for participants from the 8 Canadian ICUs. Defined as events after a period of stability or improvement while receiving invasive mechanical ventilation for at least 2 days in which a patient had at least 1 of the following indicators of worsening oxygenation: (1) an increase in daily minimum fraction of inspired oxygen of at least 0.20 more than the daily minimum fraction of inspired oxygen during the baseline period of stability that was sustained for at least 2 days; or (2) an increase in daily minimum positive end expiratory pressure of at least 3 cm H2O more than the daily minimum positive end-expiratory pressure during the baseline period that was sustained for at least 2 days (daily minimum defined by the lowest fraction of inspired oxygen or positive end-expiratory pressure during a calendar day that is maintained for at least 1 hour).
Figure 2. Cumulative Incidence of In-Hospital Mortality.
A total of 2459 of 13 415 patients (18.3%) in the proton pump inhibitor (PPI) group and 2333 of 13 356 patients (17.5%) in the histamine-2 receptor blocker (H2RB) group died at the hospital by day 90 (risk ratio, 1.05 [95% CI, 1.00 to 1.10]; absolute risk difference, 0.93 [95% CI, −0.01 to 1.88] percentage points; P = .054). The median observation time was 7.99 days (interquartile range, 4.79 to 17.0 days) in the PPI group vs 8.03 days (interquartile range, 4.82 to 17.0 days) in the H2RB group. Curve truncated at 40 days beyond which less than 10% of the study population remained at risk.
Secondary Outcomes
Clinically important upper gastrointestinal bleeding was reported in 172 of 13 436 patients (1.3%) in the proton pump inhibitor group and in 239 of 13 392 patients (1.8%) in the histamine-2 receptor blocker group (RR, 0.73 [95% CI, 0.57 to 0.92]; absolute risk difference, −0.51 percentage points [95% CI, −0.90 to −0.12 percentage points]; P = .009). New-onset Clostridioides difficile infection diagnosed in the ICU was reported for 40 of 13 436 patients (0.30%) in the proton pump inhibitor group and for 57 of 13 392 patients (0.43%) in the histamine-2 receptor blocker group (RR, 0.74 [95% CI, 0.51 to 1.09]; absolute risk difference, −0.11 percentage points [95% CI, −0.25 to 0.03 percentage points]; P = .13). There were no statistically significant between-group differences for ICU and hospital lengths of stay (Table 2; eTable 62 in Supplement 2).
Adverse Events
One patient in the proton pump inhibitor group had an allergic reaction to omeprazole and was switched to ranitidine.
Subgroups
There was no statistically significant heterogeneity of treatment response in any prespecified subgroups for mortality during index hospitalization by day 90. For patients in the proton pump inhibitor group vs patients in the histamine-2 receptor blocker group, the RR was 1.27 (95% CI, 1.04-1.57) for those who had cardiac surgery and the RR was 1.05 (95% CI, 1.00-1.10) for other patients (P = .07 for interaction).
There was statistically significant heterogeneity of treatment response with respect to the risk of clinically important gastrointestinal bleeding by region for patients in the proton pump inhibitor group vs patients in the histamine-2 receptor blocker group (RR, 0.46 [95% CI, 0.26-0.81] for Australia and New Zealand; RR, 0.92 [95% CI, 0.71-1.19] for Canada; and RR, 0.48 [95% CI, 0.33-0.69] for England and Ireland; P = .004 for interaction). Additional results from the subgroup analyses appear in eTables 63 and 64 in Supplement 2.
Post Hoc Exploratory Analyses
Comparisons of the baseline variables for the subgroups determined by whether patients were admitted to an ICU with low, medium, or high adherence to histamine-2 receptor blockers appear in eTables 65-67 in Supplement 2. There was no statistically significant heterogeneity of treatment response among the adherence subgroups during the histamine-2 receptor blocker treatment period for either the primary outcome or any of the secondary outcomes (eTable 68 in Supplement 2).
Within each illness severity quartile, baseline variables were similarly distributed by treatment group (eTables 69-72 in Supplement 2). There was statistically significant heterogeneity of treatment response with respect to the risk of death at the hospital by day 90 according to illness severity (P = .004 for interaction). Among patients with high illness severity, randomization to proton pump inhibitors was associated with an increased risk of in-hospital mortality compared with randomization to histamine-2 receptor blockers.
Although upper gastrointestinal bleeding rates increased as illness severity increased, there was no statistically significant heterogeneity of treatment response with respect to upper gastrointestinal bleeding risk according to illness severity. Subgroup analyses by illness severity quartiles for all primary and secondary outcome variables appear in Table 3.
Table 3. Post Hoc Subgroup Analyses by Quartiles of Illness Severity Based on APACHE II Scores.
| APACHE II Score Quartilea | No. of Patients |
Proton Pump Inhibitors |
Histamine-2 Receptor Blockers |
Estimate (95% CI) | P Value for Interaction |
|---|---|---|---|---|---|
| In-Hospital Mortality, No./Total No. (%) | |||||
| 1 (Score range: 0-13) | 7711 | 142/3872 (3.7) | 155/3839 (4.0) | RR, 0.92 (0.77-1.11) | .004 |
| 2 (Score range: 14-17) | 5860 | 211/2956 (7.1) | 219/2904 (7.5) | RR, 0.96 (0.86-1.08) | |
| 3 (Score range: 18-23) | 6475 | 565/3229 (17.5) | 477/3246 (14.7) | RR, 1.15 (1.05-1.25) | |
| 4 (Score range: 24-61) | 6610 | 1493/3296 (45.3) | 1440/3314 (43.5) | RR, 1.05 (1.00-1.11) | |
| Clinically Important Upper Gastrointestinal Bleeding, No./Total No. (%)b | |||||
| 1 (Score range: 0-13) | 7730 | 14/3881 (0.4) | 13/3849 (0.3) | RR, 0.99 (0.49-1.97) | .35 |
| 2 (Score range: 14-17) | 5879 | 19/2963 (0.6) | 32/2916 (1.1) | RR, 0.58 (0.32-1.07) | |
| 3 (Score range: 18-23) | 6487 | 40/3231 (1.2) | 67/3256 (2.1) | RR, 0.62 (0.40-0.97) | |
| 4 (Score range: 24-61) | 6617 | 99/3299 (3.0) | 127/3318 (3.8) | RR, 0.80 (0.63-1.01) | |
| Clostridium difficile Infection, No./Total No. (%)c | |||||
| 1 (Score range: 0-13) | 7730 | 6/3881 (0.2) | 14/3849 (0.4) | RR, 0.44 (0.17-1.15) | .04 |
| 2 (Score range: 14-17) | 5879 | 11/2963 (0.4) | 6/2916 (0.2) | RR, 1.90 (0.73-4.94) | |
| 3 (Score range: 18-23) | 6487 | 8/3231 (0.3) | 19/3256 (0.6) | RR, 0.47 (0.21-1.04) | |
| 4 (Score range: 24-61) | 6617 | 15/3299 (0.5) | 18/3318 (0.5) | RR, 0.88 (0.45-1.70) | |
| Days Until Discharge Alive From Index ICU Admission, Median (IQR)d | |||||
| 1 (Score range: 0-13) | 7726 | 1.8 (1.0-3.9) | 1.8 (1.0-3.8) | ROM, 1.02 (0.96-1.07)e | .60 |
| 2 (Score range: 14-17) | 5875 | 2.7 (1.2-5.6) | 2.6 (1.1-5.8) | ROM, 1.00 (0.95-1.04)e | |
| 3 (Score range: 18-23) | 6482 | 4.1 (2.0-11.0) | 3.9 (1.9-9.7) | ROM, 1.03 (0.98-1.08)e | |
| 4 (Score range: 24-61) | 6611 | 11.8 (4.0-NA)f | 11.5 (4.0-NA)f | ROM, 0.98 (0.92-1.05)e | |
| Days Until Discharge Alive From Index Hospitalization, Median (IQR)d | |||||
| 1 (Score range: 0-13) | 7719 | 7.0 (4.9-14.6) | 7.0 (4.9-14.5) | ROM, 1.02 (0.98-1.06)e | .81 |
| 2 (Score range: 14-17) | 5867 | 9.2 (5.9-21.1) | 9.2 (5.9-20.7) | ROM, 1.02 (0.96-1.07)e | |
| 3 (Score range: 18-23) | 6476 | 13.9 (6.9-39.0) | 14.3 (7.0-41.5) | ROM, 0.99 (0.94-1.04)e | |
| 4 (Score range: 24-61) | 6611 | 55.2 (13.5-NA)f | 47.9 (12.8-NA)f | ROM, 1.01 (0.95-1.07)e | |
Abbreviations: APACHE, Acute Physiology and Chronic Health Evaluation; ICU, intensive care unit; IQR, interquartile range; NA, not applicable; ROM, ratio of median time to discharge (or extubation); RR, risk ratio.
Scores range from 0 to 71; higher scores indicate more severe disease and a higher risk of death. No patients had an APACHE II score greater than 61.
Defined as overt upper gastrointestinal bleeding (eg, hematemesis, melaena, or frank blood in the nasogastric tube or upper gastrointestinal endoscopy) developing as a complication in the ICU and accompanied by 1 or more of the following features within 24 hours: (1) a spontaneous decline in systolic, diastolic, or mean arterial pressure of 20 mm Hg or greater; (2) initiation of a vasopressor or a 20% increase in dose of ongoing vasopressor; (3) a decrease in hemoglobin level of 20 g/L or greater; or (4) a transfusion of at least 2 U of packed red blood cells.
Defined as a new toxin or culture-positive stool sample collected during an ICU admission (excluding any patients who had positive test results from specimens collected prior to ICU admission).
Medians and IQRs are reported with mortality regarded as a competing risk. The ICU mortality rate for quartile 1 was 2.6% of patients who received proton pump inhibitors and it was 2.7% of patients who received histamine-2 receptor blockers; quartile 2: 5.3% and 5.4%, respectively; quartile 3, 13.3% and 10.3%; and quartile 4, 39.0% and 36.8%. The hospital mortality rate for quartile 1 was 3.7% of patients who received proton pump inhibitors and it was 4.1% of patients who received histamine-2 receptor blockers; quartile 2, 7.2% and 7.6%, respectively; quartile 3, 17.7% and 14.8%; and quartile 4, 45.7% and 43.7%.
Estimated using censored linear regression models with logarithm of time to discharge as the dependent variable. Patients who died were censored at their time of death. Adjustment was made for the same variables as for binary outcomes. Heterogeneity of variance by treatment group and country was accommodated for and robust standard errors were used to account for clustering by ICU.
Less than 75% of patients were discharged alive and so there is no 75th percentile available.
Discussion
In this international open-label, cluster crossover, registry-embedded randomized clinical trial, there was no statistically significant difference in all-cause mortality within 90 days during the index hospitalization for patients requiring mechanical ventilation within 24 hours of ICU admission when proton pump inhibitors were used as the default for stress ulcer prophylaxis compared with when histamine-2 receptor blockers were used.
Although nonadherence with study treatment is often considered to be random, that may not have been the case in this study. There was asymmetric nonadherence (with very little histamine-2 receptor blocker use in the proton pump inhibitor group), suggesting a systematic reason for nonadherence; there is a reasonable potential that physicians initiated treatment with proton pump inhibitors in certain patients in the histamine-2 receptor blocker group based on some assessment of expected benefit from proton pump inhibitors rather than histamine-2 receptor blockers in these patients. Although exploratory analyses did not find heterogeneity in the study results among sites stratified by differences in nonadherence rates, the possibility remains this crossover in use of assigned study treatment introduced bias.
Furthermore, the direction of the bias is difficult to anticipate. If use of proton pump inhibitors increases mortality, a high nonadherence rate could have attenuated what might otherwise have been a statistically significant increase in mortality risk with proton pump inhibitors. Alternatively, if the use of proton pump inhibitors can, perhaps through reduced risk of stress ulceration, improve survival in a select subset of patients, and clinicians correctly identified such patients and gave them a proton pump inhibitor when they were assigned an histamine-2 receptor blocker, this would have decreased mortality in the histamine-2 receptor blocker group, potentially contributing to the between-group difference observed in this study.
Fewer patients had clinically important gastrointestinal bleeding when proton pump inhibitors rather than histamine-2 receptor blockers were used as the default stress ulcer prophylaxis in the ICU. These findings are consistent with the findings from a meta-analysis6 of randomized clinical trials comparing proton pump inhibitors with histamine-2 receptor blockers. However, it is likely that a number of patients included in this trial were taking proton pump inhibitors prior to ICU admission, and rebound acid secretion occurring when these patients were switched from proton pump inhibitors to histamine-2 receptor blockers may have contributed to the excess risk of clinically important upper gastrointestinal bleeding observed in the histamine-2 receptor blocker group.
This trial did not reproduce the results of an observational study, which suggested increased risk of Clostridioides difficile infection in association with the use of proton pump inhibitors compared with histamine-2 receptor blockers.8 Clostridioides difficile infection was rarely reported in this trial. Some cases of Clostridioides difficile infection may not have been suspected by treating clinicians, meaning appropriate specimens were not sent to the laboratory and the actual cases of infection were not documented. In addition, only new-onset infections diagnosed in the ICU were recorded and the time window available to capture these infections was therefore narrow for many patients.
There was a statistically significant interaction between treatment group and in-hospital mortality by illness severity in a post hoc analysis. Among patients with high illness severity, randomization to proton pump inhibitors was associated with an increased risk of in-hospital mortality compared with randomization to histamine-2 receptor blockers. Although these findings should be considered hypothesis-generating, the potentially increased rates of mortality with proton pump inhibitors among patients with high illness acuity are consistent with exploratory analyses from a prior randomized clinical trial.20
The possibility that using proton pump inhibitors increases mortality attributable to hospital-acquired pneumonia cannot be excluded because rates of pneumonia were not measured in this study. However, because ICU and hospital lengths of stay, duration of mechanical ventilation, and ventilator-associated conditions were not significantly different by treatment group, it appears that if proton pump inhibitors do increase pneumonia rates in ICU patients compared with histamine-2 receptor blockers as suggested in observational studies,8,9,10 then either the clinical consequences of these pneumonia episodes with respect to duration of mechanical ventilation and length of stay are minor, are offset by other effects, or both.
Using a cluster crossover design combined with use of data from registry sources allowed us to recruit a large number of patients during a short time frame and provided sufficient statistical power to enable a precise range of plausible treatment effects. The findings from this study are broadly generalizable because enrolled patients were from 50 ICUs in 5 countries.
Limitations
This study has several limitations. First, some patients who were excluded from the trial because of an ICU admission diagnosis of upper gastrointestinal bleeding may have actually had lower gastrointestinal bleeding and some patients who were diagnosed as having upper gastrointestinal bleeding in the ICU may have already been bleeding at the time of ICU admission.
Second, only data from the index hospitalization were included. Third, because mortality data were obtained from registries, these data may contain random errors.
Fourth, clinicians and research staff were aware of treatment assignments. Although mortality rates are unlikely to be subject to bias as a result of this knowledge, such bias may have affected ascertainment of secondary outcomes including upper gastrointestinal bleeding.
Fifth, clinicians were allowed to use any proton pump inhibitor or histamine-2 receptor blocker and to choose the route of administration. A range of different drugs were used, increasing the generalizability of the findings. However, it is possible that a trial using different combinations of drugs or different routes of administration would have yielded different findings.
Conclusions
Among ICU patients requiring mechanical ventilation, a strategy of stress ulcer prophylaxis with use of proton pump inhibitors vs histamine-2 receptor blockers resulted in hospital mortality rates of 18.3% vs 17.5%, respectively, a difference that did not reach the significance threshold. However, study interpretation may be limited by crossover in the use of the assigned medication.
Trial protocol and statistical analysis plan
eMethods
eResults
eDiscussion
eReferences
Data sharing statement
Section Editor: Derek C. Angus, MD, MPH, Associate Editor, JAMA (angusdc@upmc.edu).
References
- 1.Krag M, Perner A, Wetterslev J, et al. ; SUP-ICU Coauthors . Prevalence and outcome of gastrointestinal bleeding and use of acid suppressants in acutely ill adult intensive care patients. Intensive Care Med. 2015;41(5):833-845. doi: 10.1007/s00134-015-3725-1 [DOI] [PubMed] [Google Scholar]
- 2.Krag M, Marker S, Perner A, et al. ; SUP-ICU Trial Group . Pantoprazole in patients at risk for gastrointestinal bleeding in the ICU. N Engl J Med. 2018;379(23):2199-2208. doi: 10.1056/NEJMoa1714919 [DOI] [PubMed] [Google Scholar]
- 3.Barbateskovic M, Marker S, Granholm A, et al. Stress ulcer prophylaxis with proton pump inhibitors or histamin-2 receptor antagonists in adult intensive care patients: a systematic review with meta-analysis and trial sequential analysis. Intensive Care Med. 2019;45(2):143-158. doi: 10.1007/s00134-019-05526-z [DOI] [PubMed] [Google Scholar]
- 4.Litton E, Eastwood GM, Bellomo R, et al. A multicentre feasibility study evaluating stress ulcer prophylaxis using hospital-based registry data. Crit Care Resusc. 2014;16(3):158-163. [PubMed] [Google Scholar]
- 5.Eastwood GM, Litton E, Bellomo R, et al. Opinions and practice of stress ulcer prophylaxis in Australian and New Zealand intensive care units. Crit Care Resusc. 2014;16(3):170-174. [PubMed] [Google Scholar]
- 6.Alhazzani W, Alenezi F, Jaeschke RZ, Moayyedi P, Cook DJ. Proton pump inhibitors versus histamine 2 receptor antagonists for stress ulcer prophylaxis in critically ill patients: a systematic review and meta-analysis. Crit Care Med. 2013;41(3):693-705. doi: 10.1097/CCM.0b013e3182758734 [DOI] [PubMed] [Google Scholar]
- 7.Vincent JL, Marshall JC, Namendys-Silva SA, et al. ; ICON Investigators . Assessment of the worldwide burden of critical illness: the intensive care over nations (ICON) audit. Lancet Respir Med. 2014;2(5):380-386. doi: 10.1016/S2213-2600(14)70061-X [DOI] [PubMed] [Google Scholar]
- 8.MacLaren R, Reynolds PM, Allen RR. Histamine-2 receptor antagonists vs proton pump inhibitors on gastrointestinal tract hemorrhage and infectious complications in the intensive care unit. JAMA Intern Med. 2014;174(4):564-574. doi: 10.1001/jamainternmed.2013.14673 [DOI] [PubMed] [Google Scholar]
- 9.Miano TA, Reichert MG, Houle TT, MacGregor DA, Kincaid EH, Bowton DL. Nosocomial pneumonia risk and stress ulcer prophylaxis: a comparison of pantoprazole vs ranitidine in cardiothoracic surgery patients. Chest. 2009;136(2):440-447. doi: 10.1378/chest.08-1634 [DOI] [PubMed] [Google Scholar]
- 10.Bateman BT, Bykov K, Choudhry NK, et al. Type of stress ulcer prophylaxis and risk of nosocomial pneumonia in cardiac surgical patients: cohort study. BMJ. 2013;347:f5416. doi: 10.1136/bmj.f5416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kedika RR, Souza RF, Spechler SJ. Potential anti-inflammatory effects of proton pump inhibitors: a review and discussion of the clinical implications. Dig Dis Sci. 2009;54(11):2312-2317. doi: 10.1007/s10620-009-0951-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Aybay C, Imir T, Okur H. The effect of omeprazole on human natural killer cell activity. Gen Pharmacol. 1995;26(6):1413-1418. doi: 10.1016/0306-3623(94)00301-3 [DOI] [PubMed] [Google Scholar]
- 13.Capodicasa E, De Bellis F, Pelli MA. Effect of lansoprazole on human leukocyte function. Immunopharmacol Immunotoxicol. 1999;21(2):357-377. doi: 10.3109/08923979909052768 [DOI] [PubMed] [Google Scholar]
- 14.Young PJ, Bagshaw SM, Forbes A, et al. ; Australian and New Zealand Intensive Care Society Clinical Trials Group on behalf of the PEPTIC Investigators . A cluster randomised, crossover, registry-embedded clinical trial of proton pump inhibitors versus histamine-2 receptor blockers for ulcer prophylaxis therapy in the intensive care unit (PEPTIC study): study protocol. Crit Care Resusc. 2018;20(3):182-189. [PubMed] [Google Scholar]
- 15.US Centers for Disease Control and Prevention Device associated module: ventilator associated events. https://www.cdc.gov/nhsn/PDFs/pscManual/10-VAE_FINAL.pdf. Accessed October 11, 2019.
- 16.PEPTIC Investigators Statistical analysis plan for the PEPTIC study (version 1). http://wellingtonicu.com/PubResPres/Protocols/. Accessed October 11, 2019.
- 17.Forbes AB, Akram M, Pilcher D, Cooper J, Bellomo R. Cluster randomised crossover trials with binary data and unbalanced cluster sizes: application to studies of near-universal interventions in intensive care. Clin Trials. 2015;12(1):34-44. doi: 10.1177/1740774514559610 [DOI] [PubMed] [Google Scholar]
- 18.Begg MD, Parides MK. Separation of individual-level and cluster-level covariate effects in regression analysis of correlated data. Stat Med. 2003;22(16):2591-2602. doi: 10.1002/sim.1524 [DOI] [PubMed] [Google Scholar]
- 19.Localio AR, Margolis DJ, Berlin JA. Relative risks and confidence intervals were easily computed indirectly from multivariable logistic regression. J Clin Epidemiol. 2007;60(9):874-882. doi: 10.1016/j.jclinepi.2006.12.001 [DOI] [PubMed] [Google Scholar]
- 20.Marker S, Perner A, Wetterslev J, et al. ; SUP-ICU Investigators . Pantoprazole prophylaxis in ICU patients with high severity of disease: a post hoc analysis of the placebo-controlled SUP-ICU trial. Intensive Care Med. 2019;45(5):609-618. doi: 10.1007/s00134-019-05589-y [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial protocol and statistical analysis plan
eMethods
eResults
eDiscussion
eReferences
Data sharing statement