Key Points
Question
Does treatment with vitamin C, hydrocortisone, and thiamine lead to a more rapid resolution of septic shock compared with hydrocortisone alone?
Findings
In this randomized clinical trial that included 216 patients with septic shock, treatment with intravenous vitamin C, hydrocortisone, and thiamine, compared with intravenous hydrocortisone alone, did not significantly improve the duration of time alive and free of vasopressor administration over 7 days (122.1 hours vs 124.6 hours, respectively).
Meaning
The findings suggest that treatment with intravenous vitamin C, hydrocortisone, and thiamine does not lead to a more rapid resolution of septic shock compared with intravenous hydrocortisone alone.
Abstract
Importance
It is unclear whether vitamin C, hydrocortisone, and thiamine are more effective than hydrocortisone alone in expediting resolution of septic shock.
Objective
To determine whether the combination of vitamin C, hydrocortisone, and thiamine, compared with hydrocortisone alone, improves the duration of time alive and free of vasopressor administration in patients with septic shock.
Design, Setting, and Participants
Multicenter, open-label, randomized clinical trial conducted in 10 intensive care units in Australia, New Zealand, and Brazil that recruited 216 patients fulfilling the Sepsis-3 definition of septic shock. The first patient was enrolled on May 8, 2018, and the last on July 9, 2019. The final date of follow-up was October 6, 2019.
Interventions
Patients were randomized to the intervention group (n = 109), consisting of intravenous vitamin C (1.5 g every 6 hours), hydrocortisone (50 mg every 6 hours), and thiamine (200 mg every 12 hours), or to the control group (n = 107), consisting of intravenous hydrocortisone (50 mg every 6 hours) alone until shock resolution or up to 10 days.
Main Outcomes and Measures
The primary trial outcome was duration of time alive and free of vasopressor administration up to day 7. Ten secondary outcomes were prespecified, including 90-day mortality.
Results
Among 216 patients who were randomized, 211 provided consent and completed the primary outcome measurement (mean age, 61.7 years [SD, 15.0]; 133 men [63%]). Time alive and vasopressor free up to day 7 was 122.1 hours (interquartile range [IQR], 76.3-145.4 hours) in the intervention group and 124.6 hours (IQR, 82.1-147.0 hours) in the control group; the median of all paired differences was –0.6 hours (95% CI, –8.3 to 7.2 hours; P = .83). Of 10 prespecified secondary outcomes, 9 showed no statistically significant difference. Ninety-day mortality was 30/105 (28.6%) in the intervention group and 25/102 (24.5%) in the control group (hazard ratio, 1.18; 95% CI, 0.69-2.00). No serious adverse events were reported.
Conclusions and Relevance
In patients with septic shock, treatment with intravenous vitamin C, hydrocortisone, and thiamine, compared with intravenous hydrocortisone alone, did not significantly improve the duration of time alive and free of vasopressor administration over 7 days. The finding suggests that treatment with intravenous vitamin C, hydrocortisone, and thiamine does not lead to a more rapid resolution of septic shock compared with intravenous hydrocortisone alone.
Trial Registration
ClinicalTrials.gov Identifier: NCT03333278
This randomized clinical trial compares the effects of treatment with intravenous vitamin C, hydrocortisone, and thiamine compared with intravenous hydrocortisone alone on duration of time alive and free of vasopressor use over 7 days in patients with septic shock.
Introduction
Sepsis is a life-threatening illness characterized by a dysregulated host response to infection.1 It causes or contributes to between one-third and half of all hospital deaths2 and is responsible for more than 5 million deaths worldwide each year.3 Patients with septic shock are an important sepsis subgroup and have circulatory and metabolic abnormalities that substantially increase their mortality risk.4 For these patients in particular, new treatments that improve outcomes are a global public health priority.
High-dose intravenous (IV) vitamin C has recently been explored as an adjunctive therapy in sepsis because of its anti-inflammatory and antioxidant properties.5,6,7,8 A previous randomized trial of 24 patients showed that high-dose IV vitamin C attenuated organ failure associated with sepsis in a dose-dependent manner.9 Thiamine deficiency has also been reported in 20% of critically ill patients with sepsis,10 and thiamine supplementation has been shown to improve lactate clearance in patients with sepsis.11,12 The combination of high-dose IV vitamin C and hydrocortisone together with thiamine was assessed in a single-center retrospective before-and-after study of 94 patients with severe sepsis or septic shock.13 The intervention was associated with shorter duration of vasopressor administration and lower hospital mortality.13 However, hydrocortisone alone has also consistently demonstrated efficacy in hastening the resolution of shock compared with placebo in 2 large multicenter double-blind trials.14,15 It is unclear whether the combination of vitamin C, hydrocortisone, and thiamine is more effective than hydrocortisone alone.
Accordingly, this trial examined the effects of vitamin C, hydrocortisone, and thiamine combination therapy on vasopressor requirements compared with hydrocortisone monotherapy in patients with septic shock. The trial aimed to test the hypothesis that treatment with combination therapy would increase time alive and free of vasopressors compared with hydrocortisone alone.
Methods
Study Design
The Vitamin C, Hydrocortisone and Thiamine in Patients With Septic Shock (VITAMINS) trial was an investigator-initiated, multicenter, open-label, parallel-group randomized trial conducted in 10 intensive care units in Australia, New Zealand, and Brazil. The management committee developed the trial protocol with a predefined statistical analysis plan (Supplement 1), which was published before study recruitment was completed.16
Ethical approval was obtained from local ethics committees for all study sites and from Monash University, Melbourne, Australia. Written informed consent for enrollment or consent to continue and use patient data was obtained from each patient or their legal surrogate. If a patient died before consent to continue could be obtained from the patient or the legal surrogate, the patient’s data were included if the relevant ethics committee approved this.
Study Population
Patients admitted to a study intensive care unit (ICU) with a primary diagnosis of septic shock were screened for eligibility. All diagnostic criteria for septic shock based on the Sepsis-3 consensus1 had to be fulfilled within a maximum of 24 hours prior to enrollment. In brief, patients had suspected or documented infection with an acute increase of at least 2 points in the Sequential Organ Failure Assessment (SOFA) score,17 had a lactate level greater than 2 mmol/L, and were vasopressor dependent for at least 2 hours at the time of enrollment. Exclusion criteria included age younger than 18 years, a do-not-resuscitate order, imminent death, diagnosis of septic shock longer than 24 hours ago, known or suspected disease with a strong indication or contraindication for any of the study drugs, and another indication for hydrocortisone than septic shock. A list of exclusion criteria is provided in eAppendix 1 in Supplement 2.
Study Randomization and Treatment
Randomization and Allocation Concealment
Patients in the trial were randomly assigned to the intervention group or the control group. The random allocation sequence was generated at the coordinating center using computer-generated random numbers with permuted block sizes of 2, 4, and 6 in a 1:1 ratio stratified by site. The sequence was then embedded into the Research Electronic Data Capture (REDCap) system, a secure web application for managing online data collection.18 Randomization was performed using the REDCap system at each study site with the concealed allocation sequence.
Interventions
Patients in the intervention group received IV vitamin C (1.5 g every 6 hours), hydrocortisone (50 mg every 6 hours), and thiamine (200 mg every 12 hours). Patients in the control group received IV hydrocortisone (50 mg every 6 hours). Because administration of IV vitamin C is not usual practice in Australian, New Zealand, or Brazilian ICUs, administration of IV vitamin C to those randomized to the control group was not allowed. However, thiamine administration in the control group was allowed at the discretion of attending ICU clinicians. This trial was an open-label study; accordingly, all site personnel were aware of study interventions assigned to participants. The study intervention continued until cessation of vasopressor administration or when any of the other criteria for stopping the study intervention were met (eAppendix 2 in Supplement 2). Cessation of vasopressor administration was defined as discontinuation of all vasopressor drugs for 4 consecutive hours in the presence of a mean arterial pressure greater than 65 mm Hg or achievement of a target mean arterial pressure set by the treating clinician. Investigators and research coordinators collected data at the trial sites. All data entry was monitored at the coordinating center, with site visits for source data verification.
Outcome Measures
The primary outcome was time alive and free of vasopressors at day 7 (168 hours) after randomization. This was defined as the time, censored at 7 days, that a patient was both alive and had not received vasopressors for at least 4 hours. If a patient died while receiving vasopressor therapy following the index episode of septic shock, the patient was assigned zero hours for this outcome. If a patient was weaned from all vasopressors for 4 consecutive hours, then all of the remaining time through day 7 was treated as success, even if the patient died or had vasopressors restarted after weaning within the 7-day period.
Secondary outcomes were 28-day, 90-day, ICU, and hospital mortality, 28-day cumulative vasopressor-free days, 28-day cumulative mechanical ventilation-free days, 28-day renal replacement therapy–free days, change in SOFA score17 at day 3, 28-day ICU free-days, and hospital length of stay. SOFA scores in the trial ranged from 0 (normal organ function) to 20 (worst organ dysfunction). Cardiovascular, coagulation, liver, renal, and respiratory components were summed. Acute kidney injury, defined by Kidney Disease: Improving Global Outcomes (KDIGO) criteria,19 and vasopressor dose over 10 days were also prespecified as exploratory outcomes.16 Recurrence of vasopressor dependency after being free of vasopressors for at least 4 consecutive hours contributed to 28-day cumulative vasopressor-free days and vasopressor dose over 10 days. Detailed definitions of the outcomes are provided in eAppendix 3 in Supplement 2.
With a view to informing the design of a subsequent larger trial powered to detect a mortality difference, a number of feasibility outcomes, which are outlined in eAppendix 3 in Supplement 2, were also prespecified.
Post hoc analyses were performed to further explain the results. The outcomes included death or vasopressor redependence by day 7, duration of vasopressors, and change in SOFA score over the first 7 days. Full details are provided in eAppendix 4 in Supplement 2.
Statistical Analysis
Initial sample size calculations suggested that 126 patients were required based on an SD of 42 vasopressor-free hours up to day 7.13 In the absence of accurate, current data, the estimation of the SD was updated from the pooled SD for the first 60 patients enrolled in the study, and the required sample size was recalculated. Based on an updated SD of 51.6 hours, the study was estimated to require 180 patients to have 90% power (2-sided α = .05) to detect a difference in vasopressor-free hours of 25. The difference of 25 hours was two-thirds of the effect estimate reported in the previous study13 and was considered plausible as a clinically minimally important difference (>1 day) for time alive and free of vasopressors. As the distribution of the primary outcome was expected to be nonparametric, and nonparametric tests have been shown to have decreased statistical power compared with parametric tests, the sample size was inflated by 15%.20 To further account for consent withdrawal (5%), 216 patients were planned to be enrolled. The robustness of the sample size estimation was further confirmed with the same method after recruitment of 108 patients (Supplement 3).
All analyses were conducted in accordance with the published statistical analysis plan.16 Patient data were analyzed according to their randomization group, excluding those who withdrew consent. Missing data were not imputed, and the numbers of patients with available data are reported. Group comparisons were made using χ2 tests for equal proportions, t tests for normally distributed data, and Wilcoxon rank sum tests otherwise, with results presented as frequencies with percentages, means with SDs, and medians with interquartile ranges (IQRs), respectively.
Primary outcome data were analyzed using a Wilcoxon rank sum test and presented using the Hodges-Lehmann estimator of the median of all paired differences with 95% confidence intervals. A multivariable sensitivity analysis was conducted using quantile regression adjusting for site and baseline imbalance (Acute Physiology and Chronic Health Evaluation [APACHE] III score, lactate levels, white blood cell counts, and milrinone use), with results reported as differences of medians with 95% confidence intervals. Quantile regression using a simplex algorithm with confidence intervals determined by inversion of rank-score tests was also used to determine effect estimates for continuous secondary outcomes.
Epinephrine and vasopressin doses were converted to equivalent norepinephrine doses.21 Vasopressor use over the first 10 days was log-transformed and analyzed using mixed linear modeling clustered at the individual patient level, fitting main effects for treatment and time and interaction between the 2 to examine the difference in vasopressor dose over time, with results reported as medians, IQRs, and ranges in a box plot. Patient survival time was analyzed using Cox proportional hazards regression, with results reported as hazard ratios with 95% confidence intervals and presented using Kaplan-Meier curves with a log-rank test for comparison. Proportional hazards assumptions were confirmed by determining the linearity of an interaction between treatment and the logarithm of survival time.
Post hoc analysis of the duration of vasopressor use was assessed using Cox proportional hazards regression, censoring patients who died before resolution of shock at the time of death and including site as a random effect to account for within-cluster variability, with results reported as hazard ratios with 95% confidence intervals comparing the probability of becoming free from vasopressors between the 2 groups. Proportional hazards assumptions were confirmed by determining the linearity of an interaction between treatment and the logarithm of time to vasopressor liberation.
Post hoc subgroup analysis for the primary outcome was performed on subgroups determined from baseline variables, which were lactate level, SOFA score, vasopressor dose, and hydrocortisone administration prior to enrollment. All other details of post hoc analyses are described in eAppendix 4 in Supplement 2.
All analyses were performed using SAS version 9.4 (SAS Institute Inc), and a 2-tailed P < .05 was used to indicate statistical significance. Because of the potential for type I error due to multiple comparisons, findings for analyses of secondary outcomes should be interpreted as exploratory.
Results
Patient Characteristics
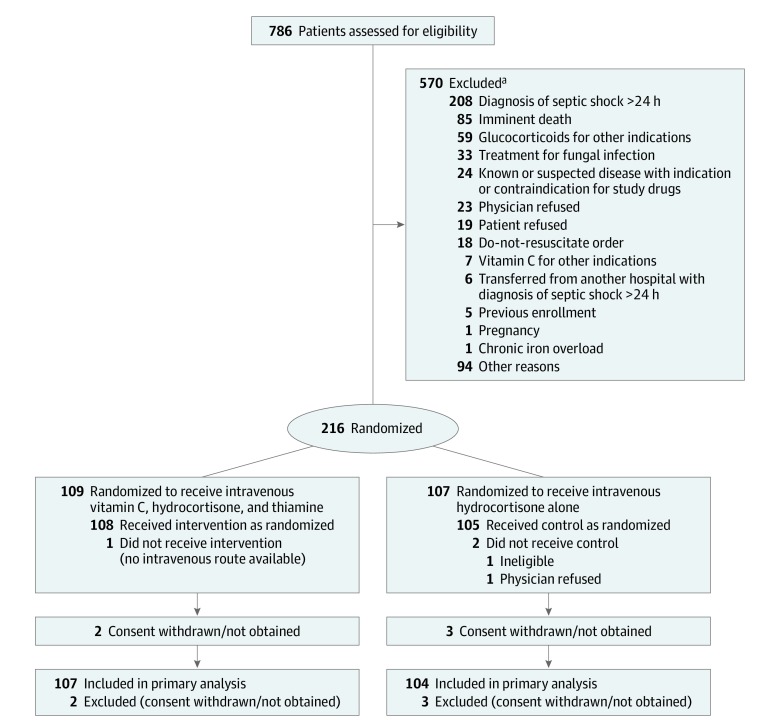
From May 2018 to July 2019, we screened 786 patients from 10 ICUs in Australia, New Zealand, and Brazil. A total of 216 were randomized. Five patients (2.3%; 2 in the intervention group and 3 in the control group) either withdrew or refused consent to continue participation and withdrew all data, leaving 211 patients (mean age, 61.7 years [SD, 15.0 years]; 133 men [63.0%] and 78 women [37.0%]). One hundred seven patients in the intervention group and 104 patients in the control group were included in the analysis for the primary outcome (Figure 1). One patient in the control group withdrew consent for follow-up at 28 days and 90 days. Three patients (2 in the intervention group and 1 in the control group) were lost to follow-up by day 90. At baseline, patients in the intervention group had lower APACHE III scores, had higher lactate and white blood cell counts, and were more likely to have received milrinone (Table 1). The primary sites of infection were predominantly pulmonary and gastrointestinal in the 2 groups (Table 1).
Figure 1. Flow of Participants in the Vitamin C, Hydrocortisone, and Thiamine in Patients With Septic Shock (VITAMINS) Trial.
aMultiple reasons for exclusion were possible.
Table 1. Baseline Participant Characteristics.
| Characteristics | Intervention (n = 107) | Control (n = 104) |
|---|---|---|
| Age, mean (SD), y | 61.9 (15.9) | 61.6 (13.9) |
| Sex, No. (%) | ||
| Men | 68 (63.6) | 65 (62.5) |
| Women | 39 (36.4) | 39 (37.5) |
| Weight, median (IQR), kg | 81.0 (66.0-95.0) | 83.0 (67.5-102.0) |
| ICU admission source, No. (%) | ||
| Emergency department | 49 (45.8) | 49 (47.1) |
| Operating room after emergency surgery | 20 (18.7) | 14 (13.5) |
| Hospital ward | 17 (15.9) | 20 (19.2) |
| Transfer from another hospital | 13 (12.1) | 10 (9.6) |
| Operating room after elective surgery | 4 (3.7) | 7 (6.7) |
| Transfer from another ICU | 4 (3.7) | 4 (3.8) |
| Chronic health condition, No. (%) | ||
| Diabetes mellitus | 22 (20.6) | 28 (26.9) |
| Chronic renal failurea | 5 (4.7) | 9 (8.7) |
| Hydrocortisone for septic shock before randomization, No. (%) | 45 (42.1) | 39 (37.5) |
| Intervention at randomization, No. (%) | ||
| Mechanical ventilation | 66 (61.7) | 65 (62.5) |
| Vasopressorsb | ||
| Norepinephrine | 99 (92.5) | 97 (93.3) |
| Vasopressin | 22 (20.6) | 22 (21.2) |
| Epinephrine | 13 (12.1) | 9 (8.7) |
| Metaraminol | 8 (7.5) | 10 (9.6) |
| Inotropesc | ||
| Milrinone | 6 (5.6) | 2 (1.9) |
| Renal replacement therapy | 12 (11.2) | 12 (11.5) |
| Physiological variables | ||
| White blood cell count, mean (SD), ×103/μLd | 17.5 (11.3) | 15.3 (10.4) |
| Platelet count, median (IQR), ×103/μLe | 162 (104-239) [n = 106] | 173 (107-251) [n = 103] |
| Lactate, median (IQR), mmol/Lf | 4.2 (2.8-5.9) | 3.3 (2.6-4.9) |
| Serum creatinine, median (IQR), mg/dLg | 1.73 (1.16-2.64) | 1.78 (1.07-2.90) |
| Acute kidney injury, No. (%)h | 74 (69.2) | 75 (72.1) |
| Stage 1 (mild) | 27 | 32 |
| Stage 2 (moderate) | 34 | 23 |
| Stage 3 (severe) | 13 | 20 |
| APACHE III score, mean (SD)i | 77.4 (29.7) | 83.3 (28.8) |
| SOFA score, mean (SD)j | 8.6 (2.7) | 8.4 (2.7) |
| Primary site of infection, No. (%) | ||
| Pulmonary | 31 (29.0) | 33 (31.7) |
| Gastrointestinal | 31 (29.0) | 31 (29.8) |
| Urinary | 18 (16.8) | 14 (13.5) |
| Skin or soft tissue | 14 (13.1) | 15 (14.4) |
| Blood | 9 (8.4) | 2 (1.9) |
| Otherk | 4 (3.7) | 9 (8.7) |
| Hospital-acquired infection, No. (%) | 18 (16.8) | 13 (12.5) |
| Time from ICU admission to randomization, median (IQR), h | 13.7 (7.1-19.3) | 11.4 (5.5-17.8) |
Abbreviations: APACHE, Acute Physiology and Chronic Health Evaluation; ICU, intensive care unit; IQR, interquartile range; SOFA, Sequential Organ Failure Assessment.
Pre-ICU glomerular filtration rate less than 30 mL/min/1.73 m2.
Some patients received more than 1 vasopressor. No patients were receiving dopamine or phenylephrine.
No patients were receiving dobutamine or levosimendan.
Highest value within 24 hours prior to randomization.
Lowest value within 24 hours prior to randomization.
Highest value, either arterial or venous, within 24 hours prior to randomization.
Highest value within 24 hours prior to randomization. To convert creatinine to μmol/L, multiply by 88.4.
As defined by Kidney Disease: Improving Global Outcomes (KDIGO) criteria.19 The 3 stages of acute kidney injury severity are defined on the basis of increases in serum creatinine from baseline levels.
APACHE III scores range from 0 (low severity of illness) to 299 (high severity of illness). The risk of death calculated using the APACHE III score (mean, 37% [SD, 27%]) indicated that the study population was seriously ill among the patients in the ICU.
SOFA scores range from 0 (normal organ function) to 20 (worst organ dysfunction). Cardiovascular, coagulation, liver, renal, and respiratory components were summed. The mean scores of 8.6 and 8.4 in the 2 groups indicated that the study population had moderate to severe organ dysfunction.
Other site of infection included unknown source.
Study Treatment
At least 1 dose of the assigned study regimen was administered to 106 of 107 patients (99.1%) in the intervention group and 102 of 104 (98.1%) in the control group. The median time from meeting eligibility criteria to the first dose of vitamin C in the intervention group was 12.1 hours (IQR, 5.7-19.0 hours), and that of hydrocortisone in the control group was 8.9 hours (IQR, 4.0-15.0 hours). Patients in the intervention group received study treatment for a mean of 3.4 days (SD, 2.1 days) and patients in the control group for a mean of 3.4 days (SD, 2.2 days). Detailed results of protocol adherence are reported in eAppendix 5 in Supplement 2.
Primary Outcome
There was no significant difference in time alive and free of vasopressors up to day 7 (168 hours) after randomization between the intervention group and the control group (median, 122.1 hours [IQR, 76.3-145.4 hours] vs 124.6 hours [IQR, 82.1-147.0 hours], respectively; median of all paired differences between groups, –0.6 hours [95% CI, –8.3 to 7.2 hours]; P = .83) (Table 2).
Table 2. Primary and Secondary Outcomes.
| Outcomes | Intervention (n = 107) | Control (n = 104) | Difference (95% CI) | P Value |
|---|---|---|---|---|
| Primary Outcome | ||||
| Time alive and free of vasopressors, median (IQR), h | 122.1 (76.3 to 145.4) | 124.6 (82.1 to 147.0) | –0.6 (–8.3 to 7.2)a | .83 |
| Secondary Outcomes | ||||
| 28-d Mortality, No. (%) | 24 (22.6) [n = 106] | 21 (20.4) [n = 103] | 2.3 (–8.9 to 13.4) | .69 |
| 90-d Mortality, No. (%) | 30 (28.6) [n = 105] | 25 (24.5) [n = 102] | 4.1 (–8.0 to 16.1) | .51 |
| ICU mortality, No. (%) | 21 (19.6) | 19 (18.3) | 1.4 (–9.2 to 11.9) | .80 |
| Hospital mortality, No. (%) | 25 (23.4) | 21 (20.4) [n = 103] | 3.0 (–8.2 to 14.1) | .60 |
| 28-d Cumulative vasopressor-free days, median (IQR) | 25.6 (17.8 to 26.8) [n = 106] | 25.8 (19.6 to 26.8) [n = 103] | –0.2 (–1.7 to 1.2) | .66 |
| 28-d Cumulative mechanical ventilation-free days, median (IQR) | 25.3 (5.2 to 28.0) [n = 106] | 24.8 (9.5 to 28.0) [n = 103] | 0.4 (–2.6 to 3.4) | .73 |
| 28-d Renal replacement therapy–free days, median (IQR) | 28.0 (23.5 to 28.0) [n = 105] | 28.0 (21.0 to 28.0) [n = 103] | 0.0 (–0.6 to 0.6) | .71 |
| Change in SOFA score at day 3, median (IQR)b | –2 (–4 to 0) [n = 82] | –1 (–3 to 0) [n = 75] | –1.0 (–1.9 to –0.1) | .02 |
| 28-d ICU-free days, median (IQR) | 21.9 (0 to 25.8) [n = 106] | 22.1 (3.9 to 25.8) [n = 103] | –0.2 (–4.1 to 3.7) | .66 |
| Hospital length of stay, median (IQR), d | 12.3 (6.2 to 26.0) | 12.3 (6.2 to 26.1) [n = 103] | 0.0 (–4.9 to 4.9) | .75 |
| Prespecified Exploratory Outcome | ||||
| Acute kidney injury, No. (%) | ||||
| Stage 1 | 18 (16.8) | 14 (13.5) | 3.4 (–6.3 to 13.0) | .80 |
| Stage 2 | 18 (16.8) | 22 (21.2) | –4.3 (–14.9 to 6.2) | |
| Stage 3 | 39 (36.4) | 39 (37.5) | –1.1 (–14.1 to 12.0) | |
Abbreviations: ICU, intensive care unit; IQR, interquartile range; SOFA, Sequential Organ Failure Assessment.
Hodges-Lehmann estimate, the median of all paired differences between observations in the intervention group minus the control group.
Change in SOFA score: score at day 3 minus score at baseline.
Secondary Outcomes
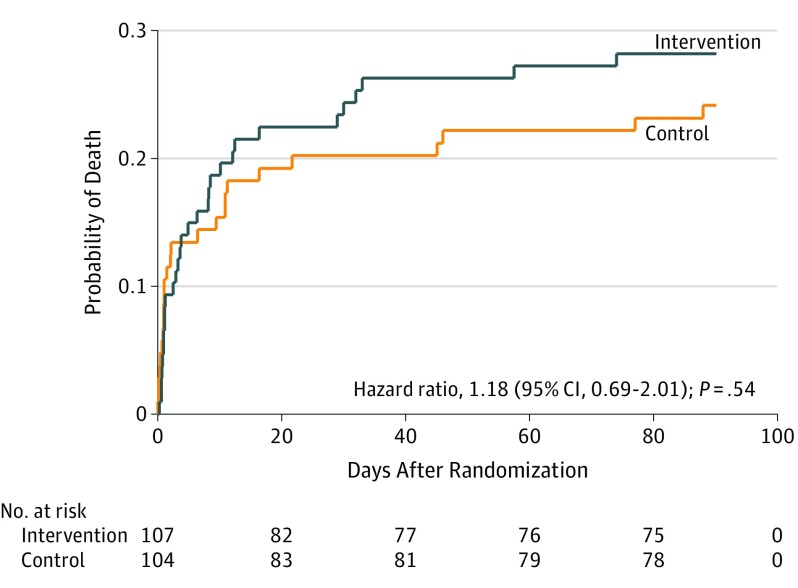
There was no significant between-group difference in all-cause mortality at 28 days after randomization (intervention, 22.6%, vs control, 20.4%; difference, 2.3%; 95% CI, –8.9% to 13.4%; P = .69) or at 90 days after randomization (intervention, 28.6%, vs control, 24.5%; difference, 4.1%; 95% CI, –8.0% to 16.1%; P = .51), or in the number of patients who survived to discharge from the ICU or the hospital (Table 2). Similarly, there was no statistically significant between-group difference in terms of 28-day cumulative vasopressor-free days, 28-day cumulative mechanical ventilation–free days, or 28-day cumulative renal replacement therapy–free days (Table 2). Change in SOFA score at day 3 was significantly greater in the intervention group than in the control group (median, –2 [IQR, –4 to 0] vs –1 [IQR, –3 to 0], respectively; difference, –1.0 [95% CI, –1.9 to –0.1]; P = .02) (Table 2). There was no statistically significant difference in 28-day ICU-free days or hospital length of stay (Table 2). Kaplan-Meier curves for the estimation of incidence of death were plotted (Figure 2), and the hazard ratio of death (intervention vs control) was 1.18 (95% CI, 0.69-2.01; P = .54). The maximum stage of acute kidney injury during the first 7 days after randomization (Table 2) and the vasopressor dose during the first 10 days were not significantly different between the 2 groups (ratio of geometric means for intervention vs control, 0.93; 95% CI, 0.65-1.32; P = .65) (Figure 3).
Figure 2. Kaplan-Meier Analysis by Randomization Group.
Proportionality assumptions were met (P = .33 for interaction of the randomization group with logarithm of time). Overall incidence of death was not significantly different between the groups (log-rank P = .55).
Figure 3. Vasopressor Use During the First 10 Days of the Trial.
Use of vasopressors was defined as any use of norepinephrine, epinephrine, vasopressin, metaraminol, dopamine, or phenylephrine. Data on doses of vasopressors were obtained every 6 hours, and the 4 doses per day were summed for the vasopressor dose on that study day. Total vasopressor doses was calculated as the sum of norepinephrine doses and converted doses of epinephrine and vasopressin. Patients receiving metaraminol monotherapy did not contribute to total vasopressor dose data, and no patients received dopamine or phenylephrine. Box center lines are medians, box tops and bottoms are interquartile ranges, and error bars are ranges. The trajectory curves connect the daily medians.
Sensitivity Analysis for the Primary Outcome
Multivariable sensitivity analysis using quantile regression adjusting for site and baseline imbalance (APACHE III score, lactate levels, white blood cell counts, and use of milrinone) confirmed the robustness of the effect estimates in the primary analysis (median of differences, –4.6 hours; 95% CI, –15.7 to 6.5 hours; P = .41).
Adverse Events
Adverse events were reported for 2 patients (2 events, fluid overload and hyperglycemia) in the intervention group and 1 patient (1 event, gastrointestinal bleeding) in the control group. No serious adverse events or suspected unexpected serious adverse reactions were reported (eAppendix 6 in Supplement 2).
Post Hoc Analysis
There was no significant difference between groups for death (intervention, 15.9%, vs control, 14.4%; P = .77) or vasopressor redependence (intervention, 33.3%, vs control, 26.7%; P = .33) by day 7. One patient from each group died between the index cessation of vasopressors and day 7. When considering duration of vasopressors accounting for death, there was no significant difference between groups for the probability of becoming free from vasopressors (hazard ratio for intervention vs control, 0.90; 95% CI, 0.67-1.21; P = .48). The results of the post hoc analyses are reported in eAppendix 7 in Supplement 2.
Discussion
In this multicenter, international, open-label, randomized clinical trial of patients with septic shock, the combination of IV vitamin C, hydrocortisone, and thiamine compared with hydrocortisone alone did not significantly affect the time alive and free of vasopressor support up to day 7.
While this study was not powered to detect any difference in secondary outcomes, mortality during any observation period and artificial organ support were not significantly different. The statistically significant difference in change in SOFA score at day 3 should be cautiously interpreted considering that there were 10 secondary outcomes without adjustment for multiple comparisons. The outcome was measured only in patients who were alive in the ICU on day 3, which was subject to the bias of competing risks in opposite directions, ie, early discharge from the ICU due to recovery or death. Furthermore, the other outcomes failed to support the observed beneficial effect. Effect estimates for mortality during any observation period point toward unfavorable effects in the intervention group; however, in light of having multiple secondary outcomes, all of which were underpowered, and the lack of evidence to support a harmful effect of the intervention, these findings should not be overinterpreted.
This trial provided the intervention for a longer period (ie, up to 10 days) than the previous observational study, which assessed the effect of 4 days of therapy.13 This provided a sufficient treatment period for the intervention to have any potential effect. No serious adverse events were reported. This trial also demonstrated that administration of vitamins in addition to hydrocortisone during the early phase of septic shock is feasible. The intervention was delivered for longer than defined in the protocol to some patients in the intervention group because of the logistics of applying the definition of shock resolution at the bedside. The extended duration of the intervention might have increased separation between the 2 groups, potentially overestimating any effect size. However, such overestimation results in bias only when the intervention shows benefit or harm, which was not the case for this trial.
The design of this trial was different from previous trials of vitamin C for sepsis in several aspects. This trial enrolled patients with septic shock within 24 hours of diagnosis to maximize the possible effects of the intervention.22 A recent placebo-controlled multicenter randomized trial (CITRIS-ALI) included 167 patients with sepsis who developed acute respiratory distress syndrome and examined the effect of IV vitamin C (50 mg/kg every 6 hours for 96 hours) on modified SOFA score and on biological markers of inflammation and vascular injury.23 The trial did not show any significant effect on changes in modified SOFA score (the primary outcome) or ventilator-free days but reported a lower 28-day mortality rate and higher number of ICU-free days up to day 28 and hospital-free days up to day 60 in the vitamin C group. However, the level of statistical significance for 46 such secondary outcomes was not adjusted for multiple comparisons.23 Patients in the current study received lower daily doses of IV vitamin C compared with CITRIS-ALI. However, in the nested cohort study within the intervention group of this trial, the median plasma concentration of vitamin C increased from 28 μmol/L at baseline to 369 μmol/L 1 hour after the first dose and achieved nearly the same plasma level at 6 hours24 as reported in CITRIS-ALI at 48 hours.23 As there is limited knowledge regarding optimal target plasma vitamin C levels to achieve clinically significant outcomes, and as there was no consistent benefit on improving organ dysfunction or mortality across these randomized trials, uncertainty remains about how different dosing might modify these effects.
Hydrocortisone monotherapy was mandated in the control group. This design allowed systematic assessment of the cardiovascular effects of vitamin C and thiamine when added to hydrocortisone and facilitated comparison with the established cardiovascular effect of hydrocortisone when used alone in septic shock.25 None of the positive findings observed in a single-center before-after study were replicated.13
This study was designed with sample size recalculation to enable adequate power to detect a clinically meaningful cardiovascular effect in a trial cohort. To minimize biases and strengthen the robustness of trial findings, the random allocation sequence was concealed, and permuted size blocks stratified by study center were used.26 Moreover, the statistical analysis plan was published before completing trial recruitment.16 Very few patients were lost to follow-up, thus minimizing attrition bias. Furthermore, this trial was conducted at 10 sites, including both high- and middle-income countries. Thus, the present results are likely to carry a degree of external validity.
Limitations
This study has several limitations. First, the trial was open label in design and lacked blinded outcome assessment, thus creating the possibility of performance and ascertainment bias. However, given the logistic complexity of double-blinding 2 interventions at multiple sites and in 3 countries, an open-label trial was considered to be a practical approach. Moreover, trial patients were cared for by more than 100 attending specialists and intensive care fellows, making systematic performance bias unlikely.
Second, because of the study design, the possible individual effects of vitamin C and thiamine were not assessed separately. Because previous studies have suggested that both vitamin C9 and thiamine10,11,12 might be beneficial for patients with septic shock and an observational study reported decreased mortality associated with combination therapy,13 research priority was allocated to examining the beneficial effect of the vitamins and hydrocortisone combination over evaluating the effect of each component.13 The effects of each vitamin and the combination are to be assessed in a network meta-analysis, which will inform future trials of promising components or combinations of the intervention.27
Third, thiamine levels were not measured in the trial, making it uncertain whether randomized patients did or did not have thiamine hypovitaminosis at randomization and whether such hypovitaminosis was corrected.
Fourth, the target mean arterial pressure set for each patient by treating clinicians was not collected. Fifth, time to the administration of antibiotics was not collected; however, all patients had already received antibiotics at enrollment. As this was a concealed allocation randomized trial and treatment allocation occurred after antibiotics had been given, the randomization would have achieved balance. Sixth, this trial was underpowered to detect differences in mortality or other patient-centered outcomes as well as differences in outcomes among specific subgroups. As such, any secondary outcome and post hoc subgroup analysis should be interpreted as exploratory. Seventh, adverse events were reported only when treating clinicians adjudicated, and patients were not systematically examined for other possible adverse effects (eg, oxaluria) that might develop with high-dose IV vitamin C.28,29
Conclusions
In patients with septic shock, treatment with intravenous vitamin C, hydrocortisone, and thiamine, compared with intravenous hydrocortisone alone, did not significantly improve the duration of time alive and free of vasopressor administration over 7 days. The finding suggests that treatment with intravenous vitamin C, hydrocortisone, and thiamine does not lead to a more rapid resolution of septic shock compared with intravenous hydrocortisone alone.
Trial Protocol
eAppendix 1. Methods: the VITAMINS Trial Inclusion and Exclusion Criteria
eAppendix 2. Methods: Criteria for Stopping Study Treatment in the VITAMINS Trial
eAppendix 3. Methods: Definitions of Secondary Outcomes in the VITAMINS Trial
eAppendix 4. Methods: Post-Hoc Analysis
eAppendix 5. Results: Other Feasibility Outcomes and Compliance With the Intervention Protocol
eAppendix 6. Results: Adverse Events, Serious Adverse Events, and Suspected Unexpected Serious Adverse Reactions
eAppendix 7. Results: Post-Hoc Analysis
eReferences
Statistical Analysis Plan for Sample Size Recalculation
Data Sharing Statement
Section Editor: Derek C. Angus, MD, MPH, Associate Editor, JAMA (angusdc@upmc.edu).
References
- 1.Singer M, Deutschman CS, Seymour CW, et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA. 2016;315(8):801-810. doi: 10.1001/jama.2016.0287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liu V, Escobar GJ, Greene JD, et al. Hospital deaths in patients with sepsis from 2 independent cohorts. JAMA. 2014;312(1):90-92. doi: 10.1001/jama.2014.5804 [DOI] [PubMed] [Google Scholar]
- 3.Fleischmann C, Scherag A, Adhikari NKJ, et al. ; International Forum of Acute Care Trialists . Assessment of global incidence and mortality of hospital-treated sepsis: current estimates and limitations. Am J Respir Crit Care Med. 2016;193(3):259-272. doi: 10.1164/rccm.201504-0781OC [DOI] [PubMed] [Google Scholar]
- 4.Shankar-Hari M, Phillips GS, Levy ML, et al. ; Sepsis Definitions Task Force . Developing a new definition and assessing new clinical criteria for septic shock: for the Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA. 2016;315(8):775-787. doi: 10.1001/jama.2016.0289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fisher BJ, Kraskauskas D, Martin EJ, et al. Mechanisms of attenuation of abdominal sepsis induced acute lung injury by ascorbic acid. Am J Physiol Lung Cell Mol Physiol. 2012;303(1):L20-L32. doi: 10.1152/ajplung.00300.2011 [DOI] [PubMed] [Google Scholar]
- 6.Kawade N, Tokuda Y, Tsujino S, et al. Dietary intake of ascorbic acid attenuates lipopolysaccharide-induced sepsis and septic inflammation in ODS rats. J Nutr Sci Vitaminol (Tokyo). 2018;64(6):404-411. doi: 10.3177/jnsv.64.404 [DOI] [PubMed] [Google Scholar]
- 7.Bornstein SR, Yoshida-Hiroi M, Sotiriou S, et al. Impaired adrenal catecholamine system function in mice with deficiency of the ascorbic acid transporter (SVCT2). FASEB J. 2003;17(13):1928-1930. doi: 10.1096/fj.02-1167fje [DOI] [PubMed] [Google Scholar]
- 8.Tyml K. Vitamin C and microvascular dysfunction in systemic inflammation. Antioxidants (Basel). 2017;6(3):49. doi: 10.3390/antiox6030049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fowler AA III, Syed AA, Knowlson S, et al. ; Medical Respiratory Intensive Care Unit Nursing . Phase I safety trial of intravenous ascorbic acid in patients with severe sepsis. J Transl Med. 2014;12(1):32. doi: 10.1186/1479-5876-12-32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Donnino MW, Carney E, Cocchi MN, et al. Thiamine deficiency in critically ill patients with sepsis. J Crit Care. 2010;25(4):576-581. doi: 10.1016/j.jcrc.2010.03.003 [DOI] [PubMed] [Google Scholar]
- 11.Woolum JA, Abner EL, Kelly A, Thompson Bastin ML, Morris PE, Flannery AH. Effect of thiamine administration on lactate clearance and mortality in patients with septic shock. Crit Care Med. 2018;46(11):1747-1752. doi: 10.1097/CCM.0000000000003311 [DOI] [PubMed] [Google Scholar]
- 12.Donnino MW, Andersen LW, Chase M, et al. ; Center for Resuscitation Science Research Group . Randomized, double-blind, placebo-controlled trial of thiamine as a metabolic resuscitator in septic shock: a pilot study. Crit Care Med. 2016;44(2):360-367. doi: 10.1097/CCM.0000000000001572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Marik PE, Khangoora V, Rivera R, Hooper MH, Catravas J. Hydrocortisone, vitamin C, and thiamine for the treatment of severe sepsis and septic shock: a retrospective before-after study. Chest. 2017;151(6):1229-1238. doi: 10.1016/j.chest.2016.11.036 [DOI] [PubMed] [Google Scholar]
- 14.Venkatesh B, Finfer S, Cohen J, et al. ; ADRENAL Trial Investigators and the Australian–New Zealand Intensive Care Society Clinical Trials Group . Adjunctive glucocorticoid therapy in patients with septic shock. N Engl J Med. 2018;378(9):797-808. doi: 10.1056/NEJMoa1705835 [DOI] [PubMed] [Google Scholar]
- 15.Annane D, Renault A, Brun-Buisson C, et al. ; CRICS-TRIGGERSEP Network . Hydrocortisone plus fludrocortisone for adults with septic shock. N Engl J Med. 2018;378(9):809-818. doi: 10.1056/NEJMoa1705716 [DOI] [PubMed] [Google Scholar]
- 16.Fujii T, Udy AA, Deane AM, et al. ; VITAMINS Trial Investigators . Vitamin C, Hydrocortisone and Thiamine in Patients With Septic Shock (VITAMINS) trial: study protocol and statistical analysis plan. Crit Care Resusc. 2019;21(2):119-125. [PubMed] [Google Scholar]
- 17.Vincent JL, Moreno R, Takala J, et al. ; Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine . The SOFA (Sepsis-Related Organ Failure Assessment) score to describe organ dysfunction/failure. Intensive Care Med. 1996;22(7):707-710. doi: 10.1007/BF01709751 [DOI] [PubMed] [Google Scholar]
- 18.Obeid JS, McGraw CA, Minor BL, et al. Procurement of shared data instruments for Research Electronic Data Capture (REDCap). J Biomed Inform. 2013;46(2):259-265. doi: 10.1016/j.jbi.2012.10.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kidney Disease: Improving Global Outcomes (KDIGO) Acute Kidney Injury Work Group KDIGO clinical practice guideline for acute kidney injury. Kidney Int Suppl. 2012;2(1):1-138. [Google Scholar]
- 20.Lehmann EL. Nonparametrics: Statistical Methods Based on Ranks, Revised. London, England: Pearson Education; 1998. [Google Scholar]
- 21.Khanna A, English SW, Wang XS, et al. ; ATHOS-3 Investigators . Angiotensin II for the treatment of vasodilatory shock. N Engl J Med. 2017;377(5):419-430. doi: 10.1056/NEJMoa1704154 [DOI] [PubMed] [Google Scholar]
- 22.Brant EB, Angus DC. Is high-dose vitamin C beneficial for patients with sepsis? JAMA. 2019;322(13):1257-1258. doi: 10.1001/jama.2019.11643 [DOI] [PubMed] [Google Scholar]
- 23.Fowler AA III, Truwit JD, Hite RD, et al. Effect of vitamin C infusion on organ failure and biomarkers of inflammation and vascular injury in patients with sepsis and severe acute respiratory failure: the CITRIS-ALI randomized clinical trial. JAMA. 2019;322(13):1261-1270. doi: 10.1001/jama.2019.11825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hudson EP, Collie JT, Fujii T, et al. Pharmacokinetic data support 6-hourly dosing of intravenous vitamin C to critically ill patients with septic shock. Crit Care Resusc. 2019;21(4):236-242. [PubMed] [Google Scholar]
- 25.Fujii T, Udy AA, Venkatesh B. Comparing apples and oranges: the vasoactive effects of hydrocortisone and studies investigating high dose vitamin C combination therapy in septic shock. Crit Care Resusc. 2019;21(3):152-155. [PubMed] [Google Scholar]
- 26.Schulz KF, Chalmers I, Hayes RJ, Altman DG. Empirical evidence of bias: dimensions of methodological quality associated with estimates of treatment effects in controlled trials. JAMA. 1995;273(5):408-412. doi: 10.1001/jama.1995.03520290060030 [DOI] [PubMed] [Google Scholar]
- 27.Fujii T, Belletti A, Carr A, et al. Vitamin C therapy for patients with sepsis or septic shock: a protocol for a systematic review and a network meta-analysis. BMJ Open. 2019;9(11):e033458. doi: 10.1136/bmjopen-2019-033458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lamarche J, Nair R, Peguero A, Courville C. Vitamin C-induced oxalate nephropathy. Int J Nephrol. 2011;2011:146927. doi: 10.4061/2011/146927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cossey LN, Rahim F, Larsen CP. Oxalate nephropathy and intravenous vitamin C. Am J Kidney Dis. 2013;61(6):1032-1035. doi: 10.1053/j.ajkd.2013.01.025 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol
eAppendix 1. Methods: the VITAMINS Trial Inclusion and Exclusion Criteria
eAppendix 2. Methods: Criteria for Stopping Study Treatment in the VITAMINS Trial
eAppendix 3. Methods: Definitions of Secondary Outcomes in the VITAMINS Trial
eAppendix 4. Methods: Post-Hoc Analysis
eAppendix 5. Results: Other Feasibility Outcomes and Compliance With the Intervention Protocol
eAppendix 6. Results: Adverse Events, Serious Adverse Events, and Suspected Unexpected Serious Adverse Reactions
eAppendix 7. Results: Post-Hoc Analysis
eReferences
Statistical Analysis Plan for Sample Size Recalculation
Data Sharing Statement