ABSTRACT
Introduction
Infection frequently complicates the treatment of combat-related wounds, impairs healing, and leads to worse outcomes. To better manage wound infections, antimicrobial therapies that are effective against biofilm and designed for direct wound application are needed. The primary objective of this work was to evaluate a chitosan matrix for delivery of two engineered antimicrobial peptides, (ASP)-1 and ASP-2, to treat biofilm-associated bacteria. A secondary objective was to determine whether replacing the levorotatory (L) form amino acids in ASP-2 with dextrorotatory (D) form amino acids would impact peptide activity.
Materials and Methods
Chitosan gels loaded with antimicrobial peptides were evaluated for peptide release over 7 days and tested for efficacy against biofilms grown both in vitro on polymer mesh and ex vivo on porcine skin.
Results
When delivered via chitosan, 70% to 80% of peptides were released over 7 days. Gels eradicated biofilms of gram-positive and gram-negative, drug-resistant bacteria in vitro and ex vivo. Under the conditions tested, no meaningful differences in peptide activity between the L and D forms of ASP-2 were detected.
Conclusions
Chitosan serves as an effective delivery platform for ASP-1 and ASP-2 to treat biofilm-embedded bacteria and warrants further development as a topical treatment.
INTRODUCTION
Infection remains a major cause of morbidity and mortality in injured military personnel.1 The ability to effectively treat combat-related injuries is hampered by the increasing prevalence of multidrug-resistant organisms and the propensity for bacteria to form biofilms.2 In many wounds, perfusion of systemically administered antibiotics is limited because of tissue and vessel damage. The impact of standard antibiotic use is further limited by bacterial resistance to certain antibiotics as well as the tolerance imposed by the structure of biofilms and behavior of bacteria within them. There is a need for a product that can deliver antimicrobial therapy directly to wounds that is effective against multidrug-resistant bacteria and their associated biofilms.
Antimicrobial peptides (AMPs) are promising candidates to address this need as many are broad spectrum, effective against drug-resistant pathogens, and capable of eradicating even dormant bacteria within biofilms.3 However, natural AMPs have some shortcomings that have limited their use as therapeutics, including lack of stability and reduced activity under conditions of high salt, divalent cations, and variable pH. These shortcomings have spurred the development of engineered peptides that mimic the function of natural peptides using sequences that have been optimized to improve stability and potency within a broader range of physiologic environments. Stability of AMP in tissue might be strongly affected by proteases, which are abundant in the skin4 and in wounds.5 It is well recognized that uncontrolled activity of both endogenous proteases and exogenous bacterial proteases are major contributing factors in nonhealing and infected wounds.6–8 Peptides comprising amino acids in dextrorotatory (D)-isoform are known to be resistant to enzymatic degradation9; therefore, there is interest in understanding the potential impact on peptide activity of replacing levorotatory (L) with D amino acids to achieve greater stability of therapeutics for potential use in the challenging wound environment.
The purpose of this work was to examine chitosan as a matrix for delivery of broad-spectrum, engineered cationic AMP directly to wounds and to evaluate the performance of peptide-loaded chitosan matrices using both in vitro and ex vivo porcine skin biofilm models. The impact of replacing L amino acids with D amino acids on antimicrobial peptide activity was also evaluated.
METHODS
Antimicrobial Peptides, Gels, and Dressings
Antimicrobial peptides, (ASP)-1 (RRWVRRVRRWVRRVVRVVRRWVRR) and ASP-2 (RWWRWWRRWWRR), were synthesized by American Peptide (now Bachem, Vista, CA) at > 96% purity using standard L-amino acids. The ASP-2D comprising all D isoform amino acids was synthesized by CASLO ApS (Kongens Lyngby, Denmark) at > 98% purity. Chitosan having an average molecular weight of 320 kDa (Siglufjordur Iceland, Primex) was used to prepare gels by first dissolving the material in acetic acid, then adjusting the pH to 5.9 ± 0.1 with sodium hydroxide. Gels were loaded with 1% peptide as well as proprietary moisturizing and viscosity modifying excipients. Chitosan sponges were prepared in the same way as gels but without excipients. These gels were dispensed into 2″ × 3″ silicone molds, frozen, and then lyophilized to form dry sponges using a Freezone 4.5 (Labconco, Kansas City, MO). The lyophilized cakes were released from molds and pressed to form 3 mm thick sheets with a density of about 30 mg/cm2.
In Vitro Release of Peptides From Gels
Release of peptides from gels was carried out in saline at 37°C for up to 7 days. Lyophilized sponges were die cut into discs, 9 mm in diameter and about 19 mg by weight. The discs were placed in 5-mL polypropylene cylindrical containers and weighed. At time zero, the dry sponges were reconstituted into gels by adding 2-mL saline, and the containers were closed and incubated at 37°C with mild shaking. At time points 1, 2, 3, 4 and 7 days, extracts above gels were collected, replaced with 1 mL of fresh saline, and placed back in the incubator at 37°C. Collected extracts were weighed and the samples were assayed for peptide content and purity by reverse phase high pressure liquid chromatography using a Waters Alliance e2695 Separations Module equipped with a Waters 2998 photodiode array detector. A Cortecs C18 + 2.7 μm column was used in conjunction with an acetonitrile/water gradient mobile phase supplemented with 0.2% trifluoroacetic acid. All tests were carried out in triplicate.
Bacterial Isolates and Growth Conditions
Methicillin-resistant Staphylococcus aureus (USA 300 ATCC BAA-1717), Pseudomonas aeruginosa (ATCC 15692), and Acinetobacter baumannii (ATCC BAA-1605) were purchased from American Type Culture Collection (ATCC, Manassas, VA). Bacteria were stored at −80°C and subcultured on trypticase soy broth (TSB) agar plates at 37°C before use. A single bacterial colony was aseptically picked from an agar overlay and released in 25 to 30 mL of prewarmed cation-adjusted Mueller Hinton Broth (CAMHB) in a culture flask. Each bacteria culture was incubated overnight in a 37°C incubator with shaking.
Minimum Bactericidal Concentration
Peptide stocks were serially diluted in acetate saline buffer, pH 6.0 within 96 well plates. An overnight bacterial culture was diluted to ~106 colony forming units (CFU)/mL using CAMHB and added to serially diluted peptide solutions for 24 hours at 37°C. The resulting solutions were serially diluted and plated on TSB agar to count the number of remaining viable bacteria. The minimum bactericidal concentrations (MBCs) were assigned to the lowest peptide concentrations at which ≥3 log10 reductions in CFU relative to controls were achieved.
Efficacy of Antimicrobial Gels Against Biofilms Grown on Mesh Substrates In Vitro
Polyethylene terephthalate (PET) mesh, 70 microns thick and 28% open area, was die cut into 9 mm discs and autoclaved (Component Supply, Sparta, TN). These PET substrates were selected because they have a high percentage of open area to allow biofilm ingrowth. A freshly prepared overnight bacteria culture was diluted with CAMHB to a concentration of 106 bacteria per mL and then 0.5 mL of the bacteria culture was added to each well within a 48 well plate. A PET disk was added to each well within the plate followed by incubation at 37°C for 24 hours to allow biofilms to form on the disk. Disks with adhered biofilms were then transferred to a fresh 48 well plate containing 150 μL of 10% porcine serum in saline per well. A 150 μL aliquot of antimicrobial gel was then applied to each disk and incubated at 37°C for 24 hours. After treatment, PET meshes were removed and sonicated in Dey/Engley neutralizing broth for 15 minutes, and sonicates were serially diluted and plated on TSB/agar to count the number of viable bacteria recovered from the mesh. Opticell Ag+, a commercially available gelling chitosan fiber dressing containing silver (Medline Industries, Inc.), and saline controls were used for comparison. All tests were performed in triplicate.
Efficacy of Antimicrobial Gels Against Biofilms Grown on Ex Vivo Porcine Skin
An ex vivo porcine skin biofilm model was previously described by Yang et al.10,11 and adapted here to allow for repeatable and higher throughput testing of antimicrobial gels. The dermal/epidermal layer of porcine skin, cut to 1.5 mm thickness, was obtained from Stellen Medical, St. Paul, Minnesota and stored frozen at −15°C. Porcine skin was die cut into 9-mm discs and sanitized in 70% ethanol overnight followed by thorough rinsing in sterile saline. A freshly prepared overnight bacteria culture was diluted with CAMHB to a concentration of 106 bacteria per mL and then 0.5 mL of the bacteria culture was added to each well within a 48 well plate. A porcine skin disk was added to each well within the plate followed by incubation at 37°C for 72 hours to allow biofilms to form on the skin. Porcine skin discs with adhered biofilms were then transferred to a fresh 48 well plate containing 150 μL of 10% porcine serum in saline per well. A 150-μL aliquot of antimicrobial gel was applied to each porcine skin disk and incubated at 37°C for 24 to 72 hours. After 1, 2, or 3 days, the porcine skin was removed, sonicated in Dey/Engley neutralizing broth for 15 minutes twice, with the first sonicate being discarded, and the second sonicate being serially diluted and plated on TSB/agar to count bacteria recovered from the skin. Opticell Ag+, a commercially available gelling chitosan fiber dressing containing silver (Medline Industries, Inc.), and saline controls were used for comparison. All tests were performed in triplicate.
Statistical Analysis
All data are presented as the mean value ± standard deviation. Datasets were evaluated for statistical significance by performing a one-way analysis of variance using SAS Statistical Software. Pairwise comparisons between groups were performed using the Tukey method. A p value of <0.05 (P < 0.05) was considered significant. For simplicity, significant differences between groups are only indicated on figures for comparisons of a group to ASP-1, ASP-2, or ASP-2D.
RESULTS
In Vitro Release of Peptides
Release of ASP-1 and ASP-2 from chitosan gels was measured over a 7-day period at 37°C in saline. The results shown in Figure 1 indicate that the gels provide consistent and strong release out to day 4 followed by slower release out to day 7. Both ASP-1 and ASP-2 demonstrated high recovery from chitosan gels in the range of 70% to 80% of the total peptide loaded and over the course of the experiment, only about 1% of the total peptide released had degraded. It should be noted that complete collection of liquid extracts from gels without disruption of gels was not possible, and therefore, the total amounts of peptides released were likely slightly higher than measured because of accumulation of sampling errors.
FIGURE 1.
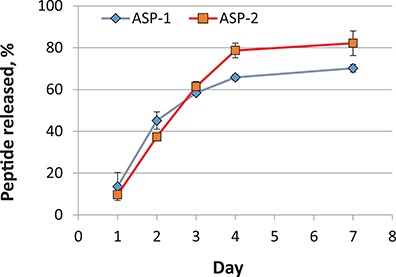
Recovery of ASP-1 and ASP-2 peptides from chitosan gels in saline at 37°C.
Minimum Bactericidal Concentration and In Vitro and Ex Vivo Efficacy
Minimum bactericidal concentration values for ASP-1, ASP-2, and ASP-2D are displayed in Table I and ranged from 4.8 to 19.3 μg/mL depending on the peptide and bacteria strain. The potencies of ASP-1 and ASP-2 against planktonic bacteria were found to be similar, and potencies for ASP-2 and ASP-2D were nearly identical.
TABLE I.
Activity of Peptides: Minimal Bactericidal Concentrations (MBCs). N = 3
| Peptide | ASP-1 | ASP-2 | ASP-2D | |
|---|---|---|---|---|
| Organism | Strain | MBC, μg/mL | MBC, μg/mL | MBC, μg/mL |
| Methicillin-resistant Staphylococcus aureus | ATCC BAA-1717 | 9.6 | 9.7 | 8.9 |
| Pseudomonas aeruginosa | ATCC 15692 | 19.2 | 19.3 | 17.8 |
| Acinetobacter baumannii | ATCC BAA-1605 | 4.8 | 9.7 | 8.9 |
The efficacies of gels loaded with the AMP, ASP-1 and ASP-2, were evaluated in vitro by application to biofilms grown for 24 hours on PET mesh substrates. The results in Figure 2 show that ASP-1 gels eradicated Pseudomonas aeruginosa and both ASP-1 and ASP-2 gels eradicated methicillin-resistant Staphylococcus aureus (MRSA) biofilms within 1 day. In the case of Pseudomonas aeruginosa biofilms, ASP-1 gels outperformed the commercially available silver-based gel.
FIGURE 2.
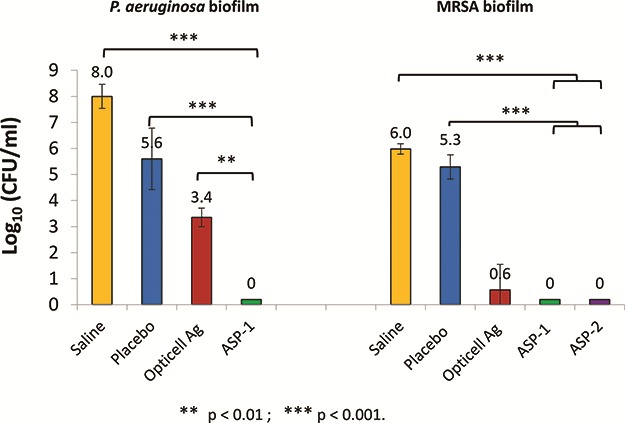
In vitro efficacy of ASP-1, ASP-2, and opticell Ag + Gels against biofilms of pseudomonas aeruginosa and MRSA grown on PET mesh for 1 day and treated for 1 day. Saline and placebo gels having no peptide served as controls. N = 3.
An ex vivo porcine skin biofilm model was employed to further evaluate antimicrobial gels under conditions thought to better simulate in vivo conditions. In this model, biofilms were allowed to develop and mature for 3 days followed by a single antimicrobial treatment that was left in place for 1, 2, or 3 days. Gels loaded with ASP-1, ASP-2, and ASP-2D reduced MRSA counts by 4 to over 7 logs within the first day of treatment with some samples showing complete or nearly complete biofilm eradication (Fig. 3). Similar results were obtained for treatments left in place for 2 or 3 days. The silver-based gels resulted in <1 log reductions over the same 3-day treatment period as shown in Figure 3. Similar results were obtained using Pseudomonas aeruginosa and Acinetobacter baumannii biofilms grown on ex vivo porcine skin as shown in Figures 4 and 5.
FIGURE 3.
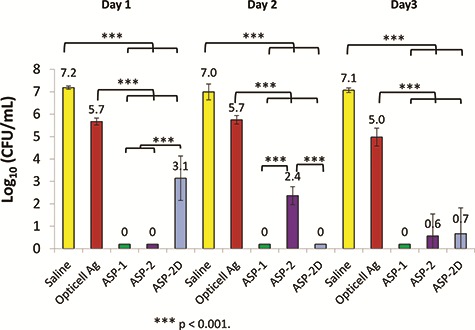
Ex vivo efficacy of ASP-1, ASP-2, ASP-2D, and opticell Ag + Gels against biofilms of MRSA grown on pig skin for 3 days and treated for 3 days. Saline served as a control. N = 3.
FIGURE 4.
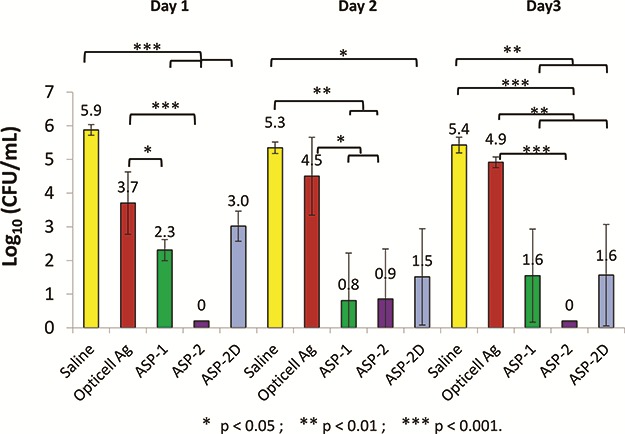
Ex vivo efficacy of ASP-1, ASP-2, ASP-2D, and opticell Ag + Gels against biofilms of pseudomonas aeruginosa grown on pig skin for 3 days and treated for 3 days. Saline served as a control. N = 3.
FIGURE 5.
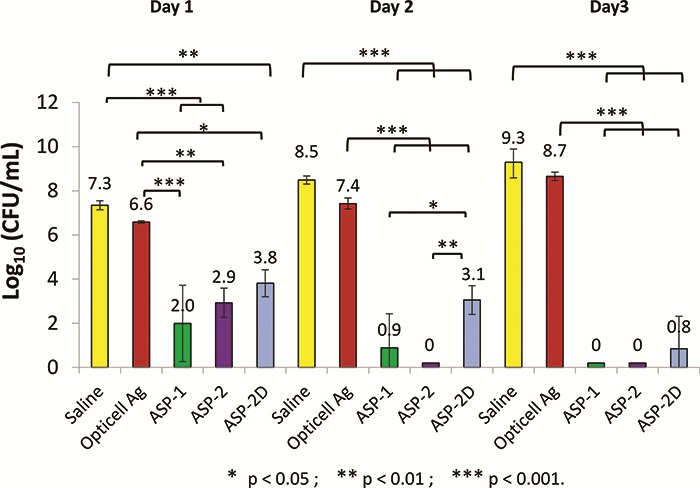
Ex vivo efficacy of ASP-1, ASP-2, ASP-2D, and opticell Ag + Gels against biofilms of acinetobacter baumannii grown on pig skin for 3 days and treated for 3 days. Saline served as a control. N = 3.
DISCUSSION
Chitosan has been studied and used extensively for wound care, tissue regeneration, and drug delivery applications largely because of its inherent biocompatibility, biodegradability, and mild antimicrobial properties.12–14 In the context of the present work, chitosan has been shown to be a suitable biodegradable platform for delivery of antibiotics to prevent wound and orthopedic device-related infections.15–19 Depending on the antibiotic and chitosan formulation, drug delivery on the order of hours to several days has been shown.17,18,20 The ASP-1 and ASP-2 chitosan gels developed herein provided sustained peptide release over a 4- to 7-day period, and the chitosan matrix met requirements for compatibility with both peptides and maintenance of peptide stability.
Although effective delivery of antibiotics from chitosan materials shows promise for inhibiting biofilm formation with prophylactic use,15,18 antibiotic-loaded materials do not appear to be effective for clearing preestablished biofilms.18 For many chronic wounds, including combat-related wounds, bacteria biofilms are thought to play a role in delayed healing. Debridement and irrigation are mainstays of wound management and are critical in the removal of bioburden from wounds. However, even with deep debridement, pockets of biofilm within the tissue have been shown to persist and result in rapid regrowth.21–23 Once established, wound biofilms are largely resistant to current antibiotic and antiseptic treatments and new technologies are needed that have potential to act on biofilm precursors and established biofilms.24 The ASP-1 and ASP-2-loaded chitosan formulations were found to be effective on biofilms that had been allowed to grow and mature for 3 days on ex vivo porcine skin before peptide exposure. Similar porcine skin models employed by others typically allow biofilm maturation for 4 to 24 hours before use for antimicrobial evaluation.25–27 In our hands, the use of biofilms allowed to mature for 3 days on pig skin followed by 1, 2, and 3 days of treatment provides more challenging conditions for evaluation relative to biofilms grown for ≤ 24 hours. This more challenging model was useful in that it allowed for better differentiation between various formulations under development as well as commercial products.
D form peptides are highly resistant to degradation by proteases, and there is potential for D form peptides to provide greater activity in vivo because of greater stability and a longer retention time.9 Several authors reported that replacement of L-peptides with D-isomers for some short AMPs led to higher in vitro antibacterial and antibiofilm activity.28,29 In the other cases, D-isomers of short AMPs had activity similar to their L-counterparts, at the same time demonstrating ~30 times lower hemolytic activity.30 Although further work is needed to evaluate the potential impact of replacing L amino acids with D amino acids, the early in vitro studies reported here indicate that gels loaded with ASP-2D perform similarly to those loaded with ASP-2 and that substitution of L amino acids with D amino acids did not adversely impact peptide activity under the conditions tested. This is consistent with the results of MBC testing, which showed similar MBC values for ASP-2 and ASP-2 D. The porcine skin used in this work was sanitized with 70% alcohol before use to avoid contamination of samples with intrinsic skin flora. Sanitization leads to reduced protease activity, and this may be one reason differences between ASP-2 and ASP-2D were not detected in this model. Further testing is needed to understand if the D form peptide would have a greater impact in vivo, where the concentration of proteases is expected to be higher, especially in the case of nonhealing and chronic wounds.
Today, combat-related infections are largely managed using systemic antibiotics, including early prophylactic administration, along with debridement, irrigation, and surgical managment.31 In the case of burn injuries, clinical practice guidelines for US Military personnel recommend the use of topical antimicrobials for infection prevention, including mafenide acetate (MFA), silver sulfadiazine (SSD) creams, and silver-impregnated dressings.31,32 All of these treatments provide broad-spectrum antibacterial activity; however, each one has limitations and there is a need for improved therapies. Although MFA has the ability to penetrate eschar, it is painful on application and pulmonary complications can occur with continuous use on large burns.33 SSD is less painful on application but does not penetrate eschar.32 Both MFA and SSD require frequent application, typically twice a day, which is labor intensive and uncomfortable for the patient. With the use of SSD and silver dressings, there is some risk of silver staining but more importantly, there is lack of evidence that these approaches reduce wound infection and some evidence indicates that they may slow healing.34 The antimicrobial peptide-based treatments described herein have potential to overcome some of these limitations. We recently demonstrated that the biocompatibility indices for both ASP-1 and ASP-2 are substantially more favorable than those for SSD and silver, which may allow for better control of bioburden without impairment of healing.35 Pathogens that commonly infect burns are known biofilm formers, and it has been shown that biofilms are present on burns.36 The strong activity of ASP-1 and ASP-2 against biofilms relevant to burn wounds combined with sustained release from a chitosan formulation has potential to allow for better infection control with less frequent treatment application relative to MFA and SSD.
Another area where the antimicrobial peptide-based gels have potential to improve care is in the treatment of musculoskeletal injuries that require fixation. The use of antibiotic-loaded beads and gels has gained acceptance for use in treating these injuries, as an adjunct to systemic antibiotics.37 In complex injuries, the local vascular anatomy is often damaged resulting in less effective delivery of systemic antibiotics to the site of injury. Under these conditions, local antibiotic delivery has been shown to reduce infection risks by enabling high local drug concentrations that would not be possible to achieve by systemic delivery without substantial side effects.37–39 The use of fixation devices increases risks for infection because they present artificial surfaces that can harbor biofilm containing bacteria that are recalcitrant to antibiotic treatment because of multiple tolerance mechanisms.40–42 Once established, biofilms formed on devices can be extremely difficult to treat, they often require surgical intervention, and in many cases, device removal.43 Current products for local delivery rely on the use of front-line clinical antibiotics and pose a threat for increasing resistance. When delivered via a local depot, the concentration of antibiotic will decrease with distance from the source and depending on the duration of delivery and timing of removal, has potential to expose bacteria to sublethal concentrations.3 Most antibiotics also display limited efficacy against bacteria within biofilms. ASP-1 and ASP-2 kill bacteria rapidly and work by a different mechanism of action relative to most front-line clinical antibiotics. As such, their use would pose little threat for promoting resistance to the limited arsenal of antibiotics. Because they are highly effective against biofilm and biodegradable, the ASP-1 and ASP-2 gels presented in this work may be considered for local delivery to prevent infection of musculoskeletal injuries that require fixation.
The work presented here focused on monospecies biofilms. However, many in vivo biofilms are polymicrobial in nature where microbial synergy can lead to increased growth, antimicrobial tolerance, and infection persistence.44,45 It has previously been shown that ASP-1 containing dressings are effective against polymicrobial biofilms comprised of MRSA, A. baumannii, K. pneumoniae, and P. aeruginosa, when studied using an in vitro poloxamer biofilm model.35 Future work will include evaluation of both ASP-1 and ASP-2 chitosan gels against mixed species biofilms established on ex vivo pig skin.
CONCLUSIONS
Chitosan-based gels provide an effective delivery platform for the engineered cationic AMPs, ASP-1 and ASP-2. The peptide-loaded gels are highly active against biofilms formed by both gram-positive and gram-negative, drug-resistant bacteria that are relevant to wound infections. Replacement of the L-form peptide (ASP-2) with its protease resistant D-form (ASP-2D) did not result in a significant change in peptide activity. Future work will include studies to evaluate efficacy against mixed species biofilms as well as the safety and efficacy of ASP treatments in a porcine model of burn wound infection.
Presented as a poster at the 2018 Military Health System Research Symposium (Abstract number: MHSRS-18-1522). The views, opinions, and/or findings contained in this article are those of the authors and should not be construed as an official Department of the Army position, policy, or decision unless so designated by other documentation
FUNDING
This work is supported in part by NIH NIAID Grant 1R43AI136195-01A1.
REFERENCES
- 1. Blyth DM, Yun HC, Tribble DR, Murray CK: Lessons of war: combat-related injury infections during the Vietnam war and operation Iraqi and enduring freedom. J Trauma Acute Care Surg 2015; 79(4 Suppl 2): S227–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Barsoumian AE, Mende K, Sanchez CJ Jr, et al. : Clinical infectious outcomes associated with biofilm-related bacterial infections: a retrospective chart review. BMC Infect Dis 2015; 15: 223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bayramov DF, Neff JA: Beyond conventional antibiotics - new directions for combination products to combat biofilm. Adv Drug Deliv Rev 2017; 112: 48–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. de Veer SJ, Furio L, Harris JM, Hovnanian A: Proteases: common culprits in human skin disorders. Trends Mol Med 2014; 20(3): 166–78. [DOI] [PubMed] [Google Scholar]
- 5. McCarty SM, Percival SL: Proteases and delayed wound healing. Adv Wound Care 2013; 2(8): 438–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Saravanan R, Adav SS, Choong YK, et al. : Proteolytic signatures define unique thrombin-derived peptides present in human wound fluid in vivo. Sci Rep 2017; 7(1): 13136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lindsay S, Oates A, Bourdillon K: The detrimental impact of extracellular bacterial proteases on wound healing. Int Wound J 2017; 14(6): 1237–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Suleman L: Extracellular bacterial proteases in chronic wounds: a potential therapeutic target? Adv Wound Care 2016; 5(10): 455–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Uppalapati M, Lee DJ, Mandal K, et al. : A potent d-protein antagonist of VEGF-A is nonimmunogenic, metabolically stable, and longer-circulating in vivo. ACS Chem Biol 2016; 11(4): 1058–65. [DOI] [PubMed] [Google Scholar]
- 10. Phillips PL, Yang Q, Davis S, et al. : Antimicrobial dressing efficacy against mature Pseudomonas aeruginosa biofilm on porcine skin explants. Int Wound J 2015; 12(4): 469–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Yang Q, Phillips PL, Sampson EM, et al. : Development of a novel ex vivo porcine skin explant model for the assessment of mature bacterial biofilms. Wound Repair Regen 2013; 21(5): 704–14. [DOI] [PubMed] [Google Scholar]
- 12. Dai T, Tanaka M, Huang YY, Hamblin MR: Chitosan preparations for wounds and burns: antimicrobial and wound-healing effects. Expert Rev Anti-Infect Ther 2011; 9(7): 857–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bhattarai N, Gunn J, Zhang M: Chitosan-based hydrogels for controlled, localized drug delivery. Adv Drug Deliv Rev 2010; 62(1): 83–99. [DOI] [PubMed] [Google Scholar]
- 14. Younes I, Rinaudo M: Chitin and chitosan preparation from marine sources: structure, properties and applications. Mar Drugs 2015; 13(3): 1133–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wells CM, Beenken KE, Smeltzer MS, Courtney HS, Jennings JA, Haggard W: Ciprofloxacin and Rifampin dual antibiotic-loaded biopolymer chitosan sponge for bacterial inhibition. J Mil Med 2018; 183(suppl_1): 433–44. [DOI] [PubMed] [Google Scholar]
- 16. Boles L, Alexander C, Pace L, Haggard W, Bumgardner J, Jennings J: Development and evaluation of an injectable chitosan/beta-Glycerophosphate paste as a local antibiotic delivery system for trauma care. J Funct Biomater 2018; 9(4): 56–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Boles LR, Awais R, Beenken KE, Smeltzer MS, Haggard WO, Jessica AJ: Local delivery of Amikacin and Vancomycin from chitosan sponges prevent Polymicrobial implant-associated biofilm. J Mil Med 2018; 183(suppl_1): 459–65. [DOI] [PubMed] [Google Scholar]
- 18. Jennings JA, Beenken KE, Parker AC, et al. : Polymicrobial biofilm inhibition effects of acetate-buffered chitosan sponge delivery device. Macromol Biosci 2016; 16(4): 591–8. [DOI] [PubMed] [Google Scholar]
- 19. Stinner DJ, Noel SP, Haggard WO, Watson JT, Wenke JC: Local antibiotic delivery using tailorable chitosan sponges: the future of infection control? J Orthop Trauma 2010; 24(9): 592–7. [DOI] [PubMed] [Google Scholar]
- 20. Sobhani Z, Mohammadi Samani S, Montaseri H, Khezri E: Nanoparticles of chitosan loaded ciprofloxacin: fabrication and antimicrobial activity. Adv Pharm Bull 2017; 7(3): 427–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Attinger C, Wolcott R: Clinically addressing biofilm in chronic wounds. Adv Wound Care (New Rochelle) 2012; 1(3): 127–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hurlow J, Blanz E, Gaddy JA: Clinical investigation of biofilm in non-healing wounds by high resolution microscopy techniques. J Wound Care 2016; 25(Suppl 9): S11–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Snyder RJ, Bohn G, Hanft J, et al. : Wound biofilm: current perspectives and strategies on biofilm disruption and treatments. Wounds 2017; 29(6): S1–17. [PubMed] [Google Scholar]
- 24. Lewis K: Multidrug tolerance of biofilms and persister cells. Curr Top Microbiol Immunol 2008; 322: 107–31. [DOI] [PubMed] [Google Scholar]
- 25. Myhrman E, Hakansson J, Lindgren K, Bjorn C, Sjostrand V, Mahlapuu M: The novel antimicrobial peptide PXL150 in the local treatment of skin and soft tissue infections. Appl Microbiol Biotechnol 2013; 97(7): 3085–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Rubinchik E, Dugourd D, Algara T, Pasetka C, Friedland HD: Antimicrobial and antifungal activities of a novel cationic antimicrobial peptide, omiganan, in experimental skin colonisation models. Int J Antimicrob Agents 2009; 34(5): 457–61. [DOI] [PubMed] [Google Scholar]
- 27. Yang Q, Schultz GS, Gibson DJ: A surfactant-based dressing to treat and prevent Acinetobacter baumannii biofilms. J Burn Care Res 2018; 39(5): 766–70. [DOI] [PubMed] [Google Scholar]
- 28. de la Fuente-Nunez C, Reffuveille F, Mansour SC, et al. : D-enantiomeric peptides that eradicate wild-type and multidrug-resistant biofilms and protect against lethal Pseudomonas aeruginosa infections. Chem Biol 2015; 22(2): 196–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Manabe T, Kawasaki K: D-form KLKLLLLLKLK-NH2 peptide exerts higher antimicrobial properties than its L-form counterpart via an association with bacterial cell wall components. Sci Rep 2017; 7: 43384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Albada HB, Prochnow P, Bobersky S, Langklotz S, Bandow JE, Metzler-Nolte N: Short antibacterial peptides with significantly reduced hemolytic activity can be identified by a systematic L-to-D exchange scan of their amino acid residues. ACS Comb Sci 2013; 15(11): 585–92. [DOI] [PubMed] [Google Scholar]
- 31. Saeed O, Tribble DR, Biever KA, Crouch HK, Kavanaugh M: Infection prevention in combat-related injuries. J Mil Med 2018; 183(Suppl 2): 137–41. [DOI] [PubMed] [Google Scholar]
- 32. D’Avignon LC, Saffle JR, Chung KK, Cancio LC: Prevention and management of infections associated with burns in the combat casualty. J Trauma 2008; 64(3 Suppl): S277–86. [DOI] [PubMed] [Google Scholar]
- 33. Dai T, Huang YY, Sharma SK, Hashmi JT, Kurup DB, Hamblin MR: Topical antimicrobials for burn wound infections. Recent Pat Antiinfect Drug Discov 2010; 5(2): 124–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Storm-Versloot MN, Vos CG, Ubbink DT, Vermeulen H: Topical silver for preventing wound infection. Cochrane Database Syst Rev 2010; 3: CD006478. [DOI] [PubMed] [Google Scholar]
- 35. Bayramov DF, Li Z, Patel E, Izadjoo MJ, Kim H, Neff JA: A novel peptide-based antimicrobial wound treatment is effective against biofilms of multi-drug resistant wound pathogens. J Mil Med 2018Mar 1; 183(Suppl 1): 481–6. [DOI] [PubMed] [Google Scholar]
- 36. Kennedy P, Brammah S, Wills E: Burns, biofilm and a new appraisal of burn wound sepsis. Burns 2010; 36(1): 49–56. [DOI] [PubMed] [Google Scholar]
- 37. Morgenstern M, Vallejo A, McNally MA, et al. : The effect of local antibiotic prophylaxis when treating open limb fractures: a systematic review and meta-analysis. Bone Joint Res 2018; 7(7): 447–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Branstetter JG, Jackson SR, Haggard WO, Richelsoph KC, Wenke JC: Locally-administered antibiotics in wounds in a limb. J Bone Joint Surg Br 2009; 91(8): 1106–9. [DOI] [PubMed] [Google Scholar]
- 39. Craig J, Fuchs T, Jenks M, et al. : Systematic review and meta-analysis of the additional benefit of local prophylactic antibiotic therapy for infection rates in open tibia fractures treated with intramedullary nailing. Int Orthop 2014; 38(5): 1025–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Ciofu O, Rojo-Molinero E, Macia MD, Oliver A: Antibiotic treatment of biofilm infections. APMIS 2017; 125(4): 304–19. [DOI] [PubMed] [Google Scholar]
- 41. Conlon BP, Rowe SE, Lewis K: Persister cells in biofilm associated infections. Adv Exp Med Biol 2015; 831: 1–9. [DOI] [PubMed] [Google Scholar]
- 42. Lewis K: Persister cells: Molecular mechanisms related to antibiotic tolerance. Handb Exp Pharmacol 2012; 211: 121–33. [DOI] [PubMed] [Google Scholar]
- 43. Zimmerli W, Sendi P: Orthopaedic biofilm infections. APMI 2017; 125(4): 353–64. [DOI] [PubMed] [Google Scholar]
- 44. Gabrilska RA, Rumbaugh KP: Biofilm models of polymicrobial infection. Future Microbiol 2015; 10(12): 1997–2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Akers KS, Mende K, Cheatle KA, et al. : Biofilms and persistent wound infections in United States military trauma patients: a case-control analysis. BMC Infect Dis 2014; 14: 190. [DOI] [PMC free article] [PubMed] [Google Scholar]


