Abstract
Objective:
To determine if first choice anti-diabetic medications are associated with reduced risk of dementia, we compared incident dementia risk among patients who initiated treatment with metformin or a sulfonylurea among Veterans Health Affairs (VHA) patients with replication in Kaiser Permanente Washington (KPW) patients.
Patients and Methods:
Cohorts contained 75,187 VHA patients and 10,866 KPW patients, ≥ 50 years of age, who initiated monotherapy with metformin or a sulfonylurea. Patients were free of dementia diagnoses and any diabetes treatment for 2 years prior to cohort entry. Variables were created from electronic health data from VHA (1999–2015) and KPW (1996–2015) which included diagnosis codes, pharmacy data, laboratory values and demographics. Propensity scores and inverse probability of treatment weighting controlled for confounding.
Results:
VHA patients were 60.8 years (SD, ±6.8) of age on average and in KPW, 63.1 years (SD, ±9.5) of age. In the VHA sample, 96.8% were male and in KPW 50.4%. After adjusting for confounding, metformin initiation was associated with a significantly lower risk of dementia in VHA (HR=0.93; 95%CI: 0.87–0.99) with a similar point estimate in KPW (HR=0.89; 95%CI: 0.74–1.07). Metformin was not associated with dementia risk among patients ≥ 75 years of age.
Conclusions:
Existing epidemiological studies of metformin and incident dementia have been inconsistent. Using a similar study design in two patient populations that differed on clinical and demographic characteristics, our results provide robust evidence that metformin use is associated with modest, lower risk of incident dementia.
INTRODUCTION
Patients with diabetes mellitus have a 70% higher risk of dementia compared to those without diabetes 1. Metformin, the first line medication for type 2 diabetes mellitus (T2DM), may reduce dementia risk. From rodent models, metformin has been shown to decrease oxidative stress, correct abnormal transport of amyloid-β through the blood brain barrier and improve memory;2–4 however, some animal studies have reported no benefit of metformin on cognitive impairment related to high fat diet 5, and others report no association between metformin and β-amyloid peptide degradation 6.
Epidemiological studies have been inconsistent, with some evidence that metformin is associated with increased risk of dementia 7,8, other studies suggesting a reduced risk 9–11, and some reporting no association 12. These prior studies are difficult to compare because of limitations and differences in study design, data quality, and methods for addressing confounding. To investigate whether metformin use may reduce the risk of incident dementia, we used electronic health data from the Veterans Health Affairs (VHA) healthcare delivery system to conduct a new-user, active-comparator cohort study comparing the initiation of treatment for diabetes with metformin vs. a sulfonylurea. We also evaluated whether findings differed by age at treatment initiation. Lastly, using a similar study design and analytic approach, we attempted to replicate our findings in a second integrated healthcare delivery system, in which patient demographics and clinical characteristics differed from VHA patients.
METHODS
Subjects:
We conducted two cohort studies using electronic health data from the Veterans Health Affairs (VHA) and Kaiser Permanente Washington (KPW). KPW is an integrated healthcare delivery system providing healthcare and insurance coverage to about 710,000 people in the Northwest United States. VHA data included patient encounters from nationally distributed community based outpatient clinics and hospitals from 10/01/1999–09/30/2015 and merged with Medicare Enrollment Data Base and Medicare claims data. Medicare Part D pharmacy data were not included because they were available for only part of the study period and only on a subset of patients. KPW data included encounters from 1996–2015. The project was approved with a waiver of consent by the Institutional Review Boards of participating institutions.
VHA cohort selection:
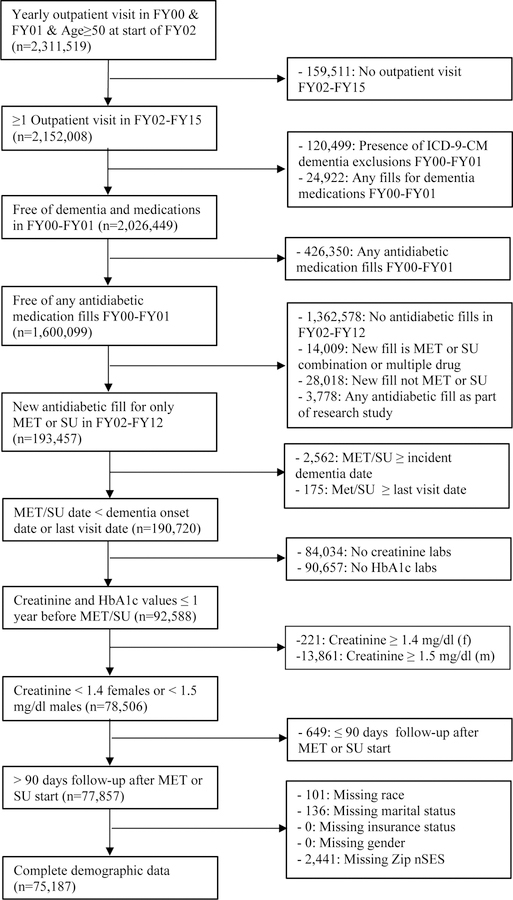
From VHA patients ≥ 50 years of age and encounters between October 1, 1999 and September 30, 2015, we selected patients with one or more yearly outpatient visit(s) in the two year “washout” period between 10/01/1999 to 09/30/2001 (fiscal years (FY) 2000-FY2001). Patients must have had one or more visits in follow-up (FY02-FY15). We excluded patients with International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) codes for dementia or dementia medication fills (donepezil, rivastigmine, galantamine or memantine) and patients who filled anti-diabetic prescriptions during the baseline period. New users of metformin or a sulfonylurea were eligible if medication use began after baseline and before 10/01/2012, which allowed for at least 3 years of follow-up after medication initiation. Patients who developed dementia after the washout period and before metformin or sulfonylurea initiation were excluded. To limit missing laboratory values important to prescribing decisions, we required patients to have had a creatinine and hemoglobin A1c (HbA1c mmol/mol) value in the year before metformin or sulfonylurea initiation. Males with creatinine values of ≥1.5 mg/dl and females with values ≥1.4 mg/dl in the year prior to metformin or sulfonylurea initiation were excluded because this is a contraindication for metformin 13. Patients must have had > 90 days of follow-up after metformin or sulfonylurea initiation to allow for an accumulation of drug exposure that would support a biologically plausible association with incident dementia. Patients with missing demographic data were excluded resulting in an analytic sample of 75,187 VHA patients.
KPW cohort selection:
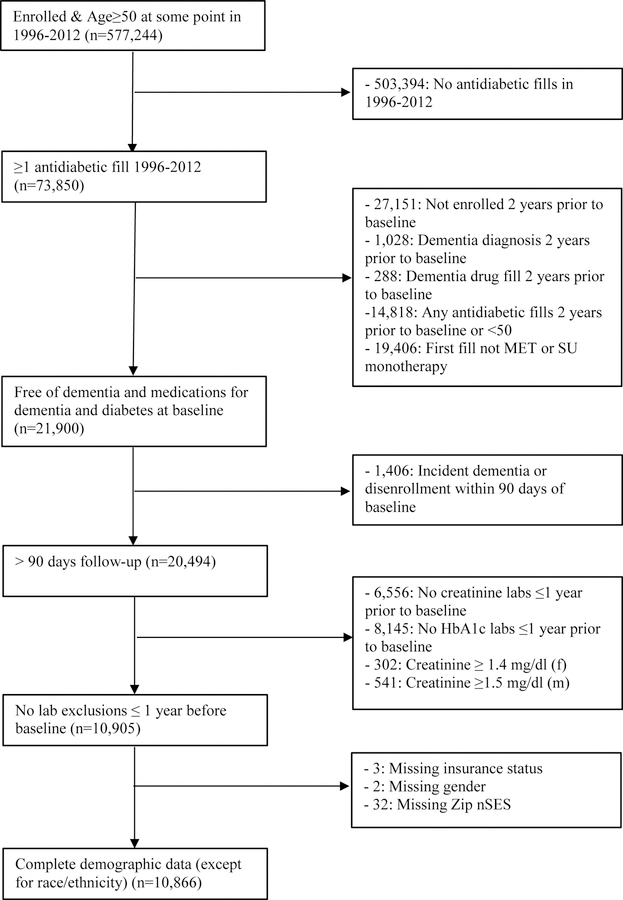
In contrast with the VHA study population, which is a fixed cohort, we sampled a dynamic cohort in KPW to maximize available sample size. Eligible patients were continuously enrolled in KPW for at least two years from January 1, 1996 to December 31, 2012, were ≥50 years of age, and initiated diabetes treatment with either metformin or a sulfonylurea. They had not filled prescriptions for diabetes or dementia treatments or been diagnosed with dementia prior to initiating treatment and, following the rationale for the VHA cohort described above, were required to have at least 90 days of follow-up after initiation. Patients with missing values for HbA1c or serum creatinine were excluded. The eligible KPW sample included 10,866 patients. The sampling designs are shown in Figures 1a and 1b.
Figure 1a.
Veterans Health Administration eligibility criteria (MET=metformin, SU=sulfonylurea; FY=fiscal year)
Figure 1b.
Kaiser Permanente Washington State eligibility criteria
Variable definitions
Use of metformin or sulfonylurea was measured from electronic records of medication prescription fills from VHA and KPW pharmacies. Sulfonylureas consisted of acetohexamide, chlorpropamide, glimepiride, glipizide, glyburide, tolazamide, or tolbutamide. We defined incident dementia as two or more ICD-9-CM diagnostic codes from any encounter type (e.g., inpatient, outpatient, or Medicare) on separate days in any 12-month period, see Appendix A. Date of dementia onset was the date of the first of the two diagnostic codes in the 12 month period. This algorithm follows the logic of Imfeld and colleagues 8 who used two or more medical record diagnoses of dementia, as well as other measures (e.g. dementia medication) to count toward a case, and found 79% agreement between medical record and clinician assessment of dementia 8.
Comorbidities during the baseline period were assessed using ICD-9-CM diagnosis codes from inpatient or outpatient encounters as well as Medicare claims (Appendix A). We defined covariates as of the time of medication initiation, using all available look-back for VHA patients and the prior 2 years for KPW patients. We identified baseline HbA1c and creatinine levels closest to and in the 12 months prior to metformin or sulfonylurea initiation. Medications that may be associated with the risk of dementia were identified (statins, anticholinergics, Nonsteroidal anti-inflammatory drug, antihypertensives), and exposure was defined as two fills in any 6-month period prior to metformin or sulfonylurea initiation. To control for detection bias, we measured volume of health services utilization prior to metformin or sulfonylurea start date. The distribution of the average number of outpatient visits per month from first visit in the available data to metformin or sulfonylurea start date (or at KPW, in the 2 years prior to initiation) was computed and the top 25th percentile was defined as high utilization and the bottom 75th percentile as “not high”.
Sociodemographic variables included age, race, gender and marital status. In VHA data, health insurance was defined as having only VHA insurance compared to VHA insurance plus other health insurance. In KPW data, federal health insurance was defined as having Medicaid or Medicare insurance compared to private insurance. We controlled for neighborhood socioeconomic status (nSES) by computing an nSES index based on all United States zip codes 14. nSES was categorized into the top 50th percentile vs. lower 50th percentile in each sample. Because prescribing rates for sulfonylurea and metformin have changed over time, we controlled for year of observation. See Appendix A for detailed variable definitions.
Propensity scores (PS) and inverse probability of treatment weighting (IPTW)
PS and IPTW were used to balance baseline variables that may confound the association between type of diabetes medication initiated and dementia risk. The PS, calculated using binary logistic regression, is the probability of initiating metformin, rather than sulfonylurea, given baseline covariates. PS were used to compute a stabilized weight 15,16 for each patient, which is the marginal probability of metformin initiation divided by the PS for metformin users or (1-marginal probability of metformin use) divided by (1-PS) for sulfonylurea users. This method reduces bias associated with extreme weights. Stabilized IPTW also retains the original sample size thereby preserving Type I error rate 17. Stabilized weights were trimmed if they were greater or equal to ten. Well-behaved weights have a mean close to one and a maximum less than ten 18,19 with extreme values indicating the PS model poorly specified predictors of the treatment exposure. After applying IPTW, we assessed adequate balance between patients who initiated metformin or sulfonylurea with standardized mean difference (SMD%) and considered variables to be well balanced when SMD was < 10%.20
Primary Analysis:
Analyses were performed with SAS v9.4 (SAS Institute, Cary, NC) at an alpha level of 0.05. Bivariate analyses estimating the association between variables associated with metformin or sulfonylurea initiation were computed using chi-square tests for categorical variables and independent samples t-tests for continuous variables, and SMD% were presented for effect size estimates 20. A Poisson regression model was used in unweighted data for descriptive purposes to estimate and compare incidence rates (per person-years, PY). In primary analyses, we used Cox proportional hazards models in unweighted and weighted data from patients 50 years of age and older with secondary analyses stratified by age group. An interaction of age group and drug in weighted models tested whether age was an effect modifier. The association between metformin or sulfonylurea and time to incident dementia was expressed by hazard ratios and 95% confidence intervals. Robust, sandwich-type variance estimators were used to calculate confidence intervals and p-values in analyses using weighted data 20. The proportional hazard assumption was tested by examining a time dependent interaction term of metformin or sulfonylurea and log follow-up time, where a significant (P<.05) interaction term indicates different hazard trends over time. The assumption was met for all models. Follow-up time was defined as days from date of metformin or sulfonylurea initiation until dementia or censoring. VHA patients were censored at the time of the last available inpatient, outpatient or Medicare encounter. KPW patients were censored when they dis-enrolled from KPW, died, or reached the end of study follow-up (September 2015).
Meta-analysis:
To maximize study power and generate the most precise estimate of incident dementia risk associated with metformin initiation, we conducted an inverse variance weighted fixed effects meta-analyses that combined results from the primary analyses in the VHA and KPW populations 21.
Moderator analysis, Average Monthly Glycemic Burden and Hypoglycemia:
Both hypo- and hyperglycemia have been associated with greater risk of cognitive decline and dementia 22,23. Following metformin or sulfonylurea medication initiation, glycemic control may partly moderate the association between metformin or sulfonylurea and incident dementia. Glycemic control was defined using average monthly glycemic burden (AMGB)24,25 from metformin or sulfonylurea initiation to dementia or censor date. We first defined total glycemic burden as the cumulative amount that HbA1c is > 7.5 (58 mmol/mol) 24,25. Each unit of total glycemic burden represents one month in which the HbA1c was one percentage point higher than 7.5 (58 mmol/mol). For example, ten units of total glycemic burden is equivalent to ten months at an HbA1c = 8.5 (69 mmol/mol) or 5 months at an HbA1c of 9.5 (80 mmol/mol). AMGB was then computed by dividing the total glycemic burden by the number of months between the first HbA1c measure after metformin or sulfonylurea to the last measure before dementia or censor date. AMGB was computed from an average of 11.6 (SD 6.4) HbA1c values for each VA patient and an average of 16.5 (SD 11.5) in KPW. In weighted data we computed a Cox proportional hazards model that adjusted for AMGB after metformin or sulfonylurea initiation.
A second sensitivity analysis adjusted for hypoglycemic events. In VHA patients, after metformin or sulfonylurea initiation to last measure before dementia or censor date, 3.2% of metformin users experienced a hypoglycemic event and 7.3% of sulfonylurea users experienced a hypoglycemic event. In KPW it was 3.4% among metformin users and 8.2% among sulfonylurea users. Hypoglycemic events were defined by previously reported ICD-9 codes (see Appendix A for details) 26. In weighted data we computed a Cox proportional hazards model that adjusted for any hypoglycemic event after metformin or sulfonylurea initiation.
Sensitivity analysis:
Diagnosis codes for mild cognitive impairment (ICD-9-CM =331.83) may be capturing impairment that is meaningful to patients but not severe enough to qualify as dementia. We computed sensitivity analysis by adding ICD-9-CM codes for mild cognitive impairment to our dementia diagnostic algorithm.
RESULTS
The mean age of VHA patients was 60.8 (±8.5) and most were male (96.8%). Approximately 84% of VHA patients were white and 14% were black. The mean age of KPW patients was 63.1 (±9.5), about half were male, and among those with known race, 83.6% were white, 4.6% black and 8.4% Asian. The prevalence of HbA1c>8 (64mmol/mol) was 53.1% in KPW and 17.8% in VHA patients. Average creatinine values were about 1.0 in both samples (Table 1).
Table 1.
Characteristics (%) of ≥ 50 years old patients initiating metformin (MET) and sulfonylurea (SU) from the Veterans Health Administration (VHA; FY2002 to FY2012) and Kaiser Permanente Washington State (KPW; 1996–2012) health care systems
| Covariates | VHA (n=75,187) | KPWa (n=10,866) |
|---|---|---|
| Index year | ||
| 1996–1998 | - | 12.8 |
| 1999–2000 | - | 10.2 |
| 2001–2002 | - | 11.2 |
| 2002–2004 | 5.6 | - |
| 2003–2004 | - | 12.2 |
| 2005–2006 | 32.5 | 14.6 |
| 2007–2008 | 26.3 | 14.0 |
| 2009–2012 | - | 25.1 |
| 2009–2010 | 20.8 | - |
| 2011–2012 | 14.8 | - |
| Sociodemographic-related | ||
| Age, mean (±sd) | 60.8 (±8.5) | 63.1 (±9.5) |
| Age category | ||
| 50–64 | 66.8 | 61.7 |
| 65–74 | 24.6 | 23.9 |
| ≥ 75 | 8.6 | 14.3 |
| Male gender | 96.8 | 50.4 |
| Race | ||
| White | 84.1 | 74.4 |
| Black | 14.0 | 4.1 |
| Asian | -- | 7.5 |
| Other | 1.9 | 3.0 |
| Unknown | -- | 11.0 |
| Race (excluding unknown) | ||
| White | -- | 83.6 |
| Black | -- | 4.6 |
| Asian | -- | 8.4 |
| Other | -- | 3.4 |
| Married | 59.3 | - |
| Low nSES b | 50.0 | 49.8 |
| VHA only insurance | 45.9 | - |
| Medicaid/Medicare | - | 38.9 |
| High healthcare utilization | 25.0 | 24.9 |
| Diabetes-related | ||
| Diabetic nephropathy | 1.2 | 1.9 |
| Diabetic retinopathy | 5.2 | 3.1 |
| Diabetic neuropathy | 7.2 | 8.3 |
| HbA1c value, mean (±sd) | 7.2 (±1.4) | 8.6 (±2.0) |
| HbA1c category | ||
| < 7 | 51.2 | 17.8 |
| 7–8 | 31.0 | 29.2 |
| > 8 | 17.8 | 53.1 |
| Creatinine value, mean (±sd) | 1.0 (±0.2) | 0.9 (±0.2) |
| Other comorbidities | ||
| Obesity | 59.5 | 50.3 |
| Hypertension | 90.9 | 57.9 |
| Hyperlipidemia | 86.9 | 40.1 |
| Stroke | 6.8 | 2.2 |
| Ischemic heart disease | 47.7 | 15.9 |
| Congestive heart failure | 17.5 | 5.4 |
| Atrial fibrillation | 13.2 | 6.7 |
| Traumatic brain injury | 6.3 | 1.4 |
| Vitamin B12 deficiency | 4.9 | 0.8 |
| Psychiatric and substance comorbidities | ||
| Depression | 28.6 | 4.4 |
| PTSD | 19.5 | 0.4 |
| Other anxiety c | 13.7 | 2.8 |
| Bipolar disorder | 7.7 | 1.7 |
| Schizophrenia | 5.5 | 0.3 |
| Nicotine abuse/dependence | 53.1 | 17.2 |
| Alcohol abuse/dependence | 16.1 | 1.9 |
| Illicit drug abuse/dependence | 8.1 | 0.6 |
| Other medications d | ||
| Statins | 69.5 | 36.9 |
| Anticholinergics | 45.0 | 23.4 |
| NSAIDs | 56.1 | 19.1 |
| Antihypertensives | 87.7 | 44.5 |
KPW dynamic cohort – covariates measured for 2 year prior to every new metformin/sulfonylurea start; VHA=Veterans Health Administration; KPW=Kaiser Permanente Washington State; FY=Fiscal Year; MET=Metformin; SU=Sulfonylurea;
nSES = top 50th vs. bottom 50th percentile of neighborhood socioeconomic status based on information from 7 measures of SES obtained from 2009–2013 5-year census estimates from the American Community Survey
Other anxiety disorders = panic disorder, OCD, social phobia, GAD, Anxiety NOS
Other medications associated with dementia= sustained use prior to MET or SU (at least 2 fills in a 6 month period)
We observed that 37.2% of VHA and 41% of KPW patients who started on metformin eventually added or switched to a sulfonylurea and 43.7% of VHA and 47% of KPW patients experienced the reverse pattern of medication use. In VHA metformin initiators, the mean duration of metformin use was 1,416 (SD ±1037) days and for sulfonylurea initiators duration of sulfonylurea use was 1,370 (SD ±1089) days. In KPW patients average duration of metformin was 1,645 (SD ±1358) and for sulfonylurea, 1,754 (SD ±1535) days. In the VA, 5,249 patients developed dementia (3,490 among metformin and 1,759 among sulfonylurea), and in KPW 673 (260 among metformin and 413 among sulfonylurea). After metformin starts, the median follow-up time was 6.4 years (interquartile range (iqr) 4.3 – 8.5) in VHA and in KPW, 6.1 years (iqr: 3.9 – 9.5). After sulfonylurea starts, the median follow-up time was 6.7 years (iqr: 4.1–9.1) in VA and 7.3 years (iqr: 3.5–11.6) for KPW. Among metformin users, the median follow-up time to dementia was 4.6 years (iqr: 2.6–6.7) in VHA and 7.6 years (iqr: 4.1–10.6) in KPW and among sulfonylurea users it was 4.7 years (iqr: 2.6–6.8) in VHA and 7.1 years (iqr: 3.8–10.3) in KPW.
The distributions of covariates by metformin and sulfonylurea use are shown in Table 2. In the VHA and KPW samples, large differences (SMD% >10) in the prevalence of metformin and sulfonylurea dispensing occurred by year of observation with sulfonylurea more prevalent in earlier years and metformin more prevalent in later years of observation. In both patient populations, older age, higher HbA1c values and higher creatinine values, ischemic heart disease, congestive heart failure and atrial fibrillation were more prevalent among sulfonylurea users. Obesity was more prevalent among metformin users. In VHA patients, post-traumatic stress disorder and non-steroidal anti-inflammatory drug use were more prevalent among metformin users. In KPW, statin use was more prevalent among metformin users.
Table 2.
Characteristics (%) of ≥ 50 years old patients initiating metformin (MET) and sulfonylurea (SU) from the Veterans Health Administration (VHA; FY2002 to FY2012) and Kaiser Permanente Washington State (KPWA; 1996–2012) health care systems before balancing characteristics using inverse probability of treatment weighting
| VHA (n=75,187) |
KPWA (n=10,866)a |
|||||||
|---|---|---|---|---|---|---|---|---|
| Covariates | MET (n=56,972) | SU (n=18,215) | p-value | SMD %b | MET (n=7,546) | SU (n=3,320) | p-value | SMD % |
| Index year | <.0001 | <.0001 | ||||||
| 1996–1998 | - | - | - | 5.9 | 28.6 | −62.8% | ||
| 1999–2000 | - | - | - | 6.2 | 19.2 | −39.6% | ||
| 2001–2002 | - | - | - | 9.7 | 14.5 | −14.6% | ||
| 2002–2004 | 4.6 | 8.8 | −16.9% | - | - | - | ||
| 2003–2004 | - | - | - | 12.3 | 11.8 | 1.4% | ||
| 2005–2006 | 28.8 | 44.0 | −31.9% | 16.2 | 11.1 | 14.9% | ||
| 2007–2008 | 26.9 | 24.1 | 6.4% | 17.2 | 6.6 | 33.4% | ||
| 2009–2012 | - | - | - | 32.5 | 8.3 | 62.7% | ||
| 2009–2010 | 22.8 | 14.7 | 20.9% | - | - | - | ||
| 2011–2012 | 16.9 | 8.4 | 25.8% | - | - | - | ||
| Sociodemographic-related | ||||||||
| Age, mean (±sd) | 60.0 (±8.0) | 63.5 (±9.4) | <.0001 | −40. 3% | 61.0 (±7.8) | 67.8 (±10.9) | <.0001 | −72.3% |
| Age category | ||||||||
| 50–64 | 70.8 | 54.2 | 34.9% | 71.0 | 40.8 | 63.9% | ||
| 65–74 | 23.0 | 29.7 | <.0001 | −15.4% | 22.3 | 27.8 | <.0001 | −12.8% |
| ≥ 75 | 6.2 | 16.1 | −31.8% | 6.8 | 31.5 | −66.2% | ||
| Male gender | 96.6 | 97.5 | <.0001 | −5.2% | 49.6 | 52.4 | .007 | −5.6% |
| Race | ||||||||
| White | 83.9 | 84.4 | −1.4% | 73.4 | 76.8 | −7.8% | ||
| Black | 14.1 | 13.8 | .076 | 0.8% | 4.3 | 3.8 | 2.3% | |
| Asian | - | - | 7.8 | 6.8 | .001 | 3.8% | ||
| Other | 2.0 | 1.7 | 1.8% | 3.3 | 2.2 | 6.7% | ||
| Unknown | - | - | 11.2 | 10.4 | 2.7% | |||
| Married | 59.1 | 59.6 | .222 | −1.0% | - | - | ||
| Low nSES c | 49.4 | 51.7 | <.0001 | −4.6% | 50.2 | 48.8 | .174 | 2.8% |
| VHA only insurance | 47.2 | 41.6 | <.0001 | 11.4% | - | - | ||
| Medicaid/Medicare | 30.4 | 58.2 | <.0001 | −58.3% | ||||
| High healthcare utilization d | 24.7 | 25.9 | .001 | −2.7% | 21.7 | 31.9 | <.0001 | −23.2% |
| Diabetes-related | ||||||||
| Diabetic nephropathy | 1.1 | 1.6 | <.0001 | −4.7% | 2.0 | 1.6 | .095 | 3.6% |
| Diabetic retinopathy | 4.8 | 6.6 | <.0001 | −7.9% | 2.6 | 4.4 | <.0001 | −9.8% |
| Diabetic neuropathy | 6.7 | 8.6 | <.0001 | −7.1% | 8.1 | 8.6 | .438 | −1.6% |
| HbA1c value, mean (±sd) | 7.2 (±1.4) | 7.4 (±1.6) | <.000 1 | −15.8% | 8.4 (±1.9) | 8.9 (±2.2) | <.0001 | −23.5% |
| HbA1c category | ||||||||
| < 7 | 53.0 | 45.4 | 15.2% | 19.3 | 14.2 | 13.7% | ||
| 7–8 | 30.7 | 32.1 | <.0001 | −3.0% | 30.7 | 25.7 | <.0001 | 11.1% |
| > 8 | 16.3 | 22.5 | −15.6% | 50.0 | 60.1 | −20.4% | ||
| Creatinine value, mean (±sd) | 1.0 (±0.2) | 1.1 (±0.2) | <.0001 | −27.3% | 0.9 (±0.2) | 1.0 (±0.2) | <.0001 | −42.6% |
| Other comorbidities | ||||||||
| Obesity | 62.2 | 51.1 | <.0001 | 22.5% | 57.9 | 33.1 | <.0001 | 51.5% |
| Hypertension | 90.6 | 91.6 | <.0001 | −3.6% | 59.5 | 54.1 | <.0001 | 11.0% |
| Hyperlipidemia | 87.6 | 84.8 | <.0001 | 8.4% | 44.6 | 29.9 | <.0001 | 25.1% |
| Stroke | 6.3 | 8.3 | <.0001 | −7.7% | 1.7 | 3.4 | <.0001 | −28.4% |
| Ischemic heart disease | 46.1 | 53.0 | <.0001 | −13.9% | 12.9 | 22.4 | <.0001 | −25.0% |
| Congestive heart failure | 15.7 | 23.4 | <.0001 | −19.7% | 3.3 | 10.3 | <.0001 | −28.4% |
| Atrial fibrillation | 11.9 | 17.4 | <.0001 | −15.6% | 4.7 | 11.4 | <.0001 | −25.0% |
| Traumatic brain injury | 6.3 | 6.1 | .376 | 0.8% | 1.3 | 1.6 | .129 | −3.1% |
| Vitamin B12 deficiency | 4.7 | 5.3 | .001 | −2.7% | 0.7 | 1.0 | .134 | −3.0% |
| Psychiatric and substance comorbidities | ||||||||
| Depression | 29.6 | 25.4 | <.0001 | 9.5% | 4.4 | 4.6 | .706 | −0.8% |
| PTSD | 20.6 | 16.2 | <.0001 | 11.3% | 0.5 | 0.2 | .012 | 5.7% |
| Other anxiety e | 14.0 | 12.7 | <.0001 | 3.8% | 3.1 | 2.1 | .008 | 5.7% |
| Bipolar disorder | 8.1 | 6.6 | <.0001 | 5.9% | 1.9 | 1.1 | .002 | 6.9% |
| Schizophrenia | 5.6 | 5.2 | .028 | 1.9% | 0.3 | 0.3 | .855 | −0.4% |
| Nicotine dependence/smoker | 53.6 | 51.6 | <.0001 | 4.0% | 17.5 | 16.5 | .184 | 2.8% |
| Alcohol abuse/dependence | 16.5 | 14.7 | <.0001 | 4.9% | 1.7 | 2.2 | .082 | −3.5% |
| Illicit drug abuse/dependence | 8.3 | 7.5 | .0004 | 3.0% | 0.6 | 0.6 | .755 | 0.7% |
| Other medications f | ||||||||
| Statins | 70.3 | 67.0 | <.0001 | 7.1% | 39.5 | 31.2 | <.0001 | 17.4% |
| Anticholinergics | 45.6 | 43.5 | <.0001 | 4.2% | 22.2 | 26.2 | <.0001 | −9.3% |
| NSAIDs | 57.6 | 51.3 | <.0001 | 12.7% | 18.7 | 20.0 | .112 | −3.3% |
| Antihypertensives | 87.4 | 88.6 | <.0001 | −3.6% | 44.4 | 44.6 | .866 | −0.4% |
KPW dynamic cohort – covariates measured for 2 year prior to every new metformin/sulfonylurea start; VHA=Veterans Health Administration; KPW=Kaiser Permanente Washington State; FY=Fiscal Year; MET=Metformin; SU=Sulfonylurea;
SMD % = Standardized mean difference percent comparing MET to SU, SMD% >10 considered a large difference; nSES = neighborhood socioeconomic status,
nSES = top 50th vs. bottom 50th percentile of neighborhood socioeconomic status based on information from 7 measures of SES obtained from 2009–2013 5-year census estimates from the American Community Survey
High healthcare utilization-top 25th percentile of mean visits per month
Other anxiety disorders = panic disorder, OCD, social phobia, GAD, Anxiety NOS
Other medications associated with dementia= sustained use prior to MET or SU (at least 2 fills in a 6 month period)
IPTW balanced potential confounding variables in VHA and KPW patients samples overall and by age stratum (age 50–64, age 65–74, age ≥75) as indicated by all SMD%’s <10% (Appendix B). Year of observation and HbA1c>8 (64mmol/mol) did not balance in KPW patients 50–64 years of age.
The unadjusted incidence of dementia was lower for metformin vs. sulfonylurea users in VHA patients (9.6/1000PY vs. 14.9/1000PY) and KPW patients (5.1/1000PY vs. 15.7/1000PY) (Appendix C1, C2). When we stratified by age, the pattern of lower risk among metformin users held true, except for VHA patients ≥75 years of age.
Results from Cox proportional hazard models (Table 3) indicated that after controlling for confounding, metformin initiation compared with sulfonylurea initiation was significantly associated with a lower risk of dementia (hazard ratio (HR)=0.93; 95%CI: 0.87–0.99) in VHA patients (see model two). In age stratified analyses, metformin was associated with a lower risk of dementia in VHA patients 50–64 years of age and 65–74 years of age but not among those ≥ 75 years of age. In the VHA sample, the interaction of age and drug was significant (P =0.04). As shown in model 2, among all KPW patients the association between metformin use, compared to sulfonylurea use, and incident dementia was of similar magnitude as found in the VHA sample but was not statistically significant (HR=0.89; 95%CI: 0.74–1.07). In KPW patients, the interaction of age and drug was not significant (P =0.23).
Table 3.
Results (HR (95%CI)) from Cox proportional hazards models estimating the association metformin (MET) vs. sulfonylurea (SU) and incident dementia in FY2002 to FY2015, overall and stratified by age among VHA patients ≥50 years old (n=75,187) and KPW patients ≥50 years old (n=10,866)
| Model 1 – Crudea MET vs. SU: | Model 2–Weightedb MET vs. SU: | Model 3 −Weighted + AMGBc MET vs. SU: | Model 4−Weighted + hypoglycemic episode MET vs. SU | |||||
|---|---|---|---|---|---|---|---|---|
| Age group | VA patients | KPW patients | VA patients | KPW patients | VA patients | KPW patients | VA patients | KPW patients |
| All ages ≥ 50 years | 0.66 (0.62–0.70) |
0.37 (0.32–0.44) |
0.93 (0.87–0.99) |
0.89 (0.74–1.07) |
0.89 (0.83–0.95) |
0.88 (0.73–1.07) |
0.95 (0.78–0.99) |
0.89 (0.73–1.07) |
| Age Strata | ||||||||
| Age 50–64 years | 0.85 (0.75–0.95) |
1.04 (0.67–1.61) |
0.86 (0.76–0.98) |
1.33 (0.78–2.25) |
0.84 (0.73–0.96) |
1.35 (0.79–2.28) |
0.89 (0.78–1.02) |
1.36 (0.80–2.31) |
| Age 65–74 years | 0.87 (0.80–0.95) |
0.76 (0.58–0.98) |
0.87 (0.80–0.96) |
0.79 (0.58–1.07) |
0.87 (0.79–0.96) |
0.78 (0.58–1.06) |
0.89 (0.81–0.98) |
0.79 (0.58–1.08) |
| Age ≥75 years | 1.05 (0.95–1.16) |
0.78 (0.62–0.99) |
1.03 (0.9–1.14) |
0.79 (0.60–1.03) |
1.02 (0.91–1.15) |
0.82 (0.62–1.07) |
1.05 (0.94–1.16) |
0.81 (0.61–1.06) |
Note: VHA=Veterans Health Administration; KPW=Kaiser Permanente Washington State; FY=Fiscal Year; MET=Metformin; SU=Sulfonylurea; AMGB=average monthly glycemic burden; HR=hazard ratio; CI=confidence interval
Unweighted data
Inverse probability of treatment weighted data with robust, sandwich-type variance estimators
Additional adjustment for glycemic burden from MET/SU initiation to end of follow-up among a subset of 71,266 VA patients and 10,743 with at least 2 HbA1c values after MET/SU initiation
Meta-analysis indicated metformin, compared to sulfonylurea use, is associated with an 8% (HR=0.92; 95%CI: 0.87–0.98) reduced risk of dementia and an 11% (HR=0.89; 95%CI: 0.83–0.95) lower risk after adjusting for AMGB.
Results of moderator analysis, adjusting for AMGB, (Table 3, model 3), and adjusting for hypoglycemic events (Table 3, model 4) indicate the associations between metformin vs. sulfonylurea and incident dementia remained similar to those observed in model 2.
Sensitivity analysis, allowing diagnoses for mild cognitive impairment to contribute to the outcome, did not change our results. Cox proportional hazard model results for VHA and KPW patients as a whole and by age stratum were nearly the same, data not shown, when compared to the primary analysis reported in Table 3.
DISCUSSION
Among VHA and KPW patients ≥50 years old, initiation of treatment for diabetes with metformin compared with a sulfonylurea was associated with a significant, modest reduction in the risk of dementia. In the KPW replication population, while not statistically significant, the hazard ratio was similar to analysis of the VHA sample (KPW: HR=0.89; 95%CI: 0.74–1.07 and VHA: HR=0.93; 95%CI: 0.87–0.99). Meta-analysis of the two patient samples indicates metformin, compared to sulfonylurea use, is associated with an 8% (HR=0.92; 95%CI: 0.87–0.98) reduced risk of dementia.
Our results are consistent with a recent study of VHA patients ≥65 that used a similar analytic approach 10. This prior study required patients to be only metformin or only sulfonylurea users for two years prior to follow-up and excluded patients who switched medications during follow-up 10. Patients 50–64 years of age accounted for 70.8% of new metformin users and 54.2% of new sulfonylurea users, therefore we can be sure 71% of our metformin users and 54.2% of our sulfonylurea users did not overlap with the prior VHA study. Despite these differences, we obtained nearly the same point estimates for our primary analysis in two separate patient populations that differ markedly in sociodemographic and clinical characteristics, and as a result we argue the present study results are the most robust evidence supporting the conclusion that metformin has a modest protective association with incident dementia. Similar to Orkaby and colleagues 10 results, we found no significant association between metformin and dementia among patients ≥ 75 years of age. The lack of an association may be due to a ceiling effect where the modest lower risk of dementia in patients who use metformin is not detected in patients at high risk for cognitive decline. Our results and those of Orkaby and colleagues 10 may differ from studies which report no association or increased risk of dementia in metformin users 7,8 because the latter did not adequately control for bias by indication.
We speculate that the association between metformin and lower risk of dementia is independent of glycemic control. Over 53% of KPW patients and only 17.8% of VHA patients had an HbA1c >8.0 (64mmol/mol) at baseline yet the hazard ratios for the two samples were similar. In addition, adjusting for AMGB and hypoglycemic events did not substantially change the magnitude of association between metformin and incident dementia. Based on animal studies, metformin may reduce dementia risk by attenuating biochemical factors such as tau phosphorylation, activated c-jun N-terminal kinase, and reduction of synaptophysin 27,28.
Limitations:
We are unable to make conclusions about effects that could be due to dose, duration of use, and medication adherence, however results are relevant to the real world patient population that includes adherent and non-adherent medication users. Our diagnostic algorithms were created to increase positive predictive value but may lack sensitivity and failed to identify some patients with incident dementia. Such false negatives could bias our results, most likely to the null resulting in conservative estimates of the association between metformin and incident dementia. We controlled for many potential confounding factors, however, unmeasured confounding is possible and may influence our findings. Though very unlikely because of our eligibility criteria, i.e. diabetes onset at age 50 or older, it is possible that we did not completely eliminate type 1 diabetes. We used established methods for measuring hyperglycemia and hypoglycemia but we realize that for the latter there may be episodes that are less severe and not diagnosed. Among all patients ≥ 50 years of age, our results from VHA patient data were replicated in KPW. However, it is not certain that findings would generalize to other regions of the United States or to other countries. We recognize that some age stratified analyses in the KPW cohort may have been under-powered.
Conclusions
Metformin is associated with a modest reduction in risk of dementia. Though modest, it warrants mentioning that the Food and Drug Administration approved treatment for dementia, donepezil, has only a modest effect on dementia progression.29 The present study provides the strongest evidence to date for an association between metformin and lower risk of dementia in patients with diabetes, a condition associated with a 70% increased risk of dementia. Dementia is expected to quadruple worldwide with >106 million cases by 2050. 30 Studies of metformin’s effect on cognitive decline in early dementia are warranted. Randomized trials may be helpful to clarify potential benefits of metformin to delay or prevent dementia in high risk populations.
Supplementary Material
ACKNOWLEDGEMENTS
Funding: This study was supported by National Institute on Aging grant R21 AG055604
Guarantors: Dr. Scherrer had full access to VHA data and Dr. Dublin had full access to KPW data. Dr. Scherrer had final responsibility for the decision to submit for publication
ABBREVIATIONS
- AMGB
average monthly glycemic burden
- FY
fiscal years
- HbA1c
hemoglobin A1c
- ICD-9-CM
International Classification of Diseases, Ninth Revision, Clinical Modification
- iqr
interquartile range
- IPTW
inverse probability of treatment weighting
- KPW
Kaiser Permanente Washington
- nSES
neighborhood socioeconomic status
- PY
person-years
- PS
propensity scores
- SMD%
standardized mean difference
- VHA
veterans health affairs
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclaimer: The views expressed to not necessarily reflect those of the Veterans Health Affairs
Declaration of interests: all authors report no conflicts of interest that could inappropriately influence this work
REFERENCES
- 1.Chatterjee S, Peters SA, Woodward M, et al. Type 2 diabetes as a risk factor for dementia in women compared with men: A pooled analysis of 2.3 million people comparising more than 100,000 cases of dementia. Diabetes Care 2016;39:300–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.DiTacchio K, Heinemann S, Dziewczapolski G. Metformin treatment alters memory function in a mouse model of Alzheimer’s disease. J Alzheimers Dis 2015;44(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen F, Dong R, Zhong K, et al. Antidiabetic drugs restore abnormal transport of amyloid-β across the blood-brain barrier and memory impairment in db/db mice. Neuropharmacology 2016;101(123–136). [DOI] [PubMed] [Google Scholar]
- 4.Mousavi S, Niazmand S, Hosseini M, et al. Beneficial Effects of Teucrium polium and Metformin on Diabetes-Induced Memory Impairments and Brain Tissue Oxidative Damage in Rats. Int J Alzheimers Dis 2015;2015(493729). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McNeilly AD, Williamson R, Balfour DJ, Stewart CA, Sutherland C. A high-fat-diet-induced cognitive deficit in rats that is not prevented by improving insulin sensitivity with metformin. Diabetologia 2012;55(11):3061–3070. [DOI] [PubMed] [Google Scholar]
- 6.Chen Y, Zhou K, Wang R, et al. Antidiabetic drug metformin (GlucophageR) increases biogenesis of Alzheimer’s amyloid peptides via up-regulating BACE1 transcription. Proceedings of the National Academy of Sciences of the United States of America 2009;106(10):3907–3912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moore E, Mander A, Ames D, et al. Increased Risk of Cognitive Impairment in Patients With Diabetes Is Associated With Metformin. Diabetes Care 2013;36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Imfeld P, Bodmer M, Jick S, Meier C. Metformin, other antidiabetic drugs, and risk of Alzheimer’s disease: a population-based case-control study. J AM Geriatr Soc 2012;60(5). [DOI] [PubMed] [Google Scholar]
- 9.Wang CP, Lorenzo C, Habib SL, Jo B, Espinoza SE. Differential effects of metformin on age related comorbidities in older men with type 2 diabetes. J Diabetes Complications 2017;31(4):679–686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Orkaby AR, Cho K, Cormack J, Gagnon DR, Driver JA. Metformin vs sulfonylurea use and risk of dementia in US veterans aged >/=65 years with diabetes. Neurology 2017;89(18):1877–1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hsu CC, Wahlqvist ML, Lee MS, Tsai HN. Incidence of dementia is increased in type 2 diabetes and reduced by the use of sulfonylureas and metformin. J Alzheimers Dis 2011;24(3):485–493. [DOI] [PubMed] [Google Scholar]
- 12.Cheng C, Lin C, Tsai Y, Tsai C, Chou P, Lan T. Type 2 diabetes and antidiabetic medications in relation to dementia diagnosis. J Gerontol A Biol Sci Med Sci 2014;69(10). [DOI] [PubMed] [Google Scholar]
- 13.Inzucchi SE, Lipska KJ, Mayo H, Bailey CJ, McGuire DK. Metformin in patients with type 2 diabetes and kidney disease: a systematic review. JAMA 2014;312(24):2668–2675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Roblin DW. Validation of a Neighborhood SES Index in a Managed Care Organization. Med Care 2013;51:e1–e8. [DOI] [PubMed] [Google Scholar]
- 15.Curtis LH, Hammill BG, Eisenstein EL, Kramer JM, Anstrom KJ. Using inverse probability-weighted estimators in comparative effectiveness analysis with observational databases. Med Care 2007;45:S103–S107. [DOI] [PubMed] [Google Scholar]
- 16.Rosenbaum PR, Rubin DB. The central role of the propensity score in observational studies for causal effects. Biometrika Trust 1983;70:41–55. [Google Scholar]
- 17.Xu S, Ross C, Raebel MA, Shetterly S, Blanchette C, Smith D. Use of stabilized inverse propensity scores as weights to directly estimate relative risk and its confidence intervals. Value Health 2010;13:273–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Harder VS, Stuart EA, Anthony JC. Propensity score techniques and the assessment of measure covariate balance to test causal associations in psychological research. Psychol Methods 2010;15:234–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sturmer T, Wyss R, Glynn RJ, Brookhart MA. Propensity scores for confounder adjustment when assessing the effects of medical interventions using nonexperimental designs. J Intern Med 2014;275:570–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Austin P, Stuart E. Moving towards best practice when using inverse probability of treatment weighting (IPTW) using the propensity score to estimate causal treatment effects in observational studies. Stat Med 2015;34:3661–3679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dominguez Islas C, Rice KM. Addressing the estimation of standard errors in fixed effects meta-analysis. Stat Med 2018;37(11):1788–1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yaffe K, Falvey C, Hamilton N, et al. Diabetes, glucose control, and 9-year cognitive decline among older adults without dementia. Arch Neur 2012;69(9):1170–1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Feinkohl I, Aung PP, Keller M, et al. Severe hypoglycemia and cognitive decline in older people with type 2 diabetes: the Edinburgh type 2 diabetes study. Diabetes Care 2014;37(2):507–515. [DOI] [PubMed] [Google Scholar]
- 24.Nichols G, Rosales A, Perrin N, Fortmann S. The association between A1c-based measures of glycemia and risk of cardiovascular disease hospitalization. Diabetes Care 2014;37:167–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brown J, Nichols G, Perry A. The burden of treatment failure in type 2 diabetes. Diabetes Care 2004;27(7). [DOI] [PubMed] [Google Scholar]
- 26.Ginde AA, Blanc PG, Lieberman RM, Camargo CA Jr. Validation of ICD-9-CM coding algorithm for improved identification of hypoglycemia visits. BMC Endocr Disord 2008;8:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kickstein E, Krauss S, Thornhill P, et al. Biguanide metformin acts on tau phosphorylation via mTOR/protein phosphatase 2A (PP2A) signaling. Proceedings of the National Academy of Sciences of the USA 2010;107(50). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li J, Deng J, Sheng W, Zuo Z. Metformin attenuates Alzheimer’s disease-like neuropathology in obese, leptin-resistant mice. Pharmacol Biochem Behav 2012;101:564–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rogers SL, Doody RS, Mohs RC, Friedhoff LT. Donepezil improves cognition and global function in Alzheimer disease: a 15-week, double-blind, placebo-controlled study. Donepezil Study Group. Arch Intern Med 1998;158(9):1021–1031. [DOI] [PubMed] [Google Scholar]
- 30.Brookmeyer R, Johnson E, Ziegler-Graham K, Arrighi HM. Forecasting the global burden of Alzheimer’s disease. Alzheimers Dement 2007;3:186–191. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.