Abstract
Objective:
To evaluate the effect of chemotherapy added to a surgical locoregional treatment (LRT) for patients with locally advanced head and neck squamous cell carcinoma (HNSCC).
Materials and Methods:
We studied the sub-group of trials with surgical LRT included in the meta-analysis on chemotherapy in head and neck cancer (MACH-NC). Data from published and unpublished randomized trials comparing the addition of chemotherapy to LRT in HNSCC patients were sought using electronic database searching for the period 1965–2000, hand searching and by contacting experts in the field. Trials with less than 60 patients, or preoperative radiotherapy or where the type of LRT could not be individually determined were excluded. All individual patient data were checked for internal consistency, compared with published reports, and validated with trialists. Data were pooled using a fixed-effect model. Heterogeneity was assessed using Cochrane test and I2 statistics.
Results:
Twenty-four trials were eligible (5000 patients). Chemotherapy improved overall survival (HR=0.92 [95%CI: 0.85 to 0.99] p=0.02). There was a significant interaction between treatment effect and timing of chemotherapy (p=0.08 at pre-specified threshold of 0.1) with a greater effect for concomitant chemotherapy (HR=0.79, 95%CI: 0.69 to 0.92). The benefit of chemotherapy was greater in women (HRwomen=0.63, 95%CI: 0.50 to 0.80) compared to men (HRmen=0.96, 95%CI: 0.89 to 1.04; p for interaction =0.001).
Conclusions:
This analysis confirmed the benefit of concomitant chemotherapy added to surgical LRT. The role of induction therapy as yet to be determined as it did not improve OS. Women may benefit more than men from chemotherapy.
Keywords: head and neck cancer, squamous cell carcinoma, chemotherapy, surgery, meta-analysis, individual patient data, randomized trial
INTRODUCTION
Every year, more than 600 000 patients are diagnosed with head and neck cancer worldwide1. Most of these cancers are squamous cell carcinomas (HNSCC); half of them diagnosed at locally advanced stage2. In the individual patient data (IPD) meta-analysis of randomized clinical trials MACH-NC (Meta-analysis of Chemotherapy in Head and Neck Cancer), we showed that the addition of chemotherapy to locoregional treatment (LRT) improved overall survival (OS) in locally advanced non-metastatic HNSCC3. This meta-analysis of 87 trials completed between 1965 and 2000 included 16 485 patients. Hazard ratio (HR) for death was 0.88 (95% confidence interval (95%CI): 0.85 to 0.92; p<.001) with an absolute benefit on survival of 4.5% at 5 years. Benefit was significantly more pronounced for chemotherapy concomitant to radiotherapy with a 6.5% benefit at 5 years (HR=0.81, 95%CI 0.78 to 0.86). In this meta-analysis, patients treated by surgery, radiotherapy, or both were analyzed together. However, patients’ characteristics usually differ between trials that included patients treated by surgery as primary LRT and trials that included patients treated by radiotherapy only. For example, in a study on oral cavity cancer, patients treated by surgery were younger and had lower stage cancer4. In a preliminary analysis of the MACH-NC database, we compared patients treated by surgery (+/− radiotherapy) to patients treated by radiotherapy only. Among the patients included in this preliminary analysis (eTable 1), 5 352 (32.9 %) had surgery as LRT. These patients had lower stage tumors (48% stage IV versus 65% for radiotherapy patients; p<.001) and had oral cavity tumors more frequently (33% versus 21% for radiotherapy patients; p<.001). Age, sex, and performance status were also significantly different. Forty per cent of patients treated by surgery received induction chemotherapy whereas only 21% of patients treated by radiotherapy did. Because patients treated by surgery are different, the effect of chemotherapy and its interaction with patient characteristics on their survival might vary. Moreover the addition of surgery in LRT changes the adverse events patients may encounter and modifies the timing of radiotherapy. Finally, although the MACH-NC meta-analysis showed no effect of induction therapy on survival, the use of induction chemotherapy is still debated, especially when surgery is considered for LRT5–7. Thus, it was decided to perform a specific analysis of patients treated by surgery in the MACH-NC database.
The primary objective was to evaluate the benefit on overall survival of chemotherapy in addition to a surgical LRT for patients diagnosed with locally advanced HNSCC. There were two secondary objectives: first, to investigate interaction between the effect of chemotherapy and patient or trial characteristics; and second, to study event-free survival and the different types of failure.
METHODS
The protocol for this meta-analysis was redacted prior to the analysis and is available at: https://www.gustaveroussy.fr/sites/default/files/protocol_mach_nc_surg.pdf
Trial selection
This meta-analysis studied the subgroup of patients treated by surgery in the MACH-NC database. Selection of included trials was described in previous publications3. All trials had to include previously untreated patients with locally advanced non-metastatic HNSCC. Accrual had to be completed between 1965 and 2000. Trials had to use a randomization method that precluded prior knowledge of treatment assignment. To be eligible in this analysis, trials had to compare curative surgical LRT (+/− radiotherapy) versus the addition of chemotherapy to the same LRT. The timing of chemotherapy could be before surgery (induction), during post-operative radiotherapy (concomitant) or after the end of the LRT (adjuvant). Trials with less than 60 patients or with systematic preoperative radiotherapy were excluded. Trials in which the patients could be treated by surgery (+/− radiotherapy) or radiotherapy alone, and in which the type of LRT could not be individually determined were excluded, except if more than 50% of patients had surgery. Both published and unpublished trials were included.
Data collection and consistency checking
The data collected for each patient were: age, sex, tumor stage, tumor site, performance status, treatment allocated, survival and failure status, date of randomization, date of first failure, date of death or date of last follow up. Information retrieved for each trial was: the timing of chemotherapy, the type of chemotherapy (number and type of drugs), and the neck dissection strategy. For induction trials, information on surgical margins strategy, planned number of chemotherapy cycles and possibility of early LRT for non-responding patients was also collected. All IPD were checked with a standard procedure3,8,9, which follows the recommendations of the Cochrane working group on meta-analysis using IPD. Results were compared with protocol (when available) and published reports, and validated with the corresponding trialist.
Outcomes
Primary endpoint was overall survival (OS), defined as the time from randomization to death from any cause. Secondary endpoints were early death and event-free survival. Death was considered early when it occurred within 6 months after randomization. Event-free survival was defined as the time from randomization to the first event10 (locoregional failure, distant failure, or death from any cause). Living patients that presented no event were censored at their date of last follow up. Events considered as locoregional failures were local failure, regional failure, or concomitant local and regional failure without concomitant distant failure. Events considered as distant failure were distant failure, either alone or combined with local or regional failure. Events considered as death without failure were death without previous locoregional or distant event.
Statistical Analysis
All randomized patients were included in an intent-to-treat analysis. Median follow up was calculated with the reverse Kaplan-Meier method11. Analyses were stratified by trial. We calculated trial and overall pooled hazard ratios (HR) using the log-rank expected number of events and variance, using a fixed effect model. Stratified survival curves were computed for control and experimental groups using Peto’s method and were used to calculate absolute benefit at 5 years12,13. Heterogeneity of chemotherapy effect among trials was assessed using χ2 heterogeneity test and I2 statistic14. Because heterogeneity test is not powerful, we chose a 0.10 significance threshold15. In case of significant heterogeneity, we performed sensitivity analysis to identify the source of heterogeneity. If heterogeneity was still significant and unexplained, we used a random-effect model15,16.
Three sensitivity analyses were planned by exclusion of some trials: with less than 100 patients, with a median follow up <5 years, and whose accrual period began before 1980. We also conducted a post hoc analysis where outlier trials (trials that had a 95%CI that did not overlap with the 95%CI of the global HR) were excluded.
In subset analyses, we used χ2 heterogeneity tests among different groups of trials to study interaction between trial characteristics and treatment effect. The residual heterogeneity within trial subgroups was the difference between the overall χ2 heterogeneity statistics and the χ2 heterogeneity statistic between groups17. Trial subsets were predefined according to: timing of chemotherapy, type of chemotherapy drugs, and neck dissection strategy (not performed because of high rate of missing data); for induction trials, surgical margins strategy, type of induction protocol (number of cycles, possibility of early LRT). We performed two post hoc analyses: the first studied the type of chemotherapy in induction trials and the second, the administration of radiotherapy in adjuvant trials. We investigated interaction between treatment effect and patients characteristics (age, stage, sex, performance status, and primary site of tumor) in a Cox model stratified by trial that included treatment arm, covariate and interaction. Trials in these analyses had to include patients in all categories of the variable under study. In case of significant interaction, the results were confirmed in a multivariate model including the other individual characteristics. Since only the first event was collected in the meta-analysis, locoregional failure, distant failure, and death without failure were analyzed using Fine and Gray models (unplanned competing risk analysis)18,19. Analyses were done using SAS, version 9.4 and RStudio (“crrsc” package for competing risk analysis), version 3.2.5.
Role of the funding source
The sponsors of this study had no role in the study design, data collection, data analysis, data interpretation, or in the writing of the report.
RESULTS
Population
Among the 87 trials included in MACH-NC database we identified 39 that proposed a surgical LRT. Fifteen of those met predefined exclusion criterions (eFigure 1). The meta-analysis included 24 trials20–43 (5 000 patients) evaluating surgical LRT versus the same LRT + chemotherapy (eTable2). One trial (UKHAN-143) had two strata comparisons based on the type of chemotherapy and was considered above as two distinct trials. There were 7 adjuvant chemotherapy trials (1 743 patients), 11 induction chemotherapy trials (1 925 patients) and 6 concomitant trials (1 332 patients). Two trials were unpublished (BNH00329, EORTC 2484432) and two were published as abstracts only (AHNTG27, GETTECadj20). Postoperative radiotherapy was planned in most of trials. Five adjuvant trials21,23–26 had only surgery as LRT (933/1 743 patients of adjuvant trials). Overall median follow up was 4.9 years (range: 1.3 to 13.7 years). Description of the overall population is available in eTable 3. Number of events in each arm is given for all endpoints in eTable 4.
Overall survival and event-free survival
There were 2 696 deaths. Chemotherapy improved OS (HR=0.92 [95%CI: 0.85 to 0.99] p=0.02, figure 1), with an absolute benefit of 4.4% (95%CI: 1.3 to 7.5%) at 5 years (figure 2). There was a significant interaction between treatment effect and timing of chemotherapy (p=0.08 at pre-specified threshold of 0.10) with a greater effect for concomitant chemotherapy (HR=0.79, 95%CI: 0.69 to 0.92) than for induction (HR=0.96, 95%CI: 0.85 to 1.08) or adjuvant chemotherapy (HR=0.98, 95%CI: 0.85 to 1.12). Heterogeneity was significant but moderate (I2=35%; p=0.04). Results of sensitivity analyses showed similar results for treatment effect and heterogeneity (eTable 5), except for the one based on trials with follow-up longer than 5 years (p for treatment effect =0.40) and the post-hoc analysis excluding two outlier trials previously identified44 (GETTECadj20 and Toulouse38; p for heterogeneity =0.39). For event-free survival, based on 23 trials and 4 501 patients (2 659 events), similar results were observed (eFigure 2), with an overall HR of 0.90 (95%CI: 0.84 to 0.98; p=0.01) and an absolute benefit of 3.3% (95%CI: 0.1 to 6.5%) at 5 years (eFigure 3). There was a significant interaction between treatment effect and timing of chemotherapy (p=0.05) with a greater effect for concomitant chemotherapy (HR=0.78, 95%CI: 0.68 to 0.90) than for induction (HR=0.98, 95%CI: 0.88 to 1.10) and adjuvant chemotherapy (HR=0.91, 95%CI: 0.78 to 1.07). Heterogeneity was also significant (I2=46%, p for heterogeneity =0.03).
Figure 1: Hazard ratio of death with loco-regional treatment plus chemotherapy versus locoregional treatment alone.

This analysis was performed using a fixed-effect model. Heterogeneity is discussed in eTable 6 and in the beginning of Discussion section. The broken line and center of the black diamond correspond to overall pooled hazard ratio (HR) and the horizontal tip of the diamond is the 95% confidence interval (95%CI). The center of the black square corresponds to the HR of trials. The area of the square and the variance of (O-E) are proportional to the number of deaths in each trial. Trials are ordered chronologically (oldest at the top of figure). CT = Chemotherapy, LRT = Loco-regional treatment; O-E = observed minus expected, I2 = Higgins statistic for heterogeneity, No. = Number. In HNCP trial, the arm on induction CT and the one on induction plus maintenance CT were pooled.
Trial group abbreviations:
AHNTG = Australian Head and neck Trial Group, BNH = B. Nanavati Hospital / Mumbai Group (India), EORTC = European Organisation for Research and Treatment of Cancer, GETTEC = Groupe d’Etude des Tumeurs de la Tête Et du Cou (France), GSTTC = Gruppo di Studio sui Tumori della Testa et del Collo (Italy), HNCP = Head and Neck Contract Program (USA), HNU = Head and Neck UFT (Japan), , INT = US INTer group trial, JHCFUS = Japanese HexyCarbanoyl 5-FluoroUracil Study, KKD = Kanto Koshinetsu District (Japan), LOHNG = Ljubljana Oncology Head and Neck Group (Slovenia), RTOG = Radiation Therapy Oncology Group (USA), SWOG = Southwest Oncology Group (USA), TMH = Tata Memorial Hospital (India), UKHAN = United Kingdom Head And Neck (UKCCR head and Neck Collaborative Group, UK), Yale = Yale University (USA).
Figure 2: Overall survival curves by treatment arm for all trials and for trial subset defined by timing of chemotherapy.

The slopes of the broken lines from year 7 to year 8 are based on the overall death rates in the seventh and subsequent years. Absolute differences are given with their 95% confidence interval. LRT = Loco-regional treatment, CT = Chemotherapy.
a) All trials; b) Adjuvant chemotherapy trials; c) Induction chemotherapy trials; d) Concomitant chemotherapy trials.
Within 6 months after randomization, 323 deaths occurred. Overall HR for the effect of chemotherapy on early death was 1.21 (95%CI: 0.97 to 1.51, p=0.08) without significant difference between concomitant chemotherapy (HR=0.98, 95%CI: 0.65 to 1.47), induction therapy (HR=1.36, 95%CI: 0.96 to 1.94), or adjuvant chemotherapy (HR=1.28, 95%CI: 0.87 to 1.90; p for interaction =0.45, eFigure 4).
Subset analyses
The effect of chemotherapy on OS was significantly different according to the type of chemotherapy (p for interaction =0.02): the HR was 0.74 (95%CI: 0.62 to 0.88) for platinum alone, 0.88 (95%CI: 0.76 to 1.02) for poly-chemotherapies based on platinum and 5-Fluorouracil (PF), 0.90 (95%CI: 0.74 to 1.11) for other mono-chemotherapies and 1.04 (95%CI: 0.92 to 1.17) for other poly-chemotherapies. The benefit of PF based chemotherapy was not significant in induction trials alone (eTable 6). No significant interaction was observed between chemotherapy effect and the type of induction protocol, or the strategy adopted for surgical margins (margin before any treatment vs. not specified) in induction trials or modalities of LRT (surgery vs.surgery + RT) for adjuvant trials.
Sub-group analyses
A significant interaction between chemotherapy effect and patients’ sex was found (Figure 3). Benefit of chemotherapy on OS was greater for women (HRwomen=0.63, 95%CI: 0.50 to 0.80) than for men (HRmen=0.96, 95%CI: 0.89 to 1.04; p for interaction <.001). Heterogeneity of interaction between treatment and sex was not significant (p for heterogeneity =0.81, eFigure5 and eTable7). Event-free survival showed similar results (HRwomen=0.63 (95%CI: 0.50 to 0.80) versus HRmen=0.95 (95%CI: 0.87 to 1.03); p for interaction =0.001).
Figure 3: Hazard ratio of death with loco-regional treatment plus chemotherapy versus loco-regional treatment alone by patient’s characteristics.

See Figure 1 Legend for more explanations.
p_inter: p-value of the test of interaction between individual characteristics and treatment effect.
p_trend: p-value of the test for trend; PS = performance status. 95%CI = 95% confidence interval O-E = observed minus expected, No. = Number.
(a) 4 980 patients included in univariate Cox model for interaction.
(b) 4 829 patients included in univariate Cox model for interaction.
(c) Missing data in 19 trials (completely missing for BNH003 (124 patients), Cologne (97), Creteil 82 (122), EORTC 24771 (231), EORTC 78-OCP (225), GETTECadj (286), JHCFUS (191), LOHNG97 (114), Pitie-74 (96), TMHR-4 (135), Toulouse (90), Yale80po (78). Only 2811 patients included in univariate Cox model for interaction (GSTTC86po and SWOG8006 had to be excluded because none of their patients had no patients included in the PS=0 category)
(d) Only 2825 patients included in univariate Cox model for interaction because all trials had not included patients in all 4 categories of interest (GETTECneo2, BNH003, Cologne, Creteil-82, EORTC24771, EORTC24844, EORTC78-OCP, GSTTC86po, HNCP, KKD-86, Pitie-74, TMHR-4 )
(e) Information on stage was not available for 2 trials (Pitie74 (96) and TMHR-4 (135)).Only 4 405 patients included in univariate Cox model for interaction (BNH003, GSTTC86po and LOHNG97 were excluded because of the absence of stage I or II patients)
The 718 (14%) women included in this study differed from the 4 262 (85%) men in age (younger), stage (lower), performance status (better) and tumor site (more oral cavity, eTable 8). As all these covariates significantly influenced survival (eTable 9), a multivariate interaction model adjusted on age, site and stage was implemented and confirmed a significant interaction (HRwomen=0.62 (95%CI: 0.49 to 0.79), versus HRmen=0.96 (95%CI: 0.88 to 1.04); p for interaction <.001). Performance status was not included because of missing data, but a sensitivity analysis including this covariate leads to similar results (HRwomen=0.59 (95%CI: 0.45 to 0.77), versus HRmen=0.99 (95%CI: 0.89 to 1.09); p for interaction <.001). Absolute benefit at 5 years was 13.3% (95%CI: 9.1 to 17.5%) for women and 3.0% (95%CI: −0.6 to 6.4%) for men (figure 4). A leave-one-out sensitivity analysis (post hoc analysis, eFigure 6), showed that the RTOG 950142 trial influenced interaction more than other trials. After exclusion of the RTOG trial42, interaction was still significant (HRwomen=0.69 (95%CI: 0.54 to 0.89) versus HRmen=0.95 (95%CI: 0.88 to 1.04); p for interaction =0.02).
Figure 4: Overall survival curves by treatment arm for all trials according to sex.
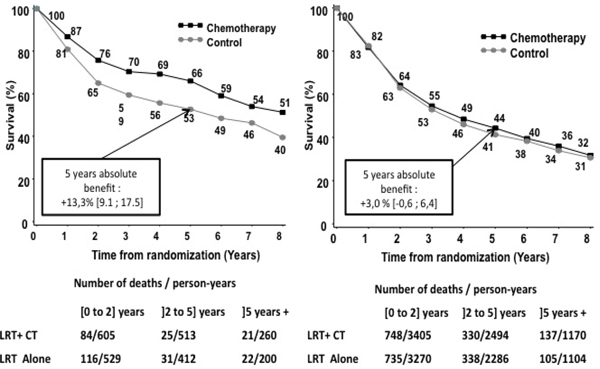
On the right: men overall survival according to treatment. On the left: female overall survival according to treatment arm. The slopes of the broken lines from year 7 to year 8 are based on the overall death rates in the seventh and subsequent years. Absolute differences are given with 95% confidence interval. LRT = Loco-regional treatment, CT = Chemotherapy.
Patterns of failure
Because two trials had no information on locations of failures (Int003422 and JHCFUS23), only 4 291 patients were included in the failure analysis (eTable 4). Chemotherapy decreased significantly the incidence of locoregional failure (HR=0.80, 95%CI: 0.70 to 0.90; p<.001) (Figure 5) but the decrease was not significant for distant failure (HR=0.87, 95%CI: 0.75 to 1.00; p=0.06). Patients treated with chemotherapy died without failure more than non-treated patients (HR=1.20, 95%CI: 1.06 to 1.37; p=0.01). Patterns of failure were different between the different chemotherapy timing, particularly on locoregional failure: significant benefit in concomitant and adjuvant trials but not on induction trials (Figure5, eTable 10). Effect of chemotherapy on distant failure was non-significant for the three chemotherapy timings.
Figure 5: Cumulative incidence by treatment arm for each type of event (for overall analysis and for each timing of chemotherapy).

Given p values correspond to the comparisons of cumulative incidence between treated and non-treated patients (stratified Fine and Gray test). The top left figure represents overall analysis. CT = Chemotherapy; LRT = Locoregional treatment
Men and women had significantly different hazards for death without failure (HRwomen=0.78 (95%CI: 0.53 to 1.15) versus HRmen=1.26 (95%CI: 1.10 to 1.45); p for interaction =0.02). Differences were not significant for locoregional failure (HRwomen=0.66 (95%CI=0.46 to 0.94) versus HRmen=0.82 (95%CI: 0.72 to 0.94); p for interaction =0.26) or for distant failure (HRwomen=0.66 (95%CI: 0.46 to 1.11) versus HRmen=0.82 (95%CI: 0.77 to 1.04; p for interaction =0.35).
DISCUSSION
This meta-analysis on individual patient data is the first to investigate the effect of chemotherapy added to surgical locoregional treatment (LRT) in HNSCC. The results confirmed those obtained in the MACH-NC overall analysis3. The addition of chemotherapy to LRT improved patients’ survival. Interaction with chemotherapy timing was significant and a benefit was particularly observed for concomitant chemotherapy.
Heterogeneity in our analysis was moderate (I2=35%). A major source of heterogeneity came from two French trials: GETTECadj20 and Toulouse38. Both trials selected patients with very high risk of failure as they only included patients with invaded surgical margins and extra capsular invasion of cervical lymph nodes. Both trials were already pointed out as heterogeneous trials in a previous work on heterogeneity in the MACH-NC database44. As we had limited information on toxicity, early deaths were analyzed as a proxy of drug induced mortality, including potential impact on postoperative mortality but no significant difference was found.
The effect of chemotherapy was consistent in all sensitivity analyses (except for trials with long follow-up), as in analyses on event-free survival. The unplanned competing risk analysis suggested that chemotherapy was most effective on locoregional failure. It showed that, for all chemotherapy timings, treatment effect on distant failure was not significant. Mono-chemotherapy using platinum increased patients’ survival more than other chemotherapies. Interaction between chemotherapy and radiotherapy by comparing the subset of trials with surgery and the subset of trials with surgery plus radiotherapy could be investigated only in adjuvant subset as the induction subset did not include trials with surgery alone: the interaction was not significant. Moreover, sensitivity analysis based only on trials with surgery plus radiotherapy (excluding trials with surgery only or mixed (surgery or surgery plus radiotherapy) locoregional treatment) showed similar results than the main analysis.
A majority of trials in this meta-analysis proposed induction chemotherapy. Despite a moderate effect on survival3, induction therapy is sometimes advocated to reduce the risk of distant metastasis and to reduce the tumor volume before surgery5. In our population of patients included in surgical trials, induction therapy showed no significant benefit on overall survival or event-free survival. There was no benefit on locoregional or distant failure. Effect was not significantly different for trials that proposed only one cycle of chemotherapy or that allowed non-responding patients to have early surgery. However, no trials proposed taxane in addition to PF, a strategy that proved significant benefit45 over induction PF. Except for a recent trial46, trials comparing taxane + PF to PF alone did not include surgery as LRT47,48 and could not be included in our meta-analysis. Finally, we could not study the benefit of induction chemotherapy on organ preservation as trials included in this meta-analysis were not designed to study organ preservation strategies.
An unexpected interaction between treatment effect and patients’ characteristics was found for sex. This differed from the overall MACH-NC analysis. In the MACH-NC analysis interaction was found only with age (chemotherapy effect was poorer for patients older than 70 years old). This may result from differences in patients’ characteristics: patients treated by surgery are younger; only 387 (7.7%) of our patients were older than 70 years and thus were analyzed in the ≥60 years old group. As the surgical subgroup only represent 28.6% (5 000/17 483) of the MACH-NC population, 23.4% (2 696/11 542) of observed deaths, and 14.3% of the patients included in concomitant trials (1 327/9 305), this interaction might have been diluted in the overall analysis (eTable 1). This interaction was consistent for OS in univariate and multivariate analyses. Results were similar for event-free survival. The study of the heterogeneity of the interaction (eFigure5) and of the hazard ratios of treatment effect by sex (eTable7) showed the consistency of the interaction throughout all trials; the leave-one-out analysis (eFigure6) showed the robustness of the results. The effect of chemotherapy on the different type of failure in men and women showed no significant difference on locoregional and distant failure. Men treated with chemotherapy had a significantly higher incidence of death without failure. Interpretation of this outcome was made difficult because of missing information on the exact cause of death. A lower rate of comorbidities and of mortality not related to cancer in women than men may explain the observed results. Despite the improvement over time of tumor control in HNSCC, survival increased moderately. Authors pointed out that patients face many competing risks of death (toxicity, comorbidities, or second malignancies)49. In a recent communication, Park and al found that women with HNSCC died less from other causes than from their tumors compared to men50. Sex effect on toxicity and efficacy of systemic treatment are debated, but often considered as understudied51. The prognostic value of sex has long been discussed in HNSCC52–55; most of the time the better survival of women was linked to lower stage tumors or better performance status. In a study evaluating multiple cancers and sex-specific survival, Cook and al found better adjusted survival for women in flour of mouth and laryngeal cancer56. Similar results were observed in the controls arms (i.e. without chemotherapy) of the whole MACH-NC database57. The study of interaction between chemotherapy effect and sex is often difficult because of the few women included in clinical trials. Only large trials or meta-analyses have sufficient power to investigate such interaction and explore the predictive value of sex. As the exploration of sex differences in medicine are actually promoted58, future HNSCC trials should plan to stratify accrual on sex and to study differences in efficacy and toxicity according to patients’ sex.
Lack of power and risk of false positive are the main limitations of this study. Our population is a subgroup of the MACH-NC patients treated with chemotherapy, but still allows an exhaustive synthesis of most surgical trials available. Negative results such as the non-significant interaction between age and chemotherapy effect, between early death and chemotherapy timing or non-significant effect of chemotherapy on distant failure could be related to the lack of power. On the other hand, the unexpected interaction with sex could be a false positive, but we found consistent results in favor of such effect in exploratory analyses. Some trials included are old, patients were accrued between 1974 and 2000, and our results may not represent contemporary treatment strategies. Among the trials eligible for the next update of MACH-NC, we have identified 2 concomitant trials59,60 and one induction trial46 (476 patients) with surgical LRT, and sensitivity analysis adding these 3 more recent trials showed similar results (data not shown). Another limitation was missing data. Some data were partially missing, such as performance status, other were totally missing, such as HPV status, tobacco and alcohol consumption, pathological characteristics, compliance to chemotherapy, or patients’ comorbidity. This may be a confounding factor in our analysis. However, all patients were included in randomized trials with homogeneous inclusion and exclusion criteria and had limited comorbidities as surgery was possible, minimizing differences between compared groups.
To conclude, this analysis confirmed the benefit of chemotherapy in addition to surgical LRT, however benefit in OS was modest and may be limited to the use of platin, concomitant timing and/or to females. The place of induction therapy has yet to be determined as it did not improved survival for patients treated with induction chemotherapy followed by surgery. Our results suggested that women benefited more from chemotherapy than men. Interaction between sex and chemotherapy should be further investigated to confirm our results. As the benefit of chemotherapy in HNSCC is now widely acknowledged, fewer trials compare chemotherapy in addition to LRT versus LRT only. Future analyses of chemotherapy effect in HNSCC will require IPD network meta-analysis to provide high-level evaluation of available treatments.
Supplementary Material
Highlights.
Addition of chemotherapy to surgery improved overall survival in head & neck cancer.
Improvement of overall survival was greater with concomitant chemotherapy.
Induction chemotherapy did not significantly improved overall survival
Women benefited from chemotherapy more than men.
Effect of sex on survival should be investigated more in head and neck cancer.
Acknowledgment
We thank the trialists and the collaborative groups who agreed to share their data. The contents of this publication and methods used are solely the responsibility of the authors and do not necessarily represent the official views of the ECOG-ACRIN Cancer Research Group, and NRG Oncology. This research was funded by grants from Institut National du Cancer (Programme Hospitalier de Recherche Clinique) and Ligue Nationale Contre le Cancer. ED was recipient of a scholarship of the Fondation ARC pour la recherche contre le cancer for his master of science. ED and JPP had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of data analysis.
Abbreviations
- HNSCC
Head and neck Squamous cell carcinoma
- HR
Hazard Ratio
- 95%CI
95% Confidence interval
- IPD
Individual patient data
- LRT
Locoregional Treatment
- OS
Overall survival
- MACH-NC
Meta-analysis of Chemotherapy in Head and Neck Cancer
Footnotes
Members of the collaborative group are listed in appendix page 1
Conflict Of Interest statement
None declared
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012: Globocan 2012. Int J Cancer. 2015;136:E359–E386. doi: 10.1002/ijc.29210 [DOI] [PubMed] [Google Scholar]
- 2.Baxi SS, Pinheiro LC, Patil SM, Pfister DG, Oeffinger KC, Elkin EB. Causes of death in long-term survivors of head and neck cancer: Competing Mortality in HNSCC Survivors. Cancer. 2014;120(10):1507–1513. doi: 10.1002/cncr.28588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pignon J-P, Le Maître A, Maillard E, Bourhis J. Meta-analysis of chemotherapy in head and neck cancer (MACH-NC): An update on 93 randomised trials and 17,346 patients. Radiother Oncol. 2009;92:4–14. doi: 10.1016/j.radonc.2009.04.014 [DOI] [PubMed] [Google Scholar]
- 4.Fujiwara RJT, Burtness B, Husain ZA, et al. Treatment guidelines and patterns of care in oral cavity squamous cell carcinoma: Primary surgical resection vs. nonsurgical treatment. Oral Oncol. 2017;71:129137. doi: 10.1016/j.oraloncology.2017.06.013 [DOI] [PubMed] [Google Scholar]
- 5.Busch C-J, Tribius S, Schafhausen P, Knecht R. The current role of systemic chemotherapy in the primary treatment of head and neck cancer. Cancer Treat Rev. 2015;41(3):217–221. doi: 10.1016/j.ctrv.2015.02.002 [DOI] [PubMed] [Google Scholar]
- 6.Ma J, Liu Y, Huang X-L, et al. Induction chemotherapy decreases the rate of distant metastasis in patients with head and neck squamous cell carcinoma but does not improve survival or locoregional control: A meta-analysis. Oral Oncol. 2012;48(11):1076–1084. doi: 10.1016/j.oraloncology.2012.06.014 [DOI] [PubMed] [Google Scholar]
- 7.Mak MP, Glisson BS. Is there still a role for induction chemotherapy in locally advanced head and neck cancer?: Curr Opin Oncol. 2014;26(3):247–251. doi: 10.1097/CCO.0000000000000073 [DOI] [PubMed] [Google Scholar]
- 8.Stewart LA, Clarke MJ. Practical methodology of meta-analyses (overviews) using updated individual patient data. Cochrane Working Group. Stat Med. 1995;14(19):2057–2079. [DOI] [PubMed] [Google Scholar]
- 9.Lacas B, Bourhis J, Overgaard J, et al. Role of radiotherapy fractionation in head and neck cancers (MARCH): an updated meta-analysis. Lancet Oncol. 2017;18(9):1221–1237. doi: 10.1016/S14702045(17)30458-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Michiels S, Le Maître A, Buyse M, et al. Surrogate endpoints for overall survival in locally advanced head and neck cancer: meta-analyses of individual patient data. Lancet Oncol. 2009;10:341–350. doi: 10.1016/S1470-2045(09)70023-3 [DOI] [PubMed] [Google Scholar]
- 11.Schemper M, Smith TL. A note on quantifying follow-up in studies of failure time. Control Clin Trials. 1996;17:343–346. [DOI] [PubMed] [Google Scholar]
- 12.Yusuf S, Peto R, Lewis J, Collins R, Sleight P. Beta blockade during and after myocardial infarction: an overview of the randomized trials. Prog Cardiovasc Dis. 1985;27:335–371. [DOI] [PubMed] [Google Scholar]
- 13.Lueza B, Rotolo F, Bonastre J, Pignon J-P, Michiels S. Bias and precision of methods for estimating the difference in restricted mean survival time from an individual patient data meta-analysis. BMC Med Res Methodol. 2016;16. doi: 10.1186/s12874-016-0137-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Higgins JPT, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539–1558. doi: 10.1002/sim.1186 [DOI] [PubMed] [Google Scholar]
- 15.Pignon J-P, Hill C. Meta-analyses of randomised clinical trials in oncology. Lancet Oncol. 2001;2:475482. doi: 10.1016/S1470-2045(01)00453-3 [DOI] [PubMed] [Google Scholar]
- 16.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–188. [DOI] [PubMed] [Google Scholar]
- 17.Chemotherapy in non-small cell lung cancer: a meta-analysis using updated data on individual patients from 52 randomised clinical trials. Non-small Cell Lung Cancer Collaborative Group. BMJ. 1995;311(7010):899–909. [PMC free article] [PubMed] [Google Scholar]
- 18.Fine JP, Gray RJ. A Proportional Hazards Model for the Subdistribution of a Competing Risk. J Am Stat Assoc. 1999;94:496. doi: 10.2307/2670170 [DOI] [Google Scholar]
- 19.Latouche A, Allignol A, Beyersmann J, Labopin M, Fine JP. A competing risks analysis should report results on all cause-specific hazards and cumulative incidence functions. J Clin Epidemiol. 2013;66(6):648653. doi: 10.1016/j.jclinepi.2012.09.017 [DOI] [PubMed] [Google Scholar]
- 20.Domenge C, Marandas P, Vignoud J. Post-surgical adjuvant chemotherapy in extracapsular spread invaded lymph node (N+R+) of epidermoid carcinoma of the head and neck: a randomized multicentric trial. Second international conference on head and neck cancer. Boston. Am Soc Head Neck Surg 1988; 74 abstr). [Google Scholar]
- 21.Tsukuda M, Ogasawara H, Kaneko S, et al. [A prospective randomized trial of adjuvant chemotherapy with UFT for head and neck carcinoma. Head and Neck UFT Study Group]. Gan To Kagaku Ryoho. 1994;21(8):1169–1177. [PubMed] [Google Scholar]
- 22.Laramore GE, Scott CB, al-Sarraf M, et al. Adjuvant chemotherapy for resectable squamous cell carcinomas of the head and neck: report on Intergroup Study 0034. Int J Radiat Oncol Biol Phys. 1992;23(4):705–713. [DOI] [PubMed] [Google Scholar]
- 23.Yoshino K, Sato T, Nakai Y, et al. [A comparative clinical study on the treatment of head and neck tumors by adjuvant chemotherapy with HCFU--Second Study by Kinki Head and Neck Tumor Study Group]. Gan To Kagaku Ryoho. 1994;21(6):777–783. [PubMed] [Google Scholar]
- 24.Kotani A, Sunada O, Tamura M, et al. [Multiple cooperative study of UFT-adjuvant chemotherapy for malignant tumor in the jaw and oral cavities. The Oral Surgery Malignant Tumor Research Association in Kanto Kohshinetsu District]. Gan To Kagaku Ryoho. 1994;21(7):987–992. [PubMed] [Google Scholar]
- 25.Rao RS, Parikh DM, Parikh HK, Bhansali MB, Deshmane VH, Fakih AR. Perioperative chemotherapy in patients with oral cancer. Am J Surg. 1994;168(3):262–267. [DOI] [PubMed] [Google Scholar]
- 26.Szpirglas H, Chastang C, Bertrand JC. Adjvant treatment of tongue and floor of the mouth cancers. Recent Results Cancer Res Fortschritte Krebsforsch Progres Dans Rech Sur Cancer. 1978;68:309–317. [DOI] [PubMed] [Google Scholar]
- 27.Dalley D, Beller E, Aroney R, et al. The value of chemotherapy (CT) prior to definitive local therapy (DTL) in patients with locally advanced squamous cell carcinoma (SCC) of the head and neck (HN). Proc ASCO 1995; 14: 297. [Google Scholar]
- 28.Domenge C, Hill C, Lefebvre JL, et al. Randomized trial of neoadjuvant chemotherapy in oropharyngeal carcinoma. French Groupe d’Etude des Tumeurs de la Tête et du Cou (GETTEC). Br J Cancer. 2000;83(12):1594–1598. doi: 10.1054/bjoc.2000.1512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Metha S BNH 03-Anterior (neoajuvant) chemotherapy in advanced resectable cancers of the oral cavity and oropharynx. Personal communication on behalf of the B. Nanavati Hospital group. Unpublished. [Google Scholar]
- 30.Volling P, Schroder M, Muller R, Ebeling O, Quirin R, Stennert E. Induction chemotherapy in primary resectable head and neck tumors - a prospective randomized trial. Int J Oncol. 1994;4(4):909–914. [DOI] [PubMed] [Google Scholar]
- 31.Mazeron JJ, Martin M, Brun B, et al. Induction chemotherapy in head and neck cancer: results of a phase III trial. Head Neck. 1992;14(2):85–91. [DOI] [PubMed] [Google Scholar]
- 32.Lefebvre JL, Sahmoud T, Kirkpatrick A. EORTC 24844 trial. Randomized trial of induction chemotherapy followed by surgery and postoperative radiotherapy versus surgery and postoperative radiotherapy alone in advanced squamous cell carcinoma of the lateral oropharynx and lateral posterior oral cavity. Personal communication on behalf of the EORTC Head and Neck Cancer Cooperative Group. [Google Scholar]
- 33.Paccagnella A, Orlando A, Marchiori C, et al. Phase III trial of initial chemotherapy in stage III or IV head and neck cancers: a study by the Gruppo di Studio sui Tumori della Testa e del Collo. J Natl Cancer Inst. 1994;86(4):265–272. [DOI] [PubMed] [Google Scholar]
- 34.Schuller DE, Metch B, Stein DW, Mattox D, McCracken JD. Preoperative chemotherapy in advanced resectable head and neck cancer: final report of the Southwest Oncology Group. The Laryngoscope. 1988;98(11):1205–1211. doi: 10.1288/00005537-198811000-00011 [DOI] [PubMed] [Google Scholar]
- 35.Jortay A, Demard F, Dalesio O, et al. A randomized EORTC study on the effect of preoperative polychemotherapy in pyriform sinus carcinoma treated by pharyngolaryngectomy and irradiation. Results from 5 to 10 years. Acta Chir Belg. 1990;90(3):115–122. [PubMed] [Google Scholar]
- 36.Richard JM, Kramar A, Molinari R, et al. Randomised EORTC head and neck cooperative group trial of preoperative intra-arterial chemotherapy in oral cavity and oropharynx carcinoma. Eur J Cancer Oxf Engl 1990. 1991;27(7):821–827. [DOI] [PubMed] [Google Scholar]
- 37.Adjuvant chemotherapy for advanced head and neck squamous carcinoma. Final report of the Head and Neck Contracts Program. Cancer. 1987;60(3):301–311. [DOI] [PubMed] [Google Scholar]
- 38.Bachaud JM, Cohen-Jonathan E, Alzieu C, David JM, Serrano E, Daly-Schveitzer N. Combined postoperative radiotherapy and weekly cisplatin infusion for locally advanced head and neck carcinoma: final report of a randomized trial. Int J Radiat Oncol Biol Phys. 1996;36(5):999–1004. [DOI] [PubMed] [Google Scholar]
- 39.Weissberg JB, Son YH, Papac RJ, et al. Randomized clinical trial of mitomycin C as an adjunct to radiotherapy in head and neck cancer. Int J Radiat Oncol Biol Phys. 1989;17(1):3–9. [DOI] [PubMed] [Google Scholar]
- 40.Bernier J, Domenge C, Ozsahin M, et al. Postoperative irradiation with or without concomitant chemotherapy for locally advanced head and neck cancer. N Engl J Med. 2004;350(19):1945–1952. doi: 10.1056/NEJMoa032641 [DOI] [PubMed] [Google Scholar]
- 41.Smid L, Budihna M, Zakotnik B, et al. Postoperative concomitant irradiation and chemotherapy with mitomycin C and bleomycin for advanced head-and-neck carcinoma. Int J Radiat Oncol Biol Phys. 2003;56(4):1055–1062. [DOI] [PubMed] [Google Scholar]
- 42.Cooper JS, Pajak TF, Forastiere AA, et al. Postoperative concurrent radiotherapy and chemotherapy for high-risk squamous-cell carcinoma of the head and neck. N Engl J Med. 2004;350(19):1937–1944. doi: 10.1056/NEJMoa032646 [DOI] [PubMed] [Google Scholar]
- 43.Tobias JS, Monson K, Gupta N, et al. Chemoradiotherapy for locally advanced head and neck cancer: 10-year follow-up of the UK Head and Neck (UKHAN1) trial. Lancet Oncol. 2010;11(1):66–74. doi: 10.1016/S1470-2045(09)70306–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Baujat B, Mahé C, Pignon J-P, Hill C. A graphical method for exploring heterogeneity in meta-analyses: application to a meta-analysis of 65 trials. Stat Med. 2002;21:2641–2652. doi: 10.1002/sim.1221 [DOI] [PubMed] [Google Scholar]
- 45.Blanchard P, Bourhis J, Lacas B, et al. Taxane-cisplatin-fluorouracil as induction chemotherapy in locally advanced head and neck cancers: an individual patient data meta-analysis of the meta-analysis of chemotherapy in head and neck cancer group. J Clin Oncol Off J Am Soc Clin Oncol. 2013;31(23):2854–2860. doi: 10.1200/JCO.2012.47.7802 [DOI] [PubMed] [Google Scholar]
- 46.Zhong L, Zhang C, Ren G, et al. Randomized Phase III Trial of Induction Chemotherapy With Docetaxel, Cisplatin, and Fluorouracil Followed by Surgery Versus Up-Front Surgery in Locally Advanced Resectable Oral Squamous Cell Carcinoma. J Clin Oncol. 2013;31(6):744–751. doi: 10.1200/JCO.2012.43.8820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ghi M, Paccagnella A, Ferrari D, et al. Induction TPF followed by concomitant treatment versus concomitant treatment alone in locally advanced Head and Neck Cancer. A phase II-III trial. Ann Oncol 2017. September 12892206–2212. doi: 10.1093/annonc/mdx299 [DOI] [PubMed] [Google Scholar]
- 48.Takácsi-Nagy Z, Hitre E, Remenár É, et al. Docetaxel, cisplatin and 5-fluorouracil induction chemotherapy followed by chemoradiotherapy or chemoradiotherapy alone in stage III–IV unresectable head and neck cancer: Results of a randomized phase II study. Strahlenther Onkol. 2015;191(8):635–641. doi: 10.1007/s00066-015-0829-z [DOI] [PubMed] [Google Scholar]
- 49.Montero-Miranda PH, Ganly I. Survivorship--competing mortalities, morbidities, and second malignancies. Otolaryngol Clin North Am. 2013;46(4):681–710. doi: 10.1016/j.otc.2013.04.008 [DOI] [PubMed] [Google Scholar]
- 50.Park A, Alabaster A, Shen H, Mell L, Katzel J. Are women with head and neck cancer undertreated? J Clin Oncol 2018. 36 Suppl Abstr LBA6002. [Google Scholar]
- 51.Özdemir BCO, Csajka C, Dotto G-P, Wagner AD. Sex Differences in Efficacy and Toxicity of Systemic Treatments: An Undervalued Issue in the Era of Precision Oncology. J Clin Oncol 2018262680–2683.:6. [DOI] [PubMed] [Google Scholar]
- 52.Ildstad ST, Tollerud DJ, Bigelow ME, Remensnyder JP. Squamous cell carcinoma of the head and neck at the Massachusetts General Hospital: a comparison of biologic characteristics in men and women. Surgery. 1986;99(1):7–14. [PubMed] [Google Scholar]
- 53.Molina MA, Cheung MC, Perez EA, et al. African American and poor patients have a dramatically worse prognosis for head and neck cancer: An examination of 20,915 patients. Cancer. 2008;113:27972806. doi: 10.1002/cncr.23889 [DOI] [PubMed] [Google Scholar]
- 54.Roberts JC, Li G, Reitzel LR, Wei Q, Sturgis EM. No Evidence of Sex-Related Survival Disparities among Head and Neck Cancer Patients Receiving Similar Multidisciplinary Care: A Matched-Pair Analysis. Clin Cancer Res. 2010;16(20):5019–5027. doi: 10.1158/1078-0432.CCR-10-0755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fakhry C, Westra WH, Wang SJ, et al. The prognostic role of sex, race, and human papillomavirus in oropharyngeal and nonoropharyngeal head and neck squamous cell cancer. Cancer. 2017;123(9):15661575. doi: 10.1002/cncr.30353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cook MB, McGlynn KA, Devesa SS, Freedman ND, Anderson WF. Sex disparities in cancer mortality and survival. Cancer Epidemiol Biomark Prev Publ Am Assoc Cancer Res Cosponsored Am Soc Prev Oncol. 2011;20(8):1629–1637. doi: 10.1158/1055-9965.EPI-11-0246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Blanchard P, Baujat B, Holostenco V, et al. Meta-analysis of chemotherapy in head and neck cancer (MACH-NC): a comprehensive analysis by tumour site. Radiother Oncol J Eur Soc Ther Radiol Oncol. 2011;100(1):33–40. doi: 10.1016/j.radonc.2011.05.036 [DOI] [PubMed] [Google Scholar]
- 58.FDA Research, Policy, and Workshops on Women in Clinical Trials. https://www.fda.gov/scienceresearch/specialtopics/womenshealthresearch/ucm131731.htm.
- 59.Racadot S, Mercier M, Dussart S, et al. Randomized clinical trial of post-operative radiotherapy versus concomitant carboplatin and radiotherapy for head and neck cancers with lymph node involvement. Radiother Oncol. 2008;87(2):164–172. doi: 10.1016/j.radonc.2007.12.021 [DOI] [PubMed] [Google Scholar]
- 60.Argiris A, Karamouzis MV, Johnson JT, et al. Long-Term Results of a Phase III Randomized Trial of Postoperative Radiotherapy With or Without Carboplatin in Patients With High-Risk Head and Neck Cancer. The Laryngoscope. 2008;118(3):444–449. doi: 10.1097/MLG.0b013e31815b48f4 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


