Abstract
Background:
We evaluated the association between maternal cytomegalovirus (CMV) viremia during pregnancy and adverse birth and infant health outcomes in HIV-infected mothers and their HIV-exposed uninfected (HEU) infants.
Methods:
HIV-positive women and their infants were followed prospectively from pregnancy through 2 years postpartum in the “Tshipidi” study in Botswana. We analysed the association between detectable CMV DNA in maternal blood at delivery and adverse birth outcomes (stillbirth, preterm delivery, small for gestational age, or birth defect), as well as infant hospitalization and mortality through 24 months.
Results:
We measured CMV DNA in blood samples from 350 (77.1%) of 454 HIV-positive women from the Tshipidi study. The median maternal CD4 count was 422 cells/mL and median HIV-1 RNA at entry was 3.2 log10 copies/mL. Fifty-one (14.6%) women had detectable CMV DNA. In unadjusted analyses, detectable CMV DNA was associated with higher maternal HIV-1 RNA (OR 1.4, 95%CI 1.1- 1.9), presence of a birth defect (OR 9.8, 95%CI 1.6-60.3), and occurrence of any adverse birth outcome (OR 2.0, 95% CI 1.04-3.95). In multivariable analysis, we observed a trend toward association between detectable maternal CMV DNA and occurrence of any adverse birth outcome (aOR 1.9, 95% CI 0.96-3.8). Maternal CMV viremia was not associated with infant hospitalization and/or death by 24 months.
Conclusion:
Approximately 1 in 6 HIV-positive women in Botswana had detectable CMV DNA in blood at delivery. Presence of maternal CMV viremia had a borderline association with adverse birth outcomes but not with 24-month morbidity or mortality in HEU children.
Keywords: Cytomegalovirus viremia, HIV, HIV positive pregnant women, HIV-exposed uninfected infants, adverse birth outcomes
INTRODUCTION
The advent of antiretroviral drugs (ARVs) to prevent mother to child transmission (PMTCT) of HIV-1 has led to a dramatic reduction in the rate of vertical transmission, including in Botswana, where MTCT rates have fallen from 35% to 2% or less1,2. As a result, HIV-exposed uninfected (HEU) infants represent almost 30% of all children in much of southern Africa3. A number of studies show that HEU children experience higher morbidity and mortality compared with their HIV-unexposed uninfected (HUU) counterparts3-5, and HIV-positive mothers appear to experience higher rates of adverse birth outcomes compared with HIV-negative mothers 6,7.
A variety of phenotypical differences between HEU and HUU children has been reported but mechanisms behind the susceptibility of HEU to higher morbidity and mortality have not been fully scrutinized. Some studies have attempted to prove this phenomenon showing poorer vaccine responses in HEU compared to HUU infants8,9. However, in Botswana we have previously reported no significant differences in antibody responses to tetanus vaccine between HEU and HUU infants10. Furthermore, opportunistic infections such as cytomegalovirus (CMV) reactivation in HIV positive women may lead to congenital infection overall poorer health outcomes in the HEU cohort; this is mostly coupled with prolonged breastfeeding in HIV positive women11,12. Congenital CMV infection is one of the leading causes of birth defects, non-genetic hearing loss, and developmental delay in children worldwide12. Congenital CMV tends to be more severe in the setting of acute maternal CMV infection during pregnancy, rather than among mothers infected with CMV prior to conception 13. In developing countries, the vast majority of adults (including 80–100% of pregnant women) are CMV-seropositive, and 95% of HIV-positive adults in Botswana are positive for CMV IgG 14,15. Immunosuppression due to uncontrolled HIV or other causes can trigger CMV reactivation and consequently could result in vertical transmission of CMV16. Congenital CMV infection occurs at high rates in HEU infants, especially when born to untreated HIV-positive women compared to women receiving ARVs17-20. Some studies have shown lower rates of vertical CMV transmission among HIV-positive women taking combination antiretroviral treatment (ART) compared to zidovudine (ZDV)14, although this association is not consistent18,21,22.
Maternal CMV co-infection and reactivation may also have implications for immune development, growth, and other health outcomes in HEU babies. For example, in a Kenyan cohort, maternal CMV DNA detection was linked to a four-fold increase in infant mortality among HEU infants16. A Zambian study has previously described that HCMV infection in HEU infants adversely impacts growth and development, in this cohort, HEU infants had higher rates of prevalence of stunting, reduced head size, as well as decreased psychomotor development compared to their HUU contemporaries23. However, most studies evaluating this association were conducted at a time when the majority of women had advanced HIV disease and very few were taking combination ART. Furthermore, very little is known about the role of maternal CMV viremia in adverse birth outcomes such as preterm delivery, stillbirth, and small for gestational age (SGA).
We tested for CMV DNA at delivery in HIV-positive women in Botswana and evaluated its association with adverse birth outcomes and with 24-month morbidity and mortality among HEU children.
METHODS
Study design and population
This study used existing samples and data from the “Tshipidi” study. Tshipidi was a prospective observational cohort study that enrolled HIV-positive and HIV-negative pregnant women and their children in one urban and one rural location in Botswana, between 2010 and 201224. During the study period, HIV-positive pregnant women in Botswana with CD4 count ≤350 cells/mL or WHO stage 3 or 4 disease were eligible for 3-drug ART; all others were eligible for prophylaxis with ZDV during pregnancy and single dose nevirapine (NVP) during labour and delivery. Women were enrolled during pregnancy (88%) or at/within 7 days of delivery (12%). HIV-1 RNA and CD4 count were measured at enrolment. Only HEU children and their mothers were included in this analysis (HIV-infected children and their mothers were excluded).
Evaluation of the role of maternal CMV infection in child health outcomes was one of the pre-specified, approved objectives of the Tshipidi study.
Maternal CMV DNA testing
The primary exposure of interest was detectable CMV DNA in maternal samples drawn between 1 day and 1 week postpartum. All available plasma and buffy coat samples were tested. CMV DNA was quantified in plasma samples using the Roche COBAS® AmpliPrep/COBAS® TaqMan® CMV Test (threshold of detection = 50 copies/mL) (Roche Diagnostics, Indianapolis, IN). CMV DNA was measured in buffy coat samples using the LightCycler-FastStart DNA Master Hybridization Probes kit (Roche Diagnostics, Indianapolis, IN) and the Light Cycler 2.0 Instrument (Roche Diagnostics, Indianapolis, IN) according to the instruction manuals (threshold of detection = 10 copies/mL). The CMV PCR on buffy coat was performed as previously described for whole blood 25,26.The only modification was diluting the buffy coat in PBS to bring it to a total volume of 800μL. A mother was defined as having CMV viremia if CMV DNA was detected in either the plasma or the buffy coat sample or both. A random sample of HIV-negative mothers participating in the Tshipidi study were also tested for CMV DNA to assess prevalence of CMV viremia among HIV-negative pregnant women.
Outcomes of interest
We determined rates of adverse birth outcomes including stillbirth, birth defects, preterm birth, very preterm birth, SGA, and very SGA. Study staff collected birth outcome data from maternal report and obstetric records. Gestational age was estimated using maternal report of last menstrual period. Infant birthweight and birth defects were recorded at delivery. Stillbirth was defined as fetal death (APGAR 0,0,0). Preterm birth was defined as <37 weeks gestational age (GA) and very preterm birth was defined as ≤32 weeks GA. SGA was defined as <10th percentile, and very SGA was defined as <3rd percentile using birthweight for gestational age norms for Botswana27. We also analyzed occurrence of any adverse birth outcome (stillbirth, preterm delivery, SGA, or birth defect) as a composite endpoint. Children were followed prospectively from birth until 2 years of age, with structured collection of growth and health outcome data. We determined rates of adverse child health outcomes, defined as the occurrence of either hospitalization or death by age 2. Children with more than one adverse child health event were counted once in the analysis. HIV positive children, children with unknown HIV status and children with missing outcomes were excluded from the analysis.
Ethical approvals
The Botswana Health Research Development Committee and the Office of Human Research Administration at Harvard T.H. Chan School of Public Health granted ethics approvals, and women provided written informed consent for study participation.
Data analysis
The exposure of interest was binary: detectable vs. undetectable CMV DNA in plasma and/or buffy coat samples. Maternal baseline characteristics were compared between women with or without detectable CMV DNA using logistic regression. Univariable and multivariable logistic regression were used to determine the association between detectable CMV DNA and adverse birth or child health outcomes. Covariates with p-values of <0.2 for association with each outcome of interest in the univariable model were included in the multivariable model. Potential covariates included maternal HIV-1 RNA, maternal CD4 count, gestational age at delivery, type of ARVs received during pregnancy (3-drug ART received for at least two weeks prior to delivery or ZDV for at least two weeks prior to delivery plus single dose NVP), and duration of ARV exposure during pregnancy. Mothers with CD4 testing done after delivery were excluded from the outcomes analysis.
In the multivariable analysis, 2 mothers who received no ARVs during pregnancy were included in the ZDV group. Alpha level was set at 0.05. All statistical analysis was performed using STATA Version 14.
RESULTS
Enrolment and testing
A total of 912 mothers and 910 of their infants were enrolled in the Tshipidi study: 454 HIV-positive mothers with 453 respective infants and 458 HIV-negative mothers with 457 respective infants. Plasma or buffy coat samples were available for 350 (77%) HIV-positive women. Fifty-two (15%) of children were excluded from the prospective health outcomes analysis (due to positive or unknown HIV status in 23 infants, or missing data; Figure 1). Thus, 350 mothers and 298 of their HEU infants were included in this analysis (Figure 1). In addition, delivery samples from 49 randomly-selected HIV-negative mothers were tested for CMV DNA. Tested samples were drawn at a median of 2 days postpartum (range, 1 to 7 days).
Figure 1: Enrollment, sample selection, CMV DNA testing and analysis.
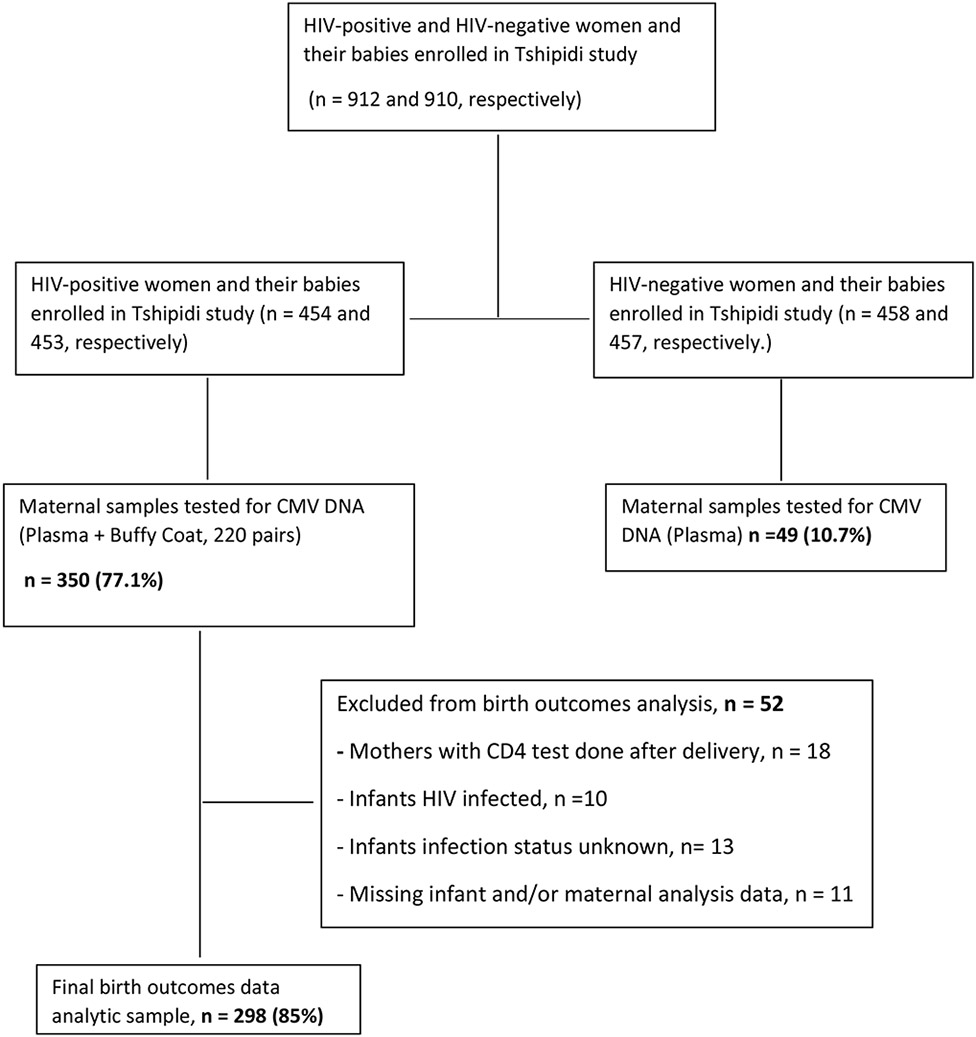
CMV, cytomegalovirus; DNA, deoxyribonucleic acid; HIV, Human Immunodeficiency virus
Prevalence of detectable CMV DNA
Fifty-one of the 350 HIV-positive women had CMV DNA detected in at least one sample type, resulting in a prevalence of 14.6% (95% CI, 11.0 −17.0). In a subset of of 49 HIV-uninfected women, three had detectable CMV DNA, resulting in a prevalence of 6.1% (95% CI, 1.28–16.8), showing a non-significant difference compared to HIV-positive women, p= 0.1. A proportion of two hundred and twenty women had both plasma and buffy coat samples tested for CMV DNA; the remaining 130 women had only 1 sample type available. The proportions of each type of sample that were positive among these 220 women are presented in Table 1. Yield of detectable CMV DNA appeared to be higher in plasma compared to buffy coat samples; however, the median CMV DNA quantity in CMV-positive samples was higher in buffy coat (254 copies/mL) compared to plasma samples (<150 copies/mL).
Table 1:
Numbers of women with positive CMV DNA in plasma vs. buffy coat (out of 220 women tested with both assays)
| BUFFY COAT Positive |
BUFFY COAT Negative |
Total tested |
|
|---|---|---|---|
| PLASMA Positive | 6 | 26 | 32 |
| PLASMA Negative | 11 | 177 | 188 |
| Total tested | 17 | 203 | 220 |
CMV, cytomegalovirus; DNA, deoxyribonucleic acid
Demographic characteristics of women with and without detectable CMV DNA
Baseline demographic and medical characteristics of HIV-positive participants are shown in Table 2, by CMV DNA status. Median plasma HIV-1 RNA was significantly higher (p=0.02) in women with detectable CMV DNA compared to women without detectable CMV DNA, although median CD4 counts did not differ significantly. Longer duration of ARV prophylaxis (either Zidovudine or 3-drug ART) was associated with lower prevalence of CMV DNA detection (p= 0.02).
Table 2:
Maternal Baseline Characteristics according to maternal CMV DNA status
| N (%) with the characteristic (or with data available) |
Detectable Maternal CMV DNA, n=51 |
No detectable Maternal CMV DNA, n = 299 |
Odds ratio (OR) for having detectable CMV DNA (95% confidence interval [CI]) |
P value |
|
|---|---|---|---|---|---|
| Maternal Baseline Characteristics | |||||
| Median maternal age in years (Q1, Q3) | 350 (100) | 27.6 (23.7,32.8) | 29.7 (25.1, 33.7) | 0.96 (0.9 - 1.0) | 0.2 |
| Median CD4 count, cells/mm3 (Q1, Q3) | 339 (96.9) | 422 (248,586) | 434 (344,574) | 1.0 (0.99-1.0) | 0.4 |
| Median HIV-1 RNA, log10 (Q1, Q3) | 337 (96.3) | 3.8 (2.6, 4.6) | 3.1 (2.6, 4.0) | 1.4 (1.1- 1.9) | 0.02 |
| Median gestational age (weeks) at delivery, (Q1, Q3) | 335 (95.7) | 40.0 (37,40) | 40.0 (38,41) | 0.9 (0.8-1.0) | 0.08 |
| Infant feeding method | 340 (97.1) | 0.3 | |||
| Breastfed | 27 (7.7) | 6/51 (11.7%) | 21/289 (7.3%) | 1.7 (0.7-4.4) | |
| Never breastfed | 313 (89.4) | 45/51 (88.2%) | 268/289 (92.7%) | ||
| Type of antepartum ARVs | 340 (97.1) | 0.06 | |||
| 3-drug ART | 107 (30.6) | 21/51 (41.2%) | 86/289 (29.8%) | ||
| Zidovudine | 231 (66.0) | 29/51 (56.9%) | 202/289 (69.6%) | ||
| No (or <2 weeks) antepartum ARVs | 2 (0.6) | 1/51 (1.96%) | 1/289 (0.3%) | ||
| Median duration of any ARVs during and prior to pregnancy (ZDV or 3-drug ART), days (Q1, Q3) | 338 (96.6) | 71(53,104) | 84(68,105) | 0.02 | |
| Median duration of 3-drug ART during and prior to pregnancy, days (Q1, Q2) | 331 (94.6) | 70(52,99) | 84(68,105) | 0.02 | |
| Median duration of any ARVs during pregnancy (ZDV or 3-drug ART), days (Q1, Q3) | 279 (79.7) | 69(49,90) | 79(64,91) | 0.08 |
ARV, antiretroviral drug; ART, antiretroviral therapy; CMV, cytomegalovirus; DNA, deoxyribonucleic acid; HIV, Human Immunodeficiency virus; Q1, 25th percentile; Q3, 75th percentile; ZDV, zidovudine
Association of detectable maternal CMV DNA with adverse birth outcome or major adverse infant health outcome
In unadjusted analysis, a higher proportion of women with detectable CMV DNA had an infant with a birth defect compared with CMV-negative mothers (7.1% vs. 0.8%; OR 9.8, 95% CI 1.6 – 60.3, p=0.01) (Table 3). Three women with detectable CMV DNA had infants with birth defects including craniosynostosis, anencephaly and a cystic abdominal mass. Two women without detectable CMV DNA had infants with craniosynostosis. A borderline association was observed between detectable CMV DNA and preterm delivery (21.4% vs. 10.5%; OR 2.3, 95% CI 1.0–5.3, p= 0.06). Finally, detectable CMV DNA was associated with the composite adverse birth outcome in univariable analysis (OR 2.0, 95% CI 1.04–3.95, p=0.04). In adjusted analyses controlling for maternal CD4 count and type of ARV prophylaxis, only the association between CMV DNA and the composite adverse birth outcome endpoint remained of borderline significance (aOR 1.9, 95% CI 0.96 – 3.8, p=0.06).
Table 3:
Pregnancy and infant health outcomes according to maternal CMV DNA status
| N | Detectable CMV DNA, n = 51 |
No detectable CMV DNA, n= 299 |
Unadjusted odds ratio (OR) of having the outcome of interest, in association with detectable CMV DNA (95%confidence interval [CI]) |
P | Adjusted odds ratio (OR) of having the outcome of interest, in association with detectable CMV DNA (95% confidence interval [CI]) |
P | |
|---|---|---|---|---|---|---|---|
| Pregnancy Outcomes | |||||||
| Stillbirth | 298 | 0/42 (0) | 0/256 (0) | - | - | - | - |
| Birth defect | 298 | 3/42 (7.1) | 2/256 (0.8) | 9.8 (1.6 -60.3) | 0.01 | * | - |
| Small for gestational age (<10th percentile) | 298 | 14/42 (33.3) | 61/256 (23.8) | 1.6 (0.79 – 3.2) | 0.2 | 1.5 (0.7 – 2.9) a | 0.3 |
| Very small for gestational age (<3rd percentile) | 298 | 10/42 (23.8) | 42/256 (16.4) | 1.6 (0.7 – 3.5) | 0.2 | - | - |
| Preterm delivery (<37 weeks gestation) | 298 | 9/42 (21.4) | 27/256 (10.5) | 2.3 (1.0- 5.3) | 0.06 | * | - |
| Very preterm delivery (≤32 weeks gestation) | 298 | 1/39 (2.3) | 3/256(1.2) | 2.2 (0.22 – 21.4) | 0.5 | - | - |
| Composite adverse birth outcome** | 298 | 19/42 (45.2) | 74/256 (28.9) | 2.0 (1.04- 3.95) | 0.04 | 1.9 (0.96 – 3.8) b | 0.06 |
| Infant Health Outcomes | |||||||
| Hospitalization | 298 | 7/42 (16.7) | 47/256 (18.3) | 0.89 (0.4 -2.1) | 0.8 | - | - |
| Infant mortality | 298 | 0/42 (0) | 1/256 (0.4) | - | - | - | - |
| Composite adverse infant health outcome*** | 298 | 7/42 (16.7) | 48/256 (18.8) | 0.9 (0.4 - 2.1) | 0.7 | 0.9 (0.4 – 2.1) c | 0.7 |
CI, confidence interval; CMV, cytomegalovirus; DNA, deoxyribonucleic acid; OR, odds ratio
Adjusted odds ratios are unavailable as co-variates did not meet the criteria for inclusion in the multivariate model
Defined as presence of one or more of the following outcomes: stillbirth, birth defect, small for gestational age or preterm delivery
Defined as hospitalization and/or death by 24 months
Adjusted for type of ARV prophylaxis (ZDV or 3-drug ART) received during current pregnancy
Adjusted for baseline CD4 count, and type of ARV prophylaxis (ZDV or 3-drug ART) received during current pregnancy
Adjusted for baseline CD4 count
The composite infant health outcome (hospitalization and/or death) occurred in 15–20% of children born to women with and without detectable CMV DNA, Table 3. Presence of detectable maternal CMV DNA was not significantly associated with the composite infant health outcome in univariable or multivariable analyses.
DISCUSSION
This study evaluated the association between detectable maternal CMV DNA in HIV-positive women at delivery and adverse birth and HEU infant health outcomes in Botswana. We found that approximately 15% of HIV-positive women and 6% of HIV-negative women had detectable CMV DNA in peripheral blood; this shows no significant difference in CMV DNA detection in HIV positive women compared to HIV negative women. Very few studies describe rates of CMV viremia among peripartum women (HIV-positive or -negative) in the southern African region14. A small study conducted in Kenya among HIV-positive pregnant women showed a 17% prevalence of detectable CMV DNA in blood samples at delivery, similar to our observation16. Of note, mothers participating in the Tshipidi study were generally healthier than women in the Kenyan study, with higher CD4 count and lower HIV-1 RNA.
Few studies of birth outcomes have been conducted among HIV-positive women with high rates of CMV seropositivity14. In our study, higher proportions of women with detectable CMV DNA had a child born with a birth defect, SGA, or preterm, compared with women without detectable CMV DNA, although this only reached statistical significance for birth defects. In addition, the occurrence of any adverse birth outcome was associated with detectable maternal CMV DNA at delivery in unadjusted analyses and maintained borderline statistical significance in adjusted analyses. In contrast, an Italian case-control study did not find any significant association between CMV viremia and adverse birth outcomes, including preterm delivery, low birthweight, major birth defects or HIV transmission, in a national cohort of pregnant women with HIV21.
Maternal CMV reactivation might lead to adverse birth outcomes through either congenital CMV infection or other mechanisms. HEU infants are known to experience high rates of congenital CMV infection12,17, although our study did not directly assess for this. CMV is known to drive profound, persistent changes in the number, phenotype and function of various immune cells, including T-cells and natural killer cells28, resulting in a state of chronic inflammation in the host29,30. This phenomenon may be exaggerated among persons co-infected with HIV, as HIV infection is characterized by chronic immune activation31-34. Pro-inflammatory maternal states have been associated with higher rates of adverse birth outcomes, including preterm delivery, in HIV-positive and HIV-negative pregnant women35,36. Thus, it is plausible that CMV reactivation could negatively impact birth outcomes among HIV-infected women through its contribution to maternal inflammation.
CMV may directly infect the placenta and cause latency in CD14+ cells, the virus is likely reactivated through cytotrophoblasts (CTB) invasion and inflammation37. This infection likely generates a pathway for prenatal CMV MTCT. Although we did not measure markers of immune activation in this study, it is possible that some of the outcomes in this study may have been due to CMV associated immune reconstitution syndrome (IRIS). Some studies have shown possible effects of IRIS in HIV infected children which leads to excess morbidity38,39, however, not much data is available on CMV associated IRIS in HEU infants. This warrants the need for more studies that look at the possible impact of CMV associated IRIS in HEU infants born to CMV DNAemic HIV positive mothers.
HEU children have higher morbidity and mortality than HUU children in resource-limited settings, largely due to increased rates of lower respiratory tract infections, sepsis, and diarrheal disease40,41. We hypothesized that exposure to maternal CMV viremia might impact fetal immune development, leading to increased susceptibility to disease in childhood. However, we did not observe an association between maternal CMV viremia and child hospitalization and/or mortality. It is possible that mechanisms other than CMV exposure are responsible for the increased rates of morbidity and mortality among HEU infants.
We found that higher maternal HIV-1 RNA was associated with the presence of detectable maternal CMV DNA. In addition, a larger proportion of women with detectable CMV DNA received 3-drug ART, compared to women without detectable CMV DNA. Because only women with CD4 counts ≤350 or WHO stage 3 or 4 disease were eligible for ART, receipt of ART is a marker of advanced HIV disease in this study. Therefore, the association of ART with CMV viremia indicates that women with more advanced HIV disease were more likely to experience CMV reactivation. Receipt of prenatal ARVs is generally thought to be protective against CMV vertical transmission22, and we found that longer duration of ARVs was associated with decreased CMV viremia. Importantly, after adjusting for maternal ARV type and CD4 count in our multivariable analysis, a borderline association with adverse birth outcomes remained; therefore, we do not believe that the association between CMV viremia and adverse birth outcome was solely due to advanced HIV disease.
There were several limitations to this study. First, it is possible that some of the trends that we observed for adverse birth outcomes in mothers with detectable CMV DNA would have reached statistical significance with a larger sample size. We did not evaluate infant CMV infection status, which could have been associated with some of the infant outcomes. The measure of morbidity in this study was limited to infant hospitalization; a limitation of our study is that we did not systematically capture neurological outcomes. We may have introduced measurement error by only evaluating samples from a single time point, as some women may have had detectable CMV DNA earlier during pregnancy but not at delivery. Moreover, testing of an equal subset of HIV negative women as a control group could add more information on whether HIV positive mothers on ART would predispose their children to CMV infection compared to HIV negative mothers. In addition, it would be ideal to compare outcomes of the HEU infants with those of HUU infants as observed in other studies in this area. Although the yield of detectable CMV DNA was higher in maternal plasma compared to buffy coat, we used plasma and buffy coat samples interchangeably to determine overall CMV infection status and we do not have sufficient data to understand whether the clinical relevance of these assays differs in the context of the analysis that we undertook.
In summary, in a cohort of HIV-positive pregnant women from Botswana with a relatively high CD4 count and low HIV-1 RNA who were receiving ARVs during pregnancy (one third of whom received 3-drug ART), the overall prevalence of CMV DNA in peripheral blood at delivery was 14.6%. Presence of maternal CMV DNA was associated with the occurrence of any adverse birth outcome in unadjusted analysis and maintained a borderline association in adjusted analysis. Repeating this study in a larger group of HIV-positive pregnant women who are universally treated with 3-drug ART would allow us to better understand the potential role of maternal CMV infection and reactivation in pregnancy outcomes. This study allowed us to begin to understand the association between CMV/HIV co-infection and adverse birth outcomes in the context of the sub Saharan African setting.
Acknowledgments
The study was funded by National Institute of Mental Health (RO1 MH087344). NOM, SM & SG were partly supported through the Sub-Saharan African Network for TB/HIV Research Excellence (SANTHE), a DELTAS Africa Initiative [grant # DEL-15-006]. The DELTAS Africa Initiative is an independent funding scheme of the African Academy of Sciences (AAS)’s Alliance for Accelerating Excellence in Science in Africa (AESA) and supported by the New Partnership for Africa’s Development Planning and Coordinating Agency (NEPAD Agency) with funding from the Wellcome Trust [grant # 107752/Z/15/Z] and the UK government. NOM was supported was supported from the National Institutes of Health (NIH) Fogarty International Center (Grant # 5D43TW009610) and OAK Foundation (Grant # OUSA-12-025). Part of SL’s effort on this project was supported by K24 AI131928.
The views expressed in this publication are those of the author(s) and not necessarily those of the funding agencies.
Footnotes
Conflicts of interest: None declared
REFERENCES
- 1.Montano M, Russell M, Gilbert P, et al. Comparative Prediction of Perinatal Human Immunodeficiency Virus Type 1 Transmission, Using Multiple Virus Load Markers. The Journal of Infectious Diseases. 2003;188(3):406–413. [DOI] [PubMed] [Google Scholar]
- 2.Shapiro RL, Hughes MD, Ogwu A, et al. Antiretroviral Regimens in Pregnancy and Breast-Feeding in Botswana. The New England journal of medicine. 2010;362(24):2282–2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Slogrove AL, Goetghebuer T, Cotton MF, Singer J, Bettinger JA. Pattern of Infectious Morbidity in HIV-Exposed Uninfected Infants and Children. Frontiers in Immunology. 2016;7:164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Afran L, Garcia Knight M, Nduati E, Urban BC, Heyderman RS, Rowland-Jones SL. HIV-exposed uninfected children: a growing population with a vulnerable immune system? Clinical and Experimental Immunology. 2014;176(1):11–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kathleen M, Powis., Gbolahan A, Jean L, et al. Decline in Early Mother-to-Child HIV Transmission (MTCT) Risk Over Time in Botswana. The annual Conference on Retroviruses and Opportunistic Infections. Boston 2016. [Google Scholar]
- 6.Brocklehurst P, French R. The association between maternal HIV infection and perinatal outcome: a systematic review of the literature and meta-analysis. BJOG: An International Journal of Obstetrics & Gynaecology. 1998;105(8):836–848. [DOI] [PubMed] [Google Scholar]
- 7.Rollins NC, Coovadia HM, Bland RM, et al. Pregnancy Outcomes in HIV-Infected and Uninfected Women in Rural and Urban South Africa. JAIDS Journal of Acquired Immune Deficiency Syndromes. 2007;44(3):321–328. [DOI] [PubMed] [Google Scholar]
- 8.Abramczuk BM, Mazzola TN, Moreno YMF, et al. Impaired humoral response to vaccines among HIV-exposed uninfected infants. Clinical and vaccine immunology : CVI. 2011;18(9):1406–1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sanz-Ramos M, Manno D, Kapambwe M, et al. Reduced Poliovirus vaccine neutralising-antibody titres in infants with maternal HIV-exposure. Vaccine. 2013/April/12/ 2013;31(16):2042–2049. [DOI] [PubMed] [Google Scholar]
- 10.Smith C, Moraka N, Ibrahim M, et al. No Difference in Antibody Responses to Tetanus Vaccine Among HIV-Exposed and -Unexposed Infants in Botswana. Open Forum Infectious Diseases. 2017;4(Suppl 1):S665–S665. [Google Scholar]
- 11.Musonda KG, Nyonda M, Filteau S, Kasonka L, Monze M, Gompels UA. Increased Cytomegalovirus Secretion and Risks of Infant Infection by Breastfeeding Duration From Maternal Human Immunodeficiency Virus Positive Compared to Negative Mothers in Sub-Saharan Africa. Journal of the Pediatric Infectious Diseases Society. 2016;5(2):138–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Manicklal S, Emery VC, Lazzarotto T, Boppana SB, Gupta RK. The “Silent” Global Burden of Congenital Cytomegalovirus. Clinical Microbiology Reviews. 2013;26(1):86–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pass RF, Anderson B. Mother-to-Child Transmission of Cytomegalovirus and Prevention of Congenital Infection. Journal of the Pediatric Infectious Diseases Society. 2014;3(suppl_1):S2–S6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bates M, Brantsaeter AB. Human cytomegalovirus (CMV) in Africa: a neglected but important pathogen. Journal of Virus Eradication. 2016;2(3):136–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wester CW, Bussmann H, Moyo S, et al. Serological Evidence of HIV-Associated Infection among HIV-1—Infected Adults in Botswana. Clinical Infectious Diseases. 2006;43(12):1612–1615. [DOI] [PubMed] [Google Scholar]
- 16.Slyker J, Lohman-Payne B, Rowland-Jones S, et al. The detection of cytomegalovirus DNA in maternal plasma is associated with mortality in HIV-1-infected women and their infants. AIDS (London, England). 2009;23(1):117–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Frederick T, Homans J, Spencer L, et al. The Effect of Prenatal Highly Active Antiretroviral Therapy on the Transmission of Congenital and Perinatal/Early Postnatal Cytomegalovirus Among HIV-Infected and HIV-Exposed Infants. Clinical Infectious Diseases. 2012;55(6):877–884. [DOI] [PubMed] [Google Scholar]
- 18.Manicklal S, van Niekerk AM, Kroon SM, et al. Birth Prevalence of Congenital Cytomegalovirus Among Infants of HIV-Infected Women on Prenatal Antiretroviral Prophylaxis in South Africa. Clinical Infectious Diseases. 2014;58(10):1467–1472. [DOI] [PubMed] [Google Scholar]
- 19.Mwaanza N, Chilukutu L, Tembo J, et al. High Rates of Congenital Cytomegalovirus Infection Linked With Maternal HIV Infection Among Neonatal Admissions at a Large Referral Center in Sub-Saharan Africa. Clinical Infectious Diseases. 2014;58(5):728–735. [DOI] [PubMed] [Google Scholar]
- 20.Duryea EL, Sánchez PJ, Sheffield JS, et al. Maternal Human Immunodeficiency Virus Infection and Congenital Transmission of Cytomegalovirus. The Pediatric Infectious Disease Journal. 2010;29(10):915–918. [DOI] [PubMed] [Google Scholar]
- 21.Floridia M, Pirillo MF, Degli Antoni A, et al. Pregnancy outcomes and cytomegalovirus DNAaemia in HIV-infected pregnant women with CMV. Clinical Microbiology and Infection. 2016;22(9):818–820. [DOI] [PubMed] [Google Scholar]
- 22.Guibert G, Warszawski J, Le Chenadec J, et al. Decreased Risk of Congenital Cytomegalovirus Infection in Children Born to HIV-1-Infected Mothers in the Era of Highly Active Antiretroviral Therapy. Clinical Infectious Diseases. 2009;48(11):1516–1525. [DOI] [PubMed] [Google Scholar]
- 23.Gompels UA, Larke N, Sanz-Ramos M, et al. Human cytomegalovirus infant infection adversely affects growth and development in maternally HIV-exposed and unexposed infants in Zambia. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2012;54(3):434–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chaudhury S, Williams PL, Mayondi GK, et al. Neurodevelopment of HIV-Exposed and HIV-Unexposed Uninfected Children at 24 Months. Pediatrics. 2017;140(4):e20170988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kroll J, Li S, Levi M, Weinberg A. Lytic and latent EBV gene expression in transplant recipients with and without post-transplant lymphoproliferative disorder. Journal of Clinical Virology. 2011/November/01/ 2011;52(3):231–235. [DOI] [PubMed] [Google Scholar]
- 26.Loechelt BJ, Boulware D, Green M, et al. Epstein-Barr and other herpesvirus infections in patients with early onset type 1 diabetes treated with daclizumab and mycophenolate mofetil. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2013;56(2):248–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Matthews LT, Ribaudo HJ, Parekh NK, et al. Birth weight for gestational age norms for a large cohort of infants born to HIV-negative women in Botswana compared with norms for U.S.-born black infants. BMC Pediatrics. December 16 2011;11(1):115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rölle A, Brodin P. Immune Adaptation to Environmental Influence: The Case of NK Cells and HCMV. Trends in Immunology. 2016;37(3):233–243. [DOI] [PubMed] [Google Scholar]
- 29.van de Berg PJ, Heutinck KM, Raabe R, et al. Human Cytomegalovirus Induces Systemic Immune Activation Characterized by a Type 1 Cytokine Signature. The Journal of Infectious Diseases. 2010;202(5):690–699. [DOI] [PubMed] [Google Scholar]
- 30.Varani S, Landini MP. Cytomegalovirus-induced immunopathology and its clinical consequences. Herpesviridae. 2011;2:6–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Freeman ML, Mudd JC, Shive CL, et al. CD8 T-Cell Expansion and Inflammation Linked to CMV Coinfection in ART-treated HIV Infection. Clinical Infectious Diseases: An Official Publication of the Infectious Diseases Society of America. 2016;62(3):392–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hunt PW, Martin JN, Sinclair E, et al. Valganciclovir Reduces T Cell Activation in HIV-infected Individuals With Incomplete CD4(+) T Cell Recovery on Antiretroviral Therapy. The Journal of Infectious Diseases. 2011;203(10):1474–1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lichtner M, Cicconi P, Vita S, et al. Cytomegalovirus Coinfection Is Associated With an Increased Risk of Severe Non–AIDS-Defining Events in a Large Cohort of HIV-Infected Patients. The Journal of Infectious Diseases. 2015;211(2):178–186. [DOI] [PubMed] [Google Scholar]
- 34.Wittkop L, Bitard J, Lazaro E, et al. Effect of Cytomegalovirus-Induced Immune Response, Self Antigen–Induced Immune Response, and Microbial Translocation on Chronic Immune Activation in Successfully Treated HIV Type 1–Infected Patients: The ANRS CO3 Aquitaine Cohort. The Journal of Infectious Diseases. 2013;207(4):622–627. [DOI] [PubMed] [Google Scholar]
- 35.López M, Figueras F, Coll O, et al. Inflammatory Markers Related to Microbial Translocation Among HIV-Infected Pregnant Women: A Risk Factor of Preterm Delivery. The Journal of Infectious Diseases. 2016;213(3):343–350. [DOI] [PubMed] [Google Scholar]
- 36.Pařízek A, Koucký M, Dušková M. Progesterone, inflammation and preterm labor. The Journal of Steroid Biochemistry and Molecular Biology. 2014/January/01/ 2014;139:159–165. [DOI] [PubMed] [Google Scholar]
- 37.Pereira L Congenital Viral Infection: Traversing the Uterine-Placental Interface. Annual Review of Virology. 2018;5(1):273–299. [DOI] [PubMed] [Google Scholar]
- 38.Smith K, Kuhn L, Coovadia A, et al. Immune reconstitution inflammatory syndrome among HIV-infected South African infants initiating antiretroviral therapy. AIDS (London, England). 2009;23(9):1097–1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Slyker JA. Cytomegalovirus and paediatric HIV infection. Journal of Virus Eradication. 2016;2(4):208–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Evans C, Jones CE, Prendergast AJ. HIV-exposed, uninfected infants: new global challenges in the era of paediatric HIV elimination. The Lancet Infectious Diseases. 2016;16(6):e92–e107. [DOI] [PubMed] [Google Scholar]
- 41.Ruck C, Reikie BA, Marchant A, Kollmann TR, Kakkar F. Linking Susceptibility to Infectious Diseases to Immune System Abnormalities among HIV-Exposed Uninfected Infants. Frontiers in Immunology. 2016;7:310. [DOI] [PMC free article] [PubMed] [Google Scholar]


