Abstract
Epilepsy is a highly prevalent disease characterized by recurrent, spontaneous seizures. Approximately one-third of epilepsy patients will not achieve seizure freedom with medical management and become refractory to conventional treatments. These patients are at greatest risk for sudden unexpected death in epilepsy (SUDEP). The exact etiology of SUDEP is unknown, but a combination of respiratory, cardiac, neuronal electrographic dysfunction, and arousal impairment is thought to underlie SUDEP. Serotonin (5-HT) is involved in regulation of breathing, sleep/wake states, arousal, and seizure modulation and has been implicated in the pathophysiology of SUDEP. This review explores the current state of the understanding of the relationship between 5-HT, epilepsy, and respiratory and autonomic control processes relevant to SUDEP in epilepsy patients and in animal models.
Keywords: SUDEP, serotonin, epilepsy, seizures, death
Introduction
The epilepsies are a heterogeneous group of diseases in which patients have unpredictable, spontaneous seizures (Fisher et al., 2017). Despite the existence of dozens of therapies to treat seizures, approximately 35% of patients will not achieve seizure freedom (Chen et al., 2018). These patients are at greatest risk for sudden unexpected death in epilepsy (SUDEP) (Nashef et al., 2007). SUDEP is the leading cause of death in patients with medically refractory epilepsy. It is second only to stroke in years of potential life lost to neurological disease, and thus poses a major public health burden (Thurman et al., 2014). The exact etiology of SUDEP is unknown. Studies in patients and animal models suggest that cardiorespiratory dysregulation and impairment of arousal contributes to SUDEP (Figure 1). Because of its role in influencing seizures, and in regulating sleep and wakefulness, arousal, circadian rhythms, breathing, and cardiac activity, serotonin (5-HT) has been implicated in the pathophysiology of SUDEP. There is large body of evidence to support this. Studies in epilepsy patients have focused on the effect of 5-HT enhancing drugs on seizure semiology, frequency, and autonomic function, whereas studies in animal models have focused on the effect of stimulating 5-HT networks and focal or systemic administration of serotonergic drugs on seizure development and death. In general, increases in 5-HT may be protective against seizures and SUDEP. Conversely, seizures and epilepsy pathologies may reduce 5-HT tone and increase risk of both seizures and SUDEP (Bagdy et al., 2007; Richerson et al., 2011). This review delves into the interplay between 5-HT, epilepsy, and SUDEP in epilepsy patients and animal models.
Figure 1.

Seizures can provoke respiratory dysfunction and blood gas derangements in epilepsy patients and animal models. (A) Oxygen saturation and ETCO2 traces from an epilepsy patient during a complex partial left temporal onset seizure without secondary generalization. Arrowheads depict duration of the seizure. Vertical lines indicate the start and end of apnea. Redrawn with permission from Seyal and Bateman 2009. (B) Terminal apnea precedes terminal asystole in monitored SUDEP cases in human epilepsy patients. Used with permission from Ryvlin et al. 2013. (C) Terminal apnea precedes terminal asystole in mice that succumb to MES induced seizures. Five min EEG, plethysmography, and ECG traces from an animal that died following a MES seizure (50 mA, 60 Hz, 200 ms). Magnified recordings taken (i) before, (ii) during, and (iii) after the seizure. Used with permission from Buchanan 2014.
5-HT in epilepsy and seizures
Effects of seizures on 5-HT
Seizure-induced alterations in 5-HT have been observed in epilepsy patients and in animal models. For example, temporal lobe epilepsy (TLE) patients exhibit decreased binding to 5-HT1A receptors within the raphe, thalamus, amygdala, neocortex, orbitofrontal cortex, fusiform gyrus, anterior cingulate cortex, and hippocampus (Assem-Hilger et al., 2010; Hasler et al., 2007; Henry et al., 2013; Martinez et al., 2013; Merlet et al., 2004; Savic et al., 2004; Toczek et al., 2003). However, increased 5-HT1A receptor density within the hippocampus is associated with longer disease duration in TLE patients (da Fonseca et al., 2017; Schlicker et al., 1996). A decrease in hippocampal 5-HT levels in TLE patients may account for this increase in receptor density but decrease in receptor binding (da Fonseca et al., 2015).
Studies in epilepsy patients have also observed seizure-induced alterations in expression of the 5-HT transporter (5-HTT) genes. 5-HTT is responsible for regulating 5-HT availability in the synaptic terminal. 5-HTT genes are less transcriptionally efficient in epilepsy patients, potentially contributing to reduced 5-HT reuptake (Esmail et al., 2015; Schenkel et al., 2011). Similarly, a reduction in 5-HTT activity in the insular cortex is observed in depressed TLE patients compared to those without depression (Martinez et al., 2013). 5-HTT binding is also reduced within the neocortex of post-mortem samples from TLE patients (Rocha et al., 2007). Changes in 5-HTT efficiency may represent an endogenous compensatory mechanism to account for seizure-induced decreases in 5-HT or 5-HT receptor binding by prolonging synaptic 5-HT availability (Martinez et al., 2013). Given that polymorphisms in the 5-HTT gene SLC6A4 may be associated with pharmaco-resistance in TLE patients, 5-HTT abnormalities may contribute to TLE pathology (Kauffman et al., 2009). However, it is unclear whether these abnormalities predispose patients to seizures or whether the seizures themselves cause changes in gene expression that lead to these polymorphisms.
Seizures may also influence levels of 5-HT metabolites. 5-hydroxyindoleacetic acid (5-HIAA), a major 5-HT metabolite, is decreased in the cerebrospinal fluid (CSF) of adults with progressive myoclonic epilepsy and children with febrile convulsions (Giroud et al., 1990; Pranzatelli et al., 1995). Levels of 5-HIAA are increased in the CSF of epilepsy patients treated with certain anti-epileptic drugs (AEDs) (Reynolds et al., 1975). Pediatric epilepsy patients also exhibit decreased concentrations of tryptophan, an amino acid essential for 5-HT production, within blood plasma and CSF (Ko et al., 1993; Marion et al., 1985). These findings may extend to adults, as polymorphisms in the tryptophan hydroxylase 2 (TPH2) gene, which encodes the major biosynthetic enzyme for 5-HT in the brain, are associated with psychiatric disorders in TLE patients and may contribute to TLE pathology (Bragatti et al., 2014; Gao et al., 2012).
Changes in 5-HT metabolite levels and receptor expression are also observed in animal models. Many genetic animal epilepsy models exhibit alterations in 5-HT receptor expression. 5-HT concentration, synaptosomal 5-HT uptake, and tryptophan hydroxylase (TpOH) activity is reduced in the genetically epilepsy prone rat (GEPR), a model of myoclonic epilepsy (Faingold, 1988) that exhibits acoustically evoked seizures (Dailey et al., 1989; Jobe et al., 1986; Jobe et al., 1982; Statnick et al., 1996). Likewise, EL (also called El or E1) mice (Imaizumi et al., 1959; Suzuki, 2004), which experience convulsive seizures upon ‘throwing’ stimulations (repeated ~10 cm vertical tosses into the air), have abnormal 5-HT levels and [3H]5-HT binding within the cortex and brainstem (Hiramatsu, 1981, 1983; Suzuki, 2013). This 5-HT deficit occurs as a direct result of seizures, as stimulated (‘thrown’) EL mice exhibit lower interictal 5-HT levels than non-stimulated EL mice.
DBA/2 mice, which are susceptible to audiogenic seizures, seizure-induced respiratory arrest (S-IRA), and death (Tupal et al., 2006), show reduced expression of 5-HT2C, 5-HT3, and 5-HT4 in the medulla (Feng et al., 2017; Uteshev et al., 2010). Similarly, 5-HT2B, 5-HT2C, and 5-HT3B expression is reduced within the brainstem of DBA/1 mice, which are also susceptible death following audiogenic seizures (Faingold et al., 2011a). Consistent with a reduction in 5-HT receptor expression influencing seizure susceptibility, 5-HT2C knock-out mice have increased spontaneous death from seizures (Brennan et al., 1997). As aforementioned in epilepsy patients, it is unclear in many models whether seizures precipitate these changes in 5-HT or whether their genetics produces changes in 5-HT that contribute to seizures.
Chemical seizure induction can likewise induce changes in 5-HT. Rats subjected to the pilocarpine-status epilepticus epilepsy model exhibit decreased 5-HT and 5-HIAA levels within the hippocampus during the chronic phase (Lin et al., 2013; Luna-Munguia et al., 2019). This may be due to seizure-induced reduction of the number of TpOH-positive cells within the raphe nuclei, where most 5-HT is produced (Lin et al., 2013), and may explain reduced hippocampal response to DRN stimulation in this model (Mazarati et al., 2008). Moreover, there is reduction in 5-HT7 expression in rats administered pilocarpine (Cavalheiro, 1995; Yang et al., 2012). In these models, it is more likely that the seizures directly produce changes in 5-HT metabolite and receptor expression.
Effects of 5-HT on seizures
Previous findings indicate 5-HT deficits are present in TLE patients. Conversely, co-administration of drugs that increase available 5-HT, such as selective serotonin reuptake inhibitors (SSRIs), may improve seizure control in epilepsy patients (Specchio et al., 2004). In epilepsy patients experiencing focal impaired awareness (formerly complex partial) seizures and focal to bilateral tonic clonic (formerly secondarily generalized) seizures (Fisher et al., 2017), adjunctive fluoxetine or citalopram both reduce seizure frequency (Albano et al., 2006; Favale et al., 2003; Favale et al., 1995). Fluoxetine and fenfluramine are also well-tolerated and reduce seizure frequency in patients with Dravet syndrome, a severe form of epilepsy with a high rate of SUDEP (Figure 2) (Ceulemans et al., 2012; Ceulemans et al., 2016; Meador, 2014; Schoonjans et al., 2017). These observations are consistent with trends seen in animal models.
Figure 2.
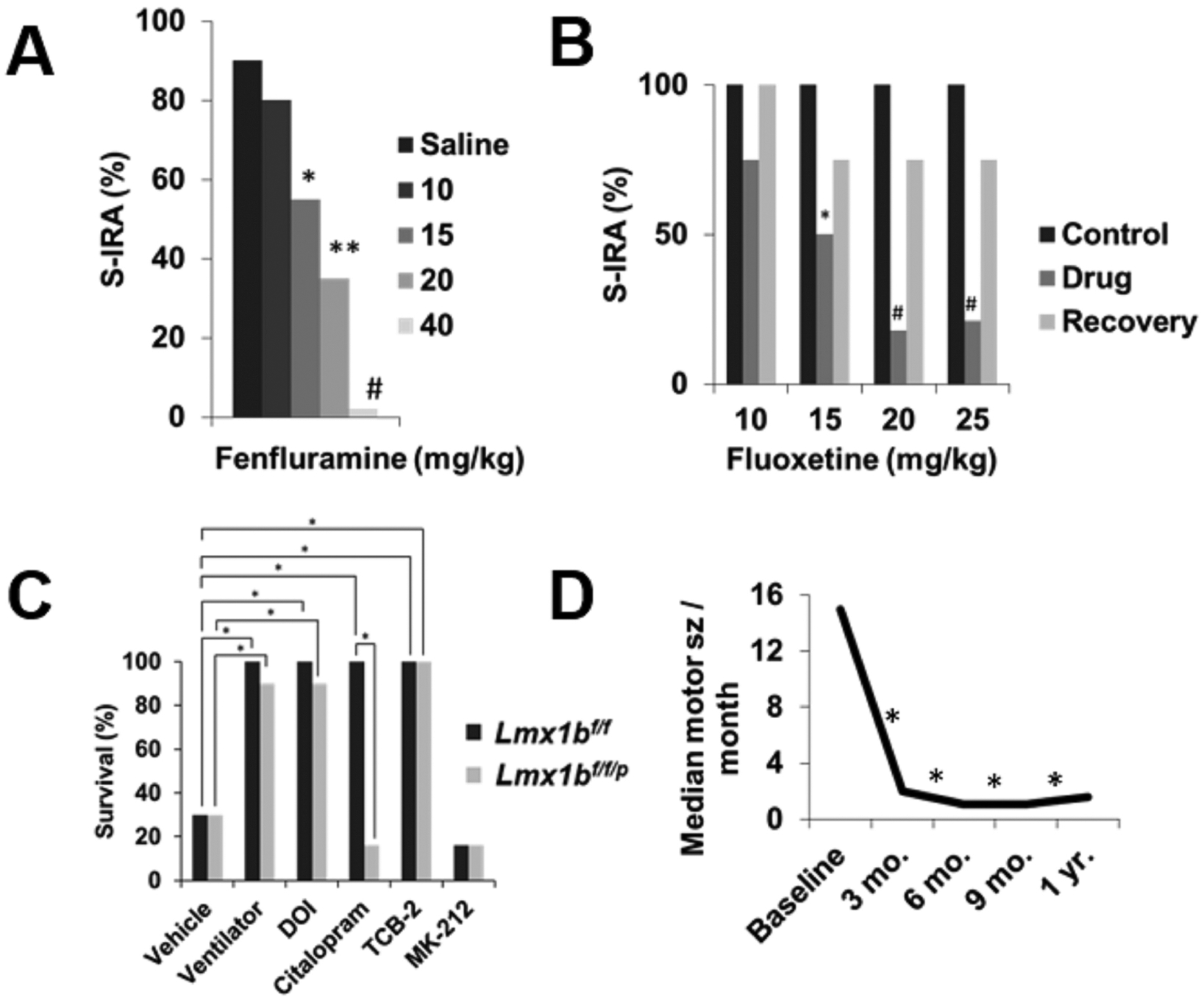
Increasing 5-HT tone reduces SUDEP risk in animal models and may protect against high-risk seizures in epilepsy patients. (A) Pretreatment with saline (n =21) or 15 (n = 9), 20 (n = 11), or 40 (n = 6) mg/kg fenfluramine 30 min before induction of audiogenic seizures reduces seizure-induced respiratory arrest in DBA/1 mice. *, p < 0.05; **, p < 0.01; #, p < 0.001. Redrawn with permission from Tupal and Faingold 2019. (B) Acute administration of 15 (n = 10), 20 (n = 12), 25 (n = 15) mg/kg fluoxetine 30 min before induction of audiogenic seizures reduces of S-IRA in DBA/2 mice. *, p < 0.05; #, p < 0.005. Redrawn with permission from Tupal and Faingold 2006. (C) Survival following a MES seizure (50 mA Lmx1bf/f; 30 mA Lmx1bf/f/p) in Lmx1bf/f and Lmx1bf/f/p is increased by mechanical ventilation or pretreatment with DOI (0.3 mg/kg Lmx1bf/f; 1 mg/kg Lmx1bf/f/p), citalopram (20 mg/kg), TCB-2 (10 mg/kg), or MK-212 (10 mg/kg) 30 min prior to seizure induction. n = 7 per drug/genotype. *, p < 0.001. Redrawn with permission from Buchanan et al. 2014. (D) Adjunctive fenfluramine reduces frequency of convulsive seizures in Dravet syndrome patients. Mo., month; Baseline, n = 9; 3 mo., n = 9; 6 mo., n = 8; 9 mo., n = 7; 1 yr., n = 6. *, p < 0.05. Redrawn with permission from Schoojans et al. 2017.
Early studies in inbred and wild type mouse strains demonstrate that strains displaying the highest levels of whole brain biogenic amines experience greater latency to maximal electroshock (MES) induced seizures (Scudder et al., 1966). Elevated intracranial 5-HT concentrations protect against pentylenetetrazol (PTZ) induced seizures in C57BL/6 and DBA/2 mice, and against audiogenic seizures in DBA/2 mice (Schlesinger et al., 1969). Conversely, decreasing 5-HT levels appears to increase seizure susceptibility. Reserpine, which reduces 5-HT levels by interfering with vesicular monoamine transporters (Giarman et al., 1964), reduces whole brain 5-HT and increases susceptibility to minimal electroshock seizures in rats (Wenger et al., 1973). This effect is prolonged with selective inhibition of 5-HT synthesis (Wenger et al., 1973). 5-HT receptor blockade with cyproheptadine, or inhibition of 5-HT synthesis with para-chloroamphetamine (PCPA) similarly decreases electroshock seizure threshold (Buterbaugh, 1978; Kilian et al., 1973).
The pro-convulsant effects of 5-HT depletion in animal models can be prevented with 5-HT enhancement (Buterbaugh, 1978; Kilian et al., 1973; Przegaliński, 1985; Truscott, 1975). Administration of 5-hydroxytryptophan (5-HTP), a 5-HT precursor, elevates whole brain levels of 5-HT and reverses reserpine induced enhancement of audiogenic seizures in DBA/2 mice (Boggan et al., 1973). 5-HTP pretreatment increases MES seizure threshold in rats (Kilian et al., 1973). This trend is recapitulated in more recent studies. For instance, 5-HTP administration reduces S-IRA following acoustic seizures in DBA/1 mice (Zhang et al., 2016). In a subset of mice 5-HTP also reduced the incidence of the tonic seizures associated with S-IRA. Effects on S-IRA by 5-HTP may be due to direct effects on breathing and/or seizure protection (Buchanan et al., 2016).
Furthermore, pharmacologically increasing extracellular 5-HT with SSRIs and other antidepressants has anticonvulsant effects across a variety of animal models (Table 1, Figure 2) (Castano-Monsalve, 2013; Igelstrom, 2012; Jobe et al., 2005; Jobe et al., 1999; Johannessen Landmark et al., 2016; Kanner, 2013, 2016; Kondziella et al., 2009; Li et al., 2018; Maguire et al., 2014; Payandemehr et al., 2012; Santos Junior et al., 2002; Vermoesen et al., 2011).
Table 1.
Review of literature supporting anticonvulsant effect of 5-HT enhancing drugs in electrical, chemical, and genetic animal models of epilepsy.
| MODEL | SPECIES | DRUG | DOSE (mg/kg) | STUDY PROTOCOL | SEIZURE OUTCOME MEASURE | REFERENCE |
|---|---|---|---|---|---|---|
| PTZ | Zebrafish | CIT, REB, BUP | 10–300 μM | 1 min before sz induction | Seizure threshold | Vermoesen et al. (2011) |
| PTZ | Mouse | REB, BUP | 5–10; 10–40 | 30 min before sz induction | Seizure threshold | Vermoesen et al. (2011) |
| PTZ | Mouse | FLX | 20 | 60 min before sz induction | Survival | Kecskemeti et al. (2005) |
| PTZ | Mouse | FLX | 1–20 | 30 min before sz induction | Seizure threshold, latency | Ugale et al. (2004) |
| PTZ | Mouse | FLX | 10, 15 | 30 min before sz induction | Seizure threshold, susceptibility | Borowicz et al. (2012) |
| PTZ | Mouse | HBK-14 | 20 | 30 min before sz induction | Seizure threshold | Pytka et al. (2017) |
| PTZ | Mouse | SRT | 10 | Drug for 14 days before sz induction | Seizure threshold | Heydari and Davoudi (2017) |
| PTZ | Rat | TNP | 20–80 | 30 min before sz induction | Seizure latency, frequency, severity | Reeta et al. (2016) |
| PTZ | Mouse | SR 57227 | 1–10 | 30 min before sz induction | Seizure latency, severity, mortality | Li et al. (2014) |
| PTZ | Mouse | TNP | 2.5–80 | 30 min before sz induction | Seizure onset | Uzbay et al. (2007) |
| PTZ | Rat | VEN | 25–50 | 30 min before sz induction | Seizure severity and latency | Santos et al. (2002) |
| PTZ | Mouse | mCPBG, CIT | 1–10; 0.1–50 | 30 min before sz induction | Seizure threshold | Payandemehr et al. (2012) |
| PTZ | Mouse | APZ | 0.5–8 | 30, 60, 120 min before sz induction | Seizure latency | Shafaroodi et al. (2015) |
| PTZ/DBA/2 | Mouse | 5-HT | 800 μg | Intracranial injection 10 min before sz induction | Seizure severity | Schlesinger et al. (1969) |
| PTZ | Mouse | REB | 4–15 | 30 min before sz induction | Seizure threshold | Poplawska et al. (2015) |
| PTZ (kindling) | Mouse | FLV | 5–20 | 30 min before sz induction | Seizure severity, brain dmg | Alhaj et al. (2015) |
| Pilocarpine | Zebrafish | CIT | 10–300 μM | 1 min before sz induction | Seizure threshold | Vermoesen et al. (2011) |
| Pilocarpine | Rat | FLX | 20 | Drug for 5 days after sz induction | Seizure frequency | Hernandez et al. (2002) |
| Pilocarpine | Rat | FLX | 20 | Drug for 10 days once epileptic | Seizure threshold | Mazarati et al. (2008) |
| Pilocarpine | Rat | PAR | 5 | Drug for 4 days after sz induction | Seizure frequency, severity, brain dmg | Lin et al. (2017) |
| KA | Mouse | CIT | 10 | Drug for 7 days before sz induction | Seizure severity | Jaako et al. (2011) |
| KA | Rat | BUP | 10, 50 | 30 min before sz induction | Seizure latency, severity, brain dmg | Lin et al. (2013) |
| KA | Rat | CIT, REB | 15, 20–30 | Drug for 4 days once epileptic | Seizure frequency, duration | Vermoesen et al. (2012) |
| MES | Mouse | APZ | 1–2 | 60 min before sz induction | Seizure mortality, tonic phase duration | Shafaroodi et al. (2015) |
| MES | Mouse | BUP | 15–30 | 30 min before sz induction | Seizure threshold | Tutka et al. (2004) |
| MES | Mouse | REB, FLX, CIT, DLX | 0.1–30; 10; 20; 10 | 30 min before sz induction | Seizure mortality | Kruse et al. (2019) |
| MES | Mouse | VEN | 12.5, 25 | 30 min before sz induction | Seizure threshold | Borowicz et al. (2011) |
| MES | Mouse | VEN | 12.5, 25 | Drug for 14 days before sz induction | Seizure threshold | Borowicz et al. (2011) |
| MES | Mouse | FLX | 2.5, 5, 10 | 30 min before sz induction | AED ED50 | Leander (1992) |
| MES | Rat | FLX, FEN | 10, 15 | 60 min before sz induction | Hind limb extension | Buterbaugh (1978) |
| MES | Mouse | DOI, CIT, TCB-2, MK-212 | 0.3, 1; 20; 10; 10 | 30 min before sz induction | Seizure mortality | Buchanan et al. (2014) |
| MES | Mouse | HBK-14, HBK-15 | 20 | 30 min before sz induction | Seizure threshold | Pytka et al. (2017) |
| MES | Mouse | FLX | 10 | 60 min before sz induction | Seizure threshold | Raju et al. (1999) |
| MES/PTZ | Mouse/Rat | 5-HTP | 100 | 30 min before sz induction | Seizure threshold | Killian and Frey (1973) |
| MES | Mouse | REB | 8–16 | 30 min before sz induction | Seizure threshold | Borowicz et al. (2014) |
| 6 Hz Corneal Stimulation | Mouse | HBK-14, HBK-15 | 20 | 30 min before sz induction | Seizure threshold | Pytka et al. (2017) |
| Ear shock | Mouse | FLX | 15–25 | 30 min before sz induction | Seizure threshold | Borowicz et al. (2006) |
| Hippocampal kindling | Rat | FLX | 10 nmol | 15 min before sz induction | Electrical threshold | Wada et al. (1993) |
| Hippocampal kindling | Rat | 5-HTP | 50 | 60 min before sz induction | Electrical threshold | Cavalheiro et al. (1981) |
| Hippocampal kindling | Rat | FLX | 10 | Drug for 21 days before sz induction | Electrical threshold | Wada et al. (1995) |
| Amygdala kindling | Cat | FLX | 2–10 | 1–49 hr before seizure induction | Electrical threshold | Siegal and Murphy (1979) |
| Amygdala kindling | Mouse | FLX | 10 | Osmotic mini pump, 30 day administration | Afterdischarge threshold | Li et al. (2018) |
| GEPR-3 and GEPR-9 | Rat | FLX | 30 | 1–5 hr before seizure induction | Seizure severity | Dailey et al. (1992) |
| GEPR-3 and GEPR-9 | Rat | FLX | 7–20 | Sz induced every 7 days for 28 days | Seizure threshold | Dailey et al. (1992) |
| GEPR-9 | Rat | FLX | 15 | 60 min before sz induction | Severity and latency | Yan et al. (1994) |
| El mice | Mouse | CIT | 0.01, 0.02, 0.04 | Drug for 14 days before sz induction | Seizure threshold | Kabuto et al. (1994) |
| El mice | Mouse | FLX | 10 | Drug for 3 or 7 days before sz induction | Seizure threshold | Richman and Heinrichs (2007) |
| DBA/1 and PTZ | Mouse | 5-HTP | 100–150 | Drug for 2 days before sz induction | S-IRA, seizure incidence | Zhang et al. (2015) |
| DBA/1 | Mouse | FLX | 30 | 30 min before sz induction | S-IRA | Zeng et al. (2015) |
| DBA/1 | Mouse | SRT | 40–75 | 30 min before sz induction | S-IRA, seizure incidence | Faingold and Randall (2013) |
| DBA/1 | Mouse | FEN | 10–40 | 30 min, 12 hr, 24 hr, or 72 hr before sz induction | Seizure severity, ED50, S-IRA | Tupal and Faingold (2019) |
| DBA/2 | Mouse | FLX | 15–25 | 30 min or 72 hr before sz induction | S-IRA, seizure incidence | Tupal and Faingold (2006) |
| DBA/1 | Mouse | SR 57227, FLX | 20–40; 40 | 30 min before sz induction | S-IRA, seizure incidence | Faingold et al. (2016) |
| DBA/1 | Mouse | FLX | 15–70 acute, 20 chronic | 30 min before or for 5 days before sz induction | S-IRA, seizure incidence | Faingold et al. (2011) |
| DBA/1 | Mouse | FLV, PAR,VEN | 50–80; 75–100; 25–100 | 30, 60, 120 min prior to sz induction | S-IRA, seizure incidence | Faingold et al. (2014) |
| Audiogenic Sz | Rat | FLX | 25 | 60 min before sz induction | Seizure severity | Ismaylova and Madzhidi (2012) |
| Audiogenic Sz | Rat | FLX | 20 | Drug for 14 days before sz induction | Seizure severity | Sarkissova et al. (2016) |
| WAG/Rij Absence Sz | Rat | FLX, DLX | 30; 10–30 | Drug administration for 7 weeks | Seizure and immobility duration | Citraro et al. (2015) |
| Flurothyl | Mouse | REB | 20 | Drug for 21 days before sz induction | Seizure latency | Ahern et al. (2006) |
| Focal pilocarpine | Rat | CIT | 1 μM | 4 hr intracranial infusion before sz induction | Seizure severity | Clinckers et al. (2004) |
| Focal bicuculline | Rat | FLX | 5–20 | 60 min before sz induction | Seizure frequency, severity | Prendiville and Gale (1993) |
| Focal bicuculline | Rat | FLX | 1.75–7 nM | 15 min before sz induction | Seizure threshold | Pasini et al. (1992) |
| Picrotoxin | Mouse | FLX | 20 | 65 min before sz induction | Seizure latency | Pericic et al. (2005) |
| Picrotoxin | Mouse | FLX | 20 | Drug for 5 days before sz induction | Seizure latency | Pericic et al. (2005) |
| 4-AP | Rat | FLX | 10 | Drug for 7 days before sz induction | Seizure latency, brain dmg | Shiha et al. (2017) |
| 4-AP | Rat | SRT | 2.5, 25 | 4 hr before sz induction | Seizure frequency, EEG changes | Sitges et al. (2012) |
| 4-AP | Rat | SRT | 0.75 | Drug for 7 days before sz induction | Seizure frequency, EEG changes | Sitges et al. (2012) |
| 4-AP | Rat | SRT | 0.75, 2.5 | 4 hr before sz induction | Seizure suppression | Sitges et al. (2016) |
BUP, bupropion; CIT, citalopram; FEN, fenfluramine; FLV, fluvoxamine; FLX, fluoxetine; GEPR, genetically epilepsy prone rats; hr, hour; KA, kainic acid; MAP, maprotiline; min, minute; PAR, paroxetine; REB, reboxetine; SRT, sertraline; sz, seizure; VEN, venlafaxine; CLM, clemizole.
In the PTZ seizure model, venlafaxine and tianeptine increase latency to convulsions and reduce seizure severity (Reeta et al., 2016; Santos Junior et al., 2002; Uzbay et al., 2007). Fluoxetine administration similarly increases seizure latency and the seizure survival rate of PTZ induced seizures (Kecskemeti et al., 2005; Ugale et al., 2004). It has been observed that concomitant fluoxetine and AED (phenytoin, carbamazepine, ameltolide) administration may be synergistic. Fluoxetine may enhance the effect of AEDS and protect against MES and PTZ seizures in mice (Borowicz et al., 2012; Borowicz et al., 2006; Leander, 1992). Fluoxetine also reduces the ED50 of phenytoin, carbamazepine, and ameltolide against MES seizures by half (Leander, 1992). Acutely enhancing 5-HT tone with other drugs such as reboxetine, citalopram, and the antidepressant bupropion also increases PTZ seizure threshold in mice (Payandemehr et al., 2012; Poplawska et al., 2015; Vermoesen et al., 2011). A similar trend is noted in zebrafish, with acute citalopram, reboxetine, and bupropion reducing behavioral PTZ seizure threshold (Vermoesen et al., 2011). Chronic 5-HT enhancement may also be seizure protective, as chronic administration of the SSRI sertraline increases PTZ seizure threshold in mice (Heydari et al., 2017).
Increases in 5-HT are anticonvulsant in several other chemical seizure models as well (Table 1). Chronic fluoxetine or paroxetine reduces seizure frequency and severity in the pilocarpine seizure model whereas 5-HT depletion increases seizure susceptibility and frequency (Hernandez et al., 2002; Lin et al., 2017; Trindade-Filho et al., 2008). Chronically increasing 5-HT tone with citalopram or reboxetine likewise reduces kainic acid seizure frequency and severity (Jaako et al., 2011; Vermoesen et al., 2012). This is supported by the observation that mutant En1Cre/+;Otx2flox/flox mice, which express 5-HT hyperinnervation in ventral midbrain, hippocampal CA3 region, and cerebral cortex, are resistant to kainic acid-induced seizures (Tripathi et al., 2015).
Genetic animal models of epilepsy also exhibit reduced seizure propensity following increases in 5-HT (Table 1). Fluoxetine and duloxetine reduce absence seizure and immobility duration in the WAG/Rij rat absence seizure model (Citraro et al., 2015). Chronic fluoxetine and citalopram administration reduces seizure frequency in EL mice (Kabuto et al., 1994; Richman et al., 2007). Similarly, audiogenic seizures are suppressed in seizure-susceptible Wistar rats administered either acute or chronic fluoxetine (Ismailova et al., 2012; Sarkissova et al., 2016). 5-HT enhancement also has anticonvulsant effects on audiogenic seizures in GEPR-3 and −9 rats and this effect is antagonized by PCPA 5-HT depletion (Browning et al., 1997; Dailey et al., 1996; Dailey et al., 1992; Yan et al., 1994).
Observations in epilepsy patients and electrical, chemical, and genetic animal models consistently demonstrate that increases in 5-HT are seizure protective and that blockade of 5-HT binding or decreases in 5-HT are proconvulsant. However, the neural mechanisms underlying this effect are not clear. Future research aimed at receptor mechanisms and underlying circuitry will be critical to discerning how 5-HT exerts its anticonvulsant effects,
Effect of anti-epileptic drugs on 5-HT
5-HT may contribute to the anticonvulsant mechanism of some AEDs (Bagdy et al., 2007; Dailey et al., 1996; Griffin et al., 2017). For instance, carbamazepine increases free blood plasma tryptophan concentrations, whereas diphenylhydantoin decreases tryptophan concentrations in epilepsy patients, although both are anticonvulsants (Pratt et al., 1984).
Bonnycastle et al. (1957) were among the first to report that some AEDs (e.g., phenytoin, methion) can increase levels of 5-HT within the rat brain (Bonnycastle et al., 1957). Carbamazepine, antiepilepsirine, and loreclezole increase 5-HT within the hippocampus, thalamus, and striatum of GEPRs (Ahmad et al., 2005; Dailey et al., 1997a; Dailey et al., 1996; Okada et al., 1992). Anticonvulsant doses of carbamazepine similarly produce hippocampal 5-HT release in non-epileptic rats (Dailey et al., 1998; Dailey et al., 1997b; Kaneko et al., 1993). At high dosages, carbamazepine promotes prolonged synaptic 5-HT by blocking uptake of 5-HT into hippocampal synaptosomes which may contribute to increased 5-HT levels (Dailey et al., 1998). 5-HT depletion decreases the anticonvulsant effect of both carbamazepine and antiepilepsirine (Dailey et al., 1996). Similarly, suppression of pilocarpine induced seizures by oxcarbazepine is associated with increases in hippocampal 5-HT in mice (Clinckers et al., 2005). However, perfusion of the 5-HT1A antagonist WAY-100635 into the hippocampus abolishes the anticonvulsant effects of oxcarbazepine, implicating a 5-HT1A receptor mechanism (Clinckers et al., 2005).
Chronic phenytoin administration increases levels of 5-HT within the motor cortex. However, it decreases levels of 5-HT within the cerebellum in rats (Meshkibaf et al., 1995). Compared to oxcarbazepine, alterations to 5-HT neurotransmission is likely not a major mechanism of phenytoin action. However, the anticonvulsant zonisamide increases 5-HIAA in the striatum and both 5-HT and 5-HIAA within the hippocampus in seizure-naïve rats, implicating enhancement of 5-HT neurotransmission in its mechanism of action (Kaneko et al., 1993; Okada et al., 1992).
Lamotrigine, an anticonvulsant and mood stabilizer, dose-dependently inhibits 5-HT uptake in human platelets and rat brain synaptosomes likely via effects on monoamine transporters (Southam et al., 1998). Another anticonvulsant and mood stabilizer, sodium valproate, dose dependently increases extracellular 5-HT within the hippocampus and caudate in seizure-naïve Wistar rats, although levels of 5-HIAA remain stable (Biggs et al., 1992; Murakami et al., 2001). Similarly, valproic acid acts as an AED and mood stabilizer and increases 5-HT levels within the amygdala of seizure-naïve rats (Kempf et al., 1982).
Consistent with the observation that increases in 5-HT are seizure protective in epilepsy patients and in animal models, 5-HT may be critical in the anticonvulsant action of a variety of AEDs. Whether the increases in 5-HT are primary or secondary to the mechanisms underlying the anticonvulsant action of AEDs is unknown. It is also possible that increases in 5-HT contribute to the mood stabilizing properties of some AEDs.
Seizure associated mortality
People with epilepsy are more likely to die prematurely than the general population due to an increased risk of motor vehicle accidents, falls, burns, drowning, aspiration pneumonia, suicide, status epilepticus, and SUDEP (Devinsky et al., 2016b; Weiss et al., 2010). Epilepsy patients, especially those with generalized tonic-clonic seizures, are also likely to endure accidental mild (dental injury, burn, head injury) and severe (skull fracture, hemorrhage, subdural/epidural hematomas, brain contusion) injuries (Asadi-Pooya et al., 2012; Dabla et al., 2018; Wirrell, 2006). These injuries put epilepsy patients at a greater risk for death or future medical complications (Chang et al., 2012). Epilepsy patients are 10× more likely to drown than the general population, with deaths occurring most commonly in the bathtub (Bain et al., 2018). Because pulmonary edema may occur in drowning and SUDEP, some cases of accidental drowning without submersion may be undiagnosed SUDEP (Cihan et al., 2018).
SUDEP is the leading cause of death in patients with refractory epilepsy (Devinsky et al., 2016b). SUDEP is diagnosed as “a sudden, unexpected, witnessed or unwitnessed, nontraumatic and non-drowning death with or without evidence for a seizure and excluding documented status epilepticus, in which postmortem examination does not reveal a toxicologic or anatomic cause of death” (Nashef et al., 2012). The American Academy of Neurology and American Epilepsy Society presently identifies SUDEP risk at 1.2/1000 patient years in adults and 1/4500 patient years in children (DeGiorgio et al., 2019; Donner et al., 2018; Harden et al., 2017; Sveinsson et al., 2017; Thurman et al., 2014). However, recent research suggests that SUDEP may have equal incidence in pediatric and adult patients (Keller et al., 2018; Sveinsson et al., 2017). SUDEP incidence is likely underestimated, as the International Classification of Disease codes used in death certificate data did not consider SUDEP a separate entity and autopsy recommendations in suspected SUDEP cases have been lacking (Middleton et al., 2018). SUDEP risk is greatest in patients with refractory epilepsy (Devinsky et al., 2016a; Dravet et al., 2013; Hesdorffer et al., 2011). Severe myoclonic epilepsy of infancy, or Dravet syndrome, is associated with a particularly high incidence of SUDEP (Dravet, 2011). The most consistent risk factor for SUDEP is frequency of generalized tonic clonic seizures (Harden et al., 2017; Hesdorffer et al., 2011; Lamberts et al., 2012; Ryvlin et al., 2019; Walczak et al., 2001). Patients with poor AED compliance and patients undergoing polytherapy are also at an increased risk of SUDEP (Hesdorffer et al., 2011; Tomson et al., 2008; Walczak et al., 2001). This is likely reflective of the fact that patients taking multiple medications have a more severe form of epilepsy (Hesdorffer et al., 2013; Ryvlin et al., 2019).
In a multi-center MORTality in Epilepsy Monitoring Units Study (MORTEMUS) of SUDEP incidents in epilepsy monitoring units, all cases of SUDEP featured terminal respiratory arrest prior to terminal asystole (Figure 1) (Ryvlin et al., 2013). This is recapitulated in animal seizure models such as the Dravet and MES seizure models, where breathing cessation precedes cardiac dysfunction and death (Figure 1) (Buchanan et al., 2014; Faingold et al., 2011b; Feng et al., 2017; Goldman et al., 2017; Hajek et al., 2016; Kim et al., 2018). Seizures frequently occur during sleep (Derry et al., 2013). SUDEP frequently occurs at night and nocturnal seizures are considered a SUDEP risk factor (Lamberts et al., 2012; Latreille et al., 2017; Nobili et al., 2011; Purnell et al., 2018). Sleep and circadian related effects on breathing and cardiac activity likely contribute to the uneven temporal distribution of SUDEP (Buchanan, 2013; Purnell et al., 2017; Purnell et al., 2018). The cardiorespiratory, circadian, time-ofday, and arousal mechanisms potentially involved in SUDEP are summarized in the following sections.
Mechanisms of death
Respiratory
Numerous animal models of epilepsy experience respiratory dysfunction, both related and unrelated to seizures. DBA/1 and DBA/2 mice experience respiratory arrest and death following audiogenic seizures (Faingold et al., 2010). Similarly, MES-induced seizures in mice often result in terminal apnea which precedes terminal asystole (Figure 1) (Buchanan et al., 2014). Mechanical ventilation can significantly reduce death in both models (Figure 2) (Buchanan et al., 2014; Venit et al., 2004). Respiratory phenotypes, including hypoventilation, apnea, and a diminished hypercapnic ventilatory response (HCVR) are observed in mouse model of Dravet syndrome caused by a mutation in the Scn1a gene (Kuo et al., 2019). Severe peri-ictal respiratory dysfunction, including ataxic breathing and prolonged hypoventilation has been observed in Dravet patients (Kim et al., 2018). Another epilepsy model, the Kcna1 null mutant mouse, experiences spontaneous seizures as well as progressive respiratory dysfunction that worsens as the animals age (Simeone et al., 2018). Similar to the SUDEP cases examined in the MORTEMUS study (Ryvlin et al., 2013), Kcna1 null mice experience seizure-induced respiratory dysfunction prior to cardiac dysfunction (Dhaibar et al., 2019).
Seizures themselves cause alterations in breathing, such as coughing, hyperventilation, apnea, increased bronchial secretions, laryngospasm, respiratory arrest, and neurogenic pulmonary edema (Kennedy et al., 2015; Nakase et al., 2016; Rugg-Gunn et al., 2016). Oxygen saturation below 90% occurs in about a third of generalized and focal seizures (Bateman et al., 2008). Moreover, post-convulsive central apnea occurs in 22% of generalized clonic seizures and is associated with SUDEP and near-SUDEP cases (Vilella et al., 2019). Although SUDEP excludes status epilepticus, status epilepticus triggers central apnea and hypoventilation in seizure prone sheep, in some cases resulting in death (Johnston et al., 1997).
Seizure duration also appears to play a major role in deleterious respiratory phenotypes. Longer seizures are associated with rises in left atrium and pulmonary artery pressures, the presence of chest x-ray abnormalities such as pulmonary edema, and are correlated with the extent of oxygen desaturation and rise of end tidal CO2 that occurs during and after seizures (Bayne et al., 1981; Kennedy et al., 2015; Seyal et al., 2010).
In humans, there is also evidence that different types of epilepsy and seizures confer different types or degrees of respiratory dysfunction. Temporal and frontal lobe epilepsy account for the majority of focal epilepsies associated with apnea, with TLE having an 8-fold greater association with apnea compared to frontal lobe epilepsy (Lacuey et al., 2018). Ictal central apneas are also particularly common when both hemispheres are involved in the seizure, but also occur in approximately half of focal impaired awareness seizures (Lacuey et al., 2017; Rugg-Gunn et al., 2016).
The exact mechanism behind respiratory failure in SUDEP is yet unknown. Central apnea and oxygen desaturation occurs in epilepsy patients when seizures spread to the amygdala and when the amygdala is directly stimulated (Dlouhy et al., 2015; Lacuey et al., 2017; Nobis et al., 2018). Patients were not aware of the stimulus-invoked apnea and could voluntarily breathe when prompted, suggesting that the apnea was due to the loss of involuntary ventilatory drive rather than dysfunction in respiratory motor output pathways or musculature (Dlouhy et al., 2015). Disruption of brainstem areas responsible for breathing output by seizures may be another mechanism of respiratory failure. This is supported by the observation that seizures provoke a decrease in the firing of medullary serotonergic neurons during and after seizures concurrent with suppression of breathing (Zhan et al., 2016). Increases in 5-HT may provide a protective mechanism against seizure-induced apneas, as lower postictal serum 5-HT has been associated with postictal central apnea (Murugesan et al., 2019).
Airway obstruction apart from central apnea may be another risk factor for postictal respiratory arrest and SUDEP. An increase in recurrent laryngeal nerve firing during a seizure can cause partial or complete obstruction of airways due to laryngospasm (Nakase et al., 2016). Postictal immobility may prevent repositioning of the head in patients that are prone, which could exacerbate respiratory distress if air exchange is impeded. These phenomena may cause a positive feedback loop, whereby the respiratory distress potentiates postictal immobility, further increasing respiratory distress (Peng et al., 2017). The hypoventilation that occurs during a seizure, when in conjunction with ictal/postictal apneas and postictal hypercapnia and hypoxia, might lead to blood gas derangements that cause fatal cardiac dysfunction and ultimately death (Massey et al., 2014).
Cardiac
Epidemiological studies have consistently shown that individuals with epilepsy have a higher prevalence of structural cardiac disease compared to those without epilepsy (Shmuely et al., 2017; Strine et al., 2005). Long QT syndrome (LQTS), which portends increased risk for cardiac arrhythmias, is common in epilepsy, with 29% of patients with LQTS exhibiting a seizure phenotype (Johnson et al., 2009). Mutations that cause LQTS are found in 7% of SUDEP cases, and another 15% have gene variants that may predispose them to cardiac arrhythmias (Bagnall et al., 2016). Oxygen desaturation below 90% greatly increases the likelihood of QT prolongation, something that may be particularly dangerous in patients who already possess a LQTS phenotype (Seyal et al., 2010).
Some specific forms of epilepsy have accompanying cardiac irregularities. Drug-naïve TLE patients have depressed apnea-mediated heart rate variability (HRV) during sleep. Reduction in HRV is a considered a marker of disease and aging. This decrease may suggest an alteration in reflex baroreceptor activation in TLE patients, predisposing them to cardiac-mediated SUDEP (Nayak et al., 2017). Mouse models of Dravet syndrome experience pathological electrocardiogram (ECG) manifestations during seizures including tachycardia, bradycardia, and a LQTS phenotype (Auerbach et al., 2013; Kalume, 2013; Kalume et al., 2013). Interictal cardiac alterations have also been observed in epilepsy patients, including faster interictal heart rate and a decrease in baroreflex sensitivity (Nayak et al., 2017; Sevcencu et al., 2010).
Seizures have numerous effects on cardiac function including asystole (Figure 3) (Schuele et al., 2008). Sinus tachycardia is seen in up to 80% of seizures (Nashef et al., 1996; Sevcencu et al., 2010). There is evidence that ictal tachycardia occurs with right-side lateralized seizures localized to the temporal lobe (Rugg-Gunn et al., 2016). Seizures may also be accompanied by less common cardiac abnormalities, such as transient myocardial ischemia and ictal bradycardia, which has a higher potential to progress to cardiac asystole than tachycardia (Nayak et al., 2017; Rugg-Gunn et al., 2016).
Figure 3.

Seizures can provoke potentially fatal cardiac arrhythmias and asystole. Twomin ECG traces from two epilepsy patients who experienced ictal asystole independent of respiratory arrest. Used with permission from Schuele et al. 2008.
One potential mechanism for cardiac dysfunction in epilepsy is channelopathy. Most patients with Dravet syndrome carry variants in Scn1a, which encodes a sodium channel expressed in the heart and brain (Depienne et al., 2009). Induced pluripotent stem cells derived from cardiac myocytes from Dravet syndrome patients exhibit increased sodium currents (INa) and spontaneous contractions (Frasier et al., 2018). The subject whose myocytes showed the largest increase in INa was clinically evaluated and cardiac abnormalities were discovered (Frasier et al., 2018). Altered INa properties in Dravet syndrome SCN1AR1407X/+ knock-in mice may be the result of an increase in the number of Nav1.5 channels, leading to altered cardiac function and a susceptibility to arrhythmogenesis (Auerbach et al., 2013).
KCNQ1 mutant mice express abnormal potassium channels in the heart and brain leading to pathological electrical discharges in both the brain and the heart (Goldman et al., 2009). In some cases these mice experience SUDEP resulting from increasingly irregular cardiac activity (Goldman et al., 2009). Similarly, Kcna1 null mice display increased interictal atrioventricular conduction blocks and bradycardia which may predispose these animals to SUDEP (Glasscock et al., 2010). It is possible that ictal or postictal hypoxemia may be sufficient to trigger life-threatening cardiac arrhythmias in susceptible human patients such as those with cardiac channelopathies (Park et al., 2017). A final factor that may augment deleterious cardiac phenotypes is, ironically, the use of antiepileptic drugs AEDs. It has been suggested that AEDs may trigger conduction abnormalities, which could contribute to the co-existence of arrhythmias in epilepsy and may likewise contribute to autonomic regulation in patients with TLE (Nayak et al., 2017). This may be less problematic if the seizures become well-controlled, but it is possible that multiple AEDs taken by patients with refractory epilepsy may contribute to seizure-induced cardiac dysfunction.
Arousal/PGES
Arousing in response to hypercapnia is an important adaptive response in normal homeostatic sleep processes (Dempsey et al., 2010; Guyenet et al., 2013). During the postictal period arousal to blood gas changes, especially a rise in CO2, may be a lifesaving reflex (Richerson et al., 2011). 5-HT neurons are thought to be important for respiratory and arousal responses to CO2. Mice with a >99% reduction in 5-HT neurons in the central nervous system do not arouse to hypercapnic challenges, but do arouse to hypoxic challenges and to auditory and tactile stimuli (Buchanan et al., 2010; Zhao et al., 2006). An individual who is prone following a seizure and whose 5-HT system either recovers quickly or is less depressed should arouse and change position when they begin to rebreathe CO2. Death can occur when seizures interfere with this ability to arouse (Blumenfeld, 2012; Tao et al., 2010). After repeated rebreathing of CO2, the prone patient would become hypoxic and acidotic, contributing to cardiorespiratory failure (Buchanan, 2019).
A potential SUDEP risk marker is the protracted postictal generalized EEG suppression (PGES) that follows some seizures (Figure 4) (Lhatoo et al., 2010). PGES is characterized by low amplitude, low frequency electrographic activity that persists after the termination of the seizure and gradually recovers (Lhatoo et al., 2010; Theeranaew et al., 2018). Accurate identification of PGES is challenging even among expert reviewers (Theeranaew et al., 2018). The origin and significance of PGES is not well understood. PGES may represent an electrographic marker of decreased arousability consequent to ictal DRN 5-HT network dysregulation. During PGES, patients experience postictal stupor and are more likely to be immobile, unresponsive, and require resuscitative measures, making the immediate environment dangerous (Kuo et al., 2016; Semmelroch et al., 2012). This hypothesis is supported by a recent study in humans indicating that interictal serum 5-HT levels are inversely related to PGES duration (Murugesan et al., 2018). Although an increase of 5-HT in serum does not reflect brain parenchymal 5-HT levels, it does support the idea that 5-HT may be involved in PGES.
Figure 4.

Postictal generalized EEG suppression (PGES) occurs following some seizures in human epilepsy patients and animal models. (A) Prolonged PGES (148 seconds) and postictal slowing following a generalized seizure in a monitored epilepsy patient who later dies of SUDEP. Used with permission from Lhatoo et al. 2010. (B) Prolonged PGES occurring following non-fatal seizures induced with MES during wake (n = 6) or NREM sleep (n = 4). Fast Fourier transform is plotted relative to baseline subtraction (1 min of wake baseline). Used with permission from Hajek and Buchanan 2016.
The relationship of PGES to SUDEP has been somewhat controversial. Early studies of SUDEP cases demonstrate that PGES duration may be an independent predictor of SUDEP with longer PGES duration correlating with increased SUDEP risk (Bozorgi et al., 2013b; Lhatoo et al., 2010). PGES preceded all instances of SUDEP captured in epilepsy monitoring units in the MORETMUS study (Ryvlin et al., 2013). Other studies have not observed a connection between PGES and SUDEP (Surges et al., 2011). However, PGES is associated with several SUDEP risk factors (Rajakulendran et al., 2015). PGES is associated with a lower postictal oxygen desaturation nadir and a higher level of end tidal CO2 in epilepsy patients following convulsive seizures (Seyal et al., 2012). Moreover, PGES often follows convulsive seizures (Lamberts et al., 2013a; Lhatoo et al., 2010; Moseley et al., 2015; Poh et al., 2012; Seyal et al., 2013; Seyal et al., 2012; Surges et al., 2011). Fitting with the nocturnal predisposition of SUDEP, PGES is more likely to occur following seizures from sleep, a time of potentially cardiorespiratory instability (Lamberts et al., 2013b; Latreille et al., 2017; Peng et al., 2017; Purnell et al., 2017). PGES following MES seizures during NREM sleep is longer than seizures induced during wake in mice (Figure 4) (Hajek et al., 2016). PGES is also associated with increased sympathetic tone and decreased parasympathetic tone, both of which can precipitate potentially fatal cardiac arrythmias (Bozorgi et al., 2013a; Moseley et al., 2013; Poh et al., 2012). However, PGES was not associated with heart rate variability in another study, but this does not preclude inadequate perfusion (Lamberts et al., 2013b).
Association between SUDEP and the night
SUDEP occurs more frequently during the night (Ali et al., 2017; Hesdorffer et al., 2011; Lamberts et al., 2012; Langan et al., 2005; Purnell et al., 2018; Ryvlin et al., 2013; van der Lende et al., 2018). There are several possible explanations as to why SUDEP may occur at night, including reduced nighttime supervision (Blachut et al., 2015; Witek et al., 2017). However, it is likely that circadian and sleep-wake influences on 5-HT contribute to SUDEP.
The nocturnality of SUDEP is often attributed to differences in seizure frequency or severity occurring across vigilance states. Vigilance state at the time of seizure onset is often inferred based on time of day and proximity of the patient to their bed (Ali et al., 2017; Kloster et al., 1999; Lamberts et al., 2012). These studies consistently find that SUDEP is sleep related, but the absence of EEG recording precludes verification that the fatal seizure emerged from sleep. However, EEG data recorded from patients who died in epilepsy monitoring units supports that SUDEP occurs more frequently during sleep (Ryvlin et al., 2013).
Animal research also supports that vigilance state may be integral to SUDEP. MES seizures in mice are universally fatal when induced during REM sleep (Hajek et al., 2016). 5-HT may be involved in the uneven temporal distribution of SUDEP, as 5-HT neuron activity is modulated by vigilance state. 5-HT neurons are most active during wakefulness, less activity during NREM sleep, and nearly quiescent during REM sleep (Trulson et al., 1979). Downregulation of 5-HT activity during sleep may result in a reduction of 5-HT tone following a seizure and an increased risk of SUDEP.
That SUDEP happens more often during the night could also speak to an independent role of circadian rhythms (Purnell et al., 2017; Purnell et al., 2018). Many aspects of mammalian physiology are regulated by circadian rhythmicity. Sleep-wake behavior is subject to circadian regulation; however, circadian rhythms occur independent of sleep (Borbely et al., 2016). Patients who die of SUDEP are more likely to have a history of seizures during the night (Borbely et al., 2016). SUDEP has a nonuniform distribution regardless of sleep-state of origin. SUDEP inferred to have happened while awake occurs between 0800 and 1200 whereas SUDEP inferred to have happened during sleep occurs between 0400 and 0800 (Borbely et al., 2016). This suggests that there is a circadian component to the timing of SUDEP.
In several animal models, day-night differences in seizure severity and susceptibility have been observed and attributed to circadian differences in 5-HT and norepinephrine (Agren et al., 1986; Schreiber et al., 1971). In both humans and rodents 5-HT is highest during the day, lower during the night, and reaches its nadir in the early pre-dawn hours (Agren et al., 1986; Mateos et al., 2009; Rao et al., 1994; Rosenwasser et al., 1985). In the Kv1.1 null mouse and the Scn1aR1407X/+ Dravet syndrome mouse, seizure induced death is more frequent during the night, with peaks at the light dark transition points (Moore et al., 2014; Teran et al., 2019). The normal circadian reduction in 5-HT during the late part of the night might make epilepsy patients more vulnerable to SUDEP during this time. Additionally, the effect of vigilance state and circadian phase may compound to cause further reductions in 5-HT tone. For example, if 5-HT tone is reduced during the late part of the night and it is reduced by sleep, sleep during the late part of the night might be a particularly hazardous time for a seizure to occur.
Similarities between SIDS and SUDEP
SUDEP shares similarities with other sudden death entities, most notably the sudden infant death syndrome (SIDS; Table 2). SIDS is defined as the unexpected, sudden death of an infant less than one year of age, for which no explanation is uncovered by exhaustive review of the clinical history, investigation of the death scene, and a complete autopsy (Willinger et al., 1991). Dysregulation of respiratory, cardiac, and arousal systems have been implicated in SIDS and SUDEP (Buchanan, 2019; Kinney et al., 2009; Richerson et al., 2011). Due to its role in regulation and/or modulation of these systems, 5-HT has been implicated in both SIDS and SUDEP (Buchanan, 2019; Richerson et al., 2011). Abnormalities in the brainstem 5-HT system have been identified on postmortem examination of the brains of several independent cohorts of babies that died of SIDS (Bright et al., 2017; Duncan et al., 2010; Haynes et al., 2017; Kinney et al., 2001; Kinney et al., 2003; Panigrahy et al., 2000; Paterson et al., 2006). As seen below, 5-HT abnormalities are beginning to be found in SUDEP cases.
Table 2.
SUDEP shares commonalities with other sudden death syndromes such as SIDS. Modified with permission from Buchanan 2019.
| SIDS | SUDEP | |
|---|---|---|
| Diagnostic method | Diagnosis of exclusion | Diagnosis of exclusion |
| Baseline health | Normal | Normal except seizures |
| Male sex | More common in males | More common in males |
| Age at death | <1 year | Any age |
| Incidence | ~0.6 per 1000 live births | 0.2–1.2 in 1000 persons with epilepsy |
| Routine autopsy findings | Normal | Normal; pulmonary edema |
| Proposed mechanism of death | Respiratory, cardiac, arousal impairment, thermoregulatory dysregulation | Respiratory, cardiac, arousal impairment |
| Circumstances of death | Often found prone | Often found prone |
| Occurrence during sleep | Common, often unwitnessed | Common, often unwitnessed |
| Link to 5-HT | Yes | Yes |
| Microscopic hippocampal abnormalities | Yes | Yes |
Additional evidence of a role for 5-HT in SUDEP
Human studies
Although it is impossible to determine if and when a patient will suffer SUDEP, it is possible to study high-risk patients and post-mortem samples to better elucidate SUDEP physiology. Volume loss is observed within the periaqueductal gray matter and 5-HT producing raphe nuclei in MRI imaging of epilepsy patients at high-risk for SUDEP. This is consistent with the role of the raphe nuclei in measures critical for SUDEP including breathing, sleep/wake regulation, and arousal. The magnitude of that deficit was greater in the two patients who later died of SUDEP and extended into the pontine and medullary brain regions (Mueller et al., 2014). Diminished TpOH and serotonin transporter (SERT) expression is also observed within the raphe nuclei in post-mortem evaluation of SUDEP cases compared to epilepsy controls (Patodia et al., 2018). Patients with TLE can express TPH2 gene polymorphisms that may affect cognition/mood, but it is also possible they affect predisposition to cardiorespiratory dysfunction (Bragatti et al., 2014; Richerson et al., 2011). Thus, preliminary findings in high-risk patients and post-mortem SUDEP victims are consistent with the epilepsy patient and animal studies implicating 5-HT broadly in seizure susceptibility.
Animal studies
5-HT may also play a role in seizure-induced mortality in animal models. Pharmacological increases in 5-HT may be protective against SUDEP in animal models. Fluoxetine suppresses S-IRA in DBA/1 and DBA/2 mice without influencing seizure severity or ventilation (Figure 2) (Tupal et al., 2006; Zeng et al., 2015). Fluoxetine may act on S-IRA in DBA/1 mice via a 5-HT3 mechanism. Administration of SR 57227, a 5-HT3 agonist, blocks S-IRA without affecting seizures. Ondansetron, a 5-HT3 antagonist, abolishes the protective effect of fluoxetine on S-IRA (Faingold et al., 2016). The SSRIs fluvoxamine and sertraline more broadly increase 5-HT tone and suppress S-IRA in DBA/1 mice (Faingold et al., 2014; Faingold et al., 2013). Fenfluramine, a serotonin releasing appetite suppressant, not only protects against S-IRA in DBA/1 mice, but reduces seizure incidence and severity (Figure 2) (Tupal et al., 2019). Conversely, blocking 5-HT transmission with cyproheptadine increases S-IRA incidence in DBA/1 and DBA/2 mice that did originally not exhibit S-IRA (Faingold et al., 2014; Tupal et al., 2006).
Lmx1bf/f/p mice lacking >99% of CNS 5-HT neurons have a lower seizure threshold and increased seizure-induced mortality in the acute pilocarpine and MES seizure models (Buchanan et al., 2014; Zhao et al., 2006). MES severity is likewise increased in C57BL/6 mice administered PCPA to reduce 5-HT to ~5–11% of original levels within the medulla and forebrain (Buchanan et al., 2014). As in the DBA mice, death occurs primarily via a respiratory mechanism in the MES model (Figure 1) (Buchanan et al., 2014). In wildtype mice, raising 5-HT levels with either citalopram, fluoxetine, duloxetine, the 5-HT2 agonist DOI, or the 5-HT2A agonist TCB-2 increased MES seizure survival (Figure 2) (Buchanan et al., 2014; Kruse et al., 2019). Similarly, administration of reboxetine, fluoxetine, DOI or TCB-2 prior to MES induction prevents death in Lmx1bf/f/p mice (Figure 2) (Buchanan et al., 2014; Kruse et al., 2019). As in prior sections, it is seen that an increase in 5-HT tone is generally anticonvulsant and lowers mortality, whereas a deficit in 5-HT promotes seizure-induced mortality that can be reversed by increases in 5-HT.
Summary/Conclusions
5-HT plays a critical modulatory role in the pathology of seizures and epilepsy. In recent years, 5-HT has become a strong topic interest within the SUDEP research community. 5-HT is uniquely positioned because of its role in biological processes relevant to SUDEP including breathing, sleep/wake regulation, circadian rhythms, and arousal. Dysregulation of 5-HT may underlie certain epilepsies. Seizures may also exacerbate pre-existing 5-HT abnormalities.
SUDEP may result from a ‘perfect storm’ of intrinsic and extrinsic factors related to the fatal seizure (Figure 5). Interference with 5-HT neurotransmission may contribute to death given its multifaceted modulatory role. Propagation to brainstem centers may depress 5-HT respiratory nuclei, producing hypoventilation and apnea, and depress 5-HT arousal networks, limiting arousal in response to blood gas changes. The additional insult of a prone position may preclude successful autoresuscitation. Moreover, prolonged central apnea can exacerbate or initiate cardiac dysfunction, which in turn can worsen respiratory outcomes until terminal apnea occurs.
Figure 5.
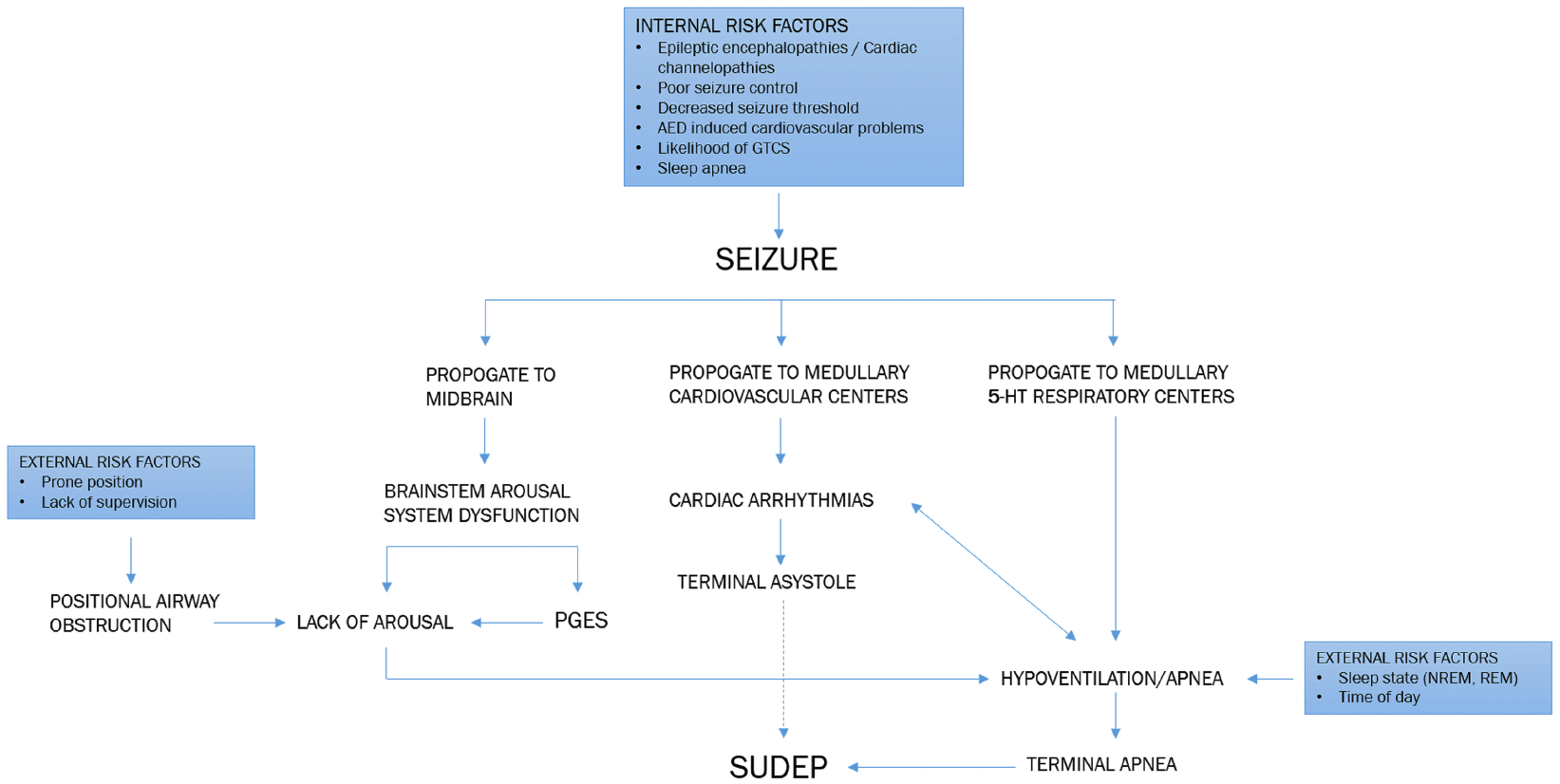
Potential pathways by which seizures can lead to SUDEP. Propagation of seizures to cardiorespiratory centers within the brain can lead to potentially fatal cardiac arrythmias or apneas that can exacerbate respiratory and cardiac dysfunction respectively. Propagation of seizures to arousal centers and the occurrence of PGES following a seizure may prevent reflexive arousal to airway obstruction and lead to potentially fatal apneas. Risk factors including a patient’s lifestyle (adherence to medication, control of triggers), the time of day of the seizure, circadian factors, vigilance state, and postictal position can increase the likelihood of a seizure becoming fatal.
It is possible that future preventative “treatments” against SUDEP may involve adjunctive SSRIs or other pharmacological agents in high risk patients to improve postictal cardiorespiratory outcomes and reduce arousal impairment. However, future research into relevant 5-HT networks may also inform targets for deep-brain stimulation. Recovering baseline activity of 5-HT networks disrupted by seizures may be one way to protect patients at greatest risk for SUDEP. Regardless of its ultimate direction, research endeavors into 5-HT will continue to yield integral findings that will both benefit the lives of epilepsy patients and perhaps reduce SUDEP.
Funding:
This work was supported by a Post Comprehensive Exam Fellowship from the Graduate College at the University of Iowa and a Pre-doctoral Fellowship from the American Epilepsy Society/LivaNova, PLC (A.N.P.); NIH/NINDS F31 NS106819 (B.S.P.); and NIH/NINDS R01 NS095842 and the Beth L. Tross Epilepsy Professorship (G.F.B.).
Abbreviations:
- SUDEP
sudden unexpected death in epilepsy
- 5-HT
serotonin
- TLE
temporal lobe epilepsy
- 5-HTT
serotonin transporter
- 5-HIAA
5-hydroxyindoleacetic acid
- CSF
cerebrospinal fluid
- AED
anti-epileptic drug
- TPH2
tryptophan hydroxylase 2
- TpOH
tryptophan hydroxylase
- GEPR
genetically epilepsy prone rat
- S-IRA
seizure-induced respiratory arrest
- SSRI
selective serotonin reuptake inhibitor
- MES
maximal electroshock seizure
- PTZ
pentylenetetrazol
- PCPA
para-chloroamphetamine
- 5-HTP
5-hydroxytryptophan
- MORTEMUS
MORTality in Epilepsy Monitoring Units Study
- LQTS
long QT syndrome
- HRV
heart rate variability
- ECG
electrocardiogram
- PGES
post-ictal generalized EEG suppression
- SIDS
sudden infant death syndrome
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of interest: None of the authors have any conflicts of interest to disclose.
References
- Agren H, Koulu M, Saavedra JM, Potter WZ, & Linnoila M (1986). Circadian covariation of norepinephrine and serotonin in the locus coeruleus and dorsal raphe nucleus in the rat. Brain Res, 397(2), 353–358. [DOI] [PubMed] [Google Scholar]
- Ahmad S, Fowler LJ, & Whitton PS (2005). Lamotrigine, carbamazepine and phenytoin differentially alter extracellular levels of 5-hydroxytryptamine, dopamine and amino acids. Epilepsy Res, 63(2–3), 141–149. [DOI] [PubMed] [Google Scholar]
- Albano C, Cupello A, Mainardi P, Scarrone S, & Favale E (2006). Successful treatment of epilepsy with serotonin reuptake inhibitors: proposed mechanism. Neurochem Res, 31(4), 509–514. [DOI] [PubMed] [Google Scholar]
- Ali A, Wu S, Issa NP, Rose S, Towle VL, Warnke P, & Tao JX (2017). Association of sleep with sudden unexpected death in epilepsy. Epilepsy Behav, 76, 1–6. [DOI] [PubMed] [Google Scholar]
- Asadi-Pooya AA, Nikseresht A, Yaghoubi E, & Nei M (2012). Physical injuries in patients with epilepsy and their associated risk factors. Seizure, 21(3), 165–168. [DOI] [PubMed] [Google Scholar]
- Assem-Hilger E, Lanzenberger R, Savli M, Wadsak W, Mitterhauser M, Mien LK, Stogmann E, Baumgartner C, Kletter K, & Asenbaum S (2010). Central serotonin 1A receptor binding in temporal lobe epilepsy: a [carbonyl-(11)C]WAY-100635 PET study. Epilepsy Behav, 19(3), 467–473. [DOI] [PubMed] [Google Scholar]
- Auerbach DS, Jones J, Clawson BC, Offord J, Lenk GM, Ogiwara I, Yamakawa K, Meisler MH, Parent JM, & Isom LL (2013). Altered cardiac electrophysiology and SUDEP in a model of Dravet syndrome. PLoS One, 8(10), e77843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagdy G, Kecskemeti V, Riba P, & Jakus R (2007). Serotonin and epilepsy. J Neurochem, 100(4), 857–873. [DOI] [PubMed] [Google Scholar]
- Bagnall RD, Crompton DE, Petrovski S, Lam L, Cutmore C, Garry SI, Sadleir LG, Dibbens LM, Cairns A, Kivity S, Afawi Z, Regan BM, Duflou J, Berkovic SF, Scheffer IE, & Semsarian C (2016). Exome-based analysis of cardiac arrhythmia, respiratory control, and epilepsy genes in sudden unexpected death in epilepsy. Ann Neurol, 79(4), 522–534. [DOI] [PubMed] [Google Scholar]
- Bain E, Keller AE, Jordan H, Robyn W, Pollanen MS, Williams AS, & Donner EJ (2018). Drowning in epilepsy: A population-based case series. Epilepsy Res, 145, 123–126. [DOI] [PubMed] [Google Scholar]
- Bateman LM, Li CS, & Seyal M (2008). Ictal hypoxemia in localization-related epilepsy: analysis of incidence, severity and risk factors. Brain, 131(Pt 12), 3239–3245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayne LL, & Simon RP (1981). Systemic and pulmonary vascular pressures during generalized seizures in sheep. Ann Neurol, 10(6), 566–569. [DOI] [PubMed] [Google Scholar]
- Biggs CS, Pearce BR, Fowler LJ, & Whitton PS (1992). Regional effects of sodium valproate on extracellular concentrations of 5-hydroxytryptamine, dopamine, and their metabolites in the rat brain: an in vivo microdialysis study. J Neurochem, 59(5), 1702–1708. [DOI] [PubMed] [Google Scholar]
- Blachut B, Hoppe C, Surges R, Stahl J, Elger CE, & Helmstaedter C (2015). Counting seizures: The primary outcome measure in epileptology from the patients’ perspective. Seizure, 29, 97–103. [DOI] [PubMed] [Google Scholar]
- Blumenfeld H (2012). Impaired consciousness in epilepsy. Lancet Neurol, 11(9), 814–826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boggan WO, & Seiden LS (1973). 5-Hydroxytryptophan reversal of reserpine enhancement of audiogenic seizure susceptibility in mice. Physiol Behav, 10(1), 9–12. [DOI] [PubMed] [Google Scholar]
- Bonnycastle DD, Giarman NJ, & Paasonen MK (1957). Anticonvulsant compounds and 5-hydroxytryptamine in rat brain. Br J Pharmacol Chemother, 12(2), 228–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borbely AA, Daan S, Wirz-Justice A, & Deboer T (2016). The two-process model of sleep regulation: a reappraisal. J Sleep Res, 25(2), 131–143. [DOI] [PubMed] [Google Scholar]
- Borowicz KK, Piskorska B, Stepniak B, & Czuczwar SJ (2012). Effects of fluoxetine on the anticonvulsant action of valproate and ethosuximide in mouse model of myoclonic convulsions. Ann Agric Environ Med, 19(3), 487–490. [PubMed] [Google Scholar]
- Borowicz KK, Stepien K, & Czuczwar SJ (2006). Fluoxetine enhances the anticonvulsant effects of conventional antiepileptic drugs in maximal electroshock seizures in mice. Pharmacol Rep, 58(1), 83–90. [PubMed] [Google Scholar]
- Bozorgi A, Chung S, Kaffashi F, Loparo KA, Sahoo S, Zhang GQ, Kaiboriboon K, & Lhatoo SD (2013a). Significant postictal hypotension: expanding the spectrum of seizure-induced autonomic dysregulation. Epilepsia, 54(9), e127–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bozorgi A, & Lhatoo SD (2013b). Seizures, Cerebral Shutdown, and SUDEP. Epilepsy Curr, 13(5), 236–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bragatti JA, Bandeira IC, de Carvalho AM, Abujamra AL, Leistner-Segal S, & Bianchin MM (2014). Tryptophan hydroxylase 2 (TPH2) gene polymorphisms and psychiatric comorbidities in temporal lobe epilepsy. Epilepsy Behav, 32, 59–63. [DOI] [PubMed] [Google Scholar]
- Brennan TJ, Seeley WW, Kilgard M, Schreiner CE, & Tecott LH (1997). Sound-induced seizures in serotonin 5–HT2c receptor mutant mice. Nat Genet, 16(4), 387–390. [DOI] [PubMed] [Google Scholar]
- Bright FM, Byard RW, Vink R, & Paterson DS (2017). Medullary Serotonin Neuron Abnormalities in an Australian Cohort of Sudden Infant Death Syndrome. Journal of Neuropathology & Experimental Neurology, 76(10), 864–873. [DOI] [PubMed] [Google Scholar]
- Browning RA, Wood AV, Merrill MA, Dailey JW, & Jobe PC (1997). Enhancement of the anticonvulsant effect of fluoxetine following blockade of 5-HT1A receptors. Eur J Pharmacol, 336(1), 1–6. [DOI] [PubMed] [Google Scholar]
- Buchanan GF (2013). Timing, sleep, and respiration in health and disease. Prog Mol Biol Transl Sci, 119, 191–219. [DOI] [PubMed] [Google Scholar]
- Buchanan GF (2019). Impaired CO2-Induced Arousal in SIDS and SUDEP. Trends in Neurosciences, 42(4), 242–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchanan GF, Murray NM, Hajek MA, & Richerson GB (2014). Serotonin neurones have anti-convulsant effects and reduce seizure–induced mortality. J Physiol, 592(19), 4395–4410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchanan GF, & Richerson GB (2010). Central serotonin neurons are required for arousal to CO2. Proc Natl Acad Sci U S A, 107(37), 16354–16359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchanan GF, & Richerson GB (2016). Epilepsy: A dietary supplement for SUDEP prevention? Nat Rev Neurol, 12(9), 495–496. [DOI] [PubMed] [Google Scholar]
- Buterbaugh GG (1978). Effect of drugs modifying central serotonergic function on the response of extensor and nonextensor rats to maximal electroshock. Life Sci, 23(24), 2393–2404. [DOI] [PubMed] [Google Scholar]
- Castano-Monsalve B (2013). [Antidepressants in epilepsy]. Rev Neurol, 57(3), 117–122. [PubMed] [Google Scholar]
- Cavalheiro EA (1995). The pilocarpine model of epilepsy. Ital J Neurol Sci, 16(1–2), 33–37. [DOI] [PubMed] [Google Scholar]
- Ceulemans B, Boel M, Leyssens K, Van Rossem C, Neels P, Jorens PG, & Lagae L (2012). Successful use of fenfluramine as an add-on treatment for Dravet syndrome. Epilepsia, 53(7), 1131–1139. [DOI] [PubMed] [Google Scholar]
- Ceulemans B, Schoonjans AS, Marchau F, Paelinck BP, & Lagae L (2016). Five-year extended follow-up status of 10 patients with Dravet syndrome treated with fenfluramine. Epilepsia, 57(7), e129–134. [DOI] [PubMed] [Google Scholar]
- Chang YH, Ho WC, Tsai JJ, Li CY, & Lu TH (2012). Risk of mortality among patients with epilepsy in southern Taiwan. Seizure, 21(4), 254–259. [DOI] [PubMed] [Google Scholar]
- Chen Z, Brodie MJ, Liew D, & Kwan P (2018). Treatment Outcomes in Patients With Newly Diagnosed Epilepsy Treated With Established and New Antiepileptic Drugs: A 30-Year Longitudinal Cohort Study. JAMA Neurol, 75(3), 279–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cihan E, Hesdorffer DC, Brandsoy M, Li L, Fowler DR, Graham JK, Donner EJ, Devinsky O, & Friedman D (2018). Dead in the water: Epilepsy-related drowning or sudden unexpected death in epilepsy? Epilepsia, 59(10), 1966–1972. [DOI] [PubMed] [Google Scholar]
- Citraro R, Leo A, De Fazio P, De Sarro G, & Russo E (2015). Antidepressants but not antipsychotics have antiepileptogenic effects with limited effects on comorbid depressive-like behaviour in the WAG/Rij rat model of absence epilepsy. Br J Pharmacol, 172(12), 3177–3188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clinckers R, Smolders I, Meurs A, Ebinger G, & Michotte Y (2005). Hippocampal dopamine and serotonin elevations as pharmacodynamic markers for the anticonvulsant efficacy of oxcarbazepine and 10,11-dihydro-10-hydroxycarbamazepine. Neurosci Lett, 390(1), 48–53. [DOI] [PubMed] [Google Scholar]
- da Fonseca NC, Joaquim HP, Talib LL, de Vincentiis S, Gattaz WF, & Valente KD (2015). Hippocampal serotonin depletion is related to the presence of generalized tonic-clonic seizures, but not to psychiatric disorders in patients with temporal lobe epilepsy. Epilepsy Res, 111, 18–25. [DOI] [PubMed] [Google Scholar]
- da Fonseca NC, Joaquim HP, Talib LL, Vincentiis S, Gattaz WF, & Valente KD (2017). 5-hydroxytryptamine1A receptor density in the hippocampus of patients with temporal lobe epilepsy is associated with disease duration. Eur J Neurol, 24(4), 602–608. [DOI] [PubMed] [Google Scholar]
- Dabla S, Puri I, Dash D, Vasantha PM, & Tripathi M (2018). Predictors of Seizure-Related Injuries in an Epilepsy Cohort from North India. J Epilepsy Res, 8(1), 27–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dailey JW, Reigel CE, Mishra PK, & Jobe PC (1989). Neurobiology of seizure predisposition in the genetically epilepsy-prone rat. Epilepsy Res, 3(1), 3–17. [DOI] [PubMed] [Google Scholar]
- Dailey JW, Reith ME, Steidley KR, Milbrandt JC, & Jobe PC (1998). Carbamazepine-induced release of serotonin from rat hippocampus in vitro. Epilepsia, 39(10), 1054–1063. [DOI] [PubMed] [Google Scholar]
- Dailey JW, Reith ME, Yan QS, Li MY, & Jobe PC (1997a). Anticonvulsant doses of carbamazepine increase hippocampal extracellular serotonin in genetically epilepsy-prone rats: dose response relationships. Neurosci Lett, 227(1), 13–16. [DOI] [PubMed] [Google Scholar]
- Dailey JW, Reith ME, Yan QS, Li MY, & Jobe PC (1997b). Carbamazepine increases extracellular serotonin concentration: lack of antagonism by tetrodotoxin or zero Ca2+. Eur J Pharmacol, 328(2–3), 153–162. [DOI] [PubMed] [Google Scholar]
- Dailey JW, Yan QS, Adams-Curtis LE, Ryu JR, Ko KH, Mishra PK, & Jobe PC (1996). Neurochemical correlates of antiepileptic drugs in the genetically epilepsy-prone rat (GEPR). Life Sci, 58(4), 259–266. [DOI] [PubMed] [Google Scholar]
- Dailey JW, Yan QS, Mishra PK, Burger RL, & Jobe PC (1992). Effects of Fluoxetine on Convulsions and on Brain-Serotonin as Detected by Microdialysis in Genetically Epilepsy-Prone Rats. Journal of Pharmacology and Experimental Therapeutics, 260(2), 533–540. [PubMed] [Google Scholar]
- DeGiorgio CM, Curtis A, Hertling D, & Moseley BD (2019). Sudden unexpected death in epilepsy: Risk factors, biomarkers, and prevention. Acta Neurol Scand, 139(3), 220–230. [DOI] [PubMed] [Google Scholar]
- Dempsey JA, Veasey SC, Morgan BJ, & O’Donnell CP (2010). Pathophysiology of sleep apnea. Physiol Rev, 90(1), 47–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Depienne C, Trouillard O, Saint-Martin C, Gourfinkel-An I, Bouteiller D, Carpentier W, Keren B, Abert B, Gautier A, Baulac S, Arzimanoglou A, Cazeneuve C, Nabbout R, & LeGuern E (2009). Spectrum of SCN1A gene mutations associated with Dravet syndrome: analysis of 333 patients. J Med Genet, 46(3), 183–191. [DOI] [PubMed] [Google Scholar]
- Derry CP, & Duncan S (2013). Sleep and epilepsy. Epilepsy Behav, 26(3), 394–404. [DOI] [PubMed] [Google Scholar]
- Devinsky O, Hesdorffer DC, Thurman DJ, Lhatoo S, & Richerson G (2016a). Sudden unexpected death in epilepsy: epidemiology, mechanisms, and prevention. Lancet Neurol, 15(10), 1075–1088. [DOI] [PubMed] [Google Scholar]
- Devinsky O, Spruill T, Thurman D, & Friedman D (2016b). Recognizing and preventing epilepsy-related mortality: A call for action. Neurology, 86(8), 779–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhaibar H, Gautier NM, Chernyshev OY, Dominic P, & Glasscock E (2019). Cardiorespiratory profiling reveals primary breathing dysfunction in Kcna1-null mice: Implications for sudden unexpected death in epilepsy. Neurobiology of Disease, 127, 502–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dlouhy BJ, Gehlbach BK, Kreple CJ, Kawasaki H, Oya H, Buzza C, Granner MA, Welsh MJ, Howard MA, Wemmie JA, & Richerson GB (2015). Breathing Inhibited When Seizures Spread to the Amygdala and upon Amygdala Stimulation. The Journal of Neuroscience, 35(28), 10281–10289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donner EJ, Stanton TF, & Richerson GB (2018). Summary of the PAME 2018 Meeting. Epilepsy Curr, 18(6), 398–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dravet C (2011). Dravet syndrome history. Developmental Medicine & Child Neurology, 53(s2), 1–6. [DOI] [PubMed] [Google Scholar]
- Dravet C, & Oguni H (2013). Chapter 65 - Dravet syndrome (severe myoclonic epilepsy in infancy) In Dulac O, Lassonde M, & Sarnat HB (Eds.), Handbook of Clinical Neurology (Vol. 111, pp. 627–633): Elsevier. [DOI] [PubMed] [Google Scholar]
- Duncan JR, Paterson DS, Hoffman JM, Mokler DJ, Borenstein NS, Belliveau RA, Krous HF, Haas EA, Stanley C, Nattie EE, Trachtenberg FL, & Kinney HC (2010). Brainstem serotonergic deficiency in sudden infant death syndrome. JAMA, 303(5), 430–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esmail EH, Labib DM, & Rabie WA (2015). Association of serotonin transporter gene (5HTT) polymorphism and juvenile myoclonic epilepsy: a case-control study. Acta Neurol Belg, 115(3), 247–251. [DOI] [PubMed] [Google Scholar]
- Faingold CL (1988). The genetically epilepsy-prone rat. Gen Pharmacol, 19(3), 331–338. [DOI] [PubMed] [Google Scholar]
- Faingold CL, Kommajosyula SP, Long X, Plath K, & Randall M (2014). Serotonin and sudden death: differential effects of serotonergic drugs on seizure-induced respiratory arrest in DBA/1 mice. Epilepsy Behav, 37, 198–203. [DOI] [PubMed] [Google Scholar]
- Faingold CL, & Randall M (2013). Effects of age, sex, and sertraline administration on seizure-induced respiratory arrest in the DBA/1 mouse model of sudden unexpected death in epilepsy (SUDEP). Epilepsy Behav, 28(1), 78–82. [DOI] [PubMed] [Google Scholar]
- Faingold CL, Randall M, Mhaskar Y, & Uteshev VV (2011a). Differences in serotonin receptor expression in the brainstem may explain the differential ability of a serotonin agonist to block seizure-induced sudden death in DBA/2 vs. DBA/1 mice. Brain Res, 1418, 104–110. [DOI] [PubMed] [Google Scholar]
- Faingold CL, Randall M, Zeng C, Peng S, Long X, & Feng HJ (2016). Serotonergic agents act on 5-HT3 receptors in the brain to block seizure-induced respiratory arrest in the DBA/1 mouse model of SUDEP. Epilepsy Behav, 64(Pt A), 166–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faingold CL, Tupal S, & Randall M (2011b). Prevention of seizure-induced sudden death in a chronic SUDEP model by semichronic administration of a selective serotonin reuptake inhibitor. Epilepsy Behav, 22(2), 186–190. [DOI] [PubMed] [Google Scholar]
- Favale E, Audenino D, Cocito L, & Albano C (2003). The anticonvulsant effect of citalopram as an indirect evidence of serotonergic impairment in human epileptogenesis. Seizure-European Journal of Epilepsy, 12(5), 316–318. [DOI] [PubMed] [Google Scholar]
- Favale E, Rubino V, Mainardi P, Lunardi G, & Albano C (1995). Anticonvulsant effect of fluoxetine in humans. Neurology, 45(10), 1926–1927. [DOI] [PubMed] [Google Scholar]
- Feng HJ, & Faingold CL (2017). Abnormalities of serotonergic neurotransmission in animal models of SUDEP. Epilepsy Behav, 71(Pt B), 174–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher RS, Cross JH, French JA, Higurashi N, Hirsch E, Jansen FE, Lagae L, Moshe SL, Peltola J, Roulet Perez E, Scheffer IE, & Zuberi SM (2017). Operational classification of seizure types by the International League Against Epilepsy: Position Paper of the ILAE Commission for Classification and Terminology. Epilepsia, 58(4), 522–530. [DOI] [PubMed] [Google Scholar]
- Frasier CR, Zhang H, Offord J, Dang LT, Auerbach DS, Shi H, Chen C, Goldman AM, Eckhardt LL, Bezzerides VJ, Parent JM, & Isom LL (2018). Channelopathy as a SUDEP Biomarker in Dravet Syndrome Patient-Derived Cardiac Myocytes. Stem Cell Reports, 11(3), 626–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao J, Pan Z, Jiao Z, Li F, Zhao G, Wei Q, Pan F, & Evangelou E (2012). TPH2 gene polymorphisms and major depression--a meta–analysis. PLoS One, 7(5), e36721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giarman NJ, Freedman DX, & Schanberg SM (1964). Drug-induced Changes in the Subcellular Distribution of Serotonin in Rat Brain with Special Reference to the Action of Reserpine In Himwich HE & Himwich WA (Eds.), Biogenic Amines (Vol. 8, pp. 72–80): Elsevier. [Google Scholar]
- Giroud M, Dumas R, Dauvergne M, D’Athis P, Rochette L, Beley A, & Bralet J (1990). 5-Hydroxyindoleacetic acid and homovanillic acid in cerebrospinal fluid of children with febrile convulsions. Epilepsia, 31(2), 178–181. [DOI] [PubMed] [Google Scholar]
- Glasscock E, Yoo JW, Chen TT, Klassen TL, & Noebels JL (2010). Kv1.1 potassium channel deficiency reveals brain-driven cardiac dysfunction as a candidate mechanism for sudden unexplained death in epilepsy. J Neurosci, 30(15), 5167–5175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman AM, Buchanan G, Aiba I, & Noebels JL (2017). SUDEP Animal Models In Pitkänen A, Buckmaster PS, Galanopoulou AS, & Moshé SL (Eds.), Models of Seizures and Epilepsy (pp. 1007–1018): Academic Press. [Google Scholar]
- Goldman AM, Glasscock E, Yoo J, Chen TT, Klassen TL, & Noebels JL (2009). Arrhythmia in heart and brain: KCNQ1 mutations link epilepsy and sudden unexplained death. Sci Transl Med, 1(2), 2ra6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin A, Hamling KR, Knupp K, Hong S, Lee LP, & Baraban SC (2017). Clemizole and modulators of serotonin signalling suppress seizures in Dravet syndrome. Brain, 140(3), 669–683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyenet PG, & Abbott SB (2013). Chemoreception and asphyxia-induced arousal. Respir Physiol Neurobiol, 188(3), 333–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajek MA, & Buchanan GF (2016). Influence of vigilance state on physiological consequences of seizures and seizure-induced death in mice. J Neurophysiol, 115(5), 2286–2293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harden C, Tomson T, Gloss D, Buchhalter J, Cross JH, Donner E, French JA, Gil-Nagel A, Hesdorffer DC, Smithson WH, Spitz MC, Walczak TS, Sander JW, & Ryvlin P (2017). Practice guideline summary: Sudden unexpected death in epilepsy incidence rates and risk factors: Report of the Guideline Development, Dissemination, and Implementation Subcommittee of the American Academy of Neurology and the American Epilepsy Society. Neurology, 88(17), 1674–1680. [DOI] [PubMed] [Google Scholar]
- Hasler G, Bonwetsch R, Giovacchini G, Toczek MT, Bagic A, Luckenbaugh DA, Drevets WC, & Theodore WH (2007). 5-HT1A receptor binding in temporal lobe epilepsy patients with and without major depression. Biol Psychiatry, 62(11), 1258–1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haynes RL, Frelinger AL, Giles EK, Goldstein RD, Tran H, Kozakewich HP, Haas EA, Gerrits AJ, Mena OJ, Trachtenberg FL, Paterson DS, Berry GT, Adeli K, Kinney HC, & Michelson AD (2017). High serum serotonin in sudden infant death syndrome. Proceedings of the National Academy of Sciences, 201617374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry TR, & Juhasz C (2013). Serotonergic PET in temporal lobe epilepsy: biomarking or etiologic mapping? Neurology, 80(16), 1450–1451. [DOI] [PubMed] [Google Scholar]
- Hernandez EJ, Williams PA, & Dudek FE (2002). Effects of fluoxetine and TFMPP on spontaneous seizures in rats with pilocarpine-induced epilepsy. Epilepsia, 43(11), 1337–1345. [DOI] [PubMed] [Google Scholar]
- Hesdorffer DC, & Tomson T (2013). Sudden unexpected death in epilepsy. Potential role of antiepileptic drugs. CNS Drugs, 27(2), 113–119. [DOI] [PubMed] [Google Scholar]
- Hesdorffer DC, Tomson T, Benn E, Sander JW, Nilsson L, Langan Y, Walczak TS, Beghi E, Brodie MJ, Hauser A, Epidemiology I. C. o., & Subcommission on M (2011). Combined analysis of risk factors for SUDEP. Epilepsia, 52(6), 1150–1159. [DOI] [PubMed] [Google Scholar]
- Heydari A, & Davoudi S (2017). The effect of sertraline and 8-OH-DPAT on the PTZ_induced seizure threshold: Role of the nitrergic system. Seizure, 45, 119–124. [DOI] [PubMed] [Google Scholar]
- Hiramatsu M (1981). Brain Monoamine Levels and El Mouse Convulsions. Folia Psychiatrica Et Neurologica Japonica, 35(3), 261–266. [DOI] [PubMed] [Google Scholar]
- Hiramatsu M (1983). Brain 5-hydroxytryptamine level, metabolism, and binding in E1 mice. Neurochem Res, 8(9), 1163–1175. [DOI] [PubMed] [Google Scholar]
- Igelstrom KM (2012). Preclinical antiepileptic actions of selective serotonin reuptake inhibitors--implications for clinical trial design. Epilepsia, 53(4), 596–605. [DOI] [PubMed] [Google Scholar]
- Imaizumi K, Ito S, Kutukake G, Takizawa T, Fujiwara K, & Tutikawa K (1959). Epilepsy Like Anomaly of Mice (Vol. 8). [Google Scholar]
- Ismailova K, & Miadzhidi MB (2012). [Study on the effect of selective serotonin reuptake inhibitor fluoxetine on seizures and inborn behavior of rats with different phenotype of the nervous system]. Zh Vyssh Nerv Deiat Im I P Pavlova, 62(6), 745–752. [PubMed] [Google Scholar]
- Jaako K, Aonurm-Helm A, Kalda A, Anier K, Zharkovsky T, Shastin D, & Zharkovsky A (2011). Repeated citalopram administration counteracts kainic acid-induced spreading of PSA-NCAM-immunoreactive cells and loss of reelin in the adult mouse hippocampus. Eur J Pharmacol, 666(1–3), 61–71. [DOI] [PubMed] [Google Scholar]
- Jobe PC, & Browning RA (2005). The serotonergic and noradrenergic effects of antidepressant drugs are anticonvulsant, not proconvulsant. Epilepsy Behav, 7(4), 602–619. [DOI] [PubMed] [Google Scholar]
- Jobe PC, Dailey JW, & Reigel CE (1986). II. Noradrenergic and serotonergic determinants of seizure susceptibility and severity in genetically epilepsy-prone rats. Life Sciences, 39(9), 775–782. [DOI] [PubMed] [Google Scholar]
- Jobe PC, Dailey JW, & Wernicke JF (1999). A noradrenergic and serotonergic hypothesis of the linkage between epilepsy and affective disorders. Crit Rev Neurobiol, 13(4), 317–356. [DOI] [PubMed] [Google Scholar]
- Jobe PC, Laird HE 2nd, Ko KH, Ray T, & Dailey JW (1982). Abnormalities in monoamine levels in the central nervous system of the genetically epilepsy-prone rat. Epilepsia, 23(4), 359–366. [DOI] [PubMed] [Google Scholar]
- Johannessen Landmark C, Henning O, & Johannessen SI (2016). Proconvulsant effects of antidepressants - What is the current evidence? Epilepsy Behav, 61, 287–291. [DOI] [PubMed] [Google Scholar]
- Johnson JN, Hofman N, Haglund CM, Cascino GD, Wilde AA, & Ackerman MJ (2009). Identification of a possible pathogenic link between congenital long QT syndrome and epilepsy. Neurology, 72(3), 224–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston SC, Siedenberg R, Min JK, Jerome EH, & Laxer KD (1997). Central apnea and acute cardiac ischemia in a sheep model of epileptic sudden death. Ann Neurol, 42(4), 588–594. [DOI] [PubMed] [Google Scholar]
- Kabuto H, Yokoi I, Takei M, Kurimoto T, & Mori A (1994). The anticonvulsant effect of citalopram on El mice, and the levels of tryptophan and tyrosine and their metabolites in the brain. Neurochem Res, 19(4), 463–467. [DOI] [PubMed] [Google Scholar]
- Kalume F (2013). Sudden unexpected death in Dravet syndrome: respiratory and other physiological dysfunctions. Respir Physiol Neurobiol, 189(2), 324–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalume F, Westenbroek RE, Cheah CS, Yu FH, Oakley JC, Scheuer T, & Catterall WA (2013). Sudden unexpected death in a mouse model of Dravet syndrome. J Clin Invest, 123(4), 1798–1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneko S, Okada M, Hirano T, Kondo T, Otani K, & Fukushima Y (1993). Carbamazepine and Zonisamide Increase Extracellular Dopamine and Serotonin Levels In Vivo, and Carbamazepine Does Not Antagonize Adenosine Effect In Vitro: Mechanisms of Blockade of Seizure Spread. Psychiatry and Clinical Neurosciences, 47(2), 371–373. [DOI] [PubMed] [Google Scholar]
- Kanner AM (2013). The treatment of depressive disorders in epilepsy: what all neurologists should know. Epilepsia, 54 Suppl 1, 3–12. [DOI] [PubMed] [Google Scholar]
- Kanner AM (2016). Most antidepressant drugs are safe for patients with epilepsy at therapeutic doses: A review of the evidence. Epilepsy Behav, 61, 282–286. [DOI] [PubMed] [Google Scholar]
- Kauffman MA, Consalvo D, Gonzalez-Moron D, Aguirre F, D’Alessio L, & Kochen S (2009). Serotonin transporter gene variation and refractory mesial temporal epilepsy with hippocampal sclerosis. Epilepsy Res, 85(2–3), 231–234. [DOI] [PubMed] [Google Scholar]
- Kecskemeti V, Rusznak Z, Riba P, Pal B, Wagner R, Harasztosi C, Nanasi PP, & Szucs G (2005). Norfluoxetine and fluoxetine have similar anticonvulsant and Ca2+ channel blocking potencies. Brain Res Bull, 67(1–2), 126–132. [DOI] [PubMed] [Google Scholar]
- Keller AE, Whitney R, Li SA, Pollanen MS, & Donner EJ (2018). Incidence of sudden unexpected death in epilepsy in children is similar to adults. Neurology, 91(2), e107–e111. [DOI] [PubMed] [Google Scholar]
- Kempf E, Mack G, Schleef C, & Mandel P (1982). Effect of valproic acid on brain serotonin metabolism in isolated and grouped rats. Pharmacol Biochem Behav, 17(1), 49–52. [DOI] [PubMed] [Google Scholar]
- Kennedy JD, Hardin KA, Parikh P, Li CS, & Seyal M (2015). Pulmonary edema following generalized tonic clonic seizures is directly associated with seizure duration. Seizure, 27, 19–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilian M, & Frey HH (1973). Central Monoamines and Convulsive Thresholds in Mice and Rats. Neuropharmacology, 12(7), 681–692. [DOI] [PubMed] [Google Scholar]
- Kim Y, Bravo E, Thirnbeck CK, Smith-Mellecker LA, Kim SH, Gehlbach BK, Laux LC, Zhou X, Nordli DR Jr., & Richerson GB (2018). Severe peri-ictal respiratory dysfunction is common in Dravet syndrome. J Clin Invest, 128(3), 1141–1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinney HC, Filiano JJ, & White WF (2001). Medullary Serotonergic Network Deficiency in the Sudden Infant Death Syndrome: Review of a 15-Year Study of a Single Dataset. Journal of Neuropathology & Experimental Neurology, 60(3), 228–247. [DOI] [PubMed] [Google Scholar]
- Kinney HC, Randall LL, Sleeper LA, Willinger M, Belliveau RA, Zec N, Rava LA, Dominici L, Iyasu S, Randall B, Habbe D, Wilson H, Mandell F, McClain M, & Welty TK (2003). Serotonergic brainstem abnormalities in Northern Plains Indians with the sudden infant death syndrome. J Neuropathol Exp Neurol, 62(11), 1178–1191. [DOI] [PubMed] [Google Scholar]
- Kinney HC, Richerson GB, Dymecki SM, Darnall RA, & Nattie EE (2009). The brainstem and serotonin in the sudden infant death syndrome. Annu Rev Pathol, 4, 517–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kloster R, & Engelskjon T (1999). Sudden unexpected death in epilepsy (SUDEP): a clinical perspective and a search for risk factors. J Neurol Neurosurg Psychiatry, 67(4), 439–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko FJ, Chiang CH, Liu WJ, & Chiang W (1993). Alteration of amino acid in plasma and cerebrospinal fluid of children with seizure disorders. Gaoxiong Yi Xue Ke Xue Za Zhi, 9(3), 131–142. [PubMed] [Google Scholar]
- Kondziella D, & Asztely F (2009). Don’t be afraid to treat depression in patients with epilepsy! Acta Neurol Scand, 119(2), 75–80. [DOI] [PubMed] [Google Scholar]
- Kruse SW, Dayton KG, Purnell BS, Rosner JI, & Buchanan GF (2019). Effect of monoamine reuptake inhibition and alpha1 blockade on respiratory arrest and death following electroshock-induced seizures in mice. Epilepsia, 60(3), 495–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo J, Zhao W, Li CS, Kennedy JD, & Seyal M (2016). Postictal immobility and generalized EEG suppression are associated with the severity of respiratory dysfunction. Epilepsia, 57(3), 412–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacuey N, Zonjy B, Hampson JP, Rani MRS, Zaremba A, Sainju RK, Gehlbach BK, Schuele S, Friedman D, Devinsky O, Nei M, Harper RM, Allen L, Diehl B, Millichap JJ, Bateman L, Granner MA, Dragon DN, Richerson GB, & Lhatoo SD (2018). The incidence and significance of periictal apnea in epileptic seizures. Epilepsia, 59(3), 573–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacuey N, Zonjy B, Londono L, & Lhatoo SD (2017). Amygdala and hippocampus are symptomatogenic zones for central apneic seizures. Neurology, 88(7), 701–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamberts RJ, Gaitatzis A, Sander JW, Elger CE, Surges R, & Thijs RD (2013a). Postictal generalized EEG suppression: an inconsistent finding in people with multiple seizures. Neurology, 81(14), 1252–1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamberts RJ, Laranjo S, Kalitzin SN, Velis DN, Rocha I, Sander JW, & Thijs RD (2013b). Postictal generalized EEG suppression is not associated with periictal cardiac autonomic instability in people with convulsive seizures. Epilepsia, 54(3), 523–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamberts RJ, Thijs RD, Laffan A, Langan Y, & Sander JW (2012). Sudden unexpected death in epilepsy: people with nocturnal seizures may be at highest risk. Epilepsia, 53(2), 253–257. [DOI] [PubMed] [Google Scholar]
- Langan Y, Nashef L, & Sander JW (2005). Case-control study of SUDEP. Neurology, 64(7), 1131–1133. [DOI] [PubMed] [Google Scholar]
- Latreille V, Abdennadher M, Dworetzky BA, Ramel J, White D, Katz E, Zarowski M, Kothare S, & Pavlova M (2017). Nocturnal seizures are associated with more severe hypoxemia and increased risk of postictal generalized EEG suppression. Epilepsia, 58(9), e127–e131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leander JD (1992). Fluoxetine, a selective serotonin-uptake inhibitor, enhances the anticonvulsant effects of phenytoin, carbamazepine, and ameltolide (LY201116). Epilepsia, 33(3), 573–576. [DOI] [PubMed] [Google Scholar]
- Lhatoo SD, Faulkner HJ, Dembny K, Trippick K, Johnson C, & Bird JM (2010). An electroclinical case-control study of sudden unexpected death in epilepsy. Ann Neurol, 68(6), 787–796. [DOI] [PubMed] [Google Scholar]
- Li C, Silva J, Ozturk E, Dezsi G, O’Brien TJ, Renoir T, & Jones NC (2018). Chronic fluoxetine treatment accelerates kindling epileptogenesis in mice independently of 5-HT2A receptors. Epilepsia, 59(7), e114–e119. [DOI] [PubMed] [Google Scholar]
- Lin WH, Huang HP, Lin MX, Chen SG, Lv XC, Che CH, & Lin JL (2013). Seizure-induced 5–HT release and chronic impairment of serotonergic function in rats. Neurosci Lett, 534, 1–6. [DOI] [PubMed] [Google Scholar]
- Lin WH, Li XF, Lin MX, Zhou Y, & Huang HP (2017). Novel insights into the effect of paroxetine administration in pilocarpineinduced chronic epileptic rats. Mol Med Rep, 16(6), 8245–8252. [DOI] [PubMed] [Google Scholar]
- Luna-Munguia H, Zestos AG, Gliske SV, Kennedy RT, & Stacey WC (2019). Chemical biomarkers of epileptogenesis and ictogenesis in experimental epilepsy. Neurobiol Dis, 121, 177–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire MJ, Weston J, Singh J, & Marson AG (2014). Antidepressants for people with epilepsy and depression. Cochrane Database Syst Rev(12), CD010682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marion JL, Bigot JC, & Goas JY (1985). Alcoholic Epilepsy - Low Tryptophan Levels in Plasma and Cerebrospinal-Fluid. Presse Medicale, 14(12), 681–683. [PubMed] [Google Scholar]
- Martinez A, Finegersh A, Cannon DM, Dustin I, Nugent A, Herscovitch P, & Theodore WH (2013). The 5-HT1A receptor and 5-HT transporter in temporal lobe epilepsy. Neurology, 80(16), 1465–1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massey CA, Sowers LP, Dlouhy BJ, & Richerson GB (2014). Mechanisms of sudden unexpected death in epilepsy: the pathway to prevention. Nat Rev Neurol, 10(5), 271–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mateos SS, Sanchez CL, Paredes SD, Barriga C, & Rodriguez AB (2009). Circadian levels of serotonin in plasma and brain after oral administration of tryptophan in rats. Basic Clin Pharmacol Toxicol, 104(1), 52–59. [DOI] [PubMed] [Google Scholar]
- Mazarati A, Siddarth P, Baldwin RA, Shin D, Caplan R, & Sankar R (2008). Depression after status epilepticus: behavioural and biochemical deficits and effects of fluoxetine. Brain, 131(Pt 8), 2071–2083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meador KJ (2014). Seizure Reduction with Fluoxetine in Dravet Syndrome. Epilepsy Behav Case Rep, 2, 54–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merlet I, Ostrowsky K, Costes N, Ryvlin P, Isnard J, Faillenot I, Lavenne F, Dufournel D, Le Bars D, & Mauguiere F (2004). 5-HT1A receptor binding and intracerebral activity in temporal lobe epilepsy: an [18F]MPPF-PET study. Brain, 127(Pt 4), 900–913. [DOI] [PubMed] [Google Scholar]
- Meshkibaf MH, Subhash MN, Lakshmana KM, & Rao BS (1995). Effect of chronic administration of phenytoin on regional monoamine levels in rat brain. Neurochem Res, 20(7), 773–778. [DOI] [PubMed] [Google Scholar]
- Middleton O, Atherton D, Bundock E, Donner E, Friedman D, Hesdorffer D, Jarrell H, McCrillis A, Mena OJ, Morey M, Thurman D, Tian N, Tomson T, Tseng Z, White S, Wright C, & Devinsky O (2018). National Association of Medical Examiners position paper: Recommendations for the investigation and certification of deaths in people with epilepsy. Epilepsia, 59(3), 530–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore BM, Jerry Jou C, Tatalovic M, Kaufman ES, Kline DD, & Kunze DL (2014). The Kv1.1 null mouse, a model of sudden unexpected death in epilepsy (SUDEP). Epilepsia, 55(11), 1808–1816. [DOI] [PubMed] [Google Scholar]
- Moseley B, Bateman L, Millichap JJ, Wirrell E, & Panayiotopoulos CP (2013). Autonomic epileptic seizures, autonomic effects of seizures, and SUDEP. Epilepsy Behav, 26(3), 375–385. [DOI] [PubMed] [Google Scholar]
- Moseley BD, & DeGiorgio CM (2015). The SUDEP Risk Inventory: Association with postictal generalized EEG suppression. Epilepsy Res, 117, 82–84. [DOI] [PubMed] [Google Scholar]
- Mueller SG, Bateman LM, & Laxer KD (2014). Evidence for brainstem network disruption in temporal lobe epilepsy and sudden unexplained death in epilepsy. Neuroimage Clin, 5, 208–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami T, Okada M, Kawata Y, Zhu G, Kamata A, & Kaneko S (2001). Determination of effects of antiepileptic drugs on SNAREs-mediated hippocampal monoamine release using in vivo microdialysis. Br J Pharmacol, 134(3), 507–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murugesan A, Rani MRS, Hampson J, Zonjy B, Lacuey N, Faingold CL, Friedman D, Devinsky O, Sainju RK, Schuele S, Diehl B, Nei M, Harper RM, Bateman LM, Richerson G, & Lhatoo SD (2018). Serum serotonin levels in patients with epileptic seizures. Epilepsia, 59(6), e91–e97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murugesan A, Rani MRS, Vilella L, Lacuey N, Hampson JP, Faingold CL, Friedman D, Devinsky O, Sainju RK, Schuele S, Diehl B, Nei M, Harper RM, Bateman LM, Richerson G, & Lhatoo SD (2019). Postictal serotonin levels are associated with peri-ictal apnea. Neurology, 93(15), e1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakase K, Kollmar R, Lazar J, Arjomandi H, Sundaram K, Silverman J, Orman R, Weedon J, Stefanov D, Savoca E, Tordjman L, Stiles K, Ihsan M, Nunez A, Guzman L, & Stewart M (2016). Laryngospasm, central and obstructive apnea during seizures: Defining pathophysiology for sudden death in a rat model. Epilepsy Res, 128, 126–139. [DOI] [PubMed] [Google Scholar]
- Nashef L, Hindocha N, & Makoff A (2007). Risk factors in sudden death in epilepsy (SUDEP): the quest for mechanisms. Epilepsia, 48(5), 859–871. [DOI] [PubMed] [Google Scholar]
- Nashef L, So EL, Ryvlin P, & Tomson T (2012). Unifying the definitions of sudden unexpected death in epilepsy. Epilepsia, 53(2), 227–233. [DOI] [PubMed] [Google Scholar]
- Nashef L, Walker F, Allen P, Sander JW, Shorvon SD, & Fish DR (1996). Apnoea and bradycardia during epileptic seizures: relation to sudden death in epilepsy. J Neurol Neurosurg Psychiatry, 60(3), 297–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nayak CS, Sinha S, Nagappa M, Thennarasu K, & Taly AB (2017). Lack of heart rate variability during sleep-related apnea in patients with temporal lobe epilepsy (TLE)-an indirect marker of SUDEP? Sleep Breath, 21(1), 163–172. [DOI] [PubMed] [Google Scholar]
- Nobili L, Proserpio P, Rubboli G, Montano N, Didato G, & Tassinari CA (2011). Sudden unexpected death in epilepsy (SUDEP) and sleep. Sleep Med Rev, 15(4), 237–246. [DOI] [PubMed] [Google Scholar]
- Nobis WP, Schuele S, Templer JW, Zhou G, Lane G, Rosenow JM, & Zelano C (2018). Amygdala-stimulation-induced apnea is attention and nasal-breathing dependent. Ann Neurol, 83(3), 460–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada M, Kaneko S, Hirano T, Ishida M, Kondo T, Otani K, & Fukushima Y (1992). Effects of zonisamide on extracellular levels of monoamine and its metabolite, and on Ca2+ dependent dopamine release. Epilepsy Res, 13(2), 113–119. [DOI] [PubMed] [Google Scholar]
- Panigrahy A, Filiano J, Sleeper LA, Mandell F, Valdes-Dapena M, Krous HF, Rava LA, Foley E, White WF, & Kinney HC (2000). Decreased serotonergic receptor binding in rhombic lip-derived regions of the medulla oblongata in the sudden infant death syndrome. J Neuropathol Exp Neurol, 59(5), 377–384. [DOI] [PubMed] [Google Scholar]
- Park KJ, Sharma G, Kennedy JD, & Seyal M (2017). Potentially high-risk cardiac arrhythmias with focal to bilateral tonic-clonic seizures and generalized tonic-clonic seizures are associated with the duration of periictal hypoxemia. Epilepsia, 58(12), 2164–2171. [DOI] [PubMed] [Google Scholar]
- Paterson DS, Trachtenberg FL, Thompson EG, Belliveau RA, Beggs AH, Darnall R, Chadwick AE, Krous HF, & Kinney HC (2006). Multiple serotonergic brainstem abnormalities in sudden infant death syndrome. Jama, 296(17), 2124–2132. [DOI] [PubMed] [Google Scholar]
- Patodia S, Somani A, O’Hare M, Venkateswaran R, Liu J, Michalak Z, Ellis M, Scheffer IE, Diehl B, Sisodiya SM, & Thom M (2018). The ventrolateral medulla and medullary raphe in sudden unexpected death in epilepsy. Brain, 141(6), 1719–1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payandemehr B, Bahremand A, Rahimian R, Ziai P, Amouzegar A, Sharifzadeh M, & Dehpour AR (2012). 5-HT(3) receptor mediates the dose-dependent effects of citalopram on pentylenetetrazole-induced clonic seizure in mice: involvement of nitric oxide. Epilepsy Res, 101(3), 217–227. [DOI] [PubMed] [Google Scholar]
- Peng W, Danison JL, & Seyal M (2017). Postictal generalized EEG suppression and respiratory dysfunction following generalized tonic-clonic seizures in sleep and wakefulness. Epilepsia, 58(8), 1409–1414. [DOI] [PubMed] [Google Scholar]
- Poh MZ, Loddenkemper T, Reinsberger C, Swenson NC, Goyal S, Madsen JR, & Picard RW (2012). Autonomic changes with seizures correlate with postictal EEG suppression. Neurology, 78(23), 1868–1876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poplawska M, Wroblewska D, & Borowicz KK (2015). Interactions between an antidepressant reboxetine and four classic antiepileptic drugs in the mouse model of myoclonic seizures. Pharmacol Rep, 67(6), 1141–1146. [DOI] [PubMed] [Google Scholar]
- Pranzatelli MR, Tate E, Huang Y, Haas RH, Bodensteiner J, Ashwal S, & Franz D (1995). Neuropharmacology of progressive myoclonus epilepsy: response to 5-hydroxy-L-tryptophan. Epilepsia, 36(8), 783–791. [DOI] [PubMed] [Google Scholar]
- Pratt JA, Jenner P, Johnson AL, Shorvon SD, & Reynolds EH (1984). Anticonvulsant drugs alter plasma tryptophan concentrations in epileptic patients: implications for antiepileptic action and mental function. Journal of Neurology, Neurosurgery & Psychiatry, 47(10), 1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Przegaliński E (1985). Monoamines and the Pathophysiology of Seizure Disorders In Frey H-H & Janz D (Eds.), Antiepileptic Drugs (pp. 101–137). Berlin, Heidelberg: Springer Berlin Heidelberg. [Google Scholar]
- Purnell BS, Hajek MA, & Buchanan GF (2017). Time-of-day influences on respiratory sequelae following maximal electroshock-induced seizures in mice. J Neurophysiol, 118(5), 2592–2600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purnell BS, Thijs RD, & Buchanan GF (2018). Dead in the Night: Sleep-Wake and Time-Of-Day Influences on Sudden Unexpected Death in Epilepsy. Front Neurol, 9, 1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajakulendran S, & Nashef L (2015). Postictal generalized EEG suppression and SUDEP: a review. J Clin Neurophysiol, 32(1), 14–20. [DOI] [PubMed] [Google Scholar]
- Rao ML, Gross G, Strebel B, Halaris A, Huber G, Braunig P, & Marler M (1994). Circadian rhythm of tryptophan, serotonin, melatonin, and pituitary hormones in schizophrenia. Biol Psychiatry, 35(3), 151–163. [DOI] [PubMed] [Google Scholar]
- Reeta K, Prabhakar P, & Gupta YK (2016). Anticonvulsant activity of the antidepressant drug, tianeptine, against pentylenetetrazole-induced seizures mitigates cognitive impairment in rats. Behav Pharmacol, 27(7), 623–632. [DOI] [PubMed] [Google Scholar]
- Reynolds EH, Chadwick D, Jenner P, & Chanarin I (1975). Folate and monoamine metabolism in epilepsy. J Neurol Sci, 26(4), 605–615. [DOI] [PubMed] [Google Scholar]
- Richerson GB, & Buchanan GF (2011). The serotonin axis: Shared mechanisms in seizures, depression, and SUDEP. Epilepsia, 52 Suppl 1, 28–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richman A, & Heinrichs SC (2007). Seizure prophylaxis in an animal model of epilepsy by dietary fluoxetine supplementation. Epilepsy Res, 74(1), 19–27. [DOI] [PubMed] [Google Scholar]
- Rocha L, Lorigados-Pedre L, Orozco-Suarez S, Morales-Chacon L, Alonso-Vanegas M, Garcia-Maeso I, Villeda-Hernandez J, Osorio-Rico L, Estupinan B, & Quintana C (2007). Autoradiography reveals selective changes in serotonin binding in neocortex of patients with temporal lobe epilepsy. Prog Neuropsychopharmacol Biol Psychiatry, 31(6), 1208–1218. [DOI] [PubMed] [Google Scholar]
- Rosenwasser AM, Trubowitsch G, & Adler NT (1985). Circadian rhythm in metabolic activity of suprachiasmatic, supraoptic and raphe nuclei. Neurosci Lett, 58(2), 183–187. [DOI] [PubMed] [Google Scholar]
- Rugg-Gunn F, Duncan J, Hjalgrim H, Seyal M, & Bateman L (2016). From unwitnessed fatality to witnessed rescue: Nonpharmacologic interventions in sudden unexpected death in epilepsy. Epilepsia, 57 Suppl 1(S1), 26–34. [DOI] [PubMed] [Google Scholar]
- Ryvlin P, Nashef L, Lhatoo SD, Bateman LM, Bird J, Bleasel A, Boon P, Crespel A, Dworetzky BA, Hogenhaven H, Lerche H, Maillard L, Malter MP, Marchal C, Murthy JM, Nitsche M, Pataraia E, Rabben T, Rheims S, Sadzot B, Schulze-Bonhage A, Seyal M, So EL, Spitz M, Szucs A, Tan M, Tao JX, & Tomson T (2013). Incidence and mechanisms of cardiorespiratory arrests in epilepsy monitoring units (MORTEMUS): a retrospective study. Lancet Neurol, 12(10), 966–977. [DOI] [PubMed] [Google Scholar]
- Ryvlin P, Rheims S, & Lhatoo SD (2019). Risks and predictive biomarkers of sudden unexpected death in epilepsy patient. Curr Opin Neurol, 32(2), 205–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos Junior JG, Do Monte FHM, Russi M, Agustine PE, & Lanziotti VMNB (2002). Proconvulsant effects of high doses of venlafaxine in pentylenetetrazole-convulsive rats. Brazilian Journal of Medical and Biological Research, 35, 469–472. [DOI] [PubMed] [Google Scholar]
- Sarkissova KY, Fedotova IB, Surina NM, Nikolaev GM, Perepelkina OV, & Poletaeva II (2016). Effect of chronic fluoxetine treatment on audiogenic epilepsy, symptoms of anxiety and depression in rats of four lines. Dokl Biol Sci, 467(1), 55–58. [DOI] [PubMed] [Google Scholar]
- Savic I, Lindstrom P, Gulyas B, Halldin C, Andree B, & Farde L (2004). Limbic reductions of 5-HT1A receptor binding in human temporal lobe epilepsy. Neurology, 62(8), 1343–1351. [DOI] [PubMed] [Google Scholar]
- Schenkel LC, Bragatti JA, Torres CM, Martin KC, Gus-Manfro G, Leistner-Segal S, & Bianchin MM (2011). Serotonin transporter gene (5HTT) polymorphisms and temporal lobe epilepsy. Epilepsy Res, 95(1–2), 152–157. [DOI] [PubMed] [Google Scholar]
- Schlesinger K, Stavnes KL, & Boggan WO (1969). Modification of audiogenic and pentylenetetrazol seizures with gamma-aminobutyric acid, norepinephrine and serotonin. Psychopharmacologia, 15(3), 226–231. [DOI] [PubMed] [Google Scholar]
- Schlicker E, Fink K, Zentner J, & Gothert M (1996). Presynaptic inhibitory serotonin autoreceptors in the human hippocampus. Naunyn Schmiedebergs Arch Pharmacol, 354(3), 393–396. [DOI] [PubMed] [Google Scholar]
- Schoonjans A, Paelinck BP, Marchau F, Gunning B, Gammaitoni A, Galer BS, Lagae L, & Ceulemans B (2017). Low–dose fenfluramine significantly reduces seizure frequency in Dravet syndrome: a prospective study of a new cohort of patients. Eur J Neurol, 24(2), 309–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiber RA, & Schlesinger K (1971). Circadian rhythms and seizure susceptibility: relation to 5-hydroxytryptamine and norepinephrine in brain. Physiol Behav, 6(6), 635–640. [DOI] [PubMed] [Google Scholar]
- Schuele SU, Bermeo AC, Locatelli E, Burgess RC, & Luders HO (2008). Ictal asystole: a benign condition? Epilepsia, 49(1), 168–171. [DOI] [PubMed] [Google Scholar]
- Scudder CL, Karczmar AG, Everett GM, Gibson JE, & Rifkin M (1966). Brain catecholamines and serotonin levels in various strains and genera of mice and a possible interpretation for the correlations of amine levels with electroshock latency and behavior. Neuropharmacology, 5(5), 343–351. [DOI] [PubMed] [Google Scholar]
- Semmelroch M, Elwes RD, Lozsadi DA, & Nashef L (2012). Retrospective audit of postictal generalized EEG suppression in telemetry. Epilepsia, 53(2), e21–24. [DOI] [PubMed] [Google Scholar]
- Sevcencu C, & Struijk JJ (2010). Autonomic alterations and cardiac changes in epilepsy. Epilepsia, 51(5), 725–737. [DOI] [PubMed] [Google Scholar]
- Seyal M, Bateman LM, Albertson TE, Lin TC, & Li CS (2010). Respiratory changes with seizures in localization-related epilepsy: analysis of periictal hypercapnia and airflow patterns. Epilepsia, 51(8), 1359–1364. [DOI] [PubMed] [Google Scholar]
- Seyal M, Bateman LM, & Li CS (2013). Impact of periictal interventions on respiratory dysfunction, postictal EEG suppression, and postictal immobility. Epilepsia, 54(2), 377–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seyal M, Hardin KA, & Bateman LM (2012). Postictal generalized EEG suppression is linked to seizure-associated respiratory dysfunction but not postictal apnea. Epilepsia, 53(5), 825–831. [DOI] [PubMed] [Google Scholar]
- Shmuely S, van der Lende M, Lamberts RJ, Sander JW, & Thijs RD (2017). The heart of epilepsy: Current views and future concepts. Seizure, 44, 176–183. [DOI] [PubMed] [Google Scholar]
- Simeone KA, Hallgren J, Bockman CS, Aggarwal A, Kansal V, Netzel L, Iyer SH, Matthews SA, Deodhar M, Oldenburg PJ, Abel PW, & Simeone TA (2018). Respiratory dysfunction progresses with age in Kcna1-null mice, a model of sudden unexpected death in epilepsy. Epilepsia, 59(2), 345–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southam E, Kirkby D, Higgins GA, & Hagan RM (1998). Lamotrigine inhibits monoamine uptake in vitro and modulates 5-hydroxytryptamine uptake in rats. Eur J Pharmacol, 358(1), 19–24. [DOI] [PubMed] [Google Scholar]
- Specchio LM, Iudice A, Specchio N, La Neve A, Spinelli A, Galli R, Rocchi R, Ulivelli M, de Tommaso M, Pizzanelli C, & Murri L (2004). Citalopram as treatment of depression in patients with epilepsy. Clin Neuropharmacol, 27(3), 133–136. [DOI] [PubMed] [Google Scholar]
- Statnick MA, Dailey JW, Jobe PC, & Browning RA (1996). Abnormalities in brain serotonin concentration, high-affinity uptake, and tryptophan hydroxylase activity in severe–seizure genetically epilepsy-prone rats. Epilepsia, 37(4), 311–321. [DOI] [PubMed] [Google Scholar]
- Strine TW, Kobau R, Chapman DP, Thurman DJ, Price P, & Balluz LS (2005). Psychological distress, comorbidities, and health behaviors among U.S. adults with seizures: results from the 2002 National Health Interview Survey. Epilepsia, 46(7), 1133–1139. [DOI] [PubMed] [Google Scholar]
- Surges R, Strzelczyk A, Scott CA, Walker MC, & Sander JW (2011). Postictal generalized electroencephalographic suppression is associated with generalized seizures. Epilepsy Behav, 21(3), 271–274. [DOI] [PubMed] [Google Scholar]
- Suzuki J (2004). Investigations of epilepsy with a mutant animal (EL mouse) model. Epilepsia, 45 Suppl 8, 2–5. [DOI] [PubMed] [Google Scholar]
- Suzuki J (2013). Neuronal mechanism of epileptogenesis in EL mouse. Proc Jpn Acad Ser B Phys Biol Sci, 89(6), 270–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sveinsson O, Andersson T, Carlsson S, & Tomson T (2017). The incidence of SUDEP: A nationwide population-based cohort study. Neurology, 89(2), 170–177. [DOI] [PubMed] [Google Scholar]
- Tao JX, Qian S, Baldwin M, Chen XJ, Rose S, Ebersole SH, & Ebersole JS (2010). SUDEP, suspected positional airway obstruction, and hypoventilation in postictal coma. Epilepsia, 51(11), 2344–2347. [DOI] [PubMed] [Google Scholar]
- Teran FA, Kim Y, Crotts MS, Bravo E, Emaus KJ, & Richerson GB (2019). Time of Day and a Ketogenic Diet Influence Susceptibility to SUDEP in Scn1a (R1407X/+) Mice. Front Neurol, 10, 278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theeranaew W, McDonald J, Zonjy B, Kaffashi F, Moseley BD, Friedman D, So E, Tao J, Nei M, Ryvlin P, Surges R, Thijs R, Schuele S, Lhatoo S, & Loparo KA (2018). Automated Detection of Postictal Generalized EEG Suppression. IEEE Trans Biomed Eng, 65(2), 371–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thurman DJ, Hesdorffer DC, & French JA (2014). Sudden unexpected death in epilepsy: assessing the public health burden. Epilepsia, 55(10), 1479–1485. [DOI] [PubMed] [Google Scholar]
- Toczek MT, Carson RE, Lang L, Ma Y, Spanaki MV, Der MG, Fazilat S, Kopylev L, Herscovitch P, Eckelman WC, & Theodore WH (2003). PET imaging of 5-HT1A receptor binding in patients with temporal lobe epilepsy. Neurology, 60(5), 749–756. [DOI] [PubMed] [Google Scholar]
- Tomson T, Nashef L, & Ryvlin P (2008). Sudden unexpected death in epilepsy: current knowledge and future directions. Lancet Neurol, 7(11), 1021–1031. [DOI] [PubMed] [Google Scholar]
- Trindade-Filho EM, de Castro-Neto EF, de A. C. R, Lima E, Scorza FA, Amado D, Naffah-Mazzacoratti Mda G, & Cavalheiro EA (2008). Serotonin depletion effects on the pilocarpine model of epilepsy. Epilepsy Res, 82(2–3), 194–199. [DOI] [PubMed] [Google Scholar]
- Tripathi PP, & Bozzi Y (2015). The role of dopaminergic and serotonergic systems in neurodevelopmental disorders: a focus on epilepsy and seizure susceptibility. Bioimpacts, 5(2), 97–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trulson ME, & Jacobs BL (1979). Raphe unit activity in freely moving cats: correlation with level of behavioral arousal. Brain Res, 163(1), 135–150. [DOI] [PubMed] [Google Scholar]
- Truscott TC (1975). Effects of phenylalanine and 5-hydroxytryptophan on seizure severity in mice. Pharmacol Biochem Behav, 3(5), 939–941. [DOI] [PubMed] [Google Scholar]
- Tupal S, & Faingold CL (2006). Evidence supporting a role of serotonin in modulation of sudden death induced by seizures in DBA/2 mice. Epilepsia, 47(1), 21–26. [DOI] [PubMed] [Google Scholar]
- Tupal S, & Faingold CL (2019). Fenfluramine, a serotonin-releasing drug, prevents seizure-induced respiratory arrest and is anticonvulsant in the DBA/1 mouse model of SUDEP. Epilepsia, 60(3), 485–494. [DOI] [PubMed] [Google Scholar]
- Ugale RR, Mittal N, Hirani K, & Chopde CT (2004). Essentiality of central GABAergic neuroactive steroid allopregnanolone for anticonvulsant action of fluoxetine against pentylenetetrazole-induced seizures in mice. Brain Res, 1023(1), 102–111. [DOI] [PubMed] [Google Scholar]
- Uteshev VV, Tupal S, Mhaskar Y, & Faingold CL (2010). Abnormal serotonin receptor expression in DBA/2 mice associated with susceptibility to sudden death due to respiratory arrest. Epilepsy Res, 88(2–3), 183–188. [DOI] [PubMed] [Google Scholar]
- Uzbay T, Kayir H, & Ceyhan M (2007). Effects of Tianeptine on Onset Time of Pentylenetetrazole-Induced Seizures in Mice: Possible Role of Adenosine A1 Receptors (Vol. 32). [DOI] [PubMed] [Google Scholar]
- van der Lende M, Hesdorffer DC, Sander JW, & Thijs RD (2018). Nocturnal supervision and SUDEP risk at different epilepsy care settings. Neurology, 91(16), e1508–e1518. [DOI] [PubMed] [Google Scholar]
- Vermoesen K, Massie A, Smolders I, & Clinckers R (2012). The antidepressants citalopram and reboxetine reduce seizure frequency in rats with chronic epilepsy. Epilepsia, 53(5), 870–878. [DOI] [PubMed] [Google Scholar]
- Vermoesen K, Serruys AS, Loyens E, Afrikanova T, Massie A, Schallier A, Michotte Y, Crawford AD, Esguerra CV, de Witte PA, Smolders I, & Clinckers R (2011). Assessment of the convulsant liability of antidepressants using zebrafish and mouse seizure models. Epilepsy Behav, 22(3), 450–460. [DOI] [PubMed] [Google Scholar]
- Vilella L, Lacuey N, Hampson JP, Rani MRS, Sainju RK, Friedman D, Nei M, Strohl K, Scott C, Gehlbach BK, Zonjy B, Hupp NJ, Zaremba A, Shafiabadi N, Zhao X, Reick-Mitrisin V, Schuele S, Ogren J, Harper RM, Diehl B, Bateman L, Devinsky O, Richerson GB, Ryvlin P, & Lhatoo SD (2019). Postconvulsive central apnea as a biomarker for sudden unexpected death in epilepsy (SUDEP). Neurology, 92(3), e171–e182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walczak TS, Leppik IE, D’Amelio M, Rarick J, So E, Ahman P, Ruggles K, Cascino GD, Annegers JF, & Hauser WA (2001). Incidence and risk factors in sudden unexpected death in epilepsy: a prospective cohort study. Neurology, 56(4), 519–525. [DOI] [PubMed] [Google Scholar]
- Weiss J, American Academy of Pediatrics Committee on Injury, V., & Poison P (2010). Prevention of drowning. Pediatrics, 126(1), e253–262.20498167 [Google Scholar]
- Wenger GR, Stitzel RE, & Craig CR (1973). The role of biogenic amines in the reserpine-induced alteration of minimal electroshock seizure thresholds in the mouse. Neuropharmacology, 12(7), 693–703. [DOI] [PubMed] [Google Scholar]
- Willinger M, James LS, & Catz C (1991). Defining the Sudden Infant Death Syndrome (Sids): Deliberations of an Expert Panel Convened by the National Institute of Child Health and Human Development. Pediatric Pathology, 11(5), 677–684. [DOI] [PubMed] [Google Scholar]
- Wirrell EC (2006). Epilepsy-related injuries. Epilepsia, 47 Suppl 1(s1), 79–86. [DOI] [PubMed] [Google Scholar]
- Witek N, Cornes S, & Hegde M (2017). Staff Response Times in the Epilepsy Monitoring Unit: A Study of Diurnal/Nocturnal Variability. Neurodiagn J, 57(4), 269–275. [DOI] [PubMed] [Google Scholar]
- Yan QS, Jobe PC, & Dailey JW (1994). Evidence That a Serotonergic Mechanism Is Involved in the Anticonvulsant Effect of Fluoxetine in Genetically Epilepsy-Prone Rats. European Journal of Pharmacology, 252(1), 105–112. [DOI] [PubMed] [Google Scholar]
- Yang Z, Liu X, Yin Y, Sun S, & Deng X (2012). Involvement of 5-HT(7) receptors in the pathogenesis of temporal lobe epilepsy. Eur J Pharmacol, 685(1–3), 52–58. [DOI] [PubMed] [Google Scholar]
- Zeng C, Long X, Cotten JF, Forman SA, Solt K, Faingold CL, & Feng HJ (2015). Fluoxetine prevents respiratory arrest without enhancing ventilation in DBA/1 mice. Epilepsy Behav, 45, 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhan Q, Buchanan GF, Motelow JE, Andrews J, Vitkovskiy P, Chen WC, Serout F, Gummadavelli A, Kundishora A, Furman M, Li W, Bo X, Richerson GB, & Blumenfeld H (2016). Impaired Serotonergic Brainstem Function during and after Seizures. J Neurosci, 36(9), 2711–2722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Zhao H, Yang X, Xue Q, Cotten JF, & Feng HJ (2016). 5-Hydroxytryptophan, a precursor for serotonin synthesis, reduces seizure-induced respiratory arrest. Epilepsia, 57(8), 1228–1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao ZQ, Scott M, Chiechio S, Wang JS, Renner KJ, Gereau R. W. t., Johnson RL, Deneris ES, & Chen ZF (2006). Lmx1b is required for maintenance of central serotonergic neurons and mice lacking central serotonergic system exhibit normal locomotor activity. J Neurosci, 26(49), 12781–12788. [DOI] [PMC free article] [PubMed] [Google Scholar]


