Abstract
Purpose
Ribociclib, an orally bioavailable small-molecule CDK4/6 inhibitor is currently undergoing evaluation to treat pediatric central nervous system (CNS) tumors. However, it is crucial that it penetrates the brain and tumor. Thus, the objectives of the present study were to derive a clinically relevant mouse dosage for cerebral microdialysis studies, and to characterize ribociclib CNS penetration in non-tumor bearing mice and in mice bearing DIPG×7 (glioma) cortical allograft tumors.
Methods
A plasma pharmacokinetic study of ribociclib (100 mg/kg, orally) was performed in CD1 nude mice bearing glioma cortical allografts to obtain initial plasma pharmacokinetic parameters and to derive D-optimal plasma sampling time-points for microdialysis studies. Using a cerebral microdialysis technique, the extracellular fluid (ECF) disposition of ribociclib was evaluated after a single oral ribociclib dose (100 mg/kg) in non-tumor bearing mice and in mice bearing glioma cortical allografts. A one-compartment plasma model with absorption and ECF compartments were fit to plasma and ECF concentration-time data using a nonlinear mixed effects modeling approach (NONMEM 7.2).
Results
The mean unbound ribociclib plasma exposure (6812 ng/ml*hr) was similar to that observed clinically at recommended dosages in adults. The median ribociclib ECF to plasma partition coefficient (Kp,uu) in non-tumor bearing and glioma mice was 0.10 and 0.07, respectively, and was not statistically different (t-test, p=0.19).
Conclusions
The CNS penetration observed was encouraging enough to move ribociclib forward with preclinical efficacy studies in models of pediatric brain tumors.
Introduction:
Malignant central nervous system (CNS) tumors carry a poor prognosis and are the most common solid tumors in children [1]. Surgical resection and radiotherapy have been the cornerstones of treatment for most brain tumors, but in some instances, chemotherapy is an important component of therapy. For children less than three years old, chemotherapy is preferentially given to avoid or delay irradiation of the developing brain [2]. Because drugs shown active to treat brain tumors in adults, such as temozolomide, have not shown efficacy in pediatric trials [3], new agents are needed to treat childhood brain tumors.
Ribociclib is an orally bioavailable small molecule inhibitor of both cyclin-dependent kinases 4 and 6 (CDK4/6) currently approved for the treatment of breast cancer [4]. CDK4/6 regulate the mammalian cell cycle progression through G1 phase and initiation of S phase [5]. They respond to mitogenic or pro-proliferative stimuli, complex with D-type cyclin, and phosphorylate the retinoblastoma (RB) tumor suppressor protein. This phosphorylation releases RB from E2F transcription factors and enables E2F to transcribe the genes that are required for G1-S phase cell cycle progression and ultimately cell proliferation [5].
Recent studies of diffuse intrinsic pontine glioma (DIPG) and pediatric non-brainstem high-grade glioma tissues have unraveled genetic alterations associated with tumor proliferation [6]. These studies reported that ~25 – 30% of these tumors contain alterations in cell cycle regulatory genes such as amplification of D-type cyclins and CDK4/6, which are associated with abnormal proliferation [7]. Beside glioma, D-type cyclins and CDK4/6 genes were also found to be amplified in other pediatric brain tumors highlighting the clinical potential of CDK4/6 inhibitors such as ribociclib in this therapeutic area [8].
If ribociclib has adequate CNS exposure (i.e., concentration vs. time), it could be an important molecularly targeted agent to treat childhood brain tumors. Thus, the objectives of the present study were to derive a clinically equivalent mouse dosage for the cerebral microdialysis studies, to establish a limited sampling model (LSM) for plasma sample collection during the cerebral microdialysis studies, and to characterize ribociclib CNS penetration in non-tumor bearing mice (i.e., normal blood brain barrier) and in mice bearing DIPG×7 (glioma) cortical allograft tumors.
Materials and Methods
Drugs and chemicals
Ribociclib succinate (purity, > 99%) was provided by Novartis Oncology (East Hanover, NJ). Methyl cellulose was purchased from Sigma-Aldrich (St. Louis, MO). Ribociclib was formulated at concentration of 10 mg/mL in 0.5% w/v methyl cellulose. Acetonitrile, ethyl acetate, and formic acid were purchased from Fisher Scientific (Fair Lawn, NJ, USA). All solvents used were HPLC grade. Water was purified using Milli-Q Advantage A10 system (Millipore, Billerica, MA, USA). Mouse plasma (CD1 strain) in sodium heparin was purchased from BioIVT (Westbury, NY, USA). Ringer’s solution was purchased from Frey Scientific (Nashua, NH, USA).
Animals and cell lines
Female CD1 nude mice (Charles River, Wilmington, MA, USA) were kept under a controlled environment where temperature, humidity, and 12-h day and night cycles were maintained artificially. One cohort of mice were cortically implanted with 1·106 cells of SJ-DIPGx7 (Line 7 passage 26 with luciferase-YFP marker). Line 7 was established from a patient autopsy DIPG sample, and carries the H3.3K27M mutation. All procedures were approved by the St. Jude Institutional Animal Care and Use Committee and met the guidelines of the Association for Assessment and Accreditation of Laboratory Animal Care (AALAC).
Ribociclib protein binding in mouse plasma
A plasma protein binding study of ribociclib was performed using a rapid equilibrium dialysis procedure. Ribociclib was added to mouse plasma (BioIVT, Westbury, NY) to make final concentrations of 500, 1000, 2500, and 5000 ng/ml. 1 mL of spiked plasma was added to Centrifree® ultrafiltration devices [molecular weight cut off: 10 K Daltons; MilliporeSigma, Darmstadt, Germany]. The plasma samples were centrifuged for 30 min at 3600 rpm at 37°C. The filtrate was saved and stored at −80°C until analysis. Fraction unbound of ribociclib in plasma was calculated as ratio of unbound ribociclib concentration in the filtrate to total ribociclib concentration in the plasma sample.
Plasma pharmacokinetic study
CD1 nude mice (n=9) bearing SJ-DIPGx7 cortical allograft tumors were dosed with 100 mg/kg ribociclib (oral; 10 mg/ml) formulated in 0.5% methyl cellulose. Multiple samples were collected per mouse for up to 24 h post-dose using a population-based study design. Blood samples (~ 75 μL) were collected by retro-orbital eye bleed using heparin coated capillary tubes (Fisher Sci, Pittsburgh, PA), except for the terminal samples, which was collected by cardiac stick with a 1 ml heparin pre-coated syringe. Immediately after collection, blood samples were centrifuged, plasma was separated, and stored at −80°C until analysis with an LC MS/MS method [9].
Development of pharmacokinetic limited sampling modeling for ribociclib
During microdialysis, only three plasma samples per mouse could be obtained due to blood sampling volume restrictions and plasma volume required for bioanalysis. Thus, a pharmacokinetic LSM was selected to provide the most statistically informative time points for the plasma sample collection during microdialysis. The LSM was developed based on the pharmacokinetic parameters obtained from the plasma disposition study, using the D-optimality method implemented in ADAPT 5 (BSMR, Los Angeles, CA, USA).
In vitro probe recovery studies
To assess the dialyzability of ribociclib, the microdialysis probe recovery was determined using in vitro recovery studies as previously described [10]. Specifically, each probe used for the in vivo microdialysis study was placed in a beaker containing a ribociclib solution prepared in Ringer’s solution at a concentration of 1 μg/mL and maintained at 37°C. The probe was perfused with blank Ringer’s solution at a flow-rate of 0.5 μL/min. After equilibration for 1 hr, three consecutive fractions each of 1 hr interval were collected. Dialysate samples were stored at −80°C until further analysis using a mass spectrometry method developed in our laboratory validated for use with Ringer’s solution. Probe recovery was calculated using the ratio of ribociclib concentration in the dialysate sample to that in the stock solution. The ECF concentration was calculated by dividing the concentration observed in the microdialysate sample by the recovery ratio of the probe used for that experiment.
Ribociclib cerebral microdialysis studies
In vivo microdialysis experiments were conducted to sample unbound ribociclib in the mouse brain ECF or tumor ECF after a single ribociclib dose (100 mg/kg). Cerebral microdialysis studies were performed in non-tumor bearing CD1 nude mice (n=12), and in CD1 nude mice bearing SJDIPGx7 cortical allograft tumors (n=16). DIGP×7 tumor cells and microdialysis guide cannula (MD-2255, BASi) were implanted separately into the cerebral cortex of mice using a stereotactic instrument as described previously (see References and Supplemental Materials for additional details [10]).
Pharmacokinetic analysis of plasma and microdialysis studies
Ribociclib plasma and ECF concentration-time data were sequentially fitted using a population-based pharmacokinetic compartmental approach using non-linear mixed effect modeling (NONMEM 7.2, ICON development solutions). See Supplemental Materials for additional details.
First, a one-compartment model consisting of a central compartment with an oral dosing compartment was fitted to the total plasma concentration-time data obtained from the plasma disposition study using (Supplemental Figure 1). Then, the same model with a compartment linked to the central compartment (Supplemental Figure 1) was fitted to the plasma LSM and ECF concentration-time data obtained during the microdialysis study. During model fitting of the microdialysis data, the mean plasma parameters were fixed to values estimated in the first step.
The area under concentration-time curve up to 24 hours after the dosing (AUC0–24h) for plasma and ECF profiles in an individual animal were estimated by integration of the predicted concentration-time profile in the NONMEM control stream. Plasma exposure of unbound ribociclib (AUC0–24h,u) was estimated as a fraction of total plasma AUC0–24h using ribociclib fraction unbound in mouse plasma. Brain/tumor ECF to plasma partition coefficient (Kp,uu) of ribociclib was estimated as a ratio of brain/tumor ECF AUC0–24h and plasma AUC0–24h,u.
Results
Ribociclib plasma protein binding
The median (range) ribociclib fraction unbound in CD1 mouse plasma was 0.23 (0.14 to 0.26) with no significant differences observed among the range of ribociclib concentrations evaluated (ANOVA, p=0.44).
Ribociclib plasma pharmacokinetic study
A plasma pharmacokinetic study of ribociclib was performed using mice bearing cortical allograft glioma tumors after a single oral dose of 100 mg/kg. As shown in Figure 1, a one-compartment model well-represented the ribociclib total plasma concentration-time data. Ribociclib was rapidly absorbed after oral administration. All model parameter estimates were reported in Table 1. The ribociclib elimination half-life in mice was 4.67 ± 0.64 h and plasma AUC0–24h,u was 6341 ± 753 ng/mL*h.
Figure 1:
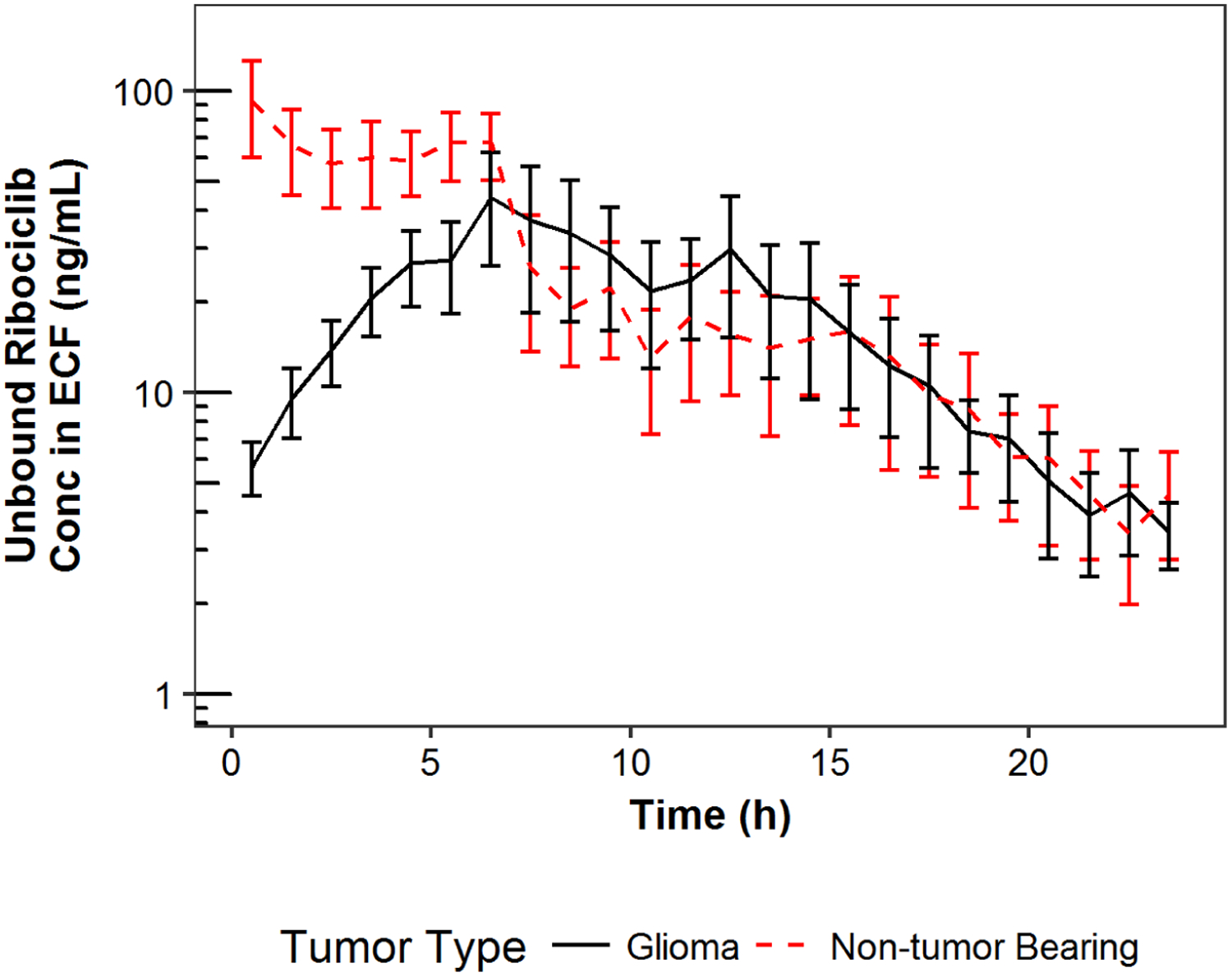
Ribociclib mean concentrations in extracellular fluid plotted against time by tumor type.
Note: Error bar represents standard error of mean at a particular time point
Table 1:
Parameter estimates and standard errors for the pharmacokinetic analysis.
| Estimate (%RSE) | ||||
|---|---|---|---|---|
| Parameters | Parameter Description | Full-plasma PK Study | Microdialysis Studies | |
| Glioma | Non-tumor mice | Glioma | ||
| θKa (1/hr) | First-order absorption constant | 2.75 (5.26) | 2.75 FIXED | 2.75 FIXED |
| θCL (L/hr/kg) | Clearance | 2.78 (1.8) | 2.78 FIXED | 2.78 FIXED |
| θVc (L/kg) | Volume of central compartment | 18.6 (3.87) | 18.6 FIXED | 18.6 FIXED |
| θCL23 (L/hr/kg) | Influx clearance from plasma to ECF compartment | NE | 0.124 (26.8) | 0.000000838 (24) |
| θCL32 (L/hr/kg) | Efflux clearance from ECF to plasma compartment | NE | 7.78 (8.81) | 0.0000808 (11.5) |
| θV3 (L/kg) | Volume of ECF compartment | NE | 0.00033 FIXED | 0.00033 FIXED |
| ωKa (Exp) | Exponential inter-individual variance in Ka | 1.33 (70.3) | 0.676 (49.6) | 0.712 (42.8) |
| ωCL (Exp) | Exponential inter-individual variance in CL | 0.0315 (90.6) | 0.217 (53.5) | 0.0367 (45.4) |
| ωCL23 (Exp) | Exponential inter-individual variance in CL23 | NE | 1.02 (42.7) | 0.765 (25.1) |
| σPlasma (Prop) | Proportional residual variance in plasma | 0.0277 (7.63) | 0.276 (16.1) | 0.217 (16.9) |
| σtECF (Prop) | Proportional residual variance in ECF | NE | 0.232 (18.4) | 0.25 (15.3) |
| Plasma* AUC0–24h |
27569 ± 3274 | 33226 ± 9375** | 28059 ± 3961*** | |
| Plasma* AUC0–24h,u |
6341 ± 753 | 7642 ± 2156** | 6454 ± 911*** | |
| Brain/tumor ECF* |
736 ± 610** | 433 ± 451*** | ||
| AUC0–24h | ||||
| Kp,uu^ | 0.105 ± 0.085** | 0.065 ± 0.064*** | ||
ng/mL*h;
n=12;
n=16;
t-test, p=0.19
The most statistically informative time-points for plasma sampling during microdialysis study determined by the pharmacokinetic LSM were 0.08, 1.5, and 7 hours. For selected studies a sample was also obtained at 24 hours.
Penetration of ribociclib in brain/tumor ECF
Cerebral microdialysis was performed to characterize the distribution of ribociclib in the brain/tumor ECF relative to unbound plasma in non-tumor bearing mice and mice bearing glioma tumor. The mean (SD) in vitro recovery of microdialysis probes used for cerebral microdialysis was 8.9 ± 6.9%. Ribociclib plasma and ECF concentration-time data were well characterized by the pharmacokinetic model as shown in Figure 1. The model parameter estimates as well as the plasma and brain/tumor ECF exposures and Kp,uu for non-tumor bearing mice and mice bearing glioma are reported in Table 1. The mean (SD) Kp,uu for ribociclib was 0.10 ± 0.09 and 0.07 ± 0.06 (t-test, p=0.19) for non-tumor bearing mice and mice bearing glioma, respectively.
Discussion
In the current study, results of the ribociclib plasma pharmacokinetic provided for the development of a pharmacokinetic LSM that was used in subsequent microdialysis studies. The results of the protein binding studies showed that the median ribociclib fraction unbound in CD1 mouse plasma was 0.23. Using cerebral microdialysis techniques with pharmacokinetic modeling, we demonstrated that the CNS penetration of unbound drug in mice bearing glioma tumors was 0.07 and in non-tumor bearing mice was 0.10, which was not statistically different (p=0.19). Lastly, the brain ECF concentrations in both models (NTB mice and mice bearing glioma tumors) were in above the IC50 for CDK-cyclin D1 (i.e., 10 nM) for approximately 10 hours.
Ribociclib is one of three CDK4/6 inhibitors with abemaciclib and palbociclib approved by the FDA for the treatment of breast cancer. These compounds are also in development as single agents and in combination for hematological malignancies, solid tumors, and for CNS malignancies. To treat a CNS malignancy, either primary or metastatic will require that the compound have adequate CNS penetration. Using a tissue homogenization method, Raub and colleagues evaluated the unbound plasma and brain abemaciclib exposures over 24 hours after an oral dosage of 30 mg/kg and reported a Kp,brain of 0.21 ± 0.04 and a Kp,uu of 0.03 ± 0.01 [11]. At this dosing, the plasma AUC reported by Raub (i.e., 69,300 ng/ml*hr) was well above the plasma AUC (i.e., 5520 ng/ml*hr) that has been reported in adults at the recommended dose [12]. Using a mouse brain uptake assay, which employs a single plasma and brain sample obtained 5 minutes after injection of a radiolabeled dose of palbociclib, the authors assessed palbociclib CNS penetration in CF1 mice and reported a Kp,brain of 0.13 ± 0.04 (dose 0.5 μmole/kg) and a Kp,uu of 0.01 ± 0.003. This is similar to what other investigators have reported for palbociclib [13,14].
As noted above, the abemaciclib CNS penetration studies were conducted at a dosage that far exceeded clinically relevant plasma systemic exposures. The results of our plasma ribociclib pharmacokinetic studies showed that at 100 mg/kg the mean unbound AUC0→24 was 6812 ng/ml*hr, which compares favorably with that reported from a phase I study by Infante and colleagues (i.e., ~6000 ng/ml*hr assuming a fraction unbound of 0.30) [15]. We have shown that this approach has been successful in translating preclinical results from the lab to the clinic for myelosuppressive agents [16], and would suggest that it would also work for other groups of drugs such as molecularly targeted agents (e.g., ribociclib).
Although cerebral microdialysis has many advantages over other methods used to assess CNS drug penetration, its use in the evaluation of ECF drug disposition may have possible limitations. Insertion of the microdialysis probe into tissues can be associated with tissue damage, hemorrhage, and immune reactivity [17]. Thus, we allow at least 1 hour for probe equilibration prior to the start of microdialysis studies. Moreover, as a quality control step at the conclusion of each microdialysis study, the animal was euthanized, the brain was removed with the probe intact, sectioned, stained to evaluate for probe placement, inflammation, and hemorrhage. Any study with excessive inflammation or hemorrhage was not included in the results.
The microdialysis membrane is semipermeable and microdialysis is a dynamic process in which substances are continuously removed from the tissue ECF by diffusion into the probe. However, a true equilibrium is never reached requiring that recovery studies be performed for each drug and probe [18]. During our developmental studies with ribociclib and prior to performing the actual microdialysis studies, we conducted in vitro, in vitro retrodialysis, and in vivo retrodialysis recovery studies with the same group of probes. We used these results in a decision tree (i.e., algorithm) to determine which approach to recovery will be used during the actual microdialysis studies. We obtained negative values for our in vivo recovery studies, and wide inter-probe variability with in vitro retrodialysis. Thus, we chose to use in vitro retrodialysis procedure to calculate recovery for our actual microdialysis experiments. Obviously, in vivo recovery has advantages over in vitro recovery, however, in general it is usually much lower than the in vitro recovery and, in that regard, we consider the in vitro recovery that we used to be a conservative approach to normalizing the ribociclib ECF concentrations. Thus, we used the well-accepted in vitro approach, which is performed for each probe and is independent of the tissue in which the probe is implanted. Probes used in the non-tumor or tumor-bearing mice had similar recovery values.
In summary, results of our plasma pharmacokinetic study of ribociclib 100 mg/kg orally in CD1 mice showed that the mouse systemic exposure was similar to that previously published for humans at the recommended adult dosage. Data from that pharmacokinetic study also enabled us to derive a pharmacokinetic LSM for use in our microdialysis studies. Lastly, the median ribociclib Kp,uu in NTB and mice bearing DIPG×7 glioma was 0.10 and 0.06, respectively.
Supplementary Material
Funding:
This work was supported in part by grants CA23099, CA096832, and by the American Lebanese Syrian Associated Charities (ALSAC).
Footnotes
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
Conflict of Interest:
The authors have no conflict of interest of commitment related to the conduct of this study to disclose.
Ethical Approval:
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
References
- 1.Gottardo NG, Gajjar A (2008) Chemotherapy for malignant brain tumors of childhood. J Child Neurol 23 (10):1149–1159. doi:23/10/1149 [pii] 10.1177/0883073808321765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rutkowski S, Bode U, Deinlein F, Ottensmeier H, Warmuth-Metz M, Soerensen N, Graf N, Emser A, Pietsch T, Wolff JE, Kortmann RD, Kuehl J (2005) Treatment of early childhood medulloblastoma by postoperative chemotherapy alone. N Engl J Med 352 (10):978–986. doi:352/10/978 [pii] 10.1056/NEJMoa042176 [DOI] [PubMed] [Google Scholar]
- 3.Broniscer A, Iacono L, Chintagumpala M, Fouladi M, Wallace D, Bowers DC, Stewart C, Krasin MJ, Gajjar A (2005) Role of temozolomide after radiotherapy for newly diagnosed diffuse brainstem glioma in children: results of a multiinstitutional study (SJHG-98). Cancer (journal) 103 (1):133–139 [DOI] [PubMed] [Google Scholar]
- 4.Ribociclib Approved for Advanced Breast Cancer (2017). Cancer Discov 7 (5):OF3. doi: 10.1158/2159-8290.CD-NB2017-043 [DOI] [PubMed] [Google Scholar]
- 5.Sherr CJ (2016) A New Cell-Cycle Target in Cancer - Inhibiting Cyclin D-Dependent Kinases 4 and 6. N Engl J Med 375 (20):1920–1923. doi: 10.1056/NEJMp1612343 [DOI] [PubMed] [Google Scholar]
- 6.Warren KE, Killian K, Suuriniemi M, Wang Y, Quezado M, Meltzer PS (2012) Genomic aberrations in pediatric diffuse intrinsic pontine gliomas. Neuro Oncol 14 (3):326–332. doi: 10.1093/neuonc/nor190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barton KL, Misuraca K, Cordero F, Dobrikova E, Min HD, Gromeier M, Kirsch DG, Becher OJ (2013) PD-0332991, a CDK4/6 inhibitor, significantly prolongs survival in a genetically engineered mouse model of brainstem glioma. PLoS One 8 (10):e77639. doi: 10.1371/journal.pone.0077639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li M, Lockwood W, Zielenska M, Northcott P, Ra YS, Bouffet E, Yoshimoto M, Rutka JT, Yan H, Taylor MD, Eberhart C, Hawkins CE, Lam W, Squire JA, Huang A (2012) Multiple CDK/CYCLIND genes are amplified in medulloblastoma and supratentorial primitive neuroectodermal brain tumor. Cancer Genet 205 (5):220–231. doi: 10.1016/j.cancergen.2012.03.002 [DOI] [PubMed] [Google Scholar]
- 9.Kala A, Patel YT, Davis A, Stewart CF (2017) Development and validation of LC-MS/MS methods for the measurement of ribociclib, a CDK4/6 inhibitor, in mouse plasma and Ringer’s solution and its application to a cerebral microdialysis study. J Chromatogr B Analyt Technol Biomed Life Sci 1057:110–117. doi: 10.1016/j.jchromb.2017.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhuang Y, Fraga CH, Hubbard KE, Hagedorn N, Panetta JC, Waters CM, Stewart CF (2006) Topotecan central nervous system penetration is altered by a tyrosine kinase inhibitor. Cancer Res 66 (23):11305–11313. doi: 10.1158/0008-5472.CAN-06-0929 [DOI] [PubMed] [Google Scholar]
- 11.Raub TJ, Wishart GN, Kulanthaivel P, Staton BA, Ajamie RT, Sawada GA, Gelbert LM, Shannon HE, Sanchez-Martinez C, De Dios A (2015) Brain Exposure of Two Selective Dual CDK4 and CDK6 Inhibitors and the Antitumor Activity of CDK4 and CDK6 Inhibition in Combination with Temozolomide in an Intracranial Glioblastoma Xenograft. Drug Metab Dispos 43 (9):1360–1371. doi: 10.1124/dmd.114.062745 [DOI] [PubMed] [Google Scholar]
- 12.Tate SC, Sykes AK, Kulanthaivel P, Chan EM, Turner PK, Cronier DM (2018) A Population Pharmacokinetic and Pharmacodynamic Analysis of Abemaciclib in a Phase I Clinical Trial in Cancer Patients. Clin Pharmacokinet 57 (3):335–344. doi: 10.1007/s40262-017-0559-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.de Gooijer MC, Zhang P, Thota N, Mayayo-Peralta I, Buil LC, Beijnen JH, van Tellingen O (2015) P-glycoprotein and breast cancer resistance protein restrict the brain penetration of the CDK4/6 inhibitor palbociclib. Invest New Drugs 33 (5):1012–1019. doi: 10.1007/s10637-015-0266-y [DOI] [PubMed] [Google Scholar]
- 14.Parrish KE, Pokorny J, Mittapalli RK, Bakken K, Sarkaria JN, Elmquist WF (2015) Efflux transporters at the blood-brain barrier limit delivery and efficacy of cyclin-dependent kinase 4/6 inhibitor palbociclib (PD-0332991) in an orthotopic brain tumor model. J Pharmacol Exp Ther 355 (2):264–271. doi: 10.1124/jpet.115.228213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Infante JR, Cassier PA, Gerecitano JF, Witteveen PO, Chugh R, Ribrag V, Chakraborty A, Matano A, Dobson JR, Crystal AS, Parasuraman S, Shapiro GI (2016) A Phase I Study of the Cyclin-Dependent Kinase 4/6 Inhibitor Ribociclib (LEE011) in Patients with Advanced Solid Tumors and Lymphomas. Clin Cancer Res 22 (23):5696–5705. doi: 10.1158/1078-0432.CCR-16-1248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zamboni WC, Stewart CF, Thompson J, Santana VM, Cheshire PJ, Richmond LB, Luo X, Poquette C, Houghton JA, Houghton PJ (1998) Relationship between topotecan systemic exposure and tumor response in human neuroblastoma xenografts. J Natl Cancer Inst 90 (7):505–511 [DOI] [PubMed] [Google Scholar]
- 17.Morgan ME, Singhal D, Anderson BD (1996) Quantitative assessment of blood-brain barrier damage during microdialysis. J Pharmacol Exp Ther 277 (2):1167–1176 [PubMed] [Google Scholar]
- 18.Jacus MO, Throm SL, Turner DC, Patel YT, Freeman BB 3rd, Morfouace M, Boulos N, Stewart CF (2014) Deriving therapies for children with primary CNS tumors using pharmacokinetic modeling and simulation of cerebral microdialysis data. Eur J Pharm Sci 57:41–47. doi: 10.1016/j.ejps.2013.11.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


