Abstract
Background:
Adolescents and young adults (AYA) are underrepresented in cancer clinical trials (CCTs). Limited trial enrollment slows progress in improving survival rates and prevents the collection of valuable biospecimens. A systematic literature review was conducted to assess barriers and facilitators to AYA enrollment onto CCTs and identify opportunities to improve enrollment.
Methods:
PubMed Medline, Web of Science, Scopus, and PsychInfo databases were searched to identify studies relevant to AYA CCT enrollment. Eligibility criteria included qualitative and/or quantitative evaluation of barriers and facilitators to AYA enrollment.
Results:
One-hundred and fifty-five unique manuscripts were identified; 13 manuscripts were included in the final analysis. Barriers to AYA enrollment on CCTs included lack of existing trials applicable to the patient population, limited access to available CCTs, and lack of physician awareness of relevant trials. Facilitators to enrollment included optimization of research infrastructure, improving awareness of available CCTs amongst providers, and enhancing communication about CCTs between providers and patients.
Conclusions:
The limited available research reports institution- and patient-level barriers and facilitators to AYA CCT enrollment. Given persistent disparities in AYA enrollment, there is an urgent need to further identify the barriers and facilitators to AYA CCT enrollment to determine actionable areas for intervention.
Keywords: Adolescents and young adults, Cancer, Clinical Trials, Barriers, Facilitators, Community-based
PRECIS SUMMARY
Adolescents and young adults (AYAs) remain underrepresented in cancer clinical trials. This systematic review summarizes evidence-based barriers and facilitators to AYA enrollment at all steps in the clinical trial enrollment process.
INTRODUCTION
Approximately 70,000 adolescents and young adults (AYAs; ages 15-39 at diagnosis) are diagnosed with cancer each year in the United States, and cancer is the leading cause of disease-related death in this age group.1, 2 AYA cancer biology, the ability to tolerate intensive anti-cancer treatment, and the survival outcomes for specific malignancies among AYAs differ compared to older adults and younger children, strongly supporting the need to study optimal approaches to treatment separately in the AYA population.3-6
Cancer clinical trials (CCTs) help determine the most effective treatments to improve patient survival and health-related quality of life.7, 8 Lack of enrollment of specific patient populations on CCTs prevents determination of optimal treatment regimens and collection of essential biospecimens needed to target the underlying disease biology in future studies. AYA enrollment on CCTs has significantly lagged behind enrollment of pediatric patients.9 Enrollment is estimated to range between 3-14% for all AYAs, but enrollment rates vary within the AYA population.7-9 Previous research has consistently found higher enrollment rates in adolescent patients compared with patients in their 20s and 30s.8, 10
The reasons for low AYA enrollment numbers compared to other patient subgroups are not well understood .11 Suggested barriers include treatment institution, lack of available trials for cancer types or certain age groups (older AYAs), and insurance status.12 Two previous reviews have examined barriers and/or facilitators to CCT enrollment in AYA patients.13, 14f The first review examined trends in CCT enrollment among AYAs across time and included a brief overview of facilitators and barriers to CCT enrollment in AYAs.14 That review was conducted in May 2015 and did not include keywords for “facilitator” or “barrier” in the literature search. Therefore, an updated and more comprehensive review of the barriers and facilitators to CCT enrollment is warranted. The second review examined AYA patients’ perceptions and attitudes towards CCTs and found that extended hospital stays and concerns about treatment side effects were common barriers.13 This systematic review focused exclusively on patients’ attitudes and beliefs towards CCT enrollment, and did not consider systemic or site-level barriers or facilitators that are operative before a patient is even presented the option of a CCT (e.g. a CCT does not exist for the patient’s cancer).Therefore, the current systematic review sought to describe the multilevel barriers and facilitators to AYA CCT enrollment and summarize those amenable to intervention.
METHODS
Search Strategy
We conducted a systematic review of the literature following the Preferred Reporting Items for Systematic Review and Meta-analysis (PRISMA) guidelines.15, 16 We searched PubMed, Web of Science (including both science and social science citation indexes), Scopus, and PsychInfo databases for all studies indexed before July 11, 2019. The search strategy utilized a combination of keywords focused on AYAs and clinical trial enrollment. The keywords; were tailored to each database (e.g. using MeSH terms in PubMed; Supplemental Table 1).
Eligibility Criteria
Studies were eligible for inclusion if: (1) full-text publication was written in English, (2) included quantitative or qualitative data analysis, (3) examined measured or perceived barriers and/or facilitators to CCT enrollment, and (4) the target population was AYAs with cancer. AYA cancer patients were defined as a first cancer diagnosis between the ages of 15 to 39.1 To allow for the most comprehensive review of relevant articles, samples were considered for inclusion if: (1) at least 75% of the sample fell within the 15-39 year age range, or (2) the mean/median age of the sample fell within the 15-39 year range. Studies were excluded if they were a review, commentary, or not peer-reviewed (e.g. dissertation).
Study Selection, Conceptual Framework, and Data Abstraction
All article titles and abstracts identified were reviewed by one reviewer (E.J.S., N.S.T., or H.A.L.), and all potentially relevant articles were retrieved. The retrieved full-text articles were reviewed by at least one reviewer and 5% were reviewed by at least two reviewers to limit bias in study inclusion and disagreements were resolved by consensus.17 We utilized the kappa (κ) statistic to describe agreement for study inclusion between each pair of reviewers. Using a predefined table, one reviewer (E.J.S., N.S.T., H.A.L.) extracted the following for each study: (1) cancer type, (2) study location, (3) population (e.g. healthcare providers, AYA patients, etc.) and sample size, (4) type of study design and data collection methods, and (5) barriers and facilitators to CCT enrollment.
We utilized a narrative approach to synthesize the data identified in this review. The conceptual framework of the CCT enrollment process proposed by Freyer et al. was utilized to organize the results.12 This framework details the CCT enrollment process as a series of steps, where at each step potential barriers may prevent a cancer patient from enrolling on a CCT. To successfully enroll on a CCT, a trial must exist for the cancer patient’s diagnosis (Step 1), the patient must have access to the CCT (Step 2), the patient must be eligible for the CCT (Step 3), the patient must be presented the CCT (Step 4), and the patient must decide to enroll in the CCT (Step 5).
To reduce the potential for bias in assigning the extracted barriers and facilitators to steps within the conceptual framework, all three reviewers independently reviewed each article and assigned the barriers/facilitators to the appropriate step in the conceptual framework. Following independent review, the three reviewers met multiple times to discuss and resolve discrepancies by consensus.
RESULTS
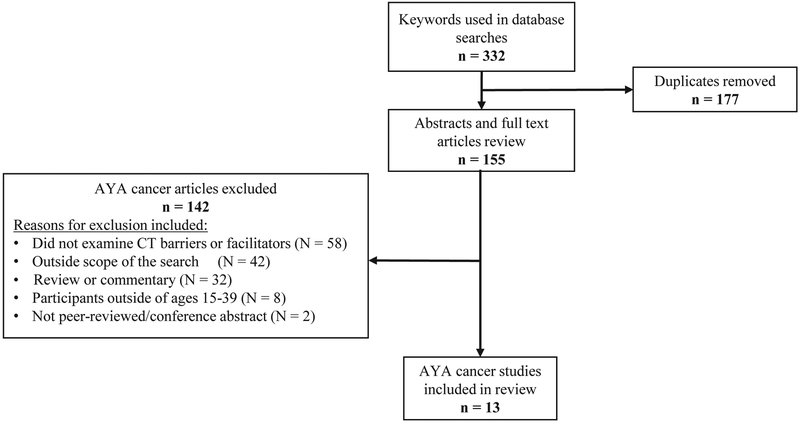
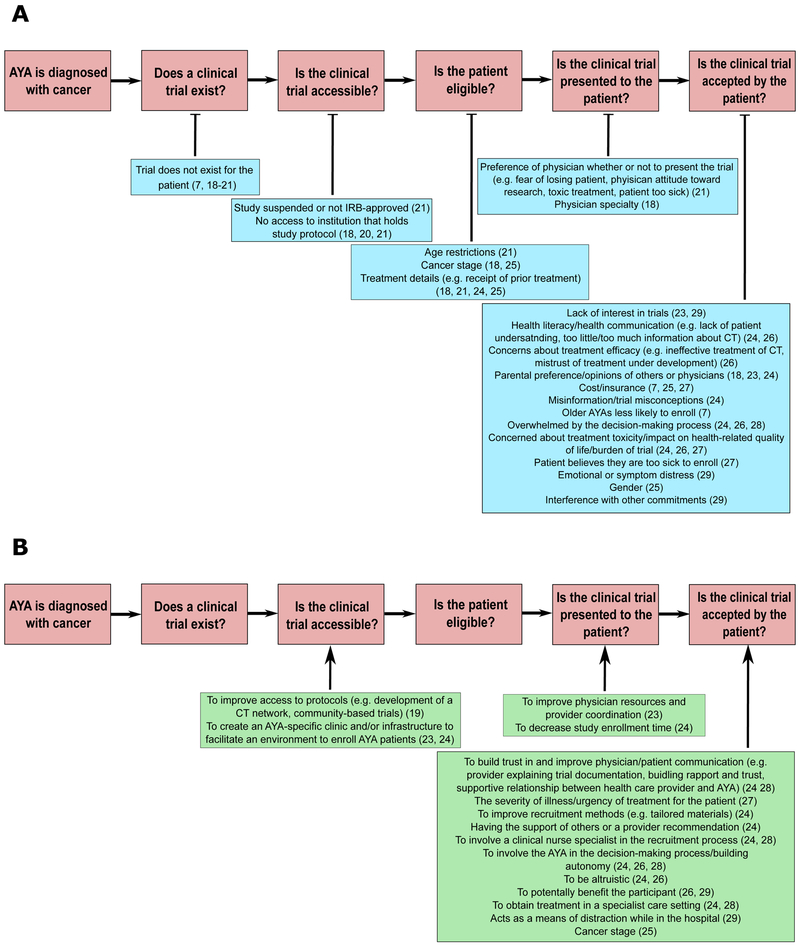
Figure 1 describes, in detail, the literature search process. The initial search yielded a total of 332 citations (date range: 1976-2019), and we ultimately included 13 manuscripts in our review. A detailed description of the reasons for exclusion for each reviewed manuscript is included in Supplemental Table 2. Agreement on study inclusion between reviewers was high (N.S.T., E.J.S.: κ=100%; N.S.T., H.A.L.: κ=100%; E.J.S., H.A.L.: κ=100%). Table 1 provides detailed information about each manuscript. The barriers and facilitators to CCT enrollment among AYA patients identified in the literature search are summarized in Figures 2A (barriers) and 2B (facilitators).
Figure 1.
Flow diagram of study selection for the systematic analysis.
Table 1.
Descriptive information for included articles (N=13)
| Citation | Study Location | Study Population | Study Objective | Data Collection Method | Type of Cancer |
|---|---|---|---|---|---|
| Shaw, P. H., & Ritchey, A. K. (2007)18 | Children's Hospital of Pittsburgh (USA) | Adolescents age 15 to 22 (n=139) | To determine if and why pediatric patients over the age of 15 had a low clinical trial enrollment rate | Secondary analysis of Children's Hospital of Pittsburgh database | Mixed (majority acute lymphoblastic leukemia, Ewing sarcoma, Hodgkin disease, non-Hodgkin lymphoma) |
| Downs-Canner, S., & Shaw, P. H. (2009)21 | Children's Hospital of Pittsburgh, University of Pittsburgh Cancer Institute (USA) | New AYA diagnosis age 15 to 22 (n=91); Patients enrolled (n=24) | To determine if and why pediatric patients over the age of 15 had a low clinical trial enrollment rate | Secondary analysis of University of Pittsburgh Cancer Institute clinical trial database | Acute lymphoblastic leukemia, acute myelogenous leukemia, central nervous system tumors, chronic myelogenous leukemia, Ewing sarcoma, Hodgkin lymphoma, melanoma, non-Hodgkin lymphoma, osteosarcoma, sarcomas |
| Parsons, H. M., Harlan, L. C., et al. (2011)7 | National Cancer Institute Patterns of Care Study, Surveillance, Epidemiology, and End Results (SEER) program data (USA) | AYA patients age 15 to 39 (n=1,358) | To examine patterns of clinical trial participation, time to treatment, and provider characteristics in a population-based sample of AYA patients with cancer | Secondary analysis of SEER cancer registry and National Cancer Institute Patterns of Care study | Multiple cancers, including acute lymphoblastic leukemia and sarcoma |
| Shaw, P. H., Boyiadzis, M., et al. (2012)23 | Children's Hospital of Pittsburgh, University of Pittsburgh Cancer Institute (USA) | AYA patients age 15 to 22 (n=57) | To determine if clinical trial enrollment improved among AYA patients who attended both pediatric and adult cancer centers since the inception of a joint AYA Oncology Program | Secondary analysis of University of Pittsburgh Cancer Institute clinical trial database | Mixed (majority: acute lymphoblastic leukemia, Ewing sarcoma, osteosarcoma, rhabdomyosarcoma) |
| Hendricks-Ferguson, V. L., et al. (2013)29 | Six pediatric and three adult hospitals across six medical centers throughout the Midwest and southern portions of the United States | AYA patients age 11-24 (n=118) | To describe recruitment strategies, participants’ reasons for consent or refusal, and the final recruitment rates of AYA with cancer into a research study called “Stories and Music for Adolescent/Young Adult Resilience during Transplant” (SMART) | Intervention recruitment to understand strategies to improve recruitment of AYA | Allogeneic or autologous HSCT for cancer with a myeloablative regimen |
| Barakat, L. P., Schwartz, L. A., et al. (2014)26 | East coast children's hospital at a large cancer center (USA) | AYA patients age 15 to 23 (n=13); Caregivers (n=16); Healthcare providers (n=11) | To describe key components of phase III clinical trial enrollment and evaluate a measure of attitudes | One-on-one interviews; focus groups | Acute lymphoblastic leukemia, acute promyelocytic leukemia, non-Hodgkin lymphoma, osteosarcoma, rhabdomyosarcoma |
| Collins, C. L., Malvar, J., et al. (2015)8 | Los Angeles County (USA) | USC Norris Comprehensive Cancer Center by institution (n=1699) versus Los Angeles County SEER incidence (n=11,358) | To compare the percentage of AYAs, children, and older adults enrolled onto cancer clinical trials and determine predictors of enrollment | Secondary analysis of California Cancer Registry and institutional trial enrollment database | Acute leukemia, breast carcinoma, central nervous system malignancies, cervical carcinoma, colorectal cancer, lymphoma, melanoma, sarcoma, thyroid carcinoma |
| Bell, J. A., Forcina, V., et al. (2018)27 | AYA Program at Princess Margaret Cancer Centre (Canada) | AYA patients age 18 to 39 (n=21) | To explore unique factors influencing the cancer clinical trial decision-making process, AYA perceptions, and attitudes towards cancer clinical trials | One-on-one interviews | Breast cancer, leukemia, lymphoma, sarcoma, testicular cancer |
| Pearce, S., Brownsdon, A., et al. (2018)24 | AYA Cancer | Teenage and Young Adult treatment center in the UK | AYA patients (n=21); Health professionals (n=18) | Qualitative; interpretive interviews | Ewing's sarcoma, Osteosarcoma |
| Sreeraman Kumar, R. Thapa, R., et al. (2018)25 | Moffitt Cancer Center in Tampa, FL (USA) | AYA patients age 15-39 (n=1,831) | To examine AYA enrollment into therapeutic melanoma clinical trials at Moffitt and examine relevant factors impacting enrollment | Retrospective review of de-identified records of patients | Melanoma |
| Thomas, S. M., Malvar, J., et al. (2018)19 | Children's Hospital Los Angeles (USA) | Total participants (n=216); children age 14 and younger (n=158); early AYA age 15 to 21 (n=58) | To assess whether differences in cancer clinical trial availability explain low cancer clinical trial enrollment for early AYAs | Quantitative analysis of an observational cohort | Mixed (majority for early AYA: leukemia/lymphoma, non-brain solid tumor, solid tumors) |
| Thomas, S. M., Malvar, J., et al. (2018)20 | Children’s Hospital Los Angeles (USA) | Children age 0 to 14; eAYAs age 15 to 21 | To compare the proportions of eAYAs and children for whom an appropriate CCT existed, was available locally, and was used for enrollment; secondary objectives were to evaluate the effects of age and other factors on CCT enrollment. | Secondary analysis of pathology reports from CHLA and Norris Cancer Hospital, and available trials listed on clinicaltrails.gov | Leukemia/lymphoma and solid tumor |
| Lavender, V., Gibson, F., et al. (2019)28 | Large AYA treatment center (UK) | Healthcare professionals who care for AYAs age 15 to 24 years (n=18) | To explore professional expertise, specialist knowledge and skill development and strategies described by the health professionals for communicating with AYAs about participating in clinical trials | Semi-structured narrative interviews | Osteosarcoma |
Figure 2.
Identified cancer clinical trial barriers (A) and facilitators (B) identified in the systematic review. No studies were found addressing facilitators for clinical trial existence or eligibility.
Does the clinical trial exist?
Barriers.
Four of the thirteen AYA CCT studies discussed trial existence as a possible barrier. Two studies found that lack of an existing trial was a primary barrier for between 16-57% of AYA patients.7, 18 However, two articles found that the proportion of AYAs and children treated at a children’s hospital with an existing CCT were similar (48.3% AYAs vs. 53.8% children).19 Additionally, the existence of a CCT for AYA patients was similar between a children’s hospital (52.9%) and their affiliated adult institution (53.6%).20
Facilitators.
The literature search did not identify any facilitators to improving CCT enrollment at this step.
Is a clinical trial accessible?
Barriers.
Six of thirteen AYA CCT studies addressed accessibility of clinical trials and found that institutional- or system-level barriers to CCT enrollment made it difficult for AYAs to access trials.8, 18-22 In one study comparing AYA CCT at a children’s hospital and its affiliated adult hospital, only 4.1% of AYAs treated at the adult institution enrolled in a CCT compared with 26.4% at the children’s hospital.21 Additionally, AYAs treated at the adult institution for “AYA” cancers (e.g. Hodgkin lymphoma) were not enrolled in any CCTs, but 27.3% of AYAs treated for the same disease at the children’s hospital were enrolled in a CCT.21 Thomas et al. (2018) found that although AYAs treated at a children’s institution and the affiliated adult hospital had similar proportions of existing trials, a significantly lower proportion of AYAs treated at the adult hospital had the existing trials activated at their treating institution (16.7%) compared to AYAs treated at the children’s hospital (44.1%).20 In contrast, when AYAs and children (≤ 14 years) were both treated at a children’s hospital, the accessibility of CCTs was similar (39.7% AYAs vs. 47.5% children).19 Additional barriers to CCT accessibility included lack of IRB approval at the treating institution, the study was suspended at the institution, the patients’ age prevented them from receiving care at the institution with the CCT (e.g. too old for care at a children’s hospital), or they lacked a referral to an institution with the CCT open.8, 18, 22 Collins et al. examined AYA CCT enrollment across three affiliated institutions – a local children’s hospital and two adult institutions (a cancer hospital and a public hospital).8 They noted that Children’s Oncology Group (COG) trials were only available to AYAs being treated at the children’s hospital, and not available to those being treated at any of the adult institutions examined.8
Facilitators.
Three of thirteen AYA CCT studies addressed efforts to increase trial accessibility.19, 23, 24Two single institution studies reported that the creation of AYA-specific oncology programs improved research/CCT participation after the implementation of dedicated staff to aid in connecting AYA patients with open CCTs.23, 24 Following establishment of an AYA oncology program at the University of Pittsburgh Cancer Institute and Children’s Hospital of Pittsburgh, participation in CCTs by referred AYA patients significantly improved (4% versus 32%, p<0.01).23 The results of this study highlight the importance of how clinical settings (e.g. pediatric, adult academic) may influence accessibility of CCTs. However, we cannot draw specific inferences because the program components responsible for the improvement in CCT enrollment were not described.
Is the patient eligible?
Barriers.
Three of the thirteen AYA studies addressed barriers to patient eligibility.7, 18, 23, 25 In their study, Shaw and Ritchey found that 6% of patients (8/139) age 15-22 where not enrolled onto a study due to ineligibility. Reasons for ineligibility included: patient began treatment at a different institution, did not complete the tests required for CCT enrollment prior to initiation of treatment, treatment was delayed, or the patient had low functional status.7, 18, 23
Facilitators.
We did not identify studies describing any facilitators to overcoming barriers to enrollment related to AYA patient eligibility.
Is the CT presented to the patient?
Barriers.
Two of thirteen AYA CCT studies addressed the extent to which lack of presentation of CCTs to eligible patients was a barrier to enrollment.7, 18 Physicians are commonly responsible for presenting patients with CCT options. However, AYA patients treated by pediatric oncologists or a pediatric oncology team were more likely to enroll on CCTs (verified by medical record extraction and treating physician), compared to AYA patients treated by medical oncologists only (pediatric oncology only: OR 7.39, 95% CI 2.49, 21.93; pediatric oncology and other: OR 3.69, 95% CI 1.63, 8.36).7
Facilitators.
The establishment of a formalized AYA Oncology Program at the University of Pittsburgh overcame barriers to enrollment related to lack of clinical trial presentation. Investigators met regularly to discuss trials available at the children’s hospital and/or adult cancer center, total accrual, and consider new means to improve enrollment.23
Is the CT accepted by the patient?
Barriers.
Nine of thirteen AYA CCT studies addressed the extent to which lack of AYA acceptance of a CCT was a barrier to enrollment. 7, 18, 23-29 CCT enrollment discussions that occurred close to diagnosis were overwhelming and impaired decision-making.24, 26, 28 The time of CCT randomization was seen as the point where the most imperative decisions were made.25 Three studies indicated patient disinterest and parental preference as barriers.18, 23, 29 Additionally, the terminology used when describing the cancer or treatment, such as “poor response” and “rare cancer”, directed AYAs’ perception of CCTs.8 Other reasons cited by AYA patients for not enrolling in CCTs were a parent or other member of their social support network suggesting against enrolling, interference of the presented CCT with other studies, other life commitments (e.g. employment), emotional or symptom distress, and cost or lack of adequate health insurance coverage.18, 23-25, 27, 29, 30 In two included studies, multivariate analysis demonstrated that uninsured individuals were about 75% less likely to enroll as individuals with private insurance (OR: 0.25, 95% CI 0.08, 0.767; OR: 0.3, 95% CI 0.20, 0.4325).7, 25 Furthermore, age at diagnosis/enrollment and gender were significantly associated with the likelihood of AYA participation in CCTs.7 In the population-based National Cancer Institute’s Patterns of Care study, AYAs ages 25-29 (OR: 0.28, 95% CI 0.11, 0.73), 30-34 (OR: 0.43, 95% CI 0.19, 0.96), and 35-39 (OR: 0.32, 95% CI 0.15, 0.69) were less likely to enroll compared to AYAs ages 15-19.7
Concerns about toxicity or potential side effects from the experimental treatment and overall burden of the trial were also commonly reported barriers in AYA cancer.24, 26, 27, 31 AYA cancer patients were concerned that the trial may cause more side effects than the standard of care and worsen their health-related quality of life.24, 27
Facilitators.
Patient-level facilitators to CCT enrollment among AYAs focused on including them in the CCT enrollment decision, building strong, trusting relationships between AYAs and their healthcare providers, altruism, having a form of distraction while at the hospital, and the desire to further science.24, 26-29 Providers reported that supporting AYAs’ growing autonomy, being sensitive to AYAs’ concerns regarding trial enrollment, and providing opportunities for AYAs to ask questions were important for establishing trusting provider-patient relationships.24, 26, 28 Including the AYA in the decision making process facilitated enrollment.26
DISCUSSION
CCTs are an essential component to studying disease biology and improving survival and health-related quality of life outcomes; however, only between 3-14% of all AYAs with cancer enroll on a CCT.7, 8, 10, 32 Previous reviews on this topic have identified patient-level barriers to CCT enrollment, including concerns about treatment side effects and a lack of patient awareness.13, 14 Our review extended this body of literature to encompass site-level barriers that may arise before the patient is ever presented with a CCT (e.g. physician preference to present trial).21 Despite a growing focus on AYA cancer patients and disparities in care and outcomes, few studies have adequately addressed factors contributing to the low enrollment of AYAs onto CCTs and even fewer studies have assessed the efficacy of interventions enhancing AYA enrollment. The current systematic review identified only thirteen eligible AYA CCT studies, ten of which were single institution reports. Given the importance of CCTs in improving patient treatment options and overall outcomes, additional research is needed to fully understand the barriers and facilitators to AYA CCT enrollment. However, this review identified several barriers to enrollment and potential areas for intervention.
AYA access to CCTs is limited by the lack of existence of trials for this age group, institutional barriers, and restrictive age of eligibility. With the relative rarity of AYA cancers and the challenges of lower-resourced settings, recruiting AYAs to CCTs faces numerous challenges.33, 34 The reorganization of the NCI National Clinical Trials Network (NCTN) and the development of the NCI Cancer Trials Support Unit (CTSU) have the potential to foster cross enrollment of patients through consolidation of the cooperative groups, increasing collaboration, maximizing patient access to trials, and expanding or eliminating age eligibility criteria.35-37 However, cross-enrollment of AYAs has been limited, and the development of intergroup trials (e.g. ARST1321) has been challenging due to differences in historical treatment approaches and institutional limits on patient age (e.g. pediatric hospitals).38-40 Additionally, the impact of CT networks improving enrollment in smaller, lower-resourced settings has been mixed.23 Our review identified the development of AYA-specific programs as one method for improving AYA CCT enrollment, however, this program was developed in an academic medical center and may not be reproducible in settings with fewer resources or different organizational priorities.23
Provider and patient factors may be more challenging to address given the diversity of clinical practices and patient populations. However, these barriers are critical to address because provider-patient discussions are one of the most common ways in which patients learn about CCTs. Lack of time and resources often prevent physicians from regularly participating in CCT enrollment.7, 18, 21, 41, 42 Incentivizing physicians, improving educational resources, learning how to best advise AYA patients about CCTs, and conducting trials in line with the physician’s interests may help overcome some of these barriers.43, 44 A means to alleviate the initial pressure on the physician may be the development of a nurse navigator program or a formalized AYA program.8, 23, 25 Previous research has found that nurse navigator programs work as a conduit for identifying eligible patients for CCTs.45, 46 Formalized AYA oncology programs increase communication between pediatric and medical oncology and has facilitated AYA CCT enrollment.51 These approaches may be particularly helpful because physicians may not initially be aware of trials for which their AYA patient is eligible. However, improving physician knowledge and awareness of trials does not alleviate patient burden. Studies assessing barriers to CCTs for older adults (ages 65 and older) and racial/ethnic minority populations suggest that life constraints and obligation of travel to the study site may substantially influence patient enrollment.47-49 These barriers may also impact AYA populations.
The current review has a few limitations. First, there is a paucity of studies that have assessed barriers and facilitators to AYA enrollment, and most assessed enrollment factors at single institutions, limiting our ability to draw generalizable conclusions. Second, due to the limited existing evidence, it is likely that not all barriers and facilitators to CCT enrollment have been captured. This may be a result of restricted response options in surveys or questionnaires, or qualitative assessment of barriers and facilitators gathered from small, targeted focus groups. Finally, many of the studies reviewed were conducted prior to or right after the initiation of the NCTN or centralized IRBs, and these systemic changes may improve AYA CCT enrollment. The current review, however, lays the groundwork to perform a further, in-depth analysis of barriers, as well as identify initial targetable areas for intervention.
CONCLUSIONS
Few studies examining the barriers and facilitators to AYA CCT enrollment currently exist in the literature. The few existing studies suggest that the barriers to AYA CCT enrollment are multifactorial and include system-, institution-, and individual-level factors. These barriers need to be further understood with the goal of identifying interventions with high potential to enhance AYA enrollment, particularly for those cancers with the most need (e.g. sarcomas, brain tumors). The NCTN and the NCI Community Oncology Research Program, a well-resourced community research network, are well-positioned to conduct this research.50, 51 Increasing enrollment of AYAs onto CCTs is urgently needed to determine optimal treatment approaches and maximize outcomes for AYAs with cancer.
Supplementary Material
ACKNOWLEDGEMENTS
The authors would like to thank Nancy Terry (National Institutes of Health, Office of the Director, Educational Services Branch) for assisting with creation of the literature search strategy, including keyword and database selection.
FUNDING
Not applicable
Footnotes
CONFLICT OF INTEREST
The authors have no conflicts of interest to declare.
REFERENCES
- 1.Institute NC. Adolescents and Young Adults with Cancer. Available from URL: www.cancer.gov/types/aya [accessed December 28, 2018, 2018].
- 2.Nass SJ, Beaupin LK, Demark-Wahnefried W, et al. Identifying and addressing the needs of adolescents and young adults with cancer: summary of an Institute of Medicine workshop. Oncologist. 2015;20: 186–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bleyer A, Barr R, Hayes-Lattin B, et al. The distinctive biology of cancer in adolescents and young adults. Nat Rev Cancer. 2008;8: 288–298. [DOI] [PubMed] [Google Scholar]
- 4.Fidler MM, Frobisher C, Hawkins MM, Nathan PC. Challenges and opportunities in the care of survivors of adolescent and young adult cancers. Pediatr Blood Cancer. 2019;66: e27668. [DOI] [PubMed] [Google Scholar]
- 5.Keegan TH, Ries LA, Barr RD, et al. Comparison of cancer survival trends in the United States of adolescents and young adults with those in children and older adults. Cancer. 2016;122: 1009–1016. [DOI] [PubMed] [Google Scholar]
- 6.Gaspar N, Marshall LV, Binner D, et al. Joint adolescent-adult early phase clinical trials to improve access to new drugs for adolescents with cancer: proposals from the multi-stakeholder platform-ACCELERATE. Ann Oncol. 2018;29: 766–771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Parsons HM, Harlan LC, Seibel NL, Stevens JL, Keegan TH. Clinical trial participation and time to treatment among adolescents and young adults with cancer: does age at diagnosis or insurance make a difference? J Clin Oncol. 2011;29: 4045–4053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Collins CL, Malvar J, Hamilton AS, Deapen DM, Freyer DR. Case-linked analysis of clinical trial enrollment among adolescents and young adults at a National Cancer Institute-designated comprehensive cancer center. Cancer. 2015;121: 4398–4406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Parsons HM, Penn DC, Li Q, et al. Increased clinical trial enrollment among adolescent and young adult cancer patients between 2006 and 2012-2013 in the United States. Pediatr Blood Cancer. 2019;66: e27426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bleyer A. Adolescent and young adult (AYA) oncology: the first A. Pediatr Hematol Oncol. 2007;24: 325–336. [DOI] [PubMed] [Google Scholar]
- 11.Hematology ASo. Dr. Theresa Keegan on Barriers, Facilitators to Clinical Trial Participation Among AYAs, 2019.
- 12.Freyer DR, Seibel NL. The Clinical Trials Gap for Adolescents and Young Adults with Cancer: Recent Progress and Conceptual Framework for Continued Research. Curr Pediatr Rep. 2015;3: 137–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Forcina V, Vakeesan B, Paulo C, et al. Perceptions and attitudes toward clinical trials in adolescent and young adults with cancer: a systematic review. Adolesc Health Med Ther. 2018;9: 87–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Friend BD, Baweja A, Schiller G, et al. Clinical trial enrollment of adolescnet and young adult patients with cancer: a systematic reivew of the literature and proposed solutions. Clinical Oncology in Adolescents and Young Adults. 2017;6: 51–59. [Google Scholar]
- 15.Shamseer L, Moher D, Clarke M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: elaboration and explanation. BMJ. 2015;350: g7647. [DOI] [PubMed] [Google Scholar]
- 16.Moher D, Shamseer L, Clarke M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Systematic Reviews. 2015;4: 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brown B, Carcamo C, Blas MM, Valderrama M, Halsey N. Peruvian FSWs: understanding HPV and barriers to vaccination. Vaccine. 2010;28: 7743–7747. [DOI] [PubMed] [Google Scholar]
- 18.Shaw PH, Ritchey AK. Different rates of clinical trial enrollment between adolescents and young adults aged 15 to 22 years old and children under 15 years old with cancer at a children’s hospital. J Pediatr Hematol Oncol. 2007;29: 811–814. [DOI] [PubMed] [Google Scholar]
- 19.Thomas SM, Malvar J, Tran H, Shows J, Freyer DR. A prospective, observational cohort study comparing cancer clinical trial availability and enrollment between early adolescents/young adults and children. Cancer. 2018;124: 983–990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thomas SM, Malvar J, Tran HH, Shows JT, Freyer DR. A Prospective Comparison of Cancer Clinical Trial Availability and Enrollment Among Adolescents/Young Adults Treated at an Adult Cancer Hospital or Affiliated Children’s Hospital. Cancer. 2018;124: 4064–4071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Downs-Canner S, Shaw PH. A comparison of clinical trial enrollment between adolescent and young adult (AYA) oncology patients treated at affiliated adult and pediatric oncoogy centers. J Pediatr Hematol Oncol. 2009;31. [DOI] [PubMed] [Google Scholar]
- 22.Shaw PH, Hayes-Lattin B, Johnson R, Bleyer A. Improving enrollment in clinical trials for adolescents with cancer. Pediatrics. 2014;133 Suppl 3: S109–113. [DOI] [PubMed] [Google Scholar]
- 23.Shaw PH, Boyiadzis M, Tawbi H, et al. Improved clinical trial enrollment in adolescent and young adult (AYA) oncology patients after the establishment of an AYA oncology program uniting pediatric and medical oncology divisions. Cancer. 2012;118: 3614–3617. [DOI] [PubMed] [Google Scholar]
- 24.Pearce S, Brownsdon A, Fern L, Gibson F, Whelan J, Lavender V. The perceptions of teenagers, young adults and professionals in the participation of bone cancer clinical trials. Eur J Cancer Care (Engl). 2018;27: e12476. [DOI] [PubMed] [Google Scholar]
- 25.Sreeraman Kumar R, Thapa R, Kim Y, Khushalani NI, Sondak VK, Reed DR. Higher than reported adolescent and young adult clinical trial enrollment during the “Golden Age” of melanoma clinical trials. Cancer Med. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Barakat LP, Schwartz LA, Reilly A, Deatrick JA, Balis F. A Qualitative Study of Phase III Cancer Clinical Trial Enrollment Decision-Making: Perspectives from Adolescents, Young Adults, Caregivers, and Providers. J Adolesc Young Adult Oncol. 2014;3: 3–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bell JAH, Forcina V, Mitchell L, et al. Perceptions of and decision making about clinical trials in adolescent and young adults with Cancer: a qualitative analysis. BMC Cancer. 2018;18: 629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lavender V, Gibson F, Brownsdon A, Fern L, Whelan J, Pearce S. Health professional perceptions of communicating with adolescents and young adults about bone cancer clinical trial participation. Supportive Care in Cancer. 2019;27: 467–475. [DOI] [PubMed] [Google Scholar]
- 29.Hendricks-Ferguson VL, Cherven BO, Burns DS, et al. Recruitment strategies and rates of a multi-site behavioral intervention for adolescents and young adults with cancer. J Pediatr Health Care. 2013;27: 434–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Barthel EM, Spencer K, Banco D, Kiernan E, Parsons S. Is the Adolescent and Young Adult Cancer Survivor at Risk for Late Effects? It Depends on Where You Look. J Adolesc Young Adult Oncol. 2016;5: 159–173. [DOI] [PubMed] [Google Scholar]
- 31.Vozy A, Blanga Charriel P, Geoerger B, Massard C, Gaspar N. Inclusion of adolescents in early phase trials and young adults in paediatric early phase trials: a reality or a myth? Annals of Oncology. 2018;29. [Google Scholar]
- 32.Bleyer A, Budd T, Montello M. Adolescents and young adults with cancer: the scope of the problem and criticality of clinical trials. Cancer. 2006;107: 1645–1655. [DOI] [PubMed] [Google Scholar]
- 33.Yeager ND, Hoshaw-Woodard S, Ruymann FB, Termuhlen A. Patterns of care among adolescents with malignancy in Ohio. J Pediatr Hematol Oncol. 2006;28: 17–22. [PubMed] [Google Scholar]
- 34.Albritton KH, Wiggins CH, Nelson HE, Weeks JC. Site of oncologic specialty care for older adolescents in Utah. J Clin Oncol. 2007;25: 4616–4621. [DOI] [PubMed] [Google Scholar]
- 35.Nass SJ, Balogh E, Mendelsohn J. A National Cancer Clinical Trials Network: recommendations from the Institute of Medicine. Am J Ther. 2011;18: 382–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kim ES, Bruinooge SS, Roberts S, et al. Broadening eligibility criteria to make clinical trials more representative: American Society of Clinical Oncology and Friends of Cancer Research Joint Research Statement. . J Clin Oncol. 2017;35: 3737–3744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chuk MK, Mulugeta Y, Roth-Cline M, Mehrotra N, Reaman GH. Enrolling Adolescents in Disease/Target-Appropriate Adult Oncology Clinical Trials of Investigational Agents. Clin Cancer Res. 2017;23: 9–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Roth ME, O’Mara AM, Seibel NL, et al. Low Enrollment of Adolescents and Young Adults Onto Cancer Trials: Insights From the Community Clinical Oncology Program. J Oncol Pract. 2016;12: e388–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Weiss AR, Hayes-Lattin B, Kutny MA, Stock W, Stegenga K, Freyer DR. Inclusion of Adolescents and Young Adults in Cancer Clinical Trials. Semin Oncol Nurs. 2015;31: 197–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Institute NC. Cancer Trials Support Unit. Available from URL: https://www.ctsu.org/Public/Default.aspx?ReturnUrl=%2f [accessed June 21, 2019, 2019].
- 41.Joseph G, Dohan D. Recruiting minorities where they receive care: Institutional barriers to cancer clinical trials recruitment in a safety-net hospital. Contemp Clin Trials. 2009;30: 552–559. [DOI] [PubMed] [Google Scholar]
- 42.Harlan LC, Lynch CF, Keegan TH, et al. Recruitment and follow-up of adolescent and young adult cancer survivors: the AYA HOPE Study. J Cancer Surviv. 2011;5: 305–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rahman S, Majumder MA, Shaban SF, et al. Physician participation in clinical research and trials: issues and approaches. Adv Med Educ Pract. 2011;2: 85–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chen DT, Miller FG, Rosenstein DL. Clinical research and the physician-patient relationship. Annals of Internal Medicine. 2003;138: 669–672. [DOI] [PubMed] [Google Scholar]
- 45.Holmes DR, Major J, Lyonga DE, Alleyne RS, Clayton SM. Increasing minority patient participation in cancer clinical trials using oncology nurse navigation. Am J Surg. 2012;203: 415–422. [DOI] [PubMed] [Google Scholar]
- 46.Wujcik D, Wolff SN. Recruitment of African Americans to National Oncology Clinical Trials through a clinical trial shared resource. J Health Care Poor Underserved. 2010;21: 38–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bernard-Davila B, Aycinena AC, Richardson J, et al. Barriers and facilitators to recruitment to a culturally-based dietary intervention among urban Hispanic breast cancer survivors. J Racial Ethn Health Disparities. 2015;2: 244–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Guadagnolo BA, Petereit DG, Helbig P, et al. Involving American Indians and medically underserved rural populations in cancer clinical trials. Clin Trials. 2009;6: 610–617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Javid SH, Unger JM, Gralow JR, et al. A prospective analysis of the influence of older age on physician and patient decision-making when considering enrollment in breast cancer clinical trials (SWOG S0316). Oncologist. 2012;17: 1180–1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Institute NC. NCI Community Oncology Research Program. Available from URL: https://ncorp.cancer.gov/ [accessed June 21, 2019, 2019].
- 51.Christie D, Viner R. ABC of adolescence: adolescent development. BMJ. 2005;330: 301–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.