Abstract
Background:
Patients often use complementary and alternative herbal medicines; hence potential exists for adverse herb-drug interactions. Fentanyl is metabolized by hepatic CYP3A4 and considered transported by blood-brain barrier P-glycoprotein. Both disposition processes could be upregulated by the herbal St. John’s wort. This investigation evaluated effects of St. John’s wort on fixed-dose and apparent steady-state intravenous fentanyl pharmacokinetics, pharmacodynamics and clinical effects.
Methods:
Healthy volunteers received a fentanyl fixed-dose infusion and an individually-tailored target controlled infusion on separate days, before and after 30-day St. John’s wort (300 mg thrice daily, n=8) or placebo control (n=8) in a randomized parallel-group design. Fentanyl plasma concentrations, pupil diameter, analgesic response to experimental pain (cold pressor), subjective side effects, and cognitive effects were measured. Plasma fentanyl concentrations and changes in pupil diameter were subjected to pharmacokinetic-pharmacodynamic modeling.
Results:
St. John’s wort did not alter fentanyl pharmacokinetics. Clearance (L/min) before and after St. John’s wort (1.13±0.29 and 1.24±0.26) or placebo (0.96±0.28 and 1.12±0.27) were not different. St. John’s wort also did not affect fentanyl pharmacodynamics as measured by pupil constriction after fixed-dose and tailored fentanyl infusions. EC50 (ng/mL) was 1.1±0.7 and 1.4±0.9 before and after St. John’s wort versus 1.2±0.8 and 1.4±1.7 before and after placebo. Effect site equilibration time, T½,ke0 (min), was 12.8±5.3 and 11.3±6.4 before and after St. John’s wort versus 11.4±6.4 and 11.1±5.6 before and after placebo. St. John’s wort had no influence on analgesia, cognitive performance, or somatic cognitive-affective effects of fentanyl.
Conclusions:
St. John’s wort did not alter fentanyl pharmacokinetics, pharmacodynamics or clinical effects, suggesting no effect on hepatic clearance or blood-brain barrier efflux. Patients taking St. John’s wort will likely not respond differently to intravenous fentanyl for anesthesia or analgesia.
INTRODUCTION
Surveys have found that 22% to 32 % of surgical patients report using herbal supplements.1,2 In addition, patients are often reluctant to disclose the use of herbal medications to their health care providers.2,3 One study found that as many as 70% of patients taking herbals were not forthcoming during preoperative evaluation.2 Pre-surgical use of herbal therapy together with multiple drug use during anesthesia present a high potential for adverse herb-anesthetic interaction. Currently, there is little data regarding the many potential herb-anesthetic interactions.
St. John’s wort has gained popularity as an antidepressant whose clinical effectiveness is supported by randomized, controlled trials in patients with mild to moderate depression.4 However, serious adverse interactions have been observed between St. John’s wort and a number of prescription drugs.5 These interactions involve the up-regulation of two major drug disposition mechanisms: cytochrome P450 enzymes (CYP) and the active efflux transporter P-glycoprotein, which could lead to a lower oral bioavailability, accelerated clearance, and/or altered organ distribution of the affected drugs, and in turn can result in a potential loss of therapeutic efficacy.
Hyperforin, a major bioactive constituent of St. John’s wort, is the most potent known agonist of the Pregnane X Receptor (PXR, NR1I2), which activates the transcription of CYP3A4 and P-glycoprotein.6 Studies have demonstrated induction of CYP3A4 in the human intestine and liver following St. John’s wort treatment.7 St. John’s wort has also been shown to induce the intestinal expression of the active efflux transporter P-glycoprotein.7,8 P-glycoprotein is constitutively expressed in human tissues other than intestinal mucosa, notably the blood-brain barrier.9 P-glycoprotein at the blood-brain barrier functions to restrict drug entry into the brain and has been shown to govern the delivery of a variety of neuropharmacological agents, including opioids.10,11 The role of P-glycoprotein in the distribution kinetics of opioids to the brain depends on the opioid and the competing processes of entry and efflux across the blood-brain barrier.12,13 There is evidence from animal studies that brain P-glycoprotein is inducible.14,15 Fentanyl, one of the most commonly used intraoperative opioids, is a known substrate of CYP3A in vitro and in vivo; it has also been shown to be a P-glycoprotein substrate in vitro.10,16,17 Thus, potential drug interactions may occur between fentanyl and St. John’s wort via multiple mechanisms involving hepatic CPY3A4 and blood-brain barrier P-glycoprotein activity.16,18,19
The overall objective of this study was to test the hypothesis that pretreatment with St. John’s wort, using a formulation and dose that is known to induce hepatic and intestinal CYP3A activity (i.e., increased midazolam clearance after oral and intravenous administration) and increase intestinal P-glycoprotein function, will alter the pharmacokinetics and pharmacodynamics of intravenous (IV) fentanyl with resultant changes in clinical effects as objectively measured and subjectively reported.20 The study was designed to evaluate two potential mechanisms of St. John’s wort-fentanyl interaction. First, that St. John’s wort may increase the systemic clearance of fentanyl through induction of hepatic CYP3A4. Second, that St. John’s wort may attenuate brain uptake of fentanyl via upregulation of P-glycoprotein expression at the blood-brain barrier. Two experimental paradigms were used: first, pharmacokinetic-pharmacodynamic modeling of the time course of pupil constriction (miosis) and arterial plasma fentanyl concentration during and following short (30 min) intravenous fixed-dose fentanyl infusion, before and after St. John’s wort; second, the influence of St. John’s wort on analgesia, miosis and side effects during a pharmacokinetically tailored infusion targeted at a pre-defined steady-state plasma concentration of fentanyl.
METHODS
Subjects and Study Protocol
The protocol was approved by the University of Washington Institutional Review Board and all subjects provided written informed consent. Sixteen healthy subjects, 8 males and 8 females, 21 to 41 years, 72 ± 9 kg (mean ± SD, range 61 to 91 kg) were enrolled in the study, which was conducted before the requirement for clinical trials registration. No subject withdrew from the study, all completed the protocol, and results from all subjects are presented. Sample size was estimated based upon miosis and response to pain stimuli observed before and following placebo treatment in an earlier study from our laboratory that deployed a similar repeated-measures, within-subject, across days design.21 For sample size calculations, a 50% decrement in opioid effect from St John’s wort treatment was sought, and α and β were set at 5% and 80%, respectively. A sample of at least 5 to 6 subjects per treatment group was deemed to provide adequate power.
The study followed a randomized, double-blinded, parallel-arm design. The sequence of fentanyl administration and St. John’s wort or control treatment is detailed in Figure 1. Subjects were randomized to either the St. John’s wort or placebo treatment arm. Each subject underwent two separate days of IV fentanyl testing 1 to 2 weeks apart: 30 min fixed-dose infusion followed by pharmacokinetically-tailored 180 min infusion. Subjects then received either St. John’s wort (one 300 mg Kira tablet, Lichtwer Pharma, Berlin, Germany) or placebo tablet three times a day from Day 1 through Day 21. Kira St John’s wort is the same formulation as the LI-160 product marketed in Germany by Lichtwer Pharma. At the same dose used in this study, LI-160 induced hepatic and intestinal CYP3A and intestinal P-glycoprotein.20 To verify the bioactive content, we assayed each batch of purchased Kira tablets for hypericin and hyperforin.22 During St. John’s wort/placebo treatment, subjects received another round of IV fentanyl testing: 30 min fixed-dose fentanyl infusion on a day between Day 14 and 18, and a pharmacokinetically-tailored 180 min infusion on Day 21.
Figure 1.
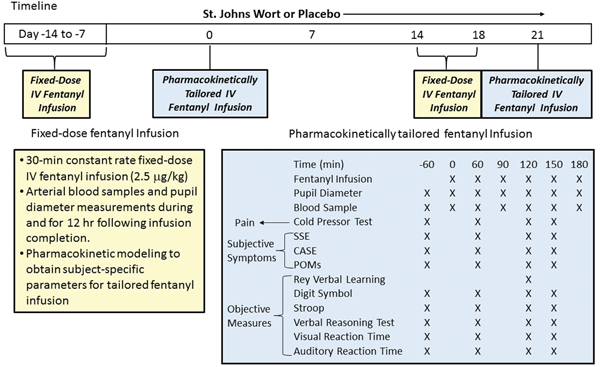
Study Protocol
Fixed-Dose IV Fentanyl Infusion.
For pharmacokinetic-pharmacodynamic modeling of pupil response, subjects received a 30-min constant rate fixed-dose intravenous infusion of fentanyl (2.5 μg/kg). Resting, dark-adapted pupil diameter was measured before, at 5, 10, 15, 30 min during infusion, and at 5, 10, 15, 20, 30, 40, 60, 75, 90, 120, 150 and 180 min and 4, 5, 6 and 12 hours after infusion was discontinued. Blood samples (5 ml each) were collected from an arterial catheter immediately following each pupil measurement. Plasma fentanyl concentration-time data were fitted to a three-compartment model with intravenous infusion input (WinNonlin 5.01, Pharsight Corp, Mountain View, CA).
Pharmacokinetically Tailored IV Fentanyl Infusion.
Effects of St. John’s wort or placebo on fentanyl analgesia, miosis, and side effects were assessed during a pharmacokinetically-tailored intravenous fentanyl infusion. The 3-compartment model parameters estimated from the preceding fixed-dose intravenous fentanyl infusion study were deployed in programming a computer-controlled intravenous infusion regimen based on the Bolus-Elimination-Transfer algorithm to achieve a steady-state plasma fentanyl concentration of 1.0 ng/ml for three hours.23 This target plasma fentanyl concentration is above the reported minimum analgesic concentration of 0.50–0.75 ng/ml.24,25 Blood samples were collected at baseline and at 60, 90, 120, 150, and 180 minutes during tailored infusion. Dark-adapted pupil diameter was measured immediately after blood sampling.
During a 60-min baseline period prior to the start of the fentanyl infusion, pain response to cold-pressor test, subjective symptoms (Somatic Side Effects, Cognitive-Affective Side Effects, Profile of Mood States) and standardized objective measures of cognitive function (Rey Verbal Learning Test, Digit Symbol Substitution Test, Stroop Color and Word Test, Verbal Reasoning Test, Visual Reaction Time, and Auditory Reaction Time) were evaluated. The test battery was repeated thrice, beginning at 60, 120 and 150 min after initiation of the tailored fentanyl infusion. Because the Rey Verbal Learning Test cannot be given more than once during each study day, it was only administered once during the second period of testing (120 min); hence, data from the training day were used as pre-opioid baseline in the statistical analysis.
Assessments of Opioid Effects
Dark-adapted pupil diameter was measured using a Pupilscan II Model 12A infrared pupillometer (Keeler Instruments, Inc., Broomall, PA). The room was kept dark for 5 minutes prior to readings. The left eye was assessed, while the right eye was covered. The average of three successive readings was recorded.
The Cold Pressor Test, a well-accepted experimental pain paradigm known to be responsive to opioids,26 was performed before and during the tailored infusion. The subjects first placed their dominant hand in a bath of warm water (35±2°C) for two min, and then submerged the hand in a container of re-circulating cold water set at 3.5±0.5°C. The subjects were instructed to verbally indicate when they first felt painful sensations (i.e., “pain threshold”). The subjects continued to hold their hand in the water until they could no longer bear the pain (i.e., “pain tolerance”) or a maximum duration of 300 sec was reached. Times to pain threshold and tolerance were recorded.
Subjective side effects were assessed on the tailored infusion day. Subjects rated side effects that could be related to opioids on a 37-item Somatic Side Effects (SSE) Scale and a 44-item Cognitive-Affective Side Effects (CASE) Scale with each item rated from 0 = “not at all” to 4 = “extremely”.27 Internal consistency reliability was strong for both the SSE (α=0.91) and the CASE (α=0.86). Subjects also completed the 30-item Profile of Mood States Short Form (POMS), a widely used, valid and reliable measure of mood disturbance to measure general dysphoric affect.28
Digit Symbol Substitution Test is a well-established tool adapted from the Wechsler Adult Intelligence Scale-Revised and has been used in a number of drug studies, including opioids.29,30 Subjects match abstract symbols with numbers according to a key provided above a long series of numbers. This timed test assesses visual-motor coordination, learning, visual scanning, response speed, and sustained attention.
The Rey Verbal Learning Test was used to assess immediate recall and delayed memory.31 Subjects were presented with 15 words and asked to repeat the words immediately. Twice more, subjects were read the list and asked to repeat the words, for a total of three trials. Next, as an ‘interference’ short delay recall test, a different list of 15 words was read and subjects were asked to recall the second list, followed by a request to recite the first list again. Twenty minutes later subjects were asked to recall the first list of words one last time.
The Verbal Reasoning Test was adapted from the Baddeley Three Minute Reasoning Test.32 Subjects read through each statement, and circled a “true” or “false” depending on whether the letter pair following each statement, accurately described the preceding statement; an example of items would be “A does not precede B: BA.” This test demonstrates adequate reliability and validity, and is a sensitive measure of verbal reasoning.
The Stroop Color and Word Test is a widely used test of cognitive flexibility and specifically the ability to inhibit cognitive interference.33 In each of 3 conditions, total reading time and errors were recorded, with reading time reported here. This test has been shown to be reliable and valid in detecting attentional deficits following numerous types of brain insult and is sensitive to cognitive changes.34
Psychomotor function was tested with two measures, simple Auditory and Visual Reaction Time. The tests are part of a computerized psychomotor battery developed for use in evaluating effects of psychoactive drugs.32 The battery has been used to assess reaction time and attention in numerous opioid and non-opioid laboratory trials.32,35 The simple reaction time tests require a subject to hit a computer key as quickly as possible after seeing or hearing a cue on the computer.
Pharmacokinetic and Pharmacodynamic Analysis
Compartmental Analysis.
A semi-logarithmic plot of the fixed-dose plasma fentanyl concentration-time data revealed a tri-exponential washout profile. Two and three-compartment models featuring elimination from central compartment were fit to plasma concentration data during and after the fixed-dose infusion from each subject using the WinNonlin program (WinNonlin 5.01, Model 19, Pharsight Corp, Mountain View, CA). The three-compartment model provided a statistically superior fit based on residual analysis and Akaike’s Information Criterion (AIC).
Pharmacokinetic-Pharmacodynamic Modeling.
A plot of the decrease in pupil diameter from pre-fentanyl baseline versus the corresponding plasma fentanyl concentration at successive sampling times demonstrated counter-clockwise hysteresis. This observation is consistent with either an equilibration delay between plasma and the effect site, or contribution of active metabolite(s) to the pupil response. The latter scenario is unlikely, since the main metabolite of fentanyl − norfentanyl is devoid of pharmacologic activity.36 An initial estimate of the effect site equilibration constant ke0 for each subject was obtained by analyzing the hysteresis data using the Ke0 program (Department of Anesthesiology, Stanford University).
An inhibitory effect Emax model (Model 103, WinNonlin 5.01, Pharsight Corp, Mountain View, CA) was used to relate the effect site concentration to pupil response as presented in Equation 1
| (1) |
Eo is the baseline pupil diameter. It should be noted that Emax is assumed equal to Eo, i.e., there is complete constriction of the pupil at very high effect-site concentration. ECe,50 is the concentration resulting in pupil constriction to 50% of the maximum. Ce is the effect site concentration at a given time. In the final output from WinNonlin, an estimate of the plasma concentration corresponding to ECe,50 at steady state (i.e., EC50) is provided and reported here.
Analytical Methods
Plasma samples were analyzed for the concentration of fentanyl using previously published methods. Sample extraction was performed according to the procedure described by Szeitz et al.37 using fentanyl-d5 (Cerrillant, Austin, TX) as the internal standard. Analysis of extracts by liquid chromatography-mass spectrometry followed a modified method of Koch et al.38 Limit of detection and limit of quantification were 0.05 ng/mL and 0.0625 ng/mL respectively. Calibration curves were linear up to a concentration of 10 ng/mL. Inter-day coefficient of variation for low (0.2 ng/mL) and high (1.5 ng/mL) quality controls were 6.2% and 3.8%, respectively.
Statistical Analysis
Generalized estimating equations (GEE) (GENMOD, SAS software version 9.0, SAS Institute Inc., Cary NC) were used to assess the effect of treatment (St. John’s wort or placebo), day (pre- or post-treatment), and time (testing at baseline or during fentanyl infusion).39 Interactions between treatment, day, time and sex (all two-factor, three-factor and four-factor interactions) were included in the model.
Analysis was primarily focused on the treatment-by-day interactions; that is, was there a difference between pre- and post-treatment day that was dependent on whether the subject received St. John’s wort or placebo treatment? Furthermore, for each of the pharmacodynamic measures, it was also important to assess whether time was a significant variable. Specifically, was there a difference between measures taken at baseline and during fentanyl infusion, i.e., was an opioid effect evident? If time was not a significant factor, any significant day-by-treatment interactions would not be pertinent as they would not be related to a measurable opioid effect.
In several cases where generalized estimating equations (GEE) would not specifically address changes during tailored fentanyl infusions, within-day comparisons were evaluated by repeated-measure analysis of variance (ANOVA).
RESULTS
Fixed-Dose IV Fentanyl Infusion
Systemic Pharmacokinetics.
Figure 2 shows the time course of mean arterial plasma fentanyl concentration on the pre- and post-treatment days. The washout of fentanyl followed a triexponential pattern, with average initial distribution half-lives of 2.4 min and 21 min and a terminal half-life of approximately 200 min. The plasma concentration-time profiles from the four study days are nearly superimposable; St. John’s wort did not appear to have altered the systemic disposition of fentanyl. Table 1 shows pharmacokinetic parameters before and after St. John’s wort and placebo. The parameters agree with previously published data for sampling durations of at least 8 hours.40 Analysis by GEE showed that none of the pharmacokinetic parameters had a significant treatment-by-day interaction; i.e., St. John’s wort treatment had no effect on fentanyl systemic pharmacokinetics.
Figure 2.
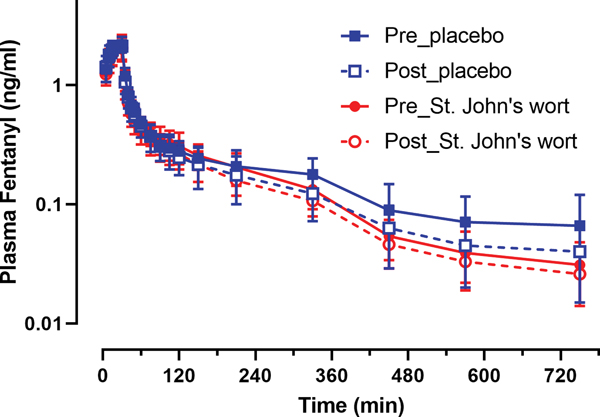
plasma concentration time course of fentanyl in healthy human subjects following a 2.5 μg/kg intravenous infusion of fentanyl on pre- (closed symbol) and post- (open symbol) treatment days in the placebo (blue squares) or St. John’s wort (red circles) treatment groups (n = 8 in each). Results are the mean ± SD.
Table 1.
Pharmacokinetic parameter estimates of fentanyl for a three-compartment model derived from the fixed-dose IV infusion pre and post placebo or St. John’s wort (n = 8 in each group).
| Parameter | Placebo | St. John’s wort | ||
|---|---|---|---|---|
| pre | post | pre | post | |
| V1 (L) | 12.4 ± 4.4 | 12.4 ± 4.0 | 14.0 ± 4.5 | 14.9 ± 5.4 |
| V2 (L) | 16.9 ± 3.6 | 23.7 ± 12.4 | 28.2 ± 24.8 | 25.6 ± 15.3 |
| V3 (L) | 186 ± 30 | 153 ± 55 | 136 ± 47 | 151 ± 44 |
| Vss (L) | 215 ± 28 | 189 ± 45 | 178 ± 46 | 191 ± 50 |
| CL10 (L/min) | 0.96 ± 0.28 | 1.12 ± 0.27 | 1.13 ± 0.29 | 1.23 ± 0.26 |
| CL12 (L/min) | 1.40 ± 0.96 | 1.19 ± 0.31 | 1.27 ± 0.60 | 1.16± 0.55 |
| CL13 (L/min) | 1.48 ± 0.47 | 1.16 ± 0.51 | 1.31 ± 0.45 | 1.35 ± 0.31 |
| k10 (min−1) | 0.083 ± 0.032 | 0.101 ± 0.051 | 0.087 ± 0.028 | 0.092 ± 0.042 |
| k12 (min−1) | 0.144 ± 0.134 | 0.103 ± 0.039 | 0.101 ± 0.059 | 0.086 ± 0.048 |
| k21 (min−1) | 0.085 ± 0.048 | 0.057 ± 0.019 | 0.063 ± 0.030 | 0.058 ± 0.030 |
| k13 (min−1) | 0.138 ± 0.073 | 0.104 ± 0.064 | 0.106 ± 0.050 | 0.099 ± 0.039 |
| k31 (min−1) | 0.0080 ± 0.0023 | 0.0078 ± 0.0021 | 0.0098 ± 0.0032 | 0.0092 ± 0.0017 |
| T½,α (min) | 2.3 ± 1.3 | 2.3 ± 0.9 | 2.5 ± 1.1 | 2.5 ± 0.7 |
| T½,β (min) | 16.5 ± 6.9 | 21.2 ± 8.6 | 24.3 ± 20 | 21.5 ± 9.5 |
| T½,γ (min) | 258 ± 72 | 212 ± 65 | 181 ± 52 | 178 ± 32 |
| MRT (min) | 250 ± 103 | 183 ± 70 | 165 ± 52 | 158 ± 37 |
Generalized estimating equation analysis did not reveal a statistically significant treatment-by-day interaction for any of the parameter estimates.
Pharmacokinetic-Pharmacodynamic Modeling.
Figure 3A shows pupil diameter verses time during and following the brief fixed-dose IV fentanyl infusion in the two treatment groups. A robust miotic response was observed with an average reduction of 58% in pupil diameter at the peak; peak pupil constriction was observed at 2.5 min after the cessation of IV infusion. St. John’s wort pretreatment did not alter the magnitude or time course of miosis after IV fentanyl infusion. A replot of the change in pupil response (miosis) versus arterial plasma fentanyl concentration (Figure 3B, 3C) revealed a counter-clockwise hysteresis, which is best explained by a delay in the equilibration of fentanyl between plasma and the opioid receptor site mediating the pupillary response. Table 2 presents the mean estimates of the pharmacodynamic model parameters. The effect compartment equilibration half-life of fentanyl averaged 12 min. The predicted steady-state EC50 was 1.3 ng/ml, which agrees with previous steady-state plasma concentration-response data.41 None of the pharmacodynamic parameters differed significantly between the placebo and St. John’s wort groups (GEE analysis revealed no treatment-by-day interaction).
Figure 3.
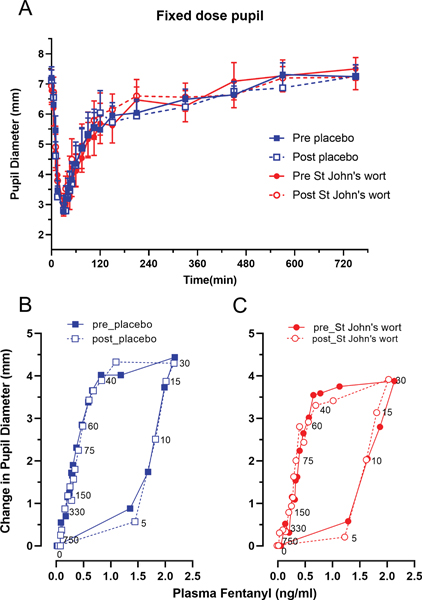
Time course of pupil diameter over 12 hours (3A) in 16 healthy human subjects following a 2.5 μg/kg intravenous infusion of fentanyl and hysteresis plots (3B, 3C) of mean change in pupil diameter versus mean plasma fentanyl concentration in the order of time on the pre- (closed symbol) and post- (open symbol) treatment days in the placebo (blue squares) and St. John’s wort (red circles) groups.
Table 2.
Pharmacodynamic parameter estimates for fentanyl derived from effect site modeling of pupil diameter during and after a fixed-dose IV infusion pre- and post-treatment in the placebo and St. John’s wort group (n = 8 in each). Results are mean± SD.
| Parameter | Placebo | St. John’s wort | ||
|---|---|---|---|---|
| Pre | Post | Pre | Post | |
| Emax (mm) | 7.64 ± 0.86 | 7.49 ± 0.88 | 7.53 ± 1.63 | 7.50 ± 1.29 |
| EC50 (ng/mL) | 1.22 ± 0.83 | 1.16 ± 1.56 | 1.13 ± 0.74 | 1.38 ± 0.89 |
| ke0 (min−1) | 0.080 ± 0.044 | 0.070 ± 0.059 | 0.062± 0.023 | 0.090 ± 0.060 |
| T½,ke0 (min) | 12.9 ± 5.5 | 10.9 ± 6.3 | 11.9 ± 5.8 | 13.0 ± 9.3 |
Generalized estimating equation analysis did not reveal a statistically significant treatment-by-day interaction for any of the parameter estimates.
Individually Tailored Target-Concentration IV Fentanyl Infusion
The pharmacokinetically tailored intravenous fentanyl infusion day was intended to provide a confirmation of a change in steady-state plasma fentanyl concentration-effect relationship, if St. John’s wort treatment were found to have altered fentanyl pharmacokinetics. We also anticipated that in the event of no pharmacokinetic effect, it would still be necessary to investigate any change in the fentanyl concentration-effect relationship since a pharmacodynamic interaction between St. John’s wort and fentanyl at the blood-brain barrier, opioid receptor, and neural circuitry level is still possible.
By tailoring or customizing the bolus-elimination-transfer-based intravenous infusion regimen using each subject’s fentanyl pharmacokinetic parameters obtained from the preceding fixed-dose intravenous infusion study, we could achieve the same target plasma fentanyl concentration in each subject, thereby reducing inter-subject variation. The tailoring approach further allowed us to normalize any effect of St. John’s wort on systemic fentanyl kinetics.
Plasma Fentanyl.
In most subjects, plasma fentanyl concentration reached an apparent steady-state by the first blood sampling time of 60 min after initiation of the tailored infusion; in a few subjects, steady-state was achieved between 60 and 90 min (Figure 4). It is possible that using arterial plasma data to develop the tailored intravenous infusion led to lower than expected venous plasma concentrations at the first sampled time point due to longer than expected arterial-venous equilibration. Repeated-measure ANOVA on pooled data for each treatment-day combination indicated no statistically significant difference in the observed plasma fentanyl concentration across the duration of infusion. The overall average plasma fentanyl achieved during tailored infusion was 0.90 ± 0.18 ng/mL (n = 32 subject-days) as compared to the intended target of 1 ng/mL. The mean prediction error (bias) was −0.10 ng/mL, and the root mean squared prediction error (precision) was 0.20 ng/mL; hence, our target-controlled infusion was fairly accurate and reproducible across subjects.42 Comparable mean steady-state plasma concentrations of fentanyl, ranging between 0.8 and 1.0 ng/mL, were achieved in both the placebo and St. John’s wort groups (GEE analysis showed no treatment-by-day interaction).
Figure 4.
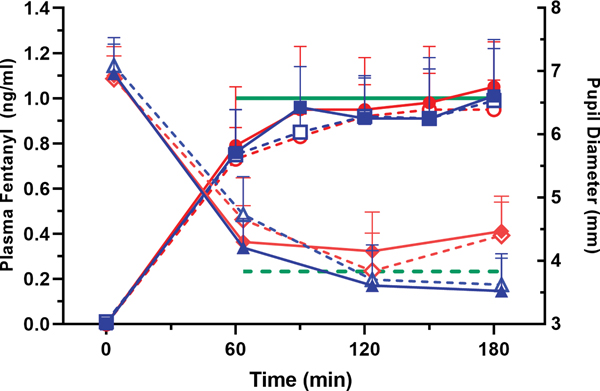
Plasma fentanyl concentrations and pupil diameter during the pharmacokinetically tailored IV infusion in the placebo (blue squares for concentration, blue triangles for pupil diameter) and St. John’s wort (red circles for concentration, red diamonds for pupil diameter) groups. Solid symbols represent St. John’s wort/placebo pre-treatment values and open symbols post-treatment values. Solid green line denotes the target plasma fentanyl at 1 ng/mL, and dashed green line represents the overall predicted pupil diameter based upon the baseline pupil diameter, achieved plasma fentanyl, and EC50 derived from the fixed-dose fentanyl study day for all 32 subject-days. Results are the mean ± SD.
Fentanyl Effects.
Pupil constriction quickly reached a steady-state by 60 min following initiation of the tailored intravenous fentanyl infusion as would be expected given the rather short equilibration half-life observed in the fixed-dose infusion study. A remarkable miotic response was observed during tailored intravenous fentanyl infusion (Figure 4); on average, pupil diameter decreased to about 60% from baseline, which was statistically significant (GEE time variable, p = 0.003). Observed miotic response was compared to response predicted by applying the baseline pupil diameter and average plasma fentanyl level achieved on each tailored infusion day to the pharmacodynamic model using the EC50 estimated on the preceding fixed dose infusion day. The mean prediction error (bias) was −0.41 mm across the 32 subject-days, or the observed miosis was on average 7.5% lower than predicted. The root mean squared prediction error (precision) was 1.05 mm, which reflects the variability in prediction error across subjects and between days. Pupillary response to pharmacokinetically tailored fentanyl infusion was not statistically different between the St. John’s wort and placebo treatments.
Pain tolerance to Cold Pressor Test increased significantly during tailored IV infusion of fentanyl (GEE time variable, p = 0.022) (Figure 5), indicating significant analgesia at the apparent steady-state plasma fentanyl concentration of 0.9 ng/ml. Mean pain tolerance did not vary across the three testing periods for all four treatment-day combinations (repeated-measure ANOVA); analgesic response appeared to equilibrate as rapidly as pupil response. There was no significant difference in response to Cold Pressor Test before and after treatment with either placebo or St. John’s wort (GEE showed no treatment-by-day interaction). Steady-state analgesic effect of fentanyl was not altered by concomitant St. John’s wort therapy.
Figure 5.
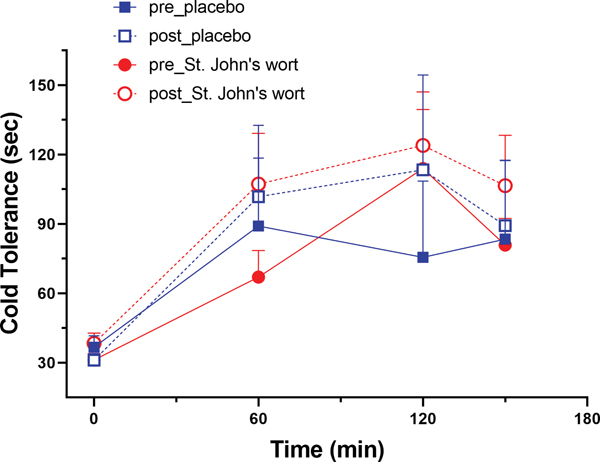
Pain tolerance in the Cold Pressor Test at baseline and the three testing periods during pharmacokinetically tailored IV infusion of fentanyl on pre- (closed symbol) and post- (open symbol) treatment days in the placebo (blue squares) and St. John’s wort (red circles) groups (n = 8 in each). Results are the mean ± SD.
Subjective side effects are shown in Figure 6 for the three side effect scales: The Somatic Side Effects scale for physical symptoms, Cognitive-Affective Side Effects scale for mental effects, and Profile of Mood States Short Form for negative mood state. Subjects reported significant opioid-related effects at the first test period. For all three scales, the mean scores remain relatively stable across the three testing periods. As expected, all three side effect scales showed a significant opioid effect (GEE time variable, p-values ranged from 0.003 to 0.01). The mean scores for the three testing periods did not differ between pre- and post-treatment in both the placebo and St. John’s wort group (GEE showed no treatment-by-day interaction).
Figure 6.
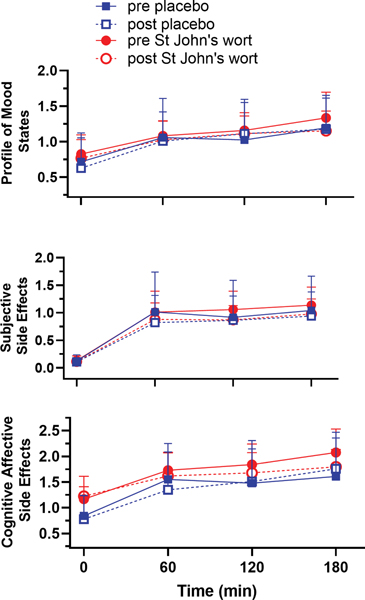
Subjective side effects scales: the Somatic Side Effects (SSE) scale for physical symptoms, Cognitive Affective Side Effects (CASE) scale for mental symptoms, and Profile of Mood States (POMS) for negative mood state at baseline and the three testing periods during pharmacokinetically tailored IV infusion of fentanyl on pre- (closed symbol) and post- (open symbol) treatment days in the placebo ( blue squares) and St. John’s wort (red circles) groups (n = 8 in each). Results are the mean ± SD. All three side effect scales showed a significant opioid effect (SSE p= 0.003, CASE p= 0.01, POMS-SF p=0.005), from baseline to fentanyl infusion with no further changes over time or by pre and post treatment.
Of the six cognitive tests in our battery (Figure 7), only Digit Symbol Substitution Test, Stroop, and Auditory Reaction Time showed a significant change in response to fentanyl with longer response time or fewer items completed on fentanyl (GEE time variable, p-values ranged from 0.0007 to 0.004). Visual Reaction Time, Rey Verbal Learning Test, and Verbal Reasoning Test were not responsive to opioid treatment. The test scores for the three fentanyl-responsive cognitive tests were stable across the three testing periods (repeated-measures ANOVA). Moreover, St. John’s wort treatment did not alter the opioid-related side effects of fentanyl at the apparent steady-state plasma concentration of 0.9 ng/ml for any cognitive tests (GEE showed no treatment-by-day interaction).
Figure 7.
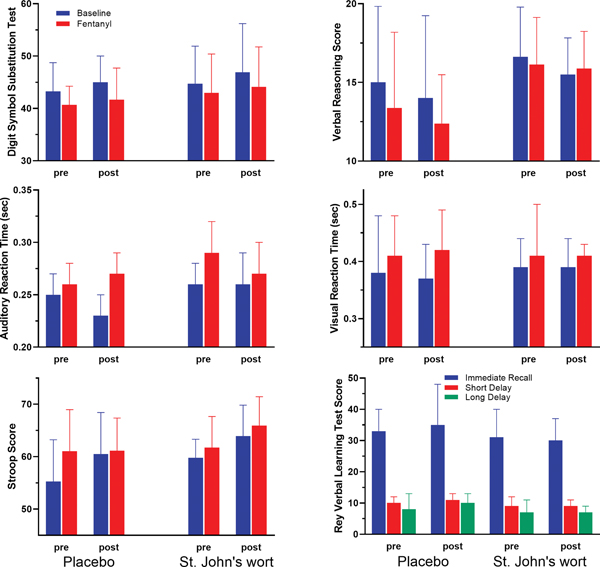
Digit Symbol Substitution Test, Auditory Reaction Time, Stroop, Verbal Reasoning, and Visual Reaction Time prior to fentanyl (blue) and the average score of three test periods after the start of the pharmacokinetically tailored IV fentanyl infusion (red) on pre and post treatment days in the placebo and St. John’s wort groups (n = 8 in each). Rey Verbal Learning test scores are sum of words remembered during 3 trials; immediate (blue), short delay (red) and long delay (green). Pre results are from training day as test may only be given once a day. Verbal learning was not significantly affected by fentanyl. Results are the mean ± SD.
DISCUSSION
The first aim of this study was to determine if St. John’s wort would induce CYP3A-mediated fentanyl metabolism, thereby accelerating plasma clearance. Reported clearance of fentanyl varies over a large range. Results of our analysis are very similar to those previously reported by McClain and Hug; they indicate a high clearance of fentanyl, approaching liver blood flow.40 As such, our findings that the systemic pharmacokinetics of fentanyl are unaffected by St. John’s wort treatment is not entirely unexpected. Traditional pharmacokinetic theory predicts that increase in intrinsic metabolic clearance will have minimal impact on the hepatic clearance of high extraction ratio drugs since it is rate-limited by liver blood flow. In this regard, it is noteworthy that treatment with two other strong CYP inducers — rifampin and carbamazepine have been reported to increase the respective clearance of transmucosal and intravenous fentanyl; a combination of increased intrinsic metabolic clearance and liver blood flow was hypothesized as the underlying mechanism, blood flow being the predominant effect.43,44 We surmise that St. John’s wort treatment most likely resulted only in an increase in intrinsic metabolic clearance of fentanyl and had no effect on liver blood flow. On another note, the lack of change in the systemic disposition fentanyl presents an advantage from our study standpoint; any changes in pharmacodynamics could be attributed to either perturbation in transport of fentanyl across the blood-brain barrier or the central actions of the opioid.
Our second aim was to explore if a regimen of St. John’s wort, known to increase intestinal and hepatic CYP3A and up-regulate intestinal P-glycoprotein expression and function is also capable of up-regulating P-glycoprotein activity at the blood brain barrier and effectively decrease blood-brain barrier permeability of fentanyl, slow the time course of onset of opioid response, accelerate offset of response during washout, and decrease the apparent potency of fentanyl. Opioid-induced miosis was utilized as a convenient reporter of fentanyl access to the brain. We did not detect a change in the magnitude and time course of pupillary response, suggesting that St. John’s wort treatment did not have a measurable effect on the efflux action of P-glycoprotein at the blood-brain barrier. Several explanations can be offered for the apparent lack of an effect on the brain uptake and pharmacodynamics of fentanyl following St. John’s wort treatment.
First, circulating concentrations of the bioactive ingredient hyperforin, which is the principal P-glycoprotein inducer, may not have been high enough to activate Pregnane X Receptor at the blood brain barrier. We used the manufacturer’s recommended dose of 300 mg, extract three times a day, which has previously been shown to increase P-glycoprotein expression and activity at the intestinal mucosa.7 In the only human study that has investigated St. John’s wort’s ability to induce P-glycoprotein in systemic tissues beyond the intestinal mucosa and liver, a St. John’s wort dose (1800 mg/day) twice that of the present study was shown to induce P-glycoprotein expression in circulating lymphocytes.45 The reported in vitro EC50 of hyperforin as an agonist of Pregnane X Receptor is 12 ng/mL.6 Our laboratory previously measured plasma hyperforin concentrations in a group of healthy volunteers (n=17) during the recommended dosing schedule, we observed a steady-state plasma hyperforin concentration of 76.8 ± 29.6 ng/mL (unpublished data). Although total circulating hyperforin concentrations appear sufficient for activation of gene transcription, the more relevant variable is the unbound or free concentration of hyperforin in plasma. Plasma protein binding of hyperforin has not been previously investigated. Preliminary study in our laboratory using equilibrium dialysis indicate that hyperforin is indeed extensively bound to plasma proteins (> 96 % at 1000– 3000 ng/ml of hyperforin). Applying a free fraction of 0.04, would lower the effective circulating concentrations of hyperforin below the reported EC50 for activation of the Pregnane X Receptor. Additionally, there may be differential tissue sensitivity to Pregnane X Receptor activation. An investigation, with another prototypal Pregnane X Receptor transcription activator and CYP3A inducer — rifampin suggest that induction of blood brain barrier P-glycoprotein does not occur readily at dose levels that elicit a robust induction of hepatic CYP3A.46
Second, there is some debate as to whether Pregnane X Receptor is expressed in the human brain capillary endothelium. Early animal studies, as well as two recent human studies, did not detect expression of Pregnane X Receptor in whole brain homogenates.47–49 However, more recent studies looking at brain micro vessels, as well as at the whole brain using more sensitive techniques have demonstrated Pregnane X Receptor gene expression at both the rodent and human blood-brain barrier.48,50
Third, it is possible that there was simultaneous induction and inhibition of P-glycoprotein by St. John’s wort resulting in little or no net change in P-glycoprotein activity at the blood-brain barrier. There are a number of in vitro studies indicating that St. John’s wort can inhibit P-glycoprotein.51,52
Finally, P-glycoprotein may not be a critical determinant of fentanyl uptake at the human blood-brain barrier as suggested by earlier in vitro study. It is possible that, despite P-glycoprotein induction, passive diffusion of fentanyl remains the predominant means by which fentanyl gains passage across the blood-brain barrier in vivo. Using primary cultured bovine brain micro vessel endothelial cell monolayers, Henthorn et al. showed that in addition to being a P-glycoprotein substrate, fentanyl uptake was mediated by an active carrier mediated process. They concluded that in their endothelial cell culture system active P-glycoprotein mediated extrusion of fentanyl was overshadowed by an active inward transport process, mediated by an as of yet unidentified transporter.14 Whether the same situation exists in humans in vivo remains an unknown. If true, it is possible to speculate that this unidentified transporter is also induced by St. John’s wort and negates any effect St. John’s wort has on P-glycoprotein induction.
The third aim of the study was to determine if treatment with St. John’s wort attenuates fentanyl analgesia and side effects. The steady-state intravenous infusion study allowed us to assess whether there is a pharmacodynamic interaction between fentanyl and St. John’s wort at the opioid receptor, the down-stream signaling processes, and actions at the level of neural nociceptive or antinociceptive circuitries. Hyperforin is an inhibitor of monoamine reuptake by synaptosomes; it also activates central benzodiazepine receptors.53,54 The crude extract, as well as, the flavonoid fraction of St. John’s wort extract have been shown to elicit antinociceptive activity in mice.55,56 In fact, the ability of St. John’s wort extract to potentiate the antinociceptive action of morphine in the rat has been reported.57 In our study, St. John’s wort did not appear to have any intrinsic analgesic effect, given our observation that baseline pain tolerance to the Cold Pressor Test did not differ before and after chronic St. John’s wort treatment at standard therapeutic levels.
We elected to study such pharmacodynamic interactions during a pharmacokinetically tailored intravenous fentanyl infusion, which allowed us to directly determine the effect of St. John’s wort on the pharmacodynamics of fentanyl at the same plasma concentration within and between subjects and would presumably minimize the confounding influence of variable systemic pharmacokinetics on the assessments of analgesia and side effects. We did not find any evidence that St. John’s wort interacts with fentanyl at the pharmacodynamic level based on no significant differences in analgesia or subjective reports or objective testing of side effects.
In summary, a two-week treatment with the recommended daily dose of St. John’s wort did not alter intravenous fentanyl pharmacokinetics or pharmacodynamics. Furthermore, the treatment did not affect the rate of effect site equilibration, nor did it affect the steady state plasma concentration-effect relationship of fentanyl. From these results we surmise that there is no significant change in P-glycoprotein function at the blood-brain barrier and brain uptake of fentanyl, nor did we find an effect on hepatic clearance of fentanyl despite a known robust induction of CYP3A enzymes at the chosen therapeutic regimen of St. John’s wort. Moreover, St. John’s wort did not alter the analgesia or side effects of fentanyl during steady-state intravenous fentanyl infusion. Collectively, these results suggest that patients who are chronic users of St. John’s wort will not respond differently to intravenous fentanyl whether used for anesthesia or analgesia.
Acknowledgments
We are grateful to David K. Blough, PhD for his expert assistance in the statistical analysis of the trial data. The skillful assistance of Linda Risler and Brian Phillips in the analysis of plasma fentanyl is also appreciated.
Funding Statement:
This work was supported in part by the National Institutes of Health: the National Center for Complementary and Alternative Medicine (NCCAM) [Grant R01 AT00864], and the National Center for Research Resources [Grant M01-RR00037]
Footnotes
Conflicts of Interest:
The authors declare no competing interests.
Contributor Information
Michael J. Loughren, Department of Anesthesia and Operative Services, Madigan Army Medical Center, Tacoma, Washington; Department of Pharmaceutics, School of Pharmacy, University of Washington, Seattle, Washington.
Evan D. Kharasch, Department of Anesthesiology, Duke University School of Medicine, Durham, North Carolina.
Megan C. Kelton-Rehkopf, Clinical Research Division, Fred Hutchinson Cancer Research Center, Seattle, Washington.
Karen L Syrjala, Clinical Research Division, Fred Hutchinson Cancer Research Center, Seattle, Washington.
Danny D Shen, Department of Pharmaceutics, School of Pharmacy, University of Washington, Seattle, Washington; Clinical Research Division, Fred Hutchinson Cancer Research Center, Seattle, Washington.
REFERENCES
- 1.Tsen LC, Segal S, Pothier M and Bader AM; Alternative medicine use in presurgical patients. Anesthesiology 2000; 93:148–151. [DOI] [PubMed] [Google Scholar]
- 2.Kaye AD, Clarke RC, Sabar R, Vig S, Dhawan KP, Hofbauer R and Kaye AM; Herbal medicines: current trends in anesthesiology practice--a hospital survey. J Clin Anesth 2000; 12:468–471. [DOI] [PubMed] [Google Scholar]
- 3.Eisenberg DM, Davis RB, Ettner SL, Appel S, Wilkey S, Van Rompay M and Kessler RC; Trends in alternative medicine use in the United States 1990–1997 results of a follow-up national survey. Jama 1998; 280:1569–1575. [DOI] [PubMed] [Google Scholar]
- 4.Linde K and Mulrow CD; St John’s wort for depression. Cochrane Database Syst 2000; Rev:CD000448. [DOI] [PubMed] [Google Scholar]
- 5.Zhou S, Chan E, Pan SQ, Huang M and Lee EJ; Pharmacokinetic interactions of drugs with St John’s wort. J Psychopharmacol 2004; 18:262–276. [DOI] [PubMed] [Google Scholar]
- 6.Moore LB, Goodwin B, Jones SA, Wisely GB, Serabjit-Singh CJ, Willson TM, Collins JL and Kliewer SA; St. John’s wort induces hepatic drug metabolism through activation of the pregnane X receptor. Proc Natl Acad Sci U S A 2000; 97:7500–7502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Durr D, Stieger B, Kullak-Ublick GA, Rentsch KM, Steinert HC, Meier PJ and Fattinger K; St John’s Wort induces intestinal P-glycoprotein/MDR1 and intestinal and hepatic CYP3A4. Clin Pharmacol Ther 2000; 68:598–604. [DOI] [PubMed] [Google Scholar]
- 8.Gurley BJ, Swain A, Williams DK, Barone G and Battu SK; Gauging the clinical significance of P-glycoprotein-mediated herb-drug interactions: Comparative effects of St. John’s wort, Echinacea, clarithromycin, and rifampin on digoxin pharmacokinetics. Mol Nutr Food Res 2008; 52:772–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fromm MF; Importance of P-glycoprotein at blood-tissue barriers. Trends Pharmacol Sci 2004; 25:423–429. [DOI] [PubMed] [Google Scholar]
- 10.Henthorn TK, Liu Y, Mahapatro M and Ng KY; Active transport of fentanyl by the blood-brain barrier. J Pharmacol Exp Ther 1999; 289:1084–1089. [PubMed] [Google Scholar]
- 11.Thompson SJ, Koszdin K and Bernards CM; Opiate-induced analgesia is increased and prolonged in mice lacking P-glycoprotein. Anesthesiology 2000; 92:1392–1399. [DOI] [PubMed] [Google Scholar]
- 12.Meissner K, Avram MJ, Yermolenka V, Francis AM, Blood J, Kharasch ED: Cyclosporine-inhibitable blood-brain barrier drug transport influences clinical morphine pharmacodynamics. Anesthesiology 2013;119:941–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Meissner K, Blood J, Francis AM, Yermolenka V, Kharasch ED: Cyclosporine-inhibitable cerebral drug transport does not influence clinical methadone pharmacodynamics. Anesthesiology 2014;121:1281–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bauer B, Yang X, Hartz AM, Olson ER, Zhao R, Kalvass JC, Pollack GMand Miller DS; In vivo activation of human pregnane X receptor tightens the blood-brain barrier to methadone through P-glycoproteinup-regulation. Mol Pharmacol 2006; 70:1212–1219. [DOI] [PubMed] [Google Scholar]
- 15.Ott M, Fricker G, Bauer B.; Pregnane X receptor (PXR) regulates P-glycoprotein at the blood-brain barrier: functional similarities between pig and human PXR. J Pharmacol Exp Ther 2009; 329:141–9. [DOI] [PubMed] [Google Scholar]
- 16.Stanley TH.; The fentanyl story. J Pain 2014; 2–15:1215–26. [DOI] [PubMed] [Google Scholar]
- 17.Labroo RB, Paine MF, Thummel KE, Kharasch ED.; Fentanyl metabolism by human hepatic and intestinal cytochrome P450 3A4: implications for interindividual variability in disposition, efficacy, and drug interactions. Drug Metab Dispos. 1997; 25:1072–80. [PubMed] [Google Scholar]
- 18.Ibrahim AE, Feldman J, Karim A, Kharasch ED.; Simultaneous assessment of drug interactions with low- and high-extraction opioids: application to parecoxib effects on the pharmacokinetics and pharmacodynamics of fentanyl and alfentanil. Anesthesiology 2003; 98(4):853–61. [DOI] [PubMed] [Google Scholar]
- 19.Kharasch ED, Hoffer C, Altuntas TG and Whittington D; Quinidine as a probe for the role of p-glycoprotein in the intestinal absorption and clinical effects of fentanyl. J Clin Pharmacol 2004; 44:224–233. [DOI] [PubMed] [Google Scholar]
- 20.Dresser GK, Schwarz UI, Wilkinson GR, Kim RB; Coordinate induction of both cytochrome P4503A and MDR1 by St. John’s wort in healthy subjects. Clin Pharmacol Ther. 2003; 73:41–50. [DOI] [PubMed] [Google Scholar]
- 21.Erjavec MK1, Coda BA, Nguyen Q, Donaldson G, Risler L, Shen DD; Morphine-fluoxetine interactions in healthy volunteers: analgesia and side effects. J Clin Pharmacol. 2000; 40:1286–95. [PubMed] [Google Scholar]
- 22.Wang Z, Gorski JC, Hamman MA, Huang SM, Lesko LJ and Hall SD; The effects of St John’s wort (Hypericum perforatum) on human cytochrome P450 activity. Clin Pharmacol Ther 2001; 70:317–326. [PubMed] [Google Scholar]
- 23.Schwilden H; A general method for calculating the dosage scheme in linear pharmacokinetics. Eur J Clin Pharmacol 1981; 20:379–386. [DOI] [PubMed] [Google Scholar]
- 24.Gourlay GK, Kowalski SR, Plummer JL, Cousins MJ and Armstrong PJ; Fentanyl blood concentration-analgesic response relationship in the treatment of postoperative pain. Anesth Analg 1988; 67:329–337. [PubMed] [Google Scholar]
- 25.Hill HF, Chapman CR, Saeger LS, Bjurstrom R, Walter MH, Schoene RB and Kippes M; Steady-state infusions of opioids in human. II. Concentration-effect relationships and therapeutic margins. Pain 1990; 43:69–79. [DOI] [PubMed] [Google Scholar]
- 26.Koltzenburg M, Pokorny R, Gasser UE and Richarz U; Differential sensitivity of three experimental pain models in detecting the analgesic effects of transdermal fentanyl and buprenorphine. Pain 2006; 126:165–174. [DOI] [PubMed] [Google Scholar]
- 27.Roth-Roemer S, Coda B, Davies PS, Dikmen S, Cowan J, Risler L, Phillips B, Schroeder T, Shen DD, Syrjala KL. Cognitive and psychomotor side effects of morphine and hydromorphone during sustained equianalgesic infusions. American Pain Society Sixteenth Annual Scientific Meeting Abstracts; 1997:120. [Google Scholar]
- 28.McNair D, Lorr M and Doppleman L; POMS Manual: Profile of Mood State. Educational and Testing Service; 1992. [Google Scholar]
- 29.Hindmarch I; Psychomotor function and psychoactive drugs. Br J Clin Pharmacol 1980; 10:189–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bruera E, Macmillan K, Hanson J and MacDonald RN; The cognitive effects of the administration of narcotic analgesics in patients with cancer pain. Pain 1989; 39:13–16. [DOI] [PubMed] [Google Scholar]
- 31.Shapiro AM, Benedict RH, Schretlen D and Brandt J; Construct and concurrent validity of the Hopkins Verbal Learning Test-revised. Clin Neuropsychol 1999; 13:348–358. [DOI] [PubMed] [Google Scholar]
- 32.Nuotto EJ and Korttila KT; Evaluation of a new computerized psychomotor test battery: effects of alcohol. Pharmacol Toxicol 1991; 68:360–365. [DOI] [PubMed] [Google Scholar]
- 33.Stroop J; Studies of interference in serial verbal reactions. Journal of Experimental Psychology 1935; 643–662. [Google Scholar]
- 34.Stuss DT, Floden D, Alexander MP, Levine B and Katz D; Stroop performance in focal lesion patients: dissociation of processes and frontal lobe lesion location. Neuropsychologia 2001; 39:771–786. [DOI] [PubMed] [Google Scholar]
- 35.Zacny JP, Lichtor JL, Zaragoza JG and de Wit H; Subjective and behavioral responses to intravenous fentanyl in healthy volunteers. Psychopharmacology (Berl) 1992b; 107:319–326. [DOI] [PubMed] [Google Scholar]
- 36.Schneider E and Brune K; Opioid activity and distribution of fentanyl metabolites. Naunyn Schmiedebergs Arch Pharmacol 1986; 334:267–274. [DOI] [PubMed] [Google Scholar]
- 37.Szeitz A, Riggs KW and Harvey-Clark C; Sensitive and selective assay for fentanyl using gas chromatography with mass selective detection. J Chromatogr B Biomed Appl 1996; 675:33–42. [DOI] [PubMed] [Google Scholar]
- 38.Koch DE, Isaza R, Carpenter JW, Hunter RP; Simultaneous extraction and quantitation of fentanyl and norfentanyl from primate plasma with LC/MS detection. J Pharm Biomed Anal. 2004; 34:577–84. [DOI] [PubMed] [Google Scholar]
- 39.Diggle PJ, Liang KY and Zeger SL; Analysis of Longitudinal Data. Clarendon Press; 1994. [Google Scholar]
- 40.McClain DA and Hug CC Jr.,; Intravenous fentanyl kinetics. Clin Pharmacol Ther 1980; 28:106–114. [DOI] [PubMed] [Google Scholar]
- 41.Hill HF, Chapman CR, Saeger LS, Bjurstrom R, Walter MH, Schoene RB and Kippes M; Steady-state infusions of opioids in human. II. Concentration-effect relationships and therapeutic margins. Pain 1990; 43:69–79. [DOI] [PubMed] [Google Scholar]
- 42.Sheiner LB, Beal SL; Some suggestions for measuring predictive performance. J Pharmacokinet Biopharm. 1981; 9:503–12. [DOI] [PubMed] [Google Scholar]
- 43.Kharasch ED, Whittington D, Hoffer C; Influence of hepatic and intestinal cytochrome P4503A activity on the acute disposition and effects of oral transmucosal fentanyl citrate. Anesthesiology 2004; 101:729–37. [DOI] [PubMed] [Google Scholar]
- 44.Nozari A, Akeju O, Mirzakhani H, Eskandar E, Ma Z, Hossain MA, Wang Q, Greenblatt DJ, Martyn JAJ; Prolonged therapy with the anticonvulsant carbamazepine leads to increased plasma clearance of fentanyl. J Pharm Pharmacol. 2019; 71:982–987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hennessy M, Kelleher D, Spiers JP, Barry M, Kavanagh P, Back D, Mulcahy F and Feely J; St John’s wort increases expression of P-glycoprotein: implications for drug interactions. Br J Clin Pharmacol 2002; 53:75–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Liu L, Collier AC1, Link JM, Domino KB, Mankoff DA, Eary JF, Spiekerman CF, Hsiao P, Deo AK, Unadkat JD; Modulation of P-glycoprotein at the Human Blood-Brain Barrier by Quinidine or Rifampin Treatment: A Positron Emission Tomography Imaging Study. Drug Metab Dispos. 2015; 43:1795–804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang H, LeCulyse E, Liu L, Hu M, Matoney L, Zhu W and Yan B; Rat pregnane X receptor: molecular cloning, tissue distribution, and xenobiotic regulation. Arch Biochem Biophys 1999; 368:14–22 [DOI] [PubMed] [Google Scholar]
- 48.Lamba V, Yasuda K, Lamba JK, Assem M, Davila J, Strom S and Schuetz EG; PXR (NR1I2): splice variants in human tissues, including brain, and identification of neurosteroids and nicotine as PXR activators. Toxicol Appl Pharmacol 2004; 199:251–265. [DOI] [PubMed] [Google Scholar]
- 49.Miki Y, Suzuki T, Tazawa C, Blumberg B and Sasano H; Steroid and xenobiotic receptor (SXR), cytochrome P450 3A4 and multidrug resistance gene 1 in human adult and fetal tissues. Mol Cell Endocrinol 2005; 231:75–85. [DOI] [PubMed] [Google Scholar]
- 50.Bauer B, Hartz AM, Fricker G and Miller DS; Pregnane X receptor up-regulation of P-glycoproteinexpression and transport function at the blood-brain barrier. Mol Pharmacol 2004; 66:413–419. [DOI] [PubMed] [Google Scholar]
- 51.Tian R, Koyabu N, Morimoto S, Shoyama Y, Ohtani H and Sawada Y; Functional induction and de-induction of P-glycoproteinby St. John’s wort and its ingredients in a human colon adenocarcinoma cell line. Drug Metab Dispos 2005; 33:547–554. [DOI] [PubMed] [Google Scholar]
- 52.Ott M, Huls M, Cornelius MG, Fricker G.; St. John’s Wort constituents modulate P-glycoproteintransport activity at the blood-brain barrier. Pharm Res. 2010; 27(5):811–22. [DOI] [PubMed] [Google Scholar]
- 53.Wonnemann M, Singer A and Muller WE; Inhibition of synaptosomal uptake of 3H-L-glutamate and 3H-GABA by hyperforin, a major constituent of St. John’s Wort: the role of amiloride sensitive sodium conductive pathways. Neuropsychopharmacology 2000; 23:188–197. [DOI] [PubMed] [Google Scholar]
- 54.Zanoli P, Truzzi C and Cannazza G; Evidence that hypericum perforatum extracts exert anxiolytic effects in rats. Fitoterapia 1998; 69:30. [Google Scholar]
- 55.Ozturk Y, Aydin S and Beis R; Effects of Hypericum perforatum L. and Hypericum caycinum L. extracts on the central nervous system in mice. Phytomedicine 1996; 3:139–146. [DOI] [PubMed] [Google Scholar]
- 56.Vasilchenko E; Analgesic action of flavenoids of Rhododendron luteum Sweet, Hypericum perforatum L, Lespedeza bicolor Turcz. and L. hedysaroides (Pall). Kitsag. Rastit. Resur. 1986; 22:12–21. [Google Scholar]
- 57.Uchida S, Hirai K, Hatanaka J, Hanato J, Umegaki K and Yamada S; Antinociceptive effects of St. John’s wort, Harpagophytum procumbens extract and Grape seed proanthocyanidins extract in mice. Biol Pharm Bull 2008; 31:240–245. [DOI] [PubMed] [Google Scholar]


