Abstract
Background
Intra-abdominal hypertension (IAH) is associated with high morbidity and mortality. IAH leads to intra-abdominal tissue damage and causes dysfunction in distal organs such as the brain. The effect of a combined injury due to IAH and traumatic brain injury (TBI) on the integrity of the blood–brain barrier (BBB) has not been investigated.
Material/Methods
Intracranial pressure (ICP) monitoring, brain water content, EB permeability detection, immunofluorescence staining, real-time PCR, and Western blot analysis were used to examine the effects of IAH and TBI on the BBB in rats, and to characterize the protective effects of basic fibroblast growth factor (bFGF) on combined injury-induced BBB damage.
Results
Combined injury from IAH and TBI to the BBB resulted in brain edema and increased intracranial pressure. The effects of bFGF on alleviating the rat BBB injuries were determined, indicating that bFGF regulated the expression levels of the tight junction (TJ), adhesion junction (AJ), matrix metalloproteinase (MMP), and IL-1β, as well as reduced BBB permeability, brain edema, and intracranial pressure. Moreover, the FGFR1 antagonist PD 173074 and the ERK antagonist PD 98059 decreased the protective effects of bFGF.
Conclusions
bFGF effectively protected the BBB from damage caused by combined injury from IAH and TBI, and binding of FGFR1 and activation of the ERK signaling pathway was involved in these effects.
MeSH Keywords: Blood–Brain Barrier; Brain Injuries; Intra-Abdominal Hypertension; Receptor, Fibroblast Growth Factor, Type 1
Background
Intra-abdominal hypertension (IAH) refers to continuous or repeated intra-abdominal pressure (IAP) ≥12 mmHg [1]. IAH often occurs in trauma patients with severe abdominal damage and can also occur in patients with significant edema of the abdominal organs after a large amount of fluid resuscitation. IAH has been shown to have adverse effects on many organs, such as the brain, heart, lungs, kidneys, and gastrointestinal tract [2]. Due to the complex relationship between the abdomen and brain, treatment for pathological changes of a single organ will have a profound impact on distant organs, especially in multiple-trauma patients with IAH and traumatic brain injury (TBI).
Increased acute IAP leads to increased intracranial pressure (ICP) and decreased cerebral perfusion pressure (CPP); an acute increase in IAP during laparoscopic surgery can even lead to increased ICP [3,4]. However, the specific mechanism is unclear and may be due to the transmission of abdominal pressure to the chest cavity, leading to jugular venous return disorder, resulting in congestion of the cerebral venous system and brain swelling [5,6]. During IAH, cytokines such as interleukin-1β (IL-1β) and tumor necrosis factor-α (TNF-α) are released, resulting in blood–brain barrier (BBB) injury, increased capillary permeability in brain, and increased extracellular fluid, causing cerebral white matter edema, and, finally, increased intracranial pressure [7–9].
Previous studies have confirmed that local ischemia after TBI leads to an inflammatory reaction, and vascular endothelial cells automatically adjust to the dysfunction, leading to destruction of BBB permeability and integrity [10–13]. Furthermore, basic fibroblast growth factor (bFGF) can increase the tight protein expression of BBB endothelial cells (ZO-1 and occludin) to maintain vascular integrity, improve BBB function, activate the PI3K-Akt signaling pathway after TBI, reduce RhoA activity, improve neurological deficits, and maintain BBB integrity [14,15].
In clinical practice, refractory intracranial hypertension, when combined with IAH, is a difficult problem for TBI patients. It is currently mainly treated by decompressive craniectomy and open decompression [6,16,17], but the mechanism responsible for increased intracranial pressure caused by IAH combined with TBI has rarely been studied. Our study used an innovative model of IAH combined with TBI to simulate IAH after fluid resuscitation following TBI combined with shock, to observe changes in BBB and to characterize the effect of IAH combined with TBI on intracranial pressure, as well as to explore its underlying mechanism.
Material and Methods
Animals
Male and female Sprague-Dawley (SD) rats, weighing approximately 220–240 g, were purchased from the Animal Center of Daping Hospital. The animals were randomly divided into experimental and control groups. The researchers were blinded to the treatment information. The rats were housed in animal quarters with a 12-h light/dark cycle, and the temperature (22±1°C) and humidity (60–65%) were controlled. The animal procedures were approved by the Institutional Animal Care and Use Committee of the Army Medical University.
Pharmacological treatments
Animals were randomly assigned to the following groups: the sham-operated (sham group, n=6), the IAH+TBI group (combined group, n=6), the combined+bFGF group (n=6), the combined+bFGF+PD173074 group (n=6), and the combined+bFGF+PD98059 group (n=6). Before blood was drawn, rats in the combined+bFGF group were treated with bFGF (10 μg/kg; intraperitoneal (IP) injection; RayBiotech, Peachtree Corners, GA, USA) for 2 min. The rats in the combined+bFGF+PD173074 group were treated with the FGFR1 antagonist, PD173074 (10 mg/kg; IP injection; APExBIO, Houston, TX, USA) 2 min before the administration of bFGF. The rats in the combined+bFGF+PD98059 group were administrated PD98059 (ERK antagonist; 20 mg/kg; IP injection; APExBIO) 2 min before bFGF administration.
The TBI and IAH model
The procedures to induce TBI were performed as previously reported [18,19]. Briefly, after anesthetization with 3% sodium pentobarbital (IP injection, 30 mg/kg), the rats underwent craniotomy with the central position between the bregma and lambdoid sutures, in a stereotaxic frame. Then, the rats were placed in a pneumatic impact device (PSI, Fairfax Station, VA, USA), which was controlled electronically, and then received a unilateral cortical impact. For moderate TBI, the impact depth was set as 2.0 mm, the dwell time was set as 100 ms, and the velocity was set as 3.5 m/s. Body temperature was maintained at 37°C during the entire operation, using a heating pad. After the TBI, the ICP monitor (Codman®; Integra Life Sciences, Princeton, NJ, USA) was inserted into the cortex and fixed in the skin, which was then sutured. All rats were closely monitored after surgery. Sham-operated animals also underwent craniotomy, but without TBI.
A model of secondary IAH was used as previously described [20,21]. The rats were immobilized in the supine position with the forearm in abduction at 90° to the body. The bilateral femoral vein was cannulated using polyethylene tubing for the infusion, and closely monitored (mercury manometer) using inferior vena cava pressure that represented the IAP. The unilateral femoral artery was cannulated for bleeding. Blood samples were taken (0.5 mL/min) within 10 min after the surgery, blood pressure was maintained at 30–40 mmHg for 1.5 h, then the reperfusion was induced by Ringer’s solution (30 mL/h, using an infusion pump). After 5 min, nitrogen was peritoneally injected until the IAP reached 12 mmHg.
Examination of the brain water content (BWC)
The whole brain of the rat was first weighed to assess wet weight, and then it was dried for 3 days at 80°C to assess the dry weight. The BWC was then determined using the following equation: wet/dry ratio=[(wet weight–dry weight)/wet weight]×100%.
BBB permeability detection
To determine the changes in BBB permeability, we used an extravasation method with Evans Blue (EB) [21,22]. Briefly, Evans Blue tetra sodium salt from APExBIO (Houston TX, USA; 2%, 4 mL/kg) was first injected (>2 min time of injection) into the left femoral vein, and then we waited for 60 min to allow the dye to circulate before the animal was sacrificed under anesthesia. After removal, the brains were soaked in 3 mL of 50% trichloroacetic acid, then homogenized with a sonicator. The homogenate was centrifuged at 10 000 rpm for 30 min, then the resulting supernatants were spectrophotometrically quantified at 620 nm to detect Evans Blue dye extravasation.
Immunofluorescence staining
The brain tissue was post-fixed (4% paraformaldehyde for 72 h), dehydrated, embedded in paraffin, and sectioned at 4-μm thickness. The sections were dried at 40°C, followed by deparaffinization and rehydration using a series of ethanol solutions. The hematoxylin and eosin (H&E) staining was performed according to the manufacture’s protocol.
For in vivo protein evaluation, paraffin-embedded sections were deparaffinized and rehydrated as described above. Immunofluorescence staining was performed as previously described [13,23]. The primary antibodies used in the present study were against claudin-5 (1: 200; Genetex, Irvine, CA, USA), ZO-1 (1: 100; Novus Biological, Centennial, CO, USA), occludin (1: 200; Abcam, Cambridge, MA, USA), or β-catenin (1: 100, CST, Danvers, MA, USA), followed by incubation with goat anti-rabbit or goat anti-mouse secondary fluorescein isothiocyanate-labeled antibody (1: 500; ZhongShan Golden Bridge Bio-technology, Beijing, China). Cellular nuclei were counterstained with Hoechst 33258 (Beyotime-Bio, Shanghai, China). The samples were imaged under ×200 magnification with a Zeiss Imager A2 (Zeiss, Jena, Germany).
Isolation of brain microvascular endothelial cells (BMECs)
Brain microvascular endothelial cells were isolated from rat brains using a previously described method [24,25] with some modifications. Briefly, the rats were euthanized by decapitation, and the brains were immediately removed and cleared of meninges and superficial large blood vessels, and then the brain tissues were homogenized. The tissues were digested by papain (10 mL of 2 mg/mL solution) and DNAse (1 mL of 10 mg/ml), and placed in a 37°C CO2 incubator for 70 min. The tissues were collected after filtering with a 74-μm filter. After centrifugation (1000 rpm for 5 min), the pellets isolated from the brain microvascular and endothelial cells were collected and used in subsequent tests.
Western blot assay
The isolated BMECs were extracted with buffer containing 50 mM Tris-HCl, pH 7.4, 150 mM NaCl, 1% Nonidet P-40, and 0.1% SDS supplemented with a cocktail of protease inhibitors (Roche, Basel, Switzerland). Equal amounts of protein were separated on 10% SDS-PAGE followed by transfer to a 0.45-μm polyvinylidene fluoride membrane (Millipore, Burlington, MA, USA). The membranes were blocked with 5% bovine serum albumin (BSA; Sigma-Aldrich, St. Louis, MO, USA) in PBS (pH 7.2) at 4°C overnight, then incubated with primary antibodies (claudin-5, ZO-1, occludin, β-catenin, MMP9, MMP12, IL-1β, or TNF-α). The membranes were then incubated with secondary horseradish peroxidase-conjugated antibodies (ZhongShan Golden Bridge Bio-technology, Beijing, China) for 1 h at 37°C at a 1: 1000–1: 2000 dilution. After repeated washings, the protein expression was visualized using enhanced chemiluminescence (Tannon-5200, Shanghai, China). Quantification was assessed by densitometry with ultraviolet spectrophotometry (TU-1900; Beijing, China).
RT-PCR
Total RNA was extracted from BMECs using TRIzol reagent (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s instructions. After DNase treatment, RNA was reverse transcribed using the ReverTra AceH kit (Toyobo, Osaka, Japan) according to the manufacturer’s instructions. The cDNA was subjected to real-time PCR using an Applied Biosystems 7500 PCR System (Applied Biosystems, Foster City, CA, USA). The primer sequences and expected sizes of the PCR products are listed in Table 1. The expression levels for each gene of interest were normalized to their corresponding β-actin values. The reactions were run in triplicate.
Table 1.
Primers sequences used for RT-PCR in this study.
| Gene | Sense primer | Antisense primer |
|---|---|---|
| ZO-1 | 5′-GAGCGGGCTACCTTATTGAATGTC-3′ | 5′-GGGCTGCTGTGGAGACTGTGTG-3′ |
| Occludin | 5′-TGGCTTCGGAGGTTACGGTTAC-3′ | 5′-CCCGATCTAATGACGCTGGTAAC-3′ |
| Claudin-5 | 5′-GGCGCTTGTGGCSCTCTTTGTTAC-3′ | 5′-CCGCGCCCAGCTCGTACTTC-3′ |
| β-catenin | 5′-CCGTTCGCCTTCATTATGGACTAC-3′ | 5′-CGCCGCTGGGTGTCCTGAT-3′ |
| β-actin | 5′-ACCCCGTGCTGCTGACCGAG-3′ | 5′-TCCCGGCCAGCCAGGTCCA-3′ |
Statistics
The data are reported as the mean±standard deviation (SD). SPSS statistical software for Windows, version 22.0 (SPSS, Chicago, IL, USA) was used for statistical analyses. Differences between the groups were determined by one-way analysis of variance (ANOVA) or Student’s t-test. A value of p<0.05 was considered statistically significant.
Results
IAH combined with TBI leads to increased intracranial pressure and cerebral edema in a rat model, which may be related to the destruction of the BBB
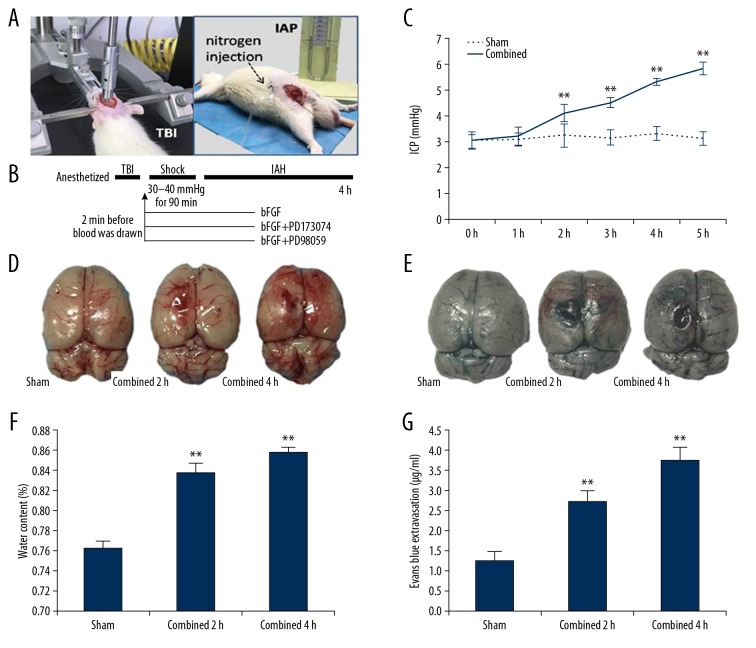
In our study, we constructed an animal model of secondary IAH combined with TBI by the innovative use of hemorrhage reperfusion and a controlled cortical impact technique (Figure 1A, 1B). The intracranial pressure increased after combined injury in rats, increasing significantly at 2 h after injury and exceeding the normal value of intracranial pressure at 4 h, indicating intracranial hypertension (Figure 1C). Further observations showed that a longer animal injury time (4 h) was associated with cerebral cortex subarachnoid hemorrhage, brain contusion, laceration, and cerebral edema (Figure 1D). In the experimental animals, the water content of brain tissues was significantly higher than that of the control group (Figure 1F). The permeability of the brain barrier evaluated by comparing the EB osmolality showed that the BBB permeability of the model animals increased, suggesting that the barrier function was destroyed (Figure 1E, 1G). We hypothesize that this may have disrupted the BBB, causing vasogenic edema and finally increasing the intracranial pressure. After construction of the IAH model, combined with TBI, the animal respiration and heart rates were affected, and most animals died within 6 h.
Figure 1.
Combined injury leads to increased intercranial pressure (ICP) and obvious cerebral edema, which may be related to the destruction of the blood–brain barrier. (A) The combined injury model (the controlled cortical impact technique was used, followed by hemorrhagic shock/resuscitation and then the injection of nitrogen.) (B) Experimental schedule and treatments. (C) ICP of the combined group was higher than that of the sham group. (D) The morphology observation revealed the combined group was associated with brain injury and cerebral edema. (F) The water content of the brain tissues in the combined group was increased compared with that in the sham group. (E, G) Representative effects of Evans Blue extravasation. Quantification of Evans Blue dye extracted from the brain at 2 h and 4 h after injury showed the barrier function was destroyed. ** P<0.01 Combined group vs. Sham group. Data are presented as the mean±SD.
Changes in p-FGFR1 and p-ERK expressions in the brain microvascular endothelial cells of the BBB
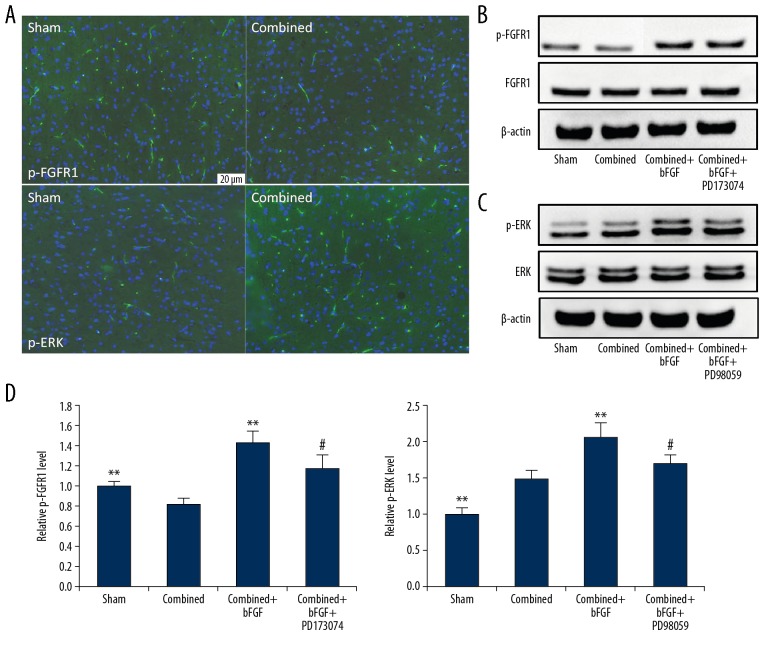
To identify the cause of BBB destruction, we used immunofluorescence staining to show that in the model of IAH combined with TBI, the expression of p-FGFR1 was downregulated and p-ERK was upregulated (Figure 2A). Western blotting of the BMECs extracted from the BBB further indicated decreased expression of p-FGFR1 and increased expression of p-ERK, but the expressions of FGFR1 and ERK proteins were unchanged (Figure 2B, 2C). The expressions of p-FGFR1 and p-ERK were increased by bFGF, and p-FGFR1 and p-ERK expressions were inhibited after the use of the corresponding blockers (Figure 2B–2D).
Figure 2.
Changes in p-FGFR1 and p-ERK expression in the brain microvascular endothelial cells of the blood–brain barrier (BBB). (A) Immunofluorescence staining of p-FGFR1 and p-ERK in the BBB; the expressions were changed after combined injury. (B) Representative image of a Western blot for FGFR1 and p-FGFR1. The expression of p-FGFR1 was affected by agonist (bFGF) and antagonist (PD173074). (C) Representative image of a Western blot for ERK and p-ERK. The expression of p-ERK was affected by agonist (bFGF) and antagonist (PD98059). (D) Quantification of data regarding expression of p-FGFR1 and p-ERK after treatment. ** P<0.01 Sham or combined+bFGF group vs. Combined group. # P<0.05 Combined+bFGF+PD173074 or Combined+bFGF+PD98059 group vs. Combined+bFGF group. Data are presented as the mean±SD.
FGFR1 enhancer bFGF reduces BBB permeability, brain edema, and intracranial pressure
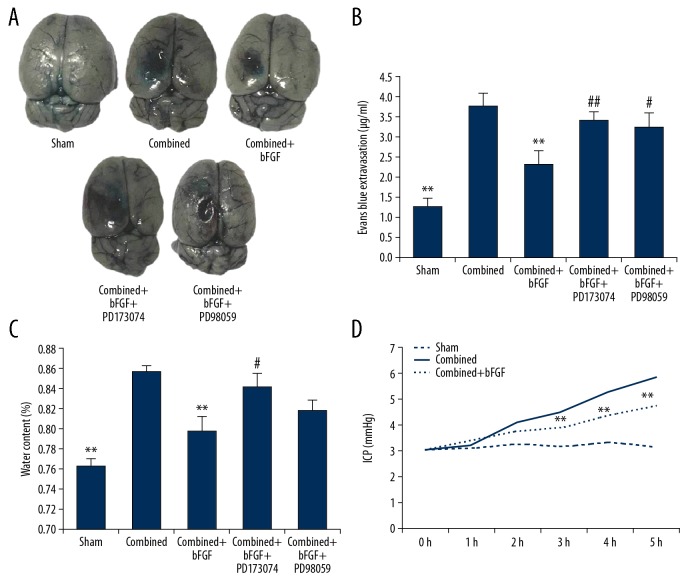
After intraperitoneal injection of the FGFR1 ligand, bFGF, the permeabilities of the EB dye were reduced in the rats. However, after the injection of the FGFR1 and ERK blockers, the protective effect of bFGF was weakened, showing significantly increased EB permeability compared with the combined+bFGF group (Figure 3A, 3B). In a similar manner, monitoring of the brain tissue water content and intracranial pressure indicated that bFGF reduced edema caused by combined injury and reduced intracranial pressure. However, after treatment with the FGFR1 or the ERK blocker, the protective effects of bFGF were significantly weakened (Figure 3C, 3D).
Figure 3.
bFGF reduces blood–brain barrier permeability, brain edema, and intracranial pressure. (A) Exogenous bFGF administration significantly decreased the permeability of Evans Blue dye (EB) 4 h after combined injury, which was reversed by treatment with PD173074 or PD95098. (B) Summary data showing EB extravasation. (C) Treatment with bFGF decreased the brain water content in the combined injury group samples. The protective effect of bFGF was prevented by pretreatment with PD173074 and PD95098, consistent with the EB extravasation results. (D) Treatment with bFGF decreased the intracranial pressure in combined+bFGF group rats. ** P<0.01 Sham or combined+bFGF group vs. Combined group. ## P<0.01, # P<0.05 Combined+bFGF+PD173074 or Combined+bFGF+PD98059 group vs. Combined+bFGF group. Data are presented as the mean±SD.
Immunofluorescence of tissue sections shows that bFGF increased the expression of tight junction (TJ) and adhesion junction (AJ) proteins, while FGFR1 and ERK blockers decreased the protective role of bFGF
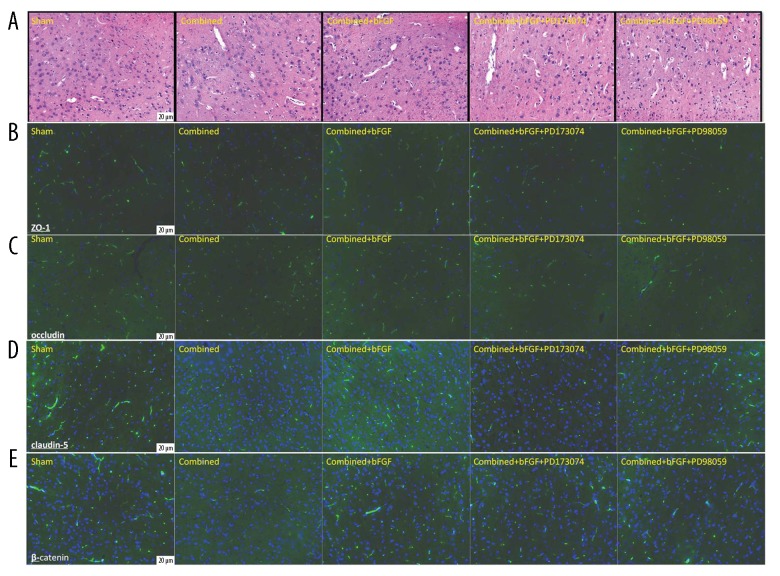
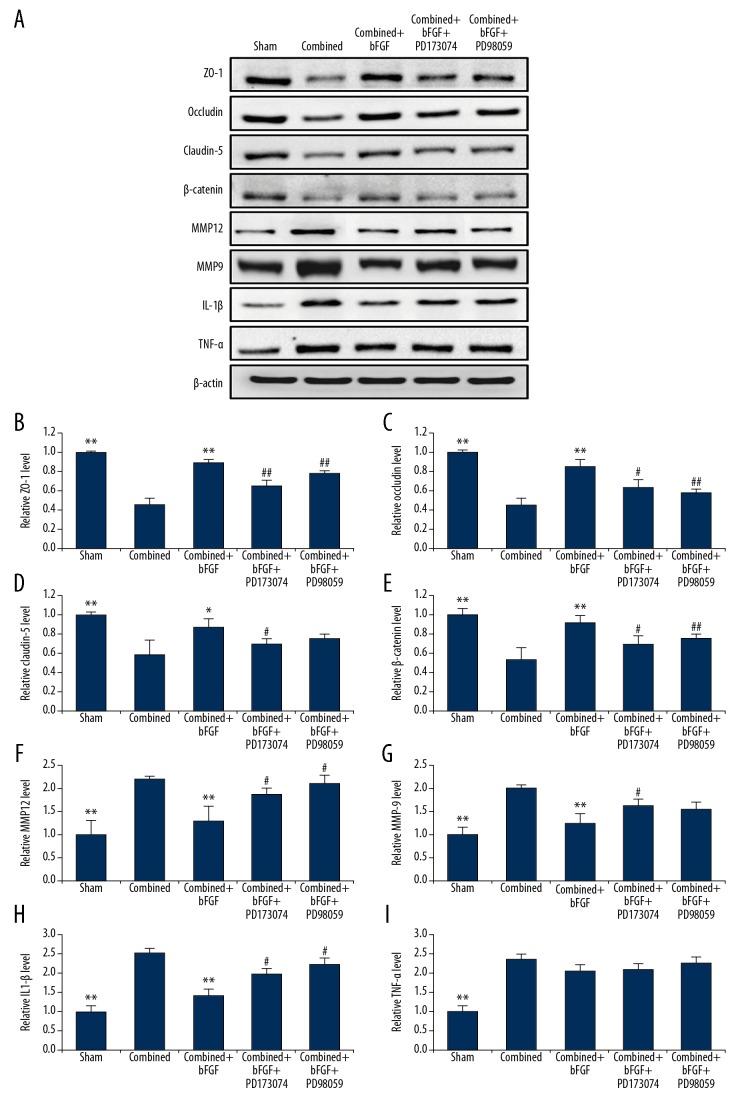
H&E staining of injured brain tissues showed that subarachnoid hemorrhage and cortical contusion were present after combined injury, microvascular endothelial cells were swollen, gaps increased, and edema around nerve cells was present. The above damage was all ameliorated by bFGF treatment, and the use of PD173074 and PD98059 blockers reversed the protective effects of bFGF (Figure 4A). In a similar manner, TJ and AJ protein expressions were observed to reflect the integrity of the BBB; the expression levels of ZO1, occludin, claudin-5, and β-catenin were significantly decreased after combined injury, while bFGF increased the expressions of TJ and AJ proteins. Similarly, the FGFR1 and ERK blockers reversed the protective effect of bFGF (Figure 4B–4E).
Figure 4.
Immunofluorescence shows that bFGF increases the expression of the TJ and AJ proteins. (A) Effects of bFGF on damaged brain tissue after combined injury, visualized with hematoxylin and eosin staining. (B–E) Exogenous bFGF rescues ZO-1, occludin, claudin-5, and β-catenin expressions at 4 h in the rat brain after combined injury. The FGFR1 and ERK blockers reversed the protective effect of bFGF.
Western blotting and PCR data confirmed that bFGF enhanced the expression of TJ and AJ proteins and genes in BMECs, and decreased matrix metalloproteinase (MMP) and IL-1β levels, but had no obvious effect on TNF-α, while FGFR1 and ERK blockers inhibited the protective effect of bFGF
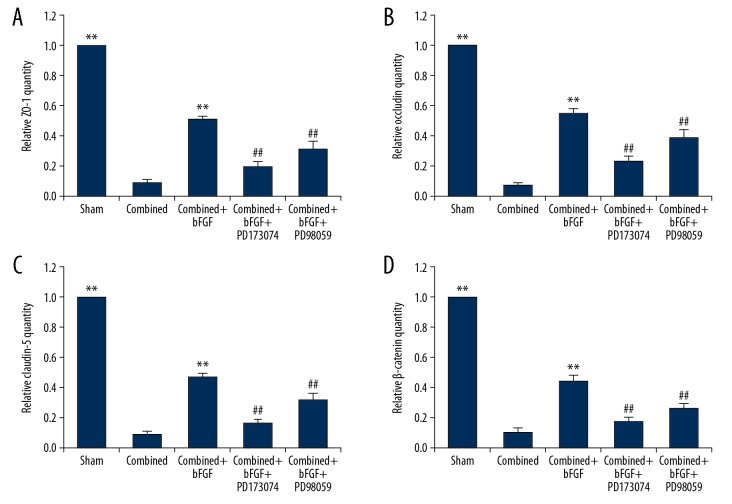
It was possible that bFGF might protect the integrity of the BBB after the combined injury by increased expressions of TJ and AJ proteins. We therefore collected BMECs that constituted the main structure of the BBB, and reconfirmed the decreased expressions of AJ and TJ proteins after combined injury, using Western blotting (Figure 5A–5E). The PCR showed that the AJ and TJ genes had decreased expressions after combined injury, bFGF upregulated the expression of related genes, and PD17073 and PD9058 blockers reduced the gene expressions of TJs and AJs, resulting in the disappearance of the protective effect of bFGF (Figure 6A–6D).
Figure 5.
Western blotting confirmed that bFGF enhances the expression of the TJ and AJ proteins and decreases MMP and IL-1β levels in brain microvascular endothelial cells, and that FGFR1 and ERK blockers inhibit the protective effect of bFGF. (A) Representative Western blots of ZO-1, occludin, claudin-5, β-catenin, MMP12, MMP9, IL-1β and TNF-α. (B–I) Quantification of data regarding the expression of the ZO-1, occludin, claudin-5, β-catenin, MMP12, MMP9, IL-1β, and TNF-α. ** P<0.01,* P<0.05 Sham or combined+bFGF group vs. Combined group. ## P<0.01, # P<0.05 Combined+bFGF+PD173074 or Combined+bFGF+PD98059 group vs. Combined+bFGF group. Data are presented as the mean±SD.
Figure 6.
PCR data confirm that bFGF enhances the expression of the TJ and AJ genes in brain microvascular endothelial cells (BMECs), while FGFR1 and ERK blockers inhibit the protective effect of bFGF. (A–D) Relative expression of genes in BMECs treated with bFGF, PD173074, or PD98059. The expression levels of the ZO-1, occludin, claudin-5, and β-catenin genes were significantly increased in the Combined+bFGF group; however, all the levels were decreased after PD173074 or PD98059 treatment. ** P<0.01 Sham or combined+bFGF group vs. Combined group. ## P<0.01 vs. Combined+bFGF+PD173074 or Combined+bFGF+PD98059 group vs. Combined+bFGF group. Data are presented as the mean±SD.
The level of MMP (MMP9, MMP12) increased after combined injury, the administration of bFGF decreased the levels of MMP protein, and FGFR1 and ERK blockers reversed the protective effect (Figure 5A, 5F, 5G).
The results showed that the inflammatory cytokines IL-1β and TNF-α were significantly increased in the combined group and that the administration of bFGF was able to reduce IL-1β levels, but had no obvious effect on TNF-α. The protective effect of bFGF was prevented by pretreatment with the FGFR1 antagonist PD173074 or the ERK antagonist PD98059 (Figure 5A, 5H, 5I).
Discussion
IAH is a pressure higher than 12 mmHg, and is currently defined as a sustained or recurrent pathological increase in intra-abdominal pressure [1]. IAH usually occurs after abdominal trauma (primary IAH) or a large amount of fluid resuscitation (secondary IAH). Epidemiological studies have shown that the incidence of IAH can exceed 50% in multicenter surgical intensive care units [26]. IAH not only adversely affects the abdominal organs, but also adversely affects distant organs, and the adverse effects are “dose-related,” indicating that the higher the abdominal pressure, the more serious the damage to distant organs. In the abdominal, thoracic, and cranial cavities, the 3 cavities interact with each other, and the increased intra-abdominal pressure causes an increase in intracranial pressure [6,27].
The factors involved in IAH causing ICP elevation are as follows: (1) IAH causes increased intrathoracic pressure, resulting in decreased cerebral venous return, venous congestion, and cerebral edema; increases in intrathoracic pressure lead to decreased venous return, decreases in cardiac output, and decreases in cerebral perfusion pressure, causing cerebral ischemia [28]. (2) Increased intra-abdominal pressure leads to decreased absorption of cerebrospinal fluid in the lumbar cistern and spinal nerve dura mater, resulting in blocked cerebrospinal fluid circulation and increases in intracranial pressure [29]. (3) Cerebral venous congestion leads to high venous pressure and its hemodynamic changes, and inflammatory factor release causes BBB destruction, which can result in cerebral edema, increased ICP, and aggravated neurological dysfunction [6,30].
In animal models and clinical studies, the mechanism responsible for destruction of the BBB after IAH leading to cerebral edema and increased intracranial pressure has been rarely reported. In our study, we constructed a rat model of IAH combined with TBI to evaluate the effect of IAH and TBI combined injury on the BBB. Because of anatomical and physiological differences, the rat model cannot completely simulate the pathophysiological process of human IAH, but it has the characteristics of simple surgery, low cost, and good reproducibility, making it a valuable animal model [20,21]. We used hemorrhagic shock resuscitation and peritoneal nitrogen injection to increase the IAP, so as to simulate secondary IAH due to excess fluid resuscitation, and then added TBI to simulate events occurring in clinical multiple-trauma patients, to identify the mechanism of BBB destruction.
In the present study, the expression of p-FGFR1 was downregulated after combined injury. FGFRs is a family of tyrosine kinase receptors and contains FGFR1 to FGFR4, which allows bFGF to bind. FGFR1-3 are mainly localized to the central nervous system [31]. It was found that FGFR1 could be the key mediator for bFGF signaling [32]. Furthermore, during cortex development in the nervous system, bFGF is important for cell proliferation and neurogenesis, and its gene regulation is in synchronization with FGFR1, which suggests their functional connection [33,34]. We proved that administrated of bFGF increased FGFR1 phosphorylation to protect BBB, and PD173074, the specific inhibitor of FGFR1, can inhibit the protective effect of bFGF in combined injury-induced BBB. The above results suggest FGFR1 plays an important role in bFGF signaling.
Our study results show the important role of the BBB in the pathogenesis of IAH combined with TBI, which could be a potential therapeutic target [35]. We found that the permeability of the BBB was significantly impaired after injury, while the expressions of TJ and AJ proteins in BMECs constituting the BBB were significantly decreased. We confirmed that bFGF reduced the exudation of the BBB and decreased the occurrence of cerebral edema. In this mechanistic study, we also found that bFGF activated endothelial cells to express p-FGFR1 and upregulated microvascular endothelial cells to express specific proteins (ZO-1, claudin-5, occludin, and β-catenin) involved in the early injury stages [36].
MMP expression was previously found to be responsible for increased BBB permeability [37]. An increase in MMP-9 is correlated with the peri-hematoma edema and early neurological deterioration after intracerebral hemorrhage in humans, which suggests that MMP-9 is therefore closely associated with edema formation [38,39]. MMP-12 is a dependable marker of TBI in animal models [40]. Western blotting results showed that the protein expressions of MMP9 and MMP12 were sharply increased in combined-injury BMECs. After the administration of bFGF, the levels of MMP9 and MMP12 were significantly decreased in the combined group, and the EB dye permeability test showed that BBB damage was ameliorated. These results suggest that bFGF protects the integrity of the BBB by affecting the protein levels of AJ, TJ, and MMP.
Previous studies found that the inflammatory cytokines IL-1β and TNF-α were significantly increased in IAH patients and caused further damage to brain tissue [7]. The present results showed that inflammatory cytokines IL-1β and TNF-α were significantly increased in the combined group and that the administration of bFGF was able to reduce IL-1β levels, but had no obvious effect on TNF-α.
Because we found that bFGF played a protective role, in addition to binding to FGFR1, we continued to assess the downstream signaling pathway. Our team previously found permeability and apoptosis of human brain microvascular endothelial cells (HBMECS) challenged by OGD/R was reduced by bFGF by activation of the FGFR1 and the ERK pathway [41]. In a model of traumatic brain injury, Liu et al. showed that sesamin could reduce damage to the BBB by reducing the activity of the ERK pathway, with anti-oxidative and anti-apoptotic effects [42]. Here, we focused on the role of the ERK pathway and found the expression of p-ERK was upregulated after combined injury. The inhibitor PD98059 was pretreated in combination with bFGF rat model, and we found more brain water content and EB extravasation than in animals treated with bFGF. Western blotting results showed that PD98059 treatment prevented the increases of TJ and AJ the decreases of MMP and IL-1β induced by bFGF. Additionally, immunostaining results confirmed the results of Western blotting. Taken together, these results suggest that PD98059 reversed the neuroprotective effect of bFGF. In addition, our in vivo study demonstrated that bFGF ameliorated combined injury-induced BBB destruction via the ERK pathway.
Conclusions
IAH combined with TBI led to increased ICP and cerebral edema, which may be related to the destruction of the BBB. The expression of p-FGFR1 in microvascular endothelial cells that constituted the BBB was altered after combined injury. Administration of bFGF to activate p-FGFR1 significantly reduced the destruction of the BBB. The protective effect of bFGF on the BBB was associated with the levels of AJ, TJ, MMP, and IL-1β. In addition, our in vivo study showed that activation of the downstream signaling pathway component, ERK, was necessary for the protective effect of bFGF on BBB integrity. Therefore, bFGF may provide a therapeutic opportunity for recovery from the combined injury of IAH and TBI.
Acknowledgments
We thank Sixu Chen (Department of Orthopedics, the 906th Hospital of the Chinese People’s Liberation Army, Wenzhou, China) and Hongming Miao (Department of Biochemistry and Molecular Biology, Army Medical University, Chongqing, China) for their help with experiment design.
Footnotes
Source of support: The study was supported by the Foundation Program of Technology of Education Committee of Chongqing (KJQN201800122) and the Program of Technology of Science and Technology Committee of Yuzhong District in Chongqing (20180131)
Ethics approval and consent to participate
This study was performed in accordance with the Declaration of Helsinki and was approved by the Institutional Animal Care and Use Committee of the Army Medical University (SYXK20170002).
Conflicts of interest
None.
References
- 1.Cheatham ML, Malbrain ML, Kirkpatrick A, et al. Results from the International Conference of Experts on Intra-abdominal Hypertension and Abdominal Compartment Syndrome. II. Recommendations. Intensive Care Med. 2007;33(6):951–62. doi: 10.1007/s00134-007-0592-4. [DOI] [PubMed] [Google Scholar]
- 2.Burchard KW. Cardiovascular, pulmonary, and renal effects of massively increased intra-abdominal pressure in critically ill patients. Crit Care Med. 1990;18(1):120. doi: 10.1097/00003246-199001000-00027. [DOI] [PubMed] [Google Scholar]
- 3.Kamine TH, Elmadhun NY, Kasper EM, et al. Abdominal insufflation for laparoscopy increases intracranial and intrathoracic pressure in human subjects. Surg Endosc. 2016;30(9):4029–32. doi: 10.1007/s00464-015-4715-7. [DOI] [PubMed] [Google Scholar]
- 4.Kamine TH, Papavassiliou E, Schneider BE. Effect of abdominal insufflation for laparoscopy on intracranial pressure. JAMA Surg. 2014;149(4):380–82. doi: 10.1001/jamasurg.2013.3024. [DOI] [PubMed] [Google Scholar]
- 5.Deeren DH, Dits H, Malbrain ML. Correlation between intra-abdominal and intracranial pressure in nontraumatic brain injury. Intensive Care Med. 2005;31(11):1577–81. doi: 10.1007/s00134-005-2802-2. [DOI] [PubMed] [Google Scholar]
- 6.Scalea TM, Bochicchio GV, Habashi N, et al. Increased intra-abdominal, intrathoracic, and intracranial pressure after severe brain injury: Multiple compartment syndrome. J Trauma. 2007;62(3):647–56. doi: 10.1097/TA.0b013e31802ee542. discussion 656. [DOI] [PubMed] [Google Scholar]
- 7.Marinis A, Argyra E, Lykoudis P, et al. Ischemia as a possible effect of increased intra-abdominal pressure on central nervous system cytokines, lactate and perfusion pressures. Crit Care. 2010;14(2):R31. doi: 10.1186/cc8908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hamidian Jahromi A, Freeland K, Youssef AM. Intra-abdominal hypertension causes disruption of the blood–brain barrier in mice, which is increased with added severe head trauma. J Trauma Acute Care Surg. 2012;73(5):1175–79. doi: 10.1097/TA.0b013e31825dec35. [DOI] [PubMed] [Google Scholar]
- 9.Youssef AM, Hamidian Jahromi A, Vijay CG, et al. Intra-abdominal hypertension causes reversible blood–brain barrier disruption. J Trauma Acute Care Surg. 2012;72(1):183–88. doi: 10.1097/TA.0b013e31822a3254. [DOI] [PubMed] [Google Scholar]
- 10.Price L, Wilson C, Grant G. Blood–brain barrier pathophysiology following traumatic brain injury. In: Laskowitz D, Grant G, editors. Translational research in traumatic brain injury. Boca Raton (FL): 2016. (Frontiers in Neuroscience). [PubMed] [Google Scholar]
- 11.Readnower RD, Chavko M, Adeeb S, et al. Increase in blood–brain barrier permeability, oxidative stress, and activated microglia in a rat model of blast-induced traumatic brain injury. J Neurosci Res. 2010;88(16):3530–39. doi: 10.1002/jnr.22510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li QX, Shen YX, Ahmad A, et al. Mesencephalic astrocyte-derived neurotrophic factor prevents traumatic brain injury in rats by inhibiting inflammatory activation and protecting the blood–brain barrier. World Neurosurg. 2018;117:e117–29. doi: 10.1016/j.wneu.2018.05.202. [DOI] [PubMed] [Google Scholar]
- 13.Wang ZG, Cheng Y, Yu XC, et al. bFGF protects against blood–brain barrier damage through junction protein regulation via PI3K-Akt-Rac1 pathway following traumatic brain injury. Mol Neurobiol. 2016;53(10):7298–311. doi: 10.1007/s12035-015-9583-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bendfeldt K, Radojevic V, Kapfhammer J, Nitsch C. Basic fibroblast growth factor modulates density of blood vessels and preserves tight junctions in organotypic cortical cultures of mice: A new in vitro model of the blood–brain barrier. J Neurosci. 2007;27(12):3260–67. doi: 10.1523/JNEUROSCI.4033-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Reuss B, Dono R, Unsicker K. Functions of fibroblast growth factor (FGF)-2 and FGF-5 in astroglial differentiation and blood–brain barrier permeability: Evidence from mouse mutants. J Neurosci. 2003;23(16):6404–12. doi: 10.1523/JNEUROSCI.23-16-06404.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Joseph DK, Dutton RP, Aarabi B, Scalea TM. Decompressive laparotomy to treat intractable intracranial hypertension after traumatic brain injury. J Trauma. 2004;57(4):687–93. doi: 10.1097/01.ta.0000140645.84897.f2. discussion 693–95. [DOI] [PubMed] [Google Scholar]
- 17.Dorfman JD, Burns JD, Green DM, et al. Decompressive laparotomy for refractory intracranial hypertension after traumatic brain injury. Neurocrit Care. 2011;15(3):516–18. doi: 10.1007/s12028-011-9549-0. [DOI] [PubMed] [Google Scholar]
- 18.Bai W, Li P, Ning YL, et al. Reduction in blood glutamate levels combined with the genetic inactivation of A2AR significantly alleviate traumatic brain injury-induced acute lung injury. Shock. 2019;51(4):502–10. doi: 10.1097/SHK.0000000000001170. [DOI] [PubMed] [Google Scholar]
- 19.Li W, Dai S, An J, et al. Genetic inactivation of adenosine A2A receptors attenuates acute traumatic brain injury in the mouse cortical impact model. Exp Neurol. 2009;215(1):69–76. doi: 10.1016/j.expneurol.2008.09.012. [DOI] [PubMed] [Google Scholar]
- 20.Chang M, Yu J, Zhang L, et al. A new model for the study of secondary intra-abdominal hypertension in rats. J Surg Res. 2014;187(1):244–51. doi: 10.1016/j.jss.2013.09.038. [DOI] [PubMed] [Google Scholar]
- 21.Liu D, Zhang HG, Zhao ZA, et al. Melanocortin MC4 receptor agonists alleviate brain damage in abdominal compartment syndrome in the rat. Neuropeptides. 2015;49:55–61. doi: 10.1016/j.npep.2014.12.003. [DOI] [PubMed] [Google Scholar]
- 22.Li L, Tao Y, Tang J, et al. A cannabinoid receptor 2 agonist prevents thrombin-induced blood–brain barrier damage via the inhibition of microglial activation and matrix metalloproteinase expression in rats. Transl Stroke Res. 2015;6(6):467–77. doi: 10.1007/s12975-015-0425-7. [DOI] [PubMed] [Google Scholar]
- 23.Chen J, Hu J, Liu H, et al. FGF21 protects the blood–brain barrier by upregulating PPARgamma via FGFR1/beta-klotho after traumatic brain injury. J Neurotrauma. 2018;35(17):2091–103. doi: 10.1089/neu.2017.5271. [DOI] [PubMed] [Google Scholar]
- 24.Rosas-Hernandez H, Cuevas E, Escudero-Lourdes C, et al. Characterization of biaxial stretch as an in vitro model of traumatic brain injury to the blood–brain barrier. Mol Neurobiol. 2018;55(1):258–66. doi: 10.1007/s12035-017-0738-5. [DOI] [PubMed] [Google Scholar]
- 25.Ge X, Li W, Huang S, et al. Increased miR-21-3p in injured brain microvascular endothelial cells following traumatic brain injury aggravates blood–brain barrier damage by promoting cellular apoptosis and inflammation through targeting MAT2B. J Neurotrauma. 2019;36(8):1291–305. doi: 10.1089/neu.2018.5728. [DOI] [PubMed] [Google Scholar]
- 26.Malbrain ML, Chiumello D, Pelosi P, et al. Prevalence of intra-abdominal hypertension in critically ill patients: a multicentre epidemiological study. Intensive Care Med. 2004;30(5):822–29. doi: 10.1007/s00134-004-2169-9. [DOI] [PubMed] [Google Scholar]
- 27.Malbrain ML, Wilmer A. The polycompartment syndrome: Towards an understanding of the interactions between different compartments! Intensive Care Med. 2007;33(11):1869–72. doi: 10.1007/s00134-007-0843-4. [DOI] [PubMed] [Google Scholar]
- 28.De Laet IE, Ravyts M, Vidts W, et al. Current insights in intra-abdominal hypertension and abdominal compartment syndrome: Open the abdomen and keep it open! Langenbeck’s. Arch Surg. 2008;393(6):833–47. doi: 10.1007/s00423-008-0347-x. [DOI] [PubMed] [Google Scholar]
- 29.Halverson AL, Barrett WL, Iglesias AR, et al. Decreased cerebrospinal fluid absorption during abdominal insufflation. Surg Endosc. 1999;13(8):797–800. doi: 10.1007/s004649901102. [DOI] [PubMed] [Google Scholar]
- 30.Halverson A, Buchanan R, Jacobs L, et al. Evaluation of mechanism of increased intracranial pressure with insufflation. Surg Endosc. 1998;12(3):266–69. doi: 10.1007/s004649900648. [DOI] [PubMed] [Google Scholar]
- 31.Ford-Perriss M, Abud H, Murphy M. Fibroblast growth factors in the developing central nervous system. Clin Exp Pharmacol Physiol. 2001;28(7):493–503. doi: 10.1046/j.1440-1681.2001.03477.x. [DOI] [PubMed] [Google Scholar]
- 32.Schlessinger J, Plotnikov AN, Ibrahimi OA, et al. Crystal structure of a ternary FGF-FGFR-heparin complex reveals a dual role for heparin in FGFR binding and dimerization. Mol Cell. 2000;6(3):743–50. doi: 10.1016/s1097-2765(00)00073-3. [DOI] [PubMed] [Google Scholar]
- 33.Raballo R, Rhee J, Lyn-Cook R, et al. Basic fibroblast growth factor (Fgf2) is necessary for cell proliferation and neurogenesis in the developing cerebral cortex. J Neurosci. 2000;20(13):5012–23. doi: 10.1523/JNEUROSCI.20-13-05012.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lin L, Wang Q, Qian K, et al. bFGF protects against oxygen glucose deprivation/reoxygenation-induced endothelial monolayer permeability via S1PR1-dependent mechanisms. Mol Neurobiol. 2018;55(4):3131–42. doi: 10.1007/s12035-017-0544-0. [DOI] [PubMed] [Google Scholar]
- 35.Shlosberg D, Benifla M, Kaufer D, Friedman A. Blood–brain barrier breakdown as a therapeutic target in traumatic brain injury. Nat Rev Neurol. 2010;6(7):393–403. doi: 10.1038/nrneurol.2010.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vajtr D, Benada O, Kukacka J, et al. Correlation of ultrastructural changes of endothelial cells and astrocytes occurring during blood–brain barrier damage after traumatic brain injury with biochemical markers of BBB leakage and inflammatory response. Physiol Res. 2009;58(2):263–68. doi: 10.33549/physiolres.931253. [DOI] [PubMed] [Google Scholar]
- 37.Yang Y, Estrada EY, Thompson JF, et al. Matrix metalloproteinase-mediated disruption of tight junction proteins in cerebral vessels is reversed by synthetic matrix metalloproteinase inhibitor in focal ischemia in rat. J Cereb Blood Flow Metab. 2007;27(4):697–709. doi: 10.1038/sj.jcbfm.9600375. [DOI] [PubMed] [Google Scholar]
- 38.Shigemori Y, Katayama Y, Mori T, et al. Matrix metalloproteinase-9 is associated with blood–brain barrier opening and brain edema formation after cortical contusion in rats. Acta Neurochir Suppl. 2006;96:130–33. doi: 10.1007/3-211-30714-1_29. [DOI] [PubMed] [Google Scholar]
- 39.Wang Z, Meng CJ, Shen XM, et al. Potential contribution of hypoxia-inducible factor-1alpha, aquaporin-4, and matrix metalloproteinase-9 to blood–brain barrier disruption and brain edema after experimental subarachnoid hemorrhage. J Mol Neurosci. 2012;48(1):273–80. doi: 10.1007/s12031-012-9769-6. [DOI] [PubMed] [Google Scholar]
- 40.Power C, Henry S, Del Bigio MR, et al. Intracerebral hemorrhage induces macrophage activation and matrix metalloproteinases. Ann Neurol. 2003;53(6):731–42. doi: 10.1002/ana.10553. [DOI] [PubMed] [Google Scholar]
- 41.Chen P, Zhang H, Zhang Q, et al. Basic fibroblast growth factor reduces permeability and apoptosis of human brain microvascular endothelial cells in response to oxygen and glucose deprivation followed by reoxygenation via the fibroblast growth factor receptor 1 (FGFR1)/ERK pathway. Med Sci Monit. 2019;25:7191–201. doi: 10.12659/MSM.918626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liu YL, Xu ZM, Yang GY, et al. Sesamin alleviates blood–brain barrier disruption in mice with experimental traumatic brain injury. Acta Pharmacol Sin. 2017;38(11):1445–55. doi: 10.1038/aps.2017.103. [DOI] [PMC free article] [PubMed] [Google Scholar]