Abstract
Introduction
Blast-induced mild traumatic brain injury was generated in a mouse model using a shock tube to investigate recovery and axonal injury from single blast.
Methods
A supersonic helium wave hit the head of anesthetized male young adult mice with a reflected pressure of 69 psi for 0.2 ms on Day 1. Subsequently, the mice were cardioperfused on Days 2, 5, or 12. The isolated brains were subjected to diffusion tensor imaging. Reduced fractional anisotropy (FA) indicated axonal injury.
Results
After single blast, FA showed a biphasic response in the corpus callosum with decrease on Days 2 and 12 and increase on Day 5.
Conclusions
Blast-induced mild traumatic brain injury in a mouse model follows a biphasic FA response within 12 days after a single blast similar to that reported for human subjects.
Introduction
Mild traumatic brain injury (TBI) is an important global public health problem. In the United States, approximately 1.5 to 2 million TBIs and 52,000 associated deaths occur each year.1 Approximately 85% of hospital-reported patients suffering from TBI have mild TBI in the civil and military sectors.2,3 Mild TBI is usually referred to as closed-head injury whose clinical severity is correlated with the duration of unconsciousness.4 This injury also has been recognized as “signature injury” of the recent wars in Afghanistan (Operation Enduring Freedom) and Iraq (Operation Iraqi Freedom).5,6 Detonation of improvised explosive devices generate very hot, dense, and high pressure gas waves that differentially damage the brain and many organs of humans and animals. In addition to explosions, falls,7 and motor vehicle crashes,8 impact sport events such as boxing, football, and soccer are receiving increased attention as possible causes for mild TBI.9,10 Initially, the main research effort was focused on axonal injury and chronic traumatic encephalopathy.11 Axonal injury was monitored with diffusion tensor imaging (DTI) providing information on fractional anisotropy (FA) and diffusivity by observing axon-related water mobility.12 In the meantime, the research target field has widened to include cellular changes inside and outside the brain, especially in response to lower pressure-associated events and processes. Integration and interaction of these different processes are still poorly understood.
In the present study, we use a mouse model exposed under anesthesia to a single blast of a high pressure helium wave delivered through a shock tube. The mouse head is hit with a reflected pressure of 69 pounds per square inch (psi). Resulting changes in FA are investigated by ex vivo DTI on Days 2, 5, and 12 after blast.
Methods
Animal Preparation and Blast-Induced Mild TBI Through a Shock Tube
All experiments were approved by the Sanford Burnham Prebys Medical Discovery Institute, Institutional Animal Care and Use Committee. No Institutional Review Board approval was required, because all experiments were conducted with wild-type mice. Male C57BL/6 N mice (7–8 weeks old), single-housed before and after the blast with free access to food and water, were anesthetized with 4% isoflurane. Anesthesia induction in a transparent induction box lasted 3.0 to 3.5 minutes. Subsequent dressing with nylon ballistic coats to protect their lungs was performed within 5 minutes under 2% isoflurane applied by a flexible tube close to their noses. At the same time, their ears were protected with plastic plugs. The mice were fixed to a vertical board with Velcro tape. The head was 1 inch away from the shock tube exit and exposed for 0.2 ms to a supersonic helium wave in a 6 feet long and 2 inches wide aluminum tube (Fig. 1). The total time the mice were exposed to isoflurane anesthesia was approximately 9 minutes. The reflected pressure was 69 psi measured in separate control experiments. A reflected pressure of 69 psi was chosen to assure that the single blast resulted in reproducible axonal injury. During the blast experiments, incident pressures were monitored. The resulting pressure wave was a Friedlander wave with 622 m/s speed at the end of the tube. The incident pressures measured on the inner wall of the shock tube are significantly lower than the reflected pressure. The 6 feet long and 2 inches wide aluminum shock tube (Fig. 1) was designed and physically characterized (Fig. 2) by Dr. Rigby. Recovery from blast and anesthesia was determined by the regained ability of the mice to walk with controlled balance using all four legs on the edges of their cages. No mortality was observed. Controls were pseudo-blasted, treated similarly like blasted mice, but without exposure to the pressure wave.
Figure 1.
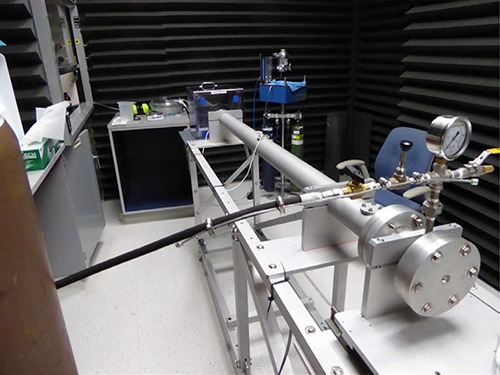
Aluminum shock tube. The tube is 6 feet long, 2 inches wide, contains a helium chamber, 4.5 inches long, serving as compression section, separated by a 5 milli-inches thick mylar diaphragm from a 65 inches long expansion section. The tube is connected with a mouse chamber in which the anesthetized mouse is attached to a vertical board. The lungs are protected by a ballistic nylon coat, the ears by foam plugs. The top of the head is 1 inch away from the tube exit facing the pressure wave. There is a 45° play for head rotation. A supersonic helium wave (622 m/s) travels through the shock tube and hits the mouse head for 0.2 ms.
Figure 2.
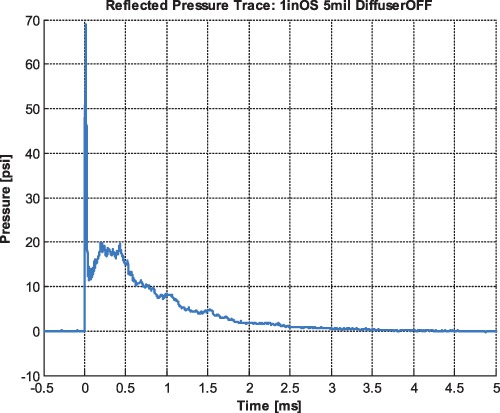
Pressure curve. During the experiment, the incident pressure is monitored with two sensors, one close at the entrance of the pressure wave into the shock tube, the other one close to the exit. The presented figure shows the reflected pressure, which is larger than the incident pressures and cannot be measured during the mouse experiment, but has to be determined in a separate experiment in the absence of the mouse. In the experiments presented here, the reflected pressure was 69 psi (shown), whereas the incident pressures (not shown) were approximately 34 and 27 psi.
Experimental Groups
For the blast experiments, 11 mice were blasted and 6 mice were pseudo-blasted. Details are described in the section ex vivo DTI.
Cardiac Perfusion
Mice were anesthetized with 4% isoflurane and fixed on a slanted board. A 25-gauge needle was inserted in the left ventricle. The right atrium was opened by incision to drain blood. Heparinized saline was flowing through the needle into the left ventricle. Perfusion was continued for 10 to 15 mL. Subsequently, saline was replaced by 4% paraformaldehyde for 10 to 15 mL. The skull was opened, the brain removed and stored in 4% paraformaldehyde.13
Ex vivo DTI
Brains were rehydrated in 0.2 M sodium phosphate buffer (pH 7.3) for 48 hours and transferred to holders filled with perfluoroether (Fomblin). DTI was performed on a Bruker Avance 14.1 T microimager at ambient temperature. Multi-slice axial images comprising the genu, body, and splenium of the corpus callosum (CC) (Fig. 3) were acquired using a spin-echo DTI pulse sequence with the following parameters: repetition time (TR)/echo time (TE) 3,000 ms/16.5 ms, 30 diffusion gradient directions, one b value equal to 1,000 s/mm2, number of slices 10, slice thickness 0.5 mm, and in-plane resolution 59 μm × 59 μm. Diffusion parameter images were calculated using Paravision 5.0 software (Bruker Biospin, Billerica, Massachusetts, USA) from which FA was measured in the CC. Regions of interest comprising medial and lateral CC in the genu, body, and splenium were drawn manually, and FA was measured in each region of interest. If a CC region was present in more than one slice, the average of two slices was calculated. Brains from Day 2 (n = 3), Day 5 (n = 5), and Day 12 (n = 3) representing acute and subacute periods of injury were analyzed. They were compared to brains from mice, which were anesthetized, but not exposed to the blast (controls, n = 6).
Figure 3.
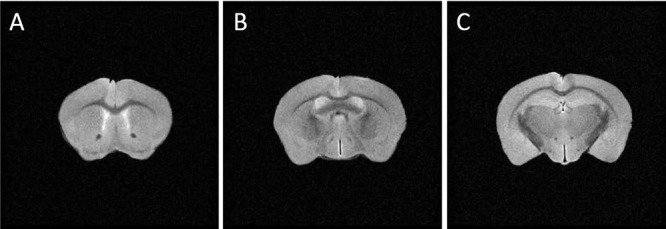
T2-weighted axial magnetic resonance (MR) images of a mouse brain. Images acquired on a 14.1T microimager show slices containing the genu (A; bregma 0.98 mm), body (B; bregma −0.34 mm), and splenium (C; bregma −1.82 mm) of CC. Imaging parameters: TR/TE 2,000 ms/40 ms, 1 average, slice thickness 0.5 mm, field of view (FOV) 1.5 cm × 1.5 cm, matrix size 256 × 256. Ex vivo DTI was acquired from these slice locations with the same slice thickness and in-plane resolution (59 μm × 59 μm).
Statistical Analysis
The DTI-measured FA values were compared between pseudo-blast- and blast-injured brains by Student t-test, and significant differences (P < 0.05) were recorded.
Results
Recovery from blast and anesthesia was significantly longer for the anesthetized and blasted mice (6.10 ± 1.79 min; n = 8) than for the anesthetized and pseudo-blasted controls (2.05 ± 0.51 min; n = 6). The one-way analysis of variance group effect was significant (P = 0.0001). The FA values from three regions of CC (genu, body, splenium) for Days 2, 5, and 12 after the blast and their pseudo-blasted controls are summarized in Table I. Since there was no significant difference in FA between left and right CC in all mice, measurements from the two hemispheres were averaged. After blast, FA values were significantly reduced on Day 2 in the medial and lateral body of the CC. On Day 5, FA was significantly higher in the medial part of the body of the CC and the medial and lateral genu, and on Day 12, FA was significantly lower in the medial splenium of blasted mice (Table I). To investigate in greater detail the nature of pathology on Day 5, we determined axial diffusivity (AD) and radial diffusivity (RD) in the medial CC for the three CC regions. Left medial CC genu of brains from blasted versus pseudo-blasted mice showed significantly elevated AD (4.32 ± 0.47 × 10−4 versus 3.69 ± 0.45 × 10−4 mm2/s, P = 0.047). The corresponding values in the right medial CC genu were 4.31 ± 0.040 × 10−4 versus 3.73 ± 0.42 × 10−4 mm2/s (P = 0.045). The slight increase of AD in the CC body was not significant, and AD was unchanged in the CC splenium. RD was not significantly changed in these CC regions. Increases in AD are consistent with edema resulting from axonal injury.14 Another pathology affecting FA values on Day 5 could be demyelination.15 It would be expected to result in increased RD. The observation of unchanged RD in any of the CC regions on Day 5 suggests that demyelination was not an acute consequence of our model of blast injury. Axonal injuries were spatially wide spread as observed on Days 2, 5, and 12 (Table I).
Table I.
Ex vivo DTI After Single Blast at 69 PSI on Day 1 and Cardio-Perfusion on Days 2, 5, and 12
| CC Genu | CC Body | CC Splenium | ||||
|---|---|---|---|---|---|---|
| Medial | Lateral | Medial | Lateral | Medial | Lateral | |
| Controls | 0.56 ± 0.02 | 0.49 ± 0.01 | 0.45 ± 0.02 | 0.43 ± 0.02 | 0.55 ± 0.01 | 0.47 ± 0.04 |
| Day 2 | 0.55 ± 0.04 | 0.50 ± 0.04 | 0.41 ± 0.02 | 0.39 ± 0.04 | 0.55 ± 0.10 | 0.49 ± 0.04 |
| Day 5 | 0.60 ± 0.02 | 0.56 ± 0.04 | 0.50 ± 0.04 | 0.45 ± 0.03 | 0.55 ± 0.08 | 0.49 ± 0.05 |
| Day12 | 0.58 ± 0.03 | 0.49 ± 0.05 | 0.45 ± 0.07 | 0.41 ± 0.04 | 0.48 ± 0.05 | 0.44 ± 0.02 |
Statistically significant reductions (P < 0.05) of FA are indicated by italic bold. Statistically significant increases of FA are indicated by bold non-italic. Controls were anesthetized and pseudo-blasted. FA values from the two hemispheres were averaged. Number of animals used for ex vivo DTI: n = 3 for Day 2; n = 5 for Day 5; n = 3 for Day 12; and n = 6 for controls.
Discussion
Several diffusion imaging studies of chronic TBI in humans have found FA changes in the CC.16,17 Our DTI results from a mouse model of blast-induced mild TBI demonstrate that acute and subacute changes of white matter structures resulting from axonal injury can be detected with DTI. Thus, DTI may have potential in detecting pathology in patients who suffer from blast injuries within days following the injury. We found that the extent of diffusion changes in the CC measured with DTI very likely depends on injury severity. Blasting young adult mice with a supersonic helium wave at a reflected pressure of 69 psi one time resulted in axonal injury in the body of the CC on Day 2. In preliminary experiments, we found that a lower reflected pressure (35 psi) did not induce axonal injury as determined by FA values (Joachim Spiess, unpublished observation). Thus, it appears that axonal diffusion changes are dependent on injury severity which in turn is dependent on blast pressure.
Previous studies of blast injury in animal models have looked predominantly at pathohistology in the cortex and the hippocampus, and therefore, little details were provided about changes in the CC.11,18–21 In this study, we have specifically examined changes in diffusion metrics in the CC, which is a major white matter structure in the brain. Our results indicate that these structures in the mouse brain are vulnerable to blast-induced mild TBI. Similar vulnerability was found in the human brain. Our data show bilateral alterations in FA and diffusivity, which vary with time after injury.
Animal studies of TBI employing intrusive injury methods such as cortical-controlled impact injury or fluid percussion injury models have reported reduced FA in the injured CC.22–26 In our blast injury model, not all regions of the CC show the same pattern of FA changes during the acute and subacute phases of blast-induced injury, which suggests differential vulnerability to injury among white matter structures. The earliest FA change occurs in the CC body as significant reduction on Day 2. At this time point, FA is not changed in the genu or splenium of the CC. A decrease of FA is generally considered to signify axonal injury. On Day 5, FA increases were found in the CC body as well as in the CC genu. These increases most likely result from an increase in AD without change in RD. In the subacute stage on Day 12, FA is only reduced in CC splenium, the other two CC regions return to control values. Thus, FA follows a biphasic response to blast-induced mild TBI in the mouse model. Similar dynamic changes were observed in humans in the first week after uncomplicated mild TBI.27 Using electron microscopy, a previous study of closed skull impact in mice detected extensive myelin damage followed by repair from 3 days to 6 weeks post-injury.15 Our DTI results, however, do not indicate any change in the myelination of CC axons in the acute phase of blast injury.
Conclusion
Mild TBI induced by a helium pressure wave at a reflected pressure of 69 psi directed at the head of an anesthetized young adult male mouse resulted in a biphasic reduced FA response with decreases on Days 2 and 12 and an increase on Day 5. A similar FA dynamics was observed in human subjects.
Presented as poster at the 2018 Military Health System Research Symposium, August 2018, Kissimmee, FL; abstract # MHSRS-18-1940.
The views expressed are solely those of the authors and do not reflect the official policy or position of the US Army, US Navy, US Air Force, the Department of Defense, or the US Government.
Funding
This study was supported by Cortrop Incorporated who funded most of the expenses for mice, blast, and single house animal holding and by the Max Planck Institute for Experimental Medicine that provided a small travel grant to facilitate cell-biological communication. (Spiess); Defense Advanced Research Projects Agency funded/Uniformed Services University of the Health Sciences award number HU00001-14-1-0023/Investigating the Neurologic Effects of Training Associated Blast (Duckworth); Defense Health Agency funded/Uniformed Services University of the Health Sciences award number HU00001-18-2-0006/Combat and Training Queryable Exposure/Event Repository (Duckworth).
References
- 1. Gardner AJ, Zafonte R: Neuroepidemiology of traumatic brain injury. Handbook Clin Neurol 2016; 138: 207–23. [DOI] [PubMed] [Google Scholar]
- 2. Agoston DV, Kamnaksh A: Modeling the neurobehavioral consequences of blast-induced traumatic brain injury spectrum disorder and identifying related biomarkers In: Brain Neurotrauma: Molecular, Neuropsychological, and Rehabilitation Aspects. Frontiers in Neuroengineering. Edited by Kobeissy FH: Boca Raton, FL, CRC Press/Taylor & Francis, 2015. [PubMed] [Google Scholar]
- 3. DePalma RG, Combat TBI: History, epidemiology, and injury modes In: Brain Neurotrauma: Molecular, Neuropsychological, and Rehabilitation Aspects. Frontiers in Neuroengineering. Edited by Kobeissy FH: Boca Raton, FL, CRC Press/Taylor & Francis, 2015. [Google Scholar]
- 4. Johnson VE, Stewart W, Smith DH: Axonal pathology in traumatic brain injury. Exp Neurol 2013; 246: 35–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ling G, Bandak F, Armonda R, Grant G, Ecklund J: Explosive blast neurotrauma. J Neurotrauma 2009; 26: 815–25. [DOI] [PubMed] [Google Scholar]
- 6. Ling G, Ecklund J: Traumatic brain injury in modern war. Curr Opin Anesthesiol 2011; 24: 124–30. [DOI] [PubMed] [Google Scholar]
- 7. Almeida OP, Hankey GJ, Yeap BB, Golledge J, Flicker L: Prevalence, associated factors, mood and cognitive outcomes of traumatic brain injury in later life: the health in men study (HIMS). Int J Geriatr Psychiatry 2015; 30: 1215–23. [DOI] [PubMed] [Google Scholar]
- 8. Sarrami P, Armstrong E, Naylor JM, Harris IA: Factors predicting outcome in whiplash injury: a systematic meta-review of prognostic factors. J Orthop Traumatol 2017; 18: 9–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mihalik JP, Lynall RC, Teel EF, Carneiro KA: Concussion management in soccer. J Sport and Health Science 2014; 3: 307–13. [Google Scholar]
- 10. Gavett BE, Stern RA, McKee AC: Chronic traumatic encephalopathy: a potential late effect of sport-related concussive and subconcussive head trauma. Clinics in Sports Medicine 2011; 30: 179–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Goldstein LE, Fisher AM, Tagge CA, et al. : Chronic traumatic encephalopathy in blast-exposed military veterans and a blast neurotrauma mouse model. Sci Trans Med 2012; 4: 134ra60. doi: 10.1126/scitranslmed.3003716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Grassi DC, Conceicao DMD, Leite CDC, Andrade CS: Current contribution of diffusion tensor imaging in the evaluation of diffuse axonal injury. Arq Neuropsiquiatr 2018; 76: 189–99. [DOI] [PubMed] [Google Scholar]
- 13. Gage GJ, Kipke DR, Shain W: Whole animal perfusion fixation for rodents. J Vis Exp 2012; 65(3564). doi: 10.3791/3564) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hutchinson EB, Schwerin SC, Avram AV, Juliano SL, Pierpaoli C: Diffusion MRI and the detection of alterations following traumatic brain injury. J Neurosci Res 2018; 96: 612–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mierzwa AJ, Marion CM, Sullivan GM, McDaniel DP, Armstrong RC: Components of myelin damage and repair in the progression of white matter pathology after mild traumatic brain injury. J Neuropathol Exp Neurol 2015; 74: 218–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ivanov I, Fernandez C, Mitsis EM, et al. : Blast exposure, white matter integrity, and cognitive function in Iraq and Afghanistan combat veterans. Front Neurol 2017; 8: 127. doi: 10.3389/fneur.2017.00127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ling JM, Pena A, Yeo RA, et al. : Biomarkers of increased diffusion anisotropy in semi-acute mild traumatic brain injury: a longitudinal perspective. Brain 2012; 135: 1281–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Rubovitch V, Ten-Bosch M, Zohar O, et al. : A mouse model of blast-induced mild traumatic brain injury. Exp. Neurol. 2011; 232: 280–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kamnaksh A, Budde MD, Kovesdi E, Long JB, Frank JA, Agoston DV: Diffusion tensor imaging reveals acute subcortical changes after mild blast-induced traumatic brain injury. Sci Rep 2014; 4: 4809. doi: 10.1038/srep04809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sosa MAG, De Gasperi R, Paulino AJ, et al. : Blast overpressure induces shear-related injuries in the brain of rats exposed to a mild traumatic brain injury. Acta Neuropathol Commun 2013; 1: 51. doi: 10.1186/2051-5960-1-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Budde MD, Shah A, McCrea M, Cullinan WE, Pintar FA, Stemper BD: Primary blast traumatic brain injury in the rat: relating diffusion tensor imaging and behavior. Front Neurology 2013; 4: 154. doi: 10.3389/fneur.2013.00154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hutchinson EB, Schwerin SC, Radomski KL, Irfanoglu MO, Juliano SL, Pierpaoli CM: Quantitative MRI and DTI abnormalities during the acute period following CCI in the ferret. Shock 2016; 46(Supp.I): 167–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hutchinson EB, Schwerin SC, Radomski KL, et al. : Detection and distinction of mild brain injury effects in a ferret model using diffusion tensor MRI (DTI) and DTI-driven tensor-based morphometry (D-TBM). Front Neurosci 2018; 12: 573. doi: 10:3389/fnins. 2018.00573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Laitinen T, Sierra A, Bolkvadze T, Pitkänen A, Gröhn O: Diffusion tensor imaging detects chronic microstructural changes in white and gray matter after traumatic brain injury in rat. Front Neurosci 2015; 9: 128. doi: 10.3389/fnins.2015.00128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wright DK, Trezise J, Kamnaksh A, et al. : Behavioral, blood, and MRI biomarkers of experimental mild traumatic brain injury. Sci Rep 2016; 6: 28713. doi: 10.1038/srep28713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Harris NG, Verley DR, Gutman BA, Sutton RL: Bi-directional changes in fractional anisotropy after experiment TBI: disorganization and reorganization? Neuroimage 2016; 133: 129–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wilde EA, McCauley SR, Barnes A, et al. : Serial measurement of memory and diffusion tensor imaging changes within the first week following uncomplicated mild traumatic brain injury. Brain Imaging Behavior 2012; 6: 319–28. [DOI] [PubMed] [Google Scholar]


