Abstract
Introduction
Previous studies of fetal effects have suggested that intrahepatic cholestasis of pregnancy is associated with a higher rate of adverse neonatal outcomes including preterm birth, neonatal respiratory distress syndrome, meconium-stained amniotic fluid, neonatal intensive care unit admission, and stillbirth. The objective was to compare the neonatal and maternal consequences in pregnancies affected by intrahepatic cholestasis and normal pregnancies.
Material and methods
This case-control study compares pregnancies affected by intrahepatic cholestasis (pruritus and bile acid ≥ 10 μmol/L) with low-risk pregnancies managed between December 2006 and December 2014 at a French university hospital center.
Results
There were 83 (59.3%) cases of mild cholestasis (10≤ BA ≤39 μmol/L), 46 (32.8%) of moderate cholestasis (40≤ BA ≤99 μmol/L), and 11 (7.9%) of severe cholestasis (BA ≥100 μmol/L). No in utero fetal deaths occurred in the 140 women with cholestasis or the 560 controls analyzed. The rate of respiratory distress syndrome was higher in neonates of women with intrahepatic cholestasis (17.1% vs. 4.6%, P<0.001; crude OR 4.46 (CI95% 2.49–8.03)). This risk was also significant after adjustment for gestational age at birth and mode of delivery, adjusted OR 2.56 (CI95%1.26–5.18). The postpartum hemorrhage rate was twice as high among the case mothers (25% versus 14.1% for controls, P = 0.002).
Conclusion
After adjustment on the confounding factors we found a higher rate of respiratory distress syndrome and neonatal morbidity among neonates of the cholestasis group.
Introduction
Intrahepatic cholestasis of pregnancy (ICP) is the most common liver disease during pregnancy, occurring most often during its second and third trimesters. Its prevalence is estimated at 0.5% in France and varies with ethnicity. ICP is defined by pruritus sine materia (without lesions), with elevated levels of serum bile acids (BA) and liver enzymes. Its pathogenesis is still unknown, but hormonal influence and the MDR3 mutation gene (multidrug resistance 3) may contribute to it. Symptoms and abnormal liver function are spontaneously reversible after delivery, with good maternal prognosis. Moreover, maternal treatment with ursodeoxycholic acid improves pruritus and laboratory abnormalities and extends pregnancy [1]. Previous studies of fetal effects have suggested that ICP is associated with a higher rate of adverse neonatal outcomes including preterm birth, neonatal respiratory distress syndrome (RDS) [2], meconium-stained amniotic fluid [3], neonatal intensive care unit admission, and stillbirth. The prevalence of in utero and perinatal mortality is estimated at 0.5%. Severe cholestasis with higher BA levels is associated with a higher risk of fetal complications [1–2]. The mechanisms relating cholestasis to stillbirth remain uncertain: in utero deaths imputable to cholestasis occur during pregnancies with other pathologies [4]. Several experimental animal studies have shown that high BA levels have a harmful effect on cardiomyocytes [5]. Thus, it has been hypothesized that ICP might induce fetal arrhythmia that may lead to stillbirth. Perez et al. reported no deleterious effects of an acute high dose of cholic acid administered to a pregnant ewe, which suggests that the harm might require exposure over some period of time [6].
Thus far, both prenatal management and optimal time to delivery remain unclear. No method of fetal monitoring has been shown to either predict adverse perinatal outcomes or reduce their risk. The recommendations of various national professional societies for time to delivery in ICP-complicated pregnancies are also divergent. The Royal College of Obstetrics and Gynaecology does not endorse routine early delivery of these pregnancies [7], while the American College of Obstetricians and Gynecologists supports active management induction of labor protocols for ICP [8].
Our level III reference center, like most French maternity units, uses active management of ICP, defined by weekly clinical and laboratory monitoring with systematic induction of labor before or by 38 weeks of gestation. The exact term depends on its severity. This active attitude aims to avoid stillbirth [9], although its imputability to cholestasis has not been clearly established [10,11]. This study aimed to evaluate neonatal and maternal outcomes of this routine induction in the ICP cases, compared with controls.
Materials and methods
Patient selection and data collection
This case-control study included 140 women identified with ICP and 560 controls from December 2006 to December 2014. Cholestasis was diagnosed by the association of pruritus and BA ≥10 μmol/L (after other causes of itching and liver dysfunction were ruled out), and by normalization of biochemical parameters after delivery. Recurrences of cholestasis (each woman was included only one time) over the study period, multiple pregnancies, congenital malformations, and chromosomal abnormalities were excluded. Eligible control women had a singleton in cephalic presentation, without congenital malformations and no obstetric disorders requiring preterm induction. The control population was matched for maternal age (one year more or less), date of delivery (same calendar year), same parity, and did not have ICP. To improve the power study, four women with low-risk pregnancies were recruited as controls for each ICP case (ratio of 1:4; following 4 patients after each case).
We collected the following data for case and control women: demographic characteristics, pregnancy history, obstetric outcomes including term at delivery, spontaneous or induced labor, mode of delivery, meconium staining of amniotic fluid during labor, birth weight, postpartum hemorrhage (defined by blood loss ≥ 500 mL) and indicated transfusion. For neonatal status, adverse neonatal outcome was defined as pHa<7.10, an Apgar score <7 at 5 minutes, intubation, neonatal intensive care unit admission or perinatal death. For cases only, we also collected the history of liver diseases including viral hepatitis or calculous pancreatitis or cholecystitis; abnormal laboratory values at diagnosis, at delivery, and highest values during pregnancy (bile acids, transaminases, total bilirubin, hemoglobin, and hemostasis); treatment initiation; and clinical and laboratory course with treatment. BA was assayed weekly with an enzymatic spectrophotometric method (Olympus AU640 chemistry analyzer). Cholestasis severity was defined by the maximum BA rate rather than by BA level at diagnosis: mild if 10 ≤ BA ≤ 39 μmol/L, moderate if 40 ≤ BA ≤ 99 μmol/L, and severe if BA ≥100 μmol/L.
Statistical analysis
Continuous data are presented as medians and interquartile ranges (1st quartile-3rd quartile) or means and their standard deviations, and categorical data as counts and percentages. Baseline characteristics, neonatal and maternal outcomes of cases and controls were compared. Mixed linear regression models were used to take the clustering effect of matching for quantitative data into account. Percentages were compared by conditional logistic regression. The cholestasis cases severity were compared by analysis of variance. The association between the composite neonatal outcome and ICP was estimated by crude and adjusted Odd ratios (adjustment on gestational age at birth and delivery mode). Analyses were conducted with R version 3.1.3. Differences were defined as significant when P<0.05.
Ethical approval
The local ethics committee of university center of Tours approved this retrospective study. All data were fully anonymized before analyze in a secure database and ethics committee waived the requirement for informed consent.
Results
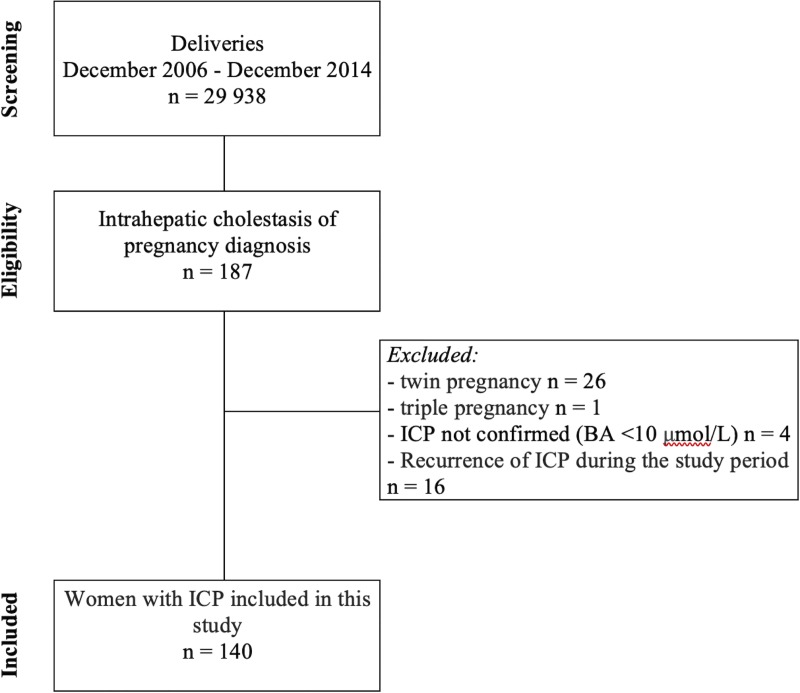
Between December 2006 and December 2014, 29 938 women gave birth at the University Hospital Center. The study finally included 140 pregnancies with a confirmed diagnosis of ICP (Fig 1) for an incidence of 0.5%.
Fig 1. Flow chart.
Baseline characteristics
Table 1 summarizes the principal characteristics of the ICP group and the control group. They did not differ significantly for the matching criteria. Six women (4.3%) from ICP group had a personal history of liver disease: 5 women with chronic or treated hepatitis and 1 with autoimmune hepatitis.
Table 1. Maternal characteristics of women with intrahepatic cholestasis of pregnancy and the control group.
| Intrahepatic cholestasis of pregnancy (ICP) N = 140 |
Controls N = 560 |
P Values | |
|---|---|---|---|
| Maternal age, years (median (IQR)) | 29 (25–34) | 29 (25–34) | 1 |
| Nulliparous, n (%) | 61 (43.6) | 246 (43.9) | 0.939 |
| White, n (%) | 118 (84.3) | 435 (77.7) | 0.086 |
| BMI, kg/m2 (median (IQR)) | 23.6 (21–27.2) | 22.4 (20–25.1) | 0.216 |
| Personal history of ICP, n (%) | 31 (22.1) | 1 (0.3) | <0.001 |
| Family history of ICP, n (%) | 4 (2.9) | 0 | <0.001 |
| MDR3 gene mutation, n (%) | 6 (4.3) | 0 | <0.001 |
| Preexisting liver disease, n (%) | 6 (4.3) | 13 (2.3) | 0.240 |
| Preexisting coagulopathy, n (%) | 5 (3.6) | 18 (3.2) | 0.957 |
| Gestational diabetes, n (%) | 22 (15.7) | 25 (5.1) | <0.001 |
| Hypertensive disorder, n (%) | 2 (1.4) | 3 (0.5) | 0.574 |
Neonatal outcomes
No stillbirth occurred during the 8-year study period in our population (Table 2). The mean birth weight was 3082 g (2825–3370 g) in the case population and 3350 g (3050–3610 g) (P <0.001) in the control population, which was the 50th percentile for term. The rate of small-for-gestational-age (SGA) fetuses did not differ between the groups, but neonatal status was worse in the cholestasis cases. Neonates exposed to cholestasis had a greater risk of having a RDS in comparison with controls (17.1% vs. 4.6%; P<0.001) crude OR 4.46 (95%CI: 2.49–8.03), even after adjustment on gestational age and delivery mode (aOR 2.56 (95%CI: 1.26–5.18)). The rates of admission to neonatal intensive care units was approximately three times higher in the cholestasis than the control group (P = 0.018). Moreover, neonatal morbidity was higher in cholestasis cases compared to controls OR 3.58 (95%CI: 2.03–6.27), and after adjustment aOR 2.28 (95%CI: 1.15–4.52) (Table 3).
Table 2. Neonatal and maternal outcomes of pregnancies with intrahepatic cholestasis of pregnancy (ICP).
| ICP N = 140 |
Controls N = 560 |
P Value | |
|---|---|---|---|
| Obstetric outcomes | |||
| Gestational age at birth, median (IQR) | 38 (37–38) | 40 (39–40) | <0.001 |
| Preterm delivery before 37 weeks, n (%) | 22 (15.7) | 27 (4.8) | <0.001 |
| Meconium-stained fluid, n (%) | 26 (18.6) | 121 (21.6) | 0.501 |
| Male, n (%) | 74 (52.9) | 282 (50.4) | 0.664 |
| Birth weight, grams, median (IQR) | 3082 (2825–3370) | 3350 (3050–3610) | <0.001 |
| Small for gestational age, n (%) | 8 (5.7) | 18 (3.2) | 0.161 |
| Arterial pH, median (IQR) | 7.29 (7.24–7.33) | 7.27 (7.22–7.31) | 0.199 |
| Venous pH, median (IQR) | 7.35 (7.30–7.38) | 7.34 (7.29–7.37) | 0.344 |
| Arterial lactates, median (IQR) | 3.5 (2.7–4.9) | 4.3 (3.1–5.7) | 0.060 |
| Maternal outcomes | |||
| Induction of labor, n (%) | 115 (82.1) | 103 (18.4) | <0.001 |
| Type of delivery, n (%) | |||
| Vaginal delivery | 104 (74.3) | 487 (86.9) | <0.001 |
| Cesarean delivery during labor | 19 (13.6) | 61 (10.9) | 0.457 |
| Scheduled cesarean delivery | 17 (12.1) | 12 (2.1) | <0.001 |
| Postpartum hemorrhage, n (%) | 29 (20.7) | 86 (15.35) | |
| Maternal transfusion, n (%) | 4 (2.5) | 6 (1.1) | 0.232 |
| Neonatal outcomes, n (%) | |||
| 5-min Apgar score <7, n (%) | 4 (2.9) | 3 (0.5) | 0.046 |
| Arterial pH<7.10, n (%) | 4 (2.9) | 8 (1.5) | 0.423 |
| Arterial pH<7.00, n (%) | 0 (0) | 2 (0.4) | 0.999 |
| Respiratory distress syndrome | 24 (17.1) | 26 (4.6) | <0.001 |
| Mechanical ventilation or intubation | 5 (3.6) | 3 (0.5) | 0.009 |
| Admission to neonatal intensive care unit | 4 (2.9) | 2 (0.4) | 0.018 |
| Stillbirth | 0 (0) | 0 (0) | 1 |
Table 3. Neonatal outcome according to antenatal exposition to cholestasis.
| ICP N = 140 |
Controls N = 560 |
Crude OR (CI95%) | aOR* (CI 95%) | |
|---|---|---|---|---|
| Respiratory distress syndrome | N = 24/140 (17.1) | N = 26/560 (4.6) |
4.46 (2.49–8.03) | 2.56 (1.26–5.18) |
| Neonatal morbidity ** | N = 25/140 (17.9) | N = 32/560 (5.7) | 3.58 (2.03–6.27) | 2.28 (1.15–4.52) |
* OR adjusted for the following confounding factors gestational age at birth and mode of delivery.
** Neonatal morbidity was defined as Apgar score at 5 min less than 7, arterial cord blood pH less than 7.10, ventilation, intubation or external cardiac massage and admission to the neonatal intensive-care unit.
Maternal outcomes
Obstetric outcomes for both groups are reported in Table 4. Active obstetric management resulted in the induction of labor in 82.1% of case women, compared to 18.4% of the control group (P <0.001). The rate of cesareans during labor did not differ between the groups (P = 0.457). In contrast, more scheduled (prelabor) cesareans took place among the case women (12% versus 2%, P <0.001). Postpartum hemorrhages were also more frequent in the case group, 25% versus 14.1% (P = 0.002), but maternal blood transfusions were not. All severe hemorrhages (blood loss > 1000 mL) occurred in the cholestasis group: six women (17%), four during cesarean deliveries. No significant differences were observed in the rates of coagulopathy, or in prothrombin times and hemoglobin concentrations at the onset of labor.
Table 4. Clinical and laboratory characteristics according to the severity of intrahepatic cholestasis of pregnancy (ICP) (N = 140).
| Mild cholestasis 10 ≤ BA ≤ 39 μmol/L N = 83 |
Moderate cholestasis 40 ≤ BA ≤ 99 μmol/L N = 46 |
Severe cholestasis BA ≥100 μmol/L N = 11 |
P Values | |
|---|---|---|---|---|
| Median gestational age at diagnosis (weeks) | 36 (33–38) | 35 (33–37) | 33 (31.5–35) | 0.185 |
| Median gestational age at delivery (weeks) | 38 (38–38) | 38 (37–38) | 36 (35–38) | 0.009 |
| Respiratory distress syndrome | 13 (15.6) | 8 (17.4) | 4 (36.4) | 0.263 |
| Biochemistry at diagnosis | ||||
| Bile acid, μmol/L | 15 (12–24) | 39,5 (21.2–55) | 59 (37–100.5) | <0.001 |
| Aspartate transaminase, IU/L | 53 (32–84) | 95.5 (46.2–170) | 220 (100.5–305) | <0.001 |
| Alanine transaminase, IU/L | 86 (40.2–148.2) | 138 (57–308) | 481 (158.5–552.5) | <0.001 |
| Most severe biochemistry | ||||
| Bile acid, μmol/L | 19 (14–26) | 55.5 (43.2–67.5) | 131 (115.5–140) | <0.001 |
| Aspartate transaminase, IU/L | 54 (31–96) | 95.5 (53.2–207.8) | 220 (113.5–323) | <0.001 |
| Alanine transaminase, IU/L | 79 (38–160) | 114 (53–296) | 481 (241–611.5) | <0.001 |
| Treatment | ||||
| Ursodeoxycholic acid treatment, % | 25 (30.1) | 30 (83.3) | 6 (66.7) | 0.252 |
| Term at BA normalization, WG | 37 (35–38) | 36 (35–38) | 32 (32–32) | 0.326 |
| Standardization time, days | 7 (0.2–12.2) | 12.5 (10–28) | 16 (16–16) | 0.070 |
| Pruritus disappearance, % | 20 (31.7) | 9 (22.5) | 0 (0) | <0.001 |
| Biochemistry at delivery | ||||
| Bile acid, μmol/L | 13 (8–23) | 30 (13–48) | 79.5 (45.8–117.2) | <0.001 |
| Aspartate transaminase, IU/L | 38 (27–89) | 58 (26–94) | 119 (33–251) | <0.001 |
| Alanine transaminase, IU/L | 59 (30–138) | 59 (27–157) | 192 (42.5–545.5) | <0.001 |
| Prothrombin time, s | 101 (92–112) | 100 (99.5–112) | 100 (50.9–100) | - |
| Postpartum hemoglobin, g/dL | 12 (11.4–13) | 11.7 (10.6–12.3) | 11.8 (11.4–12.3) | 0.170 |
Cholestasis characteristics according to severity
The gestational age at diagnosis (mean, 34.4 ± 2.1 weeks) did not differ significantly by severity, although severe ICP tended to be diagnosed around a week earlier. Hepatic cytolysis was more severe in cases with high BA levels. Hemoglobin levels and prothrombin times did not differ according to the severity of cholestasis. Ursodeoxycholic acid was administered to 43.6% of the cholestasis cases, and the rate of its prescription increased with the severity of the disease. Normalization of the BA most often occurred by 15 days after the onset of treatment. Pruritus disappeared a week after the onset of the treatment in 30% of the women with mild cholestasis and in none of the 11 with severe ICP (Table 3).
Discussion
Over the 8-year study period, no stillbirths occurred in either group. After adjustment on the confounding factors we found a higher rate of RDS and neonatal morbidity among neonates of the cholestasis group. Mothers with ICP had more postpartum hemorrhages than control women, but did not require more blood transfusions.
The RDS rate was three times higher among neonates of the cholestasis group which is consistent with findings from other studies. Zecca et al, in a case-control study (matching on gestational age) showed a risk of RDS in ICP newborns 2.5 times higher than in control infants (28.6% vs 14%), regardless of BA level [2]. Like others [12], we found that BA was higher in the ICP cases complicated by RDS. Our study also found a significant difference, with more intubation and higher special care and intensive care unit admission rates among case infants. Morbidity in the ICP cases was higher than that in the control population delivered in the same late preterm period [13]. Hypothesis to explain increased neonatal morbidity among case infants include a direct effect of BA on neonatal lung, which could be induce a “bile acid pneumonia” [2, 14]. BA have been found detectable in the bronchoalveolar lavage fluid of case neonates affected by RDS, some authors have speculated that BA inhibits surfactant activity [14]. A meta-analysis suggests that treatment with ursodesoxycholic acid is associated with a decrease in the RDS rate [3]. There were, however, no significant differences in acid-base status or meconium staining during labor, contrary to other studies [15]. The PITCHES trial outcome was to evaluate perinatal outcome in ICP-affected pregnancies of ursodesoxycholic acid versus placebo [16]. Authors funded that treatment with ursodeoxycholic acid does not reduce adverse perinatal outcomes.
The proportion of women with diabetes was higher among ICP cases compared with control, as expected from previous studies [17]. This association could increase stillbirth rates whereas confounding factors are unclear [18]. During ICP, reported stillbirth rates vary between 0.4% and 7% [18,19]. The risk of stillbirth seems to increase after 37 weeks and is rare before 34 weeks. It also increase with BA level [15], when serum bile acids concentrations are of 100 μmol/L or more [20]. Nonetheless, bile acids are not an infallible surveillance marker, and the level can rise abruptly, as shown by the serious accidents reported in this context [10]. Ethnicity (Latino, native American) is also a reported risk factor for stillbirths associated with cholestasis [21], but these ethnicities were not represented in our study. In ICP, stillbirth prevention must be weighed against the long-term consequences of “late preterm” birth [22]. Although the American College of Obstetricians and Gynecologists [8] recommends active management, it does not define an ideal term for childbirth. Two studies advocate that 36 weeks of gestation is the best compromise between the risks of preterm birth and the risk of stillbirth or neonatal death [22–24]. Nonetheless, our results do not support systematic delivery at this late preterm gestational age. Similarly, the Royal College of Obstetrics and Gynaecology does not recommend systematic active management [7]. It concludes that if ICP is associated with stillbirth, which it does not consider statistically proven, the risk is clinically insignificant [11].
Regarding maternal outcomes, the planned cesarean rate was significantly higher in ICP cases. Induction of labor for women with ICP did not increase the emergency cesarean rate [24]. On the other hand, the postpartum hemorrhage rate was higher in ICP cases, probably related to their higher rates of cesarean delivery and of oxytocin-induced labor [25]. We did not, however, observe any differences in the transfusion rates or maternal hemostasis problems. This result is consistent with Brouwers et al. [26].
Our study nonetheless has some limitations. It was a retrospective study with potential bias. Thus, we chose to report and analyze the rates of RDS rather than neonatal unit admission to limit reporting bias.
Conclusions
Weekly clinical and laboratory monitoring appears essential, although no marker can rule out the onset of in utero fetal death. Treatment with ursodeoxycholic acid does not reduce adverse perinatal outcomes [16]. The risk of RDS, appears to be more frequent in cases of cholestasis regardless of gestational age, and must be taken into account at delivery, even if the birth occurs at term.
Supporting information
(CSV)
Data Availability
All relevant data are within the manuscript and its Supporting Information files.
Funding Statement
The author(s) received no specific funding for this work.
References
- 1.Bacq Y, Sentilhes L, Reyes HB, Glantz A, Kondrackiene J, Binder T, et al. Efficacy of ursodeoxycholic acid in treating intrahepatic cholestasis of pregnancy: a meta-analysis. Gastroenterology. déc 2012;143(6):1492–501. [DOI] [PubMed] [Google Scholar]
- 2.Zecca E, De Luca D, Marras M, Caruso A, Bernardini T, Romagnoli C. Intrahepatic cholestasis of pregnancy and neonatal respiratory distress syndrome. Pediatrics. 2006;117(5):1669–72. 10.1542/peds.2005-1801 [DOI] [PubMed] [Google Scholar]
- 3.Estiú MC, Frailuna MA, Otero C, Dericco M, Williamson C, Marin JJG, et al. Relationship between early onset severe intrahepatic cholestasis of pregnancy and higher risk of meconium-stained fluid. PloS One. 2017;12(4):e0176504 10.1371/journal.pone.0176504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rioseco AJ, Ivankovic MB, Manzur A, Hamed F, Kato SR, Parer JT, et al. Intrahepatic cholestasis of pregnancy: a retrospective case-control study of perinatal outcome. Am J Obstet Gynecol. mars 1994;170(3):890–5. 10.1016/s0002-9378(94)70304-3 [DOI] [PubMed] [Google Scholar]
- 5.Gorelik J, Shevchuk A, de Swiet M, Lab M, Korchev Y, Williamson C. Comparison of the arrhythmogenic effects of tauro- and glycoconjugates of cholic acid in an in vitro study of rat cardiomyocytes. BJOG Int J Obstet Gynaecol. Août 2004;111(8):867–70. [DOI] [PubMed] [Google Scholar]
- 6.Perez R, Garcia M, Ulloa N, Jara C, Bardisa L, Rudolph MI. A single intravenous high dose of cholic acid to a pregnant ewe does not affect fetal well-being. Res Exp Med Z Gesamte Exp Med Einschl Exp Chir. 1994;194(1):63–7. [DOI] [PubMed] [Google Scholar]
- 7.Obstetric cholestasis—green-top guideline n°43, apr 2011 [Internet]. https://www.rcog.org.uk/globalassets/documents/guidelines/gtg_43.pdf
- 8.ACOG Committee Opinion No. 764: Medically Indicated Late-Preterm and Early-Term Deliveries. Obstet Gynecol. 2019. February; 133(2):e151–e155. 10.1097/AOG.0000000000003083 [DOI] [PubMed] [Google Scholar]
- 9.Shemer EW, Marschall HU, Ludvigsson JF, Stephansson O. Intrahepatic Cholestasis of Pregnancy and Associated Adverse Pregnancy and Fetal Outcomes: A 12-Year Population-Based Cohort Study. BJOG. 2013. May;120(6):717–23. 10.1111/1471-0528.12174 [DOI] [PubMed] [Google Scholar]
- 10.Sentilhes L, Verspyck E, Pia P, Marpeau L. Fetal death in a patient with intrahepatic cholestasis of pregnancy. Obstet Gynecol. févr 2006;107(2 Pt 2):458–60. [DOI] [PubMed] [Google Scholar]
- 11.Henderson CE, Shah RR, Gottimukkala S, Ferreira KK, Hamaoui A, Mercado R. Primum non nocere: how active management became modus operandi for intrahepatic cholestasis of pregnancy. Am J Obstet Gynecol. September 2014;211(3):189–96. 10.1016/j.ajog.2014.03.058 [DOI] [PubMed] [Google Scholar]
- 12.Glantz A, Marschall H-U, Mattsson L-Å. Intrahepatic cholestasis of pregnancy: Relationships between bile acid levels and fetal complication rates. Hepatology. 1 Août 2004;40(2):467–74. 10.1002/hep.20336 [DOI] [PubMed] [Google Scholar]
- 13.Sengupta S, Carrion V, Shelton J, Wynn RJ, Ryan RM, Singhal K, et al. Adverse neonatal outcomes associated with early-term birth. JAMA Pediatr. November 2013;167(11):1053–9. 10.1001/jamapediatrics.2013.2581 [DOI] [PubMed] [Google Scholar]
- 14.Zecca E, De Luca D, Baroni S, Vento G, Tiberi E, Romagnoli C. Bile acid-induced lung injury in newborn infants: a bronchoalveolar lavage fluid study. Pediatrics. Janv 2008;121(1):e146–149. 10.1542/peds.2007-1220 [DOI] [PubMed] [Google Scholar]
- 15.Kawakita T, Parikh LI, Ramsey PS, Huang C-C, Zeymo A, Fernandez M, et al. Predictors of adverse neonatal outcomes in intrahepatic cholestasis of pregnancy. Am J Obstet Gynecol. October 2015;213(4):570.e1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chappell LC, Bell JL, Smith A, Linsell L, Juszczak E, Dixon PH, et al. Ursodeoxycholic acid versus placebo in the treatment of women with intrahepatic cholestasis of pregnancy (PITCHES): a randomised controlled trial. Lancet. Sept 2019;394(10201):849–860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wikström Shemer E, Marschall HU, Ludvigsson JF, Stephansson O. Intrahepatic cholestasis of pregnancy and associated adverse pregnancy and fetal outcomes: a 12-year population-based cohort study. BJOG Int J Obstet Gynaecol. May 2013; 120(6): 717–23. [DOI] [PubMed] [Google Scholar]
- 18.Geenes V, Chappell LC, Seed PT, Steer PJ, Knight M, Williamson C. Association of severe intrahepatic cholestasis of pregnancy with adverse pregnancy outcomes: a prospective population-based case-control study. Hepatol Baltim Md. avr 2014;59(4):1482–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Williamson C, Hems LM, Goulis DG, Walker I, Chambers J, Donaldson O, et al. Clinical outcome in a series of cases of obstetric cholestasis identified via a patient support group. BJOG Int J Obstet Gynaecol. Juill 2004;111(7):676–81. [DOI] [PubMed] [Google Scholar]
- 20.Ovadia C, Seed PT, Sklavounos A, Geenes V, Di Ilio C, Chambers J, et al. Association of adverse perinatal outcomes of intrahepatic cholestasis of pregnancy with biochemical markers: results of aggregate and individual patient data meta-analyses. Lancet. 2019. March 2;393(10174):899–909. 10.1016/S0140-6736(18)31877-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Puljic A, Kim E, Page J, Esakoff T, Shaffer B, LaCoursiere DY, et al. The risk of infant and fetal death by each additional week of expectant management in intrahepatic cholestasis of pregnancy by gestational age. Am J Obstet Gynecol. Mai 2015;212(5):667.e1–5. [DOI] [PubMed] [Google Scholar]
- 22.Quigley MA, Poulsen G, Boyle E, Wolke D, Field D, Alfirevic Z, et al. Early term and late preterm birth are associated with poorer school performance at age 5 years: a cohort study. Arch Dis Child Fetal Neonatal Ed. Mai 2012;97(3):F167–173. 10.1136/archdischild-2011-300888 [DOI] [PubMed] [Google Scholar]
- 23.Lo JO, Shaffer BL, Allen AJ, Little SE, Cheng YW, Caughey AB. Intrahepatic cholestasis of pregnancy and timing of delivery. J Matern-Fetal Neonatal Med Off J Eur Assoc Perinat Med Fed Asia Ocean Perinat Soc Int Soc Perinat Obstet. 2015;28(18):2254–8. [DOI] [PubMed] [Google Scholar]
- 24.Chappell LC, Gurung V, Seed PT, Chambers J, Williamson C, Thornton JG, et al. Ursodeoxycholic acid versus placebo, and early term delivery versus expectant management, in women with intrahepatic cholestasis of pregnancy: semifactorial randomised clinical trial. BMJ. 13 Juin 2012;344:e3799 10.1136/bmj.e3799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Khireddine I, Le Ray C, Dupont C, Rudigoz R-C, Bouvier-Colle M-H, Deneux-Tharaux C. Induction of labor and risk of postpartum hemorrhage in low risk parturients. PloS One. 2013;8(1):e54858 10.1371/journal.pone.0054858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brouwers L, Koster MPH, Page-Christiaens GCML, Kemperman H, Boon J, Evers IM, et al. Intrahepatic cholestasis of pregnancy: maternal and fetal outcomes associated with elevated bile acid levels. Am J Obstet Gynecol. Janv 2015;212(1):100.e1–100.e7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(CSV)
Data Availability Statement
All relevant data are within the manuscript and its Supporting Information files.