Abstract
With polyol-synthesized silver nanoparticles (AgNPs) as raw materials, the silver electrodes with high conductivity were fabricated via a dip-coating method followed by sintering process, and the effects of the dip-coating and sintering process on the conductivity and surface roughness of silver electrodes were investigated in detail. The silver film with a thickness of 1.97 µm and a roughness of about 2 nm can be prepared after dip-coating at a pulling rate of 500 µm s−1 for 40 coating times. The non-conductive dip-coated silver films are transformed into conductive silver electrodes after conventional sintering in a muffle oven, infrared sintering and microwave sintering, respectively. Compared with high sintering temperature and long sintering time of conventional sintering and infrared sintering, microwave sintering can achieve quick sintering of silver films to fabricate high conductive silver electrodes. The silver electrodes with a sheet resistance of 0.75 Ω sq−1 and a surface roughness of less than 1 nm can be obtained after microwave sintering at 500 W for 50 s. The adjustable dip-coating method followed by quick microware sintering is an appropriate approach to prepare high conductive AgNPs-based electrodes for organic light-emitting diodes or other devices.
Keywords: AgNPs, polyol method, sinter, microwave, conductivity
1. Introduction
In recent years, organic light-emitting diodes (OLED) have attracted considerable attention owing to their advantages such as low power consumption, fast response times, good colour gamut, eco-friendly nature, self-illumination and so on [1–5]. For OLED, a lot of research has focused on its transparent and conductive anodes [6–10], while little attention is paid to the cathode. At present, vacuum evaporation is the most common method for the preparation of silver or aluminium thin-film electrodes or other films [11–13]. As is well known, the thin-film electrodes prepared by vacuum evaporation possess high conductivity, good smoothness and extremely thin thickness; however, vacuum evaporation is also limited owing to insurmountable shortcomings such as expensive equipment, complicated vacuuming processes and low economic efficiency. Therefore, it is very urgent to find an all-solution method to construct silver electrodes for cathodes of OLED or other electronic devices [14–17].
There are many methods used to prepare silver electrodes, such as solution self-assembly and ink-jet-printing, which are suitable for replacing vacuuming evaporation plating. Ishwor Khatri et al. [18] prepared silver electrodes by self-assembled silver nanowires (AgNWs) to construct organic–inorganic hybrid solar cells. The self-assembled AgNWs mesh electrodes show low sheet resistance (8 Ω sq−1) with enhanced transparency in the ultraviolet and infrared regions. Yu Chen-Chiang et al. [19] introduced a porous silver thin-film cathode fabricated by a simple ink-jet-printing process for low-temperature solid oxide fuel cell applications. However, the products obtained from these studies still have defects such as high sheet resistances and strict requirements to the precursors.
In this work, we introduce a facile preparation approach of the silver electrodes, including using synthesized silver nanoparticles (AgNPs) by a polyol method as raw materials, coating silver film by a dip-coating method and sintering the silver film by quip sintering. The dip-coating, sintering processes and sintering mechanism of AgNPs for constructing silver electrodes are discussed. The resultant AgNPs-based electrodes showed good smoothness and excellent conductivity (less than 1 Ω sq−1), which could be applied as the cathode to construct OLED, solar cells or other electronic devices.
2. Experimental procedure
2.1. Chemicals and materials
Silver nitrate (AgNO3; Aladdin Ind. Co., China, 99%) was used as the silver source. Ethylene glycol (EG; Aldrich, 99.8%) was the reducing agent as well as the solvent. Polyvinylpyrrolidone (PVP; Mw = 58000, Aladdin Ind. Co., China) was used as the stabilizer as well as the anisotropic agent. Anhydrous copper chloride (CuCl2; Aladdin Ind. Co., China, 98%) was used as the ion additive. Acetone (PA; Aldrich, 99.5%) was used as the washing solvent. All chemicals were used without further purification.
2.2. Preparation of silver nanoparticles
In the synthesis process, 50 ml EG was preheated in an oil bath at 120°C for 1 h to remove the water, and 5.0 g PVP was added into the solvent and dissolved to form a polyol solution. Twenty-five millilitres FeCl3 EG solution (600 µM) was added into the polyol solution, and subsequently, 25 ml AgNO3 EG solution (600 mM) was added into the mixed solution dropwise within 3 min. Vigorous stirring was maintained throughout the entire process. After 30 min, the one-pot reaction was quenched by cooling. The cooled solution was centrifugal at 5000 r.p.m. with PA for at least 30 min until the suspension sank to the bottom and the up-solution was clear. Then the precipitate was centrifuged for three times at 3000 r.p.m. for 10 min each time and washed with ethanol to remove excess solvent, PVP and other impurities in the supernatant.
2.3. Fabrication of silver electrodes
Silver electrode films were prepared by dip-coating on 2 × 2 cm glass slides with a dip coater (SYDC-100, Shanghai San Yan Technology Corp., Ltd). These substrates were thoroughly cleaned with detergent and washed with deionized water, sonicated in ethanol for 5 min and dried in an oven. Then the substrates were clamped to the fixture above the ethanol solution of AgNPs. After setting the cycles and the rates of pull-ups and other parameters, the dip coater was used to prepare silver film.
Three sintering processes, that is, conventional sintering, infrared sintering and microwave sintering, were used to achieve the sintering of the dip-coated silver films after dip-coating. In the conventional sintering, the silver films were sintered in a muffle furnace at different temperatures (50–400°C). In the infrared sintering, the silver films were placed under an infrared baking lamp and baked at different powers (100–250 W) for different sintering times (30–60 min). In the microwave sintering, the silver films were placed in a microwave processing instrument at different microwave powers (250–1000 W) for different sintering times (20–50 s). All three sintering processes are in air atmosphere.
2.4. Characterization
Morphologies of the AgNPs and films were characterized by using a scanning electron microscope (SEM; SU8010, Hitachi Ltd, Japan) and a transmission electron microscope (TEM; JEM-1230, JEOL Ltd, Japan). Powder X-ray diffractions (XRD) of the AgNPs were performed with an Empyrean 200895 diffractometer (XRD-6000, Shimadzu, Japan) using CuK (λ = 0.154 nm) radiation as an incident beam. The chemical composition of the AgNPs was obtained using energy-dispersive X-ray spectroscopy (EDS). The sheet resistance of the silver films was measured using a 4-point probe sheet resistance meter (RTS-9, 4 PROBES TECH). The thickness and roughness of the silver films were carried out on a step profiler (DEKTAK-XT, Bruker).
3. Result and discussion
3.1. Morphology and microstructure of silver nanoparticles
In this work, AgNPs were synthesized by a common polyol process [20–27], and the morphology and particle size of as-prepared AgNPs were investigated and are shown in figure 1a–c. The diameters of 1000 Ag particles were measured and counted by Nano Measurer software. It is demonstrated that the synthesized AgNPs have a small particle size and narrow particle size distribution, the average size of AgNPs is about 43 nm, and the particle size of about 62.2% is distributed between 32 and 54 nm. The composition of AgNPs was characterized by EDS and XRD, as shown in figure 1d,e. It can be seen that the content of silver reaches 83.77%, the contents of C and N elements are 13.69% and 2.55%, respectively, indicating the existence of PVP in the samples. AgNPs coated with PVP can be uniformly dispersed in ethanol owing to the unit group (N=C–O) of PVP. The N=C–O group has a pair of lone pair electrons, and the electrons will combine with the silver ions, which promotes the combination of PVP and silver atoms to form the complex and also improves the dispersion of AgNPs [26]. It is observed from the XRD pattern of the AgNPs that all the reflection peaks can be indexed to silver, which confirms that the as-prepared particles are pure AgNPs. The lattice constant calculated from the pattern is 4.087 Å, which is consistent with the standard value of 4.086 Å (JCPDS Card File No. 4-783) [28]. It indicates that there are no impurities detected from the AgNPs.
Figure 1.
TEM of AgNPs magnified (a) 20 000×; (b) 50 000×; (c) size statistics of AgNPs; (d) EDS diagram and element composition of AgNPs; (e) XRD pattern of AgNPs indicating the fcc structure of silver.
3.2. Dip-coating and conventional sintering preparation of silver electrodes
There are many ways to prepare thin films, such as spinning, scraping, dip-coating and so on [29–34]. Among these methods, the dip-coating method is a simple way to fabricate smooth thin film by repeated coating many times [35–37]. In this work, dip-coating is adopted to prepare AgNP-based thin film, and the process of dip-coating followed by conventional sintering is shown in figure 2a. After dip-coating, the dip-coated silver electrodes without any post-treatment are non-conductive, which indicates that the silver particles are coated by PVP. As shown in figure 2b, the dip-coated thin film becomes very bright and smooth and looks like a mirror. After conventional sintering in muffle furnace, the PVP is decomposed, and silver particles melt and coagulate together to form conductive electrodes of pure silver. The as-prepared silver electrodes are rough and become a little white.
Figure 2.
(a) Process of dip-coating and conventional sintering of silver film; (b) photographs of silver film without conventional sintering; (c) photographs of silver films after conventional sintering in a muffle oven.
The thickness and roughness of dip-coated silver films are measured by a step profiler, as shown in figure 3. The calculation of roughness was carried out in the supporting software of the step profiler. It can be seen that the thickness of silver films increases with the increase of coating times. When the number of coating times increase from 10 to 100 times, the thickness of silver films increases from 498 nm to 5.19 µm. The roughness of silver films becomes larger with the increase of pulling rate. The roughness of silver films is 2.25 nm at 250 µm s−1 of pulling rate, and becomes 1.98 nm when the pulling rate increases to 500 µm s−1. With a further increase of pulling rate, the roughness rapidly increases, and reaches 18.28 nm at 4000 µm s−1. Considering the structure of OLED or other devices, the suitable silver film with a thickness of 1.97 µm and a roughness of about 2 nm can be prepared after dip-coating at 500 µm s−1 of pulling rate for 40 coating times.
Figure 3.
(a) Thickness of silver films coated at different coating times; (b) roughness of silver films coated at different pulling rate.
The conventional sintering of dip-coated silver films was carried out in a muffle furnace to prepare conductive silver electrodes. Table 1 shows the conductivity of silver films with different numbers of coating times and after being conventionally sintered at different sintering temperatures for 30 min. It is noted that the dip-coating times and conventional sintering have an important effect on the conductivity of silver films. The silver films without sintering and sintered at 50°C are non-conductive, although they are dip-coated many times. When the sintering temperature is 100°C, the silver films dip-coated 10 or 20 times are still non-conductive, while the silver films become good conductors, after being dip-coated more than 40 times. When the sintering temperature increases from 100°C to 350°C, most of silver films with different coating times are conductive, while the silver films become non-conductive again, when the sintering temperature rises to 400°C. It can be seen from the conductive silver films at a constant sintering temperature that the conductivity of silver films increases with an increase in number of dip-coating times, and at constant number of dip-coating times, the conductivity of silver films increases with the rising of sintering temperature. After being dip-coated for 40 times and conventionally sintered at 250°C for 30 min, a silver film with a low sheet resistance (less than 1 Ω sq−1) can be obtained.
Table 1.
Conductivity of silver electrodes at different coating times and heating temperatures.
| no. of coating times |
||||||
|---|---|---|---|---|---|---|
| sintering temperature (°C) | 10 | 20 | 40 | 60 | 80 | 100 |
| without sintering | — | — | — | — | — | — |
| 50 | — | — | — | — | — | — |
| 100 | — | — | 4 | 2.4 | 1.9 | 0.7 |
| 150 | — | 10 | 2.1 | 1.6 | 1.2 | 0.5 |
| 200 | — | 4 | 1.4 | 0.95 | 0.86 | 0.44 |
| 250 | 49.6 | 1.9 | 0.94 | 0.71 | 0.57 | 0.23 |
| 300 | — | — | 3.9 | 0.5 | 0.45 | 0.16 |
| 400 | — | — | — | — | — | — |
The surface morphology of the silver electrodes after conventional sintering was characterized by SEM, shown in figure 4. It can be noted that in the silver film without sintering, the nanoparticles are piled up on the substrate, there are many pores between the AgNPs. When the sintering temperature reaches 100°C or 200°C, AgNPs are still piled up, with a few melted nanoparticles. When the sintering temperature rises to 300°C, all the nanoparticles melt together to form an individual layer, while the graininess can be still observed. When the temperature further increases to 400°C, the nanoparticles are completely deformed and wrecked, and there exist some isolated large silver particles or little silver bulk. TG and DTA tests are carried out on the AgNP powder at a heating rate of 10°C min−1 in the atmosphere. TG records the weight change of the sample, while DTA records the energy change of the sample. It is seen from the TG and DTA of AgNPs (figure 4f) that there exists a small exothermic peak at around 200°C, which indicates that the nanoparticles begin sintering at this temperature. AgNPs containing high energy atoms are highly unstable, and will diffuse and cause surface sintering at high temperature. A sharp exothermic peak near 300°C is apparently attributed to the decomposition of PVP, which suggests that the temperature of conventional sintering should be designed to more than 300°C to remove the PVP. It can be seen from the TG diagram that there is a weight increase before 200°C, which may be caused by oxidation or instrument error. An obvious weight loss between 200°C and 400°C is owing to the decomposition of PVP.
Figure 4.
SEM image of silver films without sintering (a) and sintering at 100°C (b), 200°C (c), 300°C (d) and 400°C (e); (f) DTA and TG curve of AgNPs.
3.3. Infrared sintering and conductivity of silver electrodes
Infrared sintering technology is a photonic sintering approach for metal nanoparticles [38–40]. In this work, the infrared sintering process is used to promote the deformation of PVP on the surface of AgNPs and improve the conductivity of the silver film. The schematic and physical drawings are shown in figure 5a. The silver electrode is directly irradiated and sintered by infrared light from the top side. Infrared light does not bring enough energy to melt the AgNPs and decompose the PVP. Therefore, silver particles can be in close contact, so silver electrodes have better conductivity and flatness. It can be seen from figure 5b that the silver film still reflects as smoothly as a mirror.
Figure 5.
(a) Process schematic diagram and (b) photograph of silver film after infrared sintering.
Figure 6 is the sheet resistance of the silver film obtained with different baking times and power. Under the same conditions of baking time of 60 min, the sheet resistance of the silver film decreases with the increase of power. The silver film after being baked at 100 W is relatively high, at 27.6 Ω sq−1. When the power increases to 150 W, the sheet resistance decreases to 8.65 Ω sq−1, which is still a high value. With the increase of power to 200 W, the sheet resistance continues to decrease to 1.86 Ω sq−1, a lower value. Further increasing power to 250 W, the sheet resistance becomes very low, at 0.89 Ω sq−1. Under the same condition of power of 200 W, the sheet resistance of the silver film decreases with the increase of baking time. When the baking time is 30 min, the sheet resistance is 12.31 Ω sq−1, and decreases to 2.09 Ω sq−1 when the baking time increases to 40 min. With a further increase in baking time to 50 min, the sheet resistance continuously decreases to 1.7 Ω sq−1, and slightly increases to 1.86 Ω sq−1 at the baking time of 60 min. With the increase of irradiating power and time of the infrared sintering, the conductivity of silver film increases and the sheet resistance declined, this results from the deformation effect of PVP.
Figure 6.
Sheet resistance of silver electrodes at different baking powers (a) and baking times (b) by infrared sintering.
Figure 7 shows SEM images of silver electrodes after being baked at different baking powers for 30 min and baked at 200 W for various baking times, respectively. It can be seen that the baking power and baking time do not affect the surface morphology of the silver films, and the nanoparticles do not obviously melt.
Figure 7.
SEM images of silver electrodes after being baked at (a) 100 W, (b) 150 W, (c) 200 W and (d) 250 W power for 30 min, respectively, and baked at 200 W for (e) 30 min, (f) 40 min, (g) 50 min and (h) 60 min, respectively.
Figure 8 shows the thickness and roughness of infrared sintered silver electrodes measured by a step profiler. The thicknesses of silver films after infrared sintering at 50 W for 30 min, 200 W for 30 W, and 200 W for 60 min are 1.94, 2.16 and 2.07 µm, and the roughness of the three samples is 1.3, 1.39 and 0.68 nm, respectively, indicating no significant change after infrared sintering. It can also be considered that infrared sintering has no obvious effect on the surface morphology of the silver film, while promoting the deformation of PVP to increase the conductivity of silver film.
Figure 8.
Thickness and roughness of silver electrodes baking at (a,d) 50 W for 30 min; (b,e) 200 W for 30 min; (c,f) 200 W for 60 min.
3.4. Microwave sintering and conductivity of silver electrodes
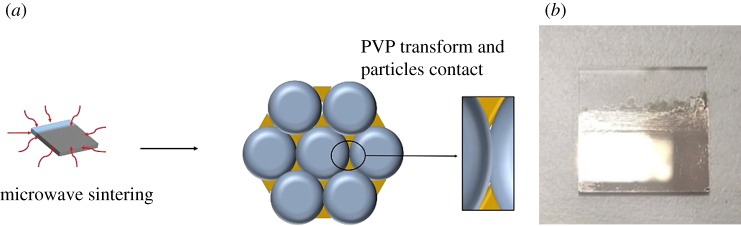
Microwave sintering is widely used for the densification of ceramic materials and in synthetic chemistry because of its advantages such as uniform, fast and volumetric heating [41,42]. In this work, the microwave sintering process is also used to promote the deformation of PVP on the surface of AgNPs and improve the conductivity of the silver film. Its schematic process diagram and physical photo diagram are shown in figure 9. The silver electrode is irradiated and sintered by microwaves in all directions, and all the nanoparticles on the surface and inside of the silver electrode will be heated. The metals can reflect the microwaves, and the PVP in silver film can be heated strongly. During microwave sintering, AgNPs do not melt under low power microwave, and the PVP is deformed by microwave energy to make silver particles contact with each other. Sintering time is very short because of the high penetration and high energy of microwaves, and the reflection of AgNPs. The characteristics of microwave sintering is uniformity, sufficiency and rapidity.
Figure 9.
(a) Process schematic diagram and (b) photograph of the silver film after microwave sintering.
Figure 10 shows the sheet resistance of silver electrodes obtained under different microwave sintering times and powers. The sheet resistance of silver electrodes decreases with the increase of microwave power at a constant sintering time, and decreases with increasing sintering time at constant microwave power. At 500 W for 50 s, the sheet resistance of silver electrodes is the lowest, at 0.75 Ω sq−1. The microwave sintering is similar to the infrared sintering, but the temperature field is more uniform because of heating from all directions. With the increase of the power and time of microwave sintering, the conductivity of silver electrodes increases and the sheet resistance declines.
Figure 10.
Sheet resistance of silver electrodes at different baking powers (a) and baking times (b) by microwave sintering.
Figure 11 shows the SEM images of silver electrodes after microwave sintering at 250, 500, 750 and 1000 W for 30 s and microwave sintering at 500 W for 20, 30, 40 and 50 s, respectively. It can be also seen that the microwave sintering power and time have no obvious effect on the surface morphology of the silver electrodes.
Figure 11.
SEM images of silver electrodes after microwave sintering at (a) 250 W, (b) 500 W, (c) 750 W and (d) 1000 W for 30 s, respectively, and microwave sintering at 500 W for (e) 20 s, (f) 30 s, (g) 40 s and (h) 50 s, respectively.
Figure 12 shows the thickness and roughness of microwave sintered silver electrodes measured by a step profiler. The thicknesses of silver electrodes after microwave sintering at 250, 1000 and 500 W for 50 s are 2.18, 1.81 and 2.54 µm respectively, and the roughness are 1.16, 0.91 and 0.90 nm respectively. It confirms that microwave sintering has no obvious effect on the surface morphology of the silver electrodes.
Figure 12.
Thickness and roughness of silver electrodes after microwave sintering at (a,d) 250 W for 30 s; (b,e) 1000 W for 30 s; and (c,f) 500 W for 50 s.
The sheet resistances of silver electrodes after suitable conventional sintering, infrared sintering and microwave sintering are compared and shown in figure 13a. It can be seen that the sheet resistance of silver electrodes after microwave sintering is the lowest among the three sintering methods, at 0.75 Ω sq−1. A silver electrode obtained by microwave sintering not only has good conductivity, but also has very short sintering time (less than 1 min). Figure 13b shows a verification circuit consisting of a power supply, a switch and a small light bulb. The lighting of the bulb proves that the microwave sintered silver electrode has good conductivity.
Figure 13.
(a) Conductivity comparison among silver electrodes by conventional sintering at 250°C for 30 min, infrared sintering at 250 W for 30 min, and microwave sintering at 500 W for 50 s; (b) photograph of verification circuit to prove the conductivity of microwave sintered silver electrodes.
4. Conclusion
In summary, the silver electrodes were facilely prepared by using synthesized AgNPs by a polyol method as raw materials, coating silver film by the dip-coating method and sintering the silver film by a sintering process. The surface morphology and conductivity of dip-coated silver film and silver electrodes sintered by conventional sintering, infrared sintering and microwave sintering were investigated in detail. The polyol-synthesized AgNPs have a narrow particle size distribution with an average diameter of 43 nm and uniformly disperse in the solution. The dip-coating process by dip-coating 40 times and at 500 µm s−1 of pulling rate are suitable to prepare silver film with good surface morphology (a thickness of 1.97 µm and a roughness of about 2 nm). Conventional sintering and infrared sintering can achieve low sheet resistance (less than 1 Ω sq−1) and good morphology of silver electrodes; however, high sintering temperature and long sintering time limit their application to some extent. The silver films obtained by microwave sintering have the lowest sheet resistance (0.75 Ω sq−1) and a low surface roughness (less than 1 nm), and can achieve quick sintering of silver films (sintering time less than 1 min) to prepare silver electrodes. This preparation route could be applied to construct OLED, solar cells or other electronic devices.
Supplementary Material
Acknowledgements
The authors thank the editors and anonymous reviewers for their constructive suggestions.
Data accessibility
Data used to produce the results in figures 3, 8 and 12 are available within the Dryad Digital Repository: https://doi.org/10.5061/dryad.9s4mw6mbc [43].
Authors' contributions
T.C., S.B. and Y.Z. contributed to experiments and tests, and T.C. wrote this manuscript. H.Y. and X.G. contributed to the theoretical guidance and the decision of the experimental plan. All authors gave final approval for publication.
Competing interests
We declare we have no competing interests.
Funding
This study was funded by the National Key Research and Development Program (grant no. 2016YFB0401305).
References
- 1.Lee GH, Choi DH, Kim YS. 2019. Thioxanthen-based blue thermally activated delayed fluorescence emitters for organic light-emitting diodes. J. Nanosci. Nanotechno. 19, 6796–6800. ( 10.1166/jnn.2019.17119) [DOI] [PubMed] [Google Scholar]
- 2.Triambulo RE, Kim J, Park J. 2019. Highly flexible organic light-emitting diodes on patterned Ag nanowire network transparent electrodes. Org. Electron. 71, 220–226. ( 10.1016/j.orgel.2019.05.035) [DOI] [Google Scholar]
- 3.Kim A, Huseynova G, Lee J, Lee J. 2019. Enhancement of out-coupling efficiency of flexible organic light-emitting diodes fabricated on an MLA-patterned parylene substrate. Org. Electron. 71, 246–250. ( 10.1016/j.orgel.2019.05.025) [DOI] [Google Scholar]
- 4.Han JW, Joo CW, Lee J, Le DJ, Kang J, Yu S, Sung WJ, Cho NS, Kim YH. 2019. Enhanced outcoupling in down-conversion white organic light-emitting diodes using imprinted microlens array films with breath figure patterns. Sci. Technol. Adv. Mat. 20, 35–41. ( 10.1080/14686996.2018.1551040) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yeh C, Lo KS, Lin W. 2019. Visual-attention-based pixel dimming technique for OLED displays of mobile devices. IEEE T. Ind. Electron. 66, 7159–7167. ( 10.1109/TIE.2018.2874582) [DOI] [Google Scholar]
- 6.Wang Y, Liu P, Wang H, Zeng B, Wang J, Chi F. 2019. Flexible organic light-emitting devices with copper nanowire composite transparent conductive electrode. J. Mater. Sci. 54, 2343–2350. ( 10.1007/s10853-018-2986-9) [DOI] [Google Scholar]
- 7.Wei M, Wang H, Wang J, Chen P, Zhao W, Chen X, Guo J, Kang B, Duan Y. 2018. Flexible transparent electrodes for organic light-emitting diodes simply fabricated with AuCl3-modied graphene. Org. Electron. 63, 71–77. ( 10.1016/j.orgel.2018.08.050) [DOI] [Google Scholar]
- 8.Niu Z, Cui F, Kuttner E, Xie C, Chen H, Sun Y, Dehestani A, Schierle-Arndt K, Yang P. 2018. Synthesis of silver nanowires with reduced diameters using benzoin-derived radicals to make transparent conductors with high transparency and low haze. Nano Lett. 18, 5329–5334. ( 10.1021/acs.nanolett.8b02479) [DOI] [PubMed] [Google Scholar]
- 9.Jung D, et al. 2015. Transparent and flexible conducting hybrid film combined with 3-Aminopropyltriethoxysilane-coated polymer and graphene. Appl. Surf. Sci. 357, 287–292. ( 10.1016/j.apsusc.2015.08.139) [DOI] [Google Scholar]
- 10.Zhang D, Wang R, Wen M, Weng D, Cui X, Sun J, Li H, Lu Y. 2012. Synthesis of ultralong copper nanowires for high-performance transparent electrodes. J. Am. Chem. Soc. 134, 14 283–14 286. ( 10.1021/ja3050184) [DOI] [PubMed] [Google Scholar]
- 11.Rogacheva EI, Menshikova SI, Sipatov AY, Nashchekina ON. 2019. Thickness-dependent quantum oscillations of the transport properties in bismuth selenide thin films. Thin Solid Films 684, 31–35. ( 10.1016/j.tsf.2019.05.046) [DOI] [Google Scholar]
- 12.Faniayeu L, Ishimatsu Y, Nakajima T. 2019. Surface plasmon resonance tuning of Ag nanoisland films using a CO2 laser. J. Phys. D Appl. Phys. 52, 29510 ( 10.1088/1361-6463/ab1b7b) [DOI] [Google Scholar]
- 13.Hartl H, East C, Xu Y, Yambem SD, Fairfull-Smith KE, Macleod J. 2019. Direct-write crosslinking in vacuum-deposited small-molecule films using focussed ion and electron beams. Nanotechnology 30, 335301 ( 10.1088/1361-6528/ab1b86) [DOI] [PubMed] [Google Scholar]
- 14.Sandstrom A, Dam HF, Krebs FC, Edman L. 2012. Ambient fabrication of flexible and large-area organic light-emitting devices using slot-die coating. Nat. Commun. 3, 1002 ( 10.1038/ncomms2002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Youn H, Jeon K, Shin S, Yang M. 2012. All-solution blade-slit coated polymer light-emitting diodes. Org. Electron. 13, 1470–1478. ( 10.1016/j.orgel.2012.04.008) [DOI] [Google Scholar]
- 16.Triambulo RE, Cheong H, Park J. 2014. All-solution-processed foldable transparent electrodes of Ag nanowire mesh and metal matrix films for flexible electronics. Org. Electron. 15, 2685–2695. ( 10.1016/j.orgel.2014.07.039) [DOI] [Google Scholar]
- 17.You J, Tseng S, Meng H, Yen F, Lin I, Horng S. 2009. All-solution-processed blue small molecular organic light-emitting diodes with multilayer device structure. Org. Electron. 10, 1610–1614. ( 10.1016/j.orgel.2009.06.020) [DOI] [Google Scholar]
- 18.Khatri I, Liu Q, Ishikawa R, Ueno K, Shirai H. 2014. Self assembled silver nanowire mesh as top electrode for organic-inorganic hybrid solar cell. Can. J. Phys. 92, 867–870. ( 10.1139/cjp-2013-0564) [DOI] [Google Scholar]
- 19.Yu C, Baek JD, Su C, Fan L, Wei J, Liao Y, Su P. 2016. Inkjet-printed porous silver thin film as a cathode for a low-temperature solid oxide fuel cell. Acs Appl. Mater. Inter. 8, 10 343–10 349. ( 10.1021/acsami.6b01943) [DOI] [PubMed] [Google Scholar]
- 20.Chen T, Wang H, Yang H, Bai S, Guo X. 2018. Mixed polyols synthesis of high aspect ratio silver nanowires for transparent conductive films. Mater Res Express. 5, 066426. [Google Scholar]
- 21.Yang H, Chen T, Wang H, Bai S, Guo X. 2018. One-pot rapid synthesis of high aspect ratio silver nanowires for transparent conductive electrodes. Mater. Res. Bull. 102, 79–85. ( 10.1016/j.materresbull.2018.02.010) [DOI] [Google Scholar]
- 22.Ding H, Zhang Y, Yang G, Zhang S, Yu L, Zhang P. 2016. Large scale preparation of silver nanowires with different diameters by a one-pot method and their application in transparent conducting films. Rsc Adv. 6, 8096–8102. ( 10.1039/C5RA25474D) [DOI] [Google Scholar]
- 23.Zong RL, Zhou J, Li Q, Du B, Li B, Fu M, Qi XW, Li LT, Buddhudu S. 2004. Synthesis and optical properties of silver nanowire arrays embedded in anodic alumina membrane. J. Phys. Chem. B. 108, 16 713–16 716. ( 10.1021/jp0474172) [DOI] [Google Scholar]
- 24.Kim SH, Choi BS, Kang K, Choi Y, Yang SI. 2007. Low temperature synthesis and growth mechanism of Ag nanowires. J. Alloy. Compd 433, 261–264. ( 10.1016/j.jallcom.2006.06.053) [DOI] [Google Scholar]
- 25.Sun YG, Yin YD, Mayers BT, Herricks T, Xia YN. 2002. Uniform silver nanowires synthesis by reducing AgNO3 with ethylene glycol in the presence of seeds and poly(vinyl pyrrolidone). Chem. Mater. 14, 4736–4745. ( 10.1021/cm020587b) [DOI] [Google Scholar]
- 26.Sun YG, Gates B, Mayers B, Xia YN. 2002. Crystalline silver nanowires by soft solution processing. Nano Lett. 2, 165–168. ( 10.1021/nl010093y) [DOI] [Google Scholar]
- 27.Sun YG, Mayers B, Herricks T, Xia YN. 2003. Polyol synthesis of uniform silver nanowires: a plausible growth mechanism and the supporting evidence. Nano Lett. 3, 955–960. ( 10.1021/nl034312m) [DOI] [Google Scholar]
- 28.Kalimuthu K, Babu RS, Venkataraman D, Bilal M, Gurunathan S. 2008. Biosynthesis of silver nanocrystals by Bacillus licheniformis. Colloid. Surface B 65, 150–153. ( 10.1016/j.colsurfb.2008.02.018) [DOI] [PubMed] [Google Scholar]
- 29.Zhang S, Zhang J, Yang J. 2009. Flue gas treatment on the combustion of scrap coated by paint, oil dirty or rubber. Environ. Eng. 27, 65–67. ( 10.13205/j.hjgc.2009.04.028) [DOI] [Google Scholar]
- 30.Bai S, Wang H, Yang H, Zhang H, Guo X. 2018. Preparation of smooth, flexible and stable silver nanowires-polyurethane composite transparent conductive films by transfer method. Mater. Res. Express 5, 026406. ( 10.1088/2053-1591/aaab26) [DOI] [Google Scholar]
- 31.Sarjidan MAM, Shuhaimi A, Majid WHA. 2019. Solution-processable vertical organic light-emitting transistors (VOLETs) with directly deposited silver nanowires intermediate source electrode. J. Nanosci. Nanotechno. 19, 6995–7003. ( 10.1166/jnn.2019.16724) [DOI] [PubMed] [Google Scholar]
- 32.Hongsith K, Wongrerkdee S, Ngamjarurojana A, Choopun S. 2019. Efficiency enhancement of perovskite solar cell by using pre-heat treatment in two-step deposition method. Thin Solid Films 684, 9–14. ( 10.1016/j.tsf.2019.05.055) [DOI] [Google Scholar]
- 33.Maeda R, Kawakami H, Shinohara Y, Kanazawa I, Mitsuishi M. 2019. Thermoelectric properties of PEDOT/PSS films prepared by a gel-film formation process. Mater. Lett. 251, 169–171. ( 10.1016/j.matlet.2019.05.005) [DOI] [Google Scholar]
- 34.Bai S, Wang H, Yang H, Zhang H, Chen T, Guo X. 2018. Fused silver nanowires with silica sol nanoparticles for smooth, flexible, electrically conductive and highly stable transparent electrodes. Rsc Adv. 8, 13 466–13 473. ( 10.1039/C8RA01569D) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chun S, Kim K, Lee S, Kim KT, Park W. 2019. A highly efficient absorbent for various organic solvents using hydrophobic graphene-sponge composite. J. Nanosci. Nanotechno. 19, 6675–6681. ( 10.1166/jnn.2019.17100) [DOI] [PubMed] [Google Scholar]
- 36.Leitzke DW, et al. 2019. Electrochemical properties of WO3 sol-gel thin films on indium tin oxide/poly(ethylene terephthalate) substrate. Thin Solid Films 683, 8–15. ( 10.1016/j.tsf.2019.05.018) [DOI] [Google Scholar]
- 37.Skolik M, Domanowska A, Karasinski P, Gondek E, Michalewicz A. 2019. Double layer sol-gel derived antireflective coatings on silicon - design, optical and auger electron spectroscopy characterization. Mater. Lett. 251, 210–213. ( 10.1016/j.matlet.2019.05.071) [DOI] [Google Scholar]
- 38.Haouanoh D, Talaighil RZ, Toubane M, Bensouici F, Mokeddem K. 2019. Effects of thermal treatment and layers' number on SnO2 thin films properties prepared by sol-gel technique. Mater. Res. Express 6, 086422 ( 10.1088/2053-1591/ab1d96) [DOI] [Google Scholar]
- 39.He X, Wang F, Liu H, Wang X, Gao J. 2019. Synthesis and coloration of highly dispersive SiO2/BiVO4 hybrid pigments with low cost and high NIR reflectance. Nanotechnology 30, 295701 ( 10.1088/1361-6528/ab0ce0) [DOI] [PubMed] [Google Scholar]
- 40.Kim K, Son E, Youn JW, Kim DU. 2019. Intense pulsed light sintering of vanadium dioxide nanoparticle films and their optical properties for thermochromic smart window. Mater. Design 176, 107838 ( 10.1016/j.matdes.2019.107838) [DOI] [Google Scholar]
- 41.Zhang L, Wan X, Duan W, Qiu H, Hou J, Wang X, Li H, Du X. 2019. One-pot synthesis of doped-polypyrrole/Fe3O4 nanosphere composites and their microwave absorption performance. J. Nanosci. Nanotechnol. 19, 7664–7672. ( 10.1166/jnn.2019.16767) [DOI] [PubMed] [Google Scholar]
- 42.Li Y, Zheng R, Lei Y, Chen W, Wan R, Zhou H, Chu PK. 2019. Microwave synthesis and enhancement of power factors of HfxTi1-xNiSn. Mater. Lett. 251, 13–17. ( 10.1016/j.matlet.2019.04.116) [DOI] [Google Scholar]
- 43.Chen T, Yang H, Bai S, Zhang Y, Guo X. 2020. Data from: Facile preparation of high conductive silver electrodes by dip-coating followed by quick sintering Dryad Digital Repository. ( 10.5061/dryad.9s4mw6mbc) [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Chen T, Yang H, Bai S, Zhang Y, Guo X. 2020. Data from: Facile preparation of high conductive silver electrodes by dip-coating followed by quick sintering Dryad Digital Repository. ( 10.5061/dryad.9s4mw6mbc) [DOI] [PMC free article] [PubMed]
Supplementary Materials
Data Availability Statement
Data used to produce the results in figures 3, 8 and 12 are available within the Dryad Digital Repository: https://doi.org/10.5061/dryad.9s4mw6mbc [43].