Abstract
Both tetrahydroisoquinolines (THIQs) and oxindoles (OXs) display a broad range of biological activities including anti-cancer activity, and are therefore recognized as two privileged scaffolds in drug discovery. In the present study, 24 3′,4′-dihydro-2′H-spiro[indoline-3,1′-isoquinolin]-2-ones, designed as molecular hybrids of THIQ and OX, were synthesized and screened in vitro against 59 cell lines in the NCI-60 screen. Twenty compounds displayed weak to moderate inhibition of cell proliferation; among them, three compounds displayed at least 50% inhibition of cell proliferation. The compounds appeared to target primarily renal cell cancer lines; however, leukaemia, melanoma, non-small cell lung cancer, prostate, ovarian and even breast cancer cell lines were also affected. Therefore, this class of spirooxindoles may provide useful leads in the search for new anti-cancer agents.
Keywords: spirooxindoles, tetrahydroisoquinolines, molecular hybridization, cancer
1. Introduction
The term cancer refers to a group of more than 100 diseases characterized by a common feature of rapid and uncontrolled cell proliferation, invasiveness and metastasis, leading to death. In 2018, worldwide mortality from cancer alone accounted for an estimated 9.6 million deaths, thereby positioning cancer as the number two leading cause of death worldwide [1]. According to statistics from the World Health Organization, five forms of cancer account for the most deaths: lung (1.76 million deaths), liver (782 000 deaths), colorectal (862 000 deaths), stomach (783 000 deaths) and breast (627 000 deaths) [1,2]. In 2010, the total economic cost of cancer was estimated at 1.16 trillion US dollars [1]. When combined with the human cost of the disease, and the increasing incidence of the disease(s), there is no disputing that cancer constitutes a major public health problem worldwide that requires continued attention.
The clinical management of cancer relies on four main pillars: surgery, hormone therapy, radiotherapy and chemotherapy [3]. While these are often used in combination, there is a heavy reliance on chemotherapy. However, the effectiveness of many anti-cancer agents is compromised by chemoresistance. The toxic side effects of some of these drugs also limit their clinical utility. Consequently, there is a continuing need to develop new agents, particularly those with novel mechanisms of action that can be deployed in the clinic.
Privileged scaffolds, as defined by Evans et al. [4], are molecular frameworks that occur in numerous biologically active low molecular weight compounds. The recurrence of these molecular frameworks in biologically active molecules has led to the idea that these scaffolds are recognized by several molecular targets. Consequently, privileged scaffolds are frequently employed as starting points in drug discovery efforts, and the combination of two or more of such scaffolds, termed molecular hybridization [5], has therefore been adopted as a useful strategy for designing biologically active molecules. The present work describes the hybridization of two privileged scaffolds, tetrahydroisoquinoline (THIQ) and oxindole (OX), to form a new class of potential anti-proliferative agents.
The THIQs display a wide range of biological activities including central nervous system, cardiovascular, anti-tumour, antibacterial, antimicrobial, antitubercular, anti-viral, antifungal, antileishmanial, antitrypanosomal and antiplasmodial activities [6–16]. Their anti-tumour activity is attributed to several mechanisms, including induction of apoptosis and increase in caspase-3, -8, -9 and p53 [17]; downregulation of CDK2 and upregulation of the tumour suppressor genes p21, p27 and p53 [18]; inhibition of the MDM2–p53 interaction [19]; reversal of multidrug resistance [20]; inhibition of histone deacetylase [21]; and induction of G2/M cell cycle arrest and mitosis through the disruption of microtubule dynamics [22]. Molecules constructed from the THIQ scaffold may therefore be expected to display anti-proliferative activity.
Naturally occurring (figure 1) and synthetic OXs have also been found to display a diverse array of biological properties, including antimalarial, antifungal, anticonvulsant, anti-HIV, radical scavenging and antibacterial activities and anti-cancer activities [23–29]. Their anti-cancer activity is attributed to a number of mechanisms such as inhibition of caspase [30,31], p53 induction [32] and inhibition of the tumour suppressor gene p53 [33].
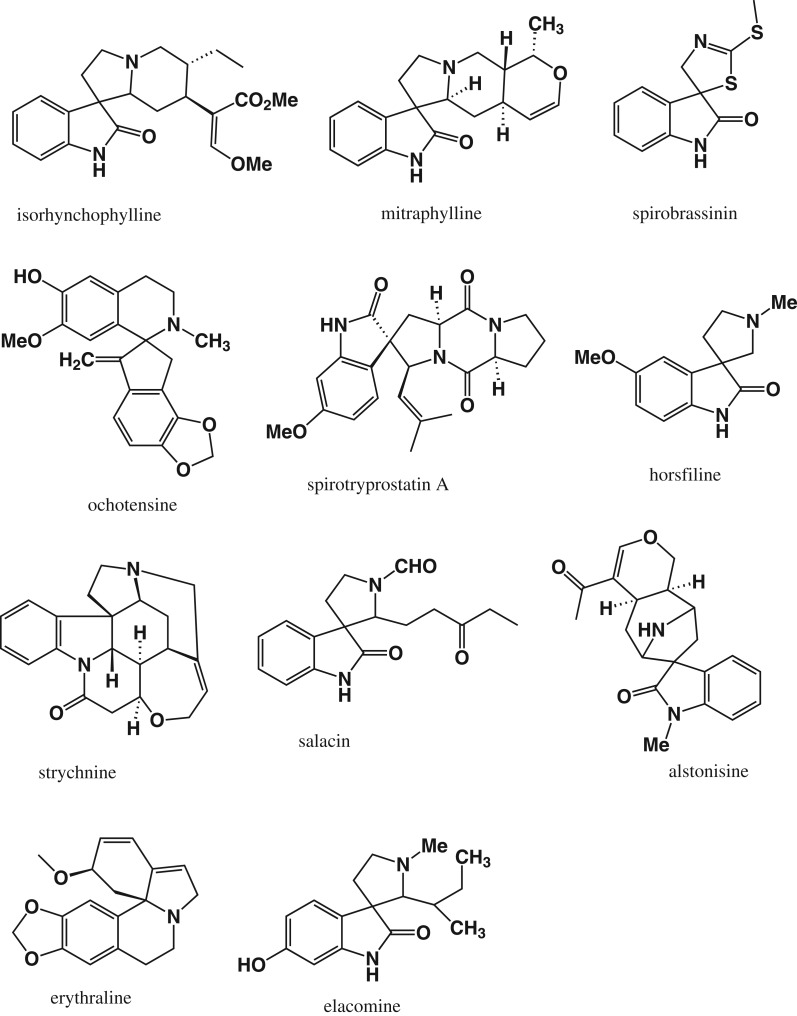
Figure 1.
Examples of naturally occurring spirooxindoles.
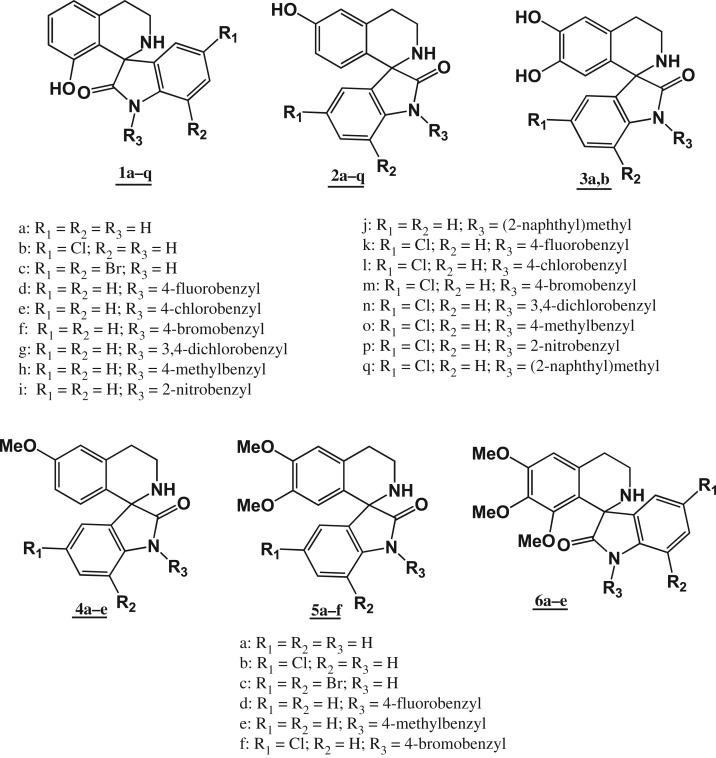
In recent years, spirocycles in general and spirooxindoles in particular have attracted much attention due to their unique structural aspects and their presence in a number of bioactive natural products [34,35]. Naturally occurring and synthetic spirooxindoles display a wide array of pharmacological activities including anti-cancer [36,37], anti-inflammatory [38], antimicrobial [35], antimalarial [39], antitubercular [40], antileishmanial [41], anti-cholinesterase [35] and anti-viral [42], activity, and therefore continue to attract interest as leads in drug discovery. Among naturally occurring spirooxindoles, the spiro[pyrrolidine-2 : 3-oxindole] scaffold (found in elacomine, horsfiline and rhynchophylline) appears quite frequently. This scaffold which may be regarded as arising from the intramolecular Mannich reaction between 3-(2-aminoethyl)indolin-2-one, an oxidation product of tryptamine, with an aldehyde has attracted considerable interest and provided many synthetic spirooxindoles [36]. However, the related class of spirooxindoles that arise from the Pictet–Spengler reaction of tryptamine with isatin (a naturally occurring compound) has been largely ignored save for work on the antimalarial agent NITD609 [39,43]. By extension, spirooxindoles, specifically 3′,4′-dihydro-2′H-spiro[indoline-3,1′-isoquinoline]-2-ones, that are obtained from the Pictet–Spengler type reaction between phenethylamines, another class of naturally occurring compounds, with isatin have also received scant attention. Formally, the latter spirooxindoles can be regarded as molecular hybrids of THIQ and OX arising from the Pictet–Spengler type reaction between a substituted phenethylamine and OX. In view of the anti-proliferative activity of both THIQs and OXs (including spirooxindoles), we proposed that this particular class of spirofused molecular hybrids of THIQ and OX would display anti-proliferative activity. A review of the literature at the beginning of the study revealed that the 3′,4′-dihydro-2′H-spiro[indoline-3,1′-isoquinolin]-2-one scaffold had been mentioned merely as a by-product of the reaction between isatin and some amines [44] and in a subsequent study of the kinetic and thermodynamic control of the Pictet–Spengler reaction of phenethylamines and isatin [45]. The present study thus focuses on the synthesis of 3′,4′-dihydro-2′H-spiro[indoline-3,1′-isoquinolin]-2-ones as molecular hybrids of THIQ and OX, and the evaluation of their anti-proliferative activity (figure 2).
Figure 2.
Structures of target compounds of the study.
2. Results and discussion
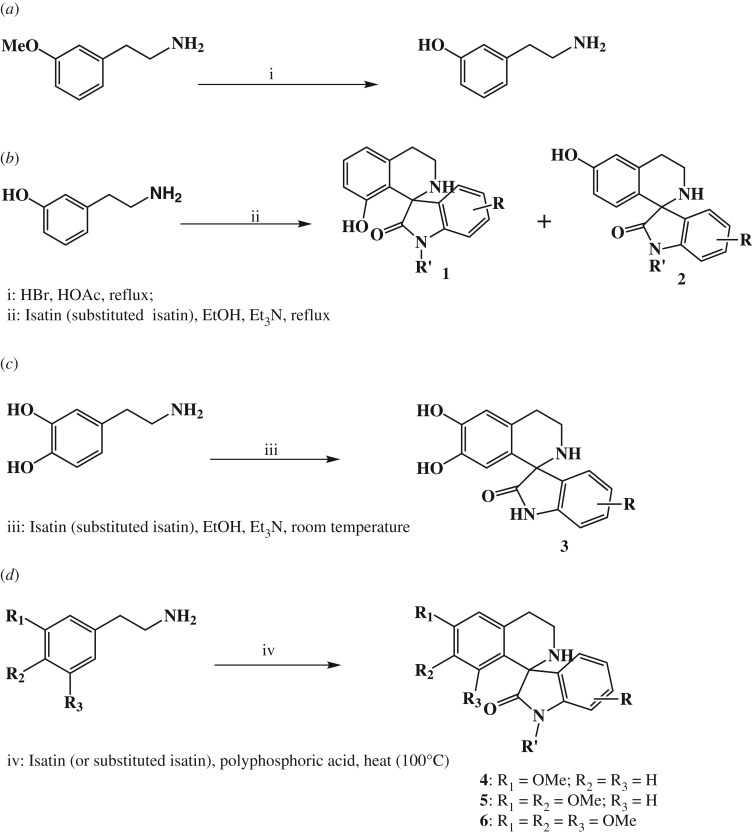
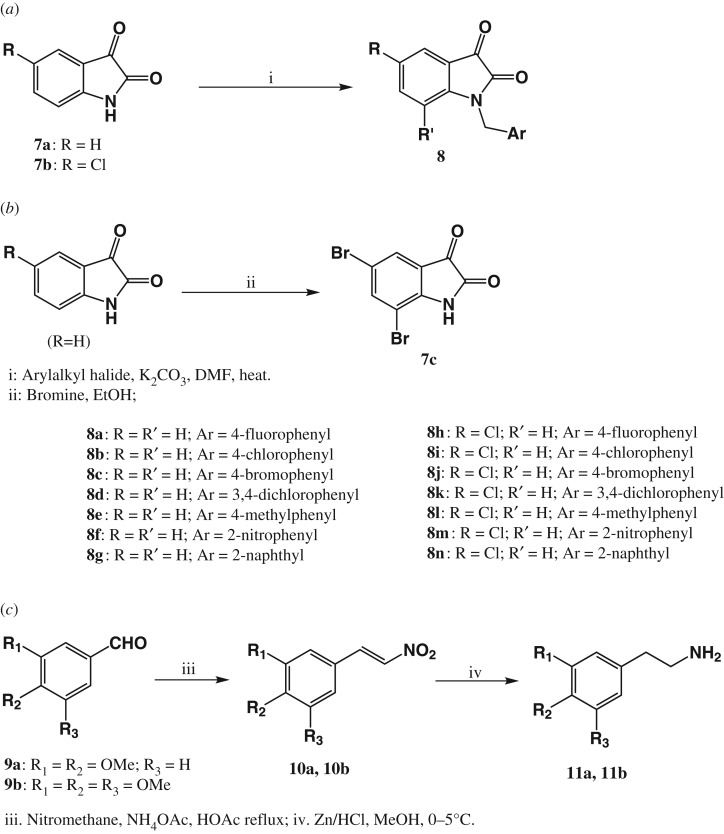
The target compounds were synthesized by the Pictet–Spengler reaction of a substituted phenethylamine with isatin or a substituted isatin. The precursors, 3,4-dimethoxyphenethylamine and 3,4,5-trimethoxyphenethylamine were prepared as previously described for related amphetamine analogues [46], while 3-hydroxyphenethylamine was obtained from the demethylation of commercially available 3-methoxyphenethylamine [47]. The N-alkylation of isatin and its analogues has also been reported [48]. For the synthesis of the hydroxylated compounds (1a–q, 2a–q and 3a,b), the phenolic Pictet–Spengler reaction, earlier described by Kametani et al. [47], was found to be quite useful as it proceeded smoothly under relatively mild conditions of heating in ethanol. This reaction generally provided two positional isomers, the 6-hydroxy (2a–q) and 8-hydroxy (1a–q) compounds, in variable ratios. In some instances, only trace amounts of the minor isomer were detected; therefore, only one isomer was isolated. The regioisomers were easily separated by medium pressure liquid chromatography or vacuum liquid chromatography on a silica gel column. To prepare the methoxyl-substituted spirooxindoles (compound series 4, 5 and 6), the Pictet–Spengler reaction of the methoxyphenethylamines with the corresponding isatins was best carried out in polyphosphoric acid at 100°C [49]. The yields were moderate to good. Under these reaction conditions, only a single regioisomer was obtained from the Pictet–Spengler reaction of 3-methoxphenethylamine with the isatins. All the compounds were converted to the corresponding hydrochlorides in methanolic HCl, recrystallized and submitted for biological testing.
The target compounds were submitted for anti-cancer screening under the NCI-60 programme of the National Cancer Institute, Bethesda, MD, USA (https://dtp.cancer.gov/discovery_development/nci-60/methodology.htm). In this protocol, test compounds are screened at a concentration of 10 µM against 59 cancer cell lines. Of 24 compounds tested, 20 were found to display weak to moderate anti-proliferative activity for at least one cancer cell line (table 1).
Table 1.
Inhibition of cancer cell growth by target spirooxindoles (concentration 10 µM).
| cancer type | compound (% inhibition of cell growth) |
|---|---|
| leukaemia (K-562) | 5f (44.6) |
| leukaemia (MOLT-4) | 5f (43.0) |
| leukaemia (RPMI-8226) | 5d (34.6); 5e (47.8); 5f (53.6) |
| leukaemia (SR) | 5f (37.7) |
| non-small cell lung cancer (HOP-92) | 1e (30.1); 2h (32.0) |
| colon cancer (HCT-116) | 5b (31.5) |
| central nervous system (SNB-75) | 4c (29.4); 5b (31.1) |
| melanoma (SK-MEL-5) | 5f (43.3) |
| melanoma (UACC-62) | 5d (30.3); 5e (33.5); 5f (45.0) |
| ovarian (IGROV1) | 1c (37.2) |
| ovarian (OVCAR-4) | 5f (39.3) |
| renal (A498) | 3b (50.2); 4b (29.6); 4c (33.0); 4d (52.9); 4e (46.1); 5a (36.7); 5c (35.7) |
| renal (CAKI-1) | 2c (35.5); 4e (39.0); 5f (30.1) |
| renal (UO-31) | 1c (35.3); 1e (35.2); 2 g (32.4); 4c (37.9); 4d (41.9); 5d (36.6); 6b (31.5); 6c (49.4); 6d (35.8); 6e (35.9) |
| prostate (PC-3) | 5d (33.8); 5e (42.2); 5f (52.1) |
| breast (MDA-MB-468) | 5f (35.7) |
Three compounds (3b, 4d, 5f) inhibited cell proliferation by at least 50% and compound 5f appeared to be the most active across a number of cell lines. Furthermore, renal cell cancer appeared to be the most susceptible to this class of compounds as up to 10 analogues displayed some anti-proliferative activity on this cancer cell line. Three compounds exerted anti-proliferative activity on the PC-3 cancer cell line, while the ovarian and breast cancer cell lines were inhibited by two and one compound, respectively.
Among the spirooxindoles showing evidence of anti-proliferative activity, analogues containing the 6,7-dimethoxy-THIQ fragment (series 5) appeared with the highest frequency while those containing the 6-methoxy-THIQ fragment (series 3) appeared with the lowest frequency. Therefore, the 6,7-dimethoxy-THIQ fragment appears to be the preferred fragment for subsequent optimization efforts of this hybrid scaffold. Of the 20 compounds showing notable anti-proliferative activity, nine (45%) were unsubstituted at the nitrogen atom of the OX fragment, while the rest featured a substituted benzyl fragment at this position. Therefore, subsequent optimization efforts may concentrate on the synthesis of either N-unsubstituted or N-substituted analogues. In the light of these findings, we conclude that the 3′,4′-dihydro-2′H-spiro[indoline-3,1′-isoquinolin]-2-one scaffold does possess anti-proliferative activity.
A recent investigation of the isomeric 2′,4′-dihydro-1′H-spiro[indoline-3,3′-isoquinolin]-2-ones, published while the current study was underway, found that the latter compounds display anti-cancer activity on colon cancer [50]. The most potent compound of that study was found to inhibit Ras-GTP and thereby suppress downstream signalling by MAPK, PI3 K-Akt and Wnt, ultimately resulting in mitochondrial apoptosis [50]. Therefore, while the mode of action of the target 3′,4′-dihydro-2′H-spiro[indoline-3,1′-isoquinolin]-2-ones of the present study is unknown, it does appear that spirofused molecular hybrids of THIQ and OX may be potentially useful leads for anti-cancer drug discovery.
Salsolinol [51] and many other naturally occurring THIQs arise from the Pictet–Spengler type reaction of a biogenic amine with an aldehyde or ketone. The design of the target compounds of this study therefore takes advantage of an endogenous pathway (biomimetic approach) that provides broad flexibility in the construction of compound libraries. Further investigation is underway to optimize the anti-proliferative activity of the target 3′,4′-dihydro-2′H-spiro[indoline-3,1′-isoquinolin]-2-ones against cell lines of interest and to elucidate their mechanism(s) of action.
3. Experimental procedure
3.1. General
All chemicals were purchased from Sigma Aldrich Chemicals Company, St Louis, MO, USA, and were used as supplied. All solvents were reagent grade. Where necessary, solvents and starting materials were purified using standard procedures. Solvent removal was performed in vacuo using a Buchi rotatory evaporator at temperatures not greater than 60°C. Melting points were measured using the Mel-Temp II apparatus with the use of open capillaries and are uncorrected.
The progress of all reactions was monitored using thin layer chromatography (TLC) on aluminium-backed sheets of Sigma Aldrich silica gel 60 F254 plates. Compounds were visualized under UV light at 254 nm or in an iodine chamber. Chromatographic purification of compounds was carried out by medium pressure liquid chromatography using silica gel 60–400 mesh. The solvent mixtures used in specific chromatographic runs are indicated where necessary.
High-resolution Fourier transform mass spectrometry electrospray ionization (FTMS-ESI) mass spectra were carried out on an LTQ Orbitrap XL mass spectrometer from Thermo Fisher Scientific (Bremen, Germany). A heated electrospray interface (H-ESI) was operated for ionization of the molecules at a spray voltage of 5 kV, whereas capillary voltage and tube lens voltages were adjusted to 20 and 100 V, respectively. The vaporizer temperature was set at 250°C and the ion transfer capillary temperature to 200°C. Measurements were carried out in the positive ion mode in a mass range of m/z 100–600 at a mass resolution of 60 000 at m/z 200. MS/MS experiments were performed using argon as collision gas in collision-induced dissociation (CID) mode, collision energies were measured at 15, 25 and 35 eV.
Nuclear magnetic resonance spectra were obtained using a Brucker Avance III spectrometer operating at 600 MHz (H1) and 150 MHz (13C). Spectra were recorded in deuterated solvents as indicated in brackets and referenced to residual solvent signals. Chemical shifts (δ) were measured in parts per million (ppm). Hydrogen and carbon peak assignments were performed using gradient correlation spectroscopy (gCOSY), heteronuclear single quantum correlation (gHSQC) spectroscopy and heteronuclear multiple bond correlation (gHMBC) techniques. For most of the compounds, signals of protons attached to oxygen and nitrogen were not observed and were attributed to exchange with solvent protons. Signal multiplicities are reported as singlet (s), doublet (d), doublet of doublets (dd), doublet of triplets (dt), triplet (t), triplet of doublets (td) and multiplet (m). Coupling constants (J) are reported in Hertz units.
Biological screening: The target compounds were tested in the NCI-60 screen following the published protocol (https://dtp.cancer.gov/discovery_development/nci-60/methodology.htm).
3.2. Synthesis of 3-hydroxyphenethylamine (method A)
This compound was synthesized following the method of Ngo Hanna et al. [52] (scheme 2a). In a typical experiment, a mixture of 3-methoxyphenethylamine (1.0 g, 6.6 mmol), hydrobromic acid (10 ml) and acetic acid (10 ml) was stirred and heated under reflux for 6 h. The cooled mixture was concentrated under reduced pressure to obtain a residue, which was used in subsequent reactions without further purification; crude yield, 97%.
Scheme 2.
Synthesis of target compounds. (a) Method A, (b) Method D, (c) Method E and (d) Method G.
3.3. General method for the synthesis of substituted N-benzylisatins (8a–n) (method B)
This was done using the method of Vine et al. [48] with some modifications (scheme 1a). The desired isatin (1 equiv) was taken up in anhydrous acetonitrile or DMF (1 ml per 0.1 mmol of isatin). Solid K2CO3 (1.2 equiv) was added in one portion, and the dark-coloured suspension was stirred at room temperature for 1 h. The appropriate benzyl halide (1.2 equiv) and KI (0.2 equiv) were added, and the reaction mixture was stirred at 80°C for 5–18 h, until the isatin starting material had been consumed as revealed by TLC. The reaction mixture was decanted into HCl (0.5 M, 50 ml) and extracted with methylene chloride (20 ml × 2), dried over anhydrous sodium sulfate and the solvent removed under reduced pressure. The crude product obtained was purified by flash chromatography using isocratic elution with hexane : ethyl acetate giving yellow to red crystals. Yields were between 80 and 95%.
Scheme 1.
Synthesis of major fragments. (a) Method B, (b) Method C and (c) Method F.
3.4. Synthesis of 5,7-dibromoisatin (7c) (method C)
The synthesis of 5,7-dibromoisatin was based on the method of Kumar et al. [25] (scheme 1b). Isatin (9.0 g, 61.2 mmol, 1 equiv) was warmed in ethanol (95%, 100 ml) with stirring until it dissolved. Bromine (3.0 equiv, 16.3 g, 183.6 mmol, 9.4 ml) was added dropwise to the stirred isatin solution while maintaining the temperature of the reaction mixture between 70 and 75°C. The solution was cooled to room temperature and placed on ice for 30 min. The resulting precipitate was washed with water and cold ethanol and then recrystallized from ethanol to yield bright orange-red crystals of 5,7-dibromoisatin (66%), m.p. 248–250°C (lit. 248–250°C).
3.5. General method for the synthesis of 6′-hydroxy-3′,4′-dihydro-2′H-spiro[indoline-3,1′-isoquinolin]-2-ones (1a–q) and 8′-hydroxy-3′,4′-dihydro-2′H-spiro[indoline-3,1′-isoquinolin]-2-ones (2a–q) (method D)
The compounds were synthesized by the phenolic Pictet–Spengler reaction reported by Kametani et al. [47] and modified by Ngo Hanna et al. [52] (scheme 2b). In a typical experiment, crude 3-hydroxyphenethylamine (0.9 g, 6.6 mmol, 1 equiv), obtained as described in Method A above, and the appropriate isatin (1 equiv, 6.6 mmol) were dissolved in absolute ethanol (10 ml) and triethylamine (1 ml) was added. The reaction mixture was stirred and heated under reflux for 7–10 h. Upon completion, the reaction was concentrated under reduced pressure to remove the solvent. Distilled water was added to the viscous mass and the product which precipitated out was extracted into ethyl acetate (3 × 30 ml). The combined organic extracts were dried over anhydrous sodium sulfate and concentrated to minimum volume. The crude product was further purified by column chromatography using the appropriate solvent system. The final product obtained was recrystallized from absolute ethanol. In most cases, the reaction provided two regioisomers as earlier documented by Kametani et al. [47]: the 8′-hydroxy compound, which had a shorter retention time, and the 6′-hydroxy isomer. The combined yields ranged between 40 and 99%.
3.6. 8′-Hydroxy-3′,4′-dihydro-2′H-spiro[indoline-3,1′-isoquinolin]-2-one (1a)
Method D. Prepared from 3-hydroxyphenethylamine (0.9 g, 6.61 mmol, 1 equiv) and isatin (0.96 g, 6.61 mmol, 1 equiv). The crude product was purified on flash chromatography (hexane : ethyl acetate—60 : 40) and recrystallized from methanol. Yield, 0.83 g, 45% (white solid). M.p. 256–258°C. 1H NMR (DMSO-d6, 700 MHz): δ ppm 2.80 (t, J = 5.8 Hz, 2H, H4′a, H4′b), 3.07 (dt, J = 12.9, 6.3 Hz, 1H, H3′a), 3.13–3.16 (m, 1H, H3′b), 6.42 (dd, J = 8.0, 1.2 Hz, 1H, H7′), 6.63 (dd, J = 7.60, 1.1 Hz, 1H, H5′), 6.80 (ddd, J = 7.3, 6.0, 1.3 Hz, 2H, H5, H7), 6.88 (m, 1H, H4), 6.98 (t, J = 7.7 Hz, 1H, H6′), 7.13 (td, J = 7.6, 1.3 Hz, 1H, H6), 9.12 (s, 1H, 8′-OH), 10.19 (s, 1H, H1). 13C NMR (DMSO-d6, 175 MHz): δ ppm 28.8 (C4′), 38.4 (C3′), 61.9 (C3/C1′), 108.8 (C7), 112.3 (C7′), 119. 6 (C5′), 120.6 (C5), 122.5 (C8′a), 123.1 (C4), 127.2 (C6′), 127.6 (C6), 135.6 (C3a), 138.4 (C4′a), 142.8 (C7a), 153.9 (C8′), 179.3 (C2). FTMS + cESI: m/z 267.11 [M + 1]+.
3.7. 5-Chloro-6′-hydroxy-3′,4′-dihydro-2′H-spiro[indoline-3,1′-isoquinolin]-2-one (2b)
Method D. Prepared from 3-hydroxyphenethylamine (1.2 g, 6.61 mmol) and 5-chloroisatin (0.9 g, 6.61 mmol). The crude product was purified by chromatography on a short column (hexane : ethyl acetate—60 : 40) and recrystallized from methanol. Yield, 1.0 g, 48% (white solid). M.p. 253–255°C. 1H NMR (DMSO-d6, 700 MHz): δ ppm 2.65 (dt, J = 16.0, 3.4 Hz, 1H, H4′a), 2.86 (ddd, J = 15.6, 9.3, 5.4 Hz, 1H, H4′b), 2.97 (dt, J = 12.1, 4.7 Hz, 1H, H3′a), 3.05 (br s, 1H, H2′), 3.60 (ddd, J = 12.8, 9.6, 4.1 Hz, 1H, H3′b), 6.24 (d, J = 8.4 Hz, 1H, H8′), 6.42 (dd, J = 8.4, 2.6 Hz, 1H, H7′), 6.55 (d, J = 2.6 Hz, 1H, H5′), 6.89 (d, J = 8.3 Hz, 1H, H7), 7.02 (d, J = 2.2, 1H, H4), 7.26 (dd, J = 8.3, 2.2 Hz, 1H, H6), 9.29 (s, 1H, 6′-OH), 10.37 (s, 1H, H1). 13C NMR (DMSO-d6, 175 MHz): δ ppm 28.8 (C4′), 38.0 (C3′), 63.3 (C3/C1′), 110.9 (C7), 113.7 (C7′), 115. 3 (C5′), 124.5 (C8′a), 125.0 (C4), 125.7 (C5), 126.9 (C8′), 128.3 (C6), 137.6 (C4′a), 138.1 (C3a), 141.1 (C7a), 156.0 (C6′), 180.0 (C2). FTMS + cESI: m/z 301.07 [M + 1]+
3.8. 5,7-Dibromo-8′-hydroxy-3′,4′-dihydro-2′H-spiro[indoline-3,1′-isoquinolin]-2-one (1c)
Method D. Prepared from 7c (1.5 g, 5 mmol, 1 equiv) and 3-hydroxyphenethylamine (0.81 g, 6 mmol). The product was separated from its 6′-OH regioisomer by column chromatography (hexane : ethyl acetate—80 : 20). Yield, 0.33 g, 16% (brown solid). M.p. 235–238°C. 1H NMR (CD3OD 600 MHz): δ ppm 2.95–2.92 (m, 2H, H4′a, H4′b), 3.16–3.22 (m, 1H, H3′a), 3.31 (d, J = 5.2 Hz, 1H, H3′b), 6.53–6.55 (m, 1H, H7′), 6.74 (d, J = 7.7 Hz, 1H, H5′), 7.06–7.11 (m, 2H, H4, H6′), 7.56 (d, J = 1.8, Hz, 1H, H6). 13C NMR (DMSO-d6, 175 MHz): δ ppm 28.1 (C4′), 38.5 (C3′), 63.5 (C3/C1′), 102.5 (C7), 112.3 (C7′), 113.9 (C5), 120 (C5′), 120.2 (C8′a), 125.3 (C4), 128.2 (C6′), 132.8 (C6), 137.9 (C3a), 138.0 (C4′a), 141.4 (C7a), 154.1 (C8′), 179.9 (C2). FTMS + cESI: m/z 426.93 [M + 1]+.
3.9. 1-(4-Fluorobenzyl)-8′-hydroxy-3′,4′-dihydro-2′H-spiro[indoline-3,1′-isoquinolin]-2-one (1d) and 1-(4-fluorobenzyl)-6′-hydroxy-3′,4′-dihydro-2′H-spiro[indoline-3,1′-isoquinolin]-2-one (2d)
Method D. Prepared from 1-(4-fluorobenzyl)indoline-2,3-dione, 8a (0.4 g, 1.6 mmol, 1 equiv), and 3-hydroxyphenethylamine (0.2 g, 1.6 mmol). The reaction afforded compounds 1d and 2d that were separated by column chromatography (hexane : ethyl acetate—80 : 20).
1d. Yield, 0.2 g, 33% (brown solid). M.p. 119–121°C. 1H NMR (CD3OD, 600 MHz): δ ppm 2.93–3.06 (m, 2H, H4′a, H4′b), 3.32–3.37 (m, 2H, H3′a, H3′b), 4.84 (d, J = 15.9 Hz, 1H, –CH2–Ar), 5.16 (d, J = 15.9 Hz, 1H, CH2–Ar), 6.54 (dd, J = 8.1, 1.1 Hz, 1H, H7′), 6.75–6.80 (m, 2H, H7, H5′), 6.97 (td, J = 7.6, 1.0 Hz, 1H, H5), 7.07 (ddt, J = 7.8, 4.0, 2.3 Hz, 4H, H4, H6′, H3″, H5″), 7.18 (td, J = 7.7, 1.3 Hz, 1H, H6), 7.49–7.53 (m, 2H, H2″, H6″). 13C NMR (CD3OD, 150 MHz): δ ppm 28.3 (C4′), 38.7 (C3′), 42.8 (–CH2–Ar), 62.3 (C3/C1′), 108.9 (C7), 112.3 (C7′), 114.9 (2C, C3″, C5″), 120.8 (C5′), 120.8 (C8′a), 122.2 (C5), 123.2 (C4), 127.9 (2C, C6, C6′), 129.0 (2C, C2″, C6″), 132.1 (C1″), 133.7 (C3a), 138.0 (C4′a), 143.1 (C7a), 154.0 (C8′), 163.2 (C4″), 179.0 (C2). FTMS + cESI: m/z 375.15 [M + 1]+.
2d. Yield, 0.2 g, 33% (brown solid). M.p. 220–222°C. 1H NMR (CD3OD, 600 MHz): δ ppm 2.88 (dt, J = 16.4, 4.5, 1H, H4′a), 3.04 (ddd, J = 15.2, 9.1, 5.5, 1H, H4′b), 3.18–3.23 (m, 1H, H3′a), 3.83–3.88 (m, 1H, H3′b), 4.90 (d, J = 15.6 Hz, 1H, –CH2–Ar), 5.01 (d, J = 15.6 Hz, 1H, CH2–Ar), 6.22 (d, J = 8.5 Hz, 1H, H8′), 6.45 (dd, J = 8.5, 2.6 Hz, 1H, H7′), 6.65 (d, J = 2.6, 1H, H5′), 6.97 (d, J = 7.9, 1H, H7), 7.06 (td, J = 7.6, 1.0 Hz, 1H, H5), 7.07–7.11 (m, 2H, H2″, H6″), 7.19 (dd, J = 7.5, 1.3 Hz, 1H, H4), 7.28 (td, J = 7.8, 1.3 Hz, 1H, H6), 7.42–7.47 (m, 2H, H3″, H5″). 13C NMR (CD3OD, 150 MHz): δ ppm 28.3 (C4′), 38.3 (C3′), 42.4 (–CH2–Ar), 63.3 (C3/C1′), 109.2 (C7), 113.6 (C7′), 115.1 (2C, C3″, C5″), 115.2 (C5′), 123.2 (C5), 124.3 (C4), 124.9 (C8′a), 127.0 (C8′), 128.8 (C6), 129.2 (2C, C2″, C6″), 132.2 (C1″), 134.6 (C3a), 137.4 (C4′a), 142.5 (C7a), 156.4 (C6′), 163.2 (C4″), 179.2 (C2). FTMS + cESI: m/z 375.15 [M + 1]+.
3.10. 1-(4-Chlorobenzyl)-8′-hydroxy-3′,4′-dihydro-2′H-spiro[indoline-3,1′-isoquinolin]-2-one (1e) and 1-(4-chlorobenzyl)-6′-hydroxy-3′,4′-dihydro-2′H-spiro[indoline-3,1′-isoquinolin]-2-one (2e)
Method D. Prepared from 1-(4-chlorobenzyl)indoline-2,3-dione, 8b (0.8 g, 3.0 mmol), and 3-hydroxyphenethylamine (0.6 g, 4.4 mmol). The regioisomeric products 1e and 2e were separated by column chromatography (hexane : ethyl acetate—80 : 20).
1e. Yield, 0.4 g, 24% (brown solid). M.p. 95–98°C. 1H NMR (CD3OD, 600 MHz): δ ppm 2.84 (dt, J = 16.7, 4.9 Hz, 1H, H4′a), 2.90 (dt, J = 16.7, 4.9 Hz, 1H, H4′b), 3.22 (m, 2H, H3′a, H3′b), 4.71 (d, J = 16.1 Hz, 1H, –CH2–Ar), 5.04 (d, J = 16.1 Hz, 1H, CH2–Ar), 6.42 (dd, J = 8.0, 1.1 Hz, 1H, H7′), 6.63–6.66 (m, 2H, H7, H5′), 6.85 (td, J = 7.5, 1.0 Hz, 1H, H5), 6.93–6.97 (m, 2H, H4, H6), 7.06 (td, J = 7.8, 1.3 Hz, 1H, H6′), 7.22–7.24 (m, 2H, H3″, H5″), 7.36 (d, J = 8.4, 2H, H2″, H6″). 13C NMR (CD3OD, 150 MHz): δ ppm 28.3 (C4′), 38.7 (C3′), 42.8 (–CH2–Ar), 62.3 (C3/C1′), 109.0 (C7), 120.0 (2C, C5′, C7′), 120.7 (C8′a), 122.3 (C4), 122.3 (C5), 128.0 (C6′), 128.1 (C6), 128.3 (2C, C3″, C5″), 128.7 (2C, C2″, C6″), 132.8 (C4″), 133.6 (C3a), 135.0 (C1″), 137.9 (C4′a), 143.0 (C7a), 154.0 (C8′), 178.8 (C2). FTMS + cESI: m/z 391.12 [M + 1]+.
2e. Yield, 0.8 g, 47% (white solid). M.p. 218–220°C. 1H NMR (CD3OD, 600 MHz): δ ppm 2.88 (dt, J = 16.4, 4.5, 1H, H4′a), 3.04 (ddd, J = 15.3, 9.2, 5.5 Hz 1H, H4′b), 3.18–3.24 (m, 1H, H3′a), 3.85 (ddd, J = 12.7, 9.1, 4.6 Hz 1H, H3′b), 4.92 (d, J = 15.8 Hz, 1H, –CH2–Ar), 5.00 (d, J = 15.8 Hz, 1H, CH2–Ar), 6.23 (d, J = 8.5 Hz, 1H, H8′), 6.46 (dd, J = 8.5, 2.6 Hz, 1H, H7′), 6.66 (d, J = 2.6, 1H, H5′), 6.94 (dd, J = 7.9, 1H, H7), 7.06 (td, J = 7.5, 1.0 Hz, 1H, H5), 7.19 (dd, J = 7.5, 1.2 Hz, 1H, H4), 7.28 (td, J = 7.7, 1.3 Hz, 1H, H6), 7.33–7.36 (m, 2H, H2″, H6″), 7.40 (m, 2H, H3″, H5″). 13C NMR (CD3OD, 150 MHz): δ ppm 28.3 (C4′), 38.3 (C3′), 42.4 (–CH2–Ar), 63.3 (C3/C1′), 109.2 (C7), 113.6 (C7′), 115.2 (C5′), 123.3 (C5), 124.3 (C4), 124.8 (C8′a), 127.0 (C8′), 128.5 (2C, C2″, C6″), 128.8 (C6), 129.1 (2C, C3″, C5″), 133.2 (C4″), 134.6 (C3a), 134.9 (C1″), 137.4 (C4′a), 142.5 (C7a), 156.4 (C6′), 179.2 (C2). FTMS + cESI: m/z 391.12 [M + 1]+.
3.11. Synthesis of 1-(4-bromobenzyl)-8′-hydroxy-3′,4′-dihydro-2′H-spiro[indoline-3,1′-isoquinolin]-2-one (1f) and 1-(4-bromobenzyl)-6′-hydroxy-3′,4′-dihydro-2′H-spiro[indoline-3,1′-isoquinolin]-2-one (2f)
Method D. Prepared from 3-hydroxyphenethylamine (1.0 g, 7.1 mmol) and 1-(4-bromobenzyl)indoline-2,3-dione, 8c (1.5 g, 4.7 mmol). The regioisomers 1f and 2f were separated by column chromatography (hexane : ethyl acetate—80 : 20).
1f. Yield, 0.3 g, 15% (brown solid). M.p. 81–83°C. 1H NMR (CD3OD, 600 MHz): δ ppm 2.97–3.01 (m, 2H, H4′a, H4′b), 3.34 (m, 2H, H3′a, H3′b), 4.81 (d, J = 16.3 Hz, 1H, –CH2–Ar), 5.14 (d, J = 16.3 Hz, 1H, CH2–Ar), 6.54 (dd, J = 8.0, 1.1 Hz, 1H, H7′), 6.75–6.78 (m, 2H, H7, H5′), 6.97 (td, J = 7.5, 1.0 Hz, 1H, H5), 7.07–7.09 (m, 1H, H4), 7.19 (td, J = 7.8, 1.3 Hz, 1H, H6′), 7.29–7.31 (m, H6), 7.41–7.44 (m, 2H, H3″, H5″), 7.50 (m, 2H, H2″, H6″). 13C NMR (CD3OD, 150 MHz): δ ppm 28.2 (C4′), 38.7 (C3′), 42.8 (–CH2–Ar), 62.3 (C3/C1′), 109.0 (C7), 112.3 (C7′), 114.6 (C4″), 120.0 (C5′), 120.7 (C8′a), 122.3 (C5), 123.3 (C4), 128.1 (C6′), 128.7 (C6), 129.0 (2C, C3″, C5″), 131.3 (2C, C2″, C6″), 133.9 (C1″), 135.5 (C3a), 137.9 (C4′a), 143.0 (C7a), 154.0 (C8′), 178.8 (C2). FTMS + cESI: m/z 435.07 [M + 1]+
2f. Yield, 0.82 g, 40% (white solid). M.p. 207–210°C. 1H NMR (CD3OD, 600 MHz): δ ppm 2.88 (dt, J = 16.4, 4.5, 1H, H4′a), 3.04(ddd, J = 15.2, 9.2, 5.5 Hz 1H, H4′b), 3.17–3.24 (m, 1H, H3′a), 3.82–3.89 (m, 1H, H3′b), 4.90 (d, J = 15.8 Hz, 1H, –CH2–Ar), 4.98 (d, J = 15.8 Hz, 1H, CH2–Ar), 6.24 (d, J = 8.5 Hz, 1H, H8′), 6.46 (dd, J = 8.5, 2.6 Hz, 1H, H7′), 6.66 (d, J = 2.6 Hz, 1H, H5′), 6.94 (dd, J = 7.8 Hz, 1H, H7), 7.06 (td, J = 7.5, 1.0 Hz, 1H, H5), 7.19 (dd, J = 7.5, 1.3 Hz, 1H, H4), 7.28 (td, J = 7.8, 1.3 Hz, 1H, H6), 7.33–7.36 (m, 2H, H2″, H6″), 7.50–7.54 (m, 2H, H3″, H5″).13C NMR (CD3OD, 150 MHz): δ ppm 28.3 (C4′), 38.3 (C3′), 42.4 (–CH2–Ar), 63.3 (C3/C1′), 109.2 (C7), 113.6 (C7′), 115.2 (C5′), 121.1 (C4″), 123.3 (C5), 124.3 (C4), 124.8 (C8′a), 127.1 (C8′), 128.8 (C6), 129.1 (2C, C3″, C5″), 131.5 (2C, C2″, C6″), 134.6 (C3a), 134.6 (C1″), 137.4 (C4′a), 142.5 (C7a), 156.4 (C6′), 179.2 (C2). FTMS + cESI: m/z 435.07 [M + 1]+.
3.12. 1-(3,4-Dichlorobenzyl)-8′-hydroxy-3′,4′-dihydro-2′H-spiro[indoline-3,1′-isoquinolin]-2-one (1g) and 1-(3,4-dichlorobenzyl)-6′-hydroxy-3′,4′-dihydro-2′H-spiro[indoline-3,1′-isoquinolin]-2-one (2g)
Method D. Prepared from 3-hydroxyphenethylamine (0.82 g, 6.0 mmol) and 1-(3,4-dichlorobenzyl)indoline-2,3-dione, 8d (1.22 g, 4.0 mmol). The reaction afforded compounds 1g and 2g that were separated by column chromatography (hexane : ethyl acetate—80 : 20).
1g. Yield, 0.4 g, 24% (brown solid). M.p. 109–113°C. 1H NMR (CD3OD, 600 MHz): δ ppm 2.93–3.06 (m, 2H, H4′a, H4′b), 3.27–3.37 (m, 2H, H3′a, H3′b), 4.85 (d, J = 16.3 Hz, 1H, –CH2–Ar), 5.12 (d, J = 16.3 Hz, 1H, CH2–Ar), 6.54 (dd, J = 8.0, 1.1 Hz, 1H, H7′), 6.76 (dd, J = 7.6, 1.2 Hz, 1H, H5′), 6.81 (d, J = 7.8 Hz, 1H, H7), 6.99 (td, J = 7.5, 1.0 Hz, 1H, H5), 7.05–7.10 (m, 2H, H4, H6′), 7.22 (td, J = 7.7, 1.3 Hz, 1H, H6), 7.42 (dd, J = 8.3, 2.1 Hz, 1H, H6″), 7.49 (d, J = 8.3, 1H, H5″), 7.70 (d, J = 2.1 Hz, 1H, H2″). 13C NMR (CD3OD, 150 MHz): δ ppm 28.3 (C4′), 38.7 (C3′), 42.4 (–CH2–Ar), 62.2 (C3/C1′), 108.8 (C7), 114.4 (C7′), 120.0 (C5′), 120.8 (C8′a), 122.4 (C5), 123.3 (C4), 127.0 (C6″), 127.9 (C6′), 128.1 (C6), 129.3 (C2″), 130.3 (C5″), 130.9 (C3″), 132.1 (C4″), 133.8 (C3a), 137.2 (C1″), 138.0 (C4′a), 142.9 (C7a), 154.0 (C8′), 179.0 (C2). FTMS + cESI: m/z 425.08 [M + 1]+.
2g. Yield, 0.9 g, 53% (white solid). M.p. 197–199°C. 1H NMR (CD3OD, 600 MHz): δ ppm 2.88 (dt, J = 16.4, 4.4 Hz, 1H, H4′a), 3.00–3.08 (m, 1H, H4′b), 3.20 (ddd, J = 12.8, 5.5, 4.3 Hz 1H, H3′a), 3.86 (ddd, J = 12.8, 9.3, 4.6 Hz 1H, H3′b), 4.90 (d, J = 16.0 Hz, 1H, –CH2–Ar), 5.00 (d, J = 16.0 Hz, 1H, CH2–Ar), 6.23 (d, J = 8.5 Hz, 1H, H8′), 6.47 (dd, J = 8.5, 2.6 Hz, 1H, H7′), 6.66 (d, J = 2.6 Hz, 1H, H5′), 6.97 (d, J = 7.9 Hz, 1H, H7), 7.08 (td, J = 7.5, 1.0 Hz, 1H, H5), 7.21 (dd, J = 7.5, 1.3 Hz, 1H, H4), 7.28 (td, J = 7.8, 1.3 Hz, 1H, H6), 7.35 (dd, J = 8.3, 2.1 Hz, 1H, H6″), 7.52 (d, J = 8.3 Hz, 1H, H5″), 7.59 (d, J = 2.1 Hz, 1H, H2″).13C NMR (CD3OD, 150 MHz): δ ppm 28.3 (C4′), 38.3 (C3′), 41.9 (–CH2–Ar), 63.2 (C3/C1′), 109.0 (C7), 113.7 (C7′), 115.2 (C5′), 123.4 (C5), 124.4 (C8′a), 124.8 (C4), 127 (C8′), 127.1 (C6″), 128.9 (C6), 129.2 (C2″), 130.5 (C5″), 131.2 (C4″), 132.3 (C1″), 134.5 (C3a), 137.1 (C3″), 137.4 (C4′a), 142.3 (C7a), 156.4 (C6′), 179.2 (C2). FTMS + cESI: m/z 425.08 [M + 1]+.
3.13. 8′-Hydroxy-1-(4-methylbenzyl)-3′,4′-dihydro-2′H-spiro[indoline-3,1′-isoquinolin]-2-one (1h) and 6′-hydroxy-1-(4-methylbenzyl)-3′,4′-dihydro-2′H-spiro[indoline-3,1′-isoquinolin]-2-one (2h)
Method D. Prepared from 1-(4-methylbenzyl)indoline-2,3-dione, 8e (053 g, 2.0 mmol), and 3-hydroxyphenethylamine (0.4 g, 2.8 mmol). The products 1h and 2h were separated by column chromatography (hexane : ethyl acetate—80 : 20).
1h. Yield, 0.1 g, 12% (brown solid). M.p. 110–112°C. 1H NMR (CD3OD, 600 MHz): δ ppm 2.33 (s, Ar–CH3), 2.96 (dt, J = 16.4, 4.7 Hz, 1H, H4′a), 3.03 (dt, J = 16.4, 4.9 Hz, 1H, H4′b), 3.34 (m, 2H, H3′a, H3′b), 4.83 (d, J = 15.8 Hz, 1H, –CH2–Ar), 5.1 (d, J = 15.8 Hz, 1H, CH2–Ar), 6.54 (dd, J = 8.0, 1.1 Hz, 1H, H7′), 6.74–6.78 (m, 2H, H7, H5′), 6.95 (td, J = 7.5, 1.0 Hz, 1H, H5), 7.05–7.09 (m, 2H, H4, H6′), 7.16–7.18 (m, 3H, H6, H3″, H5″), 7.36–7.38 (m, 2H, H2″, H6″). 13C NMR (CD3OD, 150 MHz): δ ppm 19.8 (Ar–CH3), 28.3 (C4′), 38.7 (C3′), 43.4 (–CH2–Ar), 62.3 (C3/C1′), 109.2 (C7), 112.3 (C7′), 119.9 (2C, C5′), 120.9 (C8′a), 122.1 (C5), 123.1 (C4), 127.0 (2C, C2″, C6″), 127.9 (2C, C6,C6′), 128.8 (2C, C3″, C5″), 1330 (C1″), 133.7 (C3a), 136.7 (C4″), 137.9 (C4′a), 143.3 (C7a), 154.1 (C8′), 179.0 (C2). FTMS + cESI: m/z 371.17 [M + 1]+.
2h. Yield, 0.5 g, 62% (white solid). M.p. 190–193°C. 1H NMR (CD3OD, 600 MHz): δ ppm 2.33 (s, 3H, Ar–CH3), 2.88 (dt, J = 16.4, 4.6 Hz, 1H, H4′a), 3.03 (ddd, J = 16.4, 9.0, 5.5 Hz 1H, H4′b), 3.20 (dt, J = 12.8, 5.1 Hz, 1H, H3′a), 3.84 (ddd, J = 12.8, 9.1, 4.6 Hz, 1H, H3′b), 4.82 (d, J = 15.6 Hz, 1H, –CH2–Ar), 5.01 (d, J = 15.6 Hz, 1H, CH2–Ar), 6.24 (d, J = 8.5 Hz, 1H, H8′), 6.45 (dd, J = 8.5, 2.6 Hz, 1H, H7′), 6.65 (d, J = 2.6 Hz, 1H, H5′), 6.93 (dd, J = 7.9 Hz, 1H, H7), 7.03 (td, J = 7.5, 1.0 Hz, 1H, H5), 7.17 (m, 3H, H4, H3″, H5″), 7.25 (td, J = 7.8, 1.3 Hz, 1H, H6), 7.28 (d, 2H, J = 8.0, H2″, H6″). 13C NMR (CD3OD, 150 MHz): δ ppm 28.3 (C4′), 38.3 (C3′), 42.9 (–CH2–Ar), 63.3 (C3/C1′), 109.4 (C7), 113.6 (C7′), 115.1 (C5′), 123.1 (C5), 124.2 (C4), 124.9 (C8′a), 127.1 (3C, C8′, C2″, C6″), 128.7 (C6), 129.0 (2C, C3″, C5″), 133.0 (C1″), 134.6 (C3a), 137.2 (C4″), 137.3 (C4′a), 142.7 (C7a), 156.3 (C6′), 179.2 (C2). FTMS + cESI: m/z 371.17 [M + 1]+.
3.14. 8′-Hydroxy-1-(2-nitrobenzyl)-3′,4′-dihydro-2′H-spiro[indoline-3,1′-isoquinolin]-2-one (1i) and 6′-hydroxy-1-(2-nitrobenzyl)-3′,4′-dihydro-2′H-spiro[indoline-3,1′-isoquinolin]-2-one (2i)
Method D. Prepared from 1-(2-nitrobenzyl)indoline-2,3-dione, 8f (0.8 g, 3.0 mmol), and 3-hydroxyphenethylamine (0.6 g, 4.3 mmol). The reaction afforded two regioisomers, 1i and 2i, that were separated by column chromatography (hexane : ethyl acetate—80 : 20).
1i. Yield, 0.1 g, 8% (brown solid). M.p. 122–124°C. 1H NMR (CD3OD, 600 MHz): δ ppm 2.94–3.04 (m, 2H, H4′a, H4′b), 3.36 (m, 2H, H3′a, H3′b), 5.27 (d, J = 18.0 Hz, 1H, –CH2–Ar), 5.49 (d, J = 18.0 Hz, 1H, CH2–Ar), 6.57 (dd, J = 8.0, 1.1 Hz, 1H, H7′), 6.70 (d, J = 7.9 Hz, 1H, H7), 6.78 (dd, J = 7.6 Hz, 1H, H5′), 7.02 (td, J = 7.5, 1.0 Hz, 1H, H5), 7.09 (td, J = 7.8 Hz, 1H, H6′), 7.13 (dd, J = 7.4, 1.3 Hz, 1H, H4), 7.21 (td, J = 7.8, 1.3 Hz, 1H, H6), 7.55 (td, J = 7.7, 1.4 Hz, 1H, H6′), 7.65 (td, J = 7.6, 1.3 Hz, 1H, H6″), 8.22 (dd, J = 8.2, 1.3 Hz, 1H, H3″). 13C NMR (CD3OD, 150 MHz): δ ppm 28.3 (C4′), 38.6 (C3′), 41.5 (–CH2–Ar), 62.3 (C3/C1′), 108.7 (C7), 112.3 (C7′), 120.1 (C5′), 120.7 (C8′a), 122.6 (C5), 123.4 (C4), 124.9 (C3″), 128.0 (C6′), 128.1 (C4″), 128.2 (2C, C6, C6″), 131.8 (C1″), 133.6 (C3a), 133.7 (C5″), 138.1 (C4′a), 142.9 (C7a), 148.0 (C2″), 153.9 (C8′), 179.3 (C2). FTMS + cESI: m/z 402.14 [M + 1]+.
2i. Yield, 0.6 g, 50% (reddish brown solid). M.p. 115–118°C. 1H NMR (CD3OD, 600 MHz): δ ppm 2.88 (dt, J = 16.5, 4.4, 1H, H4′a), 3.06 (ddd, J = 15.3, 9.3, 5.6 Hz 1H, H4′b), 3.22 (ddd, J = 12.8, 9.3, 4.6 Hz, 1H, H3′a), 3.87 (ddd, J = 12.8, 9.3, 4.6 Hz 1H, H3′b), 5.30–5.39 (m 2H, –CH2–Ar), 6.39 (d, J = 8.5 Hz, 1H, H8′), 6.51 (dd, J = 8.5, 2.6 Hz, 1H, H7′), 6.66 (d, J = 2.6, 1H, H5′), 6.83 (dd, J = 7.9, 1H, H7), 7.10 (td, J = 7.5, 1.0 Hz, 1H, H5), 7.25 (dd, J = 7.5, 1.2 Hz, 1H, H4), 7.28 (td, J = 7.8, 1.3 Hz, 1H, H6), 7.55 (td, J = 7.8, 1.4 Hz, 1H, H4″), 7.65 (td, J = 7.6, 1.4 Hz, 1H, H5″), 8.19 (dd, J = 8.20, 1.4 Hz, 1H, H3″). 13C NMR (CD3OD, 150 MHz): δ ppm 28.2 (C4′), 38.3 (C3′), 40.8 (–CH2–Ar), 63.4 (C3/C1′), 109.0 (C7), 113.7 (C7′), 115.2 (C5′), 123.3 (C5), 124.5 (C4), 124.7 (C8′a), 124.5 (C3″), 127.2 (C8′), 127.6 (C6″), 128.3 (C4″), 129.1 (C6), 131.2 (C1″), 134.4 (C3a), 133.7 (C5″), 137.4 (C4′a), 142.5 (C7a), 148.3 (C2″), 156.5 (C6′), 179.4 (C2). FTMS + cESI: m/z 402.14 [M + 1]+.
3.15. 8′-Hydroxy-1-(naphthalen-2-ylmethyl)-3′,4′-dihydro-2′H-spiro[indoline-3,1′-isoquinolin]-2-one (1j) and 6′-Hydroxy-1-(naphthalen-2-ylmethyl)-3′,4′-dihydro-2′H-spiro[indoline-3,1′-isoquinolin]-2-one (2j)
Method D. Prepared from 1-(naphthalen-2-ylmethyl)indoline-2,3-dione, 8 g (2.7 g, 9.4 mmol), and 3-hydroxyphenethylamine (1.54 g, 11.3 mmol). The reaction afforded compounds, 1j and 2j, that were separated by column chromatography (hexane : ethyl acetate—80 : 20).
1j. Yield, 0.1 g, 16% (brown solid). M.p. 127–129°C. 1H NMR (CD3OD, 600 MHz): δ ppm 2.97 (dt, J = 16.6, 4.8 Hz, 1H, H4′a), 3.0–3.07 (m, 1H, H4′b), 3.36 (m, 2H, H3′a, H3′b), 5.05–5.08 (m, 1H, –CH2–Ar), 5.29 (dd, J = 16.0 Hz, 1H, CH2–Ar), 6.58 (dd, J = 8.0, 1.1 Hz, 1H, H7′), 6.77 (dd, J = 7.6, 1.1 Hz, 1H, H5′), 6.82 (dd, J = 7.9, 1.1 Hz, 1H, H7), 6.95 (td, J = 7.5, 1.0 Hz, 1H, H5), 7.08–7.11 (m, 2H, H4, H6′), 7.13–7.16 (m, 1H, H6), 7.47 (td, J = 7.4, 6.7, 3.4 Hz, 2H, H6″, H7″), 7.60 (dd, J = 8.5 Hz, 1H, H3″), 7.83–7.90 (m, 3H, H4″, H5″, H8″), 7.97 (s, 1H, H2″). 13C NMR (CD3OD, 150 MHz): δ ppm 28.2 (C4′), 38.7 (C3′), 43.8 (–CH2–Ar), 62.4 (C3/C1′), 109.4 (C7), 112.2 (C7′), 120.0 (C5′), 122.2 (C8′a), 122.6 (C5), 123.3 (C4), 125.1 (C3″), 125.5 (C6″), 125.6 (C1″), 125.8 (C7″), 127.2 (C8″), 127.5 (C5″), 128.0 (C4″), 128.1 (C6′), 128.1 (C8″a), 129.3 (C6), 132.9 (C4″a), 133.5 (C2″), 133.6 (C3a), 137.8 (C4′a), 143.4 (C7a), 154.1 (C8′), 179.0 (C2). FTMS + cESI: m/z 407.18 [M + 1]+.
2j. Yield, 1.5 g, 36% (brown solid). M.p. 148–150°C. 1H NMR (CD3OD, 600 MHz): δ ppm 2.90 (dt, J = 16.4, 4.6 Hz, 1H, H4′a), 3.05 (ddd, J = 15.1, 9.0, 5.5 Hz, 1H, H4′b), 3.23 (dt, J = 12.8, 5.0 Hz, 1H, H3′a), 3.89 (ddd, J = 13.2, 9.0, 4.6 Hz, 1H, H3′b), 5.05 (d, J = 15.7 Hz, 1H, –CH2–Ar), 5.21 (d, J = 15.7 Hz, 1H, CH2–Ar), 6.30 (d, J = 8.5 Hz, 1H, H8′), 6.47 (dd, J = 8.5, 2.6 Hz, 1H, H7′), 6.67 (d, J = 2.6, 1H, H5′), 6.99 (d, J = 7.9, 1H, H7), 7.20 (dd, J = 7.4, 1.2, 1H, H4), 7.23 (td, J = 7.8, 1.2 Hz, 1H, H6), 7.45–7.52 (m, 3H, H3″, H6″, H7″), 7.82–7.87 (m, 3H, H4″, H5″, H8″), 7.89 (d, J = 1.8 Hz, 1H, H1″). 13C NMR (CD3OD, 150 MHz): δ ppm 28.3 (C4′), 38.4 (C3′), 43.3 (–CH2–Ar), 63.4 (C3/C1′), 109.4 (C7), 115.2 (C5′), 123.2 (C5), 124.3 (C4), 124.9 (C8′a), 125.0 (C7″), 125.7 (C6″), 125.9 (C1″), 126.0 (C3″), 127.1 (C8′), 127.3 (C8″), 127.4 (C5″), 128.1 (C8″a), 128.8 (C6), 133.0 (C3a), 133.5 (C2″), 134.6 (C4″a), 137.4 (C4′a), 142.7 (C7a), 156.4 (C6′), 179.3 (C2). FTMS + cESI: m/z 407.18 [M + 1]+.
3.16. 5-Chloro-1-(4-fluorobenzyl)-8′-hydroxy-3′,4′-dihydro-2′H-spiro[indoline-3,1′-isoquinolin]-2-one (1k) and 5-chloro-1-(4-fluorobenzyl)-6′-hydroxy-3′,4′-dihydro-2′H-spiro[indoline-3,1′-isoquinolin]-2-one (2k)
Method D. Prepared from 5-chloro-1-(4-fluorobenzyl)indoline-2,3-dione, 8h (1.46 g, 5.0 mmol, 1 equiv), and 3-hydroxyphenethylamine (0.7 g, 5.0 mmol). The reaction products 1k and 2k were separated by column chromatography (hexane : ethyl acetate—80 : 20).
1k. Yield, 0.7 g, 35% (brown solid). M.p. 120–123°C. 1H NMR (CD3OD, 600 MHz): δ ppm 2.97 (m, 2H, H4′a, H4′b), 3.21–3.28 (m, 1H, H3′a), 3.36 (dd, J = 13.0, 5.3 Hz, 1H, H3′b), 4.81 (d, J = 15.9 Hz, 1H, –CH2–Ar), 5.14 (d, J = 15.9 Hz, 1H, CH2–Ar), 6.56 (dd, J = 8.1, 1.1 Hz, 1H, H7′), 6.75 (d, J = 8.4 Hz, 1H, H7), 6.77 (dd, J = 7.6, 1.1 Hz, 1H, H5′), 7.04 (d, J = 2.12 Hz, 1H, H4), 7.09 (ddd, J = 11.3, 9.1, 7.0 Hz, 3H, H6′, H3″, H5″), 7.18 (dd, J = 8.4, 2.1 Hz, 1H, H6), 7.47–7.51 (m, 2H, H2″, H6″). 13C NMR (CD3OD, 150 MHz): δ ppm 28.3 (C4′), 38.7 (C3′), 42.8 (–CH2–Ar), 62.3 (C3/C1′), 110.0 (C7), 112.3 (C7′), 114.9/115.0 (2C, C3″, C5″), 120.1 (C5′), 123.4 (C4), 127.4 (C8′a), 127.7(C6), 128.2 (C6′), 129.0 (3C, C3a, C2″, C6″), 131.7 (C1″), 135.8 (C5), 138.2 (C4′a), 141.8 (C7a), 154.0 (C8′), 163.2 (C4″), 178.6 (C2). FTMS + cESI: m/z 409.11 [M + 1]+.
2k. Yield, 1.5 g, 74% (brown solid). M.p. 208–210°C. 1H NMR (CD3OD, 600 MHz): δ ppm 2.86 (dt, J = 16.4, 4.4, 1H, H4′a), 2.99–3.07 (m, 1H, H4′b), 3.18 (ddd, J = 12.7, 5.1, 4.2 Hz, 1H, H3′a), 3.85 (ddd, J = 12.7, 9.3, 4.5 Hz 1H, H3′b), 4.86 (d, J = 15.7 Hz, 1H, –CH2–Ar), 4.99 (d, J = 15.7 Hz, 1H, CH2–Ar), 6.23 (d, J = 8.5 Hz, 1H, H8′), 6.48 (dd, J = 8.5, 2.6 Hz, 1H, H7′), 6.67 (d, J = 2.6, 1H, H5′), 6.94 (d, J = 8.4, 1H, H7), 7.07–7.12 (m, 2H, H3″, H5″), 7.19 (d, J = 2.1 Hz, 1H, H4), 7.28 (dd, J = 8.4, 2.3 Hz, 1H, H6), 7.40–7.44 (m, 2H, H2″, H6″). 13C NMR (CD3OD, 150 MHz): δ ppm 28.2 (C4′), 38.3 (C3′), 42.4 (–CH2–Ar), 63.3 (C3/C1′), 110.4 (C7), 113.8 (C7′), 115.1 (2C, C3″, C5″), 115.3 (C5′), 124.2 (C8′a), 124.7 (C4), 126.9 (C8′), 128.4 (C3a), 128.6 (C6), 129.2 (2C, C2″, C6″), 131.9 (C1″), 136.5 (C5), 137.4 (C4′a), 141.3 (C7a), 156.5 (C6′), 163.2 (C4″), 178.8 (C2). FTMS + cESI: m/z 409.11 [M + 1]+.
3.17. Synthesis of 5-chloro-1-(4-chlorobenzyl)-8′-hydroxy-3′,4′-dihydro-2′H-spiro[indoline-3,1′-isoquinolin]-2-one (1l) and 5-chloro-1-(4-chlorobenzyl)-6′-hydroxy-3′,4′-dihydro-2′H-spiro[indoline-3,1′-isoquinolin]-2-one (2l)
Method D. Prepared from 5-chloro-1-(4-chlorobenzyl)indoline-2,3-dione, 8i (2.4 g, 9.5 mmol) and 3-hydroxyphenethylamine (1.3 g, 5.0 mmol). The reaction products 1l and 2l were separated by column chromatography (hexane : ethyl acetate—80 : 20).
1l. Yield, 1.1 g, 35% (brown solid). M.p. 122–124°C. 1H NMR (CD3OD, 600 MHz): δ ppm 2.97 (m, H4′a), 3.25 (m, 1H, H4′b), 3.36 (dt, J = 13.2, 5.3 Hz, 2H, H3′a, H3′b), 4.80 (d, J = 16.1 Hz, 1H, –CH2–Ar), 5.15 (d, J = 16.1 Hz, 1H, CH2–Ar), 6.56 (dd, J = 8.0, 1.1 Hz, 1H, H7′), 6.74 (d, J = 8.4 Hz, 1H, H7), 6.77 (dd, J = 7.6, 1.1 Hz, 1H, H5′), 7.05 (d, J = 2.12 Hz, 1H, H4), 7.10 (t, J = 7.8 Hz, 1H, H6′), 7.18 (dd, J = 8.4, 2.1 Hz, 1H, H6), 7.35–7.37 (d, J = 8.5 Hz, 2H, H3″, H5″), 7.45–7.47 (m, 2H, H2″, H6″). 13C NMR (CD3OD, 150 MHz): δ ppm 28.3 (C4′), 38.7 (C3′), 42.9 (–CH2–Ar), 62.3 (C3/C1′), 110.0 (C7), 112.3 (C7′), 120.1 (C5′), 123.4 (C4), 127.4 (C8′a), 127.8(C6), 128.2 (C6′), 128.3 (2C, C3″, C5″), 128.7 (2C, C2″, C6″), 132.9 (C4″), 134.7 (C1″), 135.7 (C5), 138.2 (C4′a), 141.8 (C7a), 154.0 (C8′), 178.6 (C2). FTMS + cESI: m/z 425.08 [M + 1]+.
2l. Yield, 2.0 g, 62% (brown solid). M.p. 208–210°C. 1H NMR (CD3OD, 600 MHz): δ ppm 2.86 (dt, J = 16.4, 4.4, 1H, H4′a), 3.01–3.07 (m, 1H, H4′b), 3.18 (ddd, J = 12.7, 5.5, 4.2 Hz, 1H, H3′a), 3.85 (ddd, J = 12.7, 9.4, 4.2 Hz, 1H, H3′b), 4.98 (d, J = 15.8 Hz, 1H, –CH2–Ar), 4.98 (d, J = 15.8 Hz, 1H, CH2–Ar), 6.25 (d, J = 8.5 Hz, 1H, H8′), 6.49 (dd, J = 8.5, 2.6 Hz, 1H, H7′), 6.67 (d, J = 2.6 Hz, 1H, H5′), 6.92 (d, J = 8.5 Hz, 1H, H7), 7.19 (d, J = 2.1 Hz, 1H, H4), 7.28 (dd, J = 8.4, 2.3 Hz, 1H, H6), 7.36–7.40 (m, 4H, H2″ H3″, H5″, H6″). 13C NMR (CD3OD, 150 MHz): δ ppm 28.2 (C4′), 38.2 (C3′), 42.5 (–CH2–Ar), 63.3 (C3/C1′), 110.4 (C7), 113.8 (C7′), 115.3 (C5′), 124.2 (C8′a), 124.7 (C4), 126.9 (C8′), 128.5 (C3a), 128.6 (C6), 128.7 (2C, C2″, C6″), 128.8 (2C, C3″, C5″), 133.2 (C4″), 134.6 (C1″), 136.5 (C5), 137.4 (C4′a), 141.3 (C7a), 156.6 (C6′), 178.8 (C2). FTMS + cESI: m/z 425.08 [M + 1]+.
3.18. Synthesis of 5-chloro-6′-hydroxy-1-(4-methylbenzyl)-3′,4′-dihydro-2′H-spiro[indoline-3,1′-isoquinolin]-2-one (2o)
Method D. Prepared from 5-chloro-1-(4-methylbenzyl)indoline-2,3-dione, 8l (2 g, 7 mmol), and 3-hydroxyphenethylamine (1.4 g, 10.5 mmol). The reaction product 2o was purified by column chromatography (hexane : ethyl acetate—70 : 30).
2o. Yield, 0.7 g, 25% (brown solid). M.p. 103–105°C. 1H NMR (CD3OD, 600 MHz): δ ppm 2.33 (s, 3H, Ar–CH3), 2.86 (dt, J = 16.4, 4.4, 1H, H4′a), 3.03 (ddd, J = 15.3, 9.2, 5.5 Hz 1H, H4′b), 3.15–3.20 (m, 1H, H3′a), 3.84 (ddd, J = 12.8, 9.2, 4.5 Hz 1H, H3′b), 4.79 (d, J = 15.6 Hz, 1H, –CH2–Ar), 4.99 (d, J = 15.6 Hz, 1H, CH2–Ar), 6.24 (d, J = 8.5 Hz, 1H, H8′), 6.48 (dd, J = 8.5, 2.6 Hz, 1H, H7′), 6.66 (d, J = 2.6, 1H, H5′), 6.90 (d, J = 8.5, 1H, H7), 7.16–7.19 (m, 3H, H4, H3″; H5″), 7.23–7.28 (m, 3H, H6, H2″, H6″). 13C NMR (CD3OD, 150 MHz): δ ppm 19.8 (Ar – CH3), 28.3 (C4′), 38.3 (C3′), 42.9 (–CH2–Ar), 63.4 (C3/C1′), 110.6 (C7), 113.8 (C7′), 115.1 (C5′), 124.3 (C8′a), 124.6 (C4), 127.0 (C8′), 127.2 (2C, C2″, C6″), 128.3 (C3a), 128.6 (C6), 129.1 (2C, C3″, C5″), 132.7 (C1″), 136.5 (C5), 137.4 (C4″), 137.4 (C4′a), 141.5 (C7a), 156.5 (C6′), 178.8 (C2). FTMS + cESI: m/z 405.14 [M + 1]+.
3.19. General method for the synthesis of 6′,7′-dihydroxy-3′,4′-dihydro-2′H-spiro[indoline-3,1′-isoquinolin]-2-ones (3a, 3b) (method E)
Dopamine hydrochloride (1 equiv) and the appropriate isatin (1 equiv) were dissolved in ethanol (10 ml) and triethylamine (1 ml) added (scheme 2c). The reaction mixture was stirred for 72 h at room temperature. At the end of the reaction, the solvent was removed in vacuo and distilled water added to the viscous mass. The precipitate obtained was filtered, washed repeatedly with water and dried.
3.20. 6′,7′-Dihydroxy-3′,4′-dihydro-2′H-spiro[indoline-3,1′-isoquinolin]-2-one (3a)
Method E. Prepared from isatin (0.4 g, 2.6 mmol) and dopamine (0.5 g, 2.6 mmol). Yield, 0.35 g, 50% (black solid). M.p. 235–237°C. 1H NMR (CD3OD, 600 MHz): δ ppm 2.79 (dt, J = 16.2, 4.9 Hz, 1H, H4′a), 2.93 (ddd, J = 16.2, 8.6, 5.3 Hz, 1H, H4′b), 3.19–3.23 (m, 1H, H3′a), 3.78 (ddd, J = 13.1, 8.6, 4.8 Hz, 1H, H3′b), 5.98 (s, 1H, H8′), 6.61 (s, 1H, H5′), 7.00 (d, J = 7.8 Hz, 1H, H7), 7.03 (td, J = 7.6, 1.1 Hz, 1H, H5), 7.18 (dd, J = 7.6, 1.2 Hz, 1H, H4), 7.29 (td, J = 7.7 Hz, 1.3 Hz, 1H, H6). 13C NMR (CD3OD, 150 MHz): δ ppm 27.1 (C4′), 38.5 (C3′), 63.6 (C3/C1′), 109.8 (C7), 112.2 (C8′), 115.2 (C5′), 122.7 (C5), 124.2 (C8′a), 124.6 (C4), 126.9 (C4′a), 129.0 (C6), 134.8 (C3a), 141.8 (C7a), 143.8 (C7′), 144.8 (C6′), 180.9 (C2). FTMS + cESI: m/z 283.11 [M + 1]+.
3.21. 5-Chloro-6′,7′-dihydroxy-3′,4′-dihydro-2′H-spiro[indoline-3,1′-isoquinolin]-2-one (3b)
Method E. Prepared from 5-chloroisatin (0.5 g, 2.6 mmol) and dopamine (0.5 g, 2.6 mmol). Yield, 0.82 g, 98% (black solid). M.p. 210–213°C. 1H NMR (CD3OD, 600 MHz): δ ppm 2.76 (dt, J = 16.1, 4.7 Hz, 1H, H4′a), 2.92 (ddd, J = 16.2, 8.9, 5.4 Hz, 1H, H4′b), 3.15 (dd, J = 12.7, 5.1 Hz, 1H, H3′a), 3.76 (ddd, J = 13.1, 8.9, 4.7 Hz, 1H, H3′b), 5.97(s, 1H, H8′), 6.62 (s, 1H, H5′), 6.97 (d, J = 8.3 Hz, H7), 7.17 (dd, J = 1.2 Hz, 1H, H4), 7.29 (dd, J = 8.3, 2.2 Hz, 1H, H6). 13C NMR (CD3OD, 150 MHz): δ ppm 29.2 (C4′), 38.5 (C3′), 63.8 (C3/C1′), 110.9 (C7), 112.1 (C8′), 115. 3 (C5′), 123.8 (C8′a), 124.9 (C4), 127.1 (C4′a), 127.1 (C5), 128.7 (C6), 137.0 (C3a), 140.6 (C7a), 143.8 (C7′), 145.0 (C6′), 180.6 (C2). FTMS + cESI: m/z 317.07 [M + 1]+.
3.22. Synthesis of substituted β-nitrostyrenes (10a, 10b) (method F)
The method of Maresh et al. [46] was employed here with some modification (scheme 1c). A solution of substituted benzaldehyde (1 equiv), nitromethane (2 equiv) and anhydrous ammonium acetate (0.1 equiv) in acetic acid was refluxed for 6 h. The reaction mixture was then diluted slowly while stirring, with 200 ml water. During this time, a heavy yellow crystalline mass was formed. This was removed by filtration, washed with water and sucked as dry as possible, recrystallized from boiling methanol and air dried, yielding the β-nitrostyrene as bright yellow crystals in over 95% yield. The compound was used without further purification.
3.23. Synthesis of methoxyphenethylamines (11a, 11b) (method F)
This reaction carried out following the method of Maresh et al. [46] (scheme 1c). Typically, 1.0 mmol of nitrostyrene required 2 ml of methanol, 800 mg of zinc dust (12 mmol) and 2 ml of 37% HCl (24 mmol). Methanol was stirred vigorously in an ice bath maintained between 0 and 5°C (ice/NaCl). Conc. HCl, zinc dust and the appropriate nitrostyrene were slowly added over the course of 30 min in small portions while maintaining the temperature between 0 and 5°C. After complete addition of all starting materials, any solids on the side were washed into the solution with a small amount of methanol. The reaction mixture was further stirred for 1 h until the disappearance of the initial yellow colour of the nitrostyrene was observed. The reaction was further stirred for 4–6 h after the yellow colour had disappeared. Thereafter, the reaction mixture was placed in a 4°C refrigerator overnight. Upon completion of the reaction, the excess solid zinc was removed by filtration through the filter paper. The solution was made basic by dropwise addition of saturated sodium hydroxide in methanol, while maintaining the temperature below 5°C, until the pH was greater than 11 as indicated by the pH paper. The product was then extracted into dichloromethane and the solution was dried over anhydrous sodium sulfate. The insoluble zinc hydroxide which precipitates out during the addition of sodium hydroxide solution was extracted many more times with dichloromethane and filtered. The combined organic extracts were evaporated in vacuo to yield the corresponding phenethylamine as a yellow viscous oil in about 70% yield.
3.24. General method for the synthesis of 6′-methoxy-3′,4′-dihydro-2′H-spiro[indoline-3,1′-isoquinolin]-2-ones (4a–c, 5a–c, 6a–c) and 1-(substitutedbenzyl)-6′-methoxy-3′,4′-dihydro-2′H-spiro[indoline-3,1′-isoquinolin]-2-ones (4d,e; 5d–f; 6d,e) (method G)
A mixture of the appropriate isatin (1 equiv), 3-methoxyphenethylamine (1.2 equiv) and polyphosphoric acid (2 g) was heated in an oil bath (bath temperature at 100°C), while stirring mechanically for 5 h (scheme 2d). Upon completion of the reaction, as revealed by TLC, the reaction mixture was allowed to cool to about 50°C and quenched by slow addition of water. To this mixture was added a saturated solution of sodium carbonate to make the mixture basic to pH 11. The floating product obtained was extracted into ethyl acetate (3 × 30 ml). The combined organic extracts were dried over anhydrous sodium sulfate, and concentrated under reduced pressure to obtain the crude product. This crude product was purified by flash chromatography on silica gel using suitable solvent systems. Yields ranged between 60 and 98%.
3.25. 6′-Methoxy-3′,4′-dihydro-2′H-spiro[indoline-3,1′-isoquinolin]-2-one (4a)
Method G. Prepared from isatin (0.81 g, 5.5 mmol), 3-methoxyphenethylamine (1 g, 6.6 mmol) and polyphosphoric acid (3 g). The crude product was purified by flash chromatography (hexane : ethyl acetate—60 : 40). Yield, 1.1 g, 73% (yellow oil). 1H NMR (CD3OD, 600 MHz): δ ppm 2.91 (dt, J = 16.6, 4.8 Hz, 1H, H4′a), 3.05 (ddd, J = 16.6, 8.7, 5.4 Hz, 1H, H4′b), 3.20 (dt, J = 12.9, 5.2 Hz, 1H, H3′a), 3.76 (m, 4H, OCH3, H3′b), 6.45 (d, J = 8.6 Hz, 1H, H8′), 6.61 (dd, J = 8.6, 2.7 Hz, 1H, H7′), 6.77 (d, J = 2.7 Hz, 1H, H5′), 6.98–7.03 (m, 2H, H5, H7), 7.12–7.14 (m, 1H, H4), 7.29 (td, J = 7.7, 1.3 Hz, 1H, H6). 13C NMR (CD3OD, 150 MHz): δ ppm 28.4 (C4′), 38.2 (C3′), 54.2 (OCH3), 63.7 (C3/C1′), 109.7 (C7), 112.6 (C7′), 113.4 (C5′), 122.6 (C5), 124.4 (C4), 126.1 (C8′a), 127.2 (C8′), 128.9 (C6), 135.2 (C3a), 137.3 (C4′a), 141.8 (C7a), 158.8 (C6′), 181.1 (C2). FTMS + cESI: m/z 281.13 [M + 1]+.
3.26. 5-Chloro-6′-methoxy-3′,4′-dihydro-2′H-spiro[indoline-3,1′-isoquinolin]-2-one (4b)
Method G. Prepared from 5-chloroisatin (1 g, 5.5 mmol), 3-methoxyphenethylamine (1 g, 6.6 mmol) and polyphosphoric acid (5 g). The crude product was purified by flash chromatography (hexane : ethyl acetate—50 : 50). Yield, 0.6 g, 35% (green solid). M.p. 114–116°C. 1H NMR (CD3OD, 600 MHz): δ ppm 2.90 (dt, J = 16.6, 4.6 Hz, 1H, H4′a), 3.05 (ddd, J = 16.5, 8.9, 5.4 Hz, 1H, H4′b), 3.17 (dt, J = 12.7, 5.0 Hz, 1H, H3′a), 3.77 (m, 4H, OCH3, H3′b), 6.46 (d, J = 8.6 Hz, 1H, H8′), 6.65 (dd, J = 8.7, 2.7 Hz, 1H, H7′), 6.78 (d, J = 2.7 Hz, 1H, H5′), 6.97 (d, J = 8.3 Hz, 1H, H7), 7.13 (d, J = 2.1 Hz, 1H, H4), 7.30 (dd, J = 8.3, 2.2 Hz, 1H, H6), 13C NMR (CD3OD, 150 MHz): δ ppm 28.4 (C4′), 38.2 (C3′), 54.3 (OCH3), 63.8 (C3/C1′), 110.9 (C7), 112.7 (C7′), 113.5 (C5′), 124.8 (C4), 125.5 (C8′), 127.0 (C8′), 127.6 (C3a), 128.7 (C6), 137.1 (C5), 137.4 (C4′a), 140.6 (C7a), 159.0 (C6′), 180.7 (C2). FTMS + cESI: m/z 315.09 [M + 1]+.
3.27. 5,7-Dibromo-6′-methoxy-3′,4′-dihydro-2′H-spiro[indoline-3,1′-isoquinolin]-2-one (4c)
Method G. Prepared from 5,7-dibromoisatin, 7c (2.02 g, 6–6 mmol), 3-methoxyphenethylamine (1 g, 6.6 mmol) and polyphosphoric acid (3 g). The crude product was purified by flash chromatography (hexane : ethyl acetate—90 : 10). Yield 2.03 g, 70% (brown solid). M.p. 255–257°C. 1H NMR (CD3OD, 600 MHz): δ ppm 3.52 (dt, J = 16.1, 3.9 Hz, 1H, H4′a), 3.73 (ddd, J = 15.4, 9.5, 5.5 Hz, 1H, H4′b), 3.79 (ddd, J = 12.2, 5.5, 3.6 Hz, 1H, H3′a), 4.40 (m, 1H, H3′b), 4.52 (s, 3H, OCH3), 7.43 (dd, J = 8.6, 2.8 Hz, 1H, H7′), 7.56 (d, J = 2.8 Hz, 1H, H5′), 7.94 (d, J = 1.8 1H, H4), 7.18 (d, J = 8.6 Hz, 1H, H8′), 8.48 (d, J = 1.9 Hz, 1H, H6), 11.57 (br. s, 1H, H1). 13C NMR (CD3OD, 150 MHz): δ ppm 30.1 (C4′), 39.1 (C3′), 56.3 (OCH3), 65.6 (C3/C1′), 104.1 (C7), 114.1 (C7′), 115.0 (C5′), 115.2 (C5), 127.4 (C8′a), 127.7 (C4), 128.3 (C8′), 134.5 (C6), 139.1 (C4′a), 140.7 (C3a), 142.7 (C7a), 159.3 (C6′), 181.0 (C2). FTMS + cESI: m/z 438.95 [M + 1]+.
3.28. 6′-Methoxy-1-(4-fluorobenzyl)-3′,4′-dihydro-2′H-spiro[indoline-3,1′-isoquinolin]-2-one (4d)
Method G. Prepared from 1-(4-fluorobenzyl)indoline-2,3-dione, 8a (1.5 g, 6.0 mmol), 3-methoxyphenethylamine (0.9 g, 6.0 mmol) and polyphosphoric acid (3 g). The crude product was purified by flash chromatography (hexane : ethyl acetate—70 : 30). Yield, 1.7 g, 73% (yellow oil). 1H NMR (CD3OD, 600 MHz): δ ppm 2.93 (dt, J = 16.5, 4.5 Hz, 1H, H4′a), 3.06–3.11 (m, 1H, H4′b), 3.22 (dt, J = 12.8, 5.0 Hz, 1H, H3′a), 3.76 (s, 3H, 6′-OCH3), 3.87 (ddd, J = 13.3, 9.1, 4.6 Hz, 1H, H3′b), 4.90 (d, J = 15.6, 1H, –CH2–Ar), 5.00 (d, J = 15.6 Hz, 1H, CH2–Ar), 6.32 (d, J = 8.6 Hz, 1H, H8′), 6.59 (dd, J = 8.6, 2.7 Hz, 1H, H7′), 6.79 (d, J = 2.7 Hz, 1H, H5′), 6.97 (d, J = 8.0 Hz, 1H, H7), 7.05 (td, J = 7.5, 0.9 Hz, 1H, H5) 7.07–7.11 (m, 2H, H3″,H5″), 7.18 (dd, J = 7.4, 1.2 Hz, 1H, H4), 7.28 (td, J = 7.8, 1.3 Hz, 1H, H6), 7.42–7.46 (m, 2H, H2″, H6″). 13C NMR (CD3OD, 150 MHz): δ ppm 28.4 (C4′), 38.3 (C3′), 42.4 (–CH2–Ar), 54.1 (OCH3), 63.3 (C3/C1′), 109.3 (C7), 112.6 (C7′), 113.6 (C5′), 115.0 (2C, C3″,C5″), 123.2 (C5), 124.3 (C4), 126.1 (C8′a), 127.0 (C8′), 128.9 (C6), 129.2 (2C, C2″,C6″), 132.1 (C1″), 134.5 (C3a), 137.5 (C4′a), 142.6 (C7a), 158.9 (C6′), 163.2 (C4″), 179.0 (C2). FTMS + cESI: m/z 389.17 [M + 1]+.
3.29. 6′-Methoxy-1-(4-methylbenzyl)-3′,4′-dihydro-2′H-spiro[indoline-3,1′-isoquinolin]-2-one (4e)
Method G. Prepared from 8e (1.5 g, 6.0 mmol), 3-methoxyphenethylamine (0.9 g, 0.9 ml, 6.0 mmol) and polyphosphoric acid (3 g), and purified by flash chromatography (hexane : ethyl acetate—70 : 30). Yield, 2.0 g, 87% (yellow oil). 1H NMR (CD3OD, 600 MHz): δ ppm 2.32 (s, 3H, 4″-CH3–Bz), 2.94 (dt, J = 16.5, 4.6 Hz, 1H, H4′a), 3.06–3.12 (m, 1H, H4′b), 3.21–3.26 (m, 1H, H3′a), 3.75 (s, 3H, 6′-OCH3), 3.88 (ddd, J = 13.3, 9.0, 4.7 Hz, 1H, H3′b), 4.81 (d, J = 15.5, 1H, –CH2–Ar), 5.00 (d, J = 15.5 Hz, 1H, CH2–Ar), 6.34 (d, J = 8.7 Hz, 1H, H8′), 6.58 (dd, J = 8.6, 2.7 Hz, 1H, H7′), 6.79 (d, J = 2.7 Hz, 1H, H5′), 6.95 (d, J = 7.9 Hz, 1H, H7), 7.03 (td, J = 7.5, 1.0 Hz, 1H, H5) 7.15–7.18 (m, 3H, H4, H3″,H5″), 7.25 (td, J = 7.7, 1.3 Hz, 1H, H6), 7.28 (d, J = 7.9 Hz, 2H, H2″,H6″). 13C NMR (CD3OD, 150 MHz): δ ppm 19.8 (4″–CH3), 28.3 (C4′), 38.3 (C3′), 42.9 (–CH2–Ar), 54.3 (OCH3), 63.3 (C3/C1′), 109.5 (C7), 112.7 (C7′), 113.6 (C5′), 123.2 (C5), 124.2 (C4), 126.0 (C8′a), 127.1 (C8′), 127.2 (2C, C2″, C6″), 128.9 (C6), 129.1 (2C, C3″,C5″), 133.0 (C1″), 134.2 (C3a), 137.2 (C4″), 137.3 (C4′a), 142.7 (C7a), 158.9 (C6′), 178.8 (C2). FTMS + cESI: m/z 385.19 [M + 1]+.
3.30. 6′,7′-Dimethoxy-3′,4′-dihydro-2′H-spiro[indoline-3,1′-isoquinolin]-2-one (5a)
Method G. Prepared from isatin (1.0 g, 6.8 mmol), 3,4-dimethoxyphenethylamine, 11a, (1 g, 6.6 mmol) and polyphosphoric acid (3 g). The crude product was purified by flash chromatography (hexane : ethyl acetate—70 : 30). Yield, 2.07 g, 99% (yellow solid). M.p. 188–191°C. 1H NMR (CD3OD, 600 MHz): δ ppm 2.86 (dt, J = 16.3, 4.9 Hz, 1H, H4′a), 2.95–3.03 (m, 1H, H4′b), 3.21 (dt, J = 12.9, 5.2 Hz, 1H, H3′a), 3.52 (s, 3H, 7′-OCH3), 3.76 (ddd, J = 13.1, 8.5, 4.8 Hz, 1H, H3′b), 3.83 (s, 3H, 6′-OCH3), 6.03 (s, 1H, H8′), 6.79 (s, 1H, H5′), 7.01 (dt, J = 7.8, 0.8 Hz, H7), 7.03 (td, J = 7.6, 1.1 Hz, 1H, H5), 7.15 (ddd, J = 7.5, 1,3, 0.6 Hz, 1H, H4), 7.30 (td, J = 7.7, 1.3 Hz, 1H, H6). 13C NMR (CD3OD, 150 MHz): δ ppm 27.6 (C4′), 38.4 (C3′), 54.9 (7′-OCH3), 55.0 (6′-OCH3), 63.8 (C3/C1′), 109.2 (C8′), 109.8 (C7), 112.2 (C5′), 122.6 (C5), 124.5 (C4), 125.8 (C8′a), 128.9 (C4′a), 129.0 (C6), 135.0 (C3a), 141.7 (C7a), 147.7 (C7′), 148.0 (C6′), 180.9 (C2). FTMS + cESI: m/z 311.14 [M + 1]+.
3.31. 5-Chloro-6′,7′-dimethoxy-3′,4′-dihydro-2′H-spiro[indoline-3,1′-isoquinolin]-2-one (5b)
Method G. From 5-chloroisatin (1.5 g, 8.3 mmol), 3,4-dimethoxyphenethylamine, 11a (1.5 g, 8.3 mmol) and polyphosphoric acid (3 g). The crude product was purified by flash chromatography (hexane : ethyl acetate— 70 : 30). Yield, 1.8 g, 63% (white solid). M.p. 138–140°C. 1H NMR (CD3OD, 600 MHz): δ ppm 2.83–2.88 (m, 1H, H4′a), 2.96–3.02 (m, 1H, H4′b), 3.18 (dt, J = 12.7, 5.1 Hz, 1H, H3′a), 3.56 (s, 3H, 7′-OCH3), 3.75 (ddd, J = 13.1, 8.6, 4.7 Hz, 1H, H3′b), 3.84 (s, 3H, 6′-OCH3), 6.03 (s, 1H, H8′), 6.81 (s, 1H, H5′), 6.99 (dt, J = 8.3, 1H, H7), 7.16 (d, J = 2.1 Hz, 1H, H4), 7.30–7.32 (m, 1H, H6). 13C NMR (CD3OD, 150 MHz): δ ppm 27.5 (C4′), 38.3 (C3′), 55.0 (7′-OCH3), 55.1 (6′-OCH3), 64.0 (C3/C1′), 109.0 (C8′), 111.0 (C7), 112.3 (C5′), 124.8 (C4), 125.1 (C8′a), 127.7 (C3a), 128.9 (C6), 129.0 (C4′a), 136.8 (C5), 140.6 (C7a), 147.8 (C7′), 148.9 (C6′), 180.6 (C2). FTMS + cESI: m/z 345.10 [M + 1]+.
3.32. 5,7-dibromo-6′,7′-dimethoxy-3′,4′-dihydro-2′H-spiro[indoline-3,1′-isoquinolin]-2-one (5c)
Method G. Prepared from 5,7-dibromoisatin, 7c (1.0 g, 3.3 mmol), 3,4-dimethoxyphenethylamine, 11a (0.6 g, 3.3 mmol) and polyphosphoric acid (3 g). The crude product was purified by flash chromatography (hexane : ethyl acetate—70 : 30). Yield, 1.8 g, 63% (white solid). M.p. 180–183°C. 1H NMR (CD3OD D, 600 MHz): δ ppm 2.84 (dt, J = 16.3, 4.7, 1H, H4′a), 2.96–3.03 (m, 1H, H4′b), 3.16 (dt, J = 12.8, 5.1 Hz, 1H, H3′a), 3.58 (s, 3H, 7′-OCH3), 3.73 (ddd, J = 13.1, 8.7, 4.7 Hz, 1H, H3′b), 3.84 (s, 3H, 6′-OCH3), 6.04 (s, 1H, H8′), 6.81 (s, 1H, H5′), 7.26 (d, J = 1.8 Hz, 1H, H4), 7.66 (d, J = 1.8 1H, H6). 13C NMR (CD3OD, 150 MHz): δ ppm 27.5 (C4′), 38.2 (C3′), 55.0 (7′-OCH3), 55.2 (6′-OCH3), 64.9 (C3/C1′), 103.0 (C7), 109.0 (C8′), 112.4 (C5′), 114.9 (C5), 124.6 (C8′a), 126.7 (C4), 129.1 (C4′a), 133.9 (C6), 138.2 (C5), 141.0 (C7a), 147.9 (C7′), 149.1 (C6′), 179.7 (C2). FTMS + cESI: m/z 468.96 [M + 1]+.
3.33. 1-(4-Fluorobenzyl)-6′,7′-dimethoxy-3′,4′-dihydro-2′H-spiro[indoline-3,1′-isoquinolin]-2-one (5d)
Method G. Prepared from 1-(4-fluorobenzyl)indoline-2,3-dione, 8a (2.0 g, 7.8 mmol), 3,4-dimethoxyphenethylamine, 11a (1.4 g, 7.8 mmol) and polyphosphoric acid (2.5 g). The crude product was purified by flash chromatography (hexane : ethyl acetate—90 : 10). Yield, 2.2 g, 88% (brown solid). M.p. 145–149°C. 1H NMR (CD3OD, 600 MHz): δ ppm 2.88 (dt, J = 16.2, 4.6 Hz, 1H, H4′a), 2.99–3.06 (m, 1H, H4′b), 3.21–3.26 (m, 1H, H3′a), 3.38 (s, 3H, 7′-OCH3), 3.82 (s, 4H, H3′b, 6′-OCH3), 4.82 (d, J = 15.5 Hz, 1H, –CH2–Ar), 5.09 (d, J = 15.5 Hz, 1H, CH2–Ar), 5.82 (s, 1H, H8′), 6.80 (s, 1H, H5′), 7.04–7.11 (m, 4H, H5, H7, H3″,H5″), 7.20 (dd, J = 7.5, 1.2 Hz, 1H, H4), 7.31 (td, J = 7.8, 1.3 Hz, 1H, H6), 7.45–7.48 (m, 2H, H2″, H6″). 13C NMR (CD3OD, 150 MHz): δ ppm 27.6 (C4′), 38.5 (C3′), 42.3 (–CH2–Ar), 54.8 (7′-OCH3), 55.0 (6′-OCH3), 63.3 (C3/C1′), 108.8 (C8′), 109.3 (C7), 112.2 (C5′), 115.2 (2C, C3″,C5″), 123.2 (C5), 124.3 (C4), 125.8 (C8′a), 128.9 (C6), 129.4 (2C, C2″,C6″), 132.4 (C1″), 134.3 (C3a), 137.4 (C4″), 142.4 (C7a), 147.7 (C7′), 148.8 (C6′), 163.2 (C4″), 178.8 (C2). FTMS + cESI: m/z 419.18 [M + 1]+.
3.34. 6′,7′-Dimethoxy-1-(4-methylbenzyl)-3′,4′-dihydro-2′H-spiro[indoline-3,1′-isoquinolin]-2-one (5e)
Method G. Prepared from 1-(4-methylbenzyl)indoline-2,3-dione, 8e (1.5 g, 6.0 mmol), 3,4-dimethoxyphenethylamine, 11a (1 g 6.0 mmol) and polyphosphoric acid (2.5 g). Purified by flash chromatography (hexane : ethyl acetate—90 : 10). Yield, 2.2 g, 88% (brown solid). M.p. 175–177°C. 1H NMR (CD3OD, 600 MHz): δ ppm 2.32 (s, 3H, 4″-CH3–Bz), 2.89 (dt, J = 16.2, 4.7 Hz, 1H, H4′a), 3.03 (ddd, J = 16.2, 8.7, 5.3 Hz, 1H, H4′b), 3.24 (dt, J = 12.8, 5.1 Hz, 1H, H3′a), 3.37 (s, 3H, 7′-OCH3), 3.83 (s, 4H, H3′b, 6′-OCH3), 4.75 (d, J = 15.4 Hz, 1H, –CH2–Ar), 5.13 (d, J = 15.4 Hz, 1H, CH2–Ar), 5.82 (s, 1H, H8′), 6.81 (s, 1H, H5′), 7.04 (d, J = 7.9 Hz, 1H, H7), 7.06 (td, J = 7.6, 1.0 Hz, 1H, H5), 7.17 (d, J = 7.9 Hz, 2H, H3″, H5″), 7.20 (dd, J = 7.5, 1.2 Hz, 1H, H4), 7.30 (td, J = 7.8, 1.3 Hz, 1H, H6), 7.32 (m, 2H, H2″, H6″). 13C NMR (CD3OD, 150 MHz): δ ppm 19.7 (4″-CH3), 27.5 (C4′), 38.5 (C3′), 42.8 (–CH2–Ar), 54.7 (7′-OCH3), 55.0 (6′-OCH3), 63.4 (C3/C1′), 108.8 (C8′), 109.5 (C7), 112.2 (C5′), 123.2 (C5), 124.2 (C4), 125.8 (C8′a), 127.4 (2C, C2″, C6″), 128.8 (C4′a), 128.9 (C6), 129.0 (2C, C3″,C5″), 133.2 (C1″), 134.3 (C3a), 137.4 (C4″), 142.6 (C7a), 147.7 (C7′), 148.7 (C6′), 178.9 (C2). FTMS + cESI: m/z 415.20 [M + 1]+.
3.35. 1-(4-Bromobenzyl)-5-chloro-6′,7′-dimethoxy-3′,4′-dihydro-2′H-spiro[indoline-3,1′-isoquinolin]-2-one (5f)
Method G. Prepared from 1-(4-bromobenzyl)-5-chloroindoline-2,3-dione, 8c (0.7 g, 4.1 mmol), 3,4-dimethoxyphenethylamine, 11a (1.2 g 3.4 mmol) and polyphosphoric acid (2.0 g). Product purified by flash chromatography (hexane : ethyl acetate—80 : 20). Yield, 1.2 g, 69% (brown solid). M.p. 198–201°C. 1H NMR (CD3OD, 600 MHz): δ ppm 2.84–2.90 (m, 1H, H4′a), 3.02 (ddd, J = 9.7, 5.3, 4,5 Hz, 1H, H4′b), 3.18–3.23 (m, 1H, H3′a), 3.42 (s, 3H, 7′-OCH3), 3.82 (d, J = 5.9 Hz, 1H, H3′b), 3.84 (s, 3H, 6′-OCH3), 4.83 (d, J = 15.7 Hz, 1H, –CH2–Ar), 5.06 (d, J = 15.7 Hz, 1H, CH2–Ar), 5.80 (s, 1H, H8′), 6.82 (s, 1H, H5′), 7.04 (d, J = 8.4 Hz, 1H, H7), 7.21(d, J = 72.2 Hz, 1H, H4), 7.33 (dd, J = 8.4, 2.2 Hz, 1H, H6), 7.37 (d, J = 8.4 Hz, 2H, H2″, H6″), 7.50–7.54 (m, 2H, H3″, H5″). 13C NMR (CD3OD, 150 MHz): δ ppm 27.5 (C4′), 38.4 (C3′), 42.5 (–CH2–Ar), 54.9 (7′-OCH3), 55.0 (6′-OCH3), 63.4 (C3/C1′), 108.6 (C8′), 110.5 (C7), 112.4 (C5′), 121.4 (C4″), 124.7 (C4), 125.0 (C8′a), 127.8 (C4′a), 128.5 (C6), 129.0 (C5), 129.4 (2C, C2″,C6″), 131.6 (2C, C3″,C5″), 135.3 (C1″), 136.2 (C3a), 141.1 (C7a), 147.8 (C7′), 149.0 (C6′), 178.5 (C2). FTMS + cESI: m/z 513.06 [M + 1]+.
3.36. 6′,7′,8′-Trimethoxy-3′,4′-dihydro-2′H-spiro[indoline-3,1′-isoquinolin]-2-one (6a)
Method G. Prepared from isatin (1.6 g, 10.9 mmol) and 3,4,5-trimethoxyphenethylamine, 11b (2.8 g, 13 mmol), and polyphosphoric acid (3 g). The crude product was purified by flash chromatography (hexane : ethyl acetate—80 : 20). Yield, 1.24 g, 34% (white solid). M.p. 238–240°C. 1H NMR (CD3OD, 600 MHz): δ ppm 2.85–2.97 (m, 2H, H4′a, H4′b), 3.21 (s, 3H,8′-OCH3), 3.26–3.30 (m, 2H, H3′a, H3′b), 3.69 (s, 3H, 7′-OCH3), 3.86 (s, 3H, 6′-OCH3), 6.65 (s, 1H, H5′), 6.96 (dt, J = 7.8, 0.8 Hz, H7), 6.99 (dt, td, J = 7.8, 0.8 Hz, 1H, H4), 7.06 (ddd, J = 7.4, 1.2, 0.6 Hz, 1H, H5), 7.25 (td, J = 7.7, 1.3 Hz, 1H, H6). 13C NMR (CD3OD, 150 MHz): δ ppm 28.0 (C4′), 38.6 (C3′), 55.0 (6′-OCH3), 58.5 (8′-OCH3), 59.5 (7′-OCH3), 62.4 (C3/C1′), 107.5 (C5′), 109.7 (C7), 120.4 (C8′a), 121.7 (C4), 121.7 (C5), 128.4 (C6), 132.5 (C4′a), 135.1 (C3a), 140.0 (C7a), 142.2 (C6′), 150.1 (C8′), 153.2 (C7′), 181.0 (C2). FTMS + cESI: m/z 341.15 [M + 1]+.
3.37. 5-Chloro-6′,7′,8′-trimethoxy-3′,4′-dihydro-2′H-spiro[indoline-3,1′-isoquinolin]-2-one (6b)
Method G. Prepared from 5-chloroisatin (1.5 g, 8.3 mmol), 3,4,5-trimethoxyphenethylamine, 11b (2.1 g, 9.9 mmol) and polyphosphoric acid (3 g). Purified by flash chromatography (hexane : ethyl acetate—80 : 20). Yield, 1.8 g, 58% (brown solid). M.p. 102–105°C. 1H NMR (CD3OD, 600 MHz): δ ppm 2.86–2.98 (m, 2H, H4′a, H4′b), 3.18–3.24 (m, 1H, H3′a), 3.28–3.32 (m, 4H, H3′b, 8′-OCH3), 3.70 (s, 3H, 7′-OCH3), 3.87 (s, 3H, 6′-OCH3), 6.65 (s, 1H, H5′), 6.96 (dt, J = 8.3, 1H, H7), 7.05 (d, td, J = 2.1 Hz, 1H, H4), 7.26 (dd J = 8.3, 2.1 Hz, 1H, H6).13C NMR (CD3OD, 150 MHz): δ ppm 27.9 (C4′), 38.5 (C3′), 55.1 (6′-OCH3), 58.6 (8′-OCH3), 59.5 (7′-OCH3), 62.4 (C3/C1′), 107.5 (C5′), 110.7 (C7), 119.6 (C8′a), 123.9 (C4), 126.8 (C5), 128.2 (C6), 132.6 (C4′a), 137.0 (C3a), 140.0 (C8′), 140.9 (C7a), 150.0 (C6′), 153.4 (C7′), 181.0 (C2). FTMS + cESI: m/z 375.11 [M + 1]+.
3.38. 5,7-Dibromo-6′,7′,8′-trimethoxy-3′,4′-dihydro-2′H-spiro[indoline-3,1′-isoquinolin]-2-one (6c)
Method G. Prepared from 5,7-dibromoisatin, 7c, (1.5 g, 5.0 mmol), 3,4,5-trimethoxyphenethylamine, 11b (1.3 g, 6 mmol) and polyphosphoric acid (2 g). The crude product was purified by flash chromatography (hexane : ethyl acetate—80 : 20). Yield, 2.1 g, 84% (brown solid). M.p. 200–203°C. 1H NMR (CD3OD, 600 MHz): δ ppm 2.89 (td, J = 6.5, 5.5, 2H, H4′a, H4′b), 3.18 (ddd, J = 13.1, 7.4, 5.6, 1H, H3′a,), 3.27–3.31 (m, 1H, H3′b), 3.37 (s, 8′-OCH3), 3.70 (s, 3H, 7′-OCH3), 3.87 (s, 3H, 6′-OCH3), 6.65 (s, 1H, H5′), 7.15 (d, J = 1,8 Hz, 1H, H4), 7.60 (d, J = 1.8, 1H, H6). 13C NMR (CD3OD, 150 MHz): δ ppm 27.8 (C4′), 38.4 (C3′), 55.1 (6′-OCH3), 58.6 (8′-OCH3), 59.5 (7′-OCH3), 63.3 (C3/C1′), 102.8 (C7), 107.5 (C5′), 114.0 (C5), 119.2 (C8′a), 125.6 (C4), 132.7 (C4′a), 133.1 (C6), 138.4 (C3a), 139.9 (C8′), 141.2 (C7a), 150.0 (C6′), 153.6 (C7′), 179.8 (C2). FTMS + cESI: m/z 498.97 [M + 1]+.
3.39. 1-(4-Fluorobenzyl)-6′,7′,8′-trimethoxy-3′,4′-dihydro-2′H-spiro[indoline-3,1′-isoquinolin]-2-one (6d)
Method G. Prepared from 1-(4-fluorobenzyl)indoline-2,3-dione, 8a (2.0 g, 7.8 mmol), 3,4,5-trimethoxyphenethylamine, 11b (1.7 g, 7.8 mmol) and polyphosphoric acid (2.5 g). The crude product was purified by flash chromatography (hexane : ethyl acetate—80 : 20). Yield, 3.0 g, 62% (white solid). M.p. 173–175°C. 1H NMR (CD3OD, 600 MHz): δ ppm 2.87 (s, 3H, 8′-OCH3), 2.89–3.0 (m,, 2H, H4′a, H4′b), 3.27 (ddd, J = 13.2, 7.7, 5.1 Hz, 1H, H3′a), 3.36 (m. 1H, H3′b), 3.70 (s, 3H, 7′-OCH3), 3.87 (s, 6′-OCH3), 4.91 (d, J = 15.6 Hz, 1H, –CH2–Ar), 5.08 (d, J = 15.6 Hz, 1H, CH2–Ar), 6.67 (s, 1H, H5′), 6.95 (dt, J = 7.8, 0.7 Hz, 1H, H7), 7.01 (td, J = 7.5, 1.0 Hz, 1H, H5), 7.08–7.13 (m, 3H, H4, H3″, H5″), 7.25 (td, J = 7.8, 1.3 Hz, 1H, H6), 7.54–7.58 (m, 2H, H2″, H6″). 13C NMR (CD3OD, 150 MHz): δ ppm 28.1 (C4′), 38.7 (C3′), 42.7 (–CH2–Ar), 55.1 (6′-OCH3), 58.3 (8′-OCH3),59.5 (7′-OCH3) 62.0 (C3/C1′), 107.6 (C5′), 109.2 (C7), 114.9 (2C, C3″, C5″), 120.0 (C8′a), 122.4 (C4), 123.4 (C5), 128.4 (C6), 127.6 (2C, C2″, C6″), 132.2 (C3a), 132.8 (C4′a), 134.7 (C1″), 140.1 (C8′), 142.8 (C7a), 150.0 (C7′), 153.3 (C6′), 163.2 (C4″), 178.9 (C2). FTMS + cESI: m/z 449.19 [M + 1]+.
3.40. 6′,7′,8′-Trimethoxy-1-(4-methylbenzyl)-3′,4′-dihydro-2′H-spiro[indoline-3,1′-isoquinolin]-2-one (6e)
Method G. Prepared from 1-(4-methylbenzyl)indoline-2,3-dione, 8e (1.6 g, 6.4 mmol), 3,4,5-trimethoxyphenethylamine, 11b (1.6 g, 6 mmol) and polyphosphoric acid (2 g). The crude product was purified by flash chromatography (hexane : ethyl acetate—80 : 20). Yield, 2.3 g, 80% (brown solid). M.p. 135–138°C. 1H NMR (CD3OD, 600 MHz): δ ppm 2.34 (s, 3H, 4″-CH3–Bz), 2.89 (s, 3H, 8′-OCH3), 2.90–2.96 (m, 2H, H4′a, H4′b), 3.24–3.30 (m, 1H, H3′a), 3.34–3.37 (m, 1H, H3′b), 3.71 (s, 3H, 7′-OCH3), 3.87 (s, 4H, H3′b, 6′-OCH3), 4.87 (d, J = 15.5 Hz, 1H, –CH2–Ar), 5.06 (d, J = 15.5 Hz, 1H, CH2–Ar), 6.67 (s, 1H, H5′), 6.92 (d, J = 7.9 Hz, 1H, H7), 6.99 (td, J = 7.6, 1.0 Hz, 1H, H5), 7.11 (dd, J = 7.4, 1.2 Hz, 1H, H4), 7.17–7.20 (m, 2H, H3″, H5″), 7.22 (td, J = 7.8, 1.3 Hz, 1H, H6), 7.40–7.42 (m, 2H, H2″, H6″). 13C NMR (CD3OD, 150 MHz): δ ppm 19.8 (4″-CH3), 28.1 (C4′), 38.7 (C3′), 43.3 (–CH2–Ar), 55.1 (6′-OCH3), 58.3 (8′-OCH3),59.5 (7′-OCH3) 62.0 (C3/C1′), 107.5 (C5′), 109.4 (C7), 120.1 (C8′a), 122.3 (C5), 123.3 (C4), 127.6 (2C, C2″,C6″), 128.8 (2C, C3″,C5″), 132.7 (C4′a), 133.1 (C3a), 134.1 (C1″), 137.1 (C4″), 140.1 (C8′), 143.0 (C7a), 150.1 (C7′), 153.2 (C6′), 178.9 (C2). FTMS + cESI: m/z 445.21 [M + 1]+.
Supplementary Material
Acknowledgements
The authors are indebted to Prof. Michael Spiteller, INFU, Technische Universität Dortmund, Germany, for providing all of the HPLC-MS, 13C and 1NMR data for this study. Anti-cancer screening was carried out by the US National Cancer Institute, Bethesda, MD under the NCI-60 screening programme.
Data accessibility
Electronic supplementary material available: 1H NMR and 13C NMR spectra of target compounds, S2–S81; LC-MS Spectra of target compounds, S82–121; biological Screening Data, electronic supplementary material, S122–S127.
Authors' contributions
S.M.N.E. conceived and designed the project, subsequently supervised the work; reviewed the data and prepared the manuscript. M.M.M.L. carried out the synthesis of the compounds, performed the interpretation of spectral data and co-wrote the manuscript.
Competing interests
We declare we have no competing interests.
Funding
Funding was provided by the Ministry of Higher Education of Cameroon under the Research Modernization Scheme.
References
- 1.World Health Organization. 2018 Cancer. See https://www.who.int/news-room/fact-sheets/detail/cancer (accessed 12 November 2018).
- 2.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. 2018. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 68, 394–424. ( 10.3322/caac.21492) [DOI] [PubMed] [Google Scholar]
- 3.National Cancer Institute. Cancer Treatment. See https://www.cancer.gov/about-cancer/treatment (accessed 12 November 2018).
- 4.Evans BE, et al. 1988. Methods for drug discovery: development of potent, selective, orally effective cholecystokinin antagonists. J. Med. Chem. 31, 2235–2246. ( 10.1021/jm00120a002) [DOI] [PubMed] [Google Scholar]
- 5.Viegas-Junior C, Danuello A, da Silva Bolzani V, Barreiro EJ, Manssour Fraga CA. 2007. Molecular hybridization: a useful tool in the design of new drug prototypes. Curr. Med. Chem. 14, 1829–1852. ( 10.2174/092986707781058805) [DOI] [PubMed] [Google Scholar]
- 6.Murahashi SI, Shiola T. 1987. Selenium dioxide catalyzed oxidation of secondary amines with hydrogen peroxide: simple synthesis of nitrones from secondary amines. Tetrahedron Lett. 28, 2383–2386. ( 10.1016/S0040-4039(00)96130-6) [DOI] [Google Scholar]
- 7.O'Reilly NJ, Derwin WS, Lin HC. 1990. Optically pure (S)-6,7-dimethoxy-1,2,3,4-tetrahydro-3-isoquinolinecarboxylic acid and asymmetric hydrogenation studies related to its preparation. Synthesis 7, 550–556. ( 10.1055/s-1990-26936) [DOI] [Google Scholar]
- 8.Strolin-Bendetti M, Doistert P, Cannitiati PJ. 1989. Influence of food intake on the enantiomeric composition of urinary salsolinol in man. J. Neural Transm. 78, 43–51. ( 10.1007/BF01247112) [DOI] [PubMed] [Google Scholar]
- 9.Yakoyama P, Ohwada T, Shudo KJ. 1999. Preparation and reactions of 3,4-bis(tributylstannyl)-2(5H)-furanone. Org. Chem. 64, 611–617. ( 10.1021/jo982019e) [DOI] [Google Scholar]
- 10.Bringman G, Brun R, Kaiser M, Neumann S. 2008. Synthesis and antiprotozoal activities of simplified analogs of naphthylisoquinoline alkaloids. Eur. J. Med. Chem. 43, 32–42. ( 10.1016/j.ejmech.2007.03.003) [DOI] [PubMed] [Google Scholar]
- 11.François G, Timperman G, Eling W, Assi LA, Hotemz J, Bringmann G. 1997. Naphthylisoquinoline alkaloids against malaria: evaluation of the curative potentials of dioncophylline C and dioncopeltine A against Plasmodium berghei in vivo. Antimicrob. Agents Chemother. 41, 2533–2539. ( 10.1128/AAC.41.11.2533) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bringmann G, Messer K, Wolf K, Mühlbacher J, Grüne M, Brun R, Louis AM. 2002. Dioncophylline E from Dioncophyllum thollonii, the first 7,3′-coupled dioncophyllaceous naphthylisoquinoline alkaloid. Phytochemistry 60, 389–397. ( 10.1016/S0031-9422(02)00109-7) [DOI] [PubMed] [Google Scholar]
- 13.Bringmann G, Günthera C, Saeb W, Mies J, Brun R, Aké Assi L. 2000. 8-O-Methyldioncophyllinol B and revised structures of other 7, 6′-coupled naphthylisoquinoline alkaloids from Triphyophyllum peltatum (Dioncophyllaceae). Phytochemistry 54, 337–346. ( 10.1016/S0031-9422(00)00107-2) [DOI] [PubMed] [Google Scholar]
- 14.Iwasawa Y, Kiyomoto A. 1967. Studies on tetrahydroisoquinolines (THI) (I) bronchodilator activity and structure–activity relationship. Jpn. J. Pharmacol. 17, 143–152. ( 10.1254/jjp.17.143) [DOI] [PubMed] [Google Scholar]
- 15.Cheng P, Huang N, Jiang Z-Y, Zhang Q, Zheng Y-T, Chen J-J, Zhang X-M, Ma Y-B. 2008. 1-Aryl-tetrahydroisoquinoline analogs as active anti-HIV agents in vitro. Bioorg. Med. Chem. Lett. 18, 2475–2478. ( 10.1016/j.bmcl.2008.02.040) [DOI] [PubMed] [Google Scholar]
- 16.Manmi P, d'Ischia M, Prota GJ. 2001. An unusual decarboxylative Maillard reaction between l-DOPA and d-glucose under biomimetic conditions: factors governing competition with Pictet−Spengler condensation. Org. Chem. 66, 5048–5053. ( 10.1021/jo010078d) [DOI] [PubMed] [Google Scholar]
- 17.Acikgoz E, Guven U, Duzagac F, Uslu R, Kara M, Soner BC, Oktem G. 2015. Enhanced G2/M arrest, caspase related apoptosis and reduced E-cadherin dependent intercellular adhesion by trabectedin in prostate cancer stem cells. PLoS ONE 10, e0141090 ( 10.1371/journal.pone.0141090) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mohammadi S, Seyedhoseini FS, Asadi J, Yazdani Y. 2017. Effects of berberine on the secretion of cytokines and expression of genes involved in cell cycle regulation in THP-1 monocytic cell line. Iran J. Basic Med. Sci. 20, 530–537. ( 10.22038/IJBMS.2017.8677) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ishiba H, Noguchi T, Shu K, Ohno H, Honda K, Kondoh Y, Osada H, Fujii N, Oishi S. 2017. Investigation of the inhibitory mechanism of apomorphine against MDM2–p53 interaction. Bioorg. Med. Chem. Lett. 27, 2571–2574. ( 10.1016/j.bmcl.2017.03.082) [DOI] [PubMed] [Google Scholar]
- 20.Zinzi L, Capparelli E, Cantore M, Contino M, Leopoldo M, Colabufo NA. 2014. Small and innovative molecules as new strategy to revert MDR. Front. Oncol. 4, 2 ( 10.3389/fonc.2014.00002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen D, et al. 2016. Tetrahydroisoquinolines as novel histone deacetylase inhibitors for treatment of cancer. Acta Pharm. Sin. B 6, 93–99. ( 10.1016/j.apsb.2015.11.002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.DeBono A, Capuano B, Scammells PJ. 2015. Progress toward the development of noscapine and derivatives as anticancer agents. J. Med. Chem. 58, 5699–5727. ( 10.1021/jm501180v) [DOI] [PubMed] [Google Scholar]
- 23.Khan FA, Maalik A. 2015. Advances in pharmacology of isatin and its derivatives: a review. Trop. J. Pharm. Res. 14, 1937–1942. ( 10.4314/tjpr.v14i10.28) [DOI] [Google Scholar]
- 24.Nesi G, et al. 2013. Synthesis of novel 3,5-disubstituted-2-oxindole derivatives as antitumor agents against human nonsmall cell lung cancer. ACS Med. Chem. Lett. 4, 1137–1141. ( 10.1021/ml400162g) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kumar D, Sharma VS, Kumar R, Singh T, Singh H, Singh AD, Roy RK. 2013. Design, synthesis and anticonvulsant activity of some new 5,7-dibromoisatin semicarbazone derivatives. EXCLI J. 12, 628–640. [PMC free article] [PubMed] [Google Scholar]
- 26.Dandia A, Saini D, Saini DK, Mathur M. 2014. A facile green synthesis of 3-substituted-3-hydroxyindolin-2-ones and investigation of their in vitro antioxidant and antimicrobial potential. Eur. Chem. Bull. 3, 85–92. [Google Scholar]
- 27.Welsch ME, Snyder SA, Stockwell BR. 2010. Privileged scaffolds for library design and drug discovery. Curr. Opin. Chem. Biol. 14, 347–361. ( 10.1016/j.cbpa.2010.02.018) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Modi NR, Shah RJ, Patel MJ, Suthar M, Cahauhan BF, Patel LJ. 2011. Design, synthesis, and QSAR study of novel 2-(2,3-dioxo-2,3-dihydro-1H-indol-1-yl)-N-phenylacetamide derivatives as cytotoxic agents. Med. Chem. Res. 20, 615–625. ( 10.1007/s00044-010-9361-y) [DOI] [Google Scholar]
- 29.Vine KL, Matesic L, Locke JM, Skropeta D. 2013. Recent highlights in the development of isatin-based anticancer agents. In Advances in anticancer agents in medicinal chemistry (ed. Prudhomme M.), pp. 254–312. Sharjah, UAE: Bentham Science Publishers. [Google Scholar]
- 30.Chu W, Zhang J, Zeng C, Rothfuss J, Tu Z, Chu Y, Reichert DE, Welch MJ, Mach RH. 2005. N-Benzylisatin sulfonamide analogues as potent caspase-3 inhibitors: synthesis, in vitro activity, and molecular modeling studies. J. Med. Chem. 48, 7637–7647. ( 10.1021/jm0506625) [DOI] [PubMed] [Google Scholar]
- 31.Chu W, Rothfuss J, d'Avignon A, Zeng C, Zhou D, Hotchkiss RS, Mach RH. 2007. Isatin sulfonamide analogs containing a Michael addition acceptor: a new class of caspase 3/7 inhibitors. J. Med. Chem. 50, 3751–3755. ( 10.1021/jm070506t) [DOI] [PubMed] [Google Scholar]
- 32.Davidovich P, et al. 2015. Discovery of novel isatin-based p53 inducers. ACS Med. Chem. Lett. 6, 856–860. ( 10.1021/acsmedchemlett.5b00011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zheng G-H, Shen J-J, Zhan Y-C, Yi H, Xue S-T, Wang Z, Ji X-Y, Li Z-R. 2014. Design, synthesis and in vitro and in vivo antitumour activity of 3-benzylideneindolin-2-one derivatives, a novel class of small-molecule inhibitors of the MDM2–p53 interaction. Eur. J. Med. Chem. 81, 277–288. ( 10.1016/j.ejmech.2014.05.027) [DOI] [PubMed] [Google Scholar]
- 34.Zheng Y-J, Tice CM. 2016. The utilization of spirocyclic scaffolds in novel drug discovery. Expert Opin. Drug Discov. 11, 831–834. ( 10.1080/17460441.2016.1195367) [DOI] [PubMed] [Google Scholar]
- 35.Kaur M, Singh M, Chadha N, Silakari O. 2016. Oxindole: a chemical prism carrying plethora of therapeutic benefits. Eur. J. Med. Chem. 123, 858–894. ( 10.1016/j.ejmech.2016.08.011) [DOI] [PubMed] [Google Scholar]
- 36.Yu B, Yu D-Q, Liu H-M. 2015. Spirooxindoles: promising scaffolds for anticancer agents. Eur. J. Med. Chem. 97, 673–678. ( 10.1016/j.ejmech.2014.06.056) [DOI] [PubMed] [Google Scholar]
- 37.Kumar A, Gupta G, Bishnoi AK, Saxena R, Saini KS, Konwar R, Kumar S, Dwivedi A. 2015. Design and synthesis of new bioisosteres of spirooxindoles (MI-63/219) as anti-breast cancer agents. Bioorg. Med. Chem. 23, 839–848. ( 10.1016/j.bmc.2014.12.037) [DOI] [PubMed] [Google Scholar]
- 38.Sun Y, Liu J, Sun T, Zhang X, Yao J, Kai M, Jiang X, Wang R. 2014. Anti-cancer small molecule JP-8 g exhibits potent in vivo anti-inflammatory activity. Sci. Rep. 4, 4372 ( 10.1038/srep04372) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Turner H. 2016. Spiroindolone NITD609 is a novel antimalarial drug that targets the P-type ATPase PfATP4. Future Med. Chem. 8, 227–238. ( 10.4155/fmc.15.177) [DOI] [PubMed] [Google Scholar]
- 40.Davis HJ, Kavanagh ME, Balan T, Abell C, Coyne AG. 2016. Spirooxindoles as novel 3D-fragment scaffolds: synthesis and screening against CYP121 from M. tuberculosis. Bioorg. Med. Chem. Lett. 26, 3735–3740. ( 10.1016/j.bmcl.2016.05.073) [DOI] [PubMed] [Google Scholar]
- 41.Saha S, Acharya C, Pal U, Chowdhury SR, Sarkar K, Maiti NC, Jaisankar P, Majumdera HK. 2016. A novel spirooxindole derivative inhibits the growth of Leishmania donovani parasites both in vitro and in vivo by targeting type IB topoisomerase. Antimicrob. Agents Chemother. 60, 6281–6293. ( 10.1128/AAC.00352-16) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ye N, Chen H, Wold EA, Shi P-Y, Zhou J. 2016. Therapeutic potential of spirooxindoles as antiviral agents. ACS Infect. Dis. 2, 382–392. ( 10.1021/acsinfecdis.6b00041) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zou B, Yap P, Sonntag L-S, Leong SY, Yeung BKS, Keller TH. 2012. Mechanistic study of the spiroindolones: a new class of antimalarials. Molecules 17, 10 131–10 141. ( 10.3390/molecules170910131) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Brouwer WG, Craig WA, Jeffreys JAD, Munro A. 1972. The reaction between isatin and some amines. J. Chem. Soc., Perkin Trans. 1, 124–129. ( 10.1039/p19720000124) [DOI] [Google Scholar]
- 45.Velez Y, Díaz-Oviedo C, Quevedo R. 2017. Kinetic and thermodynamic control in β-phenylethylamines reaction with isatin. J. Mol. Struct. 1133, 430–435. ( 10.1016/j.molstruc.2016.12.039) [DOI] [Google Scholar]
- 46.Maresh JJ, et al. 2014. Chemoselective Zinc/HCl reduction of halogenated β-nitrostyrenes: synthesis of halogenated dopamine analogues. Synlett 25, 2891–2894. ( 10.1055/s-0034-1379481) [DOI] [Google Scholar]
- 47.Kametani T, Fukumoto K, Agui H, Yagi H, Kigasawa TK, Sugahara H, Hiiragi M, Hayasaka T, Ishimaru H. 1968. Phenolic cyclisation. Part I. Novel synthesis of 1-substituted isoquinoline and spiro[cycloalkane-1,1′-isoquinoline] derivatives and its application to the total synthesis of isoquinoline alkaloids. J. Chem. Soc. C 1968, 112–118. ( 10.1039/J39680000112) [DOI] [Google Scholar]
- 48.Vine KL, Locke JM, Ranson M, Pyne SG, Bremner JB. 2007. An investigation into the cytotoxicity and mode of action of some novel N-alkyl-substituted isatins. J. Med. Chem. 50, 5109–5117. ( 10.1021/jm0704189) [DOI] [PubMed] [Google Scholar]
- 49.Berger L, Corraz AJ, Lee J. 1967. US Patent no. 3,301,857.
- 50.Zhao Q, Peng C, Huang H, Liu S-J, Zhong Y-J, Huang W, He G, Han B. 2018. Asymmetric synthesis of tetrahydroisoquinoline-fused spirooxindoles as Ras-GTP inhibitors that inhibit colon adenocarcinoma cell proliferation and invasion. Chem. Commun. 54, 8359–8362. ( 10.1039/C8CC04732D) [DOI] [PubMed] [Google Scholar]
- 51.Mravec B. 2006. Salsolinol, a derivate of dopamine, is a possible modulator of catecholaminergic transmission: a review of recent developments. Physiol. Res. 55, 353–364. [DOI] [PubMed] [Google Scholar]
- 52.Ngo Hanna J, Ntie-Kang F, Kaiser M, Brun R, Efange SMN. 2014. 1-Aryl-1,2,3,4-tetrahydroisoquinolines as potential antimalarials: synthesis, in vitro antiplasmodial activity and in silico pharmacokinetics evaluation RSC Adv. 4, 22 856–22 865. ( 10.1039/C3RA46791K) [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Electronic supplementary material available: 1H NMR and 13C NMR spectra of target compounds, S2–S81; LC-MS Spectra of target compounds, S82–121; biological Screening Data, electronic supplementary material, S122–S127.