Abstract
The breakdown of long-lived proteins is associated with aging, as well as disease, however our understanding of the molecular processes involved is still limited. Of particular relevance, crosslinked proteins are often reported in aged tissues but the mechanisms for their formation are poorly understood. In this study, sites of protein crosslinking in human ocular lenses were characterized using proteomic techniques. In long-lived lens proteins, several sites of crosslinking were found to involve the addition of Lys to Asp or Asn residues. Using model peptides containing Asp or Asn, a mechanism was elucidated that involves a succinimide intermediate. Succinimides formed readily from Asn at neutral pH, whereas a higher rate of formation from Asp peptides was observed at more acidic pHs. Succinimides were found to be relatively stable in the absence of nucleophiles. Since racemization of Asp residues, as well as deamidation of Asn, involves a succinimide intermediate, sites of D-Asp and isoAsp in long-lived proteins should also be considered as potential sites of protein covalent crosslinking.
Keywords: Long-lived proteins, Isopeptide bonds, Aging, Lens, Racemization
Introduction
Some amino acid residues in long-lived proteins (LLPs) are susceptible to modification over time. Asn, Asp, Gln and Ser appear to be particularly prone to spontaneous modification [1–3] and their breakdown products are responsible for the major features associated with LLPs in aged animals, including racemization, deamidation and cleavage [4]. Covalent crosslinking is another phenomenon that has been observed in a number of aged proteins [5]; however, the mechanisms underpinning the formation of such non-disulfide crosslinks are still poorly understood.
Crosslinking can be broadly broken down into two types. Crosslinking can occur via reactive small metabolites, for example glucose or other sugars that are involved in the formation of advanced glycation end products, such as pentosidine [6]. Secondly, as we age, intrinsically unstable amino acids break down forming reactive intermediates that can result in protein-protein crosslinks e.g. lanthionine and lysinoalanine etc [5]. Protein crosslinking has been shown to occur in many long-lived proteins, for example collagen [7], dentin [8] lens crystallins [5] and extracellular matrix proteins [9]. Many conditions of aging and disease have been linked to increased protein crosslinking such as arterial hardening [10], aggregation and lipofuscin formation [11], diabetes [12], osteoarthritis [13] and cataract [14] although the mechanisms underlying these have not been well characterized.
Recently the formation of dehydroalanine intermediates from the breakdown of Cys [15, 16] and phosphoserine [5] residues has been found to be a significant pathway responsible for protein-protein crosslinking in adult lenses of the eye. The human ocular lens is an ideal tissue to use for investigation of the decomposition of LLPs because there is no turnover of lens proteins in the center of the lens after they are synthesized pre-natally [17].
As part of a program to characterize the chemical processes responsible for the covalent crosslinking of LLPs, proteins from adult lenses have been examined using proteomic methods with the aim of detecting sites of novel crosslinks. An investigation of aquaporin 0 (AQP0), an abundant water channel protein with important roles in the maintenance of lens transparency and homeostasis [18], revealed sites of crosslinking involving the attachment of Lys to Asp and Asn residues. Peptide model studies were employed in an effort to understand the molecular basis for this biochemical process.
Materials and Methods
Lens membrane protein preparation, trypsin digestion and crosslinked peptide enrichment
A 68 year-old cataract lens was obtained from NDRI (Philadelphia, PA) and stored at −80°C until use. To separate the cortex region from lens nucleus, 1.0mm thickness tissue was peeled from the surface of the lens and considered the cortex region. The remaining tissue was considered as the lens nucleus. The lens fiber cell urea-insoluble fraction (UIF) was prepared from the lens nucleus and the proteins present in this fraction were reduced, alkylated and digested with trypsin as described previously [5]. Tryptic peptides were offline-fractionated by strong cation exchange as described previously [19]. Briefly, bound peptides were step-eluted sequentially from SCX resin by 40%, 60%, and 100% buffer B (5 mM potassium phosphate buffer containing 30% CH3CN, 350 mM KCl, pH 2.5) balanced with buffer A (5 mM potassium phosphate buffer containing 30% CH3CN, pH 2.5). The 60% buffer B eluate was dried in a speedvac, reconstituted in 0.1% formic acid and desalted using a C18 Ziptip (Millipore, Billerica, MA, USA). Peptides were eluted from the Ziptip using 70% CH3CN (0.1% formic acid), dried in a speedvac, and reconstituted in 0.1% formic acid. The peptides were loaded onto a custom-packed SCX trap column (6 cm × 150 μm, Jupiter C18, 5 μm, 300 Å) and 20 uL of 750 mM ammonium acetate was flowed through the column. The remaining bound peptides were eluted by 30 μL of 3 M ammonium acetate and used for crosslinked peptide identification by LC-MS/MS.
LC-MS/MS
For crosslinked peptide identification by LC-MS/MS, the sample was loaded onto a custom packed biphasic C18/SCX trap column (4 cm × 150 μm, Jupiter C18, 5 μm, 300 Å media followed by 6 cm × 150 μm, Luna SCX, 5 μm, 100 Å media) and analyzed using 3-step salt pulse gradient (1 M, 1.5 M, 2 M ammonium acetate) as described previously[5]. The eluate was directly infused into a Velos Orbitrap mass spectrometer (ThermoFisher Scientific, San Jose, CA, USA). Dynamic exclusion (repeat count 2, exclusion list size 300, and exclusion duration 60 s) was enabled to allow the detection of low abundance ions. For in vitro peptide experiments, the sample was loaded on a C18 trap column (50 mm x 100 μm) packed with Phenomenex Jupiter resin (5 μm mean particle size, 300 Å pore size) and separated on a one-dimensional fused silica capillary column (250 mm x 100 μm) packed with Phenomenex Jupiter resin (3 μm mean particle size, 300 Å pore size). One-dimensional liquid chromatography was employed using the following gradient at a flow rate of 0.5 μL/min: 0–60 min, 2–45% CH3CN (0.1% formic acid); 60–70 min, 45–95% CH3CN (0.1% formic acid) balanced with 0.1% formic acid. The eluate was directly infused into an LTQ Velos Pro mass spectrometer (ThermoFisher Scientific, San Jose, CA) with a nanoelectrospray ionization source. The LTQ Velos Pro was operated in a 17-step data dependent mode with one precursor scan event to identify the top 16 most abundant ions in each MS scan that were then selected for fragmentation. Dynamic exclusion (repeat count 2, exclusion list size 300, and exclusion duration 60s) was enabled to allow detection of less abundant ions.
In vitro peptide crosslinking
To study whether the crosslinking reaction can occur spontaneously under physiological conditions, the human AQP0 peptide 224–241 (FPRLKSISERLSVLKGAK) and AQP0 peptide 239–263 (GAKPDVSNGQPEVTGEPVELNTQAL) with an acetylated N-terminus were synthesized by Biotechnology Core Facility at the Medical University of South Carolina. These peptides were mixed in PBS solution at a final concentration of 1 mg/mL. The sample was incubated at 37°C and aliquots taken every three days. Each sample was digested by trypsin for 4 hours and analyzed by LC-MS/MS.
Data Analysis
All MS/MS spectra were converted to mzML files by Scansifter, a tool under development at Vanderbilt University Medical Center. The data were searched on a 2,500 node Linux cluster supercomputer using the TagRecon Blind searching algorithm against a custom human lens protein database [5]. Trypsin specificity was used with a maximum two missed cleavage sites. The search was done with a static modification of carbamidomethylation of cysteine and variable modification of oxidation of methionine and deamination of asparagine and glutamine. Blind searches revealed peptides with mass shifts corresponding to unknown modifications. Based on the TagRecon Blind search results, the manual analysis was then performed to assign the mass shifts as posttranslational modifications or crosslinks.
Formation and stability of succinimide intermediates
All synthetic peptides were purchased from GME biochemicals (Shanghai, China) at 95% purity. The Asp residues in these peptides were converted to the corresponding succinimide (Asu) form by incubation at 60°C in 50mM sodium acetate at pH 4.0 for 48h. The reaction mixture was injected onto a C18 RP HPLC column (Sygeni, 5μ C18 300Å, Phenomenex) and Asu peptides purified using a acetonitrile/TFA gradient on a Shimadzu HPLC monitored at 216nm and 256nm. Peaks were collected, dried down and the Asu peak confirmed by ESI mass spectrometry on a Fusion LTQ Orbitrap (ThermoFisher Scientific). Asu peptides were lyophilized and stored at −20°C until further use.
The rate of succinimide formation from Ac-PDVF, Ac-PNVF, Ac-PDGF and Ac-PNGF was examined at pH 3.0 & pH 4.0 (50mM sodium acetate), pH 5.0 (50mM citric acid), pH 6.0 and pH 7.0 (50mM sodium phosphate). All samples were incubated at 60°C and the rate of succinimide formation determined by the increase in Asu peak area as observed by HPLC.
The stabilities of Ac-P(Asu)GF, Ac-P(Asu)VF and Ac-YS(Asu)GF were examined by incubation (1mg/mL) in 50mM phosphate buffer pH 6.7 or 7.4 at 37°C. Aliquots were separated by RP-HPLC on a Sygeni column (Sygeni, 5μm C18 300Å, Phenomenex). Peaks were collected and identified by mass spectrometry. The decrease in Asu peak area observed by HPLC was used to determine succinimide half-life.
Formation and breakdown of amide crosslinks
Ac-P(Asu)GF, Ac-P(Asu)VF and Ac-YS(Asu)GF were incubated at 37°C with a 5-molar excess of phenylethylamine (PE) in either 50mM phosphate pH 6.7 or 90% CH3CN. Aliquots were injected onto a C18 RP HPLC column (Luna, 5μ C18 300Å, Phenomenex) A 60-minute gradient was performed, consisting of the following: 0–10 min, 5% CH3CN; 10–40 min, 5–40% CH3CN, 40–50min 40–80% CH3CN, 50–60min 5% CH3CN. HPLC peaks were collected and crosslinks were confirmed by MS/MS analysis using an Orbitrap Fusion mass spectrometer. Ac-P(Asu)GF was also incubated at 60°C with N-Ac-Lys in 50mM phosphate pH 6.7 and the P(Asu)GF N-Ac-Lys crosslink confirmed by MS/MS analysis. The crosslink was quantified as a percentage of starting peptide from HPLC profiles.
The stability of the Ac-PDGF-PE crosslink was examined by incubation at 37°C in 50mM phosphate buffer pH 7.4 or 50mM ammonium bicarbonate buffer pH 8.0. Aliquots were removed and peaks examined by HPLC and ESI MS/MS.
Results
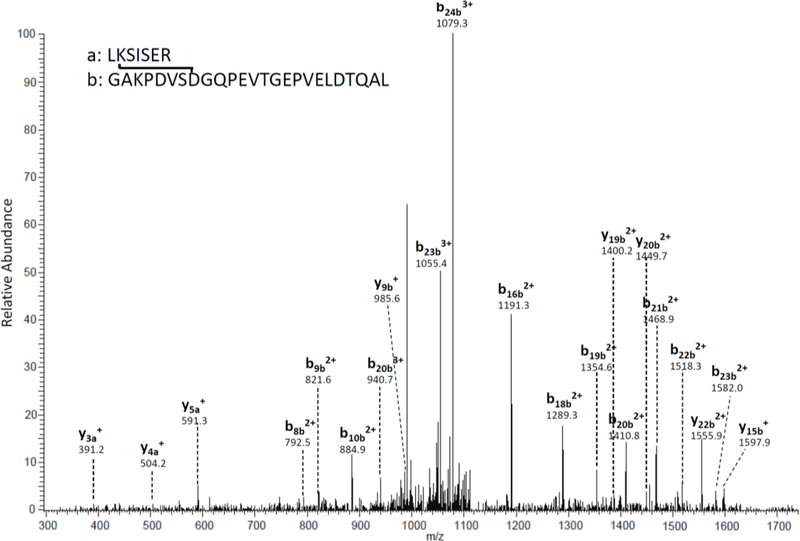
AQP0 is an abundant transmembrane protein that does not turnover in the lens and it undergoes a number of posttranslational modifications such as cleavage [20, 21], racemization[21] and deamidation [21], with age. Using a blind database search approach to identify mass shifts on tryptic peptides, a series of previously unidentified mass shifts were observed for AQP0 peptides and peptides from other major lens proteins. Manual analysis of the data revealed four crosslinked peptides occurring between lysine and asparagine/aspartate residues. These crosslinked peptides are listed in Table 1. The measured parent masses of these peptides are within 3 ppm of the theoretical masses. Crosslinking between AQP0 239–263 and AQP0 227–233 was confirmed by the tandem mass spectrum shown in Figure 1. Sequence specific b- and y-ions clearly show the presence of the b-peptide (AQP0 239–263). In addition, the three ions at m/z 391.2, 504.2, and 591.3 help to assign the a-peptide (AQP0 227–233).
Table 1:
Lys-Asp and Lys-Asn Crosslinked Peptides from the Human Lens
| Peptide | [MH] +measured | [MH] +theoretical | Error (ppm) |
|---|---|---|---|
| AQP0 (227–233):LK*SISER AQP0 (239–263):GAKPDVSD*GQPEVTGEPVELDTQAL |
3365.7052 | 3365.7071 | 0.56 |
| AQP0 (239–263):GAKPDVSDGQPEVTGEPVELD*TQAL αB crystallin?: K* |
2680.3348 | 2680.3312 | 1.34 |
| βB1 crystallin (111–123): GEM(oxidation)FILEK*GEYPR **Spectrin (1207–1210) (Q9H254–1): D*LR |
1968.9818 | 1968.9848 | 1.51 |
| βB2 crystallin: GEQFVFEK*GEYPR **Spectrin (1207–1210) (Q9H254–1): D*LR |
1969.9717 | 1969.9767 | 2.51 |
Asterisks indicate the residues that are involved in crosslinking. All masses listed are monoisotopic masses.
Probably protein in crosslink
Figure 1. MS/MS spectrum of a crosslinked peptide detected in the tryptic digest of human lens proteins.
The crosslink involves Asn 246 and Lys228 from AQP0.
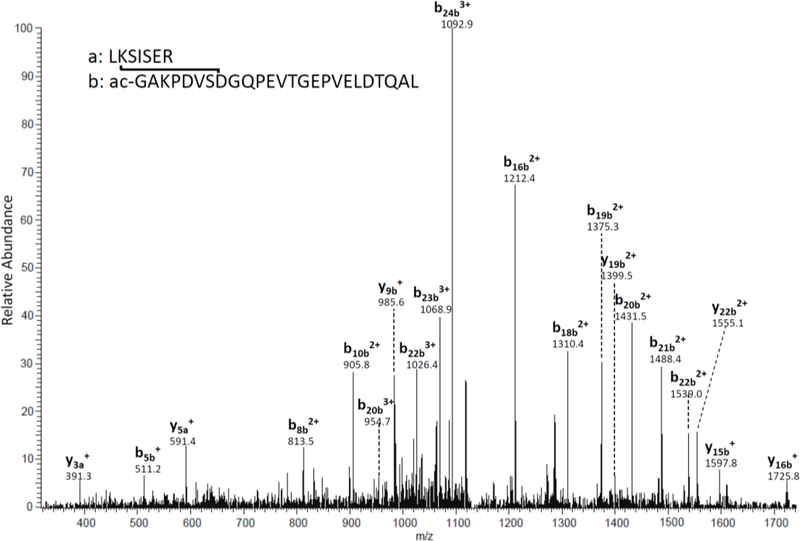
Although most processes characterized to date in LLPs involve spontaneous reactions [4], whether these protein-protein crosslinks occur spontaneously is unknown. To examine this possibility, two AQP0 peptides that contain the crosslinking regions were synthesized and incubated together. The sample was digested by trypsin after incubation to generate the same crosslinked peptide as the peptide identified in human lens. After 25 days of incubation, weak signal of crosslinked peptides can be detected and the tandem mass spectrum is shown in Figure 2. Note that the N-terminus of the synthetic b-peptide is acetylated causing a shift of 42Da for observed b ions compared to the peptide observed from lens tissue (Figure 1). The high similarity between MS/MS spectra of the in vivo and in vitro crosslinked peptides confirmed the identity and crosslink site of human lens AQP0. Moreover, these data demonstrate that this crosslinking chemistry can occur spontaneously and that enzymes are not required.
Figure 2. MS/MS spectrum of the crosslinked peptide formed after incubation of AQP0 peptides FPRLKSISERLSVLKGAK (residues 224–241) and Ac-GAKPDVSNGQPEVTGEPVELNTQAL(residues 239–263).
Peptides were incubated in PBS for 25 days at 37°C.
Another crosslinked peptide identified in this analysis involved AQP0 239–263 and lysine. The lysine could be from the free amino acid in the lens or from other lens proteins and freed by tryptic digestion. Since trypsin does not cleave lysine residues that have been crosslinked, the likely protein source of a crosslinked lysine is a C-terminal lysine residue preceded by a basic residue. Such a sequence exists in αB crystallin (Lys175) and in spectrin beta chain, brain 1 (SPTBN1) (Lys2364). The other two crosslinked peptides identified involve AQP0 239–263 and a peptide that has a sequence of DLR. In the lens, the most abundant protein containing the DLR sequence preceded by a basic residue, is spectrin beta chain, erythrocytic (SPTB); however, whether spectrin is the protein that involved in this crosslinking needs further study. Tandem mass spectra for other crosslinked peptides in Table 1 can be found in Supplementary Figures S1–S3.
To elucidate the mechanism responsible for this spontaneous crosslinking, Asn- and Asp-containing peptides were synthesized and their properties investigated under a variety of conditions. N-acetyl Tyr Ser Asn Gly Phe (Ac-YSNGF) was employed as it contains an Asn sequence with the same two flanking residues, as Asn 246 from AQP0. This peptide was acetylated to preclude reactions involving free amino groups, and a Tyr and Phe residue added at the N- and C-terminus respectively to aid in the HPLC detection of any fragments that formed.
In complementary studies, an Asp peptide N-acetyl Pro Asp Val Phe (Ac-PDVF) was examined since the Pro Asp Val sequence is also found in AQP0 (242–244) and the reactions compared with a peptide with identical sequence, except Asp was replaced by Asn.
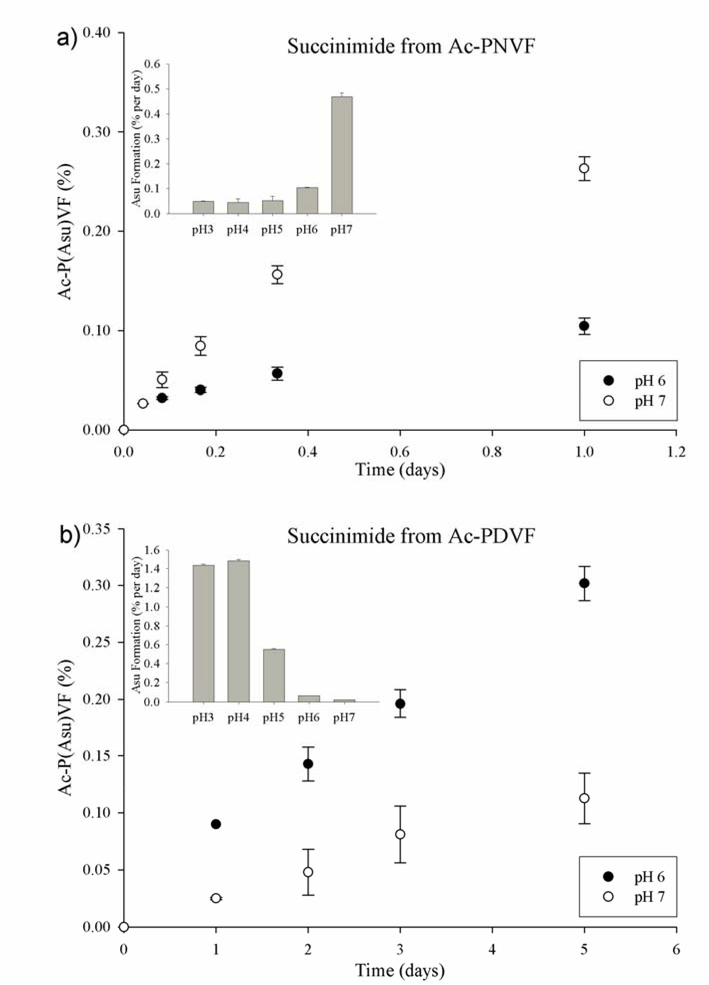
A comparison of Ac-PDVF and Ac-PNVF incubations revealed that both the Asp and Asn peptides cyclized, producing an internal succinimide (Ac-P(Asu)VF), over a range of pHs (Figure 3). For Ac-PDVF, the most rapid formation was observed between pH 3 and pH 4 (Figure 3). In contrast, the Asn-containing peptide Ac-PNVF, formed a succinimide more readily at pH 7. Despite this difference, the Asp and Asn peptides formed succinimides at similar rates at pH 6 and 7 (Figure 3).
Figure 3. Succinimide formation from Asp and Asn as a function of pH.
Time course of succinimide formation from a) Ac-PNVF and b) Ac-PDVF as a function of pH. Ac-PNVF and Ac-PDVF were incubated in 50mM phosphate at pH 6 and pH 7 at 60°C. Succinimide formation was monitored by HPLC and confirmed by mass spectrometry. Each time point, n=3 mean +/− SD. Insert; relative rates of formation of Ac-P(Asu)VF as a function of pH (see Methods for conditions).
To investigate the effect of the C-terminal residue on reactivity, N-acetyl Pro Asp Gly Phe (Ac-PDGF) was included in the experimental protocol and the data compared with that obtained from Ac-PDVF. Ac-PDGF was found to generate a higher yield of the succinimide intermediate than Ac-PDVF. Despite the greater overall rate of succinimide formation when Gly replaced Val as the C-terminal residue, Ac-P(Asu)GF formation from Ac-PDGF and Ac-PNGF showed a similar pH dependence to that of the homologous Val peptides (Supplementary Figure S4). At pH 7 Asn formed a succinimide ~20 times more rapidly than comparable Asp residues. This pH profile is consistent with literature data for the formation of succinimides from Asn and Asp residues [22]. Of relevance to the lens, succinimide formation could be detected at pH 6, as well as pH 7 for both the Asn and Asp peptides. The exterior of the lens has a pH of 7 whereas the lens interior is pH 6.7–6.9 [23–25].
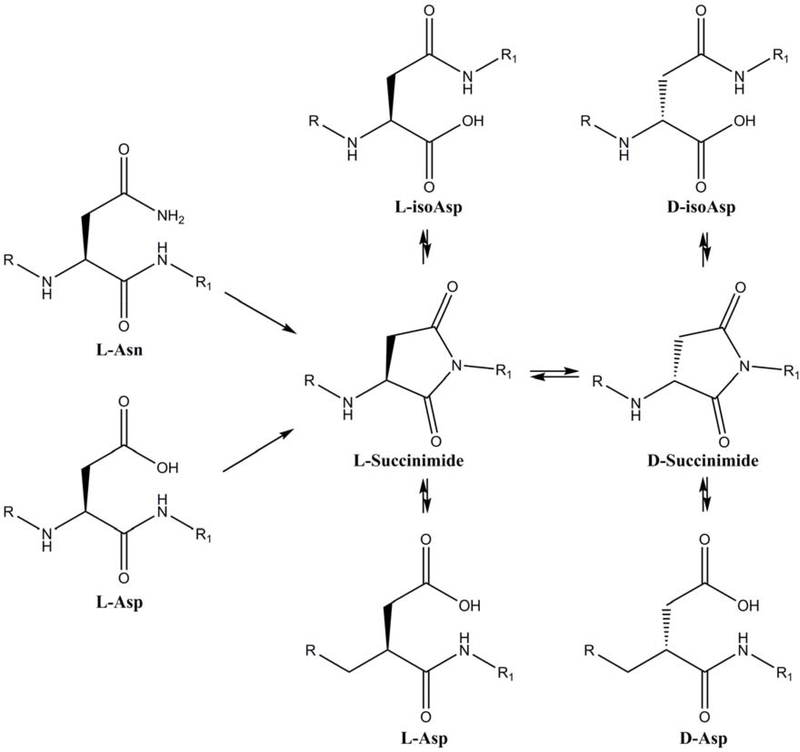
Succinimide formation and subsequent hydrolysis are known to be involved in the deamidation of Asn residues [26], as well as the isomerization of L-Asp residues, in proteins and peptides [26]. These processes are summarized schematically in Figure 4. Model peptide studies in the literature [27, 28] clearly show that flanking residues, particularly on the C-terminal side, can markedly affect the rate of succinimide-mediated isomerization/deamidation. In agreement with this, peptides Ac-PDGF and Ac-PDVF both generated succinimides, albeit at quite different rates (Figure 3 and Supplementary Figure S4).
Figure 4. Asn and Asp residues can undergo spontaneous cyclization forming a succinimide intermediate.
Racemization of the succinimide can occur with the succinimides then hydrolyzing to yield four different Asp isomers: L-Asp, D- Asp, L-isoAsp, and D-isoAsp.
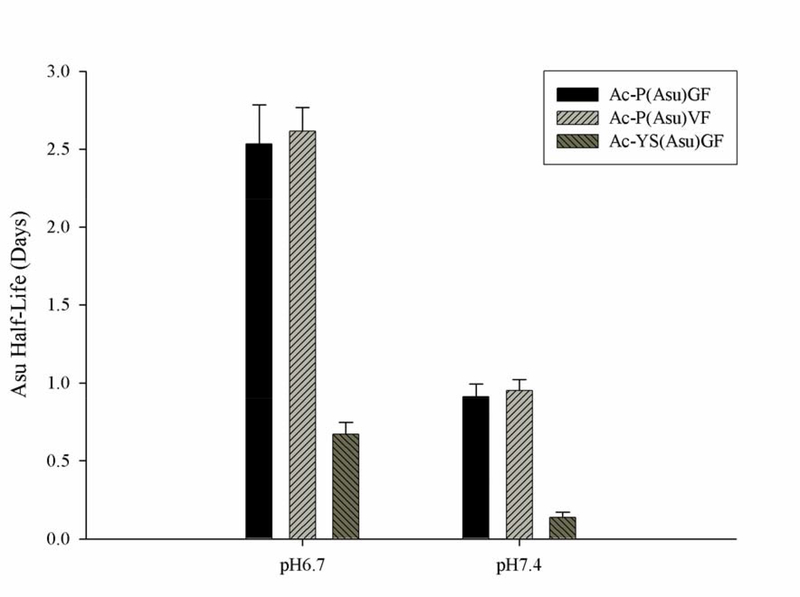
Subsequent studies on the properties of purified peptide succinimides focused on the two pHs that characterize the lens interior and exterior. One surprising result was that the succinimides were quite stable at neutral pH (Figure 5). In the absence of nucleophiles, the half-life of both Ac-P(Asu)GF and Ac-P(Asu)VF at pH 6.7 was approximately 2.5 days at 37°C. Stability was markedly pH–dependent, with a rise in pH of just 0.7 units reducing the half-life of the succinimides to approximately one day (Figure 5). A similar result was obtained with Ac-YS(Asu)GF which had a half-life of ~12hrs at pH 6.7, whereas at pH 7.4 it was less than 3 hrs. These limited data suggest that succinimide stability is affected more by the nature of the N-terminal residue than the C-terminal amino acid, although more sequences would need to be examined to verify this.
Figure 5. Stability of Ac-P(Asu)GF, Ac-P(Asu)VF and Ac-YS(Asu)GF under biological conditions.
Succinimide stability was determined by incubating the purified succinimides at 37°C in buffer at either pH 6.7 or 7.4. Half-life was determined by loss of Asu peak as observed by HPLC. Half-life is the time point when 50% of the succinimide had decomposed (n=2, mean +/− SD).
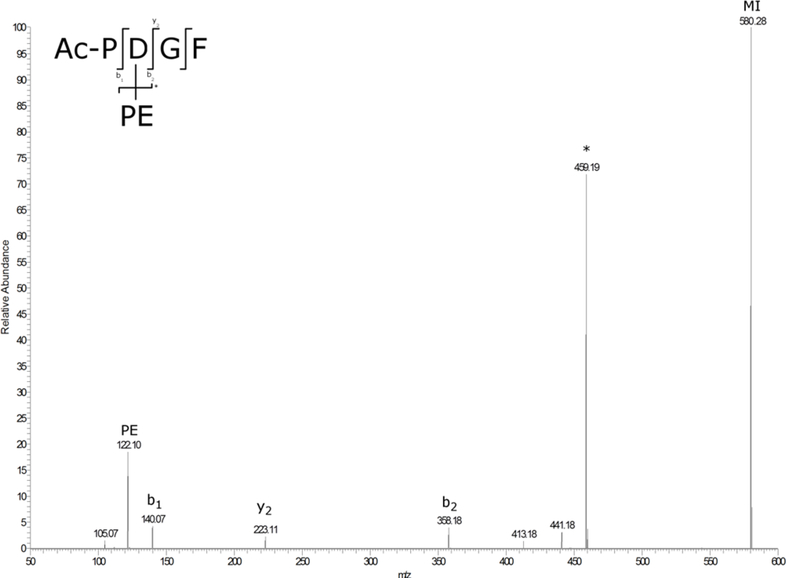
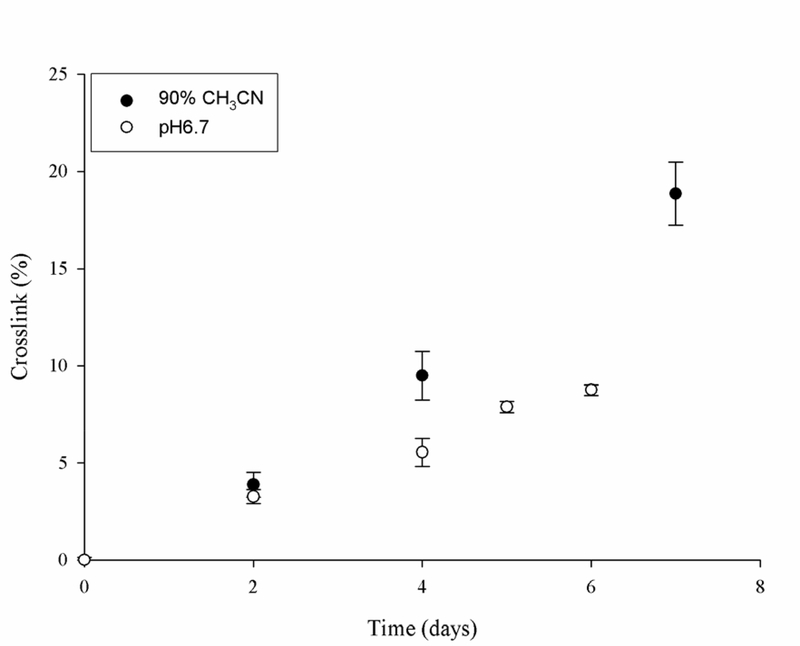
In contrast to the stability of Asu peptides in the absence of nucleophiles, when the Lys analogue, phenylethylamine (PE), was added, a new crosslinked product appeared. The time course of formation of the PE-adduct together with its MS/MS spectrum is shown in Figures 6 and 7. Crosslinking was observed at pH 6.7, although it was found that the reaction occurred more rapidly in mixed organic solvents. 90% CH3CN was chosen as a “membrane-mimicking’ solvent in which the peptide and products remained soluble. The nucleophilic addition of amines to succinimides is a well-recognized reaction [29] and the crosslink was also formed with N-Ac-Lys (Supplementary Figure S5). When Ac-YS(Asu)GF was incubated under the same membrane-mimicking conditions the PE crosslink was observed (Data not shown). The process involved in the covalent crosslinking of Lys residues to Asp residues in proteins is summarized in Figure 8.
Figure 6. MS/MS spectrum of Ac-PDGF crosslinked to PE.
Ac-P(Asu)GF was incubated with PE at pH 6.7 for 72h. * indicates loss of PE from the molecular ion. MI = molecular ion.
Figure 7. Ac-PDGF-PE crosslink formation as a function of time.
Ac-P(Asu)GF was incubated in either 90% CH3CN (closed circles) or 50mM phosphate pH 6.7 (open circles) at 37°C with a 5-molar excess of PE. Crosslink formation is expressed as a % of the original peptide and was monitored by HPLC and the product confirmed by MS/MS. Each time point, n=3 mean +/− SD.
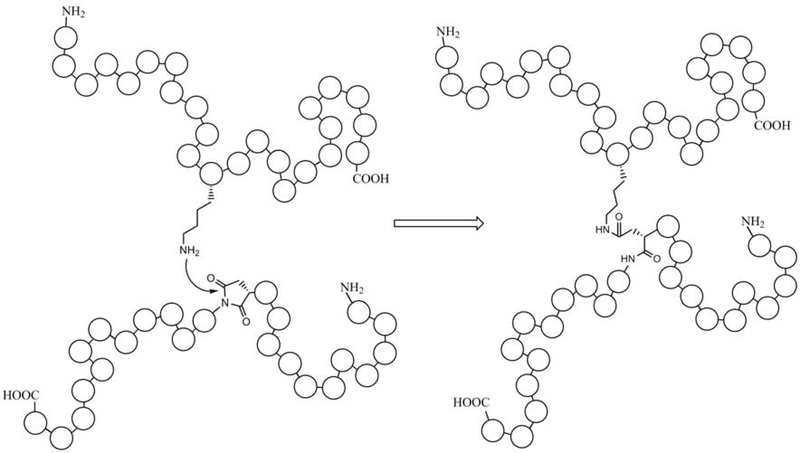
Figure 8. Spontaneous protein-protein crosslinks can form via succinimides.
A schematic illustrating the formation of a protein covalent crosslink between a Lys and a succinimide intermediate derived from Asn or Asp.
Discussion
A novel mechanism of protein crosslinking involving Asp and Asn residues has been detected. The key intermediate appears to be a succinimide which is susceptible to nucleophilic attack by the side chain of Lys. A scheme highlighting this mechanism is shown in Figure 8. In addition to Asn 246 in AQP0 being crosslinked to Lys 228 of AQP0 in the human lens, Lys residues from other proteins can also attack succinimides that presumably form spontaneously from other Asp or Asn sites. Some are depicted in Table 1. For example, Lys 118 of βB1 crystallin and Lys 76 of βB2 crystallin were each found to be crosslinked to the tryptic peptide DLR which is probably derived from the cytoskeletal protein, spectrin (Table 1).
The nucleophilic addition of amines to succinimides is a well-characterized reaction [29] so it is perhaps not surprising that it can also occur in the body. This is particularly so since succinimide formation is one of the most widespread reactions involved in the breakdown of long-lived proteins. Succinimide formation is the mechanism responsible for deamidation of Asn residues and it also underpins isomerization of Asp residues [26]. The adult human lens contains several proteins where Asp residues have been modified. α-Crystallin is one of the best characterized of these lens proteins and it contains multiple sites of Asp isomerization. For example, Asp-58, −76, −84, and −151 of αA-crystallin and Asp-62 and −96 of αB-crystallin exist as D-Asp as well as L-isoAsp and D-isoAsp isomers in older human lenses [30–32]. These modified Asp residues result from hydrolysis of a succinimide intermediate (Figure 3). Several Asn residues in crystallins from aged lenses are also deamidated [33] and isomerized [34] with the degree of deamidation of Asn 76 in γS crystallin being significantly greater in cataract lenses. These data suggest that there may be many potential sites of crosslinking however the local environment may well determine the final outcome of succinimide formation. In old proteins, it is likely that hydrolysis of any succinimide that forms from either Asn or Asp can be viewed as a competing reaction with crosslinking. If the succinimide reacts with water to form e.g. isoAsp residues, then it will be unavailable for nucleophilic addition by a nearby Lys. This factor may play a part in the observation that some crosslinks detected in our study involve AQP0, a transmembrane protein, parts of which reside in a hydrophobic environment where water is less available.
While the crosslink involving Asp and Asn residues is novel in a biological context, the mechanism is not without precedent. Similar crosslinks have been reported in recombinant proteins that had been stored for extended periods, typically at low pH [35]. As shown in Figure 3 under low pH conditions, succinimide formation from Asp occurs more rapidly, although, under these conditions, the nucleophilicity of amino groups will be less. At pH 3–4, there is very little unprotonated Lys ε-amino group available to act as a nucleophile. In some cellular environments (e.g. membranes), the pKa of the Lys side chain can be altered considerably [36]. In accord with this environment, most of the reactions observed with the recombinant proteins involved crosslinking of Asp residues with α-amino groups, since their pKa is lower than those of Lys ε-amino groups. Interestingly the pH dependence of succinimide formation was quite different for peptide-bound Asp and Asn. Starting with an Asp residue, the pH optimum was pH 3–4, whereas succinimides were found to form most readily from Asn at pH 6–7 (Figure 3). Some succinimide formation was detectable from both Asn and Asp across the range pH 3 to 7. Together, these findings suggest that in cells, both Asn and Asp are potential sites of succinimide formation however these will form at different rates. At neutral pH succinimide formation from Asn will be favored (Figure 3 and Supplementary Figure S4) however protein conformation [37] and neighboring residues [27] will also have a marked effect on the process. Crosslinking of such protein succinimides with the ε-amino group Lys may take place, as documented in this investigation, and linkage with free α-amino groups of amino acids and peptides (e.g. glutathione) could also occur.
The type of crosslink elucidated here involves an isopeptide bond, which is an amide bond incorporating the ε-amino group of Lys. Self-generated isopeptide bonds between Lys and Asn residues are a hallmark of surface proteins of gram-positive bacteria [38, 39]. All such isopeptides involve Asn, rather than the Asp described here and appear to require for their formation, a triad of Lys, Asn, and a catalytic carboxyl group e.g. from Glu, within a hydrophobic core. While the succinimide intermediate is a likely mode of formation of the crosslinks elucidated here, we cannot rule out the possibility of other mechanisms. For example, it is theoretically possible to spontaneously form a Lys-Asp isopeptide bond [40].
Interestingly the PE crosslink in our study was unstable under some experimental conditions. For example, at pHs above 7, it formed a compound that, on the basis of MS/MS data, appears to be a gem diol (Supplementary Figures S6 and S7). This diol increased with time of incubation at neutral pHs (Supplementary Figure S8) but could be reverted back to the original PE adduct by heat treatment (data not shown). In addition, the PE-crosslink was observed to decompose under acidic conditions e.g. in TFA buffers. These observations suggest that the crosslinked peptides detected from lens digests in our study, while abundant may represent just a fraction of the crosslinked species present in human lens proteins. For accurate quantification in future studies, conditions of lens extraction, enzyme digestion and HPLC will need to be optimized to take into account the properties of the crosslinked peptides.
Protein succinimides may not be stable under conditions typically employed for tryptic digestion e.g. pH 8 [41]. On the other hand, peptide succinimides were found to be relatively stable under physiological conditions (Figure 5), having half-lives measured in days. The data in this paper suggest that Asp sites on proteins that have been known previously to have been isomerized, should also be examined for the presence of covalent crosslinks. The same applies to sites of Asn deamidation in long-lived proteins since deamidation also proceeds via succinimide intermediates. In this context, another piece of evidence supports the proposed succinimide mechanism. Asn 246 in AQP0 has been reported previously to exist in other isomeric forms [21] that can arise only from a succinimide intermediate.
Covalent crosslinking of long-lived proteins has been known for many years [42–44] but it is only recently that proteomic techniques to identify and characterize such links have become available. Application of such methodology, linked with peptide-based mechanistic investigations, may in the future, uncover the nature of a biological phenomenon that has largely remained a mystery.
Supplementary Material
Acknowledgements
The authors acknowledge use of the UOW Mass Spectrometry User Resource and Research Facility (MSURRF) within the School of Chemistry, University of Wollongong.
Funding information:
Funding for the present study was provided by National Institutes of Health by grants R01 EY024258 and P30 EY008126.
Abbreviations
- LLPs
Long-lived proteins
References
- 1.Clarke S (1987) Propensity for spontaneous succinimide formation from aspartyl and asparaginyl residues in cellular proteins. Int J Pept Protein Res. 30, 808–821 [DOI] [PubMed] [Google Scholar]
- 2.Lyons B, Jamie J and Truscott R (2011) Spontaneous Cleavage of Proteins at Serine Residues. International Journal of Peptide Research and Therapeutics. 17, 131–135 [Google Scholar]
- 3.Hooi MYS and Truscott RJW (2011) Racemisation and human cataract. d-Ser, d-Asp/Asn and d-Thr are higher in the lifelong proteins of cataract lenses than in age-matched normal lenses. Age. 33, 131–141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Truscott RJW, Schey KL and Friedrich MG (2016) Old Proteins in Man: A Field in its Infancy. Trends in Biochemical Sciences. 41, 654–664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang Z, Lyons B, Truscott RJW and Schey KL (2014) Human protein aging: modification and crosslinking through dehydroalanine and dehydrobutyrine intermediates. Aging Cell. 13, 226–234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Grandhee SK and Monnier VM (1991) Mechanism of formation of the Maillard protein cross-link pentosidine. Glucose, fructose, and ascorbate as pentosidine precursors. Journal of Biological Chemistry. 266, 11649–11653 [PubMed] [Google Scholar]
- 7.Haus JM, Carrithers JA, Trappe SW and Trappe TA (2007) Collagen, cross-linking, and advanced glycation end products in aging human skeletal muscle. Journal of Applied Physiology. 103, 2068–2076 [DOI] [PubMed] [Google Scholar]
- 8.Cloos PAC and Jensen AL (2000) Age-related de-phosporylation of proteins in dentin: A biological tool for assessment of protein age. Biogerontology. 1, 341–356 [DOI] [PubMed] [Google Scholar]
- 9.Sell DR, Biemel KM, Reihl O, Lederer MO, Strauch CM and Monnier VM (2005) Glucosepane Is a Major Protein Cross-link of the Senescent Human Extracellular Matrix: Relationship with Diabetes. Journal of Biological Chemistry. 280, 12310–12315 [DOI] [PubMed] [Google Scholar]
- 10.Semba RD, Najjar SS, Sun K, Lakatta EG and Ferrucci L (2009) Serum Carboxymethyl–Lysine, an Advanced Glycation End Product, Is Associated With Increased Aortic Pulse Wave Velocity in Adults. American Journal of Hypertension. 22, 74–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grune T, Jung T, Merker K and Davies KJA (2004) Decreased proteolysis caused by protein aggregates, inclusion bodies, plaques, lipofuscin, ceroid, and ‘aggresomes’ during oxidative stress, aging, and disease. The International Journal of Biochemistry & Cell Biology. 36, 2519–2530 [DOI] [PubMed] [Google Scholar]
- 12.Gautieri A, Redaelli A, Buehler MJ and Vesentini S (2014) Age- and diabetes-related nonenzymatic crosslinks in collagen fibrils: Candidate amino acids involved in Advanced Glycation End-products. Matrix Biology. 34, 89–95 [DOI] [PubMed] [Google Scholar]
- 13.Verzijl N, Bank RA, TeKoppele JM and DeGroot J (2003) AGEing and osteoarthritis: a different perspective. Current Opinion in Rheumatology. 15, 616–622 [DOI] [PubMed] [Google Scholar]
- 14.Wang Z and Schey KL (2018) Quantification of thioether-linked glutathione modifications in human lens proteins. Experimental Eye Research. 175, 83–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Friedrich MG, Wang Z, Oakley AJ, Schey KL and Truscott RJW (2017) Hotspots of age-related protein degradation: the importance of neighboring residues for the formation of non-disulfide crosslinks derived from cysteine. Biochemical Journal. 474, 2475–2487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Friedrich MG, Wang Z, Schey KL and Truscott RJW (2018) DehydroalanylGly, a new post translational modification resulting from the breakdown of glutathione. Biochimica et Biophysica Acta (BBA) - General Subjects. 1862, 907–913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lynnerup N, Kjeldsen H, Heegaard S, Jacobsen C and Heinemeier J (2008) Radiocarbon Dating of the Human Eye Lens Crystallines Reveal Proteins without Carbon Turnover throughout Life. PLoS ONE. 3, e1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kumary SS, Gupta N, Shiels A, FitzGerald PG, Menon AG, Mathias RT et al. (2015) Role of Aquaporin 0 in lens biomechanics. Biochem. Biophys. Res. Commun 462, 339–345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang Z and Schey KL (2011) Aquaporin-0 Interacts with the FERM Domain of Ezrin/Radixin/Moesin Proteins in the Ocular Lens. Investigative Ophthalmology & Visual Science. 52, 5079–5087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Korlimbinis A, Berry Y, Thibault D, Schey KL and Truscott RJW (2009) Protein aging: Truncation of aquaporin 0 in human lens regions is a continuous age-dependent process. Experimental eye research. 88, 966–973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ball LE, Garland DL, Crouch RK and Schey KL (2004) Post-translational Modifications of Aquaporin 0 (AQP0) in the Normal Human Lens: Spatial and Temporal Occurrence. Biochemistry. 43, 9856–9865 [DOI] [PubMed] [Google Scholar]
- 22.Ouellette D, Chumsae C, Clabbers A, Radziejewski C and Correia I (2013) Comparison of the in vitro and in vivo stability of a succinimide intermediate observed on a therapeutic IgG1 molecule. mAbs. 5, 432–444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bassnett S, Reinisch L and Beebe DC (1990) Intracellular pH measurement using single excitation-dual emission fluorescence ratios. American Journal of Physiology-Cell Physiology. 258, C171–C178 [DOI] [PubMed] [Google Scholar]
- 24.Bassnett S and Duncan G (1985) Direct measurement of pH in the rat lens by ion-sensitive microelectrodes. Experimental Eye Research. 40, 585–590 [DOI] [PubMed] [Google Scholar]
- 25.Greiner JV, Kopp SJ, Sanders DR and Glonek T (1981) Organophosphates of the crystalline lens: a nuclear magnetic resonance spectroscopic study. Investigative Ophthalmology & Visual Science. 21, 700–713 [PubMed] [Google Scholar]
- 26.Geiger T and Clarke S (1987) Deamidation, isomerization, and racemization at asparaginyl and aspartyl residues in peptides. Succinimide-linked reactions that contribute to protein degradation. Journal of Biological Chemistry. 262, 785–794 [PubMed] [Google Scholar]
- 27.Stephenson RC and Clarke S (1989) Succinimide formation from aspartyl and asparaginyl peptides as a model for the spontaneous degradation of proteins. Journal of Biological Chemistry. 264, 6164–6170 [PubMed] [Google Scholar]
- 28.Aquilina JA, Carver JA and Truscott RJW (1999) Elucidation of a Novel Polypeptide Cross-Link Involving 3-Hydroxykynurenine. Biochemistry. 38, 11455–11464 [DOI] [PubMed] [Google Scholar]
- 29.Hargreaves MK, Pritchard JG and Dave HR (1970) Cyclic carboxylic monoimides. Chemical Reviews. 70, 439–469 [Google Scholar]
- 30.Hooi MYS, Raftery MJ and Truscott RJW (2013) Accelerated aging of Asp 58 in αA crystallin and human cataract formation. Experimental Eye Research. 106, 34–39 [DOI] [PubMed] [Google Scholar]
- 31.Fujii N, Sakaue H, Sasaki H and Fujii N (2012) A Rapid, Comprehensive Liquid Chromatography-Mass Spectrometry (LC-MS)-based Survey of the Asp Isomers in Crystallins from Human Cataract Lenses. Journal of Biological Chemistry. 287, 39992–40002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lyons YA, Sabbah GM, and Julian RR (2018) Differences in α-Crystallin isomerization reveal the activity of protein isoaspartyl methyltransferase (PIMT) n thenucleus and cortex of human lenses. Exp. Eye Res 171, 131–141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hains PG and Truscott RJW (2010) Age-Dependent Deamidation of Lifelong Proteins in the Human Lens. Investigative Ophthalmology & Visual Science. 51, 3107–3114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hooi MYS, Raftery MJ and Truscott RJW (2012) Racemization of Two Proteins over Our Lifespan: Deamidation of Asparagine 76 in γS Crystallin Is Greater in Cataract than in Normal Lenses across the Age Range. Investigative Ophthalmology & Visual Science. 53, 3554–3561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.DeHart MP and Anderson BD (2012) Kinetics and Mechanisms of Deamidation and Covalent Amide-Linked Adduct Formation in Amorphous Lyophiles of a Model Asparagine-Containing Peptide. Pharmaceutical Research. 29, 2722–2737 [DOI] [PubMed] [Google Scholar]
- 36.Panahi A and Brooks CL (2015) Membrane environment modulates the pK(a) values of transmembrane helices. J. Phys. Chem. B 119, 4601–4607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wearne SJ and Creighton TE (1989) Effect of protein conformation on rate of deamidation: Ribonuclease A. Proteins5, 8–12 [DOI] [PubMed] [Google Scholar]
- 38.Kang HJ, Coulibaly F, Clow F, Proft T and Baker EN (2007) Stabilizing Isopeptide Bonds Revealed in Gram-Positive Bacterial Pilus Structure. Science. 318, 1625–1628 [DOI] [PubMed] [Google Scholar]
- 39.Budzik JM, Marraffini LA, Souda P, Whitelegge JP, Faull KF and Schneewind O (2008) Amide bonds assemble pili on the surface of bacilli. Proceedings of the National Academy of Sciences. 105, 10215–10220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hagan RM, Björnsson R, McMahon SA, Schomburg B, Braithwaite V, Bühl M, Naismith JH and Schwarz-Linek U (2010) NMR Spectroscopic and Theoretical Analysis of a Spontaneously Formed Lys–Asp Isopeptide Bond. Angewandte Chemie International Edition. 49, 8421–8425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nowak C, Ponniah G, Neill A and Liu H (2017) Characterization of succinimide stability during trypsin digestion for LC-MS analysis. Analytical Biochemistry. 526, 1–8 [DOI] [PubMed] [Google Scholar]
- 42.Friedrich MG, Lam J and Truscott RJW (2012) Degradation of an Old Human Protein: AGE-DEPENDENT CLEAVAGE OF γS-CRYSTALLIN GENERATES A PEPTIDE THAT BINDS TO CELL MEMBRANES. Journal of Biological Chemistry. 287, 39012–39020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vázquez de la Torre A, Gay M, Vilaprinyó-Pascual S, Mazzucato R, Serra-Batiste M, Vilaseca M and Carulla N (2018) Direct Evidence of the Presence of Cross-Linked Aβ Dimers in the Brains of Alzheimer’s Disease Patients. Analytical Chemistry. 90, 4552–4560 [DOI] [PubMed] [Google Scholar]
- 44.Atsushi W, Won-Kyoung H, Naoshi D, Koji T, Maho MK and Yasuo I (2004) Molecular aging of tau: disulfide-independent aggregation and non-enzymatic degradation in vitro and in vivo. Journal of Neurochemistry. 90, 1302–1311 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.