Abstract
Congenital cardiac malformations have been reported in 8% of patients with craniosynostosis undergoing cranial vault remodeling (CVR), but associations with surgical outcomes are unknown. This study evaluated postoperative complications in patients who underwent CVR for craniosynostosis with or without cardiac risk factors (CRF) using the National Safety Quality Improvement Program-Pediatric (NSQIP-P) database. NSQIP-P database was queried for patients < 2 years with craniosynostosis who underwent CVR from 2012–2016 based on diagnosis and procedure codes. The primary outcome was a composite of available NSQIP-P complications. Analysis compared patients with craniosynostosis based on the presence or absence of CRF. Univariate and multiple logistic regression identified risk factors associated with postoperative complications. A total of 3,293 patients met inclusion criteria (8% with CRF). Two-thirds of patients experienced at least 1 complication, though patients with CRF experienced a greater proportion (74% vs. 66%, p=0.001). Univariate analysis identified associations between post-operative complications and age, ASA class, supplemental oxygen, neuromuscular disorders, preoperative nutritional supplementation, interventricular hemorrhage, and CRF. On multivariate regression, only older age (OR 1.17, 95%CI 1.01–1.36) and longer operative duration (OR 1.01, 95% CI 1.01–1.01) were associated with greater odds of postoperative complications. The most common complication in patients with craniosynostosis who undergo CVR is bleeding requiring transfusion. Older age and longer operative duration were associated with postoperative complications. Although patients with CRF have more postoperative complications, CRF was not a risk factor on adjusted analysis.
Keywords: Craniosynostosis, Cranial Vault Remodeling, Cardiac Risk Factors
Introduction
Craniosynostosis is the pathological early fusion of cranial sutures, resulting in abnormalities in head shape. Patients with craniosynostosis can develop elevated intracranial pressure, which has been linked to learning impairment, headaches, and even visual loss or blindness.1 Cranial vault remodeling (CVR) is the standard of care for craniosynostosis, with goals of surgically expanding the constricted cranium and reconstructing the abnormal head shapes.2 CVR remains a complex surgical procedure with a risk of significant complications, including injury to the brain, airway issues, critical bleeding events, and even death.3, 4 While improvements in pediatric anesthesia and surgical technique have significantly reduced the incidence of severe complications, they have not been completely eliminated.5
Prior studies have reported that 7–72% of patients with craniosynostosis have concurrent congenital anomalies in neurological, cardiac, renal, and other systems.6–8 In particular, cardiac malformations have been reported in 4–8% of patients with craniosynostosis.6, 7 Previous research has shown elevated rates of complications in patients with cardiac risk factors (CRF) undergoing multiple types of surgeries, including gastrostomy tube placement, cleft lip, and cleft palate repair.9, 10 However, the relationship between CRF and outcomes after CVR has not been previously examined.
The National Surgical Quality Improvement Program for Pediatrics (NSQIP-P) was developed as a large clinical registry to assess outcomes following surgical procedures.11, 12 Given the large number of patients captured by NSQIP-P and the standardized outcomes reporting, researchers have been able to assess complication rates for many common surgical procedures. We hypothesized that children with craniosynostosis and CRF would have a greater rate of postoperative complications following CVR than patients without CRF. Additionally, we aimed to identify factors associated with postoperative complications, and develop and validate a model to predict postoperative complications in this population.
Materials and Methods
Patient Selection
Using the NSQIP-P participant use files for 2012–2016, a cohort of patients was developed using specific diagnosis and procedural codes. International Classification of Diseases (ICD) 9 and 10 were used to capture patients with a diagnosis of craniosynostosis (ICD-9: 756.0 or ICD-10: Q75.0). To determine which patients underwent CVR, we used Current Procedural Terminology (CPT) codes specific for craniotomy for craniosynostosis. Only patients with both the appropriate CPT and ICD codes were included. In an effort to identify patients who underwent primary repair, only patients under the age of 2 years at the time of the CVR were included.
The cohort was compared by demographics, weight, American Society of Anesthesiologist (ASA) class, operative duration, and co-morbidities thought to impact postoperative complications, such as central nervous system (CNS) abnormalities, gastrointestinal (GI) disorders, pulmonary abnormalities, and CRF. Even though NSQIP-P categorizes CRF as none, minor, major, and severe (Table 1), we elected to pool the CRF groups to generate one binary variable (CRF present or absent) given the small number of patients in each category.
Table 1:
Description of cardiac risk factors from NSQIP-P User Guide 2016.
| Cardiac Risk Factors | Description |
|---|---|
| None | No pre-existing cardiac conditions or compromise of cardiac function requiring medication. |
| Minor | 1) Cardiac condition with or without medication and maintenance (e.g. Atrial Septal Defect, Small to moderate Ventricular Septal Defect with no symptoms or symptoms of well controlled congestive heart failure, Patent Ductus Arteriosis). 2) After repair of congenital heart defect with normal cardiovascular function and no meds (e.g. Atrial Septal Defect/Patent Foramen Ovale, Ventricular Septar Defect, Patent Ductus Arteriosis, Coarctation of the aorta). |
| Major | 1) After repair of congenital heart defect with residual hemodynamic abnormality with or without medications (e.g. Tetrology of Fallot with wide open pulmonary insufficiency, Aortic valve disease with aortic stenosis or aortic insufficiency based on presence of echocardiographic gradient, all single ventricle patients [severe Atrioventricular Canal, Hypoplastic left heart syndrome (including stage 1 repair)]). |
| Severe | 1) Uncorrected cyanotic heart disease. 2) Patients with any documented pulmonary hypertension. 3) Patients with ventricular dysfunction requiring medications, may or may not be on heart transplant list (e.g. hypertrophic cardiomyopathy). |
Data Source
The NSQIP-P was developed by the American College of Surgeons (ACS) to evaluate postoperative outcomes in pediatric surgery and to improve surgical care for children.11–13 Procedures performed by multiple pediatric subspecialties, including otolaryngology, orthopedic surgery, plastic surgery, neurosurgery, urology, gynecology, general, and thoracic surgery, are captured by NSQIP-P. Cases are systematically sampled to ensure variability of procedures and prevent oversampling of common operations. Over 200 standardized perioperative variables with stringent definitions are abstracted for each case by trained surgical clinical reviewers. Postoperative outcomes within 30 days of surgery are captured. All data collected is de-identified and made available for use by the participating hospitals.
Outcomes
Our primary outcome was a composite of any postoperative complication within 30 days of surgery. The following NSQIP-P outcome variables were utilized in the composite outcome: readmissions (planned and unplanned), reoperations, reintubations, surgical site infection (including superficial space infection, deep space infection, organ space infection or abscess), wound dehiscence, bleeding event requiring postoperative transfusions, postoperative pneumonia, postoperative urinary tract infection, postoperative sepsis, graft failure, pulmonary embolism, venous thrombosis requiring therapy, postoperative renal insufficiency or renal failure, postoperative central line-associated bloodstream infection, postoperative coma longer than 24 hours, postoperative seizure, postoperative peripheral nerve injury, postoperative cerebral intra-ventricular hemorrhage, postoperative stroke or intracranial hemorrhage, and postoperative cardiac arrest requiring CPR.
Development and validation cohorts
Prior to any analyses, the cohort was partitioned to create a model development data set to build the model, and a validation data set to test the model. Using a non-replacement technique and a random number generator, two-thirds of the records were randomized to the model development cohort, meaning all model building and testing was initially performed in these patients alone. Once finalized, the predictive ability of the model was then tested in the remaining 34% of records (the validation group). Baseline characteristics for the two groups were compared to assess whether randomization properly balanced the two cohorts (Table 2).
Table 2:
Patient demographics in development and validation sets.
| Variable, N (%) | All patients | Development Set | Validation Set | P-value |
|---|---|---|---|---|
| Number of patients | 3293 (100%) | 2182 (66%) | 1111 (34%) | -- |
| Male sex | 2173 (66%) | 1442 (66%) | 732 (66%) | 0.91 |
| Race | 0.57 | |||
| White | 2600 (79%) | 1716 (79%) | 884 (80%) | |
| Asian | 47 (1%) | 31 (1%) | 16 (1%) | |
| Black | 229 (7%) | 145 (7%) | 84 (8%) | |
| American Indian/Alaska Native | 19 (1%) | 15 (1%) | 4 (<1%) | |
| Native Hawaiian/Pacific Islander | 11 (<1%) | 8 (<1%) | 3 (<1%) | |
| Unknown/Not reported | 387 (12%) | 267 (12%) | 120 (11%) | |
| Hispanic Ethnicity | 511 (16%) | 339 (16%) | 172 (15%) | 0.99 |
| Age in months, mean ± SD | 8 ± 4.6 | 7.8 ± 4.6 | 8.1 ± 4.8 | 0.05 |
| Weight in lbs, mean ± SD | 18 ± 7.3 | 17.8 ± 6.6 | 18.2 ± 8.4 | 0.05 |
| ASA Class | 0.62 | |||
| I | 637 (19%) | 429 (20%) | 208 (19%) | |
| II | 1970 (60%) | 1306 (60%) | 664 (60%) | |
| ≥ III | 684 (21%) | 445 (20%) | 239 (22%) | |
| Structural CNS abnormality | 822 (25%) | 553 (25%) | 269 (24%) | 0.478 |
| Gastrointestinal disorders | 274 (8%) | 180 (8%) | 94 (8%) | 0.835 |
| Impaired cognitive status | 261 (8%) | 169 (8%) | 92 (8%) | 0.591 |
| Cardiac risk factors | 254 (8%) | 168 (8%) | 86 (8%) | 0.966 |
| Structural pulmonary abnormality | 102 (3%) | 60 (3%) | 42 (4%) | 0.107 |
| Previous cardiac surgery | 56 (2%) | 29 (1%) | 27 (2%) | 0.02 |
| Asthma | 55 (2%) | 30 (1%) | 25 (2%) | 0.06 |
| Neuromuscular disorder | 54 (2%) | 39 (2%) | 15 (1%) | 0.35 |
| Tracheostomy prior to surgery | 29 (1%) | 17 (1%) | 12 (1%) | 0.382 |
| Required oxygen support | 26 (1%) | 16 (1%) | 10 (1%) | 0.609 |
| Ventilator support prior to surgery | 13 (<1%) | 7 (<1%) | 6 (1%) | 0.343 |
| Operative time, min, Mean ± SD | 178 ± 96 | 176 ± 93 | 183 ± 101 | 0.032 |
| Any adverse event | 2,214 (67%) | 1,472 (67%) | 742 (67%) | 0.697 |
Analysis
Descriptive statistics were utilized to characterize our patient population. Inferential statistics, such as student’s t-test, chi-square, and Wilcoxon rank sum test, were used to compare demographics, comorbidities, operative details and outcomes between patient groups. A p-value of <0.05 was considered significant for any inferential testing regarding sample characteristics. Univariate logistic regression was used to determine whether an association between each variable and any postoperative outcome existed. A p-value of less than 0.20 was utilized for variable selection in the multiple logistic regression. Univariate and multivariate regression analyses were completed using the development data set alone. We evaluated for interactions between age at surgery and operative duration because endoscopic CVR is typically performed in infants < 5 months, takes less time to complete, and is associated with less blood loss. Performance of the multiple logistic regression model was evaluated in both data sets using receiver operating characteristic (ROC) curves, area under the curve (AUC), goodness of fit and Akaike information criteria. An AUC of 0.5 is equivalent to the flip of a coin, and an AUC greater than 0.7 is considered predictive. All analyses were conducted with Stata 14 (College Station, TX) statistical software.
Results
Descriptive Statistics
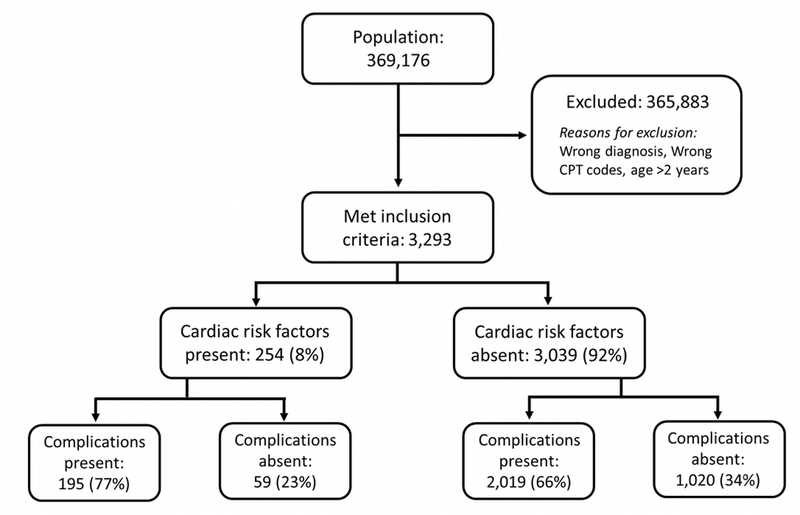
A total of 369,176 patients were captured by NSQIP-P from 2012 to 2016, of which 3,293 patients met inclusion criteria (Figure 1). A mean age at the time of surgery of 8 ± 4.6 months was observed. The majority of patients were Caucasian (73%) and male (66%). Patients in the development and validation datasets were similar except for slight differences in age and weight (Table 2). The presence of 15 comorbidities was evaluated in the study sample with 12% of patients having a secondary comorbidity. The most common comorbidity was structural central nervous system (CNS) abnormality (25%). Esophageal and gastrointestinal disorders, impaired cognitive status and CRF were each observed in 8% of the study population. Other comorbidities, such as pulmonary abnormalities and neuromuscular disorders, were present in less than 5% of the population.
Figure 1 –
CONSORT Diagram of patient selection
Sixty-seven percent of patients experienced at least 1 post-operative complication. The most common complication was a bleeding event requiring blood transfusion (66%), followed by readmissions and reoperations. Wound complications, such as surgical site infection and dehiscence, were rare (1%). Of note, there were no graft failures or mortalities.
Cardiac Risk Factors
There were 254 patients (8%) with CRF. Patients with and without CRF differed in age, race, and ASA class. Comorbidities were more common in patients with cardiac abnormalities (Table 3). The most common comorbidities for patients with CRF included: structural CNS abnormalities (39%), impaired cognitive status (33%), gastrointestinal disorders (30%), previous cardiac surgery (22%), and structural pulmonary (18%). Patients with CRF had significantly longer operative times (p=0.003) and length of stay (p<0.001) compared to patients without CRF. A greater proportion of patients with CRF (74%) experienced a complication in the postoperative period compared to those without CRF (66%, p=0.001). Patients with CRF had significantly more transfusions, readmissions, reoperations, SSIs, and surgical wound dehiscence (Table 4).
Table 3:
Patient characteristics with and without cardiac risk factors (CRF).
| Variable, N (%) | All patients (n = 3293) | No CRF (n = 3039) | CRF present (n = 254) | P-value |
|---|---|---|---|---|
| Male Sex | 2173 (66%) | 2009 (66%) | 165 (65%) | 0.70 |
| Race | 0.05 | |||
| White | 2600 (79%) | 2401 (79%) | 199 (78%) | |
| Asian | 47 (1%) | 38 (1%) | 9 (4%) | |
| Black | 229 (7%) | 112 (7%) | 17 (7%) | |
| American Indian/Alaska Native | 19 (1%) | 16 (1%) | 3 (1%) | |
| Native Hawaiian/Pacific Islander | 11 (<1%) | 10 (<1%) | 1 (<1%) | |
| Unknown/Not reported | 387 (12%) | 362 (12%) | 25 (10%) | |
| Hispanic Ethnicity | 511 (16%) | 462 (15%) | 49 (19%) | 0.08 |
| Age in months, mean ± SD | 8 ± 4.6 | 8 ± 4.6 | 9 ± 4.9 | <0.001 |
| Weight in lbs, mean ± SD | 18 ± 7.3 | 18 ± 6.2 | 18 ± 15.1 | 0.95 |
| ASA Class | <0.001 | |||
| I | 637 (19%) | 625 (21%) | 12 (5%) | |
| II | 1970 (60%) | 1887 (62%) | 83 (33%) | |
| ≥ III | 684 (21%) | 525 (17%) | 159 (63%) | |
| Structural CNS abnormality | 822 (25%) | 724 (24%) | 98 (39%) | <0.001 |
| Gastrointestinal disorders | 274 (8%) | 197 (6%) | 77 (30%) | <0.001 |
| Impaired cognitive status | 261 (8%) | 178 (6%) | 83 (33%) | <0.001 |
| Structural pulmonary abnormality | 102 (3%) | 57 (2%) | 45 (18%) | <0.001 |
| Previous cardiac surgery | 56 (2%) | 1 (<1%) | 55 (22%) | <0.001 |
| Asthma | 55 (2%) | 41 (1%) | 14 (6%) | <0.001 |
| Neuromuscular disorder | 54 (2%) | 32 (1%) | 22 (9%) | <0.001 |
| Tracheostomy prior to surgery | 29 (1%) | 17 (1%) | 12 (5%) | <0.001 |
| Required oxygen support | 26 (1%) | 9 (<1%) | 17 (7%) | <0.001 |
| Ventilator support prior to surgery | 13 (<1%) | 4 (<1%) | 9 (4%) | <0.001 |
| Cerebral palsy | 9 (<1%) | 6 (<1%) | 3 (1%) | 0.03 |
| Hepatobiliary disease | 4 (<1%) | 2 (<1%) | 2 (1%) | 0.02 |
| Operative time, min, Mean ± SD | 178 ± 96 | 177 ± 96 | 196 ± 101 | 0.003 |
| Length of stay, days, Median (IQR) | 3 (2 – 4) | 3 (2 – 4) | 4 (3 – 5) | <0.001 |
Table 4:
Incidence of complications comparing patients with and without cardiac risk factors (CRF).
| Variable, N (%) | All patients (n = 3293) | No CRF (n = 3039) | CRF present (n = 254) | P-value |
|---|---|---|---|---|
| Postoperative transfusion | 2178 (66%) | 1991 (66%) | 187 (74%) | 0.01 |
| Readmission | 95 (3%) | 82 (3%) | 13 (5%) | 0.03 |
| Reoperations | 54 (2%) | 45 (1%) | 9 (4%) | 0.01 |
| Surgical site infection | 48 (1%) | 39 (1%) | 9 (4%) | 0.004 |
| Dehiscence | 27 (1%) | 22 (1%) | 5 (2%) | 0.04 |
| Sepsis | 12 (<1%) | 11 (<1%) | 1 (<1%) | 1.0 |
| Reintubation | 9 (<1%) | 7 (<1%) | 2 (1%) | 0.15 |
| Seizures | 8 (<1%) | 8 (<1%) | 0 (0%) | 1.0 |
| Urinary Tract Infection | 5 (<1%) | 4 (<1%) | 1 (<1%) | 0.33 |
| Venous Thrombosis | 4 (<1%) | 3 (<1%) | 1 (<1%) | 0.28 |
| Cardiac Arrest | 3 (<1%) | 2 (<1%) | 1 (<1%) | 0.21 |
| Postoperative pneumonia | 2 (<1%) | 2 (<1%) | 0 (0%) | 1.0 |
| Strokes | 2 (<1%) | 1 (<1%) | 1 (<1%) | 0.15 |
| Graft failure | 0 (0%) | 0 (%) | 0 (0%) | -- |
| Any adverse event | 2214 (67%) | 2019 (66%) | 195 (77%) | 0.001 |
Risk Factors for Postoperative Complications
Univariate logistic regression revealed associations between any postoperative complication with age at the time of surgery (OR 1.13, 95% CI 1.10–1.16) and ASA classification. Patients with an ASA class 2 conferred 1.3 times (95% CI 1.02–1.60) greater odds of an adverse event compared to ASA class 1. While ASA class of ≥3 was associated with 1.7 times (95% CI 1.27–2.26) greater odds of an adverse event compared to ASA Class 1. Comorbidities associated with an adverse event included: neuromuscular disorders (OR 4.30, 95% CI 1.52–12.14), interventricular hemorrhage (OR 2.44, 95% CI 0.93–6.39), and presence of CRF (OR 1.71, 95% CI 1.18–2.49). Operative duration (OR 1.01, 95% CI 1.01–1.01) was also associated with having an adverse event (Table 3).
On adjusted analysis, older age at the time of surgery increased the odds of postoperative complications in patients undergoing CVR by 13% for each additional month of life. Longer operative duration (OR 1.01, 95% CI 1.01–1.01) also remained significantly associated with postoperative complications in patients undergoing CVR (Table 5). There was a significant interaction between age at surgery and operative duration (OR 0.99, 95% CI 0.99–0.99). Model performance was evaluated using ROC curves. Similar model performance was observed between the development and validation data sets, with AUCs >0.70 (Figure 2).
Table 5:
Results of univariate and multiple logistic regression evaluating the association of risk factors with any adverse event.
| Covariate | Unadjusted Odds Ratio | 95% Confidence Intervals | Adjusted Odds Ratio | 95% Confidence Intervals |
|---|---|---|---|---|
| Age at surgery (months) | 1.13 | 1.10 – 1.16 | 1.15 | 1.09 – 1.21 |
| Operative duration (minutes) | 1.01 | 1.01 – 1.01 | 1.01 | 1.01 – 1.02 |
| Age at surgery * Operative duration | - | - | 0.99 | 0.99 – 0.99 |
| Supplemental oxygen | 7.30 | 0.96 – 55.37 | 3.48 | 0.44 – 27.68 |
| Neuromuscular disorders | 4.30 | 1.52 – 12.14 | 2.22 | 0.75 – 6.54 |
| Esophageal or gastrointestinal disorder | 1.54 | 1.08 – 2.19 | 1.44 | 0.92 – 1.45 |
| Interventricular hemorrhage | 2.44 | 0.93 – 6.39 | 1.47 | 0.52 – 4.15 |
| Cardiac risk factors present | 1.71 | 1.18 – 2.49 | 1.21 | 0.80 – 1.82 |
| Structural CNS abnormality | 1.37 | 1.11 – 1.70 | 1.15 | 0.92 – 1.45 |
| Constant | - | - | 0.2 | 0.13 – 0.29 |
Interaction Term
Figure 2 -.
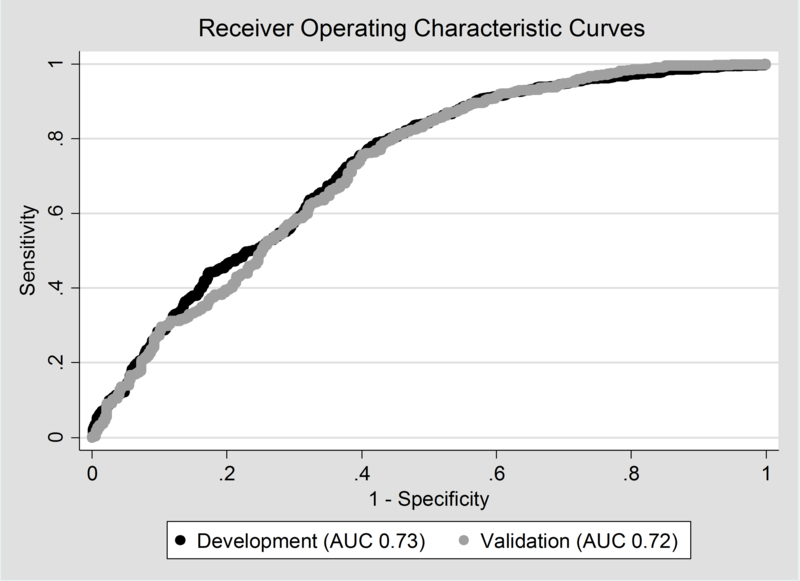
Model performance of development and validation data sets. Area under the curve (AUC) was 0.73 for the development data set, and 0.72 for validation data set.
Discussion
We found that after controlling for covariates, patients who were older at the time of surgery and those who endured longer operative times were significantly associated with postoperative complications in patients <2 years of age undergoing CVR for craniosynostosis using the NSQIP-P database. While significant on univariate analysis, the presence of CRF was not significantly associated with postoperative complications in the multivariable analysis as we had hypothesized. Likewise, a study evaluating risk factors for reoperation after CVR using 2012 – 2014 NSQIP-P data failed to identify CRF as a predictor of reoperation.7 The postoperative adverse event rate was 67%, with the most common event being need for transfusions. In a similar study using the 2004 – 2010 National Inpatient Sample (NIS) database, the presence of syndromic disorders, intraoperative blood transfusions, intermediate age of 7–12 months, and surgeries conducted at teaching hospitals were associated with increased odds of postoperative complications, with the most common complication being stroke.14 Transfusions were not included in postoperative complications in their study. Differences between the risk factors identified in the NIS study and our findings may largely be attributable to analysis of different perioperative variables – which may be a result of dissimilarities in data availability in the NIS and NSQIP-P.
The high postoperative adverse event rate of 67% reported in our study far surpasses the complication rates of 10 – 14% quoted by other studies,14–16 however these studies did not consider postoperative transfusions as an adverse event/complication, while in our study, transfusion accounted for 99% of postoperative complications. Lin et al compared postoperative complications in the 2012 Kids’ Inpatient Database (KID) to those in the 2012 NSQIP-P database after CVR, and found that rates of cardiac events, infections, wound dehiscence, seizures, and strokes/intracranial bleeds were comparable to each other and to the published literature, but the rates of blood transfusions (36% in KID versus 64% in NSQIP-P) differed significantly between the databases, as well as with the published literature.17 These differences may be a result of different definitions of transfusions (intraoperative and postoperative versus postoperative only), as well as data extraction techniques between NSQIP-P and KID (trained reviewers versus claims data).
Postoperative transfusion was by far the most common complication identified in patients undergoing CVR in this study, which is consistent with several published studies on intra- and postoperative bleeding during CVR. Perioperative transfusion rates range from 79 – 95% in children and infants undergoing CVR, with the most commonly transfused blood product being red blood cells. 18 Interventions to reduce transfusions after CVR are limited. Tranexamic acid (TXA) is one modality that has been tested to reduce perioperative and postoperative transfusions for CVR; however, the literature regarding its effectiveness is conflicted. In a single-center, retrospective review of 25 patients, intraoperative TXA use was associated with higher postoperative hemoglobin, but did not reduce perioperative transfusion rates.19 On the other hand, a single-center, retrospective review of 259 patients before and after implementation of a TXA protocol found that TXA was not associated with higher postoperative hemoglobin, but significantly reduced intraoperative transfusion requirements.20 Additionally, a single-center, retrospective study of pre- and post-implementation of postoperative furosemide administration in CVR patients found a significant reduction in transfusion rates.21 The use of furosemide was implemented because the perceived anemia was thought to be mostly attributable to hemodilution.
Controversy exists regarding whether duration of surgery is associated with postoperative complications after CVR. Lee et al reported longer duration of surgery to be positively associated with complications after CVR in a single-institution, 30-year review of 796 patients,16 while Lu et al reported that surgical duration was not a statistically significant determinant in postoperative complications after CVR in a single-center review of 65 patients.22 Our study found an association between longer operative lengths and increased postoperative complications. Longer operative times may be a surrogate indicator for more complex surgeries, problems with anesthesia, and surgeon inexperience – all of which are unable to be determined using NSQIP-P due to unavailability of these variables – but may increase the risk of postoperative complications.
Older patient age was associated with increased postoperative complications in our study. Several studies found age to be a significant risk factor for postoperative complications, but the “high-risk age” differed from study to study. Age ranges of < 9 months, 7 – 12 months, and 1–3 years have all been associated with postoperative complications in CVR.16, 23 Given the discordant results in the published literature on the age range associated with postoperative complications, there may be mediating factors associated with specific age groups that predispose those patients to adverse events. Data from our institution showed that younger patients (< 5 months) are candidates for endoscopic CVR, which was associated with a 50% decrease in transfusion rates compared to open CVR.24 Additionally, children > 1 year old tend to have thicker crania, which is technically more challenging and requires longer operative times.
The significant interaction between age at surgery and operative duration was not unexpected since younger patients tend to undergo endoscopic CVR, which has shorter operative times. Endoscopic surgery has also been associated with decreased transfusion requirements, and is likely a mediator for both older age and longer operative duration being associated with postoperative complications (namely transfusions). However, the surgical approach to CVR was not available as part of NSQIP-P, which limits the interpretation of this study’s findings.
Another limitation of this study is that the analysis could only be as granular and comprehensive as the available variables in NSQIP-P. There may be external factors that influence postoperative complications after CVR, such as surgeon experience and hospital case volume, which are not captured or reported in NSQIP-P. However, we felt that NSQIP-P was the best database to evaluate our study question because it does provide clinically abstracted data from multiple institutions utilizing rigorous variable definitions. Lastly, long-term outcomes are unknown, and therefore implications of having a postoperative complication on quality of life after the initial postoperative period cannot be determined. Further studies are needed to assess postoperative outcomes beyond 30 days in patients with craniosynostosis who undergo CRV and evaluate the effects of the high rate of post-operative transfusions.
Conclusions
Older age and longer operative duration were associated with increased postoperative complications in patients < 2 years undergoing cranial vault remodeling for craniosynostosis using the NSQIP-P database. The presence of CRFs was not associated with increased rates of complications after adjusting for other risk factors. The most common complication was need for transfusions. Further research is warranted for interventions that could reduce intraoperative bleeding and/or provide safer transfusion environments in this patient population.
Acknowledgments
Presented at: A portion of this manuscript was presented at the American Cleft Palate-Craniofacial Association meeting in Pittsburgh, PA April 2018.
Footnotes
Financial Disclosure Statement: We have no disclosures or financial support to discuss.
References
- 1.Bonfield CM, Tamber MS, Losee JE. Blindness from late presenting undiagnosed pancraniosynostosis mimicking pseudotumor cerebri. J Child Neurol. 2014;29(8):NP24–27. [DOI] [PubMed] [Google Scholar]
- 2.Fearon JA. Evidence-based medicine: Craniosynostosis. Plast Reconstr Surg. 2014;133(5):1261–1275. [DOI] [PubMed] [Google Scholar]
- 3.Morrison KA, Lee JC, Souweidane MM, et al. Twenty-Year Outcome Experience With Open Craniosynostosis Repairs: An Analysis of Reoperation and Complication Rates. Ann Plast Surg. 2018. [DOI] [PubMed] [Google Scholar]
- 4.Greives MR, Ware BW, Tian AG, et al. Complications in Posterior Cranial Vault Distraction. Ann Plast Surg. 2016;76(2):211–215. [DOI] [PubMed] [Google Scholar]
- 5.Birgfeld CB, Dufton L, Naumann H, et al. Safety of Open Cranial Vault Surgery for Single-Suture Craniosynostosis: A Case for the Multidisciplinary Team. J Craniofac Surg. 2015;26(7):2052–2058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tahiri Y, Paliga JT, Wes AM, et al. Perioperative complications associated with intracranial procedures in patients with nonsyndromic single-suture craniosynostosis. J Craniofac Surg. 2015;26(1):118–123. [DOI] [PubMed] [Google Scholar]
- 7.Jubbal KT, Agrawal N, Hollier LH Jr., Analysis of Morbidity, Readmission, and Reoperation After Craniosynostosis Repair in Children. J Craniofac Surg. 2017;28(2):401–405. [DOI] [PubMed] [Google Scholar]
- 8.Boulet SL, Rasmussen SA, Honein MA. A population-based study of craniosynostosis in metropolitan Atlanta, 1989–2003. Am J Med Genet A. 2008;146A(8):984–991. [DOI] [PubMed] [Google Scholar]
- 9.Putnam LR, Anderson KT, Tsao K, et al. The impact of cardiac risk factors on short-term outcomes for children undergoing a Ladd procedure. J Pediatr Surg. 2017;52(3):390–394. [DOI] [PubMed] [Google Scholar]
- 10.Goodenough CJ AK, Smith KE, Hanfland RA, Wadhwa N, Teichgraeber JF, Greives MG. Impact of cardiac risk factors in the post-surgical outcomes of palatoplasty patients: Analysis of the 2012–2014 NSQIP database. . Cleft Palate Craniofac J. 2018:In Submission. [DOI] [PubMed] [Google Scholar]
- 11.Raval MV, Dillon PW, Bruny JL, et al. Pediatric American College of Surgeons National Surgical Quality Improvement Program: feasibility of a novel, prospective assessment of surgical outcomes. J Pediatr Surg. 2011;46(1):115–121. [DOI] [PubMed] [Google Scholar]
- 12.Raval MV, Dillon PW, Bruny JL, et al. American College of Surgeons National Surgical Quality Improvement Program Pediatric: a phase 1 report. J Am Coll Surg. 2011;212(1):1–11. [DOI] [PubMed] [Google Scholar]
- 13.Dillon P, Hammermeister K, Morrato E, et al. Developing a NSQIP module to measure outcomes in children’s surgical care: opportunity and challenge. Semin Pediatr Surg. 2008;17(2):131–140. [DOI] [PubMed] [Google Scholar]
- 14.Abraham P, Brandel MG, Dalle Ore CL, et al. Predictors of Postoperative Complications of Craniosynostosis Repair in the National Inpatient Sample. Ann Plast Surg. 2018;80(5S Suppl 5):S261–S266. [DOI] [PubMed] [Google Scholar]
- 15.Goobie SM, Zurakowski D, Proctor MR, et al. Predictors of clinically significant postoperative events after open craniosynostosis surgery. Anesthesiology. 2015;122(5):1021–1032. [DOI] [PubMed] [Google Scholar]
- 16.Lee HQ, Hutson JM, Wray AC, et al. Analysis of morbidity and mortality in surgical management of craniosynostosis. J Craniofac Surg. 2012;23(5):1256–1261. [DOI] [PubMed] [Google Scholar]
- 17.Lin Y, Pan IW, Mayer RR, et al. Complications after craniosynostosis surgery: comparison of the 2012 Kids’ Inpatient Database and Pediatric NSQIP Database. Neurosurg Focus. 2015;39(6):E11. [DOI] [PubMed] [Google Scholar]
- 18.Stricker PA, Goobie SM, Cladis FP, et al. Perioperative Outcomes and Management in Pediatric Complex Cranial Vault Reconstruction: A Multicenter Study from the Pediatric Craniofacial Collaborative Group. Anesthesiology. 2017;126(2):276–287. [DOI] [PubMed] [Google Scholar]
- 19.Hansen JK, Lydick AM, Wyatt MM, et al. Reducing Postoperative Bleeding After Craniosynostosis Repair Utilizing a Low-Dose Transexamic Acid Infusion Protocol. J Craniofac Surg. 2017;28(5):1255–1259. [DOI] [PubMed] [Google Scholar]
- 20.Martin DT, Gries H, Esmonde N, et al. Implementation of a Tranexamic Acid Protocol to Reduce Blood Loss During Cranial Vault Remodeling for Craniosynostosis. J Craniofac Surg. 2016;27(6):1527–1531. [DOI] [PubMed] [Google Scholar]
- 21.Harroud A, Weil AG, Turgeon J, et al. Association of postoperative furosemide use with a reduced blood transfusion rate in sagittal craniosynostosis surgery. J Neurosurg Pediatr. 2016;17(1):34–40. [DOI] [PubMed] [Google Scholar]
- 22.Lu Y, Bao N, Ghanem A, et al. Early Complications and Associated Perioperative Factors in Nonsyndromic Craniosynostosis. J Craniofac Surg. 2017;28(6):1425–1430. [DOI] [PubMed] [Google Scholar]
- 23.Nguyen C, Hernandez-Boussard T, Khosla RK, et al. A national study on craniosynostosis surgical repair. Cleft Palate Craniofac J. 2013;50(5):555–560. [DOI] [PubMed] [Google Scholar]
- 24.Teichgraeber JF, Baumgartner JE, Waller AL, et al. Microscopic minimally invasive approach to nonsyndromic craniosynostosis. J Craniofac Surg. 2009;20(5):1492–1500. [DOI] [PubMed] [Google Scholar]