Abstract
PURPOSE
Phase III adjuvant trials have reported significant benefits in both relapse-free survival (RFS) and overall survival (OS) for high-dose interferon alfa (HDI) and ipilimumab at 10 mg/kg (ipi10). E1609 evaluated the safety and efficacy of ipilimumab at 3 mg/kg (ipi3) and ipi10 versus HDI.
PATIENTS AND METHODS
E1609 was a phase III trial in patients with resected cutaneous melanoma (American Joint Committee on Cancer 7th edition stage IIIB, IIIC, M1a, or M1b). It had 2 coprimary end points: OS and RFS. A 2-step hierarchic approach first evaluated ipi3 versus HDI followed by ipi10 versus HDI.
RESULTS
Between May 2011 and August 2014, 1,670 adult patients were centrally randomly assigned (1:1:1) to ipi3 (n = 523), HDI (n = 636), or ipi10 (n = 511). Treatment-related adverse events grade ≥ 3 occurred in 37% of patients receiving ipi3, 79% receiving HDI, and 58% receiving ipi10, with adverse events leading to treatment discontinuation in 35%, 20%, and 54%, respectively. Comparison of ipi3 versus HDI used an intent-to-treat analysis of concurrently randomly assigned patient cases (n = 1,051) and showed significant OS difference in favor of ipi3 (hazard ratio [HR], 0.78; 95.6% repeated CI, 0.61 to 0.99; P = .044; RFS: HR, 0.85; 99.4% CI, 0.66 to 1.09; P = .065). In the second step, for ipi10 versus HDI (n = 989), trends in favor of ipi10 did not achieve statistical significance. Salvage patterns after melanoma relapse showed significantly higher rates of ipilimumab and ipilimumab/anti–programmed death 1 use in the HDI arm versus ipi3 and ipi10 (P ≤ .001).
CONCLUSION
Adjuvant therapy with ipi3 benefits survival versus HDI; for the first time to our knowledge in melanoma adjuvant therapy, E1609 has demonstrated a significant improvement in OS against an active control regimen. The currently approved adjuvant ipilimumab dose (ipi10) was more toxic and not superior in efficacy to HDI.
INTRODUCTION
Melanoma is a major public health challenge, with a rising incidence rate.1 In the United States, an estimated 96,480 new cases of melanoma and 7,230 deaths will occur in 2019.1 Significant advances over the past 8 years have radically improved outcomes in unresectable metastatic melanoma. For patients with resectable regional or distant metastases, the risk of disease relapse justifies investigation of adjuvant systemic therapy.2 Phase III adjuvant trials have reported significant benefits in relapse-free survival (RFS) for 6 US Food and Drug Administration (FDA) –approved regimens and in overall survival (OS) for high-dose interferon alfa-2b (IFNα-2b; HDI; v observation) and ipilimumab at 10 mg/kg (ipi10; v placebo).3-8 IFNα has been tested in low-, intermediate-, and high-dose regimens for patients with intermediate- and high-risk melanoma (American Joint Committee on Cancer [AJCC] 8th edition stage II, III, or IV).9 Improvements in RFS and OS have been reported with HDI as tested in Eastern Cooperative Oncology Group (ECOG) and intergroup trials E1684, E1690, and E1694.3,10,11 Significant reductions in relapse risk were reported in all 3 trials, and significant improvements in OS were demonstrated in 2 of these studies: E1684, which evaluated HDI versus observation,3 and E1694, which evaluated HDI versus a ganglioside vaccine.11 A Cochrane meta-analysis of adjuvant IFN trials at various dose levels and regimens showed significant reductions in the risk of relapse (hazard ratio [HR], 0.83; 95% CI, 0.78 to 0.87; P < .00001) and death (HR, 0.91; 95% CI, 0.85 to 0.97; P = .003).12 ipi10 was tested as adjuvant therapy in European Organisation for Research and Treatment of Cancer (EORTC) 18071, which reported HRs of 0.76 (P < .001) and 0.72 (P = .001) for recurrence and death, respectively, compared with placebo.4 ipi10 was approved as adjuvant therapy for stage III melanoma in 2015; however, its use as adjuvant therapy has been limited by the significant risk of immune-related toxicities.13,14 For unresectable metastatic melanoma, ipilimumab at 3 mg/kg (ipi3) was approved in 2011.15 After the regulatory approval of adjuvant ipi10, the relative safety and efficacy of ipilimumab at the 2 dose levels became important to evaluate compared with HDI, a standard adjuvant treatment for high-risk melanoma available since 1996. Intergroup trial E1609 compared ipi3 and ipi10 with HDI.
PATIENTS AND METHODS
Patients
Adult patients with histologically confirmed melanoma of cutaneous or unknown primary origin (AJCC 7th edition stage IIIB, IIIC, or IV [M1a or M1b]) who were rendered disease free surgically, as documented by physical examination and imaging studies within 4 weeks before random assignment, were eligible for this study. Patients with stage IIIB or IIIC disease were required to have complete lymph node dissection. Patients had to be randomly assigned within 84 days of resection. No prior systemic adjuvant therapy was permitted, but previous radiation therapy was allowed. Patients were required to have an ECOG performance status of 0 or 1 and meet screening laboratory test criteria. Patients with autoimmune disorders or conditions that required systemic corticosteroids or other immunosuppressants were ineligible.
Trial Design and Treatments
This open-label, multicenter, multinational, 3-arm, phase III study randomly assigned patients to adjuvant therapy with either ipi3, HDI, or ipi10, stratified by AJCC 7th edition stage (IIIB, IIIC, M1a, or M1b). ipi3 or ipi10 was administered intravenously every 3 weeks for 4 doses (induction), followed by the same dose every 12 weeks for up to 4 additional doses (maintenance). HDI was administered intravenously at 20 million units/m2 of body surface area per day, 5 days per week, for 4 weeks (induction), followed by 10 million units/m2 per day subcutaneously every other day, 3 days per week, for 48 weeks (maintenance). Treatment continued for a maximum of 60 weeks with ipilimumab or 52 weeks with HDI, or until unacceptable toxic effects, disease progression, or withdrawal of consent. Guidelines for the management of immune-related adverse events (AEs) and delay or discontinuation of treatment are described in the protocol.
Random Assignment
Participant registration was performed centrally at the ECOG–American College of Radiology Imaging Network (ACRIN) headquarters in Boston, Massachusetts. The stratified central random assignment was based on the permuted block method. E1609 was originally designed to evaluate ipi10 versus HDI, and when first activated in May 2011, participants were randomly assigned (1:1) centrally to either ipi10 (arm A) or HDI (arm B). A third arm evaluating ipi3 versus HDI was added in February 2012, at which time participants were randomly assigned (1:1:1) centrally to either ipi10 (arm A), HDI (arm B), or ipi3 (arm C). When ipi10 (arm A) target enrollment was reached in April 2014, random assignment (1:1) continued to ipi3 (arm C) and HDI (arm B) until target participant accrual for arm C was reached in August 2014.
Study End Points and Assessments
The study had 2 coprimary end points: OS and RFS of patients randomly assigned to receive ipi3 or ipi10, each compared with outcomes of those patients randomly assigned to receive HDI. Secondary end points were safety and tolerability of adjuvant ipilimumab and quality-of-life assessments.
Disease recurrence status was determined by serial physical examinations and radiographic assessments at baseline, every 3 months up to 2 years from study entry, every 6 months for years 2 to 5, and once per year after 5 years from study entry. Histologic or cytologic confirmation of recurrence was required to be attempted in all patient cases except for those involving brain metastases. Criteria of treatment failure that constituted acceptable evidence of disease recurrence are described in the protocol. The active version of the National Cancer Institute (NCI) Common Terminology Criteria for Adverse Events was used to evaluate and report AEs for all treated patients.
Trial Oversight
The study protocol was approved by the institutional review board (IRB) of each participating institution and conducted in accordance with Good Clinical Practice guidelines as defined by the International Conference on Harmonisation. All patients provided IRB-approved written informed consent. This study was monitored by the ECOG-ACRIN Data Safety Monitoring Committee and the NCI. Comprehensive safety monitoring and educational measures were implemented during the conduct of the study, including a mandatory immune-related AE management training module, a toxicity management hotline, and toxicity management reminders.
Statistical Analysis
E1609 was originally designed to evaluate ipi10 versus HDI. After regulatory approval of ipi3 for unresectable melanoma,15 the study protocol was amended to add a third arm evaluating ipi3 versus HDI in February 2012. With the addition of the ipi3 arm, a 2-step hierarchic analytic approach was adopted. In the first step, ipi10 was initially to be compared with HDI, and if significantly better than HDI, ipi3 was to be compared with HDI as a second step. However, the order of the comparison was revised in favor of ipi3 versus HDI as the first-step comparison, followed by ipi10 versus HDI in May 2015. This revision was made after regulatory approval of ipi10 as adjuvant therapy for high-risk melanoma.16 Because no interim analyses had been conducted before this revision, this change did not affect the overall type I error rate of the study design.
Random assignment was planned with a goal of 500 concurrently randomly assigned patient cases per arm for the intended comparisons of ipi3 versus HDI and ipi10 versus HDI. For the coprimary end points, a cure rate model was used.17 On the basis of data from a prior adjuvant study (E4697),18 the cure rate and median time to event for those not cured in the HDI arm were estimated as shown in Appendix Figure A2. Interim analyses in the first step (ipi3 v HDI) were planned for OS only, including efficacy (O’Brien-Fleming boundaries)19 and futility (conditional power), beginning at 50% OS information time (IT). The second-step comparison of ipi10 versus HDI was planned to occur only if the first-step comparison was significant for each coprimary end point. This design provided 80% power with a 1-sided type I error rate of 0.022 for OS and 0.003 for RFS. Full information corresponded to 416 deaths for OS and 655 events for RFS at each step. A conditional power of < 10% was used to guide early termination of the study. Because information for OS accumulated slower than anticipated, a review by the NCI and an independent ECOG-ACRIN statistician concluded that the study was not likely to obtain the required number of events with > 4.5 years of follow-up time. After this recommendation, a final analysis was conducted with a data cutoff date of February 15, 2019. All changes in the statistical considerations section were reviewed and approved by the NCI. Intent-to-treat (ITT) analyses using the stratified log-rank test were performed for the coprimary end points. Distributions of OS and RFS were estimated using the Kaplan-Meier method20 and compared using the stratified log-rank test. HRs were estimated based on the Cox proportional hazards model while adjusting for the stratification factors.21 Repeated CIs (RCIs)22 were generated for the first-step OS comparison HR, adjusting for previous interim analyses. Other CIs on HRs were generated using prespecified significance levels. Binomial data were compared using Fisher’s exact test. All P values were based on 2-sided tests. Analyses were conducted using SAS software (version 9.4; SAS Institute, Cary NC). RFS was defined as the time from random assignment to the time of recurrence or death. Individuals without a documented event were censored at the last assessed recurrence-free time point.
RESULTS
Patient Characteristics and Treatment
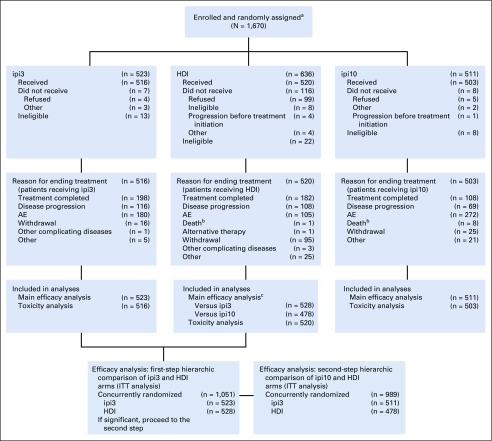
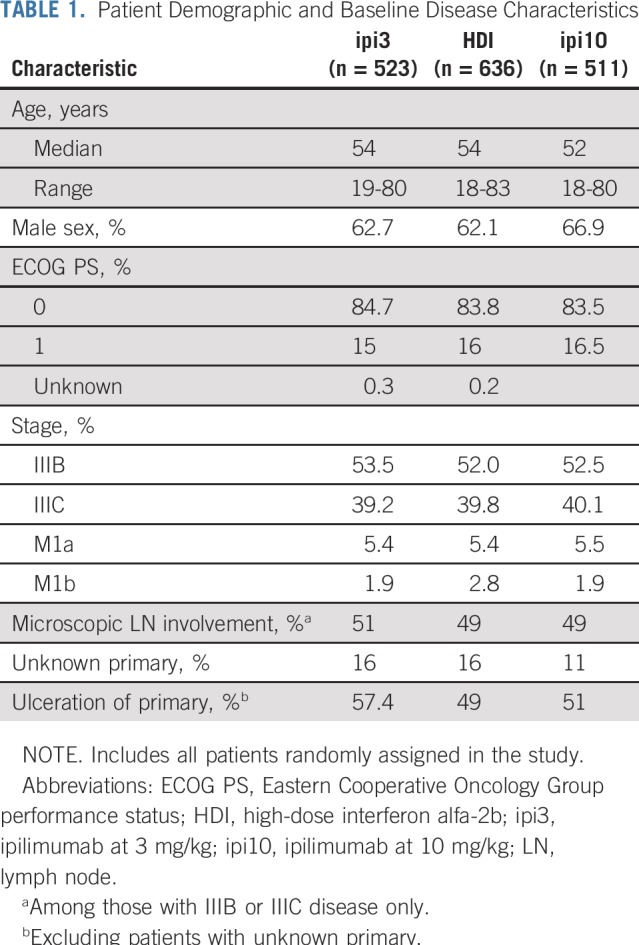
This National Clinical Trials Network study was initiated by ECOG-ACRIN, with participation from 850 sites across the United States and Canada. The trial was activated on May 25, 2011, and reached adult patient target accrual and closure on August 15, 2014. In the interim, a temporary suspension of the ipi10 arm for toxicity evaluation occurred between September and November 2013, after observation of a series of fatal (grade 5) events. A total of 1,670 adult patients were enrolled, including 523 randomly assigned to receive ipi3, 636 to receive HDI, and 511 to receive ipi10. Patient disposition is described in the CONSORT diagram (Fig 1). Baseline patient demographics and disease characteristics are listed in Table 1. Treatment details by study arm and reasons for discontinuation are listed in the Data Supplement.
FIG 1.
E1609 CONSORT diagram. AE, adverse event; HDI, high-dose interferon alfa-2b; ipi3, ipilimumab at 3 mg/kg; ipi10, ipilimumab at 10 mg/kg; ITT, intent to treat. aAdult population. E1609 included a pediatric component (age 12-17 years) consisting of 3 separate cohorts randomly assigned to the 3 treatment regimens and analyzed separately for safety per study protocol. Total pediatric accrual was 3 patients. bThese overlap but are not identical with treatment-related grade 5 events reported in Table 2. cConcurrently randomly assigned patient cases.
TABLE 1.
Patient Demographic and Baseline Disease Characteristics
Efficacy
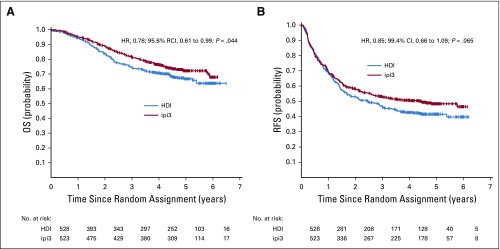
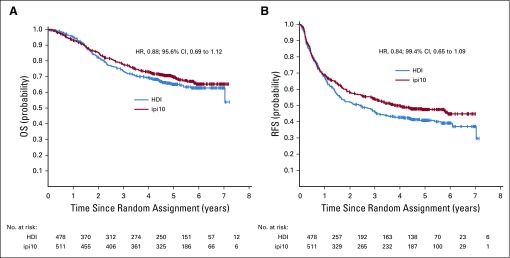
Using concurrently randomly assigned patient cases (n = 1,051) in the ipi3 and HDI arms, 4 interim analyses for the first-step OS comparison were conducted between April 2017 and September 2018. For the final analysis, a data cutoff date of February 15, 2019, was used, with a median follow-up time of 57.4 months (range, 0.03-86.6 months). Overall, we had fewer total numbers of events than originally planned based on the previous ECOG study (E4697), which enrolled a similar patient population with longer follow-up time. For ipi3 versus HDI, there were 486 RFS and 264 OS events of 655 and 416 planned events, respectively. Similarly, for ipi10 versus HDI, there were 477 RFS and 287 OS events of 655 and 416 planned events, respectively. First-step comparison of OS and RFS of ipi3 versus HDI used an ITT analysis of concurrently randomly assigned patient cases and showed significant OS difference in favor of ipi3, adjusted for previous analyses (HR, 0.78; 95.6% RCI, 0.61 to 0.99; P = .044). Five-year OS rate was 72% (95% CI, 68% to 76%) with ipi3 and 67% (95% CI, 62% to 72%) with HDI (Fig 2A). The efficacy boundary was crossed. For RFS, the HR was 0.85 (99.4% CI, 0.66 to 1.09; P = .065). Median RFS was 4.5 years (95% CI, 2.6 years to not reached) for ipi3 and 2.5 years (95% CI, 1.7 to 3.3 years) for HDI (Fig 2B). On the basis of protocol criteria, the study was positive and was allowed to proceed to the second-step OS comparison of ipi10 versus HDI (n = 989 concurrently randomly assigned patient cases). There were trends toward improvement in OS (formal comparison; HR, 0.88; 95.6% CI, 0.69 to 1.12) and RFS (exploratory; HR, 0.84; 99.4% CI, 0.65 to 1.09) in favor of ipi10 that were not statistically significant (Figs 3A and 3B). Five-year OS rate was 70% (95% CI, 65% to 74%) with ipi10 and 65% (95% CI, 60% to 70%) with HDI. Median RFS was 3.9 years (95% CI, 2.9 years to not reached) for ipi10 and 2.4 years (95% CI, 1.6 to 3.0 years) for HDI. We previously reported the results of an exploratory comparison of RFS with ipi3 versus ipi10, where we found no significant differences.23 Similarly, an exploratory analysis of OS and RFS with ipi3 versus ipi10 in concurrently randomly assigned patients (n = 773) showed no significant differences. In reviewing salvage patterns after disease progression, there were differences in salvage therapies in the HDI arm compared with the other arms, with significantly higher rates of salvage ipilimumab use after HDI (22.8%) versus after ipi3 (7.6%; P < .001) or ipi10 (4.8%; P < .001) and of ipilimumab with anti–programmed death 1 (PD1) after HDI (12.7%) versus after ipi3 (6.1%; P = .001) or ipi10 (3.9%; P < .001). Salvage use of anti-PD1 immunotherapy was 25% (ipi3), 22.1% (HDI), and 18.7% (ipi10), respectively.
FIG 2.
Kaplan-Meier plots of (A) overall survival (OS; hazard ratio [HR], 0.78; 95.6% repeated CI [RCI], 0.61 to 0.99; P = .044) and (B) relapse-free survival (RFS; HR, 0.85; 99.4% CI, 0.66 to 1.09; P = .065) with 3 mg/kg of ipilimumab (ipi3) versus high-dose interferon alfa-2b (HDI). Includes intent-to-treat concurrently randomly assigned patient cases in the 2 arms being compared.
FIG 3.
Kaplan-Meier plots of (A) overall survival (OS; hazard ratio [HR], 0.88; 95.6% CI, 0.69 to 1.12) and (B) relapse-free survival (RFS; HR, 0.84; 99.4% CI, 0.65 to 1.09) with 10 mg/kg of ipilimumab (ipi10) versus high-dose interferon alfa-2b (HDI).
Safety
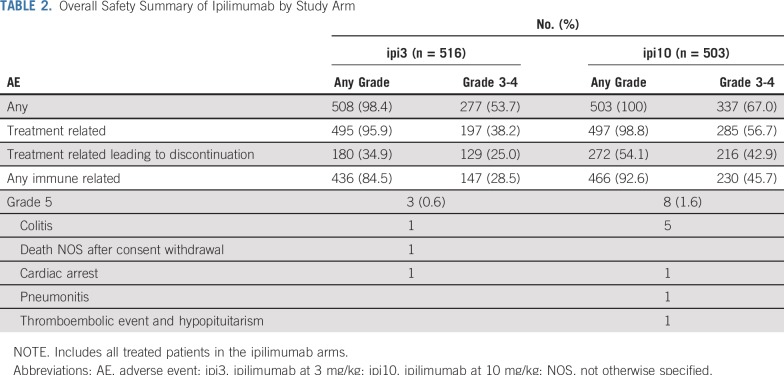
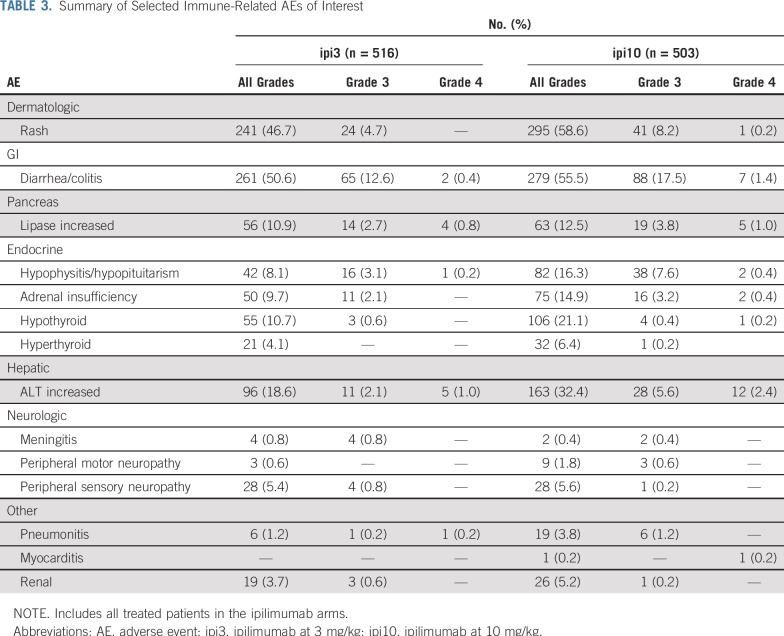
The toxicity rate for the worst-degree (grade ≥ 3) AEs was 38.6% (95% CI, 34.3% to 42.9%) with ipi3, 78.8% (95% CI, 75.1% to 82.3%) with HDI, and 57.9% (95% CI, 53.4% to 62.2%) with ipi10. Grade ≥ 3 toxicity rates were significantly different between ipi3 and ipi10 (P < .001; Table 2). The incidence rates of immune-related toxicity in the ipilimumab arms (defined as select AEs of interest considered consistent with immune checkpoint inhibitors) of grade ≥ 3 were 28.5% (95% CI, 24.6% to 32.6%) and 46.3% (95% CI, 41.9% to 50.8%) with ipi3 and ipi10, respectively, and these were significantly different between the 2 arms (P < .001; Table 3). Fatal AEs considered at least possibly treatment related occurred in 3 patients receiving ipi3, 2 receiving HDI, and 8 receiving ipi10. Toxicities encountered with HDI were consistent with the known AE profile, as listed in the Data Supplement. Corticosteroids to manage toxicities were required in 57.2% of patients receiving ipi3, 10.4% receiving HDI, and 75.7% receiving ipi10. Similarly, additional immunosuppressants were required in 5.4% of patients receiving ipi3, 0.6% receiving HDI, and 8.2% receiving ipi10. Hormone replacement therapy (primarily thyroid and adrenal) was needed by 17.2% of patients receiving ipi3, 6.3% receiving HDI, and 28.8% receiving ipi10. As listed in the Data Supplement, 38.4% of patients completed the planned treatment with ipi3, compared with 35.1% with HDI and 21.5% with ipi10. Maintenance therapy was initiated in 53.7% of patients receiving ipi3, 85.8% receiving HDI, and 31.6% receiving ipi10.
TABLE 2.
Overall Safety Summary of Ipilimumab by Study Arm
TABLE 3.
Summary of Selected Immune-Related AEs of Interest
DISCUSSION
E1609 targeted a population of patients with melanoma with a high risk of recurrence and death after standard surgical management, appropriate for the consideration of potentially toxic adjuvant therapy. These included patients with regional lymph node metastases and/or in-transit lymphatic lesions, as well as patients with completely resected stage IV (M1a or M1b) disease. Randomized phase II data had suggested a dose-dependent effect of ipilimumab, where increasing the dose seemed to increase efficacy in terms of OS but not other end points (and at the expense of greater toxicity). This supported the original design of E1609 as a 2-arm study evaluating ipi10 versus HDI.24 After the FDA approval of ipi3 for patients with unresectable metastatic melanoma, based on significant improvement in OS and considering the relative toxicity, it became important to evaluate ipi3 as adjuvant therapy. The pivotal study EORTC 18071 tested ipi10 versus placebo and reported improvement in the primary RFS end point and the secondary OS end point, leading to FDA approval in 2015.16 However, the use of ipi10 as adjuvant therapy in clinical practice has been limited by the high incidence of serious AEs.14 These factors taken together prompted the change in the E1609 hierarchic design to assess ipi3 as the first step, where the value of adjuvant ipi3 became the key question in the trial.
E1609 is unique in using an active control arm (HDI) that was previously shown to improve OS and RFS, and results of this study are important to view within this context.9 However, it is also relevant to acknowledge that HDI studies were conducted in a different era of surgical management and systemic therapy that may have limited relevance to present-day melanoma management. With ipi3 in E1609, there was a strong trend that approached significance for RFS improvement compared with HDI. A potential explanation for this finding is that both ipilimumab and HDI improve RFS to levels where a significant difference cannot be demonstrated with the E1609 sample size. The clinical impact of ipi3 seems to be more durable, translating into a greater OS benefit. Salvage therapy in patients developing recurrent disease showed significantly higher use rates for ipilimumab and the combination of anti-PD1 and ipilimumab among patients in the HDI arm versus those receiving ipi3 or ipi10. These differences raise the possibility that the OS advantage of ipi3 versus HDI might have been greater absent crossover. This also emphasizes the fact that immune-related AEs in the adjuvant setting may compromise therapeutic options in the event of recurrence. There were nonsignificant trends toward improvement in OS and RFS with ipi10 versus HDI, which raises the question of whether increased toxicity with ipi10 affected outcomes. The protocol had strict toxicity-specific criteria that mandated treatment delay for moderate immune-related AEs and discontinuation for more severe AEs. This affected treatment exposure with ipi10 compared with ipi3, as listed in the Data Supplement, with less treatment exposure and higher discontinuation rates with ipi10. A smaller percentage of patients received any salvage therapy after ipi10 compared with the other 2 arms. If we consider the salvage use of anti-PD1, anti–PD1 ligand, BRAF inhibitors, MEK inhibitors, ipilimumab, and combinations of these agents, the overall postrecurrence use was 69.7% after ipi3, 86.2% after HDI, and 51.6% after ipi10. This may also have affected the OS differences observed. Furthermore, it was notable that significantly more patients randomly assigned to the HDI arm had early censoring because of treatment refusal. Of 636 patients randomly assigned to HDI, 99 refused treatment, and a majority (78%) of these patients were censored within 6 months with incomplete follow-up time. However, taking into account the significant crossover toward ipilimumab and other forms of salvage therapy in the HDI arm, it is likely that our results represent a conservative estimate of ipi3 benefits.
AEs were mostly immune related and consistent with the known toxicity profiles of these agents. Toxicity with ipi10 was significantly greater than with ipi3, which was expected and consistent with what is known in metastatic melanoma, although the rates seem to be higher in the adjuvant setting than in the metastatic setting.25 This may be related to the quality of the host immune response and its susceptibility to immunologic interventions that may differ between earlier and more advanced disease settings.26 E1609 was conducted at a time when relatively little experience with immune checkpoint blockade was present among treating oncologists. The unprecedented comprehensive educational and training efforts provided by the study team involving hundreds of sites across the United States and Canada may have improved overall experience in the management of adjuvant immunotherapy toxicities.
After the activation of E1609, the CheckMate-238 study demonstrated RFS benefit with adjuvant nivolumab versus ipi10.6 Significant RFS benefits versus placebo were reported for pembrolizumab in KeyNote-054 and the combination of dabrafenib and trametinib in patients with BRAF-mutated melanoma in COMBI-AD.7,8 The adjuvant standard of care has clearly shifted in favor of these new agents based on the magnitude of RFS benefits seen and the demonstrated superiority of nivolumab over ipi10 in RFS and toxicity, but the question of OS benefit remains to be answered as the trials mature. E1609 supports the survival value of adjuvant immune checkpoint blockade, demonstrating significant OS benefit in comparison with the active control HDI, previously shown to benefit OS and RFS. The data support the use of ipi3 over HDI based on improved survival and similar RFS and roughly comparable toxicity. In cases where adjuvant therapy with ipilimumab may represent an option, such as in patients who experience progression during anti-PD1 therapy with resectable disease, ipi3 has an advantage over the approved dose of ipi10. Taking into account E1609 data as well data from adjuvant anti-PD1 studies, it is safe to state that HDI is no longer an acceptable initial standard adjuvant therapy option for resected stage III or IV melanoma.
In conclusion, adjuvant therapy with ipi3 improved the survival of patients with resected high-risk melanoma compared with HDI. An OS benefit was seen despite a postprotocol salvage pattern that significantly favored the use of more active agents known to affect survival in the HDI arm. It is notable that, for the first time to our knowledge in melanoma adjuvant therapy, E1609 has demonstrated a significant improvement in OS by ipi3 against an active control regimen.
ACKNOWLEGEMENT
We thank the patients and their families and the investigators who participated in the E1609 study.
Footnotes
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health, nor does mention of trade names, commercial products, or organizations imply endorsement by the US government.
Presented at the 55th ASCO Annual Meeting, Chicago, IL, May 31-June 4, 2019.
Supported by the National Cancer Institute, National Institutes of Health, under award numbers CA180820, CA180794, CA180799, CA180802, CA180821, CA180844, CA180847, CA180853, CA180863, CCSRI021039, CA180867, CA180888, CA180835, and CA189859 and by Bristol-Myers Squibb and was coordinated by the ECOG-ACRIN Cancer Research Group (Peter J. O’Dwyer, MD, and Mitchell D. Schnall, MD, PhD, group co-chairs).
See accompanying Editorial on page 529
AUTHOR CONTRIBUTIONS
Conception and design: Ahmad A. Tarhini, Sandra J. Lee, F. Stephen Hodi, Gary I. Cohen, Jeffrey A. Sosman, Lawrence E. Flaherty, Howard Streicher, Vernon K. Sondak, John M. Kirkwood
Administrative support: Ahmad A. Tarhini, Howard Streicher, Vernon K. Sondak, John M. Kirkwood
Provision of study material or patients: Ahmad A. Tarhini, Gary I. Cohen, Laura F. Hutchins, Jeffrey A. Sosman, Henry B. Koon, David R. Minor, Carrie B. Lee, Lawrence E. Flaherty, Teresa M. Petrella
Collection and assembly of data: Ahmad A. Tarhini, Sandra J. Lee, F. Stephen Hodi, Uma N.M. Rao, Omid Hamid, Harriett M. Kluger, Zeynep Eroglu, Kari L. Kendra, David R. Minor, Carrie B. Lee, Lawrence E. Flaherty, John M. Kirkwood
Data analysis and interpretation: Ahmad A. Tarhini, Sandra J. Lee, F. Stephen Hodi, Gary I. Cohen, Omid Hamid, Laura F. Hutchins, Jeffrey A. Sosman, Harriett M. Kluger, Zeynep Eroglu, Henry B. Koon, Kari L. Kendra, Mark R. Albertini, Lawrence E. Flaherty, Teresa M. Petrella, Howard Streicher, Vernon K. Sondak, John M. Kirkwood
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Phase III Study of Adjuvant Ipilimumab (3 or 10 mg/kg) Versus High-Dose Interferon Alfa-2b for Resected High-Risk Melanoma: North American Intergroup E1609
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/journal/jco/site/ifc.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Ahmad A. Tarhini
Consulting or Advisory Role: Bristol-Myers Squibb, Merck, Genentech/Roche, Incyte, Newlink Genetics, Array BioPharma, Novartis, OncoSec, HUYA Bioscience International, Immunocore, Pfizer/EMD Serono, Sanofi/Regeneron, BioNTech
Research Funding: Incyte (Inst), Prometheus Laboratories (Inst), Bristol-Myers Squibb (Inst), Amgen (Inst), Incyte (Inst), Novartis (Inst), GreenPeptide (Inst), Merck (Inst)
Sandra J. Lee
Employment: NantKwest (I)
Stock and Other Ownership Interests: NantKwest (I)
Consulting or Advisory Role: Roche/Genentech
F. Stephen Hodi
Employment: Dana-Farber Cancer Institute
Stock and Other Ownership Interests: Apricity, Torque
Consulting or Advisory Role: Merck Sharp & Dohme, Novartis, Genentech/Roche, EMD Serono, Sanofi, Bayer, Aduro Biotech, Pfizer, Verastem, Bristol-Myers Squibb, Takeda Pharmaceuticals, Surface, Compass Therapeutics, Partners Therapeutics, Pionyr, 7Hills Pharma, Torque, Rheos
Research Funding: Bristol-Myers Squibb (Inst), Merck Sharp & Dohme (Inst), Genentech/Roche (Inst), Novartis (Inst)
Patents, Royalties, Other Intellectual Property: Patent pending as per institutional policy; patent-pending royalties received on MICA-related disorder application to institution per institutional IP policy; angiopoietin-2 biomarkers predictive of anti-immune checkpoint response (Inst); compositions and methods for identification, assessment, prevention, and treatment of melanoma using PD-L1 isoforms; methods of using pembrolizumab and trebananib (Inst)
Travel, Accommodations, Expenses: Novartis, Bristol-Myers Squibb
Other Relationship: Bristol-Myers Squibb, Genentech/Roche
Gary I. Cohen
Stock and Other Ownership Interests: Nymox, Celgene, AbbVie
Honoraria: Amgen
Omid Hamid
Honoraria: Bristol-Myers Squibb, Novartis, Array BioPharma, Sanofi/Regeneron
Consulting or Advisory Role: Amgen, Novartis, Roche, Bristol-Myers Squibb, Merck, Aduro Biotech, Akeso Biopharma, Array BioPharma, BeiGene, Genentech, GlaxoSmithKline, Immunocore, Incyte, Janssen, NextCure, Regeneron, Sanofi, Seattle Genetics, Tempus, Zelluna
Speakers’ Bureau: Bristol-Myers Squibb, Novartis, Array BioPharma, Sanofi/Regeneron
Research Funding: AstraZeneca (Inst), Bristol-Myers Squibb (Inst), Genentech (Inst), Immunocore (Inst), Incyte (Inst), Merck (Inst), Merck Serono (Inst), MedImmune (Inst), Novartis (Inst), Pfizer (Inst), Roche (Inst), Amgen (Inst), CytomX Therapeutics (Inst), Iovance Biotherapeutics (Inst), NextCure (Inst), GlaxoSmithKline (Inst), Arcus Biosciences (Inst), Aduro Biotech (Inst), Akeso Biopharma (Inst), Array BioPharma (Inst), Exelixis (Inst), Moderna Therapeutics (Inst), Regeneron (Inst), Sanofi (Inst), Seattle Genetics (Inst), Torque (Inst), Zelluna (Inst)
Laura F. Hutchins
Research Funding: Abbott/AbbVie (Inst), Celldex (Inst), Amgen (Inst), Bristol-Myers Squibb (Inst), Polynoma (Inst)
Jeffrey A. Sosman
Honoraria: Bristol-Myers Squibb, Genentech, MSD Oncology
Consulting or Advisory Role: Bristol-Myers Squibb, Genentech, MSD Oncology
Harriett M. Kluger
Consulting or Advisory Role: Genentech/Roche, Corvus Pharmaceuticals, Nektar, Biodesix, Pfizer, Celldex, Iovance Biotherapeutics, Merck, Immunocore, Array BioPharma
Research Funding: Merck (Inst), Bristol-Myers Squibb (Inst), Apexigen (Inst)
Travel, Accommodations, Expenses: Apexigen
Zeynep Eroglu
Consulting or Advisory Role: Compugen, Array BioPharma, Regeneron
Research Funding: Novartis (Inst)
Travel, Accommodations, Expenses: Genentech/Roche, Tesaro
Henry B. Koon
Employment: Bristol-Myers Squibb
Stock and Other Ownership Interests: Bristol-Myers Squibb
Kari L. Kendra
Research Funding: Novartis (Inst), Bristol-Myers Squibb (Inst), GlaxoSmithKline (Inst), Karyopharm Therapeutics (Inst), Merck (Inst)
David R. Minor
Stock and Other Ownership Interests: Bristol-Myers Squibb (I), Nektar
Consulting or Advisory Role: Theravance
Carrie B. Lee
Consulting or Advisory Role: Delcath Systems
Travel, Accommodations, Expenses: Novartis
Mark R. Albertini
Research Funding: Bristol-Myers Squibb (Inst), MedImmune (Inst), Apeiron Biologics (Inst), Merck Sharp & Dohme (Inst)
Lawrence E. Flaherty
Consulting or Advisory Role: Merck/Schering Plough, HUYA Bioscience International
Travel, Accommodations, Expenses: Merck/Schering Plough
Teresa M. Petrella
Honoraria: Astellas Pharma (I), AbbVie (I), Bayer (I), Janssen (I), Sanofi Canada (I), Incyte, Bristol-Myers Squibb, Merck, Novartis, Genzyme
Consulting or Advisory Role: Janssen (I), AbbVie (I), Bayer (I), Astellas Pharma (I), Incyte, Genzyme, Merck, Bristol-Myers Squibb, Novartis
Research Funding: Roche Canada, Novartis Canada Pharmaceuticals
Vernon K. Sondak
Consulting or Advisory Role: Merck/Schering Plough, Novartis, Bristol-Myers Squibb, Array BioPharma, Genentech/Roche, Pfizer, Polynoma, Regeneron, Replimune
John M. Kirkwood
Consulting or Advisory Role: Bristol-Myers Squibb, Novartis, Array BioPharma, Immunocore, Iovance, Elsevier, Checkmate Pharmaceuticals (Inst), Bristol-Myers Squibb (Inst), Immunocore (Inst), Merck (Inst)
Research Funding: Merck (Inst), Prometheus Laboratories (Inst), Immunocore (Inst)
No other potential conflicts of interest were reported.
REFERENCES
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69:7–34. doi: 10.3322/caac.21551. [DOI] [PubMed] [Google Scholar]
- 2.Romano E, Scordo M, Dusza SW, et al. Site and timing of first relapse in stage III melanoma patients: Implications for follow-up guidelines. J Clin Oncol. 2010;28:3042–3047. doi: 10.1200/JCO.2009.26.2063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kirkwood JM, Strawderman MH, Ernstoff MS, et al. Interferon alfa-2b adjuvant therapy of high-risk resected cutaneous melanoma: The Eastern Cooperative Oncology Group trial EST 1684. J Clin Oncol. 1996;14:7–17. doi: 10.1200/JCO.1996.14.1.7. [DOI] [PubMed] [Google Scholar]
- 4.Eggermont AM, Chiarion-Sileni V, Grob JJ, et al. Prolonged survival in stage III melanoma with ipilimumab adjuvant therapy. N Engl J Med. 2016;375:1845–1855. doi: 10.1056/NEJMoa1611299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Eggermont AM, Suciu S, Testori A, et al. Long-term results of the randomized phase III trial EORTC 18991 of adjuvant therapy with pegylated interferon alfa-2b versus observation in resected stage III melanoma. J Clin Oncol. 2012;30:3810–3818. doi: 10.1200/JCO.2011.41.3799. [DOI] [PubMed] [Google Scholar]
- 6.Weber J, Mandala M, Del Vecchio M, et al. Adjuvant nivolumab versus ipilimumab in resected stage III or IV melanoma. N Engl J Med. 2017;377:1824–1835. doi: 10.1056/NEJMoa1709030. [DOI] [PubMed] [Google Scholar]
- 7.Long GV, Hauschild A, Santinami M, et al. Adjuvant dabrafenib plus trametinib in stage III BRAF-mutated melanoma. N Engl J Med. 2017;377:1813–1823. doi: 10.1056/NEJMoa1708539. [DOI] [PubMed] [Google Scholar]
- 8.Eggermont AMM, Blank CU, Mandala M, et al. Adjuvant pembrolizumab versus placebo in resected stage III melanoma. N Engl J Med. 2018;378:1789–1801. doi: 10.1056/NEJMoa1802357. [DOI] [PubMed] [Google Scholar]
- 9.Tarhini AA, Kirkwood JM. How much of a good thing? What duration for interferon alfa-2b adjuvant therapy? J Clin Oncol. 2012;30:3773–3776. doi: 10.1200/JCO.2012.44.9975. [DOI] [PubMed] [Google Scholar]
- 10.Kirkwood JM, Ibrahim JG, Sondak VK, et al. High- and low-dose interferon alfa-2b in high-risk melanoma: First analysis of intergroup trial E1690/S9111/C9190. J Clin Oncol. 2000;18:2444–2458. doi: 10.1200/JCO.2000.18.12.2444. [DOI] [PubMed] [Google Scholar]
- 11.Kirkwood JM, Ibrahim JG, Sosman JA, et al. High-dose interferon alfa-2b significantly prolongs relapse-free and overall survival compared with the GM2-KLH/QS-21 vaccine in patients with resected stage IIB-III melanoma: Results of intergroup trial E1694/S9512/C509801. J Clin Oncol. 2001;19:2370–2380. doi: 10.1200/JCO.2001.19.9.2370. [DOI] [PubMed] [Google Scholar]
- 12.Mocellin S, Lens MB, Pasquali S, et al. Interferon alpha for the adjuvant treatment of cutaneous melanoma. Cochrane Database Syst Rev. 2013;6:CD008955. doi: 10.1002/14651858.CD008955.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tarhini A, Atzinger C, Gupte-Singh K, et al. Treatment patterns and outcomes for patients with unresectable stage III and metastatic melanoma in the USA. J Comp Eff Res. 2019;8:461–473. doi: 10.2217/cer-2019-0003. [DOI] [PubMed] [Google Scholar]
- 14.Tarhini A, Ghate SR, Ionescu-Ittu R, et al. Postsurgical treatment landscape and economic burden of locoregional and distant recurrence in patients with operable nonmetastatic melanoma. Melanoma Res. 2018;28:618–628. doi: 10.1097/CMR.0000000000000507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hodi FS, O’Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711–723. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eggermont AM, Chiarion-Sileni V, Grob JJ, et al. Adjuvant ipilimumab versus placebo after complete resection of high-risk stage III melanoma (EORTC 18071): A randomised, double-blind, phase 3 trial. Lancet Oncol. 2015;16:522–530. doi: 10.1016/S1470-2045(15)70122-1. [DOI] [PubMed] [Google Scholar]
- 17.Berkson J, Gage RP. Survival curve for cancer patients following treatment. J Am Stat Assoc. 1952;47:501–515. [Google Scholar]
- 18.Lawson DH, Lee S, Zhao F, et al. Randomized, placebo-controlled, phase III trial of yeast-derived granulocyte-macrophage colony-stimulating factor (GM-CSF) versus peptide vaccination versus GM-CSF plus peptide vaccination versus placebo in patients with no evidence of disease after complete surgical resection of locally advanced and/or stage IV melanoma: A trial of the Eastern Cooperative Oncology Group-American College of Radiology Imaging Network Cancer Research Group (E4697) J Clin Oncol. 2015;33:4066–4076. doi: 10.1200/JCO.2015.62.0500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gordon Lan KK, DeMets DL: Discrete sequential boundaries for clinical trials. Biometrika 70:659-663, 1983 . [Google Scholar]
- 20. Kaplan EL, Meier P: Nonparametric estimation from incomplete observations. J Am Stat Assoc 53:457-481, 1958. [Google Scholar]
- 21.Cox DR. Regression models and life tables (with discussion) J R Stat Soc B. 1972;34:187–220. [Google Scholar]
- 22.Jennison C, Turnbull BW. Interim analyses: The repeated confidence interval approach. J R Stat Soc B. 1989;51:305–361. [Google Scholar]
- 23. doi: 10.1200/JCO.19.01381. Tarhini AA, Lee SJ, Hodi FS, et al: A phase III randomized study of adjuvant ipilimumab (3 or 10 mg/kg) versus high-dose interferon alfa-2b for resected high-risk melanoma (U.S. Intergroup E1609): Preliminary safety and efficacy of the ipilimumab arms. J Clin Oncol 35, 2017 (suppl; abstr 9500) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wolchok JD, Neyns B, Linette G, et al. Ipilimumab monotherapy in patients with pretreated advanced melanoma: A randomised, double-blind, multicentre, phase 2, dose-ranging study. Lancet Oncol. 2010;11:155–164. doi: 10.1016/S1470-2045(09)70334-1. [DOI] [PubMed] [Google Scholar]
- 25.Ascierto PA, Del Vecchio M, Robert C, et al. Ipilimumab 10 mg/kg versus ipilimumab 3 mg/kg in patients with unresectable or metastatic melanoma: A randomised, double-blind, multicentre, phase 3 trial. Lancet Oncol. 2017;18:611–622. doi: 10.1016/S1470-2045(17)30231-0. [DOI] [PubMed] [Google Scholar]
- 26.Tatsumi T, Kierstead LS, Ranieri E, et al. Disease-associated bias in T helper type 1 (Th1)/Th2 CD4(+) T cell responses against MAGE-6 in HLA-DRB10401(+) patients with renal cell carcinoma or melanoma. J Exp Med. 2002;196:619–628. doi: 10.1084/jem.20012142. [DOI] [PMC free article] [PubMed] [Google Scholar]