Abstract
PURPOSE
In patients taking tamoxifen, the CYP2D6 genotype causes different exposure of active metabolite endoxifen. The objective of this randomized, open-label, multicenter, phase II study was to prospectively evaluate whether CYP2D6 genotype–guided tamoxifen dosing in patients with hormone receptor–positive metastatic breast cancer could have an impact on the clinical outcome.
METHODS
Patients who needed first-line tamoxifen therapy were enrolled. Based on individual CYP2D6 genotype, patients heterozygous (wild type [wt]/variant [V]) or homozygous (V/V) for variant alleles of decreased or no function were randomly assigned to receive tamoxifen at an increased dose (ID arm; 40 mg daily) or regular dose (RD arm; 20 mg daily), and patients homozygous for wild-type alleles (wt/wt) received tamoxifen at 20 mg daily. The primary endpoint was the progression-free survival (PFS) rate at 6 months. The secondary endpoints included PFS and correlation of Z-endoxifen concentration with clinical outcomes.
RESULTS
Between December 2012 and July 2016, 186 patients were enrolled in Japan. Of 184 evaluable patients, 136 carried wt/V or V/V (ID arm, 70; RD arm, 66), and 48 carried wt/wt. PFS rates at 6 months were not significantly different between the ID and RD arms (67.6% v 66.7%). The serum trough concentrations of Z-endoxifen in the ID arm were significantly higher than those in the RD arm (median, 89.2 nM v 51.1 nM; P < .0001) and were also higher compared with wt/wt patients (72.0 nM; P = .045). No significant difference in Z-endoxifen concentrations was observed between patients with disease progression and those who were progression free at 6 months (P = .43).
CONCLUSION
In patients with CYP2D6-variant alleles, increasing tamoxifen dosing did not achieve a higher PFS rate at 6 months. The CYP2D6 genotype solely cannot explain individual variability in the efficacy of tamoxifen.
INTRODUCTION
Tamoxifen, a selective estrogen receptor (ER) modulator, is metabolized to active antiestrogenic metabolites by cytochrome P450s (CYPs). Compared with tamoxifen, 4-hydroxytamoxifen and 4-hydroxy-N-desmethyl-tamoxifen (endoxifen) exhibit 100-fold higher affinity to ER and 30- to 100-fold higher potency in suppressing estrogen-dependent cell proliferation in vitro.1,2 Because the blood concentration of endoxifen is approximately 6-fold higher than that of 4-hydroxytamoxifen, endoxifen is considered a key active metabolite contributing to the treatment efficacy with tamoxifen for hormone receptor–positive (HR+) breast cancer.3 Coadministration of paroxetine, a potent inhibitor of CYP2D6, with tamoxifen reduces the plasma concentration of endoxifen.1 In a large cohort study, paroxetine use during tamoxifen treatment correlated with an elevated risk of death from breast cancer.4 Thus, the clinical benefit of tamoxifen has been thought to arise from its conversion to endoxifen by CYP2D6.
The enzymatic activity of CYP2D6 is markedly variable because of genetic polymorphisms, including single-nucleotide polymorphisms and total copy number, in the CYP2D6 gene. To date, > 80 different alleles that decrease or impair the enzymatic activity of CYP2D6 have been reported,5 and their frequencies vary among ethnic groups.6 Reportedly, the plasma concentration of endoxifen is lower in patients with variant-type CYP2D6 alleles compared with those in patients with wild-type alleles.1.3.7 However, despite the consistent pharmacogenetic effects of CYP2D6 on endoxifen exposure, there has been considerable controversy over the years regarding the impact of CYP2D6 polymorphisms on the efficacy of tamoxifen for breast cancer treatment.7-10
Previously, we reported that decreased-function or no-function CYP2D6 variants significantly correlated with shorter recurrence-free survival in 282 patients with breast cancer treated with adjuvant tamoxifen.7 In addition, CYP2D6 variants correlated with lower plasma concentrations of E/Z-endoxifen and Z-4-hydroxytamoxifen at steady state in 98 independent patients with breast cancer receiving 20 mg of tamoxifen daily. Consequently, we conducted a pharmacokinetic dose-adjustment study of tamoxifen based on CYP2D6 genotypes in Japanese patients with breast cancer, demonstrating that increasing tamoxifen dosing in patients CYP2D6 heterozygous (wt/V) or homozygous (V/V) for variant alleles achieved increased plasma concentrations of Z-endoxifen and Z-4-hydroxytamoxifen, which were comparable to those in patients CYP2D6 homozygous for wild-type alleles (wt/wt).11 Based on these previous studies, we hypothesized that increased tamoxifen dosing (40 mg daily) for patients with variant-type alleles of CYP2D6 (wt/V or V/V excluding homozygous for nonfunctional alleles; null/null) could improve clinical outcomes, which is equal to regular dosing (20 mg daily) in patients with wild-type alleles (wt/wt). Hence, this prospective study aimed to evaluate CYP2D6 genotype–guided tamoxifen dosing in patients with HR+ metastatic breast cancer.
METHODS
Study Design
In this randomized, open-label, multicenter, phase II study, all eligible patients started taking tamoxifen at 20 mg daily for 2 weeks pending the CYP2D6 genotype results. Based on individual CYP2D6 genotype, patients with wt/V or V/V were randomly assigned at a 1:1 ratio to receive tamoxifen at an increased dose (ID arm; 40 mg daily) or regular dose (RD arm; 20 mg daily). Patients with wt/wt were not randomly assigned but continued to receive tamoxifen at 20 mg daily. The random assignment was based on dynamic allocation (minimization method) with the factors of (1) CYP2D6 genotype (wt/V, V/V excluding null/null, or null/null); (2) proportion of ER-positive cells (10%-49% or ≥ 50%); (3) menopausal status (premenopause, tamoxifen alone, or concurrent luteinizing hormone–releasing hormone agonist therapy; postmenopause); (4) status of breast cancer (stage IV disease or recurrence after primary surgery); and (5) metastatic sites (bone only or others). Allocated tamoxifen dosing was continued until disease progression, unacceptable toxicity, death, or discontinuation for any other reason until 6 months after randomization.
All patients were asked to record tamoxifen intake in a provided diary. At least 2 weeks since prior and concurrent systemic medications, taking strong or moderate inhibitors of CYP2D6, including paroxetine, quinidine, amiodarone, cimetidine, diphenhydramine, duloxetine, sertraline, and terbinafine, were not permitted.12 Notably, other strong or moderate inhibitors of CYP2D6, such as fluoxetine, bupropion, and thioridazine, are not marketed in Japan.
The study protocol and any amendments thereof were approved by the institutional review board at each institution. Written informed consent from each patient was obtained before participation. An independent data-monitoring committee assessed the safety data. This study was registered with the University Hospital Medical Information Network Clinical Trials Registry (UMIN000009155).
Patients
Both premenopausal and postmenopausal women aged ≥ 20 years with ER-positive (proportion of ER-positive cells ≥ 10% by immunohistochemical testing), regardless of the progesterone receptor status, human epidermal growth factor receptor 2 (HER2)–negative (≤ 2 by immunohistochemical testing or < 2 by fluorescence in situ hybridization testing) breast cancer were eligible for enrollment if they were candidates to receive tamoxifen therapy as first-line endocrine treatment of their advanced disease (stage IV or recurrence after primary surgery). Patients previously untreated with any systemic therapy for advanced disease were also eligible. If the patients had received adjuvant endocrine therapy before and/or after surgery, > 1 year of endocrine therapy was needed. Postmenopausal women for whom receipt of an aromatase inhibitor as the first-line endocrine treatment was not recommended, for example, because of their low bone density, were eligible. Moreover, the eligibility criteria included the Eastern Cooperative Oncology Group Performance Status of 0-1, measurable disease according to RECIST (version 1.1),13 and adequate organ function as follows: WBC, ≥ 2,000/mm3; hemoglobin, ≥ 9 g/dL; platelets, ≥ 75,000/mm3; AST and/or ALT, ≤ 100 IU/L; and serum creatinine, ≤ 1.5 mg/dL. However, patients with advanced, symptomatic, visceral spread (ie, spread to the viscera or main organs of the body) who were at risk for short-term, life-threatening complications were excluded from the study.
CYP2D6 Genotyping
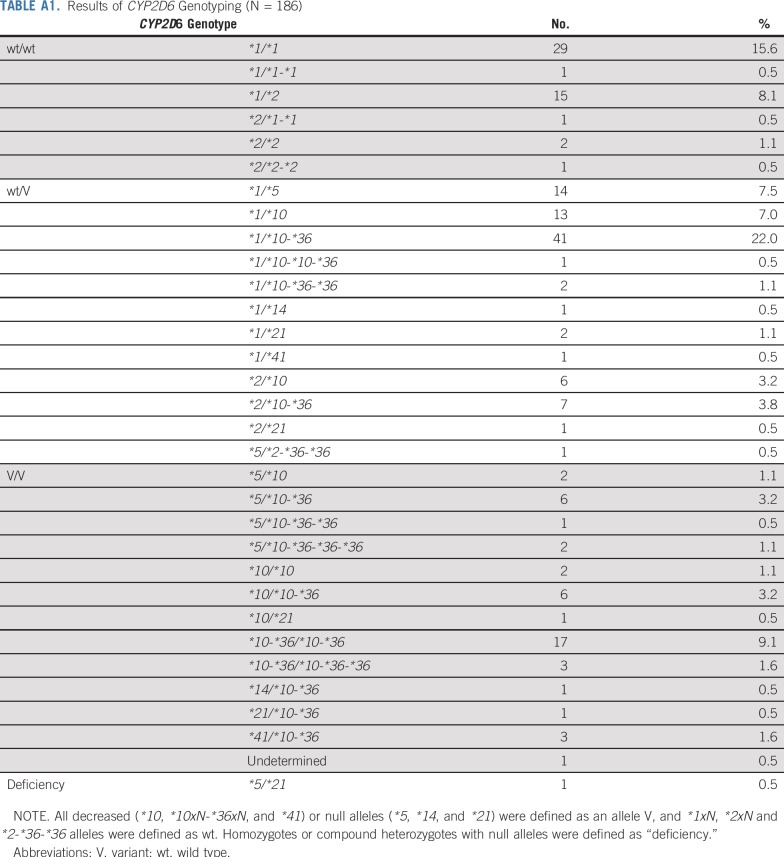
Peripheral blood samples (5 mL) were drawn into vacuumed tubes containing EDTA-2K at the baseline. Using the QuickGene-Auto240L with the QuickGene DNA Whole Blood Kit L (Kurabo Industries, Osaka, Japan), genomic DNA was extracted from the whole blood. We performed genotyping for key polymorphisms for CYP2D6*2 (2850C>T), CYP2D6*4 (1846G>A), CYP2D6*6 (1707delT), CYP2D6*10 (100C>T), CYP2D6*14 (1758G>A), CYP2D6*18 (4125_4133dupGTGCCCACT), CYP2D6*21 (2573_2574insC), CYP2D6*36 (gene conversion to CYP2D7 in exon 9), CYP2D6*41 (2988G>A), and CYP2D6*44 (2950G>C) using a multiplex polymerase chain reaction (PCR)-based real-time invader assay (mPCR-RETINA).14 Then, we determined the allelic ratio and copy number of the CYP2D6 gene using ABI PRISM 7900HT (Thermo Fisher Scientific, Waltham, MA), as demonstrated previously.15 Next, the whole-gene deletion (CYP2D6*5) was detected by long-range PCR following reported protocols.16 We defined all decreased (*10, *10xN-*36xN, and *41) and null alleles (*5, *14, and *21) as an allele V, and *1xN, *2xN, and *2-*36-*36 alleles as an allele wt to evaluate the effects of all CYP2D6 alleles tested in this study. We defined “deficiency” as homozygotes or compound heterozygotes for null alleles. These procedures were performed under the Clinical Laboratory Improvement Amendments certification.
Pharmacokinetic Assay
Serum trough concentrations of tamoxifen and its metabolites at steady state were assessed at 12 weeks after randomization or allocation. Patients did not take tamoxifen in the morning, and their peripheral blood samples (3 mL) were drawn into vacuum tubes without anticoagulants, and centrifuged at 3,000 rpm for 10 min at room temperature. The resulting serum was frozen and stored at −20°C until analysis.
Concentration of tamoxifen, N-desmethyltamoxifen, Z-endoxifen, and Z-4-hydroxytamoxifen were determined using the ultra-performance liquid chromatography–tandem mass spectrometry method,11 which we modified based on a recent report,17 per guidelines of the US Food and Drug Administration Guidance for Industry Bioanalytical Method Validation.18 The interday and intraday variabilities in precision (expressed as the coefficient of variation) for all compounds ranged from 0.7%-6.8% and 2.4%-6.2%, respectively. The average accuracies were 102.3%-107.8%.
Assessments
The primary endpoint was progression-free survival (PFS) rate at 6 months after randomization because a short-term endpoint for this phase II study was to establish proof of concept of CYP2D6 genotype–guided tamoxifen dosing and decide whether to conduct a phase III study. The secondary endpoints included the PFS, overall response rate (ORR), clinical benefit rate (CBR), safety, and correlation of serum trough concentrations of Z-endoxifen and Z-4-hydroxytamoxifen with clinical outcomes. The tumor response was assessed locally per RECIST (version 1.1). To support the primary endpoint, a blinded independent review committee performed a central assessment of disease progression at 6 months in a randomly selected subgroup (approximately 28%) of randomly assigned patients in the full analysis set. We assessed disease progression, death, or loss of follow-up in March 2017 for PFS, 1 of the secondary endpoints. Furthermore, adverse events were graded per the National Cancer Institute Common Terminology Criteria for Adverse Events (version 4.0).19
Statistical Analysis
Patients with wt/V or V/V were randomly allocated to either the ID arm or RD arm. Assuming a PFS rate at 6 months of 60% and 40% in the ID and RD arms, respectively, the planned sample size was 180 patients (90 patients/each arm), with a 1-sided α of .05 and a power of > 80%. Based on CYP2D6 allele frequencies in Japanese patients (wt/wt: wt/V or V/V = 30:70), we expected to enroll approximately 80 patients with wt/wt for enrolling 180 patients with wt/V or V/V. Hence, approximately 260 patients (180 patients with wt/V or V/V) were scheduled. The primary analysis involved comparing the PFS rate at 6 months between the ID arm and the RD arm by test statistic based on Kaplan-Meier estimates and Greenwood variance. Secondary analyses included PFS, ORR, and CBR. Patients with wt/wt who were reported to be tamoxifen responders7,8 were included as a reference for addressing the assumption of the similarity of PFS rate at 6 months for the ID arm. Therefore, the assessment of the similarity between the ID arm and patients with wt/wt was planned when the PFS rate at 6 months in the ID arm was observed to be significantly higher than in the RD arm.
On October 1, 2015, the steering committee revised the sample size to 136 patients (68 patients/arm) with a 1-sided α of .05 and a power of 70%, because of slow accrual. Accordingly, we expected to enroll approximately 44 patients with wt/wt to enroll 136 patients with wt/V or V/V based on the current frequencies of CYP2D6 genotypes in this study (wt/wt: wt/V or V/V = 25:75). Hence, approximately 180 patients (136 patients with wt/V or V/V) were rescheduled.
To compare binomial and continuous variables, χ2 tests and Wilcoxon tests were applied, respectively. Hazard ratios for survival curves were estimated by Cox proportional hazards model. All P values are reported as 2-tailed. Statistical analyses were performed using SAS 9.4 (SAS Institute, Cary, NC).
RESULTS
Patients and Treatment
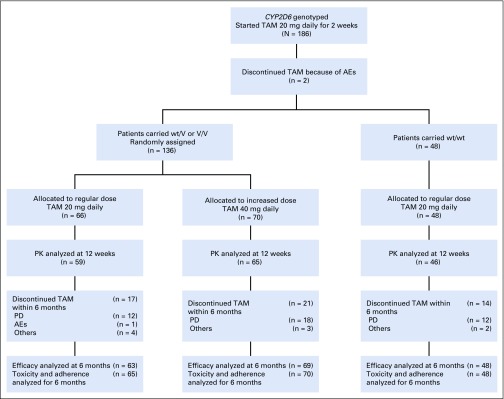
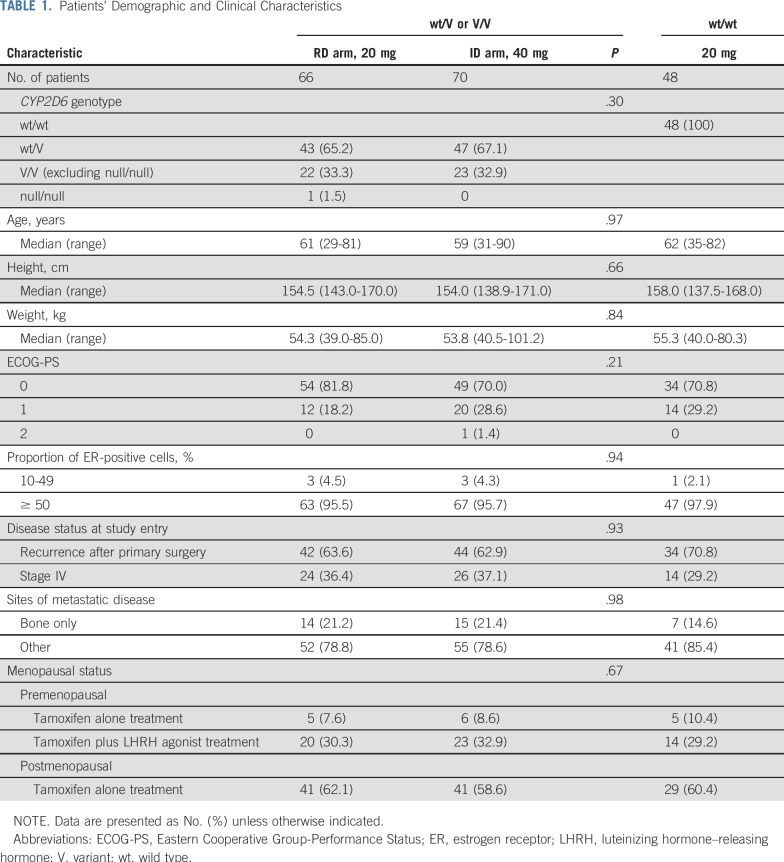
Between December 2012 and July 2016, we enrolled 186 Japanese patients from 54 institutions; 49, 90, and 47 patients carried wt/wt, wt/V, and V/V including 1 patient with null/null, respectively (Table A1). Figure 1 shows the trial profile. Two patients were withdrawn because of adverse events pending the CYP2D6 genotype results. Of 184 patients, 136 with wt/V or V/V were randomly assigned to either the ID arm (n = 70) or RD arm (n = 66), and 48 with wt/wt were not randomly assigned and continued taking 20 mg of tamoxifen daily. One patient in the RD arm who did not receive the assigned tamoxifen dose because of progressive disease was excluded from all analyses. One patient in the RD arm who did not meet eligibility criteria was also excluded from the efficacy analyses. Moreover, 1 patient in the RD arm and 1 patient in the ID arm for whom adequate images were unavailable were excluded from the efficacy analyses. Patient and tumor characteristics did not differ significantly between randomly assigned arms (Table 1). One patient with null/null was assigned to the RD arm.
FIG 1.
Trial profile. AEs, adverse events; PD, progressive disease; PK, pharmacokinetics; TAM, tamoxifen; V, variant; wt, wild type.
TABLE 1.
Patients’ Demographic and Clinical Characteristics
Efficacy
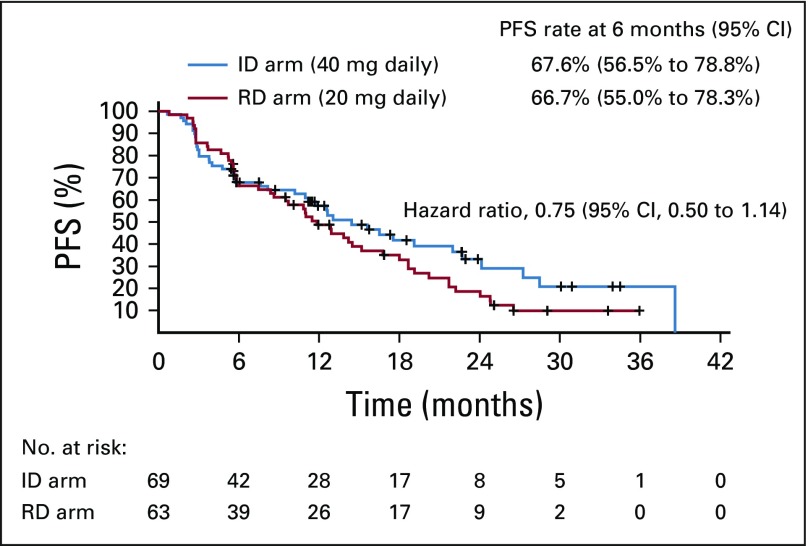
In patients with wt/V or V/V, the PFS rates at 6 months did not differ significantly between the ID arm (67.6%; 95% CI, 56.5% to 78.8%) and the RD arm (66.7%; 95% CI, 55.0% to 78.3%). The median PFS was 14.4 months (95% CI, 10.2 to 22.0 months) in the ID arm and 11.8 months (95% CI, 8.4 to 15.2 months) in the RD arm, with a median follow-up of 22.9 months (Fig 2). The hazard ratio was 0.75 (95% CI, 0.50 to 1.14), and the P value of stratified log-rank test to compare 2 survival curves was 0.15. The ORR (24.6% in the ID arm v 25.4% in the RD arm; P = .92) and CBR (66.7% in the ID arm v 66.7% in the RD arm; P = 1.00) also exhibited no significant differences between the 2 arms. In patients with wt/wt, the PFS, ORR, and CBR rates at 6 months were 63.0% (95% CI, 49.1% to 77.0%), 18.8%, and 60.4%, respectively.
FIG 2.
The Kaplan-Meier estimate of progression-free survival (PFS) in randomized arms. ID, increased dose; RD, regular dose.
Safety
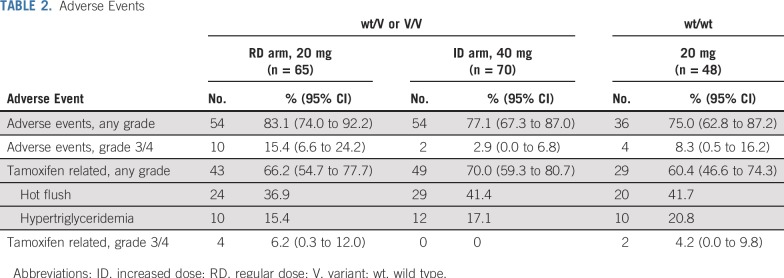
The incidence of adverse events, including hot flush and hypertriglyceridemia (common tamoxifen-related adverse events), did not differ significantly between the ID and RD arms (Table 2). No tamoxifen-related grade ≥ 3 adverse events were noted in the ID arm, whereas 4 patients in the RD arm and 2 patients with wt/wt, respectively, experienced them.
TABLE 2.
Adverse Events
Serum Concentrations of Tamoxifen and Its Active Metabolites
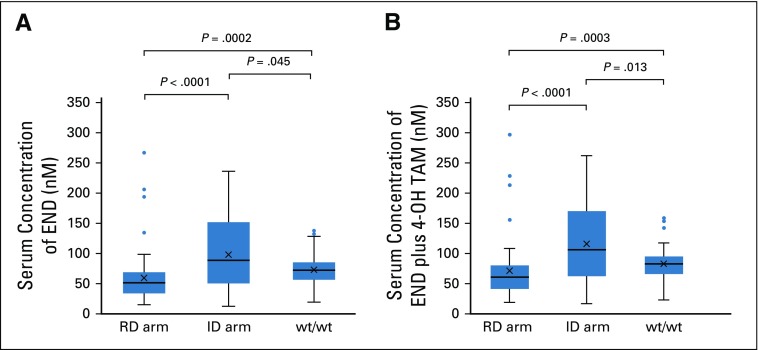
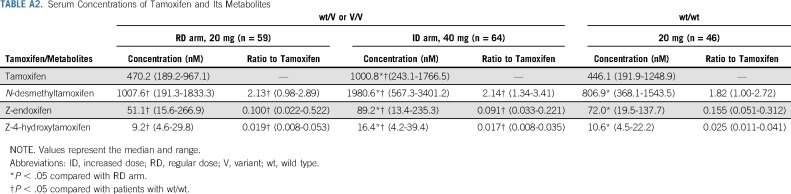
In the ID arm, the serum trough concentrations of Z-endoxifen and Z-4-hydroxytamoxifen at steady state were 13.4-488.6 (median, 91.1) nM and 4.2-62.5 (median, 16.7) nM, respectively. A patient with both maximum concentrations of Z-endoxifen (488.6 nM) and Z-4-hydroxytamoxifen (62.5 nM) in the ID arm was excluded from the pharmacokinetics comparison among groups (RD arm, ID arm, wt/wt; Fig 3; Table A2) as an outlier who might have a rare variant of tamoxifen pharmacokinetics–related genes. The serum trough concentrations of Z-endoxifen were significantly higher in the ID arm than in the RD arm (median, 89.2 nM v 51.1 nM; P < .0001; Fig 3A; Table A2). Compared with the concentrations of Z-endoxifen in patients with wt/wt (median, 72.0 nM), those in patients in the ID arm were significantly higher (P = .045), and those in patients in the RD arm were significantly lower (P = .0002). In addition, the serum trough concentrations of the sum of Z-endoxifen and Z-4-hydroxytamoxifen in the ID arm were significantly higher compared with those in the RD arm (median, 106.8 nM v 61.5 nM; P < .0001; Fig 3B). Likewise, compared with the concentrations of the sum of Z-endoxifen and Z-4-hydroxytamoxifen in patients with wt/wt (median, 83.9 nM), those in patients in the ID arm were significantly higher (P = .013), and those in patients in the RD arm were significantly lower (P = .0003). As anticipated, the concentration ratios of Z-endoxifen to tamoxifen were significantly lower (P < .0001) in patients with wt/V or V/V (RD arm: median, 0.100 [range, 0.022-0.522]; ID arm: median, 0.091 [range, 0.033-0.221]) than in patients with wt/wt (median, 0.155 [range, 0.051-0.312]; Table A2).
FIG 3.
The steady-state serum trough concentrations of (A) Z-endoxifen (END) and (B) the sum of END and Z-4-hydroxytamoxifen (4-OH TAM). ID, increased dose; RD, regular dose; wt, wild type.
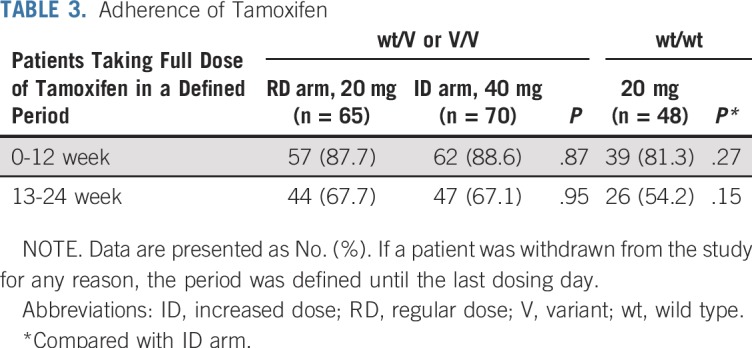
Tamoxifen Adherence
In the first 12 weeks (1-12 weeks) after randomization or allocation, the rates of patients taking a full dose of tamoxifen were 87.7%, 88.6%, and 81.3% in patients in the RD arm, in the ID arm, and with wt/wt, respectively. In the next 12 weeks (13-24 weeks), the rates declined in each group, as shown in Table 3. Overall, patients with wt/wt exhibited poor adherence (compared with the ID arm; P = .27 and .15 at 1-12 weeks and 13-24 weeks, respectively), which perhaps led to the unexpected observation in patients with wt/wt that the serum trough concentrations of Z-endoxifen or the sum of Z-endoxifen and Z-4-hydroxytamoxifen were statistically significantly lower than those in the ID arm, and the PFS rate at 6 months was relatively lower.
TABLE 3.
Adherence of Tamoxifen
Exposure-Response Relationship
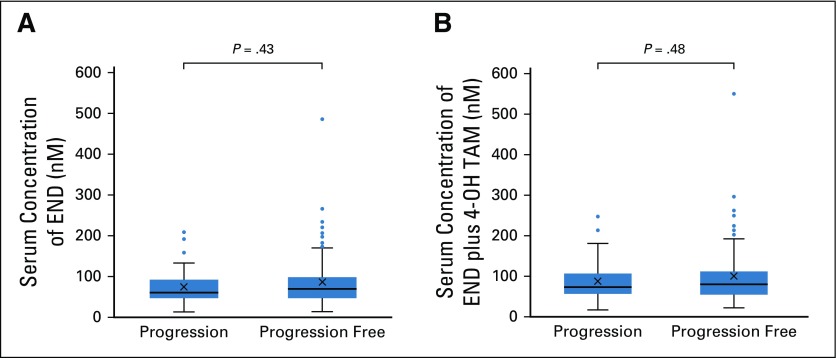
Of 170 evaluable patients for both efficacy and serum trough concentrations after 12 weeks, no significant difference was noted in the exposure of Z-endoxifen or the sum of Z-endoxifen and Z-4-hydroxytamoxifen between 53 patients with progression and 117 patients who were progression free at 6 months (median endoxifen, 61.4 nM v 69.8 nM; P = .43; median sum, 73.4 nM v 80.7 nM; P = .48; Fig 4). A patient with Z-endoxifen of 488.6 nM and Z-4-hydroxytamoxifen of 62.5 nM, excluded from comparison among groups as an outlier in the ID arm, achieved a confirmed partial response.
FIG 4.
The steady-state serum trough concentrations of (A) Z-endoxifen (END) and (B) the sum of END and Z-4-hydroxytamoxifen (4-OH TAM) in patients with progression and who were progression free at 6 months after being randomly assigned or allocated.
DISCUSSION
This study demonstrated that CYP2D6 genotype–guided tamoxifen dosing affects the serum endoxifen concentration, although with no clinical impact. The genetic polymorphisms of CYP2D6 exhibit ethnic differences, because an inactive variant allele *4 is most common (approximately 5%-10%) among individuals of European descent, whereas *4 is rare (< 1%) among those of Japanese, Korean, and Chinese descent, but a decreased functional variant allele *10 occurs commonly (approximately 50%). Accordingly, approximately 50% of patients of European descent and 70% of those of Asian descent are CYP2D6 intermediate metabolizers (IMs),6,20 who reportedly might be poor responders to tamoxifen treatment.7,8 Thus, the CYP2D6 variant is an important issue in Asian countries, and it should be determined whether the CYP2D6 genotype affects tamoxifen efficacy. In addition, we thought that the inconsistency in the results of a pharmacokinetic study for CYP2D6 genotype–guided tamoxifen dose adjustment between the United States21 and Japan11 was attributable to the ethnic difference. Namely, it suggests that the CYP2D6 genotype–guided tamoxifen dose-adjustment strategy has a potential for working for IMs but does not work for poor metabolizers who carry null/null.
A meta-analysis of data obtained from 4,973 tamoxifen-treated patients with breast cancer in retrospective studies (12 globally distributed sites) suggested that strict eligibility criteria (DNA source and target alleles for CYP2D6 genotyping, menopausal status, ER status, dose of tamoxifen, and periods of tamoxifen dosing) are needed to assess the CYP2D6 status and clinical outcomes in tamoxifen adjuvant therapy.22 Hence, our study was planned adequately to eliminate factors that affect the assessment of the correlation between clinical outcome and tamoxifen active metabolites exposure based on the individual CYP2D6 genotype. Nevertheless, even in our strict setting, an increased dose of 40 mg daily did not achieve a higher PFS rate at 6 months compared with a regular dose of 20 mg daily in patients with wt/V or V/V (Fig 2), although expected serum active metabolites exposure based on the CYP2D6 genotype and tamoxifen dose was observed (Fig 3; Table A2). The PFS rate at 6 months was an endpoint to obtain results earlier but might not be adequate to assess the response by endocrine treatment of HR+ metastatic breast cancer.
The correlation between endoxifen concentrations and tamoxifen treatment efficacy has been identified in the metastatic setting or adjuvant setting by retrospective analyses,23,24 both of which are hypothesis generating, not confirmatory. Conversely, a prospective observational study reported that clinical outcomes did not correlate with endoxifen trough concentrations at steady state in neoadjuvant or metastatic and adjuvant settings.25,26 Also, in our exposure-efficacy analysis, the exposure of either Z-endoxifen or the sum of Z-endoxifen and Z-4-hydroxytamoxifen did not correlate with PFS at 6 months in patients with metastatic breast cancer (Fig 4). It suggests that tamoxifen and its many metabolites, with varying degrees of antiestrogenic activity2,27,28 and serum concentrations, could contribute clinical outcome. Besides pharmacokinetic properties, pharmacodynamic factors, such as somatic gene mutations, other than the ER expression in the tumor could mediate variability of the tamoxifen therapy response.
In conclusion, to the best of our knowledge, this is the world’s first prospective randomized trial to assess the clinical outcome by CYP2D6 genotype–guided tamoxifen dosing. Although we observed a gene effect on the serum active metabolites exposure, the PFS rates at 6 months did not significantly differ between the increased and regular doses in patients with wt/V or V/V. In addition, no correlation existed between Z-endoxifen exposure and PFS at 6 months. Thus, this study suggests that the tamoxifen efficacy is determined by the exposure of not only endoxifen but also tamoxifen and its many metabolites with varying degrees of antiestrogenic activity, as well as pharmacodynamic factors like ER expression and somatic gene mutations in the tumor. Hence, the CYP2D6 genotype solely cannot explain individual variability in tamoxifen efficacy.
The effect of CYP2D6 genotypes on endoxifen exposure indicates that CYP2D6 is the key enzyme for generating endoxifen in metabolic activation of tamoxifen. In contrast, little impact of not only CYP2D6 genotypes but also endoxifen exposure on PFS at 6 months suggests that CYP2D6 genotype–guided dosing of tamoxifen is not likely to improve clinical outcomes. Furthermore, avoiding inhibitors of CYP2D6 would not be necessary, because endoxifen exposure was not clearly correlated with tamoxifen efficacy.
ACKNOWLEDGMENT
We thank the patients and their families, as well as the investigators and study teams, for their participation in this study. A list of investigators is given in the Appendix.
Appendix
The TARGET-1 Study Group includes the following investigators: H. Kawabata, MD, Toranomon Hospital; M. Saito, MD, Juntendo University Hospital; N. Masuda, MD, Osaka National Hospital; T. Kawasoe, MD, Japanese Red Cross Kumamoto Hospital; H. Hayashi, MD, Nagoya University Hospital; J. Horiguchi, MD, Gunma University Hospital; S. Shimizu, MD, Kanagawa Cancer Center; M. Kashiwaba, MD, Iwate Medical University Hospital; Y.I. Kim, MD, Seirei Hamamatsu General Hospital; Y. Kijima, MD, Kagoshima University Hospital; T. Toyama, MD, Nagoya City University Hospital; S. Nakamura, MD, Showa University Hospital; K. Kamei, MD, Ogaki Municipal Hospital; H. Minami, MD, Kobe University Hospital; K. Matsumoto, MD, Hyogo Cancer Center; Y. Hozumi, MD, Jichi Medical University Hospital; K. Tsugawa, MD, St Marianna University Hospital; H. Ishiguro, MD, Kyoto University Hospital; M. Futamura, MD, Gifu University Hospital; T. Ikeda, MD, Teikyo University Hospital; D. Yotsumoto, MD, Sagara Hospital; M. Ito, MD, Japanese Red Cross Ishinomaki Hospital; S. Tokunaga, MD, Osaka City General Hospital; E. Tokunaga, MD, Kyushu Cancer Center; Y. Miyoshi, MD, Hyogo College of Medicine Hospital; H. Jinno, MD, Keio University Hospital; Y. Kikawa, MD, Kobe City Medical Center General Hospital; T. Shibayama, MD, Cancer Institute Hospital of Japanese Foundation for Cancer Research; H. Iwata, MD, Aichi Cancer Center Hospital; M. Doi, MD, Hiroshima Prefectural Hospital; T. Watanabe, MD, Hamamatsu Oncology Center; S. Ozaki, MD, Kure Medical Center and Chugoku Cancer Center; J. Hashimoto, MD, St Luke’s International Hospital; Y. Saito, PhD, National Institutes of Health Sciences; T. Furuta, MD, Hamamatsu University School of Medicine; T. Satoh, MD, Osaka University Graduate School of Medicine; T. Kurata, MD, Kansai Medical University; and Y. Ariyoshi, MD, Aichi Cancer Center Hospital.
TABLE A1.
Results of CYP2D6 Genotyping (N = 186)
TABLE A2.
Serum Concentrations of Tamoxifen and Its Metabolites
Footnotes
Presented at the Annual Meeting of the American Society of Clinical Oncology, Chicago, IL, June 1-5, 2018.
Supported by grants from Project for Development of Innovative Research on Cancer Therapeutics (M.K.) and from Tailor-Made Medical Treatment Program (BioBank Japan Project; M.K.), funded by the Ministry of Education, Culture, Sports, Science, and Technology of Japan, and by Practical Research for Innovation Cancer Control, Japan Cancer Research Project funds (JP17ck0106191) from the Japan Agency for Medical Research and Development (Y.F.).
See accompanying Editorial on page 525
AUTHOR CONTRIBUTIONS
Conception and design: Kenji Tamura, Chiyo K. Imamura, Toshimi Takano, Shigehira Saji, Takeharu Yamanaka, Naoto T. Ueno, Yusuke Tanigawara
Financial support: Michiaki Kubo, Yasuhiro Fujiwara
Administrative support: Kenji Tamura, Chiyo K. Imamura
Provision of study materials or patients: Kenji Tamura, Toshimi Takano, Shigehira Saji, Kan Yonemori, Masato Takahashi, Junji Tsurutani, Reiki Nishimura, Kazuhiko Sato, Akira Kitani, Yasuhiro Fujiwara
Collection and assembly of data: Kenji Tamura, Chiyo K. Imamura, Toshimi Takano, Shigehira Saji, Kan Yonemori, Taisei Mushiroda, Michiaki Kubo, Yusuke Tanigawara
Data analysis and interpretation: Kenji Tamura, Chiyo K. Imamura, Toshimi Takano, Shigehira Saji, Takeharu Yamanaka, Naoto T. Ueno, Taisei Mushiroda, Yasuhiro Fujiwara, Yusuke Tanigawara
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
CYP2D6 Genotype–Guided Tamoxifen Dosing in Hormone Receptor–Positive Metastatic Breast Cancer (TARGET-1): A Randomized, Open-Label, Phase II Study
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/journal/jco/site/ifc.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Kenji Tamura
Research Funding: Daiichi Sankyo, Pfizer, Eli Lilly
Chiyo K. Imamura
Employment: Amgen (I)
Honoraria: Chugai Pharma
Consulting or Advisory Role: Ono Pharmaceutical
Toshimi Takano
Honoraria: Kyowa Hakko Kirin, Daiichi Sankyo, Eisai, Pfizer, Eli Lilly, Chugai Pharma (Inst), Taiho Pharmaceutical (Inst), Novartis (Inst), Ono Pharmaceutical (Inst), MSD (Inst), Merck Serono (Inst), Daiichi Sankyo (Inst), Kyowa Hakko Kirin (Inst), Eisai (Inst), Bristol-Myers Squibb (Inst)
Shigehira Saji
Honoraria: Chugai Pharma, Eisai, AstraZeneca, Ono Pharmaceutical, Taiho Pharmaceutical, Takeda, Novartis, Pfizer, Kyowa Kirin, Daiichi Sankyo
Consulting or Advisory Role: Chugai Pharma, Novartis, Kyowa Kirin
Research Funding: Chugai Pharma, Novartis, Taiho, Eisai, Takeda
Takeharu Yamanaka
Employment: Merck (I)
Honoraria: Chugai Pharma, Takeda, Taiho Pharmaceutical, Boehringer Ingelheim
Consulting or Advisory Role: Gilead Sciences, HUYA Bioscience International, Sysmex
Research Funding: Takeda (Inst), Taiho Pharmaceutical (Inst), Chugai/Roche (Inst), Bayer (Inst), Ono Pharmaceutical (Inst)
Kan Yonemori
Honoraria: Eisai, Pfizer, AstraZeneca, Novartis, Taiho Pharmaceutical
Consulting or Advisory Role: Chugai Pharma, Ono Pharmaceutical, Novartis
Research Funding: Ono Pharmaceutical
Masato Takahashi
Honoraria: AstraZeneca, Eisai, Pfizer, Eli Lilly, Chugai, Nippon Kayaku
Research Funding: Taiho Pharmaceutical (Inst), Kyowa Hakko Kirin (Inst), Eisai (Inst), Nippon Kayaku (Inst)
Junji Tsurutani
Honoraria: Kyowa Kirin, Eisai, Novartis, Chugai Pharma, Taiho Pharmaceutical, AstraZeneca, Nihon Kayaku, Lilly Japan, Pfizer, Daiichi Sankyo
Consulting or Advisory Role: Asahikasei, Daiichi Sankyo
Research Funding: Eisai (Inst), Boehringer Ingelheim (Inst)
Travel, Accommodations, Expenses: Daiichi Sankyo, Chugai Pharma, Eisai, Novartis
Reiki Nishimura
Honoraria: Pfizer, Chugai Pharma, Novartis
Naoto T. Ueno
Honoraria: Kyowa Hakko Kirin, Roche Taiwan, Chugai Pharma, Amgen
Consulting or Advisory Role: Samsung Bioepis
Research Funding: Medivation, Bayer, Amgen, Puma Biotechnology, Merck, Daiichi Sankyo, Celgene, GlaxoSmithKline, Kyowa Hakko Kirin, Bio-Path Holdings, Novartis, Sysmex
Travel, Accommodations, Expenses: Kyowa Hakko Kirin
Yasuhiro Fujiwara
Honoraria: AstraZeneca KK, Daiichi Sankyo, Chugai Pharma, Bristol-Myers Squibb Japan, SRLSantenPharmaceutical
Yusuke Tanigawara
Consulting or Advisory Role: Fujimoto
Honoraria: Chugai Pharma, Taiho Pharmaceutical, Dainippon Sumitomo Pharma, Novartis, Ono Pharmaceutical, Bristol-Myers Squibb Japan, Roche Diagnostics, Astellas Pharma
Research Funding: Taiho Pharmaceutical (Inst), Asahi Kasei (Inst), JIMRO (Inst), JSR (Inst)
No other potential conflicts of interest were reported.
REFERENCES
- 1.Stearns V, Johnson MD, Rae JM, et al. Active tamoxifen metabolite plasma concentrations after coadministration of tamoxifen and the selective serotonin reuptake inhibitor paroxetine. J Natl Cancer Inst. 2003;95:1758–1764. doi: 10.1093/jnci/djg108. [DOI] [PubMed] [Google Scholar]
- 2.Johnson MD, Zuo H, Lee KH, et al. Pharmacological characterization of 4-hydroxy-N-desmethyl tamoxifen, a novel active metabolite of tamoxifen. Breast Cancer Res Treat. 2004;85:151–159. doi: 10.1023/B:BREA.0000025406.31193.e8. [DOI] [PubMed] [Google Scholar]
- 3.Borges S, Desta Z, Li L, et al. Quantitative effect of CYP2D6 genotype and inhibitors on tamoxifen metabolism: Implication for optimization of breast cancer treatment. Clin Pharmacol Ther. 2006;80:61–74. doi: 10.1016/j.clpt.2006.03.013. [DOI] [PubMed] [Google Scholar]
- 4.Kelly CM, Juurlink DN, Gomes T, et al. Selective serotonin reuptake inhibitors and breast cancer mortality in women receiving tamoxifen: A population based cohort study. BMJ. 2010;340:c693. doi: 10.1136/bmj.c693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gaedigk A, Sangkuhl K, Whirl-Carrillo M, et al. The evolution of PharmVar. Clin Pharmacol Ther. 2019;105:29–32. doi: 10.1002/cpt.1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kurose K, Sugiyama E, Saito Y. Population differences in major functional polymorphisms of pharmacokinetics/pharmacodynamics-related genes in Eastern Asians and Europeans: Implications in the clinical trials for novel drug development. Drug Metab Pharmacokinet. 2012;27:9–54. doi: 10.2133/dmpk.dmpk-11-rv-111. [DOI] [PubMed] [Google Scholar]
- 7.Kiyotani K, Mushiroda T, Imamura CK, et al. Significant effect of polymorphisms in CYP2D6 and ABCC2 on clinical outcomes of adjuvant tamoxifen therapy for breast cancer patients. J Clin Oncol. 2010;28:1287–1293. doi: 10.1200/JCO.2009.25.7246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schroth W, Goetz MP, Hamann U, et al. Association between CYP2D6 polymorphisms and outcomes among women with early stage breast cancer treated with tamoxifen. JAMA. 2009;302:1429–1436. doi: 10.1001/jama.2009.1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Regan MM, Leyland-Jones B, Bouzyk M, et al. CYP2D6 genotype and tamoxifen response in postmenopausal women with endocrine-responsive breast cancer: The breast international group 1-98 trial. J Natl Cancer Inst. 2012;104:441–451. doi: 10.1093/jnci/djs125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rae JM, Drury S, Hayes DF, et al. CYP2D6 and UGT2B7 genotype and risk of recurrence in tamoxifen-treated breast cancer patients. J Natl Cancer Inst. 2012;104:452–460. doi: 10.1093/jnci/djs126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kiyotani K, Mushiroda T, Imamura CK, et al. Dose-adjustment study of tamoxifen based on CYP2D6 genotypes in Japanese breast cancer patients. Breast Cancer Res Treat. 2012;131:137–145. doi: 10.1007/s10549-011-1777-7. [DOI] [PubMed] [Google Scholar]
- 12. A guide for breast cancer patients and physicians; drug-interactions with tamoxifen. https://static.medicine.iupui.edu/divisions/clinpharm/content/Tamoxifen%20and%202D6v7.pdf.
- 13.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 14.Hosono N, Kubo M, Tsuchiya Y, et al. Multiplex PCR-based real-time invader assay (mPCR-RETINA): A novel SNP-based method for detecting allelic asymmetries within copy number variation regions. Hum Mutat. 2008;29:182–189. doi: 10.1002/humu.20609. [DOI] [PubMed] [Google Scholar]
- 15.Hosono N, Kato M, Kiyotani K, et al. CYP2D6 genotyping for functional-gene dosage analysis by allele copy number detection. Clin Chem. 2009;55:1546–1554. doi: 10.1373/clinchem.2009.123620. [DOI] [PubMed] [Google Scholar]
- 16.Johansson I, Lundqvist E, Dahl ML, et al. PCR-based genotyping for duplicated and deleted CYP2D6 genes. Pharmacogenetics. 1996;6:351–355. doi: 10.1097/00008571-199608000-00008. [DOI] [PubMed] [Google Scholar]
- 17.Arellano C, Allal B, Goubaa A, et al. An UPLC-MS/MS method for separation and accurate quantification of tamoxifen and its metabolites isomers. J Pharm Biomed Anal. 2014;100:254–261. doi: 10.1016/j.jpba.2014.07.033. [DOI] [PubMed] [Google Scholar]
- 18. US Department of Health and Human Services, Food and Drug Administration: Bioanalytical method validation, guidance for industry. https://www.fda.gov/files/drugs/published/Bioanalytical-Method-Validation-Guidance-for-Industry.pdf.
- 19. US Department of Health and Human Services: Common Terminology Criteria for Adverse Events (CTCAE): Version 4.0. https://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03/CTCAE_4.03_2010-06-14_QuickReference_5x7.pdf.
- 20.Garte S, Gaspari L, Alexandrie AK, et al. Metabolic gene polymorphism frequencies in control populations. Cancer Epidemiol Biomarkers Prev. 2001;10:1239–1248. [PubMed] [Google Scholar]
- 21.Irvin WJ, Jr, Walko CM, Weck KE, et al. Genotype-guided tamoxifen dosing increases active metabolite exposure in women with reduced CYP2D6 metabolism: A multicenter study. J Clin Oncol. 2011;29:3232–3239. doi: 10.1200/JCO.2010.31.4427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Province MA, Goetz MP, Brauch H, et al. CYP2D6 genotype and adjuvant tamoxifen: Meta-analysis of heterogeneous study populations. Clin Pharmacol Ther. 2014;95:216–227. doi: 10.1038/clpt.2013.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Madlensky L, Natarajan L, Tchu S, et al. Tamoxifen metabolite concentrations, CYP2D6 genotype, and breast cancer outcomes. Clin Pharmacol Ther. 2011;89:718–725. doi: 10.1038/clpt.2011.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Saladores P, Mürdter T, Eccles D, et al. Tamoxifen metabolism predicts drug concentrations and outcome in premenopausal patients with early breast cancer. Pharmacogenomics J. 2015;15:84–94. doi: 10.1038/tpj.2014.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Neven P, Jongen L, Lintermans A, et al. Tamoxifen metabolism and efficacy in breast cancer: A prospective multicenter trial. Clin Cancer Res. 2018;24:2312–2318. doi: 10.1158/1078-0432.CCR-17-3028. [DOI] [PubMed] [Google Scholar]
- 26.Sanchez-Spitman A, Dezentjé V, Swen J, et al. Tamoxifen pharmacogenetics and metabolism: Results from the prospective CYPTAM study. J Clin Oncol. 2019;37:636–646. doi: 10.1200/JCO.18.00307. [DOI] [PubMed] [Google Scholar]
- 27.Coezy E, Borgna JL, Rochefort H. Tamoxifen and metabolites in MCF7 cells: Correlation between binding to estrogen receptor and inhibition of cell growth. Cancer Res. 1982;42:317–323. [PubMed] [Google Scholar]
- 28.Wiebe VJ, Osborne CK, McGuire WL, et al. Identification of estrogenic tamoxifen metabolite(s) in tamoxifen-resistant human breast tumors. J Clin Oncol. 1992;10:990–994. doi: 10.1200/JCO.1992.10.6.990. [DOI] [PubMed] [Google Scholar]