Abstract
PURPOSE
High CD33 expression in acute myeloid leukemia (AML) with mutated NPM1 provides a rationale for the evaluation of gemtuzumab ozogamicin (GO) in this AML entity. We conducted a randomized trial to evaluate GO in combination with intensive induction and consolidation therapy in NPM1-mutated AML.
PATIENTS AND METHODS
Between May 2010 and September 2017, patients ≥ 18 years old and considered eligible for intensive therapy were randomly assigned up front for induction therapy with idarubicin, cytarabine, etoposide, and all-trans-retinoic acid with or without GO. The early (P = .02) primary end point of event-free survival (EFS) was evaluated 6 months after completion of patient recruitment.
RESULTS
Five hundred eighty-eight patients were randomly assigned (standard arm, n = 296; GO arm, n = 292). EFS in the GO arm was not significantly different compared with that in the standard arm (hazard ratio, 0.83; 95% CI, 0.65 to 1.04; P = .10). The early death rate during induction therapy was 10.3% in the GO arm and 5.7% in the standard arm (P = .05). Causes of death in both arms were mainly infections. The cumulative incidence of relapse (CIR) in patients achieving a complete remission (CR) or CR with incomplete hematologic recovery (CRi) was significantly reduced in the GO arm compared with the standard arm (P = .005), with no difference in the cumulative incidence of death (P = .80). Subgroup analysis revealed a significant beneficial effect of GO in female, younger (≤ 70 years), and FLT3 internal tandem duplication–negative patients with respect to EFS and CIR.
CONCLUSION
The trial did not meet its early primary end point of EFS, mainly as a result of a higher early death rate in the GO arm. However, in patients achieving CR/CRi after induction therapy, significantly fewer relapses occurred in the GO compared with the standard arm.
INTRODUCTION
Mutations in the nucleophosmin-1 (NPM1) gene occur in approximately 20%-33% of adult patients with acute myeloid leukemia (AML)1-4 and are present in all age groups.4,5 AML with mutated NPM1 has been included in the 2008 WHO classification as an AML disease entity.6 In the pivotal study describing NPM1 mutations in AML,1 an association between high CD33 expression and the mutation was reported and confirmed in subsequent work.7,8 Furthermore, AML with mutated NPM1 in the absence of an FLT3 internal tandem duplication (ITD) has been reported to be associated with a favorable prognosis2 and grouped in the low-risk group of current guidelines.9,10 Even in older patients, mutated NPM1 is associated with a favorable prognosis, and these patients may particularly benefit from intensive induction chemotherapy.3,11-14 Beyond chemotherapy sensitivity, AML with mutated NPM1 may benefit from the addition of all-trans-retinoic acid (ATRA) to induction and consolidation therapy.3,15
Recently, gemtuzumab ozogamicin (GO) was approved by the US Food and Drug Administration (FDA) and the European Medicines Agency (EMA) for the treatment of newly diagnosed CD33-positive AML in combination with induction and consolidation therapy.16,17 Of note, subset analysis of the pivotal ALFA 0701 study indicated that AML with mutated NPM1, in particular, may benefit from GO with regard to event-free survival (EFS) and overall survival (OS).17 The aim of this prospective, randomized, open-label, multicenter, phase III trial (AMLSG 09-09) was to determine the effect of GO added to induction and first consolidation therapy in adult patients with NPM1-mutated AML.
PATIENTS AND METHODS
Patients
Screening for NPM1 mutations was performed in patients with newly diagnosed AML within the AMLSG BiO study.4 Between May 2010 and September 2017, 1,619 adult patients age ≥ 18 years screened positive for mutated NPM1, and 600 patients (37%) were enrolled in the AMLSG 09-09 study. Two hundred thirty patients exhibiting mutated NPM1 in combination with FLT3-ITD participated in the AMLSG 16-10 study, which was open for accrual between June 2012 and May 201618; 144 patients exhibiting mutated NPM1 not eligible for intensive chemotherapy were enrolled in the AMLSG 15-10 trial (ClinicalTrials.gov identifier: NCT01237808). Details of inclusion and exclusion criteria are available in the Appendix (online only). The protocol was approved by the lead ethics review committee and registered at clinicaltrialsregister.eu (EudraCT No.: 2009-011889-28) and ClinicalTrials.gov (ClinicalTrials.gov identifier: NCT00893399).
Genetic Analyses
Details of genetic analysis are available in the Appendix.
Study Design
Induction therapy.
From May 2010 to September 2017, patients were randomly assigned (1:1) to receive induction chemotherapy without GO (standard arm) or with GO (3 mg/m2 intravenously [IV] on day 1; GO arm). Induction therapy consisted of 2 cycles idarubicin (12 mg/m2 IV on days 1, 3, and 5 [in induction cycle 2 and for patients > 60 years old, reduced to days 1 and 3]), cytarabine (100 mg/m2 continuously IV on days 1 to 7), and etoposide (100 mg/m2 IV on days 1 to 3 [in induction cycle 2 and for patients > 60 years old, reduced to days 1 and 3]; ICE) plus ATRA (45 mg/m2 orally [PO] on days 6 to 8 and 15 mg/m2 PO on days 9 to 21) with or without GO.15 Etoposide was reduced from 3 to 2 days for all patients in induction cycle 2 by amendment in April 2011 as a result of prolonged hematologic recovery in both arms. Patients with refractory disease after the first induction cycle went off study treatment and entered observational follow-up.
Consolidation therapy.
Patients who achieved a complete remission (CR) or CR with incomplete hematologic recovery (CRi) after induction therapy received consolidation therapy. Patients were assigned to 3 cycles of high-dose cytarabine plus ATRA, with cytarabine (age 18-60 years, 3 g/m2 every 12 hours on days 1 to 3; age > 60 years, 1 g/m2 every 12 hours on days 1 to 3),19 ATRA (15 mg/m2/d PO on days 4 to 21), and pegfilgrastim (6 mg subcutaneously) on day 8 in both study arms. Patients in the GO arm received GO (3 mg/m2 IV on day 1) in first consolidation therapy.
Definition of Response Criteria, Survival End Points, and Hematologic Recovery
Events for EFS defined according to the protocol were no CR or CRi within 60 days, relapse from CR or CRi, or death from any cause; patients not known to have any of these events were censored at the date they were last examined. The definition of EFS was modified for this analysis according to the European LeukemiaNet (ELN) recommendations,9 replacing the criterion of no CR or CRi within 60 days with no CR or CRi after induction therapy. Cumulative incidence of relapse (CIR) and cumulative incidence of death (CID) were defined according to ELN 2017 recommendations.9 Definitions of response criteria and hematologic recovery are available in the Appendix.
Sample Size Planning and Statistical Analysis
Originally, the study was planned to show an improvement in EFS of 15% after 2 years from 37% to 52% (hazard ratio [HR], 0.66; 2-sided log-rank test; α = .05; power, 80%; dropout rate, 5%), resulting in a number of 179 required events and a corresponding sample size of 276 patients. Given the dose reduction in induction and consolidation chemotherapy for patients older than 60 years, randomization and subsequent analysis were stratified for this age cutoff. In 2013, the study was amended based on data from the ALFA 0701 trial published in 2012,17 with OS as primary end point and an interim futility analysis after 276 patients were randomly assigned (October 31, 2013) based on conditional power (cutoff for continuation, > 30%; observed, 39.7%). Corresponding sample size planning for an OS improvement of 10% from 40% to 50% after 4 years as clinically meaningful estimated a number of 405 required events and hence a sample size of 588 patients (α = .05; power, 80%; dropout rate, 5%). Sequential testing was implemented using the fallback procedure of Wiens20 of EFS at 6 months at a significance level of 2% and OS at 4 years at a significance level of 3% after inclusion of the last patient. Hence, EFS was tested at a significance level of 2% 6 months after the last patient was randomly assigned. If the null hypothesis for EFS could be rejected at this time point, OS was tested at a significance level of 5% 4 years after the last patient was randomly assigned; otherwise, it was tested at a significance level of 3%. Here, we report on the EFS evaluation 6 months after the last patient was randomly assigned.
Pairwise comparisons were performed using the Mann-Whitney or Kruskal-Wallis test for continuous variables and the Fisher’s exact test for categorical variables. Univariable and multivariable logistic regression models were applied to investigate the influence of covariates on response to induction therapy. The primary end point of this analysis was EFS; exploratory end points were CIR, CID, and toxicity. To evaluate the primary objective, a stratified log-rank test was performed with a significance level of 2% and age as a stratification factor. For CIR and CID, equality of cumulative incidences between the treatment groups was evaluated using Gray’s test21 stratified by age. Effect estimates for EFS are given by HRs plus corresponding 95% CIs. The median duration of follow-up was calculated using the reverse Kaplan-Meier estimate,22 and the Kaplan-Meier method was used to estimate the distributions of EFS.23 Estimation of CIR and CID was done using the Aalen-Johansen estimator.24
In addition to the univariable test, exploratory analyses were performed to investigate whether the treatment effect differed for subgroups. To this aim, a multivariable Cox proportional hazards model and a cause-specific hazards models were fitted including the following covariates: age, sex, WBC count, type of AML (de novo v therapy-related or secondary AML), normal karyotype, FLT3-ITD, FLT3 tyrosine kinase domain, and DNMT3A. Modified covariates25 were used to model interaction effects of the covariates of interest and treatment without having to consider main effects. The subgroup results of (cause-specific) proportional hazards models are summarized in forest plots.
For all hypotheses that were not tested using the fallback procedure of Wiens,20 P < .05 was considered statistically significant. All statistical analyses were performed with the statistical software environment R, version 3.5.1, using the R packages rms, version 5.1-2; prodlim, version 2018.04.18; survival, version 2.43-3; riskRegression, version 2018.10.03; and cmprsk, version 2.2-7.26
RESULTS
Patients and Baseline Characteristics
Of 600 patients enrolled, 12 patients (2%) were excluded from the analysis, 6 as a result of violation of inclusion or exclusion criteria and 6 as a result of early (within 5 days) withdrawal of informed consent (Fig 1). Of 588 patients, 296 were randomly assigned to the standard arm, and 292 were assigned to the GO arm. Table 1 lists patient demographics and presenting laboratory and genetic characteristics according to treatment arms, which were well balanced except for hemoglobin, which had significantly higher values in the standard arm compared with the GO arm. Median age was 58.7 years (range, 18.4-82.3 years). Before initiation of first induction therapy, 3 patients died, 2 in the GO arm and 1 in the standard arm.
FIG 1.
CONSORT diagram. ATRA, all-trans-retinoic acid; GO, gemtuzumab ozogamicin; HCT, hematopoietic cell transplantation.
TABLE 1.
Patient and Disease Characteristics According to Randomization
Induction Therapy
First induction therapy was started in 295 and 290 patients in the standard arm and GO arm, respectively. GO was not added to induction therapy in 3 patients in the GO arm. The rates of observed adverse events categorized according to Common Terminology Criteria for Adverse Events during first induction therapy (Table 2) were in the expected range and severity of intensive induction chemotherapy15 and not different between the 2 treatment arms. After first induction therapy, 276 patients (93.2%) and 257 patients (88.0%) responded, including CR, CRi, and partial remission, and a second induction therapy was started in 258 and 239 patients in the standard arm and GO arm, respectively. GO was not applied in the second cycle in 15 patients mainly because of toxicity observed during the first induction cycle (n = 8). Overall response to induction therapy in the standard and GO arms was CR/CRi in 262 patients (88.5%) and 249 patients (85.3%), refractory disease in 16 patients (5.4%) and 12 patients (4.1%), and death during induction therapy in 17 patients (5.7%) and 30 patients (10.3%), respectively. There was no difference between the arms in CR/CRi rate (P = .27), but a higher death rate (P = .05) was observed in the GO arm. In particular, more patients > 70 years old died during induction therapy in the GO arm (20.4%, n = 10) compared with the standard arm (4%, n = 2; Appendix Table A1, online only).
TABLE 2.
AEs According to CTCAE (version 3.0) Category Occurring During First Induction Therapy According to Treatment With or Without GO
WBC recovery was faster in the GO arm after first induction therapy (median recovery, 23 days in GO arm v 24 days in standard arm; P = .003) and similar after second induction therapy (median recovery, 21 days in GO arm v 20 days in standard arm; P = .51). No difference in platelet recovery was noted after the first induction therapy (median recovery, 24 days in GO arm v 23 days in standard arm; P = .18). In contrast, platelet recovery was significantly prolonged after second induction therapy (median recovery, 25 days in GO arm v 20 days in standard arm; P < .001). In a Cox model stratified for dosage of etoposide (3 days v 2 days), factors associated with prolonged platelet recovery after second induction therapy were GO treatment (HR, 0.63; P < .001), age > 60 years (HR, 0.72; P = .002), female sex (HR, 0.62; P < .001), and prolonged platelet recovery after induction cycle 1 (HR, 0.58; P = .03).
Consolidation Therapy
For patients in CR/CRi after induction therapy, 3 cycles of consolidation therapy were intended. One, 2, and 3 cycles of consolidation therapy were applied in 19, 23, and 197 patients in the standard arm (total cycles, n = 656) and in 23, 16, and 172 patients in the GO arm (total cycles, n = 571), respectively. A higher number of consolidation therapy cycles was applied in the standard arm (83%, 656 of 786 cycles) compared with the GO arm (76%, 571 of 747 cycles). In the GO arm, 211 patients received first consolidation therapy. In 26 patients, GO was not added to first consolidation again mainly as a result of toxicity (n = 15) in previous treatment cycles. As with second induction therapy, platelet recovery in the GO arm after first consolidation therapy was significantly prolonged compared with the standard arm (median recovery, 23 v 19 days, respectively; P < .001). Allogeneic hematopoietic cell transplantation was performed in first CR/CRi in 48 patients (24 in each treatment arm) after first (n = 2) and second induction therapy (n = 13) and after first (n = 19), second (n = 7), and third (n = 7) consolidation therapy.
Survival Analysis
Data were locked as of September 28, 2018. The median follow-up time was 40.0 months (95% CI, 35.5 to 47.0). Median EFS time and 2-year EFS rate defined according to ELN 2017 recommendations were 39.4 months (95% CI, 27.1 to 59.2 months) and 55.3% (95% CI, 51.3% to 59.7%), respectively. Of 588 randomly assigned patients, 523 achieved a first CR/CRi; of these, 178 experienced relapse, and 48 died in CR/CRi.
Univariable survival analyses (age stratified: ≤ v > 60 years) on an intent-to-treat basis revealed no significant differences in EFS defined according to 2017 ELN criteria (HR, 0.83; 95% CI, 0.65 to 1.04; P = .10; Fig 2) and according to protocol (HR, 0.81; 95% CI, 0.65 to 1.01; P = .05). The 2-year EFS rates were 52.6% (95% CI, 47.0% to 58.9%) and 58.1% (95% CI, 52.5% to 64.4%) in the standard and GO arms, respectively.
FIG 2.
Kaplan-Meier plot illustrating event-free survival defined according to European LeukemiaNet 2017 recommendations in the gemtuzumab ozogamicin (GO) arm and standard arm as randomized (age-stratified hazard ratio [HR], 0.83; 95% CI, 0.65 to 1.04; P = .10). Overall, 289 events were observed (standard arm, n = 157; GO arm, n = 132).
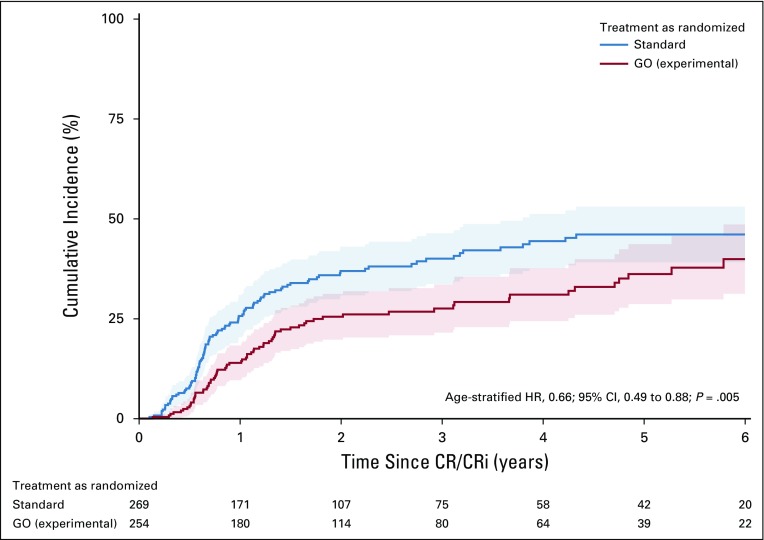
In patients achieving CR/CRi within the protocol, CIR was significantly higher in the standard arm compared with the GO arm (P = .005, age stratified; Fig 3; Appendix Fig A1, online only), with CIR rates at 2 years of 36.9% (95% CI, 30.8% to 43.0%) and 25.5% (95% CI, 19.7% to 31.2%), respectively. No difference was observed for CID (P = .80), with rates at 2 years of 7.1% (95% CI, 3.9% to 10.3%) and 8.3% (95% CI, 1.8% to 11.8%) in the standard and GO arms, respectively.
FIG 3.
Cumulative incidence plot illustrating cumulative incidence of relapse (CIR) in the gemtuzumab ozogamicin (GO) arm and standard arm as randomized (age-stratified hazard ratio [HR], 0.66; 95% CI, 0.49 to 0.88; P = .005). CR, complete remission; CRi, complete remission with incomplete hematologic recovery.
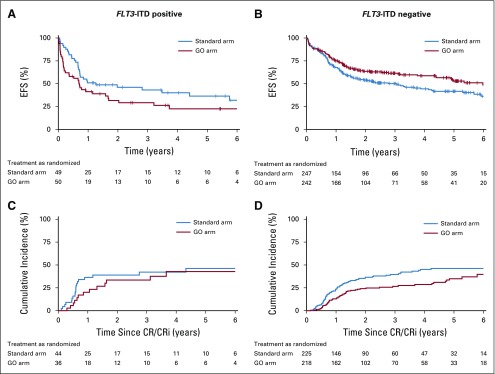
Subset analysis revealed substantial heterogeneity for the end points of EFS and CIR. In this analysis, CD33 expression was not included because it was not systematically assessed within the study. There was a strong interaction between sex and treatment arm (P = .03). Female patients had significantly improved EFS (HR, 0.67; 95% CI, 0.49 to 0.92) with the addition of GO (Fig 4; Appendix Fig A2, online only), whereas this was not seen in male patients (HR for EFS, 1.08; 95% CI, 0.77 to 1.51). Similar results were observed for CIR (Appendix Fig A1). Furthermore, the interaction between FLT3-ITD and treatment arm was significant (P = .002) in that patients with concurrent presence of FLT3-ITD did not benefit from the addition of GO in terms of EFS (HR, 1.53; 95% CI, 0.95 to 2.48), whereas patients without FLT3-ITD did benefit (HR, 0.72; 95% CI, 0.56 to 0.95; Fig 4; Appendix Fig A3, online only). Finally, older patients (age > 70 years) did not benefit from GO with regard to EFS (HR, 1.22; 95% CI, 0.76 to 1.95) and CIR (HR, 1.01; 95% CI, 0.55 to 1.86). In contrast, an explorative post hoc analysis in younger patients (age ≤ 70 years) revealed significantly improved EFS defined according to 2017 ELN recommendations (P = .05) and according to protocol (P = .02) with the addition of GO (Appendix Fig A4, online only).
FIG 4.
Forest plot of cause-specific proportional hazards models for event-free survival. DNMT3A, DNA methyltransferase 3A; FLT3, FMS-like tyrosine kinase 3 gene; GO, gemtuzumab ozogamicin; HR, hazard ratio; ITD, internal tandem duplication; LQ, lower quartile; s-AML, secondary acute myeloid leukemia after previous myelodysplastic syndrome or myeloproliferative neoplasm; t-AML, therapy-related acute myeloid leukemia; TKD, tyrosine kinase domain; UQ, upper quartile.
DISCUSSION
After the approval of GO by the FDA and EMA for patients with CD33-positive AML, our randomized trial is, to our knowledge, the first to focus on the distinct WHO 2016 disease entity of AML with mutated NPM1. Consistent with previous reports, GO did not improve the response rate after induction therapy.27 On the contrary, we observed a higher death rate in the GO arm. A higher death rate death rate was also observed in a previous trial, in which GO was added to induction therapy at day 4 in a dosage of 6 mg/m2 in adult patients up to the age of 60 years.28 On the basis of a meta-analysis, GO dosages > 3 mg/m2 per application were associated with a higher early mortality rate.27 Although we adhered to the 3 mg/m2 dosage without capping the dose at 5 mg, we observed a mortality rate of up to 20% in patients older than 70 years. In addition, we combined GO with the intensive ATRA-ICE regimen, which may have contributed further to an unfavorable safety evaluation.
By design of our study, EFS as early end point was analyzed 6 months after the last patient was randomly assigned. The trial failed to show a significant improvement in EFS, although the 2-year EFS was numerically improved from 52.6% (95% CI, 47.0% to 58.9%) to 58.1% (95% CI, 52.5% to 64.4%). Reasons for this lack of benefit are, among others, the higher early mortality rate in the GO arm in older patients and the better outcome overall in both arms compared with the available historical data at the time of sample size planning with an estimated 2-year EFS of 37%. An explorative post hoc analysis of EFS in younger patients (age ≤ 70 years) revealed improved EFS defined according to 2017 ELN recommendations (P = .05), indicating a better risk-benefit profile of GO in younger patients.
CIR was significantly reduced by the addition of GO. This observation is in line with previous reports17,27 in the total trial population as well as in subset analysis focusing on AML with mutated NPM1.17 On the basis of subgroup effect estimates, we identified good efficacy in female patients with an HR for CIR of 0.49 (95% CI, 0.32 to 0.74) and an HR for EFS of 0.67 (95% CI, 0.49 to 0.92), whereas in male patients, no significant effect was present for CIR (HR, 0.91; 95% CI, 0.59 to 1.41) or EFS (HR, 1.08; 95% CI, 077 to 1.51). This observation is in contrast to the ALFA 0701 study using fractionated dosing and the meta-analysis in which comparable effects were reported for both sexes.17,27 Of note, similar sex-specific differences were also noted in a large randomized study in older patients.29 In this study, patients were randomly assigned to GO 6 mg/m2 on day 1 and 3 mg/m2 on day 8 or to best supportive therapy. The improvement in OS was unequally distributed in women (HR, 0.53; 95% CI, 0.35 to 0.79) and men (HR, 0.90; 95% CI, 0.63 to 1.28). Whether these sex-specific differences are a result of differences in antibody-drug conjugate clearance in men and women previously reported for rituximab30 remains elusive. Another subset of patients who did not benefit from the addition of GO with respect to relapse risk reduction were patients older than age 70 years. This may be attributed to age-specific factors, independent of prognostic factors such as the NPM1 genotype31 and the intensive induction therapy regimen (ATRA-ICE) used in this study.
Consistent with previous reports,16,17 we observed a substantial prolongation of platelet recovery after repeated GO exposure in second induction and first consolidation therapy, leading to omission of GO in 15 and 26 patients, respectively. In contrast, no difference between treatment arms in adverse events and hematologic recovery in first induction therapy was noticed. On the background of lack of efficacy of GO in postremission therapy,32 this argues for restriction of GO to first induction therapy. In summary, our study failed to show a significant benefit in EFS by the addition of GO to intensive therapy in patients with NPM1-mutated AML. There was a significant reduction in CIR with GO, providing evidence for antileukemic activity of the antibody-drug conjugate.
ACKNOWLEDGMENT
We are grateful to all members of the German-Austrian Acute Myeloid Leukemia Study Group (AMLSG) for providing leukemia clinical data and specimens.
Appendix
Inclusion and Exclusion Criteria
Diagnoses included de novo acute myeloid leukemia (AML), secondary AML with a preceding history of myelodysplastic syndrome or myeloproliferative neoplasms, and therapy-related AML. Patients with acute promyelocytic leukemia and core-binding factor AML; patients with concomitant renal (creatinine > 1.5 × upper normal serum level), liver (AST or ALT > 2.5 × upper normal serum level), or cardiac dysfunction (New York Heart Association class III or IV); and patients with uncontrolled infection, primary coagulation disturbance, or Eastern Cooperative Oncology Group performance status > 2 were excluded. Written informed consent was obtained from all patients.
Definition of Response Criteria and Hematologic Recovery
In accordance with standard criteria, complete remission (CR) was defined as < 5% bone marrow blasts, an absolute neutrophil count of ≥ 1.0 × 10e9/L, a platelet count of ≥ 100 × 10e9/L, no blasts in the peripheral blood, and no extramedullary leukemia. CR with incomplete hematologic recovery was defined as CR except for residual neutropenia (neutrophils < 1.0 × 10e9/L) or thrombocytopenia (platelets < 100 × 10e9/L).9 Partial remission was defined by all hematologic criteria of CR, decrease of bone marrow blast percentage to 5%-25%, and decrease of pretreatment bone marrow blast percentage by at least 50% and no extramedullary leukemia.9 Relapse was defined as > 5% bone marrow blasts unrelated to recovery from the preceding course of chemotherapy or new extramedullary leukemia in patients with previously documented CR.
Times to WBC, neutrophil, and platelet recovery were measured from the first day of chemotherapy of each cycle until the first day with unsupported values ≥ 1, ≥ 0.5, and ≥ 20 × 10e9/L for WBC, neutrophils, and platelets, respectively. Toxicities were graded according to the National Cancer Institute Common Toxicity Criteria, version 3.0.
Genetic Analyses
Screening for NPM1 mutations was performed in 2 reference laboratories at Ulm University Hospital and Hannover Medical School.2 Leukemia samples were also analyzed for FLT3 internal tandem duplication, mutations in FLT3 tyrosine kinase domain at codons D835 and I836, and mutations in DNMT3A, as previously described (Gaidzik VI, et al: Blood 121:4769-4777, 2013).2,3 Karyotypes were designated according to the International System for Human Cytogenetic Nomenclature (Mitelman F: Basel, Switzerland, Karger, 1995).
FIG A1.
Forest plot of cause-specific proportional hazards models for cumulative incidence of relapse. DNMT3A, DNA methyltransferase 3A; FLT3, FMS-like tyrosine kinase 3 gene; GO, gemtuzumab ozogamicin; HR, hazard ratio; ITD, internal tandem duplication; LQ, lower quartile; s-AML, secondary acute myeloid leukemia after previous myelodysplastic syndrome or myeloproliferative neoplasm; t-AML, therapy-related acute myeloid leukemia; TKD, tyrosine kinase domain; UQ, upper quartile.
FIG A2.
Kaplan-Meier plots illustrating (A and B) event-free survival (EFS) defined according to European LeukemiaNet 2017 recommendations and (C and D) cumulative incidence plots according to sex in the GO arm and standard arm as randomized. CR, complete remission; CRi, complete remission with incomplete hematologic recovery.
FIG A3.
Kaplan-Meier plots illustrating (A and B) event-free survival (EFS) defined according to European LeukemiaNet 2017 recommendations and (C and D) cumulative incidence plots according to FLT3 internal tandem duplication (ITD) status in the GO arm and standard arm as randomized. CR, complete remission; CRi, complete remission with incomplete hematologic recovery.
FIG A4.
Kaplan-Meier plots illustrating event-free survival (EFS) defined (A) according to 2017 European LeukemiaNet (ELN) recommendations and (B) according to protocol in younger patients (≤ 70 years old) in the GO arm and standard arm as randomized.
TABLE A1.
Response to Induction Therapy

SUPPORT
Supported by Grant No. P80/08//A65/08 of the Else-Kröner-Fresenius Stiftung and an unrestricted grant from Pfizer and Amgen. Presented in part at the 60th Annual Meeting and Exposition of the American Society of Hematology, San Diego, CA, December 1-4, 2018.
AUTHOR CONTRIBUTIONS
Conception and design: Richard F. Schlenk, Hartmut Döhner
Financial support: Richard F. Schlenk, Hartmut Döhner
Administrative support: Richard F. Schlenk, Claudia Leis, Daniela Weber, Hartmut Döhner
Provision of study materials or patients: All authors
Collection and assembly of data: All authors
Data analysis and interpretation: Richard F. Schlenk, Julia Krzykalla, Axel Benner, Hartmut Döhner
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Gemtuzumab Ozogamicin in NPM1-Mutated Acute Myeloid Leukemia: Early Results From the Prospective Randomized AMLSG 09-09 Phase III Study
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/journal/jco/site/ifc.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Richard F. Schlenk
Consulting or Advisory Role: Daiichi Sankyo, Pfizer
Speakers' Bureau: Pfizer, Daiichi Sankyo, Novartis
Research Funding: PharmaMar, AstraZeneca, Pfizer, Daiichi Sankyo
Travel, Accommodations, Expenses: Daiichi Sankyo
Peter Paschka
Consulting or Advisory Role: Agios, Astex Pharmaceuticals, Astellas Pharma, Celgene, Jazz Pharmaceuticals, Novartis, Otsuka, Pfizer, Sunesis Pharmaceuticals
Speakers' Bureau: Astellas Pharma, Agios, Jazz Pharmaceuticals, Novartis, Pfizer, Jazz Pharmaceuticals (Inst)
Travel, Accommodations, Expenses: AbbVie, Amgen, Celgene, Janssen Oncology
Silke Kapp-Schwoerer
Honoraria: Jazz Pharmaceuticals
Travel, Accommodations, Expenses: Jazz Pharmaceuticals
Walter Fiedler
Consulting or Advisory Role: Amgen, ARIAD, Pfizer, Novartis, Jazz Pharmaceuticals, Celgene, AbbVie
Research Funding: Amgen
Patents, Royalties, Other Intellectual Property: Patent on immunotherapy in acute myeloid leukemia obtained together with Amgen
Travel, Accommodations, Expenses: Amgen, Daiichi Sankyo, Jazz Pharmaceuticals, SERVIER
Thomas Kindler
Other Relationship: AbbVie
Thomas Schroeder
Consulting or Advisory Role: Pfizer
Karin Mayer
Stock and Other Ownership Interests: Bristol-Myers Squibb, Roche Pharma AG (I)
Michael Lübbert
Consulting or Advisory Role: Hexal (Inst), Pfizer (Inst)
Research Funding: Johnson & Johnson (Inst), Teva (Inst), Cheplapharm (Inst)
Travel, Accommodations, Expenses: Astex (Inst)
Katharina Götze
Honoraria: Celgene, Janssen-Cilag, Novartis
Consulting or Advisory Role: Jazz Pharmaceuticals, AbbVie
Heinz A. Horst
Stock and Other Ownership Interests: Novartis
Consulting or Advisory Role: Pfizer, Amgen, Jazz Pharmaceutical
Speakers' Bureau: Celgene
Research Funding: Amgen, Regeneron
Travel, Accommodations, Expenses: Amgen, Jazz Pharmaceutical, Janssen-Cilag, Celgene
Elisabeth Koller
Consulting or Advisory Role: AbbVie, Celgene, Novartis, Astellas Pharma
Gerald Wulf
Consulting or Advisory Role: Gilead Sciences, Novartis
Speakers' Bureau: Takeda
Jan Schleicher
Honoraria: Celgene, Janssen-Cilag, Pfizer, Ipsen
Consulting or Advisory Role: Pfizer (I), Ipsen, Takeda, MSD, Novartis
Research Funding: Bristol-Myers Squibb (Inst), Essai (Inst), AstraZeneca (Inst), Novartis (Inst)
Martin Bentz
Consulting or Advisory Role: Roche, AbbVie, Janssen Oncology
Travel, Accommodations, Expenses: AbbVie
Richard Greil
Honoraria: Celgene, Roche, Merck, Takeda, AstraZeneca, Novartis, Amgen, Bristol-Myers Squibb, MSD, Sandoz, AbbVie, Gilead, Daiichi Sankyo
Consulting or Advisory Role: Celgene, Novartis, Roche, Bristol-Myers Squibb, Takeda, AbbVie, AstraZeneca, Janssen, MSD, Merck, Gilead, Daiichi Sankyo
Research Funding: Celgene (Inst), Merck (Inst), Takeda (Inst), AstraZeneca (Inst), Novartis (Inst), Amgen (Inst), Bristol-Myers Squibb (Inst), MSD (Inst), Sandoz (Inst), Gilead (Inst), Roche (Inst)
Travel, Accommodations, Expenses: Roche, Amgen, Janssen-Cilag, AstraZeneca, Novartis, MSD, Celgene, Gilead, Bristol-Myers Squibb
Bernd Hertenstein
Honoraria: Novartis, Janssen-Cilag
Travel, Accommodations, Expenses: Amgen, Ipsen, Novartis
Jürgen Krauter
Honoraria: Pfizer, Daiichi Sankyo, Astellas Pharma, Bristol-Myers Squibb, Amgen
Consulting or Advisory Role: Pfizer, Astellas Pharma, Amgen, Daiichi Sankyo, Bristol-Myers Squibb
Travel, Accommodations, Expenses: Daiichi Sankyo, Amgen
Uwe Martens
Consulting or Advisory Role: MSD Oncology, Roche, Bristol-Myers Squibb, Celgene
Travel, Accommodations, Expenses: Amgen, Bristol-Myers Squibb, Celgene
Maisun Abu Samra
Honoraria: Novartis
Travel, Accommodations, Expenses: AbbVie
Michael Girschikofsky
Honoraria: Kite/Gilead, Novartis
Consulting or Advisory Role: Janssen, Roche
Travel, Accommodations, Expenses: Pfizer
Axel Benner
Travel, Accommodations, Expenses: Sanofi
Felicitas Thol
Honoraria: Novartis
Consulting or Advisory Role: AbbVie, Daiichi Sankyo
Michael Heuser
Honoraria: Novartis, Pfizer
Consulting or Advisory Role: Novartis, Pfizer, Janssen Oncology, Stemline Therapeutics
Research Funding: Pfizer (Inst), Daiichi Sankyo (Inst), BerGenBio (Inst), Bayer (Inst), Novartis (Inst), Astellas Pharma (Inst)
Konstanze Döhner
Honoraria: Novartis, Jazz Pharmaceutical, Celgene, Daiichi Sankyo
Consulting or Advisory Role: CTI BioPharma, Celgene, Daiichi Sankyo, Novartis, Janssen, Roche, Amgen
Research Funding: Novartis (Inst), Celgene (Inst)
Hartmut Döhner
Honoraria: Celgene, AbbVie, Jazz Pharmaceuticals, Novartis
Consulting or Advisory Role: AbbVie, Agios, Amgen, Astellas Pharma, Astex Pharmaceuticals, Celgene, Jazz Pharmaceuticals, Roche
Research Funding: Arog (Inst), Amgen (Inst), Bristol-Myers Squibb (Inst), Novartis (Inst), Pfizer (Inst), Sunesis Pharmaceuticals (Inst), Jazz Pharmaceuticals (Inst)
No other potential conflicts of interest were reported.
REFERENCES
- 1.Falini B, Mecucci C, Tiacci E, et al. Cytoplasmic nucleophosmin in acute myelogenous leukemia with a normal karyotype. N Engl J Med. 2005;352:254–266. doi: 10.1056/NEJMoa041974. [DOI] [PubMed] [Google Scholar]
- 2.Schlenk RF, Döhner K, Krauter J, et al. Mutations and treatment outcome in cytogenetically normal acute myeloid leukemia. N Engl J Med. 2008;358:1909–1918. doi: 10.1056/NEJMoa074306. [DOI] [PubMed] [Google Scholar]
- 3.Schlenk RF, Döhner K, Kneba M, et al. Gene mutations and response to treatment with all-trans retinoic acid in elderly patients with acute myeloid leukemia: Results from the AMLSG Trial AML HD98B. Haematologica. 2009;94:54–60. doi: 10.3324/haematol.13378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nagel G, Weber D, Fromm E, et al. Epidemiological, genetic, and clinical characterization by age of newly diagnosed acute myeloid leukemia based on an academic population-based registry study (AMLSG BiO) Ann Hematol. 2017;96:1993–2003. doi: 10.1007/s00277-017-3150-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bullinger L, Döhner K, Döhner H. Genomics of acute myeloid leukemia diagnosis and pathways. J Clin Oncol. 2017;35:934–946. doi: 10.1200/JCO.2016.71.2208. [DOI] [PubMed] [Google Scholar]
- 6.Vardiman JW, Thiele J, Arber DA, et al. The 2008 revision of the World Health Organization (WHO) classification of myeloid neoplasms and acute leukemia: Rationale and important changes. Blood. 2009;114:937–951. doi: 10.1182/blood-2009-03-209262. [DOI] [PubMed] [Google Scholar]
- 7.De Propris MS, Raponi S, Diverio D, et al. High CD33 expression levels in acute myeloid leukemia cells carrying the nucleophosmin (NPM1) mutation. Haematologica. 2011;96:1548–1551. doi: 10.3324/haematol.2011.043786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ehninger A, Kramer M, Röllig C, et al. Distribution and levels of cell surface expression of CD33 and CD123 in acute myeloid leukemia. Blood Cancer J. 2014;4:e218. doi: 10.1038/bcj.2014.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Döhner H, Estey E, Grimwade D, et al. Diagnosis and management of AML in adults: 2017 ELN recommendations from an international expert panel. Blood. 2017;129:424–447. doi: 10.1182/blood-2016-08-733196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. National Comprehensive Care Network: NCCN guidelines. https://www.nccn.org/professionals/physician_gls/default.aspx.
- 11.Tassara M, Döhner K, Brossart P, et al. Valproic acid in combination with all-trans retinoic acid and intensive therapy for acute myeloid leukemia in older patients. Blood. 2014;123:4027–4036. doi: 10.1182/blood-2013-12-546283. [DOI] [PubMed] [Google Scholar]
- 12.Becker H, Marcucci G, Maharry K, et al. Favorable prognostic impact of NPM1 mutations in older patients with cytogenetically normal de novo acute myeloid leukemia and associated gene- and microRNA-expression signatures: A Cancer and Leukemia Group B study. J Clin Oncol. 2010;28:596–604. doi: 10.1200/JCO.2009.25.1496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Büchner T, Berdel WE, Haferlach C, et al. Age-related risk profile and chemotherapy dose response in acute myeloid leukemia: A study by the German Acute Myeloid Leukemia Cooperative Group. J Clin Oncol. 2009;27:61–69. doi: 10.1200/JCO.2007.15.4245. [DOI] [PubMed] [Google Scholar]
- 14.Röllig C, Thiede C, Gramatzki M, et al. A novel prognostic model in elderly patients with acute myeloid leukemia: Results of 909 patients entered into the prospective AML96 trial. Blood. 2010;116:971–978. doi: 10.1182/blood-2010-01-267302. [DOI] [PubMed] [Google Scholar]
- 15.Schlenk RF, Lübbert M, Benner A, et al. All-trans retinoic acid as adjunct to intensive treatment in younger adult patients with acute myeloid leukemia: Results of the randomized AMLSG 07-04 study. Ann Hematol. 2016;95:1931–1942. doi: 10.1007/s00277-016-2810-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lambert J, Pautas C, Terré C, et al. Gemtuzumab ozogamicin for de novo acute myeloid leukemia: Final efficacy and safety updates from the open-label, phase III ALFA-0701 trial. Haematologica. 2019;104:113–119. doi: 10.3324/haematol.2018.188888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Castaigne S, Pautas C, Terré C, et al. Effect of gemtuzumab ozogamicin on survival of adult patients with de-novo acute myeloid leukaemia (ALFA-0701): A randomised, open-label, phase 3 study. Lancet. 2012;379:1508–1516. doi: 10.1016/S0140-6736(12)60485-1. [DOI] [PubMed] [Google Scholar]
- 18.Schlenk RF, Weber D, Fiedler W, et al. Midostaurin added to chemotherapy and continued single-agent maintenance therapy in acute myeloid leukemia with FLT3-ITD. Blood. 2019;133:840–851. doi: 10.1182/blood-2018-08-869453. [DOI] [PubMed] [Google Scholar]
- 19.Jaramillo S, Benner A, Krauter J, et al. Condensed versus standard schedule of high-dose cytarabine consolidation therapy with pegfilgrastim growth factor support in acute myeloid leukemia. Blood Cancer J. 2017;7:e564. doi: 10.1038/bcj.2017.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wiens BL. A fixed sequence Bonferroni procedure for testing multiple endpoints. Pharm Stat. 2003;2:211–215. [Google Scholar]
- 21.Gray RJ. A class of k-sample tests for comparing the cumulative incidence of a competing risk. Ann Stat. 1988;16:1141–1154. [Google Scholar]
- 22.Schemper M, Smith TL. A note on quantifying follow-up in studies of failure time. Control Clin Trials. 1996;17:343–346. doi: 10.1016/0197-2456(96)00075-x. [DOI] [PubMed] [Google Scholar]
- 23.Kaplan E, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 24.Aalen O, Johansen S. An empirical transition matrix for non-homogeneous Markov chains based on censored observations. Scand J Stat. 1978;5:141–150. [Google Scholar]
- 25.Tian L, Alizadeh AA, Gentles AJ, et al. A simple method for estimating interactions between a treatment and a large number of covariates. J Am Stat Assoc. 2014;109:1517–1532. doi: 10.1080/01621459.2014.951443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.R Development Core Team . R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing; 2009. [Google Scholar]
- 27.Hills RK, Castaigne S, Appelbaum FR, et al. Addition of gemtuzumab ozogamicin to induction chemotherapy in adult patients with acute myeloid leukaemia: A meta-analysis of individual patient data from randomised controlled trials. Lancet Oncol. 2014;15:986–996. doi: 10.1016/S1470-2045(14)70281-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Petersdorf SH, Kopecky KJ, Slovak M, et al. A phase 3 study of gemtuzumab ozogamicin during induction and postconsolidation therapy in younger patients with acute myeloid leukemia. Blood. 2013;121:4854–4860. doi: 10.1182/blood-2013-01-466706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Amadori S, Suciu S, Selleslag D, et al. Gemtuzumab ozogamicin versus best supportive care in older patients with newly diagnosed acute myeloid leukemia unsuitable for intensive chemotherapy: Results of the randomized phase III EORTC-GIMEMA AML-19 trial. J Clin Oncol. 2016;34:972–979. doi: 10.1200/JCO.2015.64.0060. [DOI] [PubMed] [Google Scholar]
- 30.Müller C, Murawski N, Wiesen MH, et al. The role of sex and weight on rituximab clearance and serum elimination half-life in elderly patients with DLBCL. Blood. 2012;119:3276–3284. doi: 10.1182/blood-2011-09-380949. [DOI] [PubMed] [Google Scholar]
- 31.Ostronoff F, Othus M, Lazenby M, et al. Prognostic significance of NPM1 mutations in the absence of FLT3-internal tandem duplication in older patients with acute myeloid leukemia: A SWOG and UK National Cancer Research Institute/Medical Research Council report. J Clin Oncol. 2015;33:1157–1164. doi: 10.1200/JCO.2014.58.0571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schlenk RF, Jaramillo S, Müller-Tidow C. What’s new in consolidation therapy in AML? Semin Hematol. 2019;56:96–101. doi: 10.1053/j.seminhematol.2018.08.005. [DOI] [PubMed] [Google Scholar]