Abstract
PURPOSE
In this phase I study (BLOOM), osimertinib, a third-generation epidermal growth factor receptor (EGFR) tyrosine kinase inhibitor (TKI), was evaluated in patients with leptomeningeal metastases (LMs) from EGFR-mutated (EGFRm) advanced non–small-cell lung cancer (NSCLC) whose disease had progressed on previous EGFR-TKI therapy.
PATIENTS AND METHODS
Patients with cytologically confirmed LM received osimertinib 160 mg once daily. Objectives were to assess confirmed objective response rate (ORR), duration of response (DoR), progression-free survival (PFS), overall survival (OS), pharmacokinetics (PK), and safety. Additional efficacy evaluations included changes from baseline in CSF cytology and neurologic examination. Measurable lesions were assessed by investigator according to RECIST version 1.1. LMs were assessed by neuroradiologic blinded central independent review (BICR) according to Response Assessment in Neuro-Oncology LM radiologic criteria and by investigator.
RESULTS
Forty-one patients were enrolled. LM ORR and DoR by neuroradiologic BICR were 62% (95% CI, 45% to 78%) and 15.2 months (95% CI, 7.5 to 17.5 months), respectively. Overall, ORR by investigator was 41% (95% CI, 26% to 58%), and median DoR was 8.3 months (95% CI, 5.6 to 16.5 months). Median investigator-assessed PFS was 8.6 months (95% CI, 5.4 to 13.7 months) with 78% maturity; median OS was 11.0 months (95% CI, 8.0 to 18.0 months) with 68% maturity. CSF tumor cell clearance was confirmed in 11 (28%; 95% CI, 15% to 44%) of 40 patients. Neurologic function was improved in 12 (57%) of 21 patients with an abnormal assessment at baseline. The adverse event and PK profiles were consistent with previous reports for osimertinib.
CONCLUSION
Osimertinib showed meaningful therapeutic efficacy in the CNS and a manageable safety profile at 160 mg once daily in patients with EGFRm NSCLC and LM.
INTRODUCTION
Leptomeningeal metastases (LMs), the spread of tumor cells into the leptomeninges and CSF, occurs in approximately 3%-4% of patients with advanced non–small-cell lung cancer (NSCLC).1,2 The incidence of LM rises to approximately 9% in epidermal growth factor receptor-mutated (EGFRm) NSCLC.2,3 Survival for patients with NSCLC and LM is dismal, with a median overall survival (OS) of 3-10 months from diagnosis.2,4,5
Several therapeutic options, including whole-brain radiotherapy (WBRT) and intrathecal chemotherapy, have been applied to manage LM. However, WBRT has limited antitumor activity,6-8 and intrathecal chemotherapy is associated with significant toxicity and complications.9 More recently, EGFR tyrosine kinase inhibitors (TKIs) have shown potential as a treatment option for patients with EGFRm NSCLC and LM; however, results are difficult to interpret because most studies to date have been retrospective,1,2,4,10 and limited data exist from prospective studies.11 In 2017, the Response Assessment in Neuro-Oncology LM (RANO-LM) working group developed a proposal for evaluating responses in patients with LM.12 However, the RANO-LM criteria have not yet been validated, and standardization currently is lacking with respect to LM response criteria (clinical, neuroimaging, and CSF analysis).12,13
Osimertinib, a third-generation, irreversible, EGFR-TKI, is an approved treatment option (80 mg once daily) for patients with EGFRm advanced NSCLC and for patients with T790M-positive NSCLC after disease progression on EGFR-TKIs.14,15 Although osimertinib is a substrate for the permeability glycoprotein and breast cancer resistance protein efflux transporters, in vitro data have shown that unlike other EGFR-TKIs, the permeability of osimertinib is sufficient to overcome this efflux.16 Preclinical evidence in nonhuman primates shows that osimertinib has greater penetration of the blood-brain barrier and higher brain exposure compared with other EGFR-TKIs.16,17 In a positron emission tomography (PET) study of healthy human volunteers, a single microdose of [11C]osimertinib demonstrated rapid and widespread distribution in the brain.18 From this phase I study, we report the antitumor efficacy, pharmacokinetics (PK), and safety of osimertinib 160 mg once daily in patients with EGFRm NSCLC and LM whose disease had progressed on previous EGFR-TKI therapy. The higher 160 mg dose was chosen for this study because limited data with regard to exposure versus response were available at the time of study initiation, treatment of patients with LM has been challenging with other EGFR-TKIs, and early preclinical data16,19 suggest that a higher exposure of osimertinib may increase CNS tumor shrinkage.
PATIENTS AND METHODS
Study Design and Patients
The BLOOM study (ClinincalTrials.gov identifier: NCT02228369) was a 2-part, phase I, open-label, multicenter study in patients with EGFRm NSCLC. Part A, which comprised a dose-escalation and dose-expansion phase, investigated the primary study outcome: the safety and tolerability of AZD3759 in patients with EGFRm NSCLC, the results of which have been published.20 Part B, reported here, assessed osimertinib 160 mg once daily (without a dose-escalation phase) in patients with EGFRm NSCLC and LM whose disease had progressed on EGFR-TKI therapy. Objectives included antitumor efficacy, OS, neurologic status, the PK of osimertinib and its metabolites in blood and CSF, EGFRm copy number in CSF, and safety.
Patients were enrolled sequentially into 2 cohorts: unselected and T790M positive (see the Data Supplement, online only, for details). Key inclusion criteria were ≥ 18 years of age with a histologically or cytologically confirmed diagnosis of NSCLC and exon 19 deletions or L858R mutations, confirmed LM diagnosis by positive CSF cytology (up to 28 days before first dosing), ≥ 1 LM site that could be assessed repeatedly by magnetic resonance imaging (MRI), progression on EGFR-TKI therapy, and WHO/Eastern Cooperative Oncology Group (ECOG) performance status of 0-2. For additional inclusion and exclusion criteria and ethical conduct of the study, see the Data Supplement.
Treatment and Assessments
Osimertinib 160 mg was administered orally once daily to all patients until disease progression or unmanageable drug-related toxicity. Patients were permitted to continue study treatment beyond disease progression, as defined by RECIST version 1.1 (RECIST 1.1), as long as they were considered by the investigator to be deriving clinical benefit and tolerating treatment.
Before the first dosing of osimertinib, plasma and CSF samples were obtained from patients for assessment of EGFR mutations. EGFR mutation status was determined retrospectively at central and local laboratories using droplet digital polymerase chain reaction.21 Radiologic assessments were performed at baseline and every 6 weeks ± 1 week until progression or patient withdrawal. Required scans included computed tomography or MRI of the chest and abdomen and MRI of the brain and spine. Non-CNS lesions and measurable CNS lesions were assessed by investigator according to modified RECIST 1.1. Investigators assessed LM response using their institutional standard (most often RECIST 1.1); however, a quantitative measurement of LM cannot be accurately performed using RECIST. Therefore, LMs were also assessed by neuroradiologic blinded central independent review (BICR), either in parallel or retrospectively with other reviews, according to RANO-LM working group criteria (see Data Supplement).12 For CSF response assessment (100% CSF clearance of tumor cells), CSF was taken at baseline, day 22, and every 6 weeks ± 1 week until progression; responses were confirmed at least 4 weeks after initial CSF clearance.
Patients underwent a detailed neurologic examination by investigator to document neurologic status at baseline and every 21 days until discontinuation (Data Supplement). Safety was assessed by investigator throughout the study. Adverse events (AEs) were classified according to the Medical Dictionary for Regulatory Activities version 20.0 and graded using Common Terminology Criteria for Adverse Events (CTCAE), version 4.0. For patients who discontinued treatment for reasons other than disease progression, RECIST 1.1 assessments and/or CSF cytology assessments were performed by investigator every 6 weeks until disease progression. Survival was evaluated every 6 weeks after discontinuation of study treatment. Details of PK sample collection and analysis are provided in the Data Supplement.
Statistical Procedure
A sample size of up to approximately 40 patients was determined to provide an 80% chance of observing at least 1 incidence of a safety signal that occurs in 4% of patients in the target population. In addition, on the basis of 20 efficacy responses out of 40, the 2-sided 80% CI for the true objective response rate (ORR) would be 39% to 61%. Details of end point definitions and analysis sets are provided in the Data Supplement.
Estimated time-to-event end points (progression-free survival [PFS], duration of response [DoR], and OS) and associated 95% CIs were calculated using the Kaplan-Meier method. ORR 95% CIs were calculated using the Clopper-Pearson method. Data obtained up until progression, or last evaluation in the absence of progression, were included in the ORR assessment. No formal statistical analyses were conducted on the safety and PK data; these were summarized using appropriate descriptive statistics.
RESULTS
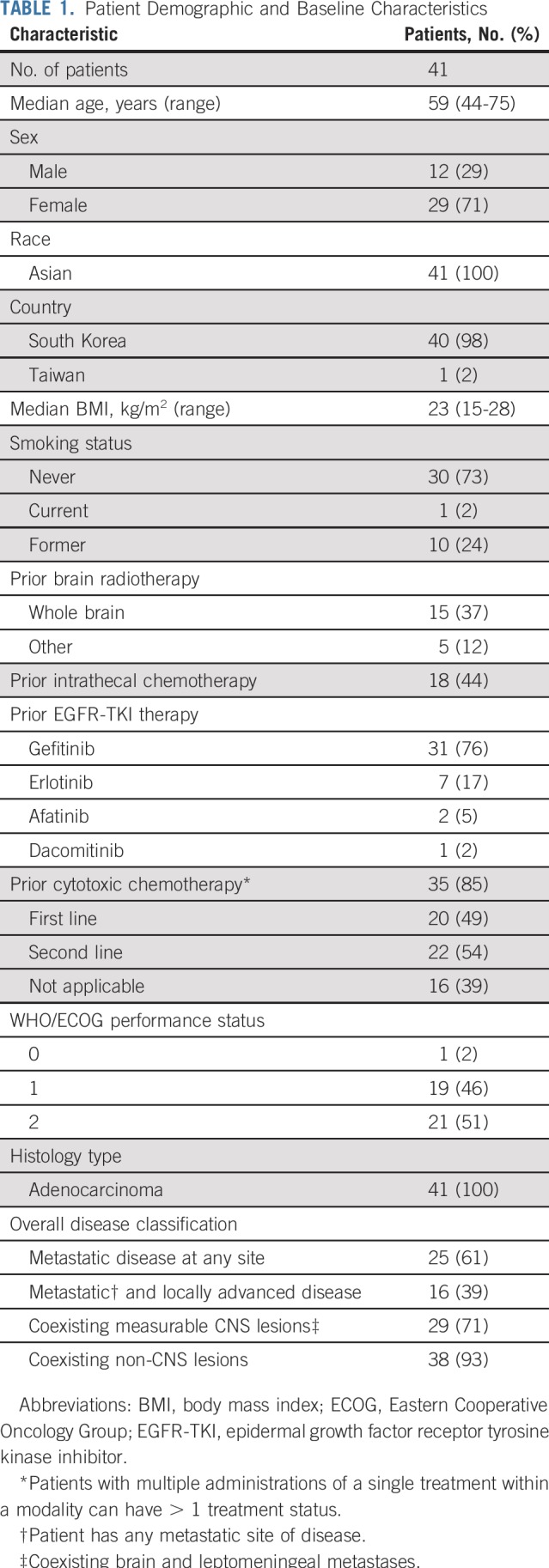
Patients
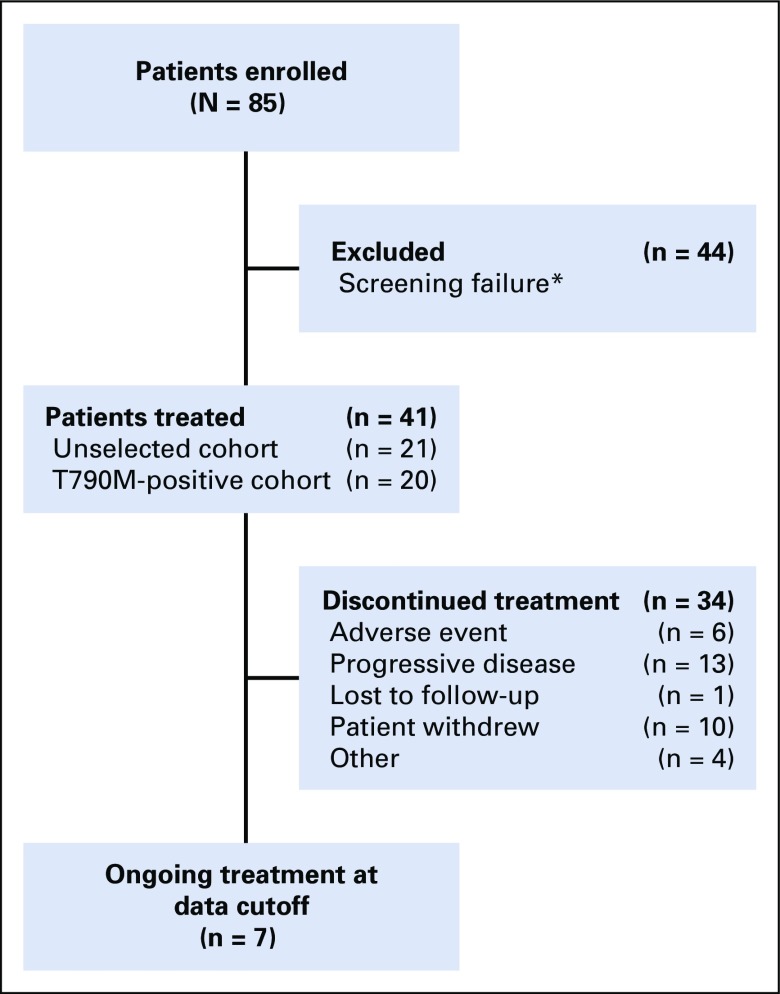
Between April 2015 and October 2017, 41 patients were enrolled sequentially and treated with osimertinib 160 mg once daily (Fig 1). Patients were enrolled at 5 sites in South Korea and 1 site in Taiwan. All patients were Asian; most were female (71%) with a WHO/ECOG performance status of 2 (51%; Table 1; for EGFR mutations status, see Data Supplement). Patient demographics and characteristics by cohort (unselected cohort, n = 21; T790M-positive cohort, n = 20) are shown in the Data Supplement. Twenty-nine patients (71%) had coexisting brain metastases, and 20 (49%) had prior radiotherapy to the brain, with 15 (37%) having received WBRT. Duration and timing of prior radiotherapy for individual patients are listed in the Data Supplement. At data cutoff (October 15, 2017), 7 patients were still receiving osimertinib (Fig 1). Median total treatment duration was 8.6 months (range, 0.1-29.7 months; Data Supplement).
FIG 1.
Patient disposition. (*)Reasons for screening failure included T790M status negative or not assessed (n = 30), negative CSF cytology (n = 1), screening failure as a result of other inclusion/exclusion criteria (n = 10), consent withdrawn (n = 1), and death (n = 2).
TABLE 1.
Patient Demographic and Baseline Characteristics
Efficacy
Tumor response.
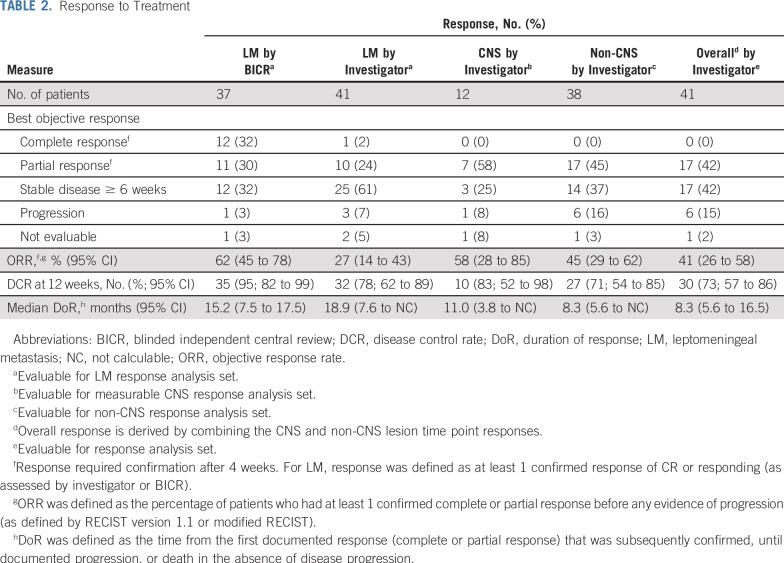
Confirmed LM ORR (excluding 4 patients without available BICR data) was observed in 23 (62%) of 37 by BICR (95% CI, 45% to 78%) and in 11 (27%) of 41 by investigator (95% CI, 14% to 43%). Twelve patients had LM complete response (CR), and 11 had LM partial response (PR) by BICR; 1 patient had LM CR, and 10 had PR by investigator. There was a higher proportion of stable disease by investigator (61%) than by BICR (32%). Median LM DoR was 15.2 months by BICR (95% CI, 7.5 to 17.5 months) and 18.9 months by investigator (95% CI, 7.6 months to not calculable). Details of LM ORR in patients with and without prior brain radiotherapy are provided in the Data Supplement. The confirmed CNS ORR was 58% (7 of 12; 95% CI, 28% to 85%). The confirmed overall ORR was 41% (17 of 41; 95% CI, 26% to 58%; Table 2). The LM and overall disease control rates were 78% and 73%, respectively. For efficacy end points by cohort, see the Data Supplement. CNS tumor shrinkage was observed in 8 (73%) of 11 evaluable patients with measurable CNS lesions and postbaseline RECIST target lesion assessment scans, with a mean reduction of 39% (Data Supplement). Non-CNS tumor shrinkage was observed in 26 (84%) of 31 evaluable patients, with a mean reduction of 36% (Data Supplement). Confirmed CSF clearance was observed in 11 (28%) of 40 evaluable patients (95% CI, 15% to 44%) during treatment.
TABLE 2.
Response to Treatment
Neurologic function.
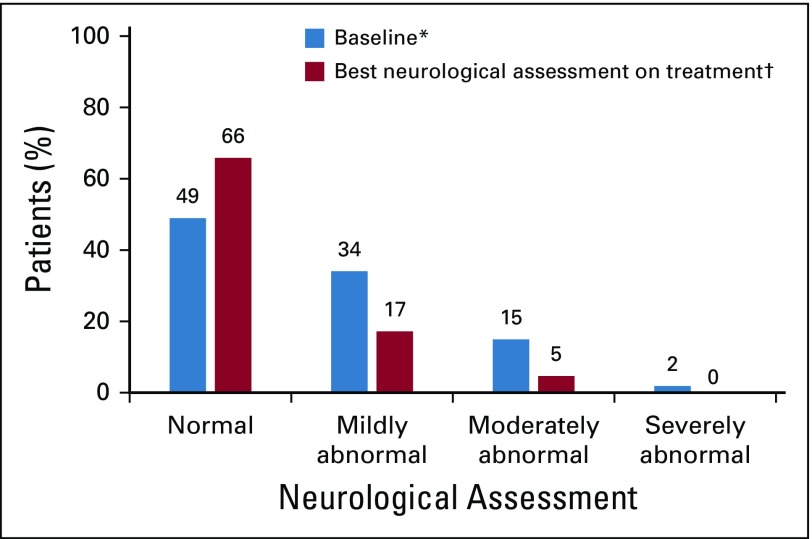
Baseline neurologic assessments were normal in 20 patients (49%), mildly abnormal in 14 (34%), moderately abnormal in 6 (15%), and severely abnormal in 1 (2%). Improved neurologic function was observed in 12 (57%) of the 21 patients who had an abnormal assessment at baseline (Fig 2). Twenty-two patients (54%) did not progress or worsen during treatment. Only 2 patients (5%) experienced regression of their neurologic functions. After treatment, information for 5 patients (12%) was missing (Data Supplement). Results of neurologic assessment by cohort are shown in the Data Supplement.
FIG 2.
Neurologic assessment. The best neurologic assessment relative to baseline in the safety analysis set is shown. All patients had baseline assessments; 5 (12%) of 41 patients had no postbaseline neurologic assessments. (*) Baseline is defined as the last result obtained before the start of study treatment. (†) Best overall neurologic assessment is the maximum improvement from baseline or the minimum worsening from baseline in the absence of an improvement recorded at 2 consecutive visits where better or equal values were recorded.
PFS and OS.
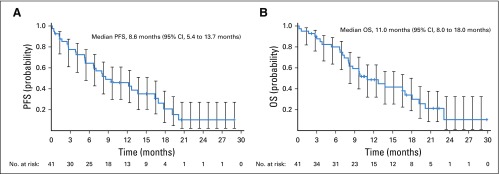
At data cutoff, 32 (78%) of 41 patients had progressed or died (Data Supplement). Median investigator-assessed PFS was 8.6 months (95% CI, 5.4 to 13.7 months; Fig 3A). At 12 months, 42% (95% CI, 27% to 57%) of patients were progression free. Of the 23 LM responders by BICR, site of first progression was most commonly non-CNS (n = 5; 22%), followed by CNS (n = 2; 9%) and LM progression (n = 2; 9%). A similar trend was observed in LM nonresponders, with 6 patients (43%) experiencing progression in non-CNS regions, 3 (21%) in the CNS, and 3 (21%) LM (Data Supplement). Fifteen LM responders (65%) and 6 LM nonresponders (43%) did not experience progression. With a median follow-up of 9.9 months, 28 patients (68%) died. Median OS was 11.0 months (95% CI, 8.0 to 18.0 months; Fig 3B), and the 12-month survival rate was 48% (95% CI, 32% to 63%). Longer exposure and OS were observed in patients who responded according to the RANO-LM criteria compared with nonresponders, particularly in the unselected cohort (Data Supplement). PFS and OS by cohort are shown in the Data Supplement.
FIG 3.
Investigator-assessed (A) progression-free survival (PFS) and (B) overall survival (OS). Evaluable for response set. Censored data are indicated by tick marks, and 95% CIs are shown. For the PFS analysis, patients who had not progressed or died at the time of analysis were censored at the time of their last evaluable RECIST assessment. For the OS analysis, any patients not known to have died at the time of analysis were censored at the last recorded date the patient was known to be alive.
PK
The PK analysis set included 35 patients who had reportable concentrations on day 22 (see Data Supplement for PK parameters). Plasma concentrations of osimertinib and its active metabolites (AZ5104 and AZ7550) reached steady state by day 15 and had a relatively flat PK profile with limited fluctuation in the peak-to-trough steady-state drug concentration in plasma ratio, which suggests that steady-state concentrations were maintained across the dosing interval (Data Supplement). This is similar to previous reports of the PK of osimertinib 80 mg.22 AZ5104 and AZ7550 were observed at steady state at approximately 10% of the exposure of osimertinib in plasma and approximately 9% (AZ5104) and 7% (AZ7550) of osimertinib exposure in CSF. The CSF-to-free plasma ratio for osimertinib was approximately 16%.
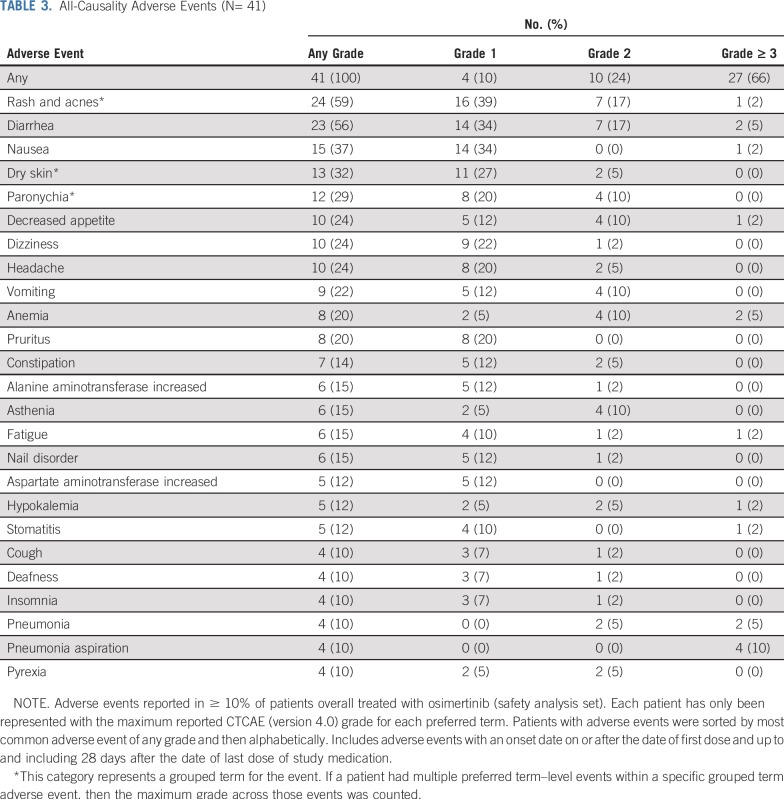
Safety
The safety data in this study indicate that osimertinib 160 mg was generally well tolerated, with a tolerability profile consistent with the known profile of osimertinib and the characteristics of the disease under investigation.23-25 All patients had at least 1 AE, 27 patients (66%) had an AE grade ≥ 3, and 10 (24%) had AEs possibly causally related to osimertinib as judged by the investigator. AEs that led to discontinuation were reported in 9 patients (22%; Data Supplement), and 5 patients (12%) had AEs that led to dose reduction. Serious AEs were reported in 21 patients (51%; Data Supplement). The most commonly reported AEs are listed in Table 3. There was 1 event of pneumonitis (grade 4) that was possibly causally related to osimertinib according to the investigator. Seven AEs (17%) were fatal, none of which were considered by the investigator to be causally related to the study drug. No AEs of QT interval prolongation, cardiomyopathy, or decreased ejection fraction were reported during the study, and the observed QT interval prolongation effects were as expected for treatment with osimertinib and for this patient population.24,25 No patients experienced elevated alanine aminotransferase, aspartate aminotransferase, or bilirubin, and no potential Hy’s law cases were reported.
TABLE 3.
All-Causality Adverse Events (N= 41)
DISCUSSION
In the BLOOM study, osimertinib demonstrated a clinically meaningful benefit across all efficacy end points tested in patients with EGFRm NSCLC and cytologically confirmed LM who had progressed on EGFR-TKIs. To our knowledge, this is the largest prospective study to test the effects of an EGFR-TKI in patients with EGFRm NSCLC and LM.
Osimertinib showed clinically meaningful LM ORR by both investigator and BICR assessment; however, LM ORR was lower by investigator (27%) than by BICR (62%). Fewer patients had CRs by investigator than by BICR (2% v 32%), and more patients had stable disease by investigator than by BICR (61% v 32%). These discrepancies may be due to different specialists with different levels of expertise who assessed scans using different radiologic criteria (Data Supplement). The data showed no positive effect of prior brain radiotherapy on LM response to osimertinib. LM ORR was 55% in patients who received prior brain radiotherapy compared with 57% in patients who did not receive prior brain radiotherapy.
Confirmed CSF response was observed in 28% of patients during treatment; however, our definition of CSF response (complete CSF clearance of tumor cells, in line with previous definitions)12 was stringent and did not capture patients who may have had a PR. Because 100% CSF clearance is seldom achieved; recent studies have defined partial CSF response as a ≥ 50% reduction in CSF tumor cells.5
The clinical benefit of osimertinib was also shown by improvements in neurologic performance. Given that most neurologic deficits caused by LMs are considered irreversible,12 the improvement in neurologic status with osimertinib (57%) is encouraging. Of note, 49% of patients had a normal neurologic examination status at baseline, despite cytologically positive LM. While abnormal neurologic symptoms are usually one of the first indicators of leptomeningeal involvement,9 neurologic symptoms related to LM, such as headache or vomiting, were not recorded in this study. Given the lack of standardized radiologic and neurologic response assessment criteria for the evaluation of LM, further prospective studies using standardized criteria, such as those proposed by the RANO-LM working group, are needed. In this study, patients with a response (as assessed by the RANO-LM criteria) had improved OS compared with nonresponders, which supports the validity of using these criteria in this study.
After the initiation of the BLOOM study, encouraging clinical activity with osimertinib 80 mg once daily in the CNS has been reported, including in patients with radiologically-detected LMs.23,26-30 In a retrospective analysis of 22 EGFR-TKI–pretreated patients with T790M mutation–positive advanced NSCLC and radiologically-detected LMs treated with osimertinib 80 mg once daily across the AURA program (AURA, AURA2, AURA17, and AURA3; ClinicalTrials.gov identifiers: NCT01802632, NCT02094261, NCT02442349, and NCT02151981, respectively), a 55% LM ORR according to RANO-LM radiologic criteria, a median LM PFS of 11.1 months, and a median OS of 18.8 months were reported.31 In the phase III FLAURA (ClinicalTrials.gov identifier: NCT02296125) study of EGFR-TKI–naïve patients with EGFRm advanced NSCLC (exon 19 deletion/L858R), 4 of 5 patients with radiologically-detected LM treated with osimertinib 80 mg once daily had a complete radiologic LM response.32 Moreover, a prospective pilot study (n = 13)33 and small retrospective study (n = 20)34 have reported efficacy with osimertinib 80 mg in patients with pretreated EGFRm NSCLC and LM, which suggests that the 80-mg dose may have similar efficacy to the 160-mg dose. Of note, the AURA phase I study compared varying doses (20-240 mg) of osimertinib and showed no increase in efficacy at 160 mg compared with 80 mg, with a higher frequency of AEs at higher doses and no maximum tolerated dose (up to 240 mg).35,36 Additional prospective studies to evaluate osimertinib 80 mg in the treatment of LM are warranted.
Most studies of EGFR-TKIs for patients with EGFRm NSCLC and LM have been retrospective,1,2,4,10 with limited data from prospective studies.11 In a phase II prospective study that tested the effects of erlotinib to treat patients with NSCLC and LM (n = 17), ORR was 35%, and median time to LM progression and OS were 2.7 and 4.0 months, respectively.37 A prospective study of afatinib (n = 11) reported an ORR of 27%, and median PFS and OS were 2.0 and 3.8 months, respectively.38 High-dose or pulsatile dosing has been reported as an attempt to increase EGFR-TKI concentrations in the CNS. In a phase I prospective study, median neurologic PFS and OS with high-dose gefitinib (750-1,000 mg) were reported as 2.3 and 3.5 months, respectively (n = 7).39 Several case studies have reported successful treatment of LM with high-dose pulsatile administration of erlotinib40; however, a retrospective study of patients with LM refractory to standard-dose EGFR-TKIs found no difference in median OS between patients treated with high-dose erlotinib (n = 12) and those treated with standard-dose EGFR-TKIs (n = 23; 6.2 v 5.9 months, respectively); median time to CNS progression was 2.3 months.41 Given the very limited PFS and OS data in patients with LM treated with EGFR-TKIs, the median PFS and OS results (8.6 and 11.0 months, respectively) observed in this study are promising. These data, however, should be interpreted with caution because of study limitations, including the small number of patients enrolled, of whom 40 were from South Korea, and the heterogeneity of prior anticancer treatments, including intrathecal and systemic chemotherapy and radiotherapy to the brain. However, these are all prior anticancer therapies that are expected in this patient setting, and exclusion of patients who received such therapies would greatly affect patient recruitment in any LM study.
Analysis of response by cohort showed that patients in the unselected cohort had higher response rates and numerically longer DoR, PFS, and OS than patients in the T790M-positive cohort. Patients in the unselected cohort, unlike those in the T790M-positive cohort, were required to have stable non-CNS disease, which is likely to have contributed to this observation. In addition, more patients in the unselected cohort received prior WBRT, a recommended therapeutic approach for patients with LM with good risk status, and had a WHO/ECOG performance status of 0-1 compared with patients in the T790M-positive cohort, which suggests that these patients may have had a better good-risk status or prognosis. Because not all patients in the unselected cohort had confirmed T790M, the high response rate in this cohort suggests CNS progression as a frequent failure site for EGFR-TKIs and osimertinib activity in the CNS regardless of T790M mutation status.
The flat PK profile of osimertinib 160 mg was in line with previous osimertinib PK studies.22,36 Furthermore, the CSF concentration of osimertinib and its metabolites indicated good penetration into the CNS, consistent with results from a PET study in healthy volunteers that showed that [11C]osimertinib distributed rapidly to all regions of the brain.18
As expected and consistent with safety findings from the 160-mg dose group in the AURA phase I study,36 the incidence and severity of AEs were higher than reported for the 80-mg dose. However, no new safety concerns were identified, and overall, safety findings with the 160-mg dose were consistent with the known profile of osimertinib and the characteristics of the disease under study. Treatment discontinuation was reported in 9 patients (22%), with dose reductions reported in 5 patients (12%) and interruptions reported in 24 (59%). In patients who received 160 mg osimertinib who required a dose reduction, the dose was reduced to 80 mg, which is the currently approved osimertinib dose. However, in consideration of the severity of LM and that 51% of patients had a WHO/ECOG performance status of 2, these incidences would be considered acceptable. The actual median treatment exposure (8.1 months; range, 0.1-29.6 months), which excluded treatment interruptions, was similar to the intended treatment exposure, with a mean relative dose intensity of 94% (standard deviation, 11%), which indicates that toxicity was manageable through dose interruptions without a need for treatment discontinuation. Furthermore, previous studies of exposure-response to osimertinib have shown that while exposure affects the safety profile, it has a minimal impact on efficacy.35 Indeed, as noted previously here, the ORR and OS outcomes in BLOOM are encouraging compared with historical published data.
In conclusion, osimertinib 160 mg once daily showed meaningful therapeutic efficacy in terms of radiologic response, neurologic improvement, CSF clearance, and a manageable safety profile in patients with LM from EGFRm advanced NSCLC whose disease had progressed on EGFR-TKI therapy. Given the lack of standardized treatment as well as the current treatment limitations and/or invasive nature of the available experimental therapies, osimertinib has the potential to become a treatment option for patients with EGFRm NSCLC and LM previously treated with an EGFR-TKI. Given the success of the FLAURA phase III study with first-line osimertinib, it is likely that osimertinib may also play an important role in the treatment of EGFR-TKI–naïve patients with EGFRm NSCLC and LM. Additional studies are required to confirm these findings using the 80-mg dose.
ACKNOWLEDGMENT
We thank the patients and their families as well as the investigators and staff at all study sites. We acknowledge Ellen Maxwell, PhD, of iMed Comms, Macclesfield, United Kingdom, an Ashfield Company, part of UDG Healthcare, for medical writing assistance under the direction of the authors. We also acknowledge Haiyi Jiang, MD (AstraZeneca, Gaithersburg, MD), and Zhenfan Yang, MD, PhD (Dizal Pharmaceutical, Shanghai, Peoples Republic of China), for their contributions to the development of the protocol.
Data underlying the findings described in this article may be obtained in accordance with AstraZeneca’s data sharing policy described at https://astrazenecagrouptrials.pharmacm.com/ST/Submission/Disclosure.
Listen to the podcast by Dr Wolf at http://ascopubs.org/jco/podcasts
PRIOR PRESENTATION
Presented at the AACR-NCI-EORTC Congress, Boston, MA, November 5-9, 2015; ASCO Annual Meeting, Chicago, IL, June 3-7, 2016; and the ASCO Annual Meeting, Chicago, IL, June 2-6, 2017.
SUPPORT
Supported by AstraZeneca (Cambridge, United Kingdom), the manufacturer of osimertinib. Writing assistance was funded by AstraZeneca in accordance with Good Publications Practice guidelines.
CLINICAL TRIAL INFORMATION
AUTHOR CONTRIBUTIONS
Conception and design: James C.H. Yang, Sang-We Kim, Byoung Chul Cho, Jin-Seok Ahn, Karthick Vishwanathan, Myung-Ju Ahn
Provision of study material or patients: James C.H. Yang, Sang-We Kim, Dong-Wan Kim, Byoung Chul Cho, Dae H. Lee, Tae Min Kim, Jonathan W. Goldman, Ronald B. Natale
Collection and assembly of data: James C.H. Yang, Sang-We Kim, Jong-Seok Lee, Byoung Chul Cho, Jin-Seok Ahn, Dae H. Lee, Jonathan W. Goldman, Ronald B. Natale, Andrew P. Brown, Karthick Vishwanathan, Myung-Ju Ahn
Data analysis and interpretation: James C.H. Yang, Sang-We Kim, Dong-Wan Kim, Byoung Chul Cho, Jin-Seok Ahn, Dae H. Lee, Tae Min Kim, Jonathan W. Goldman, Ronald B. Natale, Andrew P. Brown, Barbara Collins, Juliann Chmielecki, Karthick Vishwanathan, Ariadna Mendoza-Naranjo, Myung-Ju Ahn
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Osimertinib in Patients With Epidermal Growth Factor Receptor Mutation–Positive Non–Small-Cell Lung Cancer and Leptomeningeal Metastases: The BLOOM Study
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/journal/jco/site/ifc.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
James C.H. Yang
Honoraria: Boehringer Ingelheim, Roche, MSD, AstraZeneca, Novartis, Bristol-Myers Squibb, Ono Pharmaceutical, Takeda Pharmaceuticals, Eli Lilly, Pfizer
Consulting or Advisory Role: Boehringer Ingelheim, Novartis, AstraZeneca, Roche, Genentech, Clovis Oncology, Eli Lilly, MSD Oncology, Merck Serono, Celgene, Astellas Pharma, Bayer AG, Pfizer, Ono Pharmaceutical, Bristol-Myers Squibb, Boehringer Ingelheim (Inst), AstraZeneca (Inst), Yuhan, Hansoh, Blueprint Medicines, Daiichi Sankyo, G1 Therapeutics, AbbVie, Amgen, Takeda Oncology
Travel, Accommodations, Expenses: Pfizer
Dong-Wan Kim
Research Funding: Alpha Biopharma (Inst), AstraZeneca (Inst), MedImmune (Inst), Hanmi (Inst), Janssen Pharmaceuticals (Inst), Merus (Inst), Mirati Therapeutics (Inst), MSD (Inst), Novartis (Inst), Ono Pharmaceutical (Inst), Pfizer (Inst), Roche (Inst), Genentech (Inst), Takeda Pharmaceuticals (Inst), TP Therapeutics (Inst), Xcovery (Inst), Yuhan (Inst),
Byoung Chul Cho
Stock and Other Ownership Interests: TheraCanVac
Honoraria: Novartis, Bayer AG, AstraZeneca, MOGAM Institute, Don-A ST, Champions Oncology, Janssen Pharmaceuticals, Yuhan, Ono Pharmaceutical, Dizal Pharma, MSD
Consulting or Advisory Role: Novartis, AstraZeneca, Boehringer Ingelheim, Roche, Bristol-Myers Squibb, Yuhan, Pfizer, Eli Lilly, Janssen Pharmaceuticals, Takeda Pharmaceuticals, MSD, Ono Pharmaceutical
Speakers’ Bureau: Novartis
Research Funding: Novartis, Bayer AG, AstraZeneca, MOGAM Institute, Dong-A ST, Champions Oncology, Janssen Pharmaceuticals, Yuhan, Ono Pharmaceutical, Dizal Pharma, MSD
Patents, Royalties, Other Intellectual Property: Champions Oncology
Jin-Seok Ahn
Honoraria: Menarini, Amgen, Pfizer, AstraZeneca, Roche, Janssen Pharmaceuticals, MSD, Bristol-Myers Squibb, Ono Pharmaceutical, Eisai, Boehringer Ingelheim
Consulting or Advisory Role: Samsung Bioepis
Dae H. Lee
Honoraria: AstraZeneca, MedImmune, Boehringer Ingelheim, Bristol-Myers Squibb, Chong Kun Dang Pharmaceutical, CJ Healthcare, Eli Lilly, Janssen Pharmaceuticals, Merck, MSD, Mundipharma, Novartis, Ono Pharmaceutical, Pfizer, Roche, Genentech, Samyang, ST Cube, Takeda Pharmaceuticals, AbbVie, Menarini
Consulting or Advisory Role: ST Cube
Tae Min Kim
Research Funding: AstraZeneca
Uncompensated Relationships: AstraZeneca, MedImmune, Novartis, Takeda Pharmaceuticals, Bayer AG, Sanofi
Jonathan W. Goldman
Consulting or Advisory Role: AstraZeneca, Bristol-Myers Squibb, Clovis Oncology, Genentech, Roche, Eli Lilly, Trovagene, Vortex Biosciences, Amgen, Celgene
Speakers’ Bureau: Merck
Research Funding: Eli Lilly, Genentech, Roche, Astex Pharmaceuticals, Clovis Oncology, Bristol-Myers Squibb, AstraZeneca, MedImmune, Threshold Pharmaceuticals, Array BioPharma, Celgene, AbbVie, Astellas Pharma, Corvus Pharmaceuticals, Spectrum Pharmaceuticals
Ronald B. Natale
Employment: AstraZeneca (I), MedImmune (I)
Consulting or Advisory Role: AstraZeneca, Eli Lilly
Research Funding: AstraZeneca (Inst), Bristol-Myers Squibb (Inst), Merck (Inst)
Andrew P. Brown
Employment: AstraZeneca, AstraZeneca (I)
Stock and Other Ownership Interests: AstraZeneca, AstraZeneca (I)
Travel, Accommodations, Expenses: AstraZeneca, AstraZeneca (I)
Barbara Collins
Other Relationship: AstraZeneca
Juliann Chmielecki
Employment: AstraZeneca
Stock and Other Ownership Interests: AstraZeneca, Foundation Medicine
Patents, Royalties, Other Intellectual Property: Patent pending
Karthick Vishwanathan
Employment: AstraZeneca
Stock and Other Ownership Interests: AstraZeneca
Ariadna Mendoza-Naranjo
Employment: AstraZeneca
Stock and Other Ownership Interests: AstraZeneca
Travel, Accommodations, Expenses: AstraZeneca
Myung-Ju Ahn
Honoraria: AstraZeneca, Eli Lilly, MSD, Takeda Pharmaceuticals
Consulting or Advisory Role: AstraZeneca, Boehringer Ingelheim, Eli Lilly, MSD, Takeda Pharmaceuticals, Alpha Pharmaceutical
No other potential conflicts of interest were reported.
REFERENCES
- 1.Liao BC, Lee JH, Lin CC, et al. Epidermal growth factor receptor tyrosine kinase inhibitors for non-small-cell lung cancer patients with leptomeningeal carcinomatosis. J Thorac Oncol. 2015;10:1754–1761. doi: 10.1097/JTO.0000000000000669. [DOI] [PubMed] [Google Scholar]
- 2.Li YS, Jiang BY, Yang JJ, et al. Leptomeningeal metastases in patients with NSCLC with EGFR mutations. J Thorac Oncol. 2016;11:1962–1969. doi: 10.1016/j.jtho.2016.06.029. [DOI] [PubMed] [Google Scholar]
- 3.Kuiper JL, Hendriks LE, van der Wekken AJ, et al. Treatment and survival of patients with EGFR-mutated non-small cell lung cancer and leptomeningeal metastasis: A retrospective cohort analysis. Lung Cancer. 2015;89:255–261. doi: 10.1016/j.lungcan.2015.05.023. [DOI] [PubMed] [Google Scholar]
- 4.Umemura S, Tsubouchi K, Yoshioka H, et al. Clinical outcome in patients with leptomeningeal metastasis from non-small cell lung cancer: Okayama Lung Cancer Study Group. Lung Cancer. 2012;77:134–139. doi: 10.1016/j.lungcan.2012.03.002. [DOI] [PubMed] [Google Scholar]
- 5.Xu Y, Hu M, Zhang M, et al. Prospective study revealed prognostic significance of responses in leptomeningeal metastasis and clinical value of cerebrospinal fluid-based liquid biopsy. Lung Cancer. 2018;125:142–149. doi: 10.1016/j.lungcan.2018.08.017. [DOI] [PubMed] [Google Scholar]
- 6.Cheng H, Perez-Soler R. Leptomeningeal metastases in non-small-cell lung cancer. Lancet Oncol. 2018;19:e43–e55. doi: 10.1016/S1470-2045(17)30689-7. [DOI] [PubMed] [Google Scholar]
- 7.Morris PG, Reiner AS, Szenberg OR, et al. Leptomeningeal metastasis from non-small cell lung cancer: Survival and the impact of whole brain radiotherapy. J Thorac Oncol. 2012;7:382–385. doi: 10.1097/JTO.0b013e3182398e4f. [DOI] [PubMed] [Google Scholar]
- 8.Tan CS, Cho BC, Soo RA. Treatment options for EGFR mutant NSCLC with CNS involvement—can patients BLOOM with the use of next generation EGFR TKIs? Lung Cancer. 2017;108:29–37. doi: 10.1016/j.lungcan.2017.02.012. [DOI] [PubMed] [Google Scholar]
- 9.Chamberlain MC. Leptomeningeal metastasis. Curr Opin Oncol. 2010;22:627–635. doi: 10.1097/CCO.0b013e32833de986. [DOI] [PubMed] [Google Scholar]
- 10.Du C, Hong R, Shi Y, et al. Leptomeningeal metastasis from solid tumors: A single center experience in Chinese patients. J Neurooncol. 2013;115:285–291. doi: 10.1007/s11060-013-1228-x. [DOI] [PubMed] [Google Scholar]
- 11.Yufen X, Binbin S, Wenyu C, et al. The role of EGFR-TKI for leptomeningeal metastases from non-small cell lung cancer. Springerplus. 2016;5:1244. doi: 10.1186/s40064-016-2873-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chamberlain M, Junck L, Brandsma D, et al. Leptomeningeal metastases: A RANO proposal for response criteria. Neuro-oncol. 2017;19:484–492. doi: 10.1093/neuonc/now183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Remon J, Le Rhun E, Besse B. Leptomeningeal carcinomatosis in non-small cell lung cancer patients: A continuing challenge in the personalized treatment era. Cancer Treat Rev. 2017;53:128–137. doi: 10.1016/j.ctrv.2016.12.006. [DOI] [PubMed] [Google Scholar]
- 14. https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/208065s008lbl.pdf TAGRISSO [package insert]. Wilmington, DE, AstraZeneca, 2018. Available at .
- 15. https://www.ema.europa.eu/en/documents/product-information/tagrisso-epar-product-information_en.pdf European Medicines Agency: Summary of product characteristics: TAGRISSO, 2018.
- 16.Ballard P, Yates JW, Yang Z, et al. Preclinical comparison of osimertinib with other EGFR-TKIs in EGFR-mutant NSCLC Brain metastases models, and early evidence of clinical brain metastases activity. Clin Cancer Res. 2016;22:5130–5140. doi: 10.1158/1078-0432.CCR-16-0399. [DOI] [PubMed] [Google Scholar]
- 17.Colclough N, Ballard PG, Barton P, et al. Preclinical comparison of the blood brain barrier (BBB) permeability of osimertinib (AZD9291) with other irreversible next generation EGFR TKIs. Eur J Cancer. 2016;69:S28. [Google Scholar]
- 18. Vishwanathan K, Varrone A, Varnas K, et al: Osimertinib displays high brain exposure in healthy subjects with intact blood-brain barrier: A microdose positron emission tomography (PET) study with 11C-labelled osimertinib. Cancer Res 78, 2018 (suppl; abstr CT013) [Google Scholar]
- 19. Kim DW, Yang CH, Cross D, et al: Preclinical evidence and clinical cases of AZD9291 activity in EGFR-mutant non-small cell lung cancer (NSCLC) brain metastases. Ann Oncol 25, 2014 (suppl; abstr 456P) [Google Scholar]
- 20.Ahn MJ, Kim DW, Cho BC, et al. Activity and safety of AZD3759 in EGFR-mutant non-small-cell lung cancer with CNS metastases (BLOOM): A phase 1, open-label, dose-escalation and dose-expansion study. Lancet Respir Med. 2017;5:891–902. doi: 10.1016/S2213-2600(17)30378-8. [DOI] [PubMed] [Google Scholar]
- 21.Zhu G, Ye X, Dong Z, et al. Highly sensitive droplet digital PCR method for detection of EGFR-activating mutations in plasma cell-free DNA from patients with advanced non-small cell lung cancer. J Mol Diagn. 2015;17:265–272. doi: 10.1016/j.jmoldx.2015.01.004. [DOI] [PubMed] [Google Scholar]
- 22.Planchard D, Brown KH, Kim DW, et al. Osimertinib western and Asian clinical pharmacokinetics in patients and healthy volunteers: Implications for formulation, dose, and dosing frequency in pivotal clinical studies. Cancer Chemother Pharmacol. 2016;77:767–776. doi: 10.1007/s00280-016-2992-z. [DOI] [PubMed] [Google Scholar]
- 23.Goss G, Tsai CM, Shepherd FA, et al. CNS response to osimertinib in patients with T790M-positive advanced NSCLC: Pooled data from two phase II trials. Ann Oncol. 2018;29:687–693. doi: 10.1093/annonc/mdx820. [DOI] [PubMed] [Google Scholar]
- 24.Mok TS, Wu Y-L, Ahn M-J, et al. Osimertinib or platinum-pemetrexed in EGFR T790M-positive lung cancer. N Engl J Med. 2017;376:629–640. doi: 10.1056/NEJMoa1612674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Soria JC, Ohe Y, Vansteenkiste J, et al. Osimertinib in untreated EGFR-mutated advanced non-small-cell lung cancer. N Engl J Med. 2018;378:113–125. doi: 10.1056/NEJMoa1713137. [DOI] [PubMed] [Google Scholar]
- 26.Chan OS, Leung WK, Yeung RM. Sustained response to standard dose osimertinib in a patient with plasma T790M-positive leptomeningeal metastases from primary lung adenocarcinoma. Asia Pac J Clin Oncol. 2017;13:428–430. doi: 10.1111/ajco.12673. [DOI] [PubMed] [Google Scholar]
- 27.Pareek V, Welch M, Ravera E, et al. Marked differences in CNS activity among EGFR inhibitors: Case report and mini-review. J Thorac Oncol. 2016;11:e135–e139. doi: 10.1016/j.jtho.2016.07.010. [DOI] [PubMed] [Google Scholar]
- 28.Sakai H, Hayashi H, Iwasa T, et al. Successful osimertinib treatment for leptomeningeal carcinomatosis from lung adenocarcinoma with the T790M mutation of EGFR. ESMO Open. 2017;2:e000104. doi: 10.1136/esmoopen-2016-000104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Takeda T, Itano H, Takeuchi M, et al. Osimertinib administration via nasogastric tube in an EGFR-T790M-positive patient with leptomeningeal metastases. Respirol Case Rep. 2017;5:e00241. doi: 10.1002/rcr2.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Uemura T, Oguri T, Okayama M, et al. Dramatic intracranial response to osimertinib in a poor performance status patient with lung adenocarcinoma harboring the epidermal growth factor receptor T790M mutation: A case report. Mol Clin Oncol. 2017;6:525–528. doi: 10.3892/mco.2017.1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ahn MJ, Chiu CH, Cheng Y, et al: Osimertinib for patients (pts) with leptomeningeal metastases (LM) associated with EGFRm advanced NSCLC. Ann Oncol 29, 2018 (suppl; abstr 492O) [Google Scholar]
- 32.Reungwetwattana T, Nakagawa K, Cho BC, et al. CNS response to osimertinib versus standard epidermal growth factor receptor tyrosine kinase inhibitors in patients with untreated EGFR-mutated advanced non-small-cell lung cancer. J Clin Oncol. 2019;33:3290–3297. doi: 10.1200/JCO.2018.78.3118. [DOI] [PubMed] [Google Scholar]
- 33.Nanjo S, Hata A, Okuda C, et al. Standard-dose osimertinib for refractory leptomeningeal metastases in T790M-positive EGFR-mutant non-small cell lung cancer. Br J Cancer. 2018;118:32–37. doi: 10.1038/bjc.2017.394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Saboundji K, Auliac JB, Pérol M, et al. Efficacy of osimertinib in EGFR-mutated non-small cell lung cancer with leptomeningeal metastases pretreated with EGFR-tyrosine kinase inhibitors. Target Oncol. 2018;13:501–507. doi: 10.1007/s11523-018-0581-2. [DOI] [PubMed] [Google Scholar]
- 35.Dearden S, Brown H, Jenkins S, et al. EGFR T790M mutation testing within the osimertinib AURA phase I study. Lung Cancer. 2017;109:9–13. doi: 10.1016/j.lungcan.2017.04.011. [DOI] [PubMed] [Google Scholar]
- 36.Jänne PA, Yang JC, Kim DW, et al. AZD9291 in EGFR inhibitor-resistant non-small-cell lung cancer. N Engl J Med. 2015;372:1689–1699. doi: 10.1056/NEJMoa1411817. [DOI] [PubMed] [Google Scholar]
- 37.Ota K, Shiraishi Y, Harada T, et al. Phase II study of erlotinib in advanced non-small cell lung cancer patients with leptomeningeal metastasis (LOGIK1101) J Thorac Oncol. 2017;12:S271–S272. [Google Scholar]
- 38.Tamiya A, Tamiya M, Nishihara T, et al. Cerebrospinal fluid penetration rate and efficacy of afatinib in patients with EGFR Mutation-positive non-small cell lung cancer with leptomeningeal carcinomatosis: A multicenter prospective study. Anticancer Res. 2017;37:4177–4182. doi: 10.21873/anticanres.11806. [DOI] [PubMed] [Google Scholar]
- 39.Jackman DM, Cioffredi LA, Jacobs L, et al. A phase I trial of high dose gefitinib for patients with leptomeningeal metastases from non-small cell lung cancer. Oncotarget. 2015;6:4527–4536. doi: 10.18632/oncotarget.2886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.How J, Mann J, Laczniak AN, et al. Pulsatile erlotinib in EGFR-positive non-small-cell lung cancer patients with leptomeningeal and brain metastases: Review of the literature. Clin Lung Cancer. 2017;18:354–363. doi: 10.1016/j.cllc.2017.01.013. [DOI] [PubMed] [Google Scholar]
- 41.Kawamura T, Hata A, Takeshita J, et al. High-dose erlotinib for refractory leptomeningeal metastases after failure of standard-dose EGFR-TKIs. Cancer Chemother Pharmacol. 2015;75:1261–1266. doi: 10.1007/s00280-015-2759-y. [DOI] [PubMed] [Google Scholar]