Sustained IFN-I dampens the antibacterial activity of MAIT cells by inducing IL-10 production during chronic HIV-1 infection.
Abstract
Mucosal-associated invariant T (MAIT) cells in HIV-1–infected individuals are functionally impaired by poorly understood mechanisms. Single-cell transcriptional and surface protein analyses revealed that peripheral MAIT cells from HIV-1–infected subjects were highly activated with the up-regulation of interferon (IFN)–stimulated genes as compared to healthy individuals. Sustained IFN-α treatment suppressed MAIT cell responses to Escherichia coli by triggering high-level interleukin-10 (IL-10) production by monocytes, which subsequently inhibited the secretion of IL-12, a crucial costimulatory cytokine for MAIT cell activation. Blocking IFN-α or IL-10 receptors prevented MAIT cell dysfunction induced by HIV-1 exposure in vitro. Moreover, blocking the IL-10 receptor significantly improved anti–Mycobacterium tuberculosis responses of MAIT cells from HIV-1–infected patients. Our findings demonstrate the central role of the IFN-I/IL-10 axis in MAIT cell dysfunction during HIV-1 infection, which has implications for the development of anti–IFN-I/IL-10 strategies against bacterial coinfections in HIV-1–infected patients.
INTRODUCTION
Mucosal-associated invariant T (MAIT) cells are innate-like T cells expressing a semi-invariant T cell receptor (TCR) composed of a conserved α chain, Vα7.2, joined to Jα33, and a limited repertoire of β chains, predominantly composed of Vβ13 or Vβ2 (1). Unlike conventional T (Tconv) cells, MAIT cells recognize the microbial metabolite of riboflavin presented by MHC-I (major histocompatibility complex I)–related protein 1 (MR1) and respond to bacteria and fungi (2). In addition to the TCR signal, some cytokines, including interleukin-12 (IL-12), IL-15, and IL-18, are also necessary for the induction of a robust response against bacteria by MAIT cells (3). In particular, blocking IL-12R, rather than IL-18R, substantially reduced granzyme B (GzmB) production in MAIT cells upon Escherichia coli stimulation (4). MAIT cells can also be activated by cytokines such as IL-12 and IL-18 in a TCR-independent matter (5), especially during viral infection (6). A recent study reported that MAIT cells could also be activated by IL-23, which shares the receptor chain IL-12Rβ1 with IL-12 (7).
Activated MAIT cells produce proinflammatory cytokines, including tumor necrosis factor (TNF) and interferon-γ (IFN-γ), as well as cytotoxic products such as granzymes and perforin (4). However, in vivo studies on the functional role of MAIT cells in protection against clinically important human pathogens remain sparse. Recently, a study has shown that, as compared to wild-type mice, MAIT cell–deficient MR1−/− mice have increased mortality to severe (H1N1) influenza, which is ameliorated by previous adoptive transfer of pulmonary MAIT cells (8). Another study using Rag2−/−γC−/− mice has shown that the adoptive transfer of MAIT cells rescues mice from lethal Legionella infection, which is dependent on IFN-γ and GM-CSF (granulocyte-macrophage colony-stimulating factor), rather than IL-17A, TNF, or perforin (9). The protection was more pronounced in mice lacking CD4+ T cells than in immune competent mice, indicating a crucial role of MAIT cells in situations of compromised adaptive immunity, such as HIV-1 infection.
A loss of peripheral MAIT cells has been reported in individuals infected with HIV-1, although these cells do not appear to be infected by HIV-1 (10, 11). It is still in debate whether MAIT cells die or migrate from the peripheral blood to intestinal mucosal sites during HIV-1 infection (10, 11). Residual circulating MAIT cells from HIV-1–infected people are functionally impaired in response to E. coli, and effective antiretroviral therapy (ART) treatment fails to fully restore the number and function of MAIT cells (11, 12). It has been observed that MAIT cell dysfunction in HIV-1 infection is associated with a lower expression of transcription factors T-bet and Eomes, which could be restored by IL-7 treatment in vitro (12). Further study of this strategy in a small cohort of HIV-1–infected patients showed an increase of circulating CD8+ MAIT cells upon IL-7 treatment (13). It remains unclear, however, why reconstitution of MAIT cells fails even after prolonged ART, while homeostasis of conventional CD4+ and CD8+ T cells normalizes. The persistent defect in MAIT cells highlights the need to understand the mechanism of MAIT cell dysfunction.
HIV-1 infection induces sustained production of type I IFN (IFN-I), as evidenced by a marked increase in both IFN-α mRNA in peripheral plasmacytoid dendritic cells (pDCs) and IFN-α protein level in serum from untreated acutely and chronically infected patients (14). Accumulating evidence has implicated IFN-I in immune impairment and disease progression during chronic HIV-1 infections (15, 16), by affecting multiple immune cell compartments, including T cells (17), innate lymphoid cells (18), and hematopoietic stem cells (19). Moreover, abnormal activation induced by IFN-I persists in some patients even under suppressive ART (15). A recent study showed that IFN-I enhances MAIT cell effector responses to TCR stimulation by boosting IFN-γ and GzmB production (20), suggesting a complex role of IFN-I in the regulation of MAIT cells.
Here, we hypothesized that IFN-I may contribute to MAIT cell impairment during chronic HIV-1 infection. By performing transcriptional and proteomic analysis, as well as functional assays of MAIT cells from HIV-infected patients, we show that sustained IFN-I stimulation induces IL-10 production by monocytes, which inhibits the secretion of IL-12, thus dampening MAIT cell immunity against bacteria.
RESULTS
MAIT cells from HIV-1–infected subjects show an impaired response to E. coli but normal reactivity to IL-12/18 stimulation
MAIT cells were analyzed in peripheral blood mononuclear cells (PBMCs) and were compared among 17 viremic HIV-1–infected subjects (VIR) without receiving ART treatment, 17 HIV-1–infected patients under suppressive ART for 2 to 5 years, and 16 uninfected healthy blood donors as controls (HC) (table S1). As shown in Fig. 1A, MAIT cells were gated as CD3+Vα7.2+CD161high cells in PBMCs. The frequency of MAIT cells was significantly lower in HIV-1–infected patients, irrespective of ART treatment, compared to HC. Note that long-term effective ART treatment did not lead to the recovery of MAIT cells in peripheral blood (Fig. 1B).
Fig. 1. Functional activity of MAIT cells from healthy donors and HIV-infected patients with or without ART treatment in response to E. coli or IL-12/18 stimulation.
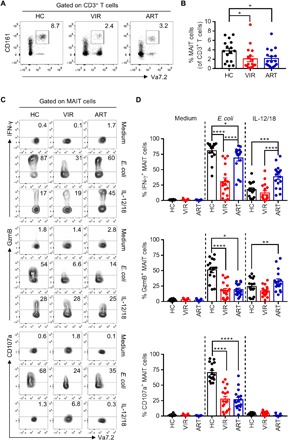
(A and B) The frequency of MAIT cells was determined in PBMCs from healthy control donors (HC; n = 16) and HIV-1–infected patients who did not receive ART (VIR; n = 17) or were treated with ART (ART; n = 17). Representative FACS (fluorescence-activated cell sorting) plots (A) and statistical analysis (B) are shown. (C and D) PBMCs freshly isolated from HC, VIR, or ART subjects were stimulated with paraformaldehyde (PFA)–fixed E. coli [multiplicity of infection (MOI), 10] or combined IL-12 and IL-18 (IL-12/18; 100 ng/ml for each) for 24 hours and then assessed for the expression level of IFN-γ, GzmB, and CD107a in MAIT cells. Representative FACS plots (C) and statistical analyses (D) of MAIT cell responses are shown.
The function of MAIT cells was evaluated by their capacity to produce IFN-γ, the cytotoxic protein GzmB, and the degranulation marker CD107a in response to paraformaldehyde (PFA)–fixed E. coli or IL-12 plus IL-18 (IL-12/18) stimulation (Fig. 1, C and D). Consistent with previous findings (11, 12), MAIT cells from HIV-1–infected subjects produced much lower levels of IFN-γ, GzmB, and CD107a in response to E. coli stimulation as compared to the HC group. Effective ART treatment did not fully restore the functionality of MAIT cells, as indicated by partial induction of IFN-γ as well as unchanged low levels of GzmB and CD107a production by MAIT cells in the ART group compared to the VIR group. In contrast, there was no significant difference in the magnitude of MAIT cell responses to IL-12/18 stimulation between HC and VIR subjects. Unexpectedly, ART increased IFN-γ and GzmB secretion by MAIT cells upon IL-12/18 stimulation.
We longitudinally analyzed MAIT cells in HIV-1–infected patients at baseline (pre-ART) and at 6 and 12 months post-ART. All five patients had complete suppression of viral load and recovery of CD4+ T cells at 6 and 12 months post-ART (fig. S1, A and B). However, the decreased proportion of MAIT cells induced by HIV-1 infection failed to be restored by effective ART (fig. S1C). Similarly, the function of MAIT cells in response to E. coli remained largely unchanged (fig. S1D), despite increased expression of CD107a at 12 months post-ART as compared to that at pre-ART. The IFN-γ and GzmB production by MAIT cells in response to IL-12/18 stimulation declined transiently. Together, MAIT cells from HIV-1–infected patients display impaired function in response to E. coli but normal reactivity to IL-12/18 stimulation, and the effective ART only partially restored their function.
MAIT cells show an activated state with up-regulation of inhibitory receptors and IFN-stimulated genes during HIV-1 infection
To define the phenotypic and molecular features of MAIT cells during HIV-1 infection, we used oligo-conjugated antibodies (Abseq) in combination with single-cell mRNA sequencing (scRNAseq) on the BD Rhapsody platform to compare the expression level of 29 surface proteins (table S2) and the mRNA level of 259 genes (table S3) in total peripheral T cells between 2 HC and 4 VIR subjects (table S1). Uniform manifold approximation and projection (UMAP) and unsupervised graph-based clustering partitioned cells into 18 clusters (Fig. 2A). On the basis of their transcriptional programs and surface protein expression profiles, a total of six CD8+ (C0 to C2, C8, C11, and C13) and five CD4+ (C3, C5, C6, C9, and C12) T cell subsets and natural killer (NK) cell (C4) and MAIT/γδT cell (C7) clusters were identified (Fig. 2, A and C, and fig. S2, B and C). C10 and C14 to C17 were contaminating non-T cells that remained after CD3+ T cell purification based on their distance from the aforementioned clusters, as well as their low expression of CD3 at both the mRNA and protein level. These clusters were excluded from downstream analyses. Compared to HC individuals, VIR subjects displayed a decreased proportion of CD4+ T cells and a simultaneously increased frequency of CD8+ T cells (Fig. 2B and fig. S1A). Cluster 7, composed of MAIT cells characterized by a high expression of CD8A, KLRB1, DPP4, and RORC and of γδT cells, which were CD4 and CD8 double negative but expressed TRDC, was also decreased in viremic HIV-1–infected patients (Fig. 2, B and C).
Fig. 2. Single-cell transcriptional and surface protein expression profiles of T cell subsets in HIV-1 infection.
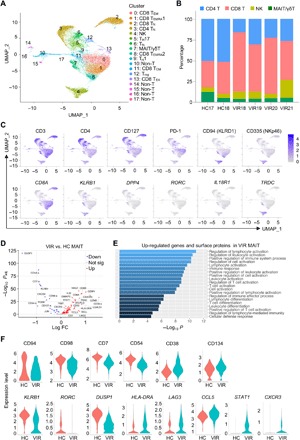
(A) UMAP plot of 23,424 T cells from two healthy donors (HC) and four viremic HIV-1–infected subjects (VIR) determined by Seurat v.3. A total of 18 clusters (clusters 0 to 17) were identified and color-coded. (B) Percentages of the major T cell subsets for each sample. (C) Projection of cells expressing the chosen surface proteins (top row) or transcripts (bottom row) to the UMAP plots. (D) Volcano plot showing the differentially expressed genes and surface proteins (-a) between MAIT cells from VIR and HC subjects. (E) GO analysis using DAVID for genes that were up-regulated in MAIT cells from VIR versus HC subjects. (F) Violin plots of the cell surface protein (top row) or gene (bottom row) expression in MAIT cells from HC and VIR-infected subjects.
To exclude γδT cells and more accurately examine MAIT cells, CD8+ cells were selected in C7 for further analyses. We sought to identify genes and surface proteins in MAIT cells that were differentially expressed between HIV+ and HC subjects (table S4). A total of 72 molecules were significantly up-regulated, and 20 molecules were down-regulated (P < 0.01). Among the up-regulated proteins and genes in MAIT cells from HIV+ versus HC individuals were activation markers such as CD38, CD134, and HLA-DRA; exhaustion associated genes including LAG3 and TIGIT; and IFN-stimulated genes (ISGs) including CXCR3, STAT1, and CCL5 (Fig. 2, D and F). In contrast, proteins and genes that are related to T cell function, including CD7, CD98, CD54, DUSP1, KLRB1, and JUN, were down-regulated in MAIT cells from HIV-1–infected patients. The Gene Ontology (GO) enrichment analysis further showed that the up-regulated genes in MAIT cells from VIR subjects were enriched in cellular activation pathways (Fig. 2E). Thus, MAIT cells from HIV-1–infected patients showed a gene signature characterized by immune activation, associated with inhibitory receptor up-regulation and increased ISG expression.
Sustained IFN-I signaling was associated with MAIT cell dysfunction during HIV-1 infection
To determine whether MAIT cell dysfunction during HIV-1 infection was dependent on inhibitory receptors, we first detected the expression of inhibitory receptors including PD-1, TIM-3, and LAG-3 by flow cytometry (fig. S3, A and B, and table S1). Consistent with our scRNAseq and Abseq data, LAG-3 expression was slightly increased at the surface of MAIT cells but not of Tconv cells, whereas an elevated level of PD-1 was detected on Tconv cells but not on MAIT cells when comparing untreated viremic HIV+ patients to HC individuals. TIM-3 expression remained comparable between VIR and HC subjects. We then used the anti–PD-1 or anti–LAG-3 blocking antibody, which could improve HIV-1–specific CD8+ T cell responses in PBMCs from untreated HIV+ patients (fig. S3D and table S1). Unexpectedly, the blocking antibodies did not increase the production of IFN-γ, GzmB, or CD107a in MAIT cells from either HC or VIR subjects in response to E. coli (fig. S3C and table S1), indicating that the increased expression of inhibitory receptors may not account for MAIT cell dysfunction during HIV-1 infection.
The up-regulation of ISGs in MAIT cells from HIV+ patients, as indicated by the scRNAseq and Abseq results, raised the possibility that IFN-I signaling could play a role in MAIT cell dysfunction. Therefore, we assessed the levels of IFN-α and IFN-β, as well as IFN-γ–inducible protein-10 (IP-10) and IL-10, which was also induced by IFN-I (21), in the plasma of subjects in the same HC, VIR, and ART cohorts as those studied in Fig. 1. VIR individuals had markedly higher concentrations of IFN-α and IP-10 in plasma than HC and ART subjects (Fig. 3A). Although ART patients and HC had a similarly low amount of IFN-α, a slightly but significantly higher level of IP-10 was detected in ART compared to HC subjects. No significant difference was observed in IFN-β among the three groups. IL-10 was induced in patient plasma after HIV-1 infection and remained elevated even after long-term effective ART treatment. Notably, the VIR patients displayed a negative correlation between IP-10 plasma levels and IFN-γ or GzmB production by MAIT cells in response to E. coli stimulation (Fig. 3B). These results suggest that IFN-I signaling could be involved in the impaired function of MAIT cells during HIV-1 infection.
Fig. 3. The relevance of IFN-I signaling to MAIT cell dysfunction in HIV-1 infection.
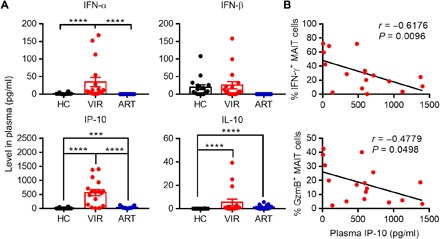
(A) The level of IFN-α, IFN-β, IP-10, and IL-10 in the plasma was detected by enzyme-linked immunosorbent assay (ELISA). (B) Correlation between the proportion of IFN-γ+ or GzmB+ MAIT cells in response to PFA-fixed E. coli and the level of IP-10 in the plasma from HIV-1–infected patients receiving (blue) or not receiving (red) ART. P values were determined by Spearman rank correlation tests.
Sustained IFN-α pretreatment suppresses the function of MAIT cells
To explore whether IFN-I influences MAIT cell response to E. coli, PBMCs were treated with IFN-α for 24, 48, or 72 hours before exposure to E. coli in vitro (Fig. 4A). Consistent with the previous report (20), MAIT cells were activated by IFN-α, indicated by the increased expression of CD69, CD38, and HLA-DR (human leukocyte antigen–DR) on the cell surface (Fig. 4B). Despite no IFN-γ or GzmB elicited, CD107a expression increased after IFN-α treatment for 24 to 48 hours. However, the level of CD107a declined when treated for 72 hours, indicating the negative regulatory effect of sustained IFN-α treatment. This was further confirmed by the observation of functional suppression in MAIT cells in response to E. coli after the sustained IFN-α pretreatment (Fig. 4, C and D). The production of IFN-γ in MAIT cells declined with treatment longer than 48 hours, while GzmB induction was suppressed as early as 24 hours of IFN-α treatment and was almost abolished after 72 hours of IFN-α treatment. A trend of decrease in CD107a expression in response to E. coli stimulation was found after 72 hours of IFN-α treatment, but it did not reach statistical significance. In addition, IFN-α treatment for 72 hours did not increase the proportion of Zombie+ or annexin V+ MAIT cells (fig. S4), excluding the possibility of cell death or apoptosis induced by IFN-α.
Fig. 4. MAIT cell response to E. coli is suppressed by IFN-α pretreatment.
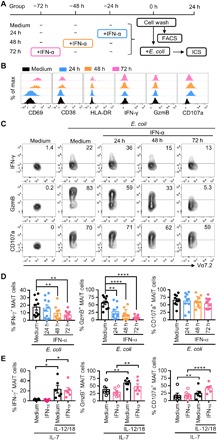
(A) Schematic diagram of the experimental setup. (B) Freshly isolated PBMCs from healthy donors were left untreated or treated with IFN-α at 100 ng/ml for 24, 48, or 72 hours and then evaluated for the expression of CD69, CD38, or HLA-DR on the surface of MAIT cells. (C and D) PBMCs pretreated with IFN-α for 24, 48, or 72 hours were subsequently stimulated with PFA-fixed E. coli at 10 MOI and then assessed for the production of IFN-γ and GzmB in MAIT cells. Representative FACS plots (C) and statistical analyses (D) are shown. (E) PBMCs pretreated with IFN-α for 72 hours in the presence of IL-7 (100 ng/ml) were then stimulated with IL-12/18 for 24 hours and then assessed for the expression level of IFN-γ and GzmB in MAIT cells.
We next set to determine whether sustained IFN-α treatment affects MAIT cell response to IL-12/18 stimulation. Because MAIT cells in PBMCs failed to respond upon IL-12/18 stimulation after 72 hours of culture in vitro, IL-7 was added at the beginning of the culture to maintain the viability of MAIT cells (Fig. 4E). In line with the previous study (12), IL-7 alone induced GzmB and CD107a but not IFN-γ production in MAIT cells, while the addition of IL-12/18 significantly induced all the IFN-γ, GzmB, and CD107a secretion. Pretreatment with IFN-α for 72 hours failed to alter MAIT cell responses to IL-7 and IL-12/18 stimulations in vitro. Together, sustained IFN-α treatment suppressed MAIT cell responses to E. coli but not to cytokine stimulation.
IFN-α–induced IL-10 inhibits MAIT cell function in response to E. coli
To explore how IFN-α impairs MAIT cell response to E. coli, we divided PBMCs obtained from healthy donors into CD3+ and CD3− populations and treated them with or without IFN-α separately for 72 hours. Then, the two cell populations were mixed together and stimulated with E. coli (Fig. 5A). The treatment of CD3− cells (Fig. 5B, blue), but not CD3+ cells (green), with IFN-α led to a similar degree of suppression in IFN-γ and GzmB production in MAIT cells as the treatment of both CD3+ and CD3− populations simultaneously (red). These results indicate that IFN-α inhibits MAIT cell function in an indirect manner through its action on CD3− cells, rather than the direct IFN-α receptor (IFNAR) signaling in MAIT cells. Because monocytes represent a major subset in CD3− cells, we asked whether this subset mediated IFN-α–induced MAIT cell dysfunction. CD3+ T cells and CD14+ monocytes were purified and cocultured in the presence or absence of IFN-α for 72 hours, followed by E. coli stimulation. IFN-γ and GzmB production were suppressed, as what we observed in PBMCs (Fig. 5C), indicating that monocytes play a crucial role in MAIT cell dysfunction induced by sustained IFN-α treatment.
Fig. 5. IFN-α induces IL-10 production from monocytes and inhibits MAIT cell function.
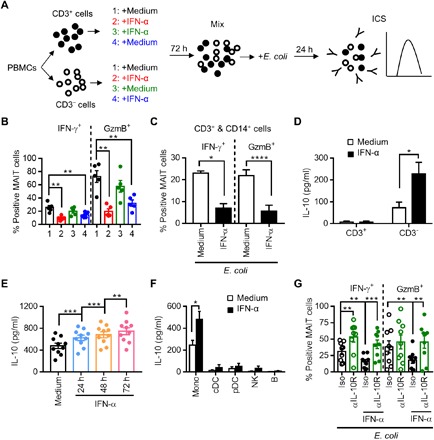
(A) Schematic of the experiment. PBMCs from healthy donors were separated into CD3+ and CD3− populations, and each population was treated or not with IFN-α at 100 ng/ml for 72 hours. The CD3+ and CD3− cell populations from each donor were then mixed back together and stimulated by E. coli for 24 hours. (B) Cells were assessed for the production of IFN-γ and GzmB in MAIT cells. (C) CD3+ and CD14+ cells were purified from PBMCs and cultured together, followed by treatment with or without IFN-α for 72 hours. The cells were then stimulated with E. coli for 24 hours and assessed for the production of IFN-γ and GzmB in MAIT cells. (D) Levels of IL-10 in the supernatant of the CD3+ or CD3− population treated with or without IFN-α for 72 hours. (E) Level of IL-10 in the supernatant of PBMCs treated with IFN-α for 24 to 72 hours. (F) Levels of IL-10 in the supernatant of different subsets of PBMCs treated with or without IFN-α for 72 hours. (G) Levels of IFN-γ and GzmB expression in MAIT cells after a 72-hour treatment of PBMCs with IFN-α in the presence of an anti–IL-10R monoclonal antibody (αIL-10R; 10 μg/ml) or isotype control, followed by E. coli stimulation.
Previous studies suggested that myeloid cell–derived IL-10 was responsible for the inhibition of T lymphocyte function by chronic IFN-I stimulation (22). Consistent with this notion, an increased level of IL-10 was detected in the supernatant of CD3− cells after IFN-α treatment, but not in that of CD3+ cells (Fig. 5D). In addition, the increasingly higher levels of IL-10 were detected in the supernatant of PBMCs treated with IFN-α for longer times (Fig. 5E). To determine the source of IL-10 induced by IFN-α, different cell subsets, including CD14+ monocytes, CD11c+ conventional dendritic cells (cDCs), BDCA2/4+ pDCs, CD56+ NK, and CD19+ B cells, were separately isolated from PBMCs, stimulated with IFN-α for 72 hours, and assessed for IL-10 production in the culture supernatant. The results showed that monocytes were the major source of both spontaneous and IFN-α–induced IL-10 (Fig. 5F). We then determined whether IL-10 acted as the mediator in IFN-α–induced MAIT cell dysfunction. Blocking the IL-10 signaling by using an anti–IL-10 receptor (IL-10R) blocking antibody effectively prevented IFN-α–induced suppression of MAIT cell response to E. coli (Fig. 5G). Together, these data indicate that the sustained IFN-α stimulation inhibits MAIT cell function mainly through IL-10 produced by monocytes.
Dysregulation in IL-12 secretion by IFN-I/IL-10 played a part in MAIT cell dysfunction
It has been known that IFN-I signaling induces the secretion of cytokines such as IL-15 (23) but inhibits the production of IL-12 in response to bacteria (24). In addition, IL-10 also inhibits IL-12 production (25). Therefore, we asked whether dysregulation in IL-12 secretion by IFN-I/IL-10 played a part in MAIT cell dysfunction. A progressive loss of IL-12 production was detected in the supernatant of PBMCs treated with IFN-α for 24 to 72 hours before E. coli stimulation (fig. S5A). Blocking IL-10R significantly restored IL-12 production, even after suppression by IFN-α treatment (fig. S5B). Moreover, supplement with IL-12 effectively prevented IL-10–induced impairment of MAIT cell response to E. coli (fig. S5C). Similarly, the addition of IL-12 fully reversed the suppression of IFN-γ production in MAIT cells induced by IFN-α and partially restored their ability to produce GzmB (fig. S5D). These results indicate that IFN-α–induced IL-10 inhibits IL-12 secretion and that the paucity of IL-12 contributes to MAIT cell dysfunction.
Blockade of IFNAR or IL-10R restores the antibacterial response of MAIT cells during HIV-1 infection
On the basis of the aforementioned results, we hypothesized that HIV-1 infection caused MAIT cell dysfunction through the IFN-I/IL-10 axis. To test this notion, we infected PBMCs with or without enriched pDCs by HIV-1 virions in vitro. An increased level of IFN-α was detected in PBMCs with enriched pDCs when inoculated with HIV-1 (Fig. 6A). Consistent with our results, significantly increased levels of IP-10 and IL-10 were induced by HIV-1 infection, which was abolished by an anti-IFNAR2 antibody (Fig. 6B), confirming that IFN-I signaling was activated in the presence of HIV-1. MAIT cells from HIV-1–infected PBMCs had significantly lower production of IFN-γ and GzmB in response to E. coli as compared to those in uninfected PBMCs (Fig. 6C). Blocking IFNAR or IL-10R during HIV-1 infection in vitro restored the responsiveness of MAIT cells against E. coli. We then analyzed the effects of the IL-12 and IL-10 pathways on the responsiveness of MAIT cells from viremic untreated (n = 6) and ART-treated (n = 8) patients (Fig. 6D and table S1). The supplement with IL-12 in the culture improved IFN-γ and GzmB but not CD107a production in MAIT cells from VIR subjects and increased the expression of all these three proteins in those from ART subjects. Blocking IL-10R also elevated IFN-γ, GzmB, and CD107a production in MAIT cells from both VIR and ART subjects.
Fig. 6. Restoration of the antibacterial function of MAIT cells in HIV-1 infection.
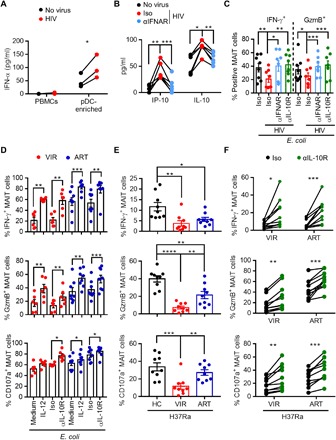
(A) PBMCs with or without pDC enrichment were stimulated with HIVNL4-3 for 24 hours before the detection of IFN-α in the supernatant by ELISA. (B) Levels of IP-10 and IL-10 in the supernatant of PBMCs stimulated with HIVNL4-3 in the presence of an anti-IFNAR2 (αIFNAR; 5 μg/ml) or isotype control antibody for 24 hours. (C) Responses of MAIT cells in PBMCs stimulated with HIVNL4-3 in the presence of an anti-IFNAR2 or anti–IL-10R antibody for 3 days before stimulation with E. coli. (D) Responses of MAIT cells from ART-treated (ART; n = 8) or untreated (VIR; n = 6) HIV-1–infected patients upon E. coli stimulation in the presence of IL-12 or the anti–IL-10R antibody. (E) Responses of MAIT cells in PBMCs from HC, VIR, or ART subjects (n = 9 in each group) to the H37Ra strain of Mtb. (F) Responses of MAIT cells from VIR or ART subjects (n = 10 in each group) upon H37Ra stimulation in the presence of an anti–IL-10R or isotype control antibody.
Patients living with HIV-1 infection have a high rate of coinfection with Mycobacterium tuberculosis (Mtb), which is one of the leading causes of AIDS-related death (26). We asked whether our strategy to restore the MAIT cell function could improve the anti-Mtb activity of MAIT cells in HIV-1–infected patients. PBMCs from HC, VIR, and ART subjects (n = 9 in each group; table S1) were stimulated with H37Ra, an attenuated tubercle bacillus, and assessed for the response of MAIT cells. MAIT cells from VIR subjects generated markedly lower levels of IFN-γ, GzmB, and CD107a compared to HC, whereas ART treatment partially restored GzmB and CD107a production (Fig. 6E). These data indicate a compromised MAIT cell function against Mtb in HIV-1–infected patients. Blockade of IL-10R markedly increased the response of MAIT cells from both VIR and ART subjects (n = 10 in each group; table S1) against H37Ra (Fig. 6F), suggestive of an improved anti-Mtb response capacity of MAIT cells in HIV-1–infected patients.
DISCUSSION
The irreversible decay of peripheral MAIT cell frequency and function in HIV-1–infected patients despite effective ART treatment poses a challenge to immune restoration attempts (10–12). Our study confirms that the functional damage of MAIT cells persists over time in treated patients and identifies chronic activation of IFN-I–dependent pathways as a driver of MAIT cell dysfunction. Immune checkpoint induction proved minimal on the surface of MAIT cells of HIV-1–infected patients and did not impair responses to bacterial antigen, emphasizing differences in the pathogenic mechanisms targeting MAIT cells and Tconv cells.
We, among others, observed a decline in the frequency of MAIT cells, accompanied by an increase in the proportion of Vα7.2+CD161− cells (data not shown), in the peripheral blood of HIV-1–infected patients as compared to HC subjects (11, 27). It was suggested that these Vα7.2+CD161− cells might originate from MAIT cells by CD161 down-regulation (11). A further study has shown that the absolute number of MAIT cells also markedly decreased in patients with HIV-1, confirming a depletion of circulation of MAIT cells during HIV-1 infection (27). In contrast, the concomitant expansion of Vα7.2+CD161− cells was only in the frequency, but not in the absolute number. In addition, Vα7.2+CD161− cells were not stained by the 5-OP-RU tetramer (27), indicating that Vα7.2+CD161− cells are a different population from MAIT cells. Moreover, by analyzing the surface immunoproteome and transcription factor profiles of MAIT cells, Dias et al. (28) have further corroborated that MAIT cells represent a distinct cell population compared with the other three T cell subsets defined by Vα7.2 and CD161.
By analyzing MAIT cell responses to E. coli antigens, we obtained evidence for a profound dysfunction of MAIT cells in viremic patients and showed that ART treatment for 2 to 5 years only partially restored IFN-γ production in MAIT cells but had little impact on GzmB or CD107a expression defects, suggesting that MAIT cells are still characterized by a deficient antibacterial activity. These findings are consistent with previous studies showing that 5 years of ART led to only partial recovery of MAIT cell function (11). Persistent MAIT cell dysfunction may be associated with the high susceptibility to tuberculosis (TB) infection characteristic of HIV-1–infected patients, which results in a major disease burden particularly in developing countries such as South Africa and China (26). Therefore, understanding the mechanism underlying MAIT cell dysfunction is pivotal for the development of immunotherapeutic intervention strategies to decrease opportunistic bacterial infections in individuals living with HIV-1.
Like other unconventional T cells, MAIT cells can be activated in both TCR-dependent and TCR-independent manners (6). Recent studies have also suggested that combined TCR signal and proinflammatory cytokines, such as IL-12 and IL-18, are essential for eliciting a robust response of MAIT cells (3, 4). Therefore, we asked which of these two major signaling pathways were impaired in MAIT cell activation during HIV-1 infection. A previous study reported that MAIT cells from treated HIV-1–infected patients had an equivalent IFN-γ production capacity upon stimulation with IL-12/18 as those from healthy donors but did not explore the response of MAITs to bacterial antigens (29). Here, we report that while MAIT cells of viremic patients maintained a response to IL-12/18 stimulation comparable to those of healthy donors, they showed markedly decreased response to E. coli antigens. Because similar antigen-specific impairment has been reported in other chronic infections, such as hepatitis C virus (HCV) and hepatitis D virus (HDV) infections (30, 31), as well as conditions such as cirrhosis (32) and autoimmune diseases (33), our study provides insights into shared mechanisms of MAIT cell dysfunction applicable to diverse diseases associated with chronic inflammation.
To explore the mechanism of MAIT cell dysfunction during HIV-1 infection, we performed scRNAseq and Abseq. Note that here we only selected CD8+ MAIT cells for analysis, without including a smaller but significant fraction of MAIT cells lacking both CD4 and CD8, which exhibit a partially distinct functional profile (34). The data showed that MAIT cells in HIV-1–infected individuals were highly activated, with up-regulation of some inhibitory receptors and ISGs. We first explored whether MAIT cell dysfunction was due to the expression of the inhibitory inhibitors PD-1, TIM-3, and LAG-3, which have been reported to be elevated on the surface of MAIT cells in patients infected with HIV-1 or HCV (11, 31), and linked to the dysfunction of MAIT cells during these chronic infections. However, we did not observe an increase in the expression of PD-1 or TIM-3 on the surface of MAIT cells from HIV-1–infected subjects compared to HC subjects. Although LAG-3 showed a slight up-regulation by both Abseq and flow cytometry, the blockade of this pathway did not improve the MAIT cell function. Thus, the functional impairment of MAIT cells during HIV-1 infection did not appear dependent on these inhibitory receptors.
Instead, we found that sustained IFN-I signaling played a crucial role in MAIT cell dysfunction during HIV-1 infection. HIV-1 infection is known to induce persistent production of IFN-I, leading to chronic activation of multiple types of immune cells, even in patients who received effective ART treatment (15, 16). The regulation of IFN-I signaling in T cells is complex. Direct and indirect effects of IFN-I can either promote or inhibit T cell activation, proliferation, differentiation, and function (35). This regulation, to a large extent, depends on the timing of IFN-I exposure relative to TCR signaling. Here, by dissecting the IFN-I effect on T and non-T cells, we found that MAIT cells were regulated by IFN-I in both direct and indirect manners. On the one hand, IFN-α could directly activate MAIT cells as shown by increasing CD69 and CD107a expression after short-term treatment. On the other hand, persistent pretreatment with IFN-α substantially inhibited the functional activity of MAIT cells in response to E. coli through an indirect manner dependent on monocytes.
We then demonstrated that the negative regulation of IFN-α on MAIT cells was mediated by IL-10, an immune regulatory cytokine known to play a critical role in the inhibition of T cell function during chronic viral infections, including HIV-1 infection, largely by altering monocyte function (22). Blockade of IL-10R was shown to rescue the in vitro proliferative capacity of HIV-1–specific CD4+ and CD8+ T cells in viremic patients and increased cytokine secretion by HIV-1–specific CD4+, but not CD8+, T cells (36). IL-10 is also involved in limiting inflammatory responses against infection with bacteria such as Mycobacterium avium (37). Here, we report that IFN-α–induced IL-10 inhibits the secretion of IL-12, an important costimulatory cytokine for MAIT cells to react robustly to TCR stimulation (4). The lack of IL-12 compromised the response of MAIT cells to bacterial stimulation. Blocking IFN-I or IL-10 signaling not only restored MAIT cell function during HIV-1 infection in vitro but also significantly improved the function of patients’ MAIT cells ex vivo. It was relevant that IL-10 blockade could rescue MAIT cell responses against TB, as these findings suggest approaches to prevent TB acquisition and pathogenesis in HIV-1–infected patients. However, it is possible that other anti-inflammatory ISG proteins, such as IL-1 receptor antagonist (IL-1RA) (38), may also contribute to the suppression of monocytes, which remains to be determined in the future.
Effective ART treatment suppresses HIV-1 replication and restores plasma IFN-α to an undetectable level. However, ISG expression is not normalized by long-term ART (>34 months) (39). Earlier studies have shown that chronic high expression of ISGs potentially distinguishes not only simian immunodeficiency virus (SIV) infection of natural hosts from pathogenic SIV infection (40) but also long-term nonprogressors from rapid progressors among HIV-1–infected individuals (41). Moreover, it is known that the levels of sCD14 and sCD163, the markers of monocyte activation, remained elevated despite ART treatment (42), indicating that activation and abnormality of monocytes, caused, in part, by IFN-α, persists throughout the course of HIV-1 infection. Here, we observed that plasma IP-10 and IL-10 levels in ART patients, although markedly decreased compared to VIR subjects, were still significantly higher than HCs. The blockade of IL-10R or supplement of IL-12 significantly improved the antibacterial activity of MAIT cells from ART patients. Therefore, we speculated that monocytes in ART patients were still suppressed by IL-10 and deficient in IL-12 production, which should be determined in further studies.
MAIT cells are abundant in mucosal sites, such as the intestine and the respiratory tract where foreign microbes have the potential to gain access to the body (43, 44). The numerical and functional changes of MAIT cells in human mucosal sites are limited during HIV-1 infection. A previous study found the frequency of MAIT cells in rectal mucosa of HIV-infected patients to be comparable to that of healthy individuals (11). Another study using human MR1–5-OP-RU tetramers found lower frequencies of MAIT cells in bronchoalveolar lavage fluid of SIV-infected versus SIV-uninfected rhesus macaques, but no significant difference in the jejunum (45). Further studies that determine the number, phenotype, and functionality of MAIT cells in mucosal sites will reveal their fates and roles in mucosal infection.
In conclusion, our findings indicate that the IFN-I/IL-10 axis plays a central role in MAIT cell dysfunction during HIV-1 infection. This study has important implications for the development of adjuvant therapy to reduce bacterial coinfections in people living with HIV-1 infection.
MATERIALS AND METHODS
Study design
The aim of this study was to understand the mechanism of MAIT cell dysfunction in HIV-1 infection. The study protocol for humans was approved by the Ethics Committee of Shenzhen Third People’s Hospital, and written informed consent was obtained from each subject. HIV-1–infected patients were recruited at the AIDS Outpatient Clinic at Shenzhen Third People’s Hospital. All patients had been infected with HIV-1 via sexual transmission. HIV-1–uninfected healthy individuals were recruited at the Physical Examination Center at Shenzhen Third People’s Hospital. Clinical characteristics and demographic information of participants are shown in table S1.
Viruses and bacteria
HIV-1NL4-3 virus stocks were prepared by expansion in CEM × 174 5.25 M7 cells and used for infection at 50 ng of p24 [determined using the ZeptoMetrix p24 enzyme-linked immunosorbent assay (ELISA) kit] for 5 × 106 cells per milliliter of RPMI 1640 with 10% fetal bovine serum, as previously described. Freshly isolated PBMCs that were not stimulated with phytohemagglutinin or IL-2 stimulation were used for infection. The Mtb strain H37Ra was prepared as previously described (46). The E. coli strain DH5α was cultured and fixed in 1% PFA for exactly 3 min.
Assay of MAIT cell function
PBMCs were incubated with PFA-fixed E. coli [10 multiplicity of infection (MOI)] for 24 hours or live H37Ra (10 MOI) for 48 hours in the presence of anti-CD28 monoclonal antibody (1.25 μg/ml) (BioLegend). For cytokine stimulation, a combination of IL-12 and IL-18 (PeproTech) was added at 100 ng/ml for 24 hours. Anti-CD107a phycoerythrin (PE)/Cy7 (1 μg/ml; BioLegend) was added at the start of culture for E. coli and IL-12/18 stimulation or for the last 24 hours for TB stimulation. Brefeldin A (BioLegend) was added during the last 6 hours of stimulation.
Antibodies and flow cytometry
Anti–CD3-APC (allophycocyanin)/Cy7 (clone SK7), anti–CD161-PE (clone HP-3G10), anti–TCR Vα7.2 (clone 3C10), anti–GzmB-FITC (fluorescein isothiocyanate) (clone QA16A02), anti–CD107a-PE/Cy7 (clone H4A3), anti–LAG-3–FITC (clone 7H2C65), anti–PD-1–PE/Cy7 (clone EH12.2H7), anti–TNF-α–APC (clone MAb11), anti–HLA-DR–FITC (clone L243), annexin V–APC, and Zombie Green Fixable Viability Kit were purchased from BioLegend. Anti–IFN-γ–APC (clone B27), anti–TIM-3–Alexa Fluor 647 (clone 7D3), anti–CD8-PE (clone RPA-T8), anti–CD69-PE/Cy7 (clone FN50), and anti–CD38-APC (clone HIT2) were from BD Biosciences.
For surface marker staining, cells were incubated with antibodies on ice for 30 min and then washed and fixed for further analysis. Intracellular staining was performed using the relevant antibodies in Perm/Wash buffer (BD Biosciences). Samples were acquired on a FACSCanto II flow cytometer (BD Biosciences), and data were further analyzed with FlowJo software v.10 (Tree Star).
Plasma and supernatant ELISA
ELISA kits were used according to the manufacturer’s instructions for assessing IFN-α (PBL Assay Science), IFN-β (PBL Assay Science), IP-10 (R&D Systems), IL-10 (BioLegend), and IL-12 (BioLegend) concentrations in the plasma from healthy subjects or HIV-1–infected patients, or in the supernatant of cultured cells.
Blocking antibody treatment
PBMCs were stimulated with PFA-fixed E. coli in the presence of anti–PD-1 (clone EH12.2H7; BioLegend), anti–LAG-3 (clone 17B4; Abcam), anti-IFNAR2 antibody (clone MMHAR-2; PBL Assay Science), or purified anti–IL-10R antibody (clone 3F9; BioLegend) at 10 μg/ml. Mouse IgG2a κ and rat IgG2a κ isotype control antibodies were used as controls.
Single-cell targeted mRNA and antibody sequencing
To capture single-cell transcriptomic and surface proteomic profiles of T cells, the BD Rhapsody system was used following the manufacturer’s instruction. Briefly, T cells were isolated from PBMCs using the Pan T Cell Isolation Kit (Miltenyi) and then labeled with 29 antibodies conjugated with polyadenylated antibody-specific barcodes (table S2), which were captured in the same manner as cellular polyadenylated RNAs. The transcriptome of single T cells was split for the construction of the Abseq library and the targeted mRNA library, which was amplified by multiplexed polymerase chain reaction with the T Cell Expression Panel for the detection of 259 genes (table S3) and then sequenced using an Illumina NovaSeq platform.
scRNAseq and Abseq analysis
The sequenced raw reads were processed with the BD Rhapsody analysis pipeline. Cell labels and molecular indices were identified, and gene identity was determined by alignment against the BD Rhapsody T Cell Expression Panel reference. Expression levels of 29 cell surface proteins were also generated for all of the cells. Seurat v.3 was used to integrate the individual mRNA and cell surface protein expression profiles for six samples, generating an integrated matrix of transcriptomic and proteomic features. The integrated matrix was analyzed by principal components analysis (PCA) and then UMAP for the top 20 principal components chosen for visualization. Meanwhile, graph-based clustering was performed on the PCA data with reduced dimension. Differential expression analysis for each cluster was performed with MAST. GO analysis was performed with DAVID, a platform for gene functional annotation.
General statistical analysis
Statistical analysis was performed using the Prism software v.7 (GraphPad). The data are represented as means with SEM. D’Agostino-Pearson omnibus or Shapiro-Wilk normality tests were used to test whether the data points were normally distributed. Paired or unpaired t tests were used to compare differences between matched or unmatched samples with a normal distribution. When data were not normally distributed, Mann-Whitney tests were used for unpaired data, whereas Wilcoxon tests were used for paired data. Spearman or Pearson tests were used for correlation analyses. All tests were two tailed. P values less than 0.05 were considered statistically significant. Asterisks indicate the degree of significance, with *P < 0.05, **P < 0.01, ***P < 0.001, or ****P < 0.0001.
Supplementary Material
Acknowledgments
We sincerely thank M. Liao and Q. Yang for providing the H37Ra strain of Mtb and X. Zhong for technical assistance in the Abseq and scRNAseq experiments. We also thank the clinical staff at Shenzhen Third Peoples’ Hospital and all study participants. Funding: We thank the National Natural Science Foundation of China (81701636 to X.T.), the National Science and Technology Major Project of Infectious Diseases (2017ZX10201101001007 to X.T.), the Science and Technology Innovation Committee of Shenzhen Municipality (JCYJ20170412151722110 to Z.Z. and JCYJ20170412151650600 to H.W.), the National Program on Key Basic Research Project (2015CB554300 to S.Z.), and the San-Ming Project of Medicine in Shenzhen (SZSM201512029 to H.W. and Z.C.) for financial support. Author contributions: X.T., S.Z., and Z.Z. conceived the project. X.T., Q.P., L.L., H.S., and L.C. performed the experiments. S.Z., Q.P., L.L., L.X., Z.C., H.W., and Z.Z. provided critical resources. X.T., S.Z., Q.P., L.L., Y.L., and Z.Z. analyzed the data. X.T., S.Z., Y.L., and Z.Z. drafted the manuscript. X.T., S.Z., L.C., L.A.C., and Z.Z. edited the manuscript. All authors read and approved the final manuscript. Competing interests: The authors declare that they have no competing interests. Data and materials availability: All data needed to evaluate the conclusions in the paper are present in the paper and/or the Supplementary Materials. Additional data related to this paper may be requested from the authors.
SUPPLEMENTARY MATERIALS
Supplementary material for this article is available at http://advances.sciencemag.org/cgi/content/full/6/8/eaaz0374/DC1
Fig. S1. Frequency and function of MAIT cells in HIV-1–infected patients during ART treatment.
Fig. S2. T cell analysis using Abseq and scRNAseq.
Fig. S3. MAIT cell dysfunction during HIV-1 infection is not ascribed to inhibitory receptors.
Fig. S4. IFN-α does not induce MAIT cell death or apoptosis.
Fig. S5. IFN-α–induced IL-10 inhibits IL-12, thus dampening MAIT cell function.
Table S1. Clinical characteristics of the enrolled participants.
Table S2. BD Rhapsody Abseq antibody panel.
Table S3. BD Rhapsody T cell expression panel.
Table S4. List of significantly up-regulated or down-regulated genes in MAIT cells from VIR versus HC subjects.
References and Notes
- 1.Tilloy F., Treiner E., Park S. H., Garcia C., Lemonnier F., de la Salle H., Bendelac A., Bonneville M., Lantz O., An invariant T cell receptor α chain defines a novel TAP-independent major histocompatibility complex class Ib-restricted α/β T cell subpopulation in mammals. J. Exp. Med. 189, 1907–1921 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Treiner E., Duban L., Bahram S., Radosavljevic M., Wanner V., Tilloy F., Affaticati P., Gilfillan S., Lantz O., Selection of evolutionarily conserved mucosal-associated invariant T cells by MR1. Nature 422, 164–169 (2003). [DOI] [PubMed] [Google Scholar]
- 3.Slichter C. K., McDavid A., Miller H. W., Finak G., Seymour B. J., McNevin J. P., Diaz G., Czartoski J. L., McElrath M. J., Gottardo R., Prlic M., Distinct activation thresholds of human conventional and innate-like memory T cells. JCI Insight 1, e86292 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kurioka A., Ussher J. E., Cosgrove C., Clough C., Fergusson J. R., Smith K., Kang Y. H., Walker L. J., Hansen T. H., Willberg C. B., Klenerman P., MAIT cells are licensed through granzyme exchange to kill bacterially sensitized targets. Mucosal Immunol. 8, 429–440 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ussher J. E., Bilton M., Attwod E., Shadwell J., Richardson R., de Lara C., Mettke E., Kurioka A., Hansen T. H., Klenerman P., Willberg C. B., CD161++ CD8+ T cells, including the MAIT cell subset, are specifically activated by IL-12+IL-18 in a TCR-independent manner. Eur. J. Immunol. 44, 195–203 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Saeidi A., Ellegård R., Yong Y. K., Tan H. Y., Velu V., Ussher J. E., Larsson M., Shankar E. M., Functional role of mucosal-associated invariant T cells in HIV infection. J. Leukoc. Biol. 100, 305–314 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Martinez-Barricarte R., Markle J. G., Ma C. S., Deenick E. K., Ramírez-Alejo N., Mele F., Latorre D., Mahdaviani S. A., Aytekin C., Mansouri D., Bryant V. L., Jabot-Hanin F., Deswarte C., Nieto-Patlán A., Surace L., Kerner G., Itan Y., Jovic S., Avery D. T., Wong N., Rao G., Patin E., Okada S., Bigio B., Boisson B., Rapaport F., Seeleuthner Y., Schmidt M., Ikinciogullari A., Dogu F., Tanir G., Tabarsi P., Bloursaz M. R., Joseph J. K., Heer A., Kong X. F., Migaud M., Lazarov T., Geissmann F., Fleckenstein B., Arlehamn C. L., Sette A., Puel A., Emile J. F., van de Vosse E., Quintana-Murci L., Di Santo J. P., Abel L., Boisson-Dupuis S., Bustamante J., Tangye S. G., Sallusto F., Casanova J. L., Human IFN-γ immunity to mycobacteria is governed by both IL-12 and IL-23. Sci. Immunol. 3, aau6759 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.van Wilgenburg B., Loh L., Chen Z., Pediongco T. J., Wang H., Shi M., Zhao Z., Koutsakos M., Nüssing S., Sant S., Wang Z., D’Souza C., Jia X., Almeida C. F., Kostenko L., Eckle S. B. G., Meehan B. S., Kallies A., Godfrey D. I., Reading P. C., Corbett A. J., McCluskey J., Klenerman P., Kedzierska K., Hinks T. S. C., MAIT cells contribute to protection against lethal influenza infection in vivo. Nat. Commun. 9, 4706 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang H., D’Souza C., Lim X. Y., Kostenko L., Pediongco T. J., Eckle S. B. G., Meehan B. S., Shi M., Wang N., Li S., Liu L., Mak J. Y. W., Fairlie D. P., Iwakura Y., Gunnersen J. M., Stent A. W., Godfrey D. I., Rossjohn J., Westall G. P., Kjer-Nielsen L., Strugnell R. A., McCluskey J., Corbett A. J., Hinks T. S. C., Chen Z., MAIT cells protect against pulmonary Legionella longbeachae infection. Nat. Commun. 9, 3350 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cosgrove C., Ussher J. E., Rauch A., Gärtner K., Kurioka A., Hühn M. H., Adelmann K., Kang Y. H., Fergusson J. R., Simmonds P., Goulder P., Hansen T. H., Fox J., Günthard H. F., Khanna N., Powrie F., Steel A., Gazzard B., Phillips R. E., Frater J., Uhlig H., Klenerman P., Early and nonreversible decrease of CD161++/MAIT cells in HIV infection. Blood 121, 951–961 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Leeansyah E., Ganesh A., Quigley M. F., Sönnerborg A., Andersson J., Hunt P. W., Somsouk M., Deeks S. G., Martin J. N., Moll M., Shacklett B. L., Sandberg J. K., Activation, exhaustion, and persistent decline of the antimicrobial MR1-restricted MAIT-cell population in chronic HIV-1 infection. Blood 121, 1124–1135 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Leeansyah E., Svärd J., Dias J., Buggert M., Nyström J., Quigley M. F., Moll M., Sönnerborg A., Nowak P., Sandberg J. K., Arming of MAIT cell cytolytic antimicrobial activity is induced by IL-7 and defective in HIV-1 Infection. PLOS Pathog. 11, e1005072 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sortino O., Richards E., Dias J., Leeansyah E., Sandberg J. K., Sereti I., IL-7 treatment supports CD8+ mucosa-associated invariant T-cell restoration in HIV-1-infected patients on antiretroviral therapy. AIDS 32, 825–828 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lehmann C., Harper J. M., Taubert D., Hartmann P., Fätkenheuer G., Jung N., Lunzen J. M., Stellbrink H. J., Gallo R. C., Romerio F., Increased interferon alpha expression in circulating plasmacytoid dendritic cells of HIV-1-infected patients. J. Acquir. Immune Defic. Syndr. 48, 522–530 (2008). [DOI] [PubMed] [Google Scholar]
- 15.Cheng L., Ma J., Li J., Li D., Li G., Li F., Zhang Q., Yu H., Yasui F., Ye C., Tsao L. C., Hu Z., Su L., Zhang L., Blocking type I interferon signaling enhances T cell recovery and reduces HIV-1 reservoirs. J. Clin. Invest. 127, 269–279 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhen A., Rezek V., Youn C., Lam B., Chang N., Rick J., Carrillo M., Martin H., Kasparian S., Syed P., Rice N., Brooks D. G., Kitchen S. G., Targeting type I interferon-mediated activation restores immune function in chronic HIV infection. J. Clin. Invest. 127, 260–268 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cheng L., Yu H., Li G., Li F., Ma J., Li J., Chi L., Zhang L., Su L., Type I interferons suppress viral replication but contribute to T cell depletion and dysfunction during chronic HIV-1 infection. JCI Insight 2, (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang Z., Cheng L., Zhao J., Li G., Zhang L., Chen W., Nie W., Reszka-Blanco N. J., Wang F. S., Su L., Plasmacytoid dendritic cells promote HIV-1-induced group 3 innate lymphoid cell depletion. J. Clin. Invest. 125, 3692–3703 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li G., Zhao J., Cheng L., Jiang Q., Kan S., Qin E., Tu B., Zhang X., Zhang L., Su L., Zhang Z., HIV-1 infection depletes human CD34+CD38- hematopoietic progenitor cells via pDC-dependent mechanisms. PLOS Pathog. 13, e1006505 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lamichhane R., Galvin H., Hannaway R. F., de la Harpe S. M., Munro F., Tyndall J. D., Vernall A. J., McCall J. L., Husain M., Ussher J. E., Type I interferons are important co-stimulatory signals during T cell receptor mediated MAIT cell activation. Eur. J. Immunol., (2019). [DOI] [PubMed] [Google Scholar]
- 21.Aman M. J., Tretter T., Eisenbeis I., Bug G., Decker T., Aulitzky W. E., Tilg H., Huber C., Peschel C., Interferon-alpha stimulates production of interleukin-10 in activated CD4+ T cells and monocytes. Blood 87, 4731–4736 (1996). [PubMed] [Google Scholar]
- 22.Said E. A., Dupuy F. P., Trautmann L., Zhang Y., Shi Y., el-Far M., Hill B. J., Noto A., Ancuta P., Peretz Y., Fonseca S. G., van Grevenynghe J., Boulassel M. R., Bruneau J., Shoukry N. H., Routy J. P., Douek D. C., Haddad E. K., Sekaly R. P., Programmed death-1-induced interleukin-10 production by monocytes impairs CD4+ T cell activation during HIV infection. Nat. Med. 16, 452–459 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mattei F., Schiavoni G., Belardelli F., Tough D. F., IL-15 is expressed by dendritic cells in response to type I IFN, double-stranded RNA, or lipopolysaccharide and promotes dendritic cell activation. J. Immunol. 167, 1179–1187 (2001). [DOI] [PubMed] [Google Scholar]
- 24.Cousens L. P., Orange J. S., Su H. C., Biron C. A., Interferon-α/β inhibition of interleukin 12 and interferon-γ production in vitro and endogenously during viral infection. Proc. Natl. Acad. Sci. U.S.A. 94, 634–639 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.D’Andrea A., Aste-Amezaga M., Valiante N. M., Ma X., Kubin M., Trinchieri G., Interleukin 10 (IL-10) inhibits human lymphocyte interferon gamma-production by suppressing natural killer cell stimulatory factor/IL-12 synthesis in accessory cells. J. Exp. Med. 178, 1041–1048 (1993). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bell L. C. K., Noursadeghi M., Pathogenesis of HIV-1 and Mycobacterium tuberculosis co-infection. Nat. Rev. Microbiol. 16, 80–90 (2018). [DOI] [PubMed] [Google Scholar]
- 27.Fernandez C. S., Amarasena T., Kelleher A. D., Rossjohn J., McCluskey J., Godfrey D. I., Kent S. J., MAIT cells are depleted early but retain functional cytokine expression in HIV infection. Immunol. Cell Biol. 93, 177–188 (2015). [DOI] [PubMed] [Google Scholar]
- 28.Dias J., Leeansyah E., Sandberg J. K., Multiple layers of heterogeneity and subset diversity in human MAIT cell responses to distinct microorganisms and to innate cytokines. Proc. Natl. Acad. Sci. U.S.A. 114, E5434–E5443 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Spaan M., Hullegie S. J., Beudeker B. J. B., Kreefft K., van Oord G. W., Groothuismink Z. M. A., van Tilborg M., Rijnders B., de Knegt R. J., Claassen M. A. A., Boonstra A., Frequencies of circulating MAIT cells are diminished in chronic HCV, HIV and HCV/HIV co-infection and do not recover during therapy. PLOS ONE 11, e0159243 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bolte F. J., O’Keefe A. C., Webb L. M., Serti E., Rivera E., Liang T. J., Ghany M., Rehermann B., Intra-hepatic depletion of mucosal-associated invariant T cells in hepatitis C virus-induced liver inflammation. Gastroenterology 153, 1392–1403.e2 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hengst J., Strunz B., Deterding K., Ljunggren H. G., Leeansyah E., Manns M. P., Cornberg M., Sandberg J. K., Wedemeyer H., Björkström N. K., Nonreversible MAIT cell-dysfunction in chronic hepatitis C virus infection despite successful interferon-free therapy. Eur. J. Immunol. 46, 2204–2210 (2016). [DOI] [PubMed] [Google Scholar]
- 32.Bolte F. J., Rehermann B., Mucosal-associated invariant T cells in chronic inflammatory liver disease. Semin. Liver Dis. 38, 60–65 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bottcher K., Rombouts K., Saffioti F., Roccarina D., Rosselli M., Hall A., Luong T., Tsochatzis E. A., Thorburn D., Pinzani M., MAIT cells are chronically activated in patients with autoimmune liver disease and promote profibrogenic hepatic stellate cell activation. Hepatology 68, 172–186 (2018). [DOI] [PubMed] [Google Scholar]
- 34.Dias J., Boulouis C., Gorin J. B., van den Biggelaar R. H. G. A., Lal K. G., Gibbs A., Loh L., Gulam M. Y., Sia W. R., Bari S., Hwang W. Y. K., Nixon D. F., Nguyen S., Betts M. R., Buggert M., Eller M. A., Broliden K., Tjernlund A., Sandberg J. K., Leeansyah E., The CD4−CD8− MAIT cell subpopulation is a functionally distinct subset developmentally related to the main CD8+ MAIT cell pool. Proc. Natl. Acad. Sci. U.S.A. 115, E11513–E11522 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Crouse J., Kalinke U., Oxenius A., Regulation of antiviral T cell responses by type I interferons. Nat. Rev. Immunol. 15, 231–242 (2015). [DOI] [PubMed] [Google Scholar]
- 36.Brockman M. A., Kwon D. S., Tighe D. P., Pavlik D. F., Rosato P. C., Sela J., Porichis F., le Gall S., Waring M. T., Moss K., Jessen H., Pereyra F., Kavanagh D. G., Walker B. D., Kaufmann D. E., IL-10 is up-regulated in multiple cell types during viremic HIV infection and reversibly inhibits virus-specific T cells. Blood 114, 346–356 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Denis M., Ghadirian E., IL-10 neutralization augments mouse resistance to systemic Mycobacterium avium infections. J. Immunol. 151, 5425–5430 (1993). [PubMed] [Google Scholar]
- 38.McNab F., Mayer-Barber K., Sher A., Wack A., O’Garra A., Type I interferons in infectious disease. Nat. Rev. Immunol. 15, 87–103 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fernandez S., Tanaskovic S., Helbig K., Rajasuriar R., Kramski M., Murray J. M., Beard M., Purcell D., Lewin S. R., Price P., French M. A., CD4+ T-cell deficiency in HIV patients responding to antiretroviral therapy is associated with increased expression of interferon-stimulated genes in CD4+ T cells. J. Infect. Dis. 204, 1927–1935 (2011). [DOI] [PubMed] [Google Scholar]
- 40.Harris L. D., Tabb B., Sodora D. L., Paiardini M., Klatt N. R., Douek D. C., Silvestri G., Muller-Trutwin M., Vasile-Pandrea I., Apetrei C., Hirsch V., Lifson J., Brenchley J. M., Estes J. D., Downregulation of robust acute type I interferon responses distinguishes nonpathogenic simian immunodeficiency virus (SIV) infection of natural hosts from pathogenic SIV infection of rhesus macaques. J. Virol. 84, 7886–7891 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rotger M., Dalmau J., Rauch A., McLaren P., Bosinger S. E., Martinez R., Sandler N. G., Roque A., Liebner J., Battegay M., Bernasconi E., Descombes P., Erkizia I., Fellay J., Hirschel B., Miró J. M., Palou E., Hoffmann M., Massanella M., Blanco J., Woods M., Günthard H. F., de Bakker P., Douek D. C., Silvestri G., Martinez-Picado J., Telenti A., Comparative transcriptomics of extreme phenotypes of human HIV-1 infection and SIV infection in sooty mangabey and rhesus macaque. J. Clin. Invest. 121, 2391–2400 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cha L., Berry C. M., Nolan D., Castley A., Fernandez S., French M. A., Interferon-α, immune activation and immune dysfunction in treated HIV infection. Clin. Transl. Immunol. 3, e10 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Napier R. J., Adams E. J., Gold M. C., Lewinsohn D. M., The role of mucosal associated invariant T cells in antimicrobial immunity. Front. Immunol. 6, 344 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sun H., Sun C., Xiao W., Sun R., Tissue-resident lymphocytes: From adaptive to innate immunity. Cell. Mol. Immunol. 16, 205–215 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vinton C., Wu F., Rossjohn J., Matsuda K., McCluskey J., Hirsch V., Price D. A., Brenchley J. M., Mucosa-associated invariant T cells are systemically depleted in simian immunodeficiency virus-infected rhesus macaques. J. Virol. 90, 4520–4529 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chen X., Zhang M., Liao M., Graner M. W., Wu C., Yang Q., Liu H., Zhou B., Reduced Th17 response in patients with tuberculosis correlates with IL-6R expression on CD4+ T Cells. Am. J. Respir. Crit. Care Med. 181, 734–742 (2010). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material for this article is available at http://advances.sciencemag.org/cgi/content/full/6/8/eaaz0374/DC1
Fig. S1. Frequency and function of MAIT cells in HIV-1–infected patients during ART treatment.
Fig. S2. T cell analysis using Abseq and scRNAseq.
Fig. S3. MAIT cell dysfunction during HIV-1 infection is not ascribed to inhibitory receptors.
Fig. S4. IFN-α does not induce MAIT cell death or apoptosis.
Fig. S5. IFN-α–induced IL-10 inhibits IL-12, thus dampening MAIT cell function.
Table S1. Clinical characteristics of the enrolled participants.
Table S2. BD Rhapsody Abseq antibody panel.
Table S3. BD Rhapsody T cell expression panel.
Table S4. List of significantly up-regulated or down-regulated genes in MAIT cells from VIR versus HC subjects.


