Abstract
The number of people affected by heart disease such as coronary artery disease and myocardial infarction increases at an alarming rate each year. Currently, the methods to treat these diseases are restricted to lifestyle change, pharmaceuticals, and eventually heart transplant if the condition is severe enough. While these treatment options are the standard for caring for patients who suffer from heart disease, limited regenerative ability of the heart restricts the effectiveness of treatment and may lead to other heart-related health problems in the future. Because of the increasing need for more effective therapeutic technologies for treating diseased heart tissue, cardiac patches are now a large focus for researchers. The cardiac patches are designed to be integrated into the patients’ natural tissue to introduce mechanical support and healing to the damaged areas. As a promising alternative, synthetic biodegradable polymer based biomaterials can be easily manipulated to customize material properties, as well as possess certain desired characteristics for cardiac patch use. This comprehensive review summarizes recent works on synthetic biodegradable cardiac patches implanted into infarcted animal models. In addition, this review describes the basic requirements that should be met for cardiac patch development, and discusses the inspirations to designing new biomaterials and technologies for cardiac patches.
Keywords: biodegradable polymer, cardiac patch, heart disease, heart infarction
1 |. INTRODUCTION
Heart disease is one of the leading causes of death and hospitalization in the US and other countries around the world. According the American Heart Association (AHA), about 1 in every 3 deaths in the US is a direct result of heart related diseases. Approximately 2,300 Americans die each day from cardiac-related illness, and nearly 92.1 billion adults are currently living with some form of cardiovascular disease or their after-effects (Benjamin et al., 2018). Diseases such as myocardial infarction (MI), or heart attack, and coronary artery disease can decrease or completely restrict blood flow to the heart, causing the surrounding myocardium to die. Challenges in treating such diseases arise because, unlike other tissues within the body, the heart lacks any significant regenerative abilities. Instead, the body’s inflammatory system produces a fibrotic response, replacing the dead myocardium with collagen-laden scar tissue. Depending on the degradation rate of dead myocardium and the synthesis rate of the new collagenous tissue, the ventricle wall can become either too thin and rupture or too thick and stiff. This stiffer tissue inhibits the heart ability to pump the blood throughout the body at a physiologically optimal pressure and volume. Because of the heart’s poor regenerative properties, treatment options for heart disease are very limited. Most patients who suffer from heart disease are permanently prescribed drugs such as ACE inhibitors and Beta blockers to lower blood pressure and relieve the heart workload. Blood thinners are prescribed to prevent future clots within the arteries, but none of these solutions promotes healing and regeneration of the heart tissue itself. While heart transplant remains an option for patients with severe enough conditions, this approach is extremely invasive and is often used as a last resort. The risk associated with this procedure coupled with the difficulty of finding donors has led researchers to look for better ways to introduce proper healing within the heart.
While preventative and medicinal techniques such as diet and lifestyle change and prescription drugs seem to be the best ways to control heart disease to date, there is an ever-increasing need for an effective treatment response that induces healing within the heart to prevent future heart disease and improve prognosis. Cardiac patches are a new and promising technology to introduce healing and repair to diseased and damaged myocardium. Utilizing a fairly basic concept, researchers are engineering scaffold constructs to act as a bandage on the heart after damage to the native cardiac tissue. Creating a cardiac patch is dependent on three main requirements: (a) a physiologically accurate scaffold microstructure, (b) a mechanical structure that supports dynamics of a beating heart, and (c) proper biocompatibility and degradation rate. Synthetic polymeric materials are particularly of interest because of their ability to be produced to such a high degree of specificity. Synthetic polymers are typically biodegradable, biocompatible, and their degradation rates can be highly controlled. Important to note is that the topics covered in this review only extend to synthetic polymeric materials for cardiac patches. Other natural and synthetic materials are being investigated for cardiac patches, but synthetic polymeric materials can be tailored on a molecular level to fit any requirement that should be met to function as an integral part of a beating heart. These considerations are just a fraction of the factors affecting the success and overall biocompatibility of a fully functional cardiac patch. In this comprehensive review, we will give a detailed overview covering the current processes and technology being utilized to create synthetic polymeric cardiac patches for successfully inducing cardiac tissue healing and regeneration after injury.
2 |. CARDIAC TISSUE MICROSTRUCTURE AND COMPOSITION AND CARDIAC PATCH REQUIREMENTS
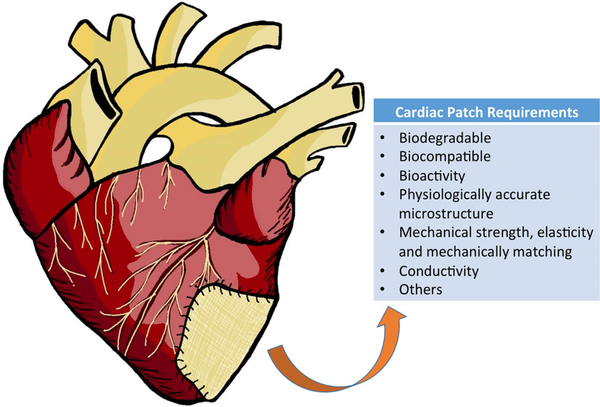
Cardiac patches are at the forefront of research, using a relatively simple concept to fulfill one of the most urgent needs in healthcare currently. Heart patches are designed to be attached to the surface of the heart; more specifically, they are meant to “patch” the dying region of the heart after MI (Figure 1). To create a therapeutic construct that will support cardiac tissue regeneration, the structure and characteristics of healthy native myocardium must be mimicked as closely as possible. Along with being both biocompatible and biodegradable, the patch microstructure, porosity, and mechanical properties should all reflect the heart’s physiological standards well enough to integrate into the heart as a functioning therapeutic component. Once implanted, the patch can host a variety of cells and biological components that dwell within the tissue. Along with being both biodegradable and biocompatible, the scaffold can also reduce the harshness of the environment around the implant, ensuring the integration into the native tissue (Cui, Yang, & Li, 2016). To design an engraftment that meets these standards, the native structure and function of the tissue itself must first be studied and understood.
FIGURE 1.
Illustration to show a cardiac patch placed on an infarcted heart, and general requirements to design a biodegradable polymeric cardiac patch
The ventricular walls of the heart consist of three distinctive layers of varying thickness, structure, and function related to mechanical strength and/or protection. The endocardium is the thin layer lining the inside of the left ventricles. This layer is collagen and elastindominant, serving to protect the inner surface of the ventricles. In a similar sense, the epicardium is a thin, protective extracellular matrix (ECM) protein-dominant layer that spans the outside surface of the ventricles. Together, these two layers encase the third and middle layer, known as myocardium. The myocardium, made up of mostly densely aligned cardiomyocytes within an intricate network of ECM components, binds directly to the surrounding two layers via the connections made by the mutually shared structural proteins. The ECM within the myocardium is mostly Types I and III collagen, mixed with a sparse distribution of elastin fibers. The final structural component affecting overall heart function is the pericardial sac, a thin collagenous membrane encasing the ventricles, separated from them by a thin layer of fluid (Shi et al., 2019). One of the many challenges in designing cardiac patches is taking the important structural components into consideration for fabricating a material that will integrate well into the complex anatomy of the heart. To do this, many researchers are attempting to mimic and improve functionality of the heart as well. Conductive polymers may integrate more successfully since they are able to participate in the pumping of the heart beginning at implantation. The success of these patches is directly related to their integration within host tissue and their ability to guide tissue development and maintain biological function. A fully integrated cardiac patch begins with materials that can support biological activity, avoid adverse host immune response, and withstand the dynamic forces of the heart including contraction, torsion, tension, and shear stresses (Jawad et al., 2007).
3 |. BIODEGRADABLE POLYMERIC CARDIAC PATCHES
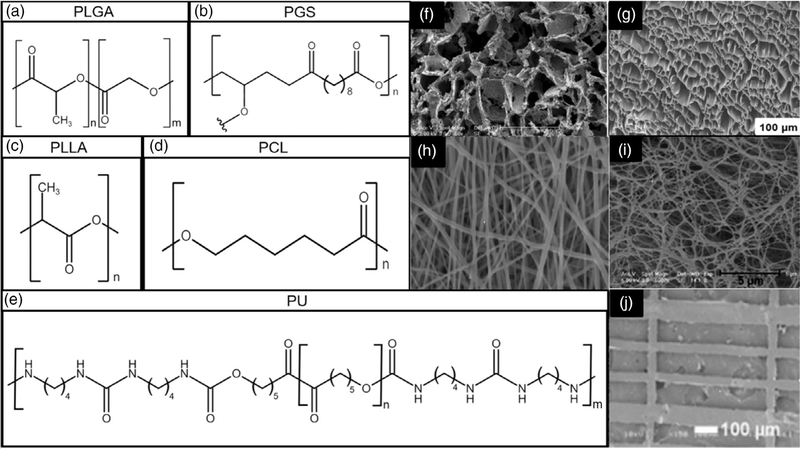
Synthetic polymeric scaffolds are excellent candidates for cardiac patch tissue engineering because they are easily tailored and fabricated to fit particular needs of the native tissues. Polymers have a wide range of mechanical properties and good biocompatibility, and their degradation rate can be easily manipulated. Additionally, synthetic polymers are known for their durability, porosity, and microstructure that can be tailored to meet the specifications of natural cardiac tissues. Though polymers as a biomaterial themselves can lead to reduced cell adhesion and scaffold integration, there are certain modifications such as the addition of stimuli and growth factors that maintain their popularity as a candidate for tissue scaffolds. The polymer chain variabilities lie in the chemical structures, molecular weights and molecular weight distribution, and functional groups that can be attached to the polymer. Cardiac scaffolds require a certain degree of elasticity and mechanical strength to withstand the dynamic nature of the heart. Many cardiac scaffolds developed incorporate a combination of polymers to achieve these properties. Blending polymers in scaffolds is utilized to incorporate the mechanical properties of different polymers to form a scaffold that contains many desirable characteristics. Through research, the development of turning polymers into biodegradable polymers have enabled a wider diversity in polymer selection (Guan, Sacks, Beckman, & Wagner, 2002). Several biodegradable polymers, such as poly(ε-caprolactone) (PCL), poly(glycerol sebacate) (PGS), poly(lactic-co-glycolic acid) (PLGA), biodegradable polyurethane (PU), and poly(l-lactide) (PLLA), are common polymers of interest for cardiac patch application research (Figure 2a–e). The fabrication methods for these materials as patches vary widely depending on a combination of material properties and physiological requirements. They can be processed scaffolds with various morphologies using different methods. Phase separation and salt leaching fabrication methods can process them into porous scaffolds (Figure 2f–g; (Hashizume, Fujimoto, et al., 2013; Ravichandran et al., 2015). Electrospinning is a common way to fabricate nano or sub-micro fibrous scaffolds (Figure 2h; He et al., 2018), while phase separation can also achieve nano-fibrous morphology (Figure 2i; Liu et al., 2015). Additionally, patches with pattern surface were fabricated using film casting (Figure 2j; Cristallini et al., 2019). The reported polymeric cardiac patches that were placed on the heart infarction animal models are summarized in Table 1.
FIGURE 2.
(a) Chemical structure of PLGA. (b) Chemical structure of PGS. (c) Chemical Structure of PLLA. (d) Chemical structure of PCL. (e) Chemical structure of a typical biodegradable PU synthesized from PCL, 1,4-diisocyanatobutane and putrescine (Guan et al., 2002). (f) PGS fabricated with salt leaching. Reproduced with permission (Radisic, M., Park, H., Martens, T. P., Salazar-Lazaro, J. E., Geng, W., Wang, Y., … Vunjak-Novakovic, G. (2008)). Copyright 2008, published by Wiley. (g) PEUU scaffold fabricated using phase separation. Reproduced with permission (Hashizume et al., 2013). Copyright 2013, published by Elsevier Ltd. (h) Electrospun GelMA/PCL. Reproduced with permission (He et al., 2018). Copyright 2018, published by Theranostics. (i) Nanofiber phase separation scaffold of PLLA. Reproduced with permission (Liu et al., 2015). Copyright 2015, published by Elsevier Ltd. (j) PLGA film cast scaffold on patterned surface. Reproduced with permission (Cristallini et al., 2019). Copyright 2019, published by Wiley
TABLE 1.
Summary of current biodegradable polymer based cardiac patches in heart infarction animal model
| Polymer | Scaffold type | Animal study results | Animal model | References |
|---|---|---|---|---|
| PCL/chitosan/ECM (porcine heart) | Film | Ingrowth and full-thickness ventricular wall repair, fast degradation. | Rat | (Pok et al., 2017) |
| PGS | Channel | Significant increase in vascularization and ventricular wall thickness. | Mouse | (Marsano et al., 2013) |
| PLGA + rMSC | Fibrous | Significant increase in cell density and local production of VEGF. | Rat | (Zhou, Zhou, Zheng, Zhang, & Hu, 2010) |
| PEUU | Porous | Maintained a similar end-diastolic, fractional area change, and ejection fraction as compared to the control that lost functionality. | Porcine | (Hashizume, Fujimoto, et al., 2013) |
| PECUU/porcine heart ECM | Fibrous | Improvement in wall thickness and gene expression, and limited heart remodeling. | Rat | (D’Amore et al., 2016) |
| PLGA/albumin | Channeled | Increase in blood vessels, improved wall thickness. | Rat | (Fleischer, Shapira, Feiner, & Dvir, 2017) |
| PCL/Col/elastin | Fibrous | High cell proliferation rate, overall improved cardiac function. | Mouse | (Liu et al., 2016) |
| PLGA/gelatin | Channeled | Improved cell presence and host integration, decrease in fibrotic response | Porcine | (Cristallini et al., 2019) |
| PLCL + hiPSC | Woven | Increased actin presence, more muscle formation in infarction area | Rat | (Sugiura, Hibino, Breuer, & Shinoka, 2016) |
| PGS/fibrinogen | Fibrous | Restored LV, high actin concentrations present, differentiation of correct cells. | Porcine | (Ravichandran, Venugopal, Mukherjee, Sundarrajan, & Ramakrishna, 2015) |
| PEUU/laminin-1 | 3D printed | Cell migration to and from patch, complete degradation. | Mouse | (Boffito et al., 2018) |
| PLLA/GCSF | Fibrous | Decreased infarction size, improved LV volume. | Rabbit | (Spadaccio et al., 2017) |
| PLLA | Porous | Improved cell differentiation, increased presence of cardiomyocytes | Mouse | (Liu et al., 2015) |
| PEEUU | Fibrous | Improved differentiation and thickness. | Rabbit | (Gu et al., 2017) |
| PLLA + VEGF | Fibrous | Improved thickness, decrease in fibrotic response | Rat | (Chung et al., 2015) |
| PCL + rMSC | Fibrous | Scaffold integration, decrease in fibrotic response | Rat | (Guex et al., 2014) |
| PCL | Fibrous | Increase in angiogenesis related growth factors | Rat | (Chen & Kan, 2018) |
| PCL/Gelatin | Fibrous | Improved cardiac function | Rat | (Wang et al., 2017) |
| PCL/Col | Fibrous | Increase in recruitment of CD29-positive cells, decrease in remodeling | Rat | (Shafiq et al., 2018) |
| PCL/GelMA–Ppy | Fibrous | Improved conductivity, uniform contraction | Rat | (He et al., 2018) |
| PGS/Gelatin | Film | Decrease in fibrotic response, improved wall thickness, reduced infarction area | Rat | (Chen et al., 2010) |
| PLLA/PCL | Porous | Decrease in fibrotic response, increase in angiogenesis related growth factors | Dog | (Ichihara, Shinoka, Matsumura, & Ikada, 2015) |
| PU (PEUU, PECUU, PCUU) | Porous | Improved LV contractility, scaffold integration | Rat | (Hashizume et al., 2013) |
| PEUU | Porous | Scaffold integration, less immune-response | Rat | (Fujimoto, Guan, Oshima, Sakai, & Wagner, 2007) |
| PEUU | Porous | Improved wall thickness | Rat | (Fujimoto et al., 2007) |
| PEUU | Porous | Contractile reserve improved | Rat | (Fujimoto et al., 2012) |
| PGS | Porous | Presence of blood vessels in acellular scaffold, presence of collagen and host myocardium in scaffold | Rat | (Radisic et al., 2008) |
| PLLA/PLGA + Triculture | Porous | Interconnected vascular from scaffold to host, increased presence of smooth muscle actin, and cell migration from host to scaffold. | Rat | (Lesman et al., 2010) |
Abbreviations: Col, collagen; ECM, extracellular matrix; GCSF, granulocyte-colony stimulating factor; GelMA, methacrylated gelatin; hiPSC, human-induced pluripotent stem cells; PCL, poly(ε-caprolactone); PCUU, poly(carbonate urethane) urea; PECUU, poly(ester carbonate urethane) urea; PEEUU, poly(ether ester urethane) urea; PEUU, poly(ester urethane) urea; PGS, poly(glycerol sebacate); PLCL, poly(L-lactide-co-caprolactone); PLGA, poly (lactic-co-glycolic acid); PLLA, poly(L-lactide); Ppy, polypyrrole; PU, polyurethane; rMSC, rat mesenchymal stem cells; Triculture, human embryonic stem cells, human umbilical vein endothelial and embryonic fibroblasts; VEGF, vascular endothelial growth factor; LV, left ventricle.
3.1 |. PCL based patches
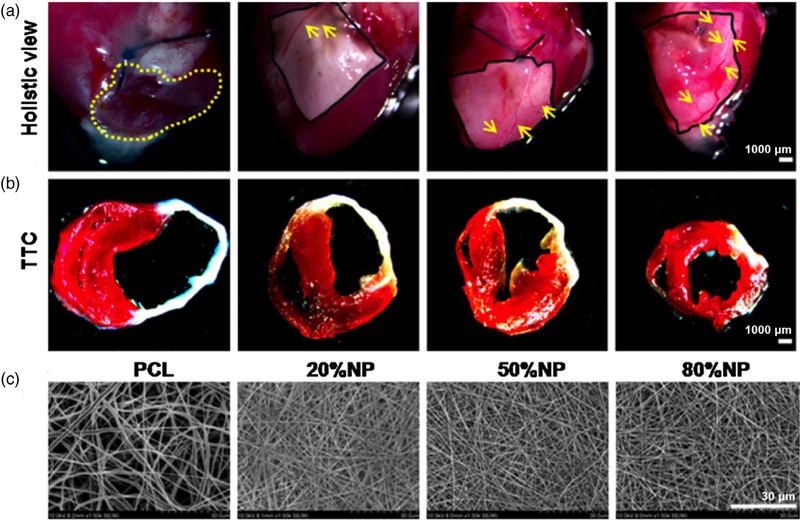
PCL is commonly used due to its rapid availability, low price, and high structural support. Earlier iterations of scaffolds involved forming meshes with PCL to support cardiomyocytes, enabling these cells to contract 3 days after seeding (Shin, Ishii, Sueda, & Vacanti, 2004). Over the course of 14 days the order of contractions and strength improved, however, cells were only able to survive on the outer portion of the patch due to poor nutrient flow into the center of the scaffold. The envelope of innovations continues to be pushed with new functionalization methods being developed and tested. Plasma functionalization has been performed on PCL scaffolds to improve and stabilize cardiac function (Guex et al., 2014). Results from the study indicate improvement in the amount of stem cells present in scar tissue. Cellular signaling indicated higher concentrations of CD68 positive cells compared to the sham, meaning gene expression in the scaffold was higher than in the untreated post-MI control group. A study using a PCL electrospun scaffold loaded with cells reported similar improvements in recovery (Chen & Kan, 2018). Rat bone marrow stem cells within the scaffold survived with high expression of VEGF. The cells were capable of surviving within the center of the scaffold because of adequate nutrient exchange. Further improving upon previous scaffold designs, a collagen/elastin/PCL scaffold was evaluated using a mouse model (Liu et al., 2016). The infarct area was significantly decreased in the scaffold with the highest concentration of natural protein and C-kit+ cells, compared to the sham operation group, the untreated MI group, the cell free PCL group, and the natural protein only group. When comparing the cell-seeded scaffold to the other experimental scaffold groups, significant improvements were seen in ejection fraction, fractional shortening, and wall thickness (Figure 3).
FIGURE 3.
(a) Macroscopic view and measurement of infarct size after 28 days post-implantation into mice. The yellow dashed line indicates the area of ventricle aneurysms. Yellow arrows point to new blood vessel branches. (b) 2,3,5-Triphenyltetrazolium chloride (TTC) staining performed on the mice groups 28 days after transplantation. Red staining denoted healthy myocardium and white staining represents the infarcted area. (c) Topography of natural proteins (elastin and collagen)/PCL electrospun sheets via scanning electron microscopy (SEM). Reprinted with permission (Liu et al., 2016). Copyright 2016, published by e-Century Publishing Corporation
Optimizations of PCL scaffolds have examined a wide array of factors on the life of cells. All of the following groups aim to improve the scaffold design either through synthesizing new combinations of polymers or utilizing different fabrication methods. For example, its effect on cellular adhesion and function are heavily influenced by fiber diameter size (Chen, Patra, Warner, & Bhowmick, 2007). Conversely, it was found that uniformity has a bigger impact on cell proliferation rather than the size of the fibers. Fabrication methods for PCL scaffolds are widely variable, with most groups focusing on electrospinning as the primary fabrication method. However, Yeong et al. (2010) used selective laser sintering to form a porous scaffold. The mechanical strengths of the highly porous structures were evaluated based on the porosity of the structure. A linear relationship between compressive stiffness and porosity was established to predict how porosity impacts the mechanical attributes. Various groups have moved from pure PCL scaffolds to scaffolds with an amalgam of polymers, both natural and synthetic, to overcome the downfalls of PCL. Incorporating both collagen and elastin into the electrospun fibrous scaffold has proven to increase cellular attachment, proliferation, and elasticity (Liu et al., 2016). The hydrophilicity is improved as well, decreasing the contact angle when more of the natural polymers are added. Additionally, fiber diameter has been shown to decrease with the addition of higher concentrations of natural polymers to PCL. The addition of collagen and elastin improved Young’s modulus and tensile strength compared to the pure PCL scaffold; however, these variables decreased when the natural polymer concentration was 50% and higher. Cell proliferation measured via Ki67 immunofluorescence staining has indicated that higher concentrations of natural polymers greatly increased cell proliferation. Alternatively, gelatin has also been used in a PCL based scaffold blend to improve cellular compatibility using electrospinning with slightly decreased tensile strength (Zhang, Ouyang, Lim, Ramakrishna, & Huang, 2005). However, the blended scaffold was capable of withstanding a greater strain than the pure PCL or pure gelatin scaffolds. Pok et al. have also examined improving the biocompatibility of PCL using chitosan in non-cardiac scaffolds (Hadjianfar, Semnani, & Varshosaz, 2018). One of the core features of the heart is the uniformity of contractions performed based on electrical pulses. Improving conductivity of scaffolds promotes better cellular signaling between the scaffold and the host. Further improving upon the gelatin–PCL scaffold, Talebi, Labbaf, & Karimzadeh (2019) synthesized a conductive PCL film by adding polypyrrole. The mechanical structure of the construct, synthesized via film casting, was improved with the introduction of polypyrrole, and its crystalline nature improved the stiffness of the scaffold while simultaneously enhancing the conductivity. Other alternatives to improving conductivity include incorporating polyaniline (PANi) as the conductive polymer to a PCL-based scaffold (Borriello, Guarino, Schiavo, Alvarez-Perez, & Ambrosio, 2011). Borriello et al. created a scaffold with 1E-5 S/cm using only 10% polyaniline in the scaffold blend. Overall, PCLbased scaffolds have shown continual improvement in complexity and as a staple material in scaffold production for cardiac patches. A complex PCL patch made by one group involved a multi-layered patch with a core of PCL coated in a chitosan and ECM gel (Pok et al., 2017). The patch was implanted and monitored for 8 weeks in a full-thickness post-MI rat model, with significant host tissue incorporation at Week 4. The multilayered patch experienced a fast degradation of the gel portion at Week 4, while the PCL core had a much slower degradation rate. The multilayer patch induced significant muscular and vascular remodeling and achieved a significantly higher right ventricular ejection fraction compared to a commercial pericardium patch.
3.2 |. PGS based patches
PGS is a synthetic polymer that has also gathered a following as a scaffold for cardiac patches (Chen et al., 2010; Marsano et al., 2013; Radisic et al., 2008; Ravichandran et al., 2015). Mechanical and degradation properties are controlled by the ratio of glycerol and sebacic acid, leading to an easily customizable polymer (Wang, Ameer, Sheppard, & Langer, 2002). PGS is a common polymer used in cardiac patches due to its mechanical and structural properties that are like that of the myocardium (Kharaziha et al., 2013; Qazi et al., 2014). Laser patterning modifications to PGS scaffolds have improved biomimic parameters by incorporating anisotropic morphologies into the scaffold. This more closely resembles native cardiac tissue, improving cell alignment. The grafts showed mechanical properties comparable to native myocardium stiffness (Engelmayr et al., 2008). Marsano et al. (2013) examined the effects of the PGS patch with VEGF on remodeling of the heart post-MI in a mouse model. The patches were implanted into the mice 1 week after an induced MI. Four weeks after implantation, a significant improvement in cardiac function in the mice with cardiac patches and growth factors was observed. Engraftment of the patch was also measured by the amount of ingrowth and thickness of the wall, with the VEGF and patch showing the most improvement over the groups with neonatal rat cardiomyocytes (RCM) alone or RCM and control skeletal muscles (SM) together. Chen et al also designed a PGS porous scaffold to examine the efficacy of this patch as a biomechanically secure cell delivery vehicle (Chen et al., 2010). The scaffolds were seeded with human embryonic stem cell-derived cardiomyocytes. This cellularized PGS scaffold produced beating cardiomyocytes suitable for implantation. The patches were implanted on the left ventricle of adult male Sprague–Dawley rats, and it indicated no alteration of important systolic or diastolic parameters after a period of 2 weeks. Similarly, Radisic et al designed a PGS cardiac patch scaffold seeded with multiple cell types (Sprague–Dawley RCM and cardiac fibroblasts) to promote cardiomyocyte proliferation, differentiation, and contractility in a nude rat model. These PGS scaffolds were prepared by porogen leaching and die-punched into disks (5 mm diameter by 2 mm thickness; Radisic et al., 2008). The RCM and fibroblasts were seeded onto the constructs using Matrigel to ensure even cell distribution. The implantation of this patch induced cardiac fibroblast attachment, which, in turn, assisted with cardiomyocyte proliferation, differentiation, and contractility. PGS has even shown success in larger animal models, where Ravichandran et al. (2015) used a porcine infarction model to test the efficacy of a PGS scaffold as biomimetic support of porcine infarcted myocardium. The PGS and fibrinogen scaffolds preceded with bone marrow-derived mesenchymal stem cells were implanted in the infarct bed of the left ventricle. This combination of materials increased the left ventricular function, as opposed to the infarct-only control group. Additionally, the presence of cardiac marker proteins showed that this scaffold was instrumental in the differentiation and proliferation of the bone marrow-derived mesenchymal stem cells into cardiac cells.
3.3 |. PLGA based patches
PLGA is a popular block copolymer used in a wide array of scaffolds. PLGA is also highly biodegradable, biocompatible, and easily customizable (Joachim et al., 2008). Zhou et al. (2010) used a PLGA patch to observe the effects on cell proliferation and ventricular remodeling. A PLGA scaffold was electrospun, and growth factors were employed to help the proliferation of cardiac cells. The PLGA patch with mesenchymal stem cells was implanted into the rat infarcted area. This scaffold had a significant increase in mesenchymal stem cell survival rate post-MI. Additionally, cardiac function and ventricular remodeling were improved in response to the patch. To improve PLGA bioactivity, Cristallini et al produced PLGA/gelatin cardiac patches functionalized with adenosine and examined their efficacy for heart healing of ischemic-reperfused hearts in a pig model (n = 4; Cristallini et al., 2019). To create the patch, blends were evenly distributed onto a mold, produced by soft lithography, to produce a microstructure like that of myocardium. After inducing heart ischemia in porcine, these 3D bioartificial patches were implanted in the affected area. The functionalization of these 3D cardiac patches promoted signaling pathways, cellularization, and a nonfibrotic outcome after 3 months of implantation.
Lesman et al. (2010) created a 50:50 PLGA:PLLA cardiac patch to promote functional vascularization. Three different cell types (human embryonic stem cell derived cardiomyocytes, endothelial cells, and embryonic fibroblasts) were suspended in a mixture of cell culture medium with Matrigel and were seeded onto the PLGA:PLLA porous scaffold for 2 weeks. Once these constructs were able to spontaneously and synchronously beat, they were implanted into the left ventricle of immunosuppressed rats. Two weeks after implantation into the left ventricle of the rats, the results of this scaffold with triple cell culture indicated that a mature tissue graft was implanted with enhanced vascularization in vivo.
3.4 |. Biodegradable PU based patches
Biodegradable PU is another popular polymer used in cardiac patch development. Expanding the validity of poly(ester urethane urea) (PEUU) as a scaffold material, rat post-infection model testing has been performed (Fujimoto, Guan, et al., 2007). Implanting PEUU scaffolds showed that after 4 weeks, the PEUU exhibited host fibroblast penetration as well as endocardial endothelization and low amounts of inflammation as compared to the polytetrafluoroethylene scaffolds used as the control. PEUU was almost completely absorbed by the host at the 12-week time point, indicating the patch was able to grow cells capable of withstanding pressure without additional structural support. Another study using PEUU in a rat model reported similar results with host integration, as well as the presence of connective tissue throughout the scaffolding and improved angiogenesis (Fujimoto, Tobita, et al., 2007). The scaffold was shown to improve the contractile function compared to the untreated infarction control group. More stiffness was also observed in the untreated infarction group, while the scaffold maintained pressure–strain curves between the treatment and both control groups (untreated healthy and untreated infarction groups). Moreso, the wall thickness of the patched hearts were nearly twice that of the control group (untreated post infarct) and contained dense, muscle-like bundles. Wagner’s group has progressed into large animal studies using polyurethane and has examined the limitations of their scaffold designs (Hashizume, Fujimoto, et al., 2013). The PEUU porous patches were implanted in pigs 2 weeks after induced MI. After 8 weeks, the wall thickness of the cardiac patch hearts was much thicker with more vascularization and less scar tissue formation. Mechanical tests showed the cardiac patch exhibited a good degree of elasticity and had low deformation.
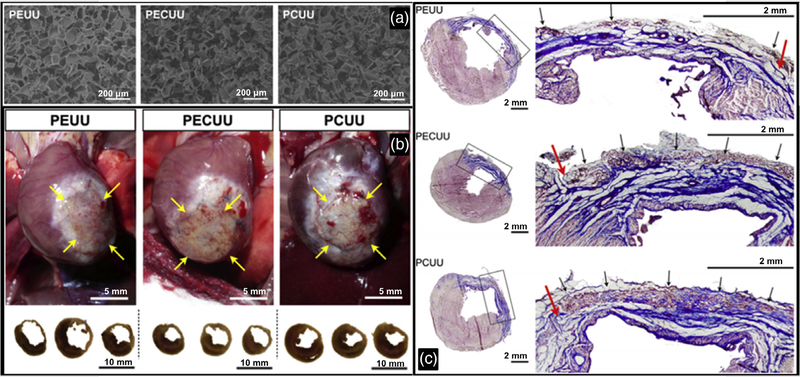
Polyurethane’s degradation and other physical and chemical properties change based on the type of polyurethane present in the polymer chain. Tests have been performed to evaluate the limitations of the various scaffold types in rat models (Hashizume, Hong, et al., 2013). Poly (ester urethane) urea (PEUU), poly(ester carbonate urethane) urea (PECUU), and poly (carbonate urethane) urea (PCUU) have progressively longer degradation times, with PCUU having the longest degradation time. Rat postinfarction model results indicate that PECUU scaffolds have significantly better cardiac output compared to the other scaffold designs. Similarly, the scaffold with the highest elastin content in scar tissue was PECUU, with a statistically significant increase compared to all other scaffold models and controls. Wall thicknesses of the three scaffold designs were not statistically different from each other, indicating all three scaffolds were viable in structural support (Figure 4). In more recent studies, a bi-layered (polyurethane and porcine heart ECM) patch was implanted in a rat model (D’Amore et al., 2016). The bilayered design had a polyurethane layer for mechanical strength with an ECM-rich layer to provide ample cellular support. This scaffold showed an improvement in vascularization with the ECM gel-coated scaffold compared to either of the aligned pure polyurethane scaffolds. Additionally, the left ventricular wall thickness was much thicker than that of the untreated infarct group. Like the healthy, untreated control group, the cardiac patch-treated group had a lower end systolic area (ESA) and end diastolic area (EDA), as well as a higher fractional area change (FAC) when compared to the untreated infarct group. Although the patch-treated heart results were similar to that of the healthy group, it is noted that the cardiac patch group was not quite as functional as the healthy group.
FIGURE 4.
(a) Scaffold cross-section electron micrograph images from PEUU, PECUU, and PCUU, respectively. Scaffolds were prepared using salt-leeching method. (b) Macroscopic images of rat heart implant sites for PEUU, PECUU, and PCUU scaffolds and their trans-sectional views at 16 weeks post-MI. (c) Representative views of Masson’s trichrome stained cross-sections of mouse hearts at 16 weeks post-MI for PEUU, PECUU, and PCUU scaffolds, where black boxes indicate the area shown in higher magnifications in the right panels, red arrows indicate suture lines placed at the time of implantation, and black arrows indicate regions with trace amount of remaining scaffold material. Reprinted with permission (Hashizume, Hong, et al., 2013). Copyright 2013, published by Elsevier Ltd
Scaffolds loaded with adeno-associated virus (AAV) have been designed to test long-term gene delivery to improve cardiac function after MI (Chung et al., 2015). Using biodegradable PEEUU and PEUU, core-sheath fibers were loaded with AAV. The patch was implanted on heart infarcted Lewis rats. The AAV was labeled with green fluorescent protein (GFP) to assess the transfection rate of the cardiac patch, with most transfection occurring during the first week, and some indications of transfection occurring up to the eighth week of study. After 12 weeks, extensive patch integration with the host body was seen with a majority of connective tissue. Muscle bundles were developed underneath the patch at the 12-week mark indicating improved regeneration of the heart. Echocardiograms showed a lower EDA, higher FAC, and higher ejection fraction (EF) in the PEEUU patch groups than the control group, with no significant difference between the unloaded and AAV-loaded PEEUU patch.
PU exhibits high elasticity and has been shown to support cardiac gap junction formation (McDevitt, Woodhouse, Hauschka, Murry, & Stayton, 2003). Alperin, Zandstra, and Woodhouse (2005) made a PU film coated in various proteins to mimic the natural microenvironment of the heart to improve biocompatibility. Collagen IV-coated PU had the best cellular proliferation on the scaffold, and cellular contractions were observed. Park et al. (2013) aimed to improve the biocompatibility of PU patches by incorporating a silk fibroin blend. The addition of silk fibroin decreased the contact angle and reduced the maximum tensile stress of the scaffold. Although pure PU proved to have superior mechanical properties, the scaffold was still within the acceptable limits required for cardiac patches. In all ratios of silk fibroin:PU, the gene expression indicating maturity was increased compared to that of pure PU. Other groups also have focused on improving the electro-conductivity of PU scaffolds. Bahrami et al incorporated graphene nanocomposites into the scaffold (Bahrami, Solouk, Mirzadeh, & Seifalian, 2019). The inclusion of graphene to the scaffold made the material semi-conductive; however, the casting film’s conductivity was greatly increased compared to the electrospun scaffold. The mechanics of the scaffold were also improved and proved to be more structurally sound when graphene was added. Cell viability was largely unaffected by the addition of graphene to the scaffold. Another example of a nanoscale additive in scaffolds is gold nano-rod (Ganji et al., 2016). The one displaying the best cell confluence contained 50 ppm of gold nano-rods, while the 100 ppm gold:PU scaffold was similar to that of pure PU.
3.5 |. PLLA based patches
PLLA has shown promise in multiple scaffold designs from gut to cardiac applications (Chung et al., 2015; Liu et al., 2015; Spadaccio et al., 2017; Xu et al., 2013). Despite the wide array of applications, a few groups have been able to use animal models to assess its capabilities (Chung et al., 2015; Liu et al., 2015; Spadaccio et al., 2017). One scaffold was composed of a PLLA mat loaded with VEGF to improve angiogenesis in the area of heart defect (Chung et al., 2015). When placed in a rat model, they determined a statistically significant increase in enjection fraction for the scaffold loaded with VEGF and cardiac stem cells (CSC) isolated from 8 week old rats. These results were compared to the PLLA mat only and control, an untreated infarct heart. Fractional shortening also exhibited a statistically significant increase compared to the control. The amount of fibrosis was significantly decreased by the presence of the cell-loaded patch, suggesting that the inclusion of the cells was the main factor in the decrease of fibrosis. One study using rabbits as their model tested a PLLA scaffold functionalized with granulocyte colony-stimulating factor (GCSF; Spadaccio et al., 2017). The scaffold was shown to improve the capillary density, indicating angiogenesis was more prevalent in the functionalized scaffold compared to that of the control and PLLA-only scaffold. The size of infarction was also decreased by a statistically significant amount in the functionalized scaffold compared to that of the control.
Porous designs have also been tested with PLLA based scaffolds. One porous design was made using sucrose leaching from a phaseseparated solution. Mice models indicated proper cell differentiation via cell markers during 7 days of implantation (Liu et al., 2015). The native mice cells migrated into the scaffold providing evidence of integration into the porous patch in the mice models. Since cardiac patches require materials that are resistant to high pressure systems, slower degrading patches have been developed using multiple layers of polymers in an effort to improve the structural support of the patch (Ichihara et al., 2015). Using a sheet of PLLA with a copolymer of lactide acid and poly(lactide-co-caprolactone) (PLCL) to make a longlasting polymeric patch. Over the course of a 6-month animal study using beagles, the collagen content of the scaffolds had increased well over the native amount, while the elastin content in the scaffold was significantly less than the native tissue. The prevalence of growth factors over the course of the 6 months decreased to levels comparable to native tissue.
4 |. FUTURE PERSPECTIVES AND CONCLUSIONS
Proper material selection is an important basis for the eventual success of the cardiac patch. While the polymers covered in this review are currently being considered by many research groups, they have varying properties that may play a large role in how the patch performs. These polymers have vastly different structures that make degradation variable. Each of these can be tailored to certain structures and molecular weights to fit many degradation time frames. All of the above-mentioned polymers (PCL, PGS, PLGA, PU, and PLLA) have excellent biocompatibility, making them desirable options for cardiac patches. Of these polymers, PGS and PLGA are known to support extensive cell growth (Rai et al., 2013; Stout, Basu, & Webster, 2011). Polyurethane (PU) promotes a cardiomyocyte friendly environment via its microbial- and thrombotic-resistance (Jaganathan, Supriyanto, Murugesan, Balaji, & Asokan, 2014; Kitsara, Agbulut, Kontziampasis, Chen, & Menasché, 2017). PCL was shown to have the higher cell retention and spreading when compared to PLLA (Castellano et al., 2014). PU and PGS are also known for its excellent physiochemical and mechanical properties with its increased shear strength and elasticity (Jaganathan et al., 2014; Rai et al., 2013). Conversely, PLGA, PLLA, and PCL are relatively mechanically rigid (Castellano et al., 2014; Park, Radisic, Lim, Chang, & Vunjak-Novakovic, 2005). PGS’s biodegradability is excellent and allows it to be completely resorbed after implantation (Kitsara et al., 2017). All of these polymers are hydrophobic without bioactivity, and it is generally required to modify the patches and improve their bioactivities. The ability to functionalize these polymers allows for easy customization depending on the needs of the individual patches.
Several of the major challenges of creating a structurally acceptable and biocompatible cardiac patch have been made clear. However, this review does not address all of the challenges that researchers face with adding cells, growth factors, and other biomaterials to achieve cardiac tissue repair. On top of the other considerations, the implant’s success is also dependent on the signaling pathways that must be activated to achieve the proper inflammatory responses, inhibit tissue degradation, and reduce adverse remodeling that leads to scar formation. These are all factors that must be considered when designing such a novel and targeted treatment to reduce heart-related death and illness by inducing healing ability where there once was none. This construct must be integrated seamlessly into the native tissue to support the proper response from the body.
Other hurdles to overcome concern commercial production and storage of these tissue engineered constructs. The production of this technology must comply with Industrial Engineering ethics and standardization so that these patches can be produced with efficiency and consistency and stored in a way that maintains a reasonable shelf-life (Gandhimathi, Muthukumaran, & Srinivasan, 2017). With heart disease affecting so many people world-wide and growing in prevalence every year, regenerative therapies are at the forefront of research to improve the outcome for patients who suffer from these diseases. Although new possibilities arise each day with so much research and funding now focusing on heart disease and cardiac patches, there are still many obstacles between research, clinical trials, and eventual wide-scale treatment.
Currently, there are no bioresorbable synthetic polymeric cardiac patches on the market or in ongoing clinical trials. The majority of post-MI treatments deal with stenting or drug administration and the patches are used only for structural support. One past clinical trial () tested a product called Anginera, a cardiac patch intended for coronary artery bypass grafting, but there have been no updates since March of 2009. Still, numerous cardiovascular patches are sold on the market for their mechanically supportive properties, non-biodegradable polytetrafluoroethylene (PTFE) and biodegradable bovine pericardium. Decellularized bovine pericardium tissue is a patch material sold on the market, produced and sold by the following companies: Neovasc Inc. (PeriPatch™), Edwards Lifesciences, Baxter (Supple Peri-Guard™), St. Jude Medical, Inc. (SJM™ Pericadial Patch with EnCap™), and LeMaitre vascular (XenoSure®). This natural material is popular for its nontoxicity and biodegradability, emphasizing its success on the market. While cardiac patches have been developed to the point of being a relatively common treatment for cardiovascular diseases, the lack of resorbable polymeric patches on market today is indicative that more research and innovation must occur before the market shifts to rely more on biodegradable polymeric-based patches.
Current and future directions of cardiac patches include the use of hybrid scaffolds; for example, polymer and polymer, polymer and decellularized ECM, polymer and hydrogel, and so forth to alter the mechanical properties and to improve the scaffold bioactivity, which can further enhance their function for heart infarction treatment. For example, PLLA/PLGA (Lesman et al., 2010), PCL/collagen/elastin (Liu et al., 2016), PCL/chitosan/ECM gel (Pok et al., 2017), and polyurethane/heart ECM (D’Amore et al., 2016) exhibited significant improvement in heart function restoration. Countless combinations exist so that tailoring the best scaffold for cardiac tissue engineering can become more of a possibility. Future efforts may focus on options such as mixing polymeric scaffolds with tissue-derived scaffolds or solid fibrous networks with hydrogels to improve the outcome. Patch conductivity has been more emphasized recently as well. Conductive polymers or particles can be combined with current cardiac patches, which can show better electric conduction. Similar to developing new conductive patches, involving other biofunctional factors with patches is also important. In addition, the development of new biodegradable polymers is also being explored. Many groups focused on using current well-known polymers, with the lack of good alternatives being a major reason. New polymer design has to be well considered in biosafety, mechanics, degradation, and processing, as well as functional involvement. For example, new polyurethanes, including low-initial modulus polyurethane (Xu, Huang, Tang, & Hong, 2017) and conductive polyurethanes (Xu et al., 2016; Xu et al., 2016), may be promising as a potential cardiac patch material. Current patch implantation requires an open-chest surgery, which presents a host of new factors that must be considered. Radisic’s group developed an minimal-invasively surgery to place an elastic patch on the heart surface (Montgomery et al., 2017), bringing new inspiration for cardiac patch evolution. Although many challenges remain in cardiac patch development progressing to clinical trials and eventually hospitals and clinics, enthusiasm in this method of treatment for heart infarction will be significantly increased with new concepts in material development, cell biology and medicine, and new surgical technique involvement.
ACKNOWLEDGMENT
We greatly thanks the partial supports from NSF CAREER (#554835, to Y.H.) and NIH R15HL140503 (to Y.H.). A.T. and K.M. are NIH T32 supported fellows (T32HL134613).
Funding information
Division of Materials Research, Grant/Award Number: 554835; National Institutes of Health, Grant/Award Numbers: R15HL140503, T32HL134613
REFERENCES
- Alperin C, Zandstra PW, & Woodhouse KA (2005). Polyurethane films seeded with embryonic stem cell-derived cardiomyocytes for use in cardiac tissue engineering applications. Biomaterials, 26(35), 7377–7386. [DOI] [PubMed] [Google Scholar]
- Bahrami S, Solouk A, Mirzadeh H, & Seifalian AM (2019). Electroconductive polyurethane/graphene nanocomposite for biomedical applications. Composites. Part B, Engineering, 168, 421–431. [Google Scholar]
- Benjamin EJ, Virani SS, Callaway CW, Chamberlain AM, Chang AR, Cheng S, … American Heart Association Council on Epidemiology and Prevention Statistics Committee and Stroke Statistics Subcommittee. (2018). Heart disease and stroke statistics-2018 update: A report from the American Heart Association. Circulation, 137(12), e67–e492. [DOI] [PubMed] [Google Scholar]
- Boffito M, Di Meglio F, Mozetic P, Giannitelli SM, Carmagnola I, Castaldo C, … Chiono V (2018). Surface functionalization of polyurethane scaffolds mimicking the myocardial microenvironment to support cardiac primitive cells. PLoS One, 13(7), e0199896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borriello A, Guarino V, Schiavo L, Alvarez-Perez MA, & Ambrosio L (2011). Optimizing PANi doped electroactive substrates as patches for the regeneration of cardiac muscle. Journal of Materials Science. Materials in Medicine, 22(4), 1053–1062. [DOI] [PubMed] [Google Scholar]
- Castellano D, Blanes M, Marco B, Cerrada I, Ruiz-Saurí A, Pelacho B, … Sepúlveda P (2014). A comparison of electrospun polymers reveals poly(3-hydroxybutyrate) fiber as a superior scaffold for cardiac repair. Stem Cells and Development, 23(13), 1479–1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen M, Patra PK, Warner SB, & Bhowmick S (2007). Role of fiber diameter in adhesion and proliferation of NIH 3T3 fibroblast on electrospun polycaprolactone scaffolds. Tissue Engineering, 13(3), 579–587. [DOI] [PubMed] [Google Scholar]
- Chen Q-Z, Ishii H, Thouas GA, Lyon AR, Wright JS, Blaker JJ, … Knowles JC (2010). An elastomeric patch derived from poly (glycerol sebacate) for delivery of embryonic stem cells to the heart. Biomaterials, 31(14), 3885–3893. [DOI] [PubMed] [Google Scholar]
- Chen WL, & Kan CD (2018). Using cell-seeded electrospun patch for myocardial injury: In-vitro and in rat model. Conference Proceedings: Annual International Conference of the IEEE Engineering in Medicine and Biology Society, 2018, 5338–5341. [DOI] [PubMed] [Google Scholar]
- Chung HJ, Kim JT, Kim HJ, Kyung HW, Katila P, Lee JH, … Lee SJ (2015). Epicardial delivery of VEGF and cardiac stem cells guided by 3-dimensional PLLA mat enhancing cardiac regeneration and angiogenesis in acute myocardial infarction. Journal of Controlled Release, 205, 218–230. [DOI] [PubMed] [Google Scholar]
- Cristallini C, Vaccari G, Barbani N, Cibrario Rocchietti E, Barberis R, Falzone M, … Giachino C (2019). Cardioprotection of PLGA/gelatine cardiac patches functionalised with adenosine in a large animal model of ischaemia and reperfusion injury: A feasibility study. Journal of Tissue Engineering and Regenerative Medicine, 13(7), 1253–1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui Z, Yang B, & Li R-K (2016). Application of biomaterials in cardiac repair and regeneration. Engineering, 2(1), 141–148. [Google Scholar]
- D’Amore A, Yoshizumi T, Luketich SK, Wolf MT, Gu X, Cammarata M, … Wagner WR (2016). Bi-layered polyurethane—Extracellular matrix cardiac patch improves ischemic ventricular wall remodeling in a rat model. Biomaterials, 107, 1–14. [DOI] [PubMed] [Google Scholar]
- Engelmayr GC, Cheng M, Bettinger CJ, Borenstein JT, Langer R, & Freed LE (2008). Accordion-like honeycombs for tissue engineering of cardiac anisotropy. Nature Materials, 7(12), 1003–1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleischer S, Shapira A, Feiner R, & Dvir T (2017). Modular assembly of thick multifunctional cardiac patches. Proceedings of the National Academy of Sciences of the United States of America, 114(8), 1898–1903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujimoto KL, Guan J, Oshima H, Sakai T, & Wagner WR (2007). In vivo evaluation of a porous, elastic, biodegradable patch for reconstructive cardiac procedures. The Annals of Thoracic Surgery, 83(2), 648–654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujimoto KL, Tobita K, Guan J, Hashizume R, Takanari K, Alfieri CM, … Wagner WR (2012). Placement of an elastic biodegradable cardiac patch on a subacute infarcted heart leads to cellularization with early developmental cardiomyocyte characteristics. Journal of Cardiac Failure, 18(7), 585–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujimoto KL, Tobita K, Merryman WD, Guan J, Momoi N, Stolz DB, … Wagner WR (2007). An elastic, biodegradable cardiac patch induces contractile smooth muscle and improves cardiac remodeling and function in subacute myocardial infarction. Journal of the American College of Cardiology, 49(23), 2292–2300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandhimathi C, Muthukumaran P, & Srinivasan DK (2017). Nanofiber composites in cardiac tissue engineering In Ramalingam M & Ramakrishna S (Eds.), Nanofiber composites for biomedical applications (pp. 411–453). Amsterdan, Netherlands: Elsevier. [Google Scholar]
- Ganji Y, Li Q, Quabius ES, Bottner M, Selhuber-Unkel C, & Kasra M (2016). Cardiomyocyte behavior on biodegradable polyurethane/gold nanocomposite scaffolds under electrical stimulation. Materials Science & Engineering. C, Materials for Biological Applications, 59, 10–18. [DOI] [PubMed] [Google Scholar]
- Gu X, Matsumura Y, Tang Y, Roy S, Hoff R, Wang B, & Wagner WR (2017). Sustained viral gene delivery from a microfibrous, elastomeric cardiac patch to the ischemic rat heart. Biomaterials, 133, 132–143. [DOI] [PubMed] [Google Scholar]
- Guan J, Sacks MH, Beckman EJ, & Wagner WR (2002). Synthesis, characterization, and cytocompatibility of elastomeric, biodegradable poly(ester-urethane)ureas based on poly(caprolactone) and putrescine. Journal of Biomedical Materials Research, 61(3), 493–503. [DOI] [PubMed] [Google Scholar]
- Guex AG, Frobert A, Valentin J, Fortunato G, Hegemann D, Cook S, … Giraud MN (2014). Plasma-functionalized electrospun matrix for biograft development and cardiac function stabilization. Acta Biomaterialia, 10(7), 2996–3006. [DOI] [PubMed] [Google Scholar]
- Hadjianfar M, Semnani D, & Varshosaz J (2018). Polycaprolactone/chitosan blend nanofibers loaded by 5-fluorouracil: An approach to anticancer drug delivery system. Polymers for Advanced Technologies, 29(12), 2972–2981. [Google Scholar]
- Hashizume R, Fujimoto KL, Hong Y, Guan J, Toma C, Tobita K, & Wagner WR (2013). Biodegradable elastic patch plasty ameliorates left ventricular adverse remodeling after ischemia-reperfusion injury: A preclinical study of a porous polyurethane material in a porcine model. The Journal of Thoracic and Cardiovascular Surgery, 146(2), 391–399 e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashizume R, Hong Y, Takanari K, Fujimoto KL, Tobita K, & Wagner WR (2013). The effect of polymer degradation time on functional outcomes of temporary elastic patch support in ischemic cardiomyopathy. Biomaterials, 34(30), 7353–7363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Y, Ye G, Song C, Li C, Xiong W, Yu L, … Wang L (2018). Mussel-inspired conductive nanofibrous membranes repair myocardial infarction by enhancing cardiac function and revascularization. Theranostics, 8(18), 5159–5177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichihara Y, Shinoka T, Matsumura G, & Ikada Y (2015). Yamazaki K. A new tissue-engineered biodegradable surgical patch for high-pressure systems dagger. Interactive Cardiovascular and Thoracic Surgery, 20(6), 768–776. [DOI] [PubMed] [Google Scholar]
- Jaganathan SK, Supriyanto E, Murugesan S, Balaji A, & Asokan MK (2014). Biomaterials in cardiovascular research: Applications and clinical implications. BioMed Research International, 2014, 459465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jawad H, Ali NN, Lyon AR, Chen QZ, Harding SE, & Boccaccini AR (2007). Myocardial tissue engineering: A review. Journal of Tissue Engineering and Regenerative Medicine, 1(5), 327–342. [DOI] [PubMed] [Google Scholar]
- Joachim SCL, Jason Tan WL, Khoa SM, Chia NK, Venkatraman S, & Boey F (2008). Hydrolytic degradation characteristics of irradiated multi-layered PLGA films. International Journal of Pharmaceutics, 360(1–2), 228–230. [DOI] [PubMed] [Google Scholar]
- Kharaziha M, Nikkhah M, Shin SR, Annabi N, Masoumi N, Gaharwar AK, … Khademhosseini A (2013). PGS:Gelatin nanofibrous scaffolds with tunable mechanical and structural properties for engineering cardiac tissues. Biomaterials, 34(27), 6355–6366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitsara M, Agbulut O, Kontziampasis D, Chen Y, & Menasché P (2017). Fibers for hearts: A critical review on electrospinning for cardiac tissue engineering. Acta Biomaterialia, 48, 20–40. [DOI] [PubMed] [Google Scholar]
- Lesman A, Habib M, Caspi O, Gepstein A, Arbel G, Levenberg S, & Gepstein L (2010). Transplantation of a tissue-engineered human vascularized cardiac muscle. Tissue Engineering. Part A, 16(1), 115–125. [DOI] [PubMed] [Google Scholar]
- Liu Q, Tian S, Zhao C, Chen X, Lei I, Wang Z, & Ma PX (2015). Porous nanofibrous poly(L-lactic acid) scaffolds supporting cardiovascular progenitor cells for cardiac tissue engineering. Acta Biomaterialia, 26, 105–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Xu Y, Wang Z, Wen D, Zhang W, Schmull S, Li H, Chen Y, Xue S (2016). Electrospun nanofibrous sheets of collagen/elastin/-polycaprolactone improve cardiac repair after myocardial infarction. American Journal of Translational Research, 8(4), 1678–1694. [PMC free article] [PubMed] [Google Scholar]
- Marsano A, Maidhof R, Luo J, Fujikara K, Konofagou EE, Banfi A, & Vunjak-Novakovic G (2013). The effect of controlled expression of VEGF by transduced myoblasts in a cardiac patch on vascularization in a mouse model of myocardial infarction. Biomaterials, 34(2), 393–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDevitt TC, Woodhouse KA, Hauschka SD, Murry CE, & Stayton PS (2003). Spatially organized layers of cardiomyocytes on biodegradable polyurethane films for myocardial repair. Journal of Biomedical Materials Research. Part A, 66(3), 586–595. [DOI] [PubMed] [Google Scholar]
- Montgomery M, Ahadian S, Davenport Huyer L, Lo Rito M, Civitarese RA, Vanderlaan RD, … Radisic M (2017). Flexible shape-memory scaffold for minimally invasive delivery of functional tissues. Nature Materials, 16(10), 1038–1046. [DOI] [PubMed] [Google Scholar]
- Park H, Radisic M, Lim JO, Chang BH, & Vunjak-Novakovic G (2005). A novel composite scaffold for cardiac tissue engineering. In Vitro Cellular & Developmental Biology- Animal, 41(7), 188–196. [DOI] [PubMed] [Google Scholar]
- Park HS, Gong MS, Park JH, Moon SI, Wall IB, Kim HW, … Knowles JC (2013). Silk fibroin-polyurethane blends: Physical properties and effect of silk fibroin content on viscoelasticity, biocompatibility and myoblast differentiation. Acta Biomaterialia, 9(11), 8962–8971. [DOI] [PubMed] [Google Scholar]
- Pok S, Stupin IV, Tsao C, Pautler RG, Gao Y, Nieto RM, … Jacot JG (2017). Full-thickness heart repair with an engineered multilayered myocardial patch in rat model. Advanced Healthcare Materials, 6(5), 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qazi TH, Rai R, Dippold D, Roether JE, Schubert DW, Rosellini E, … Boccaccini AR (2014). Development and characterization of novel electrically conductive PANI-PGS composites for cardiac tissue engineering applications. Acta Biomaterialia, 10(6), 2434–2445. [DOI] [PubMed] [Google Scholar]
- Radisic M, Park H, Martens TP, Salazar-Lazaro JE, Geng W, Wang Y, … Vunjak-Novakovic G (2008). Pre-treatment of synthetic elastomeric scaffolds by cardiac fibroblasts improves engineered heart tissue. Journal of Biomedical Materials Research. Part A, 86(3), 713–724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rai R, Tallawi M, Barbani N, Frati C, Madeddu D, Cavalli S, … Boccaccini AR (2013). Biomimetic poly(glycerol sebacate) (PGS) membranes for cardiac patch application. Materials Science & Engineering. C, Materials for Biological Applications, 33(7), 3677–2687. [DOI] [PubMed] [Google Scholar]
- Ravichandran R, Venugopal JR, Mukherjee S, Sundarrajan S, & Ramakrishna S (2015). Elastomeric core/shell nanofibrous cardiac patch as a biomimetic support for infarcted porcine myocardium. Tissue Engineering. Part A, 21(7–8), 1288–1298. [DOI] [PubMed] [Google Scholar]
- Shafiq M, Zhang Y, Zhu D, Zhao Z, Kim DH, Kim SH, & Kong D (2018). In situ cardiac regeneration by using neuropeptide substance P and IGF-1C peptide eluting heart patches. Regenerative Biomaterials, 5(5), 303–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi X, Liu Y, Copeland KM, McMahan SR, Zhang S, Butler JR, … Liao J (2019). Epicardial prestrained confinement and residual stresses: A newly observed heart ventricle confinement interface. Journal of the Royal Society Interface, 16(152), 20190028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin M, Ishii O, Sueda T, & Vacanti JP (2004). Contractile cardiac grafts using a novel nanofibrous mesh. Biomaterials, 25(17), 3717–3723. [DOI] [PubMed] [Google Scholar]
- Spadaccio C, Nappi F, De Marco F, Sedati P, Taffon C, Nenna A, … Rainer A (2017). Implantation of a poly-L-Lactide GCSF-functionalized scaffold in a model of chronic myocardial infarction. Journal of Cardiovascular Translational Research, 10(1), 47–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stout DA, Basu B, & Webster TJ (2011). Poly(lactic-co-glycolic acid): Carbon nanofiber composites for myocardial tissue engineering applications. Acta Biomaterialia, 7(8), 3101–3112. [DOI] [PubMed] [Google Scholar]
- Sugiura T, Hibino N, Breuer CK, & Shinoka T (2016). Tissue-engineered cardiac patch seeded with human induced pluripotent stem cell derived cardiomyocytes promoted the regeneration of host cardiomyocytes in a rat model. Journal of Cardiothoracic Surgery, 11(1), 163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talebi A, Labbaf S, & Karimzadeh F (2019). A conductive film of chitosan-polycaprolcatone-polypyrrole with potential in heart patch application. Polymer Testing, 75, 254–261. [Google Scholar]
- Wang QL, Wang HJ, Li ZH, Wang YL, Wu XP, & Tan YZ (2017). Mesenchymal stem cell-loaded cardiac patch promotes epicardial activation and repair of the infarcted myocardium. Journal of Cellular and Molecular Medicine, 21(9), 1751–1766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Ameer GA, Sheppard BJ, & Langer R (2002). A tough biodegradable elastomer. Nature Biotechnology, 20(6), 602–606. [DOI] [PubMed] [Google Scholar]
- Xu B, Rollo B, Stamp LA, Zhang D, Fang X, Newgreen DF, & Chen Q (2013). Non-linear elasticity of core/shell spun PGS/PLLA fibres and their effect on cell proliferation. Biomaterials, 34(27), 6306–6317. [DOI] [PubMed] [Google Scholar]
- Xu C, Huang Y, Tang L, & Hong Y (2017). Low-initial-modulus biodegradable polyurethane elastomers for soft tissue regeneration. ACS Applied Materials & Interfaces, 9(3), 2169–2180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu C, Huang Y, Yepez G, Wei Z, Liu F, Bugarin A, … Hong Y (2016). Development of dopant-free conductive bioelastomers. Scientific Reports, 6, 34451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu C, Yepez G, Wei Z, Liu F, Bugarin A, & Hong Y (2016). Synthesis and characterization of conductive, biodegradable, elastomeric polyurethanes for biomedical applications. Journal of Biomedical Materials Research. Part A, 104(9), 2305–2314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeong WY, Sudarmadji N, Yu HY, Chua CK, Leong KF, Venkatraman SS, … Tan LP (2010). Porous polycaprolactone scaffold for cardiac tissue engineering fabricated by selective laser sintering. Acta Biomaterialia, 6(6), 2028–2034. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Ouyang H, Lim CT, Ramakrishna S, & Huang ZM (2005). Electrospinning of gelatin fibers and gelatin/PCL composite fibrous scaffolds. Journal of Biomedical Materials Research. Part B, Applied Biomaterials, 72(1), 156–165. [DOI] [PubMed] [Google Scholar]
- Zhou Q, Zhou JY, Zheng Z, Zhang H, & Hu SS (2010). A novel vascularized patch enhances cell survival and modifies ventricular remodeling in a rat myocardial infarction model. The Journal of Thoracic and Cardiovascular Surgery, 140(6), 1388–1396. [DOI] [PubMed] [Google Scholar]