Abstract
Objectives
This study investigated the indirect effect of calcium-enriched mixture (CEM) cement and mineral trioxide aggregate (MTA), as 2 calcium silicate-based hydraulic cements, on human dental pulp stem cells (hDPSCs) through different dentin thicknesses.
Materials and Methods
Two-chamber setups were designed to simulate indirect pulp capping (IPC). Human molars were sectioned to obtain 0.1-, 0.3-, and 0.5-mm-thick dentin discs, which were placed between the 2 chambers to simulate an IPC procedure. Then, MTA and CEM were applied on one side of the discs, while hDPSCs were cultured on the other side. After 2 weeks of incubation, the cells were removed, and cell proliferation, morphology, and attachment to the discs were evaluated under scanning electron microscopy (SEM). Energy-dispersive X-ray (EDXA) spectroscopy was performed for elemental analysis. Alkaline phosphatase (ALP) activity was assessed quantitatively. The data were analyzed using the Kruskal-Wallis and Mann-Whitney tests.
Results
SEM micrographs revealed elongated cells, collagen fibers, and calcified nucleations in all samples. EDXA verified that the calcified nucleations consisted of calcium phosphate. The largest calcifications were seen in the 0.1-mm-thick dentin subgroups. There was no significant difference in ALP activity across the CEM subgroups; however, ALP activity was significantly lower in the 0.1-mm-thick dentin subgroup than in the other MTA subgroups (p < 0.05).
Conclusions
The employed capping biomaterials exerted biological activity on hDPSCs, as shown by cell proliferation, morphology, and attachment and calcific precipitations, through 0.1- to 0.5-mm-thick layers of dentin. In IPC, the bioactivity of these endodontic biomaterials is probably beneficial.
Keywords: Calcium-enriched mixture cement, Dental pulp, Dental pulp capping, Endodontics, Mineral trioxide aggregate, Stem cells
INTRODUCTION
Restoration of deep carious lesions that extend close to the dental pulp is a challenge for clinicians. A common approach for the management of dental caries, irrespective of their extent, is to remove the entire infected/affected dentin and provide a well-mineralized dentin base for the restorative material [1]. However, recent evidence supports less invasive carious lesion management [2]. Therefore, preventive and conservative approaches have become increasingly popular, and pulp-preserving techniques have been deemed a priority for the management of deep carious lesions [3].
Pulp capping techniques can be considered as ways to protect the dental pulp from bacterial, mechanical, chemical, and thermal stimulation caused by caries, attrition, erosion, and restorative treatments [4]. In direct pulp capping (DPC) procedures, a pulp-protecting agent with pharmaceutical properties is directly applied over the exposed pulp [3]. Instead, in indirect pulp capping (IPC), caries-affected dentin close to the pulp is not removed to prevent pulp exposure; instead, it is capped with a suitable biomaterial [5]. In addition, evidence has shown that teeth can remain asymptomatic for years after IPC, and that the pulp can maintain its reparative potential, thereby inducing tertiary dentin that generates a barrier against bacterial invasion and the extension of caries [5]. The 1-year results of a recent randomized clinical trial revealed that among 4 vital pulp therapy techniques, the radiographic success rate of IPC was 100% [6]. Moreover, IPC is simple, favored by patients, and cheaper than root canal treatment.
Several (bio)materials are employed for pulp capping procedures. An ideal antibacterial capping agent must be able to stimulate the dentinogenic potential of dental pulp cells to promote tertiary dentin secretion [7]. The bioactivity of various pulp capping materials is assessed by measuring the thickness of the formed dentinal bridge, its morphology, the severity of pulp inflammation, and the presence of odontoblasts [4]. Bioactive calcium silicate-based endodontic materials such as mineral trioxide aggregate (MTA) and calcium-enriched mixture (CEM) cement are among the commonly used pulp-protecting agents for primary and permanent teeth [8,9].
MTA induces the proliferation and differentiation of stem cells and results in the formation of a dentinal bridge, which thickens over time [10,11,12]. Moreover, MTA decreases pulpal inflammation and has an inhibitory effect on osteoclast differentiation and activity [13,14]. However, MTA has some drawbacks, such as a long setting time, high cost, and potential for discoloration [15]. To overcome the disadvantages of MTA, other calcium silicate-based hydraulic cements, such as CEM cement, have been introduced. Histological findings have shown that the efficacy of CEM cement in the formation of dentinal bridges is similar to that of MTA [16].
The thickness of the remaining dentin is an important factor in IPC, since it affects the quantity of molecular penetration of filling/capping biomaterials towards the dental pulp [17]. In addition, it may inhibit the bioactivity of the capping agent on dental pulp cells and subsequent formation of the dentinal bridge [18]. Therefore, the passage of bioactive ingredients through dentin and their efficacy in the presence of different thicknesses of remaining dentin are important parameters that need to be further evaluated.
This aim of this study was to assess the effects of MTA and CEM cement, as bioactive capping materials, through different thicknesses of dentin on the proliferation and differentiation of human dental pulp stem cells (hDPSCs).
MATERIALS AND METHODS
This study was approved by the Ethics Committee of Shahid Beheshti University of Medical Sciences (ir.sbmu.irds.rec.1394.96).
Preparation of dentin discs
Forty sound human molar teeth that had been extracted due to periodontal complications were collected. The teeth were rinsed in water and soft tissue residue was removed. Afterwards, they were immersed in 0.5% chloramine T solution, and then, mounted in a transparent acrylic resin up to the cementoenamel junction in order to prevent fracture during sectioning. The teeth were sectioned into 0.1-, 0.3-, and 0.5-mm-thick slices (± 0.05 mm) using Accutom-50 (Struers, Ballerup, Denmark) with a crosshead speed of 0.005–3 mm/sec. All discs were obtained from the middle third of the teeth. After sectioning, each disc was measured by a caliper (Velleman, Ghent, Belgium) with an accuracy of 0.001 mm. The appropriate slices were then selected and divided into 3 groups with thicknesses of 0.10 ± 0.05 mm, 0.30 ± 0.05 mm, and 0.50 ± 0.05 mm. Each group was randomly divided into 2 subgroups (n = 5).
Fabrication of setups
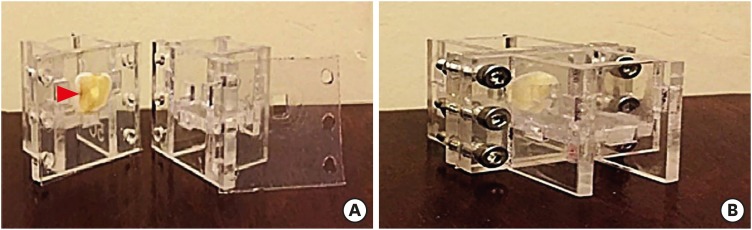
The setups needed for the experiment included 1) 2 plexiglass rectangular containers, measuring 2 × 2 × 2 cm for the placement of materials and cells, and 2) a circular hole measuring 4 × 4 mm for the placement of the dentin discs (Figure 1). The dentin discs and containers were autoclaved (121°C for 10 minutes). The containers, along with silicone sheets, were placed under a class B hood and subjected to ultraviolet (UV) irradiation for 30 minutes from each side. All instruments were placed under a sterile hood and subjected to UV irradiation for 20 minutes.
Figure 1. The setup for the experiment. (A) Separated view; (B) Assembled view. The setup had 1) 2 plexiglass rectangular chambers for the placement of materials/cells and 2) a hole (red arrowhead) for placement of the dentin discs.
The powder and liquid of ProRoot MTA (Dentsply, Tulsa, OK, USA) or CEM cement (BioniqueDent, Tehran, Iran) were mixed on glass slabs according to manufacturers' instructions and then applied to the designated holes. A sterile cotton pellet was placed over the cement and compressed to obtain a smooth surface. Each group (consisting of 5 setups) was separately transferred from the hood to an incubator (95% humidity, 5% CO2, and 37°C). In the incubator, the containers were opened. The setups remained in the incubator for 24 hours to absorb moisture to promote adequate setting of the biomaterials.
Cell culture
Dental pulp mesenchymal stem cells were isolated from a freshly sound extracted third molar. The cells were isolated by enzymatic digestion of pulpal tissue using of 0.1% collagenase type I (3 mg/mL, 30 minutes, and 37°C) (Sigma-Aldrich, St. Louis, MO, USA). After reaching 70%–80% confluency, cells were collected and passaged. Third-passage cells were used for the experiment. Surface markers were defined at the third passage through flow cytometry, using antibodies against CD29, CD44, CD49b, CD90, and STRO1. The isolated hDPSCs were then suspended in ‘Dulbecco's Modified Eagle's Medium (Sigma-Aldrich)’ containing 15% fetal bovine serum and 1% penicillin-streptomycin, and subsequently incubated at 37°C, 5% CO2, and 95% humidity.
The hole containing CEM cement or MTA was then filled with a sterile cotton pellet, and 2.5 mL of the same culture medium was poured over it. All setups were stored in large containers and incubated. Every 2 days, all setups were placed under the hood and the culture medium of the cells was carefully refreshed. The culture medium for the biomaterials was added or refreshed whenever required.
Observation of cell morphology under scanning electron microscopy (SEM)
After 2 weeks, 1 setup in each group was rinsed with phosphate-buffered saline (PBS) and immersed in 2.5% glutaraldehyde for 2 hours. The samples were then rinsed with PBS 3 times and fixed in 1% osmium tetroxide for 2 hours. They were rinsed again with PBS 3 times and dehydrated using graded ethanol (30%–100%). They were immersed in each concentration for 15 minutes. The solutions were then rinsed and the samples were placed under the hood to dry. The samples were then gold-coated and observed under SEM (EM3200, KYKY, Beijing, China) through 4-mm2 square windows onto the dentin discs.
Energy-dispersive X-ray (EDXA) spectroscopy for elemental analysis
Since SEM revealed numerous nodules on the samples of MTA and CEM cement with 0.1-mm-thick dentin, EDXA spectroscopy was performed for further analysis of the elements [19].
Assessment of alkaline phosphatase (ALP) activity
To assess ALP activity, an ALP assay kit (Abcam, Cambridge, MA, USA) was used and prepared according to the manufacturer's instructions. After detachment of cells from the dentin discs and their subsequent lysis a solution for further analysis was prepared using the ALP assay kit. The solution was then divided into 5 different ALP concentrations (30%, 40%, 50%, 60%, and 80% activity) and added to the microplates for each setup; thus, 5 samples for each group were obtained. Optical density was read by a microplate reader (ELX-800, Biotek, Winooski, VT, USA) at a wavelength of 405 nm. The values were recorded/calculated to determine ALP activity.
Statistical analysis
The collected data were analyzed using SPSS version 22 (IBM Corp., Armonk, NY, USA). The Kruskal-Wallis test was used for the comparison of the 6 groups, and the Mann-Whitney test was used for pairwise comparisons. The statistical significance threshold for between-group comparisons was set at 5% (p < 0.05).
RESULTS
SEM
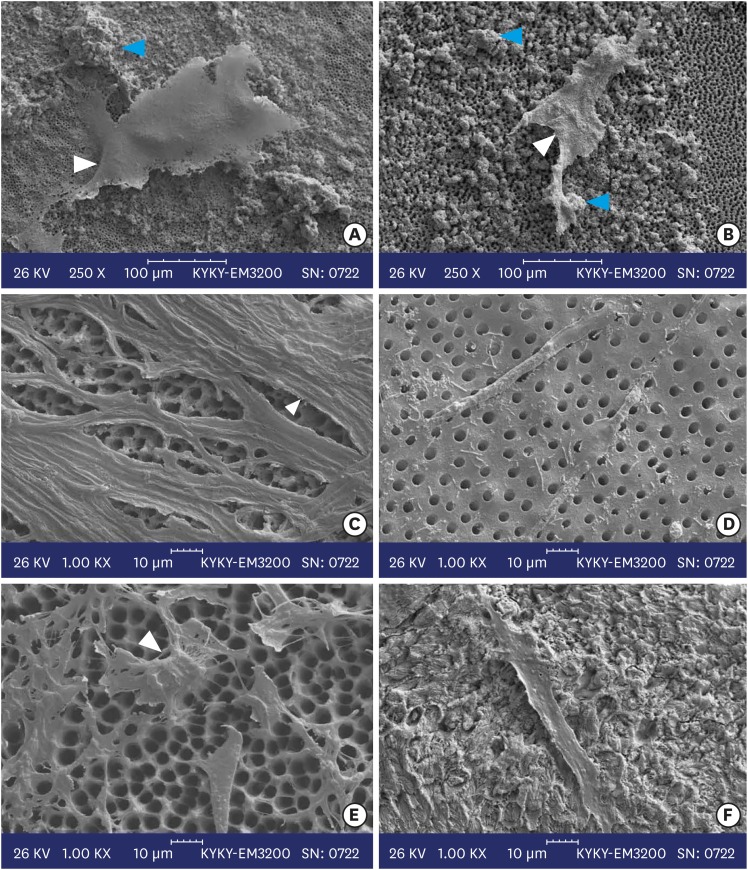
SEM micrographs of cultured hDPSCs in the MTA and CEM subgroups with 0.1-, 0.3-, and 0.5-mm-thick dentin were evaluated (Figure 2). In all the experimental samples, elongated cells were noted. In the CEM subgroups, the density of cells was greater than in the MTA subgroups; especially in the subgroup with 0.3-mm-thick dentin. In both the CEM and MTA subgroups with 0.1-mm-thick dentin, collagen fibers, calcified nodules, and mineralized foci were seen and the border between the 4 mm2 square-shaped window and the surrounding area (dentin disc without cells and biomaterials) was completely identifiable. In the group with 0.5-mm-thick dentin, relative to the group with a 0.1 mm thickness of dentin, lower cell density, a higher number of scattered cells, and smaller, limited calcified areas were identified.
Figure 2. Scanning electron micrographs of calcium-enriched mixture cement (left column) with (A) 0.1 mm, (C) 0.3 mm, and (E) 0.5 mm dentin thicknesses and mineral trioxide aggregate cement (right column) with (B) 0.1 mm, (D) 0.3 mm, and (F) 0.5 mm dentin thicknesses. White arrowheads indicate elongated cells and blue arrow heads show calcified nodules.
EDXA
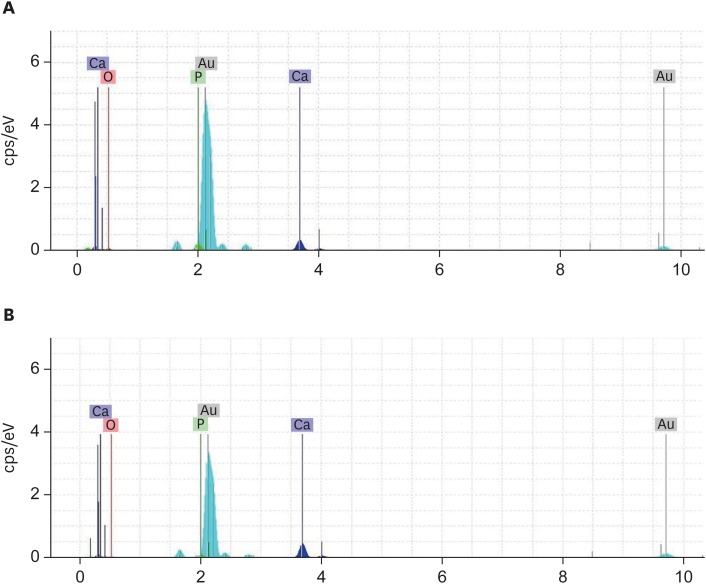
As shown in Figure 3, calcium and phosphorus ions were predominant in the examined nodules in both subgroups.
Figure 3. Energy-dispersive X-ray spectroscopy of the (A) Calcium-enriched mixture and (B) Mineral trioxide aggregate groups with 0.1-mm-thick dentin. Calcium and phosphorus ions were the predominant ions in the experimental groups.
ALP activity
The Kruskal-Wallis test showed a significant difference in ALP activity among the 6 groups (p < 0.05, Table 1). The Mann-Whitney test was used for pairwise comparisons (Table 1) and showed that in a comparison between CEM cement and MTA, there were significant differences for the 5 concentrations between the CEM and MTA groups. ALP activity was significantly higher in the CEM group with 0.1-mm-thick dentin discs (p < 0.05).
Table 1. Alkaline phosphatase (ALP) activity at various concentrations.
| Group | Activity 30 | Activity 40 | Activity 50 | Activity 60 | Activity 80 |
|---|---|---|---|---|---|
| CEM 0.1 (n = 4) | 0.0012 ± 0.0006 (14.88)a | 0.0012 ± 0.0004 (13.75)a | 0.0012 ± 0.0005 (14.00)a | 0.0013 ± 0.0006 (14.88)a,c | 0.0012 ± 0.0005 (13.75)a |
| CEM 0.3 (n = 3) | 0.0018 ± 0.0003 (17.50)a | 0.0018 ± 0.0003 (18.00)a,b | 0.0018 ± 0.0002 (17.33)a,b | 0.0018 ± 0.0001 (16.67)a,c | 0.0019 ± 0.0003 (17.50)a,b |
| CEM 0.5 (n = 4) | 0.0007 ± 0.0014 (10.25)a,b,c | 0.0005 ± 0.0011 (10.50)a,c,d | 0.0007 ± 0.0013 (10.25)a,c,d | 0.0006 ± 0.0011 (11.25)a,c | 0.0007 ± 0.0013 (10.50)a,c,d |
| MTA 0.1 (n = 5) | 0.0000 ± 0.0000 (5.00)b | 0.0000 ± 0.0000 (5.00)c,e | 0.0000 ± 0.0000 (4.50)c | 0.0000 ± 0.0000 (4.00)b | 0.0000 ± 0.0000 (4.00)c |
| MTA 0.3 (n = 4) | 0.0002 ± 0.0003 (8.25)b | 0.0004 ± 0.0008 (9.50)a,e | 0.0005 ± 0.0010 (11.13)a,e | 0.0005 ± 0.0009 (10.00)c | 0.0006 ± 0.0010 (11.25)a,e |
| MTA 0.5 (n = 3) | 0.0027 ± 0.0006 (21.67)c | 0.0024 ± 0.0007 (20.67)b,d | 0.0022 ± 0.0006 (20.00)b,d,e | 0.0022 ± 0.0005 (20.50)a | 0.0025 ± 0.0006 (20.50)b,d,e |
The values are presented as mean ± standard deviation, and the numbers in parentheses are the mean ranks in the group. Activity 30, activity 40, activity 50, activity 60, and activity 80 refer to 30%, 40%, 50%, 60%, and 80% levels of ALP activity, respectively.
Different lowercase letters in the same column indicate the presence of statistically significant differences.
CEM, calcium-enriched mixture; MTA, mineral trioxide aggregate.
DISCUSSION
The effects of MTA and CEM cement on the odontogenic differentiation of hDPSCs through dentin discs may shed light on the basic mechanisms involved in their therapeutic dentinogenesis after IPC procedures. The present study introduced a novel setup to simulate such a setting.
Evidence has shown that MTA and CEM cement have comparable effects on hard tissue formation and induced osteogenesis, cementogenesis, and dentinogenesis [20,21,22]. Furthermore, biomaterials, when used as DPC agents, can induce dentinal bridge formation via odontoblast-like cells [16]. Moreover, CEM and MTA have been shown to induce phenotypic differentiation of hDPSCs to odontogenic cells when hDPSCs are seeded directly over the biomaterials [23]. Since these endodontic biomaterials are commonly used as pulp-protecting agents in DPC and pulpotomy procedures with promising outcomes [8,9], they were chosen for the current study to evaluate their indirect effects on hDPSCs through human dentin.
SEM images exhibited elongated odontoblast-like cells, collagen fibers, calcified nodules, and mineralized foci in all samples. These findings are in accordance with those of a study by Asgary et al. [23], who reported similar SEM findings in canine dental pulp; showing that after 2 months of DPC with CEM and MTA, odontogenic cells secreted reparative dentin. In addition, calcified nodules and mineralized foci could suggest the bioactivity of the employed endodontic biomaterials; however, further bioactivity tests are recommended to obtain more definitive results.
The results of EDXA revealed predominance of calcium and phosphorus ions in the examined nodules in both the CEM and MTA subgroups. Moreover, the amount of calcium was higher than that of phosphorus in both samples, a pattern reminiscent of the composition and structure of standard hydroxyapatite (HA) [24]. In the CEM subgroup, the observed nodules were closer to HA. These results agree with the findings reported by Asgary et al. [25], who investigated the topography of the 2 above-mentioned root-end filling materials, and Sarkar et al. [24], who studied the biological properties of MTA.
Stem cells and fibroblasts isolated from dental pulp have the ability to differentiate into odontoblast-like cells [26]. Odontoblastic differentiation, either in vitro or in vivo, is divided into the following 3 phases: 1) cell proliferation, 2) secretion, and 3) maturation/mineralization of the matrix. The maturity of the matrix is characterized by the increased expression of ALP. In tissue engineering, induction of ALP activity and formation of mineralized nodules indicate differentiation and activity of odontoblast-like cells. The expression of ALP plays an important role in the early stages of differentiation. Subsequently, later in the differentiation phase, the expression of ALP decreases. The mechanism of action of ALP in the early phase is highly complex. However, since it is among the first functional genes expressed in the process of calcification, it is highly probable that it plays an important role in the initiation of mineralization [27]. Calcium silicate–based biomaterials (i.e., MTA and CEM cement) have an alkaline pH during setting and continuously release calcium ions. Research has shown that an increase in pH boosted the expression of ALP and the subsequent formation of calcified nodules. In our study, ALP activity was evaluated, and a significant difference was found across the various subgroups. These differences may have been related to the different thicknesses of the dentin discs, as well as variation in the speed of ion release/exchange, the concentration of ions, and changes in pH. Further studies are still required to better elucidate these differences.
Researchers have used several techniques to overcome the limitations of experiments and to assess the effects of applied biomaterials on hDPSCs in terms of cytotoxicity, cell viability, and differentiation [28]. The placement of some barriers between the chosen biomaterials and the employed cells is among the techniques that have been suggested. It seems that the results of such models are more suitable than other methodologies for the prediction of in vivo responses. Dentin discs are the best type of filter to be used in these studies; since they allow more accurate generalization of the results to clinical settings [29]. Thus, we used dentin discs in the present study in order to simulate IPC procedures as closely as possible.
In the present study, discs with 3 different thicknesses—0.1 (thinnest), 0.3, and 0.5 mm—were used. However, it is clear that accurate quantification of the remaining dentin thickness in a clinical setting is not possible [30]. Nevertheless, a decrease in the remaining dentin thickness (< 0.5 mm) is an important factor affecting the dental pulp tissue response to cavity restorations. Therefore, a bioactive material is probably required to protect the dental pulp and induce dentin formation [31].
CONCLUSIONS
MTA and CEM cements demonstrated bioactivity on hDPSCs as shown by cell proliferation, morphology, attachment, and calcific precipitations through 0.1- to 0.5-mm-thick dentin layers. Thus, MTA and CEM cement may be beneficial in IPC procedures.
ACKNOWLEDGEMENTS
The authors are grateful to the team at the Research Institute of Dental Sciences for their laboratory assistance. Furthermore, the authors would like to express their thanks and appreciation to Dr. Alireza Akbarzadeh Baghban for his contribution and revision of statistical analysis and related issues.
Footnotes
Funding: This project was supported, in part, by the Vice-Chancellor of Research, Shahid Beheshti University of Medical Sciences (grant No. 416/62).
Conflict of Interest: No potential conflict of interest relevant to this article was reported.
- Conceptualization: Panahandeh N, Torabzadeh H, Nazarian H, Asgary S.
- Data curation: Javid B, T Panahandeh N, Torabzadeh H, Nazarian H.
- Formal analysis: Javid B.
- Funding acquisition: Panahandeh N.
- Investigation: Javid B, Torabzadeh H, Nazarian H.
- Methodology: Javid B, Panahandeh N, Torabzadeh H, Nazarian H, Asgary S.
- Project administration: Panahandeh N, Torabzadeh H.
- Supervision: Torabzadeh H, Asgary S.
- Validation: Torabzadeh H, Nazarian H, Asgary S.
- Visualization: Javid B, Torabzadeh H.
- Writing - original draft: Javid B, Panahandeh N, Torabzadeh H, Asgary S.
- Writing - review & editing: Torabzadeh H, Parhizkar A, Asgary S.
References
- 1.Thompson V, Craig RG, Curro FA, Green WS, Ship JA. Treatment of deep carious lesions by complete excavation or partial removal: a critical review. J Am Dent Assoc. 2008;139:705–712. doi: 10.14219/jada.archive.2008.0252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schwendicke F, Frencken JE, Bjørndal L, Maltz M, Manton DJ, Ricketts D, Van Landuyt K, Banerjee A, Campus G, Doméjean S, Fontana M, Leal S, Lo E, Machiulskiene V, Schulte A, Splieth C, Zandona AF, Innes NP. Managing carious lesions: consensus recommendations on carious tissue removal. Adv Dent Res. 2016;28:58–67. doi: 10.1177/0022034516639271. [DOI] [PubMed] [Google Scholar]
- 3.Ricketts D. Management of the deep carious lesion and the vital pulp dentine complex. Br Dent J. 2001;191:606–610. doi: 10.1038/sj.bdj.4801246. [DOI] [PubMed] [Google Scholar]
- 4.Chogle SM, Goodis HE, Kinaia BM. Pulpal and periradicular response to caries: current management and regenerative options. Dent Clin North Am. 2012;56:521–536. doi: 10.1016/j.cden.2012.05.003. [DOI] [PubMed] [Google Scholar]
- 5.Gruythuysen RJ, van Strijp AJ, Wu MK. Long-term survival of indirect pulp treatment performed in primary and permanent teeth with clinically diagnosed deep carious lesions. J Endod. 2010;36:1490–1493. doi: 10.1016/j.joen.2010.06.006. [DOI] [PubMed] [Google Scholar]
- 6.Asgary S, Hassanizadeh R, Torabzadeh H, Eghbal MJ. Treatment outcomes of 4 vital pulp therapies in mature molars. J Endod. 2018;44:529–535. doi: 10.1016/j.joen.2017.12.010. [DOI] [PubMed] [Google Scholar]
- 7.Bergenholtz G. Factors in pulpal repair after oral exposure. Adv Dent Res. 2001;15:84. doi: 10.1177/08959374010150012201. [DOI] [PubMed] [Google Scholar]
- 8.Asgary S, Eghbal MJ, Bagheban AA. Long-term outcomes of pulpotomy in permanent teeth with irreversible pulpitis: a multi-center randomized controlled trial. Am J Dent. 2017;30:151–155. [PubMed] [Google Scholar]
- 9.Fallahinejad Ghajari M, Asgharian Jeddi T, Iri S, Asgary S. Treatment outcomes of primary molars direct pulp capping after 20 months: a randomized controlled trial. Iran Endod J. 2013;8:149–152. [PMC free article] [PubMed] [Google Scholar]
- 10.Araújo LB, Cosme-Silva L, Fernandes AP, Oliveira TM, Cavalcanti BD, Gomes Filho JE, Sakai VT. Effects of mineral trioxide aggregate, Biodentine™ and calcium hydroxide on viability, proliferation, migration and differentiation of stem cells from human exfoliated deciduous teeth. J Appl Oral Sci. 2018;26:e20160629. doi: 10.1590/1678-7757-2016-0629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Agrafioti A, Taraslia V, Chrepa V, Lymperi S, Panopoulos P, Anastasiadou E, Kontakiotis EG. Interaction of dental pulp stem cells with Biodentine and MTA after exposure to different environments. J Appl Oral Sci. 2016;24:481–486. doi: 10.1590/1678-775720160099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kamali A, Javadpour S, Javid B, Kianvash Rad N, Naddaf Dezfuli S. Effects of chitosan and zirconia on setting time, mechanical strength, and bioactivity of calcium silicate-based cement. Int J Appl Ceram Technol. 2017;14:135–144. [Google Scholar]
- 13.Kim M, Kim S, Ko H, Song M. Effect of ProRoot MTA® and Biodentine® on osteoclastic differentiation and activity of mouse bone marrow macrophages. J Appl Oral Sci. 2019;27:e20180150. doi: 10.1590/1678-7757-2018-0150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moazzami F, Ghahramani Y, Tamaddon AM, Dehghani Nazhavani A, Adl A. A histological comparison of a new pulp capping material and mineral trioxide aggregate in rat molars. Iran Endod J. 2014;9:50–55. [PMC free article] [PubMed] [Google Scholar]
- 15.Parirokh M, Torabinejad M. Mineral trioxide aggregate: a comprehensive literature review--part III: clinical applications, drawbacks, and mechanism of action. J Endod. 2010;36:400–413. doi: 10.1016/j.joen.2009.09.009. [DOI] [PubMed] [Google Scholar]
- 16.Tabarsi B, Parirokh M, Eghbal MJ, Haghdoost AA, Torabzadeh H, Asgary S. A comparative study of dental pulp response to several pulpotomy agents. Int Endod J. 2010;43:565–571. doi: 10.1111/j.1365-2591.2010.01711.x. [DOI] [PubMed] [Google Scholar]
- 17.Tak O, Usumez A. Diffusion of HEMA from resin cements through different dentin thicknesses in vitro . Am J Dent. 2015;28:285–291. [PubMed] [Google Scholar]
- 18.Murray PE, About I, Lumley PJ, Franquin JC, Remusat M, Smith AJ. Cavity remaining dentin thickness and pulpal activity. Am J Dent. 2002;15:41–46. [PubMed] [Google Scholar]
- 19.Asgary S, Nazarian H, Khojasteh A, Shokouhinejad N. Gene expression and cytokine release during odontogenic differentiation of human dental pulp stem cells induced by 2 endodontic biomaterials. J Endod. 2014;40:387–392. doi: 10.1016/j.joen.2013.09.017. [DOI] [PubMed] [Google Scholar]
- 20.Rahimi S, Mokhtari H, Shahi S, Kazemi A, Asgary S, Eghbal MJ, Mesgariabbasi M, Mohajeri D. Osseous reaction to implantation of two endodontic cements: mineral trioxide aggregate (MTA) and calcium enriched mixture (CEM) Med Oral Patol Oral Cir Bucal. 2012;17:e907–e911. doi: 10.4317/medoral.18136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Asgary S, Eghbal MJ, Ehsani S. Periradicular regeneration after endodontic surgery with calcium-enriched mixture cement in dogs. J Endod. 2010;36:837–841. doi: 10.1016/j.joen.2010.03.005. [DOI] [PubMed] [Google Scholar]
- 22.Zarrabi MH, Javidi M, Jafarian AH, Joushan B. Histologic assessment of human pulp response to capping with mineral trioxide aggregate and a novel endodontic cement. J Endod. 2010;36:1778–1781. doi: 10.1016/j.joen.2010.08.024. [DOI] [PubMed] [Google Scholar]
- 23.Asgary S, Parirokh M, Eghbal MJ, Ghoddusi J. SEM evaluation of pulp reaction to different pulp capping materials in dog's teeth. Iran Endod J. 2006;1:117–123. [PMC free article] [PubMed] [Google Scholar]
- 24.Sarkar NK, Caicedo R, Ritwik P, Moiseyeva R, Kawashima I. Physicochemical basis of the biologic properties of mineral trioxide aggregate. J Endod. 2005;31:97–100. doi: 10.1097/01.don.0000133155.04468.41. [DOI] [PubMed] [Google Scholar]
- 25.Asgary S, Eghbal MJ, Parirokh M, Ghoddusi J. Effect of two storage solutions on surface topography of two root-end fillings. Aust Endod J. 2009;35:147–152. doi: 10.1111/j.1747-4477.2008.00137.x. [DOI] [PubMed] [Google Scholar]
- 26.Tomson PL, Grover LM, Lumley PJ, Sloan AJ, Smith AJ, Cooper PR. Dissolution of bio-active dentine matrix components by mineral trioxide aggregate. J Dent. 2007;35:636–642. doi: 10.1016/j.jdent.2007.04.008. [DOI] [PubMed] [Google Scholar]
- 27.Aubin JE, Triffitt JT. Chapter 4 - mesenchymal stem cells and osteoblast differentiation. In: Bilezikian JP, Raisz LG, Rodan GA, editors. Principles of bone biology. 2nd ed. San Diego, CA: Academic Press; 2002. pp. 59–81. [Google Scholar]
- 28.Kim MJ, Kim KN, Lee YK, Kim KM. Cytotoxicity test of dentin bonding agents using millipore filters as dentin substitutes in a dentin barrier test. Clin Oral Investig. 2013;17:1489–1496. doi: 10.1007/s00784-012-0840-z. [DOI] [PubMed] [Google Scholar]
- 29.Sengün A, Yalçın M, Ülker HE, Öztürk B, Hakkı SS. Cytotoxicity evaluation of dentin bonding agents by dentin barrier test on 3-dimensional pulp cells. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2011;112:e83–e88. doi: 10.1016/j.tripleo.2011.02.023. [DOI] [PubMed] [Google Scholar]
- 30.Dunne SM. The limitation of visual perception in restorative dentistry. Dent Update. 1993;20:198–201. [PubMed] [Google Scholar]
- 31.Camps J, Déjou J, Rémusat M, About I. Factors influencing pulpal response to cavity restorations. Dent Mater. 2000;16:432–440. doi: 10.1016/s0109-5641(00)00041-5. [DOI] [PubMed] [Google Scholar]