Abstract
Purpose
Schizophrenia patients show cognitive and mood impairments, including memory loss and depression, suggesting damage in the brain regions. The hippocampus is a brain structure that is significantly involved in memory and mood function and shows impairment in schizophrenia. In the present study, we examined the regional hippocampal changes in schizophrenia patients using voxel-based morphometry (VBM), Freesurfer, and proton magnetic resonance spectroscopy (1H MRS) procedures.
Methods
1H MRS and high-resolution T1-weighted magnetic resonance imaging were collected in both healthy control subjects (N = 28) and schizophrenia patients (N = 28) using 3-Tesla whole body MRI system. Regional hippocampal volume was analyzed using VBM and Freesufer procedures. The relative ratios of the neurometabolites were calculated using linear combination model (LCModel).
Results
Compared to controls, schizophrenia patients showed significantly decreased gray matter volume in the hippocampus. Schizophrenia patients also showed significantly reduced glutamate (Glu) and myo-inositol (mI) ratios in the hippocampus. Additionally, significant positive correlation between gray matter volume and Glu/tCr was also observed in the hippocampus in schizophrenia.
Conclusion
Our findings provide an evidence for a possible association between structural deficits and metabolic alterations in schizophrenia patients.
Keywords: Proton magnetic resonance spectroscopy, Voxel-based morphometry, Schizophrenia, Hippocampus
Introduction
Schizophrenia is a severe mental disorder that involves a range of problems with thinking, perception, behavior, and social problems specially delusions, hallucinations, and disorganized speech [1]. Schizophrenia patients exhibit a wide range of cognitive and mood problems, including working memory and executive functions deficit, depression, and anxiety [2–5]. These characteristics indicate the presence of brain dysfunction and specifically to damage of the hippocampus, a brain structure integrally involved in such neurocognitive functions in schizophrenia [6–9].
Neuroimaging techniques provide evidences about the abnormality of the hippocampus in schizophrenia. Previous studies have shown structural abnormalities, including gray matter volume loss, in the hippocampus of schizophrenia patients [10–13]. Proton magnetic resonance spectroscopy (1H MRS) has shown decreased N-acetyl-aspartate (NAA) level [14, 15] and increased [16] or no change [17, 18] in glutamate (Glu) levels in the hippocampus of patients affected with schizophrenia. There are few studies that have investigated the association between metabolic and structural measures in the hippocampus of schizophrenia patients [19, 20].
Our aim in this study was to examine regional hippocampal changes at structural and metabolite levels in schizophrenia patients, using VBM, Freesurfer, and 1H MRS procedures. We hypothesized that schizophrenia patients would show structural and metabolic alterations in the hippocampus compared to healthy controls.
Methods
Subjects
Twenty-eight schizophrenia patients and 28 healthy controls were taken in the study. All schizophrenia patients were recruited from the Department of Psychiatry, Dr. Ram Manohar Lohia Hospital (RMLH), New Delhi, India. The diagnosis of the disorder was confirmed by interviewing the patients using the Hindi version of the Diagnostic Interview for Genetic Studies (DIGS) [21, 22]. All patients were on antipsychotic medications at the time of MRI acquisition.
All control subjects chosen for the study were recruited from the local community. Participants, both controls and patients, with a history of any clinical evidence of stroke, head injury, cardiovascular diseases, history of smoking, alcohol, or drug dependence were not included in the study.
The study was approved by the Institutional Ethics Committee of PGIMER, RMLH, New Delhi, India. Written informed consent was obtained from all the participants after complete description of the study to the participants.
Magnetic resonance imaging
Brain imaging was acquired using a 3.0-Tesla MR scanner (Magnetom Skyra, Siemens, Germany) with a 20-channel head and neck coil. The subject’s head was immobilized using expandable ear cushions while subjects lay supine. High-resolution T1-weighted images were acquired using a magnetization prepared rapid acquisition gradient-echo (MPRAGE) sequence (TR = 1900 ms, TE = 2.07 ms, inversion time = 900 ms, matrix size = 256 × 256, FOV = 256 × 256 mm, slice thickness = 1 mm, number of slices = 160). For positioning the MRS voxel, anatomical images were acquired in all the three orthogonal planes. We also collected T2-weighted multislice images (TR = 5600 ms, TE = 100 ms, NEX = 1, 312 × 512 matrix, and FOV = 180 × 220 mm, 25 slices, slice thickness = 4.0 mm, distance factor = 1.2 mm) covering the entire brain to look for any neurological abnormalities.
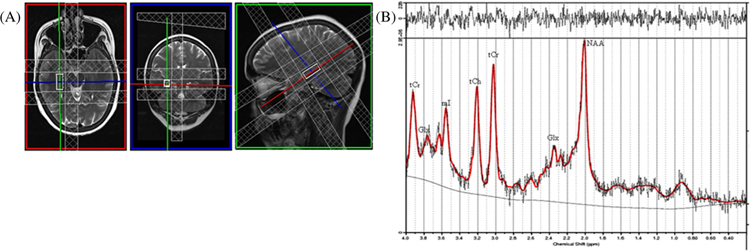
We collected MRS data from the right hippocampus of voxel of 25 × 10 × 10 mm3 (Fig. 1a). The voxel was carefully placed in the right hippocampus so as to maximize the amount of gray matter (GM) while avoiding adjoining major vessels and cerebrospinal fluid (CSF). Automated global and localized shimming were performed to minimize the Bo inhomogeneities and to optimize field homogeneity across the voxel of interest. Water-suppressed spectra were acquired using single-volume point-resolved spectroscopy sequence (PRESS) with the following acquisition parameters: TR/TE = 2000/33 ms, 2048 spectral points, 1200-Hz spectral bandwidth, and 196 averages. We used chemically selective suppression (CHESS) pulse sequence for water suppression.
Fig. 1.
a Location of 2.5 cm3 voxel and b its representative 1H MRS spectrum acquired from the right hippocampus as analyzed by LCModel of a control subject
Data processing
Structural data analysis
Voxel-based morphometry analysis
We used the statistical parametric mapping package SPM12 (Wellcome Department of Cognitive Neurology, UK; http://www.fil.ion.ucl.ac.uk/spm/) and MATLAB-based (The MathWorks Inc., Natick, MA) custom software to process images. All the steps for structural data processing were followed as described in detail [23]. We used Diffeomorphic Anatomic Registration Through Exponentiated Lie Algebra (DARTEL) algorithm toolbox in order to improve the registration of the MRI images. Firstly, the anatomical images were segmented into GM and white matter (WM) probability maps using the “new segment” option of SPM 12. Then, the flow fields and a series of template images were generated by running “DARTEL (create templates)” option. Finally, the anatomical images were smoothed (10-mm full width at half maximum), modulated, and spatially normalized into MNI space by using the flow fields and final template generated in the previous step.
FreeSurfer analysis
FreeSurfer software (v 6.0.0) (http://surfer.nmr.mgh.harvard.edu/) was used to obtain hippocampus volumes for all subjects. The methods of the automated volumetric approach have been described in detail previously [24]. FreeSurfer analyses were performed on Ubuntu 16.04.3 LTS, which allowed the FreeSurfer “recon-all” function for cortical reconstruction and brain segmentation (http://surfer.nmr.mgh.harvard.edu/fswiki/recon-all) to complete 56 participants in less than 30 h. After the “recon-all” function, the neuroanatomical labels were inspected for accuracy in all patients and controls. All subjects’ processed data were manually evaluated by an investigator to ensure no brain areas were excluded. No errors in the automatic labelling were observed for any subject, and so all data obtained from FreeSurfer analyses were 100% automated and not influenced by manual intervention.
Magnetic resonance spectroscopy data analysis
The spectra were processed using linear combination model (LCModel) software [25, 26]. Spectral peaks were assigned with reference to the water peak (4.7 ppm). Only those metabolites are included which show Cramer-Rao lower bound (CRLB) less than or equal to 20% for quantitative reliability [27]. In our study, we used metabolite ratios to reduce the variability in absolute values for different metabolites as observed in subjects. We used total creatine (tCr) values as the internal reference for relative quantification [26].
Statistical analyses
We used statistical package for social sciences (SPSS v22) for assessment of demographic data. The demographic data of schizophrenia patients and controls were compared using independent samples t test. Statistical threshold values of p < 0.05 were considered a significant difference.
For regional GM volume differences between groups, the smoothed whole-brain GM maps of schizophrenia patients and controls were compared using analysis of covariance (ANCOVA), with age and gender as covariates. A whole-brain analysis was performed, with a significance level of p < 0.001, uncorrected for multiple comparisons. The statistical parametric maps showing gray matter volume difference were set at threshold of p < 0.001 for each voxel and a minimum cluster size of 1056 voxels, which give a corrected threshold of p < 0.05 by using AlphaSim in REST software.
Regional hippocampal volumes were examined for significant differences between schizophrenia and controls using ANCOVA, with age and gender included as covariates. We considered a p < 0.05 value statistically significant.
Differences in metabolite ratios were compared with multivariate analysis of covariance (MANCOVA) using general linear model (GLM). To control the effect of age and sex between the two groups, age and sex were taken as covariates of no interest. In schizophrenia patients as well as healthy controls, Pearson’s correlation coefficient was also computed to study the relationship between metabolite ratios and gray matter volumes from the hippocampus region. The level of significance was set at p ≤ 0.05.
Results
Demographic and clinical characteristics
Demographic and clinical variables of schizophrenia patients and control subjects are summarized in Table 1. No significant differences in age (p = 0.238) and gender (p = 0.794) appeared between groups.
Table 1.
Demographic and clinical characteristics of subjects
| Schizophrenic patients (N = 28) (mean ± SD) | Healthy controls (N = 28) (mean ± SD) | p value | |
|---|---|---|---|
| Age (years) | 33.89 ± 9.34 | 31.44 ± 7.36 | 0.238 |
| Gender (male/female) | 12/16 | 14/14 | 0.794 |
| Years of education | 9.64 ± 3.43 | 12.3 ± 3.4 | |
| Duration of illness (in weeks) | 479.65 ± 323.65 | NA | |
| SANS total score | 11.8 ± 5.9 | ||
| SAPS total score | 8.1 ± 4.4 | ||
| Antipsychotic equivalent dosage of CPZ, mg/day | 488.14 ± 328.94 | ||
| Duration of antipsychotic drug taken (in weeks) | 451.47 ± 318.13 | ||
| Hippocampal volumes (in mm3) | |||
| Right hippocampus | 3786.8 ± 426.2 | 4354.4 ± 457.6 | < 0.001 |
| Left hippocampus | 3610.7 ± 402.7 | 4089.2 ± 444.5 | < 0.001 |
SANS Scale for the Assessment of Negative Symptoms, SAPS Scale for the Assessment of Positive Symptoms, CPZ chlorpromazine
Regional hippocampus volume differences
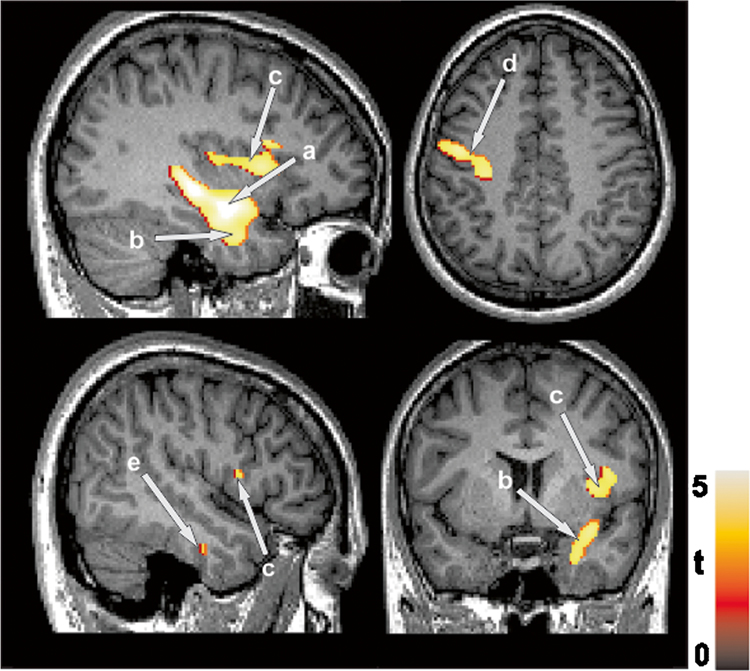
Schizophrenia patients showed significantly reduced gray matter volume in the multiple brain regions including the right hippocampus, right parahippocampal gyrus, right inferior temporal gyrus, left precentral gyrus, and right insula lobe, compared to healthy controls (Fig. 2 and Table 2). After controlling for age and gender, schizophrenia patients showed significantly reduced hippocampal volumes compared to healthy controls (Table 1.)
Fig. 2.
Gray matter volume loss in brain areas including the a right hippocampus, b right parahippocampal gyrus, c right insula lobe, d left precentral gyrus, and e right inferior temporal gyrus, in schizophrenia patients compared to healthy controls
Table 2.
Regions of gray matter volume loss in schizophrenia patients compared to healthy controls
| Brain regions | MNI coordinates | z-score | t value | p fwe-corr | Cluster size (voxels) |
|---|---|---|---|---|---|
| R hippocampus | 36 − 9 − 17 | 4.64 | 5.19 | 0.019 | |
| R parahippocampal gyrus | 33 − 8 − 29 | 4.26 | 4.68 | 0.025 | 3815 |
| R inferior temporal gyrus | 48 − 12 − 29 | 4.12 | 4.22 | 0.031 | |
| L precentral gyrus | − 42 − 18 65 | 4.28 | 4.71 | 0.032 | 1617 |
| R insula lobe | 36 9 5 | 4.40 | 4.59 | 0.012 | 1080 |
| R rolandic operculum | 44 8 12 | 4.38 | 4.48 | 0.042 |
MNI Montreal Neurological Institute, L left, R right
Magnetic resonance spectroscopy results
Our results showed a significantly reduced Glu/tCr and mI/tCr ratios in the hippocampus of schizophrenia patients compared to controls (p = 0.032 for Glu/tCr and p = 0.009 for mI/tCr). No significant differences were observed in NAA/tCr, tCho/tCr, NAA + NAAG/tCr, and Glx/tCr. The metabolite ratios with reference to tCr from the right hippocampus region of schizophrenia patients (N = 28) and healthy controls (N = 28) were provided in Table 3. The representative spectra acquired from the right hippocampus region of a control subject are shown in Fig. 1b.
Table 3.
LCModel analysis of metabolite ratios normalized to tCr (Cr + PCr) resonance calculated from the spectra obtained from the right hippocampus of both controls and schizophrenia patients (N = 28)
| Metabolite ratios | Healthy controls mean ± SD | Schizophrenia mean ± SD | p value |
|---|---|---|---|
| Glu/tCr | 1.24 ± 0.21 | 1.15 ± 0.16 | 0.032* |
| mI/tCr | 0.98 ± 0.22 | 0.85 ± 0.19 | 0.009* |
| NAA/tCr | 0.94 ± 0.18 | 0.94 ± 0.12 | 0.978 |
| GPC + PCh/tCr | 0.30 ± 0.03 | 0.31 ± 0.05 | 0.225 |
| NAA + NAAG/tCr | 1.09 ± 0.12 | 1.04 ± 0.13 | 0.148 |
| Glx/tCr | 2.07 ± 0.40 | 1.97 ± 0.43 | 0.213 |
Asterisk indicates significant difference between groups (*p < 0.05)
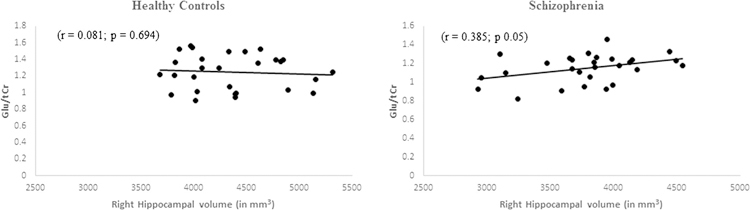
Correlation between hippocampal volume and neurometabolites
Only Glu/tCr showed significant positive correlation with reduced hippocampus volume in schizophrenia patients (r = 0.385, p = 0.05) (Fig. 3). In healthy controls, no significant correlation was observed between metabolite ratios and hippocampus volume (r = 0.081, p = 0.694) (Fig. 3).
Fig. 3.
Scatter plot showing correlation between hippocampus volume and Glu/tCr ratio quantified from the right hippocampus region in controls and schizophrenia patients
Discussion
In the present study, regional gray matter volume loss was observed in the hippocampus of schizophrenia patients compared to healthy controls. Schizophrenia patients showed decreased metabolite ratios, i.e., Glu/tCr and mI/tCr ratios in the right hippocampus compared with healthy controls. Also, Gu/ tCr ratio showed positive correlation with reduced hippocampus volume in the hippocampus in schizophrenia patients.
Reduced regional hippocampal volume in schizophrenia
Schizophrenia patients showed regional gray matter volume loss in the hippocampus compared to healthy controls. The pattern of the localized volume loss in the hippocampus may indicate specific mechanisms of damage in schizophrenia. The hippocampal volume loss may also impact other brain structures that receive input from the hippocampus. In our study, VBM showed reduced gray matter volume in the parahippocampal gyrus, inferior temporal gyrus, and insular cortex, brain regions that communicates directly or indirectly with the hippocampus. Previous meta-analyses and reviews have shown gray matter volume loss in the hippocampus in schizophrenia [10–13]. Therefore, the gray matter volume loss in the hippocampus along with other brain regions may also explain clinical manifestations such as memory and mood impairments in schizophrenia patients.
Neurometabolite alterations in schizophrenia
1H MRS showed a significantly reduced Glu/tCr and mI/tCr ratios in the hippocampus in schizophrenia patients compared to healthy controls. The reduced Glu/tCr and mI/tCr ratios likely result, in part, from decreased glutamate activity or abnormal Glu-Gln cycle [28] and decreased glial content or dysfunctional glia which might result from glutamate-mediated toxicity [29], which is consistent with the tissue damage in the right hippocampus observed in our study. Most studies showed no difference in glutamate levels in medicated schizophrenia patients and healthy controls [17, 18]. Few studies have shown elevated Glu level in unmedicated schizophrenia patients and suggested the possible normalization of Glu levels with antipsychotic medication in other brain regions [30–32]. In our study, we did not find any correlation between metabolite ratios and chlorpromazine (CPZ) antipsychotic dose in schizophrenia.
Positive correlation between metabolite ratios and regional hippocampal volume
Schizophrenia patients showed positive correlation between Glu/tCr ratio and hippocampal volume in the hippocampus. Few studies have shown the correlation of metabolic and structural measures in the hippocampus of schizophrenia patients [19, 33]. Kragulic et al. have shown negative correlation between hippocampal VBM measure and Glx/tCr in unmedicated schizophrenia patients but not in healthy controls [19]. Nenadic et al. provided evidence of a structural impact on different brain regions in relation to hippocampal Glu and NAA levels and demonstrates that these associations are different between ultra-high risk and first-episode schizophrenia [33]. In line with these studies, our findings suggest an association between structural deficits and metabolic alterations in the hippocampus in patients with schizophrenia.
Limitations
There are certain limitations in the present study. The first limitation is the measurement of metabolites is in ratios, not in absolute concentrations. Secondly, all the patients in this study were on medication. But we did not find any correlations between metabolite ratios and gray matter volumes and CPZ antipsychotic dose in schizophrenia patients. It would have been beneficial if a third group consisting of first-episode patients without treatment can be included to determine the potential effect of pharmacological treatment.
Conclusion
The overall findings of this study provide an evidence for a possible association between structural deficits and metabolic alterations in schizophrenia patients as compared to controls. These findings also suggest regional hippocampal damage in schizophrenia.
Acknowledgments
Funding This study was funded by DRDO R&D Project No. INM-311(4.1) and funded in part by grant from the Fogarty International Centre, NIH, The Impact of Yoga Supplementation on Cognitive Function Among Indian Outpatients Grant #1R01TW008289 to TB.
Footnotes
Conflict of interest The authors declare that they have no conflict of interest.
Compliance with ethical standards
Ethical approval All procedures performed in the studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed consent Informed consent was obtained from all individual participants included in the study.
References
- 1.Mueller S, Keeser D, Reiser MF, Teipel S, Meindl T (2012) Functional and structural MR imaging in neuropsychiatric disorders, part 2: application in schizophrenia and autism. AJNR Am J Neuroradiol 33(11):2033–2037. 10.3174/ajnr.A2800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Penn DL, Spaulding W, Reed D, Sullivan M, Mueser KT, Hope DA (1997) Cognition and social functioning in schizophrenia. Psychiatry 60(4):281–291. 10.1080/00332747.1997.11024806 [DOI] [PubMed] [Google Scholar]
- 3.Goldberg TEGM (2002) Neurocognitive functioning in patients with schizophrenia: an overview In: Davis KLCD, Coyle JT, Nemeroff C (eds) Neuropsychopharmacology—fifth generation of progress, pp 657–669 [Google Scholar]
- 4.Mulholland CCS (2000) The symptom of depression in schizophrenia and its management. Adv Psychiatr Treat 6(3):169–177. 10.1192/apt.6.3.169 [DOI] [Google Scholar]
- 5.Harvey PD (2011) Mood symptoms, cognition, and everyday functioning: in major depression, bipolar disorder, and schizophrenia. Innov Clin Neurosci 8(10):14–18 [PMC free article] [PubMed] [Google Scholar]
- 6.Harrison PJ (2004) The hippocampus in schizophrenia: a review of the neuropathological evidence and its pathophysiological implications. Psychopharmacology 174(1):151–162. 10.1007/s00213-003-1761-y [DOI] [PubMed] [Google Scholar]
- 7.Heckers S (2001) Neuroimaging studies of the hippocampus in schizophrenia. Hippocampus 11(5):520–528. 10.1002/hipo.1068 [DOI] [PubMed] [Google Scholar]
- 8.Wible CG (2013) Hippocampal physiology, structure and function and the neuroscience of schizophrenia: a unified account of declarative memory deficits, working memory deficits and schizophrenic symptoms. Behav Sci (Basel) 3(2):298–315. 10.3390/bs3020298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tamminga CA, Stan AD, Wagner AD (2010) The hippocampal formation in schizophrenia. Am J Psychiatry 167(10):1178–1193. 10.1176/appi.ajp.2010.09081187 [DOI] [PubMed] [Google Scholar]
- 10.Shepherd AM, Laurens KR, Matheson SL, Carr VJ, Green MJ (2012) Systematic meta-review and quality assessment of the structural brain alterations in schizophrenia. Neurosci Biobehav Rev 36(4):1342–1356. 10.1016/j.neubiorev.2011.12.015 [DOI] [PubMed] [Google Scholar]
- 11.Glahn DC, Laird AR, Ellison-Wright I, Thelen SM, Robinson JL, Lancaster JL, Bullmore E, Fox PT (2008) Meta-analysis of gray matter anomalies in schizophrenia: application of anatomic likelihood estimation and network analysis. Biol Psychiatry 64(9):774–781. 10.1016/j.biopsych.2008.03.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Asami T, Bouix S, Whitford TJ, Shenton ME, Salisbury DF, McCarley RW (2012) Longitudinal loss of gray matter volume in patients with first-episode schizophrenia: DARTEL automated analysis and ROI validation. NeuroImage 59(2):986–996. 10.1016/j.neuroimage.2011.08.066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Williams LM (2008) Voxel-based morphometry in schizophrenia: implications for neurodevelopmental connectivity models, cognition and affect. Expert Rev Neurother 8(7):1049–1065. 10.1586/14737175.8.7.1049 [DOI] [PubMed] [Google Scholar]
- 14.Steen RG, Hamer RM, Lieberman JA (2005) Measurement of brain metabolites by 1H magnetic resonance spectroscopy in patients with schizophrenia: a systematic review and meta-analysis. Neuropsychopharmacology 30(11):1949–1962. 10.1038/sj.npp.1300850 [DOI] [PubMed] [Google Scholar]
- 15.Deicken RF, Zhou L, Schuff N, Fein G, Weiner MW (1998) Hippocampal neuronal dysfunction in schizophrenia as measured by proton magnetic resonance spectroscopy. Biol Psychiat 43(7): 483–488. 10.1016/S0006-3223(97)00490-3 [DOI] [PubMed] [Google Scholar]
- 16.van Elst LT, Valerius G, Buchert M, Thiel T, Rusch N, Bubl E, Hennig J, Ebert D, Olbrich HM (2005) Increased prefrontal and hippocampal glutamate concentration in schizophrenia: evidence from a magnetic resonance spectroscopy study. Biol Psychiatry 58(9):724–730. 10.1016/j.biopsych.2005.04.041 [DOI] [PubMed] [Google Scholar]
- 17.Rusch N, van Elst LT, Valerius G, Buchert M, Thiel T, Ebert D, Hennig J, Olbrich HM (2008) Neurochemical and structural correlates of executive dysfunction in schizophrenia. Schizophr Res 99(1–3):155–163. 10.1016/j.schres.2007.05.024 [DOI] [PubMed] [Google Scholar]
- 18.Hutcheson NL, Reid MA, White DM, Kraguljac NV, Avsar KB, Bolding MS, Knowlton RC, den Hollander JA, Lahti AC (2012) Multimodal analysis of the hippocampus in schizophrenia using proton magnetic resonance spectroscopy and functional magnetic resonance imaging. Schizophr Res 140(1–3):136–142. 10.1016/j.schres.2012.06.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kraguljac NV, White DM, Reid MA, Lahti AC (2013) Increased hippocampal glutamate and volumetric deficits in unmedicated patients with schizophrenia. Jama Psychiat 70(12):1294–1302. 10.1001/jamapsychiatry.2013.2437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hasan A, Wobrock T, Falkai P, Schneider-Axmann T, Guse B, Backens M, Ecker UKH, Heimes J, Galea JM, Gruber O, Scherk H (2014) Hippocampal integrity and neurocognition in first-episode schizophrenia: a multidimensional study. World J Biol Psychia 15(3):188–199. 10.3109/15622975.2011.620002 [DOI] [PubMed] [Google Scholar]
- 21.Nurnberger JI, Blehar MC, Kaufmann CA, Yorkcooler C, Simpson SG, Harkavyfriedman J, Severe JB, Malaspina D, Reich T, Miller M, Bowman ES, Depaulo JR, Cloninger CR, Robinson G, Modlin S, Gershon ES, Maxwell E, Guroff JJ, Kirch D, Wynne D, Berg K, Tsuang MT, Faraone SV, Pepple JR, Ritz AL (1994) Diagnostic interview for genetic studies—rationale, unique features, and training. Arch Gen Psychiat 51(11):849–859. 10.1001/archpsyc.1994.03950110009002 [DOI] [PubMed] [Google Scholar]
- 22.Deshpande SN, Mathur MNL, Das SK, Bhatia T, Sharma S, Nimgaonkar VL (1998) A Hindi version of the diagnostic interview for genetic studies. Schizophrenia Bull 24(3):489–493. 10.1093/oxfordjournals.schbul.a033343 [DOI] [PubMed] [Google Scholar]
- 23.Ashburner J (2010) VBM Tutorial
- 24.Fischl B, Salat DH, Busa E, Albert M, Dieterich M, Haselgrove C, van der Kouwe A, Killiany R, Kennedy D, Klaveness S, Montillo A, Makris N, Rosen B, Dale AM (2002) Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron 33(3):341–355. 10.1016/S0896-6273(02)00569-X [DOI] [PubMed] [Google Scholar]
- 25.Provencher SW (1993) Estimation of metabolite concentrations from localized in vivo proton NMR spectra. Magnet Reson Med 30(6):672–679. 10.1002/mrm.1910300604 [DOI] [PubMed] [Google Scholar]
- 26.Provencher SW (2001) Automatic quantitation of localized in vivo 1H spectra with LCModel. NMR Biomed 14(4):260–264. 10.1002/nbm.698 [DOI] [PubMed] [Google Scholar]
- 27.Cavassila S, Deval S, Huegen C, van Ormondt D, Graveron-Demilly D (2001) Cramer-Rao bounds: an evaluation tool for quantitation. NMR Biomed 14(4):278–283. 10.1002/nbm.701 [DOI] [PubMed] [Google Scholar]
- 28.da Silva Alves F, Boot E, Schmitz N, Nederveen A, Vorstman J, Lavini C, Pouwels PJ, de Haan L, Linszen D, van Amelsvoort T (2011) Proton magnetic resonance spectroscopy in 22q11 deletion syndrome. PLoS One 6(6):e21685 10.1371/journal.pone.0021685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chang L, Friedman J, Ernst T, Zhong K, Tsopelas ND, Davis K (2007) Brain metabolite abnormalities in the white matter of elderly schizophrenic subjects: implication for glial dysfunction. Biol Psychiatry 62(12):1396–1404. 10.1016/j.biopsych.2007.05.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kegeles LS, Mao X, Stanford AD, Girgis R, Ojeil N, Xu X, Gil R, Slifstein M, Abi-Dargham A, Lisanby SH, Shungu DC (2012) Elevated prefrontal cortex gamma-aminobutyric acid and glutamate-glutamine levels in schizophrenia measured in vivo with proton magnetic resonance spectroscopy. Arch Gen Psychiatry 69(5):449–459. 10.1001/archgenpsychiatry.2011.1519 [DOI] [PubMed] [Google Scholar]
- 31.de la Fuente-Sandoval C, Leon-Ortiz P, Azcarraga M, Favila R, Stephano S, Graff-Guerrero A (2013) Striatal glutamate and the conversion to psychosis: a prospective 1H-MRS imaging study. Int J Neuropsychopharmacol 16(2):471–475. 10.1017/S1461145712000314 [DOI] [PubMed] [Google Scholar]
- 32.Olbrich HM, Valerius G, Rusch N, Buchert M, Thiel T, Hennig J, Ebert D, Van Elst LT (2008) Frontolimbic glutamate alterations in first episode schizophrenia: evidence from a magnetic resonance spectroscopy study. World J Biol Psychiatry 9(1):59–63. 10.1080/15622970701227811 [DOI] [PubMed] [Google Scholar]
- 33.Nenadic I, Maitra R, Basu S, Dietzek M, Schonfeld N, Lorenz C, Gussew A, Amminger GP, McGorry P, Reichenbach JR, Sauer H, Gaser C, Smesny S (2015) Associations of hippocampal metabolism and regional brain grey matter in neuroleptic-naive ultra-highrisk subjects and first-episode schizophrenia. Eur Neuropsychopharmacol 25(10):1661–1668. 10.1016/j.euroneuro.2015.05.005 [DOI] [PubMed] [Google Scholar]