Abstract
Regular consumption of fruits and vegetables, which is related to high plasma levels of lipid-soluble micronutrients such as carotenoids and tocopherols, is linked to lower incidences of various age-related diseases. Differences in lipid-soluble micronutrient blood concentrations seem to be associated with age. Our retrospective analysis included men and women aged 22–37 and 60–85 years from the Berlin Aging Study II. Participants with simultaneously available plasma samples and dietary data were included (n = 1973). Differences between young and old groups were found for plasma lycopene, α-carotene, α-tocopherol, β-cryptoxanthin (only in women), and γ-tocopherol (only in men). β-Carotene, retinol and lutein/zeaxanthin did not differ between young and old participants regardless of the sex. We found significant associations for lycopene, α-carotene (both inverse), α-tocopherol, γ-tocopherol, and β-carotene (all positive) with age. Adjusting for BMI, smoking status, season, cholesterol and dietary intake confirmed these associations, except for β-carotene. These micronutrients are important antioxidants and associated with lower incidence of age-related diseases, therefore it is important to understand the underlying mechanisms in order to implement dietary strategies for the prevention of age-related diseases. To explain the lower lycopene and α-carotene concentration in older subjects, bioavailability studies in older participants are necessary.
Keywords: Carotenoids, Tocopherols, Micronutrients, Age, Plasma, Food frequency questionnaire
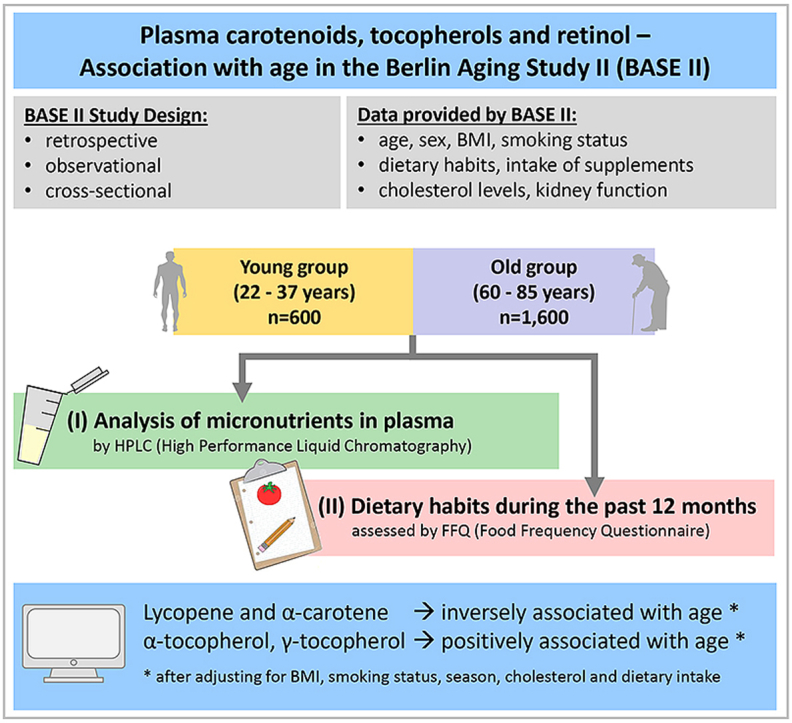
Graphical abstract
Highlights
-
•
Eating fruits and vegetables is linked to lower incidence of age-related diseases.
-
•
Different patterns of plasma antioxidant micronutrients in young vs. old subjects.
-
•
Lycopene, α-carotene, α-, and γ-tocopherol are associated with age.
-
•
Adjusting for BMI, smoking, season, cholesterol and diet confirmed associations.
-
•
Underlying mechanism could be altered bioavailability of micronutrients in aging.
1. Introduction
Higher dietary intake as well as higher plasma levels of lipid-soluble micronutrients such as carotenoids, tocopherols and retinol, especially the tomato-derived lycopene have been shown to act as antioxidants, thus counteracting oxidative stress, and to be protective against various age-related diseases such as tumors, cardiovascular diseases, metabolic syndrome, atherosclerosis, and cognitive disorders [[1], [2], [3], [4], [5], [6], [7]]. In contrast, low blood concentrations might be related to disease outcome. Previously, both low plasma micronutrient concentrations of lycopene or β-carotene, and high protein carbonyl concentrations (as biomarker for oxidative stress) were associated with the frailty syndrome in participants from the FRAILOMIC Initiative (n = 1700 from Spain, Italy, France; [8]). However, there appear to be distinct age-related differences in lipid-soluble micronutrient blood concentrations that may potentially affect disease risk.
Plasma lycopene has been shown to be significantly lower in older subjects compared to younger subjects while other carotenoids (α- and β-carotene, β-cryptoxanthin, lutein/zeaxanthin), tocopherols and retinol, showed opposite associations or even no difference [9,10]. Additionally, we have previously demonstrated that plasma lycopene was significantly lower in older participants, even after adjusting for several confounders (MARK-AGE Project; n = 2118 aged 35–75 years) in both men and women [11]. However, it is not clear, whether the observed lower blood lycopene concentrations are due to lower dietary intake or an altered age-related metabolism. It is likely that older persons consume lower amounts of processed-tomato-based products (sauce, ketchup, pizza, etc.) than younger persons. However, data on intake-adjusted relationships of plasma carotenoids, tocopherols and retinol with age are limited so far. Therefore, we aimed to assess whether there are age-related associations in micronutrient concentrations and if adjusting to possible confounders as well as to dietary factors has an influence on appearing associations.
2. Materials and methods
2.1. Study population and participant characteristics
Our retrospective analysis included men and women aged 22–37 years (n = 465, termed “young”) and 60–85 years (n = 1508, termed “old”) living independently in the Berlin metropolitan area that had been recruited within the Berlin Aging Study II (BASE-II).
All subjects gave their informed consent for inclusion before they participated in the study. The study was conducted in accordance with the Declaration of Helsinki, and the protocol was approved by the Ethics Committee of the Charité – Universitätsmedizin Berlin (EA2/029/09) and registered with the German Clinical Trials Register (DRKS00009277). The description of the BASE-II study including participant recruitment, ethical approval, study sample and variable assessment has been reported elsewhere in detail [12,13]. Participant's recruitment was carried out between June 2009 and July 2015, where anthropometrics, demographic data and nutritional data were assessed and blood samples were taken. Fasting blood samples were collected, and plasma samples were stored at −80 °C and transported on dry ice.
Participants’ information included age (years), sex, weight (kg), height (cm), body mass index (BMI; kg/m2) current smoking status (yes/no) and self-reported frequency of servings of raw tomatoes, tomato sauce, ketchup, carrots, carrot juice, multivitamin juice, nuts, orange juice, tangerine, flax seeds, spinach and use of vitamin/mineral supplements were assessed by food frequency questionnaires (FFQs). Season of blood collection was additionally included as a variable due to its relation to carotenoid status. Frequency of reported medication intake and Mini Mental State Examination (MMSE) were available only for the old group.
2.2. Analyses of micronutrients and dietary data
The analysis of plasma carotenoids, tocopherols and retinol by HPLC has been described elsewhere [11]. Estimated glomerular filtration rate (eGFR) was calculated based on serum creatinine measurements, sex and age according to the full age spectrum (FAS) equation [14] as this formula has been demonstrated valid in this population [15].
A two-day study protocol was carried out where each participant's health status was examined and an FFQ on dietary habits was filled out [12]. Participants completed the validated, self-administered 146-item EPIC-Potsdam-FFQ from Potsdam, Germany (European Prospective Investigation into Cancer and Nutrition). The EPIC-Potsdam-FFQ records the nutritional intake of the previous year [16] and has been validated and reproducibility of the results has been demonstrated [17].
It includes questions regarding food intake, such as the frequency of consumption and fat content of specific food items (e.g., meat and dairy products), and seasonal consumption of fruit and vegetables. The frequency of intake was measured using 10 categories ranging from “one time per month or less” to “five times per day or more”. Serving size for fruits and vegetables was one portion (for carrots it was two carrots), one spoon of ketchup, one scoop of tomato sauce, for juice it was 200 mL (1 glass) and for nuts one handful. Frequencies are reported in Supplemental Table 1.
2.3. Statistical analyses
Of the whole BASE-II study sample of 2171 participants, we selected only those with simultaneously available plasma samples (n = 2001) and FFQ data (n = 2126), thus our statistical analyses include data from a total of n = 1973 participants. Participants were grouped into four groups according to age and sex: young male, young female, old male and old female. Group comparisons are not specifically mentioned for young males vs. old females and young females vs. old males since these comparisons are not relevant for this study.
Demographic characteristics are described as follows: means with 95% confidence intervals (95% CI) or means with standard deviation for continuous variables, and frequencies (% (n)) for categorical variables. Differences in characteristics between study groups were assessed by Pearson's chi-squared test for categorical variables and general linear models (GLM) for continuous variables. When necessary, micronutrient concentrations were transformed (squared root (SR) and logarithmically (LN)) to achieve normal distribution and are described by geometric means with 95% CI.
Multiple regression analyses (forward stepwise approach) with age (years) as the dependent variable were carried out with all measured lipid-soluble micronutrients as covariates in the initial model to retain those micronutrients with the strongest association with age.
Differences in micronutrient concentrations between the four study groups were assessed by one-way ANOVA with Tukey's post-hoc test (unadjusted) and by GLMs with Bonferroni post-hoc test adjusted for BMI, smoking status and season.
For linear regression analyses the following age-groups (ca. 10 year-intervals) were formed to determine associations of age-groups with micronutrients: 22.0–29.9 years, 30.0–36.9 years, 60.0–69.9 years, and 70.0–84.6 years. Age-group as a determinant of those micronutrients with highest correlations with age was finally assessed and confirmed using GLMs adjusted for season of blood collection, participant characteristics (sex, BMI, smoking status), plasma cholesterol, and dietary intake (fruits, vegetables, nuts, juice, and use of vitamin/mineral supplements); partial Eta squared (ηp2) was used as a measure of effect size. For those micronutrients significantly associated with age, relevant food items were selected from FFQs. The following items were selected: raw tomato, tomato sauce, ketchup, carrot juice, multivitamin juice, nuts, orange juice, carrots, tangerine, flax seeds, spinach, vitamin/mineral supplements. Frequencies of servings were reported as servings per day, per week, per month or per year (see Supplemental Table 1).
Statistically significant differences were considered present at P < 0.05. All statistical analyses were carried out using SPSS software (SPSS Inc., Chicago, IL, USA; Version 20.0.0). Microsoft PowerPoint was additionally used for figure preparation.
3. Results
Characteristics of the study population are shown in Table 1. Men's weight, height, BMI and eGFR were significantly higher compared to women's in both, the old as well as the young group. The prevalence of smoking was higher in men compared to women in both age-groups. In contrast, cholesterol was higher in women than in men (both age groups).
Table 1.
Characteristics of the study population.
| young male (n = 221) | young female (n = 244) | old male (n = 761) | old female (n = 747) | P | |
|---|---|---|---|---|---|
| Age (years) | 29.2 (28.8; 29.6)a | 28.6 (28.2; 29.0)a | 68.9 (68.7; 69.2)b | 68.3 (68.0; 68.5)c | <0.001 |
| Weight (kg)a | 77.9 (76.1; 79.7)a | 64.5 (62.8; 66.2)b | 84.0 (83.1; 84.9)c | 70.1 (69.2; 71.0)d | <0.001 |
| Height (cm) | 180.8 (179.9; 181.7)a | 168.5 (167.7; 169.3)b | 175.6 (175.1; 176.0)c | 162.9 (162.5; 163.3)d | <0.001 |
| BMI (kg/m2)a | 23.8 (23.3; 24.3)a | 22.7 (22.1; 23.2)b | 27.2 (27.0; 27.5)c | 26.5 (26.1; 26.8)d | <0.001 |
| BMI-Groups | |||||
| < 18.5 | 4.1 (9) | 9.0 (22) | 0.0 (0) | 0.7 (5) | |
| 18.5–24.9 | 64.3 (142) | 70.9 (173) | 28.2 (214) | 43.4 (324) | <0.001 |
| 25.0–29.9 | 27.1 (60) | 13.5 (33) | 54.9 (417) | 35.5 (265) | |
| ≥ 30.0 | 4.5 (10) | 6.6 (16) | 17.0 (129) | 20.5 (153) | |
| Current smoker | 34.8 (77) | 27.5 (67) | 10.4 (79) | 8.2 (61) | <0.001 |
| Cholesterol (μmol/L) | 4460 (4356; 4563)a | 4698 (4588; 4808)b | 5284 (5214; 5355)c | 5866 (5794; 5938)d | <0.001 |
| eGFR | 117.9 (115.8; 120.0)a | 113.9 (112.0; 115.8)b | 70.2 (69.4; 71.1)c | 68.4 (37.7; 69.3)d | <0.001 |
| MMSE | n.a. | n.a. | 28.31 ± 1.40 | 28.78 ± 1.60 | <0.001 |
| Medication | n.a. | n.a. | 2.66 ± 2.41 | 2.93 ± 2.34 | 0.038 |
Weight/BMI was missing for n = 1 old male. GLM with Bonferroni post-hoc test for continuous variables, Chi-squared test for categorical variables; n.a.: not available; continuous variables are expressed as mean (95% CI) or mean ± SD and categorical variables are expressed as % (n). Cholesterol (n = 1965), MMSE (n = 1409) and Medication (n = 1235).
We evaluated self-reported intake of food items via the EPIC-Potsdam-FFQ; selected food items are shown in Supplemental Table 1. All food items differed significantly between the four groups except in serving of carrots.
In multiple regression analyses, significant associations with age (as the dependent variable) were determined for lycopene, α-tocopherol, γ-tocopherol, β-carotene and α-carotene in the whole study population (Table 2). An inverse association with age was observed for lycopene and α-carotene, whereas both tocopherols and β-carotene were positively associated with age. Retinol, β-cryptoxanthin and lutein/zeaxanthin were not associated with age and are therefore not included in the forward analyses.
Table 2.
Associations of carotenoids, α- and γ-tocopherol, and retinol with age.
| (B) | 95% CI | partial R | P | |
|---|---|---|---|---|
| (μmol/L)a | 33.58 | (28.51; 38.64) | <0.001 | |
| (SR) Lycopene | −19.47 | (-21.28; −17.66) | −0.430 | <0.001 |
| (SR) α-Tocopherol | 8.111 | (7.199; 9.023) | 0.366 | <0.001 |
| (SR) γ-Tocopherol | 5.455 | (3.132; 7.778) | 0.103 | <0.001 |
| (LN) β-Carotene | 1.855 | (1.008; 2.702) | 0.096 | <0.001 |
| (LN) α-Carotene | −1.484 | (-2.512; −0.449) | −0.063 | 0.005 |
Multiple regression analyses (forward stepwise approach) with age (years) as the dependent variable; all measured lipid-soluble micronutrients were assessed as covariates in the initial model (n = 1973), sum R = 0.550, sum R2 = 0.303.
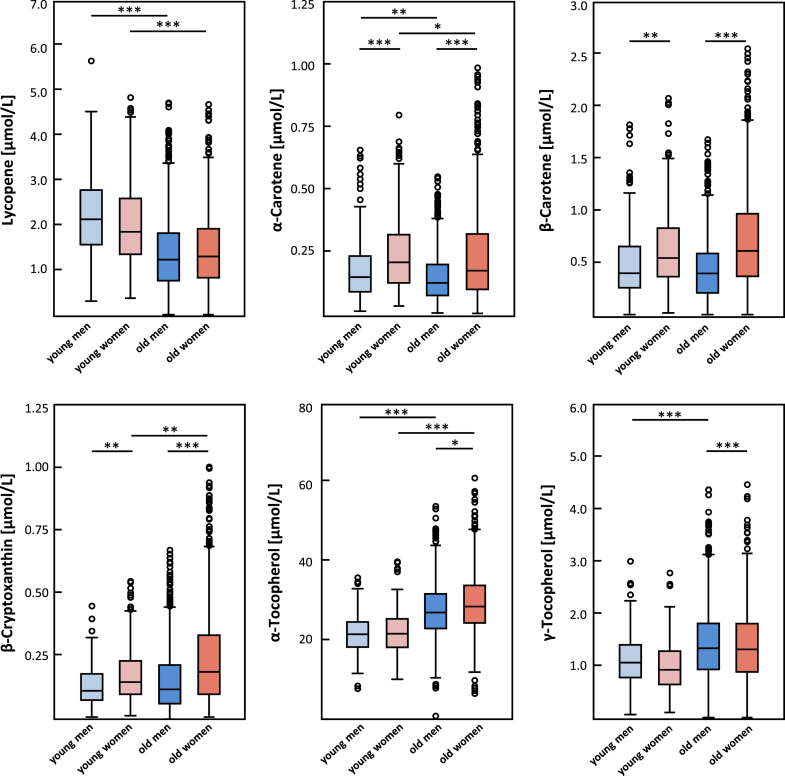
When comparing micronutrient concentrations between the four groups, statistically significant differences between men and women were observed, except for lycopene where no sex differences appeared (Fig. 1 and Table 3). For retinol, α- and γ-tocopherol differences between men and women were found only in the old group.
Fig. 1.
Lipid-soluble micronutrient concentrations in the study population.
Results are shown as boxplots and statistically significant differences between the groups were assessed by one-way ANOVA with Tukey's post-hoc test; extreme values were included in the analyses but are not shown in the figure for better visibility; *P < 0.05, **P < 0.01, ***P < 0.001.
Table 3.
Lipid-soluble micronutrient concentrations (μmol/L) in the study population.a
| young men (n = 221) | young women (n = 244) | old men (n = 761) | old women (n = 747) | P | |
|---|---|---|---|---|---|
| Lycopeneb | 4.31 (3.34; 5.57) | 4.04 (3.11; 5.24) | 3.04 (2.52; 3.65)d | 3.11 (2.54; 3.80)d | <0.001 |
| Adjusted GLMc | 2.07 (1.91; 2.24)e | 1.83 (1.69; 1.98) | 1.25 (1.19; 1.31)d | 1.26 (1.20; 1.32)d | <0.001 |
| α-Tocopherolb | 21.4 (11.1; 35.0) | 21.7 (10.9; 36.0) | 27.3 (18.2; 38.2)d,e | 28.4 (19.2; 39.5)d | <0.001 |
| Adjusted GLMc | 21.8 (20.7; 22.9) | 22.1 (21.1; 23.2) | 27.1 (26.5; 27.7)d | 28.3 (27.8; 29.0)d | <0.001 |
| γ-Tocopherolb | 1.047 (0.136; 2.813) | 0.914 (0.113; 2.481) | 1.344 (0.530; 2.532)d,e | 1.285 (0.480; 2.481)d | <0.001 |
| Adjusted GLMc | 1.090 (0.994; 1.191) | 0.972 (0.886; 1.063) | 1.304 (1.252; 1.358)d | 1.261 (1.211; 1.312)d | <0.001 |
| β-Caroteneb | 0.382 (0.080; 1.831)e | 0.526 (0.193; 1.432) | 0.315 (0.100; 0.997)e | 0.548 (0.225; 1.334) | <0.001 |
| Adjusted GLMc | 0.361 (0.430; 1.435) | 0.439 (0.518; 1.551) | 0.327 (0.356; 1.387)e | 0.549 (0.597; 1.732)d | <0.001 |
| α-Caroteneb | 0.142 (0.053; 0.383)e | 0.200 (0.093; 0.430) | 0.117 (0.064; 0.212)d,e | 0.166 (0.081; 0.338)d | <0.001 |
| Adjusted GLMc | 0.133 (0.153; 1.142)e | 0.171 (0.195; 1.186) | 0.122 (0.131; 1.130)e | 0.167 (0.179; 1.182) | <0.001 |
Geometric mean (95% CI) of back-transformed data.
One-way ANOVA with Tukey's post-hoc test; P < 0.05.
Adjusted general linear model (GLM): univariate general linear model adjusted for BMI, smoking status and season; Bonferroni post-hoc test; P < 0.05.
Statistically significant difference between age-groups: old men compared to young men; old women compared to young women.
Statistically significant difference between sexes: men compared to women within the same age group.
Differences between the young and old groups were found for lycopene, α-carotene, α-tocopherol, β-cryptoxanthin (only in women), and γ-tocopherol (only in men) (Fig. 1). β-Carotene, retinol and lutein/zeaxanthin did not differ between young and old participants regardless of the sex.
Subsequently, lycopene, α- and γ-tocopherol, α- and β-carotene were used in further adjusted GLMs and (multiple) linear regression models showing that the associations remained after adjusting for BMI, smoking status and season.
Means of these five micronutrients adjusted for BMI, smoking status and season remained significantly different between the four study groups (Table 3, adjusted GLM).
Linear regression analysis with age-groups as covariates confirmed the inverse association of lycopene with age and the positive associations of both tocopherols and α-carotene with age (Table 4). These associations remained for lycopene and both tocopherols even after adjusting for co-factors and covariates (Table 4). In detail, for every increase in age-group, lycopene concentration was lower by 21.9% (−0.312 μmol/L) after adjusting for BMI, sex, smoking status and season of blood collection. Significant associations of higher α-tocopherol (+2.270 μmol/L or 8.6%) and γ-tocopherol (0.071 μmol/L or 5.8%) with increasing age-group after adjustment for co-factors and covariates were found whereas there were no associations of α-carotene or β-carotene with age-groups after adjustment for BMI, sex, smoking status and season. After adjusting for dietary habits, cholesterol and eGFR (for tocopherols) the associations for lycopene, α-tocopherol and α-carotene remained significantly associated with age-groups, with the highest change (%) observed for lycopene.
Table 4.
Association of carotenoids and tocopherols with age groups.
| B | 95% CI | P | ηb | Change (μmol/L) a | Change (%) b | |
|---|---|---|---|---|---|---|
| (SR) Lycopene | ||||||
| Age groups c | −0.123 | −0.140; −0.107 | <0.001 | 0.100 | −0.305 | 21.4 |
| Age groups d | −0.113 | −0.131; −0.096 | <0.001 | 0.076 | −0.312 | 21.9 |
| Age groups e | −0.123 | −0.141; −0.106 | <0.001 | 0.087 | −0.218 | 15.3 |
| (SR) α-Tocopherol | ||||||
| Age groups c | 0.267 | 0.234; 0.301 | <0.001 | 0.111 | 2.686 | 10.2 |
| Age groups d | 0.255 | 0.219; 0.291 | <0.001 | 0.090 | 2.270 | 8.6 |
| Age groups f | 0.051 | 0.002; 0.099 | 0.039 | 0.002 | 0.299 | 1.1 |
| (SR) γ-Tocopherol | ||||||
| Age groups c | 0.061 | 0.047; 0.074 | <0.001 | 0.038 | 0.132 | 10.7 |
| Age groups d | 0.045 | 0.031; 0.059 | <0.001 | 0.019 | 0.071 | 5.8 |
| Age groups f | 0.006 | −0.017; 0.028 | 0.623 | 0.000 | 0.007 | 0.6 |
| (LN) β-Carotene | ||||||
| Age groups c | −0.026 | −0.078; 0.026 | 0.323 | 0.000 | −0.011 | 2.6 |
| Age groups d | 0.044 | −0.008; 0.097 | 0.100 | 0.001 | 0.052 | 12.3 |
| Age groups g | −0.033 | −0.114; 0.048 | 0.423 | 0.001 | −0.007 | 1.7 |
| (LN) α-Carotene | ||||||
| Age groups c | −0.076 | −0.117; −0.034 | <0.001 | 0.007 | −0.011 | 7.8 |
| Age groups d | −0.014 | −0.057; 0.028 | 0.509 | 0.000 | −0.006 | 4.0 |
| Age groups g | −0.066 | −0.124; −0.008 | 0.026 | 0.005 | −0.004 | 2.7 |
(SR) square root transformed; (LN) logarithmic transformed. Regression coefficient B represents the increase/decrease in respective compound for each (multiple) linear regression model.
Mean change of back-transformed data (μmol/L) per age group.
Change in unit increase as percentage (%) of the geometric means (lycopene: 1.425 μmol/L, α-tocopherol 26.29 μmol/L, γ-tocopherol 1.230 μmol/L, β-carotene: 0.423 μmol/L, α-carotene: 0.146 μmol/L).
Linear regression analysis with age groups (n = 4) as a covariate with micronutrient as dependent variable.
Multiple linear regression analyses with age group, BMI, sex and smoking status as covariates, and season as random factor.
Lycopene: Multiple linear regression analyses with age group, BMI, sex and smoking status as covariates, and season as random factor; cholesterol, frequency of raw tomato, frequency of ketchup, frequency of tomato sauce; cholesterol n = 1409.
α- and γ-tocopherol: Multiple linear regression analyses with age group, BMI, sex and smoking status as covariates, and season as random factor; cholesterol, frequency of reported dietary habits (frequency of intake of nuts, flax seeds, spinach and vitamin/mineral supplements (yes/no); eGFR, cholesterol n = 1409.
α- and β-carotene: Multiple linear regression analyses with age group, BMI, sex and smoking status as covariates, and season as random factor; cholesterol, frequency of reported dietary habits (carrots, carrot juice, multivitamin juice, and vitamin/mineral supplements (yes/no); cholesterol n = 1409.
4. Discussion
Several studies have examined lipid-soluble micronutrient status in the context of aging. Most of these publications relate to measurements in plasma/serum and/or on dietary assessments. Some of these studies found an inverse association between lycopene and age while other lipid-soluble micronutrients were positively or not associated with age [9,10,[18], [19], [20]].
Brady et al. reported that age was positively associated with all serum carotenoids, except with lycopene (inverse association) in 400 participants older than 50 years [18]. Even after adjusting for several cofactors (smoking, intake of supplements, alcohol consumption, BMI, HDL, non-HDL cholesterol), age was inversely associated with serum and dietary lycopene [18]. Significantly lower plasma lycopene was shown in participants aged ≥80 years (n = 491) in contrast to those aged < 79 years (n = 148; P < 0.05) [9]. In the total sample, women had higher concentrations of carotenoids than men, except for lycopene which was higher in men [10]. Furthermore, multivariate analyses revealed that in male non-smokers (n = 121) lycopene was inversely associated with age (β-coefficient for age (10-year interval) r = −0.23, P < 0.001), whereas in women (n = 186) no significant associations with age were observed [19]. In contrast to most other carotenoids, lycopene seems to decline during aging. Data from the European MARK-AGE Project show that the inverse relationship of lycopene with age is already observed in healthy participants starting at 35 years of age [11]. This inverse association was observed in men and in women equally.
In contrast, multiple regression analyses showed that only region and season of blood collection were significant predictors of plasma lycopene (EPIC study, n = 3011) [21]. Furthermore, it was demonstrated that BMI was inversely associated with serum concentrations of α- and β-carotene in unadjusted analyses. After adjusting for confounders, BMI was inversely associated with all carotenoids except with lycopene [18]. These observations may reflect changes in behavior or physiology, which in turn may be attributed to age [18]. Except for zeaxanthin, age was not an important predictor in regression analyses in the EPIC cohort (n = 3043), which is most probably due to the narrow age-range among the subjects (45–64 years) [21].
Plasma concentrations of lipid-soluble micronutrients rely on their dietary intake. The observed correlation between plasma lycopene with tomato products assessed by food questionnaires was r = 0.38 [10]. Total fruits and fruits and vegetables intake correlated with β-cryptoxanthin (r = 0.52 and r = 0.46, respectively). Fruits and vegetables intake correlated also with lutein (r = 0.38) and zeaxanthin (r = 0.36) as did root vegetables and total carrots with α-carotene (r = 0.39 and r = 0.38, respectively) [10]. Furthermore, significant positive correlations of fruit, vegetable and juice intake with carotenoid concentrations in plasma were found in men (n = 284) and women (n = 287) aged 20–59 years [22]. Additionally, in a small study with men and women aged >50 years, the adjusted correlation (for age, BMI, cholesterol and triglycerides) between intake and plasma concentrations for lycopene was r = 0.47 [19]. Similar results with adjusted (for age, sex, energy intake, BMI, serum cholesterol, smoking status and month of FFQ administration) correlations of r = 0.14–0.35 were found in 402 participants aged approximately 60–62 years [23]. These results imply that there is a correlation between intake and plasma.
Thus, there is no clear explanation for the inverse age association for lycopene, up to date. In addition to a possibly reduced intake in advanced-aged people, it has been suggested that the bioavailability of lycopene - in contrast to other carotenoids - is impaired during aging. This might be due to more pronounced effects of age-associated physical or chemical alterations of the gastrointestinal tract [24,25]. Furthermore, physiological changes and lifestyle factors might influence serum/plasma carotenoid concentrations. Since lycopene is derived mainly from tomato products it is possible that some persons (e.g. young persons or men) consume more lycopene-rich products (ketchup, tomato sauce, pizza, etc.) than foods which are rich in other carotenoids (other fruits and vegetables). Food processing can increase the accessibility of carotenoids to the metabolism and thus can lead to a higher bioavailability [26]. It is known that lycopene bioavailability is better from processed tomato products than from raw tomatoes [27,28]. This is also true for β-cryptoxanthin that is better bioavailable from orange juice compared to fresh oranges [29] and for β-carotene from processed carrots compared to fresh ones [30]. Beside possible beneficial effects, processing or cooking of fruits and vegetables can also lead to reduced contents of carotenoids [28,31,32]. However, the addition of oils during food processing or while eating can enhance bioavailability of carotenoids by potentially better incorporation of these into micelles, which are needed for absorption [26,33].
Few studies have examined the bioavailability of lycopene. The chylomicron-responses of lycopene, α-, β-carotene and lutein do not differ markedly between older (60–75 years) and younger (20–35 years) adults, respectively [34]. But unfortunately, only 16 subjects were included in this study and they were only men. When results of the chylomicron-carotenoid response were standardized to chylomicron-triacylglycerol response there was a remarkable difference observed for lycopene. In fact, the significantly reduced triacylglycerol-standardized lycopene response (−40%, P < 0.04) indicates that the bioavailability of lycopene is reduced in older men.
It is possible that the intake of lycopene in older subjects is lower; however, this effect may be masked partially in non-standardized chylomicron response curves since it is known that the chylomicron clearance is reduced in older persons [34]. The standardized β-carotene response after a tomato meal did not differ between the groups. Therefore, the effect on the carotenoid bioavailability is likely to be independent of the food matrix (tomato). In contrast to other carotenoids the cellular uptake of lycopene seems to be more strongly influenced by age [24,35] which was demonstrated by a low absorption efficiency [36].
Regarding the significantly age-associated micronutrients (Table 2, Table 3) in our study, we observed comparable or different concentrations to other studies. Compared to young individuals (n = 349; aged 25–45 years) from five European countries (France, Northern Ireland, Republic of Ireland, The Netherlands and Spain) [37] young individuals (aged 22–37 years) in our study had higher lycopene, similar α- and β-carotene, and lower α- and γ-tocopherol concentrations. Similar concentrations for α-tocopherol (young) and α-carotene (young and old) were found compared to young (35–44 years) and old (60–74 years) individuals from the MARK-AGE study (Austria, Belgium, Germany, Greece, Italy, and Poland) [11]. However, in our study, lycopene in young and old individuals was higher and α-tocopherol in old individuals was lower compared to MARK-AGE. These differences might appear due to regional and thus differences in eating habits between study populations. Additionally, none of the groups in our study showed any deficiencies regarding α-tocopherol (<12 μmol/L [38]) or retinol (<0.7 μmol/L [39]) concentrations. For carotenoids, no cut-off concentrations are defined, yet.
The limitations of our study include its cross-sectional nature which does not allow any conclusion on causality. Additionally, no participants between 40 and 60 years were included in the study although this would have been an interesting age-span as shown in the MARK-AGE Project [11]. Data on intake of micronutrients from foods were limited to raw tomatoes, tomato sauce, ketchup, carrots, carrot juice, multivitamin juice, nuts, flax seeds, spinach, orange juice and tangerine, and not considering other seeds, leafy green vegetables or even animal products that potentially contain micronutrients, especially tocopherols and retinol. Thus, adjusted tocopherol concentrations might be over- or underestimated. However, lycopene, α-tocopherol and α-carotene all showed significant age associations, and thus the adjustment to their main dietary sources strengthens our results. For supplement use, we were only able to include the information whether supplements were consumed but unfortunately not which type of supplements or which dosages were used; this information could have improved analyses.
We are aware that especially for older populations the use of BMI is discussed critically [40]. Therefore, we adjusted models in Table 3 for weight instead of BMI (not shown) and achieved similar results, except for β-carotene where associations with age-group became statistically significant by replacing BMI with weight.
Cognitive function and medication were not available for the young participants, and are therefore missing as possible confounder for micronutrients for the young participants. Nevertheless, the large sample size (n = 1973), the availability of several confounders that were used for adjusting statistical analyses for sex, BMI, season of blood sampling, smoking status, dietary intake of fruits and vegetables and cholesterol are strengths of our study. Additionally, all lipid-soluble micronutrients were measured within one laboratory by well-experienced personnel, thus, preventing analytical bias.
It is well known that tocopherols are transported in lipoproteins. Therefore, vitamin E concentration is closely correlated with cholesterol and total lipids [41,42] and adjusting to cholesterol showed that α- and γ-tocopherol remained significantly positively associated with age. Kidney function is also related to circulating levels of tocopherols. Gamma-tocopherol concentrations were shown to be significantly higher in hemodialysis patients (n = 15) compared to healthy controls (n = 15), whereas no difference appeared for α-tocopherol [43]. Additionally, both tocopherol concentrations were significantly higher in healthy compared to hemodialysis patients, but did not differ between healthy controls and chronic renal failure patients [44]. Furthermore, α-tocopherol was higher in healthy than in pre-dialysis patients as well as end-stage renal disease patients, which simultaneously had higher α-tocopherol than pre-dialysis patients [45]. The prevalence of impaired kidney function in our study population ranged from 7.7 to 19.6%, depending on the equation used [15]. However, since this was not in the focus of our study we did not adjust for eGFR. When including eGFR in the models for associations between tocopherols and age groups (Table 4) we observed that statistical significance remained for α-tocopherol after including eGFR to the respective models, but not for γ-tocopherol.
Absorption and post-prandial metabolism of β-carotene, β-cryptoxanthin and lutein/zeaxanthin are most likely not significantly affected in healthy older persons. In contrast, the bioavailability of lycopene and α-carotene seem to be impaired in older subjects.
Larger studies on the bioavailability of lycopene especially in old participants are lacking. In addition, an effect of participant's on bioavailability of lycopene sex cannot be excluded since previous studies have only been carried out with men. Furthermore, previous studies are difficult to compare since they differ not only in study design (number of participants, duration of intake, type of supplements, food, carotenoids vs. lycopene alone), but also in measurements (plasma/serum vs. chylomicron).
5. Conclusions
We observed significantly lower lycopene and α-carotene concentrations with age and confirmed the inverse associations between age and lycopene and α-carotene, as well as the positive association between tocopherols and age in multiple linear regression analyses. Since lycopene and α-carotene are important antioxidants, low concentrations might lead to higher oxidative stress, which might lead to an increased susceptibility for diseases. Furthermore, lycopene and α-carotene are associated with lower incidence of various age-related diseases such as tumors, cardiovascular diseases, metabolic syndrome, atherosclerosis, and cognitive disorders, thus it is important to understand the underlying mechanisms in order to implement dietary strategies for the prevention of these age-related diseases.
For the first time we were able to show that age-associated differences of plasma concentrations of micronutrients, in particular lycopene, are related to factors other than dietary habits and most likely bioavailability is altered during aging.
To explain the lower lycopene concentration in older subjects, bioavailability studies are necessary. These should take into consideration the following aspects: sufficient number of participants, inclusion of both sexes, comparison of lycopene and β-carotene (which does not seem to be associated with age in adjusted models) availability, sufficient age difference (approx. 20–25 years vs. 60–65 years) and exclusion of persons with gastrointestinal diseases. These data will serve as basis for further planned studies on bioavailability of lycopene in old age.
Author contributions
Writing—original draft preparation, statistical analyses, preparation of figures, D.W. and B.K.; writing—review and editing, T.G, I.D., E.S.-T. and K.N.; provision of resources, I.D. and E.S.-T.
Funding
The BASE-II research project (Co-PIs are Lars Bertram, Ilja Demuth, Denis Gerstorf, Ulman Lindenberger, Graham Pawelec, Elisabeth Steinhagen-Thiessen, and Gert G. Wagner) is supported by the German Federal Ministry of Education and Research (Bundesministerium für Bildung und Forschung, BMBF) under grant numbers #16SV5536K, #16SV5537, #16SV5538, #16SV5837, #01UW0808, 01GL1716A and 01GL1716B. Another source of funding is the Max Planck Institute for Human Development, Berlin, Germany. Additional contributions (e.g., equipment, logistics, and personnel) was provided by each of the participating sites.
Declaration of competing interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Acknowledgments
The authors thank Martina Scholtyssek for excellent technical assistance.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.redox.2020.101461.
Contributor Information
Daniela Weber, Email: daniela.weber@dife.de.
Bastian Kochlik, Email: bastian.kochlik@dife.de.
Ilja Demuth, Email: ilja.demuth@charite.de.
Elisabeth Steinhagen-Thiessen, Email: e.steinhagen-thiessen@charite.de.
Tilman Grune, Email: scientific.director@dife.de.
Kristina Norman, Email: kristina.norman@dife.de.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Bazzano L.A., He J., Ogden L.G., Loria C.M., Vupputuri S., Myers L., Whelton P.K. Fruit and vegetable intake and risk of cardiovascular disease in US adults: the first national health and nutrition examination survey epidemiologic follow-up study. Am. J. Clin. Nutr. 2002;76(1):93–99. doi: 10.1093/ajcn/76.1.93. [DOI] [PubMed] [Google Scholar]
- 2.Sesso H.D., Liu S., Gaziano J.M., Buring J.E. Dietary lycopene, tomato-based food products and cardiovascular disease in women. J. Nutr. 2003;133(7):2336–2341. doi: 10.1093/jn/133.7.2336. [DOI] [PubMed] [Google Scholar]
- 3.Hak A.E., Stampfer M.J., Campos H., Sesso H.D., Gaziano J.M., Willett W., Ma J. Plasma carotenoids and tocopherols and risk of myocardial infarction in a low-risk population of US male physicians. Circulation. 2003;108(7):802–807. doi: 10.1161/01.CIR.0000084546.82738.89. [DOI] [PubMed] [Google Scholar]
- 4.Agudo A., Cabrera L., Amiano P., Ardanaz E., Barricarte A., Berenguer T., Chirlaque M.D., Dorronsoro M., Jakszyn P., Larranaga N. Fruit and vegetable intakes, dietary antioxidant nutrients, and total mortality in Spanish adults: findings from the Spanish cohort of the European Prospective Investigation into Cancer and Nutrition (EPIC-Spain) Am. J. Clin. Nutr. 2007;85(6):1634–1642. doi: 10.1093/ajcn/85.6.1634. [DOI] [PubMed] [Google Scholar]
- 5.Sluijs I., Beulens J.W., Grobbee D.E., van der Schouw Y.T. Dietary carotenoid intake is associated with lower prevalence of metabolic syndrome in middle-aged and elderly men. J. Nutr. 2009;139(5):987–992. doi: 10.3945/jn.108.101451. [DOI] [PubMed] [Google Scholar]
- 6.Shardell M.D., Alley D.E., Hicks G.E., El-Kamary S.S., Miller R.R., Semba R.D., Ferrucci L. Low-serum carotenoid concentrations and carotenoid interactions predict mortality in US adults: the Third National Health and Nutrition Examination Survey. Nutr. Res. (N.Y.) 2011;31(3):178–189. doi: 10.1016/j.nutres.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boeing H., Bechthold A., Bub A., Ellinger S., Haller D., Kroke A., Leschik-Bonnet E., Muller M.J., Oberritter H., Schulze M. Critical review: vegetables and fruit in the prevention of chronic diseases. Eur. J. Nutr. 2012;51(6):637–663. doi: 10.1007/s00394-012-0380-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kochlik B., Stuetz W., Pérès K., Pilleron Sf C., García García F.J., Bandinelli S., Gomez-Cabrero D., Rodriguez-Manas L., Grune T., Weber D. Associations of lipid-soluble micronutrients and redox biomarkers with frailty status in the FRAILOMIC initiative. J. Cachexia Sarcopenia Muscle. 2019;10(6):1339–1346. doi: 10.1002/jcsm.12479. Epub 2019 Aug 21, PMID: 31436047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vogel S., Contois J.H., Tucker K.L., Wilson P.W., Schaefer E.J., Lammi-Keefe C.J. Plasma retinol and plasma and lipoprotein tocopherol and carotenoid concentrations in healthy elderly participants of the Framingham Heart Study. Am. J. Clin. Nutr. 1997;66(4):950–958. doi: 10.1093/ajcn/66.4.950. [DOI] [PubMed] [Google Scholar]
- 10.Al-Delaimy W.K., Ferrari P., Slimani N., Pala V., Johansson I., Nilsson S., Mattisson I., Wirfalt E., Galasso R., Palli D. Plasma carotenoids as biomarkers of intake of fruits and vegetables: individual-level correlations in the European Prospective Investigation into Cancer and Nutrition (EPIC) Eur. J. Clin. Nutr. 2005;59(12):1387–1396. doi: 10.1038/sj.ejcn.1602252. [DOI] [PubMed] [Google Scholar]
- 11.Stuetz W., Weber D., Dolle M.E., Jansen E., Grubeck-Loebenstein B., Fiegl S., Toussaint O., Bernhardt J., Gonos E.S., Franceschi C. Plasma carotenoids, tocopherols, and retinol in the age-stratified (35-74 Years) general population: a cross-sectional study in six European countries. Nutrients. 2016;8(10) doi: 10.3390/nu8100614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bertram L., Bockenhoff A., Demuth I., Duzel S., Eckardt R., Li S.C., Lindenberger U., Pawelec G., Siedler T., Wagner G.G. Cohort profile: the Berlin aging study II (BASE-II) Int. J. Epidemiol. 2014;43(3):703–712. doi: 10.1093/ije/dyt018. [DOI] [PubMed] [Google Scholar]
- 13.Gerstorf D., Bertram L., Lindenberger U., Pawelec G., Demuth I., Steinhagen-Thiessen E., Wagner G.G. Editorial. Gerontology. 2016;62(3):311–315. doi: 10.1159/000441495. [DOI] [PubMed] [Google Scholar]
- 14.Pottel H., Hoste L., Dubourg L., Ebert N., Schaeffner E., Eriksen B.O., Melsom T., Lamb E.J., Rule A.D., Turner S.T. An estimated glomerular filtration rate equation for the full age spectrum. Nephrol. Dial. Transplant. 2016;31(5):798–806. doi: 10.1093/ndt/gfv454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Konig M., Gollasch M., Demuth I., Steinhagen-Thiessen E. Prevalence of impaired kidney function in the German elderly: results from the Berlin aging study II (BASE-II) Gerontology. 2017;63(3):201–209. doi: 10.1159/000454831. [DOI] [PubMed] [Google Scholar]
- 16.Nöthlings U., Hoffmann K., Bergmann M.M., Boeing H. Fitting portion sizes in a self-administered food frequency questionnaire. J. Nutr. 2007;137(12):2781–2786. doi: 10.1093/jn/137.12.2781. [DOI] [PubMed] [Google Scholar]
- 17.Kroke A., Klipstein-Grobusch K., Voss S., Moseneder J., Thielecke F., Noack R., Boeing H. Validation of a self-administered food-frequency questionnaire administered in the European Prospective Investigation into Cancer and Nutrition (EPIC) Study: comparison of energy, protein, and macronutrient intakes estimated with the doubly labeled water, urinary nitrogen, and repeated 24-h dietary recall methods. Am. J. Clin. Nutr. 1999;70(4):439–447. doi: 10.1093/ajcn/70.4.439. [DOI] [PubMed] [Google Scholar]
- 18.Brady W.E., Mares-Perlman J.A., Bowen P., Stacewicz-Sapuntzakis M. Human serum carotenoid concentrations are related to physiologic and lifestyle factors. J. Nutr. 1996;126(1):129–137. doi: 10.1093/jn/126.1.129. [DOI] [PubMed] [Google Scholar]
- 19.Michaud D.S., Giovannucci E.L., Ascherio A., Rimm E.B., Forman M.R., Sampson L., Willett W.C. Associations of plasma carotenoid concentrations and dietary intake of specific carotenoids in samples of two prospective cohort studies using a new carotenoid database. Canc. Epidemiol. Biomarkers Prev. : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 1998;7(4):283–290. [PubMed] [Google Scholar]
- 20.Buiatti E., Munoz N., Kato I., Vivas J., Muggli R., Plummer M., Benz M., Franceschi S., Oliver W. Determinants of plasma anti-oxidant vitamin levels in a population at high risk for stomach cancer. Int. J. Canc. 1996;65(3):317–322. doi: 10.1002/(SICI)1097-0215(19960126)65:3<317::AID-IJC7>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 21.Al-Delaimy W.K., van Kappel A.L., Ferrari P., Slimani N., Steghens J.P., Bingham S., Johansson I., Wallstrom P., Overvad K., Tjonneland A. Plasma levels of six carotenoids in nine European countries: report from the European Prospective Investigation into Cancer and Nutrition (EPIC) Publ. Health Nutr. 2004;7(6):713–722. doi: 10.1079/phn2004598. [DOI] [PubMed] [Google Scholar]
- 22.Jansen M.C., Van Kappel A.L., Ocke M.C., Van 't Veer P., Boshuizen H.C., Riboli E., Bueno-de-Mesquita H.B. Plasma carotenoid levels in Dutch men and women, and the relation with vegetable and fruit consumption. Eur. J. Clin. Nutr. 2004;58(10):1386–1395. doi: 10.1038/sj.ejcn.1601981. [DOI] [PubMed] [Google Scholar]
- 23.Talegawkar S.A., Johnson E.J., Carithers T.C., Taylor H.A., Bogle M.L., Tucker K.L. Carotenoid intakes, assessed by food-frequency questionnaires (FFQs), are associated with serum carotenoid concentrations in the Jackson Heart Study: validation of the Jackson Heart Study Delta NIRI Adult FFQs. Publ. Health Nutr. 2008;11(10):989–997. doi: 10.1017/S1368980007001310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Evans M.A., Triggs E.J., Cheung M., Broe G.A., Creasey H. Gastric emptying rate in the elderly: implications for drug therapy. J. Am. Geriatr. Soc. 1981;29(5):201–205. doi: 10.1111/j.1532-5415.1981.tb01766.x. [DOI] [PubMed] [Google Scholar]
- 25.Ikuma M., Hanai H., Kaneko E., Hayashi H., Hoshi T. Effects of aging on the microclimate pH of the rat jejunum. Biochim. Biophys. Acta. 1996;1280(1):19–26. doi: 10.1016/0005-2736(95)00261-8. [DOI] [PubMed] [Google Scholar]
- 26.Stahl W., van den Berg H., Arthur J., Bast A., Dainty J., Faulks R.M., Gartner C., Haenen G., Hollman P., Holst B. Bioavailability and metabolism. Mol. Aspect. Med. 2002;23(1–3):39–100. doi: 10.1016/s0098-2997(02)00016-x. [DOI] [PubMed] [Google Scholar]
- 27.Stahl W., Sies H. Uptake of lycopene and its geometrical isomers is greater from heat-processed than from unprocessed tomato juice in humans. J. Nutr. 1992;122(11):2161–2166. doi: 10.1093/jn/122.11.2161. [DOI] [PubMed] [Google Scholar]
- 28.Gartner C., Stahl W., Sies H. Lycopene is more bioavailable from tomato paste than from fresh tomatoes. Am. J. Clin. Nutr. 1997;66(1):116–122. doi: 10.1093/ajcn/66.1.116. [DOI] [PubMed] [Google Scholar]
- 29.Aschoff J.K., Rolke C.L., Breusing N., Bosy-Westphal A., Hogel J., Carle R., Schweiggert R.M. Bioavailability of beta-cryptoxanthin is greater from pasteurized orange juice than from fresh oranges - a randomized cross-over study. Mol. Nutr. Food Res. 2015;59(10):1896–1904. doi: 10.1002/mnfr.201500327. [DOI] [PubMed] [Google Scholar]
- 30.Rock C.L., Lovalvo J.L., Emenhiser C., Ruffin M.T., Flatt S.W., Schwartz S.J. Bioavailability of β-carotene is lower in raw than in processed carrots and spinach in women. J. Nutr. 1998;128(5):913–916. doi: 10.1093/jn/128.5.913. [DOI] [PubMed] [Google Scholar]
- 31.Maiani G., Caston M.J., Catasta G., Toti E., Cambrodon I.G., Bysted A., Granado-Lorencio F., Olmedilla-Alonso B., Knuthsen P., Valoti M. Carotenoids: actual knowledge on food sources, intakes, stability and bioavailability and their protective role in humans. Mol. Nutr. Food Res. 2009;53(Suppl 2):S194–S218. doi: 10.1002/mnfr.200800053. [DOI] [PubMed] [Google Scholar]
- 32.Colle I., Lemmens L., Van Buggenhout S., Van Loey A., Hendrickx M. Effect of thermal processing on the degradation, isomerization, and bioaccessibility of lycopene in tomato pulp. J. Food Sci. 2010;75(9):C753–C759. doi: 10.1111/j.1750-3841.2010.01862.x. [DOI] [PubMed] [Google Scholar]
- 33.Salvia-Trujillo L., Verkempinck S.H., Sun L., Van Loey A.M., Grauwet T., Hendrickx M.E. Lipid digestion, micelle formation and carotenoid bioaccessibility kinetics: influence of emulsion droplet size. Food Chem. 2017;229:653–662. doi: 10.1016/j.foodchem.2017.02.146. [DOI] [PubMed] [Google Scholar]
- 34.Cardinault N., Tyssandier V., Grolier P., Winklhofer-Roob B.M., Ribalta J., Bouteloup-Demange C., Rock E., Borel P. Comparison of the postprandial chylomicron carotenoid responses in young and older subjects. Eur. J. Nutr. 2003;42(6):315–323. doi: 10.1007/s00394-003-0426-2. [DOI] [PubMed] [Google Scholar]
- 35.Ikuma M., Hanai H., Kaneko E., Hayashi H., Hoshi T. Effects of aging on the regulation of intracellular pH in the rat jejunum. J. Gerontol. Biol. Med. Sci. 1996;51(5):B346–B353. doi: 10.1093/gerona/51a.5.b346. [DOI] [PubMed] [Google Scholar]
- 36.Tyssandier V., Lyan B., Borel P. Main factors governing the transfer of carotenoids from emulsion lipid droplets to micelles. Biochim. Biophys. Acta. 2001;1533(3):285–292. doi: 10.1016/s1388-1981(01)00163-9. [DOI] [PubMed] [Google Scholar]
- 37.Olmedilla B., Granado F., Southon S., Wright A.J., Blanco I., Gil-Martinez E., Berg H., Corridan B., Roussel A.M., Chopra M. Serum concentrations of carotenoids and vitamins A, E, and C in control subjects from five European countries. Br. J. Nutr. 2001;85(2):227–238. doi: 10.1079/bjn2000248. [DOI] [PubMed] [Google Scholar]
- 38.Medicine Io. 1998. Dietary reference intakes for vitamins C, E, selenium and carotenoids, Institute of medicine, NIH. Washington, D.C. [Google Scholar]
- 39.Medicine Io. 1998. Dietary reference intakes for vitamins A, K and trace elements, Institute of medicine, NIH. Washington, D.C. [Google Scholar]
- 40.Batsis J.A., Mackenzie T.A., Bartels S.J., Sahakyan K.R., Somers V.K., Lopez-Jimenez F. Diagnostic accuracy of body mass index to identify obesity in older adults: NHANES 1999-2004. Int. J. Obes. 2016;40(5):761–767. doi: 10.1038/ijo.2015.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Horwitt M.K., Harvey C.C., Dahm C.H., Jr., Searcy M.T. Relationship between tocopherol and serum lipid levels for determination of nutritional adequacy. Ann. N. Y. Acad. Sci. 1972;203:223–236. doi: 10.1111/j.1749-6632.1972.tb27878.x. [DOI] [PubMed] [Google Scholar]
- 42.Thurnham D.I., Davies J.A., Crump B.J., Situnayake R.D., Davis M. The use of different lipids to express serum tocopherol: lipid ratios for the measurement of vitamin E status. Ann. Clin. Biochem. 1986;23(Pt 5):514–520. doi: 10.1177/000456328602300505. [DOI] [PubMed] [Google Scholar]
- 43.Himmelfarb J., Kane J., McMonagle E., Zaltas E., Bobzin S., Boddupalli S., Phinney S., Miller G. Alpha and gamma tocopherol metabolism in healthy subjects and patients with end-stage renal disease. Kidney Int. 2003;64(3):978–991. doi: 10.1046/j.1523-1755.2003.00151.x. [DOI] [PubMed] [Google Scholar]
- 44.Galli F., Floridi A.G., Floridi A., Buoncristiani U. Accumulation of vitamin E metabolites in the blood of renal failure patients. Clin. Nutr. (Edinburgh, Scotland) 2004;23(2):205–212. doi: 10.1016/S0261-5614(03)00128-6. [DOI] [PubMed] [Google Scholar]
- 45.Karamouzis I., Sarafidis P.A., Karamouzis M., Iliadis S., Haidich A.B., Sioulis A., Triantos A., Vavatsi-Christaki N., Grekas D.M. Increase in oxidative stress but not in antioxidant capacity with advancing stages of chronic kidney disease. Am. J. Nephrol. 2008;28(3):397–404. doi: 10.1159/000112413. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.