Highlights
-
•
Ca-alginate-chitosan and eudragit S100 nanoparticles were used for encapsulation.
-
•
The encapsulation increased the viability of probiotics into Iranian Doogh beverage.
-
•
The encapsulation increased the viability of probiotics under GI conditions.
Keywords: Microencapsulation, Probiotics, Lactobacilli, Doogh, Alginate-Chitosan, Eudragit S100
Abstract
The survival rate of free and encapsulated L. acidophilus and L. rhamnosus into Doogh beverage and simulated gastrointestinal conditions during 42-day were studied. Microencapsulation considerably protected both L. acidophilus and L. rhamnosus in Doogh beverage storage and in gastrointestinal conditions. Microencapsulation provided better protection to L. acidophilus than to L. rhamnosus during Doogh storage. In beverages containing the free form of bacteria, pH and acidity changes were greater than those of microencapsulated and control groups. More activity of the free probiotic bacteria (during a 42-day period especially after 21-day) produced more acid and metabolites inside the product, thereby reducing the organoleptic properties scores, However, acidity, pH and organoleptic characteristics of Doogh containing microencapsulated bacteria did not change considerably. In conclusion, this study suggests that the encapsulation and double coating of L. acidophilus and L. rhamnosus can increase the viability of them in Doogh beverage and in simulated GI conditions.
1. Introduction
Probiotics are live microorganisms that they provide health advantageous when consumed, commonly via improving or refurbishing the gastrointestinal (GI) flora [1,2]. These microorganisms via several identified and unidentified mechanisms increase human metabolism, relieve chronic intestinal inflammatory and functional disorders, infections, allergy, and also detoxification of several toxins like aflatoxins in products [[3], [4], [5], [6], [7]]. Foods containing probiotic bacteria fall within the “functional foods’’ class and these foods should contain at least 107 cfu/g probiotic bacteria and consumed at levels higher than 100 g/day to have helpful effects on health [8]. Different dairy products have long been used as carriers for probiotic bacteria [[9], [10], [11]]. Nevertheless, there are still problems encountered with the application of probiotic bacteria in dairy foods; one of them is survival of the probiotics in dairy foods and during GI transit to the site of action in the human gut. Cold stress, exposure to acid and bile and osmotic and oxidative stress may reduce the number of probiotic bacteria below the effective threshold ([[12], [13], [14], [15], [16]]).
Different techniques are available for improving the survival of probiotics and microencapsulation is one of the best and most outstanding techniques. This technique can be effective in both product storage as well as GI condition [14,17]. The majority of studies conclude to the benefit of microencapsulation on raising the continued existence of the probiotics in dissimilar manufacture techniques and GI undesirable circumstances ([12,[18], [19], [20], [21]]). Microencapsulation via calcium alginate (a liner anionic heteropolysaccharide) is an efficient technique for the immobilization of lactic acid bacteria (LAB). The simplicity of handling, its non-toxic nature, and its low cost have made it one of the most widely used techniques for microencapsulation [8,22]. However, there are limitations for using alginate because of its low stability in the presence of chelating agents and in acidic conditions below pH 2. Chitosan (a liner cationic polysaccharide) is able to improve the strength of alginate beads and develop survivability of probiotic microorganisms in undesirable situations. Chitosan can be used as a coat to support micro-coverage over other negative-charge micro-covers [[23], [24], [25], [26]].
Eudragit (Eu) S100 (an anionic copolymer of methacrylic acid and methyl methacrylate that it is soluble in pH 7, but is non-soluble in acidic conditions and in water) has been used for micro/nano encapsulation. The Eu S100 can prevent the rapid dissolution of beads through the gastric cavity and the upper small intestine, and it may use for the targeted release of probiotic microorganisms in the colon (pH 7) [27,28]. Eu polymers are non-toxic and food-grade material and have previously been used in pharmaceuticals. [29,30]. Eu S100 as a secondary coating can strengthen the capsules containing probiotics (after first coating of chitosan over the alginate beads) [28,[31], [32], [33]]. Also, Nanoparticles normally have a trend to accumulate and stick to the mucosal wall mainly in the inflammatory situation, therefore, Eu nanoparticles comprise two most important benefits; stick to the swollen district and releasing the probiotics in that area (mainly in the colon). Another advantage of nanoparticles in preference to Eu powder is the founding of a thin nano-size layer in surrounding the beads. This extremely slight thin layer is probably able to increase the strength of beads without expanding the size of them. Smaller beads may decrease the oral sense of beads in a foodstuff carrier and also diminish the utilization of costly Eu powder [[33], [34], [35]].
Lactic acid bacteria, especially Lactobacilli are the main probiotic microorganisms of the human gastrointestinal (GI) lumen. The proper adhesion of Lactobacilli to the enterocytes and their advanced health effects have led this genus of probiotic bacteria to be the most illustrated and applicable among other probiotic genera [[36], [37], [38], [39]]. Lactobacillus acidophilus is a probiotic microorganism existing in common yogurt [40,41] and Lactobacillus rhamnosus has been newly added to yogurt as a beneficial probiotic [42,43]. Iranian Doogh is a beverage made by yogurt, water, and salt and is a traditional and popular beverage in Iran and some other countries including Azerbaijan, Turkey, Iraq, Balkan, and Iceland. In fact, Iranian Doogh consists of milk fat (maximum 25 % of total dry non-fat in last manufactured goods), non-fat milk solids (minimum 3.2 % W/W), salt (0.2–1 % W/W) with a maximum pH of 4.50. Also, Carbon dioxide (minimum 0.4 % W/W) perhaps unnaturally or naturally has been added during fermentation time to the manufactured Iranian Doogh. Furthermore, thickening agents and/or anti-whey division compounds can be added (maximum 10 % of the total dry non-fat matter in last manufactured goods) ([44,45]). In this study, we attempted a comprehensive examination of Iranian Doogh beverage containing probiotics bacteria (Lactobacillus rhamnosus and Lactobacillus acidophilus). Free and microencapsulated bacteria are compared for organoleptic characteristics and survivability in Doogh production and subsequently in simulated GI condition.
2. Material and methods
2.1. Preparation of probiotic bacteria
Probiotic bacteria cultures of L. acidophilus (PTCC 4356, Iranian Research Organization for Science and Technology) and L. rhamnosus PTCC 1469, Iranian Research Organization for Science and Technology were inoculated into MRS-broth and incubated at 37 ± 2 °C for 24 h in aerobic condition. Then the bacteria were collected by centrifugation Eppendorf, Centrifuge 5810 R, Germany at 5000 rpm 2800 G-force for 10 min, and afterward, it was washed by distilled water before applying in the encapsulation process [46].
2.2. Preparation of Eudragit S100 nanoparticles and chitosan solution
Eu S100 powder was obtained from Evonik Pharma Polymers (Evonik, D-64275, Darmstadt, Germany). To prepare the Eu S100 nanoparticles, we applied a modified Supercritical Antisolvent Technique (SAS) progression according to the method that already described by Ansari et al. [47]. In this method, 4 mg ml−1 of Eu solution added in distilled water gently as a supercritical fluid that had been held under homogenization pressure Wisetise, DAIHAN Scientific Co., Ltd, Korea at 26,000 rpm 75,712 G-force at 35 °C for 10 min. Also, distilled water as a surfactant included 15 mg L−1 Tween 80 (Merk, Hohenbrunn, Germany). Finally, the acetone solvent was evaporated. The particle size of the Eu and PDI (polydispersibility/polydispersivity index) were evaluated by means of the Laser Particle Size Analyzer device (Brookhaven Instruments Corporation, USA) [28,31,47]. For the preparation of the chitosan solution, we also used the method described earlier by Ansari et al. [47]. 0.4 g low-molecular-weight chitosan (Sigma, USA) mixed with 90 ml distilled water and acidified by use of 0.4 ml of glacial acetic acid (Merk, Darmstadt, Germany). Then, the pH was regulated in 5.6–5.8 using 1 mol L−1 NaOH, and the solution was filtered through Whatman #4 paper filter and the extent was adjusted to 100 ml before sterilizing into the autoclave (121 °C, 15 min). Lastly, the chitosan solution was held at 5 °C overnight.
2.3. Microencapsulation and the double coating of probiotic bacteria
In this study, the extrusion method with sodium alginate and calcium chloride was performed for the microencapsulation process described earlier by Mirzaei et al. [48]. The first coating of beads with chitosan was performed by Chávarri et al. [25] and Kanmani et al. [49], and the second coating with Eudragit S100 nanoparticles was prepared as described by Badhana et al. [27] methods respectively. Finally, these double-coated beads were collected and rinsed thoroughly with distilled water and used on the same day. In this process, 4 g 100 ml−1 sodium alginate (Sigma, USA) was mixed with distilled water, sterilized and kept at 5 °C overnight. Following day, 10 ml of probiotic suspension (2 × 1010 cfu ml−1) was added to the sodium alginate liquid. Then, the combination of the bacterial suspension and sodium alginate was injected into the sterile calcium chloride (CaCl2) solution 0.1 mol/ l - 0.1 M (Merk, Darmstadt, Germany) solution via sterile insulin syringes (0.2 mm). After applying the drops into CaCl2 solution, the drops immediately turned into clot balls (the space between the CaCl2 solution and syringe needle was roughly 20 cm, and we applied as much pressure as possible to the syringe to force the solution out extremely fast), and in 60 min, the all of beads were collected. For the first coating, the beads were submerged in 100 ml of chitosan solution slowly shaken at 100 rpm (1 G-force) for 40 min on a stirrer. Then, the chitosan-coated beads (single coated) were gathered and rinsed with distilled water. For the second coating, the beads were immersed in 100 ml Eu S100 nanoparticles solution (4 mg 100 ml−1 and held for 4 h on the stirrer IKA Labortechnik, Model 79219 Staufen, KG, Germany 100 rpm or 1 G-force. Lastly, the double-coated beads were washed thoroughly with distilled water and used on the same day.
2.4. Characterization of beads
Approximately 10 beads were randomly sampled (In terms of shape and size) from the Doogh beverages and then, the diameter, outside morphology, shape, and position of the external wall and inside appearance of beads were studied by an optical microscope [47]. Furthermore, a scanning electron microscope (SEM) technique was used to discriminate between surfaces of the beads with or without nanoparticle coating [47].
2.5. Iranian Doogh beverage preparation
Doogh milk with 5 % of solid nonfat was prepared via reconstituted skim milk powder and sterilized filtered water. This combination as well included 0.5 % sodium chloride. The milk was heated at 95 °C for 15 min and it was inoculated with lyophilized powder of the traditional yogurt starters the following incubation at 42 °C until pH of 4.50 was reached. [50,51]. We then freed and encapsulated the mentioned probiotic bacteria (2 × 1010 cfu ml or g−1) and inoculated into Doogh beverage. In fact, centrifuged of 10 ml of free bacteria (1 mL), 1 g of beads containing 1 ml (centrifuged of 10 ml of free bacteria) of free bacteria. 1 ml of free bacteria and 1 g of beads added to 10 ml of product. The samples (every sample contains 10 ml of Doogh with 1 ml of free bacteria and with 1 g of beads) were kept at 5 °C for 42 days.
2.6. Survey of the survivability of free and microencapsulated probiotic bacteria into Iranian Doogh beverage during storage time
The survivability of free and encapsulated bacteria into Doogh beverage examined in 0, 7, 14, 21, 28, 35 and 42 days with one week as interval time. Bacterial enumeration was determined according to the methods described earlier by Ansari et al. [47], and Shah [52]. The counting of every probiotic was done directly following the production of probiotic Doogh beverage and during 42 days with one week as interval time. The samples of Doogh (10 ml) were diluted into 90 ml distilled water and then 1 ml aliquot dilutions were dispensed to each plate of the MRS-Salicin-agar for L. rhamnosus and MRS-Glucose-Vancomycin-agar for L. rhamnosus [18,53]. All Counting plates were incubated at 37 °C for 48 h in aerobic conditions. To count the encapsulated bacteria inside Doogh beverage, the captured bacteria were released from beads described earlier by Ansari et al. [47]. Ten milliliters of Doogh (containing 1 g beads) were blended with 90 ml of phosphate buffer (0.1 mol L-1, pH 7.0) followed by 60 min shaking in a bag blender (netech-laboratory, Bag Tech®). The Doogh sample including free probiotic bacteria was treated in a similar approach so to remain the same analogous action order.
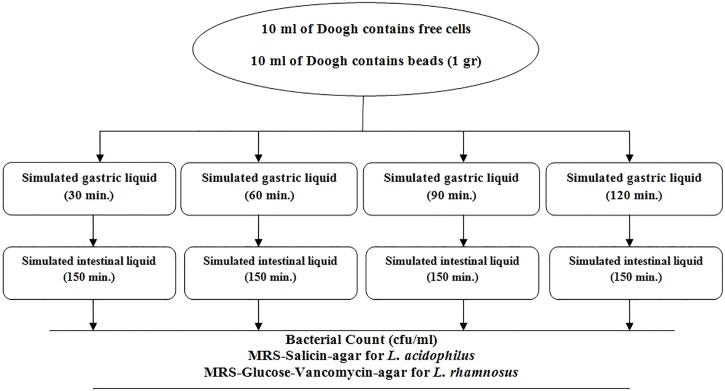
2.7. Survey of survivability of free and microencapsulated probiotic bacteria following sequential incubation in simulated gastric and intestinal liquid (in vitro)
The effectiveness of bacteria in GI simulation milieu was examined in 14, 28 and 42 days after the inoculation of bacteria (in two forms; free and microencapsulated with double coating) into Doogh beverage. In every mentioned day (14, 28, and 42) the samples (10 ml of Doogh contains free cells and 10 ml of Doogh contains beads (1 g)) were put distinctly in a tube counted by 100 ml of sterilized simulated gastric liquid (pH 1.5, 0.08 mol L−1 HCl, including 2 g L−1 NaCl, and 3 g L−1 pepsin) and incubated for 30, 60, 90, and 120 min at 37 ± 2 °C. Following the incubation, aliquots of 1 g of beads or 1 ml of free cell suspensions from the prior phase were transferred to 10 ml of sterilized simulated intestinal liquid (pH 7.5, 0.05 mol L−1 KH2PO4, and 10 g L−1 bile). Then, these tubes were incubated for 150 min at 37 ± 2 °C. Finally, samples were diluted into sterilized peptone water and 1 ml aliquot dilutions were dispensed to each plate of the MRS-Salicin-agar for L. acidophilus and MRS-Glucose-Vancomycin-agar for L. rhamnosus. All Counting plates were incubated at 37 ± 2 °C for 48 h in aerobic circumstances (See Fig. 1). ([[54], [55], [56]]). To enumerate the encapsulated bacteria, the arrested cells were released from the beads. The beads re-suspended in 90 ml of phosphate buffer (pH 7.0, 0.1 mol L−1) followed by 60 min shaking in a bag blender (netech-laboratory, Bag Tech®).
Fig. 1.
The diagram of the experimental process of survival of microencapsulated bacteria following sequential incubation in simulated gastric and intestinal juice.
2.8. Evaluation of pH, acidity, and organoleptic properties
pH and acidity of every product (10 ml of every sample of Doogh beverages in three groups: containing 1 g of beads, containing 1 ml of free bacteria, and control) were measured in 0, 7, 21 and 42 days (at the same time as the survey of survivability of free and microencapsulated probiotic bacteria). For pH and acidity measurement, pH meter (AZ-8601, Taiwan) and Dornic method were used respectively. The evaluations of organoleptic properties of every product (10 ml of every sample of Doogh beverages in three groups: containing 1 g of beads, containing free bacteria, control) were carried out by 32 taste panel (25–35 years old; 16 male and 16 female) in the same situation as location, lightness, temperature (25 °C), and containers in 7, 21 and 42 days. [26]
2.9. Statistical analysis
All statistical analyses were performed by SPSS (ver. 22) software. The survivability of bacteria in Doogh samples was examined in this period using a Repeated Measures ANOVA test. The survivability of bacteria in the GI simulation environment was examined in 14, 28 and 42 days after the inoculation of the bacteria by Repeated Measures ANOVA test. The mean of organoleptic scores on different days was compared using the Friedman Statistic test. Kruskal-Wallis none parametric test carried out for comparison of free, coated and control Doogh beverage samples. Acidity and pH of Doogh samples were assessed on days 0, 7, 21 and 42 after incubation. Each measurement has been repeated twice. The ANOVA statistic test and Bonferroni Post Hoc test performed to compare different experimental groups.
3. Results and discussion
In this study, during the study period of 42 days, we assessed several characteristics of Iranian Doogh samples containing free and microencapsulated bacteria as follows:
3.1. Production of Eudragit S100 nanoparticles
In this study, 100–150 nm-sized nanoparticles were prepared by homogenization of Eu S100 powder (26000 rpm or 75712 G-force, 10 min). Hu et al. also used Eu S100 powder and acetone solvent with the SAS method to produce nanoparticles of Eudragit. They attained uniform nanoparticles with an acceptable size (147 nm). This study performed at 35 °C temperature and 15 MPa pressure. In our study, we used the homogenization method to break down particles instead of increasing environmental pressure, and the size of obtained nanoparticles by our method was similar to Hu et al. study. After the preparation of the Eu S100 via modified SAS processing, the particle size and PDI of Eu S100 particles was 100 nm and 0.410 respectively. PDI was dimensionless and ranged in the rates less than 0.05 and is rarely able to be seen apart from extremely monodisperse standards. Concerning the DLS normally the producer announces that the PDI should be less than 0.6-0.7 to have a reliable dimension, at least for Zetasizer [57,58].
3.2. External and internal characteristics of double-coated beads
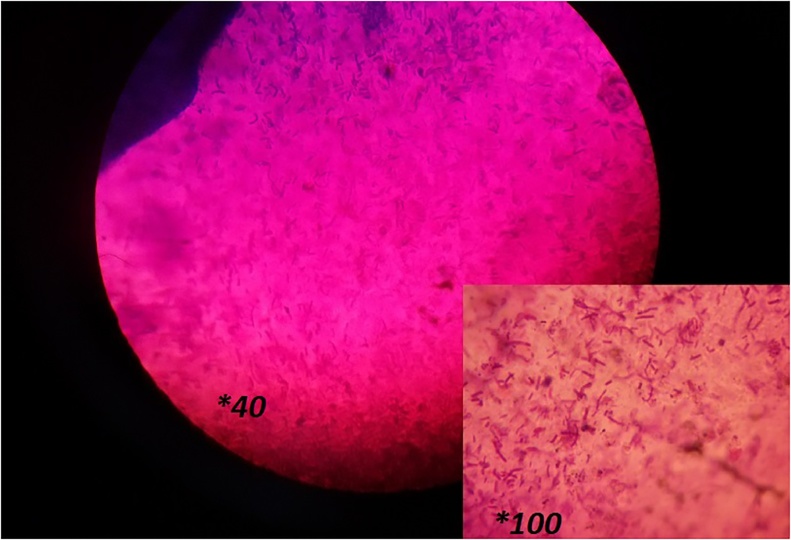
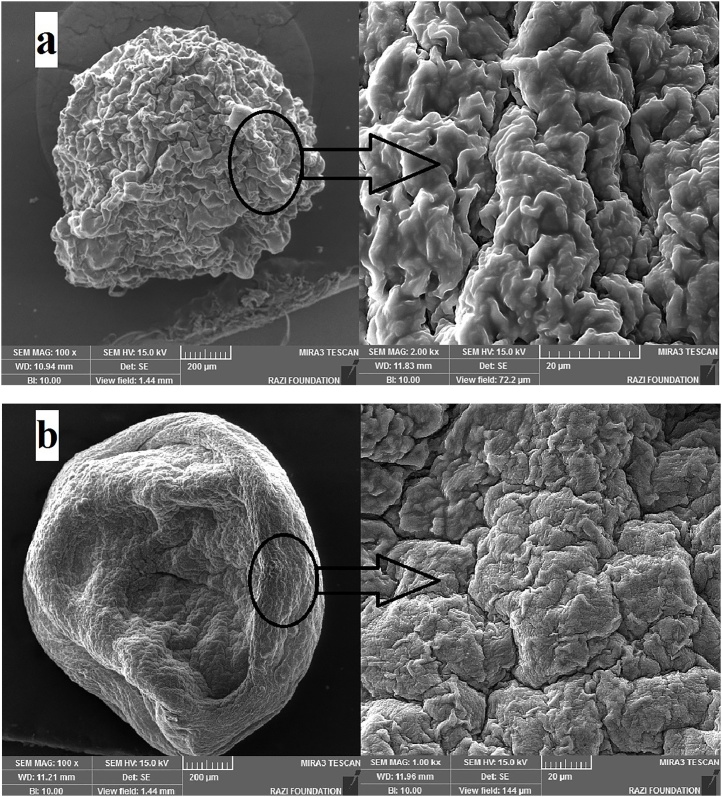
The interior appearance of the beads is shown in Fig. 2. The image of the beads underneath optical microscope at 10× magnification showed that the beads were sphere-shaped with a mean diameter about 1 mm, and as well the cross-section and interior appearance of beads at 40× and 100× magnification demonstrated that the bacterial cells were located haphazardly in the alginate matrix (See Fig. 2). Furthermore, a scanning electron microscope (SEM) was employed for distinguishing between surfaces of beads with or without Eu nanoparticle coating (Fig. 3a, b). In cases where the Eu S100 nanoparticles were used as a second coat for microencapsulation, a smooth surface was observed, which may indicate greater densities and strength of the beads against adverse environmental conditions. But, in the beads without this coating, a rough, irregular surface was created (See Fig. 3a, b). In addition to the type of material used in the coating, various studies have shown that the smoother the surface of the beads, the stronger it is against destructive conditions, and the rougher and more porous it is, the weaker it is [8,[59], [60], [61]].
Fig. 2.
Vertical cross-section and internal appearance of the bead at 40× and 100× magnification following gram staining (See the positive gram lactobacilli are distributed arbitrarily in the alginate medium).
Fig. 3.
a, b. Scanning electron microscope photomicrographs of beads. Beads encapsulated form only with chitosan coating (a) and beads encapsulated form with chitosan and Eu S100 nanoparticles coating (b).
3.3. Survival of probiotic bacteria during storage into Iranian Doogh beverage
The measurement in each day (seven days from 0 to 42 days, each with 7 days interval) was selected as the within-subject factor with 7 levels, the species of bacteria and the form of them (free or microencapsulated) were selected as between-subject factors. Bacterial count in Doogh containing free and encapsulated bacteria is demonstrated in Table 1. The Bacterial count was repeated twice for each sample and the mean of these repetitions is shown.
Table 1.
Bacterial viability (cfu ml−1) (Mean ± SD of duplicate samples) comparison of Doogh in lab environment.
| Experimental Group | Bacteria | Day 0 | Day 7 | Day 14 | Day 21 | Day 28 | Day 35 | Day 42 |
|---|---|---|---|---|---|---|---|---|
| Free | Lactobacillus acidophilus | 5.5 × 108±2.1 × 108 | 8.2 × 107±1.3 × 107 | 4.2 × 106±2.5 × 106 | 3.4 × 106±1.3 × 106 | 4.4 × 106±7.1 × 104 | 7.7 × 105±2.2 × 105 | 5.8 × 104±4.4 × 104 |
| Lactobacillus rhamnosus | 3.8 × 108±4.9 × 107 | 6.3 × 108±1.2 × 108 | 8.6 × 107±1.9 × 107 | 3.8 × 107±1.9 × 107 | 1.5 × 106±9.0 × 105 | 8.7 × 105±2.0 × 105 | 1.5 × 104±8.5 × 103 | |
| Microencapsulated | Lactobacillus acidophilus | 5.0 × 109±1.3 × 109 | 2.3 × 108±2.1 × 106 | 3.3 × 108±1.8 × 108 | 2.6 × 108±8.5 × 107 | 2.3 × 108±1.8 × 108 | 1.9 × 109±5.5 × 108 | 7.4 × 107±1.8 × 107 |
| Lactobacillus rhamnosus | 7.8 × 109±2.0 × 109 | 7.0 × 108±8.5 × 107 | 5.4 × 108±2.5 × 108 | 5.1 × 108±3.0 × 108 | 6.1 × 107±2.7 × 107 | 1.4 × 107±1.7 × 107 | 9.0 × 105±1.3 × 105 |
During the storage period (42 days), bacterial count (cfu g−1) of L. acidophilus in the free form reduced from 5.5 × 108 to 5.8 × 104; in the microencapsulated form reduced from 5.0 × 109 to 7.4 × 107. L. rhamnosus in the free form reduced from 3.8 × 108 to 1.5 × 104; in the microencapsulated form reduced from 7.8 × 109 to 9.0 × 105.
There was not any significant difference between survivability of two species of bacteria (P = 0.408) but the survivability of the microencapsulated form of bacteria was higher in comparison to the free form of bacteria (P = 0.001). The survivability of bacteria reduced significantly during the study (P < 0.001) and microencapsulated bacteria were more stable in comparison to free forms (P = 0.002).
In a similar study, the effects of encapsulation of L. acidophilus and B. lactis with calcium alginate on cell survival in Iranian Doogh during storage at 4 °C for 42 days were investigated. At day 42, the viable counts of L. acidophilus and B. lactis in the products containing encapsulated bacteria were 5.5 and 4.0 log cycles upper than those containing free bacteria, respectively [62].
Chitosan has been examined as a first or second coating for probiotic microencapsulation in beverages. In a study, Obradović et al. [63] investigated the influence of chitosan coating on the mechanical stability of biopolymer carriers with probiotic starter culture in fermented whey beverages. The results showed that adding of chitosan as a coating on the beads as well as the fermentation procedure enhanced the elastic modulus of the calcium alginate-whey beads and bacterial survival. Although, the coating did not considerably improve the viability of probiotics [63]. In a similar study, Shu et al. [64] studied the effect of xanthan–chitosan encapsulation on the survival of L. acidophilus in simulated GI condition and dairy drink. Encapsulated L. acidophilus showed significantly higher resistance to simulated gastric fluid and simulated intestinal fluid than non-encapsulated samples. Also, this study showed that xanthan–chitosan–xanthan and xanthan–chitosan significantly improved the bacterial viability of L. acidophilus in yogurt drink throughout storage time, when compared to free bacteria [64].
Chitosan itself is susceptible to degeneration by acids in low pH conditions, so we coated the second layer of anionic Eu around cationic chitosan layer for first time. This second layer is thin and improves the resistance of beads in acidic condition without significant change in the size of beads. Beads with this second layer, release in special pH conditions and in target places like colon [27,65,66]. The results of this study show that Eu S100 nanoparticles on chitosan layer can properly protect probiotic bacteria and maintain the acceptable number of probiotics in Doogh beverage during the storage period.
3.4. Survival of probiotic bacteria throughout GI simulation condition
The measurement in each day (days 14, 28 and 42 days) was selected as the within-subject factor with 3 levels, the time of sampling was also selected as a within-subject factor with 5 levels (from 0−120 min, each with 30 min interval). The species of bacteria and the form of them (free or microencapsulated) were selected as between-subject factors. The results of the bacterial count are shown in Table 2. As a previous experiment bacterial count was repeated twice for each sample and the mean of these repetitions is shown.
Table 2.
Bacterial viability (cfu ml−1) (Mean ± SD of duplicate samples) comparison of Doogh in gastrointestinal simulation environment.
| Day | Experimental Group | Bacteria | 0 min | 30 min | 60 min | 90 min | 120 min |
|---|---|---|---|---|---|---|---|
| 14 | Free | Lactobacillus acidophilus | 8.7 × 106±3.5 × 105 | 3.3 × 105±2.1 × 104 | 7.8 × 104±3.3 × 104 | 1.3 × 104±2.1 × 103 | 1.1 × 103±0.7 × 101 |
| Lactobacillus rhamnosus | 5.3 × 107±2.1 × 106 | 2.9 × 106±5.7 × 105 | 5.1 × 104±2.1 × 104 | 4.9 × 104±6.4 × 103 | 8.0 × 103±2.8 × 101 | ||
| Microencapsulated | Lactobacillus acidophilus | 3.4 × 108±4.2 × 107 | 2.0 × 108±1.4 × 108 | 7.8 × 107±4.2 × 106 | 1.0 × 107±7.1 × 105 | 5.3 × 106±1.1 × 106 | |
| Lactobacillus rhamnosus | 4.3 × 108±1.2 × 108 | 1.3 × 108±2.8 × 107 | 7.2 × 107±2.0 × 107 | 3.4 × 106±7.1 × 104 | 1.2 × 106±1.4 × 105 | ||
| 28 | Free | Lactobacillus acidophilus | 4.3 × 106±1.2 × 106 | 7.6 × 105±7.0 × 104 | 4.3 × 104±2.8 × 103 | 6.8 × 102±2.2 × 102 | 8.6 × 101±1.2 × 101 |
| Lactobacillus rhamnosus | 3.3 × 106±2.8 × 105 | 9.3 × 104±2.2 × 103 | 6.4 × 103±2.3 × 103 | 6.3 × 102±3.0 × 102 | 2.0 × 101±2.8 × 10° | ||
| Microencapsulated | Lactobacillus acidophilus | 4.9 × 108±3.1 × 108 | 3.4 × 108±1.5 × 108 | 1.6 × 107±2.1 × 104 | 6.2 × 106±2.4 × 106 | 3.7 × 105±2.1 × 104 | |
| Lactobacillus rhamnosus | 5.5 × 107±0.0 × 101 | 5.1 × 107±2.1 × 106 | 3.8 × 106±6.4 × 105 | 8.1 × 105±4.2 × 104 | 5.7 × 104±4.0 × 104 | ||
| 42 | Free | Lactobacillus acidophilus | 6.0 × 104±0.7 × 104 | 4.3 × 104±2.8 × 102 | 5.5 × 101±4.2 × 10° | 1.7 × 101±0.8 × 101 | 1.0 × 10°±0.0 × 10° |
| Lactobacillus rhamnosus | 1.4 × 102±1.8 × 101 | 8.0 × 101±1.4 × 10° | 1.1 × 101±0.7 × 10° | 2.5 × 10°±0.7 × 10° | 1.0 × 10°±0.0 × 10° | ||
| Microencapsulated | Lactobacillus acidophilus | 4.2 × 107±4.2 × 106 | 1.2 × 106±1.4 × 105 | 1.2 × 105±3.5 × 104 | 1.0 × 105±0.0 × 10° | 1.0 × 102±0.7 × 101 | |
| Lactobacillus rhamnosus | 8.7 × 105±9.1 × 104 | 3.2 × 105±1.3 × 105 | 2.1 × 104±5.7 × 103 | 4.4 × 103±2.1 × 104 | 1.9 × 101±5.7 × 10° |
In simulated gastrointestinal condition (during 120 min), on day 14, bacterial count of L. acidophilus in the free form reduced from 8.7 × 106 to 1.1 × 103; in the microencapsulated form reduced from 3.4 × 108 to 5.3 × 106. On the other hand, L. rhamnosus in the free form reduced from 5.3 × 107 to 8.0 × 103; in the microencapsulated form reduced from 4.3 × 108 to 1.2 × 106. On day 42, the reduction of the bacterial count of L. acidophilus in the free form was from 6.0 × 104 to 1.0 × 10°; in the microencapsulated form was from 4.2 × 107 to 1.0 × 102. The reduction of L. rhamnosus in the free form was from 1.4 × 102 to 1.0 × 10°; in the microencapsulated form was from 8.7 × 105 to 1.9 × 101. The viability of bacteria reduced significantly during the study period (P < 0.001) and during measurement time (P < 0.001). Lactobacillus acidophilus was more stable in comparison with Lactobacillus rhamnosus (P < 0.001) and miro-coated bacteria were more stable than free bacteria (P < 0.001).
In a similar study, the effects of encapsulation L. acidophilus and B. lactis with calcium alginate on bacterial viability in Iranian Doogh during storage at 4 °C for 42 days, as well as under simulated GI situations, were studied. The survivability of the probiotic bacteria enhanced from 0.6 % and 0.2 % (L. acidophilus and B. lactis, respectively) as free cells to 18.0 % and 9.5 % under the extreme GI situations, after microencapsulation. Under normal GI situation, the probiotic survival rates were 16.1 % for L. acidophilus and 21 % for B. lactis before microencapsulation, and 26.3 and 34.0 % (L. acidophilus and B. lactis, respectively) after microencapsulation [62].
Calcium alginate coated with chitosan has been used for microencapsulation of probiotic bacteria in several studies e.g. Kanmani et al. [49]Chávarri et al. [25], and Ghasemnezhad et al. [67] too. These studies showed that this method of microencapsulation can improve the survivability of probiotic bacteria in simulated GI conditions in comparison with free forms [25,49,67,68]. In our study, we had an effective novel approach in producing beads. In these studies, beads were transferred to simulated GI condition right after production. However, in our study, we initially added beads to the beverage and monitored the survivability of probiotics into the product itself. We also collected beads from the beverage in selected days and examined the viability of probiotics in simulated GI conditions at the same time.
3.5. Organoleptic characteristic
Organoleptic characteristic of 7, 21 and 42 days old beverages was tested by 32 students and are shown in Table 3. The results show that on day 3, all cases of the organoleptic characteristics (color, texture, flavor, and total acceptability) in all three Doogh products were in better condition than day 21 and day 42. Also on day 3, the score of the product containing free probiotics was higher than the product containing the encapsulated bacteria and the control product, and the same was true on day 21. But on day 42, the product containing free bacteria declined and was reported lower than the other two. Therefore, it can be argued that during the 42-day of product storage, more activity of the free probiotic bacteria (during this period especially after 21-day) produced more lactic acid and other bacterial metabolites inside the product (increasing acidity and decreasing pH, See Table 4), thereby reducing the organoleptic properties scores. Obviously, the increased acidity and decrease of pH the increase of bacterial metabolites and some micro materials due to the breakdown of macromolecules in the product by free bacteria, decrease the organoleptic properties of the product. The activity of the encapsulated bacteria is very low resulting in minimal changes in organoleptic properties.
Table 3.
Organoleptic scores of 7, 21 and 42 days old Doogh beverage (Mean ± SD of 32 examiners) in different experimental groups.
| Time of measuring (day) | Group | Color (from 5) | Texture (from 5) | Flavor (from 10) | Total (from 20) |
|---|---|---|---|---|---|
| 7 | Free bacteria | 4.75 ± 0.44a | 4.78 ± 0.42 a | 9.03 ± 0.78 b | 18.56 ± 1.01 a |
| Microencapsulated bacteria | 4.62 ± 0.49a | 4.00 ± 0.51b | 8.00 ± 0.76 a | 16.53 ± 1.19 b | |
| Control | 4.66 ± 0.48a | 4.59 ± 0.56 a | 8.25 ± 0.88 a | 17.50 ± 1.37 c | |
| P value of between group analysis | P = 0.547 | P < 0.001d | P < 0.001e | P < 0.001f | |
| 21 | Free bacteria | 4.03 ± 0.53 a | 4.66 ± 0.54 a | 8.84 ± 0.57 b | 17.53 ± 0.87 a |
| Microencapsulated bacteria | 4.00 ± 0.56 a | 4.09 ± 0.53 b | 8.16 ± 0.85 a | 16.25 ± 1.37 b | |
| Control | 4.03 ± 0.47 a | 4.75 ± 0.44 a | 8.12 ± 0.55 a | 16.94 ± 0.91a | |
| P value of between group analysis | P = 0.963 | P < 0.001g | P < 0.001h | P < 0.001i | |
| 42 | Free bacteria | 4.00 ± 0.51 a | 3.19 ± 0.74b | 6.90 ± 0.58 a | 14.09 ± 1.03 a |
| Microencapsulated bacteria | 4.06 ± 0.50 a | 4.00 ± 0.51a | 7.97 ± 0.59 b | 16.03 ± 0.82 b | |
| Control | 4.00 ± 0.51a | 4.00 ± 0.57a | 7.00 ± 0.47 a | 15.00 ± 0.80 c | |
| P value of between group analysis | P = 0.850 | P < 0.001j | P < 0.001k | P < 0.001l | |
| P value of within group analysis | 7, 21, 42 | <0.001m | <0.001n | <0.001° | <0.001p |
Different letters indicate significant differences between groups in each day.
Table 4.
pH and acidity of 0, 7, 21 and 42 days old Doogh beverage (Mean ± SD of two replications) in different experimental groups.
| Time of measuring (day) | Group | pH | Acidity |
|---|---|---|---|
| 0 | Free bacteria | 4.11 ± 0.01b | 70.25 ± 0.35 a |
| Microencapsulated bacteria | 4.15 ± 0.01a | 69.75 ± 1.06a | |
| Control | 4.16 ± 0.00a | 69.00 ± 1.41 a | |
| P value of between group analysis | P = 0.008d | P=0.551 | |
| 7 | Free bacteria | 3.72 ± 0.11a | 77.25 ± 0.07 a |
| Microencapsulated bacteria | 3.84 ± 0.05a | 75.55 ± 0.07a | |
| Control | 3.89 ± 0.01a | 73.50 ± 0.71b | |
| P value of between group analysis | P = 0.173 | P = 0.007e | |
| 21 | Free bacteria | 3.50 ± 0.01b | 88.95 ± 0.07 a |
| Microencapsulated bacteria | 3.63 ± 0.02a | 79.30 ± 0.14b | |
| Control | 3.64 ± 0.02a | 78.00 ± 0.00 c | |
| P value of between group analysis | P = 0.007f | P < 0.001g | |
| 42 | Free bacteria | 2.82 ± 0.03 b | 110.10 ± 0.14b |
| Microencapsulated bacteria | 3.34 ± 0.06 a | 90.10 ± 0.14a | |
| Control | 3.37 ± 0.03a | 89.35 ± 0.21a | |
| P value of between group analysis | P = 0.002h | P<0.00i | |
| P value of within group analysis | 0,7, 21, 42 | <0.001j | <0.001k |
Different letters indicate significant differences between groups in each day.
3.6. Acidity and pH of Doogh samples during 42 days storage
Acidity and pH of Doogh samples were assessed on days 0, 7, 21 and 42 after incubation and results are demonstrated in Table 4.
During the storage period, the pH and acidity were decreased and increased respectively. In beverages containing the free form of bacteria, pH and acidity changes were greater than those of microencapsulated and control groups. As discussed in the previous section (section 3.5), the high activity of free probiotic bacteria inside the product results in higher production of lactic acid and other bacterial metabolites, resulting in an increase in acidity and a decrease in pH during the product shelf life. Changes in products containing encapsulated bacteria are negligible due to the low activity of the bacteria. It seems that increasing the acidity and decreasing pH in addition to major changes in the organoleptic properties of the product also affects the viability of the probiotic bacteria in the Doogh during 42-day storage (See Tables 3,4, and 1).
In a similar study, the impacts of microencapsulation of L. acidophilus and B. lactis with calcium alginate on cell survival in Iranian Doogh during storage at 4 °C for 42 days were investigated. The pH of the Doogh at the beginning of storing was 4.53 and the final pH at the end of storage was 4.52 and 3.78 for the products enclosing microencapsulated and free probiotics, respectively. The acetic acid content in the Doogh containing encapsulated probiotics enhanced by 0.01 % (from 0.05 to 0.06 %) throughout the storage era, although for Doogh containing free probiotics the enhancement was 0.04 % (from 0.05 to 0.09 %) [62].
4. Conclusions
In this investigation, we applied chitosan and Eu S100 nanoparticles for coating encapsulated Lactobacilli probiotics in Iranian Doogh beverage and evaluated the survivability of containing the bacteria and final product characteristics throughout a 42 days investigation era. The consequences of this study suggest that the encapsulation of L. acidophilus and L. rhamnosus with calcium-alginate and then double layer coating of these beads via chitosan and Eu S100 nanoparticles can increase the survivability of these probiotics in Iranian Doogh beverage as well as in human GI lumen. This method of microencapsulation can also prevent bacteria from generating acids and metabolites that contribute to unwanted variations in taste and flavor of Doogh beverage. These metabolites may also damage the probiotic bacteria and decrease their survivability. The second coating layer-Eu S100 nanoparticles not only guards beads in the acidic milieu but also can cause beads to release in the colon zone which is the target place for the best function of probiotics.
Author statement
These authors Hadi Pourjafar, Negin Noori, Hasan Gandomi, Afshin Akhondzadeh Basti, and Fereshteh Ansari declare that the all of descriptions in the manuscript are accurate and agreed by all authors.
CRediT authorship contribution statement
Hadi Pourjafar: Conceptualization, Methodology, Investigation, Validation, Writing - review & editing, Software. Negin Noori: Supervision, Validation, Data curation. Hasan Gandomi: Supervision, Visualization. Afshin Akhondzadeh Basti: Supervision, Visualization, Data curation. Fereshteh Ansari: Software, Writing - review & editing.
Declaration of Competing Interest
The authors declare thaconf there are no conflicts of interest.
Acknowledgments
This article is related to a PhD thesis (No. 612- Hadi Pourjafar) from the University of Tehran; its preparation and investigattion work was supported by the Department of Food Hygiene, Tehran, Iran.
References
- 1.Gibson G.R., Roberfroid M.B. Dietary modulation of the human colonic microbiota: introducing the concept of prebiotics. J. Nutr. 1995;125:1401–1412. doi: 10.1093/jn/125.6.1401. [DOI] [PubMed] [Google Scholar]
- 2.FAO/WHO . World Health Organization; London: 2002. Guidelines for the Evaluation of Probiotics in Food. ON, Canada: Food and Agriculture Organization. [Google Scholar]
- 3.Rijkers G.T., Bengmark S., Enck P., Haller D., Herz U., Kalliomaki M., Kudo S., Lenoir-Wijnkoop I., Mercenier A., Myllyluoma E., Rabot S., Rafter J., Szajewska H., Watzl B., Wells J., Wolvers D., Antoine J.-M. Guidance for substantiating the evidence for beneficial effects of probiotics: current status and recommendations for future research. J. Nutr. 2010;140:671S–676S. doi: 10.3945/jn.109.113779. [DOI] [PubMed] [Google Scholar]
- 4.Huang C.-H., Shen C.-C., Liang Y.-C., Jan T.-R. The probiotic activity of Lactobacillus murinus against food allergy. J. Funct. Foods. 2016;25:231–241. [Google Scholar]
- 5.Hill C., Guarner F., Reid G., Gibson G.R., Merenstein D.J., Pot B., Morelli L., Canani R.B., Flint H.J., Salminen S. Expert consensus document: the International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastro. Hepat. 2014;11:506–514. doi: 10.1038/nrgastro.2014.66. [DOI] [PubMed] [Google Scholar]
- 6.Ansari F., Pourjafar H., Christensen L. A study on the aflatoxin M1 rate and seasonal variation in pasteurized cow milk from northwestern Iran. Environ. Monit. Assess. 2019;191:6. doi: 10.1007/s10661-018-7141-1. [DOI] [PubMed] [Google Scholar]
- 7.Elsanhoty R.M., Salam S.A., Ramadan M.F., Badr F.H. Detoxification of aflatoxin M1 in yoghurt using probiotics and lactic acid bacteria. Food Control. 2014;43:129–134. [Google Scholar]
- 8.Anal A.K., Singh H. Recent advances in microencapsulation of probiotics for industrial applications and targeted delivery. Trends Food Sci. Technol. 2007;18:240–251. [Google Scholar]
- 9.Prado F.C., Parada J.L., Pandey A., Soccol C.R. Trends in non-dairy probiotic beverages. Food Res. Int. 2008;41:111–123. [Google Scholar]
- 10.Homayouni A., Javadi M., Ansari F., Pourjafar H., Jafarzadeh M., Barzegar A. Advanced methods in ice cream analysis: a review. Food Anal. Method. 2018;11:3224–3234. [Google Scholar]
- 11.Kandylis P., Pissaridi K., Bekatorou A., Kanellaki M., Koutinas A. Dairy and non-dairy probiotic beverages. Curr. Opin. Food Sci. 2016;7:58–63. [Google Scholar]
- 12.Dimitrellou D., Kandylis P., Petrović T., Dimitrijević-Branković S., Lević S., Nedović V., Kourkoutas Y. Survival of spray dried microencapsulated Lactobacillus casei ATCC 393 in simulated gastrointestinal conditions and fermented milk. LWT- Food Sci. Technol. 2016;71:169–174. [Google Scholar]
- 13.Dimitrellou D., Kandylis P., Lević S., Petrović T., Ivanović S., Nedović V., Kourkoutas Y. Encapsulation of Lactobacillus casei ATCC 393 in alginate capsules for probiotic fermented milk production. LWT- Food Sci. Technol. 2019;116 [Google Scholar]
- 14.Tripathi M., Giri S. Probiotic functional foods: survival of probiotics during processing and storage. J. Funct. Foods. 2014;9:225–241. [Google Scholar]
- 15.Shori A.B. Microencapsulation improved probiotics survival during gastric transit. HAYATI J. Biosci. 2017;24:1–5. [Google Scholar]
- 16.Chen K.N., Chen M.J., Liu J.R., Lin C.W., Chiu H.Y. Optimization of incorporated prebiotics as coating materials for probiotic microencapsulation. J. Food Sci. 2005;70:M260–M266. [Google Scholar]
- 17.Sarkar S. Approaches for enhancing the viability of probiotics: a review. Br. Food J. 2010;112:329–349. [Google Scholar]
- 18.Pourjafar H., Noori N., Gandomi H., Akhondzadeh Basti A. Study of protective role of double coated beads of calcium alginate-chitosan-eudragit s100 nanoparticles achieved from microencapsulation of Lactobacillus acidophilus as a predominant flora of human and animals gut. J. Vet. Res. 2016;71:311–320. [Google Scholar]
- 19.Krasaekoopt W., Watcharapoka S. Effect of addition of inulin and galactooligosaccharide on the survival of microencapsulated probiotics in alginate beads coated with chitosan in simulated digestive system, yogurt and fruit juice. LWT-Food Sci. Technol. 2014;57:761–766. [Google Scholar]
- 20.de Araújo Etchepare M., Nunes G.L., Nicoloso B.R., Barin J.S., Moraes Flores E.M., de Oliveira Mello R., Cristiano Ragagnin de Menezes C.R. Improvement of the viability of encapsulated probiotics using whey proteins. LWT-Food Sci. Technol. 2020;117 [Google Scholar]
- 21.Yin J., Xiang C., Song X. Nanoencapsulation of psoralidin via chitosan and Eudragit S100 for enhancement of oral bioavailability. Int. J. Pharm. 2016;510(1):203–209. doi: 10.1016/j.ijpharm.2016.05.007. [DOI] [PubMed] [Google Scholar]
- 22.de Araújo Etchepare M., Raddatz G.C., De Moraes Flores É.M., Queiroz Zepka L., Jacob-Lopes E., Smanioto Barin J., Raimundo Ferreira Grosso C., Ragagninde Menezes C. Effect of resistant starch and chitosan on survival of Lactobacillus acidophilus microencapsulated with sodium alginate. LWT-Food Sci. Technol. 2016;65:511–517. [Google Scholar]
- 23.Abouhussein D.M., El-bary A.A., Shalaby S.H., El Nabarawi M.A. Chitosan mucoadhesive buccal films: effect of different casting solvents on their physicochemical properties. Int. J. Pharm. Pharm. Sci. 2016;8:206–213. [Google Scholar]
- 24.Ahmed T.A., Aljaeid B.M. Preparation, characterization, and potential application of chitosan, chitosan derivatives, and chitosan metal nanoparticles in pharmaceutical drug delivery. Drug Des. Dev. Ther. 2016;10:483. doi: 10.2147/DDDT.S99651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chávarri M., Marañón I., Ares R., Ibáñez F.C., Marzo F., del Carmen Villarán M. Microencapsulation of a probiotic and prebiotic in alginate-chitosan capsules improves survival in simulated gastro-intestinal conditions. Int. J. Food Microbiol. 2010;142:185–189. doi: 10.1016/j.ijfoodmicro.2010.06.022. [DOI] [PubMed] [Google Scholar]
- 26.Abdolhosseinzadeh E., Dehnad A.R., Pourjafar H., Homayouni A., Ansari F. Production of probiotic Scallion Yogurt: viability of Lactobacillus acidophilus freely and microencapsulated in the product. Carpath. J. Food Sci. Technol. 2018;10(3):72–80. [Google Scholar]
- 27.Badhana S., Garud N., Garud A. Colon specific drug delivery of mesalamine using eudragit S100-coated chitosan microspheres for the treatment of ulcerative colitis. Int. Curr. Pharm. J. 2013;2(3):42–48. [Google Scholar]
- 28.Hu D., Liu L., Chen W., Li S., Zhao Y. A novel preparation method for 5-aminosalicylic acid loaded Eudragit S100 nanoparticles. Int. J. Mol. Sci. 2012;13:6454–6468. doi: 10.3390/ijms13056454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gibson P., Fixa B., Pekarkova B., Bátovský M., Radford‐Smith G., Tibitanzl J., Gabalec L., Florin T., Greinwald R. Comparison of the efficacy and safety of Eudragit‐L‐coated mesalazine tablets with ethylcellulose‐coated mesalazine tablets in patients with mild to moderately active ulcerative colitis. Aliment. Pharm.Ther. 2006;23:1017–1026. doi: 10.1111/j.1365-2036.2006.02861.x. [DOI] [PubMed] [Google Scholar]
- 30.Thakral S., Thakral N.K., Majumdar D.K. Eudragit®: a technology evaluation. Expert Opin. Drug Del. 2013;10:131–149. doi: 10.1517/17425247.2013.736962. [DOI] [PubMed] [Google Scholar]
- 31.Yoo J.-W., Giri N., Lee C.H. pH-sensitive Eudragit nanoparticles for mucosal drug delivery. Int. J. Pharm. 2011;403:262–267. doi: 10.1016/j.ijpharm.2010.10.032. [DOI] [PubMed] [Google Scholar]
- 32.Coco R., Plapied L., Pourcelle V., Jérôme C., Brayden D.J., Schneider Y.-J., Préat V. Drug delivery to inflamed colon by nanoparticles: comparison of different strategies. Int. J. Pharm. 2013;440:3–12. doi: 10.1016/j.ijpharm.2012.07.017. [DOI] [PubMed] [Google Scholar]
- 33.Patel N.V., Sheth N.R., Mohddesi B. Formulation and evaluation of genistein–a novel isoflavone loaded chitosan and eudragit® nanoparticles for cancer therapy. Mater. Today Proc. 2015;2:4477–4482. [Google Scholar]
- 34.Pandey S., Swamy S., Ulla U., Gupta A., Patel H., Yadav J. Cell Line and Augument Cellular Uptake Study of statistically optimized sustained release capecitabine loaded Eudragit S100/PLGA (poly (lactic-co-glycolic acid)) Nanoparticles for colon targeting. Curr. Drug Deliv. 2016;14(6):887–899. doi: 10.2174/1567201813666160817150621. [DOI] [PubMed] [Google Scholar]
- 35.Younis N., Shaheen M.A., Abdallah M.H. Silymarin-loaded Eudragit® RS100 nanoparticles improved the ability of silymarin to resolve hepatic fibrosis in bile duct ligated rats. Biomed. Pharmacother. 2016;81:93–103. doi: 10.1016/j.biopha.2016.03.042. [DOI] [PubMed] [Google Scholar]
- 36.Bernet M.F., Brassart D., Neeser J.R., Servin A. Lactobacillus acidophilus LA 1 binds to cultured human intestinal cell lines and inhibits cell attachment and cell invasion by enterovirulent bacteria. Gut. 1994;35:483–489. doi: 10.1136/gut.35.4.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kandasamy S., Vlasova A.N., Fischer D., Kumar A., Chattha K.S., Rauf A., Shao L., Langel S.N., Rajashekara G., Saif L.J. Differential effects of Escherichia coli Nissle and Lactobacillus rhamnosus strain GG on human rotavirus binding, infection, and B cell immunity. J. Immunol. 2016;196:1780–1789. doi: 10.4049/jimmunol.1501705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Grimoud J., Durand H., Courtin C., Monsan P., Ouarné F., Theodorou V., Roques C. In vitro screening of probiotic lactic acid bacteria and prebiotic glucooligosaccharides to select effective synbiotics. Anaerobe. 2010;16:493–500. doi: 10.1016/j.anaerobe.2010.07.005. [DOI] [PubMed] [Google Scholar]
- 39.Hotel A.C.P., Cordoba A. Health and nutritional properties of probiotics in food including powder milk with live lactic acid bacteria. Prevention. 2001;5:1–10. [Google Scholar]
- 40.Kailasapathy K., Harmstorf I., Phillips M. Survival of Lactobacillus acidophilus and Bifidobacterium animalis ssp. Lactis in stirred fruit yogurts. LWT - Food Sci. Technol. 2008;41:1317–1322. [Google Scholar]
- 41.Kos B., Šušković J., Vuković S., Šimpraga M., Frece J., Matošić S. Adhesion and aggregation ability of probiotic strain Lactobacillus acidophilus M92. J. Appl. Microbiol. 2003;94:981–987. doi: 10.1046/j.1365-2672.2003.01915.x. [DOI] [PubMed] [Google Scholar]
- 42.Hekmat S., Soltani H., Reid G. Growth and survival of Lactobacillus reuteri RC-14 and Lactobacillus rhamnosus GR-1 in yogurt for use as a functional food. Innov. Food Sci. Emerg. Technol. 2009;10:293–296. [Google Scholar]
- 43.Salminen M.K., Tynkkynen S., Rautelin H., Saxelin M., Vaara M., Ruutu P., Sarna S., Valtonen V., Järvinen A. Lactobacillus bacteremia during a rapid increase in probiotic use of Lactobacillus rhamnosus GG in Finland. Clin. Infect. Dis. 2002;35:1155–1160. doi: 10.1086/342912. [DOI] [PubMed] [Google Scholar]
- 44.Azarikia F., Abbasi S. On the stabilization mechanism of Doogh (Iranian yoghurt drink) by gum tragacanth. Food Hydrocoll. 2010;24:358–363. [Google Scholar]
- 45.Gorji E.G., Mohammadifar M.A., Ezzatpanah H. Influence of gum tragacanth, Astragalus gossypinus, addition on stability of nonfat Doogh, an Iranian fermented milk drink. Int. J. Dairy Technol. 2011;64:262–268. [Google Scholar]
- 46.Norouzi S., Pourjafar H., Ansari F., Homayouni A. Survey the survival of Lactobacillus paracasei in fermented and non-fermented frozen soy dessert. Biocatal. Agric. Biotechnol. 2019 [Google Scholar]
- 47.Ansari F., Pourjafar H., Jodat V., Sahebi J., Ataei A. Effect of Eudragit S100 nanoparticles and alginate chitosan encapsulation on the viability of Lactobacillus acidophilus and Lactobacillus rhamnosus. AMB Express. 2017;7:144. doi: 10.1186/s13568-017-0442-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mirzaei H., Pourjafar H., Rad A.H. The effect of microencapsulation with calcium alginate and resistant starch on the Lactobacillus acidophilus (La5) survival rate in simulated gastrointestinal juice conditions. J. Vet. Res. 2011;66:337–377. [Google Scholar]
- 49.Kanmani P., Kumar R.S., Yuvaraj N., Paari K., Pattukumar V., Arul V. Cryopreservation and microencapsulation of a probiotic in alginate-chitosan capsules improves survival in simulated gastrointestinal conditions. Biotechnol. Bioprocess Eng. 2011;16:1106–1114. [Google Scholar]
- 50.Anonymous . 2008. Iran National Standard for Doogh.www.isiri.org Online available on. [Google Scholar]
- 51.Hashemi S.M.B., Shahidi F., Mortazavi S.A., Milani E., Eshaghi Z. Synbiotic potential of Doogh supplemented with free and encapsulated Lactobacillus plantarum LS5 and Helianthus tuberosus inulin. J. Food Sci. Technol. 2015;52:4579–4585. doi: 10.1007/s13197-014-1511-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shah N. Probiotic bacteria: selective enumeration and survival in dairy foods. J. Dairy Sci. 2000;83:894–907. doi: 10.3168/jds.S0022-0302(00)74953-8. [DOI] [PubMed] [Google Scholar]
- 53.Saxelin M., Lassig A., Karjalainen H., Tynkkynen S., Surakka A., Vapaatalo H., Järvenpää S., Korpela R., Mutanen M., Hatakka K. Persistence of probiotic strains in the gastrointestinal tract when administered as capsules, yoghurt, or cheese. Int. J. Food Microbiol. 2010;144:293–300. doi: 10.1016/j.ijfoodmicro.2010.10.009. [DOI] [PubMed] [Google Scholar]
- 54.Shima M., Morita Y., Yamashita M., Adachi S. Protection of Lactobacillus acidophilus from the low pH of a model gastric juice by incorporation in a W/O/W emulsion. Food Hydrocoll. 2006;20:1164–1169. [Google Scholar]
- 55.Pourjafar H., Noori N., Gandomi H., Basti A.A., Ansari F. Stability and efficiency of double-coated beads containing lactobacillus acidophilus obtained from the calcium alginate-chitosan and Eudragit S100 nanoparticles microencapsulation. Int. J. Probiotics Prebiotics. 2018;13(2/3):77–84. [Google Scholar]
- 56.Ansari F., Pourjafar H. Comment on Traditional fermented fish harbors bacteria with potent probiotic and anticancer properties. Biocatal. Agric. Biotechnol. 2019;17:269–270. [Google Scholar]
- 57.Khatak S., Dureja H. Recent techniques and patents on solid lipid nanoparticles as novel carrier for drug delivery. Recent Patents Nanotechnol. 2015;9:150–177. doi: 10.2174/1872210510999151126105754. [DOI] [PubMed] [Google Scholar]
- 58.Salem H.F., Kharshoum R.M., Abdel Hakim L.F., Abdelrahim M.E. Edge activators and a polycationic polymer enhance the formulation of porous voriconazole nanoagglomerate for the use as a dry powder inhaler. J. Liposome Res. 2016:1–12. doi: 10.3109/08982104.2016.1140182. [DOI] [PubMed] [Google Scholar]
- 59.Poshadri A., Aparna K. Microencapsulation technology: a review. J. Res. ANGRAU. 2010;38:86–102. [Google Scholar]
- 60.Gbassi G.K., Vandamme T. Probiotic encapsulation technology: from microencapsulation to release into the gut. Pharmaceutics. 2012;4:149–163. doi: 10.3390/pharmaceutics4010149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Martín M.J., Lara-Villoslada F., Ruiz M.A., Morales M.E. Microencapsulation of bacteria: a review of different technologies and their impact on the probiotic effects. Innov. Food Sci. Emerg. Technol. 2015;27:15–25. [Google Scholar]
- 62.Mortazavian A., Ehsani M., Azizi A., Mousavi M. Viability of calcium-alginate-microencapsulated probiotic bacteria in Iranian yogurt drink (Doogh) during refrigerated storage and under simulated gastrointestinal conditions. Aust. J. Dairy Technol. 2008;63:24–28. [Google Scholar]
- 63.Obradović N.S., Krunić T.Ž., Trifković K.T., Bulatović M.L., Rakin M.P., Rakin M.B., Bugarski B.M. Influence of chitosan coating on mechanical stability of biopolymer carriers with probiotic starter culture in fermented whey beverages. Int. J. Polym. Sci. 2015 [Google Scholar]
- 64.Shu G., He Y., Chen L., Song Y., Cao J., Chen H. Effect of xanthan–Chitosan microencapsulation on the survival of Lactobacillus acidophilus in simulated gastrointestinal fluid and dairy beverage. Polymers. 2018;10:588. doi: 10.3390/polym10060588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Boeris V., Romanini D., Farruggia B., Picó G. Interaction and complex formation between catalase and cationic polyelectrolytes: chitosan and Eudragit E100. Int. J. Biol. Macromol. 2009;45:103–108. doi: 10.1016/j.ijbiomac.2009.04.009. [DOI] [PubMed] [Google Scholar]
- 66.Quan J.-S., Jiang H.-L., Kim E.-M., Jeong H.-J., Choi Y.-J., Guo D.-D., Yoo M.-K., Lee H.-G., Cho C.-S. pH-sensitive and mucoadhesive thiolated Eudragit-coated chitosan microspheres. Int. J. Pharm. 2008;359:205–210. doi: 10.1016/j.ijpharm.2008.04.003. [DOI] [PubMed] [Google Scholar]
- 67.Ghasemnezhad R., Razavilar V., Pourjafar H., Khosravi-Darani K., Ala K. The viability of free and encapsulated Lactobacillus casei and Bifidobacterium animalis in chocolate milk, and evaluation of its pH changes and sensory properties during storage. Annu. Res. Rev. Biol. 2017;21:1–8. [Google Scholar]
- 68.Liserre A.M., Re M.I., Franco B.D. Microencapsulation of Bifidobacterium animalis subsp. lactis in modified alginate-chitosan beads and evaluation of survival in simulated gastrointestinal conditions. Food Biotechnol. 2007;21:1–16. [Google Scholar]