Highlights
-
•
Although very rare, hydatid cyst can primarily involve the subcutaneous tissue of the gluteal region.
-
•
Patients with a gluteal subcutaneous hydatid cyst usually present with a painless palpable mass that is usually larger than 3 cm at first admission.
-
•
Radiological imaging plays a key role in the diagnosis of gluteal hydatid cyst.
Keywords: Hydatid cyst, Gluteal region, Soft tissue, Subcutaneous, MRI
Abstract
Hydatid disease is a parasitic zoonosis caused by Echinococcus granulosus larvae. While it can affect almost any part of the human body, liver and lung are the two organs where the disease is most frequently detected. Subcutaneous hydatid cyst, which mostly develops secondary to iatrogenic spillage of cyst contents into incision area during a visceral hydatid cyst surgery, accounts for only 1.5 % of all cases of hydatid cyst. With only a limited number of reported cases, primary involvement of subcutaneous tissue by hydatid cyst is a much more rare occurrence as compared with the secondary form. Subcutaneous hydatid cysts tend to involve trunk and limb roots, and mostly present as a slowly-growing, painless, mobile mass with a normal overlying skin. To our knowledge, only a few cases of primary subcutaneous hydatid cyst in the gluteal region have been reported to date. Here, we present a 72-year-old farmer who presented with a painless lump in the gluteal region and diagnosed as having primary subcutaneous hydatid cyst.
Introduction
Hydatid disease is a zoonosis caused by tapeworm larvae of Echinococcus species and the most common type that affects humans is Echinococcus granulosus. The definite hosts of the parasite are carnivores, mostly dogs, and intermediate hosts are sheep. The disease is transmitted to humans through contact with dogs, and since intermediate hosts are sheep, the disease is endemic in areas with large grasslands such as the Mediterranean, Africa, South America, the Middle East, Australia and New Zealand [1]. While hydatid cyst (HC) can affect almost anywhere in the human body, the two most common involvement sites of the disease are the liver (66.4–89.3 %) and the lungs (7.1–21.6 %). Less commonly, other areas such as the spleen, kidney, heart, brain, and musculoskeletal system may be involved (15 %) [2,3]. Subcutaneous HC, which accounts for only 1.5 % of all HC cases (range: 0.6 %–1.6 %), is a rare entity that mostly develops secondary to iatrogenic spillage of cyst contents into incision area during a visceral HC surgery. Rarely, it may also develop due to spontaneous rupture of a visceral HC. However, primary involvement of subcutaneous tissue by HC is an exceptionally rare condition, even in endemic regions [4]. Here, we present a 72-year-old farmer who presented with a painless lump in the gluteal region and diagnosed as having primary subcutaneous HC.
Case presentation
A 72-year-old male patient presented with a painless mass in his left gluteal region which has been growing slowly over the past six months. He was a farmer living in a rural area with many dogs and sheep. He reported no fever, weight-loss, pruritus, rash or a history of any trauma or surgery. There was no history of any intramuscular injection to the gluteal region. Physical examination revealed a non-tender, mobile, subcutaneous mass of about 4 cm with normal overlying skin in the left gluteal region. Ultrasonography (US) showed a well-circumscribed, thick-walled, multiseptated, 44 × 34 × 46 mm (anteroposterior x craniocaudal x transverse) cyst. ELISA (enzyme linked immunosorbent assay) for hydatid disease was positive. Magnetic resonance imaging (MRI) demonstrated that the lesion was limited to the left gluteal subcutaneous tissue without any infiltration to the neighboring muscles (Fig. 1). It was a hyperintense lesion with multiple septae and a hypointense peripheral rim on T2-weighted images. No restriction of diffusion was noted on diffusion-weighted images and apparent diffusion coefficient (ADC) mapping (Fig. 2). Following intravenous gadolinium administration, enhancement was recorded in the rim and septae (Fig. 3). Thoracic and abdominopelvic computed tomography (CT) examination revealed no additional lesions. Based on the clinical, laboratory and imaging findings, the patient was diagnosed as having primary subcutaneous HC in the gluteal region.
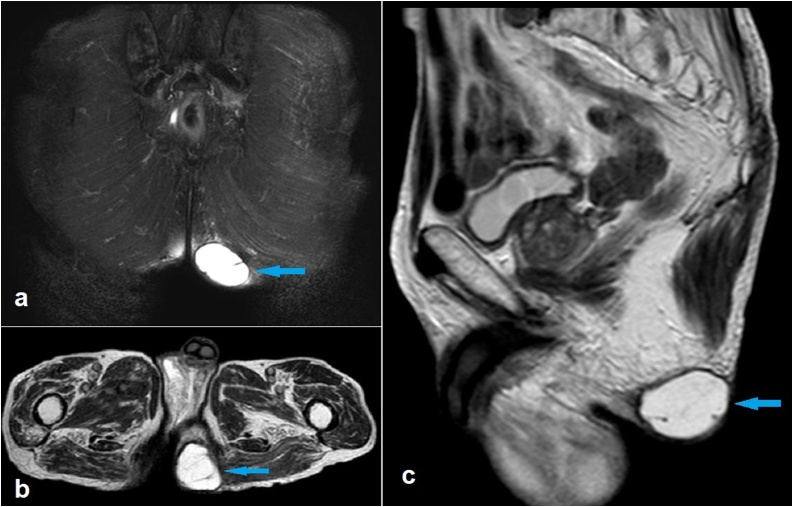
Fig. 1.
Coronal fat-suppressed T2-weighted (a), axial T2-weighted (b) and sagittal T2-weighted magnetic resonance images showing a hydatid cyst limited in the subcutaneous tissue of the left gluteal region (arrows).
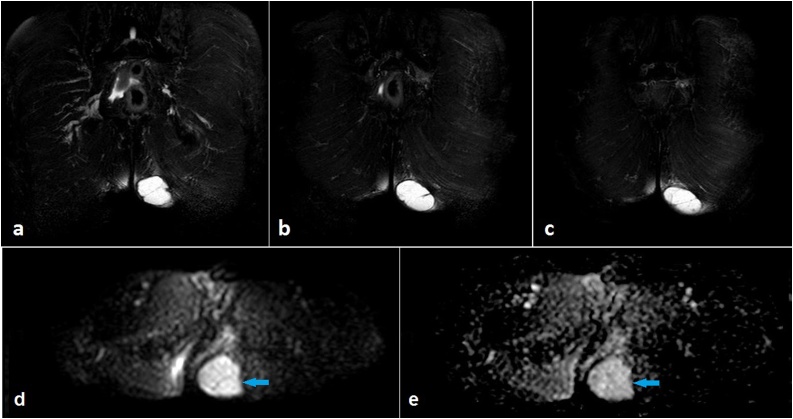
Fig. 2.
Consecutive coronal fat-suppressed T2-weighted magnetic resonance images (a to c) demonstrate that the cyst displays high internal signal intensity and shows multiple septae and a hypointense peripheral rim. Diffusion-weighted magnetic resonance image (DWI) (d) and apparent diffusion coefficient (ADC) mapping (e) depict no restriction of diffusion (arrows).
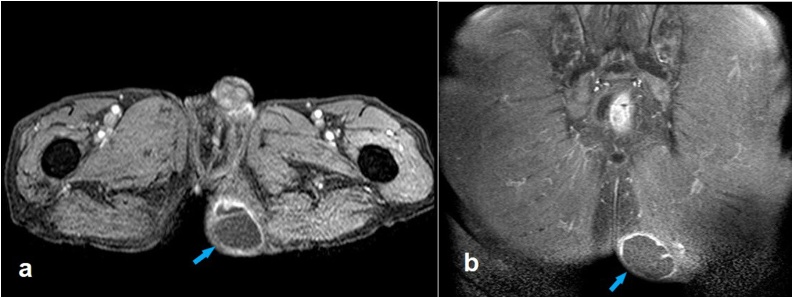
Fig. 3.
Post-contrast axial (a) and coronal (b) fat-saturated T1-weighted magnetic resonance images showing the enhancement of the rim as well as some of the thick septae (arrows).
Following five days of anthelmintic therapy with albendazole, the cyst was surgically excised. Pathologic evaluation of the surgical specimen showed laminated membranes and inner germinal layers, which are characteristic for HC (Fig. 4). No surgical complications occurred. With the follow-up of liver function, 6-month albendazole treatment was planned and the patient was discharged.
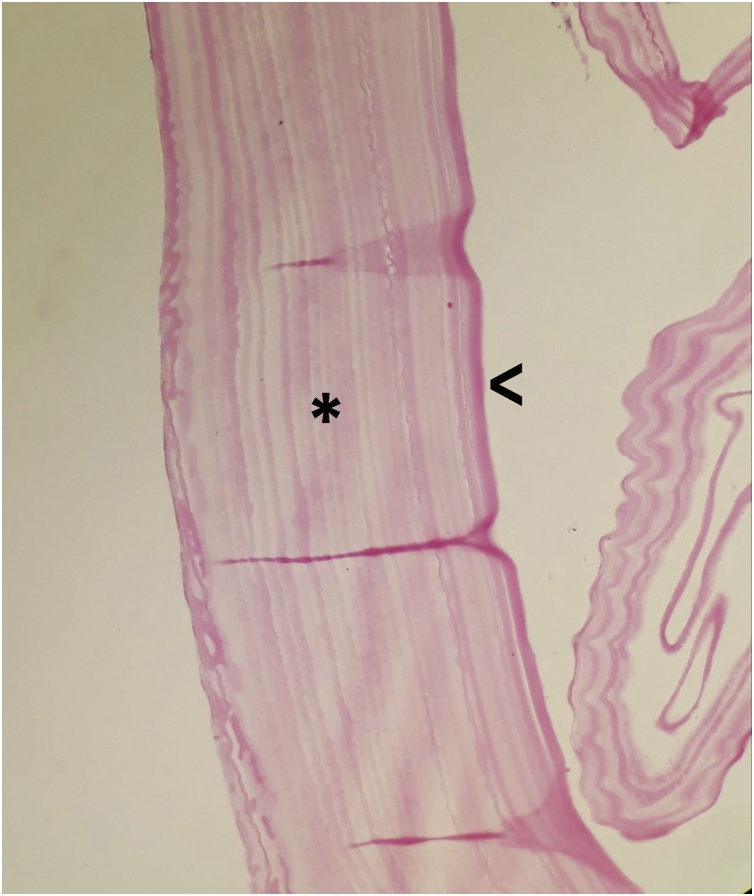
Fig. 4.
Hydatid cyst. The cyst wall; inner germinal layer (v) and laminated membrane (*). (H&E stain, x40).
Discussion
The development mechanism of primary subcutaneous HCs has not yet been fully understood. It has been suggested that subcutaneous involvement may develop secondary to contamination through an injured skin or to the subcutaneous colonization of the ingested eggs after they have passed the liver and lungs. Regardless of the development mechanism, subcutaneous HCs tend to involve trunk and limb roots, possibly due to the rich vascularization and relatively less muscle activity in these areas. They mostly present as a slowly-growing, painless, mobile mass with a normal overlying skin [4]. According to the previous case reports of gluteal subcutaneous HC, which are of limited number, the patients usually has a palpable mass history for at least three months, and the mass is usually larger than 3 cm at the initial admission [[5], [6], [7], [8], [9]]. Similar to the previously reported cases, our patient came to the hospital with a 4.5 cm mass, six months after he first noticied the gluteal mass.
There are no pathognomonic clinical signs and symptoms of musculoskeletal hydatid disease, including subcutaneous HC [10]. Because of the high false-negative result rates, serology has only a small contribution to the diagnosis [11]. Further, since the fluid inside the HCs contains large amounts of protein which may be highly toxic to the host, incisional biopsy is contraindicated in cases where HC is suspected [10]. Therefore, radiological imaging plays a key role in the diagnosis of HC. US, CT and MRI are the imaging modalities that can be used for the diagnosis and treatment determination of the disease. HC can be seen as a unilocular cyst, a cyst containing detached membranes, a multiseptated cyst, a multivesicular cyst or a complex cystic lesion on US [10,11]. It has been reported that, of all subcutaneous HCs, 61 % present as a multiloculated and 33 % as a uniloculated cyst, on imaging studies [4]. CT, another imaging option, is not a preferable method for evaluating the texture of the cyst matrix, but is the method of choice for the assessment of calcified HCs. MRI is an excellent imaging method not only to evaluate the internal structure of the cyst, but also to identify muscles, fascial borders and cyst extensions [10,11].
Treatment of the musculoskeletal hydatid disease, including that of subcutaneous HCs, is based on surgical excision combined with anthelmintic chemotherapy. Anthelmintic chemotherapy reduces the number of live cysts and the risk of recurrence. During surgery, HCs should be removed to maintain their integrity to prevent infection from spreading to healthy tissue. In case of rupture of the cyst during the operation, it is recommended to irrigate the pouch with protoscoloidal solutions after removal of the cyst contents [4,10].
Conclusion
Although very rare, HC can primarily involve the subcutaneous tissue of the gluteal region. Therefore, HC should be added to the differential diagnosis list in cases with a soft tissue mass with a normal overlying skin in this location. Although the texture of the cyst matrix can be easily evaluated using US, MRI is the method of choice in identifying muscles, facial borders and cyst extensions, prior to surgery.
Consent
Informed consent for publication was obtained from the patient.
Contribution statement
MÖ: Analysis and interpretation of data, drafting the article, final approval of the version to be published.
RPK: Acquisition of data, final approval of the version to be published.
NK: Acquisition of data.
NCA: Analysis and interpretation of data.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Declaration of Competing Interest
The authors declare that they have no conflict of interest.
Contributor Information
Meltem Özdemir, Email: meltemkaan99@gmail.com.
Rasime Pelin Kavak, Email: drrpelindemir6@hotmail.com.
Nezih Kavak, Email: nezih_kavak@hotmail.com.
Noyan Can Akdur, Email: noyanakdur@msn.com.
References
- 1.Pedrosa I., Saíz A., Arrazola J., Ferreirós J. Hydatid disease: radiologic and pathologic features and complications. Radiographics. 2000;20(3):795–817. doi: 10.1148/radiographics.20.3.g00ma06795. [DOI] [PubMed] [Google Scholar]
- 2.Mehta P., Prakash M., Khandelwal N. Radiological manifestations of hydatid disease and its complications. Trop Parasitol. 2016;6(2):103–112. doi: 10.4103/2229-5070.190812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Keser S.H., Selek A., Ece D. Review of hydatid cyst with focus on cases with unusual locations. Turk Patoloji Derg. 2017;33(1):30–36. doi: 10.5146/tjpath.2016.01369. [DOI] [PubMed] [Google Scholar]
- 4.Kayaalp C., Dirican A., Aydin C. Primary subcutaneous hydatid cysts: a review of 22 cases. Int J Surg. 2011;9:117–121. doi: 10.1016/j.ijsu.2010.10.009. [DOI] [PubMed] [Google Scholar]
- 5.Ramos Pascua L., Santos Sanchez J.A., Samper Wamba J.D. Atpical image findings in a primary subcutaneous hydatid cyst in the gluteal area. Radiography (Lond) 2017;23(3):e65–e67. doi: 10.1016/j.radi.2017.03.017. [DOI] [PubMed] [Google Scholar]
- 6.Çiçekli Ö., Öncel F., Kochai A multıple mydatıd cyst in gluteal region: a case report. Ann Clin Pathol. 2016;4(1):1060. [Google Scholar]
- 7.Kullolli G., Chavan D.R., Kannur S. A rare case of an isolated subcutaneous hydatid cyst in the gluteal region. J Evol Med Dent Sci. 2014;3(19):5276–5278. 9. [Google Scholar]
- 8.Yıldız ÖS., Özden R., Duman İG. Subcutaneous hydatid cyst in the left gluteal region: case report. CausaPedia. 2014;3:900. [Google Scholar]
- 9.Mushtaque M., Mir M.F., Lone M.A. Solitary subcutaneous gluteal hydatid cyst: a case report. East J Med. 2010;15:76–79. [Google Scholar]
- 10.Arkun R., Mete B.D. Musculoskeletal hydatid disease. Semin Musculoskelet Radiol. 2011;15(5):527–540. doi: 10.1055/s-0031-1293498. [DOI] [PubMed] [Google Scholar]
- 11.Stojkovic M., Rosenberger K., Kauczor H.U. Diagnosing and staging of cystic echinococcosis: how do CT and MRI perform in comparison to ultrasound? PLoS Negl Trop Dis. 2012;6(10):e1880. doi: 10.1371/journal.pntd.0001880. [DOI] [PMC free article] [PubMed] [Google Scholar]