Abstract
Puberty is marked by substantial increases and emerging sex differences in psychological disorders and risky behaviors. However, few studies have examined these effects beyond adolescence, and the previous literature has been dominated by samples of White girls. The current study examines the broadest known set of health sequelae related to traditional pubertal markers and peer-relative pubertal timing in a representative sample of 14,545 U.S. youth from the National Longitudinal Study of Adolescent to Adult Health.
Maturational timing was assessed by age at menarche for girls and physical development for boys (e.g., facial hair, voice change), and then categorized as early (1 SD below mean), on-time, or late (1 SD above mean) within-sex. Early and late peer-relative timing was assessed by a self-report of looking “much older” or “much younger” than one's peers. We examined psychological (depressive symptoms, antisocial behavior), behavioral (number of sex partners, drug use, physical activity, screen time, sleep hours), and physical health (self-reported health, BMI) outcomes during adolescence and young adulthood in a series of sex-stratified regression analyses using survey weights and a comprehensive set of sociodemographic covariates.
Results indicated that, overall, earlier pubertal timing (i.e., maturational timing and peer-relative timing) put both girls and boys at risk during adolescence, while later timing was protective. However, longitudinal models revealed mixed results. For instance, early maturational timing was associated with higher young adult BMI (girls: β = 0.139, p < .01; boys: β = 0.107, p < .01), but later timing for boys was associated with both risky (e.g., more screen time; β = 0.125, p < .05) and health promoting (e.g., more sleep; β = .296, p < .01) behaviors. Analysis of this holistic set of outcomes with sex differences in mind allows for more careful evidence-based recommendations for adolescent health promotion.
Keywords: Puberty, Gender differences, Internalizing behaviors, Externalizing behaviors, BMI, Add health
Highlights
-
•
Early or late puberty predicts health, but there is little longitudinal research.
-
•
Perceived pubertal development relative to peers also predicts health outcomes.
-
•
Links between pubertal timing and long-term health were more pervasive for girls.
-
•
Adolescent psychological symptoms and health behaviors explain long-term links.
-
•
Results point to the need for high-quality puberty education early in life.
1. Introduction
Puberty is marked by substantial increases and emerging sex differences in psychological disorders, physical activity patterns, and risky behaviors, with implications for health and health disparities. However, few studies have examined these effects beyond adolescence, and the previous literature has been dominated by samples of White girls, with limited research on pubertal processes in ethnic/racial minority youth and boys (Marceau, Hottle, & Yatcilla, 2019). The current study investigates the broadest known set of social, behavioral, and physical health sequelae related to pubertal timing in the National Longitudinal Study of Adolescent to Adult Health (Add Health), the most comprehensive nationally representative sample of adolescent development in the United States (U.S.).
1.1. Pubertal timing for girls and boys
The most consistent finding to emerge from the research on girls supports the early timing hypothesis, which posits that early maturing girls find pubertal adjustment especially challenging and are more likely to experience adverse outcomes (Caspi & Moffitt, 1991). Research from developmental science suggests that early puberty in girls is associated with more risk-taking behaviors, earlier sexual activity, increased use of tobacco and alcohol, and heightened prevalence and intensity of depression and anxiety, relative to on-time or late development (for a review, see Mendle, Turkheimer, and Emery (2007)). However, these studies are often limited in sample size and generalizability and few have examined long-term outcomes into adulthood. There is also a large body of epidemiological research that has uncovered links between early menarche and risk of obesity and cardiovascular disease (Adair, 2008; Lakshman et al., 2009; Prentice & Viner, 2012), reproductive cancers (Ahlgren, Melbye, Wohlfahrt, & Sorensen, 2004; Jordan, Webb, & Green, 2005), and all-cause mortality (Jacobsen, Oda, Knutsen, & Fraser, 2009; Tamakoshi, Yatsuya, Tamakoshi, & Group, 2011). However, these studies often rely on a single, retrospectively reported marker of pubertal timing and typically do not account for childhood/adolescent health, body weight, or activity behaviors (e.g., physical activity, sleep), which may confound the relationship between pubertal timing and long-term health outcomes (Hoyt & Falconi, 2015). From a demographic perspective, researchers often situate pubertal development through the lens of a modern global decline in fertility (Lee & Mason, 2014; Wachter, 2008; Wachter & Bulatao, 2003), as well as in terms of social norms surrounding family formation (Berrington & Pattaro, 2014; Hakim, 2003; Hayford, 2009). However, this demographic and family research has generally not been concerned with short or long-term health behavior sequelae of pubertal timing.
The off-time hypothesis (Caspi & Moffitt, 1991; Petersen & Taylor, 1980) suggests that girls who develop either early or late might be at risk for poor health outcomes, yet, this is not often tested given that age of menarche is typically measured as a continuous, linear variable (assuming early timing is risky and later timing is protective) or on-time and late development are combined in analyses (and compared to early timing). Conceptually, this theory is based on the idea that events that occur at appropriate or expected ages allow youth to anticipate, prepare, and learn how to cope with their changing situation, whereas events that catch youth off-time (in either direction) may be associated with insecurity and heightened stress. One study that examined nonlinear effects of pubertal timing on depressive symptoms between ages of 12 and 23 years in Add Health, found that both early and late maturing girls (and boys) were at risk of experiencing elevated depressive symptoms during early adolescence (Natsuaki, Biehl, & Ge, 2009). Another study examined perceived timing relative to peers, and found that girls (and boys) who perceived themselves to be late relative to peers were at risk for body dissatisfaction (de Guzman & Nishina, 2014).
The developmental sequelae of pubertal timing are substantially less well understood in boys than girls. One culprit is that studies of adult health and disease among men lack a discrete, measurable pubertal event (like menarche in girls) that can be accurately and retrospectively reported in large epidemiological studies. Historically, in the adolescent development literature, there was a prevailing view that early maturation had psychosocial benefits for boys, such as higher self-esteem (Blyth, 1981, Simmons, Blyth, Van Cleave, & Bush, 1979)and positive body image (Crockett & Petersen, 1987), while late maturation was associated with greater feelings of inadequacy and social rejection (Clausen, 1975). However, the majority of psychology studies in the past few decades suggest that early maturation in boys is a primary risk for internalizing symptoms, including depression and anxiety, and externalizing symptoms such as attention deficit disorder, conduct disorder, aggression, delinquency, and risk-taking behavior (Ge, Conger, & Elder, 2001; Huddleston & Ge, 2003; Mendle & Ferrero, 2012). There is also some evidence that late timing is associated with depressive symptoms in boys (Conley & Rudolph, 2009)(Graber, Lewinsohn, Seeley, & Brooks-Gunn, 1998) or that both very early and very late (i.e., off-time) puberty are associated with worse mental health (Weichold, Silbereisen, & Schmitt-Rodermund, 2003).
1.2. Life course perspective on pubertal timing
Overall, most research supporting the link between pubertal timing and health has been examined in small, short-term studies, limiting our knowledge about the enduring effects of early or off-time maturation. Importantly, concurrent effects (i.e., adolescent outcomes) and long-term effects (i.e., young adult outcomes) of pubertal timing could vary. For instance, early maturing youth may experience initial challenges, yet adverse effects could dissipate over time as other youth catch up in physical development. In this case, we might expect that early or off-time maturation is associated with increased health risk in adolescence but not in young adulthood. On the other hand, some life-course models suggest that childhood experiences may not affect outcomes until later in life, owing to a latency period (Berkman, 2009). Therefore, we may observe links between early or off-time maturation in adulthood that were not detectable in adolescence. Other life-course health theories focus on cumulative exposure (Johnson, Crosnoe, & Elder, 2011; Kuh, Ben-Shlomo, Lynch, Hallqvist, & Power, 2003): using this lens, youth who develop early or off-time may be set on a risk trajectory, and accumulating risks may exacerbate negative health trajectories into adulthood (i.e., we may observe increasing effects from adolescence to young adulthood). Finally, given research that early pubertal timing may promote some advantage in the short-term, particularly for boys (Chaku & Hoyt, 2019; Taga, Markey, & Friedman, 2006), we may observe more nuanced developmental trajectories than previously hypothesized. For instance, there could be an initial advantage for early maturing boys, particularly in their psychosocial well-being, yet other health risks could accumulate over time if these youth also experience more stress in their social relationships or suffer from long-term consequences of affiliating with older peers.
Only a few puberty studies have used assessments with multiple outcomes to examine the contribution of pubertal timing to health both concurrently and over time to test these competing hypotheses and they have had mixed results. One study included 167 youth followed over four years and found that early maturation predicted stable high depression in girls while early maturing boys showed low initial levels of depression, but increased risk over time (Rudolph, Troop-Gordon, Lambert, & Natsuaki, 2014). Research from Add Health found that early menarche was associated with depressive symptoms and antisocial behaviors during adolescence with enduring (but not increasing) effects into young adulthood (Mendle, Ryan, & McKone, 2018). An earlier Add Health study found that both early and late timing predicted depressed mood during early adolescence – but effects dissipated over time from adolescence to young adulthood (Natsuaki et al., 2009). Additionally, a meta-analysis of epidemiological studies found that earlier pubertal timing was predictive of higher adult BMI and greater risk of obesity, however, of the 48 papers analyzed, only eight papers included data on childhood BMI (Prentice & Viner, 2013). It is well-known that childhood BMI is a robust predictor of pubertal timing (Biro, Khoury, & Morrison, 2006; Kaplowitz, Slora, Wasserman, Pedlow, & Herman-Giddens, 2001). Therefore, without baseline data from childhood, it is unclear whether early puberty is a risk factor for adult obesity, or a risk marker of childhood obesity (e.g., elevated childhood BMI contributes to an earlier age of menarche, which in turn leads to a higher adult BMI). This body of research is further limited by small (nonrepresentative) sample sizes, a specific focus on White girls, and/or lack of key adolescent covariates known to be associated with both pubertal timing and health (e.g., BMI, father absence, social support). Furthermore, although previous research on childhood SES and pubertal timing is mixed (Buttke, Sircar, & Martin, 2012; James-Todd, Tehranifar, Rich-Edwards, Titievsky, & Terry, 2010; Yermachenko & Dvornyk, 2014), it is important to account for how baseline SES predicts long-term health outcomes.
1.3. The current study
To address these gaps in the literature, the current study aimed to examine the contribution of pubertal timing to adolescent health and emerging adult health outcomes approximately 13 years later using a nationally representative sample of adolescents in the U.S. Further, we compare two different measures of pubertal timing for both girls and boys: intra-individual maturational timing (i.e., a personal marker of development irrespective of one's social context) and peer-relative perceived pubertal timing (i.e., a subjective evaluation of one's physical maturity based specifically on peer comparison). This latter measure has important developmental significance given that youth compare themselves to others (e.g., friends, schoolmates, siblings, media personalities) in order to make sense of who they are and develop a self-concept (Blanton, 2001; Festinger, 1954). Although previous research using Add Health shows that these self-reported measures are moderately correlated for boys and slightly correlated for girls (Kretsch, Mendle, Cance, & Harden, 2016), no research to date have used both constructs in the same study in order to discern potential mechanisms through which pubertal timing influences population health. For instance, biological explanations for the association between pubertal timing and health may be better aligned with findings that link physical changes (e.g., menarche) to long-term health, while many psychosocial perspectives on the negative sequelae of early/off-time pubertal development may rely on subjective perceptions of peer comparison. Overall, we hypothesize that early pubertal timing (peer-relative timing, in particular) will be associated with poor mental, behavioral, and physical health for girls, both in adolescence and young adulthood, while off-time puberty (either early or late timing) may increase health risk for boys.
2. Methods
2.1. Participants and procedure
The data are drawn from Add Health, a nationally representative sample of adolescents in grades 7 through 12 (aged 12–19 years) in the United States in the 1994–1995 school year (Harris, 2013). The Wave I sample included 20,745 youth who were followed up in 1996 (Wave II), 2001–2002 (Wave III), and 2008 (Wave IV), at which time respondents were between the ages of 24 and 32; thus, the young adult follow-up from adolescence is approximately 13 years from Wave 1 to Wave 4. Of the longitudinal sample, 14,798 participants had valid sampling weights. We further excluded 253 participants who were missing pubertal timing data. The final analytic sample for the current study included 7,728 female respondents and 6,817 male respondents. Procedures for data access and analysis were implemented as approved by the Institutional Review Boards at Fordham University and University of Massachusetts, and in agreement with the sensitive data security plan approved by Add Health data managers.
2.2. Measures
Independent variables (i.e., pubertal timing measures), dependent variables (i.e., psychosocial well-being, health behaviors, physical health in adolescence and adulthood), and baseline covariates are described in detail below.
2.2.1. Pubertal timing
Two self-reported measures of pubertal timing were assessed: intra-individual development (i.e., maturational timing) and inter-individual development (i.e., peer-relative pubertal timing). Self-reported age at menarche served as a proxy for maturational timing for girls. Although this event occurs later in the pubertal process, it is commonly used as a marker of pubertal timing for girls and facilitates the comparison of findings across studies. Age of menarche was measured in whole years at Wave I, Wave II, and Wave III. Of the female respondents, 89.63% reported their age at menarche at Wave I; if a respondent had not had menarche or did not report it at Wave I, we used the next available report at Wave II (5.38%) or Wave III (3.42%). Boys’ maturational timing was derived from selected questions about bodily changes that become evident in mid-to-late puberty (i.e., facial hair, body hair, and voice change) on a continuous scale that ranged from “least developed” (1) to “most developed” (5). Responses on the three items were averaged and then standardized within each whole number age group so that a higher value represents more advanced development among same-aged boys (i.e., earlier maturation). For both sexes, early/late maturational timing was defined as one standard deviation below/above the mean. These cut-offs have been used in previous research with the Add Health sample and others (e.g., (Foster, Hagan, & Brooks-Gunn, 2008)Ge et al., 2001).
Peer-relative pubertal timing was assessed by the question, “How advanced is your physical development compared to other boys/girls your age?” Responses could range from “I look younger than most” (1) to “I look older than most” (5). We defined early peer-relative timing as physical development older than most, and late as younger than most. The distributions of early/on-time/late peer-relative timing roughly match that of maturational timing in both females and males (see Table 1).
Table 1.
Descriptive statistics for the analytic sample (n = 14, 545).
| Female (n = 7,728) |
Male (n = 6,817) |
|
|---|---|---|
| M (SD) /% | M (SD) /% | |
| Maturational Timing | ||
| Early Timing | 9.4% | 15.8% |
| On-Time | 76.1% | 68.4% |
| Late Timing | 14.4% | 15.8% |
| Peer-Relative Timing | ||
| Younger Than Most | 8.5% | 11.3% |
| On-Time | 77.7% | 74.8% |
| Older Than Most | 13.8% | 13.8% |
| Adolescent Outcomes (Wave I) | ||
| Psychosocial Well-Being | ||
| Depressive Symptoms | 7.01 (4.92) | 5.27 (3.89) |
| Antisocial Behavior | 2.75 (3.40) | 3.34 (4.16) |
| Health Behaviors | ||
| Number of Sex Partners | 1.04 (3.13) | 1.68 (5.10) |
| Drug Use | 0.61 (0.98) | 0.68 (1.04) |
| Physical Activity | 3.25 (2.00) | 4.13 (2.22) |
| Screen Time | 19.90 (19.20) | 25.48 (23.39) |
| Sleep Hours | 7.78 (1.42) | 7.87 (1.41) |
| Physical Health | ||
| Self-Reported Good Health | 3.80 (0.91) | 3.95 (0.90) |
| Body Mass Index | 22.31 (4.42) | 22.73 (4.67) |
| Young Adult Outcomes (Wave IV) | ||
| Psychosocial Well-Being | ||
| Depressive Symptoms | 6.51 (4.95) | 5.70 (4.43) |
| Antisocial Behavior | 0.19 (0.86) | 0.43 1.29) |
| Health Behaviors | ||
| Number of Sex Partners | 8.61 (9.56) | 12.13 (13.52) |
| Drug Use | 0.81 (1.03) | 1.22 (1.15) |
| Physical Activity | 3.23 (2.75) | 4.05 (3.32) |
| Screen Time | 19.77 (18.54) | 24.90 (23.32) |
| Sleep Hours | 7.99 (1.26) | 7.58 (1.36) |
| Physical Health | ||
| Self-Reported Good Health | 3.63 (0.92) | 3.69 (0.92) |
| Body Mass Index | 29.16 (8.17) | 29.01 (6.86) |
| Baseline Covariates (Wave I) | ||
| Age (in Wave I) | 15.92 (1.80) | 16.08 (1.84) |
| Parent Education | 12.86 (2.61) | 12.96 (2.60) |
| Race/Ethnicity | ||
| White | 67.7% | 67.4% |
| Black | 16.0% | 15.6% |
| Hispanic | 11.8% | 11.8% |
| Asian | 2.7% | 3.30% |
| Other | 1.4% | 1.8% |
| Receipt of Public Aid | 7.9% | 6.9% |
| Parental Marital Status | ||
| Single, Never Married | 5.4% | 4.6% |
| Married | 71.9% | 72.4% |
| Windowed | 3.1% | 3.0% |
| Divorced | 14.6% | 15.8% |
| Separated | 5.0% | 4.2% |
| Father Absence | ||
| Never Absent | 63.4% | 65.5% |
| Father Left (6–13 years) | 11.2% | 11.5% |
| Father Left (0–15 years) | 11.3% | 10.6% |
| Always Absent | 14.0% | 12.4% |
| Urbanicity | ||
| Urban | 26.1% | 26.2% |
| Suburban | 58.3% | 58.4% |
| Rural | 15.5% | 15.4% |
Notes. All descriptive statistics are drawn from the final imputed dataset and weighted using Add Health survey weights.
2.2.2. Psychosocial well-being
In Waves I and IV, participants completed the Center for Epidemiological Studies Depression Scale (CES-D), which is a short, self-report scale designed to measure depressive symptomatology in the general population, and is also valid for use in adolescent populations (Perreira, Deeb-Sossa, Harris, & Bollen, 2005, Radloff, 1991). Sample items include: felt blue; bothered by things that don't usually bother you; did not enjoy life (0 = never; 1 = sometimes; 2 = a lot of the time; 3 = most/all of the time). The full version was administered at Wave I; a 10-item abbreviated version was administered at Wave IV. To keep measurement consistent across waves, we used the abbreviated version. Participants' responses were summed to form the depressive symptoms scale (ranging from 0 – 30) at Waves I (α=.80) and IV (α=.84).
Antisocial behaviors were assessed by self-reported frequency of antisocial behaviors over the past 12 months (0 = never; 1 = 1 or 2 times; 2 = 3 or 4 times; 3 = 5 or more times). Five items were asked at both Waves I and IV, including property damage, stealing something worth <$50, stealing something worth >$50, selling drugs, and breaking into a building. Wave I included five additional items: running away from home, lying to parents, driving a car without the owner's permission, shoplifting, and being loud and rowdy in public. Wave IV included three additional items: deliberately writing a bad check, using others' debit card without permission, and buying or selling stolen property. Participants' responses were summed at Waves I (α=.78) and IV (α=.62).
2.2.3. Health behaviors
We assessed five key health behaviors: sexual risk-taking, drug use, physical activity, screen time, and sleep. Sexual risk-taking was conceptualized as the total number of sexual partners. Responses of 50 partners or more were winsorized (i.e., top coded) at 50 (0.23% of all responses in Wave I and 1.48% in Wave IV) to reduce skewness.
At Waves I and IV, participants reported the number of days they smoked cigarettes, the number of times they used marijuana and illicit drugs (e.g., cocaine, inhalants) in the last 30 days, and the number of days they drank five or more alcoholic drinks in a row over the past 12 months. These four variables were dichotomized as whether a participant used cigarettes, marijuana, or illicit drugs in the past 30 days or binge drank 3–5 days or more in the past 12 months (approximately once per month;1 = yes; 0 = no). A drug use index was created as the sum of these items (ranging from 0 to 4), based on previous research (McDade et al., 2011).
Physical activity was assessed in Wave I by three questions about the frequency of skating/biking, playing an active sport, and exercising in the past week (0 = not at all; 1 = 1 – 2 times; 2 = 3 – 4 times; 3 = 5 or more times). In Wave IV, physical activity was assessed by the frequency of participation in seven activities in the past week: skating/biking, snowboard/racquet/aerobics, team sports, individual sports, gymnastics/weights/strength training, golfing/fishing/baseball, and walking. Responses to the activities were summed (Simpkins, Schaefer, Price, & Vest, 2013). To measure screen time, we summed the number of hours per week spent in three behaviors in Wave I: TV hours, video hours, and screen games (Gordon-Larsen, McMurray, & Popkin, 2000) and three behaviors in Wave IV: TV hours, internet hours, and screen games.
In Wave I, participants were asked about how many hours of sleep they usually get with responses in whole hours. In Wave IV, participants were asked about weekday/weekend sleep via four items: “on days when you go to work, school, or similar activities, what time do you usually wake up?”; “what time do you usually go to bed the night before?”; “on days when you don't have to get up at a certain time, what time do you usually wake up?”; “on these days, what time do you usually go to sleep the night before?”. We calculated the hours of sleep per night on weekdays and weekends, and computed a weighted average (i.e., weighing the weekday items by 5/7 and the weekend items by 2/7) to assess sleep duration in a typical day during a week (Maslowsky & Ozer, 2014).
2.2.4. Physical health
Self-reported good health was assessed by the same, single question in Waves I and IV: “In general, how is your health?” (0 = poor; 1 = fair; 2 = good; 3 = very good; 4 = excellent). Self-reported height and weight were used to calculate baseline adolescent BMI because objective measures were not available at Wave I. Height and weight, measured by trained interviewers at Wave IV, were used to calculate adult BMI (i.e., the ratio of weight in kilograms over height in meters squared).
2.2.5. Baseline covariates
All baseline covariates were measured at Wave I. Demographic data included age in years and race/ethnicity (coded as Non-Hispanic White, Black/African American, Hispanic/Latino, Asian, or other). Measures of the family environment included family socioeconomic status (SES) – measured by approximate years of parents’ education and whether or not their family received public aid – as well as parental marital status (married, single parent, widowed, divorced, separated) and father absence (father present, father left when participant was 0–5 years, father left when aged 6–13 years). The environment was addressed by urbanicity (urban, suburban, or rural.
2.3. Analytic strategy
The general analytic strategy was to investigate a series of ordinary least-squares regression models to assess the association between pubertal timing and adolescent (Wave I) outcomes, and separately, young adult (Wave IV) outcomes. Given that pubertal timing is associated with adolescent health outcomes, longitudinal analyses could obfuscate effects over time such that it would be hard to detect significant differences in adult outcomes when adolescent health is controlled for. Therefore we ran two sets of longitudinal models. First, we tested the direct association of pubertal timing on adult health without including adolescent symptoms or behaviors; then we added the full set of adolescent covariates, to estimate whether early pubertal timing was related to adult health outcomes (above and beyond what might be predicted by adolescent levels), an approach used in previous research with Add Health (Mendle et al., 2018). We examined nine health outcomes across three domains, including: psychosocial well-being (i.e., depressive symptoms, antisocial behavior), health behaviors (i.e., number of sex partners, drug use, physical activity, screen time, sleep hours), and physical health (i.e., self-reported good health, BMI).
Analyses were conducted in Stata Version 15 (StataCorp, 2017) using survey procedures and Add Health longitudinal sampling weights, which adjust for the complex cluster sample design, unequal probability of selection, and non-response. The analytic sample included all participants with pubertal timing data but not necessarily complete data on the covariates or dependent variables. Missing values on covariates were assigned using chained multiple imputation methods. Missing covariates ranged from 0.0% for race/ethnicity to 17.4% for father absence. A total of 10 imputations were used to obtain final estimates. All independent variables, strata, sampling weights, and the outcome variable were used as predictors in the imputation process (Allison, 2000). In order to present results and sex differences across multiple models, puberty coefficients are graphically illustrated in Fig. 1, Fig. 2, Fig. 3. The full set of results, including coefficients and confidence intervals (CIs) for pubertal timing and all covariates, are presented in the online supplemental materials, as well as example code for regression models (Stata Version 15) and figure generation (R Version 3.5.3).
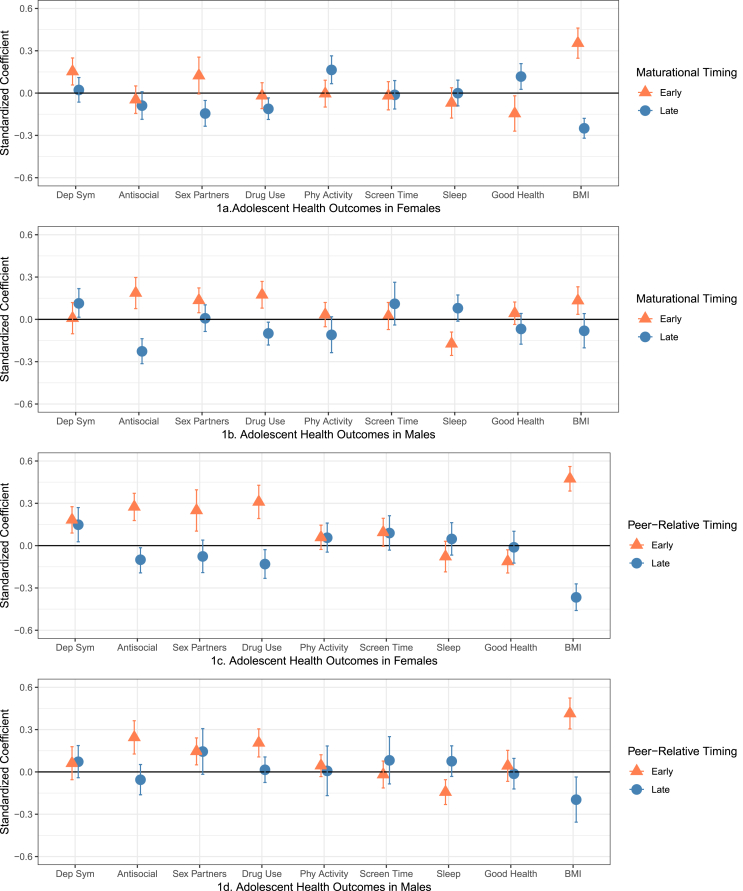
Fig. 1.
Standardized regression coefficients and 95% confidence intervals (CIs) for adolescent health outcomes controlling for sociodemographic characteristics. Coefficients are statistically significant at p < .05 where CIs do not cross the 0 line.
Fig. 2.
Standardized regression coefficients and 95% CIs for young adult health outcomes controlling for baseline sociodemographic characteristics. Coefficients are statistically significant at p < .05 where CIs do not cross the 0 line.
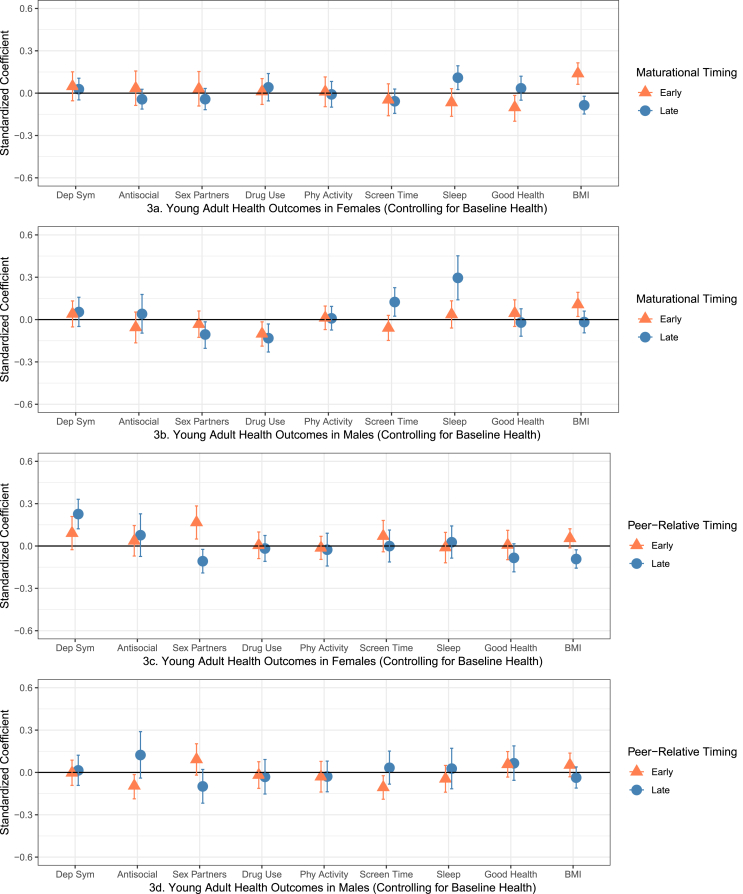
Fig. 3.
Standardized regression coefficients and 95% CIs for young adult health outcomes controlling for baseline sociodemographic characteristics as well as adolescent levels of psychosocial well-being, health behaviors, and physical health.
3. Results
3.1. Descriptive statistics
Table 1 describes the distribution of pubertal timing, outcomes, and health and demographic covariates in the analytic sample by gender with survey weights (so that estimates are nationally representative). The mean age of the full sample at Wave I was 16.00 years-old (SD= 1.82); 67.7% of the sample were non-Hispanic White, 15.8% were non-Hispanic Black and 11.8% were Hispanic. On average, parents in the sample had completed 12.88 (SD = 2.61) years of education; 7.3% of the participants had mothers who received public assistance and 64.4% had a father in the household their entire childhood. The mean age of menarche for girls was 12.19 years (SD = 1.40) and mean maturational timing for boys was 2.71 (SD = 0.82). Mean peer-relative timing was between “I look about average (3)” and “I look older than some (4)” for both girls (M = 3.30, SD = 1.10) and boys (M = 3.80, SD = 1.15).
3.2. Pubertal timing and adolescent outcomes
Standardized regression coefficients and 95% confidence intervals (CIs) are presented in Fig. 1 (coefficients are statistically significant at p < .05 where CIs do not cross the 0 line) and Supplemental Tables A1-A2 (girls) and B1-B2 (boys). Overall, early timing put both girls and boys at risk for several poor psychosocial, behavioral, and health outcomes, while late timing was mostly protective. Earlier menarche in girls was associated with more depressive symptoms, worse self-reported health, and higher BMI; later menarche was associated with fewer sex partners, less drug use, more physical activity, better self-reported health, and lower BMI during adolescence (Fig. 1; Panel 1a). For boys, earlier maturational timing was associated with significantly higher antisocial behavior, more sex partners, more drug use, less sleep, and higher BMI; later timing was associated with fewer antisocial behaviors and less drug use, but higher depressive symptoms (Panel 1b).
A similar pattern of effects was found for peer-relative pubertal timing (Panels 1c-1d). Among girls, developing earlier than most of one's peers was a consistent risk factor, significantly associated with increased depressive symptoms, antisocial behaviors, more sexual partners, drug use, worse self-reported health, and higher BMI; developing later was protective in terms of antisocial behavior, drug use, and BMI. One notable exception was that off-time peer-relative timing (self-reporting looking older or younger than most of one's peers) was associated with increased depressive symptoms. Among boys, developing earlier than most of one's peers was associated with more antisocial behaviors, more sex partners, more drug use, fewer sleep hours, and higher BMI; developing later was protective in terms of BMI.
3.3. Pubertal timing and young adult health outcomes
Standardized regression coefficients and CIs for the young adult health outcomes are presented in Fig. 2, Fig. 3 (see also Supplemental Tables A3-A6 and B3-B6). Early menarche (Fig. 2; Panel 2a) was associated with more depressive symptoms, less sleep, worse self-reported health, and higher BMI. Later menarche was still associated with fewer sex partners, more sleep hours, better self-reported health, and lower BMI in young adulthood. Examining health outcomes for boys (Panel 2b), earlier maturational timing was only statistically associated with higher BMI; later timing was associated with a number of protective health behaviors including fewer sex partners, less drug use, and more sleep – but also associated with more screen time.
When examining peer-relative pubertal timing in the girls' longitudinal models (Panel 2c), developing earlier than most of one's peers was still associated with a wide range of poor health outcomes across all domains including more depressive symptoms and antisocial behaviors; more sex partners, drug use, and screen time; worse general health and higher BMI. However, developing later (i.e., reporting looking younger than most of one's peers) was associated with both positive and negative health outcomes in young adulthood – fewer sex partners and lower BMI, but more depressive symptoms. Boys (Panel 2d) who reported looking older than most peers (earlier peer-relative timing) had more sex partners and higher BMI, but less screen time; those who reported looking younger (later timing) had lower BMI.
Next, we examined these longitudinal models controlling for baseline levels of psychosocial well-being, health behaviors, and physical health (while still controlling for demographic characteristics, including SES and family context). In this most stringent test of the longitudinal associations between pubertal timing and young adult outcomes, early menarche in girls was statistically associated with higher BMI and worse self-reported health; later menarche was associated with lower BMI and more sleep (Fig. 3, Panel 3a).
For boys (Panel 3b), earlier maturational timing was statistically associated with higher BMI; later timing for boys was associated with fewer sex partners, less drug use and more sleep but also more screen time – replicating previous models showing links between later timing and both protective and risky health behaviors. Unexpectedly, a new statistically significant association emerged between earlier maturational timing and less drug use.
In the final set of longitudinal models examining perceived pubertal timing relative to peers on young adult health outcomes for girls (controlling for baseline health), early peer-relative timing was only significantly associated with more sex partners; later peer-relative timing was associated with higher depressive symptoms, fewer sex partners and lower BMI (Panel 3c). Finally, boys who reported looking older than most peers (earlier peer-relative timing) had fewer antisocial behaviors and less screen time; BMI was no longer statistically significant (Panel 3d). Table 2 provides a visual summary of statistically significant coefficients across all models.
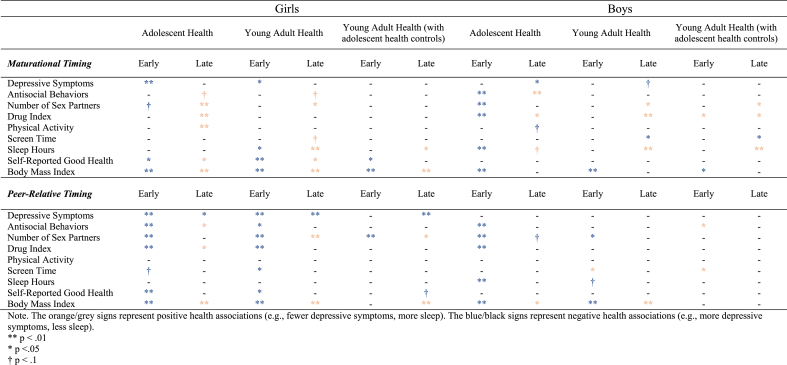
Table 2.
Summary of statistically significant regression coefficients across all models.
4. Discussion
Although the roots of many mental and physical health disorders begin in adolescence, surprisingly few studies have examined the longevity of pubertal timing effects. The current study explored the broadest known set of adolescent and young adult health outcomes related to pubertal timing in both girls and boys and examined both intra-individual development (i.e., maturational timing) and inter-individual development (i.e. peer-relative timing). Aligned with the early timing hypothesis, early pubertal timing was largely associated with increased risk for poor psychosocial, behavioral, and physical health during adolescence for both girls and boys, while later timing was generally protective. However, associations between pubertal timing and young adult outcomes were more nuanced. Further, by examining boys and girls together, we found that while pubertal timing effects on health were pervasive among both sexes during adolescence, the effects for girls were more persistent (see Table 2), with a larger impact on young adult health outcomes for women compared to men.
Results from the current study suggest that girls who experience earlier puberty (measured by menarche or peer-relative perceptions) generally continued to report poor psychological, behavioral, and physical health outcomes in young adulthood, even after accounting for demographic, social, and contextual predictors of poor health. However, in the final models (controlling for adolescent symptoms and health behaviors), we found limited direct associations with young adult health outcomes above and beyond what might be predicted by adolescent levels. Overall, this pattern of results replicates previous research using Add Health that found that girls who experienced earlier menarche were more prone to depressive symptoms in young adulthood than their on-time or later developing peers primarily because they experienced depression as adolescents, and that risk was sustained over time (Mendle et al., 2018). We also expand on these findings in several important ways.
By examining a wide range of outcomes, we observed that links between maturational timing and health extend to behavioral and physical health markers including risky health behaviors, sleep, self-reported health, and BMI. Further, while overall peer-relative pubertal timing in girls replicated the longitudinal links between menarche and health, we also identified a few key differences. Namely, girls with later peer-relative timing (i.e., girls who reported looking younger than most of their same-sex peers during adolescence) also reported more depressive symptoms. Interestingly, these results persisted in the model with the full set of baseline demographic and adolescent health controls, suggesting that these association between later peer-relative pubertal timing and young adult depressive symptoms hold above and beyond adolescent symptoms – and independent of risky behaviors and physical health. This finding highlights the important role of social perceptions in adolescent development. Adults, peers, and youth themselves may equate visible signs of physical development with social or cognitive maturity, leading to different behavioral expectations or assumptions (Carter, Mustaffa, & Leath, 2017; Mora, 2012). As a result, girls who look older or younger than their peers/classmates during adolescence, may be treated differently by teachers, parents, and other peers, with implications for future mental health, as well as social, educational, and economic trajectories across the transition to adulthood.
Overall, there was more evidence for the early timing hypothesis than the off-time hypothesis for the effects of boys’ pubertal timing on adolescent outcomes, with the exception of depressive symptoms (i.e., later maturational timing was associated with more depressive symptoms). In the longitudinal models, early maturational timing was associated with higher young adult BMI; later timing was associated with three positive (i.e., fewer sex partners, less drug use, and more sleep) and only one negative (i.e., more screen time) health behavior. Boys who reported looking older than their peers (i.e., early peer-relative timing) also reported less screen time. One possible explanation is that while later maturation provides some protection from socially deviant activities, boys who develop later also have an athletic disadvantage (e.g., smaller stature, lower muscle mass) and may turn to TV or video games for recreational activities. Interestingly, the links between pubertal timing and screen time remained statistically significant even when controlling for adolescent levels of screen time, suggesting that later maturing boys spend more time in front of screens as young men, independent of their screen time as adolescence. Understanding cumulative vulnerability to this health behavior represents an important area of future research as technology continues to advance and change the ways in which both youth and adults interact with screens.
Another noteworthy finding was that higher BMI was the most robust risk outcome associated with earlier pubertal timing, and this risk extended into young adulthood. Prior observational studies have shown inverse links between onset of menarche and BMI in females, however, most studies did not adjust for childhood obesity and antecedents of early timing, making it difficult to evaluate whether pubertal timing increases risk for obesity, or vice versa (Biro, Greenspan, & Galvez, 2012). We found that early pubertal timing effects on young adult BMI persist after adjusting for adolescent BMI (for girls but not boys) and situational factors that affect the onset of puberty (e.g., ethnicity, SES factors, education level, father absence, social support), which is consistent with a few longitudinal studies that have robustly tested the effect of pubertal timing on BMI in females. Of particular note is a recent study that found age at menarche predicted higher BMI in a sample of adult women from the UK Biobank Study, after adjusting for childhood BMI and genetic risks for early puberty (Gill et al., 2018). The literature is not consistent about this relationship in boys. While higher childhood BMI contributes to earlier onset of puberty among girls, initial evidence suggests that it may be associated with later onset of puberty among boys (Lee et al., 2010). Despite sex differences in how pre-pubertal BMI plays a role in pubertal timing, earlier pubertal onset could increase risk for higher BMI over time for both boys and girls via several psychological and behavioral pathways, including disordered eating behaviors, insufficient sleep, and stress associated with early body transformation.
4.1. Limitations and future directions
There are many benefits to using a nationally representative dataset such as Add Health, but there are also several challenges and limitations. While the longitudinal, multivariable models used in this study control for many sources of confounding variation, they do not address biases associated with unmeasured characteristics that change between Wave I and Wave IV (e.g., changes in household economic status or family structure), which may affect young people's health trajectories. Another limitation is that Wave I occurred when youth were in grades 7–12, which is later than most youth begin pubertal onset (particularly for girls), although inspection of initial variation provides ample grounds for analysis.
Additionally, our measures of physical development fail to capture the extent and tempo of the diverse morphological changes that occur during puberty for both boys and girls. Therefore, it is important that this work is replicated in future representative study samples that recruit and track a younger cohort of youth. It is also possible that the antecedents of early puberty, rather than pubertal timing itself, underlie the relationship between pubertal timing and young adult health. We examined a number of variables known to be associated with pubertal timing at Wave I (e.g., race/ethnicity, adolescent BMI, family stress), but we do not have early childhood covariates to explore.
Despite these limitations, the current study provides new insights into the relationship between pubertal timing and young adult health and suggests that we should re-examine existing theoretical models on pubertal timing. While early-onset puberty may be a stressful experience, it does not generate uniform reactions across girls and boys and may depend on social and cultural factors (Deardorff, Hoyt, Carter, & Shirtcliff, 2019; Morales-Chicas & Graham, 2015; Seaton & Carter, 2017; White, Deardorff, & Gonzales, 2012; White, Deardorff, Liu, & Gonzales, 2013(White et al., 2012)(White et al., 2013)). Additionally, particularly in more conservative settings, youth may not be adequately prepared for the physical changes of puberty and therefore important maturational events may be accompanied by feelings of shame and the need to conceal it from others (Lahme, Stern, & Cooper, 2016; Stubbs, 2008). Further, though research on SES and pubertal timing is mixed, the most recent research suggests that early maturing youth from lower SES backgrounds may have worse outcomes than early developing youth from more advantageous backgrounds (Mendle & Koch, 2019). Future studies would benefit from a biopsychosocial perspective in understanding the complex interactions between physiological changes, cultural and contextual factors, social norms, behaviors, and values in determining health trajectories across the transition from adolescence to adulthood in an ethnically and racially diverse sample of youth. For instance, research could explore the interaction between objective pubertal markers (e.g., Tanner stage, hormone levels) and peer-relative pubertal timing. A young person who begins puberty early and also perceives him/herself as more physically advanced than their peers may have different psychological and behavioral outcomes than an early maturing youth who perceives their maturation as less advanced compared to their peers.
Finally, pubertal research has largely been deficit-focused, and potential benefits associated with varying pubertal trajectories are vastly unexplored. A recent study found that pubertal maturation, controlling for age, was associated with increases in attention skills in both boys and girls (Chaku & Hoyt, 2019). Given research that physical development accompanying pubertal onset are associated with changes in brain development (Goddings, Burnett Heyes, Bird, Viner, & Blakemore, 2012), future studies should consider how cognitive and pubertal processes interact to influence health-related outcomes, and how increases in cognitive development driven by pubertal maturation may in fact prove protective for some youth.
4.2. Implications for promoting population health
The current analysis demonstrated that early pubertal timing was linked to long-term health outcomes via poor psychological functioning, risky behaviors, and higher BMI during adolescence (i.e., cumulative vulnerability over time), particularly for girls. This aligns with previous research that adolescence often contains the developmental roots of lifetime psychopathology and health habits (Harris, 2010). Therefore, it is vital to address early psychosocial and behavioral functioning in adolescence, utilizing limited resources from multiple sectors to reduce the negative effects of early/off-time pubertal timing that drive poor health outcomes, and health disparities, in the population. One important step is to ensure high-quality puberty education for all youth early in life.
Despite secular trends in the declining age of pubertal onset shifting into primary schools for girls (e.g., White and Asian girls usually start to show secondary sex characteristics by ages 9 or 10; and Black and Hispanic girls typically start developing a year or two earlier) (Lee & Styne, 2013; Parent et al., 2003), puberty education typically does not occur until middle or high school (often in combination with sexual education), if it is taught at all (Brener et al., 2012; Herbert et al., 2017). This lack of age-appropriate puberty education targeted to younger children may leave many youth uninformed and ill-prepared for pubertal transition, especially for those who mature earlier than their peers. This is especially important giving our findings, which highlight the important role of peer-relative pubertal timing (i.e., social comparison) in predicting health outcomes. Educators, clinicians, and parents could help normalize the pubertal process and underscore (and appreciate) individual differences in body shape, which become more apparent during this developmental period. Awareness, social support, and early intervention may be especially significant for girls, who experience these overt physical changes several years before same-aged boys.
The physical manifestations of puberty are observable to the developing adolescent, but they are also noticeable to others, and may be compounded by sex or racial/ethnic stereotypes. These changes may signal to adults or friends that youth are now emerging adults, despite being the same chronological age as their peers and classmates (who have more child-like appearances). For instance, more mature youth (particularly youth of color) may be perceived by others as more aggressive or violent, and more likely to have academic and social problems than their same-age peers (Carter et al., 2017; Deardorff et al., 2019). Therefore it is imperative that adults – including parents, teachers, and physicians – are knowledgeable about and sensitive to individual differences in physical development and their own implicit biases.
Finally, theories of life course health and human development suggest that humans pass through sensitive periods in which the social environment can have disproportionate impacts on future health (Halfon & Hochstein, 2002; Hertzman & Boyce, 2010). With the onset of puberty, youth gain more responsibilities from parents and spend more time outside the home, which has important implications for developmental trajectories. Therefore, while pubertal timing itself is not easily malleable at an individual level, pubertal onset (at the beginning of adolescence) may be a strategic “teachable moment” for adolescents and those who support them, as young people gain increased cognitive capacity and autonomy to make their own health-related decisions. During this time, educators, families, and health care providers could intervene to promote positive choices and behaviors that will influence long-term health.
Ethical statement
All authors (listed on the title page) have made substantial contributions to the study design/analysis and writing/revising the manuscript and have approved the final version. My coauthors and I do not have any conflicts of interest to disclose.
Procedures for data access and analysis were implemented as approved by the Institutional Review Board at the University of Massachusetts, Amherst (with an IAA agreement at Fordham University), and in agreement with the sensitive data security plan approved by Add Health data managers.
CRediT authorship contribution statement
Lindsay T. Hoyt: Conceptualization, Methodology, Writing - original draft, Supervision, Funding acquisition. Li Niu: Conceptualization, Methodology, Formal analysis, Software, Writing - review & editing, Visualization. Mark C. Pachucki: Conceptualization, Software, Resources, Data curation, Writing - review & editing, Funding acquisition. Natasha Chaku: Methodology, Formal analysis, Writing - review & editing.
Declaration of competing interest
None.
Acknowledgements
This research uses data from Add Health, a program project directed by Kathleen Mullan Harris and designed by J. Richard Udry, Peter S. Bearman, and Kathleen Mullan Harris at the University of North Carolina at Chapel Hill, and funded by grant P01- HD31921 from the Eunice Kennedy Shriver National Institute of Child Health and Human Development, with cooperative funding from 23 other federal agencies and foundations. Special acknowledgment is due to Ronald R. Rindfuss and Barbara Entwisle for assistance in the original design. Information on how to obtain the Add Health data files is available on the Add Health website (http://www.cpc.unc.edu/addhealth). No direct support was received from grant P01- HD31921 for this analysis. Research reported in this publication was supported by the National Institute of Nursing Research under Award Number R21NR017154 to Co-PIs Mark C. Pachucki & Lindsay T. Hoyt. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.The authors thank Drs. Jane Mendle and Rebecca M. Ryan for helpful advice on our analytic approach.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ssmph.2020.100549.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- Adair L.S. Child and adolescent obesity: Epidemiology and developmental perspectives. Physiology & Behavior. 2008;94(1):8–16. doi: 10.1016/j.physbeh.2007.11.016. [DOI] [PubMed] [Google Scholar]
- Ahlgren M., Melbye M., Wohlfahrt J., Sorensen T.I. Growth patterns and the risk of breast cancer in women. New England Journal of Medicine. 2004;351(16):1619–1626. doi: 10.1056/NEJMoa040576. [DOI] [PubMed] [Google Scholar]
- Allison P.D. Multiple imputation for missing data: A cautionary tale. Sociological Methods & Research. 2000;28(3):301–309. [Google Scholar]
- Berkman L.F. Social epidemiology: Social determinants of health in the United States: Are we losing ground? Annual Review of Public Health. 2009;30:27–41. doi: 10.1146/annurev.publhealth.031308.100310. [DOI] [PubMed] [Google Scholar]
- Berrington A., Pattaro S. Educational differences in fertility desires, intentions and behaviour: A life course perspective. Advances in Life Course Research. 2014;21:10–27. doi: 10.1016/j.alcr.2013.12.003. [DOI] [PubMed] [Google Scholar]
- Biro F.M., Greenspan L.C., Galvez M.P. Puberty in girls of the 21st century. Journal of Pediatric and Adolescent Gynecology. 2012;25(5):289–294. doi: 10.1016/j.jpag.2012.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biro F.M., Khoury P., Morrison J.A. Influence of obesity on timing of puberty. International Journal of Andrology. 2006;29(1):272–277. doi: 10.1111/j.1365-2605.2005.00602.x. [DOI] [PubMed] [Google Scholar]
- Blanton H. Evaluating the self in the context of another: The three-selves model of social comparison assimilation and contrast. In: Moskowitz G.B., editor. Cognitive social psychology: The princeton symposium on the legacy and future of social cognition. 2001. pp. 75–87. [Google Scholar]
- Blyth D.A. The effects of physical development on self-image and satisfaction with body-image for early adolescent males. Research in Community & Mental Health. 1981 [Google Scholar]
- Brener N.D., Roberts A.M., Mcmanus T., Trott J., Lacy K., Ngaruro A.…Song W. 2012. Results from the school health policies and practices study. [Google Scholar]
- Buttke D.E., Sircar K., Martin C. Exposures to endocrine-disrupting chemicals and age of menarche in adolescent girls in NHANES (2003–2008) Environmental Health Perspectives. 2012;120(11):1613–1618. doi: 10.1289/ehp.1104748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter R., Mustaffa F.N., Leath S. Teachers' expectations of girls' classroom performance and behavior: Effects of girls' race and pubertal timing. The Journal of Early Adolescence. 2017:1–23. [Google Scholar]
- Caspi A., Moffitt T. Individual differences are accentuated during periods of social change: The sample case of girls at puberty. Journal of Personality and Social Psychology. 1991;61(1):157–168. doi: 10.1037//0022-3514.61.1.157. [DOI] [PubMed] [Google Scholar]
- Chaku N., Hoyt L.T. Developmental trajectories of executive functioning and puberty in boys and girls. Journal of Youth and Adolescence. 2019;48:1365–1378. doi: 10.1007/s10964-019-01021-2. [DOI] [PubMed] [Google Scholar]
- Deardorff J., Hoyt L.T., Carter R., Shirtcliff E.A. Next steps in puberty research: Broadening the lens toward understudied populations. Journal of Research on Adolescence. 2019;29(1):133–154. doi: 10.1111/jora.12402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Festinger L. A theory of social comparison processes. Human Relations. 1954;7(2):117–140. [Google Scholar]
- Foster H., Hagan J., Brooks-Gunn J. Growing up fast: Stress exposure and subjective “weathering” in emerging adulthood. Journal of health and social behavior. 2008;49(2):162–177. doi: 10.1177/002214650804900204. [DOI] [PubMed] [Google Scholar]
- Ge X., Conger R.D., Elder G.H., Jr. Pubertal transition, stressful life events, and the emergence of gender differences in adolescent depressive symptoms. Developmental Psychology. 2001;37(3):404–417. doi: 10.1037//0012-1649.37.3.404. [DOI] [PubMed] [Google Scholar]
- Gill D., Brewer C.F., Fabiola Del Greco M., Sivakumaran P., Bowden J., Sheehan N.A. Age at menarche and adult body mass index: A mendelian randomization study. International Journal of Obesity. 2018;42(9):1574–1581. doi: 10.1038/s41366-018-0048-7. [DOI] [PubMed] [Google Scholar]
- Goddings A.L., Burnett Heyes S., Bird G., Viner R.M., Blakemore S.J. The relationship between puberty and social emotion processing. Developmental science. 2012;15(6):801–811. doi: 10.1111/j.1467-7687.2012.01174.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon-Larsen P., McMurray R.G., Popkin B.M. Determinants of adolescent physical activity and inactivity patterns. Pediatrics. 2000;105(6):E83. doi: 10.1542/peds.105.6.e83. [DOI] [PubMed] [Google Scholar]
- Clausen J.A. The social meaning of differential physical and sexual maturation. In: Dragastin S.E., Elder G.H., editors. Adolescence in the Life Cycle: Psychological Change and Social Context. Halstead Press; New York: 1975. [Google Scholar]
- Conley C.S., Rudolph K.D. The emerging sex difference in adolescent depression: Interacting contributions of puberty and peer stress. Development and psychopathology. 2009;21(2):593–620. doi: 10.1017/S0954579409000327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crockett L.J., Petersen A.C. Pubertal status and psychosocial development: Findings from the Early Adolescence Study. In: Lerner R.M., Foch T.T., editors. Biological-Psychosocial Interactions in Early Adolescence. Lawrence Erlbaum Associations, Publishers; Hillsdale, New Jersey: 1987. pp. 173–188. [Google Scholar]
- de Guzman N.S., Nishina A. A longitudinal study of body dissatisfaction and pubertal timing in an ethnically diverse adolescent sample. Body Image. 2014;11(1):68–71. doi: 10.1016/j.bodyim.2013.11.001. [DOI] [PubMed] [Google Scholar]
- Graber J.A., Lewinsohn P.M., Seeley J.R., Brooks-Gunn J. Is psychopathology associated with the timing of pubertal development? Annual Progress in Child Psychiatry and Child Development. 1998;36(12):1768–1776. doi: 10.1097/00004583-199712000-00026. [DOI] [PubMed] [Google Scholar]
- Hakim C. A new approach to explaining fertility patterns: Preference theory. Population and Development Review. 2003;29(3):349–374. [Google Scholar]
- Halfon N., Hochstein M. Life course health development: An integrated framework for developing health, policy, and research. The Milbank Quarterly. 2002;80(3):433–479. doi: 10.1111/1468-0009.00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris K. An integrative approach to health. Demography. 2010;47(1):1–22. doi: 10.1353/dem.0.0091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris K. 2013. The Add health study: Design and accomplishments. Retrieved from. [Google Scholar]
- Hayford S.R. The evolution of fertility expectations over the life course. Demography. 2009;46(4):765–783. doi: 10.1353/dem.0.0073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbert A.C., Ramirez A.M., Lee G., North S.J., Askari M.S., West R.L. Puberty experiences of low-income girls in the United States: A systematic review of qualitative literature from 2000 to 2014. Journal of Adolescent Health. 2017;60(4):363–379. doi: 10.1016/j.jadohealth.2016.10.008. [DOI] [PubMed] [Google Scholar]
- Hertzman C., Boyce T. How experience gets under the skin to create gradients in developmental health. Annual Review of Public Health. 2010;31:329–347. doi: 10.1146/annurev.publhealth.012809.103538. [DOI] [PubMed] [Google Scholar]
- Hoyt L.T., Falconi A.M. Puberty and perimenopause: Reproductive transitions and their implications for women's health. Social Science & Medicine. 2015;132:103–112. doi: 10.1016/j.socscimed.2015.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huddleston J., Ge X. Boys at puberty: Psychosocial implications. In: Hayward C., editor. Gender differences at puberty. Cambridge University Press; Cambridge, UK: 2003. pp. 113–134. [Google Scholar]
- Jacobsen B., Oda K., Knutsen S., Fraser G. Age at menarche, total mortality and mortality from ischaemic heart disease and stroke: The Adventist Health Study, 1976-88. International Journal of Epidemiology. 2009;38(1):245–252. doi: 10.1093/ije/dyn251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James-Todd T., Tehranifar P., Rich-Edwards J., Titievsky L., Terry M.B. The impact of socioeconomic status across early life on age at menarche among a racially diverse population of girls. Annals of Epidemiology. 2010;20(11):836–842. doi: 10.1016/j.annepidem.2010.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson M.K., Crosnoe R., Elder G.H. Insights on adolescence from a life course perspective. Journal of Research on Adolescence. 2011;21(1):273–280. doi: 10.1111/j.1532-7795.2010.00728.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan S.J., Webb P.M., Green A.C. Height, age at menarche, and risk of epithelial ovarian cancer. Cancer Epidemiology Biomarkers & Prevention. 2005;14(8):2045–2048. doi: 10.1158/1055-9965.EPI-05-0085. [DOI] [PubMed] [Google Scholar]
- Kaplowitz P.B., Slora E.J., Wasserman R.C., Pedlow S.E., Herman-Giddens M.E. Earlier onset of puberty in girls: Relation to increased body mass index and race. Pediatrics. 2001;108(2):347–353. doi: 10.1542/peds.108.2.347. [DOI] [PubMed] [Google Scholar]
- Kretsch N., Mendle J., Cance J.D., Harden K.P. Peer group similarity in perceptions of pubertal timing. Journal of Youth and Adolescence. 2016;45:1696–1710. doi: 10.1007/s10964-015-0275-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuh D., Ben-Shlomo Y., Lynch J., Hallqvist J., Power C. Life course epidemiology. Journal of Epidemiology & Community Health. 2003;57(10):778. doi: 10.1136/jech.57.10.778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahme A.M., Stern R., Cooper D. Global health promotion. 2016. Factors impacting on menstrual hygiene and their implications for health promotion. e-pub ahead of print. [DOI] [PubMed] [Google Scholar]
- Lakshman R., Forouhi N.G., Sharp S.J., Luben R., Bingham S.A., Khaw K.T.…Ong K.K. Early age at menarche associated with cardiovascular disease and mortality. Journal of Clinical Endocrinology & Metabolism. 2009;94(12):4953–4960. doi: 10.1210/jc.2009-1789. [DOI] [PubMed] [Google Scholar]
- Lee J.M., Kaciroti N., Appugliese D., Corwyn R.F., Bradley R.H., Lumeng J.C. Body mass index and timing of pubertal initiation in boys. Archives of Pediatrics & Adolescent Medicine. 2010;164(2):139–144. doi: 10.1001/archpediatrics.2009.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee R., Mason A. Is low fertility really a problem? Population aging, dependency, and consumption. Science. 2014;346(6206):229–234. doi: 10.1126/science.1250542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y., Styne D. Influences on the onset and tempo of puberty in human beings and implications for adolescent psychological development. Hormones and Behavior. 2013;64(2):250–261. doi: 10.1016/j.yhbeh.2013.03.014. [DOI] [PubMed] [Google Scholar]
- Marceau K., Hottle S., Yatcilla J.K. Puberty in the last 25 years: A retrospective bibliometric analysis. Journal of Research on Adolescence. 2019;29(1):96–114. doi: 10.1111/jora.12396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maslowsky J., Ozer E.J. Developmental trends in sleep duration in adolescence and young adulthood: evidence from a national United States sample. Journal of Adolescent Health. 2014;54(6):691–697. doi: 10.1016/j.jadohealth.2013.10.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDade T.W., Chyu L., Duncan G.J., Hoyt L.T., Doane L.D., Adam E.K. Adolescents' expectations for the future predict health behaviors in early adulthood. Social Science & Medicine. 2011;73(3):391–398. doi: 10.1016/j.socscimed.2011.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendle J., Ferrero J. Detrimental psychological outcomes associated with pubertal timing in adolescent boys. Developmental Review. 2012;32(1):49–66. doi: 10.1016/j.dr.2006.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendle J., Koch M.K. The psychology of puberty: What aren't we studying that we should? Child Development Perspectives. 2019;13(3):166–172. [Google Scholar]
- Mendle J., Ryan R., McKone K. Age at menarche, depression, and antisocial behavior in adulthood. Pediatrics. 2018;141(1) doi: 10.1542/peds.2017-1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendle J., Turkheimer E., Emery R.E. Detrimental psychological outcomes associated with early pubertal timing in adolescent girls. Developmental Review. 2007;27(2):151–171. doi: 10.1016/j.dr.2006.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mora R. “Do it for all your pubic hairs!” Latino boys, masculinity, and puberty. Gender & Society. 2012;26(3):433–460. [Google Scholar]
- Morales-Chicas J., Graham S. Pubertal timing of latinas and school connectedness during the transition to middle school. Journal of Youth and Adolescence. 2015;44(6):1275–1287. doi: 10.1007/s10964-014-0192-x. [DOI] [PubMed] [Google Scholar]
- Natsuaki M.N., Biehl M.C., Ge X. Trajectories of depressed mood from early adolescence to young adulthood: The effects of pubertal timing and adolescent dating. Journal of Research on Adolescence. 2009;19(1):47–74. [Google Scholar]
- Parent A.-S., Teilmann G., Juul A., Skakkebaek N.E., Toppari J., Bourguignon J.-P. The timing of normal puberty and the age limits of sexual precocity: Variations around the world, secular trends, and changes after migration. Endocrine Reviews. 2003;24(5):668–693. doi: 10.1210/er.2002-0019. [DOI] [PubMed] [Google Scholar]
- Perreira K.M., Deeb-Sossa N., Harris K.M., Bollen K. What are we measuring? An evaluation of the CES-D across race/ethnicity and immigrant generation. Social Forces. 2005;83(4):1567–1601. [Google Scholar]
- Petersen A.C., Taylor B. The biological approach to adolescence: Biological change and psychological adaptation. In: Adelson J., editor. Handbook of adolescent psychology. Wiley; New York, NY: 1980. pp. 117–155. [Google Scholar]
- Prentice P., Viner R. Pubertal timing and adult obesity and cardiometabolic risk in women and men: A systematic review and meta-analysis. International Journal of Obesity. 2012:1–8. doi: 10.1038/ijo.2012.177. [DOI] [PubMed] [Google Scholar]
- Prentice P., Viner R.M. Pubertal timing and adult obesity and cardiometabolic risk in women and men: A systematic review and meta-analysis. International Journal of Obesity. 2013;37(8):1036. doi: 10.1038/ijo.2012.177. [DOI] [PubMed] [Google Scholar]
- Radloff L.S. The use of the Center for Epidemiologic Studies Depression Scale in adolescents and young adults. Journal of youth and adolescence. 1991;20(2):149–166. doi: 10.1007/BF01537606. [DOI] [PubMed] [Google Scholar]
- Rudolph K.D., Troop-Gordon W., Lambert S.F., Natsuaki M.N. Long-term consequences of pubertal timing for youth depression: Identifying personal and contextual pathways of risk. Development and Psychopathology. 2014;26(4pt2):1423–1444. doi: 10.1017/S0954579414001126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seaton E.K., Carter R. Pubertal timing, racial identity, neighborhood, and school context among black adolescent females. Cultural Diversity and Ethnic Minority Psychology. 2017;24(1):40–50. doi: 10.1037/cdp0000162. [DOI] [PubMed] [Google Scholar]
- Simmons R.G., Blyth D.A., Van Cleave E.F., Bush D.M. The emerging sex difference in adolescent depression: Interacting contributions of puberty and peer stress. Development and psychopathology. 1979:948–967. doi: 10.1017/S0954579409000327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpkins S.D., Schaefer D.R., Price C.D., Vest A.E. Adolescent friendships, BMI, and physical activity: untangling selection and influence through longitudinal social network analysis. Journal of Research on Adolescence. 2013;23(3):537–549. doi: 10.1111/j.1532-7795.2012.00836.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- StataCorp . StataCorp LLC; College Station, TX: 2017. Stata statistical software: Release 15. [Google Scholar]
- Stubbs M.L. Cultural perceptions and practices around menarche and adolescent menstruation in the United States. Annals of the New York Academy of Sciences. 2008;1135:58–66. doi: 10.1196/annals.1429.008. [DOI] [PubMed] [Google Scholar]
- Taga K.A., Markey C.N., Friedman H.S. A longitudinal investigation of associations between boys' pubertal timing and adult behavioral health and well-being. Journal of Youth and Adolescence. 2006;35(3):380–390. [Google Scholar]
- Tamakoshi K., Yatsuya H., Tamakoshi A., Group J.S. Early age at menarche associated with increased all-cause mortality. European Journal of Epidemiology. 2011;26(10):771–778. doi: 10.1007/s10654-011-9623-0. [DOI] [PubMed] [Google Scholar]
- Wachter K.W. Biodemography comes of age. Demographic Research. 2008;19(40):1501–1512. doi: 10.4054/DemRes.2008.19.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wachter K.W., Bulatao R. National Academies Press; Washington, DC, USA: 2003. Offspring: Human fertility behavior in biodemographic perspective. [PubMed] [Google Scholar]
- Weichold K., Silbereisen R.K., Schmitt-Rodermund E. Short-term and long-term consequences of early versus late physical maturation in adolescents. In: Hayward C., editor. Gender differences at puberty. Cambridge Univerisity Press; New York, NY: 2003. pp. 241–276. [Google Scholar]
- White R.M.B., Deardorff J., Gonzales N.A. Contextual amplification or attenuation of pubertal timing effects on depressive symptoms among Mexican American girls. Journal of Adolescent Health. 2012;50(6):565–571. doi: 10.1016/j.jadohealth.2011.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White R.M.B., Deardorff J., Liu Y., Gonzales N.A. Contextual amplification or attenuation of the impact of pubertal timing on Mexican-origin boys' mental health symptoms. Journal of Adolescent Health. 2013;53(6):692–698. doi: 10.1016/j.jadohealth.2013.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yermachenko A., Dvornyk V. Nongenetic determinants of age at menarche: A systematic review. BioMed Research International. 2014:371583. doi: 10.1155/2014/371583. 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.