Abstract
We are immersed within an odorous sea of chemical currents that we parse into individual odors with complex structures. Odors have been posited as determined by the structural relation between the molecules that compose the chemical compounds and their interactions with the receptor site. But, naturally occurring smells are parsed from gaseous odor plumes. To give a comprehensive account of the nature of odors the chemosciences must account for these large distributed entities as well. We offer a focused review of what is known about the perception of odor plumes for olfactory navigation and tracking, which we then connect to what is known about the role odorants play as properties of the plume in determining odor identity with respect to odor quality. We end by motivating our central claim that more research needs to be conducted on the role that odorants play within the odor plume in determining odor identity.
Keywords: Olfaction, Odor Identity, Olfactory Quality, Odor Receptor, Odor Plume
1. Introduction
It is an uncontroversial claim that when an olfactory stimulus is transduced at the olfactory epithelium that the odor identified in terms of its quality (i.e. what it smells like) is determined by the molecular structure of the chemical stimulus (Keller, et al. 2007; Kermen, et al., 2011; Khan, et al. 2007; Sanz, et al. 2008). However, there is less of a consensus over how this process occurs. What has become apparent is that the atomic constituents of odors are transduced by the olfactory system in a combinatorial manner (Areneda et al. 2000; Buck and Axel, 1991; Firestein, 2001; Hallem et al. 2006; Malnic et al. 1999; Meierhenrich et al. 2004). The molecular structure of an odorant is encoded by a distribution of multiple olfactory receptor neurons (ORNs) based on their differential sensitivity to the functional groups that compose the chemical compound (Mori and Shepherd, 1994; Shepherd, 2005). Although the leading scientific theories share the view that odor identity is determined by the molecular structure of chemical compounds creating a distributed activity pattern across multiple ORNs, they differ in their accounts of both the precise structural properties and the mechanisms of stimuli transduction in olfactory perception.
The central question of this review is what role the properties of the odor plume play in determining odor identity as ascertained by odor quality. Commonly we identify the gaseous odor plumes permeating our nostrils in terms of their smell, which we shall refer to as odor quality. However, these gaseous plumes are neither uniform entities before we inhale them nor after they enter our nostrils on a sniff as they are transported to the olfactory epithelium where they are transduced by ORNs. Despite the variability of the structure and concentration of the odor plume, we nonetheless perceive odors as having the same smell (odor quality) across these variations. Even though the concentration of the odorants within the plume is constantly in flux we still smell the odor as being the same in a similar manner that fluctuations in perspective or lighting do not affect visual object identification and shifts in volume and pitch do not affect auditory object perception (Wilson et al. 2017).
While it is widely agreed that molecular structures of odorants allows us to individuate an odor, as the same smell across perceptual instances, it is still debatable whether the primary determinate of the identity of an odor (referred to throughout as odor identity), is its property of valence (being a pleasant or unpleasant scent) or olfactory quality. In a series of studies, it has been argued that valence is the perceptible property used by humans to determine odor identity (Yeshurun & Sobel, 2010). Unlike odor- quality categorization, which is similar in various respects, but varies cross-culturally, there is greater agreement on the categorization and identification of odors using their judged properties of being pleasant or unpleasant (Haddad, et al., 2008, 2010). Moreover, Snitz et al., (2013) generated a computational model of odorants that can predict perceived valence from their chemical structure alone. However, competing research indicates that humans more likely identify the perceived identity of an odor in terms of its odorous quality (i.e. what it smells like) (Olofsson et al, 2012, 2013). Additionally, Kumar et al (2015) created an alternative computational model to that of Snitz et al (2013) using descriptors of odor qualities and not judgements of valence, as well as measures of chemical structures to predict olfactory quality. Similarly, Menzel et al’s (2019) test for human olfactory change detection only yielded reliable detection for 24% of the participants, yet across all individuals olfactory quality was detected with greater frequency than concentration, which we take to further solidify the primacy of odor quality for the purposes of odor identity.
The intensity of an odor is similarly linked to the concentration of odorants composing the odor plume, but odor quality, valence, and intensity are perceptually dissociable and might be determined by different sensory mechanism.1 Research on mice further supports the claim that odor quality and intensity are dissociable, since mice display distinct cortical coding strategies for odor identity and intensity (Bolding and Franks, 2017). Moreover, the molecular features of an odorant are correlated with its perceptual qualities in a manner that allows for separable dimension of quality, pleasantness, and intensity (Keller & Vosshall, 2016). Because the perceptual properties of an odor are dissociable between its quality, pleasantness, and intensity it is an open question how the composition of an odor plume might affect our ability for odor identification along each of these dimensions. Yet, for the purposes of this review we only considers odor identity determined in accordance with odor quality following Wilson & Stevenson (2007) and Olofsson et al, 2012, 2013) and not intensity (Giaffar, Rinberg, and Koulakov, 2018) or valence (Haddad et al., 2008, 2010). We think that limiting the literature covered within this review allows for greater clarity, since there is a paucity of research even within the narrow focus on olfactory quality, while we have at best inchoate experimental knowledge of the other dimensions. Moreover, we focus upon olfactory quality, as it is most likely the primary determinate of odor identity for humans and we wish to use a perceptual property of odor identity that is prima facie scalable across animal models from insects to humans and from odor plumes down to their molecular components.
While there is evidence that some properties of the odor plume such as odor composition and concentration impact odor perception, there remains a gap in the chemoscience literature regarding how the molecular structure at the level of receptor sensitivity scales up to odor plumes. While research has been done on the receptor-odor relation relative to different types of chemical compounds (Hallem et al 2006, Mathew et al., 2013, Nara et al., 2011) and odor plumes in terms of olfactory tracking and navigation (reviewed in section 2), determining what properties of the odor plume play a role in generating odor quality is an open area of research. Thus, we wonder what are the smallest chemical changes of an odorant (the individual chemical components of an odor) that we have receptivity to, which might be manipulated to create differences in the composition of the odor plume with measurable effects on odor quality?
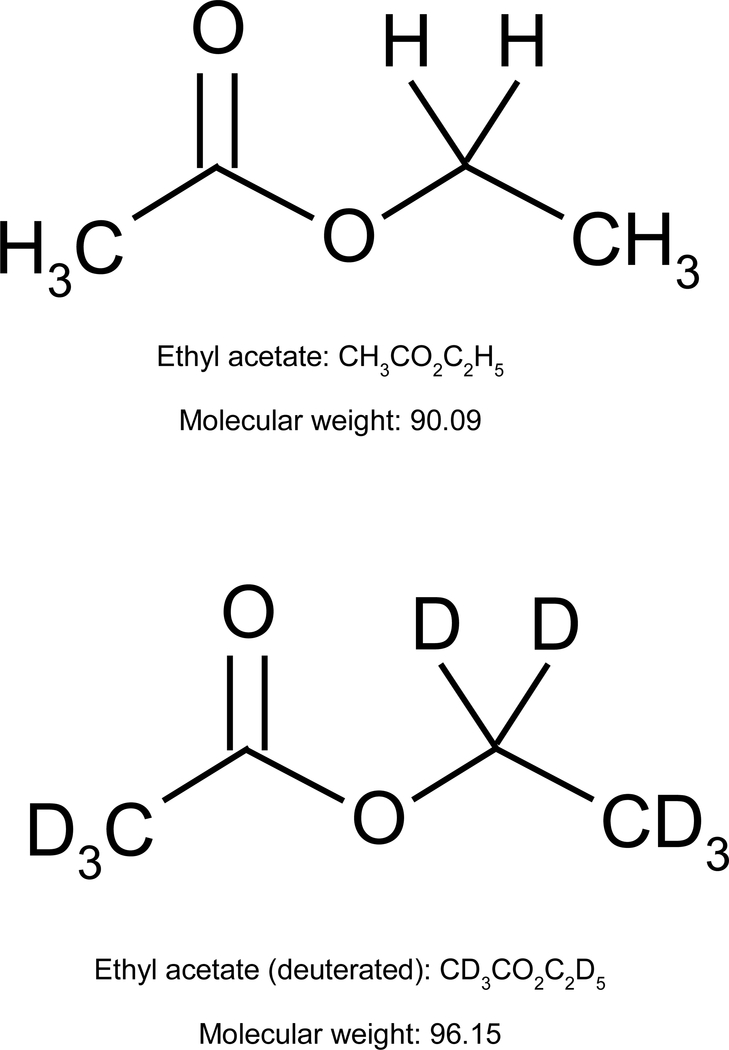
Elsewhere it was hypothesized that properties of the odor plume might play a role in generating odor quality (Young, 2016). By analogy, under laboratory conditions we truthfully assert that H2O is water, yet when considering naturally occurring samples the properties we associate with water of being clear, viscous, and liquid at room temperature are only accounted for by considering large collections of H2O that must also include the isotopes of Hydrogen (Needham, 2000; 2002). Isotopes are variants of an element that differ in the number of neutrons in their nuclei, and hence differ in their relative atomic mass. It is the microstructural features of the collection that we must advert to in any explanation of what is it about H2O that yield the properties that water appears to us as having in our normal everyday interactions with it. We wonder if we can find similar facts to be true of natural odors composed of chemical mixtures — might something as small as isotopic variation of the molecular structures (see figure 1) composing the odor plume partially determine odor quality?
Figure 1.
The figure shows 2D-chemical structures of isotopic variants of Ethyl acetate. Ethyl acetate depicted in the top panel is composed of Hydrogen atoms while the same molecule in the bottom panel is deuterated (composed of Deuterium atoms). Similar to how water is composed of a mixture of H2O and D2O molecules, an ethyl acetate odor plume is likely composed of a mixture of the hydrogenated and deuterated forms of the molecule.
Our wonder regarding the possibility of microstructural variation within the components of an odor plume generating changes in odor quality, brings up a general problem about receptor sensitivity: if as we suppose isotopes within the odorant plume yield shifts in the odors perceptual properties, then we need to first demonstrate receptor sensitivity to such properties of an odorant. To date we have found a small number of studies on olfactory receptivity to isotopic structures, which can be detected and discriminated by honeybees (Gronenberg et al. 2014a, 2014b; Paoli et al. 2016), fruit flies (Bitner et al 2012; Drimyli et al 2016; Franco et al 2011; Solov’yov et al. 2012), and humans (Gane et al. 2013; Reese et al. 2016; Solov’yov et al. 2012). However, to the best of the authors’ knowledge none of these odorants have been used in studies researching these odorants within plumes of varying concentration of the single odorant or in combinations with other components. Thus, the receptivity to microstructural properties of odorants exists, but their effects on odor identity within odor plumes remain unexamined.
The purpose of this review is to raise questions, not to answer them; to draw attention to a potential inquiry in the field of olfactory perception, rather than to survey the field fully; and to provoke discussion rather than to serve as a comprehensive or systematic review of the literature. We will primarily focus upon research on insect olfaction within the sections that review odor plume tracking and navigation, as well as perceived odor identity across shifts in concentration of the odor plume. However, we will also draw upon research on avian, aquatic, and mammalian olfaction, when these further clarify or support the central claim of a section. Given our motivation to initiate further discovery of what is known about the role that properties of the odor plume play in determining odor identity, the paper reviews three key research areas relevant to this issue. First, what is known about the role perceived concentration gradient and plume structure plays in olfactory navigation and tracking? Based on review of the literature in section 2, we conclude that successful olfactory tracking and navigation requires representation of the olfactory plume as an object over and above the mere representation of the concentration of its components. Second, we review how varying concentrations of the constituents of the odor plume affect perceived odor identity? The studies surveyed in section 3 suggest that the concentration of the odorants composing the plume affects odor identity. Third, we hone in on more minute properties that might occur within the composition of the odor plume by considering what is known about how the composition of odorants within an odor affect odor identity as they form olfactory mixtures. Section 4 highlights that the components of an odorant mixture affect odor quality, yet there is little to no research closing the gap on the determinates of odor quality as these odorous mixtures scale from objects transduced at the receptor level to the extended odorous plumes. Since it is an open question if isotopic or isotopomer (isomers having the same number of each isotope of each element but differing in their positions) variations within the composition of the odor plume might play a role in determining olfactory quality, we suggest employing these stimuli (see figure 1) as a starting point for researching the question of how the properties of odorants scale up to determine odor identity of complex odorous plumes.
2. Structure of Olfactory Plumes and Animal Navigation
Studying naturally occurring odorants requires explaining an organism’s ability to individuate and navigate through a sea of chemical currents while tracking a particular odor plume. Odor-plumes are created when odor molecules are released from their source and are taken away in the wind. Odor-plume structure is determined by the physics of atmospheric dispersion. Since air, like water, is a fluid, it is governed by the same physical laws. Thus, fluid dynamics impinge directly upon the distribution of odor molecules in time and space. Two processes working at different temporal and spatial scales are at work: (a) molecular diffusion is a slow and small-scale phenomenon in which random motion of the molecules causes them to move gradually apart; and (b) turbulent diffusion in which the cloud of molecules is physically torn apart by air turbulence. Turbulent diffusion is the major determinate of odor plume shape, size, and segregation into filaments, as it is a vigorous process that covers a wide range of temporal and spatial scales. If an odor source is smaller than the Kolmogoroff scale (at the Kolmogoroff scale, viscosity dominates and the turbulent kinetic energy is dissipated into heat), odor molecules released into the wind form a filament that expands slowly by molecular diffusion until it reaches the size of the smallest eddies. During this period of molecular diffusion, the development of the plume depends on the characteristics of the odor molecules. Thus, plumes of different materials may behave differently. After molecular diffusion, the rapid and vigorous process of turbulent diffusion takes over. The turbulent nature of the wind creates a complex flow environment and causes the filaments to propagate in the form of patches with varying concentration distributions. An early account dealing with odor plumes can be found in Dusenbery (1992), who documents how the plume and its filaments can be affected by the wind of the surrounding environment, dispersal rate, and the types, velocity, and directions of turbulence. His treatment offers a rich account of how different environments generate diverse plumes with changing turbulence dynamics and filaments. For those further interested in the physics of odor plumes, Murlis et al., (1992) provides an in-depth analysis on the topic.
The structure of the filaments of the odor as well as patches of odorless air within the plumes as generated by the size and shape of the odor sources, as well as the structure of the local environment play a role in shaping the organisms tracking behavior. While it might be thought that the organism need only track the specific odor and ignore the intervening scents, more recent research indicates that environmental odors are also used by insects and migratory birds for the purposes of odor tracking and navigation. Safi et al (2016) employing a multidisciplinary approach including computational modeling suggests that the migratory birds use encounters of gradients of localized odors from stopover sites when displaced from their normal migratory path. Their research suggests that avian olfactory navigation might employ an odor map of environmentally significant smells that can be used to maintain a migratory path.2 While avian olfactory navigation might use environmental odors as a means of localization, Webster and Carde (2017) review evidence that insects use habitat odors as cue in locating host scents during odor tracking and navigation. Moreover, they stress that research should be sensitive to the differences between laboratory conditions and natural environments as these will generate discrepancies in our knowledge of odor plume tracking and navigation
Additionally, Murlis, Elkinton, and Carde (1992) review the effects of the odor plume’s structure on insect navigation with a focus on the importance of turbulence causing the shape of the plume based on fluctuations in odor concentration. Not only do plumes composed of different odorants behave differently, but the size and structure of odor-free patches within the plume that are causally generated by the size of the odor cloud also play a significant role in insect odor tracking. While these separate pieces of research offer a wonderfully rich overview of how environmental factors generate different plume structures, they do not cover how the composition of the odorants within the odor plume affects perceived olfactory quality. In what follows, we review what is known about how insects can segregate the odor plume from amongst the sea of chemical currents composing the olfactory environment. Whenever appropriate, we cite research done in avian species, invertebrates, and mammals in order to provide further supporting evidence for our claims.
2.1. Role of Background Environmental Odors in the Olfactory Plume
One interesting factor of olfactory perception within natural odor environments are the effects that background environmental conditions have upon an organism’s ability to perceive the odor plume. For instance, increased ambient temperature negatively impacts the tracking ability of gypsy moths (Charlton et al. 1993). Similarly, shifts in the environmental prevalence of carbon dioxide negatively impact the Smooth dogfish’s (Mustelus canis) olfactory tracking abilities (Dixson et al. 2015). Also, background odorants might be used as a possible mask for the attractant. Leaf Hopper insects have difficulty detecting an attractant odor if the background odorants share chemical structures. Likewise, changing the concentration of background odorants can mask an odor plume (Cai et al. 2017). For Drosophila larvae, when the target and background odor elicit similar receptor activity patterns the stimulus becomes difficult to distinguish from the background smellscape (Kreher et al. 2008). Unsurprisingly, these studies indicate just a few of the different ways in which environmental odorants and background conditions have a causal impact upon the receptivity and ability of an organism to segregate a target from against a background of overlapping chemical or thermogenic currents.3 To address the more general issue of how the gaseous plume is perceived, we now turn to a survey of odor plume receptivity to show that organisms have the sensitivity necessary to track the concentration gradient of the components of the odor plume. Moreover, we show based on some suggestive research on olfactory tracking and navigation, that the plume is represented not just in terms of the concentration gradients of its filaments, but as a superordinate entity.
2.2. Sampling the Olfactory Plume
Perhaps the most pressing variable in determining how organisms navigate odorous environments is the base olfactory sampling rate in relation to odor plume concentration diffusion. The olfactory system encodes average concentration rates, as well as the degree of change in the concentration and the ratio of the constituents’ concentrations (Webster and Weissburg, 2001). The sensory sensitivity of the olfactory system can sample from the filaments and changes in concentrations of an odor plume. Additionally, the kinetics of insect sensory neurons for tracking filaments can occur within less than 2 ms and fluctuations of odor stimuli can be resolved at a frequency more than 100 hz (Szyszka et al. 2014). Thus, insect antennas are capable of fine-grained temporal resolution that might be necessary in tracking concentration shifts within a filament making it plausible that they have the necessary resolution to perceive the olfactory plume.
However, not all organisms have such detailed receptivity for tracking the minute changes of the concentration gradient within an olfactory plume. Yet, fine-grained resolution of minute shifts within the concentration might not be required. Boie et al (2018), using an information theoretic approach, quantified the amount of information of the concentration gradient within naturalistic odor plumes that would be required for efficient odor tracking. Their model shows that having multiple sensors increase the ability to encode information about the concentration gradient of a plume. Efficient odor tracking and navigation can be accomplished by multiple sets of receptors encoding samples of the concentration gradient at a higher rate but lower resolution. Arguably this shows that organisms do not require fine-grained sensory transduction resolution that encode the exact concentration of a filament. Given that the receptivity and encoding of odor plume concentrations is possible for organisms, we now turn to olfactory tracking and navigation, which requires the odor plume to be represented as a perceptual entity within a gaseous sea of chemical currents.
Before turning to odor plume navigation it is worth noting that organism need not only track concentration gradients and encounters with the odor plume, but they also require mechanisms for temporal processing in generating a representation of an olfactory scene. Tracking and navigating a gaseous sea of chemical currents requires representing the temporal encounters of odor plume, as well as receptivity to concentration gradients and encounters with plumes. The mechanism for encoding temporal information about turbulent odorant plumes are suggestive that the plume is not merely represented based on encounters with filaments and mean concentration of the target odor within these encounters, but the organism also represents the odor plume as a developing structure across time. For instance, during odor plume navigation the organism is sensitive to the temporal frequency between encounters with the odorant and intervening clean air of environmental odors (Vicker et al 2001, 2006; Park et al 2016), as well as the intermittency of odorant plume encounters (Crimaldi et al 2002). Building upon this research Ache et al (2016) provides evidence that animals could employ groups of rhythmically active ‘bursting’ olfactory receptor neurons (bORNs) to encode temporal information that is intrinsic to the distal olfactory stimulus. The added mechanism of tracking the temporal structure within the olfactory stimulus they claim would be a necessary component in olfactory scene analysis. Similarly, Park et al (2016) use a computational model constructed from natural turbulent plumes to show that temporal information about the plume can be used by bORNS alone to locate the odor source. Thus, temporal properties of the filament arrivals (odor intermittency) of the olfactory plume must also be taken into account for efficient olfactory navigation.
The basic organization of early olfactory circuits is conserved from insects to mammals (Ache, 2005) such that the bORNS are found in arthropods, amphibians, and mammals (Park et al, 2016). However, both Ache et al (2016) and Park et al (2016) note that insects do not exhibit bORNs and as such must employ some alternative mechanism for representing the temporal structure of the odorant plume. While insect might not employ the same neuronal mechanisms, in the next section it will be shown that representing the temporal structure of the odor plume is just as fundamental for insect odor tracking and navigation. We take it that both the necessary receptivity to the concentration gradient of the odor plume and the encoding of the temporal dynamics of the plume suggests that successful odor tracking and navigation requires representing the odor plume as an extended spatiotemporal entity across a range of properties which will be enumerated in the next section.
2.3. Tracking and Navigating the Olfactory Plume
The ability to track an odor plume through an environment requires both the ability to segregate the target stimulus from the background, as well as to track the plume through changes in its concentration gradients. Drosophila larvae can differentiate two concentrations of an odorant and track it within a Y- shaped navigation task. Additionally, larvae have distinct receptor neurons for representing and tracking sensitivity to odor concentration (Slater et al. 2015). Despite having the sensor sensitivity for all ranges of concentration, Grapholita oriental fruit moths show a preference for only tracking intermediate levels of odor concentration (Baker et al. 1981). Moths possess neurons that track the individual filaments of a plume (Lei et al. 2009). They have central processing circuitry that tracks the overall component mixture to allow the moth to track odor plumes (Martin and Hildebrand, 2010). Moreover, the temporal resolution of Helicoverpa zen moth receptors can distinguish between odor sources 1 mm apart in space and 0.001 s in time (Fadamiro et al. 1999). While moths have the sensitivity to track component filaments, for Heliothis tobacco budworm moths to effectively track an odorant the entire mixture blend must synchronically reach the sensors (Vickers and Baker, 1992). Taken together these studies suggest that some species have the sensitivity to track the concentration gradients of a filament in an odor plume and represent it as a perceptible object.
Since natural olfactory environments are dynamic and unpredictable, organisms require various sensory cues to navigate towards odor sources. One navigational strategy employed by flies is osmotropotaxis, where the measurement of concentration differences allows the fly to face the direction with the highest concentration. Another strategy employed by flies is optomotor anemotaxis, which describes the insect’s tendency to fly upwind when odors are sensed, and then turn in a zigzagging fashion known as casting (Herrero, 2012). Another instance of this type of strategy is seen in the tobacco budworm fast-acting, phasic-tonic, surging-cast, response system that reacts when in contact with pheromone filaments (Vickers and Baker, 1994). Similarly, to locate an odorant, male Pectinophora gossypiella (pink bollworm moths) sense boundaries of an odor trail during their navigational casting (Farkas and Shorey, 1972). In laboratory conditions using a sustained flight tunnel, Kuenen and Baker (1982) showed that oriental fruit moths flew at lower net-ground velocities when navigating towards higher pheromone concentration gradients. Additionally, the moths turning frequency was greater with increased pheromone concentration, while the distance of the turns relative to the plume axis decreased. While in some instances Dogs and Humans utilized a type of casting (Porter et al. 2007), terrestrial odor navigation usually requires a path selection in a familiar environment. For example, Rats employ a run-and-scan mechanism for tracking airborne odorants. Rather than casting, they run directly toward an odor source, and if incorrect, they continue to serially sample other possible odor source locations (Bhattacharyya and Bhalla, 2015). These differences between navigational strategies indicate that perception of the structure and shape of the odor plume might vary between species.
Despite the difference of navigational strategies and perceptual abilities in detecting the olfactory plume, there is preservation of an anatomical strategy to handle the perception of disparate filaments of olfactory plumes. The prevalence of two symmetrical olfactory sensors is a reoccurring structure shared by both vertebrate and invertebrate organisms (Gomez-Martin et al. 2010). The function of a stereo or bilateral olfactory input for gradient detection plays a crucial role in localizing a specific odorant source. For instance, the use of a right and left antenna in honeybees allows for the discrimination between higher and lower concentration gradients and aids in casting towards the odorant source (Martin, 1965). Honeybees with crossed left and right antennae exhibited spatial confusion in orientation tasks. Similar results have shown that fruit flies require bilateral sensory input to navigate towards odor sources.
Measurements of the physiological properties of Drosophila’s ORNs are conducted in intervals of 2s or less, yet the rate at which flies encounter an odorant pulse is not well documented. One study tested the effects of various concentrations of CO2 on fruit flies and discovered that CO2-sensitive neurons are specifically tuned to small concentration changes, despite the presence of background CO2 levels (Faucher et al. 2013). Considering that flies tend to steer laterally towards the highest concentrated odor plume, the same researchers found that by obstructing sensory input to one antenna, this ability was eliminated. Moreover, their research provides further evidence of sensory lateralization, where signals from the left antenna played a larger role in odor tracking than that of the right antenna (Duistermars et al. 2009). Unlike adult Drosophila, the larvae do not rely on bilateral input. Larval chemotaxis can occur with a single functional olfactory neuron. However, the accuracy of odor navigation is enhanced with bilateral sensory input (Louis et al. 2008). Bilateral odor input is not always necessary to track an odorant source. American cockroaches with only one antenna can locate the source of an odorant plume, but the length of the antenna influences odor tracking ability more than any particular segment of the antenna (Lockey and Willis, 2015), suggesting that bilateral detection is not the only mechanism responsible for encoding the plume structure - access to the temporal properties of the plume also play a role, which further confirms the claim in section 2.2 that encoding the temporal dynamics of the odor plume is a fundamental component of odor navigation.
The use of (symmetrical) bilateral inputs for odor tracking and navigation is employed to such an extent across species that Patzke et al (2010) provide suggestive evidence of the lateralization of homing ability relative to use of the right nostril. Their findings in homing pigeons provide further evidence for the avian olfactory navigational hypothesis, such that the left and right olfactory systems provided different information for navigational purposes. While this level of lateralization has not been documented in mammals, stereo sampling using both nostrils is also seen in mammals, Using American moles, Catania et al (2012) showed that while blocking a nostril does not disrupt the ability to locate a distant odor sources it does disrupt local odor plume sampling. And though it is mostly speculative, Jacobs (2019) provides a strong argument based on interdisciplinary research that the structure of human nose first evolved for navigational purposes then through local selection pressures shows ecological specialization and eventually decreased in size to primarily identify local odors and not distal environmental odor plumes. Based on these cross-species comparisons it becomes apparent that organisms must perceive disparate parts of the odor plume in tracking a particular odor through the environment, yet it is a further question whether organisms must represent the plume structure and not merely its component parts.
Successful navigation depends upon the temporal and spatial encoding of the plume’s properties by the olfactory system. But additionally, there is a growing body of evidence that insects represent the shape, structure, and composition of the odor plume suggesting that the plume itself is treated as a superordinate object independent of the concentration gradients of the odorants within the filaments. Moths (male Pectinophora pink bollworm moths) have been shown to sense the overall shape of a pheromone plume in navigating towards the location of an odorant (Farkas and Shorey, 1972). Additionally, olfactory navigation strategies of moths vary in accordance with the fine-grained structural characteristics of the plume (Mafra-Neto and Carde, 1994). Both the navigation strategy and success of navigating towards the odor source vary with the structure of the odor plume, which Mafra-Neto and Carde (1994) take to indicate that the moths interaction with the structure of the plume plays an integral role in their flight guidance system. Their further research on Cadra cautella (almond moths) indicates that flight tracks of male moths to point sources of a pheromone are dependent upon an increase in filament encounters. The faster the frequency of pulses and the greater the volume of the plume, the more males would respond (Mafra-Neto and Carde, 1995), which shows that fine scale changes within the plumes turbulence affected male Cadra cautella flight patterns in navigating to a point source.
The studies on moth odor navigation indicates that the structure of the odor plume plays a role in odor navigation, but it does not directly address how the components of the plume or shifts in concentration of the odorants might affect perceived plume identity. Also, their results are suggestive that the odor plume is represented as a superordinate object, yet moth navigation towards pheromones is known to be a highly selective ballistic behavior. Additionally, pheromones are often a simple chemical complex and not indicative of the highly complex scents composed of multiple components that form naturalistic odor plumes. To address these kinds of issues Dekker, Takken, and Carde (2001) studied female mosquitos’ (An. gambiae and Ae. Aegypti) olfactory navigation behavior within odor plumes of varying structures, concentrations of component parts, and types (simple and complex smells). Their results showed that both the structure of the plume (homogenous or pulsed) as well as the concentration of odor plume, within dual-choice olfactomotor, affected the mosquitos’ behavior. Plumes formed from homogenous skin odor increased the mosquitos trap capture, whereas homogenous plumes of CO2 reduced trap capture. Additionally, their findings showed that homogenous CO2 trap capture was concentration dependent and variable across mosquito species. Taken together their results indicate that the composition, structure, and type of odor plume are all variables that need to be accounted for independently in studying insect odor olfaction. For our purposes, these series of studies on insect olfaction make it clear that properties of the odor plume clearly play a determinate role in generating perceived odor identity.
The encoding of temporal information about odor plumes in natural encounters requires further research. But in a lab-made plume, researchers utilized a parameterized computational model of the Panulirus argus (caribbean spiny lobster) to show that lobsters use groups of rhythmically active neurons to encode temporal data for olfactory navigation (Park et al. 2016). Their model indicated that spatial-temporal distinctions in the odor plume signify to the organism that odorants do not share the same source. In relation to the utilization of both spatial and temporal encoding, Willis (2008) addressed the question of how plume trackers sample odor information using mobile robots as models of biological trackers. They concluded that both for walking and in-flight plume tracking the structure of the odor plume modulates tracking behavior (Willis, 2008), suggesting that successful navigation requires representing the odor plume as a spatiotemporal entity over and above the constituent odor filaments and concentration gradients. We take the host of studies above on avian, aquatic, and insect olfaction to indicate that successful navigation and odor tracking requires a host of different, yet similar, capacities that represent not only the concentration of the odorants within the plume, but also the composition of the odor, the structure of the plume, and its temporal dynamics. Thus, the odor plume is treated as a superordinate entity within a background of alternative environmental odorants. While there is a growing amount of evidence across species to support our claim that odor perception requires the representation of the odor plume as a perceptible entity, it is less clear that humans have such capacities and that they perceptually represent odor plumes.
While it is unclear if humans can perceive odor plumes as superordinate perceptual entities, we can learn specific locations using olfactory cues. We have an odor memory system for olfactory spatial representation that can be employed to relocate the placement of odors within a spatial environment. Under laboratory conditions it has been observed that we have the capacity for olfactory space representations, indicating the existence of residual directional smelling abilities for humans based on our ability for spatially encoding smells (Moessnang et al. 2011). Additionally, visually impaired individuals employ olfactory cues in locating an object’s proximity or as a point of reference in determining their spatial location within an environment, but not for the purposes of actively sampling in navigating to these locations (Koutsoklenis and Papadopoulos, 2011).
In open field environments, subjects can localize the source of an odor using just the olfactory system without trigeminal stimulation. The use of both nostrils made no difference for locating the stimulus at two meters or less from the subject. But, beyond two meters the use of both nostrils to demarcate the concentration of the plume helped in localizing the smell (Welge-Lussen et al. 2014). Moreover, an earlier study showed that humans have the ability for olfactory tracking in an open field environment (Porter et al. 2007), yet it is unclear if humans can employ this ability for navigation by only employing the olfactory system in perceiving the structure of the olfactory plume.
Jacobs (2012) argues that the primary function of olfaction is for navigation given that the scaling of the olfactory bulb does not obey the same rules as other cortical tissues across species if it were merely evolved for sensory discrimination. His theoretical claim was partially substantiated in a laboratory experiment in which humans learnt the location of a stimulus using only olfaction and navigated back to the odor’s location using multimodal cues (Jacobs et al. 2015). Moreover, it has been recently documented that olfactory identification and spatial memory are cortically associated in the orbital frontal cortex (OFC) (Dahamani et al, 2018), which is thought to be the primary olfactory cortical processing center in humans. Not only are spatial memory capacities and olfactory indemnification linked in the OFC, but Dahamani et al (2018) document cases linking deficits in olfactory identification and spatial memory as shown by lesions of the medial OFC. However, none of these studies address the role of the components of the odorant plume in determining humans’ ability to track an odor or navigate our environmental smellscape. Thus, it is an open question if properties of the odor plume considered as a superordinate object of perception yield differences in odor identity for humans. Given that tracking the concentration of the component odorants of a plume’s filament plays an essential role in olfactory tracking and navigation, we now turn to a review of how the components of an odor yield shifts in odor quality based on changes of the concentration levels of the constituents alone.
3. Olfactory Plume Properties that Impact Perceived Odor Identity: Concentration Effects
Concentration effects provide a ripe area for studying how properties of the component parts of the plume affect odor quality, since the chemical nature of the constituent odorants remain constant, while the nature of the plume is manipulated. Since the last section established that the odor plume is perceived as a superordinate entity beyond the mere concentration gradient of the component filaments, in this section we review what is known about concentration effects in multicomponent, binary, and single component odorant mixtures to establish what is currently know about how manipulations of the component properties of the plume, aside from the molecular identity of the component odorants, impact upon odor quality. The section concludes with a survey of what is known about concentration invariance and suggests that it is a learned capacity that requires the representation of types of odor plumes.
3.1. Multicomponent Odors
Odors composed of multiple different types of odorants indicate that properties of the composition of the olfactory plume impacts upon perceived odor quality. For instance, Weiss et al. (2012) showed that changes in multicomponent concentration levels yield shifts in olfactory quality for humans. Similarly, large-scale shifts of concentration using multicomponent mixtures change the perceived olfactory quality of an odor for Drosophila (Masek and Heisenberg, 2008).
While this might suggest that the concentration of all the components of the mixture are responsible for the shift in olfactory quality, Reinhard et al. (2010) showed that honeybees only tracked a subset of key components and their concentration rates among multi-component floral odorants. Additionally, increasing the concentration of an individual constituent yielded the effect that the bee treated that odorant as a key component of the mixture.
Similarly, even minute additions of a thin CO2 filament within an odor plume derived from human skin rapidly modulates mosquito flight behavior. Female Aedes aegypti mosquitos show low responsiveness to skin odor and high sensitivity to CO2, yet they become more sensitive to skin odors after a brief encounter with a CO2 filament (Dekker, Geier, and Carde, 2005). While these odor plumes might not be thought of as a complex odor mixture, nevertheless mosquitos are using the odor combination of skin odor and CO2 as indication of live human host, thus we infer that they are treating it as an odor object for their ecological purposes. In a further set of experiments Dekker and Carde (2011) measured female Aedes aegypti mosquitos’ differential flight patterns in wind tunnel relative to structure and composition of odor plumes. Their results indicate that even brief encounters of thin CO2 filaments generate rapid upwind surge. However, when presented with skin odors the mosquitos required longer continuous exposure for homogenous plumes to modulate flight behavior. These findings lead them to conclude that strategies for odor navigation and tracking are not unified even within a species and might even differ between stimuli within a species.
The research surveyed above displays how the compositional properties of complex odor mixtures play a role in segregating and demarcating the relevant components of an odor mixture. These studies taken together show that it is not just the molecular structure of odorants that play a role in generating odor identity, but also the composition and concentration of the constituents of a complex mixture as they compose the odor plume.
3.2. Binary Odor Mixtures
Similar findings to those seen in complex mixtures have been shown for mixtures of two different types of odorants. Bactrocera dorsalis (oriental fruit flies) can be conditioned to be sensitive to changes in concentration in binary mixtures (Liu et al. 2015). Additionally, Drosophila show a sensitivity to changes in concentration of binary mixtures within a hundredfold change in concentration (Borst, 1983). Changing the concentration of one or both components of binary mixtures shifts judged olfactory quality for Rats (Weiss et al. 2012). Similar results have been demonstrated looking at concentration variance based on judgments of molar ratios between components of an odor (Uchida and Mainen, 2008). These studies further substantiate the claim that compositional properties of the odor plume, in particular the concentration of the component odorants, play a role in determining odor identity.
3.3. Single Component Odors
Single component odors yield similar findings that shifts in concentration generate changes in odor identity. The additive advantage of single component odors is that we cannot attribute the shift of odor quality to the molecular features of the component odorants. Rather, the change in identity must be explained relative to the density of the odor plume as it interacts with the sensory transduction mechanism. Thus, the odor treated as a gaseous plume plays a role in generating perceived olfactory quality. One example of concentration shifts in single component odors occurs in Drosophila, who have a natural avoidance mechanism for carbon dioxide, which they learn to overcome in detecting ripening fruit. While this is a simple chemical entity, fruit flies track the changing concentrations of volatiles in fruit as it ripens (Turner and Ray, 2009). The flies track the plume of CO2 across its changes in concentration. Moreover, different species of fruit flies show distinct sensitivities to shifts in concentration for CO2, depending upon their preference of ripening stages (Pham and Ray, 2015). Additionally, in Drosophila, classical conditioning can be employed to train avoidance towards changes in concentrations (Tully and Quinn, 1985). Lastly, mosquitos show exquisite sensitivity to concentration shifts in CO2 with fluctuations as small as 40 ppm causing shifts in receptor sensitivity (Grant and O’Connell, 1996) that allows for their navigational ability upwind towards a host within a fluctuating concentration gradient. Moreover, mosquitos show differential sensitivity to the structure and concentration of a CO2 plume, such that even minute fluctuations within CO2 concentrations can generate flight tracking behavior towards a host. For instance, because of the ubiquitous human body odor, females of the malaria mosquito, Anopheles gambiae use fluctuations in CO2 concentration as means of inferring the presence of a living human (Webster, Lacey, and Carde, 2015). Similarly, shifts in concentration using aliphatic molecules yield changes in perceived olfactory qualities for Humans (Laing et al. 2003). Despite these results of concentration effects across types of mixtures and species, shifts in olfactory quality as attributable to shifts in concentration are reported as outliers rather than the norm - one explanation for this might be learnt concentration invariance.
3.4. Concentration Invariance
Concentration invariance is when the organism treats varying concentrations of the same chemical compounds as having the same odor identity. Three possible explanations (aside from those already surveyed in the introduction for intensity invariance) might be offered for concentration invariance. The first explanation is that the receptors lack sensitivity to concentrations and encode a single type of odor (Frederick et al. 2009). Another explanation is that concentration invariance is due to coarse coding either at the olfactory bulb or cortical levels of processing (Asahina et al. 2009; Fried et al. 2002; Münch et al. 2013). The most likely explanation is that it is a learned effect. Naive animals treat varying concentrations as different and based upon ecological needs learn that the different concentrations of an odor plume still indicate the presence of the olfactory source object (Uchida and Mainen, 2008). A recent study suggestively supports this explanation with the addition that organism also require the ability to encode and represent the environmental concentration of the odor plume independent of the odorants’ concentration at the receptor sites, because the act of sniffing can cause shifts in perceived concentration. Jordan et al (2018) showed that in mice fast sniffs alone can shift the concentration of an odor making the odor percept equivalent to that of a strong environmental concentration. Yet, mice employ an initial sniff modulation to sample the environmental concentration independent of future sniff variance. Not only does this show that inhalation dynamics must play a role in inferences about odor concentration and the odor identity thereof, but also that organism can learn that identical odor concentration percepts might not signify the same informational value regarding odor identity.
Concentration invariance for binary and complex odor mixtures is relative to the organism’s ability to track the molar ratios of the components of the olfactory plume. Using experimental manipulations of the ratio between the components (Cleland, 2008; Cleland et al. 2011; Uchida and Mainen, 2008) or by changing the concentration of one of the components (Le Berre et al. 2008a; Le Berre et al. 2008b; Sinding et al. 2013; Sinding et al. 2014), it can be demonstrated that the organism no longer treats the mixture as having the same odor identity. In cases of single odorant stimuli, naïve honeybees and mice consider changes in concentration as differences in olfactory quality (Choudhary, 2009; Cleland et al. 2011). However, an outlier to the hypothesis that concentration invariance is a learnt ability, is the recent work by Asahina et al. (2009) showing that naïve Drosophila demonstrate concentration invariance that is not learnt but mediated by local interneurons. Aside from the study by Asahina, concentration invariance is most likely explained as a learnt capacity, suggesting that changes in the plume generate changes in the perceptible object that require the organism to learn over time that these disparate odor plume entities are nonetheless of the same type. We take the evidence of the past two sections to suggest that the odor plume is treated as a superordinate object by the olfactory system and moreover the properties of its composition independent of the molecular features of the chemical components play a role in generating perceived odor quality. In the next section, we turn our focus to what is known about how properties of odorant mixtures yield changes in odor quality to further hone in on our wonder if minute properties of the odorants composing the plume, such as isotopic variants of individual odorants, might play a role in determining odor identity.
4. Olfactory Plume Properties that Impact Perceived Odor Identity: Olfactory Mixtures
Combinations of different types of odorants yield an olfactory mixture - mixtures can generate different odor qualities by varying the kinds of odorants used, ratio between the odorants, and concentration level of each component. Moreover, olfactory mixtures have been generated with complex combinations of up to and exceeding thirty components. Rather than start with simple mixtures and work up to larger types of olfactory mixtures, we begin with the large-scale component process for multi-molecular odor mixtures and progress to binary and unitary stimuli, given our stated motivation of attempting to show that even micro-structural chemical properties might influence odor identity.
4.1. The perception of olfactory mixtures is a component process
The perception of olfactory mixtures is a component process whereby the olfactory system organizes the constituent chemical compounds into a uniform smell. When two or more odorants are combined into a complex odor, one of two possible mixtures results: a configural mixture whose odor quality differs from the smell of the components, which are not discernable as constituents of the new smell; or an elemental mixture whose smell is the additive combination of the components, whose individual odor qualities are discernable within the complex (Berglund, 1973). Configural mixtures are particularly fascinating, since the mixture’s odor quality is not determined as an additive process such that one cannot predict the new smell from its individual components (Berglund, 1973).
Initial research on rodents indicated that perceptually similar odorants yield configural mixtures, while dissimilar odorants yield elemental mixtures (Wiltrout et al. 2003). However, it has since been established that the resultant quality of an olfactory mixture is better accounted for by receptor sensitivity to molecular features of the odorant (Kay et al. 2005). Mixtures formed by odorants with similar molecular structures activate similar sets of receptor neurons thereby generating configural mixtures, while those differing in structure yield elemental mixtures suggesting that the synergistic properties attributed to the gaseous cloud might be accounted for in terms of receptor transduction and not the plume. However, there might be reason to think that gaseous plumes also play a role, because similar and dissimilar components can yield both kinds of mixtures depending upon the concentration levels of the constituents (Kay et al. 2005). By varying the concentration of odorant components, one can influence whether the complex mixture will be perceived as configural or elemental (McNamara et al. 2007) suggesting that the overall gaseous object as demarcated by its concentration also plays a role in determining odor quality.
4.2. Olfactory transduction and perception of multi-component mixtures
Although it was commonly thought that odorants were coded in a coarse manner at the receptor and olfactory bulb (Fried et al. 2002; Asahina et al. 2009), Vincis et al. (2012) showed that these results are attributable to the odorant and anesthetized state of the organism. Under natural conditions using ordinary odorants they recorded robust fine-grained representations within the glomeruli of the olfactory bulb in mice. Despite these results, several studies using rodents show completion effects whereby in multi-component mixtures the olfactory system either shows the same coding despite the absence of a constituent or a change to the constituents. For instance, Johnson et al. (2010) showed that olfactory coding within the olfactory bulb represents the major molecular features of the stimulus. Yet, for some complex mixtures the coding was less complex than would be expected if all the molecular features were represented, which indicates that only major constituents of the complex are being represented. Their results confirm previous findings (Johnson et al. 2007) that the encoding of molecular features primarily maps the major components of an olfactory mixture. If odor identity in multi-component mixtures is primarily derived from a few major odorants this would shed doubt on our wonder if small scale microproperties of the odorants impact upon odor quality as odor mixtures scale up to environmental odor plumes. However, even if in the extreme case that only the major components of a mixture are responsible for generating odor identity, their relationship with the mixture’s composition as a complex smell must still be considered, since their ratio and concentration within the mixture have a determinate effect on perceived odor identity.
The distinction between configural and elemental mixtures is often treated as binary, yet recent studies suggest it might be treated along a continuum with some mixtures yielding only mildly configural odors. Both humans and rabbits treat the RC6 mixture (artificial strawberry smell) as configural (Sinding et al. 2013). Yet, when one of the components is removed the mixture is treated as mildly configural depending upon the identity of the constituent that has been removed, as well as the resultant ratio between the remaining components. Similar results have been shown using Rabbit pups presented with the RC6 mixture. What was interestingly demonstrated is that changing less than 50% of the components still yield a weak configural percept of olfactory quality (Romagny et al 2015) suggesting that the differences in sensory transduction reported by Johnson et al (2007, 2010) might not be perfectly preserved in perception. The continuum of perceived odor identity between elemental and configural mixtures show that even properties within the composition of the odor mixture, aside from odorant identity and their molecular features, play a role in shifting odor quality.
4.3. Olfactory transduction and perception of Unitary and Binary Odor Mixtures
In some binary mixtures, changes in concentration yield perceived differences in odor quality (Asahina et al. 2009; Malnic et al. 1999; McNamara et al. 2007; Pause et al. 1997). However, shifts in olfactory quality as brought about by changes in concentration levels are the exception and not the norm (Cleland et al. 2011; Gross-Isseroff and Lancet, 1988; Uchida and Mainen, 2008). The lack of shifts in olfactory quality as brought about by changes in concentration levels explains the dearth of literature. Nevertheless, humans use a larger set of descriptions of odor qualities for monomolecular structures with greater structural complexity (Kermen et al. 2011), which can be taken as evidence that molecular complexity plays a causal role in generating the reported odor quality even in the absence of additional odorants. Additionally, as noted in section 3, there is reason to think that the gaseous plume itself plays a role, since changes in concentration can cause variance in olfactory quality without any change in molecular structure (Gross-Isseroff and Lancet, 1988; Laing et al. 2003; Marfaing et al. 1988).
Research on the cellular and molecular basis of odor transduction further support our claim that changing the concentration of an odorant or the ratio of odorants in a simple or complex mixture may result in a change in odor perception. Genetically encoded probes have allowed researchers to observe neuronal function in real-time. Bozza and colleagues targeted expression of a pH-sensitive protein, synapto- pHluorin that reports on synaptic vesicle fusion in real-time, to olfactory neurons. Using this genetic probe, they were able to visualize glomerular activity in response to a panel of odorants. They tested responses to both increases in carbon-chain length of odorants as well as changes in odorant concentration. They found little evidence for a finely organized mapping of carbon-chain length across the olfactory bulb surface. They also found that odorant concentration could alter glomerular activation patterns such that some glomeruli highly activated by low concentrations of odors are not activated by higher concentrations. (Bozza et al., 2004). These observations could be accounted for by the physicochemical properties of odor receptors as well as cellular organization of the olfactory circuit. Odor receptors may have high or low affinity for any given odorants and may also differ in their tuning properties (Hallem and Carlson, 2006; Mathew et al., 2013). These properties could determine the distribution of glomerular activity and explain why as odorant concentration increases new receptors could potentially be recruited. Odor receptors can also be activated as well as inhibited by specific odorants further complicating the distribution of glomerular activation (Hallem and Carlson, 2006; Cao et al., 2017). Similarly, lateral inhibition processes may differentially affect the activity pattern of glomeruli depending on odor concentration (Urban, 2002; Wilson et al., 2004; McGann et al., 2005; Olsen and Wilson, 2008). Eventually, glomerular activation patterns impact odor perception (Grossman et al., 2008), however, how these cellular mechanisms scale up to account for naturally occurring odor plumes is still an open area of research.
Despite the findings of the aforementioned sections suggesting that the molecular features of the odorants composing the odor plume play a role in determining odor identity with respect to odor quality, given the variegated nature of the molecular features of these mixtures, these effects might be attributed to sensory receptivity and transduction mechanism of the olfactory system relative to the different types of molecular features of the odorants. In an attempt to control for such differences between various types of molecular features interacting with the receptor site, we think it would be prudent to study less- complex mixtures. If a single component odor plume composed of odorants sharing all the same chemical structures show shifts in perceived olfactory quality this cannot be attributed to differences in odorant’s chemical structure as composed by its functional groups and their spatial distributions. What has yet to be addressed as indicated by our focused survey of the literature, is the possibility that minute chemical properties such as isotopic or isotopomeric variations within the composition of the odor plume might play a role in generating shifts in odor identity in naturally occurring situations.
5. Conclusion:
A complete account of the nature of smell that is generalizable to occurrence outside of laboratory conditions should account for the odor plume. A comprehensive theory of olfactory perception must account for both the odorous plume’s constituents in generating the perception of odor quality at the receptor level, as well as the role that the perceptible object’s extended nature plays in generating the perception of smells. One of the key areas of unexplored research is how variations of the properties of the same chemical object composing the gaseous odor plume affects perceived odor identity. We suggest that it might be worth studying isotopic or isotopomer mixtures at varying levels of concentration to see if there are similar shifts in odor identity or invariance when compared to pure solutions. Additionally, it would be useful to understand how isotopic or isotopomer compounds within background odors affect the perception of pure odorants that form the target stimulus for olfactory tracking and navigation. Using these minuscule variations provides a means for controlling the intervening variables and alternative explanations that might arise from using more complex stimuli such as olfactory mixtures. If even in instances of variations within these microstructural chemical properties we observe shifts in perceived odor identity within varying levels of concentration, as well as masking effects, it will go some ways towards filling in the current state of our research with the added bonus of showing that the odor plume as a superordinate perceptual entity plays a determinate role in generating odor quality.
Highlights.
The components of an odorant mixture affect odor quality, but there is little to no research closing the gap on the determinates of odor quality as these odorous mixtures scale from objects transduced at the receptor level to the extended, distributed odorous plumes.
Odorant properties in an odor plume determine odor identity with respect to odor quality.
For successful navigation the odor plume must be represented by an olfactory system as a spatiotemporal entity over and above the constituent odorants and concentration gradients.
The concentration of the odorants composing the odor plume affects odor quality.
As it is an open question if isotopic or isotopomer variations within the composition of the odor plume might play a role in determining olfactory quality. We suggest using these microchemical variations for further research.
To account for natural odor perception more research needs to be conducted to account for the perception of the odor plume both at the receptor level and at the level of the odor as a gaseous plume.
Acknowledgements:
The authors are supported by startup funds and a μiCRo grant from the University of Nevada, Reno awarded to BY and DM, and by a grant from the NIGMS of the National Institute of Health under grant number P20 GM103650 awarded to DM.
Footnotes
For a good overview of the dissociation of odor quality from intensity and a theory of how odor intensity might be determined from the concentration gradient of a complex odor plume see Mainlaind et al (2014). Similarly, Giaffar et al (2018) generate a primacy model of odor identity with respect to intensity that builds upon Wilson et al ‘s (2017) model that shows how initial ORNs encode odor identity with respect to quality across a range of different concentrations. Even though Giaffar et al’s (2018) model laudably generates testable predictions of how a small set of high affinity receptors could encode odor identity within a single sniff, given their focus on odor identity as intensity and not quality we set it aside in what follows.
For those interested in avian odor navigation Wiltschko and Wiltschko (2017) provide an in-depth commentary of the debate between those claiming it requires odor maps and those that think mere atmospheric cues without a map would be sufficient for navigation. Additionally, Walcott et al’s (2018) editorial nicely summarize state of debate regarding pigeon navigation and whether it requires odor- based maps as proposed by Papi or the olfactory-activation hypothesis that atmospheric odors do not provide navigation cues.
Carde and Willis (2008) offer anin-depth article that covers navigational strategies of insects for patchy (natural) odor plumes that discussesodor dispersion as it impacts plume size and the structure of the plume, as well as how the atmospheric conditions (including wind currents) and landscape affect plume structure.
Competing Interests: We all declare no conflicting or competing interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References:
- Ache BW, Hein AM, Bobkov YV & Principe JC (2016) Smelling Time- A Neural Basis for Olfactory Scene Analysis. Trends in Neurosciences, October 2016, Vol. 39, No. 10 http://dx.doi.org/10.10167j.tins.2016.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araneda RC, Kini AD, & Firestein S (2000). The molecular receptive range of an odorant receptor. Nat Neurosci, 3(12), 1248–1255. doi: 10.1038/81774 [DOI] [PubMed] [Google Scholar]
- Asahina K, Louis M, Piccinotti S, & Vosshall LB (2009). A circuit supporting concentration-invariant odor perception in Drosophila. J Biol, 8(1), 9. doi: 10.1186/jbiol108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker TC, Meyer W, & Roelofs WL (1981). Sex pheromone dosage and blend specificity of response by oriental fruit moth males. In (Vol. 30, pp. 269–279). Entomologia Experimentalis et Applicata. [Google Scholar]
- Baker KL, Dickinson M, Findley TM et al. (2018) Algorithms for Olfactory Search across Species.The Journal of Neuroscience, 38(44):9383–9389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berglund B, et al. “Multidimensional Analysis of Twenty-One Odors.” Scand J Psychol 14.2 (1973): 131–7. Print. [DOI] [PubMed] [Google Scholar]
- Bhattacharyya U, & Bhalla US (2015). Robust and Rapid Air-Borne Odor Tracking without Casting. eNeuro, 2(6). doi: 10.1523/ENEURO.0102-15.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bingman VR (2018) can anything more be learned from homing pigeons about the sensory and spatial- representational basis of avian navigation? Journal of Experimental Biology (2018) 221, jeb163089. doi: 10.1242/jeb.163089 [DOI] [PubMed] [Google Scholar]
- Bittner ER, Madalan A, Czader A, & Roman G (2012). Quantum origins of molecular recognition and olfaction in Drosophila. J Chem Phys, 137(22), 22A551. doi: 10.1063/1.4767067 [DOI] [PubMed] [Google Scholar]
- Block E, Jang S, Matsunami H, Sekharan S, Dethier B, Ertem MZ, … Zhuang H (2015). Implausibility of the vibrational theory of olfaction. Proc Natl Acad Sci U S A, 112(21), E2766–2774. doi: 10.1073/pnas.1503054112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boie SD, Connor EG, McHugh M, Nagel KI, Ermentrout GB, Crimaldi JP, et al. (2018) Information- theoretic analysis of realistic odor plumes: What cues are useful for determining location? PLoS Comput Biol 14(7): e1006275. 10.1371/journal.pcbi.1006275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolding KA & Franks KM (2017) Complementary codes for odor identity and intensity in olfactory cortex. eLife 2017;6:e22630. DOI: 10.7554/eLife.22630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borst A (1983). Computation of olfactory signals in Drosophila melanogaster. Journal of comparative physiology, 152(3), 373–383. doi: 10.1007/BF00606242 [DOI] [Google Scholar]
- Boyle JA, Djordjevic J, Olsson MJ., Lundström JN, and Jones-Gotman M (2009): The Human Brain Distinguishes between Single Odorants and Binary Mixtures. Cereb. Cortex 19 (1): 66–71 doi: 10.1093/cercor/bhn058 [DOI] [PubMed] [Google Scholar]
- Bozza T, McGann JP, Mombaerts P, Wachowiak M (2004) In vivo imaging of neuronal activity - Neurotechnique by targeted expression of a genetically encoded probe in the mouse. Neuron 42:9–21. [DOI] [PubMed] [Google Scholar]
- Brookes JC, Horsfield AP, & Stoneham AM (2012). The swipe card model of odorant recognition. Sensors (Basel), 12(11), 15709–15749. doi: 10.3390/s121115709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buck L, & Axel R (1991). A novel multigene family may encode odorant receptors: a molecular basis for odor recognition. Cell, 65(1), 175–187. [DOI] [PubMed] [Google Scholar]
- Cai X, Bian L, Xu X, Luo Z, Li Z, & Chen Z (2017). Field background odour should be taken into account when formulating a pest attractant based on plant volatiles. Sci Rep, 7, 41818. doi: 10.1038/srep41818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao LH, Yang D, Wu W, Zeng XK, Jing BY, Li MT, Qin SS, Tang C, Tu YH, Luo DG (2017) Odor- evoked inhibition of olfactory sensory neurons drives olfactory perception in Drosophila. Nature communications 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carde RT & Willis MA (2008) Navigational Strategies Used by Insects to Find Distant, Wind-Borne Sources of Odor. J Chem Ecol, 34:854–866. [DOI] [PubMed] [Google Scholar]
- Catania KC (2012) Stereo and serial sniffing guide navigation to an odour source in a mammal. Nature Communications, 4:1441 DOI: 10.1038/ncomms2444 [DOI] [PubMed] [Google Scholar]
- Celani A, Villermaux E, & Vergassola M (2014) Odor Landscapes in Turbulent Environments. Physical Review X 4, 041015 [Google Scholar]
- Charlton Ralph E., et al. (1993). “Influence of Pheromone Concentration and Ambient Temperature on Flight of the Gypsy Moth, Lymantria Dispar (L), in a Sustained-Flight Wind Tunnel.” [Google Scholar]
- Choudhary AF (2009). Olfactory Perceptual Invariance in the Honeybee: A Psychophysical App- roach. PhD dissertation, New- castle University, Newcastle-upon- Tyne, UK. [Google Scholar]
- Cleland T (2008). The construction of olfactory representations In Holscher C & Munk M (Eds.), Information Processing by Neuronal Populations (pp. 247–280). Cambridge: Cambridge University Press. doi: 10.1017/CBO9780511541650.011 [DOI] [Google Scholar]
- Cleland TA, Chen SY, Hozer KW, Ukatu HN, Wong KJ, & Zheng F (2011). Sequential mechanisms underlying concentration invariance in biological olfaction. Front Neuroeng, 4, 21. doi: 10.3389/fneng.2011.00021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahmani L, Patel RM, Yang Y et al. (2018) An intrinsic association between olfactory identification and spatial memory in humans. Nature Communications, 9:4162 DOI: 10.1038/s41467-018-06569-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dekker T, Takken W, and Carde RT (2001). Structure of host- odour plumes influences catch of Anopheles gambiae s.s. and Aedes aegypti in a dual-choice olfactometer. Physiol. Entomol. 26–124-134. [Google Scholar]
- Dekker T Geier M, & Carde RT (2005) Carbon dioxide instantly sensitizes female yellow fever mosquitos tro human skin odors. The Journal of Experimental Biology 208, 2963–2972. [DOI] [PubMed] [Google Scholar]
- Dekker T, & Carde RT (2011) Moment-to-moment flight manoeuvres of the female yellow fever mosquito (Aedes aegypti L.) in response to plumes of carbon dioxide and human skin odour. The Journal of Experimental Biology 214, 3480–3494 [DOI] [PubMed] [Google Scholar]
- Dixson DL, Jennings AR, Atema J, & Munday PL (2015). Odor tracking in sharks is reduced under future ocean acidification conditions. Glob Chang Biol, 21(4), 1454–1462. doi: 10.1111/gcb.12678 [DOI] [PubMed] [Google Scholar]
- Drimyli E, Gaitanidis A, Maniati K, Turin L, & Skoulakis EM (2016). Differential Electrophysiological Responses to Odorant Isotopologues in Drosophilid Antennae. eNeuro, 3(3). doi: 10.1523/ENEUR0.0152-15.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duistermars BJ, Chow DM, & Frye MA (2009). Flies require bilateral sensory input to track odor gradients in flight. Curr Biol, 19(15), 1301–1307. doi: 10.1016/j.cub.2009.06.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dusenbery DB (1992) Sensory Ecology. W.H. Freeman and Company:New York. Ch: 4. [Google Scholar]
- Fadamiro HY, Cosse AA, & Baker TC (1999). Fine-scale resolution of closely spaced pheromone and antagonist filaments by flying male Helicoverpa zea. In (Vol. 185, pp. 131–141). Journal of Comparative Physiology A. [Google Scholar]
- Farkas SR, & Shorey HH (1972). Chemical trail-following by flying insects: a mechanism for orientation to a distant odor source. Science, 178(4056), 67–68. doi: 10.1126/science.178.4056.67 [DOI] [PubMed] [Google Scholar]
- Faucher CP, Hilker M, & de Bruyne M (2013). Interactions of carbon dioxide and food odours in Drosophila: olfactory hedonics and sensory neuron properties. PLoS One, 8(2), e56361. doi: 10.1371/journal.pone.0056361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng G, & Zhou W (2019) Nostril-specific and structure-based olfactory learning of chiral discrimination in human adult. eLife 2019;8:e41296. DOI: 10.7554/eLife.41296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Firestein S (2001). How the olfactory system makes sense of scents. Nature, 413(6852), 211–218. doi: 10.1038/35093026 [DOI] [PubMed] [Google Scholar]
- Franco MI, Turin L, Mershin A, & Skoulakis EM (2011). Molecular vibration-sensing component in Drosophila melanogaster olfaction. Proc Natl Acad Sci U S A, 108(9), 3797–3802. doi: 10.1073/pnas.1012293108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frederick DE, Barlas L, levins A, & Kay LM (2009). A critical test of the overlap hypothesis for odor mixture perception. Behavioral Neuroscience, 123(2), 430–437. [DOI] [PubMed] [Google Scholar]
- Fried HU et al. “Selective Imaging of Presynaptic Activity in the Mouse Olfactory Bulb Shows Concentration and Structure Dependence of Odor Responses in Identified Glomeruli.” Proc Natl Acad Sci U S A, vol. 99, no. 5, 2002, pp. 3222–3227, doi: 10.1073/pnas.052658399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giaffar H, Rinberg D, Koulakov AA (2018) Primacy model and the evolution of the olfactory receptor repertoire. bioRxiv preprint doi: http://dx.doi.org/10.110½55661. [Google Scholar]
- Gomez-Marin A, Duistermars BJ, Frye MA, S Louis M (2010). Mechanisms of odor-tracking: multiple sensors for enhanced perception and behavior. Front Cell Neurosci, 4, 6. doi: 10.3389/fncel.2010.00006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottfried JA, 2010. ‘Central mechanisms of odour object perception’. Nature Reviews Neuroscience 11, 628–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottfried JA, Winston JS, & Dolan RJ (2006). Dissociable codes of odor quality and odorant structure in human piriform cortex. Neuron, 49(3), 467–479. doi: 10.1016/j.neuron.2006.01.007 [DOI] [PubMed] [Google Scholar]
- Gronenberg W, Raikhelkar A, Abshire E, Stevens J, Epstein E, Loyola K, … Buchmann S (2014a). Honeybees (Apis mellifera) learn to discriminate the smell of organic compounds from their respective deuterated isotopomers. Proc Biol Sci, 281(1778), 20133089. doi: 10.1098/rspb.2013.3089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gronenberg W, Raikhelkar A, Abshire E, Stevens J, Epstein E, Loyola K, … Buchmann S (2014b). Honeybees (Apis mellifera) learn to discriminate the smell of organic compounds from their respective deuterated isotopomers. Proc Biol Sci, 281(1778), 20133089. doi: 10.1098/rspb.2013.3089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross-Isseroff R, and Lancet D 1988. Concentration-dependent changes of perceived odor quality. Chem. Senses 13, 191–204. [Google Scholar]
- Grossman KJ, Mallik AK, Ross J, Kay LM, Issa NP (2008) Glomerular activation patterns and the perception of odor mixtures. European Journal of Neuroscience 27:2676–2685. [DOI] [PubMed] [Google Scholar]
- Haddad R, Lapid H, Harel D, & Sobel N “Measuring smells.” Curr Opin Neurobiol, 18(4), 438–444, 2008. doi: 10.1016/j.conb.2008.09.007 [DOI] [PubMed] [Google Scholar]
- Haddad R, Medhanie A, Roth Y, Harel D, & Sobel N “Predicting odor pleasantness with an electronic nose.” PLoS Comput Biol, 6(4), 2010. e1000740. doi: 10.1371/journal.pcbi.1000740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haddad R, Weiss T, Khan R, Nadler B, Mandairon N, Bensafi M, … Sobel N “Global features of neural activity in the olfactory system form a parallel code that predicts olfactory behavior and perception.” J Neurosci, 30(27), 9017–9026, 2010. doi: 10.1523/JNEUROSCI.0398-10.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallem EA, Dahanukar A, & Carlson JR (2006). Insect odor and taste receptors. Annu Rev Entomol, 51, 113–135. doi: 10.1146/annurev.ento.51.051705.113646 [DOI] [PubMed] [Google Scholar]
- Hallem EA, Carlson JR (2006) Coding of odors by a receptor repertoire. Cell 125:143–160. [DOI] [PubMed] [Google Scholar]
- Herrero P (2012). Fruit fly behavior in response to chemosensory signals. Peptides, 38(2), 228–237. doi: 10.1016/j.peptides.2012.09.019 [DOI] [PubMed] [Google Scholar]
- Howard JD, & Gottfried JA (2014). Configural and elemental coding of natural odor mixture components in the human brain. Neuron, 84(4), 857–869. doi: 10.1016/j.neuron.2014.10.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs LF. 2012. From chemotaxis to the cognitive map: the function of olfaction. Proc Natl Acad Sci USA; 109:10693–700. doi: 10.1073/pnas.1201880109 PMID: 22723365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs LF, Arter J, Cook A, & Sulloway FJ (2015). Olfactory Orientation and Navigation in Humans. PLoS One, 10(6), e0129387. doi: 10.1371/journal.pone.0129387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs LF (2019) The navigational nose/ a new hypothesis for the function of the human external pyramid. Journal of Experimental Biology, 222, jeb186924. doi: 10.1242/jeb.186924 [DOI] [PubMed] [Google Scholar]
- Johnson BA, & Leon M (2007). Chemotopic odorant coding in a mammalian olfactory system. J Comp Neurol, 503(1), 1–34. doi: 10.1002/cne.21396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson BA, Ong J, & Leon M (2010). Glomerular activity patterns evoked by natural odor objects in the rat olfactory bulb are related to patterns evoked by major odorant components. J Comp Neurol, 518(9), 1542–1555. doi: 10.1002/cne.22289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan R, Kollo M, & Schaefer A (2018) Sniffing Fast- Paradoxical Effects on Odor Concentration Discrimination at the Levels of Olfactory Bulb Output and Behavior. eNeuro, 5(5) e0148–18.2018 1–18, 10.1523/ENEURO.0148-18.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay LM, Crk T, & Thorngate J, 2005. ‘A Redefinition of Odor Mixture Quality’, Behavioral Neuroscience 119(3): 726–733. [DOI] [PubMed] [Google Scholar]
- Keller A et al. “Genetic Variation in a Human Odorant Receptor Alters Odour Perception.” Nature, vol. 449, no. 7161, 2007, pp. 468–472, doi: 10.1038/nature06162. [DOI] [PubMed] [Google Scholar]
- Keller A, & Vosshall LB (2004). A psychophysical test of the vibration theory of olfaction. Nat Neurosci, 7(4), 337–338. doi: 10.1038/nn1215 [DOI] [PubMed] [Google Scholar]
- Kermen F, Chakirian A, Sezille C, Joussain P, Le Goff G, Ziessel A, … Bensafi M(2011). Molecular complexity determines the number of olfactory notes and the pleasantness of smells. Sci Rep, 1, 206. doi: 10.1038/srep00206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan RM, Luk CH, Flinker A, Aggarwal A, Lapid H, Haddad R, & Sobel N (2007). Predicting odor pleasantness from odorant structure: pleasantness as a reflection of the physical world. J Neurosci, 27(37), 10015–10023. doi: 10.1523/JNEUROSCI.1158-07.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koutsoklenis A, Papadopoulos K., 2011. Olfactory cues used for wayfinding in urban environments by individuals with visual impairments. J Vis Impair Blind; 105:692–702. [Google Scholar]
- Krause Pham C, and Ray A. “Conservation of Olfactory Avoidance in Drosophila Species and Identification of Repellents for Drosophila Suzukii.” Sci Rep 5 (2015): 11527 Print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreher SA, et al. “Translation of Sensory Input into Behavioral Output Via an Olfactory System.” Neuron 59.1 (2008): 110–24. Print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuenen LPS, & Baker TC (1982). The effects of pheromone concentration on the flight behavior of the oriental fruit moth, Grapholitha molesta. In (Vol. 7, pp. 423–434). Physiological Entomology. [Google Scholar]
- Kumar R, Kaur R, Auffarth B, & Bhondekar AP “Understanding the Odour Spaces: A Step towards Solving Olfactory Stimulus-Percept Problem.” PLoS One, 10(10), 2015. e0141263. doi: 10.1371/journal.pone.0141263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacey ES & Carde RT (2011) Activation, orientation and landing of female Culex quinquefasciatus in response to carbon dioxide and odour from human feet- 3-D flight analysis in a wind tunnel. Medical and Veterinary Entomology, 25, 94–103 doi: 10.1111/j.1365-2915.2010.00921.x [DOI] [PubMed] [Google Scholar]
- Laing DG, Legha PK, Jinks AL, & Hutchinson I (2003). Relationship between molecular structure, concentration and odor qualities of oxygenated aliphatic molecules. Chem Senses, 28(1), 57–69. [DOI] [PubMed] [Google Scholar]
- Le Berre E, Béno N, Ishii A, Chabanet C, Etiévant P, & Thomas-Danguin T (2008a). Just noticeable differences in component concentrations modify the odor quality of a blending mixture. Chem Senses, 33(4), 389–395. doi: 10.1093/chemse/bjn006 [DOI] [PubMed] [Google Scholar]
- Le Berre E, Thomas-Danguin T, Béno N, Coureaud G, Etiévant P, & Prescott J (2008b). Perceptual Processing Strategy and Exposure Influence the Perception of Odor Mixtures. Chemical Senses, 33(2), 193–199. [DOI] [PubMed] [Google Scholar]
- Lei H, Riffell JA, Gage SL, & Hildebrand JG (2009). Contrast enhancement of stimulus intermittency in a primary olfactory network and its behavioral significance. J Biol, 8(2), 21. doi: 10.1186/jbiol120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu JL, Chen XY, and Zeng XN. “Classical Olfactory Conditioning in the Oriental Fruit Fly, Bactrocera Dorsalis.” PLoS One 10.4 (2015): e0122155. Print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lockey JK, & Willis MA (2015). One antenna, two antennae, big antennae, small: total antennae length, not bilateral symmetry, predicts odor-tracking performance in the American cockroach Periplaneta americana. The Journal of Experimental Biology, 218(14), 2156. [DOI] [PubMed] [Google Scholar]
- Louis M, Huber T, Benton R, Sakmar TP, & Vosshall LB (2008). Bilateral olfactory sensory input enhances chemotaxis behavior. Nat Neurosci, 11(2), 187–199. [DOI] [PubMed] [Google Scholar]
- Mafra-Neto A & Carde RT (1994) Fine-scale structure of pheromone plumes modulates upwind orientation of flying moths. Nature, 369, 142–144. [Google Scholar]
- Mafra-Neto A, & Carde RT (1995). Effect of the fine-scale structure of pheromone plumes: pulse frequency modulates activation and upwind flight of almond moth males. In (Vol. 20, pp. 229–242). Physio Entom. [Google Scholar]
- Mainland JD, Lundstrom JN, Reisert J, & Lowe G (2014) From molecule to mind- an integrative perspective on odor intensity. Trends in Neurosciences, 37, 443–454. 10.1016/j.tins.2014.05.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malnic B, Hirono J, Sato T, & Buck LB (1999). Combinatorial receptor codes for odors. Cell, 96(5), 713–723. [DOI] [PubMed] [Google Scholar]
- Marfaing P, Rouault J & Laffort P 1989. Effect of the concentration and nature of olfactory stimuli on the proboscis extension of conditioned honeybees (Apis mellifera ligustica). J. Insect Physiol. 35, 949–955. (doi: 10.1016/0022-1910(89)90018-8.) [DOI] [Google Scholar]
- Martin H (1965). Osmotropotaxis in the Honey-Bee. Nature, 208(5005), 59–63. [Google Scholar]
- Martin JP, & Hildebrand JG (2010). Innate recognition of pheromone and food odors in moths: a common mechanism in the antennal lobe? Front Behav Neurosci, 4. doi: 10.3389/fnbeh.2010.00159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masek P, & Heisenberg M (2008). Distinct memories of odor intensity and quality in Drosophila. Proc Natl Acad Sci U S A, 105(41), 15985–15990. doi: 10.1073/pnas.0804086105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathew D, Martelli C, Kelley-Swift E, Brusalis C, Gershow M, Samuel AD, Emonet T, Carlson JR. Proc Natl Acad Sci U S A. 2013. June 4;110(23):E2134–43. 2013 May 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGann JP, Pirez N, Gainey MA, Muratore C, Elias AS, Wachowiak M (2005) Odorant representations are modulated by intra- but not interglomerular presynaptic inhibition of olfactory sensory neurons. Neuron 48:1039–1053. [DOI] [PubMed] [Google Scholar]
- McNamara AM, Magidson PD, & Linster C (2007). Binary mixture perception is affected by concentration of odor components. Behav Neurosci, 121(5), 1132–1136. doi: 10.1037/0735-7044.121.5.1132 [DOI] [PubMed] [Google Scholar]
- Meierhenrich UJ, Golebiowski J, Fernandez X, & Cabrol-Bass D (2004). The molecular basis of olfactory chemoreception. Angew Chem Int Ed Engl, 43(47), 6410–6412. doi: 10.1002/anie.200462322 [DOI] [PubMed] [Google Scholar]
- Menzel S, Hummel T, Schafer L, Hummel C & Croy I (2019) Olfactory change detection. Biological Psychology ,140, 75–80. 10.1016/j.biopsycho.2018.11.010 [DOI] [PubMed] [Google Scholar]
- Moessnang C, Finkelmeyer A, Vossen A, Schneider F & Habel U, 2011. Assessing implicit odor localization in humans using a cross-modal spatial cueing paradigm. PloS One 6, e29614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori K, and Shepherd GM. “Emerging Principles of Molecular Signal Processing by Mitral/Tufted Cells in the Olfactory Bulb.” Semin Cell Biol 5.1 (1994): 65–74. [DOI] [PubMed] [Google Scholar]
- Münch D, Schmeichel B, Silbering AF, & Galizia CG (2013). Weaker ligands can dominate an odor blend due to syntopic interactions. Chem Senses, 38(4), 293–304. doi: 10.1093/chemse/bjs138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murlis J, Elkington JS, and Carde RT 1992. Odor plumes and how insects use them. Annu. Rev. Entomol. 37–505-532. [Google Scholar]
- Nara K, Saraiva LR, Ye X, Buck LB. A Large-Scale Analysis of Odor Coding in the Olfactory Epithelium. J Neurosci. 2011. June 22;31(25):9179–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Needham P 2000. What is water? Analysis, 60, pp. 13–21. [Google Scholar]
- Needham P 2002. The discovery that water is H2O, International Studies in the Philosophy of Science, 16:3, 205–226 [Google Scholar]
- Olofsson JK, Bowman NE, Khatibi K, & Gottfried JA (2012) “A time-based account of the perception of odor objects and valences.” Psychological Science 23: 1224–1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olofsson JK, Bowman NE, & Gottfried JA. (2013). High and low roads to odor valence? A choice response-time study. Journal of Experimental Psychology: Human Perception and Performance 39: 1205–1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paoli M, Anesi A, Antolini R, Guella G, Vallortigara G, & Haase A (2016). Differential Odour Coding of Isotopomers in the Honeybee Brain. Sci Rep, 6, 21893. doi: 10.1038/srep21893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park IJ, Hein AM, Bobkov YV, Reidenbach MA, Ache BW, & Principe JC (2016). Neurally Encoding Time for Olfactory Navigation. PLoS Comput Biol, 12(1), e1004682. doi: 10.1371/journal.pcbi.1004682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pause BM, Sojka B, & Ferstl R (1997). Central processing of odor concentration is a temporal phenomenon as revealed by chemosensory event-related potentials (CSERP). Chem Senses, 22(1), 9–26. [DOI] [PubMed] [Google Scholar]
- Patzke N, Manns M, Gunturkun O, Ioale P, & Gagliardo A (2010) Navigation-induced ZENK expression in the olfactory system of pigeons. European Journal of Neuroscience, Vol. 31, pp. 2062–2072, 2010 doi: 10.1111/j.1460-9568.2010.07240.x [DOI] [PubMed] [Google Scholar]
- Porter J, Craven B, Khan RM, Chang SJ, Kang I, Judkewitz B, Volpe J, Settles G, and Sobel N, (2007). ‘Mechanisms of scent-tracking in humans’. Nature Neuroscience 10 (1): 27–29. [DOI] [PubMed] [Google Scholar]
- Reese A, List NH, Kongsted J, & Solov’yov IA (2016). How Far Does a Receptor Influence Vibrational Properties of an Odorant? PLoS One, 11(3), e0152345. doi: 10.1371/journal.pone.0152345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinhard J, Sinclair M, Srinivasan MV, & Claudianos C (2010). Honeybees learn odour mixtures via a selection of key odorants. PLoS One, 5(2), e9110. doi: 10.1371/journal.pone.0009110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romagny S, Thomas-Danguin T, & Coureaud G (2015). Configural processing of odor mixture: does the learning of elements prevent the perception of configuration in the newborn rabbit? Physiol Behav, 142, 161–169. doi: 10.1016/j.physbeh.2015.02.019 [DOI] [PubMed] [Google Scholar]
- Safi K, Gagliardo A, Wikelski M and Kranstauber B (2016) How Displaced Migratory Birds Could Use Volatile Atmospheric Compounds to Find Their Migratory Corridor: A Test Using a Particle Dispersion Model. Front. Behav. Neurosci. 10:175. doi: 10.3389/fnbeh.2016.00175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanz G, et al. “Relationships between Molecular Structure and Perceived Odor Quality of Ligands for a Human Olfactory Receptor.” Chem Senses 33.7 (2008): 639–53. [DOI] [PubMed] [Google Scholar]
- Schifferstein HN, Smeets MA, & Postma A, 2010. Comparing location memory for 4 sensory modalities. Chemical Senses, 35, pp. 135–145 [DOI] [PubMed] [Google Scholar]
- Shepherd GM, 2005. ‘Outline of a Theory of Olfactory Processing and its Relevance to Humans’. Chemical Senses 39 (S1): i3–i5. [DOI] [PubMed] [Google Scholar]
- Shusterman R, Sirotin YB, Smear MC, Ahmadin Y, & Rinberg D (2018) Sniff Invariant Odor Coding. eNeuro, 5(6) e0149–18.2018 1–15. 10.1523/ENEUR0.0149-18.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinding C, Puschmann L, and Hummel T. “Is the Age-Related Loss in Olfactory Sensitivity Similar for Light and Heavy Molecules?” Chem Senses 39.5 (2014): 383–90. Print. [DOI] [PubMed] [Google Scholar]
- Sinding C, Thomas-Danguin T, Chambault A, Béno N, Dosne T, Chabanet C, … Coureaud G (2013). Rabbit neonates and human adults perceive a blending 6-component odor mixture in a comparable manner. PLoS One, 8(1), e53534. doi: 10.1371/journal.pone.0053534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slater G, Levy P, Chan KL, & Larsen C (2015). A central neural pathway controlling odor tracking in Drosophila. J Neurosci, 35(5), 1831–1848. doi: 10.1523/JNEUR0SCI.2331-14.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solov’yov IA, Chang PY, & Schulten K (2012). Vibrationally assisted electron transfer mechanism of olfaction: myth or reality? Phys Chem Chem Phys, 14(40), 13861–13871. doi: 10.1039/c2cp41436h [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snitz K, Yablonka A, Weiss T, Frumin I, Khan RM, & Sobel N “Predicting odor perceptual similarity from odor structure.” PLoS Comput Biol, 9(9), 2013. e1003184. doi: 10.1371/journal.pcbi.1003184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szyszka P, Gerkin RC, Galizia CG, & Smith BH (2014). High-speed odor transduction and pulse tracking by insect olfactory receptor neurons. Proc Natl Acad Sci U S A, 111(47), 16925–16930. doi: 10.1073/pnas.1412051111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tully T, & Quinn WG (1985). Classical conditioning and retention in normal and mutant Drosophila melanogaster. J Comp Physiol A, 157(2), 263–277. [DOI] [PubMed] [Google Scholar]
- Turner RI & Laurent G (2004) Transformation of olfactory representations in the Drosophila antennal lobe. Science 303:366–370. [DOI] [PubMed] [Google Scholar]
- Uchida N, & Mainen ZF (2008). Odor concentration invariance by chemical ratio coding. Front Syst Neurosci, 1, 3. doi: 10.3389/neuro.06.003.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urban NN (2002) Lateral inhibition in the olfactory bulb and in olfaction. Physiol Behav 77:607–612. [DOI] [PubMed] [Google Scholar]
- Vickers NJ, & Baker TC (1992). Male Heliothis Virescens maintain upwind flight in response to experimentally pulsed filaments of their sex pheromone (Lepidoptera: Noctuidae). In (Vol. 5, pp. 669–687). Journal of Insect Behavior. [Google Scholar]
- Vickers NJ, & Baker TC (1994). Reiterative responses to single strands of odor promote sustained upwind flight and odor source location by moths. Proc Natl Acad Sci U S A, 91(13), 5756–5760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vickers NJ, Christensen TA, Baker TC, & Hilderbrand JG (2001) Odour-plume dynamics influence the brain’s olfactory code. Nature, 410, 466–470 [DOI] [PubMed] [Google Scholar]
- Vincis R, Gschwend O, Bhaukaurally K, Beroud J, & Carleton A (2012). Dense representation of natural odorants in the mouse olfactory bulb. Nat Neurosci, 15(4), 537–539. doi: 10.1038/nn.3057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vosshall LB (2015). Laying a controversial smell theory to rest. Proc Natl Acad Sci U S A, 112(21), 6525–6526. doi: 10.1073/pnas.1507103112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walcott C, Wiltschko W, Wiltschko R, & Zupanc GKH (2018) Olfactory navigation versus olfactory activation/ a controversy revisited. Journal of Comparative Physiology A 10.1007/s00359-018-1273-1 [DOI] [PubMed] [Google Scholar]
- Webster DR, & Weissburg MJ (2001). Chemosensory Guidance Cues in a Turbulent Chemical Odor plume. In (Vol. 46, pp. 1034–1047). Atlanta, Georgia: Limnology and Oceanography. [Google Scholar]
- Webster B, Lacey ES, & Carde RT (2015) Waiting with Bated Breath- Opportunistic Orientation to Human Odor in the Malaria Mosquito, Anopheles gambiae, is Modulated by Minute Changes in Carbon Dioxide Concentration. J Chem Ecol (2015) 41:59–66 DOI 10.1007/s10886-014-0542-x [DOI] [PubMed] [Google Scholar]
- Webster B & Carde RT (2017) Use of habitat odour by host-seeking insects.Biol. Rev, 92, 1241–1249. [DOI] [PubMed] [Google Scholar]
- Weiss T, Snitz K, Yablonka A, Khan RM, Gafsou D, Schneidman E, Sobel N. (2012) Perceptual convergence of multi-component mixtures in olfaction implies an olfactory white. Proc Natl Acad Sci U S A. 109(49):19959–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welge-Lussen A, Looser G-L, Westermann B, Hummel T., 2014. Olfactory source localization in the open field using one or both nostrils. Rhinology;52:41–7. pmid:24618627 [DOI] [PubMed] [Google Scholar]
- Willis MA (2008). Chemical Plume Tracking Behavior in Animals and Mobile Robots. In (Vol. 55, pp. 127–135). Cleveland, Ohio: Journal of the Institute of Navigation. [Google Scholar]
- Wilson DA and Stevenson RJ Learning to Smell. Baltimore, MD: The Johns Hopkins University Press; 2006. [Google Scholar]
- Wilson CD, Serrano GO, Koulakov AA, & Rinberg D (2017) A primacy code for odor identity. Nature Communications, 8:1477 DOI: 10.1038/s41467-017-01432-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiltrout C, Dogra S, and Linster C. “Configurational and Nonconfigurational Interactions between Odorants in Binary Mixtures.” Behav Neurosci 117.2 (2003): 236–45. Print. [DOI] [PubMed] [Google Scholar]
- Wiltschko R, and Wiltschko W (2017) Considerations on the role of olfactory input in avian navigation. Journal of Experimental Biology, 220, 4347–4350 doi: 10.1242/jeb.168302 [DOI] [PubMed] [Google Scholar]
- Yeshurun Y and Sobel N An Odor is Not Worth a Thousand Words: From Multidimensional Odors to Unidimensional Odor Objects. Annual Review of Psychology 61:1, 219–241, 2010. [DOI] [PubMed] [Google Scholar]
- Young BD (2016). Smelling matter. Philosophical Psychology, 29(4), 520–534. doi: 10.1080/09515089.2015.1126814 [DOI] [Google Scholar]