Abstract
Background
Monotherapy with immune checkpoint inhibitors has generally been unsuccessful in men with advanced prostate cancer. Pre-clinical data support the notion that cryotherapy may improve immune-mediated and anti-tumor responses. The objective of this study was to assess the safety and feasibility of whole-prostate gland cryotherapy combined with pembrolizumab and androgen deprivation in men with oligometastatic hormone-sensitive prostate cancer.
Methods
This single-institution, pilot trial recruited 12 patients with newly diagnosed oligometastatic prostate cancer between 2015 and 2016. Patients underwent whole-prostate cryoablation combined with short-term androgen deprivation (eight months) and pembrolizumab (6 doses). The primary clinical endpoints were the number of patients with a PSA level of <0.6ng/mL at one year and the frequency of adverse events. Other outcome measures included progression-free survival and systemic therapy-free survival. Exploratory analyses included PD-L1 protein expression.
Results
Forty two percent (5/12) of patients had a PSAs of <0.6ng/mL at one year though only 2 of these patients had recovered their testosterone at this time point. Median progression-free survival was 14 months, and median systemic therapy-free survival was 17.5 months. PD-L1 expression was not detectable by IHC in patients with evaluable tissue. All adverse events were grade ≤2, and there were no apparent complications from cryotherapy.
Conclusions
Whole-prostate cryoablation combined with short-term androgen deprivation and pembrolizumab treatment was well tolerated and no safety concerns were observed in men with oligometastatic prostate cancer. Though local disease appeared effectively treated in the majority of men, the regimen only infrequency led to sustained disease control following testosterone recovery.
Keywords: immunotherapy, cryotherapy, PD-1 checkpoint, PD-L1, abscopal, prostate cancer, oligometastatic
Introduction
Immunological therapies have substantially impacted cancer care. Notably, checkpoint inhibitors have revitalized the role of immunotherapy and are now first line options in many tumor types 1-6. Prostate cancer is not highly immunogenic and is characterized by a lower tumor mutational burden than observed in other malignancies 7. While a subset of prostate cancers harbor mismatch repair deficiencies or biallelic inactivation of CDK12, which can potentially increase responsiveness to immune checkpoint blockade, this only accounts for a small fraction of patients 8-11. Further, the use of immune checkpoint inhibitors alone has yielded minimal benefits in unselected patients with advanced prostate cancer 12.
Multiple efforts are underway to help increase the immunogenicity of prostate cancer. Among these are the manipulation of the immune system through the use of combination checkpoint blockade, targeting novel immune regulators, immune agonists, utilizing androgen axis inhibitors to upregulate immune molecules or recruit inflammatory cells, and genetic engineering of T cells 12,13. An additional approach is to attempt to induce an abscopal effect by inducing immunogenic cell death in a way that primes a systemic anti-cancer response 14. Anecdotal evidence has suggested that cyroablation in particular may trigger an abscopal effect in prostate cancer 14. Further, a recent study by our group utilizing an animal model suggested that the combination of androgen deprivation therapy (ADT), PD-1 blockade and cryoablation could elicit distant, immune-mediated, anti-tumor responses 15.
Here we conduct a pilot trial of cryoablation combined with short course androgen deprivation and PD-1 blockade in men with low volume metastatic hormone-sensitive prostate cancer. The primary goals of the study were to evaluate the feasibility and safety of this combination, as well as to assess whether this regimen could result in disease control (PSA consistent with disease control after cryoablation in the setting of a non-castrate testosterone 16,17).
Patients and Methods
Study Design and Participants
This was a single-institution, single-arm, multi-therapy study. All patients provided written informed consent prior to enrollment. Patients were eligible if they had histologically or cytologically confirmed oligometastatic hormone-sensitive prostatic adenocarcinoma. Serum testosterone level had to be non-castrate (>150 ng/dL). Oligometastatic disease was defined by ≤ 5 extra-pelvic metastases that could include lymph nodes, bones, or soft tissue involvement on conventional imaging. Exclusion criteria included prior treatment with checkpoint inhibitor therapy or metastases involving the central nervous system. The study was approved by the Johns Hopkins Institutional Review Board and was registered at Clinicaltrials.gov ().
Intervention
Patients received androgen deprivation using degarelix (80mg) subcutaneously once per month for 8 months (after an initial loading dose of 240mg). Within one month of degarelix initiation, patients received pembrolizumab (200mg) intravenously every 3 weeks for up to 6 doses. Within 3 days of pembrolizumab initiation, patients underwent whole-gland cryoablation of the prostate. At least 4 months following cryoablation, patients were required to undergo a second prostate biopsy (this was achieved in 11 of the 12 men). Peripheral blood mononuclear cells (PBMC) were cryopreserved for T-cell receptor (TCR) sequencing analyses. The timing of androgen deprivation prior to local therapy was based on previous neoadjuvant studies demonstrating that castration allows for an increased T cell infiltrate into the prostate 18,19, and that mononuclear cell infiltrates are increased by week 3 to 4 after castration 20. We also wished to determine if our regimen would provide a sustained disease response in the absence of androgen deprivation. To this end, we limited androgen deprivation to allow for testosterone recovery. A duration of 8 months (which we designate as “short-term androgen deprivation” in his manuscript) was selected empirically in collaboration with our safety monitoring board as a duration that would allow for the majority of men to have a detectable testosterone by one year without substantial risk of disease progression 21. Eight months was also the same duration of hormonal therapy used in the investigational arm of the landmark PR-7 trial of intermittent vs. continuous hormonal therapy 22.
Histopathology and Imaging
All prostate biopsy samples were assessed by a genitourinary clinical pathologist. Post-treatment biopsies were evaluated for PD-L1, CD4, CD8, and FoxP3 proteins by single-stain semi-quantitative immunohistochemistry performed by QualTek Molecular and Clinical Laboratories (Santa Barbara, CA). PD-L1 staining was performed using the monoclonal antibody 22C3 (Merck). In the majority of cases, viable tumor was not present on post-treatment biopsies, and thus tumor associated lymphocytes and other immune markers were not assessed. Semi-quantitative immunohistochemistry was therefore generally performed on non-tumor prostate tissue (scored as: negative-0, low-1, moderate-2, high-3; corresponding to 0, 1–10, 11–20 and over 20 reactive lymphocytes per high power field).
Post-contrast abdomen and pelvis computed tomography or pelvis magnetic resonance imaging and whole-body 99mTc-methylene diphosphonate bone scans were performed at baseline and every 6 months on study. Imaging evaluations were scored using PCWG3 and RECIST 1.1 criteria by a central radiologist (S.P.R.)
Data Collection
Patient data were stored in an encrypted password-protected institutional database. Baseline demographics and clinical information were recorded. Patients were assessed for adverse events, and PSA was measured at baseline, 30 days after cryoablation, and then once every 12 weeks for one year. After one year, patients were treated according to physician preferences and follow-up data were obtained from centralized electronic medical records.
Outcome measurement
Primary outcomes included assessments of safety and oncological outcomes. Regarding safety, two non-laboratory grade 4 or any grade 5 adverse events would have caused trial termination and a claim of futility to be made. Regarding efficacy, we aimed to determine if the protocol would allow for disease control defined as a PSA that remained low in the presence of a non-castrate testosterone level. Cryoablation causes periurethral scaring of prostate tissue and as such, PSA levels defining disease control are elevated above 0.2 ng/mL. The primary outcome measure was thus the number of patients with a PSA level of <0.6 ng/mL at one year. PSA at this level has been shown to be a surrogate of disease control in men with localized prostate cancer undergoing cryoablation 16,17, and the majority of men were expected have testosterone recovery (>150 ng/dL) by the one-year time point 23. We hypothesized that if the protocol were effective, it would control disease both in the primary tumor and at distant sites, and furthermore, disease control would continue after testosterone recovery due to immune surveillance. A relatively small retrospective study of stereotactic radiation in men with oligometastatic prostate cancer that included men off of androgen deprivation suggested that 29% of men would have a PSA <0.2 ng/mL at 1 year 24. For this reason, we felt that this study would warrant a follow-up phase II protocol more focused on efficacy if 30% of men with a recovered testosterone had PSAs <0.6 ng/mL.
Other outcome measures were the evaluation of PD-L1 expression in post-treatment biopsies as well as clinical endpoints including progression-free survival (including PSA progression or progression by imaging) and systemic therapy-free survival. PSA response was defined by a ≥50% fold decrease in PSA. PSA progression was defined as a ≥25% increase and ≥2 ng/mL above the nadir PSA and confirmed by a second rise in PSA (PCWG3 definition). Systemic therapy was defined by any systemic therapy prescribed for the treatment of prostate cancer such as, but not limited to, androgen deprivation therapy, chemotherapy, or AR-targeted therapy. Castration-resistant prostate cancer (CRPC) was defined as clinical, radiographic, or PSA progression despite castrate levels of serum testosterone (<50 ng/dL) while on continuous ADT.
TCR Sequencing
Whole blood or tumor tissue samples were processed, and TCR beta chain CDR3 regions were sequenced by ImmunoSeq™ (Adaptive Biotechnologies, Seattle, WA), with primers annealing to V and J segments, resulting in amplification of rearranged VDJ segments from each cell. Clonality and richness values were obtained through the ImmunoSeq Analyzer software. Clonality was measured as 1-(entropy)/log2(# of productive unique sequences). Simpson clonality was also performed to allow comparison between blood and tissue samples. Differential abundance analysis was assessed, as previously described 25, to identify clones that were significantly expanded or contracted from baseline and following 6 cycles of pembrolizumab treatment. Expanded and contracted T cell clones or clonotypes post-pembrolizumab were identified from the peripheral blood of available patients. For one patient, we then compared his expanded and contracted peripheral T cell clones to his baseline tumor sample as well as his post-cryotherapy biopsy tumor sample to determine presence and dynamics of tumor restricted T cell clones.
Statistical analysis
Study sample size rationale and calculations are described in the Supplemental Methods. PSA response rates were depicted using waterfall plots. Kaplan-Meier methods were used to estimate survival functions. Cox proportional-hazard modeling was used to estimate PSA progression-free survival, systemic therapy-free survival, and time to CRPC. Due to the small sample size PD-L1 expression was dichotomized to low (1) vs. medium (2–3). Survival curves were generated in GraphPad Prism for figure generation. Statistical analyses were performed using STATA SE/15.1. Adverse events were tabulated, and severity and attribution were graded, according to CTCAE v4.1 criteria.
Results
Patient characteristics
Between Dec. 2015 and Nov. 2016, 13 patients were enrolled in the study. One patient was excluded prior to receipt of study therapy due to his decision to withdraw consent. For the remaining 12 patients, baseline demographic and disease characteristics are summarized in Table 1. The median age at study enrollment was 65.5 years. Most patients had Gleason sum ≥ 8 (91.7%) prostate cancer, were clinical T stage ≥ T2b (75%) and had more than one site of metastasis (75%). The median PSA at study initiation was 43.3 ng/mL. Three patients had RECIST measurable nodal disease, while the remainder had bone-predominant metastases.
Table 1.
Patient Baseline Demographics and Disease Characteristics
| Characteristic | Cohort (n=12) |
|---|---|
| Age, years, median (range) | 65.5 (55-78) |
| Race, n (%) | |
| White | 7 (58.3) |
| Black | 4 (33.3) |
| Other | 1 (8.3) |
| Gleason Sum, n (%) | |
| 7 | 1 (8.3) |
| 8 | 3 (25.0) |
| 9 | 7 (58.3) |
| 10 | 1 (8.3) |
| Clinical T Stage, n (%) | |
| T1c | 3 (25.0) |
| T2b | 5 (41.7) |
| T2c | 3 (25.0) |
| T3 | 1 (8.3) |
| N Stage, n (%) | |
| N0 | 7 (58.3) |
| N1 | 5 (41.7) |
| M Stage, n (%) | |
| M1a | 1 (8.3) |
| M1b | 11 (91.7) |
| Number of Metastases | |
| 1 | 3 (25.0) |
| 2-3 | 8 (66.7) |
| 4-5 | 1 (8.3) |
| RECIST measurable disease | |
| Yes | 3 (25.0) |
| No | 9 (75.0) |
| PSA, ng/mL, median (range) | 43.3 (2.7-394.0) |
| Alkaline Phosphatase U/L, median (range) | 78.5 (50-187) |
| Follow-Up, weeks, median (range) | 136.1 (78.6-170.9) |
Adverse Events
During the study period all reported adverse events were grade ≤ 2, with the majority of events being grade 1 (Table 2). The most common adverse events were fatigue, hot flashes, pain, and urinary-related events. One patient discontinued pembrolizumab after 4 cycles due to a grade 2 rash. After the one year of follow-up, one patient developed a lung lesion consistent with pulmonary sarcoidosis, which may have been possibly exacerbated by study treatment 26.
Table 2.
Safety and Adverse Events (n=12)
| Adverse Event | Affected/At Risk n (%) Grade 1 |
Affected/At Risk n (%) Grade 2 |
|---|---|---|
| Blood and lymphatic system disorders | ||
| Edema | 2 (16.7) | 1 (8.3) |
| Anemia | 1 (8.3) | 0 (0) |
| Gastrointestinal disorders | ||
| Nausea | 0 (0) | 1 (8.3) |
| Oral Pain | 1 (8.3) | 0 (0) |
| Constipation | 1 (8.3) | 1 (8.3) |
| Mucositis | 0 (0) | 1 (8.3) |
| Dry Mouth | 1 (8.3) | 0 (0) |
| Abdominal Pain | 1 (8.3) | 0 (0) |
| Groin Pain | 1 (8.3) | 0 (0) |
| General Disorders | ||
| Fatigue | 8 (66.7) | 0 (0) |
| Injection Site Reaction | 2 (16.7) | 0 (0) |
| Chills | 1 (8.3) | 0 (0) |
| Metabolism and nutrition disorders | ||
| Night Sweats | 1 (8.3) | 0 (0) |
| Musculoskeletal and connective tissue disorders | ||
| Pain | 3 (25) | 1 (8.3) |
| Pain, extremities | 2 (16.7) | 0 (0) |
| Nervous system disorders | ||
| Dysgeusia | 2 (16.7) | 0 (0) |
| Headache | 2 (16.7) | 0 (0) |
| Psychiatric disorders | ||
| Depression | 2 (16.7) | 0 (0) |
| Insomnia | 1 (8.3) | 0 (0) |
| Agitation | 1 (8.3) | 0 (0) |
| Renal and urinary disorders | ||
| Urinary incontinence | 2 (16.7) | 0 (0) |
| Urinary urgency | 2 (16.7) | 1 (8.3) |
| Bladder spasm | 1 (8.3) | 0 (0) |
| Urinary frequency | 3 (25) | 0 (0) |
| Nocturia | 1 (8.3) | 0 (0) |
| Hematuria | 2 (16.7) | 0 (0) |
| Reproductive system disorders | ||
| Penile pain | 1 (8.3) | 0 (0) |
| Respiratory, thoracic and mediastinal disorders | ||
| Voice change | 1 (8.3) | 0 (0) |
| Sarcoidosis | 1 (8.3) | 0 (0) |
| Skin and subcutaneous tissue disorders | ||
| Pruritus | 1 (8.3) | 1 (8.3) |
| Maculo-popular rash | 1 (8.3) | 1 (8.3) |
| Skin induration | 1 (8.3) | 0 (0) |
| Vascular disorders | ||
| Hot Flashes | 6 (50.0) | 0 (0) |
Endpoints
The median follow-up time of the enrolled patients was 31.3 months, with a range between 18.1 and 39.3 months. Of the 11 evaluable patients, post-cryotherapy biopsy samples showed benign disease in 9 patients while 2 patients had residual detectable cancer (Table 3). PSA response was achieved in 11 of the 12 patients (92%) (Fig. 1A). Of the three patients with RECIST measurable disease, two patients had a partial radiographic response, while the other one patient had evidence of progression during study therapy. In accordance with the study design, we evaluated PSA at 1 year as a surrogate for disease control with the assumption that the majority of men would have recovered their testosterone by this time point. At one year following therapy initiation, 5 patients (42%) achieved a PSA level of ≤ 0.6 ng/mL. However, the majority of patients, including three of the five patients with low PSAs, had not recovered their testosterone at this time. Over the course of the entire study, median PSA progression-free survival was 14.0 months, and the median systemic therapy-free survival was 17.5 months (Fig. 1B, C). The median time to developing CRPC was not reached at last follow-up (Fig. 1D). Notably, three patients rapidly progressed and died by the time of last follow-up. Conversely, one patient had not developed progressive disease (by PSA-, clinical-, or radiographic-metrics) after 25.6 months from study initiation, despite being off all therapies for over a year with testosterone recovery (PT12; Fig. 1E). Two additional patients had slowly rising PSAs and had yet to start a new systemic therapy for over one year after finishing androgen deprivation therapy and recovering testosterone levels (Fig. 1E).
Table 3.
Immunohistochemical Staining in Prostate Biopsies Following Cryoablation
| Patient | Gleason Grade |
PD-L1 | CD8 | CD4 | FoxP3 |
|---|---|---|---|---|---|
| 1 | 3+3=6 | 1 | 1 | 1 | 1 |
| 2 | Benign | 2 | 2 | 2 | 1 |
| 3 | Benign | 1 | 1 | 1 | 1 |
| 4 | Benign | 2 | 2 | 2 | 1 |
| 5 | 5+4=9 | 2 | 2 | 2 | 1 |
| 7 | Benign | 1 | 1 | 1 | 1 |
| 8 | Benign | 1 | 2 | 2 | 1 |
| 9 | Benign | 1 | 1 | 1 | 1 |
| 10 | Benign | 2 | 2 | 2 | 1 |
| 11 | Benign | 1 | 1 | 1 | 1 |
| 12 | Benign | 3 | 2 | 2 | 1 |
Fig. 1.
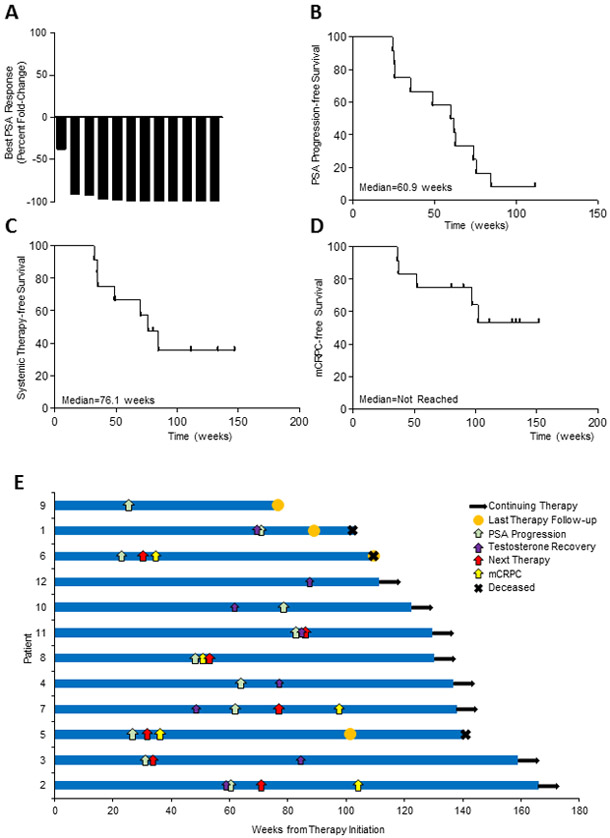
Outcomes in men with oligometastatic prostate cancer treated with whole prostate cryoablation in combination with androgen deprivation therapy and pembrolizumab. A, Waterfall plot of the best PSA response (percent fold change compared to baseline). B-D, Kaplan Meir survival analysis of (B) PSA progression-free, (C) systemic therapy-free, and (D) CRPC progression-free survival. E, Swimmers plots of PSA progression, new systemic therapy, CRPC, and death.
PD-L1 Expression and Evaluation in Post-treatment Biopsy Tissue
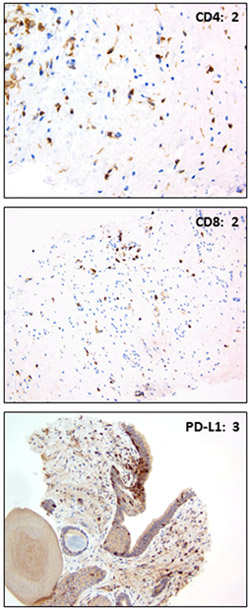
PD-L1 expression was analyzed by IHC in prostate biopsy tissue following the completion of pembrolizumab in 11 of the 12 patients. Two of the eleven patients undergoing protocol directed post treatment biopsy had viable tumor present and tumor infiltrating lymphocytes (TILs) for analysis. In both cases PD-L1 staining was absent on tumor cells. Non-tumor associated reactivity was additionally analyzed in all patients undergoing biopsy, and results are summarized in Table 3 (with representative images shown in Fig. 2). PD-L1 expression was not associated with PSA progression-free survival (P=0.434), systemic therapy-free survival (P=0.161), or time to CRPC (P=0.925).
Fig. 2.
Representative IHC staining of CD4, CD8, and PD-L1 in prostate biopsy tissue.
TCR Sequencing
Whole blood was collected prior to study initiation and following pembrolizumab treatment for TCR sequencing from 10 of the 12 patients. Diversity repertoire analyses showed that clonality and Daley-Smith richness were relatively stable at baseline and following 6 cycles of pembrolizumab overall for all patients. However, pretreatment PSA was significantly correlated with baseline T cell clonality at baseline (Spearman p=0.02, R2 = 0.46), although this correlation was not observed following 6 cycles of pembrolizumab treatment.
Of the 10 evaluable patients, one patient (PT9) appeared to have a higher frequency of contracted and expanded T cell clonotypes following 6 cycles of pembrolizumab (Fig. 3A). We thus TCR sequenced the baseline and post-treatment prostate biopsies for this patient to quantify the presence and dynamics of tumor restricted T cell clones. In this patient, T cell fraction increased in the prostate tumor biopsy tissue following 6 cycles of pembrolizumab treatment (Fig. 3B). Simpson clonality also increased in both the blood and prostate tumor tissue following 6 cycles of pembrolizumab (Fig. 3C). Looking at specific T cell clonotypes, nine of the clones expanded in the blood by cycle 6 were found in the post-treatment tumor sample and eight clones contracted in the blood were also found in the post-treatment tumor sample (Fig. 3D).
Fig. 3.
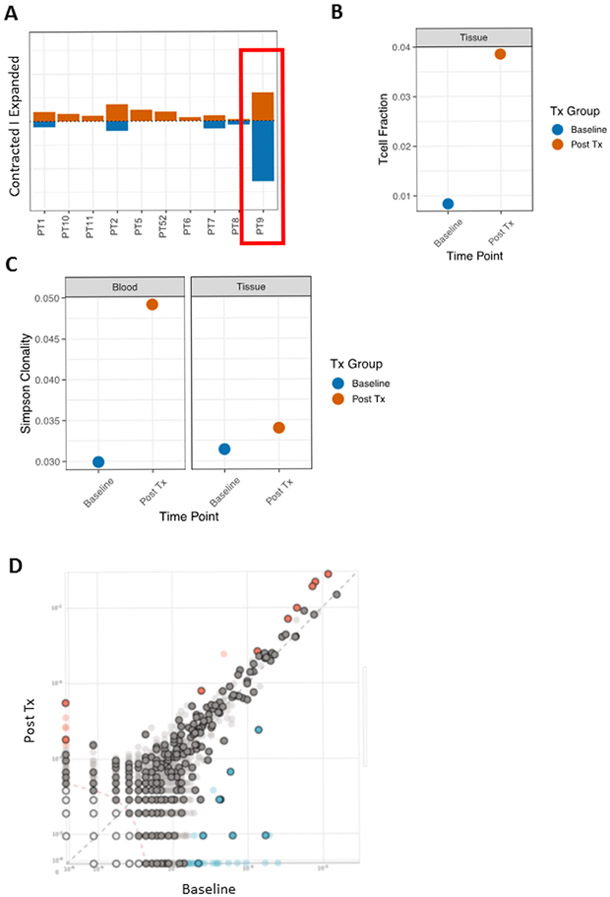
Peripheral and tumor tissue TCR clonotype dynamics following cryotherapy, ADT and pembrolizumab. A, Histogram by patient of contracted and expanded T cell clones following the treatment regimen. PT9 is highlighted. B, T cell fraction at baseline and post-treatment (Post Tx) in tumor biopsy tissue from PT9 following 6 cycles of pembrolizumab. C, Simpson clonality from PT9 in both the blood and prostate tumor tissue Post Tx. Simpson clonality allows comparisons between blood and tissue samples that have large differences in number of productive templates. For reference, median Simpson clonality in healthy adults is 0.03. D, T cell clonotype frequencies from PT9 blood are plotted at baseline and Post-Tx with the rose and blue circles denoting expanded and contracted clones. Clones that also shared in the Post-Tx tumor tissue biopsy are outlined in black.
Discussion
Immune checkpoint blockade may promote the development of CD8 memory cells, thereby potentially eliciting long-term responses in advanced disease. Certain tumor types appear more sensitive to immunotherapy as they either express dominant tumor-related antigens or have deficiencies in DNA regulation leading to a higher burden of tumor neoantigens. Established approaches to augment an anti-cancer immune response include cellular therapies that target tumor tissue type specific antigens, immune checkpoint blockade to promote and enhance T-cell responses, and the activation of stimulatory signaling molecules 13. In less immunogenic tumor types, such as prostate cancer, it is likely that combinatorial approaches will be necessary to elicit substantial tumor responses for the majority of patients 12. Here we report the results of a pilot trial in which checkpoint blockade, androgen deprivation, and prostatic cryoablation were utilized to potentially recruit immune cells, expose tumor antigens, and elicit a systemic, long-lasting anti-tumor immune response.
Our study recruited men with low volume (≤ 5 metastases) hormone-sensitive metastatic prostate cancer. We chose this population because therapy might be more efficacious in men with a lower disease burden and less immune tolerance, and it would allow for evaluation of distant tumor responses. Suggestive evidence that men with lower disease burden might have larger responses to immune based therapy comes from studies with Sipuleucel-T, the first FDA approved immune therapy for prostate cancer 27. Notably, though an initial study of checkpoint inhibition with ipilimumab in men with CRPC showed greater survival in a subset analysis of men with a greater number of better prognostic features and more limited disease treated with bone-directed radiation therapy, this was not strongly confirmed in a subsequent trial in men with better prognostic features and more limited disease who were not treated with radiation therapy 28,29.
Androgen deprivation therapy was incorporated in the treatment regimen. Aside from its direct anti-tumor activity, androgen deprivation therapy may additionally increase anti-tumor immune responses. Androgen deprivation therapy potentially increases T cell infiltration of prostate tumors 18,30 as well as mitigates immune tolerance to prostate cancer 31. Since T cell infiltration was not quantified in pre-treatment biopsies, we were unable to conclusively delineate increased infiltration, however the majority of post-treatment samples had detectable T cell infiltrates in non-tumor tissue.
Efforts to research local therapies as a means to incite an abscopal effect by causing prostate tumors to release antigens that then might allow for a systemic anti-cancer response are underway. Radiation therapy to tumor lesions has been a dominant modality tested to incite an in situ anti-tumor vaccine response. Since the initiation of this study, other clinical trials have reported oncological benefit from local therapy in men with low volume metastatic prostate cancer 32,33. These studies used external beam radiation for primary tumor treatment, and a current study is ongoing to establish the effect of cytoreductive prostatectomy in metastatic prostate cancer patients (). Cryotherapy was used for treatment of the primary tumor in our trial based on anecdotal and preclinical evidence, which identified it as potentially producing the most profound local immune response 14,15. Though reported in a retrospective series, this is the first report of cytoreductive cryoablation in a clinical trial setting 34. Patients in our study tolerated cryoablation well with all patients being treated in an outpatient setting, no grade 3 adverse events were observed, and low rates of urinary side effects ensued. In addition, when combined with a short course (8 months) of androgen deprivation, cryoablation resulted in an apparent eradication of local (intra-prostatic) disease in 9 of the 11 evaluated men. The low morbidity and low cost of cryoablation should prompt further study of this modality as a local immune-modulating treatment for men with low volume metastatic disease.
The primary objective of this study was to evaluate feasibility and safety, and future studies would be needed to determine the clinical efficacy of whole prostate cryoablation in combination with androgen deprivation therapy and pembrolizumab for men with de novo oligometastatic prostate cancer. Regardless, this study provides an indication that although the treatment strategy was well-tolerated, there were only modest long-term responses. Our initial efficacy endpoint was the evaluation of disease control at 1 year with the assumption that the majority of men would have recovered to a non-castrate testosterone during this period. This was not the case as only the minority of men had begun to normalize their testosterone by this time. Regardless, the majority of men did achieve non-castrate testosterone during follow-up and overall, prolonged treatment responses following recovery of testosterone were not common and were observed in only one patient (with two additional patients having slow PSA elevations not requiring additional therapy to date). The responding patient had Gleason grade group 3, M1b disease and showed the highest levels of PD-L1 staining in his non-tumor associated lymphocytes. It should be noted that three patients in our study progressed rapidly to castrate-resistant disease. The standard of care for hormone naïve metastatic prostate cancer has changed over the last several years with multiple studies demonstrating that intensification of androgen axis-based therapy can prolong metastasis-free and overall survival in men with low or high-volume disease 35-37.
TCR repertoire analyses from peripheral blood showed limited changes for most patients; however, one patient had an increased number of expanded and contracted T cell clonotypes in the peripheral blood, and a subset were also present in the post-treatment tumor biopsy sample following the treatment regimen. One caveat of the TCR repertoire analyses is that sequencing depth was different between the blood and tissue samples and could possibly account for the number of expanded and contracted clones shared between these two compartments. Future studies will be needed to determine correlations between TCR repertoire changes and response.
Overall, these data are hypothesis generating and highlight that alternative or modified approaches to harnessing the immune system against prostate cancer should be considered in future trials.
Conclusion
This is the first reported prospective feasibility and safety study of cryoablation to the prostate combined with short-term androgen deprivation therapy and pembrolizumab in men with oligometastatic hormone-sensitive prostate cancer. The approach was safe and provided local disease control in the majority of patients but did not give an early indication of sustained disease control following testosterone recovery in the majority of men.
Supplementary Material
Acknowledgements
We are grateful to the patients and their families for participating in this trial. This work was supported by PCF young investigator award (Ashley E.Ross) as well as partial funding from Merck and Healthtronics. Paula J. Hurley acknowledges support from the American Cancer Society (131356-RSG-17–160-01-CSM) and The National Cancer Institute/National Institute of Health RO1CA211695–01A1. Phuoc Tran acknowledges support from Ronald Rose, Joan Lazar, Movember Foundation, Prostate Cancer Foundation; NIH/NCI (R01CA166348, U01CA212007, U01CA231776 and R21CA223403). Emmanuel S. Antonarakis is partially funded by National Institutes of Health Cancer Center Support Grant P30 CA006973, and by Department of Defense grant W81XWH-16-PCRP-CCRSA.
Footnotes
Conflict of Interest
Ashley E. Ross has previously been a consultant for Healthtronics. Phuoc Tran has grant support from Astellas Pharm., RefleXion Medical, Inc and Bayer Healthcare; and has consulted for RefleXion Medical, Inc. Charles G. Drake acknowledges stock or ownership interests in Compugen, Harpoon, Kleo, Potenza, and Tizona Therapeutics, and has served as a consultant for Agenus, Dendreon, Janssen Oncology, Eli Lilly, Merck, AstraZeneca, MedImmune, Pierre Fabre, Genentech, and Genocea Biosciences. Emmanuel S. Antonarakis is a paid consultant/advisor to Janssen, Astellas, Sanofi, Dendreon, Medivation, AstraZeneca, Clovis, and Merck; he has received research funding to his institution from Janssen, Johnson & Johnson, Sanofi, Dendreon, Genentech, Novartis, Tokai, Bristol Myers-Squibb, AstraZeneca, Clovis, and Merck; and he is the co-inventor of an AR-V7 biomarker technology that has been licensed to Qiagen. No potential conflict of interests were disclosed by the other authors.
References
- 1.Postow MA, Chesney J, Pavlick AC, Robert C, Grossmann K, McDermott D et al. Nivolumab and ipilimumab versus ipilimumab in untreated melanoma. N Engl J Med 2015; 372(21): 2006–2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gandhi L, Rodriguez-Abreu D, Gadgeel S, Esteban E, Felip E, De Angelis F et al. Pembrolizumab plus Chemotherapy in Metastatic Non-Small-Cell Lung Cancer. N Engl J Med 2018; 378(22): 2078–2092. [DOI] [PubMed] [Google Scholar]
- 3.Paz-Ares L, Luft A, Vicente D, Tafreshi A, Gumus M, Mazieres J et al. Pembrolizumab plus Chemotherapy for Squamous Non-Small-Cell Lung Cancer. N Engl J Med 2018; 379(21): 2040–2051. [DOI] [PubMed] [Google Scholar]
- 4.Hellmann MD, Ciuleanu TE, Pluzanski A, Lee JS, Otterson GA, Audigier-Valette C et al. Nivolumab plus Ipilimumab in Lung Cancer with a High Tumor Mutational Burden. N Engl J Med 2018; 378(22): 2093–2104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Topalian SL. Targeting Immune Checkpoints in Cancer Therapy. JAMA 2017; 318(17): 1647–1648. [DOI] [PubMed] [Google Scholar]
- 6.Postow MA, Sidlow R, Hellmann MD. Immune-Related Adverse Events Associated with Immune Checkpoint Blockade. N Engl J Med 2018; 378(2): 158–168. [DOI] [PubMed] [Google Scholar]
- 7.Lawrence MS, Stojanov P, Polak P, Kryukov GV, Cibulskis K, Sivachenko A et al. Mutational heterogeneity in cancer and the search for new cancer-associated genes. Nature 2013; 499(7457): 214–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wu YM, Cieslik M, Lonigro RJ, Vats P, Reimers MA, Cao X et al. Inactivation of CDK12 Delineates a Distinct Immunogenic Class of Advanced Prostate Cancer. Cell 2018; 173(7): 1770–1782 e1714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Le DT, Durham JN, Smith KN, Wang H, Bartlett BR, Aulakh LK et al. Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science 2017; 357(6349): 409–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Antonarakis ES. Cyclin-Dependent Kinase 12, Immunity, and Prostate Cancer. N Engl J Med 2018; 379(11): 1087–1089. [DOI] [PubMed] [Google Scholar]
- 11.Abida W, Cheng ML, Armenia J, Middha S, Autio KA, Vargas HA et al. Analysis of the Prevalence of Microsatellite Instability in Prostate Cancer and Response to Immune Checkpoint Blockade. JAMA Oncol 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Isaacsson Velho P, Antonarakis ES. PD-1/PD-L1 pathway inhibitors in advanced prostate cancer. Expert Rev Clin Pharmacol 2018; 11(5): 475–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Comiskey MC, Dallos MC, Drake CG. Immunotherapy in Prostate Cancer: Teaching an Old Dog New Tricks. Curr Oncol Rep 2018; 20(9): 75. [DOI] [PubMed] [Google Scholar]
- 14.Abdo J, Cornell DL, Mittal SK, Agrawal DK. Immunotherapy Plus Cryotherapy: Potential Augmented Abscopal Effect for Advanced Cancers. Front Oncol 2018; 8: 85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Benzon B, Glavaris SA, Simons BW, Hughes RM, Ghabili K, Mullane P et al. Combining immune check-point blockade and cryoablation in an immunocompetent hormone sensitive murine model of prostate cancer. Prostate Cancer Prostatic Dis 2018; 21(1): 126–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Levy DA, Pisters LL, Jones JS. Prognostic value of initial prostate-specific antigen levels after salvage cryoablation for prostate cancer. BJU Int 2010; 106(7): 986–990. [DOI] [PubMed] [Google Scholar]
- 17.Levy DA, Ross AE, ElShafei A, Krishnan N, Hatem A, Jones JS. Definition of biochemical success following primary whole gland prostate cryoablation. J Urol 2014; 192(5): 1380–1384. [DOI] [PubMed] [Google Scholar]
- 18.Mercader M, Bodner BK, Moser MT, Kwon PS, Park ES, Manecke RG et al. T cell infiltration of the prostate induced by androgen withdrawal in patients with prostate cancer. Proc Natl Acad Sci U S A 2001; 98(25): 14565–14570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gannon PO, Poisson AO, Delvoye N, Lapointe R, Mes-Masson AM, Saad F. Characterization of the intra-prostatic immune cell infiltration in androgen-deprived prostate cancer patients. J Immunol Methods 2009; 348(1–2): 9–17. [DOI] [PubMed] [Google Scholar]
- 20.Mercader M, Sengupta S, Bodner BK, Manecke RG, Cosar EF, Moser MT et al. Early effects of pharmacological androgen deprivation in human prostate cancer. BJU Int 2007; 99(1): 60–67. [DOI] [PubMed] [Google Scholar]
- 21.Nam W, Choi SY, Yoo SJ, Ryu J, Lee J, Kyung YS et al. Factors associated with testosterone recovery after androgen deprivation therapy in patients with prostate cancer. Investig Clin Urol 2018; 59(1): 18–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Crook JM, O’Callaghan CJ, Duncan G, Dearnaley DP, Higano CS, Horwitz EM et al. Intermittent androgen suppression for rising PSA level after radiotherapy. N Engl J Med 2012; 367(10): 895–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Murthy V, Norman AR, Shahidi M, Parker CC, Horwich A, Huddart RA et al. Recovery of serum testosterone after neoadjuvant androgen deprivation therapy and radical radiotherapy in localized prostate cancer. BJU Int 2006; 97(3): 476–479. [DOI] [PubMed] [Google Scholar]
- 24.Ahmed KA, Barney BM, Davis BJ, Park SS, Kwon ED, Olivier KR. Stereotactic body radiation therapy in the treatment of oligometastatic prostate cancer. Front Oncol 2012; 2: 215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cha E, Klinger M, Hou Y, Cummings C, Ribas A, Faham M et al. Improved survival with T cell clonotype stability after anti-CTLA-4 treatment in cancer patients. Sci Transl Med 2014; 6(238): 238ra270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cotliar J, Querfeld C, Boswell WJ, Raja N, Raz D, Chen R. Pembrolizumab-associated sarcoidosis. JAAD Case Rep 2016; 2(4): 290–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schellhammer PF, Chodak G, Whitmore JB, Sims R, Frohlich MW, Kantoff PW. Lower baseline prostate-specific antigen is associated with a greater overall survival benefit from sipuleucel-T in the Immunotherapy for Prostate Adenocarcinoma Treatment (IMPACT) trial. Urology 2013; 81(6): 1297–1302. [DOI] [PubMed] [Google Scholar]
- 28.Kwon ED, Drake CG, Scher HI, Fizazi K, Bossi A, van den Eertwegh AJ et al. Ipilimumab versus placebo after radiotherapy in patients with metastatic castration-resistant prostate cancer that had progressed after docetaxel chemotherapy (CA184–043): a multicentre, randomised, double-blind, phase 3 trial. Lancet Oncol 2014; 15(7): 700–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Beer TM, Kwon ED, Drake CG, Fizazi K, Logothetis C, Gravis G et al. Randomized, Double-Blind, Phase III Trial of Ipilimumab Versus Placebo in Asymptomatic or Minimally Symptomatic Patients With Metastatic Chemotherapy-Naive Castration-Resistant Prostate Cancer. J Clin Oncol 2017; 35(1): 40–47. [DOI] [PubMed] [Google Scholar]
- 30.Shen YC, Ghasemzadeh A, Kochel CM, Nirschl TR, Francica BJ, Lopez-Bujanda ZA et al. Combining intratumoral Treg depletion with androgen deprivation therapy (ADT): preclinical activity in the Myc-CaP model. Prostate Cancer Prostatic Dis 2018; 21(1): 113–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Drake CG, Doody AD, Mihalyo MA, Huang CT, Kelleher E, Ravi S et al. Androgen ablation mitigates tolerance to a prostate/prostate cancer-restricted antigen. Cancer Cell 2005; 7(3): 239–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Parker CC, James ND, Brawley CD, Clarke NW, Hoyle AP, Ali A et al. Radiotherapy to the primary tumour for newly diagnosed, metastatic prostate cancer (STAMPEDE): a randomised controlled phase 3 trial. Lancet 2018; 392(10162): 2353–2366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Boeve LMS, Hulshof M, Vis AN, Zwinderman AH, Twisk JWR, Witjes WPJ et al. Effect on Survival of Androgen Deprivation Therapy Alone Compared to Androgen Deprivation Therapy Combined with Concurrent Radiation Therapy to the Prostate in Patients with Primary Bone Metastatic Prostate Cancer in a Prospective Randomised Clinical Trial: Data from the HORRAD Trial. Eur Urol 2019; 75(3): 410–418. [DOI] [PubMed] [Google Scholar]
- 34.Sheng MX, Wan LL, Liu CM, Liu CX, Chen SS. Cytoreductive cryosurgery in patients with bone metastatic prostate cancer: A retrospective analysis. Kaohsiung J Med Sci 2017; 33(12): 609–615. [DOI] [PubMed] [Google Scholar]
- 35.Chi KN, Agarwal N, Bjartell A, Chung BH, Pereira de Santana Gomes AJ, Given R et al. Apalutamide for Metastatic, Castration-Sensitive Prostate Cancer. N Engl J Med 2019; 381(1): 13–24. [DOI] [PubMed] [Google Scholar]
- 36.Davis ID, Martin AJ, Stockler MR, Begbie S, Chi KN, Chowdhury S et al. Enzalutamide with Standard First-Line Therapy in Metastatic Prostate Cancer. N Engl J Med 2019; 381(2): 121–131. [DOI] [PubMed] [Google Scholar]
- 37.James ND, de Bono JS, Spears MR, Clarke NW, Mason MD, Dearnaley DP et al. Abiraterone for Prostate Cancer Not Previously Treated with Hormone Therapy. N Engl J Med 2017; 377(4): 338–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.