Abstract
Context.
Detailed diagnostic features of acute myeloid leukemia in Down syndrome are lacking, leading to potential misdiagnoses as standard acute myeloid leukemia occurring in patients with Down syndrome.
Objective.
To evaluate diagnostic features of acute myeloid leukemia and myelodysplastic syndrome in patients with Down syndrome.
Design.
Diagnostic bone marrow samples from 163 patients enrolled in the Children’s Oncology Group study AAML0431 were evaluated by using central morphologic review and institutional immunophenotyping. Results were compared to overall survival, event-free survival, GATA1 mutation status, cytogenetics, and minimal residual disease results.
Results.
Sixty myelodysplastic syndrome and 103 acute myeloid leukemia samples were reviewed. Both had distinctive features compared to those of patients without Down syndrome. They showed megakaryocytic and erythroid, but little myeloid, dysplasia; and marked megakaryocytic hyperplasia with unusual megakaryocyte morphology. In acute myeloid leukemia cases, megakaryoblastic differentiation of blasts was most common (54 of 103, 54%); other cases showed erythroblastic (11 of 103, 11%), mixed erythroid/megakaryoblastic (20 of 103, 19%), or no differentiation (10 of 103, 10%). Myelodysplastic syndrome and acute myeloid leukemia cases had similar event-free survival and overall survival. Leukemic subgroups showed interesting, but not statistically significant, trends for survival and minimal residual disease. Cases with institutional diagnoses of French American British M1-5 morphology showed typical features of Down syndrome disease, with survival approaching that of other cases.
Conclusions.
Myelodysplastic syndrome and acute myeloid leukemia in Down syndrome display features that allow discrimination from standard cases of disease. These distinctions are important for treatment decisions, and for understanding disease pathogenesis. We propose specific diagnostic criteria for Down syndrome–related subtypes of acute myeloid leukemia and myelodysplastic syndrome.
Down syndrome (DS) is a common genetic disorder, occurring in approximately 1 in 700 births.1 The incidence of acute leukemia, both myeloid (AML) and lymphoid (ALL), is markedly increased in DS.2 ALL in DS shares genetic features with that occurring in non-DS patients, albeit with a different distribution of genotypes including a lower level of good-risk subtypes.3 A unique type of AML (DS-AML) occurs at a very high rate in DS patients younger than 4 years, estimated at 3- to 400-fold that of AML in the general pediatric population.2,4 It has been most frequently characterized as acute megakaryoblastic leukemia (AML-MK) (an otherwise uncommon variant of AML),5-7 and is often preceded by a phase of marrow failure with dysplastic marrow morphology resembling myelodysplastic syndrome (MDS),which is otherwise uncommon in children.6,8-10 It may also be preceded by neonatal transient abnormal myelopoiesis (TAM), and like TAM has a high frequency of somatic mutations in GATA binding factor 1 (GATA1), which are uncommon in AML in non-DS patients.11-15 DS-AML in patients younger than 4 years has an excellent response to modified AML therapy, as does the MDS-like syndrome that often precedes it.16-18 Common subtypes of MDS and AML that occur in non-DS patients (standard AML) also occur in DS patients, and are the predominant subtype of AML in patients older than 4 years.19 Unlike in ALL, the incidence and genetic subtypes of standard AML in DS patients are unclear. AML in DS patients older than 4 years is only rarely associated with a mutation in GATA1.19 DS patients older than 4 years with standard AML lack the good prognosis of DS-AML in young patients19; the prognostic impact of GATA1 mutation in older patients is unclear. Standard subtypes of AML presumably also occur in DS patients younger than 4 years, but at a very low percentage of cases (<1%, given the high incidence of DS-AML in these patients). These cases tend to be inaccurately diagnosed (usually overdiagnosed) and poorly characterized, in part because of imprecise diagnostic criteria for DS-AML; these cases are frequently treated the same as DS-AML, with uncertain treatment outcome owing to inaccurate recognition. More precise diagnostic criteria for DS-AML are needed.
OBJECTIVE
This study aimed to determine the morphologic and immunophenotypic spectrum of AML in DS patients, and its impact on treatment outcomes. To clarify the distinctive features of DS-AML and MDS, we reviewed morphology and institutional immunophenotyping of cases entered on a Children’s Oncology Group (COG) protocol for treatment of MDS and AML in DS patients, comparing pathologic features to treatment response and outcome.
DESIGN
Diagnostic bone marrows from 163 patients on COG protocol AAML0431 (The Treatment of DS Children with AML and MDS Under the Age of 4 Years, a Groupwide Phase III Study; clinical results reported separately17) were reviewed. Protocol eligibility was based on “intent to treat,” with subsequent central review. Submitting institutions reported French American British (FAB) morphology for their cases. Central review included evaluation of blast percentage and morphology, multilineage dysplasia, and fibrosis.20-22 Dysplasia was noted if it involved 10% or more of cells in a sublineage. A diagnosis of AML versus MDS was made by using a threshold of 20% blasts in the marrow. Cases were classified using percentage blasts and blast sublineage as MDS, AML-MK, acute erythroblastic leukemia (AML-E), AML with mixed MK and E features (AML-MK/E), and AML unclassifiable (AML-U, cases lacking demonstrable sublineage differentiation beyond generic myeloid). Blast sublineage was assigned by using morphology, as well as by review of institutional flow cytometry and immunohistochemistry, as available. Megakaryocytic differentiation was defined by the presence of blasts with cytoplasmic blebbing on morphology and/or the presence of greater than 20% cluster of differentiation (CD) 41, CD61 or CD42b expression in blasts. Erythroid differentiation was defined as blasts with deeply basophilic cytoplasm and lacking blebbing, a background of prominent erythrodysplasia, and/or the presence of greater than 20% CD36, glycophorin A, and/or CD71 expression in blasts. If features of both megakaryocytic and erythroid differentiation were present, the case was classified as AML-MK/E. Cases that had sufficient data for detailed evaluation of blast sublineage, with no discernible differentiation beyond generic myeloid, were classified as AML-undifferentiated (AML-U). For purposes of analysis “AML not otherwise specified” (AML-NOS) was used to designate cases in this study with sufficient information to confirm diagnosis of typical DS-AML but lacking sufficient data to allow subclassification; of note, this is not a specific subclassification type, but reflects insufficient data to allow subclassification. Morphologic review was conducted by 4 observers. As reported in the treatment study article, standard G-banding cytogenetic analysis was performed at COG-validated individual institutions and reviewed centrally by 2 observers.17 As also reported in the treatment study article, optional minimal residual disease (MRD) analysis at day 28 after initiation of treatment was conducted by flow cytometry on a limited number of patients in a central reference laboratory (St. Jude Children’s Research Hospital, Memphis, Tennessee); antibodies used were CD7, CD11b, CD38, CD34, CD33, CD117, CD45, Anti–HLA-Dr, CD4, CD13, CD133, CD15, CD56, CD41, and CD19.17,23 Optional GATA1 mutation analysis was performed at a central reference laboratory (The Hospital for Sick Children, Toronto, Ontario, Canada), using previously published methods.15
The National Cancer Institute’s central institutional review board and institutional review boards at each enrolling center approved the trial; patients and their families provided informed consent or assent as appropriate. The trial was conducted in accordance with the Declaration of Helsinki. The trial was registered at www.clinicaltrials.gov as NCT00369317.
Statistical analysis was performed by several methods, using data from AAML0431, which were current as of June 30, 2016. The χ2 test was used to test the significance of observed differences in proportions, and the Fisher exact test was used when data were sparse. The Kaplan-Meier method was used to estimate overall survival (OS) (defined as time from study entry to death) and event-free survival (EFS) (time from study entry until failure to achieve complete remission during induction, relapse, or death). Children lost to follow-up were censored at their date of last known contact. The log-rank test was used to compare OS and EFS. All estimates are reported with 2 times the Greenwood standard errors. A P value <.05 was considered statistically significant.
RESULTS
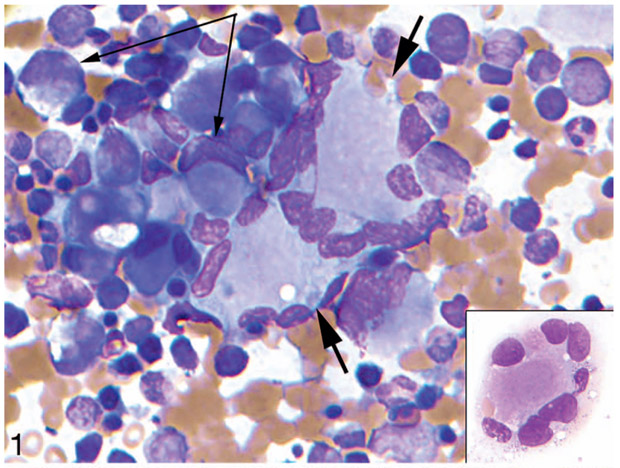
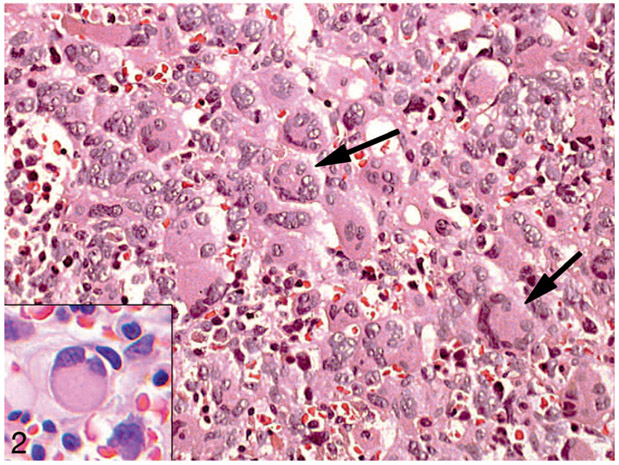
Suitable material was submitted for central evaluation on 163 cases. Of these cases, 60 were found to have MDS and 103, AML (Table 1). Both MDS and AML cases were characterized by hyperplasia and dysplasia of the megakaryocytic lineage (55 of 58 MDS cases [95%] and 89 of 92 AML cases [97%]) and erythroid lineage (43 of 57 MDS cases [75%] and 59 of 88 AML cases [67%]), both typically overt by morphology. By contrast myeloid dysplasia was infrequent (6 of 60 MDS cases [10%] and 6 of 94 AML cases [6%]) and minimal when present, at or near the minimal 10% threshold for positivity. Erythroid dysplasia consisted predominantly of megaloblastoid change. Megakaryocytic dysplasia commonly included clustering, variable cell size, and hypolobated nuclei in small megakaryocytes (Figures 1 and 2). Megakaryocytes also frequently showed distinctive morphology, including a central cytoplasmic mass (amphophilic with Wright-Giemsa staining [Figure 1 inset], eosinophilic with hematoxylin-eosin staining [Figure 2 inset]) with peripherally displaced nuclei (“ring” megakaryocytes in multinucleate cells, resembling Touton giant cells; “signet ring-like” megakaryocytes in small uninucleate cells) (Figures 1 and 2).
Table 1.
Summary of Findings in Marrow From Patients With Down Syndrome MDS and AML
| Dysplasiab,c | |||||||
|---|---|---|---|---|---|---|---|
| Diagnosis | Cases | Blastsa | MY | E | MK | MK Rings/Inclusionsb | Reticulin Fibrosisb,d |
| MDS | 60 | 11% (2%–17%) | 6/60 (10%) | 43/57 (75%) | 55/58 (95%) | 38/57 (67%) | 25/25 (100%) |
| AML-MK | 54 | 42% (20%–90%) | 5/49 (10%) | 26/46 (57%) | 47/48 (98%) | 27/46 (59%) | 11/12 (92%) |
| AML-E | 11 | 37% (22%–76%) | 0/10 (0%) | 9/10 (90%) | 10/10 (100%) | 4/8 (50%) | 4/4 (100%) |
| AML-MK/E | 20 | 54% (22%–90%) | 0/19 (0%) | 14/18 (78%) | 19/19 (100%) | 7/17 (41%) | 6/6 (100%) |
| AML-U | 10 | 51% (25%–90%) | 1/10 (10%) | 8/9 (89%) | 9/10 (90%) | 2/7 (29%) | 5/5 (100%) |
| AML-NOS | 7 | 43% (26%–75%) | 0/6 (0%) | 2/5 (40%) | 4/5 (80%) | 1/3 (33%) | 1/1 (100%) |
Abbreviations: AML, acute myeloid leukemia; AML-E, AML with erythroblastic differentiation; AML-MK, AML with megakaryoblastic differentiation; AML-MK/E, AML with mixed differentiation; AML-NOS, AML with insufficient information for sublineage assessment; AML-U, AML with no sublineage differentiation; E, erythroid; MDS, myelodysplastic syndrome; MK, megakaryocytic; MY, myeloid.
Mean (range).
Positive/evaluable (%).
Fibrosis was defined as positive results with a reticulin stain. When present, fibrosis was grade 1/3 in the grading system of Thiele et al.21
Figure 1.
Dysplastic megakaryocytic hyperplasia in bone marrow in acute myeloid leukemia in a patient with Down syndrome. Bone marrow aspirate smear. Note clustered hyperplastic megakaryocytes with a peripheral ring of nuclei (short arrows and inset) and smaller megakaryocytes with signet ring forms (long arrows) (Wright-Giemsa, original magnification ×200 [inset same]).
Figure 2.
Dysplastic megakaryocytes in bone marrow in acute myeloid leukemia in a patient with Down syndrome. Bone marrow biopsy. Note clustered hyperplastic megakaryocytes with a peripheral ring of nuclei (arrows) and smaller megakaryocytes with signet ring forms (inset) (hematoxylin-eosin, original magnifications ×100 and ×400 [inset]).
Megakaryoblastic differentiation of blasts (AML-MK) was the most frequent AML subtype observed (54 of 103 [52%]), but cases with erythroblastic (AML-E) (11 of 103 [11%]) or mixed differentiation (AML-MK/E) (20 of 103 [19%]) were frequent (Table 1). The sublineage of blasts could not be assigned in 17 cases. Of these, 10 (10 of 103 [10%]) had appropriate testing (morphology, flow immunophenotyping, or immunohistochemistry) with no discernible sublineage commitment beyond generic myeloid (AML-U). The remaining 7 cases (7 of 103 [7%]) had sufficient results to confirm DS-type disease, but insufficient testing to allow sublineage assignment (AML-NOS). AML-U cases (and insufficiently analyzed AML-NOS cases) had the lowest rate of aberrant MK morphology (MK rings or inclusions) (2 of 7 [29%] and 1 of 3 [33%], respectively), while MDS and AML-MK cases showed the highest (38 of 57 [67%] and 27 of 46 [59%], respectively). When present, fibrosis was mild, consisting of fine reticulin fibrosis (MF 1/3).21
A single case was identified with morphology and immunophenotype typical of standard (non-DS) AML, with lack of dysplasia, lack of megakaryocytic and erythroid hyperplasia, lack of megakaryoblastic or erythroblastic differentiation, and increased typical myeloblasts. This case was interpreted as a random case of standard AML occurring in a DS patient, and is not included in Table 1.
Flow cytometric results are presented in Table 2. Regardless of morphology, blasts by institutional flow cytometric analysis showed a high rate of positivity for CD13 (100 of 113 [88%]), CD33 (127 of 129 [98%]), CD38 (45 of 45 [100%]), CD7 (123 of 124 [99%]), and CD117 (110 of 113 [97%]), with a lower rate of positivity for CD34 (91 of 122 [75%]). Subsets other than AML-E and AML-U had high rates of positivity for MK markers CD41 (64 of 79 [81%]), CD42B (15 of 15 [100%]), and CD61 (71 of 89 [80%]). AML-E cases had high rates of positivity for CD36 (5 of 5 [100%]) and CD71 (5 of 5 [100%]), while AML-U cases were negative for both megakaryocytic and erythroid antigen expression.
Table 2.
Summary of Institutional Flow Results on Blasts
| Diagnosis | CD13a | CD33a | CD34a | CD117a | CD38a | CD7a | CD41a | CD42ba | CD61a | CD36a | CD71a | CD56a |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MDS | 28/34 (82) | 41/42 (98) | 28/42 (67) | 35/35 (100) | 12/12 (100) | 37/38 (97) | 21/27 (78) | 6/6 (100) | 26/34 (76) | 13/13 (100) | 6/6 (100) | 16/24 (67) |
| AML-MK | 37/41 (90) | 45/46 (98) | 36/43 (84) | 39/41 (95) | 13/13 (100) | 44/44 (100) | 31/36 (86) | 6/6 (100) | 33/40 (83) | X | 9/10 (90) | 19/22 (86) |
| AML-E | 8/9 (89) | 10/10 (100) | 5/8 (63) | 9/10 (90) | 5/5 (100) | 10/10 (100) | 0/5 (0) | X | 0/8 (0) | 5/5 (100) | 5/5 (100) | 3/5 (60) |
| AML-MK/E | 15/16 (94) | 18/18 (100) | 13/15 (87) | 14/14 (100) | 9/9 (100) | 20/20 (100) | 12/16 (75) | 3/3 (100) | 12/15 (80) | 15/15 (100) | 10/10 (100) | 7/8 (88) |
| AML-U | 9/10 (90) | 10/10 (100) | 5/10 (50) | 10/10 (100) | 6/6 (100) | 10/10 (100) | 0/6 (0) | 0/2 (0) | 0/7 (0) | X | X | 5/6 (83) |
| AML-NOS | 3/3 (100) | 3/3 (100) | 4/4 (100) | 3/3 (100) | X | 2/2 (100) | X | X | 0/1 (0) | X | X | X |
Abbreviations: AML, acute myeloid leukemia; AML-E, AML with erythroblastic differentiation; AML-MK, AML with megakaryoblastic differentiation; AML-MK/E, AML with mixed MK and E differentiation; AML-NOS, AML with insufficient information for sublineage classification; AML-U, AML with no identifiable sublineage differentiation; CD, cluster of differentiation; MDS, myelodysplastic syndrome; X, no cases tested.
Cases positive/cases tested (% positive of tested cases).
Table 3 compares institutional FAB diagnoses to central review results. Of note, submitting institutions’ diagnoses included 12 cases with diagnoses of FAB M1-5, all of which met central diagnostic criteria for DS-AML. AML-E and AML-U cases generated high levels of discordant institutional diagnoses (5 of 11 [45%] and 7 of 10 [70%], respectively).
Table 3.
Comparison of Institutional Versus Central Diagnosesa
| Inst DX | Inst Total | Cntrl DX |
||||||
|---|---|---|---|---|---|---|---|---|
| MDS | AML-MK | AML-MK/E | AML-E | AML-U | AML-NOS | AML-Other | ||
| MDS | 44 | 35 (80%) | 4 | 1 | 3 | 1 | ||
| AML-M0 | 3 | 1 | 1 | 1 | ||||
| AML-M1 | 5 | 3 | 1 | 1 | ||||
| AML-M2 | 6 | 1 | 1 | 1 | 2 | 1 | ||
| AML-M5 | 1 | 1 | ||||||
| AML-M6 | 3 | 1 | 2 | |||||
| AML-M7 | 70 | 19 (27%) | 32 (46%) | 12 (17%) | 1 | 3 | 3 | |
| AML-MX | 31 | 5 | 13 | 4 | 2 | 5 | 1 | 1 |
| Total | 163 | 60 | 54 | 20 | 11 | 10 | 7 | 1 |
Abbreviations: AML, acute myeloid leukemia; AML-E, AML with erythroblastic differentiation; AML-MK, AML with megakaryoblastic differentiation; AML-MK/E, AML with mixed MK and E differentiation; AML-M0, AML with no identifiable sublineage differentiation; AML-M1, AML with early myeloid differentiation; AML-M2, AML with greater than 20% myeloid differentiation; AML-M5, AML with monocytic differentiation; AML-M6, AML with erythroid hyperplasia, dysplasia, or differentiation; AML-M7, AML with megakaryoblastic differentiation; AML-MX, AML with no specified sublineage differentiation; AML-NOS, AML with insufficient information for sublineage classification; AML-U, AML with no identifiable sublineage differentiation; Cntrl, central; DX, diagnosis; Inst, institutional; MDS, myelodysplastic syndrome.
% = percentage of institutional diagnoses.
Central cytogenetic review results for the protocol have been presented in detail in a previous article.17 Of note, no cases were found of the typical recurring translocations and inversions found in standard AML in children.
GATA1 mutation analysis was performed on 39 samples, with 35 positive and 4 negative results (3 of 14 negative results in MDS and 1 of 25 in AML samples; P = .12).
The MRD levels of 0.01% and above on day 28 of therapy were not significantly different in MDS (4 of 40 cases were positive [10%]) versus AML (16 of 81 cases were positive [20%]) (P = .17). Likewise, AML subsets showed no statistically significant differences in MRD positivity, but of note 3 of the 7 AML-U cases tested for MRD on day 28 were positive (P = .09 versus non-AML-U cases).
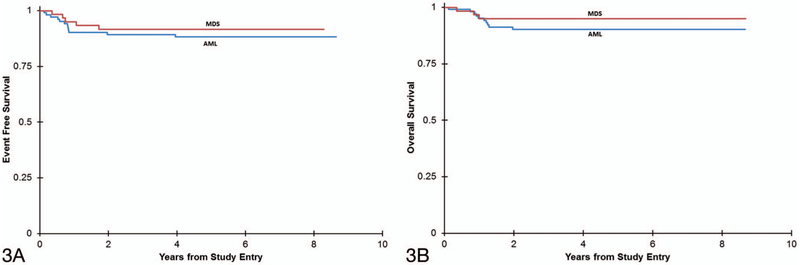
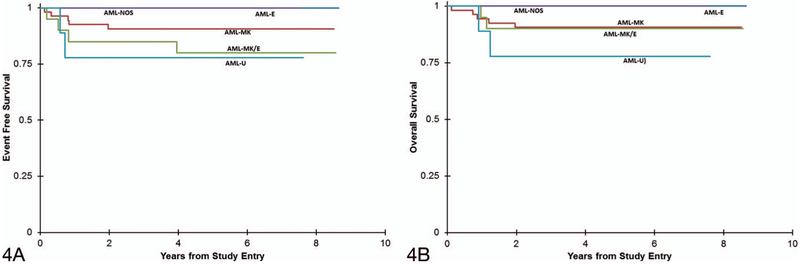
AML and MDS cases demonstrated similar rates of 5-year EFS (MDS, 92% ± 7%; AML, 88% ± 6%) (P = .60) and OS (MDS, 95% ± 6%; AML, 90% ± 6%) (P = .38) (Figure 3, A and B). Among AML subtypes, possible trends were noted (Figure 4, A and B). Patients with AML-E and AML-NOS had 100% 5-year EFS and OS. AML-MK and AML-MK/E had similar survival (AML-MK: 91% ± 8% 5-year EFS and OS; AML-MK/E: 80% ± 18% EFS and 90% ± 13% OS), while AML-U had the lowest survival (78% ± 28% 5-year EFS and OS). These results were not statistically significant for 5-year EFS or OS (EFS: P = 0.39; OS: P = .46). The single case with myeloblastic differentiation entered and remained in remission at 5 years.
Figure 3.
Kaplan-Meier curves comparing event-free survival (A) (P = .60) and overall survival (B) (P = .38) between acute myeloid leukemia (AML) and myelodysplastic syndrome (MDS) in patients with Down syndrome.
Figure 4.
Kaplan-Meier curves comparing event-free survival (A) (P = .39) and overall survival (B) (P = .46) between acute myeloid leukemia (AML) subtypes in patients with Down syndrome. Abbreviations: AML-E, acute erythroblastic leukemia; AML-MK, acute megakaryoblastic leukemia; AML-MK/E, AML with mixed MK and E features; AML-NOS, AML–not otherwise specified; AML-U, AML-undifferentiated.
Survival in the group of patients with institutional diagnoses of FAB M1-5 (EFS, 75% ± 25%; OS, 75% ± 25%) was better than expected in standard AML, but lower than that of other DS-AML cases (P = .06 for EFS, P = .02 for OS).
DISCUSSION
Myelodysplastic syndrome and AML occur at a high frequency in children with DS. Most cases comprise a unique set of myeloid diseases characterized by good response to modified cytotoxic chemotherapy, distinctive diagnostic features, and a high incidence of association with somatic GATA1 mutations. Standard (non-DS) AML also occurs in DS patients, is the predominant form of disease in patients older than 4 years, and lacks the relatively good prognosis of typical DS disease. Despite these known associations, some questions persist. Is the association of DS MDS and AML with GATA1 mutation represented by the ratio 1:1? What is the incidence of standard (non-DS) AML subtypes in DS patients younger than 4 years? How can these cases be distinguished from DS disease? What is the incidence of DS-AML in patients older than 4 years? Answers to these questions are limited in part by imprecise diagnostic criteria for DS MDS and AML.
In this review we saw frequent megakaryocytic hyperplasia, dysplasia, and clustering, as well as frequent erythroid hyperplasia and dysplasia in both MDS and AML cases, as noted by others.6 Granulocytic dysplasia was minimal or (usually) absent in DS cases. DS-AML blasts most frequently demonstrated megakaryoblastic differentiation (AML-MK),5,7,24 but there were subsets of DS-AML with erythroblastic, mixed megakaryoblastic/erythroblastic, or no definitive differentiation. Mutations in GATA1 are well recognized in DS MDS and AML. GATA-1 mediates differentiation of both megakaryocytic and erythroid, but not myeloid, progenitors.11,12,14 Our observation of megakaryocytic and erythroid (but not myeloid) sublineage abnormalities merges nicely with this known biology, and provides a more complete and concordant description of these diseases. These observations of restricted sublineage dysplastic features and differentiation of blasts may facilitate accurate diagnosis in clinical practice, including recognition of DS-AML in patients older than 4 years, allowing evaluation of their response to treatment versus patients with standard AML.19
We observed frequent unusual megakaryocytic dysplasia in both MDS and AML cases, with peripheral displacement of nuclei by a large central cytoplasmic inclusion. Although not unique, these features are not typical of MDS/AML in other clinical settings. Recognition of this distinctive dysplasia may facilitate diagnosis and monitoring of MDS and AML in DS patients. Similar morphologic abnormalities have been described in megakaryocytes in patients with heterozygous constitutional GATA2 loss of function mutations,25 suggesting that in DS patients this morphology may be a surrogate marker for GATA1 mutations. This possibility requires further study.
The variation in survival we found among AML subgroups is interesting, but requires further study to establish its clinical significance. We have previously reported that MRD positivity at day 28 correlates with poor survival in DS AML17; notably, 3 of 7 DS-AML patients lacking blast sublineage differentiation (AML-U) had MRD positivity on day 28, consistent with the apparent poorer survival of this group, but not statistically significant.
Given the much higher incidence of DS MDS and AML versus standard disease in DS patients, extreme caution should be exercised in diagnosing standard disease in patients younger than 4 years.19 To improve diagnostic precision we propose the following criteria for diagnosis of DS AML and MDS:
Dysplasia should be present and essentially limited to megakaryocytic and erythroid elements. (In the absence of both, or presence of significant myeloid dysplasia, a diagnosis of standard non-DS disease should be considered.)
Blasts should demonstrate megakaryoblastic, erythroid, mixed, or no sublineage differentiation. (If blasts demonstrate myeloid differentiation, diagnosis of standard MDS/AML should be considered; such cases, including cases with possible FAB M1-5 morphology, should be assessed by an experienced hematopathologist.)
Presence of megakaryocytic dysplasia with central eosinophilic inclusions and peripherally displaced multiple or signet-like nuclei supports diagnosis of DS myeloid disease.
Cytogenetics: Presence of a typical translocation or inversion of standard AML suggests non-DS disease, should be confirmed by fluorescence in situ hybridization, and the case should be assessed by an experienced hematopathologist.
In conclusion, we report findings of a large systematic review of cases of AML and MDS occurring in patients with DS, noting the variability of blast differentiation in these cases and the near absence of myeloid dysplasia and blast differentiation. From these findings we propose detailed diagnostic criteria for DS MDS and AML. These criteria may serve to improve institutional diagnosis of these diseases, facilitate recognition of uncommon standard AML cases in DS patients younger than 4 years and DS-AML cases in patients older than 4 years, and allow evaluation of the exact relationship of GATA1 mutations and DS myeloid disease. Future studies may also address any clinical consequences of the AML subtypes we observed in DS-AML.
Acknowledgments
This work was supported by National Institutes of Health (R01 CA120772), by the Children’s Oncology Group Operations Center Grant U10CA180886, by the Andrew McDonough B+ Foundation, by the St. Baldrick’s Foundation, and by the Children’s Oncology Group Statistics and Data Center grant (1U10CA180899).
Footnotes
Portions of our study were reported previously in an abstract presented at the annual United States-Canadian Academy of Pathology (USCAP) meeting; March 4, 2013; Baltimore, Maryland. Portions were also submitted in an abstract to the 12th International Symposium on Myelodysplastic Syndromes, May 8–11, 2013, Berlin, Germany, were not presented, but were published in symposium proceedings.
Dr Campana and Ms Coustan-Smith are inventors in a patent application on methods for detecting myelodysplastic syndrome in acute myeloid leukemia; no other relevant financial interests exist. The other authors have no relevant financial interest in the products or companies described in this article.
Contributor Information
Kelley J. Mast, Department of Pathology, Microbiology, and Immunology, Vanderbilt University Medical Center, Nashville, Tennessee.
Jeffrey W. Taub, Division of Hematology/Oncology, Children’s Hospital of Michigan, Wayne State University, Detroit.
Todd A. Alonzo, Department of Biostatistics, University of Southern California, Monrovia.
Alan S. Gamis, Division of Hematology/Oncology/Bone Marrow Transplantation, Children’s Mercy Hospital, Kansas City, Missouri.
Claudio A. Mosse, Department of Pathology, Microbiology, and Immunology, Vanderbilt University Medical Center, Nashville, Tennessee; Pathology and Laboratory Medicine Service, VA Tennessee Valley Healthcare System, Nashville.
Prasad Mathew, Department of Pediatrics, University of New Mexico, Albuquerque.
Jason N. Berman, Division of Hematology-Oncology, IWK Health Centre, Halifax, Nova Scotia, Canada.
Yi-Cheng Wang, Department of Biostatistics, University of Southern California, Monrovia.
Heath M. Jones, Department of Pathology, Microbiology, and Immunology, Vanderbilt University Medical Center, Nashville, Tennessee.
Dario Campana, Department of Oncology, St. Jude Children’s Research Hospital, Memphis, Tennessee; Department of Pediatrics, Yong Loo Lin School of Medicine, National University of Singapore, The National University Cancer Institute, NUH Medical Centre, Singapore.
Elaine Coustan-Smith, Department of Oncology, St. Jude Children’s Research Hospital, Memphis, Tennessee; Department of Pediatrics, Yong Loo Lin School of Medicine, National University of Singapore, The National University Cancer Institute, NUH Medical Centre, Singapore.
Susana C. Raimondi, Department of Pathology, St. Jude Children’s Research Hospital, Memphis, Tennessee.
Betsy Hirsch, Department of Laboratory Medicine and Pathology, University of Minnesota Medical School, Minneapolis.
Johann K. Hitzler, Department of Pediatrics, University of Toronto, Toronto, Ontario, Canada; Division of Hematology/Oncology, The Hospital for Sick Children Developmental and Stem Cell Biology, The Hospital for Sick Children Research Institute, Toronto, Ontario, Canada.
David R. Head, Department of Pathology, Microbiology, and Immunology, Vanderbilt University Medical Center, Nashville, Tennessee.
References
- 1.Parker SE, Mai CT, Canfield MA, et al. Updated National Birth Prevalence estimates for selected birth defects in the United States, 2004-2006. Birth Defects Res A Clin Mol Teratol. 2010;88(12):1008–1016. [DOI] [PubMed] [Google Scholar]
- 2.Hasle H, Clemmensen IH, Mikkelsen M. Risks of leukaemia and solid tumours in individuals with Down’s syndrome. Lancet. 2000;355(9199):165–169. [DOI] [PubMed] [Google Scholar]
- 3.Buitenkamp TD, Izraeli S, Zimmermann M, et al. Acute lymphoblastic leukemia in children with Down syndrome: a retrospective analysis from the Ponte di Legno study group. Blood. 2014;123(1):70–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ries LAG, Smith MA, Gurney JG, et al. , eds. Cancer Incidence and Survival Among Children and Adolescents: United States SEER Program 1975-1995. Bethesda, MD: National Cancer Institute, SEER Program; 1999. NIH Pub. No. 99-4649. [Google Scholar]
- 5.Zipursky A, Thorner P, De Harven E, Christensen H, Doyle J. Myelodysplasia and acute megakaryoblastic leukemia in Down’s syndrome. Leukemia Res. 1994;18(3):163–171. [DOI] [PubMed] [Google Scholar]
- 6.Baumann I, Niemeyer CM, Brunning RD, et al. Myeloid proliferations related to Down syndrome In: Swerdlow SH, Campo E, Harris NL, et al. , eds. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. 4th ed. Lyon, France: International Agency for Research on Cancer; 2008:142–144. World Health Organization Classification of Tumours; vol 2. [Google Scholar]
- 7.Zwaan CM, Reinhardt D, Hitzler J, Vyas P. Acute leukemias in children with Down syndrome. Hematol Oncol Clin North Am. 2010;24(1):19–34. [DOI] [PubMed] [Google Scholar]
- 8.Lange BJ, Kobrinsky N, Barnard DR, et al. Distinctive demography, biology, and outcome of acute myeloid leukemia and myelodysplastic syndrome in children with Down syndrome: Children’s Cancer Group Studies 2861 and 2891. Blood. 1998;91(2):608–615. [PubMed] [Google Scholar]
- 9.Webb D, Roberts I, Vyas P. Haematology of Down syndrome. Arch Dis Child Fetal Neonatal Ed. 2007;92(6):F503–F507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xavier AC, Ge Y, Taub JW. Down syndrome and malignancies: a unique clinical relationship: a paper from the 2008 william beaumont hospital symposium on molecular pathology. J Mol Diagn. 2009;11(5):371–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ahmed M, Sternberg A, Hall G, et al. Natural history of GATA1 mutations in Down syndrome. Blood. 2004;103(7):2480–2489. [DOI] [PubMed] [Google Scholar]
- 12.Gurbuxani S, Vyas P, Crispino JD. Recent insights into the mechanisms of myeloid leukemogenesis in Down syndrome. Blood. 2004;103(2):399–406. [DOI] [PubMed] [Google Scholar]
- 13.Kanezaki R, Toki T, Terui K, et al. Down syndrome and GATA1 mutations in transient abnormal myeloproliferative disorder: mutation classes correlate with progression to myeloid leukemia. Blood. 2010;116(22):4631–4638. [DOI] [PubMed] [Google Scholar]
- 14.Mundschau G, Gurbuxani S, Gamis AS, Greene ME, Arceci RJ, Crispino JD. Mutagenesis of GATA1 is an initiating event in Down syndrome leukemogenesis. Blood. 2003;101(11):4298–4300. [DOI] [PubMed] [Google Scholar]
- 15.Hitzler JK, Cheung J, Li Y, Scherer SW, Zipursky A. GATA1 mutations in transient leukemia and acute megakaryoblastic leukemia of Down syndrome. Blood. 2003;101(11):4301–4304. [DOI] [PubMed] [Google Scholar]
- 16.Taga T, Watanabe T, Tomizawa D, et al. Preserved high probability of overall survival with significant reduction of chemotherapy for myeloid leukemia in Down syndrome: a nationwide prospective study in Japan. Pediatr Blood Cancer. 2016;63(2):248–254. [DOI] [PubMed] [Google Scholar]
- 17.Taub JW, Berman JN, Hitzler JK, et al. Improved outcomes for myeloid leukemia of Down syndrome: a report from the Children’s Oncology Group AAML0431 trial. Blood. 2017;129(25):3304–3313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Uffmann M, Rasche M, Zimmermann M, et al. Therapy reduction in patients with Down syndrome and myeloid leukemia: the international ML-DS 2006 trial. Blood. 2017;129(25):3314–3321. [DOI] [PubMed] [Google Scholar]
- 19.Hasle H, Abrahamsson J, Arola M, et al. Myeloid leukemia in children 4 years or older with Down syndrome often lacks GATA1 mutation and cytogenetics and risk of relapse are more akin to sporadic AML. Leukemia. 2008;22(7):1428–1430. [DOI] [PubMed] [Google Scholar]
- 20.Foucar K, McKenna RW, Peterson LAC, Kroft SH. In: Tumors of the Bone Marrow. Vol 24 Washington, DC: American Registry of Pathology; 2016:169–202. AFIP Atlas of Tumor Pathology; series 4. [Google Scholar]
- 21.Thiele J, Kvasnicka HM, Facchetti F, Franco V, van der Walt J, Orazi A. European consensus on grading bone marrow fibrosis and assessment of cellularity. Haematologica. 2005;90(8):1128–1132. [PubMed] [Google Scholar]
- 22.Hasserjian RP, Head DR. Myelodysplastic syndromes In: Jaffe ES, Arber D, Campo E, Harris NL, Quintanilla-Martinez L, eds. Hematopathology. 2nd ed. Philadelphia, PA: Elsevier; 2017:793–816. [Google Scholar]
- 23.Rubnitz JE, Inaba H, Dahl G, et al. Minimal residual disease-directed therapy for childhood acute myeloid leukaemia: results of the AML02 multicentre trial. Lancet Oncol. 2010;11(6):543–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ravindranath Y, Abella E, Krischer JP, et al. Acute myeloid leukemia (AML) in Down’s syndrome is highly responsive to chemotherapy: experience on Pediatric Oncology Group AML Study 8498. Blood. 1992;80(9):2210–2214. [PubMed] [Google Scholar]
- 25.Spinner MA, Sanchez LA, Hsu AP, et al. GATA2 deficiency: a protean disorder of hematopoiesis, lymphatics, and immunity. Blood. 2014;123(6):809–821. [DOI] [PMC free article] [PubMed] [Google Scholar]