Abstract
Background/Aims
Alzheimer’s disease (AD) onset before 65 (early-onset AD (EOAD)) occurs in approximately 6% of cases and can affect non-memory domains. Here, we analyze patterns of impairment in amnestic EOAD individuals using data-driven statistical analyses.
Methods
Cognitive data of 146 EOAD subjects were Z-normalized to 395 cognitively-normal (CN) individuals. Domain-averaged Z-scores were adjusted for age, sex and education followed by Wald cluster analysis of residuals. MRI and PET comparisons of EOAD clusters to age-matched CN was done using SPM8. Cluster-level-family-wise error (p<0.05) correction was applied. Mixed-effect models were used to compute longitudinal change across clusters.
Results
Scree plot using the pseudo-T-squared suggested a 4-cluster solution. Cluster 1 (memory-predominant impairment) showed atrophy/hypometabolism in medial/lateral temporal, lateral parietal and posterior cingulate regions. Cluster 2 showed (memory/visuospatial-predominant) atrophy/hypometabolism of medial temporal, temporoparietal and frontal cortices. Cluster 3 (memory, language and executive function) and Cluster 4 (all domains) manifested atrophy and hypometabolism throughout the brain. Longitudinally between-cluster differences in the visuospatial and language/executive domains were significant, suggesting phenotypic variation.
Conclusion
We observed significant heterogeneity in cognitive presentation among amnestic EOAD subjects and patterns of atrophy/hypometabolism in each cluster in agreement with the observed cognitive phenotype.
Keywords: Early onset Alzheimer’s disease, Cognition, Heterogeneity, Magnetic Resonance Imaging, Positron Emission Tomography
1. Introduction
In the United States, an estimated 5.5 million people are diagnosed with Alzheimer’s disease (AD) - the leading cause of dementia world-wide[1]. Approximately 94% of patients with AD become symptomatic after age 65 [late-onset AD (LOAD)] and 6% before age 65 [early onset AD (EOAD)][2]. It has been shown that EOAD patients have more diverse disease presentations and more aggressive disease courses [3–25]. While many LOAD cases have stereotypic memory-predominant deficits, EOAD patients often have atypical presentations with non-memory impairments early in the disease course [3,5–9,11–18,25]. As many as 64% of EOAD vs. only 12.5% of LOAD patients present with focal non-amnestic symptoms [18].
Several studies have investigated the heterogeneity of EOAD compared to LOAD. Some studies [7,9,16] reported that, after memory, the language domain is most severely affected in EOAD, while visuospatial function is preserved; others indicate that praxis and visuospatial function are most affected [15,26]. This inconsistency between EOAD presentations seems to suggest high disease heterogeneity.
We aimed to use a data-driven approach to improve our understanding of the phenotypic heterogeneity among EOAD patients in the Alzheimer’s Disease Neuroimaging Initiative (ADNI) cohort. As ADNI requires all cognitively impaired individuals to manifest significant memory impairment at baseline, our analyses were limited to the amnestic EOAD subtype. We hypothesized that we would find several cognitive subtypes within the amnestic EO group and that the subsequent patterns of neurodegeneration correspond closely to their respective cognitive phenotype. Furthermore, we hypothesized that amyloid deposition would be similarly diffuse regardless of cognitive subtype. Longitudinal cognitive differences between and within clusters were also analyzed.
2. Materials and Methods
2.1. Subjects
Data used for this analysis were obtained from the Alzheimer’s Disease Neuroimaging Initiative (ADNI) database (adni.loni.usc.edu). ADNI was launched in 2003 as a public-private partnership, led by Principal Investigator Michael W. Weiner, MD. The primary goal of ADNI has been to test whether serial magnetic resonance imaging (MRI), positron emission tomography (PET), other biological markers, and clinical and neuropsychological assessments can be combined to measure the progression of mild cognitive impairment (MCI) and early Alzheimer’s disease (AD).
The clinical description of the ADNI cohort has been described previously [27–29]. Diagnosis of AD was based on the National Institute of Neurological and Communicative Disorders and Stroke and the AD and Related Disorders Association (NINCDS-ADRDA) criteria [30–32]. The full list of inclusion/exclusion criteria may be accessed on pages 23–29 of the online ADNI protocol (see http://www.adni-info.org/Scientists/ADNIScientistsHome.aspx). Written informed consent was obtained from all participants.
The ADNI cohort includes 146 subjects diagnosed with MCI or probable AD with an age of symptom onset less than 65. Of these, 143 had at least one imaging modality available. Our comparison group consisted of 395 cognitively normal (CN) subjects who had GWAS data available and were between the ages of 55 and 80.
2.2. MRI and PET Acquisition and Analyses
The protocols for MRI image acquisition and preprocessing can be found on www.adni-info.org. ADNI MRI data acquisition and preprocessing has been described elsewhere [33–35]. We downloaded the preprocessed MRI data from LONI IDA (https://ida.loni.usc.edu). All scans were analyzed using voxel-based morphometry using Statistic Parametric Mapping (SPM8), as previously described [36,37]. Scans were downloaded in NifTI format, co-registered to Montreal Neurological Institute (MNI) space and segmented into gray matter (GM), white matter, and cerebrospinal fluid. The GM maps were normalized to MNI space at 1mm×1mm×1mm voxel resolution and smoothed using a 10mm full-width half maximum Gaussian kernel resulting in GM density (GMD) maps. The intracranial volume (ICV) was extracted for each subject using FreeSurfer version 5.1 [38,39].
The F18-Fluorodeoxyglucose positron emission tomography (FDG PET) and 18F-Florbetapir PET scan acquisition and pre-processing protocols are available at www.adni-info.org. Scans were obtained using scanners and related equipment that were calibrated and standardized, as described previously [40]. Preprocessed scans were downloaded from LONI IDA (https://ida.loni.usc.edu). The downloaded scans were already averaged, aligned to standard space, re-sampled to a standard image and voxel size, and smoothed to a uniform resolution [40]. Each image was aligned to the corresponding MRI scan and normalized to MNI space using parameters from the MRI segmentation with 2mm x 2mm x 2mm resolution [40]. FDG PET scans were intensity-normalized to the pons and the Florbetapir PET scans were intensity-normalized to the whole cerebellum to create Standard Uptake Volume Ratios (SUVR) images as previously described [40]. These images were then used for voxel-wise analysis as described below.
We used the mean amyloid whole brain SUVR from the University of California Berkeley (UCB) downloaded from ADNI’s database (http://adni.loni.usc.edu). The UCB protocols for 18F-Florbetapir preprocessing, co-registration and normalization have been previously described [41].
2.3. Statistical Analyses
2.3.1. Cluster Analysis
Baseline cognitive data from the 146 EOAD subjects meeting criteria for mild cognitive impairment or dementia were Z-normalized using the mean and standard deviation of the cognitive performance of the 395 CN participants. Neuropsychological tests from the ADNI battery were grouped in four domains – memory, language, visuospatial and executive. The memory domain included the Wechsler Logical Memory Story Recall [42] and the Rey Auditory Verbal Learning Tests (RAVLT) [43]. For the RAVLT, we computed percent learned, which was derived by dividing the total score achieved at trial five by the total possible score (15) and multiplying by 100, and RAVLT percent retained, calculated by dividing the score after a 30-minute delay by the score achieved after trial 5, multiplied by 100. The language domain included the Boston Naming test [44] and animal fluency [45]. The visuospatial domain included clock drawing and clock copy [46]. The executive domain included Trailmaking A and B [47]. For the EOAD participants, all included individuals had complete test data except for a single subject, who was missing a single clock copy score. Domain averaged Z-scores were produced and adjusted for age, sex and education. Cluster analysis using the Ward method on the residuals for the 4 cognitive domains was performed [48]. Scree plot using the pseudo T-squared was used to determine the optimal number of clusters.
2.3.2. Baseline Demographic and Cognitive Comparisons
Baseline clinical and demographic between-cluster comparisons were conducted using analysis of variance (ANOVA) for continuous outcomes and chi-square tests for categorical outcomes.
2.3.4. Imaging Analyses
Using SPM8, we ran voxel-wise regression analyses to visualize the pattern of GMD, FDG PET and amyloid PET differences between each EOAD cluster and CN. EOAD cluster was used as the predictor variable with age, sex and education as covariates. ICV and MRI field strength were also used as covariates for the MRI analyses. Cluster-level family-wise error (FWE) correction for multiple comparisons was applied. Only clusters surviving FWE correction at the threshold of p < 0.05 with a minimum cluster size (k) equal to the smallest significant cluster size were displayed.
2.3.5. Longitudinal Analyses of Cognitive Decline
Longitudinal cognitive data was available on 140 of the 146 subjects. Follow-up varied between 6 months and 10 years. Due to reduction in sample sizes after 60 months, we limited our analyses at 5 years (see Table 1). Longitudinal rates of decline in each cognitive domain were examined and compared between the clusters using mixed effects models with repeated measures on all cognitive outcomes after adjusting for education, age and sex using SAS 9.4. The models included an interaction between time of evaluation from baseline treated as a continuous variable and cluster. The results were visualized by deriving predicted means and 95% confidence intervals and plotting these for each cluster over time.
Table 1.
Demographic and Z-normalized cognitive data (top) and longitudinal data, and estimated change and 95% confidence intervals in cognitive scores adjusting for age, sex and education over 60 months of follow-up (bottom). Bold values indicate cognitively significant decline and significant p-values.
| Variable | Cluster 1 (N=64) | Cluster 2 (N=31) | Cluster 3 (N=37) | Cluster 4 (N=14) | p-value |
| Number MCI/Dementia | 51/13 | 18/13 | 9/28 | 0/14 | <0.001 |
| Age, Mean Years (SD) | 63.7 (4.4) | 65.1 (6.1) | 64.8 (6.2) | 63.1 (5.6) | 0.49 |
| Age of Symptom Onset, Mean Years (SD. | 59.2 (4.5) | 59.3 (5.1) | 59.5 (4.1) | 58.3 (3.6) | 0.87 |
| Sex (Male %) | 51.6 | 45.2 | 45.9 | 57.1 | 0.84 |
| Education, Mean Years (SD) | 16.4 (2.8) | 15.8 (2.4) | 15.9 (3.1) | 16.0 (2.9) | 0.76 |
| Disease Duration, Mean Years (SD) | 4.5 (3.5) | 5.8 (4.9) | 5.3 (4.4) | 4.8 (3.0) | 0.51 |
| Amyloid PET SUVR, Mean (SD) | 1.26 (0.23) | 1.33 (0.26) | 1.34 (0.23) | 1.48 (0.09) | 0.091 |
| % APOE ε4, 0/1/2 allele(s) | 34/39/27 | 23/45/32 | 38/32/30 | 36/43/21 | 0.86 |
| Baseline MMSE, Mean (SD) | 27.0 (2.1) | 25.8 (3.1) | 24.2 (2.5) | 21.9 (1.7) | <0.001 |
| Baseline CDR-SOB, Mean (SD) | 2.0 (1.2) | 2.9 (1.9) | 3.9 (1.9) | 5.5 (1.6) | <0.001 |
| % of N with MRI/FDG/Amyloid Scans | 95.3/79.7/78.1 | 87.1/90.3/83.9 | 97.3/62.2/59.5 | 92.9/78.6/50.0 | - |
| Memory Domain Z-scores mean (SD) | −1.77 (0.95) | −2.07 (1.02) | −2.32 (0.75) | −2.89 (0.57) | <0.001 |
| Language Domain Z-scores mean (SD) | −0.35 (0.87) | −0.99 (0.74) | −1.82 (1.51) | −2.76 (1.84) | <0.001 |
| Visuospatial Domain Z-scores mean (SD) | 0.22 (0.43) | −2.91 (1.88) | −0.96 (0.97) | −6.86 (2.08) | <0.001 |
| Executive Domain Z-scores mean (SD) | 0.01 (0.70) | 0.71 (1.37) | 3.56 (1.80) | 7.45 (2.30) | <0.001 |
| Cluster 1 (N=64) | Cluster 2 (N=31) | Cluster 3 (N=37) | Cluster 4 (N=14) | time*cluster interaction p-value | |
| Memory | −0.077 (−0.47, 0.31) | 0.58 (−0.09, 1.24) | 0.37 (−0.51, 1.24) | −1.91 (−4.59, 0.76) | 0.15 |
| Language | −0.84 (−1.29, −0.39) | −0.10 (−0.86, 0.65) | −0.40 (−1.45, 0.64) | −4.87 (−7.81, −1.94) | 0.013 |
| Visuospatial | −1.60 (−2.26, −0.93) | 1.53 (0.38, 2.68) | −3.11 (−4.66, −1.56) | −3.88 (−8.46, 0.71) | <0.001 |
| Executive | 1.68 (1.00, 2.37) | 0.25 (−0.92, 1.41) | 1.21 (−0.39, 2.80) | 3.61 (−1.45, 8.67) | 0.16 |
| Number of Longitudinal visits | 299 | 114 | 99 | 31 | - |
| % 6-month visit | 98.4 | 96.8 | 94.6 | 85.7 | - |
| % 12-month visit | 93.8 | 90.3 | 78.4 | 85.7 | - |
| % 18-month visit | 31.3 | 12.9 | 5.4 | 0.0 | - |
| % 24-month visit | 82.8 | 67.7 | 54.1 | 50.0 | - |
| % 36-month visit | 70.3 | 45.2 | 16.2 | 0.0 | - |
| % 48-month visit | 59.4 | 38.7 | 10.8 | 0.0 | - |
| % 60-month visit | 31.3 | 16.1 | 8.1 | 0.0 | - |
| Mean months of follow-up (SD) | 41.44 (18.08) | 32.90 (18.72) | 21.16 (15.60) | 17.14 (7.12) | - |
3. Results
3.1. Baseline Cognitive and Imaging Analyses
The scree plot suggested a four-cluster solution. As seen in Table 1, Cluster 1 was comprised of 64 subjects (51 MCI and 13 dementia) with isolated memory deficits (Z=−1.77±0.95). Cluster 2 contained 31 subjects (18 MCI and 13 dementia) with memory and visuospatial domain deficits (Zmemory=−2.07±1.02 and Zvisuospatial=−2.91±1.88). Cluster 3 had 37 individuals (9 MCI and 28 dementia) with impairment in memory, language, and executive function (Zmemory=−2.32±0.75, Zlanguage=−1.82±1.51, and Zexecutive 3. 56±1.80). For cluster 4, there were 14 subjects (14 dementia) with deficits in all four domains (Zmemory=−2.89±0.57, Zlanguage=−2.76±1.84, Zvisuospatial=−6.86±2.08, and Zexecutive=7.45±2.30).
There were no significant between-cluster differences in age, gender, education, APOE4 genotype distribution, age at symptom onset, disease duration or mean amyloid SUVR. There was a significant between-cluster difference in baseline clinical dementia rating (CDR) and mini-mental state exam (MMSE), with cluster 1 performing the best and cluster 4 the worst (p<0.001 for both). The distribution of diagnoses between the four clusters was also significant (p<0.001). The number of subjects used in each imaging analysis varies slightly due to image availability (see Table 1).
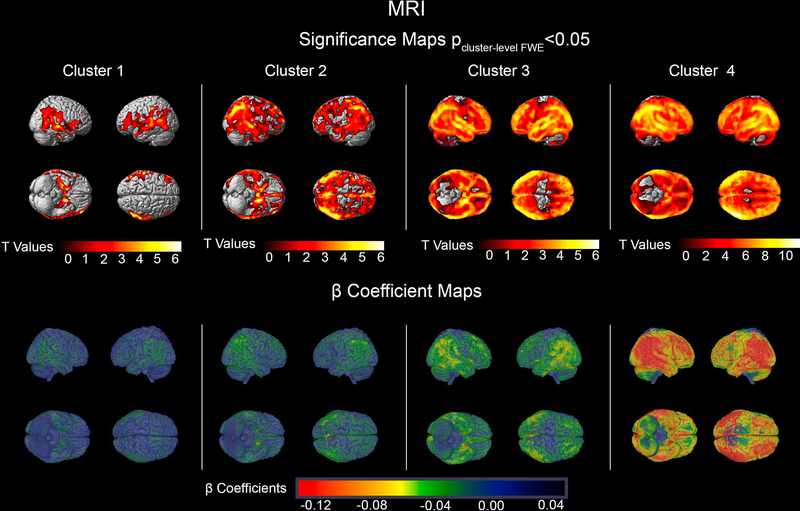
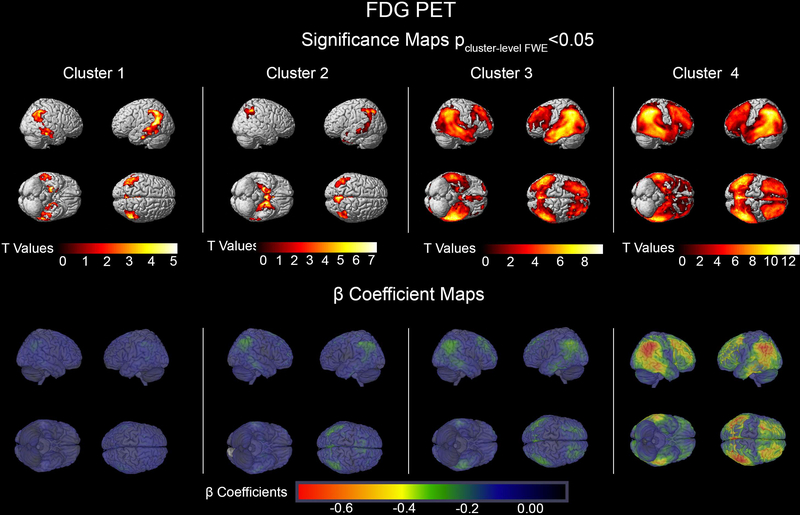
The MRI and FDG PET imaging comparisons of each cluster relative to the CN sample can be seen in Figures 1 and 2, respectively. Cluster 1 showed isolated memory impairment and reduced GMD across the temporal, parietal, and frontal lobes with cluster peaks in the left medial temporal lobe (MTL) (pFWE<0.001, k=86842 voxels), the right temporal cortex (pFWE <0.001, k=87521) and the left and right posterior cingulate cortices (PCC) (single cluster pFWE =0.002, k=5882). They also showed reduced brain metabolism in the MTL (left: pFWE =0.002, k=722; right: pFWE=0.013, k=494), the bilateral parietal cortices (both pFWE <0.001, kleft=2865, kright= 1133) and the right temporal cortex (pFWE =0.003, k=688).
Figure 1.
MRI significance and β Coefficient maps showing the comparison of each cluster to cognitively normal participants. The significance maps show p<0.05 thresholded FWE cluster-level corrected results.
Figure 2.
[18F]FDG PET significance and β Coefficient maps showing the comparison of each cluster to cognitively normal participants. The significance maps show p<0.05 thresholded FWE cluster-level corrected results.
Cluster 2 subjects demonstrated impaired memory and visuospatial function (Table 1). Compared to the CN group they showed significant reduction in GMD in one large cluster spanning the bilateral MTLs, parietal, and frontal lobes with peak in the left MTL (pFWE <0.001, k=573,388). Cluster 2 showed hypometabolism in the left MTL (pFWE<0.001, k=14,309) as well as the left and right parietal cortices (left: pFWE<0.001, k=l,457; right: pFWE=0.002, k=736).
Cluster 3 subjects had impaired memory, language, and executive function (Table 1). They showed reduced GMD throughout the brain sparing only portions of the sensorimotor cortex. The GMD cluster peak localized to the left temporal lobe (pFWE<0.001, k=l,299,556). Impaired glucose metabolism was seen in the temporal, parietal, and frontal lobes with peak in the fusiform gyrus (pFWE<0.001, k=63,532).
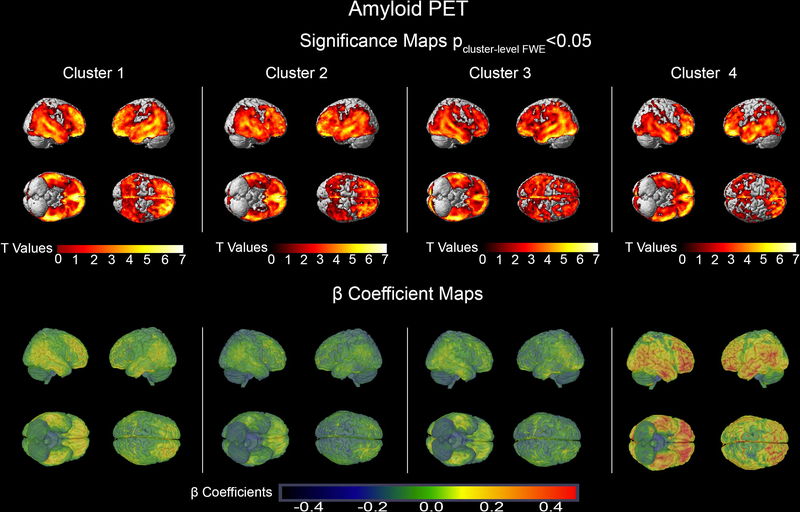
Cluster 4 individuals had impairment across all cognitive domains (Table 1). The GMD neurodegeneration spanned the entire brain including the sensorimotor cortices (peak in the right temporal cortex, pFWE<0.001, k=l,47l,637). There was also a global reduction in glucose metabolism sparing the sensorimotor cortices (peak in the left PCC pFWE <0.00l, k=97,037). Overall, the largest effect sizes were seen in cluster 4 for both GMD and brain metabolism (Figures 1 and 2, bottom rows). There were no regional differences in amyloid distribution between the clusters (Figure 3). While cluster 4 showed the highest and cluster l the lowest mean cortical amyloid SUVR, the difference only reached a trend level in the ANOVA analysis (p=0.09l, Table 1).
Figure 3.
Amyloid PET Significance and β Coefficient maps showing the comparison of each cluster to cognitively normal participants. The significance maps show p<0.05 thresholded FWE cluster-level corrected results.
3.2. Longitudinal Cognitive Decline
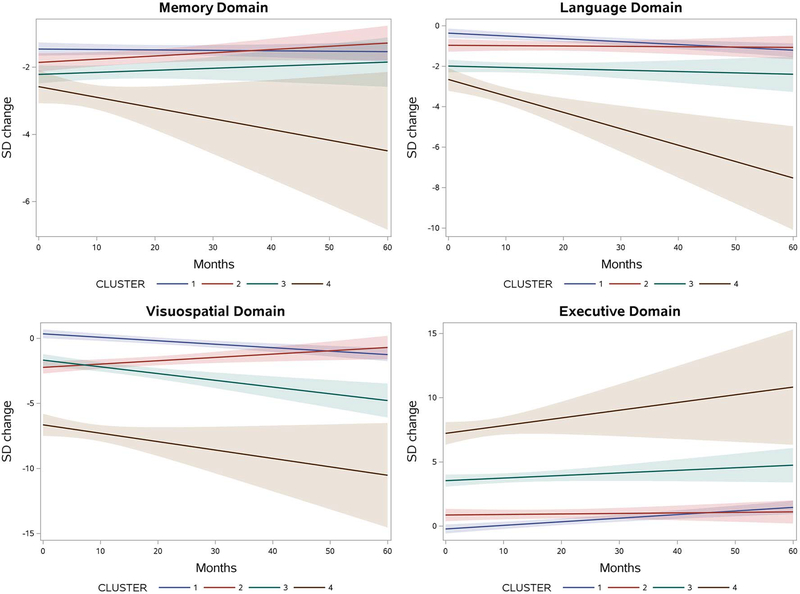
Table 1 shows the estimated domain-specific change from baseline to 60 months for subjects in each cognitive cluster. Cluster 1, who at baseline showed only memory deficits, progressed to develop significant decline in language (overall change −0.84 SD, 95% CI −1.29; −0.39), visuospatial (−1.60 SD, 95% CI −2.26; −0.93) and executive skills (6.68 SD, 95% CI 1.0;2.37) over 5 years. Cluster 2, with baseline deficits in memory and visuospatial function, showed significant improvement of visuospatial function over time (1.53 SD, 95% CI 0.38; 2.68). Cluster 3, subjects who had baseline impairments in memory, language, and executive function, showed progressive worsening in their visuospatial skills (−3.11 SD, 95% CI −4.66; −1.56). Cluster 4, which included individuals who already were impaired in all domains, showed a significant decline in language skills (−4.87 SD, 95% CI −7.81; −1.94). The longitudinal changes can be visualized in Figure 4.
Figure 4.
Longitudinal Cognitive Decline with 95% confidence interval by domain. Each figure shows the predicted change in standard deviation over 60 months of each cluster.
We further tested the hypothesis that the EOAD clusters will show differences in their pattern of cognitive change over time. We found no difference in the rates of memory and executive decline over time (Time*cluster interaction pmemory=0.15 and pexecutive=0.16). Significant differences in rate of decline were seen for the language (p=0.013) and visuospatial domains (p<0.001). In terms of language function, the overall decline of cluster 4 was significantly greater than the decline observed in other clusters (cluster 4 vs. 1 p=0.008, cluster 4 vs. 2 p=0.0021 and cluster 4 vs. 3 p=0.005). The between-clusters significant difference in visuospatial decline was driven by cluster 2 who, interestingly, improved in visuospatial performance over time (cluster 2 vs 1 p<0.001, clusters 2 vs. 3 p<0.001, clusters 2 vs. 4 p=0.0251).
4. Discussion/Conclusion
We identified several cognitive clusters among amnestic sporadic EOAD participants in the ADNI cohort. This finding agrees with previous work suggesting a heterogeneity among sporadic EOAD patients [12,49,50]. We found in addition to a cluster with a presentation of isolated memory impairment and a globally impaired cluster, two more presentations- one with additional visuospatial impairment and another with additional language + executive dysfunction.
As expected, neurodegenerative changes affected brain regions responsible for the respective cognitive functions that are impaired in each cluster (Figures 1 and 2). Such regional specificity was not observed for brain amyloidosis (Figure 3). Cluster 1 showed a purely amnestic dysfunction with a corresponding reduction in GMD and glucose metabolism centered in memory regions. The memory and visuospatial deficits of Cluster 2 corresponded to neurodegeneration of the medial temporal and parietal lobes. In Cluster 3, who had affected memory, language, and executive function, the neurodegeneration spread to the memory, language and executive centers of the brain (medial temporal, lateral temporal and frontal lobes, respectively; greater in the left hemispheric). Not surprisingly, cluster 4 with global cognitive impairment also had global reduction in GMD and glucose metabolism in all cortical regions. Invariably across all clusters, the structural neurodegenerative changes were more extensive compared to the metabolic ones.
Since all participants had an amnestic phenotype the partially overlapping patterns of neurodegeneration is not surprising. The amnestic AD variant shows the classic neurodegenerative pattern of medial and in later stages lateral temporal involvement, followed by parietal, occipital and lastly frontal lobe changes. The more strikingly different patterns of neurodegeneration such as those seen in logopenic aphasia due to AD and posterior cortical atrophy due to AD were not observed in this study as subjects with these presentations were explicitly excluded from ADNI.
Longitudinally, we found significant differences between the rate of decline in the language and visuospatial domains. For the language domain, this was primarily due to the significant decline observed in cluster 4. For the visuospatial domain, it was driven by an overall improvement in visuospatial function that was observed in cluster 2 suggesting that the participants in cluster 2 experienced a learning effect longitudinally. Such improvement has been previously observed by others in a cohort of subjects with mild dementia with a mean age 76.4 year [51]. Overall the data suggests that Cluster 4 has a more aggressive form of EOAD, as none of the other clusters reach the level or pattern of cognitive decline observed in Cluster 4 at baseline, even with a similar disease duration at baseline (4.8 years for cluster 4 vs. 4.5– 5.8 years for clusters 1–3). Further studies with larger sample size will be needed to better characterize this aggressive variant.
Several strengths and limitations of this study warrant mention. The strengths of our study include the standardization of imaging and neuropsychological data across sites in the ADNI protocol. However, the requirement that all participants have memory impairment limited our ability to study other relatively common non-amnestic EOAD presentations, such as posterior cortical atrophy, logopenic aphasia or the frontal dysexecutive variant. ADNI employs strict inclusion and exclusion criteria, which means the ADNI sample is not fully representative of all dementia patients. Also as seen in Table 1, there are large between-clusters differences in the sample sizes, both at baseline and in follow-up. The small sample sizes reduced the power of our analysis, thereby diminishing the ability to present statistically significant differences in the longitudinal decline, both within and between clusters. An ongoing large multi-site study – the Longitudinal Early-onset AD study (LEADS, Principal Investigator Liana Apostolova) is currently ongoing. LEADS will prospectively enroll and follow 600 early onset cognitively impaired individuals (CDR=0.5–1) and 100 controls (CDR=0) ages 40–64. LEADS will be able to definitively ascertain whether different patterns of cognitive decline are detectable among study participants.
In conclusion, we found 4 distinct amnestic variants of EOAD suggestive of a high degree of heterogeneity among AD patients in this age group. Larger prospective studies are needed to better characterize these subtypes of sporadic EOAD and delve deeper into any potential environmental, genetic, epigenetic and pathophysiologic differences that could potentially explain the observed clinical variability.
Acknowledgments
Funding Sources
The analyses reported in this manuscript were funded by the NIA R01 AG040770, NIA K02 AG048240, NIA P30 AG010133, NIA K01 AG049050, NIA R01 AG019771 and the Easton Consortium for Alzheimer’s Drug Discovery and Biomarker Development.
Data collection and sharing for this project was funded by the Alzheimer’s Disease Neuroimaging Initiative (ADNI) (National Institutes of Health Grant U01 AG024904) and DOD ADNI (Department of Defense award number W81XWH-12–2-0012). ADNI is funded by the National Institute on Aging, the National Institute of Biomedical Imaging and Bioengineering, and through generous contributions from the following: AbbVie, Alzheimer’s Association; Alzheimer’s Drug Discovery Foundation; Araclon Biotech; BioClinica, Inc.; Biogen; Bristol-Myers Squibb Company; CereSpir, Inc.; Cogstate; Eisai Inc.; Elan Pharmaceuticals, Inc.; Eli Lilly and Company; EuroImmun; F. Hoffmann-La Roche Ltd and its affiliated company Genentech, Inc.; Fujirebio; GE Healthcare; IXICO Ltd.; Janssen Alzheimer Immunotherapy Research & Development, LLC.; Johnson & Johnson Pharmaceutical Research & Development LLC.; Lumosity; Lundbeck; Merck & Co., Inc.; Meso Scale Diagnostics, LLC.; NeuroRx Research; Neurotrack Technologies; Novartis Pharmaceuticals Corporation; Pfizer Inc.; Piramal Imaging; Servier; Takeda Pharmaceutical Company; and Transition Therapeutics. The Canadian Institutes of Health Research is providing funds to support ADNI clinical sites in Canada. Private sector contributions are facilitated by the Foundation for the National Institutes of Health (www.fnih.org). The grantee organization is the Northern California Institute for Research and Education, and the study is coordinated by the Alzheimer’s Therapeutic Research Institute at the University of Southern California. ADNI data are disseminated by the Laboratory for Neuro Imaging at the University of Southern California.
Footnotes
Disclosure Statement: The authors have no conflicts of interest to declare
Statement of Ethics: The authors have no ethical conflicts to disclose. The research protocol was reviewed and approved by each site’s Institutional review board. Informed consent was obtained from all research participants according to the Declaration of Helsinki and the Belmont Report.
Data used in preparation of this article were obtained from the Alzheimer’s Disease Neuroimaging Initiative (ADNI) database (adni.loni.usc.edu). As such, the investigators within the ADNI contributed to the design and implementation of ADNI and/or provided data but did not participate in analysis or writing of this report.
A complete listing of ADNI investigators can be found at: http://adni.loni.usc.edu/wpcontent/uploads/how_to_apply/ADNI_Acknowledgement_List.pdf
References
- 1.Alzheimer’s Association. 2017 Alzheimer’s disease facts and figures. Alzheimer’s & Dementia 2017;13(4):325–73. [DOI] [PubMed] [Google Scholar]
- 2.Zhu X-C, Tan L, Wang H-F, Jiang T, Cao L, Wang C, Wang J, Tan C-C, Meng X-F, Yu J-T: Rate of early onset Alzheimer’s disease: a systematic review and meta-analysis. Annals of translational medicine 2015;3:38–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Koedam EL, Lauffer V, van der Vlies AE, van der Flier WM, Scheltens P, Pijnenburg YA: Early-versus late-onset Alzheimer’s disease: more than age alone. J Alzheimers Dis 2010;19:1401–1408. [DOI] [PubMed] [Google Scholar]
- 4.Ye BS, Seo SW, Lee Y, Kim SY, Choi SH, Lee YM, Kim DH, Han HJ, Na DL, Kim EJ: Neuropsychological Performance and Conversion to Alzheimer’s Disease in Early- Compared to Late-Onset Amnestic Mild Cognitive Impairment: CREDOS Study. Dementia and Geriatric Cognitive Disorders 2012;34:156–166. [DOI] [PubMed] [Google Scholar]
- 5.van der Flier WM, Pijnenburg YAL, Fox NC, Scheltens P: Early-onset versus late-onset Alzheimer’s disease: the case of the missing APOE epsilon 4 allele. Lancet Neurol 2011;10:280–288. [DOI] [PubMed] [Google Scholar]
- 6.Mendez MF, Lee AS, Joshi A, Shapira JS: Nonamnestic Presentations of Early-Onset Alzheimer’s Disease. Am J Alzheimers Dis 2012;27:413–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Seltzer B, Sherwin I: A comparison of clinical features in early- and late-onset primary degenerative dementia. One entity or two? Arch Neurol 1983;40:143–146. [DOI] [PubMed] [Google Scholar]
- 8.Loring DW, Largen JW: Neuropsychological patterns of presenile and senile dementia of the Alzheimer type. Neuropsychologia 1985;23:351–357. [DOI] [PubMed] [Google Scholar]
- 9.Filley CM, Kelly J, Heaton RK: Neuropsychologic features of early- and late-onset Alzheimer’s disease. Arch Neurol 1986;43:574–576. [DOI] [PubMed] [Google Scholar]
- 10.Migliaccio R, Agosta F, Possin KL, Canu E, Filippi M, Rabinovici GD, Rosen HJ, Miller BL, Gorno-Tempini ML: Mapping the Progression of Atrophy in Early- and Late-Onset Alzheimer’s Disease. J Alzheimers Dis 2015;46:351–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Heyman A, Wilkinson WE, Hurwitz BJ, Helms MJ, Haynes CS, Utley CM, Gwyther LP: Early-onset Alzheimer’s disease: clinical predictors of institutionalization and death. Neurology 1987;37:980–984. [DOI] [PubMed] [Google Scholar]
- 12.Binetti G, Magni E, Padovani A, Cappa SF, Bianchetti A, Trabucchi M: Neuropsychological heterogeneity in mild Alzheimer’s disease. Dementia 1993;4:321–326. [DOI] [PubMed] [Google Scholar]
- 13.Jacobs D, Sano M, Marder K, Bell K, Bylsma F, Lafleche G, Albert M, Brandt J, Stern Y: Age at Onset of Alzheimers-Disease - Relation to Pattern of Cognitive Dysfunction and Rate of Decline. Neurology 1994;44:1215–1220. [DOI] [PubMed] [Google Scholar]
- 14.Koss E, Edland S, Fillenbaum G, Mohs R, Clark C, Galasko D, Morris JC: Clinical and neuropsychological differences between patients with earlier and later onset of Alzheimer’s disease: A CERAD analysis. Neurology 1996;46:136–141. [DOI] [PubMed] [Google Scholar]
- 15.Fujimori M, Imamura T, Yamashita H, Hirono N, Ikejiri Y, Shimomura T, Mori E: Age at onset and visuocognitive disturbances in Alzheimer disease. Alz Dis Assoc Dis 1998; 12:163–166. [DOI] [PubMed] [Google Scholar]
- 16.Imamura T, Takatsuki Y, Fujimori M, Hirono N, Ikejiri Y, Shimomura T, Hashimoto M, Yamashita H, Mori E: Age at onset and language disturbances in Alzheimer’s disease. Neuropsychologia 1998;36:945–949. [DOI] [PubMed] [Google Scholar]
- 17.Mendez MF, Ghajarania M, Perryman KM: Posterior cortical atrophy: Clinical characteristics and differences compared to Alzheimer’s disease. Dement Geriatr Cogn 2002;14:33–40. [DOI] [PubMed] [Google Scholar]
- 18.Mendez MF, Lee AS, Joshi A, Shapira JS: Nonamnestic presentations of early-onset Alzheimer’s disease. Am J Alzheimers Dis Other Demen 2012;27:413–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mendez MF: Early-onset Alzheimer’s disease: nonamnestic subtypes and type 2 AD. Arch Med Res 2012;43:677–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ho GJ, Hansen LA, Alford MF, Foster K, Salmon DR, Galasko D, Thal LJ, Masliah E: Age at onset is associated with disease severity in Lewy body variant and Alzheimer’s disease. Neuroreport 2002;13:1825–1828. [DOI] [PubMed] [Google Scholar]
- 21.Barclay LL, Zemcov A, Blass JP, McDowell FH: Factors associated with duration of survival in Alzheimer’s disease. Biol Psychiatry 1985;20:86–93. [DOI] [PubMed] [Google Scholar]
- 22.Mortimer JA, Ebbitt B, Jun SP, Finch MD: Predictors of cognitive and functional progression in patients with probable Alzheimer’s disease. Neurology 1992;42:1689–1696. [DOI] [PubMed] [Google Scholar]
- 23.Rossor MN, Fox NC, Mummery CJ, Schott JM, Warren JD: The diagnosis of young-onset dementia. Lancet Neurol 2010;9:793–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Warren JD, Fletcher PD, Golden HL: The paradox of syndromic diversity in Alzheimer disease. Nat Rev Neurol 2012;8:451–464. [DOI] [PubMed] [Google Scholar]
- 25.Kim SH, Seo SW, Yoon DS, Chin J, Lee BH, Cheong HK, Han SH, Na DL: Comparison of Neuropsychological and FDG-PET Findings between Early- versus Late-Onset Mild Cognitive Impairment: A Five-Year Longitudinal Study. Dementia and Geriatric Cognitive Disorders 2010;29:213–223. [DOI] [PubMed] [Google Scholar]
- 26.Szigeti K, Doody RS: Should EOAD patients be included in clinical trials? Alzheimers Res Ther 2011;3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Petersen RC: Mild cognitive impairment. Continuum: Lifelong Learning in Neurology 2007;13:15–38. [Google Scholar]
- 28.Petersen RC, Aisen PS, Beckett LA, Donohue MC, Gamst AC, Harvey DJ, Jack CR Jr., Jagust WJ, Shaw LM, Toga AW, Trojanowski JQ, Weiner MW: Alzheimer’s Disease Neuroimaging Initiative (ADNI): clinical characterization. Neurology 2010;74:201–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Petersen RC, Smith GE, Waring SC, Ivnik RJ, Tangalos EG, Kokmen E: Mild cognitive impairment: clinical characterization and outcome. Arch Neurol 1999;56:303–308. [DOI] [PubMed] [Google Scholar]
- 30.McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM: Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology 1984;34:939–944. [DOI] [PubMed] [Google Scholar]
- 31.Dubois B, Feldman HH, Jacova C, Dekosky ST, Barberger-Gateau P, Cummings J, Delacourte A, Galasko D, Gauthier S, Jicha G, Meguro K, O’Brien J, Pasquier F, Robert P, Rossor M, Salloway S, Stern Y, Visser PJ, Scheltens P: Research criteria for the diagnosis of Alzheimer’s disease: revising the NINCDS-ADRDA criteria. Lancet Neurol 2007;6:734–746. [DOI] [PubMed] [Google Scholar]
- 32.Jack CR Jr., Albert MS, Knopman DS, McKhann GM, Sperling RA, Carrillo MC, Thies B, Phelps CH: Introduction to the recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement 2011;7:257–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wyman BT, Harvey DJ, Crawford K, Bernstein MA, Carmichael O, Cole PE, Crane PK, DeCarli C, Fox NC, Gunter JL, Hill D, Killiany RJ, Pachai C, Schwarz AJ, Schuff N, Senjem ML, Suhy J, Thompson PM, Weiner M, Jack CR Jr., Alzheimer’s Disease Neuroimaging I: Standardization of analysis sets for reporting results from ADNI MRI data. Alzheimers Dement 2013;9:332–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jack CR Jr., Bernstein MA, Borowski BJ, Gunter JL, Fox NC, Thompson PM, Schuff N, Krueger G, Killiany RJ, Decarli CS, Dale AM, Carmichael OW, Tosun D, Weiner MW, Alzheimer’s Disease Neuroimaging I: Update on the magnetic resonance imaging core of the Alzheimer’s disease neuroimaging initiative. Alzheimers Dement 2010;6:212–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jack CR Jr., Bernstein MA, Fox NC, Thompson P, Alexander G, Harvey D, Borowski B, Britson PJ, J LW, Ward C, Dale AM, Felmlee JP, Gunter JL, Hill DL, Killiany R, Schuff N, Fox-Bosetti S, Lin C, Studholme C, DeCarli CS, Krueger G, Ward HA, Metzger GJ, Scott KT, Mallozzi R, Blezek D, Levy J, Debbins JP, Fleisher AS, Albert M, Green R, Bartzokis G, Glover G, Mugler J, Weiner MW: The Alzheimer’s Disease Neuroimaging Initiative (ADNI): MRI methods. J Magn Reson Imaging 2008;27:685–691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Whitwell JL: Voxel-based morphometry: an automated technique for assessing structural changes in the brain. The Journal of neuroscience : the official journal of the Society for Neuroscience 2009;29:9661–9664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Risacher SL, Kim S, Shen L, Nho K, Foroud T, Green RC, Petersen RC, Jack CR Jr., Aisen PS, Koeppe RA, Jagust WJ, Shaw LM, Trojanowski JQ, Weiner MW, Saykin AJ, Alzheimer’s Disease Neuroimaging Initiative d: The role of apolipoprotein E (APOE) genotype in early mild cognitive impairment (E-MCI). Front Aging Neurosci 2013;5:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Risacher SL, Saykin AJ, West JD, Shen L, Firpi HA, McDonald BC, Alzheimer’s Disease Neuroimaging I: Baseline MRI predictors of conversion from MCI to probable AD in the ADNI cohort. Curr Alzheimer Res 2009;6:347–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Risacher SL, Shen L, West JD, Kim S, McDonald BC, Beckett LA, Harvey DJ, Jack CR Jr., Weiner MW, Saykin AJ, Alzheimer’s Disease Neuroimaging I: Longitudinal MRI atrophy biomarkers: relationship to conversion in the ADNI cohort. Neurobiology of aging 2010;31:1401–1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jagust WJ, Bandy D, Chen K, Foster NL, Landau SM, Mathis CA, Price JC, Reiman EM, Skovronsky D, Koeppe RA, Alzheimer’s Disease Neuroimaging I: The Alzheimer’s Disease Neuroimaging Initiative positron emission tomography core. Alzheimers Dement 2010;6:221–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jagust WJ, Landau SM, Koeppe RA, Reiman EM, Chen K, Mathis CA, Price JC, Foster NL, Wang AY: The Alzheimer’s Disease Neuroimaging Initiative 2 PET Core: 2015. Alzheimers Dement 2015;11:757–771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wechsler D: Wechsler memory scale-revised (WMS-R). Psychological Corporation, 1987. [Google Scholar]
- 43.Rey A: L’examen clinique en psychologie [The clinical psychological examination]. Paris: Presses Universitaires de France; 1964 [Google Scholar]
- 44.Kaplan E, Goodglass H, Weintraub S: The Boston naming test. Philadelphia: Lea & Febiger; 1983 [Google Scholar]
- 45.Butters N, Granholm E, Salmon DP, Grant I, Wolfe J: Episodic and semantic memory: A comparison of amnesic and demented patients. Jaournal of Clinical and Experimental Neuropsychology 1987;9:479–497. [DOI] [PubMed] [Google Scholar]
- 46.Goodglass H, Kaplan E: The assesment of aphasia and related disorders, ed 2nd Philadelphia, Lea & Febiger, 1983. [Google Scholar]
- 47.Reitan RM: Validity of the Trail Making Test as an indicator of organic brain damage. Perceptual and motor skills 1958;8:271–276. [Google Scholar]
- 48.Ward JH: Hierarchical Grouping to Optimize an Objective Function. Journal of the American Statistical Association 1963;58:236–244. [Google Scholar]
- 49.Lehmann M, Ghosh PM, Madison C, Laforce R Jr., Corbetta-Rastelli C, Weiner MW, Greicius MD, Seeley WW, Gorno-Tempini ML, Rosen HJ, Miller BL, Jagust WJ, Rabinovici GD: Diverging patterns of amyloid deposition and hypometabolism in clinical variants of probable Alzheimer’s disease. Brain 2013;136:844–858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ossenkoppele R, Cohn-Sheehy BI, La Joie R, Vogel JW, Moller C, Lehmann M, van Berckel BN, Seeley WW, Pijnenburg YA, Gorno-Tempini ML, Kramer JH, Barkhof F, Rosen HJ, van der Flier WM, Jagust WJ, Miller BL, Scheltens P, Rabinovici GD: Atrophy patterns in early clinical stages across distinct phenotypes of Alzheimer’s disease. Human brain mapping 2015;36:4421–4437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cacho J, Garcia-Garcia R, Fernandez-Calvo B, Gamazo S, Rodriguez-Perez R, Almeida A, Contador I: Improvement pattern in the clock drawing test in early Alzheimer’s disease. Eur Neurol 2005;53:140–145. [DOI] [PubMed] [Google Scholar]