Abstract
Background
M2-like macrophages are associated with the pathogenesis of castrate resistant prostate cancer (CRPC). We sought to determine if dietary omega-3 fatty acids (ω−3 FAs) delay the development and progression of CRPC and inhibit tumor associated M2-like macrophages.
Methods
MycCap cells were grown subcutaneously in immunocompetent FVB mice. Mice were castrated when tumors reached 300mm3. To study effects of dietary ω−3 FAs on development of CRPC, ω−3 or ω−6 diets were started two days after castration and mice sacrificed after early regrowth of tumors. To study ω−3 FA effects on progression of CRPC, tumors were allowed to regrow after castration before starting the diets. M2 (CD206+) macrophages were isolated from allografts to examine ω−3 FA effects on macrophage function. Omega-3 fatty acid effects on androgen-deprived RAW264.7 M2 macrophages was studied by RTqPCR and a migration/ invasion assay.
Results
The ω−3 diet combined with castration lead to greater MycCap tumor regression (182.2±33.6 mm3) than the ω−6 diet (148.3±35.2; p=0.003) and significantly delayed the time to CRPC (p=0.006). Likewise, the ω−3 diet significantly delayed progression of established castrate resistant MycCaP tumors (p=0.003). The ω−3 diet (as compared to the ω−6 diet) significantly reduced tumor associated M2-like macrophage expression of CSF-1R in the CRPC development model, and matrix metallopeptidase-9 (MMP-9) and vascular endothelial growth factor (VEGF) in the CRPC progression model. Migration of androgen depleted RAW264.7 M2 macrophages towards MycCaP cells was reversed by addition of docosahexaenoic acid (ω−3).
Conclusions
Dietary omega-3 FAs (as compared to omega-6 FAs) decreased the development and progression of CRPC in an immunocompetent mouse model, and had inhibitory effects on M2-like macrophage function. Clinical trials are warranted evaluating if a fish oil-based diet can delay the time to castration resistance in men on androgen deprivation therapy, whereas further pre-clinical studies are warranted evaluating fish oil for more advanced CRPC.
Keywords: Fish oil, castration-resistant prostate cancer, omega-3 fatty acids, tumor-associated macrophages
INTRODUCTION
Effective therapies are now available for castrate resistant prostate cancer (CRPC), but eventually the disease progresses necessitating the need for additional therapies. Whereas epidemiologic studies are inconclusive for the association between fish oil intake and risk of overall and advanced prostate cancer [1–6], preclinical studies suggest a clinical benefit for androgen sensitive and castrate resistant disease [7, 8]. Wang et al. reported that fish oil (as compared to corn oil) decreased growth of phosphatase and tensin homolog (PTEN) knockout (KO) allografts in castrated nude mice and decreased growth of native prostate tumors in castrated PTEN KO mice [9]. Tumors in the fish oil group had decreased androgen receptor levels and decreased leucocyte common antigen (CD45+) lymphocyte infiltration [9]. Likewise, Gevariya et al. recently reported that dietary fish oil delayed progression of androgen deprived TRAMP C-2 tumors in immunocompetent mice and was associated with an increased local inflammatory response [10].
Using an androgen sensitive immunocompetent allograft model we reported that fish oil delayed prostate cancer progression and inhibited the number and function of M2-like macrophages in tumor tissue [11]. Macrophages are highly plastic cells and respond to surrounding stimuli to develop tissue specific functions [12]. Distinct states of polarized TAMs include the ‘classically’ activated (M1) macrophages that have tumoricidal activity while the ‘alternatively’ activated (M2) macrophages promote tissue repair and angiogenesis, and favor tumor progression [13]. M2 tumor-associated macrophages (TAMs) make up the majority of TAMs in prostate cancer [12]. M2 polarized TAMs are associated with poor prognosis and high tumor grade in patients with prostate cancer [12–14]. Zarif and Pienta reviewed potential treatment strategies for targeting M2-like macrophages in the tumor microenvironment to treat advanced prostate cancer [15]. Since TAMs represent a functionally diverse group of macrophages with variable activation states, the terminology “M1-like” and “M2-like” is often used for in-vivo studies in recognition of the variation in functional states [16].
Androgen ablation therapy has been shown to increase the expression of M2-like macrophage markers and the number of M2-like macrophages in the tumor environment. For example, prostate cancer cells in a tumor cell-macrophage co-culture system undergoing androgen deprivation exhibited enhanced gene expression of M2-expressing cytokines VEGF-A, MMP-9, and Arg-1, and a reduction in proinflammatory M1 cytokines [17]. In addition, following androgen ablation therapy, a significant increase in M2-like (CD163+) macrophages was observed in prostate tumor tissues from hormone ablation–treated patients compared to hormone-naive tissues [18]. Zarif et al also found M2 macrophage infiltration in human mCRPC tissues from rapid autopsies [19].
In the present study, our objective was to investigate if omega-3 FAs delay the development and progression of CRPC. Based on our previous findings in androgen sensitive prostate cancer, we hypothesized that dietary omega-3 FAs will decrease the number and function of M2-like macrophages leading to a decrease in tumor growth, angiogenesis, migration, invasion, and suppression of immune cell function.
MATERIALS AND METHODS
Chemicals, Reagents and Diets
Docosahexaenoic acid (DHA) was obtained from Cayman Chemical (Ann Harbor, MI, USA), RPMI and DMEM media and fetal bovine serum (FBS) from Invitrogen (Carlsbad, CA, USA), and mouse interleukin 4 (IL-4) from Sigma Chemical (St Louis, MO, USA). Mouse diets were purchased from DYETS, Inc. (Bethlehem, PA). For the ω−6 diet, 30% of energy (134g/kg) was provided by corn oil, and the ω−6 to ω−3 ratio was 18:1. For the ω−3 diet 30% of energy was provided by menhaden oil (134g/kg) and the ω−6 to ω−3 ratio was 1:8 as previously described [11].
Allograft Tumor Models
All animal experiments were approved by the Animal Research Committee of the University of California, Los Angeles (UCLA, Los Angeles, CA). For the development of CRPC model, 40 male FVB mice (6–8 wk old) were acclimated for 7-days on a standard AIN-93 G diet (DYETS, Bethlehem, PA). 5×105 MycCap cells, derived from the FVB genetic background (provided by Dr. L. Wu, UCLA) were injected subcutaneously into the rear flank. MycCap cells were derived in the Sawyers laboratory at UCLA from Hi-Myc mouse prostate tumors [20, 21]. MycCap cells have an amplified androgen receptor gene and show androgen-dependent growth in soft agar [21]. For cell line authentication c-myc gene expression was quantified by RT qPCR. MycCap cells are tested annually for mycoplasma using the mycoplasma qPCR detection kit (Sigma-Aldrich, St. Louis, MO). Castration was performed when tumors reached 300 mm3 and the mice were assigned to the ω−3 or ω−6 diet 2 days after castration based on matching tumor volumes at the time of castration (Supplementary Figure 1). Investigators were not blinded to the group assignment. When mouse tumors in the ω−6 group regrew to 300 mm3, mice in the ω−6 group and time-matched ω−3 group mice were sacrificed. To examine the effect of fish oil on the progression of CRPC, 28 male FVB mice were acclimated for 7-days on a standard AIN- 93 G diet (DYETS, Bethlehem, PA) and subcutaneously injected with 5×105 MycCap cells. When tumors reached a volume of 300 mm3, mice were castrated and tumors were allowed to regrow until they reached 300 mm3 at which time mice were assigned to 2 groups of 14 receiving either the ω−3 or ω−6 diet (Supplementary Figure 1). Mice were assigned to diet groups based on matching tumor volumes at the time of castration. Eleven days after the diet change mice were sacrificed in both groups. For both experiments, mice were housed individually to measure food consumption. Body weights and tumor volumes were measured twice per week. At sacrifice 100 mg of the tumor tissue was snap-frozen in liquid nitrogen, and the remaining tissue used for flow cytometry and CD206+ macrophage isolation.
mRNA Isolation and Quantitative PCR
Total RNA was isolated from tumors and from isolated CD206+ macrophages using RNeasy Mini kit (Qiagen, Valencia, CA) according to the manufacturer’s protocol. The reverse-transcriptional PCR and quantitative real-time PCR were performed as previously described [11]. Briefly, first-strand cDNA was synthesized using MLV-Reverse Transcriptase and random hexamers (Promega, Madison, MI, USA). Quantitative PCR was performed using a Universal SYBR Green mastermix (Applied Biosystems, Grand Island, NY, USA) on CFX96 Real time PCR system (Bio-Rad, Hercules, CA, USA). Gene expression was calculated after normalization to GAPDH using the ΔΔCT method and expressed as relative mRNA level compared to control.
Magnetic cell sorting of M2 (CD206+) macrophage cells
CD206+ macrophages were isolated from single-cell suspensions from fresh tumor tissue using CD206-APC primary antibody and anti-APC magnetic beads with miniMACS (MiltenyiBiotec, Auburn, CA, USA) according to manufacturer’s protocol. The CD206+ macrophage fraction contained >95% macrophages, as confirmed by flow cytometry.
Flow cytometry analysis of immune cells
To prepare single-cell suspensions for flow cytometry, fresh tumor tissue was dissected into approximately 1 to 3 mm3 fragments and digested with 80 U/mL collagenase (Invitrogen) in DMEM containing 10% FBS for one hour at 37°C while shaking. After red blood cell (RBC) lysis, single-cell suspensions were filtered and incubated for 20 minutes on ice with the following antibodies (1:100): CD45-PE (eBioscience, San Diego, CA), F4/80-PE-Cy7 (eBioscience, San Diego, CA), CD11b-FITC (BD Biosciences San Jose, CA), CD206-APC (Biolegend, San Diego, CA),CD68-PerCP-Cy5.5 for macrophages (Biolegend, San Diego, CA), Cells were washed with phosphate-buffered saline (PBS) before analysis on the BD LSR-II flow cytometer (Beckman Coulter).
M2 Macrophage culture and invasion assay
RAW264.7 cells were purchased from ATCC (Manassas, VA). RAW264.7 cells are monocyte/macrophages derived from Abelson murine leukemia virus-induced tumor. To generate M2 macrophages, RAW264.7 cells were treated with 20ng/μl of murine IL-4 (Sigma, St. Louis, MO) for 24 hours as previously described [22]. M-2 type cells were grown in RPMI medium with 10% FBS medium or with 10% charcoal stripped serum (CSS medium) (Life Technologies, Grand Island, NY). Cells were treated with 1 pmol/L to 1 μmol/L of dihydrotestosterone (DHT) (Sigma, St Louis, MO) for 48 hr. At the end of incubation mRNA expression was determined as described above. 24-well plates with matrigel-coated 8 mm pore size inserts (BD Biosciences, Bedford, MA, USA) were used for the invasion assays. 1×105 M2 polarized RAW264.7 cells were seeded in the upper compartment in 500 μl FBS medium. 1×105 MycCap cells were seeded in the lower compartment in 700 μl FBS medium. After cell attachment, medium was replaced with serum-free PRMI1640 in both upper and lower compartments. After 48 hours incubation at 37°C with 5% CO2, cells on the upper insert surface of the membrane were removed with a cotton swabs. The invasive cells that grew through the membrane to the lower insert surface were fixed with 4% paraformaldehyde and quantified by staining with 0.5% crystal violet in 2% methanol and photographed with the digital microscope (Nikon).
Statistical analysis
Group size was estimated based on previous allograft studies performed in our laboratory (Liang P 2019 JNCI paper). Quantitative measures were compared between the two groups (ω−3 and ω−6 diet) using two-tailed Student’s t test calculated by GraphPadPrism6.0 software (GraphPad Software, La Jolla CA). Tumor volume over time was compared between groups (Fig 1A and Fig 3A) using Generalized Estimating Equations (GEE) models in SAS V9.4 (Cary, NC). For Figure 1A, the GEE model was constructed using terms for diet, time (in days), the interaction term, and a repeated mouse effect. For Figure 3A, the GEE model was constructed similarly and the slopes (tumor volume increase per day) were compared between groups from the interaction term. The relevant differences between groups were estimated from the models and presented with 95% confidence intervals. For each mouse, tumor regression was computed by taking the difference between their starting tumor volume and their lowest tumor volume observed and compared between groups using the two-tailed Student’s t test. Time to development of CRPC was compared between the ω−3 and ω−6 groups using the log-rank test and survival estimates (e.g. median survival by group) over time were estimated using Kaplan Meier curves (Fig 1C). For this analysis, CRPC was defined as either two consecutive increases in the tumor volume or an increase above the tumor volume prior to castration. Mice who did not reach CRPC were considered censored at the time of sacrifice. The data are presented as standard error of the mean (SEM) unless otherwise noted. In vitro experiments were performed in triplicate. A p-value <0.05 was considered statistically significant.
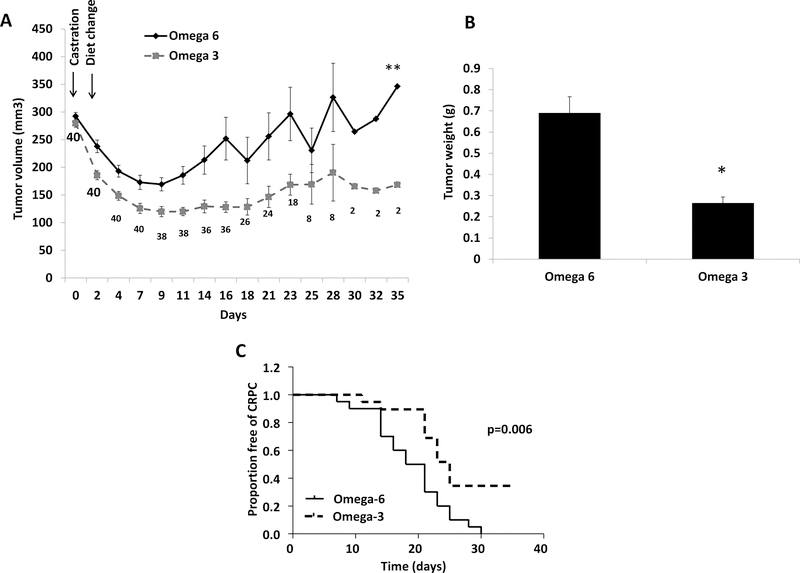
Figure 1:
Dietary ω−3 fatty acids (as compared to ω−6) delayed development of CRPC. (A) Tumor growth. Pre-castration tumor volume not shown. The numbers at each time point indicate the number of surviving mice in the two groups combined. The numbers at time points decreased as mice were sacrificed during the experiment as ω−6 group tumors reached 300 mm3. Tumor volume over time was compared between groups (Fig 1A and Fig 3A) using Generalized Estimating Equations (GEE) models. (B) Tumor weight. (C) Kaplan Meier curve showing time to development of CRPC. Data are means ±SEM (n=20 ω−3 diet, n=20 ω−6 diet); *p<0.05, **p<0.001.
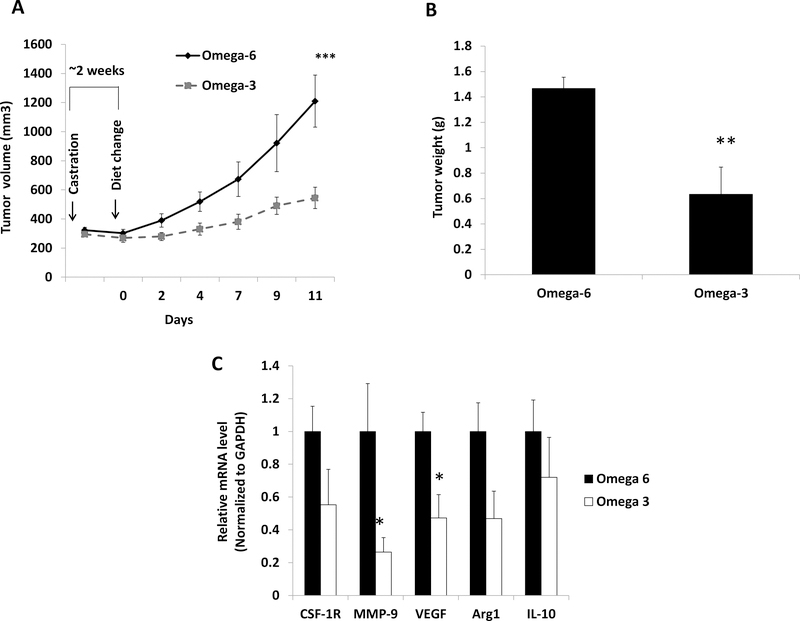
Figure 3:
Dietary ω−3 fatty acids (as compared to ω−6) decreased tumor growth in established CRPC and decreased expression of VEGF and MMP-9 in tumor infiltrating M2-like macrophages. (A) Tumor growth. Pre-castration tumor volume not shown (n=14 ω−3 diet, n=14 ω−6 diet). Tumor volume over time was compared between groups (Fig 1A and Fig 3A) using Generalized Estimating Equations (GEE) models. (B) Tumor weight (n=14 ω−3 diet, n=14 ω−6 diet). (C) Gene expression in M2-like macrophages isolated from tumor tissue (n=11 ω−3 diet, n=10 ω−6 diet). Data are means±SEM; *p<0.05, **p<0.01, ***p<0.003.
RESULTS
Effect of Dietary ω−3 FAs on the development of CRPC and tumor infiltrating immune cells
We investigated the effect of dietary ω−3 FAs on the development of CRPC by initiating the ω−3 or ω−6 diets 2-days after castration. Using the GEE model incorporating all data points, tumor volumes were significantly lower in the ω−3 vs ω−6 diet group by an average of 120 mm3 (95% CI 77.2–164.5; p<0.001) (Figure 1A). Following castration, MycCaP allograft tumor volumes decreased in all mice, but there was a greater reduction in tumor volumes in mice fed the ω−3 (tumor volume reduction: 182.3±33.6 mm3) as compared to the ω−6 diet (tumor volume reduction: 148.3±35.2 mm3; p=0.003) (Figure 1A). To compare the effects of the ω−3 and ω−6 diet on time to development of CRPC, when tumors in the ω−6 group regrew to approximately 300 mm3, mice in the ω−6 group and time-matched ω−3 group mice were sacrificed. The development of CRPC in mice fed the ω−3 diet was significantly delayed (median time to CRPC 25 days) compared to mice fed the ω−6 diet (median time to CRPC 18 days; p=0.006) (Figure 1C). Mean tumor weights at sacrifice were also significantly lower in mice fed the ω−3 (0.26±0.03g) vs the ω−6 diet (0.68±0.08g; p=0.01) (Figure. 1B). There was no significant difference in caloric intake or mouse weights between the groups (data not shown).
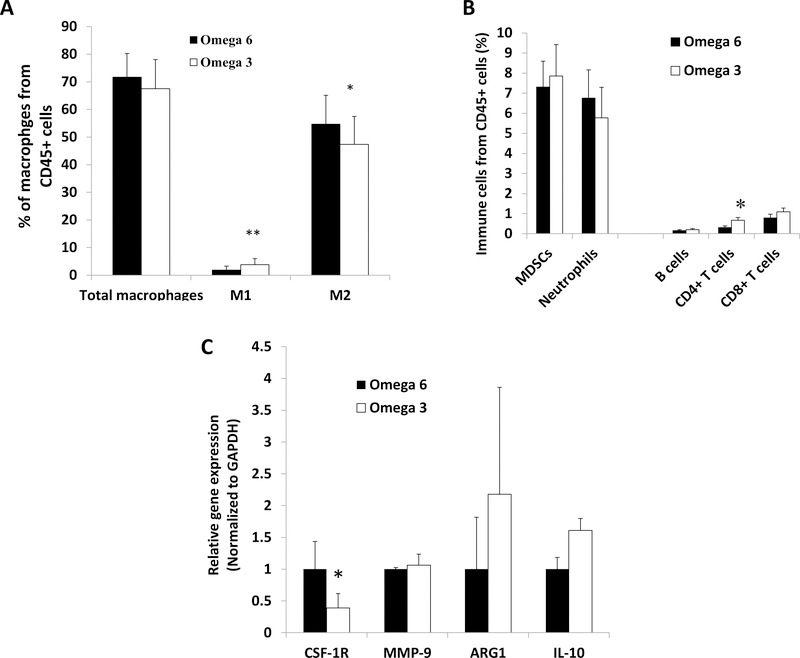
There was a significant decrease in the number of F4/80+CD206+-M2 polarized macrophages, and an increase in the number of F4/80+CD68+-M1 polarized macrophages and CD4+T cells in tumors from mice fed the ω−3 compared to ω−6 diet (Figure 2A and B). There was no significant difference in other tumor infiltrating immune cells (F4/80+CD11b+ total macrophages, CD11b+Gr1+ myeloid-derived suppressor cells (MDSCs), F4/80-CD11b+Gr1+ neutrophils, CD8+ T cells and B220+ B cells) between the groups (Figure 2B). There was a significant decrease in gene expression of colony stimulating factor 1 receptor (CSF-1R) in CD206+ (M2) macrophages isolated from the ω−3 compared to ω−6 group tumors (Figure 2C).
Figure 2:
Dietary ω−3 fatty acids (as compared to ω−6) decreased tumor infiltration of M2-like macrophages, increased infiltration of M1-like and CD4+ T cells, and decreased gene expression of CSF-1R in M2-like macrophages in the development of CRPC mouse model. (A) Macrophage quantity and type by flow cytometry of tumor tissue (n=17 ω−3 diet, n=18 ω−6 diet). (B) Quantity of other immune cells by flow cytometry of tumor tissue (n=17 ω−3 diet, n=18 ω−6 diet). (C) M2-like macrophage gene expression in sorted M2 macrophages (n=3 ω−3 diet, n=2 ω−6 diet). Data are means ±SEM; *p<0.05, **p<0.01.
Effect of Dietary ω−3 FAs on the progression of CRPC and tumor infiltrating M2 macrophages
We investigated the effect of dietary ω−3 FAs on the progression of CRPC by initiating the ω−3 or ω−6 diets, when tumors reached pre-castration volumes after castration. The ω−3 diet (as compared to ω−6 diet) significantly delayed the progression of established CRPC MycCap allografts with the estimated tumor volume slope significantly lower in the ω−3 group as compared to the ω−6 group (24.4 vs 79.8 mm3/d, respectively; 95%CI 24.6–86.1; p=0.003) (Figure 3A). Likewise, mean final tumor weight was significantly lower in mice fed the ω−3 (0.6±0.09g) vs the ω−6 diet (1.5±0.2g; p=0.001) (Figure 3B). No significant difference in mean caloric intake or mouse body weight was observed between the groups (data not shown). Gene expression of matrix metallopeptidase-9 (MMP9) and vascular endothelial growth factor (VEGF) was significantly decreased in CD206+ (M2) cells isolated from allografts from ω−3 fed mice compared to ω−6 fed mice (Figure 3C).
Effect of androgen and DHA (ω−3) on M2 macrophages in vitro
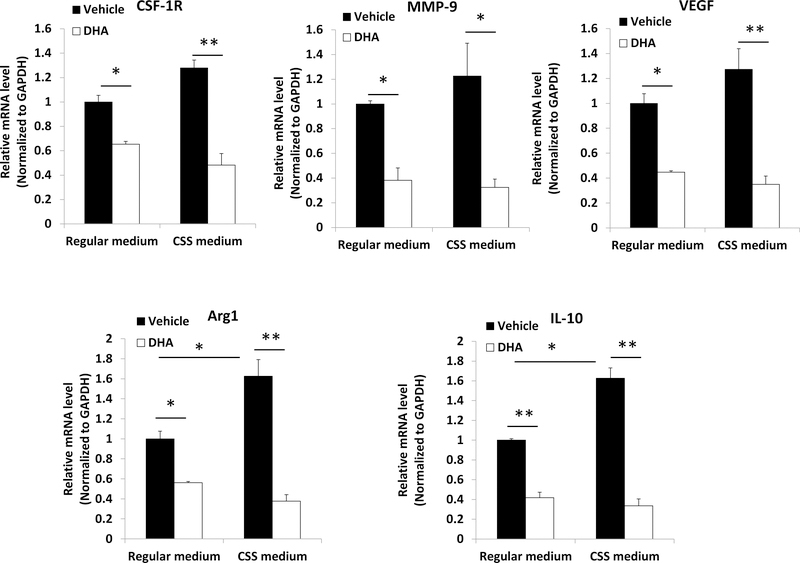
Hormone deprivation by using CCS medium significantly increased the activity of M2 polarized macrophages derived from Raw264.7 cells in vitro as measured by Arg1 and CD206 gene expression (Supplementary Figure 2), and this was partially reversed by addition of DHT. M2 macrophage gene expression of arginase 1 (Arg1), CSF-1R, MMP9, VEGF, and interleukin 10 (IL-10) was significantly reduced by DHA in both standard and charcoal stripped conditions (Figure 4).
Figure 4:
DHA inhibited gene expression in M2 macrophages in FBS medium and charcoal stripped serum (CSS) medium in vitro. Murine macrophages Raw264.7 were polarized to M2 type macrophages and cultured in androgen-deprived conditions in CSS medium or non-androgen deprived conditions in FBS medium and treated with 50 μmol/L of DHA. Mean±SD, *p<0.05, **p<0.01.
DHA inhibited M2 polarized macrophage migration towards prostate cancer cells
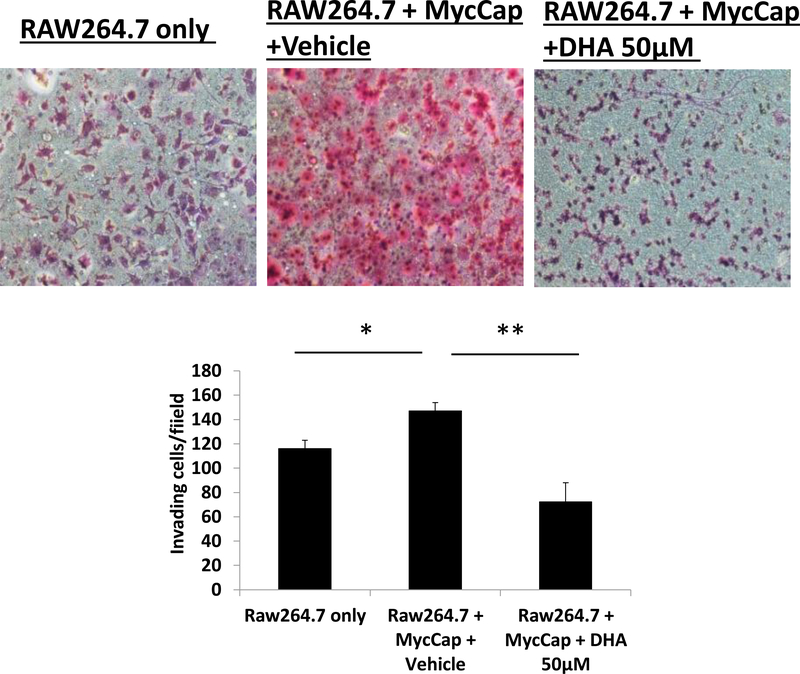
In charcoal stripped conditions, M2 polarized macrophages (derived from Raw264.7 cells) migrated towards MycCaP cells in an established co-culture assay (Figure 5). In charcoal stripped conditions, DHA treatment of M2 polarized macrophages significantly inhibited migration (Figure 5).
Figure 5:
DHA inhibited RAW264.7-derived M2 macrophage migration towards MycCap prostate cancer cells in a co-culture system. Raw264.7 were polarized to M2-type macrophages and cultured in androgen-deprived conditions with CSS medium, plated in inserts and treated with 50 μmol/L of DHA. MycCap cells were cultured in androgen-deprived conditions with CSS medium, and plated in the lower chamber.
DISCUSSION
Omega-3 fatty acid diets have previously been reported to delay progression of castrate resistant prostate cancer in pre-clinical models [7, 9, 10]. Novel in our investigation was examining ω−3 fatty acid effects on the development of CRPC, and examining ω−3 effects on M2 macrophages in early vs. established castrate resistant tumors. To examine the impact on CRPC development we started the ω−3 (or ω−6) diets immediately after castration and compared the regrowth of the tumors. Using a fully immunocompetent MycCaP allograft model, we found that dietary ω−3 (as compared to ω−6 diet) significantly increased tumor regression in the castrated mice and delayed the time to CRPC. The model we used mirrors the clinical scenario in which patients start androgen deprivation therapy for rising prostate-specific antigen (PSA). We believe our positive findings warrant translation to clinical trials in patients initiating androgen deprivation therapy to evaluate compliance and feasibility with a long-term randomized intervention. Given that the control group in our preclinical studies received a corn oil (ω−6) diet, and reduction of ω−6 intake was previously found to slow prostate cancer progression in preclinical models, it would also be rational, in clinical trials, to combine lowering ω−6 intake along with increasing ω−3 intake in the intervention group [23, 24].
In our present investigation, we also found that the ω−3 diet (compared to an ω−6 diet) decreased tumor volume of established CRPC-MycCaP allografts in immunocompetent mice. For this experimental design, after MyCaP allografts were established we performed orchiectomy and allowed tumors to regrow prior to starting the fish oil and corn oil diets. Effective therapies now exist for nonmetastatic CRPC with a PSA doubling time of 10 months or less, and for metastatic CRPC [25]. Possibly a fish oil-based intervention would be appropriate for clinical trials in nonmetastatic CRPC patients with longer PSA doubling times that elect not to take the second generation androgen receptor blocking agents. For patients with more aggressive CRPC, preclinical studies evaluating synergy between dietary fish oil and established therapies are warranted prior to initiating clinical trials.
Tumor associated macrophages, specifically M2-like macrophages, play a key role in prostate cancer progression and metastasis through a number of mechanisms including angiogenesis, immunosuppression, and migration/invasion [18, 19, 26, 27]. As previously reported, and confirmed in the present report, reducing androgen levels in tissue culture media promotes M2 activity [18, 28]. We found that M2 macrophage gene expression of CSF-1R, MMP9, VEGF, and IL-10 were significantly reduced by DHA in both standard and charcoal stripped conditions in vitro. Likewise, DHA inhibited migration of androgen depleted M2 macrophages in a migration assay. We found similar inhibitory effects of an ω−3 diet (compared to an ω−6 diet) on M2-like macrophages in castrate resistant allografts. In the MycCaP model used for our present studies, M2-like macrophages make up 55% of the immune cells infiltrating the allografts [29]. For our experiment in which the ω−3 or ω−6 diet was started 2-days after castration and tumors were harvested after early tumor regrowth, M2 macrophages in the tumor were 16.6% lower in the fish oil vs corn oil group as measured by flow cytometry. There was also a significant decrease in gene expression of CSF-1R in M2-like macrophages isolated from the allografts and a decrease in allograft CD4+ T cells. CSF-1/CSF-1R regulates the formation, differentiation, and function of M2 macrophages, which in turn supports tumor growth and metastasis [30]. M2-like macrophages isolated from established castrate resistant MycCaP allografts had decreased expression of MMP-9 and VEGF in the ω−3 vs. ω−6 diet groups. These data all point to significant anti-M2 effects of dietary fish oil and the potential for inhibition of prostate cancer progression in patients. Establishing a causal link between dietary fish oil, M2-like macrophages, and anti-prostate cancer effects will require additional experiments utilizing novel animal models.
A number of challenges and unanswered questions remain regarding the role of dietary fish oil as a potential treatment for patients with prostate cancer. In our present series of experiments we found that an ω−3 diet (as compared to an ω−6 diet) inhibited CSF-1R expression in M2-like macrophages isolated from tumors in the early CRPC model, whereas VEGF and MMP-9 were inhibited in M2-like macrophages isolated from more advanced castrate resistant tumors (Figure 6). Further research is required to explain the differing effects of fish oil in the early versus late stages of CRPC. Another complexity of applying fish oil therapies in clinical trials is the multiple anti-prostate cancer mechanisms of fish oil. Berquin et al. reviewed a number of mechanisms whereby ω−3 fatty acids inhibit prostate cancer progression in preclinical studies [31, 32]. Since this review a number of other potential anti-prostate cancer mechanism have been reported including effects on androgen receptor levels, AKT signaling, the local anti-tumor inflammatory response, and dependence on g-protein coupled receptor 120 [9, 10, 29, 33]. Multiple anti-cancer mechanisms creates a high level of complexity for devising synergistic combination therapies with existing agents. However, hitting multiple targets may also be beneficial given tumor resistance mechanisms to targeted therapy. Although there are a number of trials in clinicaltrials.gov evaluating fish oil for androgen sensitive prostate cancer, at present none are evaluating fish oil for advanced or CRPC.
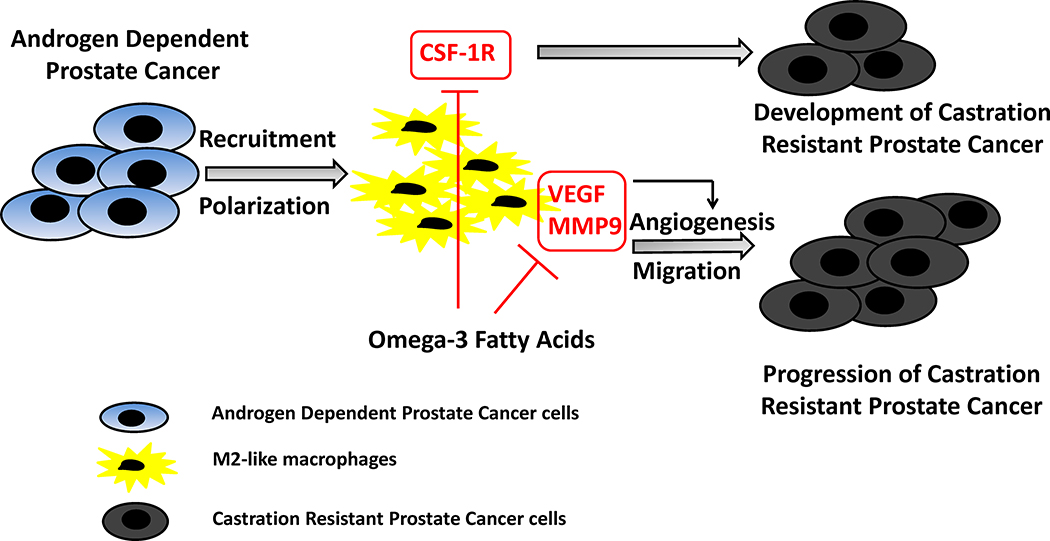
Figure 6:
Conceptual diagram of potential effects of ω−3 fatty acids on M2-like macrophages affecting CRPC development and progression. We propose that after castration, ω−3 FAs reduce CSF-1R gene expression in M2-like macrophages resulting in the inhibition of recruitment and polarization of M2 like macrophages towards tumor tissue, thus, delaying the development of CRPC. In the late stage after castration, ω−3 FAs decrease MMP9 and VEGF expression in M2-like macrophages resulting in the inhibition of migration and angiogenesis, thus, delaying the progression of CRPC.
CONCLUSION
In summary, an ω−3 diet (compared to an ω−6 diet) increased tumor regression in castrated mice, delayed the development and progression of CRPC, and had inhibitory effects on M2-like macrophage function. Clinical trials are warranted evaluating if a fish oil-based diet can delay the time to castration resistant prostate cancer in men on androgen deprivation therapy, whereas further pre-clinical combination therapy studies are warranted evaluating fish oil for more advanced CRPC.
Supplementary Material
ACKNOWLEDGEMENTS
We thank Howard B. Klein for his generous support.
Funding: This work was supported by National Institute of Health (P50CA92131 to WJA; RO1CA231219 to WJA and PC and P30CA016042 and used the flow cytometry core of the CCSG shared resource) and Department of Defense Prostate Cancer Research Program (PC141593 to PL).
Footnotes
DISCLOSURE OF POTENTIAL CONFLICTS OF INTEREST
No potential conflicts of interest were disclosed by any authors.
REFERENCES
- 1.Augustsson K, Michaud DS, Rimm EB, Leitzmann MF, Stampfer MJ, Willett WC et al. A prospective study of intake of fish and marine fatty acids and prostate cancer. Cancer Epidemiol Biomarkers Prev. 2003; 12(1):64–67. [PubMed] [Google Scholar]
- 2.Brasky TM, Darke AK, Song X, Tangen CM, Goodman PJ, Thompson IM et al. Parnes HL et al. Plasma phospholipid fatty acids and prostate cancer risk in the SELECT trial. J Natl Cancer Inst. 2013; 105(15):1132–1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brasky TM, Till C, White E, Neuhouser ML, Song X, Goodman P et al. Serum Phospholipid Fatty Acids and Prostate Cancer Risk: Results From the Prostate Cancer Prevention Trial. Am J Epidemiol. 2011; 173(12):1429–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Leitzmann MF, Stampfer MJ, Michaud DS, Augustsson K, Colditz GC, Willett WC et al. Dietary intake of n-3 and n-6 fatty acids and the risk of prostate cancer. Am J Clin Nutr. 2004; 80(1):204–216. [DOI] [PubMed] [Google Scholar]
- 5.Lovegrove C, Ahmed K, Challacombe B, Khan MS, Popert R, Dasgupta P. Systematic review of prostate cancer risk and association with consumption of fish and fish-oils: analysis of 495,321 participants. Int J Clin Pract. 2015; 69(1):87–105. [DOI] [PubMed] [Google Scholar]
- 6.Norrish AE, Skeaff CM, Arribas GL, Sharpe SJ, Jackson RT. Prostate cancer risk and consumption of fish oils: a dietary biomarker-based case-control study. Br J Cancer. 1999; 81(7):1238–1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Friedrichs W, Ruparel SB, Marciniak RA, deGraffenried L. Omega-3 fatty acid inhibition of prostate cancer progression to hormone independence is associated with suppression of mTOR signaling and androgen receptor expression. Nutrition and cancer. 2011; 63(5):771–777. [DOI] [PubMed] [Google Scholar]
- 8.Lloyd JC, Masko EM, Wu C, Keenan MM, Pilla DM, Aronson WJ et al. Fish oil slows prostate cancer xenograft growth relative to other dietary fats and is associated with decreased mitochondrial and insulin pathway gene expression. Prostate cancer and prostatic diseases. 2013; 16(4):285–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang S, Wu J, Suburu J, Gu Z, Cai J, Axanova LS et al. Effect of dietary polyunsaturated fatty acids on castration-resistant Pten-null prostate cancer. Carcinogenesis. 2012; 33(2):404–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gevariya N, Besancon M, Robitaille K, Picard V, Diabate L, Alesawi A et al. Omega-3 fatty acids decrease prostate cancer progression associated with an anti-tumor immune response in eugonadal and castrated mice. The Prostate. 2019; 79(1):9–20. [DOI] [PubMed] [Google Scholar]
- 11.Liang P, Henning SM, Schokrpur S, Wu L, Doan N, Said J et al. Effect of Dietary Omega-3 Fatty Acids on Tumor-Associated Macrophages and Prostate Cancer Progression. The Prostate. 2016; 76(14):1293–1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lundholm M, Hagglof C, Wikberg ML, Stattin P, Egevad L, Bergh A et al. Secreted Factors from Colorectal and Prostate Cancer Cells Skew the Immune Response in Opposite Directions. Scientific reports. 2015; 5:15651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Comito G, Giannoni E, Segura CP, Barcellos-de-Souza P, Raspollini MR, Baroni G et al. Cancer-associated fibroblasts and M2-polarized macrophages synergize during prostate carcinoma progression. Oncogene. 2014; 33(19):2423–2431. [DOI] [PubMed] [Google Scholar]
- 14.Lanciotti M, Masieri L, Raspollini MR, Minervini A, Mari A, Comito G et al. The role of M1 and M2 macrophages in prostate cancer in relation to extracapsular tumor extension and biochemical recurrence after radical prostatectomy. BioMed research international 2014, 2014:486798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zarif JC, Taichman RS, Pienta KJ. TAM macrophages promote growth and metastasis within the cancer ecosystem. Oncoimmunology. 2014; 3(7):e941734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Roszer T Understanding the Mysterious M2 Macrophage through Activation Markers and Effector Mechanisms. Mediators Inflamm. 2015; 2015:816460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Silva JAF, Bruni-Cardoso A, Augusto TM, Damas-Souza DM, Barbosa GO, Felisbino SL et al. Macrophage roles in the clearance of apoptotic cells and control of inflammation in the prostate gland after castration. The Prostate. 2018; 78(2):95–103. [DOI] [PubMed] [Google Scholar]
- 18.Escamilla J, Schokrpur S, Liu C, Priceman SJ, Moughon D, Jiang Z et al. CSF1 Receptor Targeting in Prostate Cancer Reverses Macrophage-Mediated Resistance to Androgen Blockade Therapy. Cancer research. 2015; 75(6):950–962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zarif JC, Yang W, Hernandez JR, Zhang H, Pienta KJ. The Identification of Macrophage-enriched Glycoproteins Using Glycoproteomics. Molecular & cellular proteomics : MCP. 2017; 16(6):1029–1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ellwood-Yen K, Graeber TG, Wongvipat J, Iruela-Arispe ML, Zhang J, Matusik R et al. Myc-driven murine prostate cancer shares molecular features with human prostate tumors. Cancer Cell. 2003; 4(3):223–238. [DOI] [PubMed] [Google Scholar]
- 21.Watson CS, Bialek P, Anzo M, Khosravi J, Yee SP, Han VK. Elevated circulating insulin-like growth factor binding protein-1 is sufficient to cause fetal growth restriction. Endocrinology. 2006; 147(3):1175–1186. [DOI] [PubMed] [Google Scholar]
- 22.Zhang C, Wang Y, Wang F, Wang Z, Lu Y, Xu Y et al. Quantitative profiling of glycerophospholipids during mouse and human macrophage differentiation using targeted mass spectrometry. Scientific reports. 2017; 7(1):412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ngo TH, Barnard RJ, Anton T, Tran C, Elashoff D, Heber D et al. Effect of isocaloric low-fat diet on prostate cancer xenograft progression to androgen independence. Cancer research. 2004; 64(4):1252–1254. [DOI] [PubMed] [Google Scholar]
- 24.Ngo TH, Barnard RJ, Cohen P, Freedland S, Tran C, deGregorio F et al. Effect of isocaloric low-fat diet on human LAPC-4 prostate cancer xenografts in severe combined immunodeficient mice and the insulin-like growth factor axis. Clinical cancer research : an official journal of the American Association for Cancer Research. 2003; 9(7):2734–2743. [PubMed] [Google Scholar]
- 25.Mateo J, Fizazi K, Gillessen S, Heidenreich A, Perez-Lopez R, Oyen WJG et al. Managing Nonmetastatic Castration-resistant Prostate Cancer. European urology. 2019; 75(2):285–293. [DOI] [PubMed] [Google Scholar]
- 26.Ammirante M, Luo JL, Grivennikov S, Nedospasov S, Karin M. B-cell-derived lymphotoxin promotes castration-resistant prostate cancer. Nature. 2010; 464(7286):302–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lin TH, Izumi K, Lee SO, Lin WJ, Yeh S, Chang C. Anti-androgen receptor ASC-J9 versus anti-androgens MDV3100 (Enzalutamide) or Casodex (Bicalutamide) leads to opposite effects on prostate cancer metastasis via differential modulation of macrophage infiltration and STAT3-CCL2 signaling. Cell death & disease. 2013; 4:e764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ren X, Fu X, Zhang X, Chen S, Huang S, Yao L et al. Testosterone regulates 3T3-L1 pre-adipocyte differentiation and epididymal fat accumulation in mice through modulating macrophage polarization. Biochemical pharmacology. 2017;140:73–88. [DOI] [PubMed] [Google Scholar]
- 29.Liang P, Henning SM, Guan J, Grogan T, Elashoff D, Olefsky JM el al. Role of Host GPR120 in Mediating Dietary Omega-3 Fatty Acid Inhibition of Prostate Cancer. Journal of the National Cancer Institute. 2019; 111(1):52–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang P, Zhao S, Wu C, Li J, Li Z, Wen C et al. Effects of CSF1R-targeted chimeric antigen receptor-modified NK92MI & T cells on tumor-associated macrophages. Immunotherapy. 2018; 10(11):935–949. [DOI] [PubMed] [Google Scholar]
- 31.Berquin IM, Edwards IJ, Kridel SJ, Chen YQ; Polyunsaturated fatty acid metabolism in prostate cancer. Cancer metastasis reviews. 2011; 30(3–4):295–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Aucoin M, Cooley K, Knee C, Fritz H, Balneaves LG, Breau R et al. Fish-Derived Omega-3 Fatty Acids and Prostate Cancer: A Systematic Review. Integrative cancer therapies. 2017; 16(1):32–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gu Z, Wu J, Wang S, Suburu J, Chen H, Thomas MJ et al. Polyunsaturated fatty acids affect the localization and signaling of PIP3/AKT in prostate cancer cells. Carcinogenesis. 2013; 34(9):1968–1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.