This study identifies the transcriptional repressor NAC052, acting in H3K4 demethylation, as a regulator of leaf anatomical and morphological traits.
Keywords: Activation tagging, C4 photosynthesis, histone modifications, Kranz anatomy, leaf development, NAC052
Abstract
In an effort to identify genetic regulators for the cell ontogeny around the veins in Arabidopsis thaliana leaves, an activation-tagged mutant line with altered leaf morphology and altered bundle sheath anatomy was characterized. This mutant had a small rosette area with wrinkled leaves and chlorotic leaf edges, as well as enhanced chloroplast numbers in the (pre-)bundle sheath tissue. It had a bundle-specific promoter from the gene GLYCINE DECARBOXYLASE SUBUNIT-T from the C4 species Flaveria trinervia (GLDTFt promoter) inserted in the coding region of the transcriptional repressor NAC052, functioning in H3K4 demethylation, in front of an alternative start codon in-frame with the natural start codon. Reconstruction of the mutation event of our activation-tagged line by creating a line expressing an N-terminally truncated sequence of NAC052 under control of the GLDTFt promoter confirmed the involvement of NAC052 in leaf development. Our study not only reveals leaf anatomic and transcriptomic effects of an N-terminally truncated NAC052 under control of the GLDTFt promoter, but also identifies NAC052 as a novel genetic regulator of leaf development.
Introduction
In C4 photosynthesis, the mesophyll cells of the leaf fix incoming CO2 into a C4 acid. Subsequently, the C4 acid is transported into specialized bundle sheath (BS) cells where it becomes decarboxylated. The CO2 that is released during the decarboxylation of the C4 acid is concentrated around the enzyme Rubisco. The increased concentration of CO2 around Rubisco reduces the rate of photorespiration, leading to enhanced photosynthetic efficiency particularly in hot and/or dry environments (Sage, 2004; Zhu et al., 2010). In terms of leaf anatomy, the BS cells in C4 plants are organized around the leaf veins in a wreath-like structure called Kranz anatomy (Haberlandt, 1896). The BS cells are interconnected with the mesophyll cells through plasmodesmata (Hatch, 1987). Compared with mesophyll cells, C4 BS cells are enlarged and enriched with chloroplasts (Welkie and Caldwell, 1970; Dengler and Nelson, 1999).
C4 photosynthesis has evolved at least 60 times independently (Sage et al., 2012). Because of these multiple independent occurrences in evolutionary history, the C4 pathway has molecularly evolved from modification to pre-existing enzymes and regulatory networks within C3 ancestors, rather than the evolution of completely new genes and traits (Monson, 1999; Gowik and Westhoff, 2011). Regarding leaf anatomy, it was shown that also in C3 plants, cells around the veins have a slightly different cellular morphology compared with the rest of the mesophyll cells, and so are termed pre-bundle sheath cells (Kinsman and Pyke, 1998). Pre-bundle sheath cell chloroplasts are smaller and occur at a lower density in the cell, and are often positioned on the cell wall distal to the vasculature (Kinsman and Pyke, 1998). Also on the physiological level, it was shown that in C3 plants, similar biochemical attributes to those needed in C4 photosynthesis are already present, being associated with photosynthesis around the vascular system of stems and petioles (Hibberd and Quick, 2002). The exact physiological role of BS cells in C3 plants is not fully understood; analysis of transcript residency on ribosomes in the Arabidopsis thaliana BS has revealed a role for the BS cells in sulfur and glucosinolate metabolism (Leegood, 2008; Aubry et al., 2014).
Quantitative modelling has shown that C4 evolution proceeded stepwise and that each evolutionary step has contributed to an increase in the general fitness of the plant (Heckmann et al., 2013). Extensive analysis of the Flaveria family, containing both C3 and C4 species within the family, as well as C3–C4 intermediates in several stages of evolution, has contributed enormously to the current knowledge on the evolutionary progression of C3 photosynthesis towards C4 photosynthesis (McKown et al., 2005; Gowik et al., 2011; Mallmann et al., 2014). The first steps believed to activate the C4 photosynthetic programme in C3 plants are the inflating of the pre-bundle sheath cells accompanied by an increase in the numbers of chloroplasts and mitochondria, and the decrease of glycine decarboxylase (GDC) activity in the mesophyll cells (Bauwe, 2011; Sage et al., 2012). GDC decarboxylates glycine that is formed in photorespiration. During the decarboxylation of glycine, CO2 is released as a by-product. The increase in the numbers of mitochondria and chloroplasts in the BS cells and the decrease of GDC activity in the mesophyll force the glycine formed by photorespiration in the mesophyll to migrate to the BS for decarboxylation, with the released CO2 accumulating and increasing Rubisco efficiency (Mallmann et al., 2014; Sage et al., 2014). This process is known as the photorespiratory CO2 pump (Bauwe, 2011)
The promoter of the bundle-specific expressed gene encoding the P-subunit of glycine decarboxylase (GLDPA) from the C4 species Flaveria trinervia maintains its bundle-specific expression when expressed in A. thaliana (Engelmann et al., 2008). Conversely, the promoter of the gene encoding the sulfate transporter SULTR2;2 from A. thaliana acts in a bundle-specific manner when expressed in the C4 species Flaveria bidentis (Kirschner et al., 2018). These two studies suggest a common transcriptional regulatory mechanism around the BS cells in C3 and C4 species. Recently, it was proven to be possible to create a reporter line in A. thaliana with chloroplast-targeted green fluorescent protein (GFP) under the control of the GLDPAFt bundle-specific promoter and to use this reporter line (the pGLDPAFt::RbcS.TP-sGFP reference line) to obtain BS anatomy mutants (Döring et al., 2019). One such mutational approach is activation tagging, in which a particular promoter is randomly inserted in a reference genome, resulting in alteration of the transcription pattern of genes in the proximity of the landing point of the inserted promoter (Tani et al., 2004). In this study, the bundle-specific promoter of the gene encoding the T-subunit of GDC from the C4 species F. trinervia, proven to also be bundle specific in Arabidopsis (Emmerling, 2018), was used for tissue-specific activation tagging in the A. thaliana pGLDPAFt::RbcS.TP-sGFP reference line.
By using activation tagging, this study identifies NAC052, a member of the NAC transcription factor gene family involved in post-transcriptional gene regulation (Butel et al., 2017), as a novel genetic regulator of leaf morphology and bundle sheath anatomy in Arabidopsis.
Materials and methods
The Arabidopsis thaliana bundle sheath reporter line
An A. thaliana BS reporter line was used that contained a construct harbouring the promoter of the GLDPA gene (NCBI accession no. Z99767), a chloroplast transit peptide (TP) of the Arabidopsis RbcS gene, and a synthetic GFP (sGFP), termed the pGLDPAFt::RbcS.TP-sGFP reporter line (Döring et al., 2019).
Activation tagging
The promoter sequence of the GLDT gene from F. trinervia (NCBI accession no. Z99769) was donated by J. Emmerling (Emmerling, 2018), amplified using PCR with restriction sites (PmeI and SacI) added to the PCR primers, and inserted in the pMDC123 vector (Curtis and Grossniklaus, 2003), as close to the T-DNA left border as a unique restriction site was found to use for inserting the GLDT promoter (the restriction site PmeI was chosen). This pMDC123-GLDT vector was transformed in the Arabidopsis pGLDPAFt::RbcS.TP-sGFP reporter line.
Cloning of pGLDTFt::NAC052 and pGLDTFt::5'truncatedNAC052 constructs
The promoter of the GLDT gene from F. trinervia was inserted in the pAUL1 vector (Lyska et al., 2013). The coding sequence of NAC052 was isolated from cDNA from the Columbia-0 accession of A. thaliana using the primers listed in Supplementary Table S1 at JXB online.
To introduce the (truncated) NAC052 coding sequence (CDS) into the Gateway entry vector pDONR221, the BP Clonase reaction (Gateway ‘BP Clonase II’ enzyme mix, ThermoFisher Scientific) was carried out as described by the manufacturer. The resulting pENTRY221-(truncated)NAC052 was subsequently used for the LR Clonase reaction (Gateway ‘LR Clonase II’ enzyme mix, ThermoFisher Scientific) to transfer the (truncated )NAC052 CDS into pAUL1-GLDTFt (pAUL1-GLDTFt::NAC052 and pAUL1-GLDTFt::5'truncatedNAC052).
CRISPR/Cas
The target site for Cas9 was chosen in the first exon of NAC052, using the primers shown in Supplementary Table S1.
The primers were annealed to produce a single-guide RNA (sgRNA). The product was ligated in the BbsI-digested sgRNA subcloning vector pFH6 (GenBank accession no. KY080689; Hahn et al., 2017). The sgRNA cassette including the 20 bp target site was amplified from pFH6 (Table S1) and integrated into the KpnI/HindIII-digested pUB-Cas9 vector (GenBank accession no. KY080691 (Hahn et al., 2017)) via Gibson Assembly.
Transformation of A. thaliana
The construct for transformation was inserted into the Agrobacterium tumefaciens strain AGL1 (Lazo et al., 1991). After confirming the vector sequence (LGC Genomics, Berlin, Germany), the A. tumefaciens bacteria were put on the plant following the floral dip protocol (Clough and Bent, 1998), as modified by Logemann et al. (2006).
Depending on the nature of the construct, plants were selected on half-strength Murashige and Skoog (1/2 MS) plates containing 0.6% agar, 1% sucrose, and 50 μg ml–1 kanamycin or on soil (Floraton 1, Floragard, Oldenburg, Germany) watered with 80 mg l–1 glufosinate-ammonium (Bayer Agrar, Germany) and 0.1% Tween-20. Positive transformants were screened under the microscope for GFP signal.
Light microscopy
The first leaf of ~2-week-old plants was analysed with a fluorescence microscope (Axio Imager M2m Zeiss, Oberkochen, Germany). The total GFP signal per leaf was quantified and normalized to leaf area with ImageJ (Version 2.0.0-rc-44/1.50e).
Leaf sectioning for internal leaf anatomy
Internal leaf anatomy was assessed on sections sampled from the middle of the second leaf pair (one leaf per plant: three plants per line). The sections were prepared for light microscopy as described by Khoshravesh et al. (2017).
Thermal asymmetric interlaced PCR (TAIL-PCR)
To determine the T-DNA insertion site after activation tagging, TAIL-PCR was performed as described by Singer and Burke (2003).
Quantitative reverse–transcription PCR (qRT–PCR)
RNA was extracted from full-grown rosette leaves (five biological replicates) from non-flowering plants grown in soil (Floraton 1, Floragard) for 28 d in a climate-controlled growth chamber (16 h, 22 °C, 110 μmol m−2 s−1 light; 8 h, 20 °C dark); the RNA was extracted according to Onate-Sánchez and Vicente-Carbajosa (2008). After normalization of RNA concentrations, cDNA was synthesized using the Qiagen Quantitect Reverse Transcription Kit (Qiagen, Hilden, Germany). qRT–PCR was performed with three technical replicates for each biological replicate using the SYBR-green mastermix from KAPA SYBR FAST (KAPA Biosystems, Roche Sequencing and Life Science). Actin (At3g18780) was used as the reference gene for normalization of samples. The primers used are given in Supplementary Table S1.
Reverse transcription–PCR (RT–PCR)
The presence of the predicted 5'truncated transcript variant was detected with agarose gel electrophoresis after RT–PCR using the primers listed in Supplementary Table S1.
RNA sequencing
RNA was extracted from plants (three biological replicates) grown in the same conditions as the plants used for qRT–PCR, using the Qiagen RNeasy Plant Mini Kit including on-column DNA digestion. Total RNA samples were quantified (Qubit RNA HS Assay, Thermo Fisher Scientific) and quality was measured by capillary electrophoresis using the Fragment Analyzer and the ‘Total RNA Standard Sensitivity Assay’ (Agilent Technologies, Inc., Santa Clara, CA, USA). The library preparation was performed according to the manufacturer’s protocol using the Illumina® ‘TruSeq Stranded mRNA Library Prep Kit’. Briefly, 200 ng of total RNA was used for mRNA capturing, fragmentation, the synthesis of cDNA, adaptor ligation, and library amplification. Bead-purified libraries were normalized and finally sequenced on the HiSeq 3000/4000 system (Illumina Inc., San Diego, CA, USA) with a read setup of 1×150 bp. The bcl2fastq tool was used to convert the bcl files to fastq files as well for adaptor trimming and demultiplexing.
Data analyses on fastq files were conducted with CLC Genomics Workbench (version 10.1.1, QIAGEN, Venlo, The Netherlands). The reads of all probes were adaptor trimmed (Illumina TruSeq) and quality trimmed (using the default parameters: bases below Q13 were trimmed from the end of the reads, ambiguous nucleotides maximal 2). Mapping was done against the A. thaliana (TAIR10) (25 May 2017) genome sequence as described by Mortazavi et al. (2008). A principal component analysis (PCA) was performed to analyse the correlation between the biological replicates. The DESeq2 R package was used to determine the differentially expressed genes (Love et al., 2014). The Gene Ontology (GO) Analysis Toolkit and Database for Agricultural Community (AgriGO) was use for GO enrichment analysis (http://systemsbiology.cau.edu.cn/agriGOv2/). The RNA sequencing data set has been deposited at NCBI with accession number GSE139503, and can be accessed through https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE139503.
Results
Activation tagging
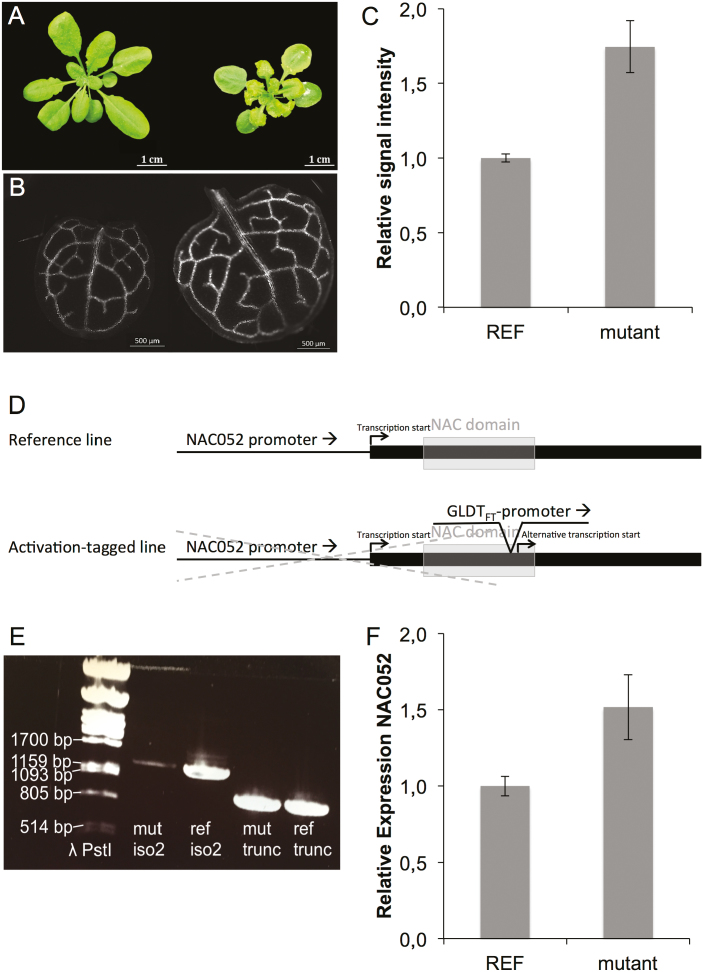
A GLDTFt promoter activation-tagged line was identified with an increased signal intensity of the GFP reporter gene expressed in the leaf bundle, and a small rosette area with wrinkled leaves and chlorotic leaf edges (Fig. 1A–C). Genomic analysis revealed that the F. trinervia-derived GLDT promoter (the activation tag) had inserted in the coding region of the gene encoding the transcription factor NAC052 (At3g10490), a transcriptional repressor functioning in H3K4 demethylation (Ning et al., 2015; Zhang et al., 2015). This gene is also known as SUPPRESSOR OF GENE SILENCING 1 (SGS1), named as such because its downstream effect is on genes that are crucial for post-transcriptional gene silencing (Butel et al., 2017). Four splice variants are known for NAC052 from published RNA sequencing experiments (Cheng et al., 2017; Supplementary Fig. S1). The different splice variants are expressed in different parts of the plant; only transcripts nr2 and nr4 were measured in the leaf, of which transcript nr2 was most prominent (Cheng et al., 2017). Our GLDTFt promoter had inserted in the end of the NAC domain; sequence analysis revealed an alternative ATG start site 30 bp downstream of the GLDTFt promoter insertion, in-frame with the coding sequence (Fig. 1D). We hypothesized the GLDTFt insertion led to production of an aberrant, 5'truncated transcript variant of NAC052. We could not detect full-length transcript nr2 in our activation-tagged mutant line (Fig. 1E). However, we could detect an increase in RNA quantity of the later exons (Fig. 1F).
Fig. 1.
C4 promoter-induced expression of NAC052 increases signal intensity of reporter gene expression in the leaf bundle and changes leaf morphology of C3Arabidopsis thaliana. (A) Leaf morphology of 28-day-old A. thaliana reference line (Col-0 transformed with GFP under control of the Flaveria trinervia-derived GLDPA promoter) and the mutant line (reference line transformed with activation tagging construct with the F. trinervia-derived GLDT promoter). (B) GFP signal overview of first leaf of 14-day-old reference line and mutant line. (C) Quantification of GFP signal of first leaf of 14-day-old reference line and mutant line, n=5. (D) Overview of the genomic landing point of the F. trinervia-derived GLDT promoter in the activation-tagged line. An alternative start codon in-frame with the other two ATGs was discovered 32 bp downstream of the genomic landing point of the GLDT promoter. The grey dotted line represents disfunction of the endogenous NAC052 promoter in the activation-tagged line. (E) Presence/absence (RT–PCR) of the wild-type transcript nr2 and of the alternative 5'truncated transcript. (F) Quantification (qRT–PCR) of the NAC052 transcripts in the reference and mutant line.
NAC052 is involved in leaf development
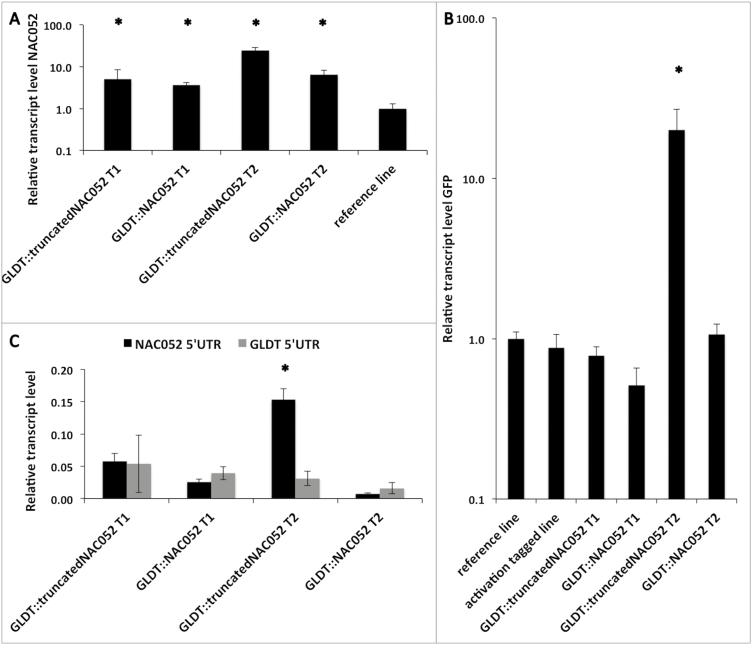
We tried to reconstruct the mutation event of our activation-tagged line by expressing pGLDTFt::5'truncatedNAC052 in the pGLDPAFt::RbcS.TP-sGFP reference line and could confirm enhanced GFP signal intensity in 1/10 T1 plants (70 T1 plants were analysed). These T1 plants were smaller than the untransformed reference line and had chlorotic and wrinkled leaf edges, similar to the original activation-tagged line (Fig. 2). The level of endogenous NAC052 transcripts was increased in the T1 plants with enhanced GFP signal intensity (Fig. 3A), suggesting that the expression of the 5'truncated NAC052 gene influences transcript levels of the endogenous NAC052. However, in the T2 plants, the GFP signal was reduced compared with the untransformed reference line and the leaf morphology phenotype was lost in all plants. This suggests that the pGLDTFt::5'truncatedNAC052 construct somehow was silenced. To confirm this, we measured the transcript level of the GFP gene itself. The pGLDTFt::5'truncatedNAC052 transgene increased the transcript level of the GFP gene only in the T2 generation, but not in T1 (Fig. 3B). However, the increased transcript level of the GFP gene did not lead to an increase in the GFP signal observed in the T2 plants. The activation-tagged line, which also accumulates a 5'truncated NAC052 transcript variant, but has no endogenous NAC052 function (Fig. 1), showed no effect on the transcript levels of the GFP transgene (Fig. 3B). However, the activation-tagged line exhibited an increased GFP signal (Fig. 1). This suggests that the endogenous native copy of NAC052 present in the genomic background of the pGLDTFt::5'truncatedNAC052 line somehow post-transcriptionally silences the GFP gene. This inference is strengthened by the observation of increased transcript levels of the endogenous NAC052 in the T2 generation of the pGLDTFt::5'truncatedNAC052 line (Fig. 3C).
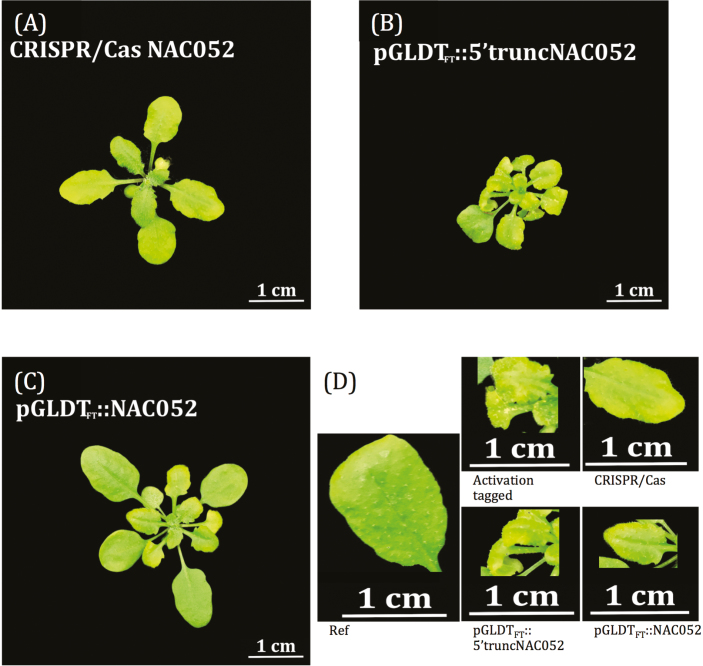
Fig. 2.
Leaf morphology of 28-day-old rosettes of Arabidopsis thaliana. (A) CRISPR/Cas line (T3), (B) pGLDTFt::5'truncatedNAC052 line (T1), and (C) pGLDTFt::NAC052 line (T1). (D) Close-ups of the leaves from the reference line, the activation-tagged line, the CRISPR/Cas line, the pGLDTFT::5'truncatedNAC052 line, and the pGLDTFt::NAC052 line to highlight leaf morphological traits.
Fig. 3.
Quantification (qRT–PCR) of transcripts in transformed lines of Arabidopsis thaliana. (A) NAC052 (normalized to reference line), (B) GFP transgene (normalized to reference line), (C) NAC052 endogene (NAC052 5'UTR) versus NAC052 transgene (GLDT 5'UTR); quantification relative to reference gene used for qRT–PCR
For further confirmation of NAC052 involvement in leaf development, we expressed the entire reading frame of NAC052 transcript nr2 under the control of the GLDTFt promoter in the pGLDPAFt::RbcS.TP-sGFP reference line, creating a pGLDTFt::NAC052 transgenic line. We could confirm enhanced GFP signal intensity in 4/10 T1 plants (40 T1 plants were analysed). In addition, these T1 plants were smaller than the untransformed reference line and had chlorotic leaf edges (Fig. 2). In contrast to the activation-tagged line and to the T1 plants from the pGLDTFt::5'truncatedNAC052 line, the T1 plants from the pGLDTFt::NAC052 line did not show wrinkled leaf edges. Again, the phenotype was completely lost in the T2 generation. The transcript levels of the GFP gene were unaltered in both the T1 and T2 generations of the pGLDTFt::NAC052 line (Fig. 3B). Unlike in the pGLDTFt::5'truncatedNAC052 line, the transcripts levels of endogenous NAC052 were not increased in the T2 generation (Fig. 3C).
For even further confirmation of the involvement of NAC052 in leaf development, we mutated the endogenous NAC052 with clustered regularly interspaced short palindromic repeats (CRISPR)/CRISPR-associated protein (Cas). In this CRISPR/Cas mutant, a thymine nucleotide was inserted in the first exon of wild-type transcript nr2 of NAC052, leading to a frameshift (Supplementary Fig. S2). Similar to the activation-tagged mutant and to the T1 plants of the pGLDTFt::5'truncatedNAC052 line, the CRISPR/Cas mutant was small and had chlorotic leaf edges (Fig. 2), but it did not show wrinkled leaf edges. However, in contrast to the activation-tagged mutant, the GFP signal intensity was decreased in the CRISPR/Cas mutant as compared with the reference line. Thus, the dysfunctional NAC052 transcript variant induced by CRISPR/Cas and the dysfunctional 5'truncated NAC052 transcript variant appear to function differently.
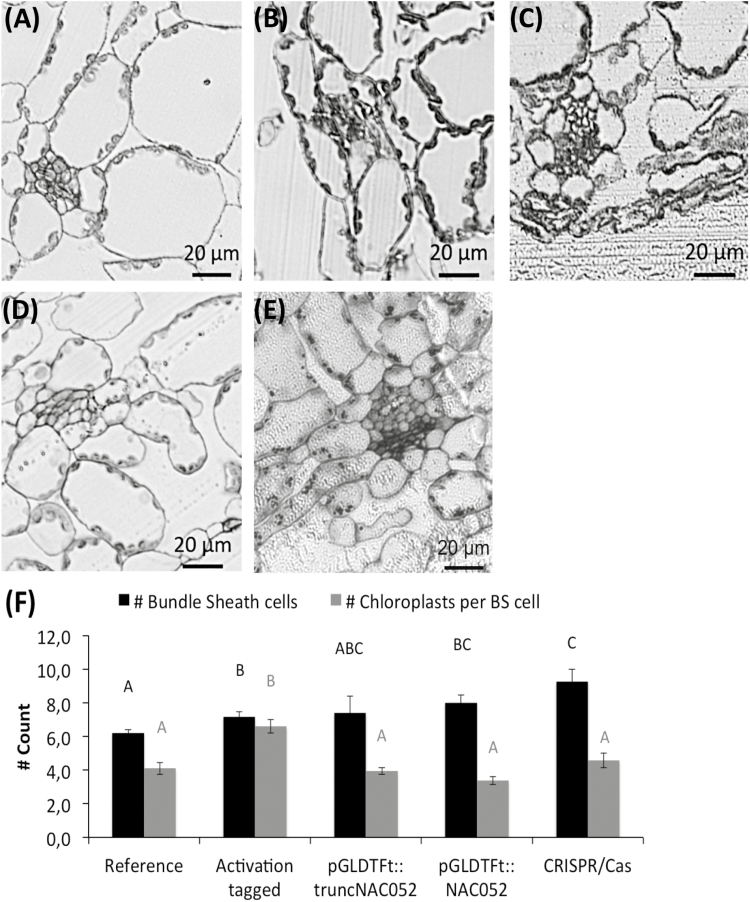
In order to fully characterize the function of NAC052 in leaf development and BS anatomy, multiple measurements were done on the transgenic plants that were created. To provide an overview, the results of those measurements are summarized in Table 1. To see the effect of the bundle-specific expression of 5'truncated NAC052 on internal leaf anatomy, transverse cross-sections of leaves of the activation-tagged, the pGLDTFt::5'truncatedNAC052, the pGLDTFt::NAC052, and the CRISPR/Cas lines were compared with cross-sections of the reference line. The activation-tagged line showed enhanced numbers of cells in the BS tissue compared with the reference line, and those cells contained a higher number of chloroplasts (Fig. 4). No such increases were observed in the mesophyll cells. Similar to the activation-tagged line, the pGLDTFt::5'truncatedNAC052, the pGLDTFt::NAC052 line (both in the T2 generation), and the CRISPR/Cas line showed increased number of cells in the BS tissue (Fig. 4). However, in contrast to the activation-tagged line, the numbers of chloroplasts in the BS cells of the pGLDTFt::5'truncatedNAC052 and the pGLDTFt::NAC052 lines were not increased compared wirtt the reference line (Fig. 4).
Table 1.
Overview of the characteristics regarding NAC052 function in the lines constructed for confirmation of NAC052 involvement in bundle sheath ontogeny
| Line | GFP signal compared with reference line | NAC052 read count (n=3) | NAC052 endogenous function? | Truncated NAC052 function? | No. of genes with differential read count compared with reference line | No. of BS cells (n=5) | BS anatomy compared with reference line |
|---|---|---|---|---|---|---|---|
| Reference | – | 657±32 | Yes | No | – | 6.2±0.2 | – |
| Activation tagged | Increased | 2106±337 | No | Yes | 69 | 7.2±0.3 | More BS cells, more chloroplasts |
| CRISPR/Cas | Reduced | 849±31 | No | No | 1028 | NM | NM |
| pGLDTFt::5'truncated NAC052 | Reduced | 1743±425 | Yes | Yes | 65 | 7.4±1.0 | More BS cells |
| pGLDTFt::NAC052 | Same | 6229±2910 | Yes | No | 1310 | 8.0±0.4 | More BS cells |
NM=not measured.
Fig. 4.
Light micrographs illustrating transverse cross-sections of a third-order vein. (A) Reference line, (B) activation-tagged line, (C) pGLDTFt::5'truncatedNAC052 line, (D) pGLDTFt::NAC052 line, (E) CRISPR/Cas line. (F) Quantification of the number of BS cells and the number of chloroplasts per BS cell. Letters indicate statistically significant differences as determined by ANOVA (n=5; P<0.05)
Downstream genes affected by NAC052
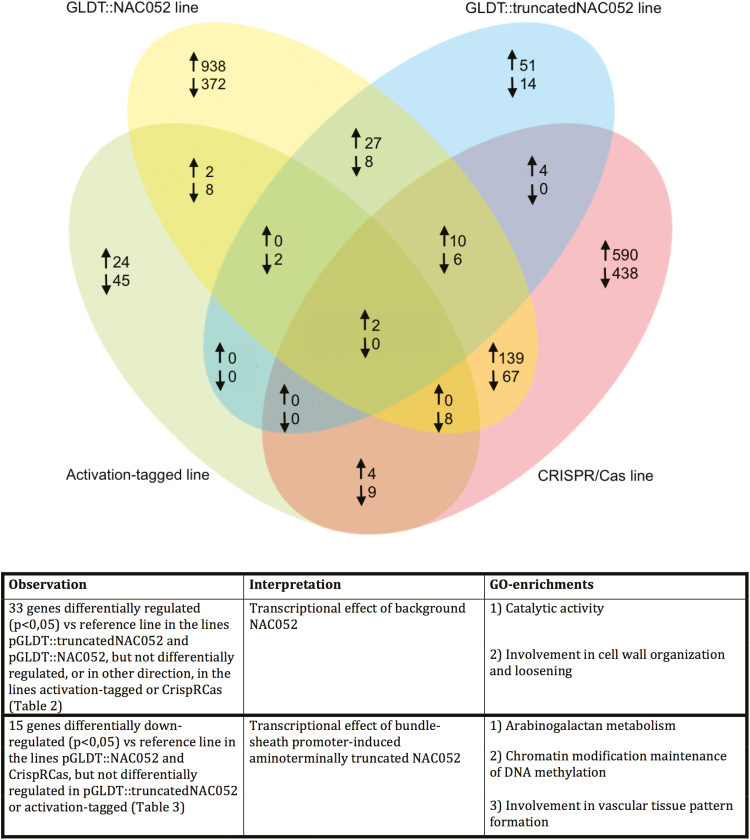
To get a clearer idea of which downstream genes and what biological processes are affected by targeted misexpression of NAC052, we performed mRNA sequencing on the activation-tagged line, the pGLDTFt::5'truncatedNAC052 transgenic line, the pGLDTFt::NAC052 transgenic line, and the CRISPR/Cas mutated line. Figure 5 shows the genes with differential transcript counts compared with the pGLDPAFt::RbcS.TP-sGFP reference line (fold change >2 or <0.5; P<0.05); the correlation between the biological replicates is shown in Supplementary Fig. S3; mapping of the reads to the four different splice variants of NAC052 as well as the expression of the splice variants is shown in Supplementary Figs S4 and S5. The activation-tagged line and the pGLDTFt::5'truncatedNAC052 line showed a low number of differentially counted transcripts compared with the reference line, whereas the pGLDTFt::NAC052 line and the CRISPR/Cas mutated line showed a relatively high number of differentially counted transcripts compared with the reference line (Fig. 5). No significant GO enrichments were found within the descriptions of the 69 genes that are differentially regulated in the activation-tagged line when compared with the reference line, nor were any GO enrichments found among the 65 genes that are differentially regulated in the pGLDTFt::5'truncatedNAC052 line when compared with the reference line. No genes respond similarly in the activation-tagged line as in the pGLDTFt::5'truncated NAC052 line when compared with the reference line (Fig. 5).
Fig. 5.
Number of genes with a differential transcript count compared with the pGLDPAFt::RbcS.TP-sGFP reference line. Venn diagrams displaying the number of significantly (P=0.05) differentially (>2.0-fold up- or down-regulated) expressed genes when comparing the pGLDTFt::NAC052, pGLDTFt::5'truncatedNAC052, activation-tagged, or CRISPR/Cas transformed line with the reference line. The panel gives an overview of the GO enrichments.
Based on the introduced mutations, both in the activation-tagged line and in the CRISPR/Cas line, the endogenous full-size NAC052 protein should be absent, while the pGLDTFt::5'truncatedNAC052 and the pGLDTFt::NAC052 lines should still accumulate endogenous NAC052 protein in the background. We compared descriptions of genes that are similarly responsive in the pGLDTFt::5'truncatedNAC052 and the pGLDTFt::NAC052 line, as well as similarly responsive in the activation-tagged line and the CRISPR/Cas line, but differently responsive between pGLDTFt::5'truncatedNAC052/pGLDTFt::NAC052 and activation-tagged/CRISPR/Cas. In such a way, we could investigate the transcriptional effect of endogenous NAC052 protein expression in the background. Thirty-three genes respond in such a way (Table 2). These genes are enriched for the GO terms ‘catalytic activity’ and ‘involvement in cell wall organization and loosening’. The genes involved in catalytic activity all encode enzymes, for example a temperature-sensitive plastidic fatty acid desaturase, a cysteine-rich receptor-like protein kinase, and a calcium-dependent phosphotriesterase superfamily protein. Among the genes involved in cell wall organization are, for example, a xyloglucan endotransglucosylase/hydrolase, an aspartyl protease, and an expansin-like protein.
Table 2.
Genes that are similarly responsive in the GLDTFt::5'truncatedNAC052 and the GLDTFt:NAC052 lines, but differently responsive in the activation-tagged line and the CRISPR/Cas line
| Gene ID | Gene description | Abbreviated gene name | Activation tagged | CRISPR/Cas | GLDT:: truncatedNAC052 | GLDT:: NAC052 | |
|---|---|---|---|---|---|---|---|
| 1 | AT1G01060 | LHY encodes a myb-related putative transcription factor (TF) involved in circadian rhythm along with another myb TF CCA1. | LHY | 2.06 | 2.37 | 0.5<FC<2.0 | 0.5<FC<2.0 |
| 2 | AT1G16730 | UNKNOWN PROTEIN 6, expressed during flowering stage, petal differentiation, and expansion stage; expressed in guard cell. | UP6 | 2.10 | 2.44 | 0.5<FC <2.0 | 0.5<FC<2.0 |
| 3 | AT4G40020 | Myosin heavy chain-related protein; involved in reciprocal meiotic recombination. | 3.34 | 2.37 | 0.5<FC<2.0 | 0.5<FC<2.0 | |
| 4 | AT5G05580 | Encodes a temperature-sensitive plastidic fatty acid desaturase. Located in chloroplast, expressed in guard cell. | FAD8 | 0.5<FC<2.0 | 0.5<FC<2.0 | 0.47 | 0.42 |
| 5 | AT5G67370 | CONSERVED IN THE GREEN LINEAGE; involved in response to iron ion starvation; located in chloroplast, integral component of membrane. | CGLD27 | 0.5<FC<2.0 | 0.5<FC<2.0 | 0.47 | 0.32 |
| 6 | AT1G72416 | Chaperone DnaJ-domain superfamily protein; located in cytoplasm, integral component of membrane; expressed in guard cell. | 0.5< FC <2.0 | 0.5< FC <2.0 | 0.44 | 0.45 | |
| 7 | AT2G34510* | Choice-of-anchor C domain protein, putative; expressed in cauline leaf, collective leaf structure, cotyledon. | 0.5<FC<2.0 | 0.5<FC<2.0 | 0.45 | 0.33 | |
| 8 | AT4G11460* | Encodes a cysteine-rich receptor-like protein kinase, involved in defence response to bacteria, protein phosphorylation. | CRK30 | 0.5<FC<2.0 | 0.5<FC<2.0 | 0.42 | 0.36 |
| 9 | AT5G01015* | Transmembrane protein. | 0.5<FC<2.0 | 0.5<FC<2.0 | 0.35 | 0.26 | |
| 10 | AT1G74010 | Calcium-dependent phosphotriesterase superfamily protein; located in cytosol, endoplasmic reticulum, extracellular region, membrane, plant-type cell wall. | 0.5<FC<2.0 | 0.5<FC<2.0 | 2.13 | 5.03 | |
| 11 | AT2G22880 | VQ motif-containing protein; involved in response to UV-B; located in nucleus. | VQ12 | 0.5<FC<2.0 | 0.5<FC<2.0 | 2.52 | 9.60 |
| 12 | AT2G39350 | Belongs to a clade of five Arabidopsis thaliana ABCG half-transporters that are required for synthesis of an effective suberin barrier in roots and seed coats (ABCG2, ABCG6, and ABCG20) and for synthesis of an intact pollen wall (ABCG1 and ABCG16). | ABCG1 | 0.5<FC<2.0 | 0.5<FC<2.0 | 2.71 | 7.97 |
| 13 | AT2G39980 | HXXXD-type acyl-transferase family protein; involved in response to karrikin; has transferase activity. | 0.5<FC<2.0 | 0.5< FC <2.0 | 2.17 | 3.71 | |
| 14 | AT4G06410 | Natural antisense transcript overlaps with AT4G16670. | 0.5<FC<2.0 | 0.5<FC<2.0 | 2.28 | 5.53 | |
| 15 | AT4G15550 | IAGLU, INDOLE-3-ACETATE BETA-d-GLUCOSYLTRANSFERASE. | IAGLU | 0.5< FC <2.0 | 0.5< FC <2.0 | 2.58 | 2.51 |
| 16 | AT4G25810* | Xyloglucan endotransglycosylase-related protein (XTR6); involved in cell wall biogenesis, cell wall organization, xyloglucan metabolic process; located in Golgi apparatus, apoplast, cell wall, extracellular region. | XTR6 | 0.5<FC<2.0 | 0.5<FC<2.0 | 2.00 | 5.71 |
| 17 | AT4G35770 | Senescence-associated gene that is strongly induced by phosphate starvation. Transcripts are differentially regulated at the level of mRNA stability at different times of day. | SEN1 | 0.5<FC<2.0 | 0.5<FC<2.0 | 2.09 | 2.66 |
| 18 | AT5G13330* | Encodes a member of the ERF (ethylene response factor) subfamily B-4 of ERF/AP2 transcription factor family. | Rap2.6L | 0.5<FC<2.0 | 0.5<FC<2.0 | 2.12 | 4.55 |
| 19 | AT1G75450 | This gene used to be called AtCKX6. It encodes a protein whose sequence is similar to cytokinin oxidase/dehydrogenase. | CKX5 | 0.50 | 0.30 | 2.00 | 2.65 |
| 20 | AT2G19800 | Encodes a myo-inositol oxygenase family gene, involved in l-ascorbic acid biosynthetic process, inositol catabolic process, oxidation–reduction process, syncytium formation. | MIOX2 | 0.36 | 0.50 | 2.00 | 2.30 |
| 21 | AT3G50970 | Belongs to the dehydrin protein family, involved in cold acclimation, defence response to fungus, response to abscisic acid, response to cold, response to water, response to water deprivation; the mRNA is cell-to-cell mobile. | LTI30 | 0.28 | 0.46 | 2.00 | 3.23 |
| 22 | AT4G20970 | Basic helix–loop–helix (bHLH) DNA-binding superfamily protein; has DNA-binding transcription factor activity. | 0.39 | 0.42 | 2.00 | 2.00 | |
| 23 | AT4G21650* | Subtilase family protein; involved in proteolysis. | SBT3.13 | 0.36 | 0.48 | 2.00 | 2.00 |
| 24 | AT5G07000 | Encodes a member of the sulfotransferase family of proteins. It may be able to act on structurally related jasmonates. | ST2B | 0.41 | 0.23 | 0.5<FC<2.0 | 0.5<FC<2.0 |
| 25 | AT5G55250 | Encodes an enzyme that specifically converts IAA to its methyl ester form MelIAA, involved in auxin homeostasis, methylation, polarity specification of adaxial/abaxial axis. | IAMT1 | 0.47 | 0.34 | 0.5<FC<2.0 | 0.5<FC<2.0 |
| 26 | AT1G04220 | Encodes KCS2, a member of the 3-ketoacyl-CoA synthase family involved in the biosynthesis of VLCFA (very long chain fatty acids). | KCS2 | 0.5<FC<2.0 | 0.5<FC<2.0 | 2.16 | 3.01 |
| 27 | AT1G64660 | Encodes a functional methionine gamma-lyase, a cytosolic enzyme catalyses the degradation of methionine into methanethiol, alpha-ketobutyrate, and ammonia. | MGL | 0.5<FC<2.0 | 0.5<FC<2.0 | 2.05 | 3.26 |
| 28 | AT2G23170 | Encodes an IAA-amido synthase that conjugates Asp and other amino acids to auxin in vitro. | GH3.3 | 0.5<FC<2.0 | 0.5<FC<2.0 | 2.57 | 4.20 |
| 29 | AT3G54400* | Eukaryotic aspartyl protease family protein; involved in protein catabolic process, proteolysis; located in apoplast, cell wall, chloroplast, extracellular region, plant-type cell wall. | 0.5<FC<2.0 | 0.5<FC<2.0 | 2.12 | 4.24 | |
| 30 | AT3G55500 | Expansin-like protein, involved in plant-type cell wall loosening, plant-type cell wall modification involved in multidimensional cell growth, plant-type cell wall organization, syncytium formation, unidimensional cell growth. | ExPA16 | 0.5<FC<2.0 | 0.5<FC<2.0 | 2.01 | 2.54 |
| 31 | AT3G62090 | PHYTOCHROME-INTERACTING FACTOR 6, encodes a novel Myc-related bHLH transcription factor, which physically associated with APRR1/TOC1 and is a member of PIF3 transcription factor family. | PIL2 | 0.5<FC<2.0 | 0.5<FC<2.0 | 2.06 | 2.27 |
| 32 | AT5G06570* | Alpha/beta-Hydrolase superfamily protein; expressed during petal differentiation and expansion stage. | 0.5<FC<2.0 | 0.5<FC<2.0 | 2.16 | 7.02 | |
| 33 | AT2G32990 | Glycosyl hydrolase 9B8; involved in cell wall organization, cellulose catabolic process. | GH9B8 | 0.45 | 0.40 | 0.5<FC<2.0 | 0.5<FC<2.0 |
Fold changes are compared with the reference line. Gene IDs in bold are involved in cell wall organization; gene IDs that include ‘*’ overlap with up-regulated genes after NAC050/052-RNAi (Ning et al., 2015); gene IDs underlined overlap with differentially expressed genes between total leaf and bundle sheath only (Aubry et al., 2014); fold changes in blue are increased compared with the reference line (P=0.05); fold changes in red are decreased compared with the reference line (P=0.05).
Both the activation-tagged line and the pGLDTFt::5'truncated NAC052 line are exposed to altered NAC052 functionality due to an N-terminal truncation of the NAC052 protein. Because NAC052 is a transcriptional repressor (Zhang et al., 2015), it is interesting to analyse descriptions of genes that are down-regulated (when compared with the reference line) in the pGLDTFt::NAC052 line as well as in the CRISPR/Cas line, but are not differentially expressed compared with the reference line in the activation-tagged line and the pGL DTFt::5'truncatedNAC052 line. Fifteen genes respond in such a way; three of those genes encode proteins functional in arabinogalactan metabolism, one is a chromatin modification maintainer of DNA methylation, and one is a carboxypeptidase involved in leaf vascular tissue pattern formation (Table 3).
Table 3.
Genes that are similarly responsive in the GLDTFt:NAC052 line and CRISPR/Cas line, but not responsive in the activation-tagged line and in the GLDTFt::5'truncatedNAC052 line
| Gene ID | Gene description | Abbreviated gene name | Acti-vation tagged | CRISPR/Cas | GLDT::5'truncatedNAC052 | GLDT::NAC052 | |
|---|---|---|---|---|---|---|---|
| 1 | AT1G02640* | Encodes a protein similar to a beta-xylosidase located in the extracellular matrix (AT5G49360). This is a member of glycosyl hydrolase family 3 and has six other closely related members. | BXL2 | 0.5<FC<2.0 | 0.36 | 0.5<FC<2.0 | 0.46 |
| 2 | AT1G26820 | Encodes ribonuclease RNS3. Involved in RNA catabolic process and ageing. | RNS3 | 0.5<FC<2.0 | 0.34 | 0.5<FC<2.0 | 0.50 |
| 3 | AT1G66040 | ORTH4, ORTHRUS 4, VARIANT IN METHYLATION 4, VIM4 Involved in chromatin organization, maintenance of DNA methylation, protein ubiquitination. Protein located in nucleus. | VIM4 | 0.5<FC<2.0 | 0.26 | 0.5<FC<2.0 | 0.44 |
| 4 | AT1G70985 | Hydroxyproline-rich glycoprotein family protein, protein located in anchored component of membrane. Expressed in seeds of first silique | 0.5<FC<2.0 | 0.44 | 0.5<FC<2.0 | 0.50 | |
| 5 | AT1G78450* | SOUL haem-binding family protein, protein located in chloroplast, expressed in hypocotyl and siliques. | 0.5<FC<2.0 | 0.41 | 0.5<FC<2.0 | 0.39 | |
| 6 | AT2G29300 | NAD(P)-binding Rossmann-fold superfamily protein, protein location unknown, expressed in seeds of first silique. | 0.5<FC<2.0 | 0.44 | 0.5<FC<2.0 | 0.48 | |
| 7 | AT3G13000 | Ubiquinone biosynthesis protein, expressed in young leaf and young flower. Protein located in nucleus and vacuole. | 0.5<FC<2.0 | 0.48 | 0.5<FC<2.0 | 0.50 | |
| 8 | AT3G54720 | ALTERED MERISTEM PROGRAM 1, AMP1, encodes glutamate carboxypeptidase. Various alleles show increased cotyledon number and rate of leaf initiation, show transformation of leaves to cotyledons, altered flowering time and photomorphogenesis, and an increased level of cytokinin biosynthesis. Protein located in endoplasmic reticulum. Involved in leaf vascular tissue pattern formation. | AMP1 | 0.5<FC<2.0 | 0.47 | 0.5<FC<2.0 | 0.39 |
| 9 | AT3G62070 | Hypothetical protein expressed in guard cell. | 0.5<FC<2.0 | 0.48 | 0.5<FC<2.0 | 0.39 | |
| 10 | AT4G03610 | Metallo-hydrolase/oxidoreductase superfamily protein, protein located in nucleus, expressed in plant embryo. | 0.5<FC<2.0 | 0.19 | 0.5<FC<2.0 | 0.45 | |
| 11 | AT4G20820 | FAD-binding Berberine family protein, electron transfer activity, protein located in chloroplast, expressed in collective leaf structure, cotyledon, guard cell, hypocotyl, root, stem, vascular leaf. | ATBBE18 | 0.5<FC<2.0 | 0.29 | 0.5<FC<2.0 | 0.34 |
| 12 | AT4G26288 | Hypothetical protein, expressed in silique, protein located in nucleus | 0.5<FC<2.0 | 0.47 | 0.5<FC<2.0 | 0.42 | |
| 13 | AT4G34790 | SAUR-like auxin-responsive protein family, protein located in mitochondrion. Expressed in guard cell. | SAUR3 | 0.5<FC<2.0 | 0.27 | 0.5<FC<2.0 | 0.33 |
| 14 | AT4G40090 | Arabinogalactan protein 3, involved in multicellular organism development, protein located in anchored component of membrane, expressed in hypocotyl, plant egg cell, root, root hair cell, shoot apex, trichoblast. | AGP3 | 0.5<FC<2.0 | 0.49 | 0.5<FC<2.0 | 0.42 |
| 15 | AT5G49360* | Encodes a bifunctional (beta)-d-xylosidase/(alpha)-l-arabinofuranosidase required for pectic arabinan modification. Located in the extracellular matrix. Gene is expressed specifically in tissues undergoing secondary wall thickening. This is a member of glycosyl hydrolase family 3 and has six other closely related members. | BXL1 | 0.5<FC<2.0 | 0.50 | 0.5<FC<2.0 | 0.46 |
Fold changes are compared with the reference line. Gene IDs in bold are involved in either chromatin organization, leaf vascular tissue pattern formation, or arabinogalactan functioning; gene IDs that include ‘*’ overlap with up-regulated genes after NAC050/052-RNAi (Ning et al., 2015); gene IDs underlined overlap with differentially expressed genes between total leaf and bundle sheath only (Aubry et al., 2014); fold changes in red are decreased compared with the reference line (P=0.05)
Discussion
In this study, activation tagging led to expression of a 5'truncated form of the NAC052 transcription factor, in which the DNA-binding domain was partly deleted. In our leaf bundle-targeted GFP reporter line, this mutation led to changes in GFP fluorescence levels, as well as anatomical changes in the BS, in addition to changes in whole-plant and leaf morphology.
NAC052 acts as a regulator of leaf development
NAC052 belongs to the NAC [no apical meristem (NAM), Arabidopsis transcription activation factor (ATAF), Cup-shaped cotyledon (CUC)] family of transcription factors. NAC transcription factors typically posses the conserved N-terminal NAC domain (~150 amino acids), which contains the DNA-binding domain, and a diversified C-terminal transcription regulatory region (Puranik et al., 2012). NAC052 is a duplicated gene of NAC050, and they together bind DNA in the form of dimers and associate with the histone demethylase JMJ14, leading to histone H3K4 demethylation (Ning et al., 2015; Zhang et al., 2015). JMJ14 acts in several biological processes, including mobile RNA silencing, DNA methylation, abundance of endogenous transposon transcripts, and flowering time genes (Deleris et al., 2010; Lu et al., 2010; Searle et al., 2010; Le Masson et al., 2012). Of the two NAC transcription factors that associate with JMJ14, mutational effects in NAC052 are similar to jmj14-mutants, whereas mutations in NAC050 have only moderate effects (Zhang et al., 2015). These mutational effects include suppression of post-transcriptional gene silencing (PTGS), leading to enhanced transcription levels of several endogenous targets of JMJ14 as well as reduced transcript levels of transgene loci (Searle et al., 2010; Le Masson et al., 2012). Within the RNA silencing process, JMJ14 was found to act downstream from the Argonaute effector complex to demethylate histone H3K4 at the RNA silencing target gene (Searle et al., 2010). Increased H3K4me3 levels at endogenous loci correlate with increased transcription at the same loci (Zhang et al., 2009). Whole-genome analysis of H3K4me3 levels and of RNA transcript levels in jmj14 revealed 130 genes that were both hypermethylated and up-regulated in jmj14; none of these genes overlapped with genes found to be transcriptionally responsive to misexpressed NAC052 in this study (Tables 2, 3) (Ning et al., 2015).
Besides identifying jmj14, forward genetic screening for mutants defective in PTGS has identified suppressor of gene silencing 1 (sgs1), found to be impaired in the NAC052 gene (Le Masson et al., 2012; Butel et al., 2017). Butel et al. (2017) showed that besides repressing transcription of endogenous genes that are involved in PTGS, the JMJ14–NAC052 module promotes transgene transcription by preventing DNA methylation, confirming that the JMJ14–NAC052 module has a dual effect (Searle et al., 2010; Le Masson et al., 2012). Whole-genome analysis of RNA transcript levels in an NAC050/052-RNAi plant identified 1470 genes with enhanced transcript levels (Ning et al., 2015), of which eight genes overlapped with the genes found in this study that are differently responsive between pGLDTFt::5'truncatedNAC052/pGLDTFt::NAC052 and activation-tagged/CRISPR/Cas (Table 2), and three genes overlapped with the genes that are differently responsive between pGLDTFt::NAC052/CRISPR/Cas and pGLDTFt::5'truncated NAC052/activation-tagged (Table 3).
In this study, an increased transcript level of an N-terminally truncated NAC052 in an activation-tagged line was observed. The N-terminally truncated NAC052 has 304 amino acids instead of the wild-type 452 amino acids, and it contains only part of the conserved NAC domain. This conserved NAC domain consists of five subdomains (A–E), and the crucial DNA-binding domain is situated within a 60 amino acid region located within subdomains D and E (Kikuchi et al., 2000; Duval et al., 2002). The N-terminally truncated NAC052 contains only subdomain E, but not A–D. Therefore, we hypothesize that the N-terminally truncated NAC052 has no functional DNA-binding domain and is an unable to associate with JMJ14, leading to a non-functional H3K4 demethylase.
When cloning the F. trinervia-derived GLDT promoter in front of the full-length CDS of NAC052, or in front of the 5'truncated NAC052, we confirmed the leaf morphology phenotype as well as the increased GFP expression in the leaf bundles. However, this confirmation was lost in the T2 generation, the reason for which could be rearrangements of the inserted T-DNA with loss of expression (Krysan et al., 1999). However, because it occurred independently in both the T2 of the GLDT::NAC052 and of the GLDT::5'truncatedNAC052, it is more likely that the GLDT::(5'truncated)NAC052 transgene post-transcriptionally silences its own protein expression, confirming that NAC052 affects PTGS. The fact that NAC052 plays a role in PTGS should be noted as potentially important to achieve cell-specific gene expression as a first step towards C4-like leaf anatomy, as post-transcriptional regulation of photosynthetic genes is a key driver of C4 leaf ontogeny (Fankhauser and Aubry, 2017). In addition, the GLDPA gene—the promoter of which was used to create the pGLDPAFt::RbcS.TP-sGFP reference line—is known to be subject to nonsense-mediated mRNA decay (NMD), (Wiludda et al., 2012). Both NMD and RNA silencing are part of the post-transcriptional gene silencing process, in which NMD is the front-line RNA quality control pathway, and RNA silencing is induced only when the capacity of the NMD becomes saturated (Christie et al., 2011). The fact that both NAC052 and GLDPA are connected to PTGS and the fact that the GLDPA gene was proven to have been important for the establishment of a photorespiratory CO2 pump in the genus Flaveria (Schulze et al., 2013) suggest that PTGS is essential for the modification of regulatory networks to go from C3 photosynthesis towards C4 photosynthesis.
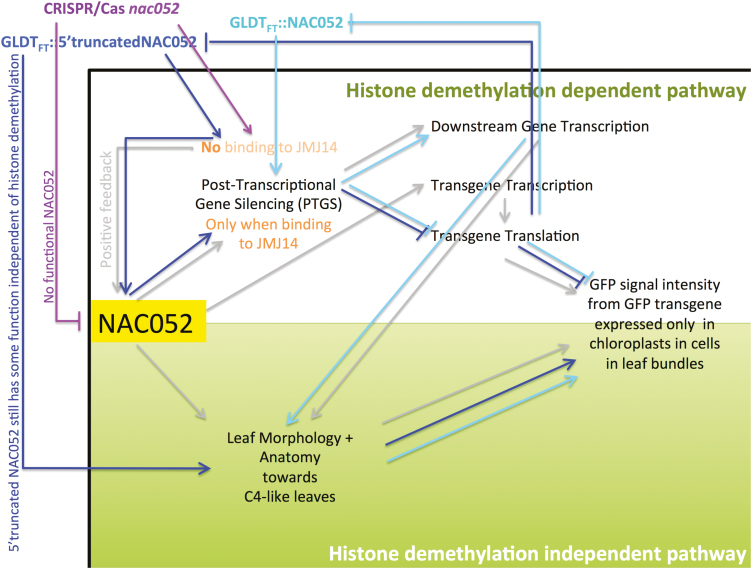
We confirmed the leaf morphology phenotype when mutating NAC052 with CRISPR/Cas. In contrast to the GLDT::(5'truncated)NAC052 transgenic lines, in the CRISPR/Cas line the leaf morphology phenotype was stable through three generations, suggesting that NAC052 has lost its PTGS effect in the CRISPR/Cas line. Also in contrast to the GLDT::(5'truncated)NAC052 transgenic lines, in the CRISPR/Cas line the GFP signal observed was significantly lower than in the reference line. Two things can be concluded from these results: the effect on PTGS and the effect on GFP expression caused by mutated NAC052 are linked, but the effect on PTGS/GFP expression and the effect on leaf morphology are not linked. NAC052 is known to be involved in transcriptional repression through both histone demethylation-dependent and demethylation-independent pathways (Ning et al., 2015). The effect of NAC052 on leaf morphology is so far unknown; we propose that the histone demethylation-independent pathway of NAC052 directs the effect on leaf morphology (Fig. 6). The effects of the diverse introduced constructs and mutations in this study on this proposed pathway of NAC052 are drawn in Fig. 6.
Fig. 6.
Proposed schematic model of NAC052 function with the effects of introduced nac052 mutations from this study. The upper half of the scheme in white represents the histone demethylation-dependent pathway for NAC052 function, as described by Ning et al. (2015) and by Butel et al. (2017); the lower half of the scheme in green represents the histone methylation-independent pathway for NAC052 function, newly described in this study. Grey arrows represent endogenous downstream effects in the NAC052 pathway; dark blue, light blue, and purple arrows represent downstream effects of the genetically introduced GLDTFt::5'truncatedNAC052 construct, the GLDTFt::NAC052 construct, and the CRISPR/Cas mutation, respectively.
In the activation-tagged line, the N-terminally truncated NAC052 leads to enhanced numbers of BS cells around the leaf vein, as well as to enhanced numbers of chloroplasts in these BS cells (Fig. 4). The presence of endogenous NAC052 function in the background of the pGLDTFt::5'truncatedNAC052 T2 line decreases the effect of the N-terminally truncated NAC052 on chloroplast numbers probably through PTGS (although the number of BS cells is still enhanced, Fig. 4). Together with post-transcriptional silencing of the GFP gene (Fig. 3), this leads to reduced observed GFP signal in the pGLDTFt::5'truncatedNAC052 T2 line. These results suggest that the increased GFP signal as a result of targeted misexpression of NAC052 after activation tagging comes internally from an increase in chloroplast numbers through the histone demethylation-independent pathway of NAC052 (the histone demethylation-dependent pathway, leading to PTGS, is dysfunctional because of dysfunctional binding between NAC052 and JMJ14). The reduced GFP signal in the pGLDTFt::5'truncatedNAC052 T2 line comes from PTGS on the GFP transgene. These observations explain how NAC052 functions in leaf development, besides functioning in PTGS.
Characterization of NAC052 downstream genes
No genes increase/decrease similarly compared with the reference line in the activation-tagged line to in the pGLDTFt::5'truncatedNAC052 line, suggesting that the few differentially counted (activated/reduced) transcripts in the activation-tagged line and the pGLDTFt::5'truncatedNAC052 line (Fig. 5) come from genes not directly influenced by (5'truncated) NAC052. Instead of actively activating/reducing downstream genes, the N-terminally truncated NAC052 only prevents activation/reduction of transcription that otherwise would have taken place following action of the endogenous NAC052, confirming the non-functionality of the 5'truncated NAC052 (Table 3).
The transcription of 33 genes was increased in response to endogenous NAC052 supplemented with bundle-specific expression of (5'truncated) NAC052, but not in response to complete knockout of the endogenous NAC052 (Table 2). These 33 genes were enriched for GO terms ‘cell wall organization and loosening’ and ‘catalytic activity’. A significant part of these 33 genes overlap with up-regulated genes in the NAC050/052-RNAi line produced by Ning et al. (2015), suggesting that they are downstream genes of endogenous NAC052. More than half of these genes were also differentially transcribed between the total leaf and BS (Aubry et al., 2014), suggesting that these downstream genes of NAC052 play a role in BS metabolism. The N-terminally truncated NAC052 under bundle-specific expression increases transcription of these genes to a lesser extent than misexpressed wild-type NAC052, probably because of the different changes in gene expression (combined gene transcription and post-transcriptional regulation) caused by the interplay of the wild-type and the 5'truncated transcripts of NAC052.
Having no endogenous NAC052 function (CRISPR/Cas line) as well as having bundle-specific misexpression of NAC052 (pGLDTFt::NAC052) decreases transcription (severely in the CRISPR/Cas line and mildly in pGLDTFt::NAC052) in the leaves of several genes involved in arabinogalactan function as well as one major gene involved in leaf vascular tissue pattern formation (ALTERED MERISTEM PROGRAM 1, AMP1) and one gene involved in chromatin modification (VARIANT IN METHYLATION 4, VIM4) (Table 3). Arabinogalactan proteins are a highly diverse class of cell surface glycoproteins, active in the biological processes of cell proliferation and survival, and of pattern formation and growth (Seifert and Roberts, 2007). Arabinogalactan proteins can be considered mediators between the cell wall, the plasma membrane, and the cytoplasm. Many arabinogalactan proteins are glycosylphosphatidylinositol (GPI) anchored, which is a form of post-translational modification common to many cell surface proteins (Seifert and Roberts, 2007). GPI modification serves as a primary plasmodesmal sorting signal (Zavaliev et al., 2016). One arabinogalactan biosynthesis gene (AT4G21060, not identified in this study) has been identified as a candidate gene underlying a quantitative trait locus controlling leaf venation patterning in Arabidopsis (Rishmawi et al., 2017). The other gene with decreased transcription in the pGLDTFt::NAC052 line and in the CRISPR/Cas line is AMP1, encoding a carboxypeptidase that is known to regulate embryo and meristem development and is linked to leaf vascular tissue pattern formation (Vidaurre et al., 2007). No effects on leaf vein density or venation patterning were found in any line produced in this study. In contrast to the arabinogalactan genes, AMP1 was also found to be differentially transcribed between the total leaf and BS by Aubry et al. (2014), as were four more genes shown in Table 3. The enrichment for expression differences in arabinogalactan genes together with the finding of AMP1 as a downstream gene of N-terminally truncated NAC052 in this study strengthens the idea that NAC052 is a regulator/initiator of leaf developmental changes in Arabidopsis.
Conclusion
We conclude that targeted misexpression of the transcription factor NAC052 leads to changes in leaf anatomical and morphological development of C3A. thaliana. This study shows that one of the biological functions of NAC052 relates to moderating specifically within the leaves the transcription pattern of cell wall organization genes as well as that of arabinogalactan genes, which are mediators between the cell wall, the plasma membrane, and the cytoplasm. As a transcription factor, NAC052 affects many genes, but the effect of NAC052 on cell wall organization and arabinogalactan genes and their link to leaf anatomical development was so far unknown.
This study was designed to look specifically into the effects of activation tagging on the leaf (pre-) BS cells, in order to find possible regulators to initiate change from C3 towards C4 photosynthesis. In earlier studies, it was suggested that a pre-existing epigenetic histone code was recruited into the C4 promoter control during the evolution of C4 metabolism, especially because cell type-specific gene expression patterns in C4 species utilize the same functional cis-regulatory elements as those in C3 species (Heimann et al., 2013; Perduns et al., 2015; Reyna-Llorens et al., 2018). In addition, it was found in maize that genes associated with the C4 trait are characterized by a unique class of highly regulated histone marks on upstream promoters (Langdale et al., 1991; Perduns et al., 2015). The fact that NAC052 is a regulator of post-transcriptional gene silencing through histone demethylation as well as a regulator of leaf morphological and anatomical traits that are related to C4 photosynthesis supports the suggestion of a histone code being recruited in C4 promoter control and provides suggestions for genes that could initiate the first steps believed to activate the C4 photosynthetic programme in the BS in C3 plants, which is the movement of chloroplasts to the (pre-) BS.
Supplementary data
Supplementary data are available at JXB online.
Table S1. Primers
Fig. S1. Splice variants for NAC052.
Fig. S2. The mutation of NAC052 in the CRISPR/Cas line.
Fig. S3. A principal component analysis (PCA) of the RNA sequencing output.
Fig. S4. Relative expression in RNA sequencing of the four splice variants of NAC052.
Fig. S5. Expression of four splice variants in all three replicates of each line.
Table S1. Primers.
Acknowledgements
This research was funded by the Deutsche Forschungsgemeinschaft through the Cluster of Excellence on Plant Sciences (EXC 1028).
References
- Aubry S, Smith-Unna RD, Boursnell CM, Kopriva S, Hibberd JM. 2014. Transcript residency on ribosomes reveals a key role for the Arabidopsis thaliana bundle sheath in sulfur and glucosinolate metabolism. The Plant Journal 78, 659–673. [DOI] [PubMed] [Google Scholar]
- Bauwe H. 2011. Photorespiration: the bridge to C4 photosynthesis. In: Raghavendra AS, Sage RF, eds. C4 photosynthesis and related CO2 concentrating mechanisms. Dordrecht: Springer, 81–108. [Google Scholar]
- Butel N, Le Masson I, Bouteiller N, Vaucheret H, Elmayan T. 2017. sgs1: a neomorphic nac52 allele impairing post-transcriptional gene silencing through SGS3 downregulation. The Plant Journal 90, 505–519. [DOI] [PubMed] [Google Scholar]
- Cheng CY, Krishnakumar V, Chan AP, Thibaud-Nissen F, Schobel S, Town CD. 2017. Araport11: a complete reannotation of the Arabidopsis thaliana reference genome. The Plant Journal 89, 789–804. [DOI] [PubMed] [Google Scholar]
- Christie M, Brosnan CA, Rothnagel JA, Carroll BJ. 2011. RNA decay and RNA silencing in plants: competition or collaboration? Frontiers in Plant Science 2, 99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clough SJ, Bent AF. 1998. Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. The Plant Journal 16, 735–743. [DOI] [PubMed] [Google Scholar]
- Curtis MD, Grossniklaus U. 2003. A gateway cloning vector set for high-throughput functional analysis of genes in planta. Plant Physiology 133, 462–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deleris A, Greenberg MV, Ausin I, Law RW, Moissiard G, Schubert D, Jacobsen SE. 2010. Involvement of a Jumonji-C domain-containing histone demethylase in DRM2-mediated maintenance of DNA methylation. EMBO Reports 11, 950–955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dengler NG, Nelson T. 1999. Leaf structure and development in C4 plants. In: Sage R, Monson RK, eds. C4 plant biology. New York: Academic Press, 133–172. [Google Scholar]
- Döring F, Billakurthi K, Gowik U, Sultmanis S, Khoshravesh R, Das Gupta S, Sage TL, Westhoff P. 2019. Reporter-based forward genetic screen to identify bundle sheath anatomy mutants in A. thaliana. The Plant Journal 97, 984–995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duval M, Hsieh TF, Kim SY, Thomas TL. 2002. Molecular characterization of AtNAM: a member of the Arabidopsis NAC domain superfamily. Plant Molecular Biology 50, 237–248. [DOI] [PubMed] [Google Scholar]
- Emmerling J. 2018. Studies into the regulation of C4 photosynthesis—towards factors controlling bundle sheath expression and Kranz anatomy development. Doctoral thesis, Heinrich Heine University Düsseldorf. [Google Scholar]
- Engelmann S, Wiludda C, Burscheidt J, Gowik U, Schlue U, Koczor M, Streubel M, Cossu R, Bauwe H, Westhoff P. 2008. The gene for the P-subunit of glycine decarboxylase from the C4 species Flaveria trinervia: analysis of transcriptional control in transgenic Flaveria bidentis (C4) and Arabidopsis (C3). Plant Physiology 146, 1773–1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fankhauser N, Aubry S. 2017. Post-transcriptional regulation of photosynthetic genes is a key driver of C4 leaf ontogeny. Journal of Experimental Botany 68, 137–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gowik U, Bräutigam A, Weber KL, Weber AP, Westhoff P. 2011. Evolution of C4 photosynthesis in the genus Flaveria: how many and which genes does it take to make C4? The Plant Cell 23, 2087–2105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gowik U, Westhoff P. 2011. The path from C3 to C4 photosynthesis. Plant Physiology 155, 56–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haberlandt G. 1896. Physiologische Pflanzenanatomie. Leipzig: Wilhelm Engelmann. [Google Scholar]
- Hahn F, Mantegazza O, Greiner A, Hegemann P, Eisenhut M, Weber AP. 2017. An efficient visual screen for CRISPR/Cas9 activity in Arabidopsis thaliana. Frontiers in Plant Science 8, 39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatch MD. 1987. C4 photosynthesis: a unique blend of modified biochemistry, anatomy and ultrastructure. Biochimica et Biophysica Acta 895, 81–106. [Google Scholar]
- Heckmann D, Schulze S, Denton A, Gowik U, Westhoff P, Weber AP, Lercher MJ. 2013. Predicting C4 photosynthesis evolution: modular, individually adaptive steps on a Mount Fuji fitness landscape. Cell 153, 1579–1588. [DOI] [PubMed] [Google Scholar]
- Heimann L, Horst I, Perduns R, Dreesen B, Offermann S, Peterhansel C. 2013. A common histone modification code on C4 genes in maize and its conservation in Sorghum and Setaria italica. Plant Physiology 162, 456–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hibberd JM, Quick WP. 2002. Characteristics of C4 photosynthesis in stems and petioles of C3 flowering plants. Nature 415, 451–454. [DOI] [PubMed] [Google Scholar]
- Khoshravesh R, Lundsgaard-Nielsen V, Sultmanis S, Sage TL. 2017. Light microscopy, transmission electron microscopy, and immunohistochemistry protocols for studying photorespiration. Methods in Molecular Biology 1653, 243–270. [DOI] [PubMed] [Google Scholar]
- Kikuchi K, Ueguchi-Tanaka M, Yoshida KT, Nagato Y, Matsusoka M, Hirano HY. 2000. Molecular analysis of the NAC gene family in rice. Molecular & General Genetics 262, 1047–1051. [DOI] [PubMed] [Google Scholar]
- Kinsman EA, Pyke KA. 1998. Bundle sheath cells and cell-specific plastid development in Arabidopsis leaves. Development (Cambridge, England) 125, 1815–1822. [DOI] [PubMed] [Google Scholar]
- Kirschner S, Woodfield H, Prusko K, Koczor M, Gowik U, Hibberd JM, Westhoff P. 2018. Expression of SULTR2;2, encoding a low-affinity sulphur transporter, in the Arabidopsis bundle sheath and vein cells is mediated by a positive regulator. Journal of Experimental Botany 69, 4897–4906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krysan PJ, Young JC, Sussman MR. 1999. T-DNA as an insertional mutagen in Arabidopsis. The Plant Cell 11, 2283–2290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langdale JA, Taylor WC, Nelson T. 1991. Cell-specific accumulation of maize phosphoenolpyruvate carboxylase is correlated with demethylation at a specific site greater than 3 kb upstream of the gene. Molecular & General Genetics 225, 49–55. [DOI] [PubMed] [Google Scholar]
- Lazo GR, Stein PA, Ludwig RA. 1991. A DNA transformation-competent Arabidopsis genomic library in Agrobacterium. Bio/technology 9, 963–967. [DOI] [PubMed] [Google Scholar]
- Le Masson I, Jauvion V, Bouteiller N, Rivard M, Elmayan T, Vaucheret H. 2012. Mutations in the Arabidopsis H3K4me2/3 demethylase JMJ14 suppress posttranscriptional gene silencing by decreasing transgene transcription. The Plant Cell 24, 3603–3612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leegood RC. 2008. Roles of the bundle sheath cells in leaves of C3 plants. Journal of Experimental Botany 59, 1663–1673. [DOI] [PubMed] [Google Scholar]
- Logemann E, Birkenbihl RP, Ülker B, Somssich IE. 2006. An improved method for preparing Agrobacterium cells that simplifies the Arabidopsis transformation protocol. Plant Methods 2, 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Love MI, Huber W, Anders S. 2014. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biology 15, 550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu F, Cui X, Zhang S, Liu C, Cao X. 2010. JMJ14 is an H3K4 demethylase regulating flowering time in Arabidopsis. Cell Research 20, 387–390. [DOI] [PubMed] [Google Scholar]
- Lyska D, Engelmann K, Meierhoff K, Westhoff P. 2013. pAUL: a gateway-based vector system for adaptive expression and flexible tagging of proteins in Arabidopsis. PLoS One 8, e53787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallmann J, Heckmann D, Bräutigam A, Lercher MJ, Weber AP, Westhoff P, Gowik U. 2014. The role of photorespiration during the evolution of C4 photosynthesis in the genus Flaveria. eLife 3, e02478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKown AD, Moncalvo JM, Dengler NG. 2005. Phylogeny of Flaveria (Asteraceae) and inference of C4 photosynthesis evolution. American Journal of Botany 92, 1911–1928. [DOI] [PubMed] [Google Scholar]
- Monson RK. 1999. The origins of C4 genes and evolutionary pattern in the C4 metabolic phenotype. In: Sage RF, Monson RK, eds. C4 plant biology. New York: Academic Press, 377–410. [Google Scholar]
- Mortazavi A, Williams BA, McCue K, Schaeffer L, Wold B. 2008. Mapping and quantifying mammalian transcriptomes by RNA-Seq. Nature Methods 5, 621–628. [DOI] [PubMed] [Google Scholar]
- Ning YQ, Ma ZY, Huang HW, Mo H, Zhao TT, Li L, Cai T, Chen S, Ma L, He XJ. 2015. Two novel NAC transcription factors regulate gene expression and flowering time by associating with the histone demethylase JMJ14. Nucleic Acids Research 43, 1469–1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oñate-Sánchez L, Vicente-Carbajosa J. 2008. DNA-free RNA isolation protocols for Arabidopsis thaliana, including seeds and siliques. BMC Research Notes 1, 93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perduns R, Horst-Niessen I, Peterhansel C. 2015. Photosynthetic genes and genes associated with the C4 trait in maize are characterized by a unique class of highly regulated histone acetylation peaks on upstream promoters. Plant Physiology 168, 1378–1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puranik S, Sahu PP, Srivastava PS, Prasad M. 2012. NAC proteins: regulation and role in stress tolerance. Trends in Plant Science 17, 369–381. [DOI] [PubMed] [Google Scholar]
- Reyna-Llorens I, Burgess SJ, Reeves G, Singh P, Stevenson SR, Williams BP, Stanley S, Hibberd JM. 2018. Ancient duons may underpin spatial patterning of gene expression in C4 leaves. Proceedings of the National Academy of Sciences, USA 115, 1931–1936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rishmawi L, Bühler J, Jaegle B, Hülskamp M, Koornneef M. 2017. Quantitative trait loci controlling leaf venation in Arabidopsis. Plant, Cell & Environment 40, 1429–1441. [DOI] [PubMed] [Google Scholar]
- Sage RF. 2004. The evolution of C4 photosynthesis. New Phytologist. 161, 341–370. [DOI] [PubMed] [Google Scholar]
- Sage RF, Khoshravesh R, Sage TL. 2014. From proto-Kranz to C4 Kranz: building the bridge to C4 photosynthesis. Journal of Experimental Botany 65, 3341–3356. [DOI] [PubMed] [Google Scholar]
- Sage RF, Sage TL, Kocacinar F. 2012. Photorespiration and the evolution of C4 photosynthesis. Annual Review of Plant Biology 63, 19–47. [DOI] [PubMed] [Google Scholar]
- Schulze S, Mallmann J, Burscheidt J, Koczor M, Streubel M, Bauwe H, Gowik U, Westhoff P. 2013. Evolution of C4 photosynthesis in the genus Flaveria: establishment of a photorespiratory CO2 pump. The Plant Cell 25, 2522–2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Searle IR, Pontes O, Melnyk CW, Smith LM, Baulcombe DC. 2010. JMJ14, a JmjC domain protein, is required for RNA silencing and cell-to-cell movement of an RNA silencing signal in Arabidopsis. Genes & Development 24, 986–991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seifert GJ, Roberts K. 2007. The biology of arabinogalactan proteins. Annual Review of Plant Biology 58, 137–161. [DOI] [PubMed] [Google Scholar]
- Singer T, Burke E. 2003. High-throughput TAIL-PCR as a tool to identify DNA flanking insertions. Methods in Molecular Biology 236, 241–272. [DOI] [PubMed] [Google Scholar]
- Tani H, Chen X, Nurmberg P, Grant JJ, SantaMaria M, Chini A, Gilroy E, Birch PR, Loake GJ. 2004. Activation tagging in plants: a tool for gene discovery. Functional & Integrative Genomics 4, 258–266. [DOI] [PubMed] [Google Scholar]
- Vidaurre DP, Ploense S, Krogan NT, Berleth T. 2007. AMP1 and MP antagonistically regulate embryo and meristem development in Arabidopsis. Development (Cambridge, England) 134, 2561–2567. [DOI] [PubMed] [Google Scholar]
- Welkie W, Caldwell M. 1970. Leaf anatomy of species in some dicotyledon families as related to the C3 and C4 pathways of carbon fixation. Canadian Journal of Botany. 48, 2135–2146. [Google Scholar]
- Wiludda C, Schulze S, Gowik U, Engelmann S, Koczor M, Streubel M, Bauwe H, Westhoff P. 2012. Regulation of the photorespiratory GLDPA gene in C4flaveria: an intricate interplay of transcriptional and posttranscriptional processes. The Plant Cell 24, 137–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zavaliev R, Dong X, Epel BL. 2016. Glycosylphosphatidylinositol (GPI) modification serves as a primary plasmodesmal sorting signal. Plant Physiology 172, 1061–1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S, Zhou B, Kang Y, et al. . 2015. C-terminal domains of a histone demethylase interact with a pair of transcription factors and mediate specific chromatin association. Cell Discovery. 1, 15003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Bernatavichute YV, Cokus S, Pellegrini M, Jacobsen SE. 2009. Genome-wide analysis of mono-, di- and trimethylation of histone H3 lysine 4 in Arabidopsis thaliana. Genome Biology 10, R62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu XG, Long SP, Ort DR. 2010. Improving photosynthetic efficiency for greater yield. Annual Review of Plant Biology 61, 235–261. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.