Abstract
The treatment of invasive fungal infections remains challenging due to limitations in currently available antifungal therapies including toxicity, interactions, restricted routes of administration, and drug resistance. This review focuses on novel therapies in clinical development, including drugs and a device. These drugs have novel mechanisms of action to overcome resistance, and some offer new formulations providing distinct advantages over current therapies to improve safety profiles and reduce interactions. Among agents that target the cell wall, 2 glucan synthesis inhibitors are discussed (rezafungin and ibrexafungerp), as well as fosmanogepix and nikkomycin Z. Agents that target the cell membrane include 3 fourth-generation azoles, oral encochleated amphotericin B, and aureobasidin A. Among agents with intracellular targets, we will review olorofim, VL-2397, T-2307, AR-12, and MGCD290. In addition, we will describe neurapheresis, a device used as adjunctive therapy for cryptococcosis. With a field full of novel treatments for fungal infections, the future looks promising.
Keywords: antifungal drugs, invasive fungal infections, novel treatment, review
The treatment of invasive fungal infections remains challenging. Current antifungal therapies have several limitations, including toxicities, interactions and drug-resistant fungi continue to arise. New antifungals in the pipeline will be discussed in this review.
Fungal infections are among the most difficult diseases to manage in humans, with approximately 1.7 billion individuals suffering from these infections worldwide [1, 2]. Invasive fungal infections (IFIs) refer to systemic fungal infections, in which fungi have infiltrated and established themselves in the deep tissues, resulting in life-threatening illness [3]. Although IFIs have a lower incidence compared with superficial fungal infections, they carry substantial human morbidity, mortality, and economic burden [1, 2, 4]. The overall mortality in patients with IFIs is as high as 45% [5]. These mortality rates remain high, and they often exceed deaths due to tuberculosis [6, 7] and malaria, despite the availability of several effective antifungal drugs [1, 2, 4].
More than 90% of all reported fungal-related deaths result from Cryptococcus, Candida, Aspergillus, Histoplasma, and Pneumocystis [2, 7]. Other emerging opportunistic fungal pathogens, including molds such as Fusarium spp, Scedosporium spp, Penicillium spp, Lomentospora spp, and the Mucorales, are becoming significantly more prevalent due to selective pressure from use of antifungals in agriculture, prophylaxis, and empirical therapy [8–10]. There has recently been a rise in resistant species and isolates, such as Candida auris, azole-resistant Aspergillus spp, and Lomentospora (previously Scedosporium) prolificans, some of which are resistant to all currently available antifungals [11–13].
Great strides were made in the 1990s after the introduction of second-generation azoles, echinocandins, and lipid formulations of amphotericin B (AmB), but drug development has largely stalled since then [1]. Currently, 3 main classes of antifungals are approved for the treatment of IFIs: polyenes, azoles, and echinocandins [14]. These agents target ergosterol in the cell membrane, lanosterol 14α-demethylase, and 1,3-β-glucan synthase, respectively [14]. The newest class of antifungal drugs, the echinocandins, was discovered in the 1970s and took almost 30 years to be approved. The first echinocandin, caspofungin, gained approval in 2001, representing the only novel class of antifungals to be approved in the 21st century [15]. Only 1 antifungal drug (isavuconazole) has been approved and marketed over the last decade [16]. Compared with the development of new antibacterials, antifungal drug development is hampered because fungal pathogens are very closely related to humans, using the same eukaryotic machinery, and therefore offer few pathogen-specific targets [5, 17]. New antifungal therapies that act through novel mechanisms are needed to reduce the high mortality of invasive fungal disease and combat the emergence of resistance to existing therapies. However, with several promising drugs in development, the future looks bright. This review focuses on novel therapies in the pipeline at various phases of clinical development.
THE NOVEL THERAPEUTICS
AGENTS TARGETING CELL WALL INTEGRITY
Rezafungin (CD101)
Mechanism of action and potential role.
Rezafungin (formerly SP3025 and CD101; developed by Cidara Therapeutics, San Diego, CA) is a novel echinocandin, currently in phase 3 development for treatment of candidemia and invasive candidiasis, with pharmacokinetic and safety qualities that distinguish it as a next-generation echinocandin [18–21]. Studies have also shown potential use for prevention of infections caused by Candida, Aspergillus, and Pneumocystis spp in bone marrow transplant patients, with a prophylaxis trial in planning [22, 23]. The US Food and Drug Administration (FDA) has designated intravenous (IV) rezafungin as a Qualified Infectious Disease Product (QIDP) with fast track status for its development program.
Rezafungin is a structural analog of anidulafungin with a modified choline moiety at the cyclic echinocandin core for greater chemical and metabolic stability and solubility. This might prevent hepatotoxicity by decreasing secondary metabolites [18] while retaining the antifungal activity of an echinocandin by inhibition of β-1,3-glucan synthesis [22] (Figure 1 and Table 1).
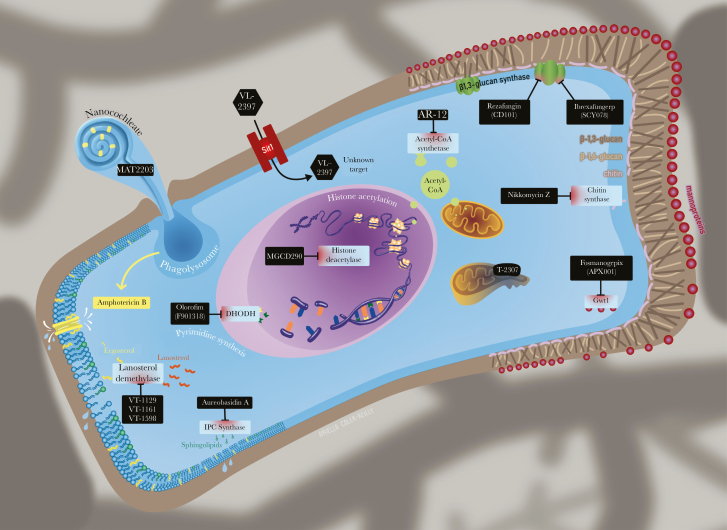
Figure 1.
Mechanism of action and target sites of the novel antifungals. The antifungal compounds currently in development have several novel mechanisms of action or new formulations to (1) improve efficacy or reduce toxicity and (2) target different sites in the cell wall, cell membrane, and also intracellular targets. DHODH, dihydroorotate dehydrogenase; IPC, inositol phosphorylceramide.
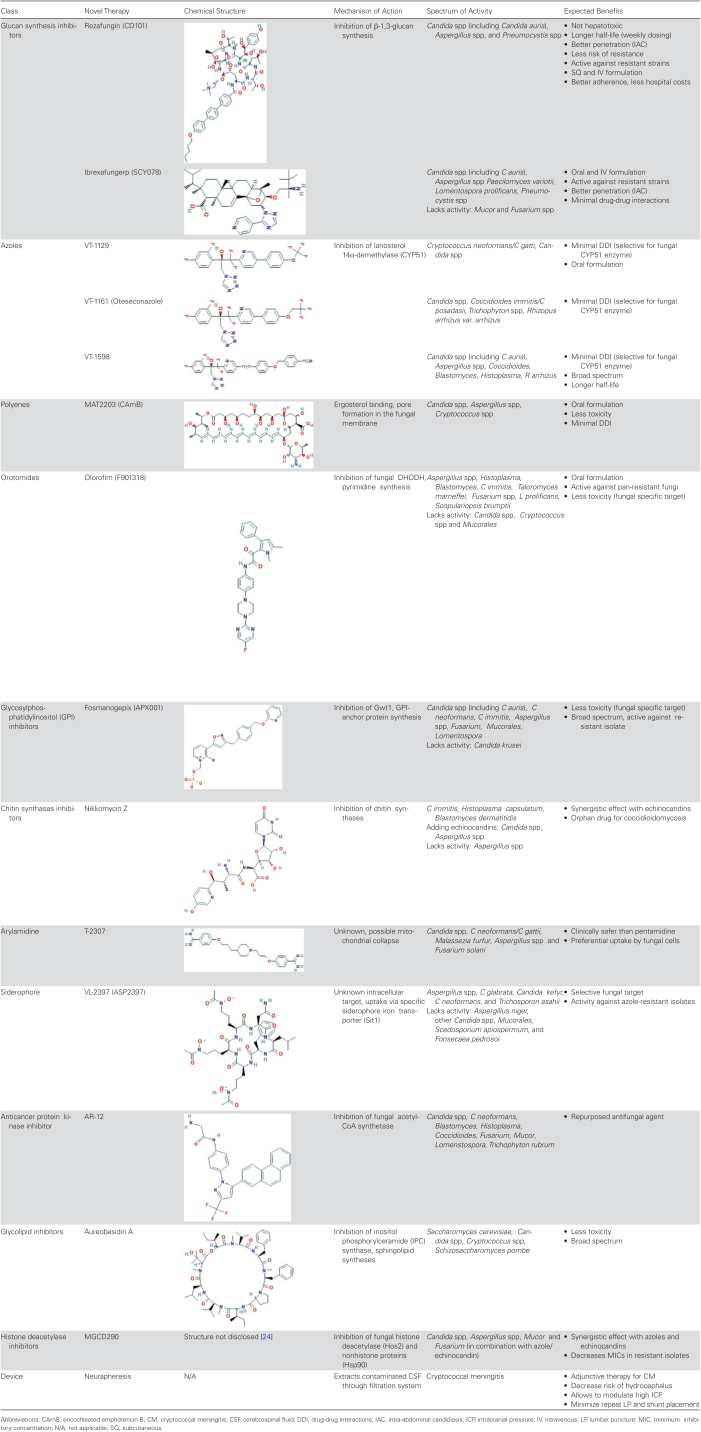
Table 1.
Novel Antifungals With Chemical Structure, Mechanism of Action, Spectrum of Activity, Expected Benefits
Pharmacokinetics/Pharmacodynamics
Pharmacologically, rezafungin has a much longer half-life (approximately 80 hours after the first dose and 150 hours after the second or third dose) than any currently available echinocandin, allowing for extended-interval IV dosing, such as weekly regimens [21]. Theoretically, this would increase pathogen killing and reduce spontaneous mutations by maximizing the drug effect early in the course of therapy when the burden of the pathogen is greatest [21, 25].
Evidence supports the use of weekly dosing for the treatment of patients with candidemia and invasive candidiasis, and higher doses might be able to overcome FKS mutant Candida isolates [26]. To evaluate the potential for resistance to rezafungin in Candida spp, spontaneous mutations to the drug were compared with caspofungin and anidulafungin, and results indicated an overall low potential for resistance development. Rezafungin has the additional advantage of higher plasma drug exposure due to the front-loaded dosing regimen, which further lowers the risk of resistance development [27]. However, the risk of development of resistance in the long “tail” of the pharmacokinetics (PK) has yet to be fully evaluated.
Activity
Rezafungin exhibits a broad in vitro potency against fungal pathogens comparable to that of other echinocandins [20, 25, 28, 29] (Figure 2). Rezafungin has concentration-dependent fungicidal activity and is highly bound to plasma proteins with extensive tissue distribution and minimal urinary excretion [21, 22, 30]. Rezafungin is being developed as a subcutaneous and IV medication [18, 31]. Previous in vivo pharmacodynamics studies demonstrated robust efficacy against Candida spp, including multidrug-resistant isolates such as C auris [32–34] and Aspergillus spp [35]. By both Clinical and Laboratory Standards Institute (CLSI) and EUCAST broth microdilution methods, the minimum inhibitory concentrations (MICs) for rezafungin were determined to range between ≤0.008 and 2 mg/L against Candida albicans, Candida glabrata, Candida tropicalis, Candida dubliniensis, and Candida krusei and between 0.5 and 2 mg/L against Candida parapsilosis [20]. Activity against Aspergillus spp, including Aspergillus fumigatus, Aspergillus terreus, Aspergillus flavus, and Aspergillus niger isolates, was comparable to other echinocandins with minimal effective concentrations (MECs) that ranged from ≤0.008 to 0.03 mg/L in one study [20] and ≤0.015–2 mg/L for all Aspergillus isolates, including azole-resistant isolates in another study [29].
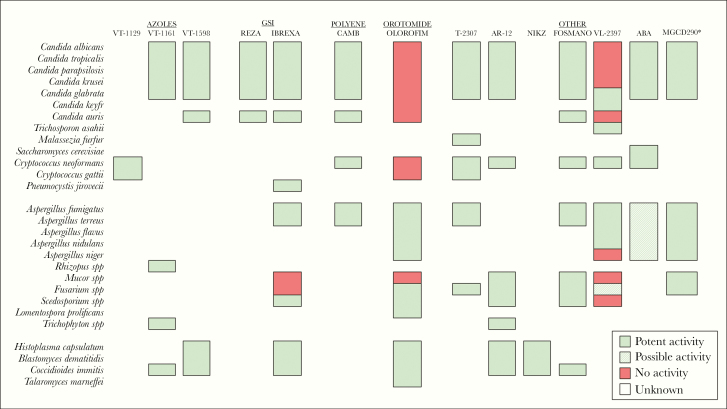
Figure 2.
Novel antifungals with spectrum of activity. New antifungal compounds show extensive spectrum of activity to overcome resistant fungi; however, several gaps still remain. *MGCD290: Potent activity in combination with azoles and/or echinocandins. ABA, aureobasidin A; CAMB, encochleated amphotericin B; FOSMANO, fosmanogepix; GSI, glucan synthase inhibitor; IBREXA, ibrexafungerp; NIKZ, nikkomycin Z; REZA, rezafungin.
Stage of Development and Ongoing Clinical Studies
A phase 1 study showed low toxicity and a favorable safety profile in humans with no serious or severe adverse events (AEs) or withdrawals from the study due to an AE, with the majority of AEs being mild and all with complete resolution [21]. Studies showed no biotransformation in liver microsomes or hepatocytes, reducing the risk of hepatotoxicity, and minimal inhibition of cytochrome P450 (CYP450) enzymes [21, 22]. Rezafungin, compared with conventional echinocandins, displays improved drug penetration for intra-abdominal candidiasis (IAC) [36].
In a phase 2 multicenter, randomized, double-blinded trial (STRIVE trial, NCT02734862), patients with candidemia and/or invasive candidiasis receiving rezafungin were compared with patients receiving a regimen of daily caspofungin with fluconazole stepdown once clinically stable. Rezafungin was well tolerated and provided clearance of blood cultures and other sterile sites, meeting all primary safety and efficacy end points [37]. Currently, patients are being recruited for a phase 3 study (ReSTORE study, NCT03667690) to determine the noninferiority of rezafungin compared with caspofungin in treatment of candidemia and/or invasive candidiasis, with the primary outcome being all-cause mortality at day 30. Future plans include a second phase 3 trial (ReSPECT study) using rezafungin in a 90-day prophylactic regimen for Candida, Aspergillus, and Pneumocystis infections in allogeneic bone marrow transplant patients, with results expected in 2020 (Figure 3). Unfavorable results from a phase 2 trial (RADIANT study, NCT02733432) investigating topical rezafungin (gel and ointment) resulted in the withdrawal of the topical formulation for the potential treatment of vulvovaginal candidiasis (VVC) [38, 39].
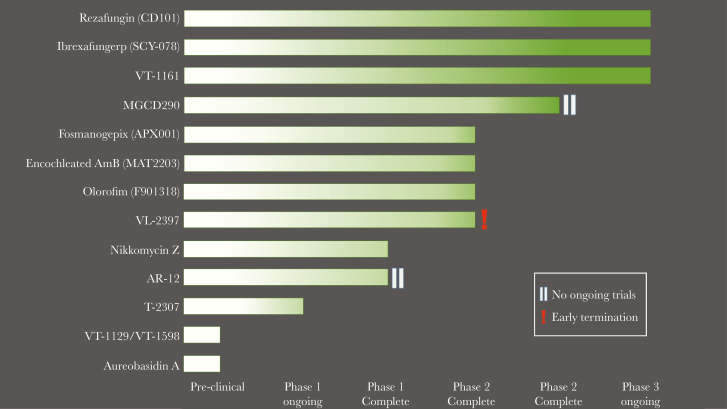
Figure 3.
Stage of clinical development of the novel antifungals. New antifungal compounds are currently in several stages of development. Most have advanced to phase 2 or 3, but a few still remain in phase 1 or preclinical development. VL-2397 had early termination of phase 2 trial, and there are currently no ongoing trials. AR-12 and MGCD290 have no ongoing trials.
Ibrexafungerp (SCY-078)
Mechanism of Action and Potential Role
Ibrexafungerp (previously MK-3118 and SCY-078; developed by Scynexis, Jersey City, NJ) is a first-in-class oral glucan synthase inhibitor, semisynthetic derivative of enfumafungin and represents the first compound of the triterpenoid antifungals to reach clinical development for the treatment of invasive candidiasis [40]. Although structurally different from echinocandins, it has the same fungal target through inhibition of β-1,3-glucan synthase [41] (Figure 1 and Table 1). Ibrexafungerp has been designated as a QIDP for oral and IV use by the FDA, for the indications of invasive candidiasis and invasive aspergillosis.
Pharmacokinetics/Pharmacodynamics
Ibrexafungerp is being developed for both oral and IV dosing, but only the oral dosing is currently in clinical trials [41]. It is characterized by high-volume distribution and extensive tissue penetration. It met efficacy end points across multiple murine models of invasive candidiasis at concentrations that have been safely achieved after oral administration in humans, but it does not achieve central nervous system (CNS) penetration [16, 40]. Despite binding to plasma proteins, the affinity may be weak, thereby allowing distribution to tissues [40]. An in vitro study showed robust drug penetration at the site of infection for IAC. Drug concentrations within the necrotic center of liver abscesses are almost 100-fold higher than the serum concentrations at corresponding time points [42]. Ibrexafungerp exhibits pH-dependent solubility, achieving the highest concentrations in acidic media consistent with simulated gastric and intestinal fluids, which may increase its efficacy in vaginal tissues as well as in abscesses [40]. Orally administered ibrexafungerp accumulates in the acidic environment of vaginal tissue in mice [43]. In vitro studies indicate that ibrexafungerp is a modest inhibitor of CYP450 (CYP2C8) with markedly lower effect over other CYP450 isozymes; however, substrates were not affected to a clinically meaningful extent in the presence of therapeutically relevant ibrexafungerp concentrations, suggesting low risk for significant CYP450 drug-drug interactions [44, 45].
Activity
In vitro and in vivo studies have demonstrated potency against the most common Candida spp, as well as resistant isolates [41, 46–51]. In addition, it demonstrates excellent in vitro activity against wild-type and azole-resistant strains of Aspergillus spp, Paecilomyces variotii, and some activity against L prolificans, but poor activity was observed against Mucor and Fusarium spp [47, 52]. It retains activity against Candida FKS1 or FKS2 mutants [53], including echinocandin-resistant isolates of C glabrata and C auris [41, 46–51]. Due to differential binding sites, the FKS mutations associated with echinocandin resistance are distinctly different from those reducing susceptibility to the enfumafungin derivatives including ibrexafungerp, limiting cross-resistance [47]. By both CLSI and EUCAST broth microdilution methodologies, MICs were determined to be 0.06–2 mg/L against C albicans and C tropicalis, 0.25–1 mg/L against C parapsilosis, and 0.5–2 mg/L against C glabrata and C krusei [46]. Against Aspergillus spp, MEC values reported were 0.03–1 mg/L and 0.015–0.25 mg/L when tested by CLSI and EUCAST methods, respectively [47]. Studies also show that oral ibrexafungerp could be a viable option for managing Pneumocystis in immunocompromised patients, because it has shown significant activity in a murine therapy and prophylaxis model [54, 55] (Figure 2).
Stage of Development and Ongoing Clinical Studies
Ibrexafungerp met primary end points in 2 phase 2 clinical trials (NCT02679456, NCT02244606) in VVC and invasive candidiasis, respectively. In VVC, oral ibrexafungerp was superior compared with oral fluconazole (78% vs 65%), and at the end of a 4-month follow-up period (88% vs 65%) with a lower recurrence rate (4% vs 15%) [56]. For invasive candidiasis, ibrexafungerp, as an oral step-down treatment after 3–10 days of echinocandin therapy, compared with fluconazole, achieved the predetermined target exposure in most subjects [57]. Mild-to-moderate gastrointestinal events such as diarrhea, nausea, vomiting, abdominal pain or discomfort were seen with ibrexafungerp, but no discontinuations due to AEs or serious AEs were observed. No mycological failure was reported in the study drug arm [57]. A recently completed phase 2 trial, the DOVE study (NCT03253094), explored 5 dosing regimens of oral ibrexafungerp versus fluconazole in patients with acute VVC to identify an optimal dose for a phase 3 trial. Results have not been published.
Currently, there are multiple ongoing phase 3 trials [58] (Figure 3). The CARES study (NCT03363841), open for enrollment in the United States and India, is assessing ibrexafungerp for the treatment of C auris infections. Initial evidence of efficacy and safety in 2 subjects was presented with promising results [59]. The FURI study (NCT03059992) is evaluating the efficacy and safety in patients with an invasive and/or severe fungal disease that are refractory or intolerant to standard-of-care antifungal treatment. Initial results of 20 patients who completed therapy were favorable [60]. The Data Review Committee adjudicated 55% of patients as achieving complete or partial response, 30% maintaining stable disease, 10% with progression of disease, and 1 case was considered as indeterminate [60]. Outcomes for 2 cases of C albicans and C tropicalis spondylodiscitis have shown clinical data for its use in bone infections [61]. Upcoming studies will evaluate oral ibrexafungerp in patients with acute VVC (VANISH306 study, NCT03987620) and as combination therapy with voriconazole in patients with invasive pulmonary aspergillosis (SCYNERGIA study, NCT03672292).
Fosmanogepix (APX001)
Mechanism of Action and Potential Role
Fosmanogepix (also known as APX001) (synthetized by Amplyx Pharmaceuticals, San Diego, CA), a first-in-class antifungal, is a prodrug of Manogepix (E1210), and it displays highly selective antifungal activity by inhibiting fungal enzyme Gwt1 and subsequently inactivating posttranslational modification of glycosylphosphatidylinositol (GPI) anchor proteins, also known as mannoproteins [62]. The GPI-anchored proteins become covalently linked to β-1,3-glucan, helping maintain the integrity of the fungal cell wall and play a role in adherence and invasion of host tissues. After GPI-anchor synthesis is disrupted, β-1,3-glucan can be exposed, increasing recognition of the fungus by immune cells [63]. The action of fosmanogepix appears to be specific to fungi, because it does not inhibit human inositol acylation, resulting in a wide therapeutic index [63] (Figure 1 and Table 1). Fosmanogepix received QIDP and fast track designations as well as orphan drug status for 4 indications: invasive candidiasis, invasive aspergillosis, coccidioidomycosis, and rare molds, including Scedosporium and Fusarium.
Activity
Fosmanogepix has in vitro activity against a broad spectrum of fungi, including yeast such as Candida spp [62–64], Cryptococcus neoformans [65], Coccidioides immitis [66], and C auris [67, 68], but it lacks activity against C krusei [62, 69], and it also has activity against many molds, including Aspergillus (even non-A fumigatus spp), Fusarium, Mucorales, and Scedosporium spp (including L prolificans) [62, 70, 71] (Figure 2). This drug has also shown efficacy in animal models of IFIs, including candidiasis caused by azole and echinocandin-resistant isolates, aspergillosis, and fusariosis [72–75]. Animal models in both rats and monkeys indicate that fosmanogepix is extensively distributed to major tissues relevant to IFIs (including liver, lung, brain, and eye) and cleared primarily by biliary and fecal excretion [76].
Stage of Development and Ongoing Clinical Studies
Phase 1 clinical studies have demonstrated >90% bioavailability for fosmanogepix, in both oral and IV formulations. These trials (NCT02957929, NCT02956499, NCT03333005) have determined the safety, tolerability, and PK of fosmanogepix in healthy volunteers and patients with acute myelogenous leukemia, who are at increased risk of IFIs. The clinical pharmacological profile, including drug-drug interactions and drug distribution, was also studied [77, 78]. Currently, phase 2 proof-of-concept studies are being conducted in patients with invasive candidiasis/candidemia and invasive aspergillosis [79] (Figure 3).
Nikkomycin Z
Mechanism of Action and Potential Role
Nikkomycin Z ([NikZ] developed by Valley Fever Solutions, Inc., Tucson, AZ) is a first-in-class antifungal derived from Streptomyces tendae, with a novel mechanism of action through inhibition of chitin synthases, an essential component of the fungal cell wall [80] (Figure 1 and Table 1). The target enzyme is absent in mammalian hosts, making NikZ selective to fungal cells with little to no toxicity in humans [80]. This agent was first described in the 1970s, but it was not until the early 2000s that NikZ gained interest for the treatment of endemic mycoses, particularly coccidioidomycosis [80]. This agent is under development as an orphan product for the treatment of coccidioidomycosis, and in 2014 it gained the QIDP designation.
Activity
Nikkomycin Z has potent in vitro and in vivo activity against dimorphic fungi with high quantities of chitin, including C immitis and Blastomyces dermatitidis. This agent has demonstrated activity in murine models of coccidioidomycosis, blastomycosis, and histoplasmosis [81–84]. Its fungicidal effect exceeded that of azoles and AmB, by sterilizing the lungs of most mice in murine models of pulmonary coccidioidomycosis and blastomycosis; however, moderate efficacy was seen for histoplasmosis [83, 85]. As monotherapy, it has limited activity against some yeasts, such as C albicans, and no activity against filamentous fungi, such as Aspergillus [83]. Despite its narrow spectrum, it has the potential to enhance other antifungal treatments when combined with other drugs. For example, NikZ in combination with echinocandins produced a synergistic effect against A fumigatus [86, 87], and it has also been productive in combination with fluconazole and itraconazole for different Candida and Aspergillus species [88] (Figure 2).
Pharmacokinetics/Pharmacodynamics
Pharmacologic studies in mice showed that NikZ administered through IV infusion was rapidly eliminated; however, oral administration resulted in slow absorption, allowing inhibitory levels to persist for hours [83]. Experimental murine studies suggest that twice-daily dosing of 250–500 mg could achieve optimal plasma concentrations in humans [89].
Stage of Development and Clinical Studies
Nikkomycin Z has completed phase 1 clinical development (NCT00834184) and showed excellent safety in healthy humans, without AEs observed [90]. A phase 2 trial (NCT00614666) to determine a safe dose in patients with pulmonary coccidioidomycosis was terminated early due to recruitment challenges and lack of funding [91, 92], but a phase 2a trial is planned for 2020 (Figure 3).
AGENTS TARGETING CELL MEMBRANE
VT-1129, VT-1161, and VT-1598
Mechanism of Action and Potential Role
One of the main limitations of the azole class of antifungals is the clinically significant drug-drug interactions experienced due to inhibition of off-target CYP450 [93]. VT-1129, VT-1161 (or Oteseconazole), and VT-1598 (developed by Viamet Pharmaceuticals, Durham, NC) are next-generation azoles, termed tetrazoles, attempting to remedy this limitation through improved binding discrimination between fungal and mammalian CYP450 enzymes. By replacing the triazole metal-binding group with a tetrazole, this group achieves greater selectivity for fungal lanosterol 14α demethylase (CYP51) [94, 95], which will hopefully result in fewer safety issues and lower MICs (Figure 1 and Table 1). The inhibition of this enzyme prevents conversion of lanosterol to ergosterol, an essential component of the fungal membrane. The FDA has granted QIDP, fast track, and orphan drug designation to VT-1598 for the treatment of coccidioidomycosis, and VT-1161 received QIDP and fast track status for recurrent VVC.
Activity
Each of these agents has potent activity against various yeast isolates, including C albicans and non-C albicans species, and Cryptococcus spp, as well as significant mold activity, including the Mucorales (Figure 2).
Potent in vitro activity has been reported for VT-1161 against Candida spp (MIC range, ≤0.03–0.25 mg/L), including wild-type isolates and some azole- and echinocandin-resistant strains, C immitis/Coccidioides posadasii (1–4 mg/L), Trichophyton spp (≤0.03–0.06 mg/L) [96–99], and against molds such as Rhizopus arrhizus var. arrhizus [100]. In vitro activity against Candida species has translated into in vivo efficacy in various animal models, including those of VVC and invasive candidiasis secondary to wild-type, azole- and echinocandin-resistant C albicans [101]. In murine models of coccidioidomycosis, VT-1161 provided prolonged suppression of fungal burden, improved survival, and prevented dissemination of infection from the original inoculation site to a greater extent than fluconazole [96]. This suggests that VT-1161 might have the ability to cure the “uncurable” syndromes such as meningitis. Efficacy has also been demonstrated in a guinea pig model of onychomycosis with either once-daily or weekly administration [102]. VT-1161 could be a potential treatment for mucormycosis because it has in vitro activity against Rhizopus arrhizus var. arrhizus. This has translated into in vivo efficacy in a mouse pulmonary infection model, but the in vitro potency of this agent appears to be reduced against R arrhizus var. delemar [100].
VT-1129, which is structurally very similar to VT-1161, was mainly designed to treat cryptococcal meningitis (CM). It has potent in vitro activity against resistant Candida spp [99], and activity is maintained against C neoformans/C gatti genotypes with reduced susceptibility to fluconazole [103, 104]. Studies have shown MIC50 that range between 0.05 and 0.25 μg/mL for dose-dependent and -resistant Cryptococcus isolates, respectively [103, 104]. In a murine model, oral administration of this agent resulted in improved survival and reduced tissue fungal burden. Due to the long half-life of this agent (more than 6 days in mice), elevated concentrations were detected in the brain long after dosing was stopped [105]. A loading dose-maintenance dose strategy has been shown to quickly reach highly efficacious concentrations [106]. No antagonism was observed when VT-1129 was combined with AmB [105].
Of these 3 investigational agents, VT-1598 has the broadest spectrum and most potent activity against molds, including various Aspergillus spp and R arrhizus, as well as endemic fungi, including Coccidioides with elevated fluconazole MICs, Blastomyces and Histoplasma isolates [105, 107]. A murine model of CNS coccidioidomycosis also showed the efficacy of VL-1598 [108]. This agent demonstrates potent in vitro and in vivo activity against Candida species [109], including resistant isolates such as C auris (MIC range, 0.03–8 mg/L). An in vivo study of neutropenic mice with invasive C auris infection treated with VT-1598 resulted in significant dose-dependent improvements in survival and reductions in kidney and brain fungal burden compared with the control [110].
Stage of Development and Ongoing Clinical Studies
Preclinical results of VT-1129, VT-1161, and VT-1598 have been reported in the literature, and VT-1161 is currently in clinical development (Figure 3). Phase 2 clinical trials have evaluated the efficacy and safety of VT-1161 for the treatment of tinea pedis, onychomycosis, and acute and recurrent VVC (NCT02267382, NCT02267356, NCT01891331, NCT01891305) [111]. Two phase 3 trials in recruitment (NCT03561701 and NCT03840616) will evaluate the effectiveness and safety of VT-1161 versus fluconazole and a placebo for the treatment of VVC.
Encochleated Amphotericin B (MAT2203)
Mechanism of Action and Potential Role
MAT2203 (developed by Matinas BioPharma, Bedminster, NJ) is a novel encochleated amphotericin B (CAmB) formulation for oral administration [4]. Encochleated AmB, like all forms of amphotericin, binds with sterols, leading to increased cell membrane permeability to ions [112, 113] (Figure 1 and Table 1). Despite the highly favorable antifungal activity of AmB, the successful treatment of IFIs remains difficult due to toxicity in critical mammalian organ systems caused by its lack of selectivity between fungal and animal sterols [112, 113]. Recent investigations have shown the safety and efficacy of the lipid-based cochleates formulation as a delivery system for AmB for use in Candida infections [114]. The cochleates consist of solid phospholipid bilayer vehicles, designed for hydrophobic drug delivery, that are arranged in rolled-up sheets composed of divalent cation precipitates, specifically phosphatidylserine and calcium. This structure protects the AmB encapsulated in the cochleates molecules from degradation in the gastrointestinal tract [114]. Cochleates result in a stable, nontoxic, highly efficacious AmB lipid particle, enabling its potential oral formulation [112]. The drug is released upon interaction of the cochleate with the target cells in the presence of the low intracellular calcium concentrations [4, 16]. There is direct interaction between CAmB and the fungus cell membrane, but the precise mechanism by which cochleates fuse with cell membranes is not yet fully understood [113]. Presumably, the drug is delivered to the target cell cytoplasm after phagocytosis by macrophages, and the fungal cells make contact with the lysosome and deliver the drug content after fusion with the lysosome [4]. Its potential for oral delivery and reduction in toxicity are the main advantages of this drug. Encochleated AmB received QIDP designation as well as fast track status from the FDA in 2015 for the treatment of invasive candidiasis, aspergillosis, and the prevention of IFIs in patients on immunosuppressive therapy. It recently received QIDP, fast track status, and orphan drug designation for treatment of CM.
Pharmacokinetics/Pharmacodynamics
The high efficiency in vitro and in vivo can be explained by increased uptake of the drug by fungus, which is mediated by a direct interaction and fusion between CAmB and the fungus cell membrane [113]. Animal models have shown extensive tissue distribution and penetration of CAmB into target organs after administration of a single dose. The liver acts as a reservoir, releasing AmB slowly, increasing its potential for the treatment of IFIs [115]. In vivo, orally administered CAmB resulted in adequate amounts of AmB reaching target organs, including the liver, spleen, and kidneys, early in treatment. Mice treated with CAmB had 100% survival and comparable efficacy with that of deoxycholate AmB (DAmB), resulting in substantial colony-forming unit count reduction in the kidneys and lungs [116]. Targeted therapy was demonstrated after the higher AmB concentrations were seen in infected mice, whereas no such accumulation occurred in the organs of healthy animals [116]. Further work will be required to assess whether immune cells are required for efficacy and concentration, because a significant portion of fungal infections occur in neutropenic patients.
Further investigations have also shown the in vivo efficacy of oral CAmB in a murine model of disseminated aspergillosis [117] and activity in visceral leishmaniasis [114, 118]. Oral CAmB has potential for treatment for CNS fungal infections, in combination with flucytosine (5FC) in a murine model of CM proving as effective as parenteral DAmB plus 5FC, and superior to oral fluconazole without inconvenient toxicity after a 28-day treatment schedule [119].
In vivo safety studies in murine models have shown 100% survival after 30 days when administering escalating daily doses of intraperitoneal CAmB, and equivalent safety of high oral CAmB doses, suggesting that CAmB could be administered in more aggressive doses (as high as 25 mg/kg) than currently available formulas [112, 113]. Encochleated AmB produced no hemolysis at concentrations as high as 500 µg/mL AmB, whereas DAmB was highly hemolytic at 10 µg/mL, possibly due to a low interaction of CAmB with red blood cells, in contrast to DAmB [113].
Stage of Development and Ongoing Clinical Studies
Preliminary results from a phase 1 study demonstrated that single doses of 200 and 400 mg were well tolerated without serious AEs, opening the way for phase 2 trials (NCT02971007) [120]. MAT2203 was well tolerated, with AEs mostly related to mild gastrointestinal symptoms, and showed a favorable safety profile without the renal and hepatic toxicities seen with IV AmB [121].
Enrollment is currently underway for a phase 2a clinical trial assessing orally administered MAT2203 (200–800 mg) in refractory mucocutaneous candidiasis (NCT02629419). Preliminary results are promising, with MAT2203 being well tolerated and effective [121]. A phase 1/2 trial is planned to start recruitment in Uganda in October 2019 to evaluate CAmB for CM (EnACT trial, NCT04031833). A phase 2 study was being planned to investigate MAT2203 for the prevention of IFIs, but the trial was recently withdrawn due to protocol redundancy (NCT03187691). Matinas plans to conduct a phase 2 trial to get MAT2203 approved for IFI prophylaxis in patients with acute lymphoblastic leukemia [121] (Figure 3).
Aureobasidin A
Aureobasidin A ([AbA] developed by Takara Bio, Shiga, Japan) is a cyclic depsipeptide antibiotic isolated from the filamentous fungus Aureobasidium pullulans R106 [122, 123]. Aureobasidin A was initially discovered in 1989, although its license patent was granted in 2007 to AureoGen Biosciences/Merck & Co. for further development [124].
Mechanism of Action and Potential Role
Aureobasidin A exhibits antifungal activity through inhibition of inositol phosphorylceramide synthase, an important enzyme expressed from the AUR1 gene involved in sphingolipid synthesis, exclusively found in the fungal cell membrane [125–127] (Figure 1 and Table 1). The absence of this enzyme in mammalian cells decreases the risk of toxicity. Resistance to AbA can be seen due to mutation of the AUR1 gene in fungi [127].
Activity
In vitro studies have shown activity against Saccharomyces cerevisiae, Candida spp, Cryptococcus spp, Schizosaccharomyces pombe, and some Aspergillus spp [122] (Figure 2). An in vivo murine model of candidiasis showed fungicidal activity of AbA with low toxicity and prolonged survival [123].
Stage of Development and Clinical Studies
This drug remains in the preclinical developmental, but it has a favorable profile to advance for clinical use (Figure 3).
AGENTS WITH INTRACELLULAR TARGETS
Olorofim (F901318)
Mechanism of Action and Potential Role
Olorofim (developed by F2G, Inc., Manchester, UK) is a novel antifungal drug in a novel class of orotomides. Its mechanism of action involves the inhibition of fungal dihydroorotate dehydrogenase (DHODH), an important enzyme in pyrimidine biosynthesis essential for deoxyribonucleic acid synthesis [128] (Figure 1 and Table 1). Olorofim has no activity against human DHODH and therefore selectively arrests fungal pyrimidine biosynthesis, resulting in limited toxicity and a favorable safety profile [128]. There are several other appealing characteristics of this drug including oral bioavailability and potent activity against triazole-resistant isolates and other pan-resistant fungal pathogens [129]. The European Medicines Agency Committee for Orphan Medicinal Products has recently granted orphan drug status to olorofim for the treatment of invasive aspergillosis and rare mold infections caused by Lomentospora spp.
Pharmacokinetics/Pharmacodynamics
Olorofim is relatively insoluble in water and highly protein bound [129], but it has good distribution to tissues, including kidney, liver, lung, and, although at lower levels, has been detected in the brain [128]. Olorofim demonstrates time-dependent killing effect [129, 130] and shows a typical pharmacokinetic profile when dosed by IV and oral routes (bioavailability >45%) [131]. Olorofim is cleared by multiple CYP450 enzymes; however, it does not induce CYP450 enzymes itself. It is a weak CYP3A4 inhibitor [132], making it prone to drug-drug interactions [16]. Olorofim has a delayed fungicidal effect if the exposure is prolonged [130].
Activity
In vitro studies have shown broad-spectrum activity against filamentous and dimorphic fungi including Aspergillus spp, Histoplasma capsulatum, B dermatitidis, C immitis, Talaromyces (formerly Penicillium) marneffei, and Fusarium spp [128], as well as organisms pan-resistant to clinically available antifungal agents such as Scedosporium apiospermum, L prolificans and Scopulariopsis spp (including Scopulariopsis brumptii) [133–135]. It is highly active against all pathogenic Aspergillus spp including wild-type and azole-resistant CYP51A mutant strains, with an MIC of <0.1 µg/mL. It is active against cryptic species of Aspergillus that can be hard to treat with current antifungal therapies, such as Aspergillus nidulans, Aspergillus tubingensis, Aspergillus lentulus, and Aspergillus calidoustus, with lower MICs than AmB, voriconazole, and posaconazole [129, 136, 137]. This agent displays potent in vitro activity against most molds with a limited variation in EUCAST susceptibility testing [138]. Organisms that remain susceptible to olorofim are grouped together under the same DHODH phylogenetic tree, whereas the lack of activity seen against Candida spp, C neoformans, and Mucorales is due to a distantly related DHODH [128]. Resistance to olorofim, as assessed by serial passage and drug gradients, does not appear to be easily induced, at least with A fumigatus [128]. Lack of cross-resistance was also demonstrated with azole-resistant Aspergillus spp mutant strains [139] (Figure 2).
Olorofim demonstrated in vivo efficacy in murine models of invasive aspergillosis caused by wild-type and azole-resistant strains [128, 129, 140]. Olorofim was also highly efficacious in prolonging survival of mice with disseminated aspergillosis [128, 129, 140]. In vivo efficacy with up to 88% survival of intraperitoneal olorofim dosing (15 mg/kg 3 times daily) was recently reported against infection with A fumigatus, A nidulans, and Aspergillus tanneri in both neutropenic and chronic granulomatous disease mouse models of invasive aspergillosis [141]. Olorofim therapy demonstrated robust antifungal activity, leading to prolonged survival and a concentration-dependent decline in circulating galactomannan levels in an immunosuppressed murine model of acute sinopulmonary infection caused by 4 clinical A flavus isolates (MIC ranged from 0.015 to 0.06 mg/L), which correlated with histological clearance of lung tissue [142].
Stage of Development and Ongoing Clinical Studies
Both oral and IV formulations are being developed, but the exact dosing regimen for both formulations is still being studied. An oral immediate release tablet evaluated in a phase 1 study (NCT03340597) showed that olorofim was well tolerated and effective [143]. The IV formulation was studied during clinical development (NCT02342574 and NCT02142153) and achieved the target steady-state plasma concentrations predicted for efficacy. Two phase 2 trials (NCT02856178 and NCT03036046) for use as fungal prophylaxis were recently withdrawn, because they were no longer required, and the drug is now in phase 2b clinical development with a global open-label study (FORMULA-OLS, NCT03583164) to evaluate olorofim for difficult-to-treat IFIs, including azole-resistant aspergillosis, scedosporiosis, lomentosporiosis, and other rare molds, in patients lacking suitable alternative treatment options [144] (Figure 3).
VL-2397
Mechanism of Action and Potential Role
VL-2397 (formerly ASP2397, developed by Vical Pharmaceuticals) potentially represents a new class of antifungal agents. It is a cyclic hexapeptide isolated from the fermentation broth of Acremonium persicinum [145]. VL-2397 is structurally related to the siderophore ferrichrome, a low molecular weight siderophore with high specificity for iron. This agent is able to chelate aluminum ions instead of iron; however, its exact mechanism of action is not fully understood, because the aluminum compound does not inhibit fungal growth [146]. Activity results from its effect on an unknown intracellular target (Figure 1 and Table 1). However, it is known that it triggers a potent and rapid antifungal effect after transport into fungal cells, specifically A fumigatus, via siderophore iron transporter 1 (Sit1), which is absent in mammalian cells. Live cell imaging suggests that VL-2397 causes arrest of hyphal elongation [147]. The FDA has granted QIDP, Orphan Drug, and fast track designations to VL-2397 for the treatment of invasive aspergillosis.
Activity
VL-2397 is active against different fungi (Figure 2). It demonstrates rapid onset of inhibition (within the first 2 to 4 hours) and potent in vitro fungicidal activity against hyphal elongation compared with existing drugs [147, 148]. It has proven activity against Aspergillus spp (including wild-type as well as azole-resistant strains), some Candida spp (only C glabrata and C kefyr), C neoformans, and Trichosporon asahii. The agent appears to have limited activity against Fusarium solani, and it is not active against A niger, Mucorales, Scedosporium apiospermum, and Fonsecaea pedrosoi [148]. This has translated into in vivo efficacy in a silkworm model and neutropenic murine models of invasive pulmonary aspergillosis, where VL-2397 caused dose-dependent prolongation of survival and reduction in fungal burden in the lung [145]. There is evidence of in vivo efficacy in a neutropenic murine model of invasive candidiasis caused by both wild-type and azole- and echinocandin-resistant C glabrata isolates [149].
Stage of Development and Ongoing Clinical Studies
A phase 1 study showed that this agent was well tolerated in healthy volunteers who received escalating single and multiple IV doses per day [150]. It was well tolerated up to 1200 mg, and no accumulation was observed for 21 days [150, 151]. There were no reported serious AEs related to the drug [150, 151]. VL-2397 demonstrated nonlinear saturable binding kinetics, most likely due to protein binding [148]. A phase 2 trial (NCT03327727) for the treatment of invasive aspergillosis in acute leukemia patients and recipients of bone marrow transplants was underway, but this was terminated early due to a business decision [152]. It is unfortunate that there are no ongoing trials at this time (Figure 3).
T-2307
Mechanism of Action and Potential Role
T-2307 (synthesized at Toyama Chemical Co., Llt., Tokyo, Japan) is an investigational arylamidine, structurally similar to a class of aromatic diamidines that includes pentamidine [153]. Its mechanism of action remains unknown; however, it appears to cause the collapse of fungal mitochondrial membrane potential in yeasts [154] (Figure 1 and Table 1). T-2307 is preferentially taken up by fungal cells via high-affinity spermine and spermidine transport systems with evidence of little effect on rat liver mitochondrial function [153, 155]. This transporter-mediated uptake contributes to its activity against Candida and makes it clinically safer than pentamidine. T-2307 inhibits the respiratory chain complexes in whole yeast cells and isolated yeast mitochondria, which is key for selective disruption of yeast mitochondrial function and antifungal activity [156].
Activity
T-2307 has potent in vitro and in vivo activity against Candida species, including azole- and echinocandin-resistant Candida spp, C neoformans, C gattii, Malassezia furfur, and F solani [157–161]. The in vitro activity of T-2307 was far superior to the activities of fluconazole, voriconazole, micafungin, and AmB. T-2307 was active against Aspergillus spp, and in vitro activity against these species was shown to be comparable to the activities of micafungin and voriconazole [157] (Figure 2).
Stage of Development and Ongoing Clinical Studies
For this potential antifungal drug, phase 1 studies are still ongoing, and no clinical efficacy data are available (Figure 3).
AR-12
Mechanism of Action
AR-12 (developed by Arno Therapeutics Inc., Flemington, NJ) is a novel molecule, antitumor celecoxib-derivative that has progressed to clinical development as an anticancer agent and has activity against a number of infectious agents including fungi, bacteria, and viruses [162]. With the goal of repurposing, a set of anticancer protein kinase inhibitors were screened for molecules with fungicidal activity towards C neoformans and C albicans, and AR-12 was found to have good antifungal activity against both species [163]. This agent acts by 2 different mechanisms of action, first by inhibiting the activity of fungal acetyl-CoA synthetase [164], and by downregulating host chaperone proteins, increasing the host immune response [16, 17] (Figure 1 and Table 1). It is interesting to note that AR-12 has also shown activity against bacteria, including Salmonella and Francisella, and hemorrhagic fever viruses [162]. The European Commission has designated AR-12 as an orphan drug for use in combination with other drugs for treatment of cryptococcosis and tularemia.
Activity
AR-12 has broad-spectrum antifungal activity against yeasts, molds, and dimorphic fungi (Figure 2). This includes Candida spp, including C glabrata and C krusei, and isolates of C neoformans with elevated fluconazole MICs [164]. It has also shown activity against dimorphic fungi including Blastomyces, Histoplasma, and Coccidioides [164]. In a murine model of cryptococcosis, AR-12 was synergistic with fluconazole [165]. However, the most promising feature of AR-12 is its in vitro activity against difficult-to-treat molds, including Fusarium, Mucor, and Scedosporium [164]. In a recent study, AR-12 was found to be highly active against Trichophyton rubrum, one of the organisms predominantly responsible for onychomycosis [166].
Stage of Development and Ongoing Clinical Studies
Target serum concentrations providing antifungal activity were safely achieved during phase 1 clinical trials as an anticancer agent (NCT00978523), indicating that AR-12 is promising candidate for repurposing as an antifungal agent [162]. However, Arno Therapeutics declared bankruptcy in 2017 leaving the future of the medication in limbo (Figure 3).
MGCD290
Mechanism of Action and Potential Role
MGCD290 (developed by MethylGene, Inc., Montreal, Canada) is a novel antifungal that inhibits fungal histone deacetylase 2 [16, 17]. Histone deacetylase is an enzyme that removes lysine residues of core histones, which control gene transcription and expression (Figure 1 and Table 1). They also control nonhistone proteins, including Hsp90, a molecular chaperone that regulates cellular stress response. In fungal cells, Hsp90 has been shown to be responsible for morphogenetic changes, stress adaptation, virulence, and antifungal resistance [167]. MGCD290 has shown a synergistic effect with other antifungals such as azoles or echinocandins [17, 167]. In cases of mutant-resistant isolates, MGCD290 produces a favorable influence on the MICs, restoring sensitivity [168].
Activity
MGCD290 only has modest activity against Candida spp [168]. However, it has demonstrated in vitro synergy with azoles and echinocandins against many azole- and echinocandin-resistant isolates including Candida spp, Aspergillus spp, Mucor, and Fusarium [168, 169] (Figure 2).
Stage of Development and Clinical Studies
This drug is currently in clinical development, and a phase 2 trial (NCT01497223) was completed that evaluated the cure rate with combination treatment of MGCD290 and fluconazole compared with fluconazole alone for patients with moderate to severe VVC [170] (Figure 3). This yielded poor results with no statistically significant benefit of combination therapy [171].
DEVICE
Neurapheresis
Mechanism of Action and Potential Role
The neurapheresis therapy system (developed by Minnetronix, Inc., St. Paul, MN) is a closed-loop system designed to filter fungal pathogens from human cerebrospinal fluid (CSF) (Table 1). It operates by extracting CSF from the subarachnoid space, removing pathogens through a filtration system, and then redepositing the CSF through the same catheter [172]. It is currently considered as a potential novel adjunctive therapy for CM.
Stage of Development and Ongoing Clinical Studies
Smilnak et al [172] demonstrated the effects both in vitro and in vivo. Filtration of C neoformans in vitro accomplished a 5-log reduction in yeasts over 24 hours, compared with AmB plus 5FC, which achieved 0.42-log clearance of colony-forming units of yeast/mL per day from CSF [173]. Another in vivo rabbit model demonstrated a large decrease (97%) in yeast concentration after 4–6 hours of filtration. Concentrations of cryptococcal antigen decreased substantially (≥90%) postfiltration. Removal of cryptococcal antigen, which is responsible for occluding arachnoid villi, as well as rapid clearing of both single and clumping yeasts from the CSF, may reverse outflow obstruction in CM patients and alleviate resulting headaches and hydrocephaly. The neurapheresis system is expected to (1) allow physicians to modulate the high ICP typical of CM patients more directly and (2) also serve as a favorable alternative to other drainage procedures, minimizing the need for repeated lumbar punctures and/or placement of shunts [172]. The system was first used in a patient with subarachnoid hemorrhage, for which she underwent lumbar catheter placement and completed 9 hours of CSF filtration without complications. Repeat imaging demonstrated interval reduction of subarachnoid hemorrhage immediately after filtration [174]. In addition, it was piloted as treatment for other conditions such as bacterial meningitis and leptomeningeal disease [175, 176]. The ability of neurapheresis to reduce C neoformans and cryptococcal antigen demonstrates its potential to efficiently treat CM as an adjunctive therapy to systemic antifungals. Given its utility and the ability to directly remove the pathogen and secondary virulence factors, this platform is an appealing choice for adjunctive treatment of other fungal meningitis, such as the coccidioidal meningitis [172].
Ongoing studies include a clinical trial (PILLAR trial, NCT02872636) evaluating the safety and feasibility of this system in patients with subarachnoid hemorrhages. Another study testing the potential of neurapheresis therapy to reduce fungal burden in human patients with CM is currently being planned. Upcoming studies will explore the ability of the neurapheresis system to (1) filter smaller mediators such as cytokines and chemokines to control the neuroinflammatory response seen in CM and (2) avoid dangerous levels of inflammation or even immune reconstitution inflammatory syndrome (IRIS) within the CNS [172].
DISCUSSION
Despite a large number of patients suffering, the global burden of IFIs remains relatively underappreciated and underfunded. We face many challenges in the treatment of these diseases due to increasing resistance to currently available antifungals, demanding the need for development of new treatment options. This review focused on several novel antifungal therapies at various clinical stages in the development pipeline, including a first device used as adjunctive therapy.
These new antifungals have many potential roles and advantages over current drugs. New drugs, including olorofim, fosmanogepix, and ibrexafungerp, are now available options for the treatment of previously pan-resistant organisms. This is incredibly important because these drugs offer options for physicians where there were previously no treatment avenues available. In the case of olorofim, physicians now have another course of action in tackling triazole-resistant Aspergillus isolates [129] and other pan-resistant fungal pathogens including Lomentospora spp [133]. Still, chemical classes for IFI treatment are so few that emergence of resistance to even 1 drug class tragically limits therapy options.
Many of the described therapies have improved safety profiles with reduced toxicity and fewer adverse effects and drug-drug interactions while preserving efficacy. A majority of IFI cases are seen in patients with other comorbid conditions [177], and a variety of treatments and procedures for other conditions are well recognized risk factors for developing IFIs, such as abdominal surgery, chemotherapy, and use central venous catheters. With these patients being treated for multiple reasons concurrently, drug-drug interactions and compound toxicities are significant concerns for physicians. A patient’s ability to tolerate the effects of treatment can limit a clinician when prescribing a course of treatment, especially in critically ill patients. For example, CAmB minimizes the toxicity seen with AmB by using cochleates as a drug delivery system to target the fungal cell membrane. Likewise, olorofim and fosmanogepix have lower risk of toxicity due to fungal-specific targets, and they also possess novel mechanisms of action by inhibition of fungal DHODH for pyrimidine synthesis and Gwt1 for GPI-anchor protein synthesis, respectively. The caveat of olorofim is related to possible drug-drug interactions given its weak inhibition of CYP3A4. Drug-drug interactions are minimized while using CamB and also with tetrazoles because they selectively target the fungal CYP51 enzyme.
The burden of administration requirements is reduced with the oral formulation of ibrexafungerp, olorofim, and CAmB decreasing barriers of IV administration, and rezafungin has a longer half-life allowing weekly dosing. Complex dosing schedules and IV administration requirements are known to negatively impact medication adherence [178, 179], and, for patients receiving antifungal treatment for extended periods, these oral formulations and reduced dosing intervals have the potential to decrease hospital and clinic visits and ultimately increase patient adherence during treatment.
CONCLUSIONS
These promising features begin to address the urgent need for improvement in available therapies, but there is work still to be done. It is unfortunate that the potential toxicities and disadvantages of these therapies have not been fully evaluated, because most of them have not reached advanced clinical development in stage 3 or 4 studies. Even with all of the drugs in development, not every compound with antifungal activity will result in an approved drug for the market. Translating the results of early studies into clinical candidates can be difficult due to efficacy and financial reasons, and only approximately 20% of the potential antifungal targets published in the literature were further developed [16]. However, given the number of new promising compounds in development, the future is brighter than it has ever been.
Acknowledgments
Disclaimer. The content is solely the responsibility of the authors and does not necessarily represent the official view of the National Institutes of Health.
Financial support. This work was funded by the Washington University Institute of Clinical and Translational Sciences grant number UL1TR002345 from the National Center for Advancing Translational Sciences of the National Institutes of Health.
Potential conflicts of interest. A. S. reports consulting fees from Viamet, Scynexis, and Minnetronix and an investigator-sponsored grant from Astellas. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Denning DW, Bromley MJ. Infectious disease. How to bolster the antifungal pipeline. Science 2015; 347:1414–6. [DOI] [PubMed] [Google Scholar]
- 2. Brown GD, Denning DW, Gow NA, et al. Hidden killers: human fungal infections. Sci Transl Med 2012; 4:165rv13. [DOI] [PubMed] [Google Scholar]
- 3. Ramana KV, Kandi S, Bharatkumar V, et al. Invasive fungal infections: a comprehensive review. Am J Infect Dis Microbiol 2013; 1:64–9. [Google Scholar]
- 4. Gonzalez-Lara MF, Sifuentes-Osornio J, Ostrosky-Zeichner L. Drugs in clinical development for fungal infections. Drugs 2017; 77:1505–18. [DOI] [PubMed] [Google Scholar]
- 5. Drgona L, Khachatryan A, Stephens J, et al. Clinical and economic burden of invasive fungal diseases in Europe: focus on pre-emptive and empirical treatment of Aspergillus and Candida species. Eur J Clin Microbiol Infect Dis 2014; 33:7–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Pasqualotto AC, Quieroz-Telles F. Histoplasmosis dethrones tuberculosis in Latin America. Lancet Infect Dis 2018; 18:1058–60. [DOI] [PubMed] [Google Scholar]
- 7. Adenis AA, Valdes A, Cropet C, et al. Burden of HIV-associated histoplasmosis compared with tuberculosis in Latin America: a modelling study. Lancet Infect Dis 2018; 18:1150–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Seyedmousavi S, Rafati H, Ilkit M, et al. Systemic antifungal agents: current status and projected future developments. In: Lion T, ed. Human Fungal Pathogen Identification. Methods in Molecular Biology. Vol. 1508 New York: Humana Press; 2017. [DOI] [PubMed] [Google Scholar]
- 9. Castelli MV, Derita MG, López SN. Novel antifungal agents: a patent review (2013 - present). Expert Opin Ther Pat 2017. [DOI] [PubMed] [Google Scholar]
- 10. Dunne K, Hagen F, Pomeroy N, et al. Intercountry transfer of triazole-resistant Aspergillus fumigatus on plant bulbs. Clin Infect Dis 2017; 65:147–9. [DOI] [PubMed] [Google Scholar]
- 11. Forsberg K, Woodworth K, Walters M, et al. Candida auris: the recent emergence of a multidrug-resistant fungal pathogen. Med Mycol 2019; 57:1–12. [DOI] [PubMed] [Google Scholar]
- 12. Chowdhary A, Sharma C, Meis JF. Azole-resistant Aspergillosis: epidemiology, molecular mechanisms, and treatment. J Infect Dis 2017; 216(Suppl 3):S436–44. [DOI] [PubMed] [Google Scholar]
- 13. Verweij PE, Chowdhary A, Melchers WJ, Meis JF. Azole resistance in Aspergillus fumigatus: can we retain the clinical use of mold-active antifungal azoles? Clin Infect Dis 2016; 62:362–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Chang YL, Yu SJ, Heitman J, et al. New facets of antifungal therapy. Virulence 2017; 8:222–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Letscher-Bru V, Herbrecht R. Caspofungin: the first representative of a new antifungal class. J Antimicrob Chemother 2003; 51:513–21. [DOI] [PubMed] [Google Scholar]
- 16. Van Daele R, Spriet I, Wauters J, et al. Antifungal drugs: what brings the future? Med Mycol 2019; 57:328–43. [DOI] [PubMed] [Google Scholar]
- 17. Perfect JR. The antifungal pipeline: a reality check. Nat Rev Drug Discov 2017; 16:603–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Krishnan BR, James KD, Polowy K, et al. CD101, a novel echinocandin with exceptional stability properties and enhanced aqueous solubility. J Antibiot (Tokyo) 2017; 70:130–5. [DOI] [PubMed] [Google Scholar]
- 19. Lakota EA, Bader JC, Ong V, et al. Pharmacological basis of CD101 efficacy: exposure shape matters. Antimicrob Agents Chemother 2017; 61:e00758-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Pfaller MA, Messer SA, Rhomberg PR, et al. Activity of a long-acting echinocandin, CD101, determined using CLSI and EUCAST reference methods, against Candida and Aspergillus spp., including echinocandin- and azole-resistant isolates. J Antimicrob Chemother 2016; 71:2868–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sandison T, Ong V, Lee J, Thye D. Safety and pharmacokinetics of CD101 IV, a novel echinocandin, in healthy adults. Antimicrob Agents Chemother 2017; 61:e01627-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ong V, Hough G, Schlosser M, et al. Preclinical evaluation of the stability, safety, and efficacy of CD101, a novel echinocandin. Antimicrob Agents Chemother 2016; 60:6872–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Cushion M, Ashbaugh A, Lynch K, et al. Efficacy of CD101, a novel echinocandin, in prevention of pneumocystis pneumonia (PCP): thwarting the biphasic life cycle of pneumocystis. Blood 2016; 128:3396. [Google Scholar]
- 24. Liu N, Tu J, Dong G, et al. Emerging new targets for the treatment of resistant fungal infections. J Med Chem 2018; 61:5484–511. [DOI] [PubMed] [Google Scholar]
- 25. Pfaller MA, Messer SA, Rhomberg PR, Castanheira M. CD101, a long-acting echinocandin, and comparator antifungal agents tested against a global collection of invasive fungal isolates in the SENTRY 2015 Antifungal Surveillance Program. Int J Antimicrob Agents 2017; 50:352–8. [DOI] [PubMed] [Google Scholar]
- 26. Lakota EA, Ong V, Flanagan S, Rubino CM. Population pharmacokinetic analyses for rezafungin (CD101) efficacy using phase 1 data. Antimicrob Agents Chemother 2018; 62:e02614-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Locke JB, Almaguer AL, Zuill DE, Bartizal K. Characterization of in vitro resistance development to the novel echinocandin CD101 in Candida species. Antimicrob Agents Chemother 2016; 60:6100–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Arendrup MC, Meletiadis J, Zaragoza O, et al. Multicentre determination of rezafungin (CD101) susceptibility of Candida species by the EUCAST method. Clin Microbiol Infect 2018; 24:1200–4. [DOI] [PubMed] [Google Scholar]
- 29. Wiederhold NP, Locke JB, Daruwala P, Bartizal K. Rezafungin (CD101) demonstrates potent in vitro activity against Aspergillus, including azole-resistant Aspergillus fumigatus isolates and cryptic species. J Antimicrob Chemother 2018; 73:3063–7. [DOI] [PubMed] [Google Scholar]
- 30. Lakota EA, Voon O, Flanagan S, Rubinoa CM. Population pharmacokinetic analyses for rezafungin (CD101) efficacy using phase 1 data. Antimicrob Agents Chemother 2018; 62:e02603-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. James KD, Laudeman CP, Malkar NB, et al. Structure-activity relationships of a series of echinocandins and the discovery of CD101, a highly stable and soluble echinocandin with distinctive pharmacokinetic properties. Antimicrob Agents Chemother 2017; 61:e01541-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lepak AJ, Zhao M, VanScoy B, et al. Pharmacodynamics of a long-acting echinocandin, CD101, in a neutropenic invasive-candidiasis murine model using an extended-interval dosing design. Antimicrob Agents Chemother 2018; 62:e02154-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lepak AJ, Zhao M, Andesa DR. Pharmacodynamic evaluation of rezafungin (CD101) against Candida auris in the neutropenic mouse invasive candidiasis model. Antimicrob Agents Chemother 2018; 62:e01572-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hager CL, Larkin EL, Long LA, Ghannoum MA. Evaluation of the efficacy of rezafungin, a novel echinocandin, in the treatment of disseminated Candida auris infection using an immunocompromised mouse model. J Antimicrob Chemother 2018; 73:2085–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ong V, Bartizal K, Miesel L. Efficacy of CD101, a novel echinocandin, in mouse models of aspergillosis and azole-resistant disseminated candidiasis. Blood 2016; 128:3400. [Google Scholar]
- 36. Zhao Y, Prideaux B, Nagasaki Y, et al. Unraveling drug penetration of echinocandin antifungals at the site of infection in an intra-abdominal abscess model. Antimicrob Agents Chemother 2017; 61:e01009-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Cidara Therapeutics. 2018. Cidara Therapeutics Reports Positive Topline Results from Phase 2 STRIVE Trial of Lead Antifungal Rezafungin. News Release. Available at: http://ir.cidara.com/news-releases/news-release-details/cidara-therapeutics-reports-positive-topline-results-phase-2. Accessed 18 July 2019. [Google Scholar]
- 38. Cidara Therapeutics. Cidara Therapeutics Reports Unfavorable Results of Phase 2 RADIANT Trial of CD101 Topical in VVC. New Release. 2017. Available at: http://ir.cidara.com/news-releases/news-release-details/cidara-therapeutics-reports-unfavorable-results-phase-2-radiant. Accessed 18 July 2019. [Google Scholar]
- 39. Nyirjesy P, Alessio C, Jandourek A, et al. CD101 topical compared with oral fluconazole for acute vulvovaginal candidiasis: a randomized controlled trial. J Low Genit Tract Dis 2019; 23:226–9. [DOI] [PubMed] [Google Scholar]
- 40. Wring SA, Randolph R, Park S, et al. Preclinical pharmacokinetics and pharmacodynamic target of SCY-078, a first-in-class orally active antifungal glucan synthesis inhibitor, in murine models of disseminated candidiasis. Antimicrob Agents Chemother 2017; 61:e02068-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Schell WA, Jones AM, Borroto-Esoda K, Alexander BD. Antifungal activity of SCY-078 and standard antifungal agents against 178 clinical isolates of resistant and susceptible Candida species. Antimicrob Agents Chemother 2017; 61:e01102-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Lee A, Prideaux B, Lee M, et al. Penetration of Ibrexafungerp (formerly SCY-078) versus micafungin at the site of infection in an intra-abdominal candidiasis mouse model. ECCMID (Amsterdam, Netherlands). 13–16 April 2019. [Google Scholar]
- 43. Larkin EL, Long L, Isham N, et al. A novel 1,3-Beta-d-glucan inhibitor, Ibrexafungerp (formerly SCY-078), shows potent activity in the lower pH environment of vulvovaginitis. Antimicrob Agents Chemother 2019; 63:e02611-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Wring S, Murphy G, Atiee G, et al. Lack of impact by SCY-078, a first-in-class oral fungicidal glucan synthase inhibitor, on the pharmacokinetics of rosiglitazone, a substrate for CYP450 2C8, supports the low risk for clinically relevant metabolic drug-drug interactions. J Clin Pharmacol 2018; 58:1305–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Wring S, Murphy G, Atiee G, et al. Clinical pharmacokinetics and drug-drug interaction potential for coadministered SCY-078, an oral fungicidal glucan synthase inhibitor, and tacrolimus. Clin Pharmacol Drug Dev 2019; 8:60–9. [DOI] [PubMed] [Google Scholar]
- 46. Pfaller MA, Messer SA, Motyl MR, et al. Activity of MK-3118, a new oral glucan synthase inhibitor, tested against Candida spp. by two international methods (CLSI and EUCAST). J Antimicrob Chemother 2013; 68:858–63. [DOI] [PubMed] [Google Scholar]
- 47. Pfaller MA, Messer SA, Motyl MR, et al. In vitro activity of a new oral glucan synthase inhibitor (MK-3118) tested against Aspergillus spp. by CLSI and EUCAST broth microdilution methods. Antimicrob Agents Chemother 2013; 57:1065–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Jiménez-Ortigosa C, Paderu P, Motyl MR, Perlin DS. Enfumafungin derivative MK-3118 shows increased in vitro potency against clinical echinocandin-resistant Candida species and Aspergillus species isolates. Antimicrob Agents Chemother 2014; 58:1248–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Larkin E, Hager C, Chandra J, et al. The emerging pathogen Candida auris: growth phenotype, virulence factors, activity of antifungals, and effect of SCY-078, a novel glucan synthesis inhibitor, on growth morphology and biofilm formation. Antimicrob Agents Chemother 2017; 61:e02396-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Berkow EL, Angulo D, Lockhart SR. In vitro activity of a novel glucan synthase inhibitor, SCY-078, against clinical isolates of Candida auris. Antimicrob Agents Chemother 2017; 61:e00435-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Pfaller MA, Messer SA, Rhomberg PR, et al. Differential activity of the oral glucan synthase inhibitor SCY-078 against wild-type and echinocandin-resistant strains of Candida species. Antimicrob Agents Chemother 2017; 61:e00161-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Lamoth F, Alexander BD. Antifungal activities of SCY-078 (MK-3118) and standard antifungal agents against clinical non-Aspergillus mold isolates. Antimicrob Agents Chemother 2015; 59:4308–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Wiederhold NP, Najvar LK, Jaramillo R, et al. Oral glucan synthase inhibitor SCY-078 is effective in an experimental murine model of invasive candidiasis caused by WT and echinocandin-resistant Candida glabrata. J Antimicrob Chemother 2018; 73:448–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Cushion M, Ashbaugh A, Borroto-Esoda K, et al. SCY-078 demonstrates antifungal activity against pneumocystis in a prophylactic murine model of pneumocystis pneumonia. ECCMID (Madrid, Spain). 21–24 April 2018. [Google Scholar]
- 55. Barat S, Borroto-Esoda K, Ashbaugh A, Cushion MT. Efficacy of Ibrexafungerp (formerly SCY-078) against pneumocystis pneumonia in a murine therapeutic model. ECCMID (Amsterdam, Netherlands). 13–16 April 2019. [Google Scholar]
- 56. Helou S, Angulo D. A multicenter, randomized, evaluator blinded, active-controlled study to evaluate the safety and efficacy of oral SCY-078 vs. oral fluconazole in 96 subjects with moderate to severe vulvovaginal candidiasis. Am J Obstet Gynecol 2017; 217:720–1. [Google Scholar]
- 57. Spec A, Pullman J, Thompson GR, et al. ; Mycoses Study Group MSG-10: a Phase 2 study of oral ibrexafungerp (SCY-078) following initial echinocandin therapy in non-neutropenic patients with invasive candidiasis. J Antimicrob Chemother 2019; 74:3056–62. [DOI] [PubMed] [Google Scholar]
- 58. SCYNEXIS I. Ibrexafungerp (formerly SCY-078): An Innovative Antifungal. Available at: https://www.scynexis.com/pipeline. Accessed 23 July 2019. [Google Scholar]
- 59. Juneja D, Singh O, Tarai B, Angulo Gonzalez D. Successful treatment of two patients with Candida auris candidemia with the investigational agent, oral Ibrexafungerp (formerly SCY-078) from the CARES study. ECCMID (Amsterdam, Netherlands). 13–16 April 2019. [Google Scholar]
- 60. Cornely OA, Ostrosky-Zeichner L, Miller R, et al. Favorable response to oral Ibrexafungerp (formerly SCY-078) in patients with refractory fungal diseases, interim analysis by pathogen from a phase 3 open-label study (FURI). ECCMID (Amsterdam, Netherlands). 13 -16 April 2019. [Google Scholar]
- 61. Köhler P, Cornely OA, Angulo Gonzalez D. Favourable clinical outcome of two patients with Candida spp spondylodiscitis treated with oral Ibrexafungerp (formerly SCY-078) from the FURI study. ECCMID (Amsterdam, Netherlands). 13 -16 April 2019. [Google Scholar]
- 62. Miyazaki M, Horii T, Hata K, et al. In vitro activity of E1210, a novel antifungal, against clinically important yeasts and molds. Antimicrob Agents Chemother 2011; 55:4652–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Watanabe NA, Miyazaki M, Horii T, et al. E1210, a new broad-spectrum antifungal, suppresses Candida albicans hyphal growth through inhibition of glycosylphosphatidylinositol biosynthesis. Antimicrob Agents Chemother 2012; 56:960–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Pfaller MA, Hata K, Jones RN, et al. In vitro activity of a novel broad-spectrum antifungal, E1210, tested against Candida spp. as determined by CLSI broth microdilution method. Diagn Microbiol Infect Dis 2011; 71:167–70. [DOI] [PubMed] [Google Scholar]
- 65. Shaw KJ, Schell WA, Covel J, et al. In vitro and in vivo evaluation of APX001A/APX001 and other Gwt1 inhibitors against Cryptococcus. Antimicrob Agents Chemother 2018; 62:e00523-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Viriyakosol S, Kapoor M, Okamoto S, et al. APX001 and other Gwt1 inhibitor prodrugs are effective in experimental Coccidioides immitis pneumonia. Antimicrob Agents Chemother 2019; 63:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Hager CL, Larkin EL, Long L, et al. In vitro and in vivo evaluation of the antifungal activity of APX001A/APX001 against Candida auris. Antimicrob Agents Chemother 2018; 62:e02319-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Zhao M, Lepak AJ, Vanscoy B, et al. In vivo pharmacokinetics and pharmacodynamics of APX001 against Candida spp. in a neutropenic disseminated candidiasis mouse model. Antimicrob Agents Chemother 2018; 62:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Jørgensen KM, Astvad KM, Arendrup MC. EUCAST susceptibility testing of APX001A: MIC data for contemporary clinical blood stream isolates. Mycoses 2017; 60:214. [Google Scholar]
- 70. Pfaller MA, Duncanson F, Messer SA, et al. In vitro activity of a novel broad-spectrum antifungal, E1210, tested against Aspergillus spp. determined by CLSI and EUCAST broth microdilution methods. Antimicrob Agents Chemother 2011; 55:5155–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Castanheira M, Duncanson FP, Diekema DJ, et al. Activities of E1210 and comparator agents tested by CLSI and EUCAST broth microdilution methods against Fusarium and Scedosporium species identified using molecular methods. Antimicrob Agents Chemother 2012; 56:352–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Zhao Y, Lee MH, Paderu P, et al. Significantly improved pharmacokinetics enhances in vivo efficacy of APX001 against echinocandin-and multidrug-resistant Candida isolates in a mouse model of invasive candidiasis. Antimicrob Agents Chemother 2018; 62:e00425-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Hata K, Horii T, Miyazaki M, et al. Efficacy of oral E1210, a new broad-spectrum antifungal with a novel mechanism of action, in murine models of candidiasis, aspergillosis, and fusariosis. Antimicrob Agents Chemother 2011; 55:4543–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Wiederhold NP, Najvar LK, Fothergill AW, et al. The investigational agent E1210 is effective in treatment of experimental invasive candidiasis caused by resistant Candida albicans. Antimicrob Agents Chemother 2015; 59:690–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Zhao M, Lepak AJ, Marchillo K, et al. APX001 pharmacokinetic/pharmacodynamic target determination against Aspergillus fumigatus in an in vivo model of invasive pulmonary aspergillosis. Antimicrob Agents Chemother 2019; 63:e02372-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Mansbach R, Shaw KJ, Hodges MR, et al. Absorption, distribution, and excretion of 14C-APX001 after single-dose administration to rats and monkeys. Open Forum Infect Dis 2017; 4(Suppl 1):S472. [Google Scholar]
- 77. Hodges MR, Ople E, Shaw KJ, et al. First-in-human study to assess safety, tolerability and pharmacokinetics of APX001 administered by intravenous infusion to healthy subjects. Open Forum Infect Dis 2017; 4(Suppl_1):S526. [Google Scholar]
- 78. Hodges MR, Ople E, Shaw KJ, et al. Phase 1 study to assess safety, tolerability and pharmacokinetics of single and multiple oral doses of APX001 and to investigate the effect of food on APX001 bioavailability. Open Forum Infect Dis 2017; 4(Suppl_1):S534. [Google Scholar]
- 79. Amplyx. APX001 Fact Sheet. Available at: https://amplyx.com/wp-content/uploads/Amplyx-APX001-Fact-Sheet-v11.pdf. Accessed 1 August 2019. [Google Scholar]
- 80. Stenland CJ, Lis LG, Schendel FJ, et al. A practical and scalable manufacturing process for an anti-fungal agent, Nikkomycin Z. Org Process Res Dev 2013; 17:265–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Goldberg J, Connolly P, Schnizlein-Bick C, et al. Comparison of nikkomycin Z with amphotericin B and itraconazole for treatment of histoplasmosis in a murine model. Antimicrob Agents Chemother 2000; 44:1624–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Graybill JR, Najvar LK, Bocanegra R, et al. Efficacy of nikkomycin Z in the treatment of murine histoplasmosis. Antimicrob Agents Chemother 1998; 42:2371–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Hector RF, Zimmer BL, Pappagianis D. Evaluation of nikkomycins X and Z in murine models of coccidioidomycosis, histoplasmosis, and blastomycosis. Antimicrob Agents Chemother 1990; 34:587–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Shubitz LF, Roy ME, Nix DE, Galgiani JN. Efficacy of Nikkomycin Z for respiratory coccidioidomycosis in naturally infected dogs. Med Mycol 2013; 51:747–54. [DOI] [PubMed] [Google Scholar]
- 85. Clemons KV, Stevens DA. Efficacy of nikkomycin Z against experimental pulmonary blastomycosis. Antimicrob Agents Chemother 1997; 41:2026–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Ganesan LT, Manavathu EK, Cutright JL, et al. In-vitro activity of nikkomycin Z alone and in combination with polyenes, triazoles or echinocandins against Aspergillus fumigatus. Clin Microbiol Infect 2004; 10:961–6. [DOI] [PubMed] [Google Scholar]
- 87. Cheung Y-Y, Hui M. Effects of Echinocandins in combination with Nikkomycin Z against invasive Candida albicans bloodstream isolates and the fks mutants. Antimicrob Agents Chemother 2017; 61:e00619-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Li RK, Rinaldi MG. In vitro antifungal activity of nikkomycin Z in combination with fluconazole or itraconazole. Antimicrob Agents Chemother 1999; 43:1401–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Shubitz LF, Trinh HT, Perrill RH, et al. Modeling nikkomycin Z dosing and pharmacology in murine pulmonary coccidioidomycosis preparatory to phase 2 clinical trials. J Infect Dis 2014; 209:1949–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Nix DE, Swezey RR, Hector R, Galgiani JN. Pharmacokinetics of nikkomycin Z after single rising oral doses. Antimicrob Agents Chemother 2009; 53:2517–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. ClinicalTrials.gov. Safety and PK of Nikkomycin Z for Coccidioides Pneumonia Treatment. Available at: https://clinicaltrials.gov/ct2/show/NCT00614666. Accessed 14 August 2019. [Google Scholar]
- 92. Valley Fever Solutions. A first-in-class compound, New Mechanism of Action. Available at: https://www.valleyfeversolutions.com/. Accessed 14 August 2019. [Google Scholar]
- 93. Yates CM, Garvey EP, Shaver SR, et al. Design and optimization of highly-selective, broad spectrum fungal CYP51 inhibitors. Bioorg Med Chem Lett 2017; 27:3243–8. [DOI] [PubMed] [Google Scholar]
- 94. Warrilow AG, Hull CM, Parker JE, et al. The clinical candidate VT-1161 is a highly potent inhibitor of Candida albicans CYP51 but fails to bind the human enzyme. Antimicrob Agents Chemother 2014; 58:7121–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Warrilow AG, Parker JE, Price CL, et al. The investigational drug VT-1129 is a highly potent inhibitor of Cryptococcus species CYP51 but only weakly inhibits the human enzyme. Antimicrob Agents Chemother 2016; 60:4530–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Shubitz LF, Trinh HT, Galgiani JN, et al. Evaluation of VT-1161 for treatment of coccidioidomycosis in murine infection models. Antimicrob Agents Chemother 2015; 59:7249–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Long L, Isham N, Ghannoun M, et al. High in vitro potency of the clinical investigational agent VT-1161 against clinical isolates of Candida spp. 25th ECCMID (Copenhagen, Denmark) , 25–28 April 2015. [Google Scholar]
- 98. Warrilow AG, Parker JE, Price CL, et al. The Tetrazole VT-1161 Is a potent inhibitor of Trichophyton rubrum through its inhibition of T. rubrum CYP51. Antimicrob Agents Chemother 2017; 61:e00333-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Schell WA, Jones AM, Garvey EP, et al. Fungal CYP51 inhibitors VT-1161 and VT-1129 exhibit strong in vitro activity against Candida glabrata and C. krusei isolates clinically resistant to azole and echinocandin antifungal compounds. Antimicrob Agents Chemother 2017; 61:e01817-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Gebremariam T, Wiederhold NP, Fothergill AW, et al. VT-1161 protects immunosuppressed mice from Rhizopus arrhizus var. arrhizus infection. Antimicrob Agents Chemother 2015; 59:7815–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Garvey EP, Hoekstra WJ, Schotzinger RJ, et al. Efficacy of the clinical agent VT-1161 against fluconazole-sensitive and -resistant Candida albicans in a murine model of vaginal candidiasis. Antimicrob Agents Chemother 2015; 59:5567–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Garvey EP, Hoekstra WJ, Moore WR, et al. VT-1161 dosed once daily or once weekly exhibits potent efficacy in treatment of dermatophytosis in a guinea pig model. Antimicrob Agents Chemother 2015; 59:1992–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Lockhart SR, Fothergill AW, Iqbal N, et al. The investigational fungal Cyp51 inhibitor VT-1129 demonstrates potent in vitro activity against Cryptococcus neoformans and Cryptococcus gattii. Antimicrob Agents Chemother 2016; 60:2528–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Nielsen K, Vedula P, Smith KD, et al. Activity of VT-1129 against Cryptococcus neoformans clinical isolates with high fluconazole MICs. Med Mycol 2017; 55:453–6. [DOI] [PubMed] [Google Scholar]
- 105. Wiederhold NP, Najvar LK, Garvey EP, et al. The fungal Cyp51 inhibitor VT-1129 is efficacious in an experimental model of cryptococcal meningitis. Antimicrob Agents Chemother 2018; 62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Wiederhold NP, Xu X, Wang A, et al. In vivo efficacy of VT-1129 against experimental cryptococcal meningitis with the use of a loading dose-maintenance dose administration strategy. Antimicrob Agents Chemother 2018; 62:e01315-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Hargrove TY, Garvey EP, Hoekstra WJ, et al. Crystal structure of the new investigational drug candidate VT-1598 in complex with Aspergillus fumigatus sterol 14α-demethylase provides insights into its broad-spectrum antifungal activity. Antimicrob Agents Chemother 2017; 61:e00570-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Wiederhold NP, Shubitz LF, Najvar LK, et al. The novel fungal Cyp51 inhibitor VT-1598 is efficacious in experimental models of central nervous system coccidioidomycosis caused by Coccidioides posadasii and Coccidioides immitis. Antimicrob Agents Chemother 2018; 62:e02258-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Break TJ, Desai JV, Healey KR, et al. VT-1598 inhibits the in vitro growth of mucosal Candida strains and protects against fluconazole-susceptible and -resistant oral candidiasis in IL-17 signalling-deficient mice. J Antimicrob Chemother 2018; 73:2089–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Wiederhold NP, Lockhart SR, Najvar LK, et al. The fungal Cyp51-specific inhibitor VT-1598 demonstrates in vitro and in vivo activity against Candida auris. Antimicrob Agents Chemother 2019; 63:e02233-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Brand SR, Degenhardt TP, Person K, et al. A phase 2, randomized, double-blind, placebo-controlled, dose-ranging study to evaluate the efficacy and safety of orally administered VT-1161 in the treatment of recurrent vulvovaginal candidiasis. Am J Obstet Gynecol 2018; 218:624.e1–9. [DOI] [PubMed] [Google Scholar]
- 112. Santangelo R, Paderu P, Delmas G, et al. Efficacy of oral cochleate-amphotericin B in a mouse model of systemic candidiasis. Antimicrob Agents Chemother 2000; 44:2356–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Zarif L, Graybill JR, Perlin D, et al. Antifungal activity of amphotericin B cochleates against Candida albicans infection in a mouse model. Antimicrob Agents Chemother 2000; 44:1463–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Cuddihy G, Wasan EK, Di Y, Wasan KM. The development of oral amphotericin b to treat systemic fungal and parasitic infections: has the myth been finally realized? Pharmaceutics 2019; 11:99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Segarra I, Movshin DA, Zarif L. Pharmacokinetics and tissue distribution after intravenous administration of a single dose of amphotericin B cochleates, a new lipid-based delivery system. J Pharm Sci 2002; 91:1827–37. [DOI] [PubMed] [Google Scholar]
- 116. Mannino R, Perlin D. Oral dosing of encochleated amphotericin B (CAmB): rapid drug targeting to infected tissues in MiOral dosing of encochleated amphotericin B (CAmB): rapid drug targeting to infected tissues in mice. ICAAC and ICC (San Diego, CA). 17–21 September 2015. [Google Scholar]
- 117. Delmas G, Park S, Chen ZW, et al. Efficacy of orally delivered cochleates containing amphotericin B in a murine model of aspergillosis. Antimicrob Agents Chemother 2002; 46:2704–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Wasan KM, Wasan EK, Gershkovich P, et al. Highly effective oral amphotericin B formulation against murine visceral leishmaniasis. J Infect Dis 2009; 200:357–60. [DOI] [PubMed] [Google Scholar]
- 119. Lu R, Hollingsworth C, Qiu J, et al. Efficacy of oral encochleated amphotericin B in a mouse model of cryptococcal meningoencephalitis. MBio 2019; 10:e00724-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Biederdorf F, Breithaupt I, Mannino R, Blum D.. Oral Administration of Amphotericin B (CAmB) in Humans: a Phase I Study of Tolerability and Pharmacokinetics. Available at: https://content.equisolve.net/_1086974c35b0aa727eebf78941ddb61c/matinasbiopharma/db/128/515/pdf/CAmB-Focus-Phase-1-Poster.pdf. Accessed 25 July 2019. [Google Scholar]
- 121. Matinas BioPharma Holdings. MAT2203: LNC Formulation of Amphotericin B. Available at: https://www.matinasbiopharma.com/lnc-pipeline/mat2203-lnc-formulation-of-amphotericin-b Accessed 26 July 2019. [Google Scholar]
- 122. Tan HW, Tay ST. The inhibitory effects of aureobasidin A on Candida planktonic and biofilm cells. Mycoses 2013; 56:150–6. [DOI] [PubMed] [Google Scholar]
- 123. Takesako K, Kuroda H, Inoue T, et al. Biological properties of aureobasidin A, a cyclic depsipeptide antifungal antibiotic. J Antibiot (Tokyo) 1993; 46:1414–20. [DOI] [PubMed] [Google Scholar]
- 124. Takara Bio Inc. Takara Bio grants a non-exclusive license of its proprietary antibiotic aureobasidin A-related patents to AureoGen Biosciences for development of novel drugs. News Release. Available at: http://www.takara-bio.com/news_e/2007/01/29.htm. Accessed 27 August 2019. [Google Scholar]
- 125. Zhong W, Jeffries MW, Georgopapadakou NH. Inhibition of inositol phosphorylceramide synthase by aureobasidin A in Candida and Aspergillus species. Antimicrob Agents Chemother 2000; 44:651–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Moraes Nicola A, Albuquerque P, Costa Paes H, et al. Antifungal drugs: new insights in research & development. Pharmacol Ther 2019; 195:21–38. [DOI] [PubMed] [Google Scholar]
- 127. Wang XH, Guo XJ, Li HY, Gou P. Characteristics of inositol phosphorylceramide synthase and effects of aureobasidin A on growth and pathogenicity of Botrytis cinerea. J Gen Appl Microbiol 2015; 61:108–16. [DOI] [PubMed] [Google Scholar]
- 128. Oliver JD, Sibley GE, Beckmann N, et al. F901318 represents a novel class of antifungal drug that inhibits dihydroorotate dehydrogenase. Proc Natl Acad Sci U S A 2016; 113:12809–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Hope WW, McEntee L, Livermore J, et al. Pharmacodynamics of the Orotomides against Aspergillus fumigatus: new opportunities for treatment of multidrug-resistant fungal disease. MBio 2017; 8:e01157-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Du Pré S, Beckmann N, Almeida MC, et al. Effect of the novel antifungal drug F901318 (olorofim) on growth and viability of Aspergillus fumigatus. Antimicrob Agents Chemother 2018; 62:e00231-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Law D, Birch M, Oliver J, et al. Pharmacokinetics of the novel antifungal agent F901318 in mice, rats and cynomolgus monkeys. 55th ICAAC (San Diego, CA). 17–21 September 2015. [Google Scholar]
- 132. Kennedy T, Graham A, Steiner J, et al. An open-label study in healthy volunteers to evaluate the potential for cytochrome P450 3A4 inhibition by F901318 using oral midazolam as a probe. ECCMID (Vienna, Austria). 22–25 April 2017. [Google Scholar]
- 133. Wiederhold NP, Law D, Birch M. Dihydroorotate dehydrogenase inhibitor F901318 has potent in vitro activity against Scedosporium species and Lomentospora prolificans. J Antimicrob Chemother 2017; 72:1977–80. [DOI] [PubMed] [Google Scholar]
- 134. Biswas C, Law D, Birch M, et al. In vitro activity of the novel antifungal compound F901318 against Australian Scedosporium and Lomentospora fungi. Med Mycol 2018; 56:1050–4. [DOI] [PubMed] [Google Scholar]
- 135. Müller C, Fietz C, Koehler P, et al. Reliable and easy-to-use liquid chromatography-tandem mass spectrometry assay for quantification of olorofim (F901318), a novel antifungal drug, in human plasma and serum. Antimicrob Agents Chemother 2018; 62:e01356-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Buil JB, Rijs AJ, Meis JF, et al. In vitro activity of the novel antifungal compound F901318 against difficult-to-treat Aspergillus isolates. J Antimicrob Chemother 2017; 72:2548–52. [DOI] [PubMed] [Google Scholar]
- 137. Rivero-Menendez O, Cuenca-Estrella M, Alastruey-Izquierdo A. In vitro activity of olorofim (F901318) against clinical isolates of cryptic species of Aspergillus by EUCAST and CLSI methodologies. J Antimicrob Chemother 2019; 74:1586–90. [DOI] [PubMed] [Google Scholar]
- 138. Jørgensen KM, Astvad KM, Hare RK, Arendrup MC. EUCAST determination of olorofim (F901318) susceptibility of mold species, method validation, and MICs. Antimicrob Agents Chemother 2018; 62:e00487-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Beckmann N, Binder U, Lass-Flörl C, et al. Activity of F901318, a member of the new orotomide class of antifungal agents, against clinical Aspergillus isolates from the UK and Austria. 55th ICAAC (San Diego, CA). 17–21 September 2015. [Google Scholar]
- 140. Lackner M, Birch M, Naschberger V, et al. Dihydroorotate dehydrogenase inhibitor olorofim exhibits promising activity against all clinically relevant species within Aspergillus section Terrei. J Antimicrob Chemother 2018; 73:3068–73. [DOI] [PubMed] [Google Scholar]
- 141. Seyedmousavi S, Chang YC, Law D, et al. Efficacy of olorofim (F901318) against Aspergillus fumigatus, A. Nidulans, and A. Tanneri in murine models of profound neutropenia and chronic granulomatous disease. Antimicrob Agents Chemother 2019; 63:e00129-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Negri CE, Johnson A, McEntee L, et al. Pharmacodynamics of the novel antifungal agent F901318 for acute sinopulmonary aspergillosis caused by Aspergillus flavus. J Infect Dis 2018; 217:1118–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Kennedy T, Allen G, Steiner J, et al. Multiple dose pharmacokinetics of an immediate-release tablet formulation of F901318 in healthy male and female subjects. 27th ECCMID (Vienna, Austria). 22–25 April 2017. [Google Scholar]
- 144. F2G. F2G expands investor syndicate and progresses Phase IIb study for novel antifungal. F2G News & Publications. Available at: https://www.f2g.com/news. Accessed 28 July 2019. [Google Scholar]
- 145. Nakamura I, Kanasaki R, Yoshikawa K, et al. Discovery of a new antifungal agent ASP2397 using a silkworm model of Aspergillus fumigatus infection. J Antibiot (Tokyo) 2017; 70:41–4. [DOI] [PubMed] [Google Scholar]
- 146. Nakamura I, Yoshimura S, Masaki T, et al. ASP2397: a novel antifungal agent produced by Acremonium persicinum MF-347833. J Antibiot (Tokyo) 2017; 70:45–51. [DOI] [PubMed] [Google Scholar]
- 147. Nakamura I, Ohsumi K, Takeda S, et al. ASP2397 is a novel natural compound that exhibits rapid and potent fungicidal activity against Aspergillus species through a specific transporter. Antimicrob Agents Chemother 2019; 63:e02689-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Kovanda LL, Sullivan SM, Smith LR, et al. Population pharmacokinetic modeling of VL-2397, a novel systemic antifungal agent: analysis of a single- and multiple-ascending-dose study in healthy subjects. Antimicrob Agents Chemother 2019; 63:e00163-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Wiederhold N, Najvar L, Jaramillo R, et al. The novel antifungal VL -2397 demonstrates efficacy in an in vivo model of invasive candidiasis caused by wild-type and multi-drug resistant Candida glabrata. ASM Microbe, New Orleans, LA,1–5 June 2017. [Google Scholar]
- 150. Mammen M, Armas D, Smith L, et al. Phase 1 safety and pharmacokinetics study of VL-2397, a novel antifungal agent. ASM Microbe; 2017. [Google Scholar]
- 151. Vical Pharmaceutics. Development of VL-2397 as an Antifungal Drug Candidate to Treat Invasive Fungal Infections. Available at: http://s1.q4cdn.com/508380786/files/doc_downloads/VL-2397_ASM_Microbe_2017/Sullivan_ASM_Microbe_2017_Oral_Presentation.pdf. Accessed 27 July 2019. [Google Scholar]
- 152. ClinicalTrials.gov. VL-2397 Compared to Standard First-Line Treatment for Invasive Aspergillosis (IA) in Adults. Available at: https://clinicaltrials.gov/ct2/show/NCT03327727. Accessed 27 July 2019. [Google Scholar]
- 153. Nishikawa H, Yamada E, Shibata T, et al. Uptake of T-2307, a novel arylamidine, in Candida albicans. J Antimicrob Chemother 2010; 65:1681–7. [DOI] [PubMed] [Google Scholar]
- 154. Shibata T, Takahashi T, Yamada E, et al. T-2307 causes collapse of mitochondrial membrane potential in yeast. Antimicrob Agents Chemother 2012; 56:5892–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Nishikawa H, Sakagami T, Yamada E, et al. T-2307, a novel arylamidine, is transported into Candida albicans by a high-affinity spermine and spermidine carrier regulated by Agp2. J Antimicrob Chemother 2016; 71:1845–55. [DOI] [PubMed] [Google Scholar]
- 156. Yamashita K, Miyazaki T, Fukuda Y, et al. The novel arylamidine T-2307 selectively disrupts yeast mitochondrial function by inhibiting respiratory chain complexes. Antimicrob Agents Chemother 2019; 63:e00374-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Mitsuyama J, Nomura N, Hashimoto K, et al. In vitro and in vivo antifungal activities of T-2307, a novel arylamidine. Antimicrob Agents Chemother 2008; 52:1318–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Nishikawa H, Fukuda Y, Mitsuyama J, et al. In vitro and in vivo antifungal activities of T-2307, a novel arylamidine, against Cryptococcus gattii: an emerging fungal pathogen. J Antimicrob Chemother 2017; 72:1709–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Wiederhold NP, Najvar LK, Fothergill AW, et al. The novel arylamidine T-2307 demonstrates in vitro and in vivo activity against echinocandin-resistant Candida glabrata. J Antimicrob Chemother 2016; 71:692–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Wiederhold NP, Najvar LK, Fothergill AW, et al. The novel arylamidine T-2307 maintains in vitro and in vivo activity against echinocandin-resistant Candida albicans. Antimicrob Agents Chemother 2015; 59:1341–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Yamada E, Nishikawa H, Nomura N, Mitsuyama J. T-2307 shows efficacy in a murine model of Candida glabrata infection despite in vitro trailing growth phenomena. Antimicrob Agents Chemother 2010; 54:3630–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Koselny K, Green J, Favazzo L, et al. Antitumor/antifungal celecoxib derivative AR-12 is a non-nucleoside inhibitor of the ANL-family adenylating enzyme acetyl CoA synthetase. ACS Infect Dis 2016; 2:268–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Baxter BK, DiDone L, Ogu D, et al. Identification, in vitro activity and mode of action of phosphoinositide-dependent-1 kinase inhibitors as antifungal molecules. ACS Chem Biol 2011; 6:502–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Koselny K, Green J, DiDone L, et al. The celecoxib derivative AR-12 has broad-spectrum antifungal activity in vitro and improves the activity of fluconazole in a murine model of cryptococcosis. Antimicrob Agents Chemother 2016; 60:7115–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Chabrier-Roselló Y, Gerik KJ, Koselny K, et al. Cryptococcus neoformans phosphoinositide-dependent kinase 1 (PDK1) ortholog is required for stress tolerance and survival in murine phagocytes. Eukaryot Cell 2013; 12:12–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Kushwaha AS, Sharma P, Shivakumar HN, et al. Trans-ungual delivery of AR-12, a novel antifungal drug. AAPS PharmSciTech 2017; 18:2702–5. [DOI] [PubMed] [Google Scholar]
- 167. Lamoth F, Juvvadi PR, Steinbach WJ. Histone deacetylase inhibition as an alternative strategy against invasive aspergillosis. Front Microbiol 2015; 6:96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Pfaller MA, Rhomberg PR, Messer SA, Castanheira M. In vitro activity of a Hos2 deacetylase inhibitor, MGCD290, in combination with echinocandins against echinocandin-resistant Candida species. Diagn Microbiol Infect Dis 2015; 81:259–63. [DOI] [PubMed] [Google Scholar]
- 169. Pfaller MA, Messer SA, Georgopapadakou N, et al. Activity of MGCD290, a Hos2 histone deacetylase inhibitor, in combination with azole antifungals against opportunistic fungal pathogens. J Clin Microbiol 2009; 47:3797–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. ClinicalTrials.gov. MGCD290 and Fluconazole Versus Fluconazole Alone for the Treatment of Moderate to Severe Vulvovaginal Candidiasis. Available at: https://clinicaltrials.gov/ct2/show/NCT01497223. Accessed 30 August 2019. [Google Scholar]
- 171. MethylGene I. MethylGene Reports Results of Phase II Trial of MGCD290. Available at: https://www.advfn.com/stock-market/TSX/MYG/stock-news/56785808/methylgene-reports-results-of-phase-ii-trial-of-mg. Accessed 30 August 2019. [Google Scholar]
- 172. Smilnak GJ, Charalambous LT, Cutshaw D, et al. Novel treatment of cryptococcal meningitis via neurapheresis therapy. J Infect Dis 2018; 218:1147–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. Day JN, Chau TT, Lalloo DG. Combination antifungal therapy for cryptococcal meningitis. N Engl J Med 2013; 368:2522–3. [DOI] [PubMed] [Google Scholar]
- 174. Blackburn SL, Swisher CB, Grande AW, et al. Novel dual lumen catheter and filtration device for removal of subarachnoid hemorrhage: first case report. Oper Neurosurg (Hagerstown) 2019; 16:E148–53. [DOI] [PubMed] [Google Scholar]
- 175. Ejikeme T, Chongsathidkiet P, Ballard C, et al. A Novel Therapeutic Approach for Leptomeningeal Metastases. Mayo Clinic Neuroscience and Oncology Innovation Summit (Orlando, FL). 14-16 December 2017. [Google Scholar]
- 176. Ballard C, Ashraf B, Ejikeme T, et al. Feasibility of NeurapheresisTM as a therapy for multidrug resistant Gram-negative bacterial meningitis. IDWeek (San Diego, CA). 4–8 October 2017. [Google Scholar]
- 177. Enoch DA, Yang H, Aliyu SH, Micallef C. The changing epidemiology of invasive fungal infections. Methods Mol Biol 2017;1508:17–65. [DOI] [PubMed] [Google Scholar]
- 178. Julius RJ, Novitsky MA Jr, Dubin WR. Medication adherence: a review of the literature and implications for clinical practice. J Psychiatr Pract 2009; 15:34–44. [DOI] [PubMed] [Google Scholar]
- 179. Krueger KP, Berger BA, Felkey B. Medication adherence and persistence: a comprehensive review. Adv Ther 2005; 22:313–56. [DOI] [PubMed] [Google Scholar]