Abstract
With the introduction of population-based bowel cancer screening, rectal cancer is diagnosed at earlier stages, yet standard treatment still requires the same extensive surgery that is used for more advanced stages. Organ preserving treatment is rapidly developing and is subject of investigation in numerous clinical trials. The STAR-TREC trial is an international, multi-centre randomised trial investigating organ preservation using (chemo)radiotherapy. Patients with small mrT1-3bN0V0M0 tumours are randomized between three arms: standard TME, organ preservation with SCRT or with CRT. In this trial, the clinical target volume has been tailored to the early staged disease of the included patients. This mesorectal irradiation volume includes the mesorectum and pre-sacral lymph nodes at the level of the tumour, two centimetres below and cranially up to the S2-3 interspace level. In contrast to conventional irradiation volumes, the lateral lymph nodes and the nodes along the superior rectal artery are excluded. As a result, the dose to the bowel, bladder, anal sphincter and the neurovascular plexus in the lower pelvis is substantially decreased, especially when combined with modern irradiation techniques, such as dynamic arc therapy. These lower doses are expected to lead to decreasing acute and late toxicity and beneficial functional outcomes. The implementation of this novel target volume will be accompanied by an extensive quality assurance program in the STAR-TREC trial. We describe the rationale behind the novel, mesorectal only radiotherapy treatment used in the STAR-TREC trial specifically tailored for early stage disease, with the goal of organ preservation.
Abbreviations: OP, organ preservation; WG, working group; RO, radiation oncologist
Keywords: Rectal neoplasms, Radiotherapy, Clinical target volumes, Organ Preservation
1. Introduction
Early stage rectal cancer has a favourable prognosis for patients treated with total mesorectal excision (TME) [1]. Only 2% and 12% of patients experience local or distant failure [2], [3], [4]. However, resection of a low rectal tumour requires a permanent stoma in approximately 40% of cases while many more patients will have a temporary stoma [5], [6], [7]. Complications of surgical resection include anastomotic leaks, autonomic nerve damage leading to urinary incontinence or retention, sexual dysfunction and faecal incontinence. Therefore, reconsidering TME as standard of care is a high research priority. There is a need for less invasive and toxic strategies, accompanied by good functional outcomes. In the last decade, research focus has shifted towards limited resections and active surveillance of good responders after chemoradiotherapy (CRT) [8], [9], [10], [11].
The STAR-TREC trial explores whether primary short-course radiotherapy (SCRT) or CRT followed by a two-stage response assessment with selective use of local excision, is a safe alternative to TME surgery (ClinicalTrials.gov Identifier: NCT02945566) [12]. Patients with small mrT1-3bN0V0M0 tumours are randomized between three arms: standard TME, organ preservation (OP) with SCRT or with CRT [12]. Patients in the OP arms with a complete clinical response (cCR) enter active surveillance without further treatment. For patients with a good partial response the residual disease will be locally excised, while in poor responders a TME resection is advocated.
In early stage tumours (C)RT can lead to OP in more than 50% when combined with local excision [13], [14], [15], [16]. Unfortunately, this (C)RT can be accompanied by toxicity, morbidity and even mortality [14], [15], [17]. However, all available data on toxicity and mortality, are based on conventional radiotherapy techniques, with large treatment volumes, including large elective LN regions. In early stage rectal cancers, such as those included in the STAR-TREC trial, a smaller tailored CTV is expected to be oncologically safe. This paper describes the rationale for such a CTV and provides delineation guidelines as used in the trial.
2. Materials and methods
2.1. Participants
A working group (WG) of three experienced radiation oncologists (ROs), three junior ROs and a medical physicist reviewed current literature and discussed the rationale of mesorectal radiotherapy. All ROs were involved in design of the STAR-TREC trial and experienced in rectal cancer treatment. The trials surgical principal investigators were involved in defining the anatomical boundaries of the clinical CTV. The WG focused on the definition and delineation of the CTV; delineation of the gross tumour volume (GTV) was beyond the scope of this project.
The open source software CERR [18] as well as Oncentra Masterplan v4.1 (Elekta AB) were used to support the consensus process and to facilitate the validation process by teleconference discussions.
2.2. Consensus process
During a kick-off meeting, the WG defined a roadmap to develop consensus guidelines on CTV delineation:
-
1.
Review of available literature and pelvic anatomy.
-
2.
Creation consensus CTV proposal.
-
3.
Selection of two eligible cases with different tumour location (mid-rectum and distal).
-
4.
Delineation of first case by all ROs.
-
5.
Discussion of results of first delineation.
-
6.
Re-delineation of re-defined consensus by all ROs.
-
7.
Discussion of results of second delineation.
-
8.
Delineation of second case by all ROs.
-
9.
Discussion of delineation of case 2 and validation of final consensus guidelines.
-
10.
Completion of delineation atlas.
3. Results
Patients eligible for participation in the STAR-TREC trial are patients with small (<4 cm) mrT1-3bN0V0M0 tumours without involvement of the mesorectal fascia (MRF) or extra-mural vascular invasion (EMVI). The a priori risk of nodal involvement, especially outside the mesorectum, in these patients is substantially lower than in patients with more advanced stages. The recommended CTV for rectal cancer normally includes the GTV and elective nodal irradiation of the presacral space, mesorectum, internal iliac nodes and if indicated, obturator nodes, external iliac nodes and ischiorectal fossa [19]. Careful evaluation of the sites at risk of nodal spread in the group eligible for inclusion can therefore facilitate reduction of the CTV.
3.1. Sites of nodal involvement – mesorectum
Patients with low risk early rectal cancers do not undergo preoperative radiotherapy in most countries. Local control rate of 98% after surgery in this group underlines that removal of only the mesorectal envelope is sufficient in most patients [2], [3], [4]. Irradiating only this mesorectal envelope should therefore also be sufficient.
In series evaluating LN distribution in resection specimens, it has been shown that most nodal metastases are located peri-tumoural in the mesorectum at the level of or a few centimetres proximal of the tumour. Metastases distal from the tumour are very rare. In an analysis of MRI and histopathological evaluation of mesorectal LNs in 16 patients with T1-3 rectal cancer, 97% of all 134 mesorectal LNs (benign and malignant) were located within 6 mm below and up to 5 cm proximal from the tumour [20]. None of the malignant mesorectal LNs (n = 12) were located below the tumour. Another study found 2 malignant LNs within 15 mm below the tumour, but both patients had numerous LN metastases at the level of the tumour [21]. Wang et al. found tumour deposits up to 3 cm distal from the primary tumour in 4/62 patients, but only in patients with stage III disease [22]. They demonstrated that the outer circumferential area of the mesorectum was involved in 38,7% of all patients, underlining the importance of treating the whole circumferential mesorectal tissue [23].
3.2. Sites of nodal involvement – common iliac and pre-sacral LNs
Recurrence pattern analyses have shown that in low and middle rectal tumours without clinically node positive disease, the cranial border can be lower than in conventional fields. Nijkamp et al. showed that in the TME trial only one of 58 pN0 patients had a recurrence just above the S2-3 interspace plane [24]. Syk et al. found no recurrences above the level of S1-2 [25]. Lowering the cranial border in low and middle rectal cancer without nodal disease has recently been implemented in the conventional CTV [19] and is certainly indicated in the primary organ preservation population.
3.3. Sites of nodal involvement – lateral LNs
In literature, varying definitions are used including only the obturator LNs, the internal iliac nodes or the combination. The recent delineation guideline by Valentini et al. defines the lateral LN region as the triangular lympho-vascular area located between the pelvic wall and the mesorectum, containing the lymphatic vessels and the nodes along the internal iliac and obturator vessels [19]. Roels et al. [26] reported a rate of lateral LN involvement (defined as LN along the middle rectal, the obturator, and the internal iliac vessels) of 5% in T1-2 tumours and 14% in T3 tumours. The overall rate was higher in patients with nodal involvement in other more prevalent areas. Socha et al. investigated the rate of lateral lymph node metastasis in pT2 tumours treated with resection only. They found a rate of 8.2% in distal tumours, located below the peritoneal fold, and 0% in tumours above the peritoneal fold [27]. It is, however, unclear what the clinical staging of these patients was. The rate is probably lower in tumours staged as N0 on MRI. In addition, these lymph node metastases, when present, clearly do not always result in disease recurrence, since the rate of lateral recurrences in T2 tumours with TME resection alone is very low, see below.
Vuong et al. reported on ~500 patients with T2-3 tumours with threatened circumferential margin, treated with brachytherapy, followed by TME [28]. Unfortunately, the group was inhomogeneous, since node positive patients received post-operative CRT and an unknown number of patients received adjuvant chemotherapy. Still, treating only a limited amount of mesorectal tissue directly around the tumour resulted in a low combined luminal and nodal recurrence rate of 4.8%.
3.4. Local recurrences after TME surgery
Analysis of local recurrence pattern in patients from the TME trial, including patients with LARC, showed lateral recurrences in 1,9% (n = 23) of the non-irradiated patients [29]. Of those, 18 had one or more risk factors, such as stage IV disease, pT4, pN2 or an involved CRM after surgery. In 996 patients without risk factors, only 5 patients (0,5%) developed lateral recurrence. Analysis of 934 non-irradiated patients undergoing TME, only 3 (0,3%) developed a recurrence in a lateral LN [25]. These data indicate that in early staged cT1-3bN0 patients the lateral LN region is not at risk.
A consistent finding is that the pre-sacral region is at considerable risk for local recurrence in all stages and tumour locations and should always be included in the radiation fields [24], [25], [26], [29].
3.5. Local recurrences after TEM surgery
In a series of 100 patients treated for low risk pT1 tumours, 14 local recurrences were reported [30]. All were intraluminal and 11/14 could be attributed to local tumour regrowth. Concomitant nodal involvement was present in 4/11 operated recurrences. In 144 patients with cT1-cT3, 44 local recurrences occurred [31]. After salvage surgery, histology showed that 24/26 recurrences were endoluminal.
This demonstrates that most recurrences after local excision are endoluminal, rarely accompanied by mesorectal nodal disease. Metastases in obturator or iliac lymph nodes were not reported. Therefore, extending the radiotherapy fields beyond the mesorectal fat seems unnecessary in this selected patient group.
In summary, there is adequate evidence in the literature that conventional clinical CTV are not required in small cT1-3bN0 tumours, and a smaller CTV can be treated, including only the mesorectum and pre-sacral region at the level of the tumour and a few centimetres caudally and cranially to the level of the S2-3 interspace.
4. Mesorectal delineation guidelines
4.1. GTV
Only the macroscopic primary tumour is delineated. It is strongly recommended that MRI images are available during delineation.
4.2. CTV
The CTV includes the mesorectum considered at risk for LN involvement and the pre-sacral LN at the same level. MRI should be used to aid. Since all tumours should be amenable for local excision, proximal anterior located tumours (above peritoneal fold) and distal tumours with extension in the anal canal are not eligible for inclusion in the STAR-TREC trial. The delineation guidelines are, therefore, not applicable for these locations.
On each slice, the mesorectum is delineated circumferentially:
4.2.1. Superior limit
-
•
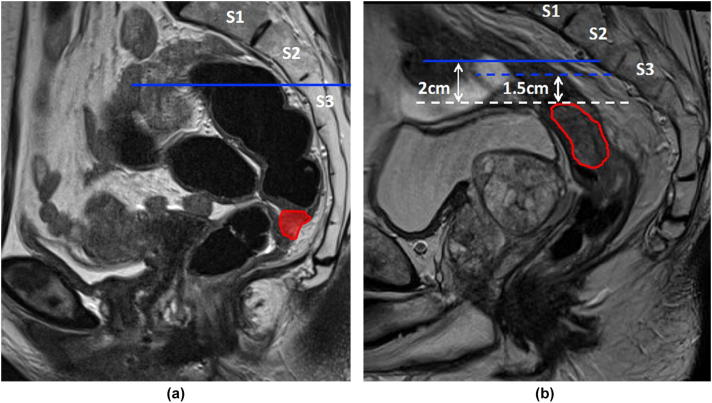
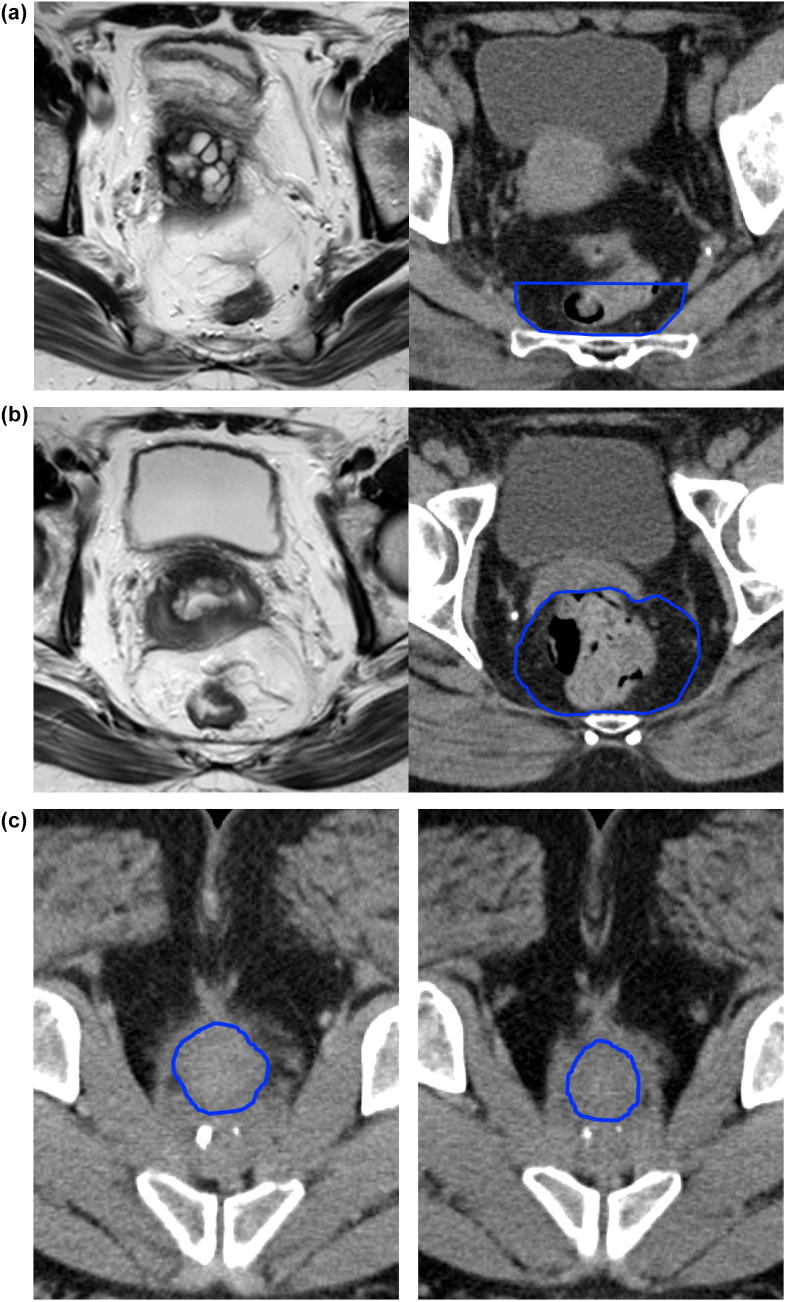
S2/S3 interspace (determined on the sagittal view). A horizontal line from the anterior edge of the S2/3 interspace (Fig. 1a).
-
•
A minimum of 2 cm is required from the superior limit of the GTV to the CTV. In proximal tumours, this may require an extension above the S2/3 interspace (Fig. 1b).
Fig 1.
(a and b) Superior limit mesorectal target volume.
4.2.2. Inferior limit
-
•
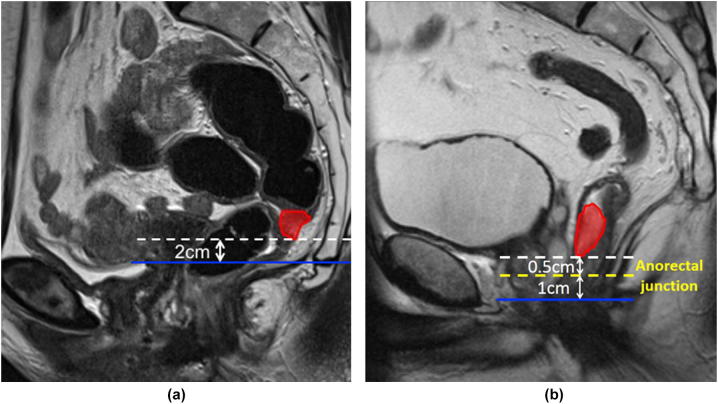
Two cm inferiorto the GTV (Fig. 2a).
In low tumours, where a 2 cm margin extends into the anal canal, this margin is reduced to 1 cm, ie. a maximum of 1 cm of the upper anal canal is delineated (Fig. 2b).
Fig 2.
(a and b) Inferior limit mesorectal target volume.
4.2.3. Anterior limit
-
•
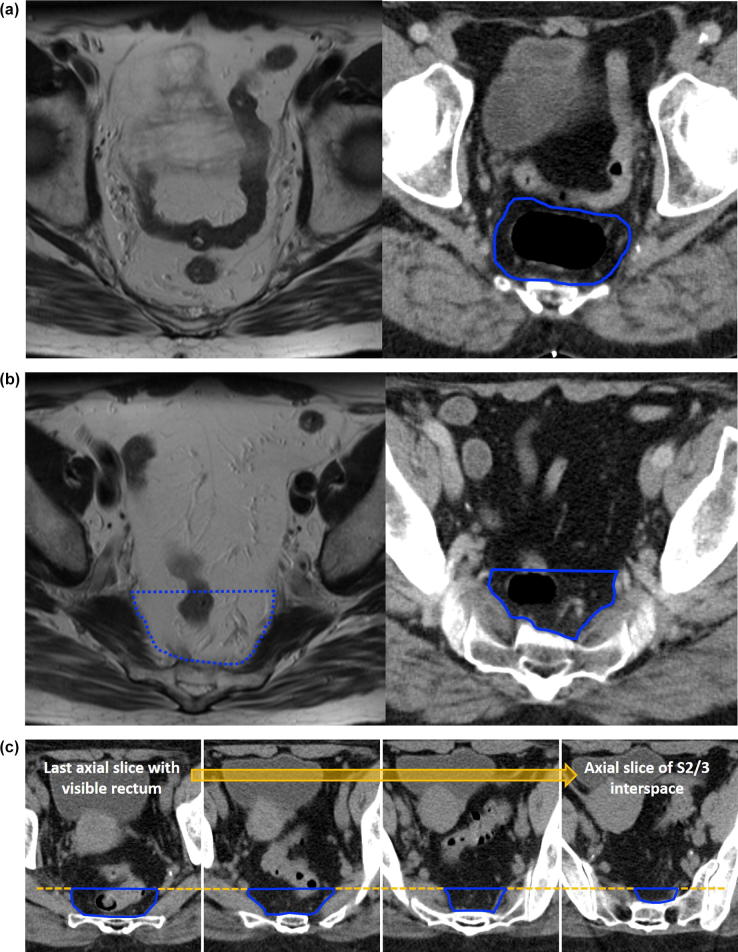
The MRF is contoured (Fig. 3a).
-
•
If the MRF disappears anteriorly, the anterior border is the anterior rectal wall (Fig. 3b).
-
•
For cranial slices with no visible rectum, the anterior border is defined by the contour used for the last cranial slice with visible rectum (Fig. 3c).
Fig 3.
(a–c) Anterior limit mesorectal target volume.
4.2.4. Posterior limit
The anterior margin of the sacrum or coccyx, or the inner border of the puborectalis muscle in caudal slices, to include the pre-sacral LN (Fig. 4a-c).
Fig 4.
(a–c) Posterior limit mesorectal target volume.
4.2.5. Lateral limit
-
•
The MRF is contoured.
-
•
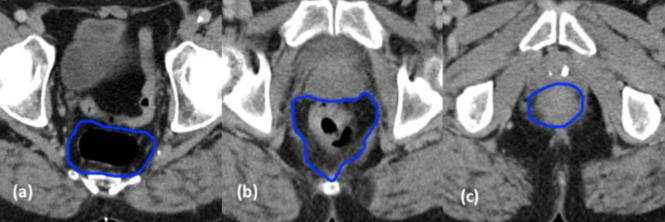
High pelvis – If the MRF disappears laterally, the inner border of the pyriformis muscle is contoured (Fig. 5a).
-
•
Mid pelvis – The MRF (Fig. 5b).
-
•
Low pelvis – The inner border of the puborectalis muscle as it converges to form the anorectal ring (Fig. 5c). When including the anal canal, only the inner sphincter is delineated, the levator ani and external sphincters are excluded (Fig. 5d).
Fig 5.
(a–d) Lateral limit mesorectal target volume.
4.3. PTV
Required PTV margins are highly dependable of local techniques, such as positioning and position verification. A strict instruction is, therefore, not in place. Within the STAR-TREC trial we recommend supine positioning and daily online position verification. In that case, a PTV margin of at least 1.5 cm anteriorly and 1 cm in all other directions should be used (according to local experience, a smaller PTV margin in the direction of the sacrum can be considered). If less than daily online position verification is used or, for example, the patient is in prone position without belly board, appropriate extra PTV margin should be added.
5. Discussion
With introduction of population-based screening for bowel cancer, incidence of early staged rectal cancer has risen substantially. Organ preservation in these early tumours warrants rethinking of conventional radiotherapy CTVs, since these patients have excellent overall survival rates, making long-term functional outcome even more important. There are no current guidelines for this specific patient category [19]. We describe the rationale for a mesorectal only CTV specifically aiming at OP in early staged disease, i.e. patients with small (<4 cm) cT1-3bN0M0 tumours without EMVI or involvement of the MRF. Irradiation of this CTV is introduced and investigated in the ongoing STAR-TREC trial, accompanied by an extensive quality assurance program [12].
The mesorectal CTV includes the mesorectum and pre-sacral lymph nodes at the level of the tumour, 2 cm below and cranially up to the S2-3 interspace level. The lateral LNs and the nodes along the superior rectal artery (SRA) are excluded, in contrast to conventional CTV.
The proposed CTV is largely corresponding with the recently proposed volumes by Socha et al. [27]. In this systematic review and meta-analysis the authors investigated the extent of extent of distal mesorectal (DMS) and distal intramural spread (DIS), the risk of lateral lymph node (LLN) metastases in pT2 tumours, and regional recurrence pattern after organ preservation, in order to propose an adapted CTV for pT2 rectal cancer. The only discrepancy between the two papers is the inclusion of the lateral lymph node region. Based on a lymph node metastasis rate in the lateral lymph nodes (internal iliac and obturator nodes) of 8.2% in tumours below the peritoneal fold, they advise to include the LLN in distal tumours. We advise not to include the LLN in cT1-3N0 tumours, based on the very low recurrence rate (<1%) in the LLN after TME resection alone. This discrepancy, could be a result of multiple causes. First of all, the rate found by Socha is based on surgical series of patients treated with resection with lateral lymph adenectomy without neo-adjuvant treatment. They selected the pT2 patients, which does not encompass the same selection as cT1-3bN0 MRF- EMVI- patients for whom we propose this CTV. The pT2 patient group will probably also include patients with cN+ status and other adverse features, such as EMVI. Secondly, it is possible that not all tumour cells that can be found in lymph nodes will always develop into recurrence if left untreated, for example due to immunological phenomena. Any risks of excluding the LLN region will become clear from the STAR-TREC data.
The differences between the resection volume with TME resection, conventional CTV based on the Valentini guideline, the CTV described in this paper and the Socha CTV are shown in Table 1.
Table 1.
Anatomic subsites included in CTV or resection volume for cT1-3bN0 rectal cancer.
| TME resection | Valentini [19] | STAR-TREC | Socha [27] | |
|---|---|---|---|---|
| Mesorectum | + | + | +, <S2-3 | +, <S2-3 |
| Presacral Nodes, pelvic | – | + | +, <S2-3 | +, <S2-3 |
| LLN post. = internal iliac LN | – | + | – | +, If < peritoneal fold |
| LLN ant. = obturator LN | – | – | – | +, If < peritoneal fold |
| Sphincter Complex | – | – | – | – |
| External Iliac Nodes | – | – | – | – |
| Ischiorectal Fossa | – | – | – | – |
| Inguinal Nodes | – | – | – | – |
| Presacral Nodes, abdominal | – | – | – | – |
TME: total mesorectal excision, LLN: lateral lymph nodes, LN: lymph nodes.
5.1. Expected benefits for toxicity
Small bowel dosages are known to be associated with acute and late bowel toxicity in conventional radiotherapy [32], [33], although this might be less pronounced with advanced radiation techniques [34].
Mesorectum only radiotherapy results in 58% smaller CTVs when compared to conventional CTVs [35], mainly cranially and anteriorly, ensuring smaller volumes of irradiated small bowel and probably less acute and late toxicity.
In addition, this smaller volume will decrease the dose to urogenital structures such as ureters, bladder and neurovascular plexus. Lower doses to bladder, bladder trigone, vagina and lumbo-sacral plexus have been shown to be associated with better functional and HRQOL outcomes in gynaecological cancer patients [36]. For rectal cancer, there is little data available. Analysis of the toxicity data in the STAR-TREC study population will provide this valuable information.
5.2. Possible risks of mesorectal target volume
The described mesorectal CTV is mainly based on expert opinion and review of the limited available literature. Since the introduction of this CTV within the trial is guided by standardised QA and meticulous follow-up of patients, reliable data regarding patterns of pelvic failure will become available. Use of a mesorectal CTV outside trial setting is not recommended until the STAR-TREC data will be available.
The theoretical risk of this substantially smaller irradiated volume is that it may result in an increase of recurrences in the lateral LNs or nodes along the SRA, although this is not supported by data from literature.
Literature on recurrences after a wait and see policy with standard chemoradiotherapy has shown that salvage TME resection is possible and leads to good results [8]. Currently it is unclear whether reduced treatment volumes will influence these results negatively, but given that most recurrences occur intraluminally, this would seem unlikely. The STAR-TREC trial results are expected to answer these questions.
5.3. Quality assurance (QA) program
Any new treatment would benefit from a QA program, to ensure correct and reproducible implementation over different centres. Reproducibility of delineation is important to ensure homogeneity of delineations within trials, but also to ensure consistent daily clinical practice. Only then clinical outcomes and toxicity can be compared. Quality of delineation is even more important when using modern irradiation techniques, such as dynamic arc therapy, because of the conformality of the high dose volume [37].
To ensure correct and consistent implementation of this novel mesorectal only CTV in all participating centres of the STAR-TREC trial, an extensive program with pre-trial workshops and direct feedback after patient inclusion has been set up.
6. Conclusions
The STAR TREC trial facilitates the controlled introduction of a novel CTV for good prognosis, early rectal cancer. This will enable the collection of high-quality data on treatment toxicity and organ preservation efficacy. Treatment of this novel target volume should preferably be combined with modern (IMRT/VMAT) treatment techniques for optimal normal tissue sparing. Recurrence patterns from the trial will inform us on the safety of this mesorectal CTV and ultimately, facilitate further treatment refinement to achieve optimum oncological efficacy with the lowest toxicity and best functional outcomes for our early rectal cancer patients.
Funding source
The STAR-TREC trial is funded in the UK by Cancer Research UK (C41557/A19393 and C41557/A28538), in the Netherlands by the Dutch Cancer Society (KWF KUN 2014-7448), and in Denmark by the Danish Cancer Society (R100-A6747 and R231-A14087). AA is supported by Yorkshire Cancer Research Academic Fellowship funding (grant L389AA). MT was supported by Academy of Medical Sciences Starter Grant for Clinical Lecturers. None of the funders have had any involvement in the study design; in the collection, analysis and interpretation of data; in the writing of the report; or in the decision to submit the article for publication.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Contributor Information
Femke P. Peters, Email: f.peters@nki.nl.
Mark T.W. Teo, Email: M.T.W.Teo@leeds.ac.uk.
Ane L. Appelt, Email: A.L.Appelt@leeds.ac.uk.
Simon Bach, Email: S.P.Bach@bham.ac.uk.
Gunnar Baatrup, Email: Gunnar.Baatrup@rsyd.dk.
Johannes H.W. de Wilt, Email: Hans.deWilt@radboudumc.nl.
Camilla Jensenius Kronborg, Email: cam.kro@auh.rm.dk.
Karen-Lise Garm Spindler, Email: K.G.Spindler@rm.dk.
Corrie A.M. Marijnen, Email: c.marijnen@nki.nl.
David Sebag-Montefiore, Email: D.SebagMontefiore@leeds.ac.uk.
References
- 1.Brouwer N.P.M., Bos A., Lemmens V., Tanis P.J., Hugen N., Nagtegaal I.D. An overview of 25 years of incidence, treatment and outcome of colorectal cancer patients. Int J Cancer. 2018;143:2758–2766. doi: 10.1002/ijc.31785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bentrem D.J., Okabe S., Wong W.D., Guillem J.G., Weiser M.R., Temple L.K. T1 adenocarcinoma of the rectum: transanal excision or radical surgery? Ann Surg. 2005;242 doi: 10.1097/01.sla.0000183355.94322.db. 472-7; discussion 7-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.van Gijn W., Marijnen C.A.M., Nagtegaal I.D., Kranenbarg E.M.-K., Putter H., Wiggers T. Preoperative radiotherapy combined with total mesorectal excision for resectable rectal cancer: 12-year follow-up of the multicentre, randomised controlled TME trial. Lancet Oncol. 2011;12:575–582. doi: 10.1016/S1470-2045(11)70097-3. [DOI] [PubMed] [Google Scholar]
- 4.Endreseth B.H., Myrvold H.E., Romundstad P., Hestvik U.E., Bjerkeset T., Wibe A. Transanal excision vs. major surgery for T1 rectal cancer. Dis Colon Rectum. 2005;48:1380–1388. doi: 10.1007/s10350-005-0044-6. [DOI] [PubMed] [Google Scholar]
- 5.Peeters K.C., van de Velde C.J., Leer J.W., Martijn H., Junggeburt J.M., Kranenbarg E.K. Late side effects of short-course preoperative radiotherapy combined with total mesorectal excision for rectal cancer: increased bowel dysfunction in irradiated patients–a Dutch colorectal cancer group study. J Clin Oncol. 2005;23:6199–6206. doi: 10.1200/JCO.2005.14.779. [DOI] [PubMed] [Google Scholar]
- 6.Erlandsson J., Holm T., Pettersson D., Berglund Å., Cedermark B., Radu C. Optimal fractionation of preoperative radiotherapy and timing to surgery for rectal cancer (Stockholm III): a multicentre, randomised, non-blinded, phase 3, non-inferiority trial. Lancet Oncol. 2017;18:336–846. doi: 10.1016/S1470-2045(17)30086-4. [DOI] [PubMed] [Google Scholar]
- 7.Guckenberger M., Saur G., Wehner D., Thalheimer A., Kim M., Germer C.T. Long-term quality-of-life after neoadjuvant short-course radiotherapy and long-course radiochemotherapy for locally advanced rectal cancer. Radiother Oncol. 2013;108:326–330. doi: 10.1016/j.radonc.2013.08.022. [DOI] [PubMed] [Google Scholar]
- 8.Habr-Gama A., Gama-Rodrigues J., Sao Juliao G.P., Proscurshim I., Sabbagh C., Lynn P.B. Local recurrence after complete clinical response and watch and wait in rectal cancer after neoadjuvant chemoradiation: impact of salvage therapy on local disease control. Int J Radiat Oncol Biol Phys. 2014;88:822–828. doi: 10.1016/j.ijrobp.2013.12.012. [DOI] [PubMed] [Google Scholar]
- 9.Glynne-Jones R., Hughes R. Critical appraisal of the 'wait and see' approach in rectal cancer for clinical complete responders after chemoradiation. Br J Surg. 2012;99:897–909. doi: 10.1002/bjs.8732. [DOI] [PubMed] [Google Scholar]
- 10.Marijnen C.A.M. Organ preservation in rectal cancer: have all questions been answered? Lancet Oncol. 2015;16:e13–e22. doi: 10.1016/S1470-2045(14)70398-5. [DOI] [PubMed] [Google Scholar]
- 11.Stijns R.C.H., Tromp M.R., Hugen N., de Wilt J.H.W. Advances in organ preserving strategies in rectal cancer patients. Eur J Surg Oncol. 2018;44:209–219. doi: 10.1016/j.ejso.2017.11.024. [DOI] [PubMed] [Google Scholar]
- 12.Rombouts A.J.M., Al-Najami I., Abbott N.L., Appelt A., Baatrup G., Bach S. Can we Save the rectum by watchful waiting or TransAnal microsurgery following (chemo) Radiotherapy versus Total mesorectal excision for early REctal Cancer (STAR-TREC study)?: protocol for a multicentre, randomised feasibility study. BMJ Open. 2017;7 doi: 10.1136/bmjopen-2017-019474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Verseveld M., de Graaf E.J., Verhoef C., van Meerten E., Punt C.J., de Hingh I.H. Chemoradiation therapy for rectal cancer in the distal rectum followed by organ-sparing transanal endoscopic microsurgery (CARTS study) Br J Surg. 2015;102:853–860. doi: 10.1002/bjs.9809. [DOI] [PubMed] [Google Scholar]
- 14.Smart C.J., Korsgen S., Hill J., Speake D., Levy B., Steward M. Multicentre study of short-course radiotherapy and transanal endoscopic microsurgery for early rectal cancer. Br J Surg. 2016;103:1069–1075. doi: 10.1002/bjs.10171. [DOI] [PubMed] [Google Scholar]
- 15.Rullier E., Rouanet P., Tuech J.-J., Valverde A., Lelong B., Rivoire M. Organ preservation for rectal cancer (GRECCAR 2): a prospective, randomised, open-label, multicentre, phase 3 trial. The Lancet. 2017;390:469–479. doi: 10.1016/S0140-6736(17)31056-5. [DOI] [PubMed] [Google Scholar]
- 16.Garcia-Aguilar J., Shi Q., Thomas C.R., Jr., Chan E., Cataldo P., Marcet J. A phase II trial of neoadjuvant chemoradiation and local excision for T2N0 rectal cancer: preliminary results of the ACOSOG Z6041 trial. Ann Surg Oncol. 2012;19:384–391. doi: 10.1245/s10434-011-1933-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stijns R.C.H., de Graaf E.J.R., Punt C.J.A., Nagtegaal I.D., Nuyttens J., van Meerten E. Long-term oncological and functional outcomes of chemoradiotherapy followed by organ-sparing transanal endoscopic microsurgery for distal rectal cancer: the CARTS study. JAMA Surg. 2018 doi: 10.1001/jamasurg.2018.3752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Deasy J.O., Blanco A.I., Clark V.H. CERR: a computational environment for radiotherapy research. Med Phys. 2003;30:979–985. doi: 10.1118/1.1568978. [DOI] [PubMed] [Google Scholar]
- 19.Valentini V., Gambacorta M.A., Barbaro B., Chiloiro G., Coco C., Das P. International consensus guidelines on Clinical Target Volume delineation in rectal cancer. Radiother Oncol. 2016;120:195–201. doi: 10.1016/j.radonc.2016.07.017. [DOI] [PubMed] [Google Scholar]
- 20.Koh D.M., Brown G., Temple L., Blake H., Raja A., Toomey P. Distribution of mesorectal lymph nodes in rectal cancer: in vivo MR imaging compared with histopathological examination. Initial observations. Eur Radiol. 2005;15:1650–1657. doi: 10.1007/s00330-005-2751-8. [DOI] [PubMed] [Google Scholar]
- 21.Engelen S.M., Beets-Tan R.G., Lahaye M.J., Kessels A.G., Beets G.L. Location of involved mesorectal and extramesorectal lymph nodes in patients with primary rectal cancer: preoperative assessment with MR imaging. Eur J Surg Oncol. 2008;34:776–781. doi: 10.1016/j.ejso.2007.10.007. [DOI] [PubMed] [Google Scholar]
- 22.Wang Z., Zhou Z., Wang C., Zhao G., Chen Y., Gao H. Microscopic spread of low rectal cancer in regions of the mesorectum: detailed pathological assessment with whole-mount sections. Int J Colorectal Dis. 2005;20:231–237. doi: 10.1007/s00384-004-0674-5. [DOI] [PubMed] [Google Scholar]
- 23.Wang Z., Zhou Z.G., Wang C., Zheng X.L., Wang R., Li F.Y. Regional micrometastasis of low rectal cancer in mesorectum: a study utilizing HE stain on whole-mount section and ISH analyses on tissue microarray. Cancer Invest. 2006;24:374–381. doi: 10.1080/07357900600705300. [DOI] [PubMed] [Google Scholar]
- 24.Nijkamp J., Kusters M., Beets-Tan R.G., Martijn H., Beets G.L., van de Velde C.J. Three-dimensional analysis of recurrence patterns in rectal cancer: the cranial border in hypofractionated preoperative radiotherapy can be lowered. Int J Radiat Oncol Biol Phys. 2011;80:103–110. doi: 10.1016/j.ijrobp.2010.01.046. [DOI] [PubMed] [Google Scholar]
- 25.Syk E., Torkzad M.R., Blomqvist L., Nilsson P.J., Glimelius B. Local recurrence in rectal cancer: anatomic localization and effect on radiation target. Int J Radiat Oncol Biol Phys. 2008;72:658–664. doi: 10.1016/j.ijrobp.2008.01.063. [DOI] [PubMed] [Google Scholar]
- 26.Roels S., Duthoy W., Haustermans K., Penninckx F., Vandecaveye V., Boterberg T. Definition and delineation of the clinical target volume for rectal cancer. Int J Radiat Oncol Biol Phys. 2006;65:1129–1142. doi: 10.1016/j.ijrobp.2006.02.050. [DOI] [PubMed] [Google Scholar]
- 27.Socha J., Pietrzak L., Zawadzka A., Paciorkiewicz A., Krupa A., Bujko K. A systematic review and meta-analysis of pT2 rectal cancer spread and recurrence pattern: implications for target design in radiation therapy for organ preservation. Radiother Oncol. 2019;133:20–27. doi: 10.1016/j.radonc.2018.12.024. [DOI] [PubMed] [Google Scholar]
- 28.Vuong T., Devic S. High-dose-rate pre-operative endorectal brachytherapy for patients with rectal cancer. J Contemp Brachytherapy. 2015;7:183–188. doi: 10.5114/jcb.2015.51402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kusters M., Marijnen C.A., van de Velde C.J., Rutten H.J., Lahaye M.J., Kim J.H. Patterns of local recurrence in rectal cancer; a study of the Dutch TME trial. Eur J Surg Oncol. 2010;36:470–476. doi: 10.1016/j.ejso.2009.11.011. [DOI] [PubMed] [Google Scholar]
- 30.Junginger T., Goenner U., Hitzler M., Trinh T.T., Heintz A., Roth W. Analysis of local recurrences after transanal endoscopic microsurgery for low risk rectal carcinoma. Int J Colorectal Dis. 2017;32:265–271. doi: 10.1007/s00384-016-2715-2. [DOI] [PubMed] [Google Scholar]
- 31.Stipa F., Giaccaglia V., Burza A. Management and outcome of local recurrence following transanal endoscopic microsurgery for rectal cancer. Dis Colon Rectum. 2012;55:262–269. doi: 10.1097/DCR.0b013e318241ef22. [DOI] [PubMed] [Google Scholar]
- 32.Chopra S., Dora T., Chinnachamy A.N., Thomas B., Kannan S., Engineer R. Predictors of grade 3 or higher late bowel toxicity in patients undergoing pelvic radiation for cervical cancer: results from a prospective study. Int J Radiat Oncol Biol Phys. 2014;88:630–635. doi: 10.1016/j.ijrobp.2013.11.214. [DOI] [PubMed] [Google Scholar]
- 33.Banerjee R., Chakraborty S., Nygren I., Sinha R. Small bowel dose parameters predicting grade >/= 3 acute toxicity in rectal cancer patients treated with neoadjuvant chemoradiation: an independent validation study comparing peritoneal space versus small bowel loop contouring techniques. Int J Radiat Oncol Biol Phys. 2013;85:1225–1231. doi: 10.1016/j.ijrobp.2012.09.036. [DOI] [PubMed] [Google Scholar]
- 34.Xu B., Guo Y., Chen Y., Lu H., Tang T., Yue Z. Is the irradiated small bowel volume still a predictor for acute lower gastrointestinal toxicity during preoperative concurrent chemo-radiotherapy for rectal cancer when using intensity-modulated radiation therapy? Radiat Oncol. 2015;10:257. doi: 10.1186/s13014-015-0566-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Appelt A.L., Teo M., Christophides D., Peters F.P., Lilley J., Spindler K.L.G. EP-1748: mesorectal-only irradiation for early stage rectal cancer: target volumes and dose to organs at risk. Radiother Oncol. 2017;123:S962–S963. [Google Scholar]
- 36.Pisani C., Deantonio L., Surico D., Brambilla M., Galla A., Ferrara E. Quality of life in patients treated by adjuvant radiotherapy for endometrial and cervical cancers: correlation with dose-volume parameters. Clin Transl Oncol. 2016;18:901–908. doi: 10.1007/s12094-015-1458-9. [DOI] [PubMed] [Google Scholar]
- 37.Chang A.T.Y., Tan L.T., Duke S., Ng W.T. Challenges for quality assurance of target volume delineation in clinical trials. Front Oncol. 2017;7:221. doi: 10.3389/fonc.2017.00221. [DOI] [PMC free article] [PubMed] [Google Scholar]