Abstract
In the present longitudinal study, we investigated the joint effect of early family factors and long-term vocabulary development on the structure of reading-related white matter pathways in adolescents. Seventy-nine children participated in this study. Family environment was measured via parental questionnaire between age 1 and age 3. From age 4 to age 10, children’s vocabulary skills were tested annually. At age 14, diffusion tensor imaging data of the children were collected. Using individual-based tractography, 10 reading-related tracts of the two hemispheres were delineated. Different family factors were found to be correlated with different pathways: Age of literacy exposure was correlated with fractional anisotropy of the direct segment of the left arcuate fasciculus, while an association trend was found between early family socioeconomic status and fractional anisotropy of the left inferior frontal occipital fasciculus. Further regression analyses showed that the age of literacy exposure modulated the relationships between vocabulary development and the structure of the left arcuate fasciculus. Specifically, in the earlier literacy exposure group, no association was found between vocabulary development and the strength of the arcuate fasciculus, whereas in the later literacy exposure group, significant associations were found between vocabulary development and the strength of the arcuate fasciculus.
Keywords: Reading, Family environment, Vocabulary development, White matter tracts
1. Introduction
Learning to read is one of the important milestones in child development. With the development of neuroimaging technology, more researchers tend to believe that reading acquisition depends on continuous communication within a brain network of visual, auditory, and semantic-processing regions; the white matter pathways that connect them lay the foundation for communication (Ramus, 2004; Vandermosten et al., 2012a; Wandell and Yeatman, 2013). Longitudinal studies have shown that the development of these white matter tracts is a long-lasting process from early childhood to adolescence (Barnea-Goraly et al., 2005; Eluvathingal et al., 2007; Lebel et al., 2008). During this long-term process, the brain is inevitably influenced by the external family environment and internal learning experience of the children (Asaridou et al., 2017; Farah, 2017; Yeatman et al., 2012). However, there is a paucity of evidence on how the family environment and individual learning experience interact in the development of white matter pathways. Therefore, the present study explored the joint effect of the family environment and language learning experience on the structure of reading-related white matter pathways.
In the external environment, the early family environment plays an essential role in children’s cognitive development. A large number of behavioral studies have highlighted the importance of socioeconomic status (SES) in children's reading and language development (Hoff, 2003; Noble et al., 2007; Rowe and Goldin-Meadow, 2009). Neuroimaging studies in recent years have also found correlations between SES and brain structure and function (Brito and Noble, 2014; Farah, 2017; Hackman et al., 2010; Noble et al., 2015; Ozernov-Palchik et al., 2019). Noble et al. (2015) reported that after controlling for genetic factors, the influence of SES on the area and thickness of gray matter remained significant, and the significant areas included the reading and language-related regions. Nevertheless, the majority of previous studies have focused on a single family index of SES. However, other factors in the environment might mediate the effects of SES on the brain (Hackman et al., 2010). These factors may include home literacy experiences like parent-child interactions (Hirsh-Pasek et al., 2015; Rowe and Goldin-Meadow, 2009; Sénéchal and Lefevre, 2002) and the home literacy environment like book resources at home (Deng et al., 2015; Manolitsis et al., 2013). There is now some evidence that certain aspects of parent-child interactions relate to the functional brain activity of children (Garcia-Sierra et al., 2016; Romeo et al., 2018a, 2018b). Garcia-Sierra et al. (2016) found that the amount of parents’ language input was related to their infants’ brain responses (mismatch negativity, MMN) in the prefrontal cortical regions. Romeo et al. (2018a, 2018b) reported that parent-child conversational turns were associated with the activation of the left inferior frontal area during a story listening fMRI task. However, the relations of these family factors and brain structures, especially the reading-related pathways, are largely unknown. Recently, researchers reported that parent-child conversational turns were significantly correlated with the connectivity of the left arcuate/superior longitudinal fasciculi, independently of SES and the amount of adult speech (Romeo et al., 2018a, 2018b). This study highlights the importance of early parent-child interaction for the development of the white matter pathways. However, this study was cross-sectional among preschool children, and it remains unclear whether the effect of the early family environment on the brain lasts for a long-time period. Therefore, in the present study, we measured various family factors (SES, age of literacy exposure and home literacy environment) in the preschool years and examined their association with brain structure in adolescence.
Apart from early environmental factors, the long-term language and reading learning experiences may influence reading and brain development (Asaridou et al., 2017; Song et al., 2015; Thiebaut de Schotten et al., 2012). As one of the most important experiences in language acquisition and reading development (Lee, 2010; Nation et al., 2010; Verhoeven et al., 2011), children’s vocabulary skill develops in step with the white matter tracts from early childhood to adolescence. In general, children’s vocabulary development can be conceptualized as a combination of the initial size and growth rate of their vocabulary. Compared with the initial vocabulary size, the growth rate of vocabulary development explains relatively more variance in subsequent language and reading skills in some studies (Rowe et al., 2012; Song et al., 2015). In recent MRI studies, researchers have also found that the growth rate of vocabulary development, rather than the initial vocabulary size, was a specific predictor of brain structure (Asaridou et al., 2017; Su et al., 2018). These studies highlight the importance of vocabulary growth rate on reading and brain development. In the present study, we also focused on vocabulary skill, particularly the rate of vocabulary development, as an indicator of children’s language learning over time.
Previous studies were mainly focused on the single predictive effect of the early family environment or language learning experience on reading performance and the reading brain. However, the early family environment and language learning experience may interact in facilitating subsequent reading and brain development. For example, Noble et al. reported that SES and phoneme awareness (PA) interacted in a multiplicative fashion with reading or reading-related brain activity (Noble, Farah, et al., 2006; Noble, Wolmetz, et al., 2006). Specifically, at higher SES backgrounds, children’s reading or reading-related brain activity was generally at an above-average level, regardless of their PA levels. However, at lower SES backgrounds, children with higher PA levels performed better than children with lower PA levels in reading and reading-related brain activity. This is the multiplicative factor model they provided to explain the interaction of the social background and cognitive skills on reading and the brain (Noble, Farah, et al., 2006; Noble, Wolmetz, et al., 2006). Until now, the investigation of this interaction effect has been limited to the behavior and brain function levels. Little is known about interaction effects on brain structures, such as white matter tracts.
In summary, the present study aimed to investigate the joint effect of early family factors (SES, age of literacy exposure, and literacy environment) and long-term vocabulary development on the structure of reading-related white matter pathways in adolescence. Different early family factors may predict the property of different tracts in the left hemisphere. Furthermore, according to the multiplicative factor model (Noble et al., 2006a), we hypothesized that the early family factors would interact with vocabulary development on the structure of the reading-related white matter tracts.
2. Methods
2.1. Participants
Seventy-nine Chinese children participated in this study. There were 39 girls and 40 boys with a mean age of 14.1 years old (SD = 0.5). All participants came from a large on-going longitudinal study of Chinese language and literacy development that has taken place since 2000 (Song et al., 2015; Tardif et al., 2008). The original sample of participants consisted of 338 one-year-old children. From 1–3 years old, the family environment was measured via parent questionnaire. From 4–11 years old, these children were tested annually on a range of language and reading related tasks. At age 14, we mailed invitation letters for the MRI study to all participants remaining at age 11 (n = 291). We then made telephone calls to the parents who signed the agreement from the invitation letter (n = 107). Of these children, 28 children had counter indications to an MRI scan for various reasons (e.g., metal braces). Finally, we ended up with 79 volunteers in the DTI study. We compared demographic and behavioral variables of the children who were included in this study vs. those excluded. Independent-samples t tests showed that there was no significant difference between the two groups with respect to age, sex, IQ, family SES, and word reading ability (tested at age 11) (all ps < 0.05). The participants were all native Mandarin speakers with a wide range of reading skills. They had normal or corrected-to-normal vision with no history of neurological abnormalities, head injury, or intellectual disability. Written informed consent was obtained from all parents. Ethical approval for the present study was obtained from the Institutional Review Board of Beijing Normal University Imaging Center for Brain Research.
2.2. Measures and analysis
2.2.1. Early family environment
The children’s early family environment was measured via parent questionnaire. There were 10 items in total. These items have been used in previous studies and have been demonstrated to be reasonable proxies representing Chinese children’s early family environment (Su et al., 2015, 2017; Zhang et al., 2013). To reduce the 10 variables, an exploratory factor analysis was conducted in a previous study (Su et al., 2017). Three factors emerged: The first component was family socioeconomic status (SES) (including 4 items), the second component was home literacy environment (including 4 items), and the third component was age of literacy exposure at home (including 2 items). The three components were then calculated as the mean standard scores of the corresponding measures. Based on the study by Su et al. (2017), we chose the same three components as the early family factors in the present study. Detailed descriptions regarding each item in each component are provided as follows.
2.2.1.1. Socioeconomic status
SES consisted of 4 items (mother’s education, father’s education, mother’s income, and father’s income). Parental education level was measured on a 7-point scale: 1 = lower than third grade; 2 = fourth to sixth grade; 3 = junior high school completion; 4 = senior high school completion; 5 = college; 6 = university graduate; and 7 = graduate school. Each parent's income level was measured on a 6-point scale: 1 = lower than 300; 2 = 300–499; 3 = 500–999; 4 = 1,000–1,999; 5 = 2,000–8,999; and 6 = greater than 9000 Chinese Renminbi RMB per month. The items of the SES component were collected from the mothers of the participants after they gave birth to the children.
2.2.1.2. Literacy environment
The literacy environment consisted of 4 items (number of adults' books at home, number of children's books at home, parents' reading frequency per week, and parents' reading frequency per day). The numbers of adults' and children's books at home were measured with two six-point scales: 1 = 0–5 books; 2 = 6–10 books; 3 = 11–25 books; 4 = 26–50 books; 5 = 51–100 books; and 6 = more than 100 books. The parents’ reading habits were assessed with two questions. First, on how many days in a week do you usually read books or magazines? The scale included 1 = 0 days; 2 = 1–2 days; 3 = 3–4 days; 4 = 5–6 days; and 5 = 7 days. Second, for how long do you read each time? The scale include 1 = 0−10 min; 2 = 11−30 min; 3 = 31−60 min; 4 = 1–2 hours; 5 = 2–4 hours; and 6 = more than 4 h. Items loading on this component were collected when children were 3 years old.
2.2.1.3. Age of literacy exposure
Age of literacy exposure consisted of 2 items (storybook reading age and character teaching age). The parents were asked about the age at which they started or intended to start reading storybooks to their child and teaching their child to read characters. The choices were 1 = have no plan; 2 = after age 4; 3 = at 3–4 years old; 4 = at 2–3 years old; 5 = at 1–2 years old; and 6 = from 0 to 1 years old. Items for the age of literacy exposure component were collected when children were 3 years old.
2.2.2. Vocabulary development
2.2.2.1. Vocabulary definitions task
Following the vocabulary subtest of the Stanford-Binet Intelligence Scale (Thorndike et al., 1986), this task was translated and adapted for Chinese children (Song et al., 2015). It was used in a series of previous studies and is a reliable proxy for vocabulary knowledge in Chinese children (Chow et al., 2005; Lei et al., 2011; McBride-Chang et al., 2008). In this task, the experimenters orally presented a two-character word to the participant. The children’s task was to provide the definition of the word. The experimenters subsequently scored the answer according to the standard scoring scheme in the test manual. The maximum score for each word was two. Each response of the children was rated by two well-trained experimenters with high inter-rater reliability in pilot tests. Overall, the children’s vocabulary knowledge was measured across six time points. There were thirty-two words for the first five time points. At the final time point, fourteen new words were added to avoid a ceiling effect for the task. From age 4 to age 10, children’s vocabulary ability was tested annually, with the exception of at age 9. As additional measures assessing children’s attention were added at age 9, we did not measure vocabulary knowledge in this year so as to avoid fatigue in the children.
2.2.2.2. Fitting vocabulary developmental trajectories
As part of a larger longitudinal study examining children’s language development (n = 264, including all participants of the present study) (Song et al., 2015), the vocabulary developmental trajectory of each participant in the present study was transformed into two parameters, the intercept (starting point) and the slope (growth rate), using linear growth models (Rogosa et al., 1982). A detailed description regarding the model fit procedure was reported in a previous study (Song et al., 2015). Therefore, in the present study, each child had two indices (intercept and slope) that represented his/her developmental trajectory from age 4 to age 10. Previous behavioral and neuroimaging studies have reported that, compared with the starting point, the growth rate of vocabulary development explained more variance in later reading behavior and brain development (Asaridou et al., 2017; Rowe et al., 2012; Song et al., 2015; Su et al., 2018). Therefore, in the present study, we selected growth rate as our proxy for children’s vocabulary development.
2.3. MRI data acquisition and analysis
A 3 T MRI system (Siemens Trio, Germany) with a 12-channel head coil was used to acquire the diffusion-weighted images (DWI) of all participants. A single-shot spin-echo echo-planar imaging sequence was applied to collect the DWI data (coverage of the whole brain; TR = 8000 ms; TE =89 ms; acquisition matrix = 128 × 128; field of view = 282 × 282 mm2; and slice thickness = 2.2 mm with no gap). The diffusion sensitizing gradients were applied along 30 noncollinear directions with a b-value of 1000s/mm2 and one image with a b-value of 0 s/mm2. The resolution was 2.2 × 2.2 × 2.2 mm3. To improve the signal-to-noise ratio, we repeated the DWI sequence twice.
The registration of the raw DWI images and correction for subject motion and geometrical distortions were performed using ExploreDTI software (http://www.exploredti.com; Leemans and Jones, 2009). The Levenberg–Marquardt nonlinear regression was then used to fit the tensor model (Marquardt, 1963). The fractional anisotropy (FA) was computed based on the eigenvalues of the diffusion tensor (Basser and Pierpaoli, 1996). The whole-brain tractography was subsequently performed using an interpolated streamline algorithm with a step length of 0.5 mm and a maximum angle threshold of 35°. Voxels with an FA below the threshold value of 0.2 were excluded from the tractography. This initial analysis yielded whole-brain tractography independently from regions-of-interest. Finally, the diffusion tensor maps and tractography data were imported into the TrackVis software tool (http://www.trackvis.org; Wedeen et al., 2008).
Tract dissections were performed for each participant’s native space using a region-of-interest (ROI) approach. Fig. 1 (right column) displays our tracts of interest. The protocol for defining the ROIs for each fiber tract has been described in detail in previous tractography studies (Catani et al., 2005, 2007; Catani and Thiebaut de Schotten, 2008): The three segments of AF (AF-anterior, AF-posterior, and AF-direct) were dissected following Catani et al. (2005), the IFOF following Catani and Thiebaut de Schotten (2008), and the ILF following Catani and Thiebaut de Schotten (2008). As in previous studies (Rojkova et al., 2016; Zhao et al., 2016), we automated steps of the tract dissection to minimize the subjective variability associated with manual dissection. We defined ROIs on the MNI152 template (provided by FSL, http://www.fmrib.ox.ac.uk/fsl/). The FA map of each child was registered to the MNI152 template using Advanced Normalization Tools combining affine with diffeomorphic deformations (ANTs, http://www.picsl.upenn.edu/ANTS/; Avants et al., 2008; Klein et al., 2009). The inverse deformation was subsequently used to bring the ROIs defined on the MNI152 template to the native space of each participant. Each dissected tract was visually inspected and corrected in the native space of each child by two anatomists (MS and AC). All visual inspections and corrections were carried out under the supervision of expert anatomist MTS. Finally, the mean fractional anisotropy (FA) along the tracts of interest was extracted for further analyses.
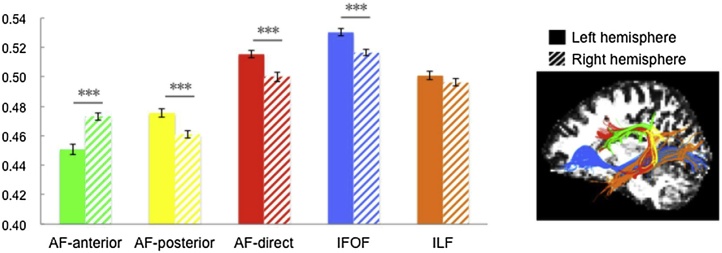
Fig. 1.
FA values of the white matter tracts between hemispheres.
Note: The solid bar represents the left hemisphere, and the striped bar represents the right hemisphere. Different tracts are displayed in different colors: green = arcuate fasciculus-anterior (AF-anterior), yellow = arcuate fasciculus-posterior (AF-posterior), red = arcuate fasciculus-direct (AF-direct), blue = inferior frontal occipital fasciculus (IFOF), and orange = inferior longitudinal fasciclus (ILF). Error bars represent ± 1 standard error of the mean, ***p< 0.001.
2.4. Statistical analysis
Statistical analyses were performed using SPSS Statistics v.20 (IBM Corporation, Somers, New York). First, demographics and differences in the FA values of the tracts between the two hemispheres were tested through paired-samples t-tests. Second, partial correlation analyses were used between the early family factors and the FA of the tracts and between the vocabulary growth rate and the FA of the tracts after controlling for age, sex, and whole brain mean FA. Furthermore, to examine the joint influence, particularly the interaction between early family factors and vocabulary development on the property of the tracts, hierarchical linear regression models were performed. FA values of the tracts that had been found in previous analyses to be significantly associated with family factors or vocabulary development were chosen as the dependent variables. Age, sex and the whole brain mean FA were statistically controlled in the first step as independent variables. In the second step, the control family factors were entered as a covariate. In the third step, the target family factor, the vocabulary growth rate, and the target family factor × growth rate interaction were entered as the independent variables of interest. Finally, to better understand the nature of the interactions, we split the target family factor and vocabulary growth rate into two groups by their group median and used ANCOVAs with FA as our dependent variable, target family factor and vocabulary developmental groups as between-subject factors, and age, sex, whole brain mean FA, and control family factors as covariates. The results were corrected for multiple comparisons of tracts using the false discovery rate (FDR) correction (Benjamini and Hochberg, 1995). We have reported uncorrected p-values and have then compared them to the FDR-corrected alpha threshold q value in the results.
3. Results
3.1. General distribution of the FA values of the tracts
The quality of the tract dissection was checked in the first step. The tractography success rates were greater than 95 % on each segment of the arcuate fasciculus in both hemispheres, with the exception of the right AF-direct, which had a success rate of 87 %, consistent with previous studies (Catani et al., 2007; Yeatman et al., 2011). The tractography success rate of the inferior frontal occipital fasciculus (IFOF) and inferior longitudinal fasciclus (ILF) reached 100 % in both hemispheres. We then compared the FA values of the tracts between the two hemispheres (Fig. 1). Paired-samples t tests showed that the FA of the left AF-posterior, AF-direct and IFOF were significantly larger than their counterparts in the right hemisphere (AF-posterior: t = 4.760, p < .001; AF-direct: t = 5.062, p < .001; and IFOF: t = 7.967, p < .001), which demonstrates leftward asymmetry, while the FA of the right AF-anterior was significantly larger than the FA of the left AF-anterior (AF-anterior: t = −6.809, p < .001), which implies rightward asymmetry. Finally, there was no significant difference in the FA of the ILF between the two hemispheres (ILF: t = 1.306, p = .196).
3.2. Influences of the family environment and vocabulary development
Before exploring the influences of the family environment and vocabulary development, we examined the descriptive statistics of the 10 original family factors. Table 1 shows the ranges (min and max), means and SDs of the early family factors. In the area of the present study (Beijing, China), the median educational attainment is 3 and the median income is 3 (National Bureau of Statistics, 2001), which indicated that the children in our sample were generally from the upper middle class families of this area. In general, the ranges for all of the measures were relatively large with reasonable standard deviations. Thus, this sample demonstrated sufficient variability in the early family environment.
Table 1.
Descriptive statistics of the early family factors.
| Measures | Range | Mean | S. D. |
|---|---|---|---|
| Mother’s education | 2-7 | 4.5 | 1.1 |
| Father’s education | 3-7 | 4.7 | 1.1 |
| Mother’s income | 1-6 | 3.8 | 1.5 |
| Father’s income | 1-6 | 4.7 | 1.1 |
| Number of adults' books at home | 1-6 | 4.8 | 1.5 |
| Number of children's books at home | 1-6 | 4.2 | 1.2 |
| Parents' reading frequency per week | 1-5 | 4.5 | 1.1 |
| Parents' reading frequency per day | 1-6 | 3.1 | 1.1 |
| Storybook reading age | 1-6 | 4.6 | 1.2 |
| Character teaching age | 1-6 | 4.0 | 1.0 |
To reduce the 10 early family variables, an exploratory factor analysis was conducted. Three factors emerged: The first component was family socioeconomic status (SES) (including mother’s education, father’s education, mother’s income and father’s income), the second component was home literacy environment (including number of adults' books at home, number of children's books at home, parents' reading frequency per week and parents' reading frequency per day), and the third component was age of literacy exposure (including storybook reading age and character teaching age). The three components were then calculated as the mean standard scores of the corresponding measures (Su et al., 2017). We subsequently performed a correlation analysis among the three early family factors and the vocabulary developmental parameter. Early family factors were significantly correlated with each other (ps < .05). SES was found to be significantly associated with the vocabulary intercept (r = .229, p = .042), while no significant correlation was found between early family factors and vocabulary growth rate (ps > .05).
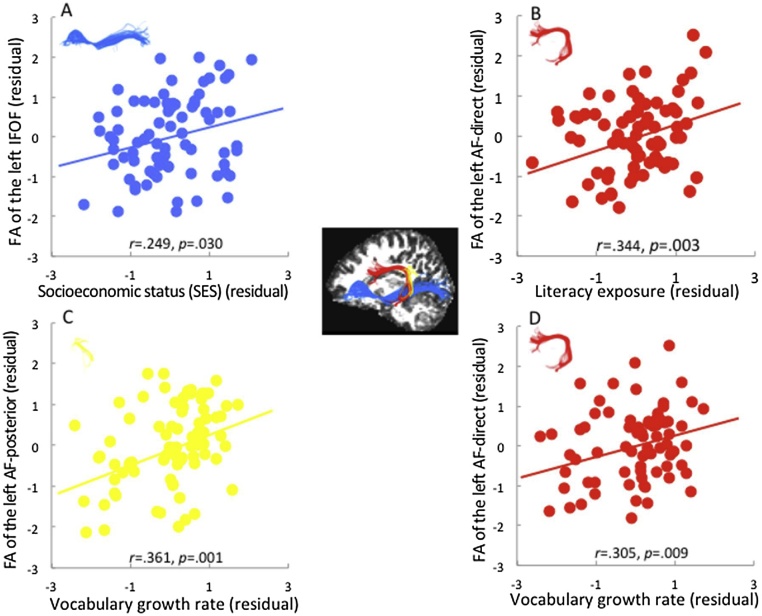
Furthermore, we performed partial correlation analyses among family factors, vocabulary growth rate, and FA of the white matter tracts, statistically controlling for age, sex and whole brain mean FA. Table 2 shows that three tracts in the left hemisphere (AF-posterior, AF-direct, and IFOF) were correlated with family factors or vocabulary development. More specifically, SES was associated with the FA of the left IFOF (r = .249, p = .030 > FDR-corrected q = .005). Further correlation analyses between individual SES factors (parental education, parental income) and FA of the white matter tracts showed that parental education was associated with the FA of the left IFOF (r = .247, p = .031), while no significant correlation was found between parental income and the left IFOF (r = .166, p = .153). Age of literacy exposure was correlated with the FA of the left AF-direct (r = .344, p = .003 < FDR-corrected q = .005), and vocabulary growth rate was associated with the FA of the left AF-posterior (r = .361, p = .001 < FDR-corrected q = .010) and left AF-direct (r = .305, p = .009 < FDR-corrected q = .010). No significant correlations were identified in the right hemisphere (ps > .05). Fig. 2 displays the individual scatter plots for the significant correlations. In general, a better early family environment and higher vocabulary growth rate were associated with larger FA values of the reading-related tracts.
Table 2.
Partial correlations between family factors, vocabulary growth rate and the FA of the white matter tracts controlling for age, sex and whole brain mean FA.
| Tracts | SES | Literacy environment | Age of literacy exposure | Vocabulary growth rate | |
|---|---|---|---|---|---|
| Left hemisphere | |||||
| AF-anterior | .076 | .182 | .196 | −.069 | |
| AF-posterior | −.017 | .026 | −.018 | .361** | |
| AF-direct | −.128 | .129 | .344** | .305** | |
| IFOF | .249* | .090 | −.053 | .158 | |
| ILF | .145 | .060 | .079 | .052 | |
| Right hemisphere | |||||
| AF-anterior | −.206 | −.180 | −.165 | .065 | |
| AF-posterior | −.076 | .049 | −.217 | −.087 | |
| AF-direct | −.057 | .054 | −.153 | .046 | |
| IFOF | .222 | .086 | −.006 | −.026 | |
| ILF | .107 | .177 | .007 | .041 | |
Note: *p < 0.05, **p < 0.01, r values surviving FDR correction are in bold.
Fig. 2.
Correlation between family factors, vocabulary growth rate and mean FA of the left arcuate fasciculus and left inferior frontal occipital fasciculus (residuals, after controlling for age, sex, and whole-brain mean FA).
(A) Correlation between socioeconomic status (SES) and mean FA of the left inferior frontal occipital fasciculus. (B) Correlation between age of literacy exposure and mean FA of the left arcuate fasciculus-direct. (C) Correlation between vocabulary growth rate and mean FA of the left arcuate fasciculus-posterior. (D) Correlation between vocabulary growth rate and mean FA of the arcuate fasciculus-direct.
3.3. Family environment × vocabulary development interaction
To explore the joint influence of the early family factors and vocabulary development on the structure of the white matter tracts, we performed hierarchical linear regression models on the tracts (left AF-posterior, left AF-direct, and left IFOF) that have been found to be significantly associated with family factors or vocabulary development in previous analyses. Correlational analyses showed that SES, age of literacy exposure, and vocabulary growth rate were correlated with the FA of these tracts (Table 2). Thus, we tested the SES-vocabulary interaction and age of literacy exposure-vocabulary interaction in further regression analyses. When age, sex, whole brain mean FA, SES and literacy environment were statistically controlled, age of literacy exposure was a significant predictor of the FA of left AF-direct (β = .256, p = .011); vocabulary growth rate significantly predicted the FA of left AF-posterior (β = .298, p = .002) and left AF-direct (β = .220, p = .014). Interestingly, two significant age of literacy exposure × vocabulary development interactions were found in the left AF-posterior (β = -.201, p = .032) and left AF-direct (β = -.220, p = .015) (Table 3). No significant SES-vocabulary interaction was identified among the previously described tracts (ps > .05). Figs. 3A and 3B illustrate the age of literacy exposure-vocabulary interactions in which age of literacy exposure was split into “earlier” and “later” groups by the group median. The interaction patterns of the AF-posterior and AF-direct were similar: In the earlier literacy exposure group, there was no correlation between vocabulary growth rate and the FA of the tracts, whereas the vocabulary growth rate was positively correlated with the FA of the tracts in the later literacy exposure group. This finding suggests that age of literacy exposure can systematically influence the vocabulary development–brain relationships. No interaction effect was identified in the left IFOF (β = -.072, p = .456), although SES remained a significant predictor of the FA of the IFOF (β = .231, p = .033).
Table 3.
Hierarchical regression models using control variables, age of literacy exposure and vocabulary growth rate to predict the FA of the left arcuate fasciculus (AF) the left inferior frontal occipital fasciculus (IFOF).
| AF-posterior | AF-direct | IFOF | |||||
|---|---|---|---|---|---|---|---|
| ΔR2 | Beta | ΔR2 | Beta | ΔR2 | Beta | ||
| 1 | Age | .300*** | −.195* | .313*** | .008 | .325*** | −.064 |
| Sex | .090 | .034 | .163 | ||||
| Whole brain FA | .548*** | .540*** | .524*** | ||||
| 2 | SES | <.001 | −.057 | .039 | −.258** | .042 | .231* |
| Literacy environment | .018 | .083 | .029 | ||||
| 3 | Age of literacy exposure | .135** | −.059 | .192*** | .256* | .031 | −.162 |
| Vocabulary growth rate | .298** | .220* | .102 | ||||
| Interaction | −.201* | −.220* | −.072 | ||||
Note: *p < 0.05, **p < 0.01, ***p < 0.001.
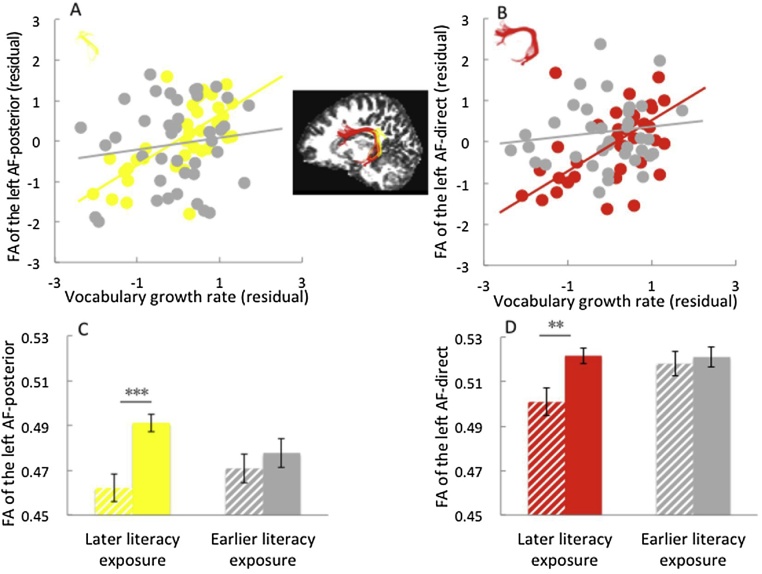
Fig. 3.
The modulation of age of literacy exposure on the relationship between vocabulary growth rate and FA values of the left arcuate fasciculus.
(A) Correlation between residuals (controlling for age, sex, whole-brain mean FA, SES and literacy environment) of vocabulary growth rate and FA values of the left arcuate fasciculus-posterior for the earlier literacy exposure group and later literacy exposure group, respectively. (B) Correlation between residuals of vocabulary growth rate and FA values of the left arcuate fasciculus-direct for the earlier and later literacy exposure groups. (C) Age of literacy exposure by vocabulary development interaction in the left arcuate fasciculus-posterior. (D)Age of literacy exposure by vocabulary development interaction in the left arcuate fasciculus-direct.
Note: In the upper panel, age of literacy exposure is split into “earlier” and “later” groups by group median. Gray dots represent children in the earlier literacy exposure group; yellow or red dots represent children in the later literacy exposure group. In the lower panel, the Y-axis indicates the mean FA of the tracts. Vocabulary growth rate is split into “high” and “low” groups by group median. The solid bar represents the high vocabulary growth rate group, and the striped bar represents the low vocabulary growth rate group. Error bars represent ± 1 standard error of the mean, **p < 0.01, ***p < 0.001.
In line with the regression models, further ANCOVAs, transforming the age of literacy exposure (group median = 4) and vocabulary development into binary variables, showed two significant interactions in the left AF-posterior (F(1, 69) = 6.395, p = .014, = .085) and left AF-direct (F(1, 66) = 5.836, p = .018, = .081). For the earlier literacy exposure group, the children had similar FA values of the two tracts irrespective of their vocabulary growth rate (AF-posterior: F(1, 34) = .057, p = .812; AF-direct (F(1, 30) = .061, p = .807). Regarding the later literacy exposure group, the children with a high growth rate had larger FA than those with a low growth rate (AF-posterior: F(1, 30) = 25.932, p < .001; AF-direct (F(1, 31) = 8.326, p = .007). Figs. 3C and 3D display these interactions.
4. Discussion
Combining detailed behavioral analyses and an individual-based tractography methodology, the present study investigated the influence of early family factors and long-term vocabulary development on the structure of reading-related white matter pathways. First, different family factors were found to be correlated with different pathways: Age of literacy exposure was correlated with the structure of the left AF-direct at 14 years old, while an association trend was found between early family SES and FA of the left IFOF at 14 years old. Furthermore, the structures of the left AF-posterior and left AF-direct at 14 years old were related to the vocabulary growth rate from 4 years old to 10 years old. Finally, significant age of literacy exposure -vocabulary development interactions were found in the left AF-posterior and left AF-direct. Thus, age of literacy exposure can systematically modulate the relationships between vocabulary development and the structure of the left arcuate fasciculus. Specifically, in the earlier literacy exposure group, no association was found between vocabulary development and the strength of the arcuate fasciculus, whereas in the later literacy exposure group, significant associations were found between vocabulary development and the strength of the arcuate fasciculus.
4.1. Long-lasting effect of the early family environment
The influence of the family environment on brain development is a long-standing issue (Farah, 2017). During previous decades, a substantial body of research has focused on the influence of SES, and found that variation in SES tends to be associated with variation in the gray matter structure (Jednoróg et al., 2012; Mackey et al., 2015; Noble et al., 2015), white matter structure (Gianaros et al., 2013; Ursache and Noble, 2016) and functional activity (Raizada et al., 2008) of children’s brains. However, relatively few studies have explored the influence of additional family factors on brain development, and most previous studies were cross-sectional. It is difficult to observe the long-lasting effect of different family factors on subsequent brain development. In the present longitudinal study, we measured different family factors from children’s birth to the ages of 3 years old and measured brain structure at 14 years old. Interestingly, we found that the age of literacy exposure was related to the property of AF-direct at 14 years old, even statistically controlling for SES, literacy environment, and long-term vocabulary development. This may highlight the long-lasting effect of the age of literacy exposure.
In the present study, we did not find significant correlations between early family factors and vocabulary growth rate. According to the previous literature (Rowe et al., 2012), early family factors, especially SES, should be associated with the vocabulary growth rate. Thus, we conducted further statistical analyses. As all the 79 participants of the present MRI study came from a larger longitudinal study (n = 262) (Su et al., 2017), we had familial and behavioral data for all 262 children. Thus, we calculated the correlations between early family factors and vocabulary growth rate in the larger sample. Results showed that SES and home literacy were both significantly associated with vocabulary growth rate. These results indicate that early family factors are related to the vocabulary growth rate, which is consistent with the previous literature. The weak association between early family factors and vocabulary growth rate may be due to the relatively small sample size of the present study, which influences the statistical power of the correlation analyses (Cohen, 2013). Therefore, it seems desirable to enlarge the sample size in further MRI studies.
4.2. Functional dissociation of different family factors
Via individual-based tractography, the current study comprehensively examined the property of 10 reading-related white matter pathways in the bilateral hemispheres. Association analyses between these pathways and early family factors showed that different family factors were associated with different pathways in the left hemisphere: Age of literacy exposure was associated with FA of the left AF-direct in the dorsal pathway (and survived the FDR correction); an association trend was also found between SES and FA of the left IFOF in the ventral pathway (did not survive the FDR correction). The association between age of literacy exposure and FA of the left AF was similar to a previous study in which a significant correlation was identified between conversational interaction and FA values of the left arcuate and superior longitudinal fasciculi (Romeo et al., 2018a,b). This is not surprising because the processes of literacy exposure and conversational interaction both incorporate auditory or phonological processing of the children, which has been found to be the function of the left arcuate fasciculus (Vandermosten et al., 2012b; Yeatman et al., 2011). The association trend between SES and IFOF was also similar to that demonstrated in several previous studies in which a significant correlation was identified between SES and the left ILF (Dufford and Kim, 2017; Gullick et al., 2016). The ILF and IFOF in the ventral pathway might serve as a route to transfer primary visual input to the Visual Word Form Area (VWFA), and it may be correlated with orthographical processing skills (Epelbaum et al., 2008). Furthermore, many studies have found different associations in both brain structure and cognitive outcomes to be partly dependent upon the SES variable used; current research favors using specific SES indicators (e.g., income or education) as variables (Brito and Noble, 2014; Duncan and Magnuson, 2003). Results of the present study showed that parental education was associated with the FA of the left IFOF, while no significant correlation was found between parental income and the left IFOF. This reflects that the association we found between the composite SES score and the reading-related tract IFOF is mainly due to the contribution of the parental education. This result is not surprising, as previous studies have shown that parental education is strongly related to the richness of children’ s language learning environment and is particularly impactful on children’ s literacy development; in contrast, parental income representing the material resources available to children may have a greater impact on social, emotional, or executive function development (Brito and Noble, 2014; Duncan and Magnuson, 2012; Noble et al., 2015).
4.3. Development of the left AF
Relative to the ventral fiber pathway, the maturation of the left AF in the dorsal pathway occurs later (Dubois et al., 2008). Newborn studies show that the ventral fiber tract is clearly present at birth. However, in contrast to this connection in adults, the dorsal fiber pathway (left AF) that connects Broca’s area and Wernicke’s area is not mature in newborns (Brauer et al., 2011; Perani et al., 2011). This may suggest that both external family environment and internal learning experience play important roles in shaping the development of the dorsal fiber pathway. Using a longitudinal design, the present study highlighted the potential influences of the early family environment, long-term vocabulary development, and the environment-vocabulary interaction on the structure of the arcuate fasciculus in adolescence, thus providing evidence for the roles of external family environment and internal learning experience in shaping the development of the dorsal pathway.
4.4. Interaction of the early family environment and vocabulary development
The early family environment, as represented by age of literacy exposure in the present study, interacted with vocabulary development on the structure of reading-related white matter tracts. In the earlier literacy exposure group, no significant difference was found in the left AF between high vocabulary growth group and low vocabulary growth group. While in the later literacy exposure group, a significant difference was found in the left AF between high vocabulary growth group and low vocabulary growth group. Specifically, children with low vocabulary growth rate had significant lower FA than children with high vocabulary growth rate. Therefore, we conjecture that children who seem to be particularly at risk to have low FA are those who have both later literacy exposure and low vocabulary growth rate. In other words, this is a kind of “double hit” situation. If the children have both disadvantages, they will have lower FA. Either earlier literacy exposure or high vocabulary growth grate can rescue the children from this unfavorable situation. Considering the influential factors of the vocabulary growth rate, it may be influenced both by genetic factors and by environmental factors such as home literacy environment and exposure to literacy. And there may be gene x environment interactions, such that a more favorable environment buffers against the effects of less favorable genetic predispositions. This is known as a vulnerability model, or double hit as well. According to the distribution of the age of literacy exposure (median = 4), children who experience literacy exposure before 2 years old are in the earlier literacy exposure group, which may suggest the importance of providing literacy exposure for child before this curtail time point. However, it is notable that the above explanations are our conjectures. In future studies, we need to combine more brain and behavioral data to clarify and refine these ideas.
Interestingly, the interaction pattern identified in this study confirmed the “multiplicative factor model” suggested by previous behavioral studies (Noble, Farah, et al., 2006). Together with a previous functional MRI study (Noble, Wolmetz, et al., 2006), the results of the present DTI study provide new evidence for this interaction model at the neural anatomical level. Moreover, the interacting factors included in previous studies were a single SES index or a 1 time-point cognitive skill, such as PA. However, reading in real life is more complicated; it is influenced by various environmental factors and long-term cognitive development. The present study found the associations between age of literacy exposure, long-term vocabulary development, and the structure of the white matter tracts, which may increase the theoretical significance of the multiplicative factor model.
4.5. The nature of the age of literacy exposure
From both the correlational analyses and the interaction analyses, we found that the age of literacy exposure was related to the white matter structure, independent of other family factors. This finding led us to further consider the nature of the age of literacy exposure. According to Teale and Sulzby’s (1986) conceptualization of the home literacy environment, children’s literacy experiences originate from three aspects: (a) experiences in which children interact with adults in reading and writing situations, (b) experiences in which children explore print on their own, and (c) experiences in which children observe adults modeling literate behaviors. In the present study, we assume that the earlier the age of literacy exposure, the more literacy experiences the child may receive. Therefore, the two items we used (storybook reading age and character teaching age) in the present study belong to the same category, namely, experiences in which children interact with adults in the reading situation. Sénéchal and Lefevre (2002) argued that storybook reading is a type of informal literacy experiences, while character or word teaching is a type of formal literacy experiences. These two types of literacy experiences can enhance children’s phonological awareness and vocabulary knowledge, increase their familiarity with the syntax of written language, and heighten awareness of written letters and words (Sénéchal and Lefevre, 2002). The present study demonstrated the association between age of literacy exposure and the left AF, thus providing neural evidence for the role of the age of literacy exposure in literacy development.
However, the age of literacy exposure in the present study is a general and implicit measure (e.g., reading onset time). We did not collect data on the actual frequency of the parent-child reading interaction. This is a limitation of the present study. Further studies should investigate more concrete and explicit measures, including the actual frequency and time of children’s reading behavior, parent-children reading interaction and direct observations and assessment of parent-child reading activities (Romeo et al., 2018a,b; Rowe and Goldin-Meadow, 2009). Another limitation of the present study is the relatively lenient correction strategy used in the correlation analyses. As an exploratory study, we did separate FDR corrections for each of the family factors, which may have increased the rate of false positives. Findings of the present study need to be validated in future research. Finally, the present study reveals the association between early family factors and the white matter structure in adolescence. However, SES and home literacy environment are dynamic construct that can change throughout a child's development. Further studies should collect more familial data at different time points and explore the dynamic relationship between early family factors and the brain development.
4.6. Practical implications
The present results may also have practical implications. The present study demonstrated that the age of literacy exposure correlated with the strength of the connectivity in the left dorsal pathways, independent of SES. This indicates that the timeliness and enrichment of literacy exposure is essential for the neural development of typical children, and this effect is independent of the effect of SES. Compared with SES, the onset time and quality of literacy exposure are more malleable. Family-based programs designed to improve children’s reading and language ability could focus on timely and high-quality parent-child literacy training. This may enhance the brain development and the language abilities in children from all backgrounds.
5. Conclusion
In the current longitudinal study, we investigated the influence of early family factors and long-term vocabulary development on the structure of reading-related white matter pathways. Age of literacy exposure and early SES were related to the structure of the left AF and left IFOF, respectively. Furthermore, age of literacy exposure and vocabulary development interacted on the structure of the left AF-posterior and left AF-direct. From the perspective of practice, this interaction may lead to insights in effectively targeting the specific needs of children in different environments, particularly children living in relatively disadvantaged backgrounds.
Declaration of Competing Interest
The authors declare no conflict of interest.
Acknowledgement
We thank all the children and their families for their collaboration in this study. We also thank Zhichao Xia for his help in the MRI data collection and Allyson Covello for the manual correction of the tract dissection. This study was supported by the National Natural Science Foundation of China (31800934, 31271082, 31671126), the National Key Basic Research Program of China (2014CB846103), Beijing Municipal Science & Technology Commission (Z151100003915122), the Key Project of Philosophical and Social Science Foundation, ministry of education (11JZD041), the Fundamental Research Funds for the Central Universities (2017XTCX04), General Research Fund of the Hong Kong Special Administrative Region Research Grants Council (14600818), the Interdiscipline Research Funds of Beijing Normal University, the Social Science Project of Beijing Municipal Education Commission (SM201910028008), the Agence Nationale de la Recherche (ANR-11-BSV4-014-01, ANR-10-LABX-0087 IEC and ANR-10-IDEX-0001-02 PSL*), the China Scholarship Council and the NSFC-CNRS Joint Research Project Grant (31611130107).
Contributor Information
Franck Ramus, Email: franck.ramus@ens.fr.
Hua Shu, Email: shuh@bnu.edu.cn.
References
- Asaridou S.S., Demir-Lira O.E., Goldin-Meadow S., Small S.L. The pace of vocabulary growth during preschool predicts cortical structure at school age. Neuropsychologia. 2017;98:13–23. doi: 10.1016/j.neuropsychologia.2016.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avants B.B., Epstein C.L., Grossman M., Gee J.C. Symmetric diffeomorphic image registration with cross-correlation: evaluating automated labeling of elderly and neurodegenerative brain. Med. Image Anal. 2008;12(1):26–41. doi: 10.1016/j.media.2007.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnea-Goraly N., Menon V., Eckert M., Tamm L., Bammer R., Karchemskiy A. White matter development during childhood and adolescence: a cross-sectional diffusion tensor imaging study. Cereb. Cortex. 2005;15(12):1848–1854. doi: 10.1093/cercor/bhi062. [DOI] [PubMed] [Google Scholar]
- Basser P.J., Pierpaoli C. Microstructural features measured using diffusion tensor imaging. J. Magn. Reson. 1996;111(3):209–219. doi: 10.1006/jmrb.1996.0086. [DOI] [PubMed] [Google Scholar]
- Benjamini Y., Hochberg Y. Controlling the false discovery Rate-A practical and powerful approach to multiple testing. J. R. Stat. Soc. 1995;57(57):289–300. [Google Scholar]
- Brauer J., Anwander A., Friederici A.D. Neuroanatomical prerequisites for language functions in the maturing brain. Cereb. Cortex. 2011;21(2):459–466. doi: 10.1093/cercor/bhq108. [DOI] [PubMed] [Google Scholar]
- Brito N.H., Noble K.G. Socioeconomic status and structural brain development. Front. Neurosci. 2014;8:1–12. doi: 10.3389/fnins.2014.00276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catani M., Thiebaut de Schotten M. A diffusion tensor imaging tractography atlas for virtual in vivo dissections. Cortex. 2008;44(8):1105–1132. doi: 10.1016/j.cortex.2008.05.004. [DOI] [PubMed] [Google Scholar]
- Catani M., Jones D.K., Ffytche D.H. Perisylvian language networks of the human brain. Ann. Neurol. 2005;57(1):8–16. doi: 10.1002/ana.20319. [DOI] [PubMed] [Google Scholar]
- Catani M., Allin M.P., Husain M., Pugliese L., Mesulam M.M., Murray R.M., Jones D.K. Symmetries in human brain language pathways correlate with verbal recall. Proc. Natl. Acad. Sci. 2007;104(43):17163–17168. doi: 10.1073/pnas.0702116104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow B.W.-Y., McBride-Chang C., Burgess S. Phonological processing skills and early reading abilities in Hong Kong chinese kindergarteners learning to read english as a second language. J. Educ. Psychol. 2005;97(1):81–87. [Google Scholar]
- Deng C., Silinskas G., Wei W., Georgiou G.K. Cross-lagged relationships between home learning environment and academic achievement in Chinese. Early Child. Res. Q. 2015;33(3):12–20. [Google Scholar]
- Dubois J., Dehaene‐Lambertz G., Perrin M., Mangin J.F., Cointepas Y., Duchesnay E. Asynchrony of the early maturation of white matter bundles in healthy infants: quantitative landmarks revealed noninvasively by diffusion tensor imaging. Hum. Brain Mapp. 2008;29(1):14–27. doi: 10.1002/hbm.20363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dufford A.J., Kim P. Family income, cumulative risk exposure, and white matter structure in middle childhood. Front. Hum. Neurosci. 2017;11 doi: 10.3389/fnhum.2017.00547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan G.J., Magnuson K.A. Off with Hollingshead: socioeconomic resources, parenting, and child development. Socioeconomic status, parenting, and child development. 2003:83–106. [Google Scholar]
- Duncan G.J., Magnuson K. Socioeconomic status and cognitive functioning: moving from correlation to causation. Wiley Interdiscip. Rev. Cogn. Sci. 2012;3(3):377–386. doi: 10.1002/wcs.1176. [DOI] [PubMed] [Google Scholar]
- Eluvathingal T.J., Hasan K.M., Kramer L., Fletcher J.M., Ewing-Cobbs L. Quantitative diffusion tensor tractography of association and projection fibers in normally developing children and adolescents. Cereb. Cortex. 2007;17(12):2760–2768. doi: 10.1093/cercor/bhm003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epelbaum S., Pinel P., Gaillard R., Delmaire C., Perrin M., Dupont S. Pure alexia as a disconnection syndrome: new diffusion imaging evidence for an old concept. Cortex. 2008;44(8):962–974. doi: 10.1016/j.cortex.2008.05.003. [DOI] [PubMed] [Google Scholar]
- Farah M.J. The neuroscience of socioeconomic status: correlates, causes, and consequences. Neuron. 2017:56–71. doi: 10.1016/j.neuron.2017.08.034. [DOI] [PubMed] [Google Scholar]
- Garcia-Sierra A., Ramírez-Esparza N., Kuhl P.K. Relationships between quantity of language input and brain responses in bilingual and monolingual infants. Int. J. Psychophysiol. 2016;110:1–17. doi: 10.1016/j.ijpsycho.2016.10.004. [DOI] [PubMed] [Google Scholar]
- Gianaros P.J., Marsland A.L., Lei K.S., Erickson K.I., Verstynen T.D. Inflammatory pathways link socioeconomic inequalities to white matter architecture. Cereb. Cortex. 2013;23(9):2058–2071. doi: 10.1093/cercor/bhs191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gullick M.M., Demirlira Ö., Booth J.R. Reading skill-fractional anisotropy relationships in visuospatial tracts diverge depending on socioeconomic status. Dev. Sci. 2016;19(4):673–685. doi: 10.1111/desc.12428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackman D.A., Farah M.J., Meaney M.J. Socioeconomic status and the brain: mechanistic insights from human and animal research. Nat. Rev. Neurosci. 2010;11(9):651–659. doi: 10.1038/nrn2897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsh-Pasek K., Adamson L.B., Bakeman R., Owen M.T., Golinkoff R.M., Pace A. The Contribution of Early Communication Quality to Low-Income Children’s Language Success. Psychol. Sci. 2015;26(7):1071–1083. doi: 10.1177/0956797615581493. [DOI] [PubMed] [Google Scholar]
- Hoff E. The specificity of environmental influence: socioeconomic status affects early vocabulary development via maternal speech. Child Dev. 2003;74(5):1368–1378. doi: 10.1111/1467-8624.00612. [DOI] [PubMed] [Google Scholar]
- Jednoróg K., Altarelli I., Monzalvo K., Fluss J., Dubois J., Billard C. The influence of socioeconomic status on children’s brain structure. PLoS One. 2012;7(8) doi: 10.1371/journal.pone.0042486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein A., Andersson J., Ardekani B.A., Ashburner J., Avants B., Chiang M.-C. Evaluation of 14 nonlinear deformation algorithms applied to human brain MRI registration. Neuroimage. 2009;46(3):786–802. doi: 10.1016/j.neuroimage.2008.12.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebel C., Walker L., Leemans A., Phillips L., Beaulieu C. Microstructural maturation of the human brain from childhood to adulthood. Neuroimage. 2008;40(3):1044–1055. doi: 10.1016/j.neuroimage.2007.12.053. [DOI] [PubMed] [Google Scholar]
- Lee J. Size matters: early vocabulary as a predictor of language and literacy competence. Appl. Psycholinguist. 2010;32(1):69–92. [Google Scholar]
- Leemans A., Jones D.K. The B‐matrix must be rotated when correcting for subject motion in DTI data. Magn. Reson. Med. 2009;61(6):1336–1349. doi: 10.1002/mrm.21890. [DOI] [PubMed] [Google Scholar]
- Lei L., Pan J., Liu H., McBride‐Chang C., Li H., Zhang Y. Developmental trajectories of reading development and impairment from ages 3 to 8 years in Chinese children. J. Child Psychol. Psychiatry. 2011;52(2):212–220. doi: 10.1111/j.1469-7610.2010.02311.x. [DOI] [PubMed] [Google Scholar]
- Mackey A.P., Finn A.S., Leonard J.A., Jacobysenghor D.S., West M.R., Gabrieli C.F., Gabrieli J.D. Neuroanatomical correlates of the income-achievement gap. Psychol. Sci. 2015;26(6):925–933. doi: 10.1177/0956797615572233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manolitsis G., Georgiou G.K., Tziraki N. Examining the effects of home literacy and numeracy environment on early reading and math acquisition. Early Child. Res. Q. 2013;28(4):692–703. [Google Scholar]
- Marquardt D.W. An algorithm for least-squares estimation of nonlinear parameters. J. Soc. Ind. Appl. Math. 1963;11(2):431–441. [Google Scholar]
- McBride-Chang C., Tardif T., Cho J.-R., Shu H., Fletcher P., Stokes S.F. What’s in a word? Morphological awareness and vocabulary knowledge in three languages. Appl. Psycholinguist. 2008;29(3):437–462. [Google Scholar]
- Nation K., Cocksey J., Taylor J.S., Bishop D.V. A longitudinal investigation of early reading and language skills in children with poor reading comprehension. J. Child Psychol. Psychiatry. 2010;51(9):1031–1039. doi: 10.1111/j.1469-7610.2010.02254.x. [DOI] [PubMed] [Google Scholar]
- National Bureau of Statistics . China Statistics Press; Beijing: 2001. China Statistical Yearbook on Price & Urban Household Income and Expenditure Survey 2000. [Google Scholar]
- Noble K.G., Farah M.J., Mccandliss B.D. Socioeconomic background modulates cognition-achievement relationships in reading. Cogn. Dev. 2006;21(3):349–368. doi: 10.1016/j.cogdev.2006.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noble K.G., Wolmetz M.E., Ochs L.G., Farah M.J., McCandliss B.D. Brain–behavior relationships in reading acquisition are modulated by socioeconomic factors. Dev. Sci. 2006;9(6):642–654. doi: 10.1111/j.1467-7687.2006.00542.x. [DOI] [PubMed] [Google Scholar]
- Noble K.G., McCandliss B.D., Farah M.J. Socioeconomic gradients predict individual differences in neurocognitive abilities. Dev. Sci. 2007;10(4):464–480. doi: 10.1111/j.1467-7687.2007.00600.x. [DOI] [PubMed] [Google Scholar]
- Noble K.G., Houston S.M., Brito N.H., Bartsch H., Kan E., Kuperman J.M. Family income, parental education and brain structure in children and adolescents. Nat. Neurosci. 2015;18:773–778. doi: 10.1038/nn.3983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozernov-Palchik O., Norton E.S., Wang Y., Beach S.D., Zuk J., Wolf M. The relationship between socioeconomic status and white matter microstructure in pre‐reading children: a longitudinal investigation. Hum. Brain Mapp. 2019;40(3):741–754. doi: 10.1002/hbm.24407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perani D., Saccuman M.C., Scifo P., Anwander A., Spada D., Baldoli C. Neural language networks at birth. Proc. Natl. Acad. Sci. 2011;108(38):16056–16061. doi: 10.1073/pnas.1102991108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raizada R.D.S., Richards T.L., Meltzoff A., Kuhl P.K. Socioeconomic status predicts hemispheric specialisation of the left inferior frontal gyrus in young children. Neuroimage. 2008;40(3):1392–1401. doi: 10.1016/j.neuroimage.2008.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramus F. The neural basis of reading acquisition. In: Gazzaniga M.S., editor. The Cognitive Neurosciences III. MIT Press; Cambridge, MA: 2004. p. 815824. [Google Scholar]
- Rogosa D., Brandt D., Zimowski M. A growth curve approach to the measurement of change. Psychol. Bull. 1982;92:726–748. [Google Scholar]
- Rojkova K., Volle E., Urbanski M., Humbert F., Dell’Acqua F., Schotten M.T.D. Atlasing the frontal lobe connections and their variability due to age and education: a spherical deconvolution tractography study. Brain Struct. Funct. 2016;221(3):1–16. doi: 10.1007/s00429-015-1001-3. [DOI] [PubMed] [Google Scholar]
- Romeo R.R., Leonard J.A., Robinson S.T., West M.R., Mackey A.P., Rowe M.L., Gabrieli J.D. Beyond the 30-million-word gap: children’s conversational exposure is associated with language-related brain function. Psychol. Sci. 2018;29(5):700–710. doi: 10.1177/0956797617742725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romeo R.R., Segaran J., Leonard J.A., Robinson S.T., West M.R., Mackey A.P. Language exposure relates to structural neural connectivity in childhood. J. Neurosci. 2018;38(36):7870–7877. doi: 10.1523/JNEUROSCI.0484-18.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe M.L., Goldin-Meadow S. Differences in early gesture explain SES disparities in child vocabulary size at school entry. Science. 2009;323(5916):951–953. doi: 10.1126/science.1167025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe M.L., Raudenbush S.W., Goldin-Meadow S. The pace of vocabulary growth helps predict later vocabulary skill. Child Dev. 2012;83(2):508–525. doi: 10.1111/j.1467-8624.2011.01710.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sénéchal M., Lefevre J.A. Parental involvement in the development of children’s reading skill: a five‐year longitudinal study. Child Dev. 2002;73(2):445–460. doi: 10.1111/1467-8624.00417. [DOI] [PubMed] [Google Scholar]
- Song S., Su M., Kang C., Liu H., Zhang Y., McBride‐Chang C. Tracing children’s vocabulary development from preschool through the school‐age years: an 8‐year longitudinal study. Dev. Sci. 2015;18:119–131. doi: 10.1111/desc.12190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su M., Wang J., Maurer U., Zhang Y., Li J., McBride C. Gene–environment interaction on neural mechanisms of orthographic processing in Chinese children. J. Neurolinguistics. 2015;33:172–186. doi: 10.1016/j.jneuroling.2014.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su M., Peyre H., Song S., McBride C., Tardif T., Li H. The influence of early linguistic skills and family factors on literacy acquisition in Chinese children: follow-up from age 3 to age 11. Learn. Instr. 2017;49:54–63. [Google Scholar]
- Su M., Thiebaut de Schotten M., Zhao J., Song S., Zhou W., Gong G. Vocabulary growth rate from preschool to school-age years is reflected in the connectivity of the arcuate fasciculus in 14-year-old children. Dev. Sci. 2018 doi: 10.1111/desc.12647. [DOI] [PubMed] [Google Scholar]
- Tardif T., Fletcher P., Zhang Z., Liang W. Peking University Medical Press; Beijing, China: 2008. Chinese Communicative Development Inventories: User’s Guide and Manual. [Google Scholar]
- Teale W.N., Sulzby E. Emergent literacy as a perspective for examining how young children become writers and readers. In: Teale W.N., Sulzby E., editors. Emergent Literacy: Writing and Reading. Ablex; Norwood, NJ: 1986. [Google Scholar]
- Thiebaut de Schotten M., Cohen L., Amemiya E., Braga L.W., Dehaene S. Learning to read improves the structure of the arcuate fasciculus. Cereb. Cortex. 2012;24(4):989–995. doi: 10.1093/cercor/bhs383. [DOI] [PubMed] [Google Scholar]
- Thorndike R.L., Hagen E.P., Sattler J.M. Riverside Publishing Company; 1986. The Stanford-binet Intelligence Scale: Guide for Administering and Scoring. [Google Scholar]
- Ursache A., Noble K.G. Socioeconomic status, white matter, and executive function in children. Brain Behav. 2016;6(10):1–13. doi: 10.1002/brb3.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandermosten M., Boets B., Poelmans H., Sunaert S., Wouters J., Ghesquière P. A tractography study in dyslexia: neuroanatomic correlates of orthographic, phonological and speech processing. Brain. 2012;135(3):935–948. doi: 10.1093/brain/awr363. [DOI] [PubMed] [Google Scholar]
- Vandermosten M., Boets B., Wouters J., Ghesquière P. A qualitative and quantitative review of diffusion tensor imaging studies in reading and dyslexia. Neurosci. Biobehav. Rev. 2012;36(6):1532–1552. doi: 10.1016/j.neubiorev.2012.04.002. [DOI] [PubMed] [Google Scholar]
- Verhoeven L., van Leeuwe J., Vermeer A. Vocabulary growth and reading development across the elementary school years. Sci. Stud. Read. 2011;15(1):8–25. [Google Scholar]
- Wandell B.A., Yeatman J.D. Biological development of reading circuits. Curr. Opin. Neurobiol. 2013;23(2):261–268. doi: 10.1016/j.conb.2012.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wedeen V.J., Wang R.P., Schmahmann J.D., Benner T., Tseng W.Y.I., Dai G. Diffusion spectrum magnetic resonance imaging (DSI) tractography of crossing fibers. Neuroimage. 2008;41(4):1267–1277. doi: 10.1016/j.neuroimage.2008.03.036. [DOI] [PubMed] [Google Scholar]
- Yeatman J.D., Dougherty R.F., Rykhlevskaia E., Sherbondy A.J., Deutsch G.K., Wandell B.A., Ben-Shachar M. Anatomical properties of the arcuate fasciculus predict phonological and reading skills in children. J. Cogn. Neurosci. 2011;23(11):3304–3317. doi: 10.1162/jocn_a_00061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeatman J.D., Dougherty R.F., Ben-Shachar M., Wandell B.A. Development of white matter and reading skills. Proc. Natl. Acad. Sci. 2012;109(44):E3045–E3053. doi: 10.1073/pnas.1206792109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Tardif T., Shu H., Li H., Liu H., McBride-Chang C. Phonological skills and vocabulary knowledge mediate socioeconomic status effects in predicting reading outcomes for Chinese children. Dev. Psychol. 2013;49(4):665–671. doi: 10.1037/a0028612. [DOI] [PubMed] [Google Scholar]
- Zhao J., Thiebaut de Schotten M., Altarelli I., Dubois J., Ramus F. Altered hemispheric lateralization of white matter pathways in developmental dyslexia: evidence from spherical deconvolution tractography. Cortex. 2016;76:51–62. doi: 10.1016/j.cortex.2015.12.004. [DOI] [PubMed] [Google Scholar]