Abstract
Objective
We aimed to establish a scoring system to predict the risk of breast cancer-related lymphedema.
Methods
From April 2017 to December 2018, 533 patients who previously underwent surgery for breast cancer were enrolled in this cross-sectional study. Univariate analysis was performed to explore and define the risk factors. A scoring system was then established on the basis of odds ratio values in the regression analysis.
Results
The additive scoring system values ranged from 6 to 22. The receiver operating characteristic (ROC) curve of this scoring system showed a sensitivity and specificity of 83.3% and 57.3%, respectively, to predict the risk of lymphedema at a cut-off of 15.5 points; the area under the curve was 0.736 (95% confidence interval: 0.662–0.811), with χ2 = 5.134 (P = 0.274) for the Hosmer–Lemeshow test.
Conclusions
The predictive efficiency and accuracy of the scoring system were acceptable, and the system could be used to predict and screen groups at high risk for breast cancer-related lymphedema.
Keywords: Breast cancer lymphedema, Breast neoplasms, China, Cross-sectional studies, Lymphedema, Risk factors, ROC curve
What is known?
-
•
Breast cancer-related lymphedema (BCRL) remains a challenge globally.
-
•
Published studies on the risk factors for BCRL have mostly explored the risk associated with either treatment-related or non-treatment-related risk factors.
-
•
Previous studies have tried to build risk prediction models or risk assessment systems for BCRL by comprehensively analyzing the relationship between different risk factors and the occurrence of lymphedema.
-
•
The existing studies mainly focused on patient demographic and clinical factors and paid less attention to the postoperative behaviors of patients.
What is new?
-
•
We establish a simple and efficient model to estimate the risk of BCRL based on combinations of demographic, medical, and behavior-related risk factors.
-
•
This scoring system can be used for predicting the risk of BCRL of patients after breast cancer surgery to identify high-risk groups. For high-risk groups, closer monitoring is needed and more accurate screening programs are recommended.
1. Introduction
Breast cancer is the most common cancer among women worldwide [1]. With the development of treatments, the outcomes of breast cancer patients are also improving. However, while these treatment technologies prolong the lifespan, distressing accompanying complications can negatively affect patient quality of life [2]. Breast cancer-related lymphedema (BCRL) is one of the most common and distressing complications among post-operative breast cancer survivors [3], the incidences of which vary from study to study due to different target population, measurement time, etc., which range from 13.5% to 42.0% [[4], [5], [6]]. BCRL is the result of damage to the lymphatic drainage system after removing lymph nodes or/and administering radiation to the axillary lymph nodes [7]. The removal of lymph nodes includes sentinel lymph node biopsy (SLNB) and axillary lymph node dissection (ALND). Generally, damage to the lymphatic system associated with ALND is greater than that of SLNB, which means that postoperative breast cancer survivors who undergo ALND are more likely to develop lymphedema; however, patients who undergo SLNB are also at risk of lymphedema [8].
BCRL can cause upper limb swelling, heaviness, immobility, and pain etc., which significantly negatively affect the quality of life [9,10]. BCRL remains a challenge globally and continues to be a potentially lifelong, irreversible, and incurable complication with chronic and negative physical, psychological, and emotional effects for breast cancer survivors [9,10].
Therefore, prevention should be a focus in BCRL and it is paramount to screen high-risk groups for early detection and early management by identifying the risk factors for developing lymphedema that place breast cancer survivors at a higher risk. Published studies on the risk factors for BCRL have mostly explored the risk associated with either treatment-related or non-treatment-related risk factors. ALND and radiotherapy are the main treatment-related risk factors for BCRL [3]. Some non-treatment-related risk factors for developing lymphedema have also been reported, including high body mass index (BMI) [11,12], lymphedema-related harmful behaviors [13], etc.
However, while each of these factors contributes independently to lymphedema, their combined effects on individual outcome have rarely been reported. Currently, there is no publicly recommended risk assessment model for BCRL. Previous studies have tried to build risk prediction models or risk assessment systems for BCRL by comprehensively analyzing the relationship between different risk factors and the occurrence of lymphedema [[14], [15], [16], [17], [18]]. For example, Bevilacqua et al. [14] developed a nomogram that incorporated age, BMI, radiotherapy fields, level of ALND, and number of cycles of neoadjuvant therapy to predict the risk of arm lymphedema in breast cancer patients after axillary node dissection. Li et al. [19] demonstrated this nomogram to be an accurate and discriminative tool in Chinese breast cancer patients. Wang et al. [18] also developed a scoring system to estimate the risk of lymphedema based on multivariate logistic regression analysis for patients with ALND, and the level of ALND, history of hypertension, surgery on dominant arm, radiotherapy, and surgical infection/seroma/early edema were included in the scoring system as the independent risk factors for developing BCRL.
Nevertheless, the existing studies mainly focused on patient demographic and clinical factors and paid less attention to the postoperative behaviors of patients. Most guidelines [20,21] strongly advise that postoperative patients avoid harmful behaviors such as blood draws, injections, blood pressure measurements, trauma, lifting heavy objects, saunas, etc., to reduce the risk of developing cellulitis and BCRL. However, with an absence of high quality evidence supporting these practices, these recommendations are based on expert opinion and physiologic principles. For example, hospital skin puncture is considered to be related to BCRL, which increases the risk of infection and inflammation of the affected arm, and then may induce or worsen lymphedema; in theory, exposure to extreme cold or heat and excessive pressure can lead to tissue damage that increases the risk of lymphedema [22]. Consequently, we should equally consider the postoperative behaviors of patients and their demographic and clinical factors. Further study is still needed to evaluate whether the performance of these models is impacted by other risk factors such as behaviors.
The main purpose of the present study was to establish a simple and efficient model to estimate the risk of BCRL based on combinations of demographic, medical, and behavior-related risk factors.
2. Methods
2.1. Study design and participants
This cross-sectional study was conducted at a tertiary hospital in Beijing between April 2017 and December 2018. Researchers took responsibility for recruiting the participants. Some were recruited when they returned to the hospital for postoperative re-examination or medication after surgery. Others were recruited by telephone. The latter participants had undergone surgery in this hospital between 2012 and 2016 and returned to the hospital after agreeing to join the study. The inclusion criteria for participants were as follows: (a) women; (b) aged 18 years or older; (c) unilateral breast cancer diagnosed for the first time and finished surgery; (d) completed SLNB or ALND; (e) self-report of no cognitive or communication impairments; (f) willing and able to provide consent. The exclusion criteria were as follows: (a) radiation and/or chemotherapy were needed but not finished; (b) the occurrence of tumor metastasis or recurrence; (c) other conditions that could lead to edema (e.g. renal disease, malnutrition, or congestive heart failure); (d) previous surgery or injury occurred in the affected side’s axilla or on the affected side’s arm. After participant recruitment, two-thirds of the recruited patients were randomly selected by rand() function in Microsoft Office Excel for model establishment; the remaining patients were used for model validation.
The research project was approved by a biomedical ethics committee of the university (IRB00001052-15073). All participants were informed of their confidentiality and voluntary participation. Each participant in this study provided informed written consent before being included.
2.2. Data variables
Demographic and medical information: We applied a self-designed questionnaire to collect demographic and medical information regarding breast cancer and lymphedema. The demographic information included age, height, weight, average household monthly income, education, marital status, employment status and methods of medical payment. The medical information included dominant arm, surgical site, tumor location, tumor staging, pathological type, type of surgery, breast reconstruction, type of axillary lymph node surgery, axillary lymph node status, number of axillary lymph nodes removed, postoperative complications, radiotherapy, chemotherapy, neoadjuvant chemotherapy, hormonal therapy, menstruation status and comorbidities.
Lymphedema-related precautionary behaviors: We used the questionnaire developed by Fu et al. [23]. This self-report checklist is used to assess breast cancer survivors’ practice of precautionary behaviors. The checklist comprises 15 behaviors, including blood draws, injections, blood pressure measurements, trauma, lifting heavy objects suddenly, etc. By choosing either “Yes” or “No” to indicate the presence or absence, each behavior can be considered as a categorical variable.
BCRL status: In this study, lymphedema was defined by circumferential measurements. We used a well-established protocol for sequential circumferential arm measurements to measure the bilateral limbs: at the hand proximal to the metacarpals, the wrist, and every 4 cm from the wrist to the axilla [24]. An increase in arm circumference of 2 cm or more in the ipsilateral arm was considered diagnostic of BCRL [25].
2.3. Data collection
Circumferential measurements were performed according to the protocol by a single researcher to avoid errors between the evaluators and the measurement results were recorded accurately and faithfully. The participants included in the study completed the self-report questionnaires, including demographic information and information on lymphedema-related harmful behaviors. The researchers retrieved and checked information from electronic medical records.
2.4. Data analysis
Analysis was performed with IBM SPSS Statistics for Windows, version 21.0 (IBM Corp., Armonk, N.Y.). Categorical variables were summarized as frequencies and percentages; continuous data were presented as means with standard deviation (SD). Chi-square test and fisher’s exact test were used to perform univariate comparisons of qualitative variables. Significant factors identified in the univariate analysis were entered into the logistic regression model to produce a multivariate regression equation. An additive scoring system of independent factors was then developed on the basis of odds ratio (OR) value to predict the risk of BCRL.
2.5. Establishment and evaluation of the scoring system
Generally, we can build a function based on multivariate logistic regression analysis that can be used to estimate the risk of illness. However, since the regression coefficient is often small and the decimal number large, the memorization and calculation are troublesome and a computer is usually required, which is inconvenient to use. Therefore, for the convenience of calculation and use, a simple scoring system can be established according to the results of the logistic regression analysis [26]. In this study, we established an additive scoring system to predict the risk of BCRL based on the OR value from the logistic regression analysis. The additive scoring system was developed on the basis of the OR value, and the scores of the variables contained in the scoring system were set as their corresponding integral value of the OR value. The total score of all variables can predict the risk of lymphedema in breast cancer survivors. The cut-off values, sensitivity, and specificity of this additive scoring system were determined by receiver operating characteristic (ROC) curve method, the predictive efficiency was assessed by the area under the curve (AUC), and the predictive accuracy was assessed by the Hosmer–Lemeshow test.
3. Results
We enrolled 533 breast cancer survivors. Their mean age was 57.95 ± 11.28 (range: 30–85) years and their mean BMI was 24.89 ± 3.33 (range: 16.44–36.81) kg/m2. Two-thirds of the 533 survivors (355 patients) were used for model establishment and the remaining 178 patients were used for model validation. Of the 355 patients, 102 (28.7%, lymphedema group) developed lymphedema, while 253 (71.3%, non-lymphedema group) did not. Of the 178 patients in the model validation group, 54 (30.3%) and 124 (69.7%) did and did not develop lymphedema. There was no statistically significant difference in the demographic and medical characteristics between the patients for model establishment and patients for model validation.
3.1. Demographic characteristics
Among the 355 patients, except for marital status, there was no statistically significant difference in the demographic characteristics between the two groups. Univariate analysis of the demographic characteristics is shown in Table 1.
Table 1.
Univariate comparisons of demographic characteristics [n (%)].
| Variable | System establishment |
P | System validation |
P | System |
P | |||
|---|---|---|---|---|---|---|---|---|---|
| Lymphedema (N1 = 102) | Non-lymphedema (N2 = 253) | Lymphedema (N3 = 54) | Non-lymphedema (N4 = 124) | Establishment (N5 = 355) | Validation (N6 = 178) | ||||
| Age, years | 0.728 | 0.169 | 0.374 | ||||||
| <45 | 11(10.8%) | 35(13.8%) | 7(13.0%) | 18(14.5%) | 46(13.0%) | 25(14.0%) | |||
| 45–65 | 65(63.7%) | 158(62.5%) | 26(48.1%) | 75(60.5%) | 223(62.8%) | 101(56.7%) | |||
| >65 | 26(25.5%) | 60(23.7%) | 21(38.9%) | 31(25.0%) | 86(24.2%) | 52(29.2%) | |||
| Body mass index, kg/m2 | 0.442 | 0.404 | 0.698 | ||||||
| <28 | 82(80.4%) | 212(83.8%) | 42(77.8%) | 103(83.1%) | 294(82.8%) | 145(81.5%) | |||
| ≥28 | 20(19.6%) | 41(16.2%) | 12(22.2%) | 21(16.9%) | 61(17.2%) | 33(18.5%) | |||
| Average household monthly income, CNY | 0.934 | 0.717 | 0.276 | ||||||
| <2000 | 3(2.9%) | 9(3.6%) | 4(7.4%) | 6(4.8%) | 12(3.4%) | 10(5.6%) | |||
| 2000–5000 | 26(25.5%) | 67(26.5%) | 17(31.5%) | 36(29.0%) | 93(26.2%) | 53(29.8%) | |||
| >5000 | 73(71.6%) | 177(70.0%) | 33(61.1%) | 82(66.1%) | 250(70.4%) | 115(64.6%) | |||
| Education | 0.228 | 0.958 | 0.772 | ||||||
| Primary school | 7(6.9%) | 13(5.1%) | 4(7.4%) | 10(8.1%) | 20(5.6%) | 14(7.9%) | |||
| Middle school | 13(12.7%) | 59(23.3%) | 11(20.4%) | 27(21.8%) | 72(20.3%) | 38(21.3%) | |||
| Senior high school/secondary school | 32(31.4%) | 67(26.5%) | 18(33.3%) | 34(27.4%) | 99(27.9%) | 52(29.2%) | |||
| Junior college | 20(19.6%) | 40(15.8%) | 7(13.0%) | 18(14.5%) | 60(16.9%) | 25(14.0%) | |||
| College degree or above | 30(29.4%) | 74(29.2%) | 14(25.9%) | 35(28.2%) | 104(29.3%) | 49(27.5%) | |||
| Marital status | 0.010 | 0.466 | 0.356 | ||||||
| Married | 90(88.2%) | 242(95.7%) | 53(98.1%) | 117(94.4%) | 332(93.5%) | 170(95.5%) | |||
| Single/divorced/separated | 12(11.8%) | 11(4.3%) | 1(1.9%) | 7(5.6%) | 23(6.5%) | 8(4.5%) | |||
| Employment status | 0.142 | 0.924 | 0.739 | ||||||
| Unemployed | 71(69.6%) | 195(77.1%) | 40(74.1%) | 91(73.4%) | 266(74.9%) | 131(73.6%) | |||
| Employed | 31(30.4%) | 58(22.9%) | 14(25.9%) | 33(26.6%) | 89(25.1%) | 47(26.4%) | |||
| Methods of medical payment | 1.000 | 0.554 | 0.207 | ||||||
| Medical insurance | 98(96.1%) | 244(96.4%) | 54(100.0%) | 121(97.6%) | 342(96.3%) | 175(98.3%) | |||
| Self-supporting | 4(3.9%) | 9(3.6%) | 0(0.0%) | 3(2.4%) | 13(3.7%) | 3(1.7%) | |||
3.2. Medical characteristics
Among the 355 patients used for model establishment, several significant medical risk factors were identified in univariate analysis, including tumor staging (UICC), type of surgery, type of surgery (breast), type of axillary lymph node surgery, axillary lymph node status, number of lymph nodes dissected, subcutaneous hydrops, postoperative infection, early edema on affected arm, neoadjuvant chemotherapy, chemotherapy and radiotherapy (Table 2).
Table 2.
Univariate comparisons of medical characteristics [n (%)].
| Variable | System establishment |
P | System validation |
P | System |
P | |||
|---|---|---|---|---|---|---|---|---|---|
| Lymphedema (N1 = 102) | Non-lymphedema (N2 = 253) | Lymphedema (N3 = 54) | Non-lymphedema (N4 = 124) | Establishment (N5 = 355) | Validation (N6 = 178) | ||||
| Staging (UICC) | 0.004 | 0.001 | 0.545 | ||||||
| 0 | 0(0.0%) | 6(2.4%) | 1(1.9%) | 5(4.0%) | 6(1.7%) | 6(3.4%) | |||
| Ⅰ | 10(9.8%) | 69(27.3%) | 2(3.7%) | 32(25.8%) | 79(22.3%) | 34(19.1%) | |||
| Ⅱ | 55(53.9%) | 126(49.8%) | 28(51.9%) | 66(53.2%) | 181(51.0%) | 94(52.8%) | |||
| Ⅲ | 21(20.6%) | 46(18.2%) | 17(31.5%) | 18(14.5%) | 67(18.9%) | 35(19.7%) | |||
| Data Deficient | 16(15.7%) | 6(2.4%) | 6(11.1%) | 3(2.4%) | 22(6.2%) | 9(5.1%) | |||
| Type of surgery | <0.001 | 0.015 | 0.888 | ||||||
| Breast conserving surgery | 9(8.8%) | 77(30.4%) | 6(11.1%) | 36(29.0%) | 86(24.2%) | 42(23.6%) | |||
| Partial mastectomy | 1(1.0%) | 8(3.2%) | 1(1.9%) | 5(4.0%) | 9(2.5%) | 6(3.4%) | |||
| Total mastectomy | 11(10.8%) | 42(16.6%) | 7(13.0%) | 14(11.3%) | 53(14.9%) | 21(11.8%) | |||
| Modified radical mastectomy | 75(73.5%) | 126(49.8%) | 38(70.4%) | 69(55.6%) | 201(56.6%) | 107(60.1%) | |||
| Radical mastectomy | 5(4.9%) | 0(0.0%) | 2(3.7%) | 0(0.0%) | 5(1.4%) | 2(1.1%) | |||
| Extended radical mastectomy | 1(1.0%) | 0(0.0%) | 0(0.0%) | 0(0.0%) | 1(0.3%) | 0(0.0%) | |||
| Type of Surgery (breast) | <0.001 | 0.013 | 0.761 | ||||||
| Lumpectomy | 9(8.8%) | 77(30.4%) | 6(11.1%) | 35(28.2%) | 86(24.2%) | 41(23.0%) | |||
| Mastectomy | 93(91.2%) | 176(69.6%) | 48(88.9%) | 89(71.8%) | 269(75.8%) | 137(77.0%) | |||
| Type of axillary lymph node surgery | <0.001 | <0.001 | 0.577 | ||||||
| Sentinel lymph nodes biopsy | 2(2.0%) | 69(27.3%) | 1(1.9%) | 31(25.0%) | 71(20.0%) | 32(18.0%) | |||
| Axillary lymph nodes dissection | 100(98.0%) | 184(72.7%) | 53(98.1%) | 93(75.0%) | 284(80.0%) | 146(82.0%) | |||
| Axillary lymph node status | 0.002 | 0.001 | 0.797 | ||||||
| Negative | 15(14.7%) | 89(35.2%) | 6(11.1%) | 49(39.5%) | 104(29.3%) | 55(30.9%) | |||
| Positive (1–4) | 46(45.1%) | 111(43.9%) | 29(53.7%) | 53(42.7%) | 157(44.2%) | 82(46.1%) | |||
| Positive (>4) | 23(22.5%) | 38(15.0%) | 12(22.2%) | 15(12.1%) | 61(17.2%) | 27(15.2%) | |||
| Data Deficient | 18(17.6%) | 15(5.9%) | 7(13.0%) | 7(5.6%) | 33(9.3%) | 14(7.9%) | |||
| Number of lymph nodes dissected | <0.001 | 0.001 | 0.709 | ||||||
| <10 | 4(3.9%) | 67(26.5%) | 2(3.7%) | 33(26.6%) | 71(20.0%) | 35(19.7%) | |||
| 10–20 | 42(41.2%) | 93(36.8%) | 23(42.6%) | 52(41.9%) | 135(38.0%) | 75(42.1%) | |||
| >20 | 38(37.3%) | 78(30.8%) | 22(40.7%) | 32(25.8%) | 116(32.7%) | 54(30.3%) | |||
| Data Deficient | 18(17.6%) | 15(5.9%) | 7(13.0%) | 7(5.6%) | 33(9.3%) | 14(7.9%) | |||
| Subcutaneous hydrops | 0.001 | 0.781 | 0.627 | ||||||
| No | 89(87.3%) | 244(96.4%) | 51(94.4%) | 114(91.9%) | 333(93.8%) | 165(92.7%) | |||
| Yes | 13(12.7%) | 9(3.6%) | 3(5.6%) | 10(8.1%) | 22(6.2%) | 13(7.3%) | |||
| Postoperative infection | 0.003 | 0.016 | 0.641 | ||||||
| No | 93(91.2%) | 249(98.4%) | 48(88.9%) | 122(98.4%) | 342(96.3%) | 170(95.5%) | |||
| Yes | 9(8.8%) | 4(1.6%) | 6(11.1%) | 2(1.6%) | 13(3.7%) | 8(4.5%) | |||
| Early edema on affected arm | <0.001 | 0.016 | 0.137 | ||||||
| No | 76(74.5%) | 241(95.3%) | 40(74.1%) | 111(89.5%) | 317(89.3%) | 151(84.8%) | |||
| Yes | 26(25.5%) | 12(4.7%) | 14(25.9%) | 13(10.5%) | 38(10.7%) | 27(15.2%) | |||
| Neoadjuvant chemotherapy | <0.001 | 0.541 | 0.722 | ||||||
| No | 79(77.5%) | 232(91.7%) | 48(88.9%) | 106(85.5%) | 311(87.6%) | 154(86.5%) | |||
| Yes | 23(22.5%) | 21(8.3%) | 6(11.1%) | 18(14.5%) | 44(12.4%) | 24(13.5%) | |||
| Chemotherapy | 0.047 | 0.026 | 0.305 | ||||||
| No | 8(7.8%) | 40(15.8%) | 4(7.4%) | 26(21.0%) | 48(13.5%) | 30(16.9%) | |||
| Yes | 94(92.2%) | 213(84.2%) | 50(92.6%) | 98(79.0%) | 307(86.5%) | 148(83.1%) | |||
| Radiotherapy | 0.002 | 0.028 | 0.373 | ||||||
| No | 18(17.6%) | 86(34.0%) | 11(20.4%) | 46(37.1%) | 104(29.3%) | 57(32.0%) | |||
| Yes | 84(82.4%) | 167(66.0%) | 43(79.6%) | 78(62.9%) | 251(70.7%) | 121(68.0%) | |||
3.3. Lymphedema-related precautionary behaviors
Regarding lymphedema-related precautionary behaviors among the 355 patients, except for “use the affected arm to lift or carry heavy objects suddenly”, there were no statistically significant differences between the two groups. The results of the univariate analysis are shown in Table 3.
Table 3.
Univariate comparisons of precautionary behaviors [n (%)].
| Item | System establishment |
P | System validation |
P | ||
|---|---|---|---|---|---|---|
| Lymphedema (N1 = 102) | Non-lymphedema (N2 = 253) | Lymphedema (N3 = 54) | Non-lymphedema (N4 = 124) | |||
| 1. Infections in the affected limb | 5(4.9%) | 3(1.2%) | 0.082 | 1(1.9%) | 0(0.0%) | 0.303 |
| 2. Cuts and scrapes in the affected limb | 7(6.9%) | 11(4.3%) | 0.328 | 6(11.1%) | 4(3.2%) | 0.081 |
| 3. Sunburn in the affected limb | 0(0.0%) | 1(0.4%) | 1.000 | 0(0.0%) | 0(0.0%) | |
| 4. Scald by hot oil or steam in the affected limb while cooking | 9(8.8%) | 23(9.1%) | 0.937 | 7(13.0%) | 10(8.1%) | 0.307 |
| 5. Mosquito bites in the affected limb | 67(65.7%) | 143(56.5%) | 0.112 | 33(61.1%) | 72(58.1%) | 0.704 |
| 6. Get scratched by pet in the affected limb | 0(0.0%) | 5(2.0%) | 0.351 | 0(0.0%) | 2(1.6%) | 1.000 |
| 7. Measure blood pressure in the affected limb | 16(15.7%) | 58(22.9%) | 0.129 | 14(25.9%) | 24(19.4%) | 0.325 |
| 8. Blood draws/acupuncture/injections in the affected limb | 15(14.7%) | 48(19.0%) | 0.341 | 12(22.2%) | 22(17.7%) | 0.485 |
| 9. Trauma in the affected limb | 1(1.0%) | 2(0.8%) | 1.000 | 0(0.0%) | 0(0.0%) | |
| 10. Use the affected arm to lift or carry heavy objects suddenly | 26(25.5%) | 26(10.3%) | <0.001 | 13(24.1%) | 15(12.1%) | 0.044 |
| 11. Use the affected shoulder to carry shoulder bags | 12(11.8%) | 45(17.8%) | 0.162 | 2(3.7%) | 23(18.5%) | 0.009 |
| 12. Use the affected arm to hold babies | 6(5.9%) | 8(3.2%) | 0.373 | 0(0.0%) | 3(2.4%) | 0.554 |
| 13. Exposure to heat, such as hot tubs or saunas | 4(3.9%) | 8(3.2%) | 0.973 | 4(7.4%) | 7(5.6%) | 0.912 |
| 14. Cut cuticles in the affected limb | 13(12.7%) | 31(12.3%) | 0.899 | 3(5.6%) | 21(16.9%) | 0.041 |
| 15. Air travel | 48(47.1%) | 93(36.8%) | 0.073 | 23(42.6%) | 45(36.3%) | 0.426 |
3.4. Multivariable logistic regression analysis
Significant variables in univariate analysis were entered in a logistical regression analysis (Forward: LR). The result of this analysis is shown in Table 4. The included variables were type of surgery (breast) (lumpectomy = 1, mastectomy = 2), type of axillary lymph node surgery (SLNB = 1, ALND = 2), early edema on affected arm (no = 1, yes = 2), neoadjuvant chemotherapy (no = 1, yes = 2), radiotherapy (no = 1, yes = 2), and “use the affected arm to lift or carry heavy objects suddenly” (no = 1, yes = 2).
Table 4.
Multivariate logistic regression analysis testing for independent effects.
| Predictor variable | β | S.E. | Wald | P | OR | 95% CI |
|---|---|---|---|---|---|---|
| Type of Surgery (breast) | 1.063 | 0.433 | 6.035 | 0.014 | 2.895 | (1.240, 6.758) |
| Lumpectomy | ||||||
| Mastectomy | ||||||
| Type of axillary lymph node surgery | 2.056 | 0.750 | 7.510 | 0.006 | 7.811 | (1.796, 33.979) |
| SLNB | ||||||
| ALND | ||||||
| Early edema on affected arm | 1.551 | 0.409 | 14.401 | <0.001 | 4.718 | (2.117, 10.514) |
| No | ||||||
| Yes | ||||||
| Neoadjuvant chemotherapy | 0.744 | 0.367 | 4.114 | 0.043 | 2.104 | (1.025, 4.317) |
| No | ||||||
| Yes | ||||||
| Radiotherapy | 0.804 | 0.364 | 4.870 | 0.027 | 2.234 | (1.094, 4.563) |
| No | ||||||
| Yes | ||||||
| Use the affected arm to lift or carry heavy objects suddenly | 0.819 | 0.403 | 4.135 | 0.042 | 2.268 | (1.030, 4.995) |
| No | ||||||
| Yes |
Note: ß = ß-coefficient, S.E. = standard error, OR = odds ratio, CI = confidence interval, SLNB = Sentinel lymph nodes biopsy, ALND = Axillary lymph nodes dissection.
3.5. Scoring system
Based on the results of the multivariable logistic regression analysis, an additive scoring system was designed to predict the individual risk and probability of BCRL after operation among breast cancer survivors. The possible total scores of the additive scoring system ranged from 6 to 22. The variables and their corresponding scores in the additive scoring system are shown in Table 5.
Table 5.
Additive scoring system.
| Predictor variable | OR | Additive Points |
|---|---|---|
| Type of Surgery (breast) | 2.895 | |
| Lumpectomy | 1 | |
| Mastectomy | 3 | |
| Type of axillary lymph node surgery | 7.811 | |
| SLNB | 1 | |
| ALND | 8 | |
| Early edema on affected arm | 4.718 | |
| No | 1 | |
| Yes | 5 | |
| Neoadjuvant chemotherapy | 2.104 | |
| No | 1 | |
| Yes | 2 | |
| Radiotherapy | 2.234 | |
| No | 1 | |
| Yes | 2 | |
| Use the affected arm to lift or carry heavy objects suddenly | 2.268 | |
| No | 1 | |
| Yes | 2 |
Note: SLNB = Sentinel lymph nodes biopsy, ALND = Axillary lymph nodes dissection.
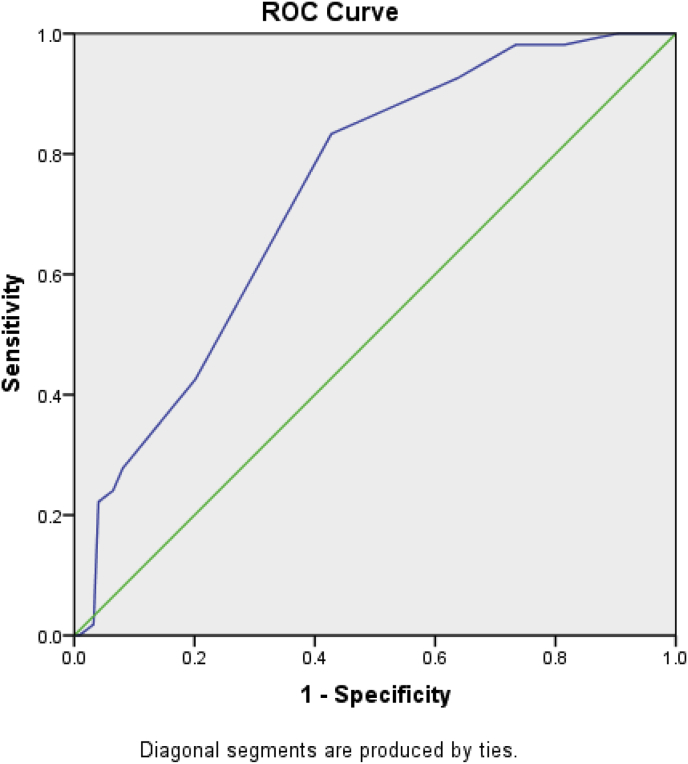
The ROC curve corresponding to the scoring system is shown in Fig. 1. For this additive scoring system, the AUC was 0.736 (95% CI = 0.662–0.811). For a cut-off value of 15.5 with the highest Youden’s index, the sensitivity was 83.3% and the specificity was 57.3%. These values indicate that the scoring system had a good discrimination ability. The result of the Hosmer–Lemeshow test was χ2 = 5.134 (P = 0.274), indicating the good fit of the model.
Fig. 1.
Receiver operating characteristic curve corresponding to the additive scoring system. Diagonal segments are produced by ties.
4. Discussion
This study applied multivariate logistic regression analysis to estimate the combined effects of demographic, medical, and behavioral-related variables to obtain a succinct set of vital and independent risk factors to include in the scoring system. Our scoring system to predict the risk of BCRL in breast cancer patients has been validated to be discriminative and accurate. The scoring system is based on multivariable statistical approaches and shares similar benefits with those of other prediction models; additionally, this tool does not require a computer, calculator, or complex statistical processing, making it convenient to obtain a predictive score.
Our study included mastectomy, ALND, early edema on the affected arm, neoadjuvant chemotherapy, radiotherapy and “use the affected arm to lift or carry heavy objects suddenly” as the independent risk factors for developing BCRL.
Well-defined risk factors for developing lymphedema include ALND, mastectomy and radiotherapy [3]. ALND is the most heavily weighted factor in scoring system and has also been identified as an independent predictor of BCRL in a prospective cohort study [3]. Lymph node removal is one of the main risk factors for BCRL. Over the past decade, the prevalence of BCRL has decreased with the practice of SLNB; however, ALND is still required for some breast cancer patients with positive axillary lymph nodes, in whom lymphedema continues to be a considerable problem. As for mastectomy, previous studies have shown the same results. A randomized trial of Ozcinar et al. [27] and a meta-analysis of Tsai et al. [28] showed that the incidence of lymphedema in patients with mastectomy was higher than that in patients with breast conserving surgery. Radiotherapy has been acknowledged as the risk factor of BCRL, which is due to the adverse effects of radiotherapy on the skin and subcutaneous tissues in the irradiated areas. Numerous studies have shown that radiotherapy does increase the risk of lymphedema [17,29].
Whether neoadjuvant chemotherapy is a risk factor for BCRL has not been determined. It has been suggested that neoadjuvant chemotherapy could, in theory, decrease BCRL incidence by reducing the number of positive lymph nodes [8]. In the study of Kim et al. [6], neoadjuvant chemotherapy was not found to be a significant risk factor associated with BCRL. More studies are needed to define the role of neoadjuvant chemotherapy in BCRL risk.
It remains undetermined as to why, among breast cancer survivors with comparable demographics and treatment-related characteristics, some patients develop BCRL while others do not. This variation has stimulated speculation on lymphedema-related precautionary behaviors.
Owing to postoperative health education and patients’ adherence to recommendations on precautionary behaviors, the prevalence of most precautionary behaviors was low, such as sunburn, trauma, injections, ipsilateral blood draws, etc. Mosquito bites in the affected limb were most common because they were not easy to avoid; however, they generally have little effect on patients and there is little relevant research demonstrating it as a risk factor of BCRL. The prevalence of air travel was in the second place. Air travel has received relatively little attention; thus, its incidence rate was relatively high. Theoretically, air travel can have a harmful effect on lymphedema. Changes in cabin pressure during an airplane’s ascent and descent and the relatively low cabin pressure at high altitude are assumed to contribute to this problem [30]. However, the indications from the published literatures are contradictory [11,[31], [32], [33]].
In the present study, among such behaviors, only “use the affected arm to lift or carry heavy objects suddenly” was associated with lymphedema. This may due to the affected limb muscles’ excessive tension caused by excessive use, which breaks the balance of lymphatic return and then induces lymphedema [34].
Precautionary behaviors in relation to lymphedema risk remains controversial, and this uncertainty influences the decision-making of those at risk of lymphedema or those with lymphedema in contrasting ways. Further research is required to determine whether they can exacerbate lymphedema in postoperative breast cancer patients.
Screening, early detection, and treatment referral are of great importance to reduce lymphedema-related morbidity. Screening programs for BCRL, such as tape measurement of arm circumferences and bioimpedance spectroscopy, are inaccessible due to their relatively high costs (including human and material resources), time, and money. If resources are limited, screening could be aimed primarily at groups at high risk for BCRL for optimal outcome and cost-effectiveness. Consequently, the identification of high-risk groups is important. A predictive scoring system incorporating several risk factors of BCRL would be quantitative and helpful. When screening programs are unavailable, the scoring system can be used as a quick and convenient screening tool. Therefore, this scoring system can be used for predicting the risk of BCRL of patients after breast cancer surgery to identify high-risk groups. For high-risk groups, closer monitoring is needed and more accurate screening programs are recommended. A patient identified as having signs of BCRL should be referred to a qualified and professional clinician, such as a certified lymphedema physical therapist, an occupational therapist, or a rehabilitation physician. Nurses who are involved in the care of cancer patients play important roles. They must collaborate across service lines and across disciplines to implement screening, early education, and early referral to lymphedema clinicians for all at-risk populations [35].
5. Study limitations
Due to the limitation of research time, this study was conducted in only one hospital in China and a convenience sampling method was used to recruit the participants, which may have limited the generalizability of this additive scoring system. In addition, data were collected retrospectively. Prospective research methods could be adopted in future studies to comprehensively collect factors related to BCRL and multi-center studies can ensure the extensibility of the model. And, the duration of post-operation was not included in the study. Despite these limitations, we consider that this additive scoring system could be used by physicians as a convenient tool to estimate the individual risk of BCRL.
6. Conclusions
In conclusion, an additive scoring system using combinations of risk factors was proposed to estimate the probability of lymphedema. Its predictive efficiency and accuracy were shown to be good, indicating that this scoring system could be used to predict risk and screen high-risk groups for BCRL. This system may help promote the screening, detection, and management of lymphedema.
Funding
This study was supported by Nursing Research Grant of Peking University Health Science Center (BMU20160517).
CRediT authorship contribution statement
Fenglian Li: Conceptualization, Investigation, Formal analysis, Writing - original draft, Writing - review & editing. Qian Lu: Conceptualization, Resources, Writing - review & editing, Supervision, Project administration, Funding acquisition. Sanli Jin: Investigation, Writing - review & editing. Quanping Zhao: Resources, Writing - review & editing. Xueying Qin: Writing - review & editing. Shuai Jin: Writing - review & editing. Lichuan Zhang: Writing - review & editing.
Conflicts of interest
The authors declare no conflict of interest.
Acknowledgments
We thank the breast surgery group of Peking University People’s Hospital for permitting access to review the medical records of breast cancer patients. We are also grateful to all those who participated in the study.
Footnotes
Peer review under responsibility of Chinese Nursing Association.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ijnss.2019.12.007.
Contributor Information
Fenglian Li, Email: lifenglian1003@126.com.
Qian Lu, Email: luqian@bjmu.edu.cn.
Sanli Jin, Email: jinsanli@bjmu.edu.cn.
Quanping Zhao, Email: zqp030424@sina.com.
Xueying Qin, Email: xueyingqin@bjmu.edu.cn.
Shuai Jin, Email: 934343227@qq.com.
Lichuan Zhang, Email: zhanglichuan@bjmu.edu.cn.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Torre L.A., Islami F., Siegel R.L., Ward E.M., Jemal A. Global cancer in women: burden and trends. Cancer Epidemiol Biomark Prev. 2017;26(4):444–457. doi: 10.1158/1055-9965.EPI-16-0858. [DOI] [PubMed] [Google Scholar]
- 2.Wanchai A., Armer J.M. Effects of weight-lifting or resistance exercise on breast cancer-related lymphedema: a systematic review. Int J Nurs Sci. 2019;6(1):92–98. doi: 10.1016/j.ijnss.2018.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zou L., Liu F.H., Shen P.P., Hu Y., Liu X.Q., Xu Y.Y. The incidence and risk factors of related lymphedema for breast cancer survivors post-operation: a 2-year follow-up prospective cohort study. Breast Canc. 2018;25(3):309–314. doi: 10.1007/s12282-018-0830-3. [DOI] [PubMed] [Google Scholar]
- 4.Ribeiro P.A., Koifman R.J., Bergmann A. Incidence and risk factors of lymphedema after breast cancer treatment: 10 years of follow-up. Breast. 2017;36:67–73. doi: 10.1016/j.breast.2017.09.006. [DOI] [PubMed] [Google Scholar]
- 5.Monleon S., Murta-Nascimento C., Bascuas I., Macia F., Duarte E., Belmonte R. Lymphedema predictor factors after breast cancer surgery: a survival analysis. Lymphatic Res Biol. 2015;13(4):268–274. doi: 10.1089/lrb.2013.0042. [DOI] [PubMed] [Google Scholar]
- 6.Kim M., Park I.H., Lee K.S., Ro J., Jung S.Y., Lee S. Breast cancer-related lymphedema after neoadjuvant chemotherapy. Cancer Res Treat. 2015;47(3):416–423. doi: 10.4143/crt.2014.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Simonavice E., Kim J.S., Panton L. Effects of resistance exercise in women with or at risk for breast cancer-related lymphedema. Support Care Cancer. 2017;25(1):9–15. doi: 10.1007/s00520-016-3374-0. [DOI] [PubMed] [Google Scholar]
- 8.Gillespie T.C., Sayegh H.E., Brunelle C.L., Daniell K.M., Taghian A.G. Breast cancer-related lymphedema: risk factors, precautionary measures, and treatments. Gland Surg. 2018;7(4):379–403. doi: 10.21037/gs.2017.11.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Delgado-Sanz M.C., Garcia-Mendizabal M.J., Pollan M., Forjaz M.J., Lopez-Abente G., Aragones N. Heath-related quality of life in Spanish breast cancer patients: a systematic review. Health Qual Life Outcomes. 2011;9(3):1–10. doi: 10.1186/1477-7525-9-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pusic A.L., Cemal Y., Albornoz C., Klassen A., Cano S., Sulimanoff I. Quality of life among breast cancer patients with lymphedema: a systematic review of patient-reported outcome instruments and outcomes. J Cancer Surviv. 2013;7(1):83–92. doi: 10.1007/s11764-012-0247-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kilbreath S.L., Refshauge K.M., Beith J.M., Ward L.C., Ung O.A., Dylke E.S. Risk factors for lymphoedema in women with breast cancer: a large prospective cohort. Breast. 2016;28:29–36. doi: 10.1016/j.breast.2016.04.011. [DOI] [PubMed] [Google Scholar]
- 12.Wanchai A., Armer J.M., Stewart B.R., Lasinski B.B. Breast cancer-related lymphedema: a literature review for clinical practice. Int J Nurs Sci. 2016;3(2):202–207. [Google Scholar]
- 13.Park J.H., Lee W.H., Chung H.S. Incidence and risk factors of breast cancer lymphoedema. J Clin Nurs. 2008;17(11):1450–1459. doi: 10.1111/j.1365-2702.2007.02187.x. [DOI] [PubMed] [Google Scholar]
- 14.Bevilacqua J.L., Kattan M.W., Changhong Y., Koifman S., Mattos I.E., Koifman R.J. Nomograms for predicting the risk of arm lymphedema after axillary dissection in breast cancer. Ann Surg Oncol. 2012;19(8):2580–2589. doi: 10.1245/s10434-012-2290-x. [DOI] [PubMed] [Google Scholar]
- 15.Basta M.N., Wu L.C., Kanchwala S.K., Serletti J.M., Tchou J.C., Kovach S.J. Reliable prediction of postmastectomy lymphedema: the risk assessment tool evaluating lymphedema. Am J Surg. 2016;213(6):1125–1133. doi: 10.1016/j.amjsurg.2016.08.016. [DOI] [PubMed] [Google Scholar]
- 16.Kim M., Kim S.W., Lee S.U., Lee N.K., Jung S.Y., Kim T.H. A model to estimate the risk of breast cancer-related lymphedema: combinations of treatment-related factors of the number of dissected axillary nodes, adjuvant chemotherapy, and radiation therapy. Int J Radiat Oncol Biol Phys. 2013;86(3):498–503. doi: 10.1016/j.ijrobp.2013.02.018. [DOI] [PubMed] [Google Scholar]
- 17.Kim M., Shin K.H., Jung S.Y., Lee S., Kang H.S., Lee E.S. Identification of prognostic risk factors for transient and persistent lymphedema after multimodal treatment for breast cancer. Cancer Res Treat. 2016;48(4):1330–1337. doi: 10.4143/crt.2015.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang L., Li H.P., Liu A.N., Wang D.B., Yang Y.J., Duan Y.Q. A scoring system to predict arm lymphedema risk for individual Chinese breast cancer patients. Breast Care. 2016;11(1):52–56. doi: 10.1159/000443491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li X., Huang H., Lin Q., Yu Q., Zhou Y., Long W. Validation of a breast cancer nomogram to predict lymphedema in a Chinese population. J Surg Res. 2017;210:132–138. doi: 10.1016/j.jss.2016.11.009. [DOI] [PubMed] [Google Scholar]
- 20.National Lymphedema Network Position statement of the National Lymphedema Network: lymphedema risk reduction practices. 2012. www.lymphnet.org/pdfDocs/nlnriskreduction.pdf Jun 7, 2018, from.
- 21.Fu M.R., Deng J., Armer J.M. Putting evidence into practice: cancer-related lymphedema. Clin J Oncol Nurs. 2014;18(6):68–79. doi: 10.1188/14.CJON.S3.68-79. [DOI] [PubMed] [Google Scholar]
- 22.McLaughlin S.A., Bagaria S., Gibson T., Arnold M., Diehl N., Crook J. Lymphedema risk reduction practices. J Am Coll Surg. 2013;216(3):380–389. doi: 10.1016/j.jamcollsurg.2012.11.004. 511-513. [DOI] [PubMed] [Google Scholar]
- 23.Fu M.R., Axelrod D., Haber J. Breast-cancer-related lymphedema: information, symptoms, and risk-reduction behaviors. J Nurs Scholarsh. 2008;40(4):341–348. doi: 10.1111/j.1547-5069.2008.00248.x. [DOI] [PubMed] [Google Scholar]
- 24.Fu M.R., Ridner S.H., Armer J. Post-breast cancer. Lymphedema: part 1. Am J Nurs. 2009;109(7):48–54. doi: 10.1097/01.NAJ.0000357172.94131.58. 55. [DOI] [PubMed] [Google Scholar]
- 25.Wang H., Shen L., Liu T., Shao P., Dylke E.S., Jia J. Circumference-based criteria for detection of secondary arm lymphedema for Chinese women. Lymphatic Res Biol. 2017;15(3):262–267. doi: 10.1089/lrb.2017.0002. [DOI] [PubMed] [Google Scholar]
- 26.Sullivan L.M., Massaro J.M., D’Agostino R.S. Presentation of multivariate data for clinical use: the Framingham Study risk score functions. Stat Med. 2004;23(10):1631–1660. doi: 10.1002/sim.1742. [DOI] [PubMed] [Google Scholar]
- 27.Ozcinar B., Guler S.A., Kocaman N., Ozkan M., Gulluoglu B.M., Ozmen V. Breast cancer related lymphedema in patients with different loco-regional treatments. Breast. 2012;21(3):361–365. doi: 10.1016/j.breast.2012.03.002. [DOI] [PubMed] [Google Scholar]
- 28.Tsai R.J., Dennis L.K., Lynch C.F., Snetselaar L.G., Zamba G.K., Scott-Conner C. The risk of developing arm lymphedema among breast cancer survivors: a meta-analysis of treatment factors. Ann Surg Oncol. 2009;16(7):1959–1972. doi: 10.1245/s10434-009-0452-2. [DOI] [PubMed] [Google Scholar]
- 29.Ibrahim A.O., Alawad A.A.M. Risk factors and management of breast cancer- related lymphedema. Int J Med. 2015;3(1):38–40. [Google Scholar]
- 30.Gavish I., Brenner B. Air travel and the risk of thromboembolism. Intern Emerg Med. 2011;6(2):113–116. doi: 10.1007/s11739-010-0474-6. [DOI] [PubMed] [Google Scholar]
- 31.Ward L.C., Battersby K.J., Kilbreath S.L. Airplane travel and lymphedema: a case study. Lymphology. 2009;42(3):139–145. [PubMed] [Google Scholar]
- 32.Ferguson C.M., Swaroop M.N., Horick N., Skolny M.N., Miller C.L., Jammallo L.S. Impact of ipsilateral blood draws, injections, blood pressure measurements, and air travel on the risk of lymphedema for patients treated for breast cancer. J Clin Oncol. 2016;34(7):691–698. doi: 10.1200/JCO.2015.61.5948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Co M., Ng J., Kwong A. Air travel and postoperative lymphedema-A systematic review. Clin Breast Canc. 2017;18(1):e151–e155. doi: 10.1016/j.clbc.2017.10.011. [DOI] [PubMed] [Google Scholar]
- 34.Fu M.R., Ridner S.H., Armer J. Post-breast cancer lymphedema: part 2. Am J Nurs. 2009;109(8):34–41. doi: 10.1097/01.NAJ.0000358492.91678.78. 42. [DOI] [PubMed] [Google Scholar]
- 35.Hutchison N.A. Diagnosis and treatment of edema and lymphedema in the cancer patient. Rehabil Nurs. 2018;43(4):229–242. doi: 10.1097/rnj.0000000000000177. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.